Summary
Solid organ transplant remains a life-saving therapy for children with end-stage heart, lung, liver, or kidney disease; however, ∼33% of allograft recipients experience acute rejection within the first year after transplant. Our ability to detect early rejection is hampered by an incomplete understanding of the immune changes associated with allograft health, particularly in the pediatric population. We performed detailed, multilineage, single-cell analysis of the peripheral blood immune composition in pediatric solid organ transplant recipients, with high-dimensional mass cytometry. Supervised and unsupervised analysis methods to study cell-type proportions indicate that the allograft type strongly influences the post-transplant immune profile. Further, when organ-specific differences are considered, graft health is associated with changes in the proportion of distinct T cell subpopulations. Together, these data form the basis for mechanistic studies into the pathobiology of rejection and allow for the development of new immunosuppressive agents with greater specificity.
Keywords: transplantation, pediatric, CyTOF, rejection, allograft
Graphical abstract

Highlights
-
•
CyTOF is used to generate immune profiles of pediatric transplant recipients
-
•
The post-transplant immune profile depends on the allograft type
-
•
Rejection is associated with changes in the proportions of CD8 T cell subpopulations
-
•
Increased CD:45RA−25+5+38− T cells are associated with stable allograft health
Rao et al. perform multilineage, single-cell analysis of the immune composition of pediatric solid-organ transplant recipients. The immune response to an allograft is strongly associated with graft type, and when organ-specific differences are considered, distinct subpopulations of T cells are found to be associated with graft health.
Introduction
For children with end-stage heart, liver, kidney, or lung disease, solid organ transplant confers a significant benefit to both quality and quantity of life. Nearly 3,000 children underwent single-organ transplant in 2021 in the United States. Receiving an allograft confers a new set of challenges, however, as the recipient will also need lifelong immunosuppression to prevent the recipient’s immune system from attacking the donor organ. Maintaining an appropriate degree of immunosuppression remains one of the major challenges in transplant medicine today: too little immunosuppression and the recipient’s immune system will attack the donor organ, leading to acute rejection, while too much immunosuppression leaves the recipient unnecessarily vulnerable to opportunistic infections,1 organ damage,2 and post-transplant lymphoproliferative disorder.3 It is estimated that between 25% and 40% of solid organ transplant recipients will have at least one episode of acute rejection in the first 12 months after transplant.4,5,6,7
The alloimmune response involves both innate and adaptive components, and the cellular populations involved in rejection have been the subject of several excellent recent reviews.8,9,10,11,12 Much of the available data are based on analysis of single lineages or a single allograft type or are taken from adult patients. Here we address this gap in the literature by performing a detailed, multilineage, single-cell analysis of the peripheral blood immune composition of pediatric solid organ transplant recipients with modern high-dimensional proteomic technologies. We constructed immune profiles from pediatric heart, liver, kidney, and small intestine recipients at post-transplant, either with stable graft function or experiencing rejection, by measuring the proportion of 29 subpopulations of peripheral blood mononuclear cells. Our goal was to determine which features of the alloimmune response were conserved across different allograft types during rejection. Using a combination of supervised and unsupervised analyses, we defined the immune features associated with allograft type and the distinct changes in immune composition associated with allograft rejection.
Results
Study population and construction of immune profiles
The experimental approach for this study is shown in Figure 1A. Immune profiles were constructed from 52 pediatric transplant recipients as described in STAR Methods. The “stable” and “rejection” cohorts were demographically similar; the characteristics of the cohorts are given in Table 1. The rejection episodes profiled in this study were treatable; none led to graft loss. The interval between blood collection and diagnosis of rejection differed slightly between allograft types; among heart recipients, the diagnosis of rejection was made on the day of sample collection, likely due to the high use of surveillance endomyocardial biopsies to detect rejection. Among kidney recipients, the interval between collection and diagnosis was 7.3 ± 10.8 days, while for liver recipients, the interval was 11.3 ± 10.8 days (p = 0.080, heart vs. kidney; p = 0.003, heart vs. liver; p > 0.999, kidney vs. liver, Kruskal-Wallis test with Dunn’s correction for multiple comparisons). Of note, we required 12 months of stability before sample collection in our stable cohort to minimize the effects of treatment for a prior rejection episode; therefore, the time between transplant and sample collection was significantly greater in the stable cohort compared with the rejection cohort. Clinical samples were processed as described in the STAR Methods, stained using the CyTOF panel in Table 2, and Figure 1B.
Figure 1.
Experimental approach and sample processing workflow
(A) Patient samples were obtained from a biobank constructed as a part of the Clinical Trials in Organ Transplantation-06 (CTOTC-06) study. Patients were classified as “stable” if they had no evidence of rejection for 12 months before and 12 months after sample collection; patients were classified as “rejection” if they developed biopsy-proven rejection 30 days or less after sample collection.
(B) PBMCs from donors were thawed, “barcoded” with palladium isotopes, stained with a combination of intracellular and extracellular markers, and analyzed on a mass cytometer. Normalized and debarcoded mass cytometer data were analyzed as described in STAR Methods.
(C) For each patient sample, live singlets were identified based on DNA content, event length, and live/dead staining. Twenty-nine subpopulations were identified using previously published combinations of surface and intracellular markers.
(D) The events in the terminally differentiated branches of the tree outlined in (C) from all clinical samples were pooled and used to construct a single-cell UMAP (clustering markers CCR7, CD8, CD45RA, CD25, CD3, CD5, CD4, FOXP3, CD56, CD38, GzmB, CD16, CD19, CD20, CD14, TCRγδ, CD11c, CD25, LAG3) to show the phenotypic relationship between the terminally differentiated populations. Cells are colored by their manually gated population.
(E) Hierarchically clustered heatmap visualizes the relationships between populations and correlations between marker expression. The median expression of each marker used for gating was then assessed for each of the populations identified in (C).
Table 1.
Characteristics of patient samples used in the study
| Stable | Rejection | p value | |
|---|---|---|---|
| Total patients | 24 (46%) | 28 (54%) | – |
| Male gender | 14 (58%) | 16 (57%) | – |
| Heart recipient | 7 (29%) | 11 (39%) | – |
| Liver recipient | 12 (50%) | 9 (32%) | – |
| Kidney recipient | 4 (17%) | 7 (25%) | – |
| Intestine recipient | 1 (4%) | 1 (4%) | – |
| Age at transplant (years) | 6.9 ± 6.8 | 8.7 ± 5.8 | 0.302 |
| Age at collection (years) | 9.3 ± 6.3 | 9.9 ± 6.0 | 0.710 |
| Time post-transplant at collection (days) | 878 ± 446 | 448 ± 450 | 0.001 |
| Time between collection and rejection diagnosis (days) | – | 6.1 ± 9.5 | – |
Table 2.
Antibodies used for CyTOF staining
| Epitope | Tag | Clone | Source | Cat. no. |
|---|---|---|---|---|
| CD45 | 089-Y | HI30 | Standard Biotools | 3089003B |
| CCR6 | 141-Pr | 11A9 | Standard Biotools | 3141014A |
| CD19 | 142-Nd | HIB19 | Standard Biotools | 3142001B |
| CD127 | 143-Nd | A019D5 | Standard Biotools | 3143012B |
| CD69 | 144-Nd | FN50 | Standard Biotools | 3144018B |
| CD4 | 145-Nd | RPA-T4 | Standard Biotools | 3145001B |
| CD8 | 146-Nd | RPA-T8 | Standard Biotools | 3146001B |
| CD11c | 147-Sm | Bu15 | Standard Biotools | 3147008B |
| CD16 | 148-Nd | 3G8 | Standard Biotools | 3148004B |
| CD25 | 149-Sm | 2A3 | Standard Biotools | 3149010B |
| LAG-3 | 150-Nd | 11C3C65 | Standard Biotools | 3150030B |
| CD107a | 151-Eu | H4A3 | Standard Biotools | 3151002B |
| TCRγδ | 152-Sm | 11F2 | Standard Biotools | 3152008B |
| CD45RA | 153-Eu | HI100 | Standard Biotools | 3153001B |
| CD3 | 154-Sm | UCHT1 | Standard Biotools | 3154003B |
| PD-1 | 155-Gd | EH12.2H7 | Standard Biotools | 3155009B |
| CD14 | 156-Gd | HCD14 | Standard Biotools | 3156019B |
| CD27 | 158-Gd | L128 | Standard Biotools | 3158010B |
| FOXP3 | 159-Tb | 259D/C7 | Standard Biotools | 3159028A |
| CD28 | 160-Gd | CD28.2 | Standard Biotools | 3160003B |
| Ki67 | 161-Dy | B56 | Standard Biotools | 3161007B |
| NKp46 | 162-Dy | BAB281 | Standard Biotools | 3162021B |
| CD57 | 163-Dy | HCD57 | Standard Biotools | 3163022B |
| CD45RO | 164-Dy | UCHL1 | Standard Biotools | 3164007B |
| NKG2C | 165-Ho | 134591 | R&D | MAB-138SP |
| NKG2D | 166-Er | ON72 | Standard Biotools | 3166016B |
| CCR7 | 167-Er | G043H7 | Standard Biotools | 3167009A |
| CD40L | 168-Er | 24-31 | Standard Biotools | 3168006B |
| NKG2A | 169-Tm | Z199 | Standard Biotools | 3169013B |
| HLA-DR | 170-Er | L243 | Standard Biotools | 3170013B |
| CD20 | 171-Yb | 2H7 | Standard Biotools | 3171012B |
| CD38 | 172-Yb | HIT2 | Standard Biotools | 3172007B |
| Granzyme B | 173-Yb | GB11 | Standard Biotools | 3173006B |
| CD5 | 174-Yb | UCHT2 | Biolegend | 300602 |
| CCR4 | 175-Lu | L291H4 | Standard Biotools | 3175035A |
| CD56 | 176-Yb | HCD56 | Standard Biotools | 3176001B |
| CD11b | 209-Bi | ICRF44 | Standard Biotools | 3209003B |
Sample quality assessment
Obtaining an accurate assessment of the immune composition of an individual requires that ample numbers of cells remain viable after collection and processing. In immunocompromised children, obtaining sufficient leukocytes is limited by both the relatively smaller volume of blood that can be collected from a child and the lower concentration of leukocytes within the blood due to immunosuppression. In this study, the number of cells that were assayed was highly variable; in 48 of 52 samples, however, at least 10,000 cells were used for analyses (Figure S1A); when the analyses were repeated with exclusion of those samples with less than 10,000 events available, no differences were observed compared with the analysis including all samples. As described in the STAR Methods, we used palladium-based barcoding to minimize differences in staining quality. Low viability after debarcoding can also compromise the analyses, so we examined the percentage viable after debarcoding in each sample. Viability ranged between 92% and 100% for all samples, with an average of 98% (Figure S1B). Together, these data suggest that sufficient numbers of cells survived the collection and processing to facilitate an accurate analysis of immune phenotype.
High-dimensional analysis identifies 29 different immune cell populations
Using an approach similar to that described in Neeland et al.,13 manual gating was used to identify 29 subpopulations of peripheral blood mononuclear cells (PBMCs) using the gating strategy described in Figure 1C. Twenty-eight of these populations have been well described in the literature; the 29th population, hereafter referred to as CD:45RA−25+5+38− cells, is a subpopulation of CD4+ T cells first described in a 2016 study as upregulated in the peripheral blood of operationally tolerant pediatric liver transplant recipients.14 To determine if the relationship between the gated populations in high-dimensional space was consistent with known phenotypic similarities between cell types, the cells in the 23 terminally differentiated branches of the tree outlined in Figure 1C from all clinical samples were pooled and used to construct a single-cell uniform manifold approximation and projection (UMAP). UMAP spatial relationships were consistent within manual CellEngine gating. The major T cell, B cell, natural killer (NK) cell, and monocyte lineages separated in a biologically meaningful manner, and their cell subtype relationships were largely consistent with known biological patterns, such as the separation between lymphoid and myeloid lineages (Figure 1D). Hierarchical clustering of median marker signal intensity for each manually gated population separated lymphoid and myeloid subsets and captured more detailed cell-type relationships between CD4 and CD8 T cells (Figure 1E).
The post-transplant immune profile depends on the allograft type
The goal of this study was to understand those features of the immune response that led to rejection that were common to multiple different allograft types. As such, our patient population included children who had received one of four different types of allografts, either with stable graft function or developing rejection. We used principal-component analysis (PCA), an unsupervised approach to exploratory data analysis, to better understand patterns present in the high-dimensional immune profiles across the entire cohort. We qualitatively observed that, when grouping by allograft health alone, there was overlap between the groups, suggesting that the high-dimensional immune profiles were globally similar (Figure 2A). When grouping by allograft type, the groups showed separation in principal component (PC) space (Figures 2B, S2A, and S2B). PC1 separates immune profiles of heart recipients from kidney and liver recipients, while PC3 separates kidney from liver recipients. We also used permutational multivariate analysis of variance (Permanova) of graft and graft health to statistically assess community-level differences in immune cell-type proportions between graft and graft health. Consistent with our observations using PCA, the immune profiles differed significantly by allograft type (p = 0.001) but not by graft health (p = 0.388). Of note, given the small number of immune profiles from intestinal transplant recipients, these data were excluded from the analysis. Proportion of variance explained by allograft health and allograft type using mixed models showed that the allograft type accounted for a greater proportion of the variance compared with allograft health (Figure S2C).
Figure 2.
The post-transplant immune profile depends on the allograft type
(A) Biaxial plot of PC1 and PC2, colored by graft health.
(B) Biaxial plot of PC1 vs. PC2 and PC1 vs. PC3, colored by graft.
(C) Proportions by lineage for each cell type across the different grafts. Error bars indicate standard deviation.
(D) Hierarchically clustered heatmap of mean cell-type proportions for each graft.
(E) Volcano plot summarizing results of differential proportions analysis between pairs of different grafts (excluding intestine). Cell-type proportions compared using Wilcoxon signed-rank test, corrected for multiple hypotheses with an adjusted p value cutoff at 0.1.
(F) Biaxial plot of LD1 and LD2 from LDA of immune cell-type proportions by graft. Lines indicate cell-type importance for the top contributing immune cell types separating grafts along LD1 and LD2.
(G) Cell-type importance results from LDA in (F) for LD1, LD2, and LD3.
Analysis of the mean and variance (standard deviation) between immune cell proportions for the 23 terminally gated populations between the liver, heart, and kidney recipients (n = 21, 18, and 11, respectively) showed that certain immune cell subpopulations had similar means and variances in proportion across different allograft types (e.g., CD45RA+ regulatory T cells [Tregs] and CD56bright CD16dim NK cells), while others varied significantly in their means and variances (e.g., plasmablasts, memory B cells, and CD4 effector memory (EM) T cells; Figure 2C). To determine whether any of the immune cell subtypes were differentially abundant in an allograft-dependent manner, we analyzed immune cell proportions for 23 terminally gated populations between liver, heart, kidney, and intestinal recipients (n = 21, 18, 11, and 2, respectively) (Figure 2C). Hierarchical clustering of the mean cell-type proportions between each two allograft types showed that the immune compositions of liver and kidney recipients were more like each other than like those of heart recipients (Figure 2D). On average, peripheral blood from heart recipients had greater enrichment of naive B cells, plasmablasts, and γδ T cells and decreased enrichment of CD4 T effector memory cells re-expressing CD45RA (TEMRA) and CD4 EM populations compared with liver and kidney recipients. Peripheral blood from kidney recipients had higher enrichment of CD4 TEMRA, CD8 TEMRA, and memory B populations and decreased enrichment of plasmablasts and CD4 central memory (CM) populations compared with the liver and heart recipients. One possible explanation for these differences in immune composition is the effects of immunosuppression. The induction and immunosuppressive regimens for the patients in the study, separated by allograft type, are shown in Table 3. There were 12 unique combinations of immunosuppressive agents in use at the time of collection across the cohort, with most patients receiving tacrolimus alone, tacrolimus plus mycophenolate, or tacrolimus plus mycophenolate and a corticosteroid. The remaining patients received other unique combinations of immunosuppressive agents (Figure S3A). Hierarchical clustering by immunosuppressive regimen did not reveal an association between regimen and allograft type. Chi-squared analysis of the three major immunosuppressive regimens compared with allograft or graft health did not identify a significant association between immunosuppressive regimen and allograft type (p = 0.1636) or graft health (p = 0.2647). For the nine remaining combinations of immunosuppressive agents in use at the time of collection, the interaction between the regimen and the immune profile could not be assessed statistically given the small numbers in each group.
Table 3.
Comparison of patient characteristics by allograft type
| Allograft type |
p value |
|||||
|---|---|---|---|---|---|---|
| Heart (18) | Kidney (11) | Liver (21) | Heart vs. kidney | Heart vs. liver | Kidney vs. liver | |
| Immunosuppression | ||||||
| Tacrolimus | 88.9% | 90.9% | 85.7% | – | – | – |
| MMF | 72.2% | 72.7% | 33.3% | – | – | – |
| Cyclosporine | 5.6% | 9.1% | 4.8% | – | – | – |
| Azathioprine | 5.6% | 0.0% | 0.0% | – | – | – |
| Cyclophosphamide | 0.0% | 0.0% | 0.0% | – | – | – |
| mTOR inhibitor | 5.6% | 0.0% | 9.5% | – | – | – |
| Corticosteroid | 33.3% | 18.2% | 9.5% | – | – | – |
| Methylprednisolone | 0.0% | 9.1% | 0.0% | – | – | – |
| Other | 16.7% | 0.0% | 4.8% | – | – | – |
| Induction medication | ||||||
| ATG (thymoglobulin) | 33% | 55% | 10% | – | – | – |
| Basiliximab | 11% | 9% | 5% | – | – | – |
| Methylprednisolone | 22% | 36% | 52% | – | – | – |
| IVIg | 17% | 0% | 0% | – | – | – |
| None | 17% | 0% | 33% | – | – | – |
| Age at collection (days) | 3,962 ± 2,345 | 3,819 ± 2,508 | 2,928 ± 2,033 | 0.985 | 0.335 | 0.543 |
| Time post-transplant (days) | 693 ± 499 | 551 ± 315 | 614 ± 487 | 0.703 | 0.857 | 0.928 |
With respect to induction therapy, most patients across all allograft types received ATG (thymoglobulin) or methylprednisolone (Table 3). We performed a proportion of variance analysis with immunosuppressive regimens and induction agents included alongside allograft type and allograft health; this demonstrated that allograft type remains the greatest contributor to variance (Figure S3B), particularly within the lymphocyte compartment (Figure S3C). There were specific T cell and NK cell populations that varied with the immunosuppressive regimen that did not vary with allograft type or allograft health. Interestingly, while CD45RA+ Tregs varied the most with the immunosuppressive regimen, the CD45RA− Tregs and CD:45RA−25+38− cells were stable. In addition, CD:45RA−25+38− were the most varied population with respect to allograft health, with subtle relative variance associated with allograft type and the induction agent. The induction agent seemed to be broadly associated with both memory and effector lymphocyte populations, classical monocytes, non-classical monocytes, and intermediate monocytes.
Patient age was not a confounding variable for these differences across cohorts, as (1) each allograft cohort had patients from a wide variety of age groups and (2) there were not significant differences in the ages of patients across cohorts (Table 3).
Given that we observed differences in the high-dimensional immune compartments of different allograft types, we performed statistical analyses to determine whether any immune cell subtypes were differentially abundant in an allograft-dependent manner. Specifically, we asked whether there were statistically significant differences in the proportions of any immune populations between two graft types. Indeed, cells in the lymphocyte compartment were significantly differentially abundant in an allograft-dependent manner, even after multiple hypothesis correction using the false discovery rate (Figure 2E). Specifically, plasmablasts, naive B cells, CD4 TEMRA, CD4 EM, and γδ T cells were differentially abundant when comparing heart with liver recipients, while CD4 TEMRA, naive B cells, and plasmablasts were differentially abundant when comparing heart with kidney recipients. Consistent with the hierarchical clustering, most cell-type proportions were differentially abundant in the heart compared with the liver and kidney.
Correlation analysis of the immune cell compartments also showed that the correlative relationships between immune cell-type proportions in each allograft were also different (Figure S2D). Specifically, we found that the magnitude and direction of the correlative structure between cell-type proportions differed between allograft type. For example, among kidney recipients, the proportion of peripheral CD4 naive cells was positively correlated with the proportion of CD4 CM, plasmablasts, and naive B cells; however, in heart or liver recipients, the abundance of these same populations was either weakly positively correlated or negatively correlated.
Our initial differential abundance analysis comparing grafts in a pairwise manner found larger differences between the post-transplant peripheral immune compartment of heart recipients compared with the liver and kidney recipients. Both the correlation analysis and the PCA suggest that there may be differences in the immune compartment of the liver and kidney recipients as well. We performed supervised dimensionality reduction using linear discriminant analysis (LDA) to further assess differences in the post-transplant peripheral immune compartment of these grafts by inputting the immune cell-type proportions for all patients (Figures 2F and S2E). LDA is a classification algorithm that identifies linear combinations of features that optimally separate previously determined class labels—such as allograft types—and has been used in both single-cell and clinical trial analysis.15,16 We exploited the inherent dimensionality reduction of LDA to visualize differences between samples. Indeed, the post-transplant peripheral immune compartment of each allograft type was strongly separated by LDA, corroborating that the immune cell-type proportions of patients with these grafts were indeed different. LDA reduces multiple cell types into linear discriminants (LDs), and each cell type is given a coefficient that has a magnitude and direction associated with the LD. The magnitude and direction of the coefficient indicates which cell types are the strongest drivers of differences between allograft types. LD2 adequately separates kidney from heart and liver recipients, while LD1 additionally separates heart from liver recipients. Consistent with our differential expression analysis, lymphocyte subpopulations were some of the strongest contributors to both LD1 and LD2, including naive B cells, CD4 TEMRA cells, CD4 EM cells, CD8 EM cells, and CD8 CM cells (Figure 2G). The CD:45RA−25+5+38− subset of CD4+ T cells was also a strong contributor to LD2, suggesting that these cells may also have differential abundance in an allograft-dependent manner.
We additionally performed a follow-up random sampling analysis to ascertain whether this allograft-dependent result was due to random patient-level heterogeneity using sparseLDA, an optimized version of LDA for datasets with small sample sizes and many features (e.g., cell types). Indeed, sparseLDA of 1,000 random samples analyzing three groups of size equal to those of the heart, kidney, and liver recipients failed to separate the three randomly sampled groups compared with the ground-truth allograft analysis (Figure S4); thus, this allograft-dependent difference in the post-transplant immune compartment of these patients was not due to random patient-level heterogeneity. This comprehensive analysis using both unsupervised and supervised methods to study cell-type proportions indicates that there are cell types in the post-transplant immune compartment of pediatric heart, kidney, and liver recipients that differ in an allograft-dependent manner.
Rejection is associated with changes in proportions of distinct PBMC subpopulations
Given the well-described role of T cells in the pathogenesis of allograft rejection, we began with an analysis of the percentage of cycling T cells based on Ki67 expression; cycling T cells were relatively rare in the peripheral blood, with more than 60% of the samples having fewer than 100 cycling cells in any given T cell subpopulation measured, so no further exploration of this idea was performed (data not shown). To address whether there were differences in marker expression across cell types between stable and rejecting patients, i.e., evaluation of GzmB expression as a marker of cytotoxic activity, we performed differential marker expression analysis. Instead of multiple hypothesis correction, we used a less stringent unadjusted p value cutoff of 0.01. Even with this less stringent cutoff, features that had large fold change differences were not significant (e.g., CD57 expression in CD4 CM cells), and features that passed the statistical cutoff did not pass fold-change cutoffs (e.g., CD69 in CD4 naive cells) (Figure S5). Unsupervised analysis by PCA showed no separation between stable and rejecting patients (Figure 2A). However, given that we saw allograft-level differences between many of these subpopulations, and that there were differences in immunogenicity across organs, we hypothesized that the abundance of peripheral immune cells as markers of allograft stability or rejection may be masked by basal differences in cell-type proportions between patients with different allograft types. For example, if the proportion of a specific cell type is generally higher among heart recipients than other allograft types but also changes with allograft health, then a traditional statistical test comparing average cell-type abundance in stable vs. rejecting patients will not capture this biology. Linear mixed models can be used to partition the variance of each feature (e.g., cell type) across experimental or clinical conditions and summarize the contribution of each cell type in terms of the fraction of variation explained (FVE).17,18 To account for this, we applied linear mixed models to measure the variance of each cell type’s proportions associated with allograft type and allograft health in our cohort of transplant patients. We found that the variance of lymphocyte populations was associated with allograft type, allograft health, or both (Figure 3A). This was consistent with our prior differential abundance analysis, where the cell-type proportions of several populations, including plasmablasts, CD4 EMs, CD4 TEMRA, and naive B cells, were significantly different between allograft types (Figure 2E). Interestingly, the total variance in cell-type proportions was more strongly associated with the allograft type than with allograft health (Figure S2C). We also found that CD8 naive, CD8 CM, and CD:45RA−25+5+38− cells were specific populations with the largest variance associated with allograft health relative to other cell types (Figure 3A). Hierarchical clustering of the mean cell-type proportion across all allograft types and allograft health states also showed that the post-transplant peripheral immune compartments of different grafts were more similar within each allograft type regardless of allograft health status (Figure 3B). Interestingly, CD:45RA−25+5+38− cell abundance was associated with both allograft type and allograft health, and the average CD:45RA−25+5+38− cell proportion was higher in patients with stable graft function (compared with those experiencing rejection) within each allograft type. Given that CD8 naive, CD8 CM, and CD:45RA−25+5+38− cells were populations whose abundance was associated with allograft health, we performed differential abundance analysis between the stable and the rejection cohorts in these three major populations. We implemented generalized linear models (GLMs), which have been commonly used in both clinical trial and single-cell analysis to compare the stable cohort with the rejection cohort, controlling for basal differences in cell-type abundance between allograft types.19,20,21 The advantage of this approach is that it allows us to leverage all samples in the same statistical test, improving statistical power. The proportions of CD8 naive, CD8 CM, and CD:45RA−25+5+38− cells were all significantly associated with allograft health (Figure 3C). Increased CD:45RA−25+5+38− cell proportion was positively associated with stable grafts, while increased CD8 naive and CD8 CM proportion was positively associated with rejecting grafts. Increased CD:45RA−25+5+38− cell proportion was also significantly associated with graft health with cell-type annotations derived with unsupervised clustering (p < 0.05) using a simple lasso-regularized logistic regression model of cell type without interaction effects (Figure 3D). We sought to further ascertain whether these cell populations were directly associated with allograft health using logistic regression models comparing the stable cohort to the rejection cohort. First, we performed a lasso-regularized logistic regression model comparing the stable to the rejection cohort across all gated populations (data not shown). The final cell types selected as important in reducing the prediction error of allograft health included CD8 naive and CD:45RA−25+5+38− cells, with coefficients of approximately −4 and +2, respectively. Classical monocytes were also selected as an important predictor; however, the coefficient was less than 0.1, suggesting that, while monocytes were selected by the model, it added negligible predictive power to the model in comparison to other selected cell types. We also performed a leave-one-out-cross-validation (LOOCV) logistic regression model comparing the stable cohort with the rejection cohort using only the CD8 naive, CD8 CM, and CD:45RA−25+5+38− cell-type abundances that were significantly associated with allograft health (data not shown). We achieved 62.5% accuracy in correctly predicting stability or rejection. Predictions better than a coin flip (62.5% > 50%) suggests these populations to be important for allograft health. These modeling results further support the importance of CD:45RA−25+5+38− cells as well as CD8 naive and CD8 CM abundance in determining allograft health in the post-transplant immune system of pediatric transplant recipients.
Figure 3.
Rejection is associated with changes in proportions of distinct lymphocyte subpopulations
(A) Biaxial plot showing results of linear mixed modeling analysis to calculate the fraction of variance explained by graft and graft health. Color indicates cell-type lineage.
(B) Hierarchically clustered heatmap of mean cell-type proportions across each clinical subset of graft and graft health. Boxes highlight key populations from (A).
(C) Confidence intervals for statistically comparing cell-type populations that vary with graft health using generalized linear models: p ≤ 0.05.
(D) Selected cell-type results from a lasso-regularized logistic regression model.
CD:45RA−25+5+38− cells have a phenotype between that of effector T cell and regulatory T cell subpopulations
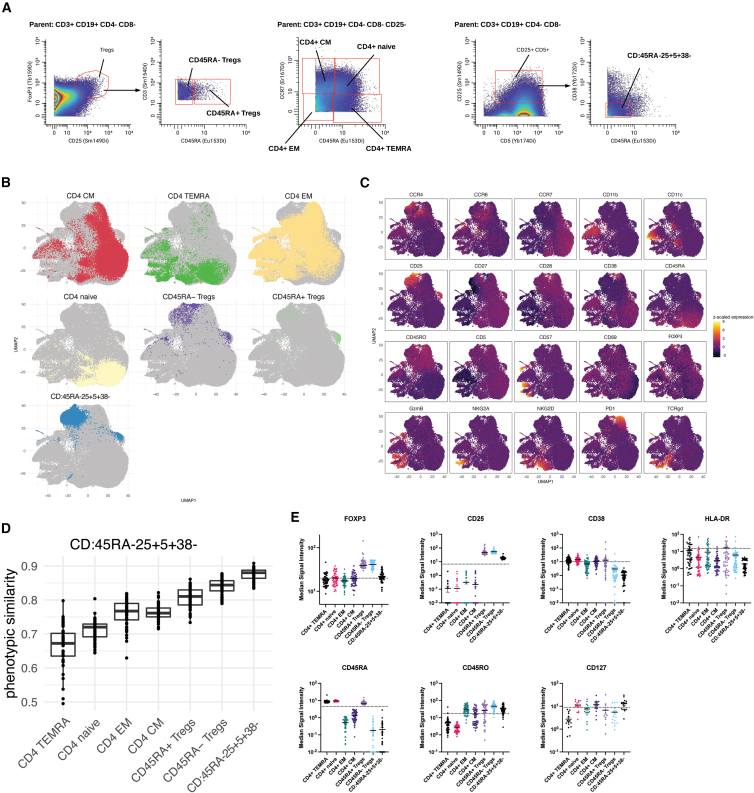
We had previously observed that CD:45RA−25+5+38− cells are more abundant in operationally tolerant liver transplant patients, i.e., liver transplant patients who maintain stable allograft function even while off immunosuppression.14 This raises the possibility that CD:45RA−25+5+38− cells have some inherent immunosuppressive function, like Tregs. Because of the small number of cells available in these patient samples, isolation of CD:45RA−25+5+38− cells for in vitro functional characterization was not feasible. As an alternate means to gain insight into the identity of CD:45RA−25+5+38− cells, we compared the phenotype of CD:45RA−25+5+38− cells with the six other CD4+ subpopulations queried in this study (Figure 1C) defined by the gating strategy shown in Figure 4A.22 The events in the seven CD4+ subpopulations from all clinical samples were pooled and used to construct a single-cell UMAP with the subset of markers in the panel known to be expressed on CD4+ T cells faceted by each of the seven CD4+ subpopulations (Figure 4B). CD:45RA−25+5+38− cells were spatially proximate to the CD45RA− Tregs, indicating phenotypic similarity to this subpopulation. We then examined the expression of each of the CD4+ T cell markers used to construct the UMAP across all the CD4+ populations. CD:45RA−25+5+38− cells had high expression of CD25 and CD45RO, had low expression of FOXP3, and did not appear to express CD45RA, PD-1, or CD38 (Figure 4C).
Figure 4.
CD:45RA−25+5+38− cells have phenotypic similarities to regulatory T cells
(A) Gating strategy used to define key subpopulations of CD4+ T cells.
(B) The events in the seven CD4+ T cell subpopulations outlined in (A) from all clinical samples were pooled and used to construct a single-cell faceted UMAP using markers known to be expressed on CD4+ T cells (CCR4, CCR6. CCR7, CD11b, CD11c, CD25, CD27, CD28, CD38, CD45RA, CD45RO, CD5, CD57, CD69, FOXP3, GzmB, NKG2A, NKG2D, PD1, and TCRγδ) to show the phenotypic relationship between these CD4+ subpopulations.
(C) A faceted UMAP using the same events in (B) shows the expression levels of various T cell markers.
(D) Cosine similarity analysis of CD4+ subpopulations identified in (A).
(E) The median signal intensity of selected markers for each of the CD4+ subpopulations identified in (A).
To quantitatively measure the phenotypic similarity between CD:45RA−25+5+38− cells and the other CD4+ populations, we performed cosine similarity analysis.23 High-dimensional phenotypic (dis)similarity was estimated as a cosine distance to an average cell in each CD4+ population for each sample. Populations that are like one another will have a cosine similarity metric closer to 1, and those that are dissimilar will have a cosine similarity metric closer to 0. The CD:45RA−25+5+38− populations across all the clinical samples were most like themselves (cosine ∼0.87). The populations that they were next most like (cosine ∼0.83) were the CD45RA− Tregs, and the populations that they were least like (cosine ∼0.65) were the CD4+ TEMRAs (Figure 4D).
We next compared the signal intensity of selected markers relevant to regulatory function or T cell activation between CD:45RA−25+5+38− populations and other CD4+ populations across all clinical samples. On average, CD:45RA−25+5+38− cells express higher levels of two key regulatory T cell markers—FOXP3 and CD25—than other CD4 naive, CM, EM, and TEMRA cells, but not as high as CD45RA+ and CD45RA− Treg cells (Figure 4E). Other T cell markers—CD45RA, CD45RO, and CD38—were expressed at similar levels between CD:45RA−25+5+38− cells and CD45RA− Tregs. Last, like CD4+ naive and CM subsets, CD:45RA−25+5+38− cells have low-level expression of CD127; this is distinct from CD45RA+ and CD45RA− Tregs, which are CD127−. Together, these data suggest that CD:45RA−25+5+38− cells are most phenotypically like the CD45RA−subset of Tregs but have distinct features that make it unlikely that they merely represent a subset of this population.
Discussion
Mass cytometry (CyTOF) was used to construct multilineage immune profiles from a cohort of pediatric heart, liver, kidney, and small bowel transplant recipients. Previous literature on the peripheral blood immune composition of pediatric transplant recipients has been limited to single-lineage or has had limited depth within each subset.24,25,26 This is largely due to technical limitations, and the use of a 37-marker mass cytometry panel allowed a much more detailed characterization at the immune composition. Stenard et al.26 examined Tregs (CD4+CD25hiFOXP3+) in pediatric liver transplant recipients and demonstrated that the percentage of Tregs was significantly lower in patients experiencing rejection compared with those with stable graft function. We observed differential abundance of both subsets of Tregs (CD45RA+ and CD45RA−) in our dataset, with higher levels in stable patients compared with rejecting patients across all allograft types, but the magnitude of the effect was not as pronounced as was observed with the other cell types highlighted in this study and did not reach statistical significance. Schulz-Juergensen et al.25 also looked at subpopulations of peripheral blood immune cells from pediatric liver transplant recipients, comparing abundance of Tregs and subsets of γδ T cells between operationally tolerant recipients and immunosuppression-dependent recipients. They noted higher abundance of Tregs and a higher ratio of Vδ1 to Vδ2 γδ T cells in operationally tolerant patients compared with those who were immunosuppression dependent, suggesting that these populations may promote immune tolerance. A key difference between their study and the one described here is that they were comparing a population receiving immunosuppression with one that was not; in our study, all patients were receiving immunosuppression. De Serres et al.24 looked at the longitudinal effects of alemtuzumab—an induction agent used to prevent rejection—on the abundance of selected immune cell subpopulations in pediatric renal transplant recipients. Among other findings, they report that treatment with alemtuzumab increases the ratio of Tregs to effector memory populations. We have similarly observed that lower levels of effector memory populations are associated with stability across different allograft types. We observed an association between a greater abundance of Tregs and graft stability, although these findings were not statistically significant.
One of the significant findings that emerged from analysis of these data is that the immune composition of the peripheral blood following solid organ transplant is correlated with the type of allograft, with the largest separation occurring between heart recipients and liver or kidney recipients. One possible explanation is that these differences existed prior to transplant. There is some evidence in the literature to support the idea that heart failure, one of the leading indications for heart transplant, is associated with alterations in immune function. For example, patients with heart failure had a higher abundance of NK subsets that expressed granzyme B or fractalkine receptor compared with healthy controls; these patients also had a higher proportion of conventional dendritic cell populations and a lower proportion of classical monocytic populations. Interestingly, no significant changes in the abundance of major T or B cell populations were observed.27 If changes in immune function led to heart failure and these changes persisted after transplant, one might expect the heart failure to recur rapidly, which is not supported by clinical data. Many of the recipients profiled in this study are months or years from transplant, and any effect of organ failure on immune composition should have resolved. Another possible explanation is that thymectomy performed at the time of heart transplant may result in alterations in immune function. It has been reported that children who undergo thymectomy at the time of transplant have lower percentages of total Tregs and naive Tregs but higher percentages of memory CD4+ cells; these patients are also more susceptible to the development of atopic conditions.28 However, the majority of thymic involution occurs by age 2, so thymectomy is not necessary for older children. The patients profiled in our study had a mean age around 7 and included toddlers, school-age children, and adolescents. Therefore, most of these children would not have required thymectomy. The distinct features of the immune composition that we observed in heart transplant recipients spanned a much broader age range than could be explained by the effects of thymectomy alone. A third possible explanation is that the allograft-specific differences result from differences in immunosuppressive treatment. As described above, the similarities in immunosuppressant use between allograft types were not correlated with the similarities in immune composition. Individual patient data on immunosuppressant target levels were not available to us, so the effects of different levels of immunosuppression (i.e., high tacrolimus levels vs. low tacrolimus levels) on immune composition cannot be excluded. Given the relative standardization of immunosuppressive regimens for a given allograft type across transplant centers, it is likely not feasible to separate the effects of immunosuppression from allograft type on immune composition. A fourth possible explanation is that the mere presence of an allograft, due to its inherent immunologic mismatch from the host, induces changes in immune cell populations, regardless of whether rejection is elicited, and that these changes are dependent on the type of allograft implanted. Differential gene expression (or, in this case, differential abundance of immune cell subpopulations) between two different healthy and diseased subjects has been commonly used to identify causes of a disease; however, whether these differences are the cause or the result of the disease can often be difficult to elicit, and newer computational methods are being developed for distinguishing between these two possibilities.29
If the immune response to an allograft is dependent on the type of the graft received, these differences may have significant implications for the development of rejection. There is some evidence in the literature consistent with this idea. For example, rates of rejection vary dramatically depending on allograft type, with the lower rates of rejection occurring in kidney (14%) or liver (27%) transplant recipients and higher rates occurring in heart or intestinal transplant recipients (40%). In addition, operational tolerance—a state wherein allograft health can be maintained without administration of exogenous immunosuppression—has been observed in kidney and liver transplant recipients but only anecdotally in heart or intestinal transplant recipients.30,31,32 We also observed that the immune compositions of kidney and liver recipients were more like each other than either was to heart recipients. The presence of organ-specific differences in post-transplant immune composition highlights the importance of first performing organ-specific analyses when looking at populations important for graft health.
When these organ-specific differences were considered, we observed that there were distinct subpopulations of T cells whose abundance in the peripheral blood was associated with graft health. First, we observed that increased frequency of CD8 naive and CD8 CM populations was associated with the development of allograft rejection. A role for CD8 T cells in the pathogenesis of allograft rejection is well established, but the roles of specific CD8 subpopulations are less well defined, particularly in pediatric transplant recipients. There is evidence that highlights a role for CCR7+CD8+ populations—which include naive CD8 and CD8 CM populations—in the prevention of rejection. In an in vitro study, CCR7+CD8+ cells from healthy humans suppressed proliferation and production of IL-2 and IL-17 by CD4+ T cells stimulated by addition of anti-CD3/CD28 antibodies.33 However, this study also observed a higher abundance of CCR7+CD8+ populations in the peripheral blood of adult kidney transplant recipients with T cell-mediated rejection compared with those with normal biopsies.33 Another study that examined the composition of the peripheral immune compartment in adult kidney transplant recipients reported higher percentages of naive CD8 T cells in patients experiencing biopsy-proven late rejection; a similar trend was seen in the abundance of CD8 CM T cells.34 The relationship between abundance of naive and CM CD8 T cells and allograft health demonstrated here further highlights the importance of these cell types in the pathogenesis of rejection.
An increased frequency of CD:45RA−25+5+38− cells was associated with stable allograft function in our study. CD:45RA−25+5+38− cells were first reported as a subpopulation of CD4+ T cells in 2016 using machine learning approaches to identify novel combinations of surface markers expressed on PBMCs in pediatric liver transplant patients.14 Importantly, combination of cell surface markers expressed by CD:45RA−25+5+38− cells had not previously been described and was identified only by using many cellular markers in combination with unsupervised analyses. This highlights the importance of performing high-dimensional analyses on patient samples and interrogating the data with unsupervised algorithms to truly appreciate the complexity of the immune response to an allograft.
CD:45RA−25+5+38− cells make up a significant portion of the CD4+ compartment (5%–10%); a higher frequency of CD:45RA−25+5+38− cells is associated with operational tolerance, the ability of an individual to maintain stable allograft function without exogenous immunosuppression, in pediatric liver transplant recipients.14 Our findings suggest a much larger role for CD:45RA−25+5+38− cells in maintenance of allograft stability, as we observed that increased frequency of CD:45RA−25+5+38− cells was associated with graft stability in heart, liver, and kidney recipients. It had been previously reported that CD:45RA−25+5+38− cells were upregulated in patients who were not receiving immunosuppression;14 here, we observe a similar degree of upregulation of CD:45RA−25+5+38− cells even in patients receiving maintenance immunosuppression. This suggests that (1) CD:45RA−25+5+38− cells are not negatively regulated by classical forms of immunosuppression and (2) augmentation of CD:45RA−25+5+38− abundance or function may represent an alternative route to maintenance of allograft stability outside of that mediated by classical immunosuppressive agents. Furthermore, since the development of rejection was associated with changes in frequency of CD:45RA−25+5+38− cells, they may represent a biomarker for the detection of rejection.
Molecular profiling of allograft tissue to identify a common signature of rejection has been described. Morgun et al.35 used microarray analysis of cardiac biopsy tissue from patients either experiencing rejection or with stable graft function and were able to identify a set of genes whose expression could discriminate between acute rejection and stable graft function; they then went on to show that this same gene set could be used to distinguish between stable and rejecting grafts in tissue obtained from kidney and lung recipients. Building on this work, Khatri et al.36 used microarray data of both tissue and blood samples and found an 11-gene signature commonly overexpressed in acute rejection across all transplanted organs. They proposed using their gene signature for diagnostic purposes and therapeutic repositioning based on how drugs interact with the signature. Of note, Khatri et al. also designed their integrative analysis to consider organ-specific bias and demonstrated that their meta-analysis approach avoided tissue-specific, organ-specific expression bias, which supports our own findings. Here, we build on the findings of these studies by identifying differentially abundant populations at the cellular level; these populations may be the functional mediators of rejection, perhaps through changes in the gene expression identified in prior work.
It is important to note that many of the differences in immune cell abundance between stable and rejecting patients were not apparent when the effects of allograft type were not considered. This suggests that previous studies of differences in immune composition that did not take the organ-specific differences into consideration may have failed to detect differential abundance in populations between stable and rejecting patients. In our dataset, we also noted that there were other cell types with 0.5–1 log fold-change differences between stable and rejecting patients, but these differences were not significantly different. We also considered the possibility that some of the changes that we observed may be influenced by the type of rejection that the patient was experiencing, i.e., T cell-mediated rejection vs. antibody-mediated rejection. The parent study under which these samples were collected was not designed to look at the relationship between immune composition and rejection. Thus, while some data on the occurrence of rejection was collected, the details of the type of rejection were not, and therefore subgroup analysis by the type of rejection is not feasible based on the clinical data that we have on these patients. A recent study that looked at the relative frequency of T cell-mediated vs. antibody-mediated rejection in 130 pediatric liver transplant recipients reported 44 cases of rejection (34%), with 31/44 (70%) classified as T cell-mediated rejection and the remainder being antibody-mediated rejection.37 A similar division was observed among pediatric heart transplant recipients,38 while kidney recipients were largely skewed toward T cell-mediated rejection.39,40 Thus, while we cannot further stratify the immune signature by rejection type, it is likely most cases in our study represent T cell-mediated rejection. An important caveat to considering these differences is that, even though all blood samples were collected within 30 days prior to rejection, there were differences in the timing across cohorts; all heart transplant recipients in this study had blood collected on the day that rejection was diagnosed, but there was often a longer period between collection and diagnosis for liver and kidney recipients. This is a function of the fact that many pediatric transplant centers perform surveillance biopsies only on heart transplant recipients, not on liver or kidney transplant recipients. It is possible, therefore, that the immune profiles generated from patients with a larger gap between collection and diagnosis of rejection may represent a different phase of rejection than those collected on the day of diagnosis. While this may affect comparison across allograft types, the comparison between stable and rejecting allografts within a given allograft type should not be affected.
CD:45RA−25+5+38− cells were determined to be phenotypically most like CD45RA− Tregs but express enough differences in expression of key markers that it is unlikely that they are a subpopulation of these cells. Furthermore, CD:45RA−25+5+38− cells are more abundant in the peripheral circulation than the CD45RA− Tregs. This suggests that CD:45RA−25+5+38− cells may represent a “ready reserve” of CD4+ T cells that can be induced to suppress immune activation when needed. An equilibrium between effector and regulatory T cell populations has been described previously in the literature, with multiple cytokine-signaling pathways implicated in driving the balance in one direction or the other. For example, TGF-β, an anti-inflammatory cytokine, induces FOXP3 and the development of Treg cells in the periphery; however, the combination of the pro-inflammatory cytokine IL-6 and TGF-β induces generation of effector Th17 populations and TGF-β is required for this process.41 The transcription factors RORγt/RORα (found in Th17 populations) and FOXP3 (found in Treg populations) can bind and inhibit each other’s function.42,43 IL-2 promotes growth of Tregs and inhibits Th17 differentiation, while IL-21 promotes Th17 differentiation and inhibits Treg expansion.44 If CD:45RA−25+5+38− cells indeed represent a ready reserve of CD4+ T cells with regulatory capabilities, the above data suggest that we may be able to “nudge” them toward a regulatory phenotype with exogenous cytokine stimulation as a novel therapy for prevention or treatment of rejection.
It is possible that clinical evidence of rejection may lag behind the immune events that accompany rejection. In the absence of a biopsy that clearly demonstrates that immune infiltration of the allograft has not occurred, one cannot be sure that there is not a low-level immune activation occurring even when the patient has no clinical evidence of rejection. To minimize the potential of misclassifying a patient with low-level rejection as stable, we stipulated that patients should not have any evidence of rejection for the 12 months after sample collection to be used for analysis in the study.
In summary, we demonstrated that the immune response to an allograft is strongly associated with the type of allograft received and that increased abundance of CD:45RA−25+5+38− cells is strongly associated with graft stability across multiple different types of allografts. Because a limited set of cell-surface markers can be used to define CD:45RA−25+5+38− cells, their abundance can be measured in individuals using clinical flow cytometry-based assays, and this may represent a minimally invasive approach to monitor for the development of allograft rejection. Measurement of CD:45RA−25+5+38− cell abundance in transplant recipients over time will need to be performed to determine whether there is an absolute threshold for CD:45RA−25+5+38− abundance that predicts rejection or whether changes in CD:45RA−25+5+38− abundance from pre-transplant or early post-transplant levels can be used to predict the development of rejection. Furthermore, because CD:45RA−25+5+38− cells have features of regulatory cells, they may represent a population that can be targeted in vivo to selectively dampen an immune response in a patient experiencing rejection.
Limitations of the study
There are three main limitations to the findings observed in this study: first, our study did not have enough subjects to perform an age-specific subanalysis of data. Our study is among the larger human immunoprofiling studies, particularly in children, published to date, and we made every effort to include patients from a wide variety of ages and from both sexes. To understand the immune events that underlie the development of rejection most rigorously, we had very specific criteria that we used to select patients, which limited our ability to recruit large numbers of patients from any one age group or perform gender-specific subanalysis. Detailed information on the type of rejection was not available and we were therefore unable to delineate differences between acute cellular rejection and antibody-mediated rejection. Second, because we lacked longitudinal samples from any given patient, we were unable to determine whether specific changes in the abundance of immune cell populations over time could be used to predict the development of rejection. Last, the findings in this study were determined based on a single cohort of patients and were not validated in an independent cohort. All these limitations will be the subject of future investigations in this area.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-human CD45-89Y (HI30) | Standard Biotools | Cat. #3089003B; RRID:AB_2938863 |
| anti-human CCR6-141Pr (11A9) | Standard Biotools | Cat. #3141014A |
| anti-human CD19-142Nd (HIB19) | Standard Biotools | Cat. #3142001B |
| anti-human CD127-143Nd (A019D5) | Standard Biotools | Cat. #3143012B; RRID:AB_2810240 |
| anti-human CD69-144Nd (FN50) | Standard Biotools | Cat. #3144018B |
| anti-human CD4-145Nd (RPA-T4) | Standard Biotools | Cat. #3145001B |
| anti-human CD8-146Nd (RPA-T8) | Standard Biotools | Cat. #3146001B |
| anti-human CD11c-147Sm (Bu15) | Standard Biotools | Cat. #3147008B |
| anti-human CD16-148Nd (3G8) | Standard Biotools | Cat. #3148004B |
| anti-human CD25-149Sm (2A3) | Standard Biotools | Cat. #3149010B; RRID:AB_2756416 |
| anti-human LAG-3-150Nd (11C3C65) | Standard Biotools | Cat. #3150030B |
| anti-human CD107a-151Eu (H4A3) | Standard Biotools | Cat. #3151002B |
| anti-human TCRgd-152Sm (11F2) | Standard Biotools | Cat. #3152008B; RRID:AB_2687643 |
| anti-human CD45RA-153Eu (HI100) | Standard Biotools | Cat. #3153001B; RRID:AB_2802108 |
| anti-human CD3-154Sm (UCHT1) | Standard Biotools | Cat. #3154003B; RRID:AB_2811086 |
| anti-human PD-1-155Gd (EH12.2H7) | Standard Biotools | Cat. #3155009B; RRID:AB_2811087 |
| anti-human CD14-156Gd (HCD14) | Standard Biotools | Cat. #3156019B |
| anti-human CD27-158Gd (L128) | Standard Biotools | Cat. #3158010B; RRID:AB_2858231 |
| anti-human FOXP3-159Tb (259D/C7) | Standard Biotools | Cat. #3159028A; RRID:AB_2811088 |
| anti-human CD28-160Gd (CD28.2) | Standard Biotools | Cat. #3160003B; RRID:AB_2868400 |
| anti-human Ki67-161Dy (B56) | Standard Biotools | Cat. #3161007B; RRID:AB_2811255 |
| anti-human NKp46-162Dy (BAB281) | Standard Biotools | Cat. #3162021B |
| anti-human CD57-163Dy (HCD57) | Standard Biotools | Cat. #3163022B; RRID:AB_2756434 |
| anti-human CD45RO-164Dy (UCHL1) | Standard Biotools | Cat. #3164007B; RRID:AB_2811092 |
| anti-human NKG2C-165Ho (134591) | R&D | Cat. #MAB-138SP |
| anti-human NKG2D-166Er (ON72) | Standard Biotools | Cat. #3166016B; RRID:AB_2892110 |
| anti-human CCR7-167Er (G043H7) | Standard Biotools | Cat. #3167009A; RRID:AB_2858236 |
| anti-human CD40L-168Er (24-31) | Standard Biotools | Cat. #3168006B |
| anti-human NKG2A-169Tm (Z199) | Standard Biotools | Cat. #3169013B; RRID:AB_2756426 |
| anti-human HLA-DR-170Er (L243) | Standard Biotools | Cat. #3170013B; RRID:AB_2888929 |
| anti-human CD20-171Yb (2H7) | Standard Biotools | Cat. #3171012B; RRID:AB_2802112 |
| anti-human CD38-172Yb (HIT2) | Standard Biotools | Cat. #3172007B; RRID:AB_2756288 |
| anti-human/mouse granzyme B-173Yb (GB11) | Standard Biotools | Cat. #3173006B; RRID:AB_2811095 |
| anti-human CD5 (UCHT2) | Biolegend | Cat. #300602; RRID:AB_314088 |
| anti-human CCR4-175Lu (L291H4) | Standard Biotools | Cat. #3175035A; RRID:AB_2921320 |
| anti-human CD56-176Yb (HCD56) | Standard Biotools | Cat. #3176001B |
| anti-human CD11b-209Bi (ICRF44) | Standard Biotools | Cat. #3209003B |
| Chemicals, peptides, and recombinant proteins | ||
| Maxpar® X8 Antibody Labeling Kit, 165Ho | Standard Biotools | Cat #201165A |
| Maxpar® X8 Antibody Labeling Kit, 174Yb | Standard Biotools | Cat #201174A |
| cisplatin | Standard Biotools | Cat #201064 |
| iridium intercalator | Standard Biotools | Cat #201192A |
| Cell Staining Buffer | Standard Biotools | Cat #201068 |
| Cell Acqusition Solution | Standard Biotools | Cat #201240 |
| Cell-ID™ 20-Plex Pd Barcoding Kit | Standard Biotools | Cat #201060 |
| Fc block | Biolegend | Cat #422301 |
| Fixation/Permabilization Concentrate | Invitrogen | Cat #00-5123-43 |
| eBioscience Fixation/Perm Diluent | Invitrogen | Cat #00-5223-56 |
| Permabilization Buffer, 10X | Invitrogen | Cat #00-8333-56 |
| Maxpar PBS | Standard Biotools | Cat #201058 |
| Software and algorithms | ||
| Mendeley Data DOI | This paper | https://doi.org/10.17632/gnmm8zcj25.1 |
| Github Repository | This paper | https://github.com/skpediatrictransplant/skpediatrictransplant2023 |
| Zenodo DOI | This paper | https://doi.org/10.5281/zenodo.7964888 |
| CellEngine | CellCarta | http://cellengine.com |
| R | R Core Team | https://www.R-project.org |
| Prism 9.0 | Graphpad Software | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Sheri M. Krams (smkrams@stanford.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Patient samples
Patient samples were obtained from a biobank generated as a part of the Clinical Trials in Organ Transplantation in Children-06 (CTOTC-06) study.45 CTOTC-06 was an NIH-sponsored prospective, observational study in which 944 pediatric subjects (neonate to 21 years of age) were enrolled pre-transplant and post-transplant at seven clinical sites. Institutional Review Board (IRB) approval was obtained, and all procedures were performed in accordance with the relevant guidelines and regulations. For participants under the age of 18 years, informed consent was obtained from a parent and/or legal guardian. Of these subjects, 872 received transplants. Immunosuppressive and anti-viral therapy were per each center’s standard protocol. Prospective blood samples were collected (n=4753) at enrollment or transplant, every 3 months during the first 2 years and twice yearly thereafter, regardless of allograft health. Follow up was for a minimum of 12 months and a maximum of four years based on the time of enrollment. Subjects enrolled post-transplant were within three years of transplantation. Peripheral blood mononuclear cells (PBMCs) were isolated from these samples and cryopreserved. Samples were obtained from the CTOTC-06 parent study, and information on race, ethnicity, and socioeconomic status was not provided to us.
In this study, a total of 52 samples were profiled (Table 1). We based our power calculations on data from a 2016 study using a similar approach in a different cohort of pediatric liver transplant recipients.14 In that study, we observed more than a two-fold difference in the percentage of a population of interest (CD:45RA-25+5+38- cells) between patient groups. Assuming a normal distribution and type I error rate=0.05, two-sided Satterthwaite’s t-tests for unequal variance were estimated to have >99% power to detect similar differences between rejection and stable patients within each organ group. Twenty-four of the samples - the “stable” cohort - were from patients who had no biochemical or biopsy evidence of rejection or infection in the 12 months before and the 12 months after sample collection; the remaining 28 samples – the “rejection” cohort - were from age-matched patients who had biopsy-proven rejection in the 30-day period after the sample was collected. Matching was performed by B.A., who was not involved in data collection or analysis. Of note, no two samples used in this study were collected from the same patient. This number of samples gave us a robust data set, particularly given the difficulties in obtaining samples from pediatric patients. In fact, the number of patients profiled in this study was greater than many other recent high-quality immunoprofiling or transplantation studies.46,47,48,49,50
Samples were collected as a part of a parent study (CTOTC-06) and provided to us as vials of cryopreserved PBMCs. Whole blood samples from all participants were collected in heparin-containing green-top tubes and shipped overnight on the day of collection to the Stanford University School of Medicine Clinical and Translational Research Unit for processing. PBMCs were isolated using a Ficoll-based procedure. Isolated PBMCs were resuspended in 100% human serum in cryovials, and an equal volume of 80% human serum/20% DMSO was added dropwise. Cryovials were then placed in a controlled-rate freezing chamber at -80C for 24 hours, then placed in liquid nitrogen until ready for thawing and analysis. Cryopreserved PBMCs were thawed and barcoded with palladium isotopes. Use of palladium-isotope barcoding permits pooling of the sample prior to staining, thereby reducing sample-to-sample data variability from slight differences in staining time, antibody concentration, etc. Samples were then pooled and stained with a panel of 37 metal-conjugated antibodies against cell surface and intracellular proteins designed to identify specific immune cell lineages. The pooled sample was then subject to analysis on a mass cytometer. The mass cytometer data were normalized and debarcoded. The data from the patient samples were collected over the course of three experiments; to account for subtle differences in staining patterns and variability in machine conditions, single aliquots from the same two healthy donors were included with each run alongside the patient samples; these are hereafter referred to as “anchor” samples. Batch correction was performed with the anchor samples included in the experiments using the approach described in Schuyler, et al.51
Method details
Reagents
All CyTOF reagents and antibodies (Table 2) were purchased from Standard Biotools (South San Francisco, CA, USA), except as noted. Cell-ID cisplatin (Standard Biotools) was used as per manufacturer’s directions to differentiate live vs dead populations. Monoclonal antibodies against NKG2C and CD5 were purchased from R&D Systems (Minneapolis, MN, USA) and Biolegend (San Diego, CA, USA), respectively, and were conjugated to various heavy metals using Maxpar Antibody Labeling Kits from Standard Biotools.
CyTOF staining
PBMCs from patient samples or anchor controls were thawed into warmed Roswell Park Memorial Institute 1640 media (RPMI) with 20% fetal bovine serum, washed once with phosphate-buffered saline (PBS), and stained with 1 ml of 0.25 mM cisplatin (Standard Biotools) for 5 minutes at room temperature for exclusion of dead cells. Samples were then washed with Cell Staining Buffer (CSB) (Standard Biotools) and barcoded using a Cell-ID™ 20-Plex Pd Barcoding Kit (Standard Biotools) of lanthanide-tagged cell-reactive metal chelators that will covalently label samples with a unique combination of palladium isotopes according to the manufacturer’s instructions, except that 4X Fixation/Permeabilization Concentrate (Invitrogen) diluted to 1X with eBioscience Fixation/Perm Diluent (Invitrogen) was used in place of the Maxpar Barcode Perm Buffer. Samples were washed twice with CSB, combined, and incubated with Fc block (Biolegend) for 10 minutes at room temperature. Cells were then stained with a heavy metal-labeled monoclonal antibody cocktail directed against cell surface markers in CSB for 30 minutes at room temperature. Cells were washed twice with 1X Permeabilization Buffer (Invitrogen) and incubated in 1X Permeabilization Buffer (Invitrogen) with an intracellular staining cocktail for 30 minutes at room temperature. Cells were twice washed with CSB, then incubated overnight at 4°C with 1 mL of 125 nM iridium intercalator (Standard Biotools) in 1.4% freshly diluted paraformaldehyde for DNA staining. Cells were washed twice with CSB and washed twice with cell acquisition solution (CAS, Standard Biotools), then passed through a 50-μm filter and analyzed on a Helios mass cytometer (250–450 events per second).
Quantification and statistical analysis
CyTOF data pre-processing
Flow cytometry standard (FCS) files were concatenated, normalized (using beads) and debarcoded using the Helios software. Normalized and debarcoded data was gated using CellEngine (http://cellengine.com). Briefly, cellular events were identified using a DNA-intercalator dye (191-iridium and 193-iridium double positive). Singlets were extracted from all cellular events based on event length, and live cells were extracted from singlets based on cisplatin staining (195-cisplatin negative). Exported FCS files of the raw counts of all markers and cell annotations were imported into R using FlowCore.52 Cells were annotated with their most confident, terminally defined manual gate in CellEngine. If cells were unable to be annotated by a terminal gate (e.g., memory B cell), they were annotated by the closest upstream gate (e.g., B cell). We removed duplicate cells from upstream FCS files but retained the most terminal gate for each cell to obtain a single-cell matrix of cells and features that includes all live cells gated within the T cell, B cell, and myeloid populations. Each marker was transformed using an arcsinh scale of 5 and percentile normalized using a quantile value of 0.999 across all cells. Cell proportions were computed based on the major lineage they occupy: T cells, B cells, NK cells or CD56- myeloid cells.
Technical effect discovery and correction
We implemented linear mixed models to unveil drivers of clinical and biological variation using the VariancePartition package.17 We identified drivers of variation by quantifying the variation in each trait that is attributable to differences in metadata such as cell type, sample, age at collection, allograft tissue, and allograft health. We input our single-cell matrix of cells and markers, and modeled cell-level metadata. The contribution of each variable is in terms of the fraction of variation explained (FVE). Due to technical effects from separate CyTOF runs on patient samples, we implemented batch correction using the CyTOF BatchAdjust algorithm on raw data to harmonize all patient samples. Anchor samples included in each batch were used to compute adjustment factors for each channel in each batch.51 Batch-corrected data was imported into CellEngine, manually gated, and then imported into R for preprocessing and transformation prior to further analysis.
Statistics
Correlations between marker expression values were generated using the Pearson correlation method. Statistical testing of independent groups was performed using the Wilcoxon Rank Sum Test. Where applicable, multiple hypothesis correction was performed using the false discovery rate. Aggregate marker expression values were computed using the median marker expression across a cell type in each sample to maintain robustness to outliers, and statistical comparisons were made at the sample-level. We used logistic regression with the generalized linear model (glm) function in the stats package of R to make comparisons with blocking effects in the model. L1 regularized regression model was done using the glmnet package in R with the alpha = 1 argument (LASSO). We used the built-in cross validation feature to tune the lambda parameter and presented the results for the lambda value that gives the minimum mean cross-validated error (lambda.min). We input the proportions of all 'terminal' celltype populations to predict stable vs rejection.
Dimensionality reduction
We used four dimensionality-reduction algorithms for analysis: two unsupervised methods - principal component analysis (PCA) and uniform manifold approximation and projection (UMAP) visualization - and two supervised methods – linear discriminant analysis (LDA) and sparse linear discriminant analysis (sparseLDA). Data was z-scaled and centered prior to PCA transformation, and PCA was done in R using prcomp in the stats package. UMAP visualization was performed using the uwot package.53 UMAP parameter tuning was done using the alpha and beta parameters. For the single-cell UMAP, we visualized only cells in the terminally gated populations. LDA was performed using the MASS and hsslda packages in R.15,54,55 Data was z-scaled and centered prior to LDA to improve feature interpretability. Feature importance was inferred using the magnitude and direction of the LD coefficient with respect to the respective class distribution in linear discriminant space. Permutation analysis of the allograft versus randomized groups was done using sparseLDA in R.
Supervised analysis with manual gating
Cells were gated into 29 populations based on surface and intracellular marker staining patterns. The abundance of each population as a percentage of the parent lineage (T cell, B cell, NK cell, CD56- myeloid cell) was computed. Comprehensive comparisons between CyTOF and flow cytometric data have been described by Bendall, et al.56 Plots were constructed using either Prism 9.0 (Graphpad Software) or the various R packages listed. Comparisons between groups were performed using the statistical test and the assumptions given in the legend below each figure. The number of asterisks indicates the p-value obtained from the appropriate statistical test (∗ = p < 0.05; ∗∗ = p < 0.001; ∗∗∗ = p < 0.0005.)
Additional resources
CTOTC-06 Biomarkers for Post-Transplant Lymphoproliferative Disorders in Children: https://clinicaltrials.gov/study/NCT02182986.
Acknowledgments
This study was funded by awards from the NIH (UO1 AI104342 [C.O.E.], U01 AI1359590 [S.M.K.]), the Stanford Maternal and Child Health Research Institute (M.R.), the Stanford Transplant and Tissue Engineering Center of Excellence (M.R.), and the Stanford University Jackson Vaughan Critical Care Research Fund (M.R.). M.A. is supported by the Stanford Immunology Training Grant (T32 AI007290-37). The authors would like to extend their special thanks to all the investigators in the CTOTC-06 consortium for their work in enrolling subjects in the parent study and providing samples for this investigation.
Author contributions
Conceptualization, S.M.K., O.M.M., C.O.E., J.O., and T.D.; methodology, S.M.K., M.R., J.T.H., M.A., and S.C.B.; software, M.A. and S.C.B.; formal analysis, A.T., M.A., and S.C.B; investigation, M.R. and J.T.H.; resources, S.M.K., O.M.M., and C.O.E.; data curation, M.A.; writing – original draft, M.R. and M.A.; writing – review & editing, M.R., M.A., S.M.K., and O.M.M.; visualization, M.R. and M.A.; supervision, S.M.K., O.M.M., C.O.E., and S.C.B.; project administration, M.G.L., B.A., and T.D.; funding acquisition, S.M.K., C.O.E., M.R., and M.A.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in their field of research or within their geographical location. One or more of the authors of this paper self-identifies as a member of the LGBTQIA+ community. One or more of the authors of this paper received support from a program designed to increase minority representation in their field of research. We support inclusive, diverse, and equitable conduct of research.
Published: August 7, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101147.
Supplemental information
Data and code availability
-
•
De-identified patient data and raw mass-cytometry data generated from patient samples have been deposited at Mendeley and are publicly available as of the date of publication. The DOI is listed in the key resources table.
-
•
All original code, processed data, metadata, and markdown-generated PDF files to reproduce results from the manuscript are deposited at Github and are publicly available as of the date of publication. The link to the Github repository is shown in the key resources table. The Github repository is also versioned and therefore citable using Zenodo. The DOI is listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Alessiani M., Kusne S., Martin F.M., Fung J.J., Jain A., Todo S., Simmons R., Starzl T.E. Infections with FK 506 immunosuppression: preliminary results with primary therapy. Transplant. Proc. 1990;22:44–46. [PMC free article] [PubMed] [Google Scholar]
- 2.Williams D., Haragsim L. Calcineurin nephrotoxicity. Adv. Chronic Kidney Dis. 2006;13:47–55. doi: 10.1053/j.ackd.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Absalon M.J., Khoury R.A., Phillips C.L. Post-transplant lymphoproliferative disorder after solid-organ transplant in children. Semin. Pediatr. Surg. 2017;26:257–266. doi: 10.1053/j.sempedsurg.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Colvin M., Smith J.M., Ahn Y., Skeans M.A., Messick E., Goff R., Bradbrook K., Foutz J., Israni A.K., Snyder J.J., Kasiske B.L. OPTN/SRTR 2019 Annual Data Report: Heart. Am. J. Transplant. 2021;21(Suppl 2):356–440. doi: 10.1111/ajt.16492. [DOI] [PubMed] [Google Scholar]
- 5.Horslen S.P., Smith J.M., Ahn Y., Skeans M.A., Cafarella M., Noreen S.M., Snyder J.J., Israni A.K. OPTN/SRTR 2019 Annual Data Report: Intestine. Am. J. Transplant. 2021;21(Suppl 2):316–355. doi: 10.1111/ajt.16498. [DOI] [PubMed] [Google Scholar]
- 6.Hart A., Lentine K.L., Smith J.M., Miller J.M., Skeans M.A., Prentice M., Robinson A., Foutz J., Booker S.E., Israni A.K., et al. OPTN/SRTR 2019 Annual Data Report: Kidney. Am. J. Transplant. 2021;21(Suppl 2):21–137. doi: 10.1111/ajt.16502. [DOI] [PubMed] [Google Scholar]
- 7.Kwong A.J., Kim W.R., Lake J.R., Smith J.M., Schladt D.P., Skeans M.A., Noreen S.M., Foutz J., Booker S.E., Cafarella M., et al. OPTN/SRTR 2019 Annual Data Report: Liver. Am. J. Transplant. 2021;21(Suppl 2):208–315. doi: 10.1111/ajt.16494. [DOI] [PubMed] [Google Scholar]
- 8.Mbiribindi B., Harden J.T., Pena J.K., Krams S.M. Natural killer cells as modulators of alloimmune responses. Curr. Opin. Organ Transplant. 2019;24:37–41. doi: 10.1097/MOT.0000000000000590. [DOI] [PubMed] [Google Scholar]
- 9.Miyairi S., Baldwin W.M., 3rd, Valujskikh A., Fairchild R.L. Natural Killer Cells: Critical Effectors During Antibody-mediated Rejection of Solid Organ Allografts. Transplantation. 2021;105:284–290. doi: 10.1097/TP.0000000000003298. [DOI] [PubMed] [Google Scholar]
- 10.Moreau A., Varey E., Anegon I., Cuturi M.C. Effector mechanisms of rejection. Cold Spring Harb. Perspect. Med. 2013;3:a015461. doi: 10.1101/cshperspect.a015461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong A.S. Mechanisms of organ transplant injury mediated by B cells and antibodies: Implications for antibody-mediated rejection. Am. J. Transplant. 2020;20(Suppl 4):23–32. doi: 10.1111/ajt.15844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duneton C., Winterberg P.D., Ford M.L. Activation and regulation of alloreactive T cell immunity in solid organ transplantation. Nat. Rev. Nephrol. 2022;18:663–676. doi: 10.1038/s41581-022-00600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neeland M.R., Andorf S., Manohar M., Dunham D., Lyu S.C., Dang T.D., Peters R.L., Perrett K.P., Tang M.L.K., Saffery R., et al. Mass cytometry reveals cellular fingerprint associated with IgE+ peanut tolerance and allergy in early life. Nat. Commun. 2020;11:1091. doi: 10.1038/s41467-020-14919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau A.H., Vitalone M.J., Haas K., Shawler T., Esquivel C.O., Berquist W.E., Martinez O.M., Castillo R.O., Krams S.M. Mass cytometry reveals a distinct immunoprofile of operational tolerance in pediatric liver transplantation. Pediatr. Transplant. 2016;20:1072–1080. doi: 10.1111/petr.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amouzgar M., Glass D.R., Baskar R., Averbukh I., Kimmey S.C., Tsai A.G., Hartmann F.J., Bendall S.C. Supervised dimensionality reduction for exploration of single-cell data by HSS-LDA. Patterns. 2022;3:100536. doi: 10.1016/j.patter.2022.100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hastie T., Tibshirani R., Friedman J.H. 2nd Edition. Springer; 2009. The Elements of Statistical Learning : Data Mining, Inference, and Prediction. [Google Scholar]
- 17.Hoffman G.E., Schadt E.E. variancePartition: interpreting drivers of variation in complex gene expression studies. BMC Bioinf. 2016;17:483. doi: 10.1186/s12859-016-1323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu R. Measuring explained variation in linear mixed effects models. Stat. Med. 2003;22:3527–3541. doi: 10.1002/sim.1572. [DOI] [PubMed] [Google Scholar]
- 19.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seiler C., Ferreira A.M., Kronstad L.M., Simpson L.J., Le Gars M., Vendrame E., Blish C.A., Holmes S. CytoGLMM: conditional differential analysis for flow and mass cytometry experiments. BMC Bioinf. 2021;22:137. doi: 10.1186/s12859-021-04067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 23.Han J., Kamber M. 3rd Edition. Elsevier; 2012. Data Mining : Concepts and Techniques. [Google Scholar]
- 24.De Serres S.A., Mfarrej B.G., Magee C.N., Benitez F., Ashoor I., Sayegh M.H., Harmon W.E., Najafian N. Immune profile of pediatric renal transplant recipients following alemtuzumab induction. J. Am. Soc. Nephrol. 2012;23:174–182. doi: 10.1681/ASN.2011040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulz-Juergensen S., Marischen L., Wesch D., Oberg H.H., Fändrich F., Kabelitz D., Burdelski M. Markers of operational immune tolerance after pediatric liver transplantation in patients under immunosuppression. Pediatr. Transplant. 2013;17:348–354. doi: 10.1111/petr.12079. [DOI] [PubMed] [Google Scholar]
- 26.Stenard F., Nguyen C., Cox K., Kambham N., Umetsu D.T., Krams S.M., Esquivel C.O., Martinez O.M. Decreases in circulating CD4+CD25hiFOXP3+ cells and increases in intragraft FOXP3+ cells accompany allograft rejection in pediatric liver allograft recipients. Pediatr. Transplant. 2009;13:70–80. doi: 10.1111/j.1399-3046.2008.00917.x. [DOI] [PubMed] [Google Scholar]
- 27.Kumar P., Lim A., Poh S.L., Hazirah S.N., Chua C.J.H., Sutamam N.B., Arkachaisri T., Yeo J.G., Kofidis T., Sorokin V., et al. Pro-Inflammatory Derangement of the Immuno-Interactome in Heart Failure. Front. Immunol. 2022;13:817514. doi: 10.3389/fimmu.2022.817514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim T.B., Ionescu L., Avdimiretz N., Murdoch F., Larsen I.M., Motyka B., West L.J., Urschel S. Alterations in the immune phenotype of thymectomized children and the development of atopic disorders after heart transplantation. Pediatr. Transplant. 2022;26:e14252. doi: 10.1111/petr.14252. [DOI] [PubMed] [Google Scholar]
- 29.Porcu E., Sadler M.C., Lepik K., Auwerx C., Wood A.R., Weihs A., Sleiman M.S.B., Ribeiro D.M., Bandinelli S., Tanaka T., et al. Differentially expressed genes reflect disease-induced rather than disease-causing changes in the transcriptome. Nat. Commun. 2021;12:5647. doi: 10.1038/s41467-021-25805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brouard S., Pallier A., Renaudin K., Foucher Y., Danger R., Devys A., Cesbron A., Guillot-Guegen C., Ashton-Chess J., Le Roux S., et al. The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am. J. Transplant. 2012;12:3296–3307. doi: 10.1111/j.1600-6143.2012.04249.x. [DOI] [PubMed] [Google Scholar]
- 31.Chandrasekharan D., Issa F., Wood K.J. Achieving operational tolerance in transplantation: how can lessons from the clinic inform research directions? Transpl. Int. 2013;26:576–589. doi: 10.1111/tri.12081. [DOI] [PubMed] [Google Scholar]
- 32.Feng S., Ekong U.D., Lobritto S.J., Demetris A.J., Roberts J.P., Rosenthal P., Alonso E.M., Philogene M.C., Ikle D., Poole K.M., et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012;307:283–293. doi: 10.1001/jama.2011.2014. [DOI] [PubMed] [Google Scholar]
- 33.Kim K.W., Kim B.M., Doh K.C., Cho M.L., Yang C.W., Chung B.H. Clinical significance of CCR7(+)CD8(+) T cells in kidney transplant recipients with allograft rejection. Sci. Rep. 2018;8:8827. doi: 10.1038/s41598-018-27141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Betjes M.G.H., Litjens N.H.R. High numbers of differentiated CD28null CD8+ T cells are associated with a lowered risk for late rejection and graft loss after kidney transplantation. PLoS One. 2020;15:e0228096. doi: 10.1371/journal.pone.0228096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgun A., Shulzhenko N., Perez-Diez A., Diniz R.V.Z., Sanson G.F., Almeida D.R., Matzinger P., Gerbase-DeLima M. Molecular profiling improves diagnoses of rejection and infection in transplanted organs. Circ. Res. 2006;98:e74–e83. doi: 10.1161/01.res.0000228714.15691.8a. [DOI] [PubMed] [Google Scholar]
- 36.Khatri P., Roedder S., Kimura N., De Vusser K., Morgan A.A., Gong Y., Fischbein M.P., Robbins R.C., Naesens M., Butte A.J., Sarwal M.M. A common rejection module (CRM) for acute rejection across multiple organs identifies novel therapeutics for organ transplantation. J. Exp. Med. 2013;210:2205–2221. doi: 10.1084/jem.20122709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W., Wang Z.L., Kang Z.Y., Xiao Y.L., Liu C., Li D.H. Liver graft injury caused by de novo donor-specific HLA antibodies in pediatric liver transplant recipients with low, moderate, and high immunologic risk. Am. J. Surg. 2023;225:275–281. doi: 10.1016/j.amjsurg.2022.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Webber S., Zeevi A., Mason K., Addonizio L., Blume E., Dipchand A., Shaddy R., Feingold B., Canter C., Hsu D., et al. Pediatric heart transplantation across a positive crossmatch: First year results from the CTOTC-04 multi-institutional study. Am. J. Transplant. 2018;18:2148–2162. doi: 10.1111/ajt.14876. [DOI] [PubMed] [Google Scholar]
- 39.Chen J., Liverman R., Garro R., Jernigan S., Travers C., Winterberg P.D. Acute cellular rejection treatment outcomes stratified by Banff grade in pediatric kidney transplant. Pediatr. Transplant. 2019;23:e13334. doi: 10.1111/petr.13334. [DOI] [PubMed] [Google Scholar]
- 40.Twombley K., Thach L., Ribeiro A., Joseph C., Seikaly M. Acute antibody-mediated rejection in pediatric kidney transplants: a single center experience. Pediatr. Transplant. 2013;17:E149–E155. doi: 10.1111/petr.12129. [DOI] [PubMed] [Google Scholar]
- 41.Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 42.Du J., Huang C., Zhou B., Ziegler S.F. Isoform-specific inhibition of ROR alpha-mediated transcriptional activation by human FOXP3. J. Immunol. 2008;180:4785–4792. doi: 10.4049/jimmunol.180.7.4785. [DOI] [PubMed] [Google Scholar]
- 43.Zhou L., Lopes J.E., Chong M.M., Ivanov I.I., Min R., Victora G.D., Shen Y., Du J., Rubtsov Y.P., Rudensky A.Y., et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laurence A., Tato C.M., Davidson T.S., Kanno Y., Chen Z., Yao Z., Blank R.B., Meylan F., Siegel R., Hennighausen L., et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Biomarkers for Post-Transplant Lymphoproliferative Disorders in Children. 2019. https://clinicaltrials.gov/ct2/show/NCT02182986 ClinicalTrials.gov Identifier: NCT02182986. [Google Scholar]
- 46.Braza F., Dugast E., Panov I., Paul C., Vogt K., Pallier A., Chesneau M., Baron D., Guerif P., Lei H., et al. Central Role of CD45RA- Foxp3hi Memory Regulatory T Cells in Clinical Kidney Transplantation Tolerance. J. Am. Soc. Nephrol. 2015;26:1795–1805. doi: 10.1681/ASN.2014050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fribourg M., Anderson L., Fischman C., Cantarelli C., Perin L., La Manna G., Rahman A., Burrell B.E., Heeger P.S., Cravedi P. T-cell exhaustion correlates with improved outcomes in kidney transplant recipients. Kidney Int. 2019;96:436–449. doi: 10.1016/j.kint.2019.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishiyama K., Arakawa-Hoyt J., Aguilar O.A., Damm I., Towfighi P., Sigdel T., Tamaki S., Babdor J., Spitzer M.H., Reed E.F., et al. Mass cytometry reveals single-cell kinetics of cytotoxic lymphocyte evolution in CMV-infected renal transplant patients. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2116588119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stern L., McGuire H.M., Avdic S., Fazekas de St Groth B., Gottlieb D., Abendroth A., Blyth E., Slobedman B. Immunoprofiling reveals cell subsets associated with the trajectory of cytomegalovirus reactivation post stem cell transplantation. Nat. Commun. 2022;13:2603. doi: 10.1038/s41467-022-29943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ung N., Goldbeck C., Man C., Hoeflich J., Sun R., Barbetta A., Matasci N., Katz J., Lee J.S.H., Chopra S., et al. Adaptation of Imaging Mass Cytometry to Explore the Single Cell Alloimmune Landscape of Liver Transplant Rejection. Front. Immunol. 2022;13:831103. doi: 10.3389/fimmu.2022.831103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuyler R.P., Jackson C., Garcia-Perez J.E., Baxter R.M., Ogolla S., Rochford R., Ghosh D., Rudra P., Hsieh E.W.Y. Minimizing Batch Effects in Mass Cytometry Data. Front. Immunol. 2019;10:2367. doi: 10.3389/fimmu.2019.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ellis B., Haaland P., Hahne F., Le Meur N., Gopalakrishnan N., Spidlen J., Jiang M., Finak G. 2022. flowCore: Basic Structures for Flow Cytometry Data. [Google Scholar]
- 53.Melville J., Lun A., Djekidel M.N., Hao Y. 2021. Uwot: The Uniform Manifold Approximation and Projection (UMAP) Method for Dimensionality Reduction. [Google Scholar]
- 54.Ripley B., Venables B., Bates D.M., Hornick K., Gebhardt A., Firth D. 2022. MASS: Support Functions and Datasets for Venables and Ripley’s MASS. [Google Scholar]
- 55.Venables W.N., Ripley B.D., Venables W.N. 4th Edition. Springer; 2002. Modern Applied Statistics with S. [Google Scholar]
- 56.Bendall S.C., Simonds E.F., Qiu P., Amir E.A.D., Krutzik P.O., Finck R., Bruggner R.V., Melamed R., Trejo A., Ornatsky O.I., et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
De-identified patient data and raw mass-cytometry data generated from patient samples have been deposited at Mendeley and are publicly available as of the date of publication. The DOI is listed in the key resources table.
-
•
All original code, processed data, metadata, and markdown-generated PDF files to reproduce results from the manuscript are deposited at Github and are publicly available as of the date of publication. The link to the Github repository is shown in the key resources table. The Github repository is also versioned and therefore citable using Zenodo. The DOI is listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.