Abstract
Sepsis is a life-threatening condition characterized by a dysregulated host response to infection. Early and accurate diagnosis of sepsis is crucial for timely intervention and improved patient outcomes. In recent years, there has been growing interest in identifying reliable biomarkers to aid in the diagnosis of sepsis. This study aims to evaluate the levels of two potential biomarkers, high-mobility group box 1 (HMGB1) and human β-defensin 3 (HBD-3), and compare their diagnostic efficacy in sepsis.
We aimed to assess HMGB-1 and HBD-3 levels in sepsis and assess the combined diagnostic validity of HMGB-1 and HBD-3. In this case-control study, the plasma concentration of HMGB-1 and HBD-3 was measured using an enzyme-linked immunosorbent assay (ELISA). Two groups, totaling 144 people, were formed; 66 patients treated in the ICU for sepsis were included in the patient group. 78 Blood donors from the Salmaniya Medical Complex Blood Bank who had no prior infection or inflammatory disease made up the Control group. The statistical computations were performed using the STATA 8® statistical software tool (StataCorp LP, College Station, TX, USA).
In patients' mean HMGB-1 levels were 2.1442 ng/ml, compared to 0.62141 ng/ml in the control group. The mean HBD-3 level was 1068.453 ng/ml in sepsis patients versus 589.935 ng/ml in controls. A significant difference between the two groups has been observed in both biomarkers (P < 0.05). The sensitivity of HMGB-1 was 75.8% and 41.3%, respectively. The sensitivity and specificity of HBD-3 were 63.6% and 93.5%, respectively.
The levels of HMGB-1 and HBD-3 between healthy and septic subjects varied significantly. HMGB-1 and HBD-3 levels in the blood tested together might accurately identify sepsis. These findings contribute to the growing body of evidence supporting the utility of biomarkers in sepsis diagnosis, and may ultimately aid in the development of more effective diagnostic strategies for sepsis management.
Keywords: Sepsis, Infection, Inflammation, Diagnosis, HMGB1, HBD3
Highlights
-
•
The present study is an attempt to assess the combined diagnostic value of HMBG1 and HBD3 and to assess the levels of HMGB1 and HBD3 in patients with acute sepsis.
-
•
There are significant differences in the levels of both HMGB1 and HBD3 between healthy and septic participants. The levels of markers were higher in individuals with sepsis. HMGB1 and HBD3 are pro-inflammatory mediators that can provide clues for the diagnosis of sepsis.\.
-
•
Patients had significantly lower cervicovaginal HBD3, compared to the healthy participants. The serum concentrations of HBD3 also increased in the acute phase of bacterial pneumonia.
-
•
The presence of HBD3 in the airways and its strong bactericidal activity suggest its role in innate mucosal defense within the respiratory tract.
1. Introduction
A state of physiological, pathological, and metabolic abnormalities brought on by infection is known as sepsis. Hippocrates coined the term “sepsis” in the fourth century B.C., which was once referred to as the disintegration or decomposition of organic materials [1,2]. A dysregulated host response to infection causes sepsis and results in a life-threatening organ failure [3]. The devastating effect of sepsis results from infection and the excessive release of inflammatory mediators from the host which is also called a ‘cytokine storm’ [4,5].
Sepsis is the most severe consequence of a systemic inflammatory response to microbial infection. Using sepsis as a model, interactions between infection and the body's inflammatory response have also been examined [6]. Sepsis can cause Multiple Organ Dysfunction Syndrome (MODS), leading to death in patients admitted to the ICU [7].
Sepsis is a catastrophe for public health and a primary contributor to the global burden of disease worldwide [8]. According to studies, there are 19–48.9 million cases of sepsis worldwide each year, and it remains the leading cause of mortality worldwide, with at least five million deaths [9,10]. Multi-organ damage due to severe sepsis is also the third leading cause of death in the USA [11].
Early diagnosis and treatment are the most efficient means of preventing mortality and enhancing patient outcomes [12]. However, the complexities that result from sepsis symptoms are questionable and occasionally non-specific. Recently, several biomarkers have been identified as candidates for the early identification of sepsis, including Procalcitonin (PCT), C-Reactive Protein (CRP), Tumor Necrosis Factor- α (TNF-α), Interleukin 6 (IL-6), Monocyte Chemoattractant Protein-1 (MCP-1), Programmed Death Receptor-1, and Programmed Death Ligand-1, Presepsin (sCD14-ST), Matrix Metalloproteinases (MMPs), etc. [13]. Among these newly recognized markers, High Mobility Group Box-1(HMGB1) and Human Beta Defensin 3 (HBD3) are two important biomarkers considered for diagnostic use in sepsis.
HMGB1 is a DNA-binding protein involved in gene transcription and nucleosome maintenance. One of the most significant chromatin proteins is HMGB1. HMGB1 interacts with histones, transcription factors, and nucleosomes in the nucleus. This nuclear protein controls transcription and arranges the DNA. Following binding, HMGB1 bends DNA, making it easier for other proteins to bind. In its interactions with numerous transcription factors, HMGB1 supports the transcription of numerous genes. Additionally, it works with nucleosomes to rearrange the chromatin and release tightly packed DNA. Nucleosome structure is altered by interaction with core histones. Posttranslational changes are necessary for HMGB1 to stay in the nucleus. The protein stays in the nucleus when it is not acetylated, but it moves into the cytoplasm when lysine residues are hyperacetylated.
HMGB1 can be phosphorylated, acetylated, and methylated. Necrosis, pyroptosis, and apoptosis passively release HMGB1 [14,15]. It serves as a pro-inflammatory cytokine mediator within and outside the cells in sepsis. Clinical and preclinical studies suggest HMGB1 modulates sepsis pathogenesis and mortality [11,16].
On the other hand, HBD3 is a potent antimicrobial peptide released by epithelial tissues and leukocytes in the innate immune system [17]. HBD3 has significant bactericidal activity. It helps with chemoattraction and adaptive immunity [18]. Studies have shown that the levels of HBD3 levels increase during the acute phase of bacterial infection [19]. Because of its broad-spectrum antibiotic action, HBD3 is believed to be one of the most potent antimicrobial peptides [20].
Previous studies that have evaluated the association of serum levels of either HMBG1 or HBD3 as markers in sepsis, none of them have studied the combined effect of both these markers. The present study is an attempt to assess the combined diagnostic value of HMBG1 and HBD3 and to assess the levels of HMGB1 and HBD3 in patients with acute sepsis.
2. Methods and materials
2.1. Study design
This case-control study was conducted in Salmaniya Medical Complex in the Kingdom of Bahrain from February 2018 to September 2019. A total of 144 participants were divided into two groups. The patient group included 66 patients diagnosed with sepsis and treated in the ICU. The Control group comprised 78 people from the Salmaniya Medical Complex Blood Bank in Bahrain, who had no history of infections or inflammatory illness.
2.2. Participant selection
The diagnosis of sepsis was performed based on organ dysfunction, which can be identified as an acute change in the total Sequential Organ Failure Assessment (SOFA) score. The value of more than two points is a consequence of the infection. Sepsis, severe sepsis, and septic shock were defined according to the criteria proposed by the American College of Chest Physicians/Society of Critical Care Medicine. Septic shock was defined as severe sepsis in addition to hypotension requiring vasopressor support, or mean arterial pressure less than 70 mm Hg for more than 30 min despite adequate fluid resuscitation. For patients with no infection or those who were not known to have pre-existing organ dysfunction, the baseline SOFA score was considered zero.
All participants were explicitly informed about the nature of the study. All patients and control participants gave their informed consent under a protocol authorized by the Research and Ethics Committee of the College of Medicine and Medical Sciences, Arabian Gulf University, Bahrain. No other chronic illnesses were reported in the participants selected and no one was immunocompromised. Individual clinical data were registered for the patients. The severity of the disease was measured by Acute Physiology and Chronic Health Evaluation (APACHE) II score at admittance and also by daily Sepsis-related Organ Failure Assessment (SOFA) scores [21,22]. Final diagnoses were determined retrospectively, based on complete patient charts and laboratory tests. The SOFA score evaluates day-by-day morbidity in critically ill patients. Respiration, coagulation, liver, cardiovascular, central nervous system, and renal dysfunction were all evaluated daily and graded accordingly.
2.3. Blood sampling
Venepuncture was used to obtain the peripheral whole blood samples from participants and was collected in ethylene diamine tetra acetic acid (EDTA) tubes. Blood samples were collected on the day of diagnosis for individuals with sepsis syndrome before using any type of available therapy. Blood was immediately collected upon arrival and confirmation of sepsis. Within 30 min of blood collection, plasma was separated by centrifuging for 15 min at 1000×g (temperature was maintained between 2 and 8 °C. The plasma sample was aliquoted and kept at −80 °C until use.
2.4. Laboratory assays
The levels of HMGB1 were determined by an Enzyme-linked Immunosorbent Assay (ELISA) kit assay according to the manufacturer's instructions (Shino-test Corporation, Kanagawa, Japan). Blood samples were taken from patients, and after allowing the blood to clot, the serum was extracted using a low-speed centrifuge at 4 °C and stored at −80 °C until the ELISA test was performed. The inter-assay and intra-assay coefficients were both about <10%. HMGB1 had a detection limit of 0.6 ng/ml. HBD3 concentrations were determined by ELISA (Pepro Tech, Rock Hill, NJ). For the HBD-3 assay, the sensitivity was 62 pg/ml, and a standard curve was constructed from 62.5 to 4000 pg/ml. The HBD-3 final concentration was expressed per microgram of the total protein concentration of each sample.
2.5. Statistical analysis
Medians and interquartile ranges (IQRs) were used to present data and means ± standard deviations. A p-value of less than 0.05 was considered statistically significant. The area under the curve (AUC) and receiver operator characteristic (ROC) curves were calculated for protein markers. For the AUC, 95% confidence intervals (CIs) were reported. For the AUC and ROC curve comparison, the method described by DeLong and colleagues was used as the significance test [21]. The detection limit of the HMGB1 ELISA was 0.6 ng/ml. HMGB1 levels below 0.6 ng/ml were therefore assigned a value of 0.6 ng/ml for calculations. STATA 8® statistical software package (StataCorp LP, College Station, TX, USA) was used to perform the statistical calculations.
3. Results
There were 66 patients in the sepsis group and 78 in the healthy group. The mean HMGB-1 level in individuals with sepsis was 2.1442 ng/ml; whereas in healthy individuals, it was 0.62141 ng/ml. The Independent student's t-test showed a significant difference (P < 0.0001). The mean HBD-3 level in individuals with sepsis was 1068.453 ng/ml; whereas in healthy individuals, it was 589.935 ng/ml. The difference between both groups is significant (P < 0.05). Table 1.
Table 1.
The HMGB1 and HBD-3 levels in the participants.
| Group | N | HMGB-1 Mean (ng/ml) | P-Value | HBD-3 Mean (ng/ml) |
P-Value |
|---|---|---|---|---|---|
| Sepsis | 66 | 2.1442 | 0.0001 | 1068.453 | 0.0343 |
| Healthy | 78 | 0.62141 | 589.935 |
Fig. 1 shows the correlation between HMGB1 and HBD3 levels in all participants. No statistically significant correlation was found which could be indicated by a low R square value of 0.001. Pearson's correlation coefficient is 0.035, and the p-value is 0.781.
Fig. 1.
Correlation between HMGB1 and HBD 3 concentration in all participants.
The correlation between HMGB 1 and HBD 3 in septic and healthy individuals are represented separately in Fig. 2, Fig. 3 respectively.
Fig. 2.
Correlation between HMGB1 and HBD 3 concentration in Sepsis patients.
Fig. 3.
Correlation between HMGB1 and HBD-3 concentration in Healthy participants.
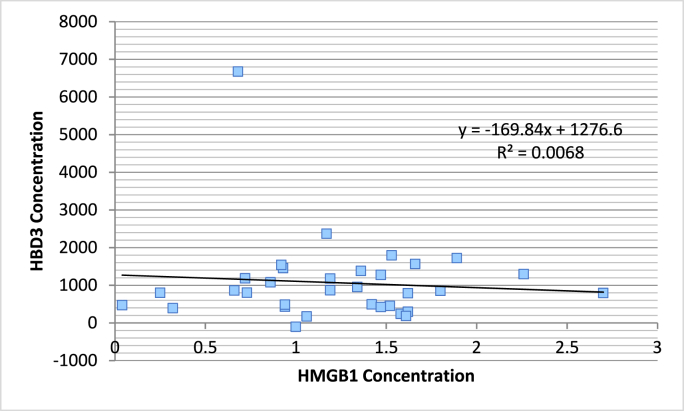
In the case of septic patients, no statistically significant correlation was found, which could be indicated by a low R square value of 0.006. Pearson's correlation coefficient is 0.082, and the p-value is 0.651.
In the case of healthy patients, a statistically significant correlation was also absent, which could be indicated by a low R square value of 0.002 between HMGB1 and HBD3 concentrations, as indicated in Fig. 3. Here, Pearson's correlation coefficient is 0.046, and the p-value is 0.808.
It is evident that despite a significant difference in the level of HMGB1 and HBD3 in septic and healthy patients, there is no significant correlation in both parameters.
The sensitivity and specificity were analyzed, and the cut-off values were chosen after balancing for both sensitivity and specificity. For HMGB1, The area under the curve is 0.684 as indicated in Fig. 4. It implies that there is a 68% chance that HMGB1 levels can help distinguish between positive and negative cases. Positive cases represent the patients diagnosed with sepsis and negative cases are those who don't have sepsis due to infection. HMGB1 concentration of 0.0145 is seen to have a sensitivity of 75.8% and a specificity of 41.3%.
Fig. 4.
Receptor Operating Characteristic Curve (ROC) curve for HMGB 1 levels.
However, in Fig. 5 we can see that for HBD3 the area under the curve is 0.679. It implies that there is a 68% chance that HBD3 levels can help distinguish between positive and negative cases. HBD3 concentration of 788.27 ng/ml is seen to have a sensitivity of 63.6% and a specificity of 93.5%.
Fig. 5.
Receptor Operating Characteristic Curve (ROC) curve for HBD-3 levels.
4. Discussion
There is a significant difference in levels of HMGB1 and HBD3 in individuals with sepsis and those without any infection. A study in accordance with the findings of our study reported that the HMGB1 level was significantly higher in patients with melioidosis who died as compared to those who survived. The median range of HMGB 1 levels in patients who died due to infection was 14.8 ng/ml as compared to 9.2 ng/ml in patients who survived [22]. Our study does not report the outcome of positive cases, but the above study has concluded that HMGB1 levels were higher in patients who died due to sepsis. There was a progressive but statistically nonsignificant decline in HMGB1 concentration among the survivors, while non-survivors showed an increase in HMGB1 level [14]. Similarly, another study discovered elevated HMGB1 blood levels during sepsis, however, they were unable to detect a link between HMGB1 concentration and infection severity or other cytokines [23].
There are many studies conducted on the effect of HMGB1 levels in patients with sepsis [21]. However, the literature on the HBD3 levels in sepsis patients is minimal. We are unable to find any studies that studied the combined effect of HMGB1 and HBD3 levels in patients with acute sepsis. Previously there were some reports presented, but they are on different types of infection and reported on different sites of infection [24]. The studies also showed a marked difference in results based on the time of analysis. However, the results were altered after day three [25]. As an intracellular protein, HMGB1 takes part in DNA replication, transcription, and repair mechanisms [26]. As an extracellular mediator, it is essential for triggering inflammatory process cascades as well as a variety of other extracellular biological functions [27].
Wang and coworkers have demonstrated the cytokine-like properties of HMGB1 and also established HMGB1 as a prototype for endogenous danger signals or the so-called “Alarmins” [18,20]. Roles of HMGB1 as an alarmin signal were also reported in a study based on a group of critically ill patients. Here, the plasma level of HMGB1 has been studied, indicating the extracellular properties of HMGB1. However, the same study did not reveal a significant association between HMGB1 levels in patients admitted to ICU and clinical outcomes in critically ill patients; as illness might be related to the late stages of the critical disease, which cannot be accurately captured at admission to the ICU [28].
It was found that the HMGB1 is high in a severe septic shock patient cohort, and the relation of HMGB1 with morbidity and mortality is proven to be consistent [21]. HMGB1 is a multi-functional protein with major homeostatic roles, as well as important roles as a Damage associated molecular pattern (DAMP) mediator of inflammation. The role that HMGB1 plays is dependent largely on its location, and it can have both protective and detrimental roles during the pathogenesis of sepsis and trauma/hemorrhagic shock [29].
Two of the studies inferred that HBD3 had a significant role in septic implant loosening and that there was a significant difference in the blood levels of HBD3 between patients with septic loosening and non-septic loosening [30]. One study found an inverse association between HBD-3 levels in Chlamydia trachomatis infection. Patients had significantly lower cervicovaginal HBD3 compared to the healthy participants. At the acute stage of bacterial pneumonia, the serum levels of HBD3 also rose. During a respiratory illness, inflammatory cytokines promote the synthesis and release of HBD3. Due to its presence in the airways and high bactericidal activity, HBD3 may play a role in the innate mucosal defence of the respiratory tract. The anti-inflammatory action of HBD3 was independent of cAMP levels and was not controlled by an increase in IL-10, indicating that HBD3 may be important in controlling inflammation and septic shock. On the other hand, other researchers indicate the role of HBD3 as a potent inhibitor of the accumulation of pro-inflammatory cytokines TNF- and IL-6.
HBD3 significantly decreased the production of pro-inflammatory biomarkers in response to lipopolysaccharide induction and exhibited a suppressive effect on the downstream NF-κB signaling pathway. Furthermore, HBD3 gene modification promotes the osteogenic differentiation of human periodontal ligament cells and bone repair in periodontitis as HBD3 was able to alleviate the inflammatory destruction of periodontitis along with the promotion of bone repair in vivo.
5. Conclusion
There are significant differences in the levels of both HMGB1 and HBD3 between healthy and septic participants. The levels of markers were higher in individuals with sepsis. HMGB1 and HBD3 are pro-inflammatory mediators that can provide clues for the diagnosis of sepsis. Our study also has some limitations as we have not reported the outcome of positive cases.
Since it is a comparative study, no matching was done. The other patient factors, including the other comorbidities not considered in the study, might have influenced the results. The sample size is relatively small. Further studies on larger samples are warranted before the results can be generalized. While these findings demonstrate the potential of HMGB1 and HBD-3 as diagnostic biomarkers for sepsis, it is important to note that they should not replace present diagnostic techniques entirely. The combination of biomarkers with existing diagnostic methods could enhance accuracy and improve sepsis diagnosis. Additionally, further validation and larger-scale studies are necessary to establish the clinical utility of these biomarkers in routine practice.
In conclusion, HMGB1 and HBD-3 show promise as diagnostic biomarkers for sepsis. However, the integration of these biomarkers with current diagnostic techniques is crucial for accurate sepsis diagnosis. The findings of this study contribute to the understanding of sepsis pathophysiology and may aid in the development of more comprehensive and effective diagnostic strategies for sepsis management.
Authors' note
Written informed consent was obtained from the patients or their legally authorized representative for anonymized patient information to be published in this article. This material is the result of work supported with resources and use of facilities at the College of Medicine and Medical Sciences, Arabian Gulf University, Kingdom of Bahrain.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Arabian Gulf University to Prof. Khalid Bindayna (E21-PI-10/17).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
None.
Data availability
No data was used for the research described in the article.
References
- 1.Majno G. The ancient riddle of σñψις (sepsis) J. Infect. Dis. 1991;163(5):937–945. doi: 10.1093/infdis/163.5.937. [DOI] [PubMed] [Google Scholar]
- 2.Kumar V. Targeting macrophage immunometabolism: dawn in the darkness of sepsis. Int. Immunopharm. 2018;58:173–185. doi: 10.1016/j.intimp.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chousterman B.G., Swirski F.K., Weber G.F., editors. Seminars in Immunopathology. Springer; 2017. Cytokine storm and sepsis disease pathogenesis. [DOI] [PubMed] [Google Scholar]
- 5.Fajgenbaum D.C., June C.H. Cytokine storm. N. Engl. J. Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 7.Toro-Pérez J., Rodrigo R. Contribution of oxidative stress in the mechanisms of postoperative complications and multiple organ dysfunction syndrome. Redox Rep. 2021;26(1):35–44. doi: 10.1080/13510002.2021.1891808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudd K.E., Kissoon N., Limmathurotsakul D., Bory S., Mutahunga B., Seymour C.W., et al. The global burden of sepsis: barriers and potential solutions. Crit. Care. 2018;22(1):1–11. doi: 10.1186/s13054-018-2157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleischmann-Struzek C., Mellhammar L., Rose N., Cassini A., Rudd K., Schlattmann P., et al. Incidence and mortality of hospital-and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1552–1562. doi: 10.1007/s00134-020-06151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleischmann C., Scherag A., Adhikari N.K., Hartog C.S., Tsaganos T., Schlattmann P., et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 11.Chen W., Qiang X., Wang Y., Zhu S., Li J., Babaev A., et al. Identification of tetranectin-targeting monoclonal antibodies to treat potentially lethal sepsis. Sci. Transl. Med. 2020;12(539) doi: 10.1126/scitranslmed.aaz3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marik P.E. Don't miss the diagnosis of sepsis. Crit. Care. 2014;18(5):1–3. doi: 10.1186/s13054-014-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang M., Cai S., Su J. The pathogenesis of sepsis and potential therapeutic targets. Int. J. Mol. Sci. 2019;20(21):5376. doi: 10.3390/ijms20215376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milić L., Grigorov I., Krstić S., Ćeranić M.S., Jovanović B., Stevanović J., et al. Serum level of HMGB1 protein and inflammatory markers in patients with secondary peritonitis: time course and the association with clinical status. J. Med. Biochem. 2017;36(1):44. doi: 10.1515/jomb-2016-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan J.Y., Zhao F., Deng S.X., Zhu H.C., Gong Y., Wang W. Glycyrrhizin affects monocyte migration and apoptosis by blocking HMGB1 signaling. Mol. Med. Rep. 2018;17(4):5970–5975. doi: 10.3892/mmr.2018.8598. [DOI] [PubMed] [Google Scholar]
- 16.Yu H., Qi Z., Zhao L., Shao R., Fang Y., Li C. Prognostic value of dynamic monitoring of cellular immunity and HMGB1 in severe sepsis: delayed chronic inflammation may be the leading cause of death in late severe sepsis. Clin. Lab. 2016;62(12):2379–2385. doi: 10.7754/Clin.Lab.2016.160530. [DOI] [PubMed] [Google Scholar]
- 17.Jung S., Mysliwy J., Br Spudy, Lorenzen I., Reiss K., Gelhaus C., et al. Human β-defensin 2 and β-defensin 3 chimeric peptides reveal the structural basis of the pathogen specificity of their parent molecules. Antimicrob. Agents Chemother. 2011;55(3):954–960. doi: 10.1128/AAC.00872-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neelima S., Archana K., Athira P., Anju M., Anooja V., Singh I.B., et al. Molecular characterization of a novel β-defensin isoform from the red-toothed trigger fish, Odonus Niger (Ruppel, 1836) Journal of Genetic Engineering and Biotechnology. 2021;19(1):1–14. doi: 10.1186/s43141-021-00175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayer A., Lammel J., Tohidnezhad M., Lippross S., Behrendt P., Klüter T., et al. The antimicrobial peptide human beta-defensin-3 is induced by platelet-released growth factors in primary keratinocytes. Mediat. Inflamm. 2017;2017 doi: 10.1155/2017/6157491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nehls C., Böhling A., Podschun R., Schubert S., Grötzinger J., Schromm A., et al. Influence of disulfide bonds in human beta defensin-3 on its strain specific activity against Gram-negative bacteria. Biochim. Biophys. Acta, Biomembr. 2020;1862(8) doi: 10.1016/j.bbamem.2020.183273. [DOI] [PubMed] [Google Scholar]
- 21.Gaïni S., Pedersen S.S., Koldkjær O.G., Møller H.J. High mobility group box-1 protein in patients with suspected community-acquired infections and sepsis: a prospective study. Crit. Care. 2007;11(2):1–10. doi: 10.1186/cc5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charoensup J., Sermswan R.W., Paeyao A., Promakhejohn S., Punasee S., Chularari C., et al. High HMGB1 level is associated with poor outcome of septicemic melioidosis. Int. J. Infect. Dis. 2014;28:111–116. doi: 10.1016/j.ijid.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Sundén-Cullberg J., Norrby-Teglund A., Rouhiainen A., Rauvala H., Herman G., Tracey K.J., et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit. Care Med. 2005;33(3):564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 24.van Zoelen M.A., Laterre P.-F., van Veen S.Q., van Till J.W., Wittebole X., Bresser P., et al. Systemic and local high mobility group box 1 concentrations during severe infection. Crit. Care Med. 2007;35(12):2799–2804. doi: 10.1097/01.CCM.0000287588.69000.97. [DOI] [PubMed] [Google Scholar]
- 25.Barnay-Verdier S., Borde C., Fattoum L., Wootla B., Lacroix-Desmazes S., Kaveri S., et al. Emergence of antibodies endowed with proteolytic activity against High-mobility group box 1 protein (HMGB1) in patients surviving septic shock. Cell. Immunol. 2020;347 doi: 10.1016/j.cellimm.2019.104020. [DOI] [PubMed] [Google Scholar]
- 26.Ding J., Cui X., Liu Q. Emerging role of HMGB1 in lung diseases: friend or foe. J. Cell Mol. Med. 2017;21(6):1046–1057. doi: 10.1111/jcmm.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang R., Chen R., Zhang Q., Hou W., Wu S., Cao L., et al. HMGB1 in health and disease. Mol. Aspect. Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yagmur E., Buendgens L., Herbers U., Beeretz A., Weiskirchen R., Koek G.H., et al. High mobility group box 1 as a biomarker in critically ill patients. J. Clin. Lab. Anal. 2018;32(8) doi: 10.1002/jcla.22584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng M., Scott M.J., Fan J., Billiar T.R. Location is the key to function: HMGB1 in sepsis and trauma‐induced inflammation. J. Leukoc. Biol. 2019;106(1):161–169. doi: 10.1002/JLB.3MIR1218-497R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levón J., Al-Samadi A., Mackiewicz Z., Coer A., Trebse R., Waris E., et al. Human beta-defensin-3 producing cells in septic implant loosening. J. Mater. Sci. Mater. Med. 2015;26(2):98. doi: 10.1007/s10856-015-5440-4. (i) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.