Abstract
Background
Sarcopenia and muscular dystrophy are two muscle diseases. In cancer patients, cancer cachexia induces continuous weight loss and muscle loss due to the disease itself or the use of anticancer drugs. Cachexia occurs in up to 80% of cancer patients. It is recognized as a direct cause of reduced quality of life, contributing to at least 20% of cancer-associated deaths and limiting therapeutic options for cancer patients. Cancer cachexia is associated with multiple chronic or end-stage conditions and develops similarly. There are various options for the treatment of cancer cachexia, but there are still many issues to be solved. Hence, to determine its potential to overcome the muscle wasting during cancer cachexia, we studied the effect of BST204, a refined dry ginseng extract, on muscle fiber regeneration.
Experimental procedure
We checked the muscle regeneration efficacy of BST204. First, BaCl2 and freeze injury models were selected to investigate muscle regeneration after BST204 administration. In addition, after inducing muscle differentiation of C2C12 cells, the efficacy of BST204 was analyzed. In this model, we analyzed the expression of the signal pathway (PI3K-AKT signal) by Western blot and imaging methods.
Results and conclusion
These results showed that BST204 induced muscle fiber regeneration in BaCl2 and freeze injury models. Also, we confirmed that BST204 could regulate the PI3K/AKT signaling pathway and regulate the differentiation of C2C12 cells. These results indicate that BST204 has the potential to facilitate the skeletal muscle regeneration during muscle wasting induced by various factors including cancer cachexia.
Keywords: BST204, Cancer cachexia, Regeneration, PI3K/AKT, Muscle differentiation, Muscle atrophy, Sarcopenia, BaCl2 and freeze injury models
Graphical abstract
Highlights
-
•
To determine the potential of BST204, a refined dry ginseng extract, to overcome the muscle wasting during cancer cachexia, we studied the effect of BST204 in the mouse muscle injury model.
-
•
BST204 promotes skeletal muscle regeneration in BaCl2 and freeze induced mouse muscle injury models by efficiently enhancing myoblast fusion and myotube maturation.
-
•
Regulation of PI3K-Akt signaling pathway by BST204 was shown in differentiated C2C12 myoblast cells.
1. Introduction
Skeletal muscle, which accounts for 40–50% of total body weight, is an anatomical component essential for movement and the physiological coordination of various organ systems and processes (e.g., glucose metabolism, etc.) [1]. Since skeletal muscle is susceptible to damage by a number of internal or external factors, its regeneration is an important process for maintaining function [2]. Therefore, following muscle injury, skeletal muscle initiates an innate and active regeneration process to maintain physiological homeostasis.
The main players in skeletal muscle regeneration are adult primary stem cells, termed satellite cells (SCs), which reside adjacent to muscle fibers. SCs are responsible for muscle development and regeneration [3,4] and are highly coordinated in a process involving necrosis of the injured muscle cell and SC activation, proliferation, and differentiation [[5], [6], [7]]. Under normal healthy conditions, satellite cells are maintained in a quiescent state. In response to various stimuli, these cells are prompted to enter the cell cycle, proliferate, and further differentiate into new myotubes or fuse with damaged myofibers to repair muscle injury [8].
Skeletal muscle wasting and regeneration defects are specific to many pathological conditions, including Duchenne muscular dystrophy, aging, sarcopenia, and cachexia [9], and the major therapeutic approaches for these muscle regeneration defective diseases are stem cell therapies (SCTs). However, due to the high complexity and vulnerability of SCTs, understanding the regulatory mechanisms of muscle regeneration remains challenging, and the preparation of sufficient numbers of stem cells and their targeted delivery are particularly large hurdles. Other approaches potentially include the direct delivery of chemokines and growth factors that stimulate the growth and differentiation of stem cells and the development of small molecules that directly promote the proliferation of satellite cells. However, despite extensive effort, recent approaches have achieved little success.
BST204, a purified dry extract of ginseng, is a natural product containing the bioactive components 5% Rh2 and 10% Rg3 [10]. Rh2 and Rg3 ginsenosides are found in Korean red ginseng root and possess various biological activities. Various biological functions of BST204 have been reported, including the inhibition of nitric oxide synthase and cyclooxygenase-2 during macrophage inflammation, inhibition of the proliferation and tumorigenesis of cancer stem cells, and regulation of fatigue and toxicity after chemotherapy [6,10]. Additionally, it has been reported that BST204 improves and prevents dexamethasone-induced muscle atrophy, indicating its likely involvement in the regeneration of skeletal muscle [11]. BST204 is known to promote myotube growth through the activation of PI3K/Akt/mTOR signaling in C2C12 myoblasts [12]. The major activator of Akt is PI3K, which is activated by various growth factors and cytokines. When activated, PI3K generates PI3P from PI2P, which activates a number of signaling proteins, including Akt, PDK1, and GRP1 [[13], [14], [15]]. Various factors, such as cytokines, hormones, and growth factors, can induce downstream activation by phosphorylation. PI3K-Akt signaling leads to the activation of mTOR, which regulates muscle protein synthesis [16].
Considering that BST204 is involved in muscle regeneration, we analyzed its effects on mouse skeletal injury models. Four mouse skeletal muscle injury models (freeze injury, notexin, cardiotoxin, and barium chloride (BaCl2) have been established thus far, with each showing notable differences in the degree of injury and the regeneration process, such as satellite cell kinetics [[17], [18], [19]]. Of these four injury models, we investigated the role of BST204 in two different mouse injury models characterized by mechanical injury and chemical injury. For traditional mechanical injury, we chose the freeze injury (FI) model, which is the most severe mouse injury model and shows approximately 90% satellite cell loss after injury [17]. For chemical injury, we chose BaCl2 injury (BI), a recently established chemical injury model with less variation among experimental animals than other chemical injury models [20]. By these studies, we found that BST204 significantly increases muscle regeneration after both mechanical and chemical injury by promoting the differentiation of satellite cells, the main drivers of muscle regeneration. These results indicate that BST204 has the potential to facilitate the skeletal muscle regeneration during muscle wasting induced by various factors including cancer cachexia.
2. Materials & methods
2.1. Preparation of BST204
The Panax dry purified extract of ginseng used in this study was described previously under the name BST204 [12]. BST204 is a purified extract of the root of the plant ginseng; crude ginseng is processed by ginsenoside-β-glucosidase and acid hydrolysis to enrich the bioactive ginsenosides Rg3 and Rh2 [7].
2.2. Plasmids and chemicals
The original constructs harboring AKT (Eevee-3548NES) and Pippi-PIP3 (Pippi-1748x) were provided by Michiyuki Matsuda (Kyoto, Japan) [21,22].
2.3. Muscle fiber regeneration after mouse muscle injury
All animal studies were conducted in compliance with animal protocols approved by the Institutional Animal Care and Use Committee at ASAN Laboratory of Animal Re-search (Reference code: IACUC 2017-14-258). All experiments were conducted with 6-week-old C57BL/6 mice (Ja bio, Suwon-si, Korea), and three animals (n = 3) were used per condition and time point. Mice were randomized into eight groups: (1) mice that received vehicle or (2) 200 mg/kg BST204 and were sacrificed on the 7th day after chemical injury (BaCl2 7- day groups); (3) mice that received vehicle or (4) 200 mg/kg BST204 and were sacrificed on the 10th day after chemical injury (BaCl2 10- day groups); (5) mice that received vehicle or (6) 200 mg/kg BST204 and were sacrificed on the 7th day after freeze injury (freeze 7 day groups); and (7) mice that received vehicle or (8) 200 mg/kg BST204 and were sacrificed on the 10th day after freeze injury (freeze 10day groups). Chemical injury was induced by an intramuscular (IM) injection into the tibialis anterior (TA) muscle of 50 μL of barium chloride (BaCl2, 1.2% in sterile demineralized water (Sigma Aldrich, MO, USA)) using a 30-gauge Hamilton microsyringe. Freeze injury was described previously (3). After skin incision and muscle exposure, TA muscles were freeze–thawed three times by a 15sec application of a liquid nitrogen-cooled metallic rod. The skin was then sutured with 4.0 suture, and the mice were kept at 37 °C on a heating pad for 2 h. Mice injured either chemically (BaCl2) or by freezing were orally administered 200 mg/kg BST204 in 5-day cycles (1st cycle: Days 0-4; 2nd cycle: Days 7-9). To compare with the injury model, non-injury model was orally administered in the same way as the test group. On the 7th or 10th day after injury, the right and left TA muscles were collected, snap frozen, and cross-sectioned at 4 μm for histopathological analysis. After immunohistochemical staining with an anti-laminin antibody (Ab11575, Abcam, Cambridge, United Kingdom) to detect muscle fiber membranes, the slides were scanned by the Vectra® PolarisTM automated quantitative pathology imaging system (Akoya Biosciences, CA, USA). Images were analyzed by HALO software (Indica Labs, Corrales, NM, USA). Additionally, mouse muscle tissues were homogenized by cryogenic grinding, lysed in RIPA lysis buffer and analyzed by western blotting. The following primary antibodies were used: anti-myogenin antibody (ab124800, Abcam), anti-myosin heavy chain antibody (MAB4470-SP, R&D Systems, Minneapolis, MN, USA), and anti-MyoD antibody (NB100-56511, Novus Biologicals, Littleton, CO, USA).
2.4. Western blot analysis
Cell lysates were harvested for 30 min on ice in RIPA lysis buffer (BRI-9001: T&I, Republic of Korea) supplemented with Xpert protease inhibitor cocktail solution (P3100-005, GenDEPOT, USA) and Xpert phosphatase inhibitor cocktail solution (100X, P3200-005, GenDEPOT, USA) and then centrifuged at 14,000 rpm for 15 min at 4 °C. The supernatant was collected, and protein concentrations were determined by a BCA protein assay kit (23225, Thermo Fisher Scientific, USA). Total protein extracts (20–30 μg) were separated by SDS–PAGE, and proteins were transferred to PVDF membranes (IPVH00010, Millipore, Burlington, MA, USA). Membranes were blocked with 5% skim milk or BSA in 0.1% Tween-20/TBS (TR-2007-000-74, Biosesang, Republic of Korea) and then incubated overnight at 4 °C with primary antibodies overnight (1:1000 in 5% milk or BSA or in 0.1% Tween 20 in TBS). The following primary antibodies were used: anti-beta actin (sc-4778; Santa Cruz-, CA, USA); and anti-mTOR (2972S), anti-phospho-mTOR (2971S), anti-Akt (9272S), anti-phospho-Akt (4060S), anti-PI3K (4292S), and anti-phospho-PI3K (4228S) (Cell Signaling Technology (Danvers, MA, USA). The membranes were then washed three times and incubated with anti-mouse or anti-rabbit HRP-conjugated secondary antibodies (ab6721 and ab6789, Abcam-) for 1 h at RT. The blots were washed three times and visualized with a manual chemiluminescent immunoblotting detection system. The films were scanned and analyzed by Image J software.
2.5. Cell culture, differentiation, and transfection
Mouse C2C12 myoblasts (American Type Culture Collection, ATCC, Manassas, VA, USA) were cultured under 5% CO2 at 37 °C in Dulbecco’s modified Eagle’s medium with high glucose (DMEM; Biowest, Riverside, MO, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Biowest) and 1% antibiotic-antimycotic (AA; Gibco, Waltham, MA, USA). C2C12 cells were plated in 35-mm glass bottom dishes (SPL, Pocheon-si, Korea) at a density of 4 x 104/cm2 and incubated for 7 days in DMEM containing 2% horse serum (HS, Gibco, Waltham, MA, USA) and 1% AA for differentiation. To introduce FRET indicator into C2C12 cells, differentiated cells were incubated for 24 h- at 37 °C with DNA(0.5 μg/ml) in a Lipofectamine solution which had been prepared with 4 μ-l of Lipofectamine2000 (Invitrogen, Waltham, MA, USA) in a 100 μl of Opti MEM (Gibco, Waltham, MA, USA) and 1ug of DNA in a 100 μl of Opti MEM for 20 min at room temperature. After 24 h, the transfection medium was changed to phenol red-free medium containing 2% horse serum and 1% AA.
2.6. Time-lapse FRET imaging
To analyze Pippi-PIP3 and Akt activation, time-lapse FRET images were obtained as previously described [19]. In brief, FRET images were obtained using a Nikon Ti-E inverted microscope (Nikon, Tokyo, Japan) equipped with a PFS and CoolSNAP HQ camera (Roper Scientific, Trenton, NJ, USA) with excitation and emission filter wheels. Images were acquired using a 2 x 2 binning mode and a 200-ms exposure time. For dual-emission ratio imaging of the intramolecular FRET probe, we obtained images for cyan fluorescent protein (CFP) and FRET. All images were processed and analyzed using MetaMorph software (Universal Imaging, Downingtown, PA, USA). After background subtraction, pseudocolor images of FRET/CFP were generated using eight colors from red to blue to represent the FRET/CFP ratio in intensity-modulated display (IMD) mode. The following items used for dual-emission ratio imaging were purchased from Semrock (Rochester, NY, USA): an FF01-438/24–25 excitation filter, an FF458-Di02-25x36 dichroic mirror, and FF01-483/32-25 CFP and FF01-542/27-25 FRET emission filters.
2.7. Statistical analysis
Quantitative data are presented as the mean ± SEM of multiple replicates from at least three independent experiments. The data were analyzed using Student’s t-test in GraphPad Prism software (GraphPad, San Diego, CA, USA), and p values were calculated to assess statistical significance.
3. Results
3.1. Generation of FI- and BI-induced mouse muscle injury models
Sarcopenia is a loss of muscle mass and function by aging or adverse effects of the cancer stage [9,23]. Cancer patients with sarcopenia have a poor prognosis associated with cancer treatment. Such sarcopenia occurs in many cancer patients [24], and there is an urgent need to develop a therapeutic agent to treat it.
Based on the ability of BST204 to enhance myoblast differentiation and multinucleated myotube formation in C2C12 mouse myoblast cells [12], we hypothesized that it may affect the processes of myoblast fusion and differentiation in injured mouse muscle. To evaluate muscle regeneration post-injury, we conducted injury model experiments according to a previously described protocol [8]. When the cancer cachexia model is used, it is expected that muscle loss is already very severe due to cachexia and various factors work due to cancer even before mechanical or chemical stimulation is given. Therefore, in order to interpret only from the perspective of muscle regeneration, experiments were conducted on normal mice.
After injury in each model, H&E staining of tibialis anterior (TA) muscles in FI and BI mice showed significant muscle injury compared to control mice at 18 h. Normal TA muscles were characterized by the even size (diameter) distribution of muscle fibers with peripheral nuclei. On the other hand, injured TA muscles exhibited uneven muscle fiber sizes and necrotic and inflammatory muscle fibers (data not shown). H&E staining further showed marked necrosis and inflammatory cell infiltration of muscle fibers seven days post-injury in both injury models. Although the number of inflammatory cells decreased ten days after injury, muscle fibers still showed morphological damage (Fig. S2). Even though BST204 was administered on Day 2 after either freezing or BaCl2 injection, necrotic and degenerated myofibers were found in TA muscle sections, and the tissues recovered most of their normal morphology and showed persistence of central nuclei on Day 14 regardless of BST204 administration (Fig. S1).
3.2. BST204 enhances muscle fiber regeneration after chemical muscle injury by inducing myocyte differentiation and myofiber fusion
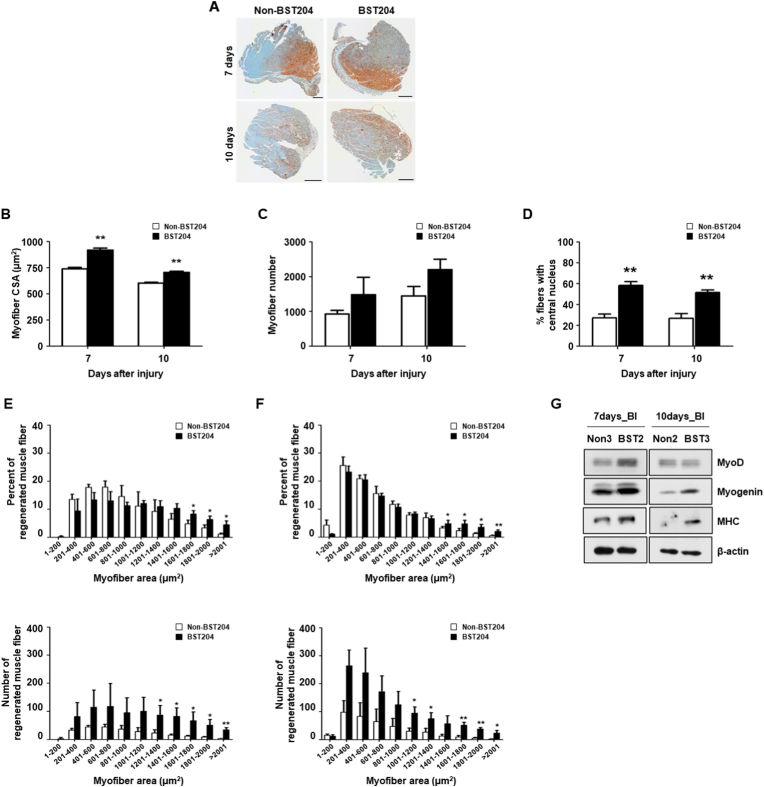
To examine the role of BST204 during skeletal muscle regeneration, it was orally administered to 6-week-old mice damaged by 1.2% BaCl2 injection, followed by the analysis of muscle regeneration on 7 and 10 days from the date of injury (Fig. S3). Typically, muscle fibers that regenerate from murine muscle can be identified by the presence of centrally located nuclei [25]. To quantify muscle fiber regeneration, we used the muscle fiber module of HALO software. Immunohistochemistry images of staining with an anti-laminin antibody, which is used to visualize fiber membranes, were analyzed to detect the total count of muscle fibers, as well as the area, diameter, and number of fibers with centrally located nuclei (Fig. 1A). 7- and 10- days following injury, most inflammatory cells in the TA muscle of BST204-treated mice were cleared, and the damaged myofibers were replaced with newly formed myofibers with centralized nuclei (Fig. S2). In addition, for comparison with the injury model, analysis was performed through immunohistochemistry after administration of BST204 in a non-injury model and there were no significant changes in the non-injury model upon BST204 treatment (Fig. S4).
Fig. 1.
Effect of BST204 on muscle fiber regeneration after BaCl2-induced muscle injury.
A. Immunohistochemical staining of laminin in tibialis anterior (TA) muscle sections from C57BL/6 mice orally administered BST204 or vehicle for 7 or 10 days after the injection of 1.2% BaCl2. Representative sections are shown. IHC images were scanned with a Vectra Polaris. The scale bar represents 300 μm.
B. Cross-sectional areas (CSAs) of myofibers in TA muscles from BST204-treated and untreated mice (n = 3 per group, Student’s t-test, *p<0.05).
C. Myofibers were quantified in TA muscles from 6-week-old male mice treated with or without BST204 (n = 3 per group).
D. Percentage of myofibers with central nuclei (mean ± SEM) in TA sections at 7 and 10 days post-injury (n = 3 per group, Student’s t-test, *p<0.05).
E-F. The total fiber area (μm2) and distribution in TA muscles after 7 (E) or 10 (F) days of the oral administration of BST204 or vehicle to C57BL/6 mice injected with 1.2% BaCl2. Data are presented as the mean ± SEM (n = 3 per group, Student’s t-test, *p<0.05, **p<0.01).
G. MyoD, myogenin, and MHC expression was analyzed by western blotting. β-Actin was used as a loading control.
Our results also showed that the cross-sectional areas (CSAs) of TA muscles from BST204-treated mice were significantly larger than those of untreated mice, both at 7 and 10 days after BI (Fig. 1B). However, no crucial differences between groups were observed in the number of total myofibers (Fig. 1C). To investigate whether the increased CSAs in BST204-treated mice were due to increased myoblast fusion, we analyzed the proportion of centrally nucleated myofibers. The ratio of fibers with centralized nuclei to total fibers was markedly increased in BST204-treated muscles at 7 but not 10 days post-BI (Fig. 1D). In agreement with the results of the myonuclei analysis, BST204-treated mice had a greater number of regenerated myofibers than did untreated mice at 7 days post-BI (Fig. 1E).
These data suggest that the impaired regeneration in muscles of untreated mice was due to defects in myoblast fusion. Meanwhile, the percentage of regenerated muscle fibers at 10 days post-BI showed no significant difference between BST204-treated and untreated mice, whereas the analysis of regenerated muscle fibers indicated a prominent increase in the total number of new muscle fibers in BST204-treated mice (Fig. 1F).
In addition, we investigated the expression of proteins that regulate myogenic differentiation by Western blot analysis of muscle tissue extracted from untreated and BST204-treated mice. Myogenic factors are known to be differentially expressed during myotube formation. When SCs commit to forming myoblasts, they express myogenic markers such as Pax7, Myf5, and MyoD. Myoblasts further differentiate into myocytes, during which Pax7 and Myf5 decrease and myogenin increases. When myocytes fuse into myotubes and further mature into myofibers, myosin heavy chain (MHC) expression gradually increases [26]. Thus, we analyzed the muscle tissues of BI mice by Western blot and found that late phase myogenic factors (e.g., myogenin and MHC) were upregulated in BST204-treated mice at 7 and 10- days post-BI (Fig. 1G). In addition, the levels of MyoD, an early differentiation marker of SC commitment, were increased at 7 days post-BI.
Collectively, these data suggest that muscle regeneration is mediated by myoblast fusion, which is increased by BST204 treatment at 7 days post-injury. However, there were no significant differences in myoblast fusion at 10 days post-BI, since natural muscle fiber regeneration occurred to the same degree in untreated and BST204-treated mice.
3.3. BST204 enhances muscle fiber regeneration after mechanical muscle injury by inducing myocyte differentiation and myofiber fusion
To compare the efficacy of BST204 in the mechanical muscle injury model, muscle regeneration after FI was analyzed. Laminin-stained IHC slides of tissues from FI mice were scanned and analyzed by HALO software (Fig. 2A). The cross-sectional areas of TA muscles from BST204-treated mice were considerably larger than those from untreated mice at 7 and 10 days after FI (Fig. 2B). However, the total number of myofibers was significantly higher in BST204-treated mice than in untreated mice at 7- and 10- days post-FI, identical to the result for BI mice (Fig. 2C). The proportion of myofibers with centrally located nuclei was notably increased in BST204-treated muscles at 7- and 10- days post-FI (Fig. 2D), and significantly greater numbers of regenerated myofibers were observed in BST204-treated mice (Fig. 2E, F).
Fig. 2.
Effect of BST204 on muscle fiber regeneration after freezing injury. A. Immunohistochemical staining of laminin in tibialis anterior (TA) muscle sections from C57BL/6 mice orally administered BST204 or vehicle for 7 or 10 days following freeze injury. Representative sections are shown. IHC images were scanned with a Vectra Polaris. The scale bar represents 300 μm.
B. Cross-sectional areas (CSAs) of myofibers in TA muscles from BST204-treated and untreated mice (n = 3 per group, Student’s t-test, *p<0.05).
C. Myofibers were quantified in TA muscles from 6-week-old male BST204-treated and untreated mice (n = 3 per group).
D. Percentage of myofibers with central nuclei in TA sections at 7 and 10 days post-injury (mean ± SEM, n = 3 per group, Student’s t-test, *p<0.05).
E-F. Percentage of myofibers with central nuclei in TA sections at 7 (E) and 10 (F) days post-injury (mean ± SEM, n = 3 per group, Student’s t-test, **p<0.01).
G. MyoD, myogenin, and MHC expression was analyzed by western blotting. β-Actin was used as a loading control.
Myogenic factors from muscle tissues of untreated and BST204-treated mice were analyzed, and the expression of MyoD, an early differentiation marker of SC commitment, was higher in the BST204-treated group at 7 days post-FI. At 10 days post-FI, MyoD and MHC were significantly and substantially increased by BST204 treatment compared to the 7- day timepoint (Fig. 2G). Taken together, these results indicate that BST204 contributes to the regeneration of skeletal muscle after mechanical injury.
3.4. BST204 promotes the differentiation of C2C12 cells by activating PI3K/Akt/mTOR signaling
To confirm whether BST204 increases Akt signaling through activated PI3K in differentiated C2C12 mouse myoblast cells, we performed live FRET (fluorescence resonance energy transfer) imaging in the differentiated C2C12 cells. Differentiation of C2C12 cells were induced by incubating the cells in 2% horse serum condition and was found to be well differentiated (Fig. S5). To monitor changes in PI3K, we used an intramolecular FRET-based Pippi-PIP3 indicator [22]. Differentiated C2C12 cells overexpressing the PIP3 indicator were treated with 30 μg/mL BST204, which we found to significantly increase FRET signaling compared to that in untreated C2C12 cells (Fig. 3A, B). Similar to the changes in PIP3, we observed that Akt FRET signaling was considerably increased upon treatment with 30 μg/mL BST204 (Fig. 3C, D) [21].
Fig. 3.
Live-cell imaging of Akt and Pippi-PIP3signaling using a FRET indicator in differentiated C2C12 cells stimulated with BST204.
A. BST204 substantially increased activated Pippi-PIP3 levels. Select images of live C2C12 cells expressing the Pippi-PIP3 FRET indicator after 7 days of differentiation. After 5 min of microscopic observation, phenol-red free medium with/without BST204 was injected, and time-lapse images were recorded at 30-sec intervals for 3 h
B. Quantification of relative fluorescence indicating activated Pippi-PIP3. Five minutes after injecting medium with/without BST204, the value was recorded, and data are presented in a line graph. The bar graph shows the relative fluorescence intensity at 10 min and 180 min in differentiated C2C12 cells treated with vehicle (n = 8) or BST204 (n = 6). The intensity was normalized to the value at 10 min. Data are presented as the mean ± SEM (Student’s t-test, *p<0.05).
C. BST204 substantially increased activated Akt levels. Select images of live C2C12 cells expressing an Akt FRET indicator after 7 days of differentiation. After 5 min of microscopic observation, phenol-red free medium with/without BST204 was injected, and time-lapse images were recorded at 30 sec intervals for 3 h.
D. Quantification of relative fluorescence indicating activated Akt. Five minutes after injecting medium with/without BST204, the value was recorded, and data are presented as a line graph. The bar graph shows the relative fluorescence intensity at 10 min and 180 min in differentiated C2C12 cells treated with vehicle (n = 6) or BST204 (n = 5). The intensity was normalized to the value at 10 min. Data are presented as the mean ± SEM (Student’s t-test, *p<0.001). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
We further demonstrated that BST204 activates PI3K/Akt/mTOR signaling in C2C12 cells by Western blot. After inducing the differentiation of C2C12 cells with differentiation medium (DM) for 7 days and treating the cells for 3 h with BST204, cell lysates were harvested and analyzed by Western blot to investigate the phosphorylation of PI3K, mTOR, and AKT (Fig. 4A). Activation of p-PI3K tend to increase by 1.3-fold, while AKT and mTOR phosphorylation tend to increase by 1.7- and 1.8-fold compared to control, respectively. These results demonstrates that BST204 could induce the myogenic differentiation of C2C12 cells through the PI3K/AKT/mTOR signaling pathway and were consistent with the live imaging data and previous results [12].
Fig. 4.
Effects of BST204 on C2C12 differentiation through the PI3K/AKT signaling pathway
A. The levels of phosphorylated PI3k, AKT, and mTOR were increased by BST204. β-Actin was used as a loading control. The result is n=2 with two repetitions, and the western blot image is representative of the results for two repetitions.
B. The bar graph shows the relative expression in the BST-treated group. The white bar shows the result of the comparative analysis of the expression of proteins (black bar) that change after BST treatment when the control is set to 1 to compare the control after BST treatment under the same conditions.
In conclusion, the results of this study suggest that the regenerative effect of BST204 could contribute to the recovery of damaged muscle fibers in various skeletal disorders related to muscle weakness and wasting based on the results of BI and FI mouse muscle injury models. In addition, BST204 could induce the activation of PI3K/Akt/mTOR signaling in C2C12 mouse myoblast cell line, which its activation is known to enhance the differentiation of myoblasts and the generation of myotubes.
4. Discussion
BST204 is a fermented and enriched ginseng extract with various physiological functions relevant to multiple pathologies. BST204 is known to regulate inflammation by inhibiting nitric oxide production and cyclooxygenase-2 -expression and to regulate the proliferation and invasion of cancer cells through cell cycle arrest and the induction of tumor suppressor genes [27]. In recent studies, BST204 was shown to improve chemotherapy-related fatigue in a cancer-related fatigue mouse model and to exhibit potential preventive and regenerative effects during muscle atrophy [11]. However, those studies were limited to demonstrating the role of BST204 in an in vitro myoblast differentiation model. Therefore, in this study, we investigated the effect of BST204 and the molecular mechanisms underlying muscle regeneration in mouse skeletal muscle injury models induced by mechanical and chemical damage.
The process of muscle regeneration is highly complex and coordinated, requiring fine regulation of the interplay between the immune system and satellite cells via multiple growth factors, intracellular signaling molecules, and transcription factors. In this process, satellite cells play a major role due to their self-renewal and differentiation capacities in damaged tissues. Under normal healthy conditions, satellite cells are maintained in a quiescent state. In response to various stimuli, such as muscle injury, these cells are activated and re-enter the cell cycle, proliferate, and further differentiate into myoblasts, thus regenerating damaged tissues [28]. Following proliferation, myoblasts exit the cell cycle and differentiate into myocytes, which fuse to construct new myotubes [29] and further mature in the final step of tissue repair [30]. This final step is characterized by central nucleated myofibers and increased muscle fiber diameter.
In both the freeze injury (FI) and BaCl2-induced (BI) mouse muscle injury models, treatment with BST204 significantly improved the regeneration of muscle fibers. BST204 led to increased numbers of central nucleated myofibers and cross-sectional areas (CSAs) at both the 7 and 10 days post-injury, indicating that BST204 facilitated sufficient fusion of muscle fibers at those time points. Additionally, the number of regenerated muscle fibers increased, demonstrating that the sufficient recovery of muscle fibers was stimulated by BST204. In BI and FI model mice, the potency of BST204 regarding myogenic differentiation and myotube formation was confirmed by increased MyoD and MHC expression (Fig. 1). These results demonstrate that BST204 promotes efficient muscle regeneration after muscle injury by enhancing the fusion of myoblasts and the maturation of myotubes into mouse skeletal muscle. However, the recovery timeline was different in BI and FI mice (Fig. 1, Fig. 2F). Much greater regeneration in response to BST204 was observed at 10 days post-FI than at 7 days post-FI, while the opposite was true for BI (i.e., the effects of BST204 were greater at 7 than 10 days in this model). Such phenomena can be explained by the inherent differences between the two models of mouse muscle damage. For example, freeze injury is the most aggressive injury and kills most satellite cells [17], while BaCl2-induced damage is milder, and the chemical treatment must be administered several times. Therefore, freeze-injured muscle requires much more time to recover than BaCI2-injured muscle. Since BST204 acts in the latter half of the muscle regeneration process, i.e., the fusion and maturation of myotubes [11], it is logical that the effects of BST204 differ in the two models. Consistent with these results, myogenic factors were differentially regulated by BST204 in the two mouse models (Fig. 1, Fig. 2G) in our study. The expression of MHC, a late phase differentiation marker, showed the greatest difference after BST204 treatment, again confirming that BST204 promotes the later stages of muscle regeneration.
During skeletal muscle regeneration, the role of mammalian target of rapamycin (mTOR) is defined quite clearly [31]. mTOR is a serine/threonine kinase that regulates diverse processes in satellite cells, including cell growth, differentiation, autophagy, survival, and metabolism. It is well known that muscle-specific mTOR inactivation leads to severe myopathy and premature death, as mTOR is essential for the proper proliferation and differentiation of satellite cells during muscle regeneration. More specifically, PI3K/Akt/mTOR is the key regulatory pathway in skeletal muscle hypertrophy and atrophy. In this study, BST204 treatment activated this pathway in C2C12 myoblasts (Fig. 4), indicating that BST204 regulates the differentiation of myoblasts through PI3K/Akt/mTOR signaling during muscle fiber regeneration, as indicated by previous studies [12].
To the best of our knowledge, this study is the first to demonstrate that BST204 promotes skeletal muscle regeneration in mouse muscle injury models by efficiently enhancing myoblast fusion and myotube maturation. These processes, which are mediated by the PI3K/Akt/mTOR pathway, were confirmed in C2C12 myoblasts. Further experiments in other injury models or assessments of the involvement of other signaling molecules in C2C12 myoblasts are potentially needed to understand the mechanism of BST204 in muscle regeneration and hence solidify the potential of this promising compound.
Ethics approval and consent to participate
All samples were obtained post-mortem from animals euthanized for non-research purposes.
Consent for publication
Not applicable.
Funding
This work was supported by the Industrial Core Technology Development Program (10063475) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea). This work was also supported by a grant (2020IP0030-1) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
CRediT authorship contribution statement
Su In Jo: Conceptualization, Writing and editing, Data performance. Yoon Sun Park: Data performance, Formal analysis, Writing. Yeeun Chang: Data performance, Formal analysis. Jai-Hee Moon: Conceptualization. Slee Lee: Data performance, Formal analysis. Hyejin Lee: Data performance, Formal analysis. MiYeon Kim: Data performance, Formal analysis. Do Yeon Kim: Data performance, Formal analysis. SangMun Bae: Data performance, Formal analysis. Se Yeong Park: Data performance, Formal analysis. Hyeseon Yun: Data performance, Formal analysis. Ji-Eun You: Data performance, Formal analysis. Minju Im: Data performance, Formal analysis. Hae-Jung Han: Data performance, Formal analysis. Sang-Yeob Kim: Conceptualization, Writing and editing, Formal analysis. Dong-Hoon Jin: Conceptualization, Writing and editing, Formal analysis.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Appendix A
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2023.101525.
Contributor Information
Sang-Yeob Kim, Email: sykim3yk@amc.seoul.kr.
Dong-Hoon Jin, Email: inno183@amc.seoul.kr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- 1.Frontera W.R., Ochala J. Skeletal muscle: a brief review of structure and function. Calcif. Tissue Int. 2015;96(3):183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosio F., Kadi F., Lexell J., Fitzgerald G.K., Boninger M.L., Huard J. The effect of muscle loading on skeletal muscle regenerative potential: an update of current research findings relating to aging and neuromuscular pathology. Am. J. Phys. Med. Rehabil. 2009;88(2):145–155. doi: 10.1097/PHM.0b013e3181951fc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forcina L., Cosentino M., Musaro A. Mechanisms regulating muscle regeneration: insights into the interrelated and time-dependent phases of tissue healing. Cells. 2020;9(5) doi: 10.3390/cells9051297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forcina L., Miano C., Pelosi L., Musaro A. An overview about the biology of skeletal muscle satellite cells. Curr. Genom. 2019;20(1):24–37. doi: 10.2174/1389202920666190116094736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaauw B., Reggiani C. The role of satellite cells in muscle hypertrophy. J. Muscle Res. Cell Motil. 2014;35(1):3–10. doi: 10.1007/s10974-014-9376-y. [DOI] [PubMed] [Google Scholar]
- 6.Park H.J., Shim H.S., Kim J.Y., Kim J.Y., Park S.K., Shim I. Ginseng purified dry extract, BST204, improved cancer chemotherapy-related fatigue and toxicity in mice. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/197459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo J.Y., Lee J.H., Kim N.W., Her E., Chang S.H., Ko N.Y., Yoo Y.H., Kim J.W., Seo D.W., Han J.W., Kim Y.M., Choi W.S. Effect of a fermented ginseng extract, BST204, on the expression of cyclooxygenase-2 in murine macrophages. Int. Immunopharm. 2005;5(5):929–936. doi: 10.1016/j.intimp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Yang W., Hu P. Skeletal muscle regeneration is modulated by inflammation. J Orthop Translat. 2018;13:25–32. doi: 10.1016/j.jot.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans W.J. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 2010;91(4):1123S–1127S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 10.Yi S.A., Lee J., Park S.K., Kim J.Y., Park J.W., Lee M.G., Nam K.H., Park J.H., Oh H., Kim S., Han J., Kim B.K., Jo D.G., Han J.W. Fermented ginseng extract, BST204, disturbs adipogenesis of mesenchymal stem cells through inhibition of S6 kinase 1 signaling. J Ginseng Res. 2020;44(1):58–66. doi: 10.1016/j.jgr.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim R., Kim H., Im M., Park S.K., Han H.J., An S., Kang J.S., Lee S.J., Bae G.U. BST204 protects dexamethasone-induced myotube atrophy through the upregulation of myotube formation and mitochondrial function. Int. J. Environ. Res. Publ. Health. 2021;18(5) doi: 10.3390/ijerph18052367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauro A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantley L.C. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 14.Lexell J., Jarvis J., Downham D., Salmons S. Quantitative morphology of stimulation-induced damage in rabbit fast-twitch skeletal muscles. Cell Tissue Res. 1992;269(2):195–204. doi: 10.1007/BF00319609. [DOI] [PubMed] [Google Scholar]
- 15.Benoit P.W., Belt W.D. Destruction and regeneration of skeletal muscle after treatment with a local anaesthetic, bupivacaine (Marcaine) J. Anat. 1970;107(Pt 3):547–556. [PMC free article] [PubMed] [Google Scholar]
- 16.Czech M.P. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100(6):603–606. doi: 10.1016/s0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- 17.Hardy D., Besnard A., Latil M., Jouvion G., Briand D., Thepenier C., Pascal Q., Guguin A., Gayraud-Morel B., Cavaillon J.M., Tajbakhsh S., Rocheteau P., Chretien F. Comparative study of injury models for studying muscle regeneration in mice. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S.J., Im M., Park S.K., Kim J.Y., So E.Y., Liang O.D., Kang J.S., Bae G.U. BST204, a Rg3 and Rh2 enriched ginseng extract, upregulates myotube formation and mitochondrial function in TNF-alpha-induced atrophic myotubes. Am. J. Chin. Med. 2020;48(3):631–650. doi: 10.1142/S0192415X20500329. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J.Y., Bass K.D. Skeletal muscle regeneration by extracellular matrix biological scaffold: a case report. J. Wound Care. 2018;27(Sup9):S11–S14. doi: 10.12968/jowc.2018.27.Sup9.S11. [DOI] [PubMed] [Google Scholar]
- 20.Morton A.B., Norton C.E., Jacobsen N.L., Fernando C.A., Cornelison D.D.W., Segal S.S. Barium chloride injures myofibers through calcium-induced proteolysis with fragmentation of motor nerves and microvessels. Skeletal Muscle. 2019;9(1):27. doi: 10.1186/s13395-019-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiaffino S., Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skeletal Muscle. 2011;1(1):4. doi: 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marte B.M., Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem. Sci. 1997;22(9):355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 23.Larsson L., Degens H., Li M., Salviati L., Lee Y.I., Thompson W., Kirkland J.L., Sandri M. Sarcopenia: aging-related loss of muscle mass and function. Physiol. Rev. 2019;99(1):427–511. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anjanappa M., Corden M., Green A., Roberts D., Hoskin P., McWilliam A., Choudhury A. Sarcopenia in cancer: risking more than muscle loss. Tech Innov Patient Support Radiat Oncol. 2020;16:50–57. doi: 10.1016/j.tipsro.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabebordbar M., Wang E.T., Wagers A.J. Skeletal muscle degenerative diseases and strategies for therapeutic muscle repair. Annu. Rev. Pathol. 2013;8:441–475. doi: 10.1146/annurev-pathol-011811-132450. [DOI] [PubMed] [Google Scholar]
- 26.Jiwlawat N., Lynch E., Jeffrey J., Van Dyke J.M., Suzuki M. Current progress and challenges for skeletal muscle differentiation from human pluripotent stem cells using transgene-free approaches. Stem Cell. Int. 2018;2018 doi: 10.1155/2018/6241681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park J.W., Park J.H., Han J.W. Fermented ginseng extract, BST204, suppresses tumorigenesis and migration of embryonic carcinoma through inhibition of cancer stem cell properties. Molecules. 2020;25(14) doi: 10.3390/molecules25143128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin H., Price F., Rudnicki M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampath S.C., Sampath S.C., Millay D.P. Myoblast fusion confusion: the resolution begins. Skeletal Muscle. 2018;8(1):3. doi: 10.1186/s13395-017-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feige P., Brun C.E., Ritso M., Rudnicki M.A. Orienting muscle stem cells for regeneration in homeostasis, aging, and disease. Cell Stem Cell. 2018;23(5):653–664. doi: 10.1016/j.stem.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang P., Liang X., Shan T., Jiang Q., Deng C., Zheng R., Kuang S. mTOR is necessary for proper satellite cell activity and skeletal muscle regeneration. Biochem. Biophys. Res. Commun. 2015;463(1–2):102–108. doi: 10.1016/j.bbrc.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.