Abstract
We present a genome assembly from an individual female Bicyclus anynana (the Squinting Bush Brown; Arthropoda; Insecta; Lepidoptera; Nymphalidae). The genome sequence is 457.2 megabases in span. Most of the assembly is scaffolded into 28 chromosomal pseudomolecules, including the Z sex chromosome. The mitochondrial genome has also been assembled and is 16.1 kilobases in length.
Keywords: Bicyclus anynana, Squinting Bush Brown, genome sequence, chromosomal, Lepidoptera
Species taxonomy
Eukaryota; Metazoa; Ecdysozoa; Arthropoda; Hexapoda; Insecta; Pterygota; Neoptera; Endopterygota; Lepidoptera; Glossata; Ditrysia; Papilionoidea; Nymphalidae; Satyrinae; Satyrini; Mycalesina; Bicyclus; Bicyclus anynana (Butler, 1879) (NCBI:txid110368).
Background
Bicyclus anynana, commonly known as the Squinting Bush Brown, is a widely distributed, medium-sized, Afrotropical satyrid butterfly, inhabiting grasslands and forest edges. Its seasonally plastic phenotype and modular pattern of wing eye spots ( Figure 1), combined with ease of rearing in captivity, have made it a model system for evo-devo and life-history research ( Brakefield et al., 2009). In nature, the primary larval host plant is Oplismenus compositus, and the adults feed on rotting fruit; the laboratory stocks have been domesticated onto maize and wheatgrass.
Figure 1. Bicyclus anynana female – wet season phenotype.
(Photograph by William Piel, source: https://lepdata.org/monteiro/PNAS%20covers%20shots.html)
The standard karyotype consists of WZ females and ZZ males ( n = 28), but the W chromosome is exceptionally small and remains to be assembled ( Beldade et al., 2009; Van’t Hof et al., 2008). The present genome is the third independent assembly ( Murugesan et al., 2022; Nowell et al., 2017), which will support ongoing research on a wide range of fundamental questions. These include the genetics and development of wing colour pattern ( Prakash et al., 2022), regulation of phenotypic plasticity ( Tian & Monteiro, 2022), responses to climate change ( Oostra et al., 2018), genetics of inbreeding depression ( Saccheri et al., 2020), and sex-determination mechanisms.
Genome sequence report
The genome was sequenced from one female Bicyclus anynana from a collection at the University of Liverpool. A total of 20-fold coverage in Pacific Biosciences single-molecule HiFi long reads was generated. Primary assembly contigs were scaffolded with chromosome conformation Hi-C data. Manual assembly curation corrected 117 missing joins or mis-joins and removed 6 haplotypic duplications, reducing the scaffold number by 32.52%.
The final assembly has a total length of 457.2 Mb in 83 sequence scaffolds with a scaffold N50 of 17.4 Mb ( Table 1). Most (99.51%) of the assembly sequence was assigned to 28 chromosomal-level scaffolds, representing 27 autosomes and the Z sex chromosome. Chromosome-scale scaffolds confirmed by the Hi-C data are named in order of size ( Figure 2– Figure 5; Table 2). While not fully phased, the assembly deposited is of one haplotype. Contigs corresponding to the second haplotype have also been deposited. The mitochondrial genome was also assembled and can be found as a contig within the multifasta file of the genome submission.
Figure 2. Genome assembly of Bicyclus anynana, ilBicAnyn1.1: metrics.
The BlobToolKit Snailplot shows N50 metrics and BUSCO gene completeness. The main plot is divided into 1,000 size-ordered bins around the circumference with each bin representing 0.1% of the 457,175,899 bp assembly. The distribution of scaffold lengths is shown in dark grey with the plot radius scaled to the longest scaffold present in the assembly (21,498,244 bp, shown in red). Orange and pale-orange arcs show the N50 and N90 scaffold lengths (17,439,807 and 13,224,086 bp), respectively. The pale grey spiral shows the cumulative scaffold count on a log scale with white scale lines showing successive orders of magnitude. The blue and pale-blue area around the outside of the plot shows the distribution of GC, AT and N percentages in the same bins as the inner plot. A summary of complete, fragmented, duplicated and missing BUSCO genes in the lepidoptera_odb10 set is shown in the top right. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/ilBicAnyn1.1/dataset/CAMWEB01/snail.
Figure 5. Genome assembly of Bicyclus anynana, ilBicAnyn1.1: Hi-C contact map of the ilBicAnyn1.1 assembly, visualised using HiGlass.
Chromosomes are shown in order of size from left to right and top to bottom. An interactive version of this figure may be viewed at https://genome-note-higlass.tol.sanger.ac.uk/l/?d=TwYnm5nYQWuSaZneX5H9vQ.
Table 1. Genome data for Bicyclus anynana, ilBicAnyn1.1.
| Project accession data | ||
|---|---|---|
| Assembly identifier | ilBicAnyn1.1 | |
| Species | Bicyclus anynana | |
| Specimen | ilBicAnyn1 | |
| NCBI taxonomy ID | 110368 | |
| BioProject | PRJEB54938 | |
| BioSample ID | SAMEA9252597 | |
| Isolate information | ilBicAnyn1, female (genome sequencing)
ilBicAnyn2, male (Hi-C scaffolding) ilBicAnyn3, female (RNA sequencing) |
|
| Assembly metrics * | Benchmark | |
| Consensus quality (QV) | 63 | ≥ 50 |
| k-mer completeness | 100% | ≥ 95% |
| BUSCO ** | C:98.2%[S:97.6%,D:0.6%],
F:0.4%,M:1.4%,n:5,286 |
C ≥ 95% |
| Percentage of assembly mapped to
chromosomes |
99.51% | ≥ 95% |
| Sex chromosomes | Z chromosome | localised homologous pairs |
| Organelles | Mitochondrial genome assembled | complete single alleles |
| Raw data accessions | ||
| PacificBiosciences SEQUEL II | ERR10008895, ERR10008896, ERR10008897 | |
| Hi-C Illumina | ERR9988140 | |
| PolyA RNA-Seq Illumina | ERR10378024 | |
| Genome assembly | ||
| Assembly accession | GCA_947172395.1 | |
| Accession of alternate haplotype | GCA_947172465.1 | |
| Span (Mb) | 457.2 | |
| Number of contigs | 770 | |
| Contig N50 length (Mb) | 1.2 | |
| Number of scaffolds | 83 | |
| Scaffold N50 length (Mb) | 17.4 | |
| Longest scaffold (Mb) | 21.5 | |
* Assembly metric benchmarks are adapted from column VGP-2020 of “Table 1: Proposed standards and metrics for defining genome assembly quality” from ( Rhie et al., 2021).
** BUSCO scores based on the lepidoptera_odb10 BUSCO set using 5.3.2. C = complete [S = single copy, D = duplicated], F = fragmented, M = missing, n = number of orthologues in comparison. A full set of BUSCO scores is available at https://blobtoolkit.genomehubs.org/view/ilBicAnyn1.1/dataset/CAMWEB01/busco.
Figure 3. Genome assembly of Bicyclus anynana, ilBicAnyn1.1: BlobToolKit GC-coverage plot.
Scaffolds are coloured by phylum. Circles are sized in proportion to scaffold length. Histograms show the distribution of scaffold length sum along each axis. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/ilBicAnyn1.1/dataset/CAMWEB01/blob.
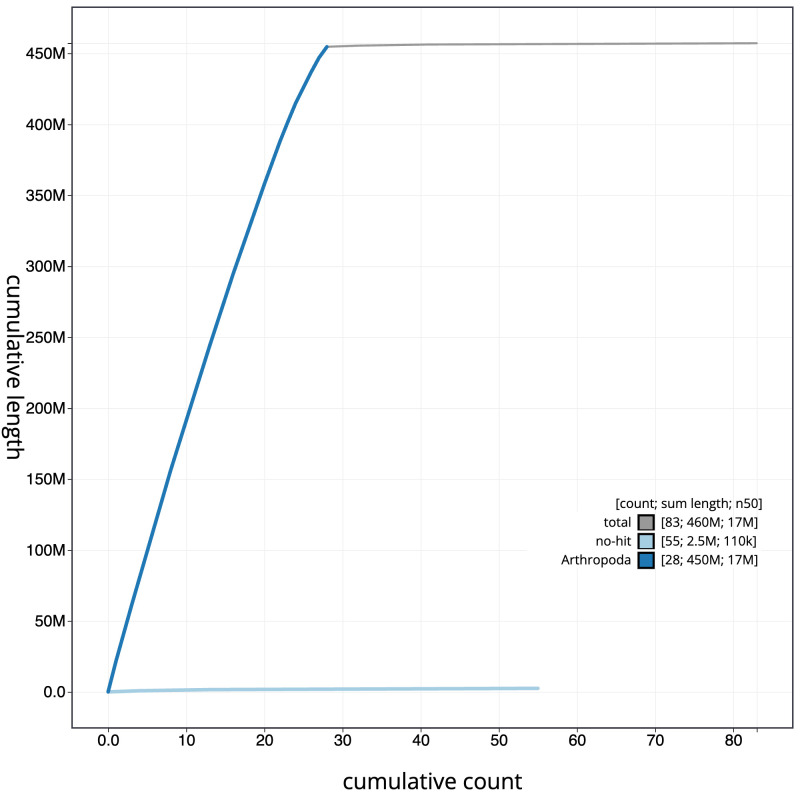
Figure 4. Genome assembly of Bicyclus anynana, ilBicAnyn1.1: BlobToolKit cumulative sequence plot.
The grey line shows cumulative length for all scaffolds. Coloured lines show cumulative lengths of scaffolds assigned to each phylum using the buscogenes taxrule. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/ilBicAnyn1.1/dataset/CAMWEB01/cumulative.
Table 2. Chromosomal pseudomolecules in the genome assembly of Bicyclus anynana, ilBicAnyn1.
| INSDC accession | Chromosome | Size (Mb) | GC% |
|---|---|---|---|
| OX359205.1 | 1 | 19.87 | 36.3 |
| OX359206.1 | 2 | 19.55 | 36.5 |
| OX359207.1 | 3 | 19.46 | 36.4 |
| OX359208.1 | 4 | 19.23 | 36.4 |
| OX359209.1 | 5 | 19.12 | 36.4 |
| OX359210.1 | 6 | 18.7 | 36.2 |
| OX359211.1 | 7 | 18.69 | 36.1 |
| OX359212.1 | 8 | 17.88 | 36.6 |
| OX359213.1 | 9 | 17.57 | 36.3 |
| OX359214.1 | 10 | 17.51 | 36.2 |
| OX359215.1 | 11 | 17.49 | 36.6 |
| OX359216.1 | 12 | 17.44 | 36.3 |
| OX359217.1 | 13 | 17.11 | 36.3 |
| OX359218.1 | 14 | 16.98 | 36.5 |
| OX359219.1 | 15 | 16.48 | 36.5 |
| OX359220.1 | 16 | 16.15 | 36.5 |
| OX359221.1 | 17 | 15.96 | 36.7 |
| OX359222.1 | 18 | 15.78 | 37.3 |
| OX359223.1 | 19 | 15.49 | 36.7 |
| OX359224.1 | 20 | 15.16 | 36.6 |
| OX359225.1 | 21 | 14.96 | 37 |
| OX359226.1 | 22 | 13.7 | 36.7 |
| OX359227.1 | 23 | 13.22 | 36.9 |
| OX359228.1 | 24 | 11.08 | 37.1 |
| OX359229.1 | 25 | 10.94 | 36.9 |
| OX359230.1 | 26 | 10.07 | 37.8 |
| OX359231.1 | 27 | 7.6 | 37.5 |
| OX359204.1 | Z | 21.5 | 36.2 |
| OX359232.1 | MT | 0.02 | 19.2 |
| - | unplaced | 2.47 | 37.7 |
The estimated Quality Value (QV) of the final assembly is 63 with k-mer completeness of 100%, and the assembly has a BUSCO v5.3.2 completeness of 98.2% (single = 97.6%, duplicated = 0.6%), using the lepidoptera_odb10 reference set ( n = 5,286).
Metadata for specimens, spectral estimates, sequencing runs, contaminants and pre-curation assembly statistics can be found at https://links.tol.sanger.ac.uk/species/110368.
Methods
Sample acquisition and nucleic acid extraction
Three Bicyclus anynana (ilBicAnyn1, ilBicAnyn2 and ilBicAnyn3) were collected from laboratory stock at the University of Liverpool on 15 August 2015. The specimens were killed and stored in a –80°C freezer.
The samples were prepared and DNA was extracted at the Tree of Life laboratory, Wellcome Sanger Institute (WSI). The ilBicAnyn1 sample was weighed and dissected on dry ice. Whole organism tissue was disrupted using a Nippi Powermasher fitted with a BioMasher pestle. High molecular weight (HMW) DNA was extracted using the Qiagen MagAttract HMW DNA extraction kit. HMW DNA was sheared into an average fragment size of 12–20 kb in a Megaruptor 3 system with speed setting 30. Sheared DNA was purified by solid-phase reversible immobilisation using AMPure PB beads with a 1.8X ratio of beads to sample to remove the shorter fragments and concentrate the DNA sample. The concentration of the sheared and purified DNA was assessed using a Nanodrop spectrophotometer and Qubit Fluorometer and Qubit dsDNA High Sensitivity Assay kit. Fragment size distribution was evaluated by running the sample on the FemtoPulse system.
RNA was extracted from whole organism tissue of ilBicAnyn3 in the Tree of Life Laboratory at the WSI using TRIzol, according to the manufacturer’s instructions. RNA was then eluted in 50 μl RNAse-free water and its concentration assessed using a Nanodrop spectrophotometer and Qubit Fluorometer using the Qubit RNA Broad-Range (BR) Assay kit. Analysis of the integrity of the RNA was done using Agilent RNA 6000 Pico Kit and Eukaryotic Total RNA assay.
Sequencing
Pacific Biosciences HiFi circular consensus DNA sequencing libraries were constructed according to the manufacturers’ instructions. Poly(A) RNA-Seq libraries were constructed using the NEB Ultra II RNA Library Prep kit. DNA and RNA sequencing were performed by the Scientific Operations core at the WSI on Pacific Biosciences SEQUEL II (HiFi) and Illumina NovaSeq 6000 (RNA-Seq) instruments. Hi-C data were also generated from whole organism tissue of ilBicAnyn2 using the Arima2 kit and sequenced on the Illumina NovaSeq 6000 instrument.
Genome assembly, curation and evaluation
Assembly was carried out with Hifiasm ( Cheng et al., 2021) and haplotypic duplication was identified and removed with purge_dups ( Guan et al., 2020). The assembly was then scaffolded with Hi-C data ( Rao et al., 2014) using YaHS ( Zhou et al., 2023). The assembly was checked for contamination as described previously ( Howe et al., 2021). Manual curation was performed using HiGlass ( Kerpedjiev et al., 2018) and Pretext ( Harry, 2022). The mitochondrial genome was assembled using MitoHiFi ( Uliano-Silva et al., 2022), which runs MitoFinder ( Allio et al., 2020) or MITOS ( Bernt et al., 2013) and uses these annotations to select the final mitochondrial contig and to ensure the general quality of the sequence.
A Hi-C map for the final assembly was produced using bwa-mem2 ( Vasimuddin et al., 2019) in the Cooler file format ( Abdennur & Mirny, 2020). To assess the assembly metrics, the k-mer completeness and QV consensus quality values were calculated in Merqury ( Rhie et al., 2020). This work was done using Nextflow ( Di Tommaso et al., 2017) DSL2 pipelines “sanger-tol/readmapping” ( Surana et al., 2023a) and “sanger-tol/genomenote” ( Surana et al., 2023b). The genome was analysed within the BlobToolKit environment ( Challis et al., 2020) and BUSCO scores ( Manni et al., 2021; Simão et al., 2015) were calculated.
Table 3 contains a list of relevant software tool versions and sources.
Table 3. Software tools: versions and sources.
| Software tool | Version | Source |
|---|---|---|
| BlobToolKit | 4.0.7 | https://github.com/blobtoolkit/blobtoolkit |
| BUSCO | 5.3.2 | https://gitlab.com/ezlab/busco |
| Hifiasm | 0.16.1-r375 | https://github.com/chhylp123/hifiasm |
| HiGlass | 1.11.6 | https://github.com/higlass/higlass |
| Merqury | MerquryFK | https://github.com/thegenemyers/MERQURY.FK |
| MitoHiFi | 2 | https://github.com/marcelauliano/MitoHiFi |
| PretextView | 0.2 | https://github.com/wtsi-hpag/PretextView |
| purge_dups | 1.2.3 | https://github.com/dfguan/purge_dups |
| sanger-tol/genomenote | v1.0 | https://github.com/sanger-tol/genomenote |
| sanger-tol/readmapping | 1.1.0 | https://github.com/sanger-tol/readmapping/tree/1.1.0 |
| YaHS | yahs-1.1.91eebc2 | https://github.com/c-zhou/yahs |
Ethics and compliance issues
The materials that have contributed to this genome note have been supplied by a Darwin Tree of Life Partner. The submission of materials by a Darwin Tree of Life Partner is subject to the Darwin Tree of Life Project Sampling Code of Practice. By agreeing with and signing up to the Sampling Code of Practice, the Darwin Tree of Life Partner agrees they will meet the legal and ethical requirements and standards set out within this document in respect of all samples acquired for, and supplied to, the Darwin Tree of Life Project. Each transfer of samples is further undertaken according to a Research Collaboration Agreement or Material Transfer Agreement entered into by the Darwin Tree of Life Partner, Genome Research Limited (operating as the Wellcome Sanger Institute), and in some circumstances other Darwin Tree of Life collaborators.
Funding Statement
This work was supported by Wellcome through core funding to the Wellcome Sanger Institute (206194, <a href=https://doi.org/10.35802/206194>https://doi.org/10.35802/206194</a>) and the Darwin Tree of Life Discretionary Award (218328, <a href=https://doi.org/10.35802/218328>https://doi.org/10.35802/218328</a>).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
Data availability
European Nucleotide Archive: Bicyclus anynana (squinting bush brown). Accession number PRJEB54938; https://identifiers.org/ena.embl/PRJEB54938 ( Wellcome Sanger Institute, 2022)
The genome sequence is released openly for reuse. The Bicyclus anynana genome sequencing initiative is part of the Darwin Tree of Life (DToL) project. All raw sequence data and the assembly have been deposited in INSDC databases. The genome will be annotated using available RNA-Seq data and presented through the Ensembl pipeline at the European Bioinformatics Institute. Raw data and assembly accession identifiers are reported in Table 1.
Author information
Members of the Wellcome Sanger Institute Tree of Life programme are listed here: https://doi.org/10.5281/zenodo.4783585.
Members of Wellcome Sanger Institute Scientific Operations: DNA Pipelines collective are listed here: https://doi.org/10.5281/zenodo.4790455.
Members of the Tree of Life Core Informatics collective are listed here: https://doi.org/10.5281/zenodo.5013541.
Members of the Darwin Tree of Life Consortium are listed here: https://doi.org/10.5281/zenodo.4783558.
References
- Abdennur N, Mirny LA: Cooler: Scalable storage for Hi-C data and other genomically labeled arrays. Bioinformatics. 2020;36(1):311–316. 10.1093/bioinformatics/btz540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allio R, Schomaker-Bastos A, Romiguier J, et al. : MitoFinder: Efficient automated large‐scale extraction of mitogenomic data in target enrichment phylogenomics. Mol Ecol Resour. 2020;20(4):892–905. 10.1111/1755-0998.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldade P, Saenko SV, Pul N, et al. : A Gene-Based Linkage Map for Bicyclus anynana Butterflies Allows for a Comprehensive Analysis of Synteny with the Lepidopteran Reference Genome. PLoS Genet. 2009;5(2):e1000366. 10.1371/journal.pgen.1000366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt M, Donath A, Jühling F, et al. : MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 2013;69(2):313–9. 10.1016/j.ympev.2012.08.023 [DOI] [PubMed] [Google Scholar]
- Brakefield PM, Beldade P, Zwaan BJ: The African Butterfly Bicyclus anynana: A Model for Evolutionary Genetics and Evolutionary Developmental Biology. Cold Spring Harb Protoc. 2009;2009(5):pdb.emo122. 10.1101/pdb.emo122 [DOI] [PubMed] [Google Scholar]
- Challis R, Richards E, Rajan J, et al. : BlobToolKit - interactive quality assessment of genome assemblies. G3 (Bethesda). 2020;10(4):1361–1374. 10.1534/g3.119.400908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Concepcion GT, Feng X, et al. : Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat Methods. 2021;18(2):170–175. 10.1038/s41592-020-01056-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tommaso P, Chatzou M, Floden EW, et al. : Nextflow enables reproducible computational workflows. Nat Biotechnol. 2017;35(4):316–319. 10.1038/nbt.3820 [DOI] [PubMed] [Google Scholar]
- Guan D, McCarthy SA, Wood J, et al. : Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics. 2020;36(9):2896–2898. 10.1093/bioinformatics/btaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry E: PretextView (Paired REad TEXTure Viewer): A desktop application for viewing pretext contact maps. 2022; (Accessed: 19 October 2022). Reference Source [Google Scholar]
- Howe K, Chow W, Collins J, et al. : Significantly improving the quality of genome assemblies through curation. GigaScience. Oxford University Press,2021;10(1):giaa153. 10.1093/gigascience/giaa153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerpedjiev P, Abdennur N, Lekschas F, et al. : HiGlass: Web-based visual exploration and analysis of genome interaction maps. Genome Biol. 2018;19(1):125. 10.1186/s13059-018-1486-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni M, Berkeley MR, Seppey M, et al. : BUSCO Update: Novel and Streamlined Workflows along with Broader and Deeper Phylogenetic Coverage for Scoring of Eukaryotic, Prokaryotic, and Viral Genomes. Mol Biol Evol. 2021;38(10):4647–4654. 10.1093/molbev/msab199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan SN, Connahs H, Matsuoka Y, et al. : Butterfly eyespots evolved via cooption of an ancestral gene-regulatory network that also patterns antennae, legs, and wings. Proc Natl Acad Sci U S A. 2022;119(8):e2108661119. 10.1073/pnas.2108661119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell RW, Elsworth B, Oostra V, et al. : A high-coverage draft genome of the mycalesine butterfly Bicyclus anynana. GigaScience. 2017;6(7):1–7. 10.1093/gigascience/gix035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra V, Saastamoinen M, Zwaan BJ, et al. : Strong phenotypic plasticity limits potential for evolutionary responses to climate change. Nat Commun. 2018;9(1):1005. 10.1038/s41467-018-03384-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A, Finet C, Banerjee TD, et al. : Antennapedia and optix regulate metallic silver wing scale development and cell shape in Bicyclus anynana butterflies. Cell Rep. 2022;40(1):111052. 10.1016/j.celrep.2022.111052 [DOI] [PubMed] [Google Scholar]
- Rao SSP, Huntley MH, Durand NC, et al. : A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159(7):1665–80. 10.1016/j.cell.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, McCarthy SA, Fedrigo O, et al. : Towards complete and error-free genome assemblies of all vertebrate species. Nature. 2021;592(7856):737–746. 10.1038/s41586-021-03451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, Walenz BP, Koren S, et al. : Merqury: Reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 2020;21(1):245. 10.1186/s13059-020-02134-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccheri IJ, Whiteford S, Yung CJ, et al. : Recessive Z-linked lethals and the retention of haplotype diversity in a captive butterfly population. Heredity (Edinb). 2020;125(1–2):28–39. 10.1038/s41437-020-0316-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, et al. : BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–2. 10.1093/bioinformatics/btv351 [DOI] [PubMed] [Google Scholar]
- Surana P, Muffato M, Qi G: sanger-tol/readmapping: sanger-tol/readmapping v1.1.0 - Hebridean Black (1.1.0). Zenodo.2023a; (Accessed: 17 April 2023). 10.5281/zenodo.7755665 [DOI] [Google Scholar]
- Surana P, Muffato M, Sadasivan Baby C: sanger-tol/genomenote (v1.0.dev). Zenodo.2023b; (Accessed: 17 April 2023). 10.5281/zenodo.6785935 [DOI] [Google Scholar]
- Tian S, Monteiro A: A Transcriptomic Atlas Underlying Developmental Plasticity of Seasonal Forms of Bicyclus anynana Butterflies. Mol Biol Evol. 2022;39(6):msac126. 10.1093/molbev/msac126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uliano-Silva M, Ferreira JGRN, Krasheninnikova K, et al. : MitoHiFi: a python pipeline for mitochondrial genome assembly from PacBio High Fidelity reads. bioRxiv. [Preprint],2022. 10.1101/2022.12.23.521667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Hof AE, Marec F, Saccheri IJ, et al. : Cytogenetic Characterization and AFLP-Based Genetic Linkage Mapping for the Butterfly Bicyclus anynana, Covering All 28 Karyotyped Chromosomes. PLoS One. 2008;3(12):e3882. 10.1371/journal.pone.0003882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasimuddin M, Misra S, Li H, et al. : Efficient Architecture-Aware Acceleration of BWA-MEM for Multicore Systems.In: 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS).IEEE,2019;314–324. 10.1109/IPDPS.2019.00041 [DOI] [Google Scholar]
- Wellcome Sanger Institute: The genome sequence of the Squinting Bush Brown, Bicyclus anynana (Butler, 1879). European Nucleotide Archive.[dataset], accession number PRJEB54938,2022.
- Zhou C, McCarthy SA, Durbin R: YaHS: yet another Hi-C scaffolding tool. Bioinformatics. Edited by C. Alkan,2023;39(1):btac808. 10.1093/bioinformatics/btac808 [DOI] [PMC free article] [PubMed] [Google Scholar]