Abstract
Recent molecular biology studies have revealed the process of nasal capsule determination. We aimed to create a fate map showing the association between the adult and embryonic components of the nasal wall and nasal capsule derivatives. We examined paraffin‐embedded histological sections between 15 mid‐term (9–16 weeks) and 12 near‐term (27–40 weeks) foetuses. Until 15 weeks, membranous ossification occurred ‘along’ the capsular cartilage, contributing to the formation of the vomer, maxilla and bony nasal septum as well as the nasal, frontal and lacrimal bones. After 15 weeks, a wide lateral part of the capsule became thin and fragmented, and degenerative cartilage was observed near the lacrimal bone, in the three conchae, and at the inferolateral end of the capsule sandwiched between the maxilla and palatine bone. The disappearing cartilages appeared to be replaced by nearby membranous bones. This type of membranous ossification did not appear to use the capsular cartilage as a ‘mould’, although the perichondrium may have a role in inducing ossification. Calcified cartilage indicated endochondral ossification in the inferior concha until 15 weeks and, later, at the bases of three conchae and around the future sphenoid sinus (i.e. the concha sphenoidalis). The capsular cartilage extended antero‐superiorly over the frontal bone and inserted into the nasal bone. At 40 weeks, the capsular cartilage remained in the cribriform plate and at the inferolateral end along the palatine bone. Consequently, less guidance from the nasal capsule seemed to provide great individual variation in the shape of the wide anterolateral wall of the nasal cavity.
Keywords: calcified cartilage, cartilage degeneration, human foetus, nasal capsule, ossification
The nasal wall is composed of three parts: (1) endochondral bones from the nasal capsule (red coloured), (2) membranous bones developing along the nasal capsule (yellow coloured) and (3) membranous bones developing during and after degeneration of the capsular cartilage (green coloured). Blue indicates parts of the nasal capsule that disappear postnatally: the inferolateral part of the capsule.

1. INTRODUCTION
The nasal capsule consists of a common median septal part, sometimes initially termed ‘interorbitonasal septum’, and two lateral regions (Standling, 2005). The free caudal borders of the lateral regions are incurved to form the inferior nasal conchae. The capsule is commonly found in living vertebrates and has been a topic of major interest in molecular developmental biology (Kuratani & Ahlberg, 2018; Swartz et al., 2012; Tang & Bronner, 2020). More than 40 years ago, Noden (1978) reported that any cranial neural crest cells could develop into normal nasal capsule cartilage when grafted to the frontal region. The shape and position of the nasal capsule are determined by the endoderm at the level of the forebrain (Pasqualetti & Rijli, 2002). Conversely, depletion of the most rostral part of the endoderm causes the absence of the nasal capsule cartilage (Couly et al., 2002). Signalling pathways have been studied, but research may not be exhaustive (Benouaiche et al., 2008; Liu et al., 2013; Yang et al., 2010). Additionally, the brain determines the shape of the superior nasal capsule (Gitton et al., 2011; Kaucka et al., 2018).
Despite recent progress in research regarding the molecular basis of nasal capsule development, descriptions of the development and growth of human capsular cartilage are very limited, possibly because of the abundance of ossification centres. Slabý (1960) described the early development of the cartilage until its tissue is established; however, he did not specify which parts of the capsule are replaced by bone. The photographs captured by Dankmeijer (1968) were limited to the nasal septum from foetuses less than 15 weeks old. Sandikcioglu et al. (1994) reported ossification centres of the vomer and nasal bone, stating that the septal cartilage disappears after puberty between a pair of vomers. The study by Vidić (1971) seems to be limited to the histology of nasal wall ossification. According to this study, initial ossification occurs in the cartilaginous medial wall of the nasal cavity of the foetus at around 18 weeks of gestational age (GA) and 160 mm of crown‐rump length (CRL). The three conchae are fully ossified by 26 weeks of GA and 210 mm CRL, except for their anterior ends. The author did not use the term ‘membranous ossification’ and paid special attention to the disappearance of cartilage, implying that he believed that ‘endochondral ossification’ (intra‐cartilaginous ossification) took place throughout the capsular cartilage. Likewise, Gray's Anatomy (Standling, 2005) gives the impression that bones replace the capsular cartilage. The textbook simply states, ‘The nasal capsule is ossified during the fifth month’. However, a combination of endochondral and membranous ossification seems to be a general rule at most head sites (Hayashi et al., 2014; Yamamoto et al., 2021).
None of the studies suggests that each part of the nasal capsule should develop into a corresponding part of the adult bony nasal wall. In humans, considerable individual variations are observed in the adult bone configuration of the lateral nasal wall made by the ethmoid (Isobe et al., 1998; Sato et al., 2000). Therefore, a significant question to be answered is ‘Does endochondral ossification of the capsular cartilage cause redundancy in development to provide bony variations?’ Rather than a single‐bone ethmoid, almost half of the lateral nasal wall is composed of the maxilla and palatine bone, which should develop ‘outside’ the nasal capsule. This brings up the question of whether the capsular cartilage determines or ‘guides’ the morphology of membranous bones. Since the nasal capsule is unlikely to have a miniature adult morphology, we aimed to create a fate map showing the association between the adult and embryonic components of the nasal wall and nasal capsule derivatives.
2. MATERIALS AND METHODS
This study was conducted in accordance with the Declaration of Helsinki of 1995 (as revised in 2013). We examined paraffin‐embedded histological sections from 15 mid‐term (38–125 mm CRL; GA of approximately 9–16 weeks) and 12 near‐term (216–350 mm CRL; GA of approximately 27–40 weeks) foetuses. The serial histological sections of the 15 mid‐term foetuses were a part of the large collection kept at the Department of Anatomy of the Universidad Complutense, Madrid and were the result of miscarriage and ectopic pregnancy cases from the Department of Obstetrics of the University. The foetuses were prepared serially and stained with haematoxylin and eosin (H&E), azan or silver impregnation. The sectional planes were frontal (nine specimens), sagittal (four specimens) or horizontal (two specimens). This study was approved by the Ethics Committee of Complutense University (B08/374).
The 12 near‐term foetuses were a part of the collection of the Department of Anatomy, Akita University, donated by their families to the Department in 1975–1985, and preserved in 10% w/w neutral formalin solution for more than 30 years. The specimens had data on the donation date and GA, but no information on the family name, obstetrician, hospital or reason for abortion. The use of these specimens for this research was approved by the Akita University Ethics Committee (no. 1428). Dr. Hiroshi Abe, Emeritus Professor at Akita University, kindly permitted the use of these materials. Before routine procedures for embedding in paraffin, the foetal head specimens were decalcified by incubation at room temperature in Plank‐Rychlo solution (AlCl2/6H2O, 7.0% w/v; hydrochloric acid [HCl], 3.6% w/v; formic acid [HCOOH], 4.6% w/v) for 3–7 days. Frontal (seven specimens), sagittal (three specimens) and horizontal (two specimens) sections of the head were prepared at 0.1–0.2 mm intervals, with 7–10 μm in thickness and stained with H&E. All photographs were captured using a Nikon Eclipse 80 microscope. Almost all sections of the near‐term foetuses were prepared for our recent study of the orbital contents and sphenoid (Naito et al., 2019; Yamamoto et al., 2021).
Section 3 defines different types of ossification using histological sections of the tibia, ilium and frontal bone from human foetuses. The sections were previously prepared for earlier studies (Hayashi et al., 2014; Kim et al., 2022, 2023).
3. RESULTS
3.1. Defining different types of ossification using H&E‐stained sections of human foetus specimens
The epiphysis of a long bone displays ‘endochondral ossification’, featuring a border of calcified cartilage between the hypertrophied cartilage cell layer and the woven bone (Figure 1a,c). The ‘periosteal ossification’ creates a thin bone plate around the woven bone in the diaphysis or shaft of a long bone, with osteocytes arranged in a line along the outer aspect adjacent to the periosteum, not the inner aspect facing the woven bone (Figure 1b). The cartilaginous ilium has a ‘perichondral bone’ at its superomedial end near the sacroiliac joint (Figure 1d). Unlike endochondral ossification, osteoblasts are arranged along the outer or superolateral aspect of the bone, not between the cartilage and the bone, and the cartilage cells are not hypertrophied (Figure 1e). The perichondral bone grows inferolaterally, creating a collar for the endochondral bone‐derived ilium (Figure 1f). This narrow meaning of perichondral ossification is likely found as a spotty focus in the growing pterygoid (Hayashi et al., 2014; Yamamoto et al., 2021). The ‘membranous bones’ appear as fragments in thick and tight fibrous tissue, such as the primitive dura mater (Figure 1g). Each of the bone fragments is surrounded by osteoblast candidates, especially after the size increases (Figure 1h,i,j,g). The term perichondral bone will be strictly discriminated from ‘membranous ossification along the nasal capsule cartilage’.
FIGURE 1.
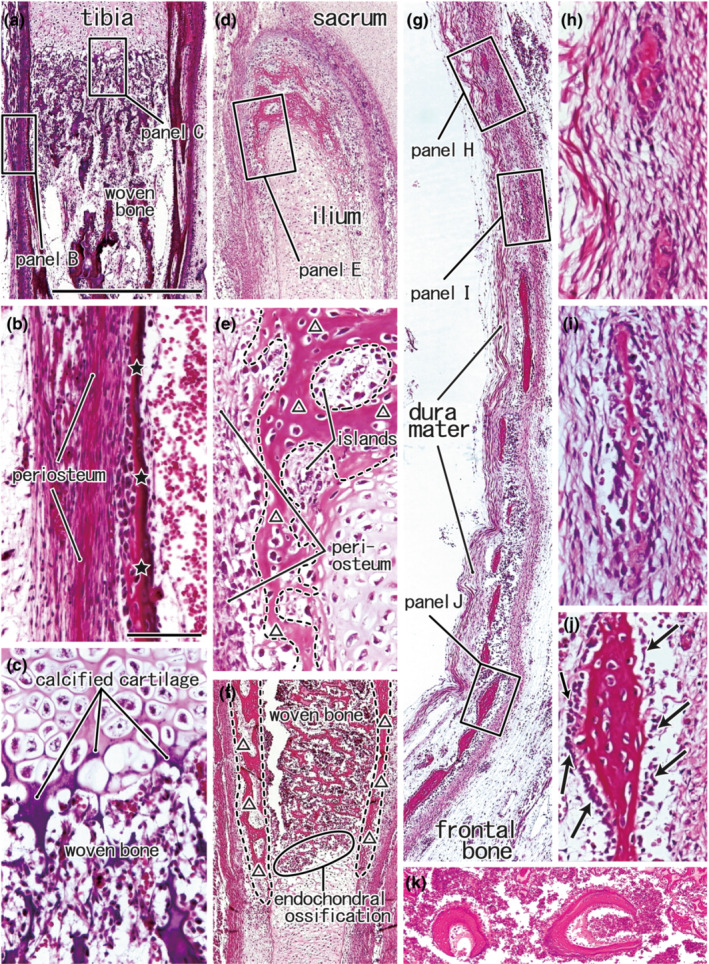
Various modes of ossification seen outside of the head. Panels (a–c), Sagittal section of the proximal part of the tibia from a foetus of 46‐mm CRL. Panels (d–f) Frontal sections of the superomedial part of the ilium near the sacroiliac joint from a foetus of 105‐mm CRL. Panels (g–j), Sagittal section of the squamous part of the frontal bone from a foetus of 125‐mm CRL. In all panels, the upper side corresponds to the superior side of the body. Squares in panels (a), (d), and (g) are shown at a higher magnification in panels (b), (c), (e), and (h–j), respectively. The periosteal ossification (stars) is shown in panel (b), while the endochondral ossification in panel (c). The perichondral ossification (triangles) is shown in panel e, and it coexists with the woven bone by the endochondral ossification in panel (f). Panel (f) displays a plane 2‐mm anterolateral to panel (d). Membranous bones, surrounded by osteoblast candidates (arrows in panel i), develop in a tight fibrous tissue (panel h) and grow to connect together (panels i and j). Panel (k) (a sagittal section from a foetus of 210‐mm CRL) exhibits a woven bone area containing ball‐like bones (osseous globules): this specific woven bone is seen in the endochondral ossification of otic capsule cartilage. Panels (a), (d), and (g) (or panels b, c, e, f and h–j) were prepared at the same magnification.
3.2. Observations in early and mid‐term foetuses
Frontal sections (Figures 2, 3, 4) demonstrate a cage‐like nasal capsule, whereas sagittal sections show the long anteroposterior extension of the capsule, including the cribriform plate (Figure 5). By a roof‐like primitive cribriform plate, the median part of the nasal capsule (a cartilaginous nasal septum) connects to a long lateral part including shelf‐like conchae. The bottom of the capsule has been opened to create a space covered by the maxilla and palatine bone, with membranous ossification. One event requires the citation of multiple figures. Therefore, multiple events are shown in one figure because of the specimen‐to‐specimen demonstrations.
FIGURE 2.
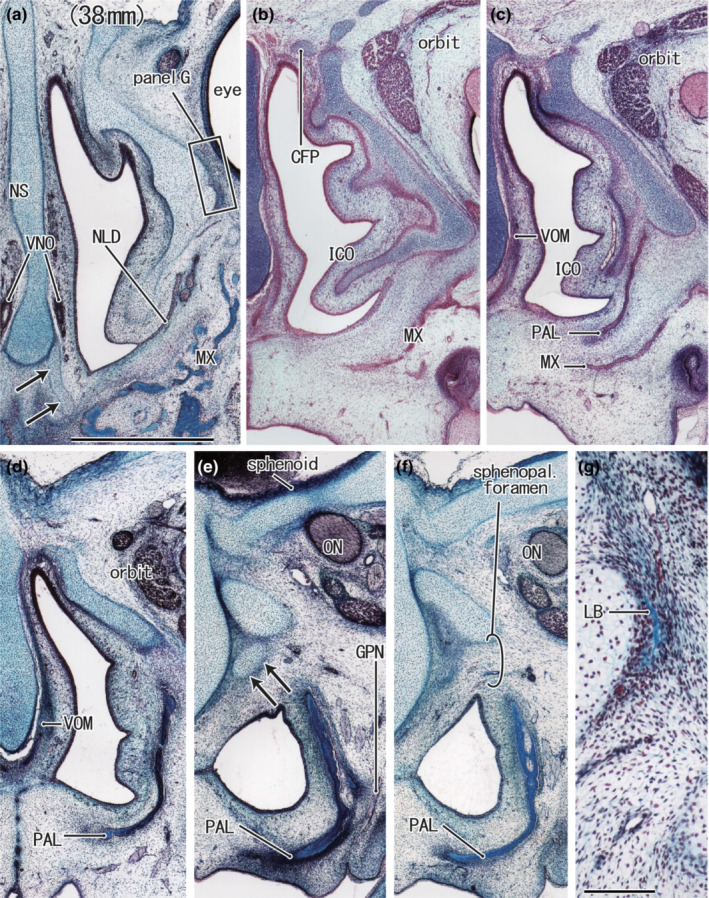
Nasal capsule in frontal sections of a foetus of 38‐mm CRL with an initial lacrimal bone. H&E staining. Frontal sections. The inferior concha contained an inferomedial continuation of the nasal capsule cartilage (panels a and b). The greater alar cartilage appears to be separated from the nasal septum (arrows in panel a). The inferolateral end of the capsule is directed towards a large space between the maxilla and the palatine bone (panel c). The posterior end of the capsular cartilage is a short process (arrows in panel e). The future sphenopalatine foramen was identified between the palatine bone (PAL) and the posterior end of the capsular cartilage (panel f). The initial lacrimal bone (LB), a membranous bone, appears along the lateral aspect of the capsular cartilage (panel g). Panels (a–f) were prepared at the same magnification (scale bars: 1 mm in panel a, 0.1 mm in panel g). Other abbreviations are common abbreviations.
FIGURE 3.

Nasal capsule in frontal sections of a foetus of 80‐mm CRL with a long inferolateral part inserted between the maxilla and palatine bone. H&E staining. Frontal sections. Panel (a) (or panel h) shows the most anterior (or posterior) plane in the figure. The nasal capsule is composed of a median thick septal cartilage (NS) and inferolaterally extending plate (arrows in panel a). Both parts were connected by the lateral nasal cartilage at the anterior site (stars in panel a). The bony maxilla (MX) appeared to develop along the outer aspect of the nasal capsule at the anterior sites (panels b and c). Owing to the wavy course, the greater alar cartilage appeared to be fragmented and separated from the nasal septum (arrows in panels c and d). The cribriform plate is underdeveloped, and the foramina are very large (stars in panels e and f). The inferolateral end of the nasal capsule (arrow in panel g) is sandwiched between the palatine bone (PAL) and the maxilla (MX). The cartilage process extends anteriorly from the inferior end (arrowhead in panels e and f). All panels were prepared at the same magnification (scale bar in a: 1 mm). Other abbreviations are common abbreviations.
FIGURE 4.
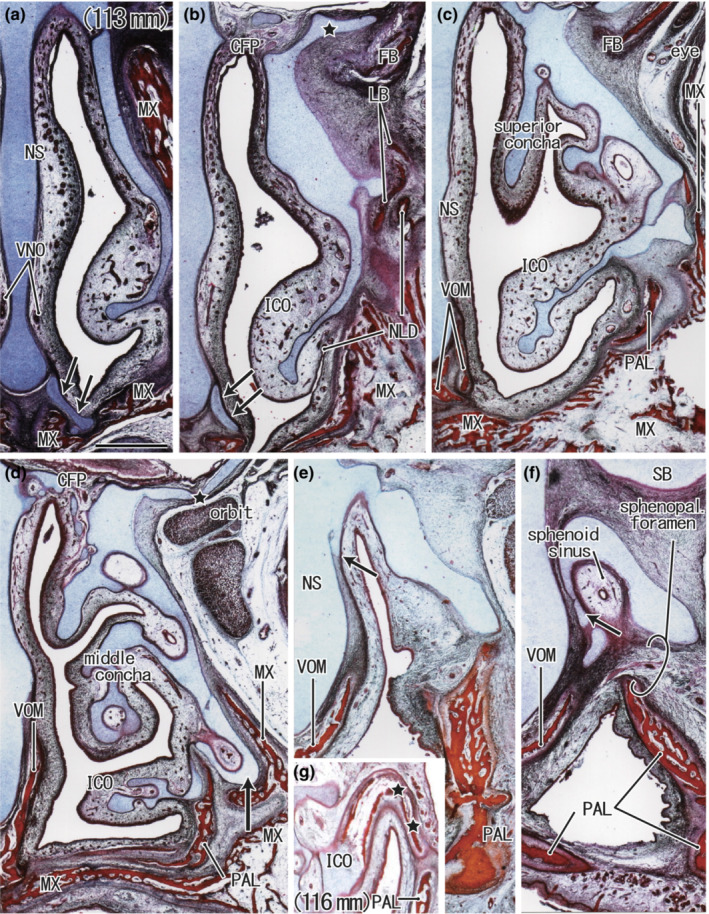
Frontal sections from two mid‐term foetuses at and just before the initial ossification of the nasal capsule. H&E staining. a–f: a foetus with a CRL of 113 mm; g: a foetus with a CRL of 116 mm. Panel (a) displays the most anterior plane in the figure, whereas panel (f) displays the most posterior plane. A long cartilage process (star in panel b) extends along the upper surface of the frontal bone (FB). The inferior concha (ICO) contains a simple inferior continuation of the nasal capsule cartilage (panels b and c). The inferolateral part of the capsule (arrow in panel d) surrounds the maxillary sinus. Multiple foramina were evident in the cribriform plate (CFP) (panel d). The posterior part of the nasal septum (NS) gives off a cartilage process (arrow in panel e) and becomes longer at the more posterior site (arrow in panel f). These cartilages surround the developing sphenoid sinus (panel f). Panel g shows the earliest ossification (stars) found in the lateral half of the inferior concha in another foetus. All panels were prepared at the same magnification (scale bar in panel a: 1 mm). Other abbreviations are common abbreviations.
FIGURE 5.

Nasal capsule seen in sagittal sections from a foetus of 100‐mm CRL with cartilage in the nasal bone. Panel (a) displays the most medial plane in the figure, whereas panel (f) displays the most lateral plane. Because of the wavy course, the posterior end of the nasal capsule appears fragmented (arrows in panel a). The cribriform plate (CFP) developed well (panel a), and the ethmoidal labyrinth started to develop (panel b). The nasal bone (NB) contains the process of the anterior part of the capsule (star in panel b). The anterior capsule tunes posteriorly and continues to the inferior concha (ICO; panels b–d). Asterisks in panel (d) indicate artefact spaces produced during the histological procedure. Panel (e) contains The upper end of the nasolacrimal duct (NLD). The lateral marginal part of the capsular cartilage is seen below the medial marginal part of the eyeball (panel f). All panels were prepared at the same magnification (scale bar in panel a: 1 mm). The other abbreviations refer to common abbreviations.
During mid‐term periods between GA of 9–16 weeks, the nasal capsule increases almost twice in size (Figure 2a vs. Figure 4a), and ossification occurs ‘along’ the outer surfaces of the capsule (Figures 2g, 3b, 4d, 5a). The inferior concha is built by the inferomedial continuation of the nasal capsule cartilage; it is not cartilage isolated from the nasal capsule but a part of the latter (Figures 2b, 3e, 4b, 5b). Being independent of the inferior concha and placed below it, the capsule issues a long inferolateral process towards the space between the bony maxilla and the palatine bone (Figures 2c and 3g). The inferolateral process of the capsule surrounds the newly developed recess of the nasal cavity (the future maxillary sinus; Figure 4d). At and near the future transverse suture of the bony palate, the palatine bone is riding over the maxilla (Figure 4d). The initial nasolacrimal duct passed through a site just below the anterolateral end of the nasal capsule (Figure 2a): thus, throughout the development, it did not penetrate the capsule.
The early cribriform plate was underdeveloped, and the foramina appeared much thicker than the nerves themselves (Figures 2a,b and 3e,f). The plate‐like cartilage thickening made the cribriform appearance evident (Figures 4d and 5a). The cribriform plate was laterally continuous with a developing ethmoidal labyrinth, and the sphenoid covered the latter from the superior side: thus, the sphenoid was riding over the ethmoid (Figure 5b). Anteriorly, the frontal bone grew along a long crista galli (Figure 5a). The initial lacrimal bone, a very thin membranous bone, appeared along the medial orbital wall, corresponding to the most lateral part of the capsular cartilage (Figure 2g). The initial lacrimal bone was located in the superomedial side of the underdeveloped nasolacrimal duct and lacrimal sac.
The posterior end of the capsular cartilage is composed of two short cartilage bars (the transverse process and the cartilage cupularis by van Gilse 1927; for details, see Section 4), which is similar to the tuberosity and head of the rib (Figures 3d,e and 4b). Both processes are attached to the thick cartilaginous nasal capsule under the sphenoid body (Figure 2e), providing a thick cartilage mass (the future sphenoidal concha) that surrounds a future sphenoid sinus (Figure 4f). The posterior part of the nasal septum gives off a cartilage process (Figure 4e,f) that elongates to become the perpendicular plate of the ethmoid at the near term (membranous bone; Figure 7). The posterior end is near the palatine bone (Figures 4f and 5b) and vomer (Figure 4f). The future sphenopalatine foramen can be identified between the palatine bone and the capsular cartilage near the palatine bone, but not between the bony palatine bone and maxilla (Figures 2f, 3h, and 4f).
After 10 weeks, the usual membranous ossification occurred along the capsular cartilage to create the vomer and nasal, frontal and lacrimal bones. Likewise, membranous ossification of the maxilla's nasal face and the frontal process began at 12 weeks along the capsular cartilage (Figure 4d). At these sites, the membranous bone used capsular cartilage as a ‘mould’. In the cartilage, the earliest endochondral ossification was observed in the lateral or proximal half of the inferior concha of a limited specimen (116 mm CRL; Figure 4g). The details of ossification are described in the next subsection. The nasal bone often contains a small cartilage mass (Figure 5b), which corresponds to a small process in the capsular cartilage. The anterior end of the nasal capsule will be shown later in combination with near‐term morphology (Figure 12).
FIGURE 12.

Anterior nose in sagittal sections from two foetuses. Panels (a–c), a foetus with a 125‐mm CRL (approximately 16 weeks); Panel (d), a foetus with 274‐mm CRL (approximately 32 weeks). Panel (a) displays an almost median plane containing the septal cartilage (NS), whereas panel (c) exhibits a plane 1.0 mm lateral to panel (a). The lateral nasal and greater alar cartilages (LNC and GAC) are observed on the lateral side of the nasal septum (panels b, c). Inset in the right‐hand margin of panel (c), a plane 0.1‐mm lateral to panel (c), containing a cartilage process of the nasal capsule (star) inserted into the nasal bone (NB). Panel (d) shows an acute curve (arrowheads) at the border between the future lateral nasal cartilage and greater alar cartilage. Inset in the inferior‐hand margin of panel (d) exhibits a rare combination of the calcified‐ and non‐calcified‐cartilages in the frontal process of the maxilla (see the ‘perichondral ossification’ in the first paragraph of Section 3). Arrows in panels (a), (b) and (d) indicate the lesser alar cartilage connected by a mesenchymal band to the nasal capsule. The triangles in panels (a) and (b) indicate unknown spaces. Panels (a–c) were prepared at the same magnification (scale bars in panels a and d: 1 mm). The other abbreviations are common abbreviations for the figures.
3.3. Observations of near‐term foetuses
During near‐term periods between 27 and 40 weeks, the nasal capsule increased almost 1.5 times in size, and ossification was advanced in the lateral nasal wall and along the cartilaginous septum (Figure 6a,d). In any near‐term specimen, the palatine bone is riding over the maxilla at and near the future transverse suture of the bony palate (Figure 6a,d). In frontal sections (Figures 6, 7, 8, 9), Figure 7 displays an anterior continuation of Figure 6a–c, whereas Figure 8 exhibits a posterior continuation of Figure 6d–g. At 27 weeks, ossification in the three conchae progressed (Figure 6d); however, the capsular cartilage still extended to the inferior concha (Figure 7b), with the peripheral parts remaining cartilaginous (Figure 7a). A bone structure surrounding the future sphenoid sinus (future concha sphenoidalis; Figures 8b–d) was connected to a membranous bone along the septal cartilage (future perpendicular plate of the ethmoid) and the bony ethmoid along the orbital wall. Ossification of the concha sphenoidalis did not begin at 27 weeks (data not shown). Figure 9 shows the degeneration and fragmentation of the capsular cartilage. Figures 10 (horizontal sections) and 11 (sagittal sections) assist in understanding the topographical anatomy in frontal sections. A single horizontal section contained endochondral ossification at multiple bases of the conchae (Figure 10c,d). Likewise, a single sagittal section contained both the bony concha sphenoidalis and the anterior nose (Figure 11d). Due to the specimen‐to‐specimen demonstrations, multiple events are shown in one figure.
FIGURE 6.

A near‐term comparison in size and ossification of the nasal capsule between small and large foetuses. H&E staining. Two frontal sections from two specimens: a foetus with a 216‐mm CRL (panels a–c) and one with a 334‐mm CRL (panels d–g). Panels (b) and (c) (or panels e–g) show higher‐magnification views of the squares in panel (a) (or panel d). The lateral wall of the nasal cavity is composed of single cartilage (panel a), whereas it contains multiple bones (panel d). In panel (a), a thick perichondrium is evident along the orbit (black arrowheads) but absent along the maxilla (MX; open arrowheads). The bony ethmoid (ETH) extends along the nasal septum cartilage (NS) in panel (d), but it is absent in panel (a). Degenerative and fragmented cartilage is seen in the lateral nasal wall (surrounded by a dotted line in panels b, c, e, and f). In panel (e), candidate calcified cartilages (arrows) suggest endochondral ossification in the posterior end of the cribriform plate of the ethmoid, but a thick perichondrium (arrowheads) and membranous bone (star) co‐exist in the area. The small cartilage mass in panel (g) extends postero‐superiorly to the future concha sphenoidalis (cont. to Figure 8). Panels (a) and (d) (or panels b, c, e, f and g) were prepared at the same magnification (scale bars: 1 mm in panel a; 0.1 mm in panel b). The other abbreviations refer to common abbreviations.
FIGURE 7.

Thin and fragmented capsular cartilage facing the orbit and maxilla in a foetus of 216‐mm CRL. H&E staining. Panel (a) (or panel b) displays a plane 4 mm (or 6 mm) anterior to Figure 6a. Panels (c–e) show higher magnification views of squares in panel (a). The nasal capsule cartilage is very thin and fragmented (arrowheads in panel a). The peripheral parts of the capsular cartilage (stars in panel a) remain in the inferior concha (ICO). The capsule (arrowheads) in panel (b) continues inferomedially to the inferior concha. Candidate calcified cartilage suggests endochondral ossification in the middle and inferior conchae (panels c and d). A cartilage bar with membranous ossification connects the palatine bone (PAL) and the inferior concha (panel e). Panels (c–e) were prepared at the same magnification (scale bars: 1 mm in panels a and b; 0.1 mm in d). The other abbreviations refer to common abbreviations.
FIGURE 8.
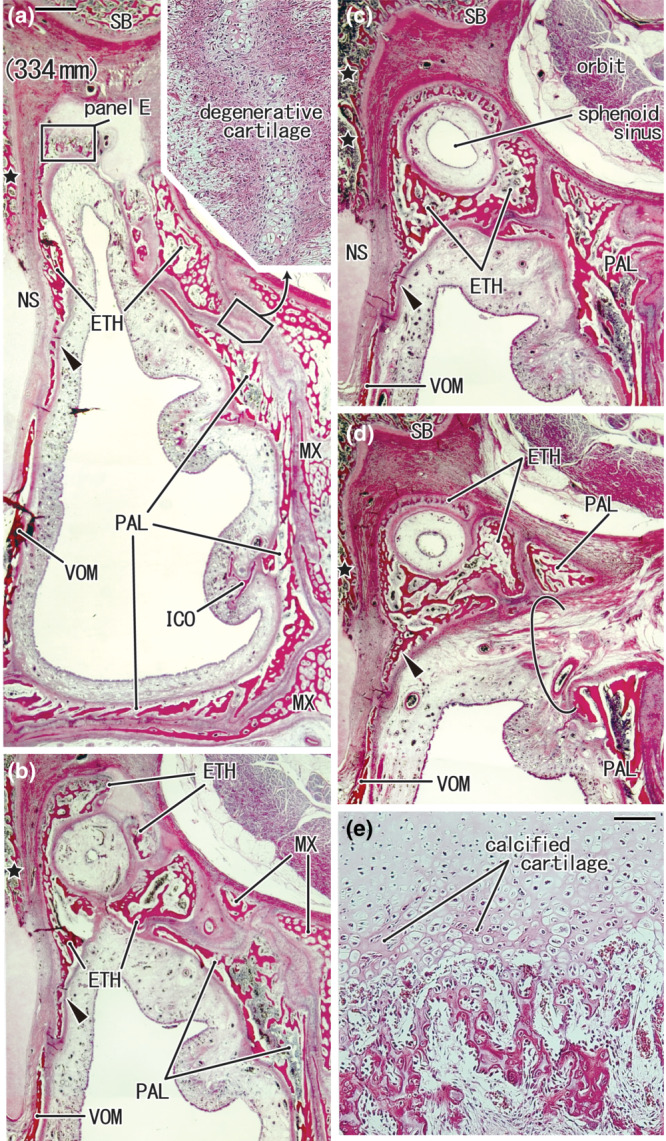
Endochondral ossification in the posterior part of the ethmoid in a foetus of 334 mm. H&E staining. Four frontal sections of the same specimen are shown in Figure 6d. Panel (a) (or d) displays the figure's most anterior (or posterior) plane. Panel (e) is a higher magnification view of a square in panel (a), showing a plane 1 mm posterior to Figure 6d, containing (1) degenerative capsular cartilage (inserted at the upper angle of panel a) and (2) endochondral ossification in the posterior end of the cribriform plate (panel e). The future concha sphenoidalis is a bulky bone mass around the sphenoid sinus (panels b–d). The perpendicular plate of the ethmoid, a membranous bone plate, is observed along the nasal septum cartilage (arrowheads in panels a–d). The sphenopalatine foramen (semicircle in panel d) appeared to pass through the palatine bone (PAL). Panels (a–d) were prepared at the same magnification (scale bars: 1 mm in panel a; 0.1 mm in panel e). The other abbreviations refer to common abbreviations.
FIGURE 9.

Anterolateral part of the nasal capsule between the nasal cavity and orbit in a foetus of 350 mm. H&E staining. Three frontal sections, including the nasolacrimal duct (NLD) and the lacrimal sac (LS). Panel (a) displays the most posterior plane, whereas panel (c) is the most anterior. Panels (d–g) are higher magnification views of the squares in panels (a–c), respectively. The nasal capsule cartilage was very thin and fragmented (arrowheads in panels d–g) near the membranous bones of the ethmoid (ETH), maxilla (MX) and lacrimal bone (LB). Ossification was complete in the middle and inferior conchae. The bony inferior concha (ICO) is connected to the maxilla. Panels (a–c) (or Panels d–g) were prepared at the same magnification (scale bars: 1 mm in panel a; 0.1 mm in panel d). The other abbreviations refer to common abbreviations.
FIGURE 10.

Horizontal sections from two near‐term foetuses show thin and fragmented capsular cartilage. H&E staining. Two horizontal sections from two foetuses of 262 mm (panels a–d) and 265 mm (panels e–h) CRL. Panels (a) and (e) show the topographical anatomy of the nasolacrimal duct (NLD) and lacrimal sac (LS), respectively. Panels (b–d) (or panels f–h) are higher magnification views of the three squares in panel a (or panel e). The lateral parts of the nasal capsule cartilage were thin and fragmented (arrowheads in panels a and e). In panels (c), (d) and (h), in combination with membranous ossification (arrows), candidate calcified cartilage suggests endochondral ossification in the middle and inferior conchae. Degenerative cartilage was also observed near the lacrimal bone (LB; panel g). Panels (b) and (f) show the inferolateral end of the nasal capsule inserted between the maxilla (MX) and the palatine bone (PAL). Scale bars: 1 mm in panels (a) and (e); 0.1 mm in the other panels. The other abbreviations refer to common abbreviations.
FIGURE 11.

Cribriform plate and nasal septum of the ethmoid in sagittal sections of a foetus of 328‐mm CRL. H&E staining. Two sagittal sections. Panel (d) is 8 mm medial to panel (a). Three squares in panel (a) are shown in panels (b), (c) and (e) at a higher magnification. The frontal bone develops along the anterosuperior process (crista galli or CG) of the nasal capsule in panel (a). Part of the capsular cartilage (star in panel a) is separated and involved in the frontal bone (future spina frontalis). Endochondral ossification in the cribriform plate (CFP) started from the inferior margin (Panel b). Another endochondral ossification is observed at the posterior end of the capsule (the future concha sphenoidalis; panel c) and at the base of the superior concha (panel e). In panel (d), the nasal septum cartilage extends posteriorly from the attachment to the frontal bone (FB) to the attachment to the vomer (VOM). Scale bars: 1 mm in panels (a) and (d); 0.1 mm in the other panels. The other abbreviations refer to common abbreviations.
The coexistence of advanced ossification, degeneration and fragmentation of the nasal capsule characterises the near‐term nasal capsule cartilage. Advanced ossification includes both membranous and endochondral ossification. The usual membranous ossification occurred along the cartilaginous nasal septum (the future perpendicular plate of the ethmoid; Figures 6d,g and 8a–d). In the three conchae (Figures 7c,d and 10c,d,h), calcified cartilages suggesting endochondral ossification appeared along with sheet‐like membranous bones, which was similar to the growing diaphysis of long bones of the extremities. In contrast, endochondral ossification was strongly suggested in the cribriform plate (Figures 6e and 11b), three conchae (Figures 7c,d, 10c,d,h and 11e), and future concha sphenoidalis (Figures 8b–d and 11c). However, there was no linear border between the calcified and non‐calcified zones (i.e. a tidemark), and the calcified cartilages were sparsely distributed.
Notably, in abundant parts of the lateral nasal wall, membranous ossification coexisted with degenerative and/or fragmented capsular cartilage: the future orbital lamina of the ethmoid or along the upper half of the nasal face of the maxilla (Figures 6b,f, and 9d–g) and near the lacrimal bone (Figures 9g and 10g). Degenerative cartilage was also seen near the cribriform plate (Figure 6e), along the nasal septum near the sphenoid concha (Figure 6g) and near the lacrimal bone (Figures 9g and 10g). The degenerative cartilage, mostly or partly, lost the perichondrium and contained large round cells. Thinning, fragmented or degenerative cartilage appeared to be replaced by growing membranous bones; this was different from the usual ones developing along the capsular cartilage. In the latter, the membranous bone uses the nasal capsule as a ‘mould’. Although it became relatively thin, the inferolateral end of the capsular cartilage remained sandwiched between the maxilla and the palatine bone (Figure 10b,f) and was often degenerative (Figure 6c,f).
The anterior margin of the cartilaginous nasal septum continued inferiorly to the greater alar cartilage and laterally to the lateral nasal cartilage (Figure 12b,c). The border between the alar and septal cartilages became wavy by the near term (Figure 12d). The lesser alar cartilage was isolated from the septum and connected by a mesenchymal band at mid‐term (Figure 12a,b,d). However, the mesenchymal band was not observed in the near term. Strangely, the upper end of the frontal process of the maxilla (Figure 12d inset) and nasal bone (Figures 5b and 12c inset) occasionally contained a small cartilage mass, whereas the latter cartilage was a process of the capsular cartilage. Even at 40 weeks, there were still three major cartilages without ossification: (1) a large mass of the cribriform plate, including a long crista galli (Figures 9b and 11a); (2) the entire part of the cartilaginous nasal septum (Figures 6d and 11d) and (3) the inferolateral end of the capsule inserting between the well‐developed palatine bone and the maxilla (Figure 10b,f). Therefore, at birth, the nasal septum is likely composed of a pair of bilateral membranous bones (perpendicular plates of the ethmoid; Figures 6d and 8a) sandwiching a thick median part of the nasal capsule cartilage.
Figure 13 shows a fate map of the nasal capsule showing site‐dependent ossification: (1) the usual membranous ossification along the capsular cartilage (yellow coloured), (2) a specific membranous ossification during and after degeneration of the capsule (green coloured) and (3) endochondral ossification without a linear border (red coloured) between the calcified and non‐calcified cartilages. Finally, at the near term, the bony inferior concha was connected to both the palatine bone (Figures 4g, 6a,d, 7a,e and 8a) and the maxilla (Figure 9b,c); the second ossification type appeared to occur in the connecting part.
FIGURE 13.

A fate map of the nasal capsule cartilage: the results and hypothesis. Panels (a) (anterior), (b) (midportion) and (c) (posterior) display schematic frontal views of the nose. Panel (d) shows a sagittal view of the anterior nose. Panel (e) shows the middle concha as a representative mosaic ossification pattern. The nasal wall is composed of three parts: (1) endochondral bones from the nasal capsule (red coloured), (2) membranous bones developing along the nasal capsule (yellow coloured) and (3) membranous bones developing during and after degeneration of the capsular cartilage (green coloured). Blue indicates parts of the nasal capsule that disappear postnatally: the inferolateral part of the capsule (arrowhead in panels a, b and e) and a major part of the nasal septum. Endochondral ossification is seen in the three conchae, especially at the base (black triangles in panels a, b and e), in the entire cribriform plate (CFP), including the anterior continuation extending over the frontal bone (FB; panels a, b and d), and in the future concha sphenoidalis surrounding the sphenoid sinus (SS; panel c). The definite septal (panel a), lateral nasal and greater alar (LNC, GAC; panels a and d) cartilages are remnants of the nasal capsule. The star in panels b and e indicates a bony bar connecting the palatine bone (PAL) and inferior concha (ICO), which is built in the third manner of ossification. Postnatally, a part of the nasal capsule in the conchae (open triangles in panel e) may disappear without bone replacement. The frontal and lacrimal bones involved parts of the nasal capsule (panels a and d). The other abbreviations are common abbreviations for the figures.
4. DISCUSSION
To make a specific three‐dimensional shape, membranous bones need guidance or moulding, such examples are seen in the squamosal bones of the skull around the growing brain (Hayashi et al., 2014) and the ala major and pterygoid of the sphenoid along the growing muscle attachments (Yamamoto et al., 2021). The nasal capsule cartilage provides a mould of the future nasal wall in two ways: (1) endochondral ossification in several parts of the capsular cartilage and (2) membranous ossification along the capsular cartilage (Figure 13). In the ethmoid bone, the first pattern was seen in the cribriform plate, concha sphenoidalis and base of the superior and middle conchae. In contrast, the second pattern was seen in the perpendicular plate. The second pattern has been suggested to be referred to as ‘perichondral ossification’, which is different from membranous ossification (Hayashi et al., 2014; Yamamoto et al., 2021). However, for instance, osteoblast candidates were likely to arrange in line along both the orbital and the nasal aspects of the developing bony plate in the nasal face of the maxilla. Thus, at the beginning of this study, we defined a bone mass entirely surrounded by osteocytes as a membranous bone.
The most striking observation in the present study was the third type of ossification, a specific membranous ossification starting near‐term during and/or after capsular cartilage degeneration. The third pattern was seen in the wide anterolateral wall of the nasal cavity, that is the orbital lamina of the ethmoid and the nasal face of the maxilla, potentially similar to the ossification in the long bony diaphysis of the extremities, that is the periosteal bone (Egawa et al., 2014). The periosteal bone should accompany osteoblasts along the ‘outer’ aspect adjacent to the periosteum. However, osteoblast candidates were likely to ‘surround’ the bone in this specific membranous ossification. The perichondrium and periosteum play a significant role in the ossification of the mandibular condyle (Shibata et al., 2013, 2014) and flat squamosal bones of the skull (Hayashi et al., 2014). Likewise, the maxilla might enlarge along the persistent perichondrium after the disappearance of the nasal capsule to provide a large nasal face of the maxilla (blue colour in Figure 13a,b). Therefore, the third method does not contradict the growth pattern of the maxilla as determined by the nasal capsule. The first description of islands of degenerating cartilages in the growing nasal wall was made by Schaeffer (1920). Smith et al. (2012) later used foetal specimens of nonhuman primates and histochemistry to describe two types of cartilage degeneration in the primates' nasal wall: (1) loss of perichondrium and cessation of matrix production and (2) active absorption of matrix by chondroclasts. Their study provided clear images showing evidence of cartilage degeneration in the nasal wall. The present study observed loss of perichondrium (Figures 6e,f and 9e,f) and abnormally large chondrocytes (Figure 6).
An individual variation in shape is known to occur in the lateral nasal wall in adults, especially at and around the uncinate process (Isobe et al., 1998; Sato et al., 2000). The three conchae were built by a mosaic of endochondral and membranous bones (Figure 13e), and the latter showed a specific type (see the paragraph above) in contrast to the usual ossification along capsular cartilage. Moreover, we were not able to deny the possibility of the disappearance of peripheral parts of the capsular cartilage after birth without replacement by bones in the middle and inferior conchae (triangles in Figure 13b,e). Therefore, limited guidance by the nasal capsule in ossification seemed to provide variations at and around the uncinate process. In adults, the inferior concha is an independent bone attached to the ethmoid, lacrimal, maxilla and palatine bones. However, the inferior concha is a simple inferomedial continuation of the nasal capsule in the foetuses. Moreover, in the near term, the attachment to the palatine bone seemed to be the largest; this difference might also be a disturbing factor against morphological stability since, in postnatal growth, the inferior concha needs to obtain the largest attachment to the maxilla. Similarly, growth of the perpendicular plate of the palatine bone, a large membranous bone in adults, seemed to require degeneration of the inferolateral end of the nasal capsule. Even at 40 weeks, the capsular end remained sandwiched between the palatine bone and maxilla. A suture between the maxilla and palatine bone may show great variation in shape and position in adults.
The bony nasal septum in adults is described to undergo endochondral ossification of the median septal part of the nasal capsule (Belden et al., 1997; Smith, Ufelle, et al., 2021). A striking thick cartilage of the nasal septum was located the near term and was sandwiched by a pair of membranous bones (the vomer and ethmoid). The most posterior part might not increase in thickness and, with postnatal endochondral ossification, would become involved in concha sphenoidalis. According to a magnetic resonance imaging study by Kim et al. (2008), the middle part of the nasal septum cartilage was sandwiched by a pair of membranous bones in children (bony perpendicular plates of the ethmoid). The posterior end of the nasal capsule (lamina transversalis by van Gilse, 1927) surrounded the future sphenoid sinus and was attached to a sphenoid‐derived nasal septum, which was accompanied by a cartilage bar (cartilage cupularis by van Gilse 1927) that was separated from the capsule itself (Figure 2e). However, the cartilage cupularis was soon involved at the base of the bony nasal septum of the ethmoid: this was different from the primates' nasal capsule in which, at the caudal end, a thick protrusion of the septal cartilage initially surrounded the olfactory recess (Smith, Reynolds, et al., 2021). An analogy of the delayed involvement of nearby cartilages into the septum in humans was found in the anterior nose: the greater alar cartilage was likely to be separated from the early nasal capsule; however, it later became fused within the cartilaginous nasal septum (Figures 2a and 3c,d). We considered these marginal cartilages to be part of the nasal capsule. It is important to note that there is significant individual variation in the topographical relationship between the cartilage cupularis and other surrounding structures such as the medial pterygoid, vomer and/or palatine bone (Yamamoto et al., 2021).
Like the thick nasal septum, the anterosuperior part of the capsular cartilage appeared longer and wider than was the corresponding adult structure (i.e. the future crista galli): the capsular cartilage rose over the frontal bone. This was similar to the future ala minor of the sphenoid riding over the near‐term frontal bone, possibly to ensure postnatal growth (Yamamoto et al., 2021). The same phenomenon can be seen in the future transverse portion of the bony palate, with the palatine bone riding over the maxilla. Moreover, parts of the nasal capsule are likely involved in the frontal bone, contributing to the formation of the spina nasalis (Figure 13d). A small cartilage mass in the upper end of the frontal process of the maxilla as well as in the nasal bone also seemed to be new findings, but they might not contribute to membranous ossification. These small masses of cartilage might correspond to a morphology that would postnatally occur the perichondral ossification like the ilium superomedial end (see the first subsection of Section 3). Sandikcioglu et al. (1994) reported details of ossification centres of the nasal bone, but they did not describe the cartilage process of the nasal capsule.
Overall, endochondral and usual membranous ossification occurred at several sites in and along the nasal capsule. Therefore, the capsular cartilage itself acts as a mould of adult morphology. In contrast, in the wide anterolateral wall of the nose, including the conchae, another type of membranous ossification occurs during or after the disappearance of cartilage. Remnant perichondrium may induce ossification even after cartilage loss. With a range of redundancy (i.e. providing a definite mould or not), the nasal capsule seemed to determine the topographical relationship among bones surrounding the nasal cavity.
4.1. Study limitations
Histological sections from the 12 near‐term foetuses were semi‐serial with 0.1–0.2‐mm intervals. Therefore, it was difficult to identify the part of nasal capsule cartilage that had disappeared without ossification (triangles in Figure 13e). Likewise, we could not rule out the possibility that the second manner of ossification (see the first paragraph of Section 4) created a short bony bar in the conchae (arrows in Figure 13e). Because all near‐term sections were limited to those stained with H&E, we described calcified cartilage as the candidate (calcified cartilage with a ‘question mark’).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENTS
This study was supported by a Grant‐in‐Aid for Scientific Research (no. 20K09895: Shinichi Abe, no. 20K10191: Masahito Yamamoto) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. We sincerely thank the staff of the Department of Anatomy, Tokyo Dental College, for their wholehearted cooperation.
Yamamoto, M. , Hayashi, S. , Honkura, Y. , Hirano‐Kawamoto, A. , Katori, Y. , Murakami, G. et al. (2023) Nasal capsule ossification: A histological study using human foetuses to find an association between the foetus and adult morphologies of the nasal wall. Journal of Anatomy, 243, 517–533. Available from: 10.1111/joa.13867
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- Belden, C. J. , Mancuso, A. A. , & Kotzur, I. M. (1997). The developing anterior skull base: CT appearance from birth to 2 years of age. American Journal of Neuroradiology, 18(5), 811–818. [PMC free article] [PubMed] [Google Scholar]
- Benouaiche, L. , Gitton, Y. , Vincent, C. , Couly, Ǵ. & Levi, G. (2008) Sonic hedgehog Signalling from foregut endoderm patterns the avian nasal capsule. Development, 135(13), 2221–2225. Available from: 10.1242/dev.020123 [DOI] [PubMed] [Google Scholar]
- Couly, G. , Creuzet, S. , Bennaceur, S. , Vincent, C. & Le Douarin, N.M. (2002) Interactions between Hox‐negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development, 129(4), 1061–1073. Available from: 10.1242/dev.129.4.1061 [DOI] [PubMed] [Google Scholar]
- Dankmeijer, J. (1968) Some remarks on the development and anatomy of the septum nasi. International Rhinology, 6, 27–33. [Google Scholar]
- Egawa, S. , Miura, S. , Yokoyama, H. , Endo, T. & Tamura, K. (2014) Growth and differentiation of a long bone in limb development, repair and regeneration. Development, Growth & Differentiation, 56(5), 410–424. Available from: 10.1111/dgd.12136 [DOI] [PubMed] [Google Scholar]
- Gitton, Y. , Benouaiche, L. , Vincent, C. , Heude, E. , Soulika, M. , Bouhali, K. et al. (2011) Dlx5 and Dlx6 expression in the anterior neural fold is essential for patterning the dorsal nasal capsule. Development, 138(5), 897–903. Available from: 10.1242/dev.057505 [DOI] [PubMed] [Google Scholar]
- Hayashi, S. , Kim, J.H. , Hwang, S.E. , Shibata, S. , Fujimiya, M. , Murakami, G. et al. (2014) Interface between intramembranous and endochondral ossification in human Foetuses. Folia Morphologica, 73(2), 199–205. Available from: 10.5603/FM.2014.0029 [DOI] [PubMed] [Google Scholar]
- Isobe, M. , Murakami, G. & Kataura, A. (1998) Variations of the Uncinate process of the lateral Nasal Wall with clinical implications. Clinical Anatomy, 11(5), 295–303. Available from: [DOI] [PubMed] [Google Scholar]
- Kaucka, M. , Petersen, J. , Tesarova, M. , Szarowska, B. , Kastriti, M.E. , Xie, M. et al. (2018) Signals from the brain and olfactory epithelium control shaping of the mammalian nasal capsule cartilage. eLife, 7, e34465. Available from: 10.7554/elife.34465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, I.S. , Lee, M.Y. , Lee, K.I. , Kim, H.Y. & Chung, Y.J. (2008) Analysis of the development of the nasal septum according to age and gender using MRI. Clinical and Experimental Otorhinolaryngology, 1(1), 29–34. Available from: 10.3342/ceo.2008.1.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.H. , Jin, Z.W. , Hayashi, S. , Murakami, G. , Abe, H. & Rodríguez‐Vázquez, J.F. (2023) Development and growth of the human fetal sacroiliac joint revisited: a comparison with the temporomandibular joint. Anatomy and Cell Biology. Available from: 10.5115/acb.22.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.H. , Sugai, N. , Suzuki, D. , Murakami, G. , Abe, H. , Rodríguez‐Vázquez, J.F. et al. (2022) Paratenon of the cruciate ligaments of the knee: a macroscopic and histological study of human Foetuses. Folia Morphologica, 81(1), 134–143. Available from: 10.5603/FM.a2021.0003 [DOI] [PubMed] [Google Scholar]
- Kuratani, S. & Ahlberg, P.E. (2018) Evolution of the vertebrate neurocranium: problems of the premandibular domain and the origin of the trabecula. Zoological Letters, 4, 1. Available from: 10.1186/s40851-017-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Jin, Y. , Li, J. , Seto, E. , Kuo, E. , Yu, W. et al. (2013) Inactivation of Cdc42 in neural crest cells causes craniofacial and cardiovascular morphogenesis defects. Developmental Biology, 383(2), 239–252. Available from: 10.1016/j.ydbio.2013.09.013 [DOI] [PubMed] [Google Scholar]
- Naito, T. , Cho, K.H. , Yamamoto, M. , Hirouchi, H. , Murakami, G. , Hayashi, S. et al. (2019) Examination of the topographical anatomy and fetal development of the tendinous annulus of Zinn for a common origin of the extraocular recti. Investigative Ophthalmology & Visual Science, 60(14), 4564–4573. Available from: 10.1167/iovs.19-28094 [DOI] [PubMed] [Google Scholar]
- Noden, D.M. (1978) The control of avian cephalic neural crest Cytodifferentiation. I. Skeletal and connective tissues. Developmental Biology, 67(2), 296–312. Available from: 10.1016/0012-1606(78)90201-4 [DOI] [PubMed] [Google Scholar]
- Pasqualetti, M. & Rijli, F.M. (2002) Developmental biology: the plastic face: developmental biology. Nature, 416(6880), 493–494. Available from: 10.1038/416493a [DOI] [PubMed] [Google Scholar]
- Sandikcioglu, M. , Mølsted, K. & Kjaer, I. (1994) The prenatal development of the human nasal and vomeral bones. Journal of Craniofacial Genetics and Developmental Biology, 14(2), 124–134. [PubMed] [Google Scholar]
- Sato, T.J. , Murakami, G. , Tsubota, H. , Isobe, M. , Akita, K. & Kataura, A. (2000) Morphometric study of the medial aspect of the human maxillary sinus with special reference to the nasal Fontanelle. Auris Nasus Larynx, 27(2), 121–130. Available from: 10.1016/s0385-8146(99)00056-5 [DOI] [PubMed] [Google Scholar]
- Schaeffer, J.P. (1920) The nose, paranasal sinuses, nasolacrimal passageways, and olfactory organ in man. Philadelphia: P. Blakiston's Son & Co. [Google Scholar]
- Shibata, S. , Sakamoto, Y. , Yokohama‐Tamaki, T. , Murakami, G. & Cho, B.H. (2014) Distribution of matrix proteins in perichondrium and periosteum during the incorporation of Meckel's cartilage into ossifying mandible in midterm human fetuses: an immunohistochemical study: perichondrium and periosteum in human fetus. The Anatomical Record (Hoboken), 297(7), 1208–1217. Available from: 10.1002/ar.22911 [DOI] [PubMed] [Google Scholar]
- Shibata, S. , Sato, R. , Murakami, G. , Fukuoka, H. & Francisco Rodríguez‐Vázquez, J. (2013) Origin of mandibular condylar cartilage in mice, rats, and humans: periosteum or separate blastema? Journal of Oral Biosciences, 55(4), 208–216. Available from: 10.1016/j.job.2013.08.001 [DOI] [Google Scholar]
- Slabý, O. (1960) Die Frühe Morphogenesis Der Nasenkapsel Beim Menschen. Cells, Tissues, Organs, 42(1–2), 105–175. Available from: 10.1159/000141638 [DOI] [Google Scholar]
- Smith, T.D. , Reynolds, R.L. , Mano, N. , Wood, B.J. , Oladipupo, L. , Hughes, G.K. et al. (2021) Cranial synchondroses of primates at birth. The Anatomical Record (Hoboken), 304(5), 1020–1053. Available from: 10.1002/ar.24521 [DOI] [PubMed] [Google Scholar]
- Smith, T.D. , Rossie, J.B. , Cooper, G.M. , Durham, E.L. , Schmeig, R.M. , Docherty, B.A. et al. (2012) Microanatomical variation of the nasal capsular cartilage in newborn primates. The Anatomical Record (Hoboken), 295(6), 950–960. Available from: 10.1002/ar.22448 [DOI] [PubMed] [Google Scholar]
- Smith, T.D. , Ufelle, A.C. , Cray, J.J. , Rehorek, S.B. & DeLeon, V.B. (2021) Inward collapse of the nasal cavity: perinatal consolidation of the midface and cranial base in primates. The Anatomical Record (Hoboken), 304(5), 939–957. Available from: 10.1002/ar.24537 [DOI] [PubMed] [Google Scholar]
- Standling, S. (2005) Gray's anatomy, 39th edition, p. 493, 610–611. London: Elsevier Churchill Livingstone. [Google Scholar]
- Swartz, M.E. , Nguyen, V. , McCarthy, N.Q. & Eberhart, J.K. (2012) Hh signaling regulates patterning and morphogenesis of the pharyngeal arch‐derived skeleton. Developmental Biology, 369(1), 65–75. Available from: 10.1016/j.ydbio.2012.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W. & Bronner, M.E. (2020) Neural crest lineage analysis: from past to future trajectory. Development, 147(20), dev193193. Available from: 10.1242/dev.193193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gilse, P.H. (1927) The development of the sphenoidal sinus in man and its homology in mammals. Journal of Anatomy, 61(Pt 2), 153–166. [PMC free article] [PubMed] [Google Scholar]
- Vidić, B. (1971) The morphogenesis of the lateral Nasal Wall in the early prenatal life of man. The American Journal of Anatomy, 130(2), 121–139. Available from: 10.1002/aja.1001300202 [DOI] [PubMed] [Google Scholar]
- Yamamoto, M. , Jin, Z.‐W. , Hayashi, S. , Rodríguez‐Vázquez, J.F. , Murakami, G. & Abe, S. (2021) Association between the developing sphenoid and adult morphology: a study using sagittal sections of the skull base from human embryos and fetuses. Journal of Anatomy, 239(6), 1300–1317. Available from: 10.1111/joa.13515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Kilgallen, S. , Andreeva, V. , Spicer, D.B. , Pinz, I. & Friesel, R. (2010) Conditional expression of Spry1 in neural crest causes craniofacial and cardiac defects. BMC Developmental Biology, 10(1), 48. Available from: 10.1186/1471-213X-10-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


