Abstract
The liver plays a crucial role in drug detoxification, and the main source of liver transplants is brain-dead patients. However, the demand for transplants exceeds the available supply, leading to controversies in selecting suitable candidates for acute liver diseases. This research aimed to differentiate mesenchymal stem cells (MSCs) into hepatocyte-like cells using galactosylated rat natural scaffolds and comparing 2-D and 3-D cell culture methods. The study involved isolating and culturing Wharton's jelly cells from the umbilical cord, examining surface markers and adipogenic differentiation potential of MSCs, and culturing mesenchymal cells on galactosylated scaffolds. The growth and proliferation of stem cells on the scaffolds were evaluated using the MTT test, and urea synthesis was measured in different culture environments. Changes in gene expression were analyzed using real-time PCR. Flow cytometry results confirmed the presence of specific surface antigens on MSCs, indicating their identity, while the absence of a specific antigen indicated their differentiation into adipocytes. The MTT test revealed higher cell attachment to galactosylated scaffolds compared to the control groups. Urea secretion was observed in differentiated cells, with the highest levels in cells cultured on galactosylated scaffolds. Gene expression analysis showed differential expression patterns for OCT-4, HNF1, ALB, AFP, and CYP genes under different conditions. The findings indicated that hepatocyte-like cells derived from 3D cultures on galactosylated scaffolds exhibited superior characteristics compared to cells in other culture conditions. These cells demonstrated enhanced proliferation, stability, and urea secretion ability. The study also supported the differentiation potential of MSCs derived from Wharton's jelly umbilical cord into liver-like cells.
Keywords: Hepatocytes, Stem cells, Scaffolds, Cell differentiation, Galactosyl
Highlights
-
•
Differentiation of mesenchymal stem cells into hepatocyte-like cells using galactosylated rat natural scaffold.
-
•
Comparison of 2-D and 3-D cell culture for differentiation.
-
•
Expression of surface markers and genes related to liver differentiation assessed.
-
•
Mesenchymal stem cells derived from Wharton's jelly umbilical cord showed potential for liver cell differentiation.
-
•
Galactosylated scaffold promoted higher urea secretion in differentiated cells.
1. Introduction
The liver is a complex and large organ whose main role is to detoxify drugs, remove waste products from the degradation and regeneration of red blood cells in the form of bile, produce blood clotting factors, store sugar in the form of glycogen, and regulate sugar and fat metabolism [1]. Several factors such as bacteria and viruses, anoxia, metabolic disorders, toxins and drugs, nutritional disorders, and allergic conditions can lead to liver cell dysfunction and liver disease in acute or chronic form [2,3]. Chronic liver diseases, including cirrhosis, are the twelfth cause of death among young and middle-aged adults. When liver diseases are completely progressed and no treatment is successful, liver transplantation is performed as the only solution to save the patient [4].
The main source of liver transplants is brain-dead patients. Due to the shortage of transplant organs and on the other hand, the growing demand for transplants, there is no balance in this regard, which is why choosing a suitable candidate for the treatment of acute liver diseases is very controversial [5]. As a result, treatment methods for liver failure are increasing day by day. Today, tissue engineering (TE) is considered one of the most practical and active fields in biomedicine [6]. TE uses the principles of engineering and life sciences together to evolve biological components in need of being repaired or replaced. TE is a science used to create biological substitutes that can restore, preserve and improve the function of damaged tissues. TE is very important in medicine, repair, and regeneration of damaged tissues and organs [7]. In general, the main components in TE are scaffolds, cells, and growth factors. Also, bioreactors are considered one of the pillars of TE [8].
The use of biological substrates compatible with the body is one of the important key factors in TE. The structure of the scaffolds is as similar as possible to the tissue of the culture area, and thus the regeneration and treatment of the damaged tissue increases qualitatively and quantitatively. The structure of the scaffold is in the form of a porous matrix, which helps with adhesion and better cell Translocation [[9], [10], [11]]. Scaffolds should not be toxic to cells. Until recently, researchers have used two-dimensional and three-dimensional substrates for differentiation, one of these substrates is collagen heparan sulfate matrices, providing a wide range of applications [12]. The science of TE deals with the construction of suitable scaffolds for the implantation of cells, creating and maintaining favorable environmental conditions for the continuation of cell life, and thus controlling all factors affecting the creation of new tissue [8]. To create a functioning liver, for cell therapy, it is very important to pay attention to the type and source of the cells, the cell scaffold, and the soluble factors which are used, all three factors are required for the growth and differentiation of cells [13,14].
The types of cells used in liver cell therapy include stem cells, liver progenitor cells, and mature hepatocytes. Mesenchymal stem cells (MSCs) are retrieved from various sources such as adipose tissue, blood, lung, liver, and amniotic fluid, and then used for differentiation into semi- Hepatocytes, which have characteristics such as cultivability, multiplication and differentiation, and secretion of vital cytokines [[15], [16], [17]].
MSCs also have anti-inflammatory and anti-apoptotic properties and can participate in the repair of liver lesions [[18], [19], [20]]. Many cytokines and growth factors such as HGF and bFGF, insulin, insulin growth factor (IGF), and leukemia inhibitory factors (LIF) are known to be involved in the liver cell's growth and their differentiation [21,22].
The goal of this research is to differentiate MSCs into hepatocytes by the Differentiation of mesenchymal stem cells to hepatocyte-like cells by use of galactosylation of rat natural scaffold and comparison between 2-D and 3-D cell cultures. In other words, in this research, the specific objective is to assess and compare the differentiation of hepatocytes in two distinct culture environments: a three-dimensional (3D) culture medium utilizing scaffolds and a two-dimensional (2D) culture medium without scaffolds. The focus is on examining the behavior and degree of differentiation exhibited by liver cells in these two environments.
The aim is to investigate whether the incorporation of scaffolds in the 3D culture medium enhances the differentiation of mesenchymal stem cells (MSCs) into hepatocyte-like cells compared to the traditional 2D culture approach. The scaffolds are designed using nano or extracellular matrix compounds and serve as a supportive structure for the MSCs to grow and differentiate.
By comparing the behavior and extent of differentiation of liver cells in the two culture environments, the researchers aim to determine whether the 3D culture system with scaffolds provides a more conducive and efficient environment for hepatocyte differentiation from MSCs. This comparison will shed light on the potential advantages and limitations of each culture method, thereby contributing to the optimization of hepatocyte differentiation protocols and potential applications in regenerative medicine and liver tissue engineering.
2. Method
2.1. Isolation and primary culture of umbilical cord Wharton's jelly (UC-WJ) cells
UC samples (n = 20) from newborn boys born by cesarean section were obtained from Zeynabieh Shiraz Hospital and placed under sterile conditions in a container having physiological saline solution and transported to the laboratory. The pieces of Wharton's jelly were divided into pieces less than half a millimeter in size and transferred to the growth medium DMEM containing high glucose with 20% FBS. Furthermore, 1% antibiotics (penicillin/streptomycin) were added to the medium to prevent contamination. The plates were placed in an incubator with 88% humidity, 5% CO2, and a temperature of 37 °C. This method of culturing small tissue fragments is commonly referred to as the “explants method.” The explants were left undisturbed for one week to allow cell migration from the edges of the tissue fragments. Once the adherent cells reached a confluency of 70%–80%, they were harvested using trypsinization with 0.05% trypsin-EDTA (Gibco, Germany). The resulting single cell suspension was utilized for subsequent experiments.
It is important to note that prior ethical approval was obtained from the Ethical Committee of the University of Isfahan by relevant laws and regulations, including The Code of Ethics (ethic code: IR.UL.REC.1400.071) of the World Medical Association (Declaration of Helsinki). Consent forms were obtained from the individuals and confirmed byvthd ebioetical committee of the University of Isfahan (under number: IR.UL.REC.1400.071)
2.2. Determination of surface markers of MSCs cells using flow cytometry method
To determine the cell-surface antigens, the cells at the fourth passage were subjected to staining with monoclonal antibodies specific to various proteins. Specifically, the following antibodies were used: CD90-FITC, CD45-FITC, CD133-PE, CD44-FITC, CD34-FITC, CD133-PE, and CD105-FITC (BioLegend, San Diego, CA, USA). In order to establish appropriate controls, cells were also treated with corresponding isotype control antibodies. Following staining, the cells were suspended in PBS and analyzed using a FACS Calibur flow cytometer (Becton Dickinson, NJ, USA). A minimum of 10,000 events were recorded for each sample.
First, the cells attached to the bottom of the flask were separated by trypsin/EDTA and centrifuged for 5 min at 1500 rpm at room temperature. After counting the cells, 105–106 cells were poured into the test and negative control tubes and centrifuged again for 5 min at 1500 rpm at room temperature. Subsequently, the supernatant was discarded and 100 μL of PBS were added to the pellet at the bottom of each tube and were pipetted. Then, 3 μL of specific antibodies were added to the test tubes in a dark environment. Antibodies were not added to control tubes. Hence, the tubes were incubated for 45–60 min at 4°Celsius. Finally, the expression rate of CD34, CD90, CD44, and CD105 markers were read by flow cytometry.
2.3. Differentiation of UCB-MSCs into adipocytes
UCB-MSCs were differentiated into fat in differentiation media after proliferation in the growth medium. To induce differentiation, the number of 103 × 5 UCB-MSCs was poured into each well of a 4-well plate and for 2 weeks they were under the influence of half a milliliter of medium containing 10% FBS, 1 μM dexamethasone, 200 μM indomethacin, 1.7 μM insulin, 500 Micromolar methylxanthine isobutanol per house. Then the cells were fixated with a 4% paraformaldehyde solution. Consequently, Oil Red staining was performed and the results were observed via an optical microscope.
2.4. Differentiation of UCB-MSCs into hepatocyte-like cells using growth factors and cytokines
To differentiate MSCs into hepatocyte-like cells, fibroblast growth factor, hepatocyte growth factor, oncostatin M, and dexamethasone were used. To induce differentiation, 100,000 MSC cells were cultured in each well of a 6-well plate containing collagen, and for 24 h, the cells were treated with Williams medium with penicillin and streptomycin antibiotics, ascorbic acid, sodium pyruvate, and nicotinamide. The medium mentioned above was introduced as the basic medium during differentiation. After reaching the concentration of 90–100%, a basic medium along with FGF-4 10 ng/ml was added to the above cells for the next two days.
Throughout three weeks the cells were differentiated. First, the cells were exposed to a basic medium with FGF-4 10 ng/ml and HGF 20 ng/ml for about one week. In the second week, a basic medium containing OSM 20 ng/ml, TSA 1 μM, Dexamethasone 0.1 μM, and ITS 0.5x was added to the cells. Finally, in the third week, a basic medium containing OSM 20 ng/ml, Dexamethasone 0.5 μM, TSA 1 μM, and ITS 0.25x was added to the cells.
2.5. Assessment of hepatic differentiation, morphological examination
Morphological changes were checked daily with an inverted microscope. The cells transformed from the fibroblastic shape of MSC to a round epithelioid shape with little cytoplasm and a medium-sized nucleus with 1–2 nucleoli. The main changes of the cells were observed on days 3, 7 and 14.
2.6. Preparation of natural scaffold from mouse liver
First, several pieces of liver tissue were prepared from 4-week-old male Sprague Dawley mice from the institute Pasteur. The decellularization process was done with the aid of 24 h treatment with 1% sodium dodecyl sulfate solution.
2.7. Scaffold construction and sterilization
In chemical crosslinking, 500 μl of solution (50 mM 2-(-N-morpholino) ethane sulfonic acid (MES), 30 mM 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide (EDC), and 30 mM N-hydroxysulfosuccinimide (NHS)) was added to each well and incubated for 24 h. After 24 h, the previous solution was removed and 0.1 M Na2HPO4 in double-deionized water was added to the scaffolds for 2 h. The resulting scaffolds should be washed several times with distilled water. Scaffolds were lyophilized and stored at −80 °C for use. The scaffolds were transferred to sterile plates and then 70% ethanol was added for 20 min. Then the ethanol was removed and the plates were washed several times with PBS to prepare for cell culture. First, each of the scaffolds prepared for cellular work was cut into circles with a diameter of 1.5 cm using a punch. To sterilize the resulting scaffolds, each side of them was exposed to ultraviolet radiation for 10 min and then, the scaffolds were immersed in 70% ethanol solution for 12 h. Finally, to ensure the absence of any microbial contamination, the sterilized scaffolds were placed in the culture medium rich in fetal bovine serum for 24 h to determine possible microbial contamination.
2.8. Cell culture on galactosylated scaffolds
To perform cell culture tests and investigate liver differentiation, after the passage of mesenchymal cells, approximately 200,000 cells were placed on scaffold plates in 24 well plates. In the end, these scaffolds were immersed in the culture medium. After one day, the basic culture medium was replaced with a differentiation culture medium containing liver factors. To check the structure of the scaffold and also to evaluate the shape and morphology of the cultured cells on the prepared scaffolds, the scanning electron microscope was used.
2.9. MTT test
In this study, the MTT test was used to compare the growth and proliferation of stem cells on the galactosylated scaffold and without it. Cut and sterilized scaffolds were placed inside the wells of a 24-well cell culture container and 4000 cells were placed on each scaffold. And for 24, 48, and 76 h after this initial culture, an amount of 50 μL of MTT solution (5 mg/liter in the basic medium) was added to each well. After the formation of formazan crystals inside the cells on the scaffold, the scaffolds were placed inside the plastic tubes and a certain amount of solvent (100 μl into each well) was added to the tubes to dissolve the formazan crystals. After the crystals were completely dissolved, the absorbance of the obtained purple solution was measured at a wavelength of 570 nm. All experiments were performed in triplicate.
2.10. Evaluation of urea concentration
Determination of urea is very useful to assess whether hepatocytes are acting as a primary means of eliminating ammonia in the culture medium. In this study, to measure urea synthesis in the two-dimensional and three-dimensional environment of cultured liver cells, a method based on a chromogenic reagent that specifically creates a colored compound with urea was used. This reagent was prepared according to the statements of Jung et al. and it was optimized according to the method of Zawada et al. (Zawada, 2009 #221}). The urea reagents were prepared according to the method described by Jung et al. (1975). The final working reagent, known as the Jung working reagent, consisted of the following components: 100 mg/L o-phthalaldehyde, 215 mg/L N-(1-naphthyl)ethylenediamine, 2.5 mol/L sulfuric acid, 2.5 g/L boric acid, and 0.03% Brij-35.A modified reagent was also used, where the 215 mg/L N-(1-naphthyl) ethylenediamine reagent was substituted with 513 mg/L primaquine bisphosphate.
For the urea assay, a urea standard was prepared using double-distilled water, with a concentration of 5.00 mg/dL urea. In a clear flat-bottom 96-well plate, 50 μL of water, 50 μL of the 5.00 mg/dL standard, and 50 μL of the samples were transferred into separate wells. Then, 200 μL of freshly prepared working reagent was added to each well, and the plate was gently rocked to ensure thorough mixing. The reaction was incubated for 1 h at room temperature. Optical densities (OD) at 430 nm and 505 nm were measured using a plate reader for assays that used the modified reagent and the original Jung reagent, respectively.
To calculate the urea concentration of the samples, the experimenter has the option to either use the slope of the standard curve or a single urea concentration. In this study, it was determined that using one blank (water) and one single urea concentration (5.00 mg/dL) is sufficient for calculating the sample urea concentrations. This approach simplifies the calculation process and provides accurate results.
Urea concentration in the sample was obtained from the OD values according to the formula Calculated below.
where ODSAMPLE, ODSTANDARD, and ODBLANK are OD430 nm values of the sample, standard, and water blank, respectively. [Standard] is the concentration of the urea standard (5.00 mg/dL or 0.83 mmol/L) and n is the dilution factor. Dilution of samples in distilled water is necessary when sample OD430 nm values are higher than the OD430 nm value for the 5.00 mg/dL urea standard. All experiments were performed in triplicate.
2.11. Examining the expression of OCT-4, HNF1, ALB, AFP and CYP genes
From the cell samples exposed to the galactose scaffold and the control sample on days 3, 7, and 14, RNA extraction was performed using the RNX-Plus solution of Synaclon Company according to its manufacturer's protocol. 500 ng of total RNA was subjected to reverse transcription. This process involved the use of random primers, which facilitate the synthesis of complementary DNA (cDNA) from the RNA template, Then cDNA was synthesized from the RNAs by the Takara kit, Dalian, Japan Cat. No. RR0378. Then, primers were designed for all four genes with GeneRunner software, and then the quality of the primers was checked by Allele ID 7 and their specificity by Primer Blast. Then, gene expression was performed by Applied Biosystems 7500 Real-Time PCR System with (SYBR Premix Ex Taq II), TaKaRa Company, Japan, and ROX dye, TaKaRa Company, Japan. The 2−ΔΔCT method was used to analyze the data obtained from Real-Time PCR. Then the raw numbers obtained from the above measurements and the raw numbers of urea concentration were analyzed and compared with each other by SPSS version 22 software and through one-way ANOVA and Tukey's post hoc test for each test. The values used are mean ± standard deviation error and significant level (p < 0.05). All experiments were performed in triplicate.
3. Results
3.1. The results of identifying specific surface markers of MSCs by flow cytometry
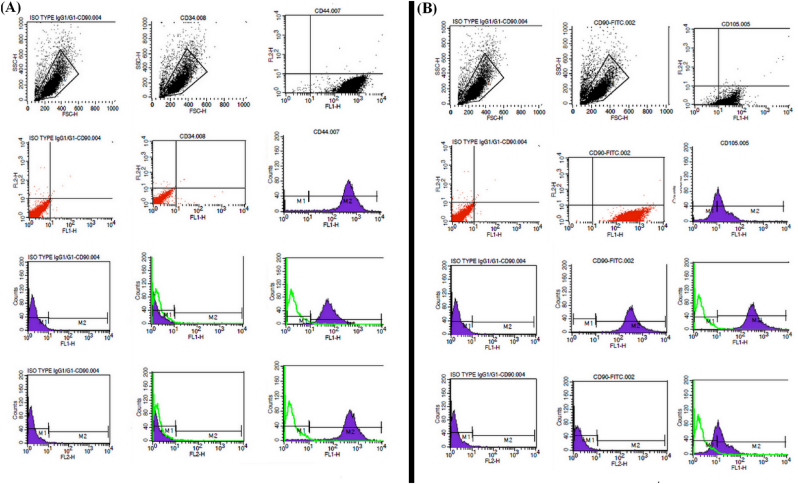
Flow cytometry results of CD44, CD105, and CD90 surface antigen markers, specific to MSCs, illustrated a positive correlation between the said cells and the aforementioned indicators. Moreover, CD34 surface antigens, distinct to hematopoietic stem cells, were identified to be not being expressed in MSCs making these cells negative for CD34.
The correct conduct of mesenchymal cell extraction from the UC tissue was confirmed by the said results (Fig. 1).
Fig. 1.
The percentage of CD90, CD105, CD44, and CD34 surface marker expression of WJUC MSCs. R1 demonstrates the Gating region of stem cells based on cell distribution in terms of cell size (FSC = Forward scatter) as well as range of complexity (SSC = Side scatter) with the X axis illustrating the fluorescence intensity and the Y axis the number of cells.
3.2. Results from the MSCs differentiation into adipocyte cell lines
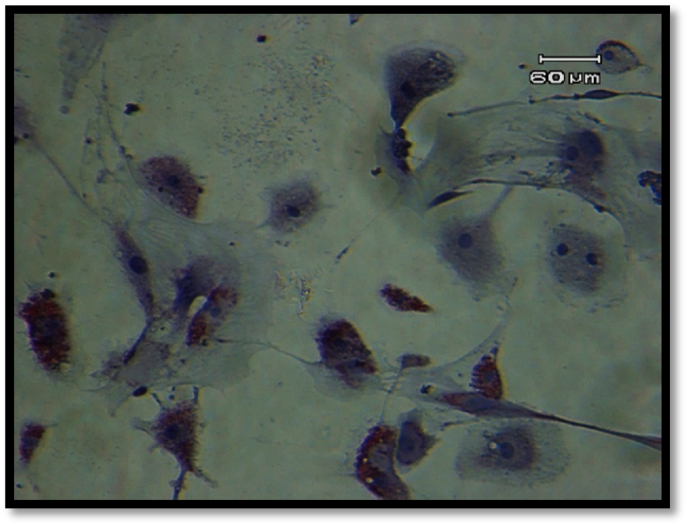
On account of MSCs lacking any primary and consistent indicator, to verify their mesenchymal nature, in addition to cell surface marker flow cytometry, it is required that these cells be induced to differentiate in differentiating media. In Fig. 2, achieved by Oil Red staining, the existence of cytoplasmic vacuoles containing fat droplets connote to the differentiation of the aforementioned cells into adipocytes of the aforementioned cells into adipocytes.
Fig. 2.
Lipid vacuoles stained with Oil Red O (×100).
3.3. Results of differentiation of UCB-MSCs into hepatocyte-like cells, a morphological examination
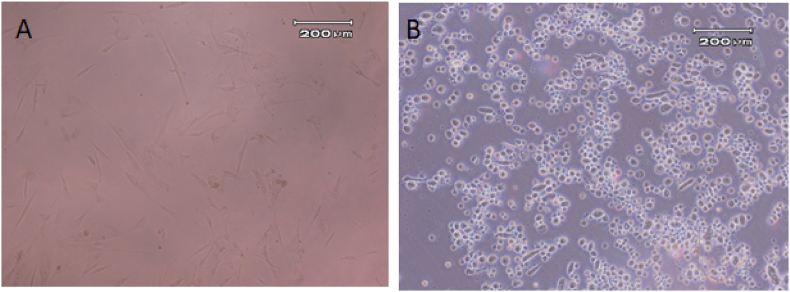
In the first week of differentiation, the differentiated cells didn't undergo any notable change in their morphology, rather after adding OSM in the second phase of differentiation, the morphology of the hepatocytes appeared which consisted of large nucleus and polyhedral cells. Before differentiation, mesenchymal cells had an elongated fibroblast-like and spindle-shaped appearance with protruding cytoplasmic appendages, however, following differentiating into hepatocyte-like cells through a differentiating medium, their morphology altered from elongated into multifaceted (Fig. 3).
Fig. 3.
Image from cell morphology by inverted microscope, (A) before differentiation and B after differentiation (×10).
3.4. Outcome of MTT
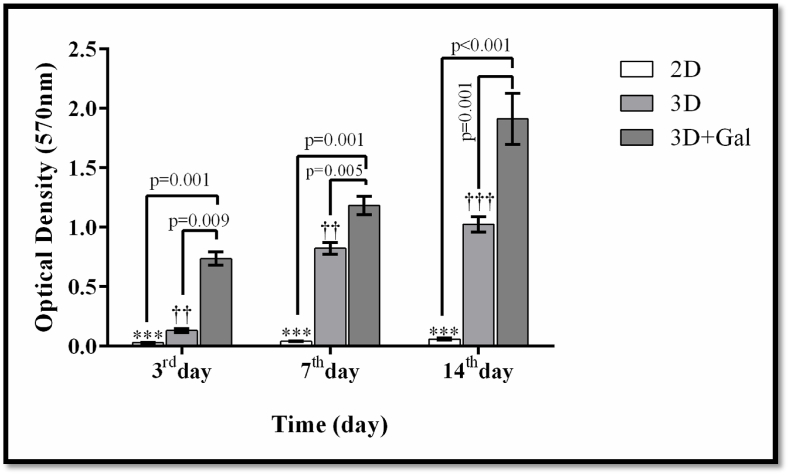
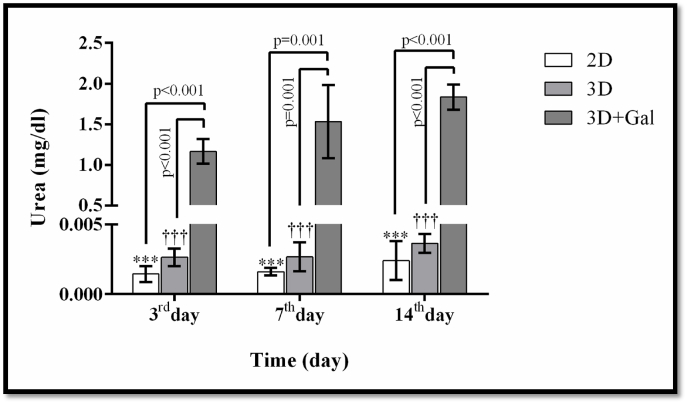
MTT test was employed to verify the lack of scaffold toxicity on the cells. Utilizing parametric ANOVA test as well as Tukey's Honest Significant Difference, the light absorption quantity between test groups, namely two-dimensional culture (2D), three-dimensional culture without scaffold galactosylation (3D), and three-dimensional culture with galactosylated scaffold (3D + Gal), were determined. The light absorption level was the highest in the 3D + Gal group with a significant difference being reported in days 3, 7, and 14 in comparison to the other two groups (p < 0.01). This result indicates a higher number of cells attached to the scaffold than other groups, which is statistically significant (Fig. 4).
Fig. 4.
The results of effects of scaffolds evaluation on the proliferation and viability of cells by MTT method. The data is shown as SD ± Mean. *** indicates a significant difference at p ≤ 0.001 level in the 3D + Gal group compared to the 2D group. †† and ††† indicates a significant difference in the 3D + Gal group compared to the 3D and 2D groups, respectively, at the level of P < 0.01 and p = 0.001.
3.5. The results of urea secretion in culture medium by differentiated cells
Examining the ability to secrete urea showed that mesenchymal cells cannot produce and excrete urea before differentiation while following their differentiation, hepatocyte-like cells derived from these stem cells become capable to secrete urea. Therefore, the synthesis and secretion of urea are unique to liver cells. The results yielded from this research demonstrated that the differentiated cells on the surface of 2D, 3D, and galactosylated 3D scaffolds possess the ability to produce a notable amount of urea. However, the urea production level gradually increased and the highest level was observed in the cells cultured on galactosylated 3D scaffold. Urea secretion in UC stem cells was regarded as a negative control.
Through parametric ANOVA along with Tukey's Honest Significant Difference, the urea secretion level between test groups, namely two-dimensional culture (2D) culture, three-dimensional culture without scaffold galactosylation (3D), and three-dimensional culture with scaffold galactosylation (3D + Gal), were investigated with the result introducing 3D + Gal with a significant increase in urea secretion in comparison to other two groups (Fig. 5).
Fig. 5.
Examining the amount of urea secretion in the culture medium by differentiated cells in different groups. - The data is shown as SD ± Mean. *** indicates a significant difference at p ≤ 0.001 level in the 3D + Gal group compared to the 2D group. †† and ††† indicates a significant difference in the 3D + Gal group compared to the 3D and 2D groups, respectively, at the level of P < 0.01 and p = 0.001.
3.6. The results of the OCT-4, HNF1, ALB, AFP and CYP gene expressions
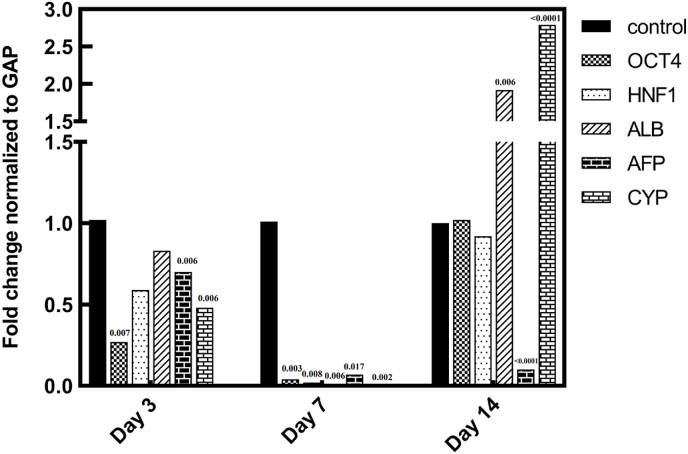
In accordance to Fig. 6 and compared to control cells, the relative modifications in OCT-4 gene expression on the third day in the cells on the scaffold decreased 0.27 times which is statistically significant with p = 0.007. On the seventh and fourteenth day, the relative expression of the OCT-4 gene in the scaffolded sample showed, respectively, a decrease of 0.04 times with a statistical significance of p: 0.003 and a slight increase of 1.02 times compared to the control.
Fig. 6.
Diagram of gene expression changes in the scaffold group and the control group on days 3, 7 and 14.
The relative alterations in HNF1 gene expression on the third day in scaffolded cells declined by 0.59 times compared to control cells. In comparison to the control group on the seventh day, the relative expression of the HNF1 gene in the sample cells on the scaffold diminished by 0.02 times, with a statistical significance of p:0.008. Additionally, the relative changes in the aforementioned gene showed a decrease of 0.92 times compared to the control in scaffolded cells on the fourteenth day.
In comparison to the control, the relative modifications in ALB gene expression on the third day in the cells on the scaffold showed a decrease of 0.83 times. On the seventh day and in comparison with the control group, a statistically significant decline (p:0.006), almost reaching zero, in ALB gene expression was reported, however, the same gene expression was reported to be elevated 1.92 times with a significant p-value of 0.006 in scaffolded cells on the fourteenth day compared to control.
Compared to the control group, the relative alterations in AFP gene expression in the cells on the scaffold on the third day diminished 0.7 times, reporting a statistical significance of p:0.006. On the seventh day, a 0.07 times decrease in AFP gene expression was detected which represented a p-value of 0.017, while the same gene expression was observed to be elevated 1.92 times with a significant p-value of 0.006 on the fourteenth day, all in comparison with the control group. Compared to control group, the relative changes in CYP gene expression in the cells on the scaffold on the third day diminished 0.48 times, representing a statistical significance of p:0.006. On the seventh day, 0.01 times decline in CYP gene expression was noted which meant a p value of 0.002, however the same gene expression was subjected to a 1.92 times elevation with a statistical significance of p < 0.0001 on the fourteenth day, all in comparison with the control group (Fig. 6).
4. Discussion
One of the recent discoveries in the field of regenerative medicine of medical science is the identification and isolation of a new type of cells from mammals called stem cells, which are considered by numerous researchers as a source for cell therapy due to their special characteristics. Regarding this recent discovery, the purpose of this study was to investigate the possibility of employing stem cells in cell therapy for liver diseases. Stem cells are present in all mature tissues including bone marrow, peripheral blood, blood vessels, skeletal muscles, fat tissue, skin, and liver [23].
Previously the best source for isolating MSCs was considered to be bone marrow, however, due to the invasive method of accessing and collecting bone marrow along with the possibility of inflicting injury to the donator during sample collection as well as other complications such as the chance of collecting an inadequate volume of sample and an apparent decline in stem cells potential in differentiating in the course of aging procedure, researchers were prompted to look for other sources for stem cells. With regards to that, fat tissues being more accessible as well as more able to provide a higher quantity of sample as a source of cells with inflicting negligible ramifications on the donator and the collected cells demonstrating higher efficiency, lead this tissue to be considered as a proper alternative to bone marrow for MSCs extraction. Subsequently, UC tissue came to be known as an ideal source for isolating MSCs, bearing great resemblance to those isolated from bone marrow in terms of morphology as well as surface markers expressions [24].
Additionally, when deposited in a culture medium containing appropriate inducers, stem cells isolated from UC demonstrated a greater potential in differentiating into various mesenchymal cell lines such as bone marrow, cartilage, and fat, a feature that illustrates little to no difference compared to bone marrow extracted MSCs [25].
Moreover, it has been shown in many studies that the differentiation capability of UC MSCs is higher than that of bone marrow in some tissues such as liver-like cells. MSCs isolated from UC tissue possess some advantages over bone marrow-derived MSCs, paving the way for these cells to be employed in the clinical field as well as research and innovative field.
The availability and accessibility to sufficient amounts are a few benefits of utilizing UC as a source for stem cell extraction. Furthermore, the UC stem cells, in comparison with those extracted from bone marrow tissue, yield more efficient cells [26].
In this study, MSCs were isolated from the UC. Following the 48 h of culture of extracted cells, the first adherent, elongated, spindle-shaped cells with uncanny resemblance to fibroblasts, were discerned at the bottom of the flask, which grew and multiplied as colonies. According to the available data, colony formation is an indicator of the potential for expansion and proliferation of MSCs.
The next step was checking and proving the stem cell nature of the extracted cells through flow cytometry analysis and exploring their differentiation ability. More evidence in support of the cultured cells being MSCs was obtained by determining the surface markers of the mentioned cells by exposing them to appropriate monoclonal antibodies and subsequent conduction of flow cytometry. Identification of the researched mesenchymal cells in this study was achieved by superficial indicators including the negative marker CD34 and two positive markers CD44 and CD105, with the obtained results showing many of the purified cells being by these markers.
In most hematopoietic stem cells (HSCs) and hematopoietic progenitor cells, CD34 is expressed. The role of CD34 is not fully defined and its expression in the blood system is limited to only the aforementioned cells, however, the expression pattern of CD34 demonstrates that this antigen plays an important role in primary hematopoiesis [27].
CD44 is a membrane receptor-dependent on cell adhesion molecules that is expressed in most cells and is involved not only in the cell's response to the surrounding microenvironment, but also in most cellular procedures such as growth regulation, survival, differentiation, and movement. CD44 is also present in the membrane of MSCs and is employed in determining the characteristics of these cells and their subsequent isolation [28].
CD105 is a proliferation-dependent and hypoxia-inducible protein expressed copiously in angiogenic endothelial cells. This protein acts as a receptor for transforming growth factor beta (TGF-β) and is involved in the signaling of TGF-β [29]. The aforementioned antigen is employed in MSCs isolation and identification. According to the data collected in this research and the expression of aforementioned superficial markers on the isolated stem cells as well as flowcytometric results of positive surface markers of MSCs, the adherent cells on the bottom of the cultural flask demonstrated an expression pattern of these markers similar to those found on MSCs, thus the MSC origin of isolated and cultured cells was verified. However, other tests were also performed for the verification of these cells, which are discussed in the following sections. One of the practical ways to determine mesenchymal cells is to examine their ability to transform into mesodermal cells in the laboratory environment. Therefore, in this research, the differentiation ability of MSCs to adipose cell lines was investigated. The results of the current study showed that the isolated purified cells are capable of differentiating into fat cells. The recognition of differentiated cells was possible by observing the accumulation of fat-rich vacuoles inside the cells. Differentiation of stem cells into adipocytes was determined by specialized Oil-Red staining. The results illustrated the multipotent capability of the aforementioned cells in giving rise to MSCs [30].
Also, the yielded results from this study confirmed the ability of the isolated mesenchymal cells in differentiating into fat cell lines. Regarding this and the presence of specific surface markers, it was deducible that the isolated MSCs possess the perfect mesenchymal phenotype.
For this research, a scaffold was utilized to stimulate an environment identical to the extracellular matrix. Mature stem cells are present in a specific niche that coordinates their regeneration and differentiation. This article has led to the birth of a theorem that imitating the stem cell niche might result in facilitating its regeneration and controlled differentiation outside the body.
Cells in two-dimensional culture are propagated in a single layer in a specific container, and due to this one-layer proliferation, numerous disparities in cell behavior and procedures, including gene expression and signaling, between 2D cultured cells and living organisms are reported. These differences emerge from the number of dimensions that cells are in contact with each other, with the cells grown in a cultural flask proliferating and surviving in a 2D manner while cells in living organisms interact in the 3D environment. Thus, the specific characteristics of intercellular communication are lost in terms of histology. Contrary to adjustable yet two-dimensional substrates, three-dimensional substrates make cell interaction with a versatile three-dimensional environment feasible and possible.
For this research, due to availability and cost efficiency, decellularized murine liver connective tissue was used as a scaffold and was coated with a low concentration of galactose. MTT test was conducted to ascertain the performance along with the viability of the cells on the scaffold. This quantitative and sensitive assay illustrates a linear association between viable cells and the color intensity emitted; the more abundant the cells, the higher the intensity of emitted color. The color intensity created in 3D culture and galactosylated 3D culture was higher than in other groups, with a significant difference observable on days third, seventh, and fourteenth, indicating the existence of more cells adhered to the scaffold in comparison to other groups. As of yet, the accumulated results of various studies on creating suitable laboratory models for diverse body tissues indicate that 3D cultures with the implementation of proper scaffolds ultimately lead to these models resembling more natural tissues [[31], [32], [33]]. Therefore, the decellularized murine liver was used as a scaffold. Through depositing growth factors as well as tissue-specific cytokines, and establishing some signaling pathways, glycosaminoglycan, one of the prominent components of the hepatic extracellular matrix, engenders the structural organization and better functioning of the liver tissue [34]. On the other hand, the presence of collagen in the natural structure of the decellularized liver causes structural preservation of the hepatic tissue, and alongside improving tissue cohesion, cell connections, and regulating mechanical parameters, it elevates the survival rate and performance of hepatocytes [35], which is in line with the results of this current research.
In 2014, Ramaiahgari and colleagues discovered that HepG2 cells cultured with Matrigel in 3D formed bile canaliculi-like structures and regained many of the lost hepatocyte characteristics [36]. In 2016, Yamada et al. demonstrated that the culture of hepatocytes in the form of spheroids and the presence of type 1 collagen spheres increased survival and improved albumin secretion [37]. These results are indicative of the critical influence of form, structure, and composition of the surrounding environment on cell behavior and performance [38]. In this research, the shape and structure of a natural murine liver scaffold, taken by electron microscope confirmed that its three-dimensional and porous structure can provide the proper nutrition, mobility, migration, and proliferation for the desired cells since the yielded results affirmed that this method of 3D culture and galactosylation of the scaffold leads to enhancement of the differentiated cells performance (including the amount of urea production, viability, and increased albumin gene expression as important hepatic indicators). In this study, through examining the capability to secrete urea, it was revealed that the MSCs are deprived of this ability and only obtain this function following differentiating to hepatocyte-like cells, thus urea synthesis and secretion is exclusive to hepatocytes. The collected results of this experiment elucidate that the differentiated cells on the surface of 2D, 3D, and 3D galactosylated scaffolds can produce a significant amount of urea. The level of urea synthesis escalated in the aforementioned cells media with the highest level of urea secretion belonging to cells cultured on the galactosylated scaffold. Urea secretion in UC basal cells was considered a negative control. Therefore, in this study, the amount of urea secretion in the 3D + Gal group demonstrated a remarkable elevation compared to the other two groups, indicating the proper function of the differentiated cells in the three-dimensional galactosylated scaffold. In 2015, Khetani et al. illustrated that, in comparison with two-dimensional culture, the cultivation of HepG2/C3A cells in the form of liver spheroids ameliorates albumin secretion, elevates metabolic activity and increases enzyme expressions of phases one and two of liver metabolism [39].
Li's work showed that a collagen-modified 3D PLGA scaffold was superior to a control without collagen modification and to a 2D culture system in supporting the growth and metabolism of primary human hepatocytes. The cell number and lifespan of primary hepatocytes were significantly greater in collagen-coated PLGA scaffolds compared with those in a control PLGA scaffold without collagen modification [40].
This study put the gene expression of albumin, OCT-4, HNF1, AFP, and CYP under investigation which is among the most salient genes involved in the development procedure of the liver. Scrutinizing albumin gene expression has captured the spotlight in exploring the field of hepatogenic differentiation of stem cells [41]. In this study as well, the expression of the albumin gene during a certain interim was investigated, and detecting its expression was one of the reasons for confirming the hepatic differentiation of the cells. Therefore, albumin, as one of the most important secretory proteins of the liver, plays a paramount role in liver function and an increase in this gene expression indicates the improvement of the proper function of liver hepatocyte-like cells.
In most hepatic laboratory models, the focus is on hepatocytes as hepatic parenchymal cells, since these cells are the most abundant in the hepatic tissue and are responsible for the majority of liver functions. However, with the liver being a complex organ, cells other than parenchyma exist in it, which have prominent roles in hepatic structural and functional maintenance.
Therefore, a suitable and functional laboratory model is comprised of a model that contains other pertinent cells in addition to hepatocytes [42]. In 2016, Gaskel et al. delineated that the presence of sinusoidal endothelial cells adjoining rat hepatocytes preserves CYPs function and increases the expression of indicators such as albumin and transferrin for up to 37 days. In 2011, Kasuya et al. illustrated that stellate cells, having a prominent role in liver homeostasis, establish communication between hepatocytes and endothelial cells [43]. Qinzi et al. not only reported the presence of albumin in a nine-day-old fetus but also illustrated the ability of nine-day-old liver cells to synthesize urea, the amount of which was interestingly 40 times less compared to the adult liver [44].
One of the indicators that determine the function of hepatocytes is the activity level of cytochrome 450 enzymes as well as their inducibility potential. The exceedingly low expression and activity of the aforementioned enzymes in hepatoma cell lines have given rise to critical complications in their utilization for pharmaceutical studies [45]. To overcome this obstacle, the performance of these enzymes in cell lines should be ameliorated and through previous studies, it has been revealed that the 3D culture of cell lines in comparison with 2D culture could notably increase the cytochromes activity [46]. Through this study, it was discovered that the level of CYP gene expression was elevated considerably on the fourteenth day in the galactosylated 3D scaffold group. In this research, a significant increase in CYP gene expression level in the galactosylated 3D scaffold group was reported on the fourteenth day. On this account, it is deducible that through galactosylation of 3D culture protocol, the performance of cytochrome enzymes could be enhanced, however, for obtaining concrete proof, either the concentration of the aforementioned enzymes must be measured or their presence proven by utilizing western blotting and or immunohistochemistry techniques, both of which due to financial constraints could not be afforded to be performed.
A prominent gene in creating self-renewal ability is the OCT-4 gene, which maintains the self-renewal characterization of stem cells through the simultaneous activation of stem cells and inhibition of genes responsible for initiating differentiation. Thus, the central network plays a major role in proliferation as well as self-renewal of embryonic stem cells [47]. The findings of the current research demonstrated that on the third day the OCT-4 gene is slightly expressed, with this expression severely reduced on the seventh day, however on the fourteenth day, a rise in expression level was reported. Research findings illustrated that the OCT-4 expression level is significantly low in normal colon samples. In 2007, Atlasi et al. demonstrated that the OCT-4 gene is expressed to a small extent in normal bladder tissue [48]. Furthermore, Tai et al. illustrated that the OCT-4 gene is expressed exclusively in the stem cells of one tissue with the other cells of the said tissue being deprived of the ability to express this gene [49].
AFP (Alpha-Fetoprotein) is a fetal liver marker the expression of which decreases with liver development [50]. The proliferation and differentiation of hepatocytes are influenced by various extracellular signals such as hormones and cytokines. In the fetal liver, the physiological concentration of dexamethasone (a synthetic glucocorticoid) suppresses AFP production and DNA synthesis [51]. In numerous studies, AFP has been considered a primary liver marker for the differentiation of stem cell types into hepatocyte lineage cells. In this research, through executing RT-PCR, not much of a change was discerned in the level of expression during the differentiation, but the expression level declined significantly on the seventh and fourteenth days compared to the third day. In the year 2011, through conducting research on the in vitro differentiation of MSCs of human UC vein into liver-like cells, Amini et al. discovered that AFP gene expression level didn't alter much during differentiation, even after executing additional PCR using PCR product, no expression of the said gene was detected [52].
Ghodsizadeh et al. demonstrated significant upregulation of hepatic specific genes, ALB, HNF4a, CYP3A4, G6P, and ASGR1, and downregulation of AFP in addition to efficient functional enhancement of hepatocyte-like cells compared to the C matrix by differentiation of hESC-hepatic endoderm on galactosylated collagen-coated plates [53].
An innumerable number of HNF genes regulate intrahepatic and extrahepatic biliary development [54]. The transcription factor HNF is responsible for regulating the competence of the endoderm in developing the liver and is essential for liver gene expression [55]. In this study, expression of the mentioned gene was discerned on the third and fourteenth days.
Transcription factor HNF binds to 40% of liver gene promoters and is required for the activation of most liver genes [56,57] and therefore is the main determinant of hepatocyte function and plays a fundamental role in establishing the normal structure of the liver during mid-pregnancy by stimulating the expression of cellular binding proteins in the hepatoblasts as well as facilitating the formation of canaliculi along with maintaining its sinusoids. Also, the aforementioned transcription factor not only effectively promotes the transformation of immature embryonic hepatocytes into mature polar epithelial hepatocytes, but also maintains the differentiated hepatocyte phenotype [52].
The findings of this study are complementary to the findings of previous ones, making it conspicuous to conclude that, Wharton jelly MSCs of human UC are capable of differentiating into liver cells under specific and appropriate laboratory conditions, which were met in this study.
The collected results of this study indicate that hepatocyte-like cells obtained from 3D culture on galactosylated scaffold have better characteristics compared to differentiated cells on 3D scaffold alone and 2D culture, which was due to firm adhesion and exceptional proliferation as well as great stability in the course of proliferation. Also, according to the gathered data, it is conducible that MSCs derived from Wharton's jelly UC can differentiate into liver-like cells.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors would like to convey their sincerest gratitude to Dr. Massoud Vosough for donation of scaffold.
Contributor Information
Massoud Vosough, Email: masvos@royaninstitute.org.
Kamran Ghaedi, Email: kamranghaedi@sci.ui.ac.ir.
Abbreviations
- (WJUC)
Wharton's jelly umbilical cord
- (MSCs)
Mesenchymal Stem Cells
- (UC)
umbilical cord
- (TE)
Tissue engineering
- (IGF)
Insulin growth factor
- (LIF)
Leukemia inhibitory factors
- (FBS)
Fetal Bovine Serum
- (PBS)
Phosphate Buffer Saline
- (NHS)
N-hydroxysulfosuccinimide
- (MES)
2-(-N-morpholino) ethanesulfonic acid
- (EDC)
1-ethyl-3-[3-dimethylaminopropyl] carbodiimide
- (DMEM)
Dulbecco's Modified Eagle Medium
Data availability
The data that has been used is confidential.
References
- 1.Söderberg C., Stål P., Askling J., Glaumann H., Lindberg G., Marmur J., et al. Decreased survival of subjects with elevated liver function tests during a 28‐year follow‐up. Hepatology. 2010;51(2):595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 2.Chivu M., Dima S.O., Stancu C.I., Dobrea C., Uscatescu V., Necula L.G., et al. In vitro hepatic differentiation of human bone marrow mesenchymal stem cells under differential exposure to liver-specific factors. Transl. Res. 2009;154(3):122–132. doi: 10.1016/j.trsl.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Lysy P.A., Smets F., Najimi M., Sokal E.M. Leukemia inhibitory factor contributes to hepatocyte-like differentiation of human bone marrow mesenchymal stem cells. Differentiation. 2008;76(10):1057–1067. doi: 10.1111/j.1432-0436.2008.00287.x. [DOI] [PubMed] [Google Scholar]
- 4.Ko I.K., Peng L., Peloso A., Smith C.J., Dhal A., Deegan D.B., et al. Bioengineered transplantable porcine livers with re-endothelialized vasculature. Biomaterials. 2015;40:72–79. doi: 10.1016/j.biomaterials.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Bowden R.A., Ljungman P., Snydman D.R. 2012. Transplant Infections. [Google Scholar]
- 6.Hutmacher D., Goh J., Teoh S. An introduction to biodegradable materials for tissue engineering applications. Ann. Acad. Med. Singapore. 2001;30(2):183–191. [PubMed] [Google Scholar]
- 7.Ikada Y. Challenges in tissue engineering. J. R. Soc. Interface. 2006;3(10):589–601. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher J.P., Mikos A.G., Bronzino J.D., Peterson D.R. CRC Press; 2012. Tissue Engineering: Principles and Practices. [Google Scholar]
- 9.Lucas P.A., Laurencin C., Syftestad G.T., Domb A., Goldberg V.M., Caplan A.I., et al. Ectopic induction of cartilage and bone by water‐soluble proteins from bovine bone using a polyanhydride delivery vehicle. J. Biomed. Mater. Res. 1990;24(7):901–911. doi: 10.1002/jbm.820240708. [DOI] [PubMed] [Google Scholar]
- 10.Masters D.B., Berde C.B., Dutta S.K., Griggs C.T., Hu D., Kupsky W., et al. Prolonged regional nerve blockade by controlled release of local anesthetic from a biodegradable polymer matrix. Anesthesiology. 1993;79(2):340–346. doi: 10.1097/00000542-199308000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Heller J. Development of poly (ortho esters): a historical overview. Biomaterials. 1990;11(9):659–665. doi: 10.1016/0142-9612(90)90024-k. [DOI] [PubMed] [Google Scholar]
- 12.Macchiarini P., Jungebluth P., Go T., Asnaghi M.A., Rees L.E., Cogan T.A., et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal V., Tottey S., Johnson S.A., Freund J.M., Siu B.F., Badylak S.F. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng. 2011;17(19–20):2435–2443. doi: 10.1089/ten.tea.2011.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wainwright J.M., Czajka C.A., Patel U.B., Freytes D.O., Tobita K., Gilbert T.W., et al. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng. C Methods. 2010;16(3):525–532. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crapo P.M., Tottey S., Slivka P.F., Badylak S.F. Effects of biologic scaffolds on human stem cells and implications for CNS tissue engineering. Tissue Eng. 2014;20(1–2):313–323. doi: 10.1089/ten.tea.2013.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mase V.J., Jr., Hsu J.R., Wolf S.E., Wenke J.C., Baer D.G., Owens J., et al. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics. 2010;33(7) doi: 10.3928/01477447-20100526-24. [DOI] [PubMed] [Google Scholar]
- 17.Valentin J.E., Badylak J.S., McCabe G.P., Badylak S.F. Extracellular matrix bioscaffolds for orthopaedic applications: a comparative histologic study. JBJS. 2006;88(12):2673–2686. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- 18.Faulk D.M., Carruthers C.A., Warner H.J., Kramer C.R., Reing J.E., Zhang L., et al. The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater. 2014;10(1):183–193. doi: 10.1016/j.actbio.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf M.T., Daly K.A., Brennan-Pierce E.P., Johnson S.A., Carruthers C.A., D'Amore A., et al. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. 2012;33(29):7028–7038. doi: 10.1016/j.biomaterials.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sicari B.M., Dearth C.L., Badylak S.F. Tissue engineering and regenerative medicine approaches to enhance the functional response to skeletal muscle injury. Anat. Rec. 2014;297(1):51–64. doi: 10.1002/ar.22794. [DOI] [PubMed] [Google Scholar]
- 21.Beck L.R., Pope V.Z. Controlled-release delivery systems for hormones: a review of their properties and current therapeutic use. Drugs. 1984;27(6):528–547. doi: 10.2165/00003495-198427060-00002. [DOI] [PubMed] [Google Scholar]
- 22.Dutta R.C., Dutta A.K. Cell-interactive 3D-scaffold; advances and applications. Biotechnol. Adv. 2009;27(4):334–339. doi: 10.1016/j.biotechadv.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 24.Romanov Y.A., Darevskaya A., Merzlikina N., Buravkova L. Mesenchymal stem cells from human bone marrow and adipose tissue: isolation, characterization, and differentiation potentialities. Bull. Exp. Biol. Med. 2005;140:138–143. doi: 10.1007/s10517-005-0430-z. [DOI] [PubMed] [Google Scholar]
- 25.Caplan A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007;213(2):341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 26.Fukuchi Y., Nakajima H., Sugiyama D., Hirose I., Kitamura T., Tsuji K. Human placenta‐derived cells have mesenchymal stem/progenitor cell potential. Stem cells. 2004;22(5):649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland D.R., Keating A. The CD34 antigen: structure, biology, and potential clinical applications. Journal of hematotherapy. 1992;1(2):115–129. doi: 10.1089/scd.1.1992.1.115. [DOI] [PubMed] [Google Scholar]
- 28.Alhadlaq A., Mao J.J. Mesenchymal stem cells: isolation and therapeutics. Stem Cell. Dev. 2004;13(4):436–448. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Elsner T., Botella L.M., Velasco B., Langa C., Bernabéu C. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-β pathways. J. Biol. Chem. 2002;277(46):43799–43808. doi: 10.1074/jbc.M207160200. [DOI] [PubMed] [Google Scholar]
- 30.Morrison S.J., Spradling A.C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotfinia M., Kadivar M., Piryaei A., Pournasr B., Sardari S., Sodeifi N., et al. Effect of secreted molecules of human embryonic stem cell-derived mesenchymal stem cells on acute hepatic failure model. Stem Cell. Dev. 2016;25(24):1898–1908. doi: 10.1089/scd.2016.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell C.C., Hendriks D.F., Moro S.M., Ellis E., Walsh J., Renblom A., et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci. Rep. 2016;6(1) doi: 10.1038/srep25187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darakhshan S., Pour A.B., Kowsari-Esfahan R., Vosough M., Montazeri L., Ghanian M.H., et al. Investigation of in vitro functionality of liver microtissues produced by Co-culture of mesenchymal stem cells, endothelial, and hepatic cell line. Research in Medicine. 2020;44(4):532–539. [Google Scholar]
- 34.De Colli M., Massimi M., Barbetta A., Di Rosario B., Nardecchia S., Devirgiliis L.C., et al. A biomimetic porous hydrogel of gelatin and glycosaminoglycans cross-linked with transglutaminase and its application in the culture of hepatocytes. Biomedical Materials. 2012;7(5) doi: 10.1088/1748-6041/7/5/055005. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Kim M.H., Shirahama H., Lee J.H., Ng S.S., Glenn J.S., et al. ECM proteins in a microporous scaffold influence hepatocyte morphology, function, and gene expression. Sci. Rep. 2016;6(1) doi: 10.1038/srep37427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramaiahgari S.C., Den Braver M.W., Herpers B., Terpstra V., Commandeur J.N., van de Water B., et al. A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Arch. Toxicol. 2014;88:1083–1095. doi: 10.1007/s00204-014-1215-9. [DOI] [PubMed] [Google Scholar]
- 37.Piryaei A., Valojerdi M.R., Shahsavani M., Baharvand H. Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis model. Stem Cell Reviews and Reports. 2011;7:103–118. doi: 10.1007/s12015-010-9126-5. [DOI] [PubMed] [Google Scholar]
- 38.Discher D.E., Janmey P., Wang Y-l. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 39.Khetani S.R., Berger D.R., Ballinger K.R., Davidson M.D., Lin C., Ware B.R. Microengineered liver tissues for drug testing. J. Lab. Autom. 2015;20(3):216–250. doi: 10.1177/2211068214566939. [DOI] [PubMed] [Google Scholar]
- 40.Li J., Li L., Yu H., Cao H., Gao C., Gong Y. Growth and metabolism of human hepatocytes on biomodified collagen poly (lactic-co-glycolic acid) three-dimensional scaffold. Am. Soc. Artif. Intern. Organs J. 2006;52(3):321–327. doi: 10.1097/01.mat.0000217794.35830.4a. [DOI] [PubMed] [Google Scholar]
- 41.Baharvand H., Hashemi S.M., Ashtiani S.K., Farrokhi A. Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int. J. Dev. Biol. 2004;50(7):645–652. doi: 10.1387/ijdb.052072hb. [DOI] [PubMed] [Google Scholar]
- 42.Skardal A., Devarasetty M., Soker S., Hall A.R. In situ patterned micro 3D liver constructs for parallel toxicology testing in a fluidic device. Biofabrication. 2015;7(3) doi: 10.1088/1758-5090/7/3/031001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasuya J., Sudo R., Mitaka T., Ikeda M., Tanishita K. Hepatic stellate cell-mediated three-dimensional hepatocyte and endothelial cell triculture model. Tissue Eng. 2011;17(3–4):361–370. doi: 10.1089/ten.TEA.2010.0033. [DOI] [PubMed] [Google Scholar]
- 44.Chinzei R., Tanaka Y., Shimizu-Saito K., Hara Y., Kakinuma S., Watanabe M., et al. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology. 2002;36(1):22–29. doi: 10.1053/jhep.2002.34136. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz R., Fleming H., Khetani S., Bhatia S. Pluripotent stem cell-derived hepatocyte-like cells. Biotechnol. Adv. 2014;32(2):504–513. doi: 10.1016/j.biotechadv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaskell H., Sharma P., Colley H.E., Murdoch C., Williams D.P., Webb S.D. Characterization of a functional C3A liver spheroid model. Toxicology research. 2016;5(4):1053–1065. doi: 10.1039/c6tx00101g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodda D.J., Chew J.-L., Lim L.-H., Loh Y.-H., Wang B., Ng H.-H., et al. Transcriptional regulation of nanog by OCT4 and SOX2. J. Biol. Chem. 2005;280(26):24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- 48.Atlasi Y., Mowla S.J., Ziaee S.A., Bahrami A.R. OCT‐4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int. J. Cancer. 2007;120(7):1598–1602. doi: 10.1002/ijc.22508. [DOI] [PubMed] [Google Scholar]
- 49.Tai M.-H., Chang C.-C., Olson L.K., Trosko J.E. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26(2):495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 50.Shiojiri N., Lemire J.M., Fausto N. Cell lineages and oval cell progenitors in rat liver development. Cancer Res. 1991;51(10):2611–2620. [PubMed] [Google Scholar]
- 51.Belanger L., Frain M., Baril P., Gingras M.C., Bartkowiak J., Sala-Trepat J.M. Glucocorticosteroid suppression of. alpha. 1-fetoprotein synthesis in developing rat liver. Evidence for selective gene repression at the transcriptional level. Biochemistry. 1981;20(23):6665–6672. doi: 10.1021/bi00526a022. [DOI] [PubMed] [Google Scholar]
- 52.Raufi A., Amini A., Azadbakht M., Aboozari M., Fathi F. In vitro differentiation of human umbilical vein mesenchymal stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Scientific Journal of Iran Blood Transfus Organ. 2010;7(1):34–40. [Google Scholar]
- 53.Ghodsizadeh A., Hosseinkhani H., Piryaei A., Pournasr B., Najarasl M., Hiraoka Y., et al. Galactosylated collagen matrix enhanced in vitro maturation of human embryonic stem cell-derived hepatocyte-like cells. Biotechnol. Lett. 2014;36:1095–1106. doi: 10.1007/s10529-014-1454-0. [DOI] [PubMed] [Google Scholar]
- 54.Saha S.K., Parachoniak C.A., Ghanta K.S., Fitamant J., Ross K.N., Najem M.S., et al. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature. 2014;513(7516):110–114. doi: 10.1038/nature13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alizadeh E., Akbarzadeh A., Eslaminejad M.B., Barzegar A., Hashemzadeh S., Nejati‐Koshki K., et al. Up regulation of liver‐enriched transcription factors HNF 4a and HNF 6 and liver‐specific Micro RNA (miR‐122) by inhibition of Let‐7b in mesenchymal stem cells. Chem. Biol. Drug Des. 2015;85(3):268–279. doi: 10.1111/cbdd.12398. [DOI] [PubMed] [Google Scholar]
- 56.Divine J.K., Staloch L.J., Haveri H., Jacobsen C.M., Wilson D.B., Heikinheimo M., et al. GATA-4, GATA-5, and GATA-6 activate the rat liver fatty acid binding protein gene in concert with HNF-1α. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287(5):G1086–G1099. doi: 10.1152/ajpgi.00421.2003. [DOI] [PubMed] [Google Scholar]
- 57.Pournasr B., Farzaneh Z., Shahsanvani M., Baharvand H. Liver development and in vitro differentiation of embryonic stem cells to hepatocytes. Cell Journal (Yakhteh) 2010;11(4):348–373. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.