Abstract
We describe the endocranial structures of Hamadasuchus, a peirosaurid crocodylomorph from the late Albian‐Cenomanian Kem Kem group of Morocco. The cranial endocast, associated nerves and arteries, endosseous labyrinths, and cranial pneumatization, as well as the bones of the braincase of a new specimen, are reconstructed and compared with extant and fossil crocodylomorphs, which represent different lifestyles. Cranial bones of this specimen are identified as belonging to Hamadasuchus, with close affinities with Rukwasuchus yajabalijekundu, another peirosaurid from the ‘middle’ Cretaceous of Tanzania. The endocranial structures are comparable to those of R. yajabalijekundu but also to baurusuchids and sebecids (sebecosuchians). Paleobiological traits of Hamadasuchus, such as alert head posture, ecology, and behavior are explored for the first time, using quantitative metrics. The expanded but narrow semi‐circular canals and enlarged pneumatization of the skull of Hamadasuchus are linked to a terrestrial lifestyle. Continuing work on the neuroanatomy of supposedly terrestrial crocodylomorphs needs to be broadened to other groups and will allow to characterize whether some internal structures are affected by the lifestyle of these organisms.
Keywords: Crocodylomorpha, Hamadasuchus, Kem Kem, Morocco, paleoneuroanatomy, Peirosauridae
We describe the endocranial structures of Hamadasuchus, a peirosaurid crocodylomorph from the late Albian‐Cenomanian Kem Kem group of Morocco. The cranial endocast, endosseous labyrinths, and cranial pneumatization of a new specimen are reconstructed and compared with extant and fossil crocodylomorphs, which represent different lifestyles. The expanded but narrow semi‐circular canals and enlarged pneumatization of the skull of Hamadasuchus are linked to a terrestrial lifestyle.

1. INTRODUCTION
Peirosaurids represent an extinct family of crocodylomorphs spanning from at least the Barremian to the Maastrichtian (Lamanna et al., 2019; Larsson & Gado, 2000). They were widely distributed in South America and Africa. However, not much is known about their ecology, as the cranial and postcranial elements are rare or remain undescribed. The group is diverse, comprising at least 16 different species (see Nicholl et al., 2021 for a complete review), but paradoxically, these taxa are mostly represented by mandibular fragments (Barrios et al., 2016; Coria et al., 2019; Filippi et al., 2018; Lamanna et al., 2019; Lio et al., 2016; Nicholl et al., 2021). Their phylogenetic relationships with other groups as well as within Peirosauridae are poorly known and subject to numerous debates. In most studies, Peirosauridae is placed close to uruguaysuchids and mahajangasuchids (Barrios et al., 2016; Carvalho et al., 2004; Coria et al., 2019; Dumont Jr et al., 2020; Fiorelli et al., 2016; Godoy et al., 2016; Kellner et al., 2014; Leardi et al., 2015, 2018; Martinelli et al., 2018; Nicholl et al., 2021; Pol et al., 2012, 2014; Sertich & O'Connor, 2014; Turner & Calvo, 2005; Turner & Sertich, 2010; Turner, 2006); other studies depict them as united with sebecids, forming the clade Sebecia (Geroto & Bertini, 2019; Larsson & Sues, 2007; Meunier & Larsson, 2017; Ruiz et al., 2021) or closely related to neosuchians (Company et al., 2005; Leardi & Pol, 2009; Nascimento & Zaher, 2011; Pol & Apesteguía, 2005; Pol & Norell, 2004; Pol & Powell, 2011; Sereno et al., 2003; Sereno & Larsson, 2009; Turner & Buckley, 2008; Zaher et al., 2006).
Like notosuchians, peirosaurids are inferred to have had terrestrial habits by extrapolation with better‐known closely related groups (e.g., sebecids; Busbey, 1986, Molnar, 2012; Pol et al., 2012 and mahajangasuchids; Sereno & Larsson, 2009). However, evidence of a more aquatic lifestyle also exists in some specimens (Sena et al., 2018). The study of the neuroanatomy and the pneumaticity of the skull could hold relevant data about the neurosensorial capabilities of the specimen studied and thus opens the possibility to infer its lifestyle.
In the last decade, numerous studies using computed tomography (CT) data have been published (see Barrios et al., 2022 for a complete review) on eusuchians (Blanco et al., 2015; Bona et al., 2013; Burke & Mannion, 2023; Erb & Turner, 2021; Holliday & Gardner, 2012; Puértolas‐Pascual et al., 2022, 2023; Serrano‐Martínez et al., 2021; Serrano‐Martínez, Knoll, Narváez, Lautenschlager, & Ortega, 2019; Serrano‐Martínez, Knoll, Narváez, & Ortega, 2019), thalattosuchians (Brusatte et al., 2016; Fernández et al., 2011; Herrera et al., 2018; Pierce et al., 2017; Schwab et al., 2021; Wilberg et al., 2021), early crocodylomorphs (Leardi et al., 2020; Melstrom et al., 2022; Ruebenstahl et al., 2022) and some notosuchians (Dumont Jr et al., 2020; Fonseca et al., 2020; Kley et al., 2010; Pochat‐Cottilloux et al., 2021; Sereno & Larsson, 2009). Those studies are based on extant crocodylian databases for comparison (Bona et al., 2017; Witmer et al., 2008), as well as detailed studies focusing on certain parts of the braincase (Dufeau, 2011; Dufeau & Witmer, 2015; George & Holliday, 2013; Hu et al., 2021; Jirak & Janacek, 2017; Klembara, 2005; Kuzmin et al., 2021; Lessner et al., 2022; Lessner & Holliday, 2020; Montefeltro et al., 2016; Perrichon et al., 2023; Porter et al., 2016; Schwab et al., 2021). The neuroanatomy of Peirosauridae has been investigated in a few members of the group such as Rukwasuchus yajabalijekundu (Sertich & O'Connor, 2014) and Hamadasuchus rebouli sensu Larsson & Sues, 2007 (ROM 52620) by Dufeau (2011) and George and Holliday (2013). However, in both cases, those structures are not described in detail, especially the endosseous labyrinths and the cranial pneumaticity, and are not used for paleobiological inferences. Therefore, the endocranial structures of peirosaurids deserve an augmented treatment.
Since the recognition of Hamadasuchus rebouli Buffetaut, 1994, more complete peirosaurids specimens have been discovered, enhancing the diversity of this poorly known genus endemic to Africa (Ibrahim et al., 2020; Larsson & Sues, 2007; Pochat‐Cottilloux, Perrier, et al., 2023; Rauhut & Lopez‐Arbarello, 2005). Here, we describe the internal and external anatomy of a new specimen of Hamadasuchus (UCBL‐FSL 532408) from the late Albian‐Cenomanian Kem Kem group of Morocco.
Using the reconstructed internal structures of this specimen (cranial endocast, endosseous labyrinths, and braincase pneumaticity), we use several proxies to infer associated paleobiological traits of Hamadasuchus, putting them into perspective with comparative data on sebecosuchians, eusuchians, and thalattosuchians. The data are also used to reconstruct the braincase osteology, allowing a robust taxonomic assessment of this specimen.
2. MATERIALS AND METHODS
2.1. CT scan
The studied material consists of a complete skull of a crocodylomorph (UCBL‐FSL 532408; Figure 1), currently curated in the geological collections of Université Lyon 1. The specimen originates from an unknown location in the Kem Kem group of Morocco (late Albian—Cenomanian; Sereno et al., 1996; Martin & de Lapparent de Broin, 2016; Ibrahim et al., 2020). The skull was CT scanned in November 2020 at the Laboratoire Mateis (INSA Lyon) to reconstruct its internal soft anatomy, as well as the bones constituting the braincase. We used a Vtomex laboratory X‐ray computed tomography (GE Phoenix X‐Ray GmbH): scanning parameters were set to 140 kV tube voltage and 80 μA current and a 0.5 mm copper filter was used at the source exit. The scan has a voxel size of 86 μm and a 1 s exposure time for each of the 1200 projections. Avizo Lite (version 9.5.0), MeshLab (version 2020.07), and Blender (version 2.91.2) were used for the volume rendering and calculations, as well as the processing of scans of the endosseous labyrinths, sinuses, and cranial endocast. Processed volumes are available in Pochat‐Cottilloux, Rinder, et al. (2023), see also Figures S1 and S2. Here, we describe the bone‐bounded spaces that house soft tissue organs as such, given that numerous recent studies on extant taxa have showed that the shape of those structures is strongly correlated with their associated organs (Watanabe et al., 2019 on reptiles and Early et al., 2020 on birds).
FIGURE 1.

Skull of Hamadasuchus (UCBL‐FSL 532408) in dorsal (a), ventral (b), lateral (c) anterior (d) and posterior (f) views, close up on a tooth (e). IX–XI, foramen for cranial nerve IX–XI; c, choana; cb, crest B; if, infratemporal fenestra; o, orbit; oc, occipital condyle; ot, otoccipital; p, parietal; q, quadrate; sf, supratemporal fenestra; so, supraoccipital; sq, squamosal. Scale bars for (a), (b), (c), (d), and (f) are 5 cm and 1 cm for (e).
2.2. Comparisons
To keep the description free of constant repetitions of references, we summarize the data on extinct and extant crocodylomorph taxa used for comparison in Table 1. In an effort to standardize descriptive studies in paleoneuroanatomy, we follow the anatomical nomenclature published by Colbert (1946a, 1946b), Kley et al. (2010), Sertich and O'Connor (2014), Dufeau and Witmer (2015), Brusatte et al. (2016), Bona et al. (2017), Pierce et al. (2017), Herrera et al. (2018), Serrano‐Martínez, Knoll, Narváez, & Ortega, (2019), Serrano‐Martínez, Knoll, Narváez, Lautenschlager, & Ortega, (2019), Serrano‐Martínez et al., (2021), Leardi et al. (2020), Lessner and Holliday (2020), Fonseca et al. (2020), Dumont Jr et al. (2020), Erb and Turner (2021), Kuzmin et al. (2021), Ruebenstahl et al. (2022), Schwab et al. (2021), Wilberg et al. (2021), Melstrom et al. (2022), Pochat‐Cottilloux et al. (2022), Puértolas‐Pascual et al. (2022, 2023), Ristevski (2022), Burke and Mannion (2023) and Perrichon et al. (2023) concerning the endocranial structures (and references therein). Table 2 compiles several important measurements following Pierce et al. (2017, figure 2).
TABLE 1.
Comparative material used in this study.
| Taxon | Specimen | Publication or database |
|---|---|---|
| Alligator mississippiensis | UCBL WB35 | This study |
| Caiman crocodilus | UMMZ HERPS 128024 | Morphosource |
| Crocodylus acutus | MZS Cro 055 | This study |
| Gavialis gangeticus | MLP 602, UF HERP 118998 | Bona et al. (2017), Pierce et al. (2017), Morphosource |
| Mecistops sp. | MHNL 50001393 | This study |
| Osteolaemus tetraspis | UCBL 2019‐1‐236 | This study |
| Paleosuchus trigonatus | MHNL 50003939 | This study |
| Tomistoma schlegelii | TMM M6342 | Morphosource |
| Tomistoma downsoni | NHMUK PV R 4769 | Burke and Mannion (2023) |
| Diplocynodon tormis | STUS‐344 | Serrano‐Martínez, Knoll, Narváez, and Ortega (2019) |
| Arenysuchus gascabadiolorum | MPZ 2011/184 | Puértolas‐Pascual et al. (2022) |
| Agaresuchus fontisensis | HUE‐02502, HUE‐03713 | Serrano‐Martínez et al. (2021) |
| Agaresuchus subjuniperus | MPZ 2012/288 | Puértolas‐Pascual et al. (2022) |
| Trilophosuchus rackhami | QMF16856 | Ristevski (2022) |
| Portugalosuchus azenhae | ML1818 | Puértolas‐Pascual et al. (2023) |
| Pelagosaurus typus | BRLSI‐M1413 | Pierce et al. (2017) |
| Plagiophthalmosuchus cf. gracilirostris | NHMUK PV OR 33095 | Brusatte et al. (2016) |
| Macrospondylus bollensis | SNSB‐BSPG 1984 1258, MCZ VPRA‐1063 | Herrera et al. (2018), Wilberg et al. (2021) |
| Cricosaurus araucanensis | MLP 72‐IV‐7‐1 | Herrera et al. (2018) |
| ‘Metriorhynchus’ cf. brachyrhynchus | NHMUK PV O 32617 | Schwab et al. (2021) |
| Rhabdognathus aslerensis |
AMNH FARB 33354 formerly CNRST‐SUNY 190 |
Erb and Turner (2021) Brochu et al. (2002) |
| Rukwasuchus yajabalijekundu | RRBP 08630 | Sertich and O'Connor (2014) |
| Simosuchus clarki | UA 8679 | Kley et al. (2010) |
| Campinasuchus dinizi | CPPLIP 1319 and 1360 | Fonseca et al. (2020) |
| Baurusuchus sp. | IFSP‐VTP/PALEO‐0002 and 0003, FEF‐PV‐R‐1/9 and FUP‐Pv 000020 and 000021 | Dumont Jr et al. (2020) |
| Sebecus icaeorhinus | AMNH 3160 | Colbert (1946a) |
| Zulmasuchus querejazus | MHNC 6672 | Pochat‐Cottilloux et al. (2021) |
| Sphenosuchus acutus | SAM 3014 | Walker (1990) |
| Almadasuchus figarii | MPEF‐PV 3838 | Leardi et al. (2020) |
| Junggarsuchus sloani | IVPP14010 | Ruebenstahl et al. (2022) |
| Eopneumatosuchus colberti | MNA V2460 | Melstrom et al. (2022) |
Note: MHNC 6672 is here identified as Zulmasuchus mainly for consistency with previous studies, although there are conflicting views on the separation of this genus with Sebecus (Fiorelli et al., 2016; Leardi et al., 2015, 2018; Pol et al., 2012).
Abbreviations: AMNH, American Museum of Natural History; BRLSI, Bath Royal Literary and Scientific Institute; CNRST, Centre National de la Recherche Scientifique et Technologique; CPPLIP, Centro de Pesquisas Paleontologicas “Llewellyn Ivor Price”; FEF‐PV, Fernandopolis Educational Foundation; FUP, University of Brasilia, campus Planaltina; IFSPVTP, Federal Institute of Education, Science and Technology of Sao Paulo; IVPP, Institute of Vertebrate Paleontology and Paleoanthropology; MCZ, Museum of Comparative Zoology; MHNC, Museo de Historia Natural “Alcide d'Orbigny”; MHNL, Musée d'Histoire Naturelle de Lyon; ML, Museu da Lourinhã; MLP, Museo de La Plata; MNA, Museum of Northern Arizona; MPEF, Museo Paleontológico Egidio Feruglio; MPZ, Museo de Ciencas Naturales de la Universidad de Zaragoza; MZS, Musée Zoologique de Strasbourg; NHMUK, Natural History Museum UK; NMT, National Museum of Tanzania; OUVC, Ohio University Vertebrate Collections; QM, Queensland Museum; RRBP, Rukwa Rift Basin Project; ROM, Royal Ontario Museum; SAM, South African Museum; SNSB‐BSPG, Staatliche Naturwissenschaftliche Sammlungen Bayerns‐Bayerische Staatssammlung für Paläontologie und Geologie; STUS, Sala de las Tortugas “Emiliano Jiménez” de la Universidad de Salamanca; SVSTUA, Collections pédagogiques du Département de Biologie de l'Ecole Normale Supérieure de Lyon; TMM, Texas Memorial Museum; UA, Université d'Antananarivo; UCBL, Université Claude Bernard Lyon 1; UF, University of Florida; UM, Université de Montpellier; UMMZ, University of Michigan Museum of Zoology.
TABLE 2.
Raw morphometric data of UCBL‐FSL 532408 (Hamadasuchus) and comparative material.
| (Rounded to nearest mm or mm2) | Pelagosaurus typus | Plagiophthalmosuchus cf. gracilirostris | Macrospondylus bollensis | Cricosaurus araucanensis | ‘Metriorhynchus’ cf. brachyrhynchus | Rhabdognathus aslerensis | Gavialis gangeticus | Tomistoma downsoni | Alligator mississippiensis | Crocodylus porosus | Diplocynodon tormis | Arenysuchus gascabadiolorum | Agaresuchus subjuniperus |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skull width at cerebrum (SW) | 52 | ? | 33 | 40 | 74 | 89 | 168 | 109 | 73 | 39 | 25 | 68 | ? |
| Cephalic flexure angle (CF) | 160 | 175 | 170 | 135 | 155 | 158 | 150 | 143 | 135 | 125 | 140 | 159 | 154 |
| Pontine flexure angle (PF) | 160 | 170 | 165 | 170 | 165 | 152 | 154 | 149 | 145 | 170 | 160 | 160 | 156 |
| Endocast length (EL) | 57 | ? | 76 | 140 | ? | 171 | 146 | 146 | 98 | 40 | 82 | 32 | 88 |
| Olfactory tract length (+ bulbs) (OL) | 21 | ? | 26 | 69 | ? | 49 | 55 | 66 | 48 | 18 | 36 | 20 | 68 |
| Cerebrum width (CW) | 15 | 28 | 19 | 25 | 40 | 26 | 32 | 30 | 21 | 18 | 23 | 19 | 27 |
| Pituitary width (PW) | 6 | 14 | 4 | 11 | 16 | 5 | 6 | 9 | 5 | 4 | 2 | 6 | 7 |
| Pituitary height (PH) | 7 | 12 | 6 | 9 | 12 | 10 | 9 | 9 | 8 | 3 | 6 | 4 | 7 |
| Pituitary length (PL) | 10 | 17 | 4.5 | 14 | 30 | 14 | 11 | 17 | 10 | 13 | 4 | 6 | 14 |
| Labyrinth height (LH) | 14 | 26 | 22 | 19 | 20 | 25 | 21 | 19 | 18 | 9 | ? | ? | ? |
| Labyrinth width (LW) | 11 | 26 | 18 | 18 | 21 | 26 | 21 | 15 | 14 | 9 | ? | ? | ? |
| Endosseous cochlear duct length (ECL) | 8 | 13 | 12 | 7 | 9 | 12 | 9 | 11 | 8 | 3 | ? | ? | ? |
| Anterior semi‐circular canal area (AA) | 9 | 38 | 18 | ? | ? | 19 | 36 | 21 | 35 | 11 | ? | ? | ? |
| Posterior semi‐circular canal area (PA) | 6 | 19 | 5 | ? | ? | 4 | 15 | 10 | 12 | 3 | ? | ? | ? |
| Lateral semi‐circular canal area (LA) | 4 | 14 | 7 | ? | ? | 8 | 22 | ? | 13 | 3 | ? | ? | ? |
| Source | Pierce et al. (2017) | Brusatte et al. (2016) | Herrera et al. (2018); Erb and Turner (2021) | Herrera et al. (2018) | Schwab et al. (2021) | Erb and Turner (2021) | Pierce et al. (2017) | Burke and Mannion (2023) | Witmer and Ridgely (2008) | Pochat‐Cottilloux et al. (2021) | Serrano‐Martínez, Knoll, Narváez, and Ortega (2019) | Puértolas‐Pascual et al. (2022) | Puértolas‐Pascual et al. (2022) |
| (Rounded to nearest mm or mm2) | Agaresuchus fontisensis | Trilophosuchus rackhami | Portugalosuchus azenhae | Simosuchus clarki | Rukwasuchus yajabalijekundu | Hamadasuchus | Campinasuchus dinizi | Baurusuchus sp. | Sebecus icaeorhinus | Zulmasuchus querejazus | Sphenosuchus acutus | Almadasuchus figarii | Eopneumatosuchus colberti |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skull width at cerebrum (SW) | 150 | 37 | ? | 58 | 37 | 94 | 32 | ? | 147 | 42 | ? | ? | ? |
| Cephalic flexure angle (CF) | 155/160 | 136 | 159 | 142 | 145 | 165 | 155 | 165 | 150 | 130 | 152 | 161 | 170 |
| Pontine flexure angle (PF) | 155 | 142 | 159 | 165 | 145 | 150 | 150 | 165 | 160 | 130 | 146 | 170 | 160 |
| Endocast length (EL) | 107/133 | 56 | 112 | 79 | 100 | 70 | 85 | 70 | 120 | ? | ? | 58 | ? |
| Olfactory tract length (+ bulbs) (OL) | 36/50 | 22 | 36 | 25 | 45 | 27 | 39 | 32 | 46 | ? | ? | ? | ? |
| Cerebrum width (CW) | 21/29 | 16 | 25 | 25 | 28 | 26 | 13 | 14 | 30 | 35 | ? | 18 | ? |
| Pituitary width (PW) | 4/6 | 3 | 5 | 5 | 7 | 6 | 4 | ? | ? | 14 | ? | ? | ? |
| Pituitary height (PH) | 7/10 | 2 | 10 | 9 | 10 | 8 | 4 | ? | 9 | 17 | ? | 4 | ? |
| Pituitary length (PL) | 4/10 | 4 | 24 | 10 | 5 | 14 | 4 | ? | 8 | 27 | ? | 7 | ? |
| Labyrinth height (LH) | ? | 13 | 19 | ? | ? | 10 | ? | 12 | ? | 22 | ? | ? | ? |
| Labyrinth width (LW) | ? | 10 | 17 | ? | ? | 14 | ? | 13 | ? | 14 | ? | ? | ? |
| Endosseous cochlear duct length (ECL) | ? | 6.5 | 6 | ? | ? | ? | ? | 6 | ? | 7 | ? | ? | ? |
| Anterior semi‐circular canal area (AA) | ? | 8 | 8 | ? | ? | 8 | ? | 2 | ? | 7 | ? | ? | ? |
| Posterior semi‐circular canal area (PA) | ? | 4 | 5 | ? | ? | 5 | ? | 4 | ? | 7 | ? | ? | ? |
| Lateral semi‐circular canal area (LA) | ? | 2 | 8 | ? | ? | 8 | ? | 1 | ? | 6 | ? | ? | ? |
| Source | Serrano‐Martínez et al. (2021) | Ristevski (2022) | Puértolas‐Pascual et al. (2023) | Kley et al. (2010) | Sertich and O'Connor (2014) | This study | Fonseca et al. (2020) | Dumont Jr et al. (2020) | Colbert (1946a) | Pochat‐Cottilloux et al. (2021) | Walker (1990) | Leardi et al. (2020) | Melstrom et al. (2022) |
2.3. Paleobiological inferences
Brain volume was estimated using the following relation: log BV = log EV * 0.7279 + 0.75624 where EV: endocranial volume and BV: brain volume (Dumont Jr et al., 2020 and references therein; see also Data S1). The volume of the sinus system was calculated using Blender v2.91 and skull width was measured as the largest skull width (including quadrates; see Data S1). We chose to use the skull width rather than the skull length to prevent us from potential convergence problems related to the size of the rostrum, as well as making it possible to include fossil specimens for which the skull length is unknown without having to estimate it.
3. RESULTS
3.1. General preservation
The cranium is complete and well‐preserved. It is altirostral (short and elevated snout) and has heterodont ziphodont teeth. In some skull areas, however, the bone surface is not clear and is sometimes covered by sediment, which was probably glued to the bones during preparation. There are also two areas in which the surface is covered by white plaster, one ventrally in the palatal area, and one at the junction of the right jugal and quadratojugal. The braincase occupies the posterior third of the skull. For this paper, we will focus only on this part of the skull, while a complete description of the skull and a reappraisal of Hamadasuchus will be the topic of a separate study. Overall, in the areas that are visible, the skull is ornamented with circular/ovoid pits dorsally.
3.1.1. Cranial openings
There are no post‐temporal fenestrae to be seen, but the area is quite damaged and there seems to be an opening, so the absence of those structures is unsure (Figures 1f and 2d).
FIGURE 2.
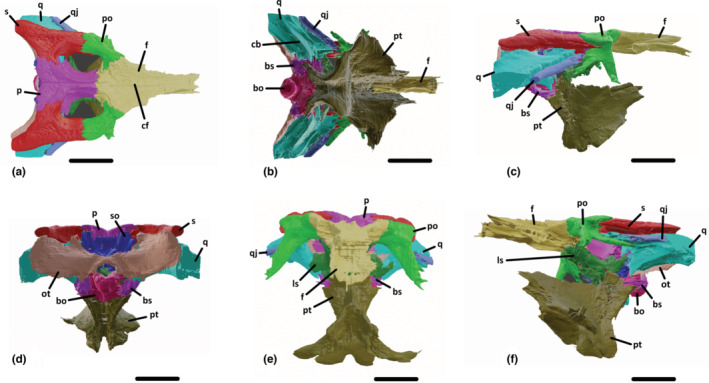
Three‐dimensional reconstruction of the posterior part of the skull of UCBL‐FSL 532408 (Hamadasuchus) based on segmented bones in dorsal (a), ventral (b), lateral (c), posterior (d), anterior (e), and ventrolateral (f) views. bo, basioccipital; bs, basisphenoid; cb, crest B; cf, crest on the frontal; f, frontal; ls, laterosphenoid; ot, otoccipital; p, parietal; po, postorbital; pt, pterygoid; q, quadrate; qj, quadratojugal; s, squamosal; so, supraoccipital. Scale bars are 5 cm.
The supratemporal fenestra is ovoid with its longest axis directed anteroposteriorly and extends posteriorly to more than half the length of the parietal. The supratemporal fossa is small and quickly disappears ventrally.
The foramen magnum is triangular shaped, about 1.5 cm at its widest part, and bordered by the otoccipital and the basioccipital.
The internal choana is contained by the pterygoids and the palatines and is situated at the level of contact between these bones, quite anteriorly relative to the whole skull.
3.2. Braincase
3.2.1. Frontal
The frontal forms a bridge between the rostrum and the post‐orbital region of the skull, it constitutes most of the medial margin of the orbit (Figures 1a and 2a–c,e,f). Posteriorly, it contacts the postorbital obliquely, the parietal transversely, and the laterosphenoid ventrally. The sutural surfaces are zigzagged. The frontal participates in the formation of the supratemporal fenestra, contributing to its anteromedial margin. It is dorsally concave and thick, with a slight depression anteriorly between the two orbits. On the midline dorsal surface, the frontal bears an anteroposteriorly directed crest (Figure 2a). The ventral surface, although degraded, is smooth and convex ventrally to accommodate for the olfactory tract (Figure 2b).
3.2.2. Postorbital
The postorbital makes for the orbit's posterior margin, as well as the supratemporal fenestra anterolateral margin (Figures 1a,d,e and 2a–c,e,f). The dorsal angle between the medial and posterior parts of the postorbital is not of 90°, as there is an anterolaterally facing edge. However, the ventral and posterior parts do form a 90° angle in lateral view. Medially, it contacts the frontal but not the parietal. Posteriorly, it has a lateromedially oriented suture with the squamosal. The ventrolaterally descending process sutures with the dorsomedially ascending process of the jugal to form the postorbital bar. Posterolaterally, the postorbital extends along the anterior margin of the quadratojugal and forms the anteroventral margin of the anterior part of the squamosal (Figures 1d,e and 2c,f).
3.2.3. Parietal
The unpaired parietal is dorsally thin and elevated but tends to widen ventrally (Figures 1a and 2a,d,e). In dorsal view, it is shaped like an hourglass with the posterior part being wider than the anterior one. Laterally, the parietal forms the medial margin of both supratemporal fenestrae and fossae. It contacts the frontal anteriorly and the squamosal laterally. The parietal is also exposed posteriorly, where it sutures with the dorsal margin of the supraoccipital (Figures 1f and 2d).
3.2.4. Squamosal
The squamosal is T‐shaped and forms the posterolateral corner of the skull roof (Figures 1a,b,d,e and 2a,c,d,f). It contacts the quadrate on both sides of the auditory meatus. This suture is anteroposteriorly straight. Anteriorly, a process connects with the postorbital to form the lateral margin of the supratemporal fenestra (Figures 1a and 2a). This process is more extended ventrally than dorsally. Medially, the contact with the parietal is oblique dorsally and straight anteriorly within the supratemporal fossa. At its posteriormost part, the squamosal contacts with the otoccipital and the quadrate laterally and with the supraoccipital medially (Figures 1f and 2d): the squamosal thus forms the posterodorsal part of the back of the skull.
3.2.5. Quadratojugal
The quadratojugal extends posteriorly from the posterior margin of the infratemporal fenestra, connecting all the way with the quadrate on its medial side (Figures 1a,c–e and 2a–c,e,f). The suture with the quadrate is straight ventrally and dorsally. This contact does not extend anteriorly beyond the level of the postorbital bar. The bone is smooth ventrally and pitted dorsally. It is thin and plate‐like, wider mediodorsally than lateroventrally.
3.2.6. Quadrate
The quadrate has a complex shape (Figures 1a–e and 2a–f). Anteriorly, it goes all the way to the posterolateral margin of the supratemporal fenestra and contacts the parietal. Dorsally, it contacts the squamosal on both sides of the auditory meatus and extends anteriorly to contact the postorbital (Figure 2c,f). Laterally, the suture with the quadratojugal is straight all the way. The state of preservation of the specimen does not allow to state if the quadrate contacts the pterygoid ventrally. Posteriorly, it contacts the otoccipital straightly and the basisphenoid ventrally. Crest B (Iordansky & Gans, 1973; Figures 1c and 2b) is clearly visible, extending in parallel to the quadrate‐quadratojugal suture from the ventrolateral margin of the supratemporal fenestra to beyond the posterolateral most part of the quadrate. This bone is globally tall in cross‐section (almost three centimetres maximum), it is flat on the dorsal surface and curved dorsally on the ventral surface.
3.2.7. Supraoccipital
The supraoccipital has a rounded triangular shape in posterior view (Figures 1b and 2a,d). It connects dorsolaterally with the squamosal and ventrolaterally with the otoccipital in an oblique suture. It does not participate in the formation of the foramen magnum. In dorsal view, it does not invade the parietal anteriorly but forms two postoccipital processes that are situated on each side of the midline.
3.2.8. Otoccipital
This bone forms most of the posterodorsal surface of the skull (Figures 1b,c and 2b,d). It connects laterally with the quadrate and the squamosal and medially with the supraoccipital, in an obtuse angle directed medially (Figures 1f and 2d). Dorsally, the contact with the squamosal is straight lateromedially. On the left bone, a foramen is visible corresponding to the exit for the cranial nerves IX to XI (Figure 1f). Ventrally, the otoccipital forms the dorsal and lateral margins of the foramen magnum, and contacts ventrally with the basioccipital on both sides of the opening. Ventrolaterally, the otoccipital also connects with the basisphenoid. The otoccipital is plate‐like and curved anteromedially.
3.2.9. Basioccipital
The basioccipital sutures dorsally with the otoccipital, laterally with the basisphenoid, and ventrally with the pterygoids (Figures 1b,c and 2b,d,f). It forms the ventral margin of the foramen magnum (Figures 1b and 2d). The occipital condyle is small and directed posteroventrally. Its dorsal surface is grooved to accommodate for the central nerve cord. The foramina for the lateral Eustachian tubes are not preserved and/or not visible on the CT slices.
3.2.10. Pterygoid
The pterygoids are fused, they suture with the basisphenoid and the basioccipital posterodorsally (Figures 1b–e and 2b–f). The posterior part is directed dorsoventrally, and the pterygoid wings are broken. In the otic region, the pterygoid connects with the laterosphenoid and the quadrate dorsally (Figure 1f), although this part was quite damaged and could not be segmented very accurately. The internal choana is ovoid with the largest axis directed anteroposteriorly and its margins are curved dorsally, which makes for a clear dorsal depression at the midline where the two pterygoids meet. There is no midline process, although the posteriormost part is damaged (Figures 1c and 2b). It is also enclosed anteriorly by the palatines. Overall, it is located anteriorly in the bone, almost at the level of the pterygoid‐palatine suture. The surface of the pterygoid is smooth, although most of it is covered by sediment.
3.2.11. Laterosphenoid
This bone is shaped like an hourglass from a ventral point of view (Figures 1c and 2e,f). It connects with the parietal to form the internal medial margin of the supratemporal fenestra. It also contacts with the pterygoid and the quadrate (Figure 2f). Unfortunately, the prootic and the basisphenoid rostrum could not be distinguished because of the poor state of preservation of this part of the specimen (even on the CT data). Dorsally, it is also sutured with the postorbital and frontal via the capitate process (Figure 2f).
3.2.12. Basisphenoid
Only the posterior part of the basisphenoid could be observed (Figures 1b,c and 2b,d,f). It contacts the posterior margin of the quadrate and the otoccipital but does not participate in the foramen magnum. Medially, it connects with the basioccipital along a sagittal suture (Figures 1b and 2d).
3.3. Cranial endocast and associated nerves and vascular structures
The anterior part of the forebrain could be reconstructed up to the anterior part of the frontal (Figures 3 and 4). This includes the posterior part of the olfactory region (Figures 3a–f and 4a–c,e,f). This part has a more tapered profile in this specimen of Hamadasuchus (as in Rukwasuchus) than it is in Pelagosaurus, Rhabdognathus, Campinasuchus, Trilophosuchus, Portugalosuchus, Tomistoma downsoni, or extant crocodylians where it is extremely thin dorsoventrally, although this could be a segmentation bias. No grooves are visible, as in other extant and extinct crocodylomorphs, except for Gavialis where this structure creates a bulbous expansion, and Pelagosaurus and Eopneumatosuchus where it is paired. The olfactory tract (Figures 3a–d, f and 4a–c,e,f) is long and flat along its dorsal margin, as is the dorsal margin of the skull bones of the interorbital area. This is different from Rukwasuchus, Trilophosuchus, Tomistoma downsoni, and Simosuchus where it is bent ventrally, and Almadasuchus where it is bent dorsally. The cerebral hemispheres are laterally expanded in an intermediate manner, as in Almadasuchus and Eopneumatosuchus (Figures 3a–d,f and 4a–c,e,f) between those of extant crocodylians, Portugalosuchus, and Tomistoma downsoni (markedly expanded) and those of extinct crocodylomorphs where they are not laterally expanded (baurusuchids, Macrospondylus, Plagiophthalmosuchus, Zulmasuchus, Sphenosuchus; see also Table 2). These structures exhibit a typical round shape and are symmetrically expanded along the midline in dorsal view. The endocast has a sigmoid shape in lateral view with a pontine and cephalic flexure angle that is similar to most extinct and extant crocodylomorphs (Figure 3a,b), unlike Rukwasuchus, Trilophosuchus, and Zulmasuchus, which have lower values, leading to more pronounced flexures (Table 2). On the other hand, Almadasuchus, Eopneumatosuchus, thalattosuchians (Pelagosaurus, Plagiophthalmosuchus, Macrospondylus, Cricosaurus, ‘Metriorhynchus’), and dyrosaurids (Rhabdognathus) have higher values, resulting in a “flatter” angle. The dorsal part of the hindbrain is compressed mediolaterally to accommodate for the endosseous labyrinths, as is seen in other crocodylomorphs with the notable exception of dyrosaurids, where this region is heavily compressed (Rhabdognathus for example). There are no signs of a depression linked with pericerebral spines (as in Zulmasuchus; Pochat‐Cottilloux et al., 2021), the caudal middle cerebral vein (as in Rhabdognathus) or an acute dorsal dural peak (as in Trilophosuchus; Ristevski, 2022) in dorsal view. However, this region has a blunt dorsal peak, as in allodaposuchids, Almadasuchus, and Baurusuchus. The ventral longitudinal venous sinus is well expanded and is ridge‐shaped, as in Alligator, Crocodylus, Portugalosuchus, Sphenosuchus, and Zulmasuchus (Figure 3e,f). Apart from the significant dorsal post‐cerebral concavity noticed in Rukwasuchus, the overall shape and features of UCBL‐FSL 532408 are remarkably close to those of this taxon.
FIGURE 3.

Three‐dimensional reconstruction of the endocranial cavities within the braincase of UCBL‐FSL 532408 (Hamadasuchus) in lateral (a), anterior ¾ (b), dorsal (c), anterior (d), posterior (e) and ventral (f) views. Blue: endocast, red: internal carotid artery, yellow: cranial nerve, purple: pituitary fossa. ch, cerebral hemisphere; el, endosseous labyrinth; fb, forebrain; hb, hindbrain; ic, internal carotid artery; IX–XI, cranial nerve IX–XI; nso, supraoccipital ramus of cranial nerve V; ntym, tympanic branch of cranial nerve V; oa, orbital artery; or: olfactory region; ot: olfacory tract; pf, pituitary fossa; V2, maxillary division of cranial nerve V; V3, mandibular division of cranial nerve V; VI, cranial nerve VI; vlvs, ventral longitudinal venous sinus; XII, cranial nerve XII. The scale bar is 3 cm.
FIGURE 4.

Three‐dimensional reconstruction of the pneumatic cavities within the braincase of UCBL‐FSL 532408 (Hamadasuchus) in anterior (a), anterior ¾ (b), lateral (c), posterior (d), dorsal (e), and ventral view (f). Blue: endocast, green: pharyngotympanic sinuses and eustachian system, orange: intertympanic diverticulum, red: internal carotid artery, yellow: cranial nerve, purple: pituitary fossa. ch, cerebral hemisphere; cq, cranioquadrate passage; eam, external auditory meatus; fb, forebrain; hb, hindbrain; ic, internal carotid artery; id, intertympanic diverticulum; IX–XI, cranial nerve IX–XI; od, otoccipital diverticulum; or, olfactory region; ot, olfactory tract; pd, parietal diverticulum; pf, pituitary fossa; pr, pterygoid recess; ps, pharyngotympanic sinus; pt, pharyngotympanic tube; qd, quadrate diverticulum; rpr, precarotid recess; uc, unidentified canal; V, cranial nerve V; VI, cranial nerve VI; vlvs, ventral longitudinal venous sinus; XII, cranial nerve XII. The scale bar is 2 cm.
Cranial nerve VI is expanded anteroposteriorly and is situated between the pituitary fossa ventrally and the ventral edge of the endocast dorsally. It comes out from the hindbrain just ventrally to the overly voluminous cranial nerve V (Figure 3f).
Cranial nerve V is a large ganglion, laterally projecting from the endocast, anteroventrally to the endosseous labyrinths. The maxillary division (V2) is the largest visible and extends anteriorly almost until the anterior end of the cerebral hemisphere (Figure 3a,b). The beginning of the mandibular division (V3) can be seen but could not be segmented further. Additionally, the two branches extending dorsally from the trigeminal ganglion are the tympanic branch (anteriorly) and the supraorbital ramus (posteriorly).
Cranial nerves IX–XI and XII are easily distinguishable (Figure 3a,c,e,f). There are two canals linked with those nerves, the anteriormost one being for cranial nerve IX–XI and probably a part of cranial nerve XII, whereas the posteriormost one is probably for a part or the totality of cranial nerve XII (Figure 3a–c,e,f).
The pituitary fossa is more extended anteroposteriorly than dorsoventrally. It is directed posteroventrally, as in Zulmasuchus, Macrospondylus, Plagiophthalmosuchus, Rhabdognathus Arenysuchus, Agaresuchus, Sebecus, Trilophosuchus, Sphenosuchus, Portugalosuchus, Eopneumatosuchus, Tomistoma downsoni, and extant crocodylians whereas it is directed anteroposteriorly in ‘Metriorhynchus’, Cricosaurus and Pelagosaurus and ventrally in baurusuchids and Rukwasuchus. The starting point of the orbital arteries can be seen in the anteriormost part (Figure 3a,f).
The internal carotid arteries have the typical shape found in all crocodylomorphs so far: they enter the posterior end of the pituitary fossa separately at the level of the midline, and the canals enclosing them (carotid pillars sensu Walker, 1990) are large. Those canals then pass through the pharyngotympanic sinus so they cannot be segmented. Finally, the posterior section runs anterodorsally from the foramen for the internal carotid artery to the ventral to the endosseous labyrinths. As in Pochat‐Cottilloux et al. (2021), the limit between the internal carotid arteries and the pituitary fossa was established where the arteries are no longer separated and merge into a single structure.
3.4. Endosseous labyrinths
Those structures are partially preserved, the cochlear duct being missing (Figures 3 and 5). However, the main structures are still available, comprising the vestibular apparatus. The right one is the best‐preserved one, so it is displayed here, whereas the left one is missing the lateral semicircular canal (Figure 3a–c). The ampullae are not clearly distinguishable from the canals as their shape is continuous with those of the semicircular canals. All the canals are also circular in cross‐section, and narrow compared to those of extant crocodylians and Portugalosuchus, but in a less extreme way than in Zulmasuchus, Almadasuchus, and Eopneumatosuchus. However, the space between those canals is still important compared to other notosuchians (see comparative metrics in Table 2). The common crus is elongated dorsoventrally, in a comparable manner to what is seen in extant crocodylians and Tomistoma downsoni but less so than in Trilophosuchus, where it is especially high. The lateral semicircular canal is straight, whereas the anterior and posterior ones are much more bent (Figure 5). As a result, the lateral canal does not extend laterally, and the anterior semicircular canal is more expanded dorsally than the posterior one, resulting in a larger area of the canal, as in modern crocodylians, Portugalosuchus, Junggarsuchus, Eopneumatosuchus, Tomistoma downsoni, and Trilophosuchus (Table 2), whereas they are equally expanded anteroposteriorly, as in Simosuchus clarki, Tomistoma downsoni, and thalattosuchians. In other words, the anterior semicircular canal has a pyramidal shape (as those of Zulmasuchus, Simosuchus, Junggarsuchus, Eopneumatosuchus, and Sphenosuchus), whereas the posterior one is more rounded (like the ones of extant crocodylians, Tomistoma downsoni, and Portugalosuchus). The angle between the anterior and the posterior semicircular canals is approximately 80° (Figure 5a), which is less than Caiman and Trilophosuchus (100°; Bona & Paulina‐Carabajal, 2013 and 98°; Ristevski, 2022) or Zulmasuchus, Eopneumatosuchus, and Simosuchus (90°). However, the angle between the anterior and the lateral semicircular canal is the same as in all other extant crocodylians, thalattosuchians, Trilophosuchus, Eopneumatosuchus, and Portugalosuchus (60°; Figure 5a), while it is higher in Almadasuchus, Zulmasuchus, Baurusuchus, and Simosuchus (70°). The lagenar section could not be segmented.
FIGURE 5.

Three‐dimensional reconstruction of the right endosseous labyrinth of UCBL‐FSL 532408 (Hamadasuchus) in lateral (a), dorsal (b), ventral (c), posterior (d), and anterior (e) views. asc, anterior semicircular canal; cc, common crus; cd, cochlear duct; lsc: lateral semicircular canal; psc, posterior semicircular canal. The scale bar is 5 mm.
3.5. Braincase pneumaticity
The braincase of crocodylomorphs is heavily pneumatized with peculiar structures only found in this group (Kuzmin et al., 2021) (Figure 4). The pharyngotympanic system and the median pharyngeal system form the paratympanic sinus system. Dorsally to these structures is the intertympanic diverticulum, which is located along the dorsal margin of the endocast. In UCBL‐FSL 532408, this structure excavates the parietal, creating the parietal diverticulum (Figure 4a,b,d,e) which has a peculiar shape. In dorsal view, it is anteriorly expanded along its midline showing two canals that emerge laterally to it (Figure 4e). Those canals extend posterolaterally in the parietal, and the central expansion is bifurcated posteriorly. This arrangement is also present in Zulmasuchus, Trilophosuchus, and extant crocodylians, however, it is absent in thalattosuchians. The structure also has a central opening, which is ovoid in shape. The intertympanic diverticulum itself is linked dorsally to the parietal diverticulum and links the two pharyngotympanic sinuses on each side of the endocast, as in all crocodyliforms (Clark, 1986; Leardi et al., 2020).
The pharyngotympanic sinuses are located just dorsolaterally to the level of the endosseous labyrinths (Figure 4b,c). As in Zulmasuchus, they are rounded and expanded laterally, surrounding the endocast and the internal carotid arteries. These structures are also indistinguishable from the otoccipital diverticula, expanding posteriorly (Figure 4d,e). Both quadrate diverticula are segmented, as well as the cranioquadrate passage on each side (Figure 4b,c,e,f). These cranioquadrate passages are not separated from the sinus by a thin bony lamina, contrary to what is seen in metriorhynchids (Herrera et al., 2018). A canal separating from the cranioquadrate passage was also identified in the posterior part of the specimen, which has not been observed before in other crocodylomorphs (Figure 4e,f), but could simply be vascularization or innervation. The expanded quadrate diverticulum exhibits an unidentified canal expanding anteriorly without contacting the rest of the paratympanic system or invading the postorbital or the squamosal, especially visible on the left side (Figure 4b,c,e). This might be a peculiar structure of Hamadasuchus or due to the lack of sampling and reconstruction of this area in other studies. The basisphenoid diverticulum, which links the pharyngotympanic system with the median pharyngeal system is also very expanded (Figure 4b,c) as in Zulmasuchus. The recessus epitubaricus and the rostral pneumatic recess are expanded and were reconstructed on the left side (Figure 4c). The pharyngotympanic tubes are not reconstructed in their entirety, but they are ventrally directed and expanded mediolaterally. The pterygoid recesses are symmetrically expanded to those structures but have a more anterior origin (Figure 4a,b,c,f). The median pharyngeal sinus is verticalized and connects with the basisphenoid diverticulum anteriorly and the pharyngotympanic system posteriorly (Figure 4c,f), which suggests that the specimen is not a juvenile, otherwise it would have been more horizontal (Dufeau & Witmer, 2015; Serrano‐Martínez, Knoll, Narváez, & Ortega, 2019).
4. DISCUSSION
4.1. Attribution to Hamadasuchus and detailed comparison with Rukwasuchus yajabalijekundu
This specimen can be attributed to Crocodylomorpha because it has no descending process of the squamosal (Parrish, 1993). It can be further attributed to Crocodyliformes because the squamosals form most of the flat skull roof, the otoccipitals do not participate in the formation of the occipital condyle (Pol & Norell, 2004), there is no incisive foramen on the palate (Pol & Powell, 2011), the frontal is sculpted with low ridges and furrows, the parietal has a subrectangular shape in dorsal view, there is a strongly concave anterior margin of the otic aperture, the quadrate contacts with the squamosal on the posteroventral border of the otic aperture, and there is a connection between the pharynx and the dorsal pneumatic system (Leardi et al., 2020).
Crocodylomorphs from the Cretaceous of Africa are numerous and diverse, but altirostral forms with a heterodont dentition are restricted to a handful of notosuchians. Kaprosuchus saharicus Sereno & Larsson, 2009 has posterodorsally projecting parietal‐squamosal horns and a squared internal choana, which the specimen described here does not have. Uruguaysuchidae sensu Gasparini, 1971 (including Araripesuchus wegeneri Buffetaut, 1981 and Araripesuchus rattoides Sereno & Larsson, 2009) is diagnosed by having a vascular opening in the postorbital bar, a non‐ornamented quadratojugal and a distinct development of the distal quadrate body ventral to the otoccipital‐quadrate contact, traits that are absent in the specimen studied here. Libycosuchus brevirostris Stromer, 1914 has a very small choana and almost round supratemporal fenestrae, which our specimen does not have.
However, the specimen described here is remarkably similar to ROM 52620, ROM 52509, and ROM 54511 described as belonging to Hamadasuchus rebouli Buffetaut, 1994 (Larsson & Sues, 2007) and RRBP 08360 described as the holotype of Rukwasuchus yajabalijekundu Sertich & O'Connor, 2014. Concerning the ROM specimens, they are in fact not directly referrable to H. rebouli for now because the overlapping part of the set of fossils does not correspond with the holotype of this taxon, which is a partial dentary (Buffetaut, 1994). However, given the close resemblance between the two, those specimens remain in an unresolved specific status, while still belonging to the genus Hamadasuchus (for more information, see discussion in Nicholl et al., 2021; Pochat‐Cottilloux, Perrier, et al., 2023). A future detailed comparison of UCBL FSL 532408 and other specimens attributable to Hamadasuchus will be beneficial on this topic (Ibrahim et al., 2020; Rauhut & Lopez‐Arbarello, 2005).
Rukwasuchus is diagnosed by having a dorsally upturned tip of the posterior process of the squamosal and a ventrally descending process of the postorbital, which all ROM specimens assigned to Hamadasuchus and UCBL FSL 532408 lack. On the other hand, all ROM specimens are diagnosed by the combination of a thin intertemporal bar, oval rather than round supratemporal fenestrae with dorsomedial edges level with the skull table, tapered distal squamosal prong, large posteroventral process on the postorbital that contacts the quadrate and quadratojugal, external auditory meatus fossa extending anteriorly over the entire length of the postorbital, prominent bilateral projections on the posterodorsal surface of the supraoccipital, no dorsal crest on the dorsal distal part of the quadrate and a supratemporal fossa covering most of the bony bar between the supratemporal fenestra and the orbit (Larsson & Sues, 2007). UCBL FSL 532408 also displays all those features, apart from the last one. We thus argue that this specimen can be attributed to Hamadasuchus rather than to Rukwasuchus. Nonetheless, those taxa remain remarkably close, as evidenced in Larsson & Sues, 2007 and Sertich & O'Connor, 2014: for example, they both share a depressed posterior parietal border and an anteroventrally projected process of the squamosal below the dorsal lamina of the postorbital.
In terms of neuroanatomy, R. yajabalijekundu exhibits a significant post‐cerebral concavity posteriorly to the cerebral hemispheres (Sertich & O'Connor, 2014, figure 6c) whereas it is not the case of the specimen studied here. The pituitary fossa is directed almost ventrally in Rukwasuchus whereas it is much more horizontal in UCBL FSL 532408. Furthermore, the ganglion of cranial nerve V is more expanded in this specimen than in Rukwasuchus, but this could also be due to a preservation or acquisition bias. Further work is needed to understand if those differences really highlight taxonomic differences or if they are more linked to ontogenetic and/or ecological differences.
4.2. Paleobiological inferences
Peirosaurids are almost exclusively known from cranial remains, which can be very fragmentary (Barrios et al., 2016; Lamanna et al., 2019; Lio et al., 2016). As such, inferences about their ecology and mobility are exceedingly difficult to make. Here we use for the first time the internal structures of a peirosaurid to infer several ecological and behavioral traits.
4.2.1. Head posture
Head posture can be inferred using the orientation of the lateral semi‐circular canal (Erichsen et al., 1989; Hullar, 2006; Sampson & Witmer, 2007; Sereno et al., 2007; Witmer et al., 2003, 2008; Witmer & Ridgely, 2008). However, this method could be subject to interspecific variation (Marugán‐Lobón et al., 2013; Taylor et al., 2009), so we also estimated it with the alignment of the maxillary toothrow (Marugán‐Lobón et al., 2013) or the endocranial surface of the parietal (Kley et al., 2010; von Baczko et al., 2018) with the horizontal plane. Those three methods recover an angle between the longitudinal axis of the skull and the horizontal plane of 7 to 9° (Figure 6), which is in line with other values inferred for other notosuchians (Dumont Jr et al., 2020; Fonseca et al., 2020; Kley et al., 2010; Pochat‐Cottilloux et al., 2021). This posture allows the nostrils to be closer to the ground and a better vision over the altirostral snout, which is in line with a terrestrial ecology (Marinho et al., 2013; Stevens, 2006) rather than a semi‐aquatic/aquatic one where the longitudinal axis of the skull is considered parallel to the horizontal plane, especially for buoyancy purposes (Witmer et al., 2008; Bona et al., 2017; Serrano‐Martínez, Knoll, Narváez, & Ortega, 2019; Figure 6d). This also implies lower energy costs compared to keeping the head on the horizontal plane, although this could only be confirmed by studying postcranial material. Apart from Uberabasuchus (de Vasconcellos & Carvalho, 2006) and osteoderms of Montealtosuchus (Tavares et al., 2015), postcranial anatomy is poorly known in Peirosauridae. This altirostral skull morphology is present in sebecosuchians as well (Dumont Jr et al., 2020; Fonseca et al., 2020; Marinho et al., 2013), which may be related to peirosaurids (Larsson & Sues, 2007; Pol et al., 2014). This morphology is also present in some theropods (Schwab et al., 2020; Stevens, 2006), which suggests a similar terrestrial ecology and predatory habits for all those organisms.
FIGURE 6.

Inferred alert head posture of UCBL FSL 532408 aligning the lateral semi‐circular (a), the endocranial surface of the parietal (b), and the maxillary toothrow (c) with the horizontal plane. Extant representative (Paleosuchus trigonatus MHNL 50003939) for comparison (d).
4.2.2. Endosseous labyrinth
The anterior semi‐circular canal of Hamadasuchus is more expanded than the posterior one, as in most other crocodylomorphs, and corresponds to a moderate sensitivity to pitching (the movement of the endolymphatic liquid inside the canal when the head moves up and down is transmitted to the brain via the cristae to control the acceleration; Hudspeth, 1983; Rabbitt et al., 2004). The same can be said for the lateral semi‐circular canal, which corresponds to the sensitivity of yaw (lateral acceleration) movements. As a result, Hamadasuchus would have been able to move its head to the full extent of its capabilities, especially up and down, which brings a further argument in favor of a terrestrial ecology. An especially interesting comparison to make here is with the internal structures of Zulmasuchus querejazus (Pochat‐Cottilloux et al., 2022): this crocodylomorph also has an extended anterior semi‐circular canal compared with the posterior one, however, its lateral semi‐circular canal is much more expanded. It can thus be hypothesized that this could be the result of a slightly different adaptation of those two crocodylomorphs to hunting or moving, with Zulmasuchus also able to perform more pronounced lateral movements of the head, whereas it was less the case in Hamadasuchus. The endosseous labyrinth of Hamadasuchus is also close in shape to those of terrestrial predatory theropods, pseudosuchians, and early crocodylomorphs (Figure 7; Sanders & Smith, 2005; Witmer & Ridgely, 2009; Paulina‐Carabajal & Succar, 2014; Xing et al., 2014; Paulina‐Carabajal & Filippi, 2018; Nesbitt et al., 2018; Cerroni & Paulina‐Carabajal, 2019; Paulina‐Carabajal & Nieto, 2019). Those hypotheses are strengthened when looking at semi‐aquatic and aquatic reptiles which have less expanded semi‐circular canals, linked with a head more in line with body movement (Figure 7; Evans, 1999; Yi & Norell, 2019; Schwab et al., 2020). Furthermore, dyrosaurids (semi‐aquatic to aquatic crocodylomorphs, mainly marine) have very expanded vestibules, which could be linked to an otolith‐mediated inner ear for underwater vibration detection rather than tympanic‐driven impedance matching (Erb & Turner, 2021). This structure is absent in Hamadasuchus (as well as Zulmasuchus) which is a further argument for a terrestrial ecology in these taxa. Several studies have looked at the relationship between the shape of the endosseous labyrinth and the living environment: Schwab et al. (2020) retrieved a significant correlation between those variables using canonical variate analysis (CVA), sampling a variety of fossil and extant crocodylomorphs but Bronzati et al. (2021), after investigating on a broader archosauriform sample and including phylogenetic relationships a priori, concluded that those variations might be more related to constraints from the development of the skull. Furthermore, Schwab et al. (2022) demonstrated that the shape of the endosseous labyrinth in extant crocodylians varies a lot throughout ontogeny, bringing more complexity to the debate, which thus remains open. As a result, no clear link between the shape of the endosseous labyrinth and ecology has been demonstrated so far.
FIGURE 7.

Three‐dimensional reconstructions of the endosseous labyrinths of different reptiles in lateral view: (a) Hamadasuchus (right, UCBL FSL 532408); (b) Zulmasuchus querejazus (left, MHNC 6672 from Pochat‐Cottilloux et al., 2021); (c) Crocodylus porosus (left, OUVC 10899); (d) Viavenator exxoni (left, MAU‐Pv‐Li‐530 from Paulina‐Carabajal & Filippi, 2018); (e) Baurusuchus sp. (right, FUP‐Pv 000021 from Dumont Jr et al., 2020, figure 9b); (f) Pelagosaurus typus (left, BRLSI M1413 from Pierce et al., 2017, figure 6b); (g) Parringtonia gracilis (left NMT RB460, from Nesbitt et al., 2018, figure 11k); (h) Platecarpus tympaniticus (left, AMNH FRAB1645 from Yi & Norell, 2019). All structures are oriented with the lateral semi‐circular canal oriented horizontally. Scale bars are 5 mm.
4.2.3. Relative brain size
When looking at the endocast of any organism, an idea of its development can be obtained by comparing the metrics of the brain it represents to the metrics of the whole body of the organism, i.e., relative brain size. It is used to infer cognition and thermoregulation of extinct vertebrates, based on extant representatives (Hopson, 1977; Jerison, 1973). The relative brain size is traditionally linked to the reptile encephalization quotient (REQ) in the case of crocodylomorphs, which consists of a linear regression between body mass and brain mass (Hurlburt, 1996). This approach has been widely used for paleobiological inferences on fossil crocodylomorphs (Puértolas‐Pascual et al., 2022; Serrano‐Martínez et al., 2021; Serrano‐Martínez, Knoll, Narváez, Lautenschlager, & Ortega, 2019; Serrano‐Martínez, Knoll, Narváez, & Ortega, 2019) but, as rightly highlighted in Dumont Jr et al. (2020), this use is problematic because the REQ changes throughout ontogeny (corresponding with changes in brain morphology), even between different adult stages (Hurlburt et al., 2013). As a result, we completed the dataset of Dumont Jr et al. (2020; Figure 8), which rather uses the raw log‐transformed measurements of body and brain mass (Figure 8; see also Data S1). Body mass is estimated from skull measurements in fossil specimens using linear regression between those metrics in extant crocodylians (Paiva et al., 2022). Brain mass can be derived from the endocranial volume (assuming a brain density of 1) from the following relation: where EV: endocranial volume and BV: brain volume (Dumont Jr et al., 2020 and references therein). Potential bias in those estimation methods exist and the results presented thereafter should thus be taken with the necessary precautions.
FIGURE 8.

Body mass vs brain mass in different specimens of extant and extinct crocodylomorphs.
As can be seen in Figure 8, across 148 extant and extinct specimens, Hamadasuchus (with the addition of the data available for ROM 52620 in George & Holliday, 2013) has an encephalization quotient in the range of modern crocodylians. With the addition of allodaposuchids (Puértolas‐Pascual et al., 2022; Serrano‐Martínez et al., 2021), the difference observed in baurusuchids originally proposed in Dumont Jr et al., 2020 now does not seem so important. The difference remains significant when looking at body mass (Wilcoxon test, p‐value = 0.019) but not for brain mass (Wilcoxon test, p‐value = 0.75). Furthermore, Campinasuchus dinizi, which is also a baurusuchid, has similar values as other baurusuchids (Fonseca et al., 2020). As a result, we argue that not many paleobiological inferences can be made using the relative brain size.
4.2.4. Cranial pneumaticity
The pneumaticity of the skull of Hamadasuchus seems to be very expanded, as in Zulmasuchus and Campinasuchus (Fonseca et al., 2020; Pochat‐Cottilloux et al., 2021). However, these structures are less expanded in extant crocodylians, allodaposuchids, Portugalosuchus, and Trilophosuchus (Bona et al., 2017; Puértolas‐Pascual et al., 2022, 2023; Ristevski, 2022; Serrano‐Martínez et al., 2021; Serrano‐Martínez, Knoll, Narváez, Lautenschlager, & Ortega, 2019; Serrano‐Martínez, Knoll, Narváez, & Ortega, 2019; Witmer et al., 2008). In fossils that show adaptations to the aquatic environment, some of those structures are even absent: for example, the intertympanic diverticulum and parietal diverticulum are missing in thalattosuchians (Brusatte et al., 2016; Herrera et al., 2018; Pierce et al., 2017; Schwab et al., 2021; Wilberg et al., 2021). Multiple hypotheses have been formulated to explain these differences and are linked to one another: the enlargement of the sinuses could improve resonance and allow for better aerial sound pickup, whereas soundwaves travel more easily in the aquatic environment (Dufeau & Witmer, 2015; Herrera et al., 2018). The impact of those structures on the buoyancy and skull density in semi‐aquatic to aquatic taxa could also be important (Brusatte et al., 2016). Finally, the development of the sinus system seems to also be linked to the shape of the rostrum (and thus the diet), which could have direct implications on the internal constraints of the skull and particularly the braincase (Dufeau & Witmer, 2015). As we previously stated, this hypothesis could be debatable because this pattern is not found in extant specimens (i.e., longirostrine Gavialis and brevirostrine Caiman have similar sinuses; Pochat‐Cottilloux et al., 2021, figure 8). Finally, there seems to be a link between the development of the sinus system and the living environment in crocodylomorphs, as terrestrial forms, such as Zulmasuchus, have a very expanded cranial pneumaticity, whereas among forms showing adaptations to the aquatic environment, such as thalattosuchians, cranial pneumaticity is, as stated above, reduced.
To get a better view of the problem, we introduced the data obtained from the current study on Hamadasuchus in a dataset consisting of extant specimens of diverse ecologies, as well as fossil representatives such as Diplocynodon, Trilophosuchus, Portugalosuchus, or Zulmasuchus, all adults (see Data S1 for more information). To get an unbiased idea of the sinus volume of each specimen, we compared the log‐transformed sinus volume (without the cranioquadrate passages) to the log‐transformed skull width and skull length for each specimen (Figure 9).
FIGURE 9.

(a) Sinus volume vs skull length in different specimens of extant crocodylians and Hamadasuchus, (b) Sinus volume vs skull width in different specimens of extant and extinct crocodylomorphs.
First, as can be seen in Figure 9a, sinus volume is in fact strongly correlated with skull length (log skull length = 0.438 ± 0.106 * log sinus volume + 0.927 ± 0.126, 2 s.d.; R2 = 0.84, p‐value = 3.33.10−9), although their overall morphology seems quite similar. However, once sinus volume is normalized to skull length for each specimen, the different snout morphologies of extant crocodylians (i.e., brevirostrine for Alligator, Caiman and Osteolaemus, mesorostrine for Crocodylus and longirostrine for Gavialis, Mecistops, and Tomistoma), which should be distinguished when considering the previous correlation, are undistinguishable under a Wilcoxon test (see Data S1). As such, sinus volume is correlated to skull length but not to snout morphology.
Second, as can be seen in Figure 9b, sinus volume is even better correlated to skull width (log skull width = 0.418 ± 0.064 * log sinus volume + 0.666 ± 0.078, 2 s.d.; R2 = 0.91; p‐value = 1.61.10−14). Finally, sinus volume could also be associated with lifestyle: when looking at a longirostrine extant specimen (Gavialis), Crocodylus, and Zulmasuchus (black square), there is a difference in sinus volume between the three groups for specimens of the same size, which could be associated with their different lifestyle (respectively aquatic, semi‐aquatic, and terrestrial). Trilophosuchus (terrestrial) also has similarly expanded sinuses compared to caimans (semi‐aquatic) of the same skull width. This would be linked with the hypotheses mentioned above, such as improved resonance and better aerial sound pickup or the impact on the buoyancy of such structures. As a result, Hamadasuchus displaying average values of sinus volume would be associated with a terrestrial lifestyle (but with some semi‐aquatic affinities), which was also recently highlighted using an independent histological approach (Pochat‐Cottilloux, Martin, et al., 2023). However, these results come only by taking into account the sinus structure and volume which is still poorly understood and will have to be compared with other proxies to reach a more robust answer.
This method calls for a deeper sampling and remains limited in fully aquatic and fully terrestrial fossil forms, mainly because the sinuses as well as skull width must be completely preserved.
5. CONCLUSION
A detailed comparison of the cranial bones allows to identify the specimen described here as belonging to Hamadasuchus, pending a future reassessment of other specimens described as belonging to this genus. Close affinities with Rukwasuchus yajabalijekundu are also put forward.
The endocranial structures of Hamadasuchus are described here for the first time. The endocast has a sigmoidal shape, the endosseous labyrinths are expanded and the pneumaticity of the skull is very enlarged, with a much higher volume of the sinuses compared to extant semi‐aquatic representatives or aquatic fossil forms.
Using the endocranial structures, the alert head posture of the specimen is inferred to be about 7–9° with the horizontal plane, which is an important clue as to the terrestrial affinities of this taxon, linked with a binocular vision over the altirostral snout. Furthermore, the morphology of the endosseous labyrinth also brings information about the lifestyle of the organism: its expanded semi‐circular canals are related to enhanced mobility of the head, which is again in line with a terrestrial lifestyle. Finally, the enlarged sinus cavities are compared with those of some sebecosuchians: the hypotheses of sinus volume related to the living environment and/or snout morphology are tested (scaled with skull size). The volume of the sinuses indeed seems to be correlated to both skull width and skull length. Although there is no significant link between sinus volume and snout morphology, cranial pneumaticity seems to be linked to the lifestyle, but these interpretations must be taken with caution, as lack of preservation in fossil forms and thus data, especially on terrestrial representatives, could represent potential biases. These data indicate a more nuanced result concerning the ecology of Hamadasuchus, with more marked semi‐aquatic affinities than in sebecosuchians for example, but while still having an overall terrestrial lifestyle.
Another proxy that could be related to the lifestyle is tested: the relative brain size. However, it does not seem to bring out relevant information, as all sampled specimens are grouped, and, as a further limitation, is based on size estimates for extinct forms.
The study and interpretation of the internal structures of crocodylomorphs need to be amplified and applied to other putatively terrestrial groups, such as atoposaurids or sphagesaurians and may be coupled with other geochemical or histological proxies in order to further aid paleoecological and paleobiological inferences.
OPEN RESEARCH BADGE
This article has earned an Open Data badge for making publicly available the digitally‐shareable data necessary to reproduce the reported results. The data is available at https://doi.org/10.18563/journal.m3.183.
Supporting information
Figure S1.
Figure S2.
Data S1.
Data S1.
ACKNOWLEDGMENTS
This work was supported by the Agence Nationale de la Recherche (SEBEK project no. ANR‐19‐CE31‐0006‐01 to Jeremy E. Martin). The authors would like to thank Céline Salaviale (Université Lyon 1, France) for help during the segmentation of the specimen; Emmanuel Robert and Nicolas Roumenoff (Université Lyon 1) for legal and conservative clarifications; Eric Buffetaut (CNRS, ENS Paris) for his contribution to making the specimen available to the scientific community; Pedro Henrique Fonseca (Universidade Federal do Rio Grande do Sul) for pictures of Campinasuchus dinizi; David Blackburn (Florida Museum of Natural History, USA), Cody Thompson (University of Michigan, USA), Pedro Henrique Morais Fonseca (UFRGS, Brazil), Lawrence Witmer (Ohio University, USA), Blandine Bartschi (Université Lyon 1, France), Timothy Rowe (University of Texas, USA), Marie Meister and Elisabeth Ludes‐Fraulob (Musée Zoologique de Strasbourg, France), Didier Berthet (Musée d'Histoire Naturelle de Lyon, France), Medhi Mouana, and Anne‐Lise Charrault (Université de Montpellier, France), Ronan Allain, Florent Goussard, and Nour‐Edine Jalil (Muséum National d'Histoire Naturelle, France), Margarethe Maillart (ENS Lyon, France) for access to diverse extant and extinct specimens as well as the sharing of CT data. They would also like to thank Jorgo Ristevski (University of Queensland) and an anonymous reviewer, as well as Philip Cox (University College London) for insightful comments that greatly improved the quality of this manuscript.
Pochat‐Cottilloux, Y. , Rinder, N. , Perrichon, G. , Adrien, J. , Amiot, R. , Hua, S. et al. (2023) The neuroanatomy and pneumaticity of Hamadasuchus (Crocodylomorpha, Peirosauridae) from the Cretaceous of Morocco and its paleoecological significance for altirostral forms. Journal of Anatomy, 243, 374–393. Available from: 10.1111/joa.13887
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Pochat‐Cottilloux et al. (2023; MorphoMuseum) at https://doi.org/10.18563/journal.m3.183.
REFERENCES
- Barrios, F. , Bona, P. , Paulina‐Carabajal, A. , Leardi, J.M. , Holliday, C.M. & Lessner, E.J. (2022) An overview on the crocodylomorpha cranial neuroanatomy: variability, morphological patterns and paleobiological implications. In: Dozo, M.T. , Paulina‐Carabajal, A. , Macrini, T.E. & Walsh, S. (Eds.) Paleoneurology of amniotes: new directions in the study of fossil endocasts. Cham: Springer, pp. 213–266. [Google Scholar]
- Barrios, F. , Paulina‐Carabajal, A. & Bona, P. (2016) A new peirosaurid (Crocodyliformes, Mesoeucrocodylia) from the Upper Cretaceous of Patagonia, Argentina. Ameghiniana, 53(1), 14–25. [Google Scholar]
- Blanco, A. , Fortuny, J. , Vicente, A. , Lujan, A.H. , García‐Marçà, J.A. & Sellés, A.G. (2015) A new species of Allodaposuchus (Eusuchia, Crocodylia) from the Maastrichtian (Late Cretaceous) of Spain: phylogenetic and paleobiological implications. PeerJ, 3, e1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona, P. , Carabajal, A.P. & Gasparini, Z. (2017) Neuroanatomy of Gryposuchus neogaeus (Crocodylia, Gavialoidea): a first integral description of the braincase and endocranial morphological variation in extinct and extant gavialoids. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 106(4), 235–246. [Google Scholar]
- Bona, P. , Degrange, F.J. & Fernández, M.S. (2013) Skull anatomy of the bizarre crocodylian Mourasuchus nativus (Alligatoridae, Caimaninae). The Anatomical Record, 296(2), 227–239. [DOI] [PubMed] [Google Scholar]
- Bona, P. & Paulina, C.A. (2013) Caiman gasparinae sp. nov., a huge alligatorid (Caimaninae) from the late Miocene of Paraná, Argentina. Alcheringa: An Australasian Journal of Palaeontology, 37(4), 462–473. [Google Scholar]
- Brochu, C.A. , Bouaré, M.L. , Sissoko, F. , Roberts, E.M. & O'Leary, M.A. (2002) A dyrosaurid crocodyliform braincase from Mali. Journal of Paleontology, 76(6), 1060–1071. [Google Scholar]
- Bronzati, M. , Benson, R.B. , Evers, S.W. , Ezcurra, M.D. , Cabreira, S.F. , Choiniere, J. et al. (2021) Deep evolutionary diversification of semicircular canals in archosaurs. Current Biology, 31(12), 2520–2529. [DOI] [PubMed] [Google Scholar]
- Brusatte, S.L. , Muir, A. , Young, M.T. , Walsh, S. , Steel, L. & Witmer, L.M. (2016) The braincase and neurosensory anatomy of an Early Jurassic marine crocodylomorph: implications for crocodylian sinus evolution and sensory transitions. The Anatomical Record, 299(11), 1511–1530. [DOI] [PubMed] [Google Scholar]
- Buffetaut, E. (1981) Die biogeographische Geschichte der Krokodilier, mit Beschreibung einer neuen Art. Araripesuchus Wegeneri. Geologische Rundschau, 70(2), 611–624. [Google Scholar]
- Buffetaut, E. (1994) A new crocodilian from the Cretaceous of southern Morocco. Comptes rendus de l'Académie des sciences. Série 2. Sciences de la Terre et Des planètes, 319(12), 1563–1568. [Google Scholar]
- Burke, P.M. & Mannion, P.D. (2023) Neuroanatomy of the crocodylian Tomistoma dowsoni from the Miocene of North Africa provides insights into the evolutionary history of gavialoids. Journal of Anatomy, 243, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busbey, A.B. (1986) New material of Sebecus cf. huilensis (Crocodilia: Sebecosuchidae) from the Miocene La Venta formation of Colombia. Journal of Vertebrate Paleontology, 6(1), 20–27. [Google Scholar]
- Carvalho, I.D.S. , Ribeiro, L.C.B. & dos Santos, A.L. (2004) Uberabasuchus terrificus sp. nov., a new Crocodylomorpha from the Bauru Basin (Upper Cretaceous), Brazil. Gondwana Research, 7(4), 975–1002. [Google Scholar]
- Cerroni, M.A. & Paulina‐Carabajal, A. (2019) Novel information on the endocranial morphology of the abelisaurid theropod Carnotaurus sastrei . Comptes Rendus Palevol, 18(8), 985–995. [Google Scholar]
- Clark, J.M. (1986) Phylogenetic relationships of the crocodylomorph archosaurs . Unpublished PhD Thesis, University of Chicago, Chicago.
- Colbert, E.H. (1946a) Sebecus, representative of a peculiar suborder of fossil Crocodilia from Patagonia. Bulletin of the AMNH, 87, 4. [Google Scholar]
- Colbert, E.H. (1946b) The Eustachian tubes in the Crocodilia. Copeia, 1946(1), 12–14. [Google Scholar]
- Company, J. , Suberbiola, X.P. , Ruiz‐Omeñaca, J.I. & Buscalioni, A.D. (2005) A new species of Doratodon (Crocodyliformes: Ziphosuchia) from the Late Cretaceous of Spain. Journal of Vertebrate Paleontology, 25(2), 343–353. [Google Scholar]
- Coria, R.A. , Ortega, F. , Arcucci, A.B. & Currie, P.J. (2019) A new and complete peirosaurid (Crocodyliformes, Notosuchia) from Sierra Barrosa (Santonian, Upper Cretaceous) of the Neuquén Basin, Argentina. Cretaceous Research, 95, 89–105. [Google Scholar]
- de Vasconcellos, F.M. & Carvalho, I.D.S. (2006) Condicionante etológico na tafônomia de Uberabasuchus terrificus (Crocodyliformes, Peirosauridae) da Bacia Bauru (Cretáceo Superior). Geociências, 25(2), 225–230. [Google Scholar]
- Dufeau, D.L. (2011) The evolution of cranial pneumaticity in Archosauria: patterns of paratympanic sinus development . Unpublished PhD thesis, Ohio University, 174.
- Dufeau, D.L. & Witmer, L.M. (2015) Ontogeny of the middle‐ear air‐sinus system in Alligator mississippiensis (Archosauria: Crocodylia). PLoS One, 10(9), e0137060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont, Jr., M.V. , Santucci, R.M. , de Andrade, M.B. & de Oliveira, C.E.M. (2020) Paleoneurology of Baurusuchus (Crocodyliformes: Baurusuchidae), ontogenetic variation, brain size, and sensorial implications. The Anatomical Record, 305(10), 1–25. [DOI] [PubMed] [Google Scholar]
- Early, C.M. , Iwaniuk, A.N. , Ridgely, R.C. & Witmer, L.M. (2020) Endocast structures are reliable proxies for the sizes of corresponding regions of the brain in extant birds. Journal of Anatomy, 237(6), 1162–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb, A. & Turner, A.H. (2021) Braincase anatomy of the Paleocene crocodyliform Rhabdognathus revealed through high resolution computed tomography. PeerJ, 9, e11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen, J.T. , Hodos, W. , Evinger, C. , Bessette, B.B. & Phillips, S.J. (1989) Head orientation in pigeons: postural, locomotor and visual determinants. Brain, Behavior and Evolution, 33(5), 268–278. [DOI] [PubMed] [Google Scholar]
- Evans, M. (1999) A new reconstruction of the skull of the Callovian elasmosaurid plesiosaur Muraenosaurus leedsii Seeley. Mercian Geologist, 14(4), 191–198. [Google Scholar]
- Fernández, M.S. , Carabajal, A.P. , Gasparini, Z. & Chong, D.G. (2011) A metriorhynchid crocodyliform braincase from northern Chile. Journal of Vertebrate Paleontology, 31(2), 369–377. [Google Scholar]
- Filippi, L.S. , Barrios, F. & Garrido, A.C. (2018) A new peirosaurid from the Bajo de la Carpa Formation (Upper Cretaceous, Santonian) of Cerro Overo, Neuquén, Argentina. Cretaceous Research, 83, 75–83. [Google Scholar]
- Fiorelli, L.E. , Leardi, J.M. , Hechenleitner, E.M. , Pol, D. , Basilici, G. & Grellet‐Tinner, G. (2016) A new Late Cretaceous crocodyliform from the western margin of Gondwana (La Rioja Province, Argentina). Cretaceous Research, 60, 194–209. [Google Scholar]
- Fonseca, P.H.M. , Martinelli, A.G. , da Silva Marinho, T. , Ribeiro, L.C.B. , Schultz, C.L. & Soares, M.B. (2020) Morphology of the endocranial cavities of Campinasuchus dinizi (Crocodyliformes: Baurusuchidae) from the Upper Cretaceous of Brazil. Geobios, 58, 1–16. [Google Scholar]
- Gasparini, Z.B. (1971) Los Notosuchia del Cretácico de América del Sur como un nuevo infraorden de los Mesosuchia (Crocodilia). Ameghiniana, 8(2), 83–103. [Google Scholar]
- George, I.D. & Holliday, C.M. (2013) Trigeminal nerve morphology in Alligator mississippiensis and its significance for crocodyliform facial sensation and evolution. The Anatomical Record, 296(4), 670–680. [DOI] [PubMed] [Google Scholar]
- Geroto, C.F.C. & Bertini, R.J. (2019) New material of Pepesuchus (Crocodyliformes; Mesoeucrocodylia) from the Bauru Group: implications about its phylogeny and the age of the Adamantina Formation. Zoological Journal of the Linnean Society, 185(2), 312–334. [Google Scholar]
- Godoy, P.L. , Bronzati, M. , Eltink, E. , Marsola, J.C.A. , Cidade, G.M. , Langer, M.C. et al. (2016) Postcranial anatomy of Pissarrachampsa sera (Crocodyliformes, Baurusuchidae) from the Late Cretaceous of Brazil: insights on lifestyle and phylogenetic significance. PeerJ, 4, e2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera, Y. , Leardi, J.M. & Fernández, M.S. (2018) Braincase and endocranial anatomy of two thalattosuchian crocodylomorphs and their relevance in understanding their adaptations to the marine environment. PeerJ, 6, e5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday, C.M. & Gardner, N.M. (2012) A new eusuchian crocodyliform with novel cranial integument and its significance for the origin and evolution of Crocodylia. PLoS One, 7(1), e30471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopson, J.A. (1977) Relative brain size and behavior in archosaurian reptiles. Annual Review of Ecology, Evolution, and Systematics, 8, 429–448. [Google Scholar]
- Hu, K. , King, J.L. , Romick, C.A. , Dufeau, D.L. , Witmer, L.M. , Stubbs, T.L. et al. (2021) Ontogenetic endocranial shape change in alligators and ostriches and implications for the development of the non‐avian dinosaur endocranium. The Anatomical Record, 304(8), 1759–1775. [DOI] [PubMed] [Google Scholar]
- Hudspeth, A.J. (1983) The hair cells of the inner ear. Scientific American, 248(1), 54–65. [PubMed] [Google Scholar]
- Hullar, T.E. (2006) Semicircular canal geometry, afferent sensitivity, and animal behavior. The Anatomical Record, 288(4), 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlburt, G.R. (1996) Relative Brain Size in Recent and Fossil Amniotes: Determination and Interpretation (PhD Dissertation). University of Toronto, Department of Zoology, Toronto.
- Hurlburt, G.R. , Ridgely, R.C. & Witmer, L.M. (2013) Relative size of brain and cerebrum in tyrannosaurid dinosaurs: an analysis using brain‐endocast quantitative relationships in extant alligators. In: Parrish, J.M. , Molnar, R.E. & Currie, P.J. (Eds.) Tyrannosaurid paleobiology. Bloomington, Indiana: Indiana University Press, pp. 1–21. [Google Scholar]
- Ibrahim, N. , Sereno, P.C. , Varricchio, D.J. , Martill, D.M. , Dutheil, D.B. , Unwin, D.M. et al. (2020) Geology and paleontology of the upper cretaceous Kem Kem group of eastern Morocco. ZooKeys, 928, 1–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordansky, N.N. & Gans, C. (1973) The skull of the Crocodilia. Biology of the Reptilia, 4, 201–262. [Google Scholar]
- Jerison, H.J. (1973) Evolution of the brain and intelligence. New York, NY: Academic Press. [Google Scholar]
- Jirak, D. & Janacek, J. (2017) Volume of the crocodilian brain and endocast during ontogeny. PLoS One, 12(6), e0178491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner, A.W. , Pinheiro, A.E. & Campos, D.A. (2014) A new Sebecid from the Paleogene of Brazil and the Crocodyliform radiation after the K–Pg boundary. PLoS One, 9(1), e81386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klembara, J. (2005) Ontogeny of the partial secondary wall of the otoccipital region of the endocranium in prehatching Alligator mississippiensis (Archosauria, Crocodylia). Journal of Morphology, 266(3), 319–330. [DOI] [PubMed] [Google Scholar]
- Kley, N.J. , Sertich, J.J. , Turner, A.H. , Krause, D.W. , O'Connor, P.M. & Georgi, J.A. (2010) Craniofacial morphology of Simosuchus clarki (Crocodyliformes: Notosuchia) from the Late Cretaceous of Madagascar. Journal of Vertebrate Paleontology, 30(1), 13–98. [Google Scholar]
- Kuzmin, I.T. , Boitsova, E.A. , Gombolevskiy, V.A. , Mazur, E.V. , Morozov, S.P. , Sennikov, A.G. et al. (2021) Braincase anatomy of extant Crocodylia, with new insights into the development and evolution of the neurocranium in crocodylomorphs. Journal of Anatomy, 239(5), 983–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamanna, M.C. , Casal, G.A. , Ibiricu, L.M. & Martínez, R.D. (2019) A new peirosaurid crocodyliform from the Upper Cretaceous Lago Colhué Huapi Formation of Central Patagonia, Argentina. Annals of Carnegie Museum, 85(3), 193–211. [Google Scholar]
- Larsson, H.C. & Gado, B. (2000) A new Early Cretaceous crocodyliform from Niger. Neues Jahrbuch für Geologie Und Paläontologie‐Abhandlungen, 217, 131–141. [Google Scholar]
- Larsson, H.C. & Sues, H.D. (2007) Cranial osteology and phylogenetic relationships of Hamadasuchus rebouli (Crocodyliformes: Mesoeucrocodylia) from the Cretaceous of Morocco. Zoological Journal of the Linnean Society, 149(4), 533–567. [Google Scholar]
- Leardi, J.M. & Pol, D. (2009) The first crocodyliform from the Chubut Group (Chubut Province, Argentina) and its phylogenetic position within basal Mesoeucrocodylia. Cretaceous Research, 30(6), 1376–1386. [Google Scholar]
- Leardi, J.M. , Pol, D. & Clark, J.M. (2020) Braincase anatomy of Almadasuchus figarii (Archosauria, Crocodylomorpha) and a review of the cranial pneumaticity in the origins of Crocodylomorpha. Journal of Anatomy, 237(1), 48–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leardi, J.M. , Pol, D. & Gasparini, Z. (2018) New Patagonian baurusuchids (Crocodylomorpha; Notosuchia) from the Bajo de la Carpa Formation (Upper Cretaceous; Neuquén, Argentina): new evidences of the early sebecosuchian diversification in Gondwana. Comptes Rendus Palevol, 17(8), 504–521. Available from: 10.1016/j.crpv.2018.02.002 [DOI] [Google Scholar]
- Leardi, J.M. , Pol, D. , Novas, F.E. & Suárez, R.M. (2015) The postcranial anatomy of Yacarerani boliviensis and the phylogenetic significance of the notosuchian postcranial skeleton. Journal of Vertebrate Paleontology, 35(6), e995187. [Google Scholar]
- Lessner, E.J. , Elsey, R.M. & Holliday, C.M. (2022) Ontogeny of the trigeminal system and associated structures in Alligator mississippiensis . Journal of Morphology, 283(9), 1210–1230. [DOI] [PubMed] [Google Scholar]
- Lessner, E.J. & Holliday, C.M. (2020) A 3D ontogenetic atlas of Alligator mississippiensis cranial nerves and their significance for comparative neurology of reptiles. The Anatomical Record, 2020, 1–29. [DOI] [PubMed] [Google Scholar]
- Lio, G. , Agnolín, F.L. , Valieri, R.J. , Filippi, L. & Rosales, D. (2016) A new peirosaurid (Crocodilyformes) from the Late Cretaceous (Turonian–Coniacian) of Patagonia, Argentina. Historical Biology, 28(6), 835–841. [Google Scholar]
- Marinho, T.D.S. , Iori, F.V. , de Souza, C.I. & de Vasconcellos, F.M. (2013) Gondwanasuchus scabrosus gen. Et sp. nov., a new terrestrial predatory crocodyliform (Mesoeucrocodylia: Baurusuchidae) from the Late Cretaceous Bauru Basin of Brazil. Cretaceous Research, 44, 104–111. [Google Scholar]
- Martin, J.E. & de Lapparent de Broin, F. (2016) A miniature notosuchian with multicuspid teeth from the Cretaceous of Morocco. Journal of Vertebrate Paleontology, 36(6), e1211534. [Google Scholar]
- Martinelli, A.G. , Marinho, T.S. , Iori, F.V. & Ribeiro, L.C.B. (2018) The first Caipirasuchus (Mesoeucrocodylia, Notosuchia) from the Late Cretaceous of Minas Gerais, Brazil: new insights on sphagesaurid anatomy and taxonomy. PeerJ, 6, e5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugán‐Lobón, J. , Chiappe, L.M. & Farke, A.A. (2013) The variability of inner ear orientation in saurischian dinosaurs: testing the use of semicircular canals as a reference system for comparative anatomy. PeerJ, 1, e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melstrom, K.M. , Turner, A.H. & Irmis, R.B. (2022) Reevaluation of the cranial osteology and phylogenetic position of the early crocodyliform Eopneumatosuchus colberti, with an emphasis on its endocranial anatomy. The Anatomical Record, 305(10), 2557–2582. [DOI] [PubMed] [Google Scholar]
- Meunier, L.M. & Larsson, H.C. (2017) Revision and phylogenetic affinities of Elosuchus (Crocodyliformes). Zoological Journal of the Linnean Society, 179(1), 169–200. [Google Scholar]
- Molnar, R.E. (2012) Jaw musculature and jaw mechanics of Sebecus icaeorhinus Simpson, 1937 (Mesoeucrocodylia, Sebecosuchia). Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 103(3–4), 501–519. [Google Scholar]
- Montefeltro, F.C. , Andrade, D.V. & Larsson, H.C. (2016) The evolution of the meatal chamber in crocodyliforms. Journal of Anatomy, 228(5), 838–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento, P.M. & Zaher, H. (2011) The skull of the pper retaceous baurusuchid crocodile Baurusuchus albertoi Nascimento & Zaher 2010, and its phylogenetic affinities. Zoological Journal of the Linnean Society, 163(suppl_1), S116–S131. [Google Scholar]
- Nesbitt, S.J. , Stocker, M.R. , Parker, W.G. , Wood, T.A. , Sidor, C.A. & Angielczyk, K.D. (2018) The braincase and endocast of Parringtonia gracilis, a Middle Triassic suchian (Archosaur: Pseudosuchia). Journal of Vertebrate Paleontology, 37(1), 122–141. [Google Scholar]
- Nicholl, C.S. , Hunt, E.S. , Ouarhache, D. & Mannion, P.D. (2021) A second peirosaurid crocodyliform from the Mid‐Cretaceous Kem Kem Group of Morocco and the diversity of Gondwanan notosuchians outside South America. Royal Society Open Science, 8(10), 211254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva, A.L.S. , Godoy, P.L. , Souza, R.B. , Klein, W. & Hsiou, A.S. (2022) Body size estimation of Caimaninae specimens from the Miocene of South America. Journal of South American Earth Sciences, 118, 103970. [Google Scholar]
- Parrish, J.M. (1993) Phylogeny of the Crocodylotarsi, with reference to archosaurian and crurotarsan monophyly. Journal of Vertebrate Paleontology, 13(3), 287–308. [Google Scholar]
- Paulina‐Carabajal, A. & Filippi, L. (2018) Neuroanatomy of the abelisaurid theropod Viavenator: the most complete reconstruction of a cranial endocast and inner ear for a south American representative of the clade. Cretaceous Research, 83, 84–94. [Google Scholar]
- Paulina‐Carabajal, A. & Nieto, M.N. (2019) Brief comment on the brain and inner ear of Giganotosaurus carolinii (Dinosauria: Theropoda) based on CT scans. Ameghiniana, 57(1), 58–62. [Google Scholar]
- Paulina‐Carabajal, A. & Succar, C. (2014) The endocranial morphology and inner ear of the abelisaurid theropod Aucasaurus garridoi . Acta Palaeontologica Polonica, 60(1), 141–144. [Google Scholar]
- Perrichon, G. , Hautier, L. , Pochat‐Cottilloux, Y. , Raselli, I. , Salaviale, C. , Dailh, B. et al. (2023) Ontogenetic variability of the intertympanic sinus distinguishes lineages within Crocodylia. Journal of Anatomy, 242, 1096–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, S.E. , Williams, M. & Benson, R.B. (2017) Virtual reconstruction of the endocranial anatomy of the early Jurassic marine crocodylomorph Pelagosaurus typus (Thalattosuchia). PeerJ, 5, e3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochat‐Cottilloux, Y. , Martin, J.E. , Amiot, R. , Cubo, J. & de Buffrénil, V. (2023) A survey of osteoderm histology and ornamentation among Crocodylomorpha: a new proxy to infer lifestyle? Journal of Morphology, 284(1), e21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochat‐Cottilloux, Y. , Martin, J.E. , Jouve, S. , Perrichon, G. , Adrien, J. , Salaviale, C. et al. (2021) The neuroanatomy of Zulmasuchus querejazus (Crocodylomorpha, Sebecidae) and its implications for the paleoecology of sebecosuchians. The Anatomical Record, 305(10), 1–21. [DOI] [PubMed] [Google Scholar]
- Pochat‐Cottilloux, Y. , Perrier, V. , Amiot, R. & Martin, J.E. (2023) A peirosaurid mandible from the Albian‐Cenomanian (Lower Cretaceous) of Algeria and the taxonomic content of Hamadasuchus (Crocodylomorpha, Peirosauridae). Papers in Palaeontology, 9(2), e1485. [Google Scholar]
- Pochat‐Cottilloux, Y. , Rinder, N. , Perrichon, G. , Adrien, J. , Amiot, R. , Hua, S. et al. (2023) 3D models related to the publication: the neuroanatomy and pneumaticity of Hamadasuchus from the Cretaceous of Morocco and its significance for the paleoecology of Peirosauridae and other altirostral crocodylomorphs. MorphoMuseum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol, D. & Apesteguía, S. (2005) New Araripesuchus remains from the Early Late Cretaceous (Cenomanian–Turonian) of Patagonia. American Museum Novitates, 2005(3490), 1–38. [Google Scholar]
- Pol, D. , Leardi, J.M. , Lecuona, A. & Krause, M. (2012) Postcranial anatomy of Sebecus icaeorhinus (Crocodyliformes, Sebecidae) from the Eocene of Patagonia. Journal of Vertebrate Paleontology, 32(2), 328–354. [Google Scholar]
- Pol, D. , Nascimento, P.M. , Carvalho, A.B. , Riccomini, C. , Pires‐Domingues, R.A. & Zaher, H. (2014) A new notosuchian from the Late Cretaceous of Brazil and the phylogeny of advanced notosuchians. PLoS One, 9(4), e93105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol, D. & Norell, M.A. (2004) A new crocodyliform from Zos Canyon, Mongolia. American Museum Novitates, 2004(3445), 1–36. [Google Scholar]
- Pol, D. & Powell, J.E. (2011) A new sebecid mesoeucrocodylian from the Rio Loro Formation (Palaeocene) of North‐Western Argentina. Zoological Journal of the Linnean Society, 163(suppl_1), S7–S36. [Google Scholar]
- Porter, W.R. , Sedlmayr, J.C. & Witmer, L.M. (2016) Vascular patterns in the heads of crocodilians: blood vessels and sites of thermal exchange. Journal of Anatomy, 229(6), 800–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puértolas‐Pascual, E. , Kuzmin, I.T. , Serrano‐Martínez, A. & Mateus, O. (2023) Neuroanatomy of the crocodylomorph Portugalosuchus azenhae from the late cretaceous of Portugal. Journal of Anatomy, 242, 1146–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puértolas‐Pascual, E. , Serrano‐Martínez, A. , Pérez‐Pueyo, M. , Bádenas, B. & Canudo, J.I. (2022) New data on the neuroanatomy of basal eusuchian crocodylomorphs (Allodaposuchidae) from the Upper Cretaceous of Spain. Cretaceous Research, 135, 105170. [Google Scholar]
- Rabbitt, R. , Damiano, E. & Grant, J.W. (2004) Biomechanics of the semicircular canals and otolith organs. In: Highstein, S.M. , Fay, R.R. & Popper, A.N. (Eds.) The vestibular system. New York: Springer, pp. 153–201. [Google Scholar]
- Rauhut, O.W.M. & Lopez‐Arbarello, A. (2005) Wirbeltierreste aus der “mittleren” Kreide des Kem Kem, Marokko. Freunde der Bayerischen Staatssamlung für Paläontologie Und Geologie, Jahresbericht Und Mitteilungen, 34, 41–45. [Google Scholar]
- Ristevski, J. (2022) Neuroanatomy of the mekosuchine crocodylian Trilophosuchus rackhami Willis, 1993. Journal of Anatomy, 241, 981–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruebenstahl, A.A. , Klein, M.D. , Yi, H. , Xu, X. & Clark, J.M. (2022) Anatomy and relationships of the early diverging Crocodylomorphs Junggarsuchus sloani and Dibothrosuchus elaphros . The Anatomical Record, 305(10), 2463–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz, J.V. , Bronzati, M. , Ferreira, G.S. , Martins, K.C. , Queiroz, M.V. , Langer, M.C. et al. (2021) A new species of Caipirasuchus (Notosuchia, Sphagesauridae) from the Late Cretaceous of Brazil and the evolutionary history of Sphagesauria. Journal of Systematic Palaeontology, 19(4), 265–287. [Google Scholar]
- Sampson, S.D. & Witmer, L.M. (2007) Craniofacial anatomy of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar. Journal of Vertebrate Paleontology, 27(S2), 32–104. [Google Scholar]
- Sanders, R.K. & Smith, D.K. (2005) The endocranium of the theropod dinosaur Ceratosaurus studied with computer tomography. Acta Palaeontologica Polonica, 50(3), 601–616. [Google Scholar]
- Schwab, J.A. , Young, M.T. , Herrera, Y. , Witmer, L.M. , Walsh, S.A. , Katsamenis, O.L. et al. (2021) The braincase and inner ear of ‘Metriorhynchus’ cf. ‘M.’brachyrhynchus– implications for aquatic sensory adaptations in crocodylomorphs. Journal of Vertebrate Paleontology, 41, e1912062. [Google Scholar]
- Schwab, J.A. , Young, M.T. , Neenan, J.M. , Walsh, S.A. , Witmer, L.M. , Herrera, Y. et al. (2020) Inner ear sensory system changes as extinct crocodylomorphs transitioned from land to water. Proceedings of the National Academy of Sciences, 117(19), 10422–10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab, J.A. , Young, M.T. , Walsh, S.A. , Witmer, L.M. , Herrera, Y. , Brochu, C.A. et al. (2021) Ontogenetic variation in the crocodylian vestibular system. Journal of Anatomy, 240(5), 821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena, M.V. , Andrade, R.C. , Sayao, J.M. & Oliveira, G.R. (2018) Bone microanatomy of Pepesuchus deiseae (Mesoeucrocodylia, Peirosauridae) reveals a mature individual from the Upper Cretaceous of Brazil. Cretaceous Research, 90, 335–348. [Google Scholar]
- Sereno, P.C. , Dutheil, D.B. , Iarochene, M. , Larsson, H.C.E. , Lyon, G.H. , Magwene, P.M. et al. (1996) Predatory dinosaurs from the Sahara and Late Cretaceous faunal differentiation. Science, 272(5264), 986–991. [DOI] [PubMed] [Google Scholar]
- Sereno, P.C. & Larsson, H.C. (2009) Cretaceous crocodyliforms from the Sahara. ZooKeys, 28, 1–143. [Google Scholar]
- Sereno, P.C. , Sidor, C.A. , Larsson, H.C.E. & Gado, B. (2003) A new notosuchian from the Early Cretaceous of Niger. Journal of Vertebrate Paleontology, 23(2), 477–482. [Google Scholar]
- Sereno, P.C. , Wilson, J.A. , Witmer, L.M. , Whitlock, J.A. , Maga, A. , Ide, O. et al. (2007) Structural extremes in a Cretaceous dinosaur. PLoS One, 2(11), e1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano‐Martínez, A. , Knoll, F. , Narváez, I. , Lautenschlager, S. & Ortega, F. (2019) Inner skull cavities of the basal eusuchian Lohuecosuchus megadontos (Upper Cretaceous, Spain) and neurosensorial implications. Cretaceous Research, 93, 66–77. [Google Scholar]
- Serrano‐Martínez, A. , Knoll, F. , Narváez, I. , Lautenschlager, S. & Ortega, F. (2021) Neuroanatomical and neurosensorial analysis of the Late Cretaceous basal eusuchian Agaresuchus fontisensis (Cuenca, Spain). Papers in Palaeontology, 7(1), 641–656. [Google Scholar]
- Serrano‐Martínez, A. , Knoll, F. , Narváez, I. & Ortega, F. (2019) Brain and pneumatic cavities of the braincase of the basal alligatoroid Diplocynodon tormis (Eocene, Spain). Journal of Vertebrate Paleontology, 39(1), e1572612. [Google Scholar]
- Sertich, J.J. & O'Connor, P.M. (2014) A new crocodyliform from the Middle Cretaceous Galula Formation, southwestern Tanzania. Journal of Vertebrate Paleontology, 34(3), 576–596. [Google Scholar]
- Stevens, K.A. (2006) Binocular vision in theropod dinosaurs. Journal of Vertebrate Paleontology, 26(2), 321–330. [Google Scholar]
- Stromer, E.V. (1914) Ergebnisse der Forschungreisen Prof. E. Stromers in den Wusten Agyptens: II. Wirbeltier‐Reste der Baharije‐Stufe (unterstes Cenoman). 1. Einleitung und 2. Libycosuchus . Abhandlungen der Koniglichen Bayerischen Akademie der Wissenschaften, Mathematisch‐Physikalischen Klasse, 27(3), 1–15. [Google Scholar]
- Tavares, S.A.S. , Ricardi‐Branco, F. & Carvalho, I.D.S. (2015) Osteoderms of Montealtosuchus arrudacamposi (Crocodyliformes, Peirosauridae) from the Turonian‐Santonian (Upper Cretaceous) of Bauru Basin, Brazil. Cretaceous Research, 56, 651–661. [Google Scholar]
- Taylor, M.P. , Wedel, M.J. & Naish, D. (2009) Head and neck posture in sauropod dinosaurs inferred from extant animals. Acta Palaeontologica Polonica, 54(2), 213–220. [Google Scholar]
- Turner, A.H. (2006) Osteology and phylogeny of a new species of Araripesuchus (Crocodyliformes: Mesoeucrocodylia) from the Late Cretaceous of Madagascar. Historical Biology, 18(3), 255–369. [Google Scholar]
- Turner, A.H. & Buckley, G.A. (2008) Mahajangasuchus insignis (Crocodyliformes: Mesoeucrocodylia) cranial anatomy and new data on the origin of the eusuchian‐style palate. Journal of Vertebrate Paleontology, 28(2), 382–408. [Google Scholar]
- Turner, A.H. & Calvo, J.O. (2005) A new sebecosuchian crocodyliform from the Late Cretaceous of Patagonia. Journal of Vertebrate Paleontology, 25(1), 87–98. [Google Scholar]
- Turner, A.H. & Sertich, J.J. (2010) Phylogenetic history of Simosuchus clarki (Crocodyliformes: Notosuchia) from the late cretaceous of Madagascar. Journal of Vertebrate Paleontology, 30(sup1), 177–236. [Google Scholar]
- von Baczko, M.B. , Taborda, J.R. & Desojo, J.B. (2018) Paleoneuroanatomy of the aetosaur Neoaetosauroides engaeus (Archosauria: Pseudosuchia) and its paleobiological implications among archosauriforms. PeerJ, 6, e5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, A.D. (1990) A revision of Sphenosuchus acutus Haughton, a crocodylomorph reptile from the Elliot Formation (late Triassic or early Jurassic) of South Africa. Philosophical Transactions: Biological Sciences, 330(1256), 1–120. [Google Scholar]
- Watanabe, A. , Gignac, P.M. , Balanoff, A.M. , Green, T.L. , Kley, N.J. & Norell, M.A. (2019) Are endocasts good proxies for brain size and shape in archosaurs throughout ontogeny? Journal of Anatomy, 234(3), 291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilberg, E.W. , Beyl, A.R. , Pierce, S.E. & Turner, A.H. (2021) Cranial and endocranial anatomy of a three‐dimensionally preserved teleosauroid thalattosuchian skull. The Anatomical Record, 2021, 1–34. [DOI] [PubMed] [Google Scholar]
- Witmer, L.M. , Chatterjee, S. , Franzosa, J. & Rowe, T. (2003) Neuroanatomy of flying reptiles and implications for flight, posture and behaviour. Nature, 425(6961), 950–953. [DOI] [PubMed] [Google Scholar]
- Witmer, L.M. & Ridgely, R.C. (2008) The paranasal air sinuses of predatory and armored dinosaurs (Archosauria: Theropoda and Ankylosauria) and their contribution to cephalic structure. The Anatomical Record, 291(11), 1362–1388. [DOI] [PubMed] [Google Scholar]
- Witmer, L.M. & Ridgely, R.C. (2009) New insights into the brain, braincase, and ear region of tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and behavior. The Anatomical Record, 292(9), 1266–1296. [DOI] [PubMed] [Google Scholar]
- Witmer, L.M. , Ridgely, R.C. , Dufeau, D.L. & Semones, M.C. (2008) Using CT to peer into the past: 3D visualization of the brain and ear regions of birds, crocodiles, and nonavian dinosaurs. In: Endo, H. & Frey, R. (Eds.) Anatomical imaging. Tokyo: Springer, pp. 67–87. [Google Scholar]
- Xing, L. , Paulina‐Carabajal, A. , Currie, P.J. , Xu, X. , Zhang, J. , Wang, T. et al. (2014) Braincase anatomy of the basal theropod Sinosaurus from the Early Jurassic of China. Acta Geologica Sinica‐English Edition, 88(6), 1653–1664. [Google Scholar]
- Yi, H. & Norell, M. (2019) The bony labyrinth of Platecarpus (Squamata: Mosasauria) and aquatic adaptations in squamate reptiles. Palaeoworld, 28(4), 550–561. [Google Scholar]
- Zaher, H. , Pol, D. , Carvalho, A.B. , Riccomini, C. , Campos, D. & Nava, W. (2006) Redescription of the cranial morphology of Mariliasuchus amarali, and its phylogenetic affinities (Crocodyliformes, Notosuchia). American Museum Novitates, 2006(3512), 1–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Figure S2.
Data S1.
Data S1.
Data Availability Statement
The data that support the findings of this study are openly available in Pochat‐Cottilloux et al. (2023; MorphoMuseum) at https://doi.org/10.18563/journal.m3.183.


