Abstract
Objective
The primary aim was to investigate the effectiveness of adding more resistance exercise to usual care on pain mechanisms (including temporal summation, conditioned pain modulation (CPM) and local pain sensitivity) and pain catastrophising in people with subacromial impingement at 16 weeks follow-up. Second, to investigate the modifying effect of pain mechanisms and pain catastrophising on the interventions’ effectiveness in improving shoulder strength and disability
Methods
200 consecutive patients were randomly allocated to usual exercise-based care or the same plus additional elastic band exercise to increase total exercise dose. Completed add-on exercise dose was captured using an elastic band sensor. Outcome measures recorded at baseline, 5 weeks, 10 weeks and 16 (primary end point) weeks included temporal summation of pain (TSP) and CPM assessed at the lower leg, pressure pain threshold at the deltoid muscle (PPT-deltoid), pain catastrophising and the Shoulder Pain and Disability Index.
Results
Additional elastic band exercise was not superior to usual exercise-based care in improving pain mechanisms (TSP, CPM and PPT-deltoid) or pain catastrophising after 16 weeks. Interaction analyses showed that pain catastrophising (median split) modified the effectiveness of additional exercises (effect size 14 points, 95% CI 2 to 25), with superior results in the additional exercise group compared with the usual care group in patients with less pain catastrophising.
Conclusion
Additional resistance exercise added to usual care was not superior to usual care alone in improving pain mechanisms or pain catastrophising. Additional exercise was, however, superior in improving self-reported disability in patients with lower levels of pain catastrophising at baseline.
Trial registration number
Keywords: Exercise, Shoulder, Rotator cuff, Pain Catastrophizing
WHAT IS ALREADY KNOWN ON THIS TOPIC
Current care for subacromial impingement may not provide an adequate exercise dose, but prescribing more exercise is not the solution for this patient group as a whole.
Pain mechanisms (temporal summation, conditioned pain modulation and local pain sensitivity), pain catastrophising, and exercise adherence have the potential to impact and attenuate treatment outcome.
WHAT THIS STUDY ADDS
Adding more exercise using a time-contingent approach supported by brief pain education does not appear useful in improving pain mechanisms and pain catastrophising.
The level of pain catastrophising modifies the effectiveness of more exercise in improving shoulder disability, meaning that patients with lower level of pain catastrophising at baseline benefit from more resistance exercise whereas those with higher level of pain catastrophising do not.
A larger exercise dose was related to larger improvements in shoulder disability but not shoulder strength.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Stratified care based on pain catastrophising seems a promising way to improve care for the large and heterogeneous population of patients with persistent subacromial impingement.
A time-contingent approach (not stopping exercise because of pain) can be used to increase resistance exercise dose and improve shoulder disability outcomes in patients with less pain catastrophising, while other initiatives seem warranted to improve care for patients with more pain catastrophising.
Introduction
Each month, one in every six adults experiences shoulder pain lasting more than 1 week.1 Subacromial impingement is the most common cause of shoulder pain, accounting for 50%–74% of shoulder cases in general practice.2 3 The condition is often persistent,4 and patients are frequently referred to orthopaedic specialist care.5 Non-operative care, including exercise therapy, is recommended as first-line treatment6–10 while subacromial decompression surgery is not recommended as standard care.11 Current non-operative care leaves many patients with unacceptable and long-standing symptoms,5 for unknown reasons.
Resistance exercise constitutes a key component in non-operative care of subacromial impingement.6 7 Previous studies that included resistance exercise in the non-operative care for subacromial impingement reported limited to non-existent improvements in shoulder strength,12–21 suggesting that they did not provide an adequate stimulus. In the pragmatic SExSI Trial (Strengthening Exercises in Shoulder Impingement Trial),22 we found that prescribing more exercise was not the solution to this problem—at least not for this patient group as a whole.23 Large heterogeneity in pain and symptom changes and treatment adherence suggests that stratified analyses could be useful to better understand this treatment response. The effect of exercise dose on shoulder pain could be attenuated by abnormal pain mechanisms (ie, increased gain of central pain mechanisms and pain hypersensitivity)24 and negative pain cognitions.25 Alterations in central pain mechanisms include increased activity of top-down pain modulatory pathways, impaired descending inhibitory mechanisms and enhanced temporal summation of pain (TSP); various mechanisms that collectively lead to hyperexcitability in the pain system.26 It has been suggested that exercise has the potential to normalise some of the abnormal pain mechanisms, by deactivating brain-orchestrated top-down pain facilitatory pathways.26 This potential benefit requires the use of a time-contingent approach where exercises are not stopped based on immediate pain response,26 which was a core element of the SExSI Trial intervention.22 23 The time-contingent approach may, however, be hindered by the presence of negative pain cognitions (such as pain catastrophising), which has been linked to increased postexercise hyperalgesia and greater perceived exertion during exercise.27 28 The SExSI Trial add-on exercise programme included a brief pain education to counteract the impact of negative pain cognitions. Further, it remains unanswered if adherence to exercise (and hence the actual exercise dose) impacts the outcome of non-operative care for subacromial impingement.
With this study we aimed to investigate if adding a large resistance exercise dose to usual care using a time-contingent approach is superior to usual care alone for improving pain mechanisms and pain catastrophising, in patients with long-standing subacromial impingement at 16 weeks follow-up. We also investigated the modifying effect of pain mechanisms and pain catastrophising on the effectiveness of additional exercise in improving shoulder strength and disability. Finally, we explored the impact of shoulder strengthening exercise dose and central pain mechanisms on changes in shoulder strength and disability from baseline to 4 months follow-up.
Methods
Study design
This study reported on predefined secondary analyses from the SExSI Trial22 23—a pragmatic, assessor-blinded and participant-blinded, randomised, controlled superiority trial, with a two-group parallel design. The trial protocol and primary trial report are available open access.22 23 End-of-treatment effect outcomes for shoulder strength, range of motion, patient-reported disability and pain, as well as quality of life, have been reported in the primary trial report.23 Conduct and presentation of statistical analyses are in accordance with the CHAMP (CHecklist for statistical Assessment of Medical Papers)29 statement.
Participants
Patients were included consecutively as part of standard procedure at our orthopaedic outpatient hospital clinic, and included both men and women. Patients were required to live in the Capital Region of Copenhagen, Denmark and be able to understand spoken and written Danish. Eligibility criteria are described in the trial protocol.22 Briefly, these include: age 18–65 years; persistent subacromial impingement (>3 months) diagnosed using predefined and valid criteria; a medically justified need for general rehabilitation, accompanied by the offer of a rehabilitation plan, including free-of-charge physiotherapy (in line with the Danish Health Act §140).
Procedures
Participants were randomised 1:1 to intervention (IG) or control (CG) groups. Blinded outcome assessors22 collected data prior to randomisation (baseline) and at follow-ups 5 weeks, 10 weeks and 16 weeks after randomisation, with 16 weeks as the primary end point. As prespecified,22 pain mechanisms (ie, central mechanisms and local pain sensitivity) were not assessed at baseline. Participants were not informed about the specific treatment content in the two groups, nor about the study hypothesis. We did not inform or discuss with participants whether they had been allocated to control or intervention (for details on blinding and allocation concealment, please see Clausen et al 23).
Interventions
Both groups received usual non-operative care—including the offer to be referred to free-of-charge general rehabilitation in a municipal clinic. In addition, participants in the IG received an add-on intervention of progressive, high-volume resistance exercise. The intervention was split in three phases of 5-6 weeks each. For each new phase, one exercise was added, and the exercise load increased. All exercises were performed unilaterally and targeted the rotator-cuff muscles through loaded external rotation and/or abduction, and were continued to contraction failure (muscular exhaustion) to facilitate an optimised physiological response.30 31 The intervention started with exercises at low relative resistance but high volume (three sets per session, every day) and was designed to produce a large total time-under-tension, i.e. the time that muscle and tendon is under tension during the resistance exercise. We instructed patients in the use of a pro- and regression algorithm, developed specifically for this study. The algorithm provides simple strategies to self-monitor symptom-response and take action when needed32 —using elements of pain neuroscience education.33 Accordingly, patients were taught that pain during exercise is not a sign of immediate danger and should be tolerated, as long as it is bearable. They were also instructed to stop the intervention if they had a flare-up lasting more than 24 hours, and to resume the intervention with lower resistance once the flare-up had settled. We did this to prepare patients for a time-contingent approach (i.e. not stopping exercise because of pain),26 by reducing fear of pain related to the intervention.33 Pro- and regression of external exercise resistance was further based on the repetition maximum (RM) principle, as described in the trial protocol.22 Supervised exercise instructions were provided at baseline and after 2, 5 and 10 weeks. A full description of the intervention, following the TIDieR checklist and guide,34 and the rationale to support it is available open access,22 and educational videos of the exercises are available from https://video.kp.dk/playlist/dedicated/418727/0_m91947x6/.
Outcomes and exposure variables
Central pain mechanisms were quantified using a user-independent and reliable computer-controlled cuff algometer35–37 to assess TSP38 and conditioned pain modulation detection and tolerance thresholds (CPM-detection and CPM-tolerance)39 measured at the leg to examine central pain mechanisms independently from local pain. Higher TSP Scores and lower CPM Scores equal a more facilitated (ie, abnormal) pain mechanism. Local pain sensitivity was quantified using reliable measures of pressure pain threshold (PPT)38 at four standardised body sites at the side of the painful shoulder: (1) The deltoid muscle, (2) The supraspinatus muscle,40 (3) The infraspinatus muscle40 and (4) At the site of worst pain as described by the patient (lower scores=more pain sensitivity). As prespecified22 only PPT for site 1 (deltoid muscle) was included as a potential effect modifier. Pain catastrophising was quantified using the valid and reliable Danish version of the Pain Catastrophizing Scale41—scored from 0 to 52 (higher scores equal more catastrophising). For further details on assessment of pain mechanism and catastrophising, see supplementary appendix and trial protocol (additional file 7).22 Shoulder disability was assessed using the valid and reliable Shoulder Pain and Disability Index (SPADI),42 43—scored from 0 to 100 (100=worst). A priori,22 we defined 10 points as the minimal clinical important difference, and SPADI was the primary outcome in the SExSI Trial. Shoulder strength was measured as maximum voluntary contraction in abduction and external rotation (in Nm/kg) using a handheld dynamometer. Completed add-on exercise dose was quantified as the total time-under-tension (in hours) for each phase of the intervention using BandCizer sensor technology—a sensor developed and validated specifically for capturing time under tension for elastic band exercises.44–46 Self-reported information regarding time spent on exercise in usual care was collected through a weekly text message using the SMS-track system and reported as minutes per week. The time spent per week for a given phase was calculated as a weighted average of the calendar weeks included in that phase. For further details on assessment of outcome and exposure variables, please see the trial protocol22 including its additional files.
bjsports-2022-106383supp001.pdf (313.3KB, pdf)
Statistics
We applied constrained linear mixed models (cLMM) to test the effectiveness of the add-on intervention on changes in pain mechanism outcomes and pain catastrophising. Baseline values (measured before randomisation) were constrained to be equal, and a compound symmetry covariance structure was used. This structure was chosen based on the MAICE (Minimum Akaike Information Criterion Estimation) procedure as prespecified in the published protocol.22 For intention-to-treat analysis, missing outcome data were imputed using multiple imputations (30 imputation sets). Multiple imputation was based on all previous scores in the relevant outcome, age, sex and allocation as described in the protocol22 using fully conditional specification.47 48 The missingness of data at the different time points is shown in online supplemental table 1 (available online). The assumptions of linear mixed models were evaluated by inspection of histograms, quantile-quantile plots and residual plots of the scaled residuals. To test the modifying effect of pain mechanisms and pain catastrophising on the effect of the add-on intervention for shoulder strength and disability, we performed cLMM analyses similar to the corresponding main analyses reported in the primary trial report,23 but included a dichotomised value (median split) for the first measurement of the relevant variable as the interaction term and reporting the unstandardised effect size. We used stratified cLMM analyses to obtain within-group changes and between-group differences for each strata.
We tested the dose-response relationship between add-on exercise dose and shoulder-specific outcomes (strength and disability) using linear mixed models. We included both exercise dose and shoulder strength and disability outcomes as repeated measurements, using the change in shoulder strength and disability outcomes between each follow-up time point (baseline to 5 weeks, 5 weeks to 10 weeks, and 10 weeks to 4 months) as the outcome variable. The total time under tension in a time period constituted the exercise dose. In addition, we performed the same analysis, adding average weekly time spent on other exercises in each time period as the independent variable.
To better understand our results, we performed two supplemental analyses based on grouping of participants using dichotomised baseline pain catastrophising scores (median split). In the first analysis we compared the groups to discover if they differed in the total amount of completed add-on exercise dose and average weekly time spent on other exercises, respectively. These analyses were conducted using negative binomial regression analyses. In the second analysis we examined if this grouping explained part of the association between exercise dose and change in SPADI Score by adding the pain catastrophising group as an independent variable to the previously described linear mixed model.
Sample size considerations
The study sample size was determined based on the primary study hypothesis,22 and was powered to detect a 10-point difference in SPADI after 4 months with an SD of 19.5 and 95% power at the 5% significance level. Hence, we consider these prespecified secondary analyses to be exploratory.
Equity, diversity and inclusion statement
Our study applied consecutive sampling, meaning that we included all cases irrespective of gender, race/ethnicity and socioeconomic level. We did not purposefully recruit people from marginalised communities but recruited from an uptake area that includes substantial socioeconomic diversity. The ability to understand spoken and written Danish was considered a requirement. The project group included nine men and three women, all from Denmark. The trial protocol and primary trial report have been published, and additional substudies are planned. For all studies related to this project we follow the ICMJE (International Committee of Medical Journal Editors) recommendations regarding authorship. For this substudy, our author team includes seven men. The rest of the project group members are not included as authors as they did not fulfil the ICMJE criteria for this publication. Author disciplines included physiotherapy, physiology, biostatistics and medicine. Data collection was similar in all cases, and we did not alter methods based on regional, educational or socioeconomic differences. Our analyses do not explore the effects of sex, gender, race/ethnicity or socioeconomic status as this was not within the scope of the current study.
Results
A total of 200 participants were randomly assigned to either IG or CG. Patient flow and characteristics have been described elsewhere.23
Effectiveness analyses for pain mechanisms and pain catastrophising
Intention-to-treat analyses revealed no between-group difference in pain mechanism outcomes at any time point and no between-group difference for the change in pain catastrophising from baseline to 4 months follow-up (mean difference, 1 point (95% CI −2 to 4)), see online supplemental tables 2 and 3. Pain catastrophising improved in both CG (−7 points (95% CI −9 to −5)) and IG (−6 points (95% CI −8 to −4)).
Modifying effects of pain mechanisms and pain catastrophising
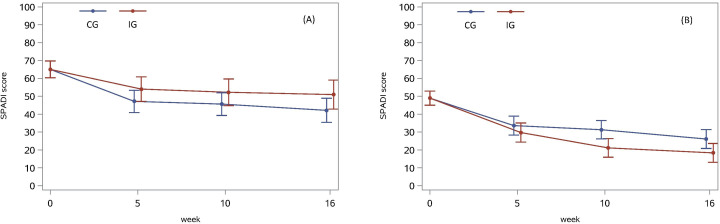
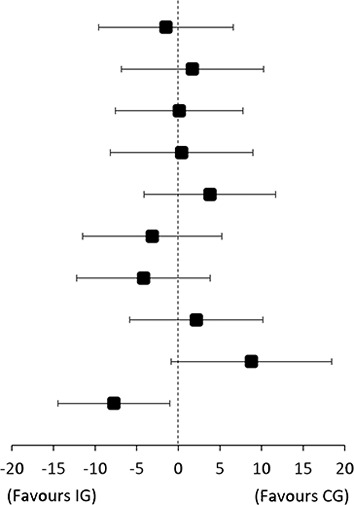
Results for the modifying effect of pain mechanisms and pain catastrophising are shown in table 1, figure 1 and online supplemental tables 4 and 5. For descriptive data on potential effect modifiers, see online supplemental table 6. Baseline level of pain catastrophising modified the effectiveness of the add-on intervention in improving SPADI Score from baseline to 4 months follow-up (effect-size 14 points (95% CI 2 to 25)), with significantly larger improvements in IG compared with CG for patients with lower levels of pain catastrophising (mean difference −8 (95% CI −14 to −1)), see figure 1. The level of CPM-detection and PPT at the deltoid muscle (PPT-deltoid) (assessed at 5 weeks follow-up) modified the effectiveness of the add-on intervention in improving external rotation strength from baseline to 4 months follow-up (effect size −0.04 Nm/kg (95% CI −0.06 to −0.01) and 0.03 Nm/kg (95% CI 0.00 to 0.06), respectively), with larger improvements in IG compared with CG for patients with lower CPM-detection Scores and higher PPT-deltoid Score.
Table 1.
The modifying effect of pain mechanisms* and pain catastrophising* on the effectiveness of the add-on intervention in improving shoulder disability and strength, and within-group changes and between-group differences in change for each stratum of potential effect modifiers
| Within-group change (baseline to week 16) | Between-group change (difference in change) | Interaction | ||||||
| Intervention | Control | Contrast | Effect | |||||
| Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | (95% CI) | ||
| SPADI, 0 (best) to 100 (worst) | ||||||||
| TSP higher | −24 | (−30 to −18) | −22 | (−28 to −16) |
|
−1 | (−10 to 7) | −3 (−15 to 8) |
| TSP lower | −21 | (−28 to −14) | −23 | (−28 to −18) | 2 | (−7 to 10) | ||
| CPM-tolerance higher | −26 | (−32 to −20) | −26 | (−32 to −21) | 0 | (−8 to 8) | 0 (−11 to 11) |
|
| CPM-tolerance lower | −17 | (−23 to −10) | −17 | (−23 to −11) | 0 | (−8 to 9) | ||
| CPM-detection higher | −21 | (−27 to −15) | −25 | (−31 to −19) | 4 | (−4 to 12) | 7 (−5 to 18) |
|
| CPM-detection lower | −22 | (−29 to −16) | −19 | (−25 to −14) | −3 | (−11 to 5) | ||
| PPT-deltoid higher | −27 | (−33 to −20) | −23 | (−28 to −17) | −4 | (−12 to 4) | −7 (−18 to 4) |
|
| PPT-deltoid lower | −20 | (−26 to −14) | −22 | (−27 to −16) | 2 | (−6 to 10) | ||
| PCS higher | −14 | (−22 to −6) | −23 | (−29 to −17) | 9 | (−1 to 18) | 14 (2 to 25) |
|
| PCS lower | −31 | (−35 to −26) | −23 | (−28 to −18) | −8 | (−14 to −1) | ||
| Abduction MVC, Nm/kg | ||||||||
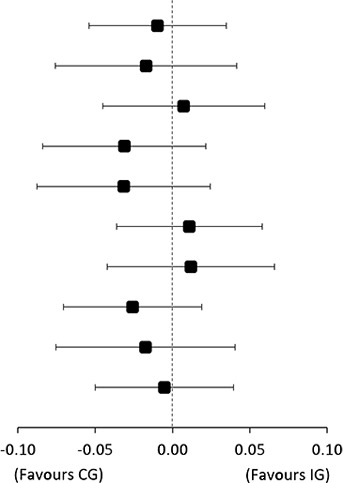
| TSP higher | 0.04 | (0.01 to 0.07) | 0.05 | (0.02 to 0.08) |
|
−0.01 | (−0.05 to 0.03) | 0.00 (−0.08 to 0.07) |
| TSP lower | 0.01 | (−0.04 to 0.06) | 0.03 | (−0.01 to 0.06) | −0.02 | (−0.08 to 0.04) | ||
| CPM-tolerance higher | 0.04 | (0.00 to 0.08) | 0.03 | (−0.01 to 0.07) | 0.01 | (−0.05 to 0.06) | 0.03 (−0.04 to 0.11) |
|
| CPM-tolerance lower | −0.01 | (−0.05 to 0.03) | 0.02 | (−0.01 to 0.05) | −0.03 | (−0.08 to 0.02) | ||
| CPM-detection higher | 0.00 | (−0.04 to 0.04) | 0.03 | (−0.01 to 0.07) | −0.03 | (−0.09 to 0.02) | −0.03 (−0.11 to 0.04) |
|
| CPM-detection lower | 0.03 | (0.00 to 0.07) | 0.02 | (−0.01 to 0.05) | 0.01 | (−0.04 to 0.06) | ||
| PPT-deltoid higher | 0.04 | (0.00 to 0.08) | 0.03 | (−0.01 to 0.06) | 0.01 | (−0.04 to 0.07) | 0.04 (−0.03 to 0.11) |
|
| PPT-deltoid lower | 0.00 | (−0.03 to 0.03) | 0.03 | (0.00 to 0.06) | −0.03 | (−0.07 to 0.02) | ||
| PCS higher | 0.00 | (−0.04 to 0.05) | 0.02 | (−0.02 to 0.06) | −0.02 | (−0.08 to 0.04) | −0.01 (−0.07 to 0.06) |
|
| PCS lower | 0.05 | (0.02 to 0.08) | 0.05 | (0.02 to 0.08) | −0.01 | (−0.05 to 0.04) | ||
| External rotation MVC, Nm/kg | ||||||||
| TSP higher | 0.00 | (−0.01 to 0.02) | −0.01 | (−0.02 to 0.01) |
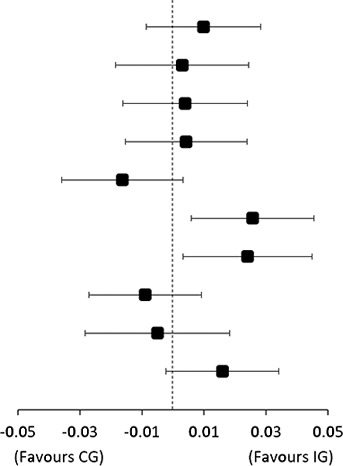
|
0.01 | (−0.01 to 0.03) | 0.00 (−0.02 to 0.03) |
| TSP lower | 0.01 | (−0.01 to 0.03) | 0.01 | (−0.01 to 0.02) | 0.00 | (−0.02 to 0.02) | ||
| CPM-tolerance higher | 0.01 | (0.00 to 0.03) | 0.01 | (−0.01 to 0.02) | 0.00 | (−0.02 to 0.02) | 0.00 (−0.03 to 0.02) |
|
| CPM-tolerance lower | 0.00 | (−0.02 to 0.02) | 0.00 | (−0.02 to 0.01) | 0.00 | (−0.02 to 0.02) | ||
| CPM-detection higher | −0.01 | (−0.02 to 0.00) | 0.01 | (−0.01 to 0.02) | −0.02 | (−0.04 to 0.00) | −0.04 (−0.06 to −0.01) |
|
| CPM-detection lower | 0.02 | (0.01 to 0.04) | 0.00 | (−0.01 to 0.01) | 0.03 | (0.01 to 0.05) | ||
| PPT-deltoid higher | 0.02 | (0.00 to 0.04) | −0.01 | (−0.02 to 0.01) | 0.02 | (0.00 to 0.04) | 0.03 (0.00 to 0.06) |
|
| PPT-deltoid lower | 0.00 | (−0.02 to 0.01) | 0.01 | (−0.01 to 0.02) | −0.01 | (−0.03 to 0.01) | ||
| PCS higher | 0.00 | (−0.02 to 0.02) | 0.00 | (−0.01 to 0.02) | 0.00 | (−0.03 to 0.02) | −0.01 (−0.04 to 0.02) |
|
| PCS lower | 0.01 | (0.00 to 0.03) | 0.00 | (−0.02 to 0.01) | 0.02 | (0.00 to 0.03) | ||
*Median split cut points: TSP=2.7 points, CPM-tolerance=1.5 kPa, CPM-detection=3.3 kPa, PPT-deltoid=176 kPa, PCS=16 points.
CPM-detection, conditioned pain modulation detection threshold; CPM-tolerance, conditioned pain modulation tolerance threshold; MVC, maximum voluntary contraction; PCS, Pain Catastrophizing Scale; PPT-deltoid, pressure pain threshold at the deltoid muscle; SPADI, Shoulder Pain and Disability Index; TSP, Temporal summation of pain.
Figure 1.
Shoulder Pain and Disability Index (SPADI) Scores with 95% CIs in the control group (CG) and intervention group (IG) before randomisation and for each follow-up time point for (A) patients with Pain Catastrophizing Scale (PCS) Score >16 and (B) patients with PCS Score ≤16.
Supplemental analyses revealed that patients with higher levels of pain catastrophising at baseline had completed less of the add-on exercise dose compared with those with lower levels of pain catastrophising (2.0 hours vs 3.7 hours, difference 1.7 hours (95% CI 0.5 to 2.7)), while the average weekly time spent on other exercises did not seem to depend on the level of pain catastrophising (43 min vs 38 min in average per week, difference 5 min (95% CI −5 to 15)).
The impact of dose and central pain mechanisms on treatment outcome
The impact of add-on exercise dose and central pain mechanisms on changes in strength and disability outcomes are presented in table 2, figure 2 and online supplemental figures 1 and 2. The dose of resistance exercise completed as part of the add-on intervention had a significant impact on change in SPADI Score, also when adjusted for the time a patient had spent on other exercises (additional improvement in SPADI per hour time under tension: −2.6 points (95% CI −4.1 to −1.1), p<0.001).
Table 2.
Difference in change in outcome per unit increase in exercise dose (time under tension) or central pain mechanism
| SPADI | Abduction strength | External rotation strength | |||||||
| mean | (95% CI) | P value | mean | (95% CI) | P value | mean | (95% CI) | P value | |
| Time under tension | −2.5 | (−4.1 to −0.9) | 0.002 | 0.01 | (−0.002 to 0.015) | 0.109 | 0.00 | (−0.002 to 0.004) | 0.485 |
| adjusted* | −2.6 | (−4.1 to −1.1) | 0.001 | 0.00 | (−0.004 to 0.011) | 0.317 | 0.00 | (−0.002 to 0.004) | 0.452 |
| TSP | 0.7 | (−0.4 to 1.8) | 0.212 | 0.00 | (−0.009 to 0.004) | 0.401 | 0.00 | (−0.006 to 0.001) | 0.137 |
| adjusted* | 0.8 | (−0.3 to 1.9) | 0.168 | 0.00 | (−0.009 to 0.004) | 0.462 | 0.00 | (−0.006 to 0.001) | 0.146 |
| CPM-tolerance | 0.1 | (−0.2 to 0.3) | 0.538 | 0.00 | (−0.001 to 0.002) | 0.704 | 0.00 | (−0.001 to 0.001) | 0.849 |
| adjusted* | 0.2 | (−0.1 to 0.5) | 0.219 | 0.00 | (−0.001 to 0.002) | 0.694 | 0.00 | (−0.001 to 0.001) | 0.810 |
| CPM-detection | 0.0 | (−0.1 to 0.2) | 0.549 | 0.00 | (−0.001 to 0.001) | 0.529 | 0.00 | (−0.001 to 0) | 0.021 |
| adjusted* | 0.0 | (−0.1 to 0.2) | 0.502 | 0.00 | (−0.001 to 0.001) | 0.506 | −0.0004 | (−0.0007 to −0.0001) | 0.009 |
*Adjusted for exercise time in usual care.
CPM-detection, conditioned pain modulation detection threshold; CPM-tolerance, conditioned pain modulation tolerance threshold; TSP, temporal summation of pain.
Figure 2.
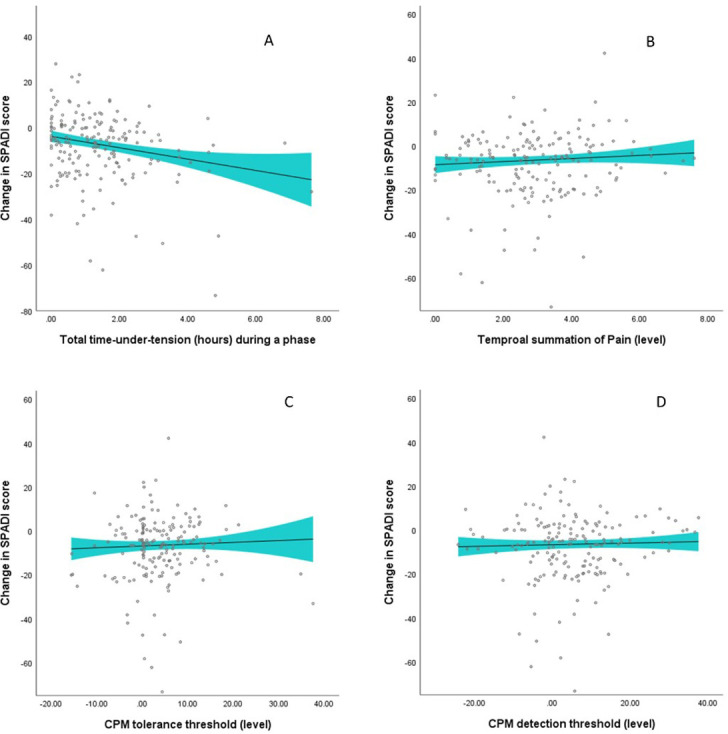
Changes in Shoulder Pain and Disability Index (SPADI) during a phase (baseline to 5 weeks, 5 weeks to 10 weeks, and 10 weeks to 16 weeks) per unit increase in (A) exercise dose, (B) level of temporal summation of pain, (C) level of CPM tolerance threshold and (D) level of CPM detection threshold for the respective phase. The curve is a smooth curve fitted to the predicted values obtained from the linear mixed model and fitted using a kernel density estimator. The shaded portion represents a smoothing of the 95% confidence limits of individual prediction intervals. CPM, conditioned pain modulation.
We also found that the time spent on usual care exercise was significantly related to changes in SPADI Score when adjusted for completed add-on exercise dose (−0.05 points in SPADI (95% CI −0.10 to −0.01) for every minute spent per week during a phase), see online supplemental figure 3. This means that an additional 60 min spent per week during a 5–6 weeks phase is associated with ~3 points improvement in SPADI during that time period. Add-on exercise dose and time spent on usual care exercise did not significantly impact the change in shoulder strength outcomes. CPM-detection was significantly related to changes in external rotation strength, also when adjusted for the time a patient had spent on other exercises (additional improvement in strength per kPa increase in CPM-detection: −0.0004 Nm/kg, 95% CI −0.0007 to −0.0001, p=0.009).
The supplemental analysis revealed that the relationship between completed add-on exercise dose and change in SPADI Score remained of a similar magnitude when adjusting for baseline pain catastrophising (higher vs lower), with −2.3 SPADI points per hour ((95% CI −3.8 to −0.8), p=0.0029).
Discussion
We found that adding a large resistance exercise dose to usual care—using a time-contingent approach with brief pain education—is not superior to usual care alone in improving pain mechanisms or pain catastrophising in patients with subacromial impingement, but the level of pain catastrophising modifies its effectiveness in improving shoulder disability. Further, we found no evidence of a modifying effect of pain mechanisms in relation to shoulder disability, but we did find that a larger exercise dose was related to larger improvements in shoulder disability but not shoulder strength.
Negative pain cognitions hinder benefit from more resistance exercise
The predefined secondary analyses reported here extends the findings of the primary report,23 revealing that the lack of overall effect from additional exercise could be explained through a differing response depending on pain cognition (ie, pain catastrophising). The effect was favourable in patients with lower level of pain catastrophising (−8 points, 95% CI −14 to −1), but absent with a tendency towards the unfavourable in patients with higher level of pain catastrophising (9 points, 95% CI −1 to 18). From our results, it appears that the brief pain education was not sufficient to improve negative pain cognitions. This may help explain our finding that patients with higher levels of pain catastrophising completed less of the additional exercise and thus provide an explanation why they did not benefit from additional exercise. Accordingly, higher levels of pain catastrophising have been linked to increased exercise-induced hyperalgesia27 while a larger pain response may reduce the willingness to complete further exercise sessions.49 Nevertheless, the difference in completed additional exercise dose (2 vs 3.7 hours) does not fully explain the difference in effect between the two strata (14 points difference in SPADI), let alone the tendency towards an unfavourable effect in patients with higher levels of pain catastrophising, especially when considering the dose-response relationship (~3 SPADI points per hour of performed exercise), regardless of pain catastrophising level. As such, other elements of the intervention might have had an unfavourable impact on patients with higher levels of pain catastrophising. We speculate that using an approach that allows exercises to be painful could be counterproductive in the presence of pain catastrophising. Regardless of this, our combined findings indicate that improving exercise adherence could be a way to improve shoulder disability outcomes but also suggests that different strategies might be relevant depending on level of pain catastrophising.
Impact of dose and pain mechanisms on shoulder strengthening
We found evidence of a modifying effect of PPT-deltoid level, with higher PPT related to a significant effect of additional exercise in increasing external rotation strength, possibly because less sensitivity to pain provides a better foundation for proper loading during exercise. Similarly, we found evidence of a modifying effect of CPM-detection with lower (ie, worse) pain modulation related to a significant effect of additional exercise in increasing external rotation strength. This could be explained by the low relative resistance and high volume used in the first phases, which is found to elicit the highest gains in muscle strength in untrained individuals,50 but only if patients with lower (ie, worse) CPM-detection are also less trained to begin with. Finally, shoulder strengthening exercise dose did not significantly impact the change in shoulder strength outcomes. This suggests that the mode of exercise was not useful to improve strength but considering the lack of improvements in many previous trials51 other explanations could be relevant, one being that the painful condition has hindered patients from achieving sufficient loading stimuli to elicit an increase in shoulder strength.
Clinical implications
Stratified care has been suggested as the solution to improve non-operative care for patients with subacromial impingement,52 53 based on the understanding that the diagnostic category is too broad to efficiently guide treatment.52 While previous studies on this subject have described a theoretical framework, ours is the first to provide clinical evidence in favour of stratified care, by showing that pain catastrophising modifies the response to additional exercise. Most importantly this study showed that adding more—while allowing exercises to be painful—was only a useful strategy for patients with lower levels of pain catastrophising.
Strengths and limitations
Important strengths of the current study are that our findings are based on prespecified analyses of data collected through a large randomised clinical trial in a clinical population of consecutive patients. Limitations include the risk of measurement bias in the intention-to-treat estimates and the fact that sample size was determined by the primary hypothesising means that we consider the analyses reported here as exploratory. Our findings have the potential to broaden our knowledge regarding the interplay between pain cognitions, behaviour and exercise response, but further confirmatory studies are needed.
Conclusion
Additional resistance exercise added to usual care did not result in superior improvements in pain mechanism or pain catastrophising outcomes when compared with usual care alone. The level of pain catastrophising modified the effectiveness of additional resistance exercise in improving shoulder disability, with additional exercise being superior in improving self-reported disability in patients with lower levels of pain catastrophising at baseline. Furthermore, larger completed add-on exercise dose is associated with larger improvements in shoulder disability.
Acknowledgments
The authors thank all orthopaedic specialists and nurses at the Sports Orthopedic Research Center–Copenhagen, Department of Orthopedic Surgery, Amager-Hvidovre Hospital, for helping with the implementation of this trial. The authors also thank Laura Krohn, Magnus Baasch Ahlfors, Mathias Fabricius Nielsen and Chanel Beldner Hansen for their assistance during the trial.
Footnotes
Twitter: @MikkelBek, @TBandholm, @KThorborg
Contributors: MC is the grant holder, primary investigator and guarantor of this trial. MC and KT conceived the study idea. MC, KT, TB and MR initiated the study design. MC, KT, TB, MR, TG, KC and PH contributed to study design. MC drafted the study protocol and all authors contributed to protocol refinement and approved the final protocol. KC provided statistical expertise in the trial design and planning of statistical analyses. MC, KT and PH took part in funding acquisition. MC was responsible for coordination, administration and monitoring of the trial. PH and KT supported implementation of the project. KC conducted all statistical analyses. MC and KT secured execution and completion of the project. MC, KT and MR conducted training of the study personnel in testing procedures. MC drafted the original manuscripts. All authors contributed significantly to the interpretation of results and all have reviewed and edited the manuscript and approved the final version.
Funding: Fysioterapipraksisfonden has partly funded the salary of MC, the salary of other study personnel and testing equipment (Kr 805 000). The Danish Rheumatism Association has funded testing equipment (Kr 50 000). TG is a part of Center for Neuroplasticity and Pain (CNAP) supported by the Danish National Research Foundation (DNRF121).
Competing interests: TB has received speaking fees from Zimmer Biomet and Novartis. All authors declare no other relationships or activities that could appear to have influenced the submitted work.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the supplement file in the primary trial report for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. With publication of this trial, a complete de-identified data set on individual patients on which the analysis, results and conclusions reported in the paper are based, will be made available to researchers who provide a methodologically sound proposal and whose proposed use of the data has been approved. Proposals should be directed to mikkelbek@gmail.com. To gain access, data requestors will need to sign a data access agreement. The data can be used only to achieve aims in the approved proposal. Use of the data to conduct analyses solely based on this data set and in relation to research aims that are closely related to those described in the published trial protocol is not allowed without approval from the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Regional Ethics Committee of the Capital Region of Denmark (approval number H-16016763). Participants gave informed consent to participate in the study before taking part.
References
- 1. Urwin M, Symmons D, Allison T, et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis 1998;57:649–55. 10.1136/ard.57.11.649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostör AJK, Richards CA, Prevost AT, et al. Diagnosis and relation to general health of shoulder disorders presenting to primary care. Rheumatology (Oxford) 2005;44:800–5. 10.1093/rheumatology/keh598 [DOI] [PubMed] [Google Scholar]
- 3. van der Windt DA, Koes BW, de Jong BA, et al. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis 1995;54:959–64. 10.1136/ard.54.12.959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Windt DA, Koes BW, Boeke AJ, et al. Shoulder disorders in general practice: prognostic indicators of outcome. Br J Gen Pract 1996;46:519–23. [PMC free article] [PubMed] [Google Scholar]
- 5. Clausen MB, Merrild MB, Holm K, et al. Less than half of patients in secondary care adheres to clinical guidelines for subacromial pain syndrome and have acceptable symptoms after treatment: a Danish nationwide cohort study of 3306 patients. Musculoskelet Sci Pract 2021;52:102322. 10.1016/j.msksp.2021.102322 [DOI] [PubMed] [Google Scholar]
- 6. Danish Health Authority . National clinical guideline on diagnostics and treatment of patients with selected shoulder conditions. 2016. Available: https://www.sst.dk/da/udgivelser/2013/~/media/FCAD4C2F10564900983B5BDC15D764DD.ashx [Accessed 15 Mar 2018].
- 7. The Royal College of Surgeons of England . Subacromial shoulder pain - commissioning guide. England; 2014. Available: https://www.rcseng.ac.uk/library-and-publications/rcs-publications/docs/subacromial-shoulder-pain/ [Google Scholar]
- 8. Diercks R, Bron C, Dorrestijn O, et al. Guideline for diagnosis and treatment of subacromial pain syndrome: a multidisciplinary review by the Dutch orthopaedic association. Acta Orthop 2014;85:314–22. 10.3109/17453674.2014.920991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. Optimizing the management of rotator cuff problems. J Am Acad Orthop Surg 2011;19:368–79. 10.5435/00124635-201106000-00007 [DOI] [PubMed] [Google Scholar]
- 10. Hopman K, Lukersmith S, McColl A, et al. Clinical practice guidelines for the management of rotator cuff syndrome in the workplace. Port Macquarie, Australia: University of South Wales, 2013. [Google Scholar]
- 11. Vandvik PO, Lähdeoja T, Ardern C, et al. Subacromial decompression surgery for adults with shoulder pain: a clinical practice guideline. BMJ 2019;364:l294. 10.1136/bmj.l294 [DOI] [PubMed] [Google Scholar]
- 12. Bang MD, Deyle GD. Comparison of supervised exercise with and without manual physical therapy for patients with shoulder impingement syndrome. J Orthop Sports Phys Ther 2000;30:126–37. 10.2519/jospt.2000.30.3.126 [DOI] [PubMed] [Google Scholar]
- 13. Başkurt Z, Başkurt F, Gelecek N, et al. The effectiveness of scapular stabilization exercise in the patients with subacromial impingement syndrome. J Back Musculoskelet Rehabil 2011;24:173–9. 10.3233/BMR-2011-0291 [DOI] [PubMed] [Google Scholar]
- 14. Bennell K, Wee E, Coburn S, et al. Efficacy of standardised manual therapy and home exercise programme for chronic rotator cuff disease: randomised placebo controlled trial. BMJ 2010;340:c2756. 10.1136/bmj.c2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dilek B, Gulbahar S, Gundogdu M, et al. Efficacy of proprioceptive exercises in patients with subacromial impingement syndrome: a single-blinded randomized controlled study. Am J Phys Med Rehabil 2016;95:169–82. 10.1097/PHM.0000000000000327 [DOI] [PubMed] [Google Scholar]
- 16. Galace de Freitas D, Marcondes FB, Monteiro RL, et al. Pulsed electromagnetic field and exercises in patients with shoulder impingement syndrome: a randomized, double-blind, placebo-controlled clinical trial. Arch Phys Med Rehabil 2014;95:345–52. 10.1016/j.apmr.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 17. Ingwersen KG, Jensen SL, Sørensen L, et al. Three months of progressive high-load versus traditional low-load strength training among patients with rotator cuff tendinopathy: primary results from the double-blind randomized controlled roctex trial. Orthop J Sports Med 2017;5:2325967117723292. 10.1177/2325967117723292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lombardi I, Magri AG, Fleury AM, et al. Progressive resistance training in patients with shoulder impingement syndrome: a randomized controlled trial. Arthritis Rheum 2008;59:615–22. 10.1002/art.23576 [DOI] [PubMed] [Google Scholar]
- 19. Maenhout AG, Mahieu NN, De Muynck M, et al. Does adding heavy load eccentric training to rehabilitation of patients with unilateral subacromial impingement result in better outcome? A randomized, clinical trial. Knee Surg Sports Traumatol Arthrosc 2013;21:1158–67. 10.1007/s00167-012-2012-8 [DOI] [PubMed] [Google Scholar]
- 20. McClure PW, Bialker J, Neff N, et al. Shoulder function and 3-dimensional kinematics in people with shoulder impingement syndrome before and after a 6-week exercise program. Phys Ther 2004;84:832–48. 10.1093/ptj/84.9.832 [DOI] [PubMed] [Google Scholar]
- 21. Struyf F, Nijs J, Mollekens S, et al. Scapular-focused treatment in patients with shoulder impingement syndrome: a randomized clinical trial. Clin Rheumatol 2013;32:73–85. 10.1007/s10067-012-2093-2 [DOI] [PubMed] [Google Scholar]
- 22. Clausen MB, Bandholm T, Rathleff MS, et al. The Strengthening Exercises in Shoulder Impingement trial (The SExSI-trial) investigating the effectiveness of a simple add-on shoulder strengthening exercise programme in patients with long-lasting subacromial impingement syndrome: Study protocol for a pragmatic, assessor blinded, parallel-group, randomised, controlled trial. Trials 2018;19:154. 10.1186/s13063-018-2509-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clausen MB, Hölmich P, Rathleff M, et al. Effectiveness of adding a large dose of shoulder strengthening to current nonoperative care for subacromial impingement: a pragmatic, double-blind randomized controlled trial (SExSI Trial). Am J Sports Med 2021;49:3040–9. 10.1177/03635465211016008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sanchis MN, Lluch E, Nijs J, et al. The role of central sensitization in shoulder pain: a systematic literature review. Semin Arthritis Rheum 2015;44:710–6. 10.1016/j.semarthrit.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 25. Chester R, Jerosch-Herold C, Lewis J, et al. Psychological factors are associated with the outcome of physiotherapy for people with shoulder pain: a multicentre longitudinal cohort study. Br J Sports Med 2018;52:269–75. 10.1136/bjsports-2016-096084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nijs J, Malfliet A, Ickmans K, et al. Treatment of central sensitization in patients with “unexplained” chronic pain: an update. Expert Opin Pharmacother 2014;15:1671–83. 10.1517/14656566.2014.925446 [DOI] [PubMed] [Google Scholar]
- 27. Brellenthin AG, Crombie KM, Cook DB, et al. Psychosocial influences on exercise-induced hypoalgesia. Pain Med 2017;18:538–50. 10.1093/pm/pnw275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. George SZ, Parr JJ, Wallace MR, et al. Biopsychosocial influence on exercise-induced injury: genetic and psychological combinations are predictive of shoulder pain phenotypes. J Pain 2014;15:68–80. 10.1016/j.jpain.2013.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mansournia MA, Collins GS, Nielsen RO, et al. A checklist for statistical assessment of medical papers (the CHAMP statement): explanation and elaboration. Br J Sports Med 2021;55:1009–17. 10.1136/bjsports-2020-103652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burd NA, Andrews RJ, West DWD, et al. Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. J Physiol 2012;590:351–62. 10.1113/jphysiol.2011.221200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Izquierdo M, Ibañez J, González-Badillo JJ, et al. Differential effects of strength training leading to failure versus not to failure on hormonal responses, strength, and muscle power gains. J Appl Physiol (1985) 2006;100:1647–56. 10.1152/japplphysiol.01400.2005 [DOI] [PubMed] [Google Scholar]
- 32. de Silva D, Health Foundation (Great Britain) . Helping people help themselves: A review of the evidence considering whether it is worthwhile to support self-management. 2011.
- 33. Nijs J, Paul van Wilgen C, Van Oosterwijck J, et al. How to explain central sensitization to patients with “unexplained” chronic musculoskeletal pain: practice guidelines. Man Ther 2011;16:413–8. 10.1016/j.math.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 34. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (tidier) checklist and guide. BMJ 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 35. Graven-Nielsen T, Wodehouse T, Langford RM, et al. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum 2012;64:2907–16. 10.1002/art.34466 [DOI] [PubMed] [Google Scholar]
- 36. Skou ST, Graven-Nielsen T, Rasmussen S, et al. Widespread sensitization in patients with chronic pain after revision total knee arthroplasty. Pain 2013;154:1588–94. 10.1016/j.pain.2013.04.033 [DOI] [PubMed] [Google Scholar]
- 37. Rathleff MS, Petersen KK, Arendt-Nielsen L, et al. Impaired conditioned pain modulation in young female adults with long-standing patellofemoral pain: a single blinded cross-sectional study. Pain Med 2016;17:980–8. 10.1093/pm/pnv017 [DOI] [PubMed] [Google Scholar]
- 38. Graven-Nielsen T, Vaegter HB, Finocchietti S, et al. Assessment of musculoskeletal pain sensitivity and temporal summation by cuff pressure algometry: a reliability study. Pain 2015;156:2193–202. 10.1097/j.pain.0000000000000294 [DOI] [PubMed] [Google Scholar]
- 39. Graven-Nielsen T, Izumi M, Petersen KK, et al. User-independent assessment of conditioning pain modulation by cuff pressure algometry. Eur J Pain 2017;21:552–61. 10.1002/ejp.958 [DOI] [PubMed] [Google Scholar]
- 40. Hidalgo-Lozano A, Fernández-de-las-Peñas C, Alonso-Blanco C, et al. Muscle trigger points and pressure pain hyperalgesia in the shoulder muscles in patients with unilateral shoulder impingement: a blinded, controlled study. Exp Brain Res 2010;202:915–25. 10.1007/s00221-010-2196-4 [DOI] [PubMed] [Google Scholar]
- 41. Kjøgx H, Zachariae R, Pfeiffer-Jensen M, et al. Pain frequency moderates the relationship between pain catastrophizing and pain. Front Psychol 2014;5:1421. 10.3389/fpsyg.2014.01421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roach KE, Budiman-Mak E, Songsiridej N, et al. Development of a shoulder pain and disability index. Arthritis Care Res 1991;4:143–9. [PubMed] [Google Scholar]
- 43. Christiansen DH, Andersen JH, Haahr JP. Cross-cultural adaption and measurement properties of the danish version of the shoulder pain and disability index. Clin Rehabil 2013;27:355–60. 10.1177/0269215512456220 [DOI] [PubMed] [Google Scholar]
- 44. Rathleff MS, Bandholm T, Ahrendt P, et al. Novel stretch-sensor technology allows quantification of adherence and quality of home-exercises: a validation study. Br J Sports Med 2014;48:724–8. 10.1136/bjsports-2012-091859 [DOI] [PubMed] [Google Scholar]
- 45. Rathleff MS, Thorborg K, Rode LA, et al. Adherence to commonly prescribed, home-based strength training exercises for the lower extremity can be objectively monitored using the bandcizer. J Strength Cond Res 2015;29:627–36. 10.1519/JSC.0000000000000675 [DOI] [PubMed] [Google Scholar]
- 46. Skovdal Rathleff M, Thorborg K, Bandholm T. Concentric and eccentric time-under-tension during strengthening exercises: validity and reliability of stretch-sensor recordings from an elastic exercise-band. PLoS ONE 2013;8:e68172. 10.1371/journal.pone.0068172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van Buuren S, Brand JPL, Groothuis-Oudshoorn CGM, et al. Fully conditional specification in multivariate imputation. J Stat Comput Simul 2006;76:1049–64. 10.1080/10629360600810434 [DOI] [Google Scholar]
- 48. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–42. 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 49. Littlewood C, Malliaras P, Mawson S, et al. Patients with rotator cuff tendinopathy can successfully self-manage, but with certain caveats: a qualitative study. Physiotherapy 2014;100:80–5. 10.1016/j.physio.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 50. Peterson MD, Rhea MR, Alvar BA. Applications of the dose-response for muscular strength development: a review of meta-analytic efficacy and reliability for designing training prescription. J Strength Cond Res 2005;19:950–8. 10.1519/R-16874.1 [DOI] [PubMed] [Google Scholar]
- 51. Clausen MB, Hölmich P, Rathleff MS, et al. Effectiveness of adding a large dose of shoulder strengthening to current nonoperative care for subacromial impingement: a pragmatic, double-blind randomized controlled trial (SExSI Trial): response. Am J Sports Med 2022;50:20–3. 10.1177/03635465211055449 [DOI] [PubMed] [Google Scholar]
- 52. Cools AM, Michener LA. Shoulder pain: can one label satisfy everyone and everything? Br J Sports Med 2017;51:416–7. 10.1136/bjsports-2016-096772 [DOI] [PubMed] [Google Scholar]
- 53. McClure PW, Michener LA. Staged approach for rehabilitation classification: shoulder disorders (STAR-shoulder). Phys Ther 2015;95:791–800. 10.2522/ptj.20140156 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2022-106383supp001.pdf (313.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. With publication of this trial, a complete de-identified data set on individual patients on which the analysis, results and conclusions reported in the paper are based, will be made available to researchers who provide a methodologically sound proposal and whose proposed use of the data has been approved. Proposals should be directed to mikkelbek@gmail.com. To gain access, data requestors will need to sign a data access agreement. The data can be used only to achieve aims in the approved proposal. Use of the data to conduct analyses solely based on this data set and in relation to research aims that are closely related to those described in the published trial protocol is not allowed without approval from the corresponding author.