Abstract
Introduction
Continuity of child and family healthcare is vital for optimal child health and development for developmentally vulnerable children. Migrant and refugee communities are often at-risk of poor health outcomes, facing barriers to health service attendance including cultural, language, limited health literacy, discrimination and unmet psychosocial needs. ‘Integrated health-social care hubs’ are physical hubs where health and social services are co-located, with shared referral pathways and care navigation.
Aim
Our study will evaluate the impact, implementation and cost-benefit of the First 2000 Days Care Connect (FDCC) integrated hub model for pregnant migrant and refugee women and their infants.
Materials and methods
This study has three components. Component 1 is a non-randomised controlled trial to compare the FDCC model of care with usual care. This trial will allocate eligible women to intervention and control groups based on their proximity to the Hub sites. Outcome measures include: the proportion of children attending child and family health (CFH) nurse services and completing their CFH checks to 12 months of age; improved surveillance of growth and development in children up to 12 months, post partum; improved breastfeeding rates; reduced emergency department presentations; and improved maternal well-being. These will be measured using linked medical record data and surveys. Component 2 will involve a mixed-method implementation evaluation to clarify how and why FDCC was implemented within the sites to inform future roll-out. Component 3 is a within-trial economic evaluation from a healthcare perspective to assess the cost-effectiveness of the Hubs relative to usual care and the implementation costs if Hubs were scaled and replicated.
Ethics and dissemination
Ethical approval was granted by the South Eastern Sydney Local Health District Human Research Ethics Committee in July 2021 (Project ID: 020/ETH03295). Results will be submitted for publication in peer-reviewed journals and presented at relevant conferences.
Trial registration number
ACTRN12621001088831.
Keywords: Community child health, Maternal medicine, Organisation of health services, PAEDIATRICS, PUBLIC HEALTH
Strengths and limitations of this study.
The study has an embedded implementation evaluation and economic evaluation in addition to the non-randomised trial component of the study.
A strength of the design of the study is the logic modelling process used to map the implementation context and intervention components to guide data collection methods.
A strength of the design of the implementation evaluation is a mixed-methods approach that will enable the triangulation of barriers and facilitators to implementing hubs with implementation success across the sites qualitatively and quantitatively.
The non-randomised design of the trial has some limitations, particularly the inability to guarantee the comparability of the intervention and control groups.
Background and rationale
In New South Wales (NSW), Australia, 25% of children from migrant and refugee families are ‘developmentally vulnerable’.1 Developmental vulnerability is measured by the Australian Early Development Census across five domains including physical health and well-being, social competence, emotional maturity, language and cognitive skills and communication skills and general knowledge. Children who are in the lowest 10% of the national population are classified as developmentally ‘vulnerable’.1 Developmental vulnerability is associated with undetected maternal postnatal depression, the early cessation of breast feeding2 and parental unmet psychosocial needs (eg, housing, domestic violence).3 4 Children who are developmentally vulnerable are twice as likely to struggle at school, experience adverse childhood events and have poorer long-term health outcomes and higher healthcare costs.1 5–12 These adverse childhood events can continue into adulthood, contributing up to 44% of adult morbidity.13 14
Continuity of care with regular child and family health (CFH) checks by local health district (LHD) employed child and family health nurses (CFHN) are the foundation for optimal child health and development. This is particularly the case for priority populations, including newly arrived migrant and refugee women, children and their families.5 However, these populations also experience significant barriers to services including cultural, language, limited health literacy, discrimination and unmet psychosocial needs.15–31 Families with greater disadvantage are at greater risk of developmental vulnerability and poorer maternal mental health and other health problems. These families are less likely to engage with health services, particularly health promotion programmes like CFH checks.2–4 15 32–36
Australian policymakers identified service areas that need improvement to optimise outcomes in the first 2000 days of a child’s life.5 37 These include the transition from maternity to CFH services; increasing uptake and length of time families stay connected with CFH services; and supporting priority populations. Unfortunately, in NSW, two-thirds of the children stop attending CFH services by 12 months of age,15 18–20 further fragmenting care.
Benefits of integrated health-social care hubs
To address the fragmented CFH services for priority populations, integrated health-social care hubs were established in multiple jurisdictions across Australia. These are physical hubs where health and social services are co-located, supported by care navigators and shared referral pathways.38 39 Co-location and navigation support aims to remove barriers that hinder engagement between families and CFH services. However, the evidence-base for their effectiveness is limited. Our recent systematic review demonstrated the dearth of experimental trial evidence in Australia regarding physical CFH Hubs.40 Yet, individual studies have found Hub models increase access to CFH services and the identification of developmental vulnerability.40 Additionally, a recent scoping review of models of care across the continuum of pregnancy, birth and the postpartum period for women from migrant and refugee backgrounds in high-income countries highlighted an evidence gap for models that improved maternal and child infant health outcomes.8
We have extended this evidence-base by showing the feasibility and efficacy of integrated CFH hubs and cross-cultural workers (CCW) models in South Eastern Sydney.8 41–43 These models support women and families to navigate maternity, CFH and community-based services, providing continuity of care across the continuum of pregnancy and transition to CFH. The pilot interventions demonstrated that for women and families from migrant and refugee populations: CFHN services embedded in integrated hubs increased the completion rate of CFH checks from 30% to 60% at 12 months and facilitated linkage with co-located non-government organisations.41 42 CCW support in pregnancy was also highly rated by staff and pregnant women regarding support for pregnancy and linkage with services.44 45
Current study: First 2000 Days Care Connect
First 2000 Days Care Connect (FDCC) is an integrated health-social care hub model that builds on these feasible and acceptable pilot interventions. The FDCC model involves co-located CFH services and non-government organisations (NGO), including psychosocial support services (eg, playgroups, domestic violence support, mental health support, early childhood education, family support). These services operate from a physical location to facilitate service collaboration, integration and a community-led approach to local needs. This Hub is supported by care navigation, increasing continuity from maternity to CFH services.
Objectives
The overall aim of the FDCC study is to evaluate: the impact of FDCC (an integrated CFH Hub) on attendance at CFHN services and completion of CFH checks, support of child growth and development, breast feeding and maternal well-being and meeting family psychosocial needs (Component 1); the process of implementing FDCC (Component 2); and the cost-effectiveness of FDCC (Component 3).
Methods and analysis
Study setting
FDCC is a multisite study, conducted across three metropolitan LHDs in Greater Sydney, NSW—namely, South Eastern Sydney Local Health District (SESLHD), South Western Sydney Local Health District (SWSLHD) and Northern Sydney Local Health District (NSLHD). Participants will be recruited from public and universally available antenatal services at participating public hospitals within the LHDs and receive services from CFHN services within each LHD.
Recruitment and consent
The study will recruit 240 women between November 2021 and April 2022. Eighty participants will be enrolled within SESLHD, NSLHD and SWSLHD, with 40 allocated to the intervention arm (FDCC Hub) and 40 to the control arm (routine care). Potential participants are women attending antenatal clinics at the participating public hospitals within each study site and fulfilling the eligibility criteria (table 1).
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
Eligible women will be expectant mothers who are:
|
|
Using three processes, midwives and CCWs (where available) will identify eligible women attending antenatal services at the intervention sites during regular consultations. The processes include: midwives and CCW introduce the project to women attending a group model of antenatal care; midwives will promote the study during individual hospital antenatal visits and provide potential participants a flyer; and midwives will identify potential participants who meet the eligibility criteria and provide study details during regular antenatal visits. If potential participants provide verbal consent, they will be introduced to the project officer. The project officer will explain the study and provide a participant information sheet and consent form (PISCF) using translated documents and/or interpreter services, if required. They will confirm eligibility at face-to-face clinical visits or via telephone consultation. If the woman is not interested in the study, there will be no further contact regarding the study.
Participants will provide informed consent via completing paper-based consent forms, via email or verbally via phone or via online electronic signature option using the Research Electronic Data Capture (REDCap) database. Participants consenting to the study can opt out of the data linkage component.
For component 2, once the FDCC trial is underway, the project implementation scientist will contact participating CFHNs, NGO staff and Hub administrative staff via telephone and/or email to invite them to an interview or focus group. Prior to the interviews and focus groups, the implementation researcher will describe the study to participants and its rationale, providing a PISCF, and obtain informed consent. Hub staff and service leaders, including LHD partners and policymakers, will be invited to complete a 32-item online survey at the completion of Component 1. The online survey will include a detailed description of the study, rationale and an opportunity to indicate informed consent before survey completion. Hub staff and managers who do not complete the survey will receive a reminder thrice via email.
Study procedures
This protocol has used the Standard Protocol Items: Recommendations for Interventional Trials (online supplemental spirit checklist) reporting guidelines.46 Following the identification of potential participants, project officers will confirm participant eligibility as part of the consent process. This is a non-randomised study whereby eligible participants will be allocated to a study arm (FDCC intervention or control group) based on their residential postcode at the time of enrolment (see below). Participation will be 12 months, including: intervention allocation; intervention delivery (12 months); and data collection (baseline, 6 months post partum, 12-month post partum). In addition to English, the study materials will be translated in the six most common community languages (Arabic, Bengali, Simplified Chinese, Korean, Hindi and Vietnamese).
bmjopen-2022-061002supp002.pdf (81.4KB, pdf)
Allocation, concealment and implementation
Women attending antenatal services from the participating hospitals who live in a defined geographical area (postcode) served by an established CFH Hub in their LHD will be allocated to the FDCC intervention group. Women attending antenatal services from the participating hospitals but do not live in the defined geographical area above will be in the control group.
Blinding
Given the nature of the study, blinding to group allocation is impractical. However, as the intervention is dependent on participant postcode of residence, there is expected to be minimal treatment contamination between the intervention and control groups. To assess for intervention contamination, women in all groups will be asked at the 12 months postpartum assessment regarding the use of any Hub and CFHN service. While the site project officers collecting survey data at each site will not be blinded to allocation, the researcher analysing data will be blinded to group allocation.
Intervention
After recruitment, the Hub navigator or key worker (ie, an individual based at the hub responsible for linking participants with services, usually the CFHN) will contact participants to introduce Hub services and support engagement with identified services, if needed. This will be followed by another contact between birth and 8 weeks post partum. Following mothers’ and infants’ discharge from birthing services, women will access CFH services via the Hub, as well as psychosocial support services suited to maternal needs and preferences. Per routine care, all women and their babies will be offered an appointment (approximately 1 hour) with a CFHN at 1–4 weeks post partum, 6–8 weeks post partum, 6 months post partum and 12 months post partum.
Hub services will be face-to-face, online and one-to-one. Some services, such as playgroup or mothers’ groups, might be in a group setting. Mothers and their babies will have access to the Hub for 12 months. Further contacts with the Hub navigator or keyworker as participants require.
The integrated FDCC Hubs are a physical building and a way of working, facilitating service collaboration, integration and a community-led approach to local needs. Hubs most commonly operate from a host building from which partner community-based or public services are delivered. In our Hub model, CFH services are co-located with NGOs. Families are linked with psychosocial support services, including playgroups, early childhood learning opportunities and family support. Within the Hub services, existing CFH and NGO services support families to navigate systems and engage with other health services. These include general practitioners, early childhood, education and psychosocial support to address their needs.
Control arm: routine care
Pregnant women attending the participating hospitals who meet eligibility criteria but do not live in the geographical area will be allocated to a control cohort and receive routine care (eg, receive information on CFHN services at discharge and follow-up as per current pathways).
Implementation evaluation
Our mixed-methods implementation evaluation will assess the barriers and facilitators to implementing the FDCC Hubs at the three sites, as guided by the consolidated framework for implementation research (CFIR).47 The CFIR is a comprehensive framework designed to ‘offer an overarching typology to promote implementation theory development and verification about what works where and why across multiple contexts’.47 The CFIR is widely used in diverse healthcare contexts, including primary care.48 The CFIR identifies five major domains and guides the consideration and assessment of factors that can impact intervention implementation and effectiveness. Additionally, the researchers will evaluate specific implementation outcomes of acceptability, appropriateness, fidelity to the implementation strategy, coverage, sustainability and cost (table 2) as guided by the taxonomy proposed by Proctor and colleagues.49
Table 2.
Proctor and colleagues implementation outcomes mapped to First 2000 Days Care Connect evaluation
| Questions addressed by each implementation factor | |
| Acceptability | Do Hub staff and families view the Hub model as acceptable? |
| Adoption | Do Hub staff intend to apply the Hub model as described in the study protocol? |
| Appropriateness | Do Hub staff perceive the Hub model as relevant and useful for their services? |
| Fidelity | Is the Hub model applied as intended? |
| Coverage | How many eligible families are reached through the Hub model and keyworker? |
| Cost | How much does it cost to implement Hubs? |
| Sustainability | What are the factors that will allow the Hubs to be sustained/scaled-up further? |
Logic model
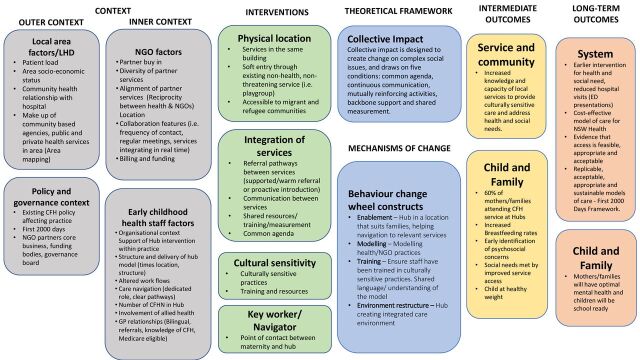
We developed a logic model to inform the FDCC implementation evaluation (figure 1). We used a modified version of existing logic model frameworks50 51 to include the inner context (ie, individual factors, organisational settings) and the outer context of each site (ie, area demographics, policy climate, relevant geographically adjacent clinical services). These contextual factors were incorporated within the logic modelling to enable implementation researchers to better describe the determinants of successful implementation in clinical practice.52
Figure 1.
First 2000 Days Care Connect implementation evaluation logic model. CFH, child and family health; CFHN, child and family health nurses; ED, emergency department; LHD, local health district; NGO, non-government organisations; NSW, New South Wales.
Additionally, we included a detailed description of the intervention to identify feasibility elements to measure during the study. These include features of the physical location of services, how services are integrated, the availability of culturally sensitive support materials and services and the navigator or keyworker. To supplement the practical elements of the intervention, we described the underlying theoretical principles of the model. These include the collective impact framework53 and the elements of the behaviour change wheel that we perceived the model to adhere.54 Collective impact is designed to inform change on complex social issues, and draws on five conditions: common agenda; continuous communication; mutually reinforcing activities; backbone support; and shared measurement.55 Collective impact and the behavioural change wheel mechanisms of change within the logic model will inform the qualitative interview schedule. Finally, we drew connections from these underlying theories of change to the specific intermediate and long-term outcomes that we hypothesised the model will produce. Principally, we hypothesise that the intervention components will work on the core principles of environmental restructure, enablement, modelling and training within the Hub sites, underpinned by the collective impact principles to support migrant and refugee parents to engage with health and social support services. This engagement will provide better outcomes for children and families. It will also create opportunities for shared knowledge between health and non-health services, as part of an acceptable and cost-effective model delivery. Table 3 provides an overview of the planned outcomes and measurement for the implementation evaluation.
Table 3.
Overview of the implementation evaluation outcomes
| Implementation evaluation outcomes | |||
| Outcome measure | Data source | Methods | Data collection |
| Description of local context and Hub | SEIFA data, search of grey literature, informal contact with Hub service leaders. | SEIFA data, search of grey literature, informal contact with Hub service leaders. | Trial commencement. |
| Fidelity of Hub model | Hub intervention log. | A bespoke log completed by site project officers. | Ongoing during the trial. |
| Acceptability of intervention measure (AIM), intervention appropriateness measure (IAM), and feasibility of intervention measure (FIM)63 | Research survey administered by project officer. | AIM, IAM and FIM measures completed by Hub staff, service leaders, participants in the intervention group. | Trial end (included in the 12-month postpartum parent survey for parents and separate staff survey). |
| Barriers and facilitators to running the FDCC Hubs | Interviews with Hub staff, service leaders, participants in the intervention group. | Qualitative interviews and focus groups, guided by the CFIR. | Pre-trial (with Hub staff and service leaders). Ongoing during and end of the trial for all participants. |
| The NoMAD tool64 to assess Hub staff buy in to the model | Research survey administered by project officer. | NoMAD tool completed by Hub staff. | Trial end. |
CFIR, consolidated framework for implementation research; FDCC, First 2000 Days Care Connect; NoMAD, NOrmalization MeAsure Development; SEIFA, Socio-Economic Indexes for Areas.
Economic evaluation
The economic evaluation will adopt a healthcare perspective beginning with a cost consequence analysis to describe the costs and all main study outcome measures (tables 4 and 5) and then generate a cost-utility analysis. The costs of Hub implementation will include: the establishment and operation of Hubs; and the flow-on cost from service use from Hub referrals. Hubs are likely to be implemented in different ways relative to local context and, as such, costs might differ. Two bespoke costing templates will be shared with Hub managers on trial commencement to be completed at 6 and 12 months, with researcher support to ensure accuracy. The templates will allow for standardisation and between-site comparison.
Table 4.
Overview of the economic evaluation outcomes
| Economic evaluation outcomes | |||
| Outcome measure | Data source | Methods | Data collection |
| Mother quality of life (EQ-5D quality of life) | Research survey administered by project officer. | Research survey administered by project officer. EQ-5D quality of life questionnaire. | Baseline (antenatal time of enrolment) 6 months post partum 12 months post partum |
| Cost of implementing Hubs and referral services | Bespoke surveys. | Bespoke surveys completed by Hub staff and participants in the intervention group. | 6 and 12 months 6 and 12 months |
EQ-5D, EuroQol five-dimension scale questionnaire.
Table 5.
Overview of the FDCC study outcome variables
| FDCC trial | |||
| Outcome measure | Data source | Methods | Data collection |
| Proportion of mothers, children and families who attend CFHN at FDCC Hub for checks. (Primary Outcome) | Electronic medical record at LHD. | Extraction of routine clinical data from electronic medical record at LHD. |
|
| Proportion of mothers, children and families who are up to date with age appropriate health checks, either via CFHN services or GP. (Primary Outcome) | Electronic medical record at LHD. | Extraction of routine clinical data from electronic medical record at LHD. |
|
| Proportion of women identified as at risk of experiencing depression on the EPDS.65 (Secondary Outcome) | Electronic medical record at LHD. | Extraction of routine clinical data from electronic medical.
Response to item 10 of EPDS. |
|
| Proportion of women identified as having more than one unmet social need on the We Care questionnaire.66 (Secondary Outcome) | Research survey administered by project officer. | Research survey administered by project officer. We Care questionnaire. |
|
| Proportion of women identified as experiencing psychosocial vulnerability on NSW Health psychosocial screening tools (Safe Start Psychosocial assessment including Domestic Violence screen).67 (Secondary Outcome) | Electronic medical record at LHD. | Extraction of routine clinical data from electronic medical. Presence/absence of psychosocial risk factors on Safe Start Psychosocial assessment including the Domestic Violence screen. |
|
| Proportion of mothers reporting poor quality of life on EQ-5D quality of life questionnaire. (Secondary Outcome) | Research survey administered by project officer. | Research survey administered by project officer. EQ-5D quality of life questionnaire. |
|
| Proportion of children monitored for growth parameters and their growth parameters (weight, height, head circumference). (Secondary Outcome) | Electronic medical record at LHD. | Extraction of routine clinical data from electronic medical.
|
|
| Proportion of women exclusively breast feeding /predominately breast feeding/partially breast feeding/ artificially feeding. (Secondary Outcome) | Electronic medical record at LHD. Data linkage with NSW Perinatal Data Collection. |
Extraction of routine clinical data from electronic medical.
|
Electronic medical record at LHD:
Data linkage with NSW Perinatal Data Collection
|
| Proportion of children identified by CFHN as at developmental risk on the Learn the Signs Act Early (LtSAE) and Ages and Stages Questionnaire Screening tools. (Secondary Outcome) | Electronic medical record at LHD. | Extraction of routine clinical data from electronic medical record at LHD.
Ages and Stages Questionnaire (ASQ and ASQ-SE) secondary screener given to families by CFHN as clinically required. |
|
| Mother and infant attendance at emergency departments from recruitment at 6-month post partum and 12-month post partum. (Secondary Outcome) | Data linkage with NSW-wide Emergency Department Data Collection (EDDC). | NSW-wide EDDC data Linkage. | At 6-month post partum and 12-month post partum. |
CFHN, child and family health nurses; EPDS, Edinburgh Postnatal Depression Scale; EQ-5D, EuroQol five-dimension scale questionnaire; FDCC, First 2000 Days Care Connect; GP, General Practitioner; LHD, local health district; NSW, New South Wales.
Establishment and operational costs
A micro-costing approach will be adopted to account for funded and in-kind expenditures.56 57 A simple template will have major generic expenditure categories, including upfront capital costs (eg, vehicles, buildings), governance arrangements to manage the Hubs (eg, staff meeting time), material costs (eg, brochures) and in-kind support from staff, including partner agencies. There might be expenditures against these categories. At this stage, there is no plan for capital expenditures. This is included for completeness. Operational costs pertain to daily Hub operation, including new staff hired (eg, salary, on-costs), in-kind costs (eg, time costs from non-salaried staff), venue costs (eg, utilities, even if in-kind) and material costs (eg, brochures).
Referral costs
Prior to Hub commencement, Hub personnel will be asked for a list of service partners to create a template where clients will be asked the services accessed and frequency; clients will be surveyed using this. Other sites will follow suit. Full client recall is not anticipated. However, it is important that the study clarifies the impact on referral services, if possible. A top-down costing estimate will then be made.56 57 Each partner service will then be contacted to generate an estimate of the average client service cost. Providers typically adopt an activity-based costing approach in accounting and funding proposals. No specific client data will be accessed. Rather, the researchers will guide service providers to generate average costs, which typically only involves dividing total funding for service(s) by total occasions of service. Researchers will only be privy to the overall average costs. Where costs are unavailable, an approximation will be made if public and research data are available. Otherwise, a list of service counts only will be made and remain uncosted. Table 4 provides an overview of the planned outcomes and measurement for the implementation evaluation.
Primary and secondary outcome measures
Outcomes will be measured from enrolment (baseline) until and including 12 months post partum (table 5). Outcomes will be gathered via: the extraction of routinely collected clinical data from electronic medical records (eMRs) at each site or LHD; surveys administered by a researcher to mothers; and data linkage of participants with administrative data sets (NSW perinatal data collection, NSW emergency department data collection). The primary outcome measure is the proportion of mothers and their respective infant who attend CFHN services for early childhood health checks at 1–4 weeks post partum, 6–8 weeks post partum, 6 months post partum and 12 months post partum. For primary and secondary variables, see table 5.
Data analysis plan
Sample size estimation
Based on pilot data, we anticipate the percentage of children to have their CFH check done by a CFHN will be 60% in the intervention group and 30% in the control group. Therefore, 72 children will be needed for each arm to provide 80% of power to detect the magnitude of such an increase with a p value<0.05. Allowing for a 40% attrition rate (ie, loss-to-follow-up) as this is a vulnerable community,15 we aim to recruit 120 children in each arm or 240 children in total across the three sites.
Statistical analysis
Statistical analysis will include descriptive analysis of participating mother and child outcomes at each assessment. We will compare outcomes between the intervention and control groups using the Fisher’s test for binary outcomes, χ2 method for categorical outcomes, non-parametric method (eg, Wilcoxon rank-sum test) and parametric methods (eg, t-test) for continuous and ordinal variables. As outcomes will be measured repeatedly, multilevel regression analysis will be undertaken to examine intervention impact on outcomes, controlling for the plausible confounders at the individual (eg, mother’s socio-demographic characteristics, geographical area of residence) and community levels at baseline (eg, neighbourhood socioeconomic factors). Generalised estimating equations method will be used in the regression analysis considering the potential clustering effect by site. Only de-identified data will be analysed. No data safety monitoring committee is needed for this study due to the known minimal risks. No interim analyses or stopping rules will be applied.
Implementation evaluation analysis
Implementation effectiveness will be evaluated using the validated scoring system of −2 to +2 with score descriptions as follows: −2 indicates the construct has negatively influenced the practice and examples of negative manifestations are indicated; −1 indicates the construct has negatively influenced the practice and general statements of negative manifestations are made; 0 indicates the construct neutrally influenced the practice; +1 indicates the construct positively influenced the practice and general statements of positive manifestations are made; and +2 indicates the construct positively influenced the practice and explicit examples of positive manifestations are described.58 Using these scores, construct scores can range from a low of −80 to a high of +80, demonstrating the key barriers and facilitators to uptake and sustain the FDCC hubs. This method of quantifying implementation effectiveness will be supplemented with an inductive analysis of qualitative data to ensure openness to emerging themes not readily captured by the CFIR and Proctor and colleague’s outcome measures.49
Economic analysis
First, a cost consequence analysis will collate and list the main costs and outcomes from the trial (tables 4 and 5) to provide transparency regarding the overall impacts of Hubs. Second, a cost-utility will then report the incremental (net) cost per change in quality adjusted life years (with health utilities derived from the EuroQol five-dimension scale questionnaire (EQ-5D))59 simulated using a decision tree, and where the threshold willingness to pay is varied between A$42 000 and $A67 000.60 Third, a probability sensitivity analysis will be undertaken and, where there is statistical uncertainty regarding cost-effectiveness a value of information analysis will assess statistical uncertainty and value for further research, including, for example, the value of longer follow-up to assess medium-to-long term impacts.61 Finally, a budget impact analysis will be undertaken where there are positive and attributable impacts regarding primary and/or secondary outcomes (captured in the cost consequences analysis). This will estimate the overall financial cost if Hubs were scaled-up across NSW to inform policy affordability considerations. The latter will involve estimating the potential Hubs would be made and an average cost (of the three Hubs) applied, with high and low estimates in a sensitivity analysis.
Data management
All participants will be allocated a randomly generated unique identifier code to be used throughout the study. Project officers will have identified information of the participants enrolled at their site, stored in password protected files. The project officer within each LHD will work with data managers to extract routinely collected clinical data from electronic medical records for all participants, per table 3. Data will be stored within a protected site-based server. Only de-identified data will be transferred from each LHD to the researchers (SW, KO, NH) for data analysis using encrypted transfer.
Project officers with support from CCWs and/or interpreters will collect surveys at baseline, 6 months post partum and 12 months post partum. The survey can be completed in hardcopy (face-to-face or telephone) or online by participants using a secure link to REDCap. Subsequently, project officers who can access the identifying information within each LHD will enter survey data into the REDCap database. REDCap is hosted on the University of NSW (UNSW) infrastructure. Permissions granted to each user within each REDCap project is controlled by and is the responsibility of the project team. Hardcopy materials will be stored in locked cabinets for the required period, either indefinitely if the participant consents to providing their data for data pooling or for 15 years after the completion of the study. After these periods, hardcopy materials will be destroyed and password-protected electronic archives will be deleted.
The identifying information collected within each LHD will be compiled into a single password-protected file and sent to The Centre for Health Record Linkage (CHeReL) for data linkage. The minimum identifying information for mothers and infants will be used to extract participant records from the administrative data. On completion of data extraction, CHeReL will transfer to UNSW administrative data of the participants who consented to data linkage. The administrative records will be de-identified by CHeReL, which will create the person project number (PPN) for each participant. The PPN will be linked to the participant’s unique project identification number to link the administrative records with the eMR and survey records that belong to the same participant.
Patient and public involvement
The research questions were developed based on qualitative research undertaken with Hub participants and community members and service providers in the pilot study.41 62 The FDCC team have a consumer representative and consultation was undertaken with local Hub partner services. The researchers also consulted multicultural health services, including cultural support workers, to ensure research materials are culturally nuanced. Patients or participants have not directly been involved in the current study design.
Ethics and dissemination
Ethical approval was granted by the SESLHD (2020/ETH03295). This trial was registered with the Australian New Zealand Clinical Trials.
Confidentiality
The researchers acknowledge that ensuring confidentiality is essential. The researchers will exercise due diligence to anonymise participants’ responses for reporting, publication and presentation purposes. Only de-identified data will be transferred from each LHD to the UNSW researchers for data analysis. The de-identified data from each LHD to the UNSW team will be securely transferred through an NSW Health-approved e-health platform.
Managing potential harms
If issues are disclosed outside of the study parameters, mandatory NSW Health policy directives will apply (eg, family and domestic violence, child protection matters). These will be managed as per current policies and practices within LHDs. The child protection and domestic violence counselling teams are readily accessible to provide advice and support if issues are identified. As the researchers are all mandatory reporters, they will inform participants that they are not able to maintain confidentiality when it relates to the safety of the participant, the child/children, the family and the wider community. These obligations are detailed in the PISCF (online supplemental appendix 1).
bmjopen-2022-061002supp001.pdf (234.2KB, pdf)
Dissemination
Data obtained for the study will be published in reports, peer-reviewed journals and presented at appropriate conferences. The de-identified data will be available to all investigators. Access by individuals’ other than the named investigators will only be permitted after consideration and agreement by all the remaining investigators. An essential element of knowledge translation are the study partners and advisors who will share findings and consider if and how to progress to trialling or implementing the programme at scale. We intend to produce at least two papers (eg, protocol, main findings) for peer-review publication, written by core research and implementation team.
Study governance
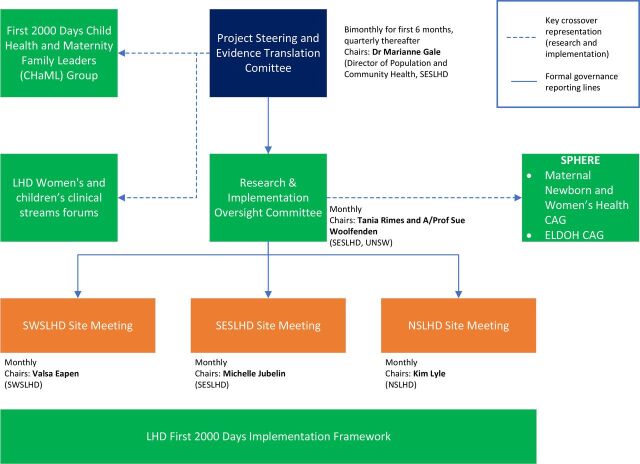
The FDCC team will support planning, implementation and governance of the project and ensure that Work Health and Safety requirements and policies are considered and actioned. There are currently no procedures for auditing trial conduct. All protocol modifications will be discussed within all levels of governance and communicated to the SESLHD Human Research Ethics Commitee. Figure 2 outlines our governance structure.
Figure 2.
First 2000 Days Care Connect governance structure. CAG, Clinical Academic Group; ELDOH, Early Life Determinants of Health; LHD, local health district; NSLHD, Northern Sydney Local Health District; SESLHD, South Eastern Sydney Local Health District; SWSLHD, South Western Sydney Local Health District. UNSW, University of New South Wales.
Supplementary Material
Acknowledgments
This protocol has been authored on behalf of the FDCC Collaborative Group. The authors would like to acknowledge the members of the group not listed as authors: Melissa Green, John Eastwood, Karen Sorensen, Kim Lyle, Catherine Jones, Vicki Blight, Amit Arora, Michelle de Vroome, Andrew Hayen, Nick Hopwood, Virginia Schmied, Rebekah Grace, Jane Kohlhoff, Fiona Brooks, Cathy Kaplun, Kathleen Baird, Myna Hua, Lisa Woodland, Ben Harris-Roxas, Brendan Goodger, Tracey Szanto, Karen Zwi and Grainne O’Loughlin.
Footnotes
Twitter: @NorasamirSYD, @amanda_henry_AK
Collaborators: The FDCC Collaborative Group: Sue Woolfenden; Tania Rimes; Katarina Ostojic; Michael Hodgins; Helen Rogers; Raghu Lingam; Valsamma Eapen; Melissa Green; Karen Sorensen; Kim Lyle; Amanda Henry; Kathleen Baird; Nick Hopwood; Michelle De Vroome; Cathy Kaplun; Teena Clerke; Nan Hu; Tracey Szanto; Amit Arora; Andrew Hayen; Antonio Mendozadiaz; Fiona Brooks; Kenny Lawson; Lisa Woodland; Myna Hua; Shanti Raman; Virginia Schmied; Ann Dadich; Rebekah Grace; Jane Kohlhoff; Grainne O’Loughlin; Brendan Goodger; Teresa Winata; Si Wang; Jodie Adams; Kim Rodgers; Amanda Webster; Hilda Ignatius; Catherine Jones; Vicki Blight; John Eastwood; Ben Harris-Roxas; Karen Zwi; Mona Asghari.
Contributors: The original trial design was conceived by SW, TR, AW, RL, VE and HR. The implementation evaluation design was conceived by MH and RL. The economic evaluation design was conceived by KDL. The statistical analysis methods were initially designed by NH. MH developed the initial draft of the protocol, which was refined by SW, TR, AW, RL, VE, HR, KO, NH, KDL, NS, AH, EM, SR, AMD and AD. All authors approved the final manuscript.
Funding: This project was funded by the NSW Health Translational Research Grants Scheme. The views expressed are those of the authors and not necessarily those of the partner organisations. SW is funded through the National Health and Medical Research Council. RL is funded through the Financial Markets Foundation for Children. The project is supported in-kind by the Early Life Determinants of Health Clinical Academic Group, Maridulu Budyari Gumal (SPHERE).
Competing interests: The views expressed are those of the author(s) and not necessarily those of the funding partners. NSW Health has no direct role in study design; data collection, analysis and interpretation, or writing of final reports, presentations or publications.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. AEDC . Australian early development census, 2020. Available: https://www.aedc.gov.au/data/data-explorer?id=135216
- 2. COAG . COAG health Council 2019 the Australian National breastfeeding strategy: 2019 and beyond was prepared under the auspices of the COAG health Council, 2019. [Google Scholar]
- 3. Moore T, Arefadib N, Deery A. The first thousand days: an evidence paper. Melbourne: Murdoch Children’s Research Institute, 2017. [Google Scholar]
- 4. Goldfeld S, D'Abaco E, Bryson H, et al. Surveying social adversity in pregnancy: the antenatal risk burden experienced by Australian women. J Paediatr Child Health 2018;54:754–60. 10.1111/jpc.13860 [DOI] [PubMed] [Google Scholar]
- 5. NSW Ministry of Health . The first 2000 days framework, 2019. [Google Scholar]
- 6. Goldfeld S, O'Connor M, Chong S, et al. The impact of multidimensional disadvantage over childhood on developmental outcomes in Australia. Int J Epidemiol 2018;47:1485–96. 10.1093/ije/dyy087 [DOI] [PubMed] [Google Scholar]
- 7. Woolfenden S, Asher I, Bauert P. Summary of position statement on inequities in child health. 54, 2018: 832–3. [DOI] [PubMed] [Google Scholar]
- 8. Rogers HJ, Hogan L, Coates D, et al. Responding to the health needs of women from migrant and refugee backgrounds-Models of maternity and postpartum care in high-income countries: a systematic scoping review. Health Soc Care Community 2020;28:1343–65. 10.1111/hsc.12950 [DOI] [PubMed] [Google Scholar]
- 9. Hiscock H, Danchin MH, Efron D, et al. Trends in paediatric practice in Australia: 2008 and 2013 national audits from the Australian paediatric research network. J Paediatr Child Health 2017;53:55–61. 10.1111/jpc.13280 [DOI] [PubMed] [Google Scholar]
- 10. Palfrey JS, Tonniges TF, Green M, et al. Introduction: Addressing the millennial morbidity--the context of community pediatrics. Pediatrics 2005;115:1121–3. 10.1542/peds.2004-2825B [DOI] [PubMed] [Google Scholar]
- 11. Woolfenden S, Galea C, Smithers-Sheedy H, et al. Impact of social disadvantage on cerebral palsy severity. Dev Med Child Neurol 2019;61:586–92. 10.1111/dmcn.14026 [DOI] [PubMed] [Google Scholar]
- 12. Brinkman S, Gregory T, Harris J, et al. Associations between the early development instrument at age 5, and reading and numeracy skills at ages 8, 10 and 12: a prospective linked data study. Child Indic Res 2013;6:695–708. 10.1007/s12187-013-9189-3 [DOI] [Google Scholar]
- 13. Merrick MT, Ford DC, Ports KA, et al. Vital Signs: Estimated Proportion of Adult Health Problems Attributable to Adverse Childhood Experiences and Implications for Prevention - 25 States, 2015-2017. MMWR Morb Mortal Wkly Rep 2019;68:999–1005. 10.15585/mmwr.mm6844e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kezelman C, Hossack N, Stavropoulos P. The cost of unresolved childhood trauma and abuse in adults in Australia, adults surviving child abuse and. Sydney: Pegasus Economics, 2015. [Google Scholar]
- 15. Woolfenden S, Eapen V, Jalaludin B, et al. Prevalence and factors associated with parental concerns about development detected by the parents' evaluation of developmental status (Peds) at 6-month, 12-month and 18-month well-child checks in a birth cohort. BMJ Open 2016;6:e012144. 10.1136/bmjopen-2016-012144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ayer C, Eapen V, Overs B, et al. Risk factors for non-participation in a universal developmental surveillance program in a population in Australia. Aust Health Rev 2020;44:512–20. 10.1071/AH18236 [DOI] [PubMed] [Google Scholar]
- 17. Eapen V, Walter A, Guan J, et al. Maternal help-seeking for child developmental concerns: associations with socio-demographic factors. J Paediatr Child Health 2017;53:963–9. 10.1111/jpc.13607 [DOI] [PubMed] [Google Scholar]
- 18. Zwi K, Rungan S, Woolfenden S, et al. Refugee children and their health, development and well-being over the first year of settlement: a longitudinal study. J Paediatr Child Health 2017;53:841–9. 10.1111/jpc.13551 [DOI] [PubMed] [Google Scholar]
- 19. Health NSW. Nsw child health survey 2009-2010 summary report; 2010.
- 20. McLean K, Goldfeld S, Molloy C. Screening and surveillance in early childhood health: rapid review of evidence for effectiveness and efficiency of models. Ultimo, NSW, Australia: The Sax Institute, 2014. [Google Scholar]
- 21. Garg P, Ha MT, Eastwood J, et al. Explaining culturally and linguistically diverse (CALD) parents' access of healthcare services for developmental surveillance and anticipatory guidance: qualitative findings from the 'Watch Me Grow' study. BMC Health Serv Res 2017;17:228. 10.1186/s12913-017-2143-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Almeida LM, Caldas J, Ayres-de-Campos D, et al. Maternal healthcare in migrants: a systematic review. Matern Child Health J 2013;17:1346–54. 10.1007/s10995-012-1149-x [DOI] [PubMed] [Google Scholar]
- 23. Fellmeth G, Fazel M, Plugge E. Migration and perinatal mental health in women from low- and middle-income countries: a systematic review and meta-analysis. BJOG 2017;124:742–52. 10.1111/1471-0528.14184 [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization (WHO) . Promoting the health of refugees and migrants: framework of priorities and guiding principles to promote the health of refugees and migrants. Geneva: WHO Secretariat, 2017: 1–4. [Google Scholar]
- 25. Higginbottom GMA, Morgan M, Alexandre M, et al. Immigrant women's experiences of maternity-care services in Canada: a systematic review using a narrative synthesis. Syst Rev 2015;4:13. 10.1186/2046-4053-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Correa-Velez I, Ryan J. Developing a best practice model of refugee maternity care. Women Birth 2012;25:13–22. 10.1016/j.wombi.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 27. Henderson S, Kendall E. Culturally and linguistically diverse peoples' knowledge of accessibility and utilisation of health services: exploring the need for improvement in health service delivery. Aust J Prim Health 2011;17:195–201. 10.1071/PY10065 [DOI] [PubMed] [Google Scholar]
- 28. Heslehurst N, Brown H, Pemu A, et al. Perinatal health outcomes and care among asylum seekers and refugees: a systematic review of systematic reviews. BMC Med 2018;16:89. 10.1186/s12916-018-1064-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boyle JA, Willey S, Abbasova G. Supporting better outcomes for migrant and refugee women. O&G Magazine 2018; 20(1). Available: https://www.ogmagazine.org.au/20/1-20/better-outcomes-migrant-refugee-women/
- 30. Small R, Roth C, Raval M, et al. Immigrant and non-immigrant women’s experiences of maternity care: a systematic and comparative review of studies in five countries. BMC Pregnancy Childbirth 2014;14:1–17. 10.1186/1471-2393-14-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Woolfenden S, Posada N, Krchnakova R, et al. Equitable access to developmental surveillance and early intervention--understanding the barriers for children from culturally and linguistically diverse (CALD) backgrounds. Health Expect 2015;18:3286–301. 10.1111/hex.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Woolfenden S, Posada N, Krchnakova R. Equitable access to developmental surveillance and early intervention - understanding the barriers for children from culturally and linguistically diverse (CALD) backgrounds. Health Expect. 10.1111/hex.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woolfenden S, Eapen V, Williams K, et al. A systematic review of the prevalence of parental concerns measured by the parents' evaluation of developmental status (Peds) indicating developmental risk. BMC Pediatr 2014;14:231. 10.1186/1471-2431-14-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Woolfenden S, Galea C, Badland H, et al. Use of health services by preschool-aged children who are developmentally vulnerable and socioeconomically disadvantaged: testing the inverse care law. J Epidemiol Community Health 2020;74:jech-2019-213384–501. 10.1136/jech-2019-213384 [DOI] [PubMed] [Google Scholar]
- 35. Chando S, Craig JC, Burgess L, et al. Developmental risk among Aboriginal children living in urban areas in Australia: the study of environment on Aboriginal resilience and child health (search). BMC Pediatr 2020;20:1–13. 10.1186/s12887-019-1902-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alrashdi M, Hameed A, Cervantes Mendez MJ, et al. Education intervention with respect to the oral health knowledge, attitude, and behaviors of refugee families: a randomized clinical trial of effectiveness. J Public Health Dent 2021;81:90–9. 10.1111/jphd.12415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Henry R. Review of health services for children young people and families within the NSW health system, 2019. [Google Scholar]
- 38. National Academies of Sciences E . Medicine Integrating Social Care Into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health. Washington, DC: National Academies Press, 2019. [PubMed] [Google Scholar]
- 39. Moore T. Using place-based approaches to strengthen child wellbeing. Developing Practice: The Child, Youth and Family Work Journal 2014:40. [Google Scholar]
- 40. Glover J, Samir N, Kaplun C, et al. The effectiveness of place-based interventions in improving development, health and wellbeing outcomes in children aged 0–6 years living in disadvantaged neighbourhoods in high-income countries – a systematic review. Wellbeing Space Soc 2021;2:100064. 10.1016/j.wss.2021.100064 [DOI] [Google Scholar]
- 41. Edwards K, Rimes T, Smith R, et al. "Improving Access to Early Childhood Developmental Surveillance for Children from Culturally and Linguistically Diverse (CALD) Background". Int J Integr Care 2020;20:3. 10.5334/ijic.4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Edwards K, Fernandez R, Rimes T. “Happy, Healthy, Ready – working with early childhood non-government organisations for developmental surveillance for vulnerable children”. Australian Journal of Advanced Nursing 2020;37. [Google Scholar]
- 43. Alrashdi M, Cervantes Mendez MJ, Farokhi MR. A randomized clinical trial preventive outreach targeting dental caries and Oral-Health-Related quality of life for refugee children. Int J Environ Res Public Health 2021;18:1686. 10.3390/ijerph18041686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rogers HJ, Hogan L, Coates D, et al. Cross cultural workers for women and families from migrant and refugee backgrounds: a mixed-methods study of service providers perceptions. BMC Womens Health 2021;21:222. 10.1186/s12905-021-01368-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rogers HJ, Hogan L, Coates D. A bridge to health: the cross cultural workers in maternity and child and family health service for women and families from migrant and refugee backgrounds: a mixed-methods study of service providers perceptions in Sydney. Australia 2020. 10.21203/rs.3.rs-200027/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50. 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kirk MA, Kelley C, Yankey N. A systematic review of the use of the consolidated framework for implementation research. Implementation Science 2016;11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65–76. 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lawton B, Brandon PR, Cicchinelli L. Logic models: a tool for designing and monitoring program evaluations. Rel 2014-007. Regional Educational Laboratory Pacific 2014. [Google Scholar]
- 51. US. Department of health and human services centres for disease control and prevention. Introduction to program evaluation for public health programs: a self-study guide. Atlanta, GA: Centers for Disease Control and Prevention, 2011. [Google Scholar]
- 52. Li S-A, Jeffs L, Barwick M, et al. Organizational contextual features that influence the implementation of evidence-based practices across healthcare settings: a systematic integrative review. Syst Rev 2018;7:72. 10.1186/s13643-018-0734-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kania J, Kramer M. Collective impact: Leland Stanford Jr. university 2011. 10.48558/5900-kn19 [DOI]
- 54. Michie S, Atkins L, West R. The behaviour change wheel. In: A guide to designing interventions. 1st ed. Great Britain: Silverback Publishing, 2014: 1003–10. [Google Scholar]
- 55. Smart JR. Collective impact: evidence and implications for practice. Melbourne: Australian Institute of family studies, 2017. [Google Scholar]
- 56. Chapko MK, Liu C-F, Perkins M, et al. Equivalence of two healthcare costing methods: bottom-up and top-down. Health Econ 2009;18:1188–201. 10.1002/hec.1422 [DOI] [PubMed] [Google Scholar]
- 57. O'Brien B, Drummond MF, Sculpher MJ. Methods for the economic evaluation of health care programmes. Oxford university press, 2015. [Google Scholar]
- 58. Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR). Implementation Science 2013;8:1–32. 10.1186/1748-5908-8-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Viney R, Norman R, King MT, et al. Time trade-off derived EQ-5D weights for Australia. Value Health 2011;14:928–36. 10.1016/j.jval.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 60. Huang L, Frijters P, Dalziel K, et al. Life satisfaction, QALYs, and the monetary value of health. Soc Sci Med 2018;211:131–6. 10.1016/j.socscimed.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 61. Briggs AH, Weinstein MC, Fenwick EAL, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling good research practices Task force working Group-6. Med Decis Making 2012;32:722–32. 10.1177/0272989X12458348 [DOI] [PubMed] [Google Scholar]
- 62. Edwards B, Mullan K, Katz I. The stronger families in Australia (SFIA) study: phase 2 the stronger families in Australia (SFIA) study. Phase 2 2014. 10.2139/ssrn.1728591 [DOI] [Google Scholar]
- 63. Weiner BJ, Lewis CC, Stanick C, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci 2017;12:108. 10.1186/s13012-017-0635-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Finch TL, Rapley T, Girling M, et al. Improving the normalization of complex interventions: measure development based on normalization process theory (NoMAD): study protocol. Implement Sci 2013;8:43. 10.1186/1748-5908-8-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry 1987;150:782–6. 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- 66. Garg A, Toy S, Tripodis Y, et al. Addressing social determinants of health at well child care visits: a cluster RCT. Pediatrics 2015;135:e296–304. 10.1542/peds.2014-2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. NSW Department of Health . Nsw Health/Families NSW supporting families early package – safe start strategic policy, 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-061002supp002.pdf (81.4KB, pdf)
bmjopen-2022-061002supp001.pdf (234.2KB, pdf)