Abstract
Background
COVID-19 has been associated with a broad range of thromboembolic, ischemic, and hemorrhagic complications (coagulopathy complications). Most studies have focused on patients with severe disease from high-income countries (HICs).
Objectives
The main aims were to compare the frequency of coagulopathy complications in developing countries (low- and middle-income countries [LMICs]) with those in HICs, delineate the frequency across a range of treatment levels, and determine associations with in-hospital mortality.
Methods
Adult patients enrolled in an observational, multinational registry, the International Severe Acute Respiratory and Emerging Infections COVID-19 study, between January 1, 2020, and September 15, 2021, met inclusion criteria, including admission to a hospital for laboratory-confirmed, acute COVID-19 and data on complications and survival. The advanced-treatment cohort received care, such as admission to the intensive care unit, mechanical ventilation, or inotropes or vasopressors; the basic-treatment cohort did not receive any of these interventions.
Results
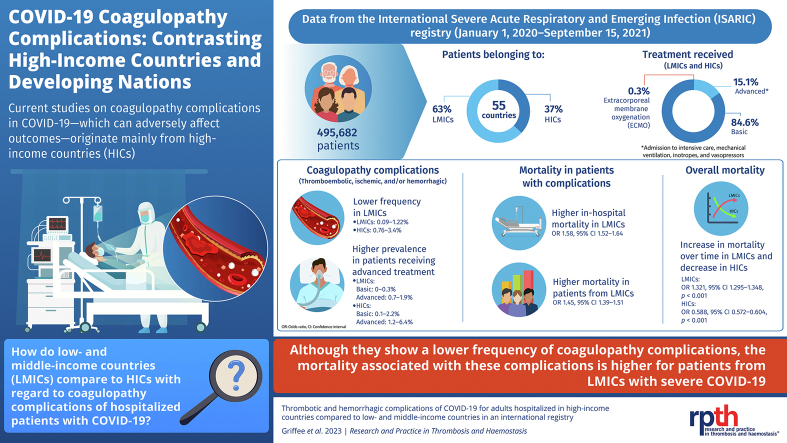
The study population included 495,682 patients from 52 countries, with 63% from LMICs and 85% in the basic treatment cohort. The frequency of coagulopathy complications was higher in HICs (0.76%-3.4%) than in LMICs (0.09%-1.22%). Complications were more frequent in the advanced-treatment cohort than in the basic-treatment cohort. Coagulopathy complications were associated with increased in-hospital mortality (odds ratio, 1.58; 95% CI, 1.52-1.64). The increased mortality associated with these complications was higher in LMICs (58.5%) than in HICs (35.4%). After controlling for coagulopathy complications, treatment intensity, and multiple other factors, the mortality was higher among patients in LMICs than among patients in HICs (odds ratio, 1.45; 95% CI, 1.39-1.51).
Conclusion
In a large, international registry of patients hospitalized for COVID-19, coagulopathy complications were more frequent in HICs than in LMICs (developing countries). Increased mortality associated with coagulopathy complications was of a greater magnitude among patients in LMICs. Additional research is needed regarding timely diagnosis of and intervention for coagulation derangements associated with COVID-19, particularly for limited-resource settings.
Keywords: COVID-19, developing countries, disseminated intravascular coagulation, hemorrhage, ischemic stroke, SARS-CoV-2, thromboembolism, thrombosis
Graphical abstract
Essentials
-
•
COVID-19 is associated with complications related to abnormal bleeding and clotting.
-
•
We investigated bleeding and clotting complications in 495,682 adult patients.
-
•
Bleeding and clotting complications were more frequent in high-income countries.
-
•
The impact of complications on mortality was greater for patients in developing countries.
1. Introduction
COVID-19 is associated with thrombotic, embolic, and hemorrhagic complications, which may lead to poor outcomes [[1], [2], [3]]. Because the viral binding target, angiotensin-converting enzyme 2 (ACE2), is present on endothelial cells, endothelial injury and inflammation may lead to thrombosis, thromboembolism, and disseminated intravascular coagulation [4]. Fatal cases of COVID-19 exhibited severe endothelial damage, including extensive pulmonary thrombosis, significantly more often than in cases of influenza [5,6]. ACE2 is also present on epithelial cells of the gastrointestinal tract, and gastrointestinal bleeding (GIB) is reported in 0.6% to 13% of patients hospitalized for COVID-19 [7]. Hypercoagulability and vascular inflammation caused by COVID-19 have been linked to markedly increased risks of ischemic stroke and myocardial infarction (MI) [8,9].
Early case series from Asia and Europe reported diagnosis of thromboembolic complications in 25% to 50% of patients with severe COVID-19 [10,11]. Similarly, a meta-analysis of studies with universal screening for venous thromboembolism revealed rates of pulmonary embolism (PE) and deep venous thrombosis (DVT) of 32% and 27%, respectively [12]. In contrast, other studies have reported substantially lower frequencies of thrombosis and bleeding among patients hospitalized for COVID-19 [13]. Clinical trials have shown that the optimal dosing of prophylactic anticoagulation varies based on disease severity, leading to consensus guideline recommendations from the International Society on Thrombosis and Hemostasis to incorporate an individualized assessment of the risk of thrombosis and bleeding into the prophylaxis strategy [14,15].
Most studies on thromboembolism, coagulopathy, and hemorrhage associated with COVID-19 are from high-income countries (HICs). Resource limitations in low- and middle-income countries (LMICs) have had an impact on treatment options for patients with COVID-19, and the outcomes of severe COVID-19 have been worse in LMICs than in HICs [[16], [17], [18]]. More information is needed regarding the prevalence of coagulopathy complications of COVID-19 across a range of economic settings, including severe and non-severe disease presentations. This may be relevant as the pandemic transitions to endemic disease, and emerging variants may evade vaccine-mediated and post-infection immunity while continuing to enter tissues with ACE2 [19,20].
Our primary aims were to compare the frequency of coagulopathy complications in LMICs with those in HICs, with stratification by treatment intensity, and determine the association between coagulopathy complications and in-hospital mortality in LMICs and HICs. The secondary aims were to review trends over time for coagulopathy complications and mortality and identify independent risk factors for coagulopathy complications and mortality.
2. Methods
This was a prospective, observational study within the International Severe Acute Respiratory and Emerging Infection (ISARIC)—World Health Organization (WHO) Clinical Characterization Protocol for Severe Emerging Infections [[21], [22], [23]]. The study enrolled patients from LMICs (developing countries) and HICs hospitalized for COVID-19. Information on patient consent, the case report form, and the study protocol are available on the ISARIC website (https://isaric.org [22]). Investigators from 52 countries used data collection instruments, which have been adapted for a range of resource settings, to collect data using the ISARIC case report form, built using Research Electronic Data Capture (version 8.11.11) hosted by the University of Oxford [22,24]. The timing of data reporting was not controlled and depended on site resources and the medical information system structure. In some cases, data were entered into case report forms on a live basis—day by day throughout admission—whereas in other centers, data were completed by reviewing charts of patients hospitalized at a prior time. In addition, some collaborating investigators collected data using local systems and submitted these data to ISARIC for centralized mapping.
All investigators retain full rights to their data. The WHO Ethics Review Committee approved the ISARIC-WHO Clinical Characterisation Protocol (RPC571 and RPC572). In addition, local ethics approval was obtained for each participating country and site according to local requirements. The economic classification of countries was based on gross national income per capita using World Bank definitions (https://data.worldbank.org/country).
2.1. Study population and measurements
The inclusion criteria were age ≥18 years, laboratory confirmation of SARS-CoV-2 infection, and admission to a hospital for acute illness due to COVID-19. Patients were excluded if they did not have a country code or admission date. Sites were required to include all patients meeting the inclusion criteria (full recruitment) or, when full recruitment was not possible due to limited resources, select a strategy to reduce bias in patient selection (Supplementary File, “Procedure to Reduce Bias in Patient Selection”). We defined “coagulopathy complications” related to acute COVID-19 as any of the following 6 diagnoses using ISARIC definitions and clinical criteria for the diagnoses in the case report completion guide (https://isaric.org/research/covid-19-clinical-research-resources/covid-19-crf/, and Supplementary File, Case Definitions): PE, DVT, stroke, MI, GIB, or coagulation disorder/disseminated intravascular coagulation. The complications occurred during hospitalization. The 6 coagulopathy complications were selected based on clinical relevance and availability in the case report form. The following comorbidities were included: diabetes mellitus, chronic cardiac disease, chronic kidney disease, chronic pulmonary disease, chronic neurological disease, malignant neoplasm, chronic hematologic disease, chronic liver disease, smoking, and pregnancy. The complications and comorbidities are described in the case report form completion guide (available at https://isaric.org/research/covid-19-clinical-research-resources/covid-19-crf/). The collection of diagnoses included in “coagulopathy complications” is similar to those used in other COVID-19 studies [9,11,25]. Note that “coagulopathy complications” refers to the 6 complications described in Supplementary Material (ISARIC case report form completion guide), whereas “coagulation disorder/DIC” is one of the 6 complications.
Three cohorts of increasing treatment intensity were defined based on criteria used for a prior ISARIC analysis of patients with COVID-19 [26]. The most aggressively treated cohort received extracorporeal membrane oxygenation (ECMO). The advanced-treatment cohort received any of the following treatments: admission to the intensive care/high-dependency unit, invasive or noninvasive mechanical ventilation, high-flow nasal cannula oxygen, or inotropes or vasopressors. The basic-treatment cohort did not require ECMO or any of the treatments defining the advanced-treatment cohort. The main outcomes evaluated in this study were prevalence of coagulopathy complications and in-hospital mortality.
2.2. Statistical analysis
The definitions of patient characteristics, complications, and outcomes are provided in Supplementary Material, “Case Report Form Completion Guide.” Clinically relevant variables were requested using the ISARIC data dictionary. The host repository at the University of Oxford converted data from contributing sites, which used a variety of local data systems, into Study Data Tabulation Model standards (version 1.7; Clinical Data Interchange Standards Consortium). The requested data fields for the data extraction period from January 1, 2020, to September 15, 2021, were sent to a secure server of the Study Design and Biostatistics Center at the University of Utah. Because the ISARIC collaboration process accommodates sites with a wide range of data collection capabilities, the number of case reports, including clinical characteristics and complications, varied substantially [22]. For example, nearly all cases had sex and age included, but very few patients (<10%) had medications reported in the case report forms. Given this variation in the number of patients with variables of interest reported, the numbers of patients with the specified entry in the case report form are presented as denominators in tables. Patients with case report forms without complications were excluded from the evaluation of the prevalence of complications. Analysis was conducted using R, version 4.1.1.
Continuous variables were expressed as mean (SD), median (IQR), and range. Categorical variables were expressed as counts (percentages). To evaluate the association between demographic characteristics, economic stratification, and the presence of a coagulopathy complication, analysis of variance testing was used for continuous variables and chi-squared testing for categorical variables. A P value of <.05 was deemed statistically significant. When the clinical characteristics of patients with vs without coagulopathy were compared, a standardized mean difference (SMD) of >0.2 was considered as the minimum difference to suggest that the characteristic warranted consideration.
The trends in coagulopathy are represented graphically as monthly proportions calculated as the number of patients with reported coagulopathy complications divided by the number of patients enrolled. To answer the question “is there a significant linear trend over time in the rate of mortality?,” a logistic interaction model was constructed, with covariates of time and economic stratification as well as separate logistic models to determine the changes in mortality over the study period for each economic cohort. Results are presented as odds ratios (ORs) with 95% confidence limits.
Bar graphs are used to show the relationship between coagulopathy complications, income status, and treatment intensity. A 2-sample test of proportions was used to generate P values.
We fitted 2 multivariable logistic regression models. The first was to identify associations between clinically relevant patient characteristics and the outcome of any coagulopathy complication. Some characteristics were selected based on established risk factors for thromboembolism (such as cancer and smoking), and other characteristics were selected as indicators of high disease severity (such as treatment with mechanical ventilation or ECMO). The goal of the first model was to define potential clinical risk factors for coagulopathy complications, with adjustment for economic setting, treatment intensity, and increasing age. The goal of the second model was to quantify the association between coagulopathy complications and in-hospital mortality, with adjustment for treatment intensity, economic stratification, sex, increasing age, and clinically relevant comorbidities. ORs are presented using forest plots. Mortality designates in-hospital mortality, as specified in the final disposition section. Patients lacking a final disposition entry (N = 1013), located in the outcomes section of the case report form, were excluded from the evaluation of mortality for various cohorts and from the multivariable model of risk factors for mortality.
3. Results
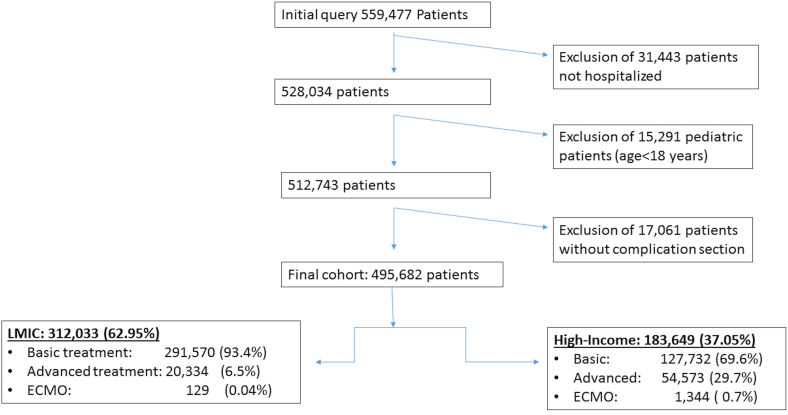
A total of 495,682 patients from 52 countries met the inclusion criteria, including 312,033 (62.9%) patients from LMICs (developing countries), within the enrollment period of January 1, 2020, to September 15, 2021 (Figure 1). Eighty percent of patients in the registry were from the United Kingdom or South Africa (Supplementary Figure: patient numbers by country). In these countries, nation-wide protocols targeted full recruitment (all patients hospitalized for COVID-19). In addition, 84.6% (419,302/495,682) of the patients were classified as receiving basic treatment, 15.1% (74,907/495,682) as receiving advanced treatment, and 0.3% (1473/495,682) as receiving ECMO (Figure 1, Table 1); 6.5% (20,334/312,033) of the patients in LMICs received advanced treatment compared with 29.7% (54,573/183,649) of patients in HICs, and 91% (1344/1473) of the patients receiving ECMO were enrolled from HICs. Comparisons of demographics and comorbidities of patients in LMICs vs those of patients in HICs are presented in Table 1.
Figure 1.
Patient Flow Diagram, showing exclusion steps and final cohorts by treatment intensity for LMIC and HIC.
Table 1.
Demographics of high-income vs low- and middle-income patients.
| Characteristic | Category | High-income countries N = 183,649 (37.1%) |
Low- and middle-income countries N = 312,033 (62.9%) |
P value |
|---|---|---|---|---|
| Treatment intensity | Basic treatments | 127,732 (69.6%) | 291,570 (93.4%) | |
| Treatment intensity | Advanced treatments | 54,573 (29.7%) | 20,334 (6.5%) | |
| Treatment intensity | Extracorporeal membrane oxygenation | 1344 (0.7%) | 129 (0.04%) | |
| Age (y) | Mean (SD) | 67.7 (17.8) | 54.1 (16.4) | <.001 |
| Median (IQR) | 71.0 (56.0-82.0) | 55.0 (42.0-66.0) | - | |
| Range | 18.0-107.0 | 18.0-109.0 | - | |
| Body mass index | Mean (SD) | 29.8 (7.2) | 30.5 (8.8) | .001 |
| Median (IQR) | 28.5 (25.2-33.0) | 28.9 (24.9-34.2) | - | |
| Ethnicity | ABORIGINALFIRSTNATIONS | 104/150,548 (0.07%) | 0/195,354 (0%) | <.001 |
| Arab | 1028/150,548 (0.68%) | 4/195,354 (0%) | - | |
| Black | 5842/150,548 (3.88%) | 127,234/195,354 (65.13%) | - | |
| East Asian | 1122/150,548 (0.75%) | 573/195,354 (0.29%) | - | |
| Latin American | 1358/150,548 (0.9%) | 1312/195,354 (0.67%) | - | |
| Mixed ethnicity | 1110/150,548 (0.74%) | 111/195,354 (0.06%) | - | |
| Other | 4618/150,548 (3.07%) | 15,361/195,354 (7.86%) | - | |
| South Asian | 10789/150,548 (7.17%) | 19,383/195,354 (9.92%) | - | |
| South East Asian | 59/150,548 (0.04%) | 3777/195,354 (1.93%) | - | |
| West Asian | 452/150,548 (0.3%) | 10/195,354 (0.01%) | - | |
| White | 124,066/150,548 (82.41%) | 27,589/195,354 (14.12%) | - | |
| Sex | Female | 81,062/183,605 (44.15%) | 166,458/312,033 (53.35%) | <.001 |
| Male | 102,334/183,605 (55.74%) | 145,525/312,033 (46.64%) | - | |
| Pregnancy | Yes | 2116/183,649 (1.15%) | 8867/312,033 (2.84%) | <.001 |
| Diabetes | Yes | 52,849/180,870 (29.22%) | 63,632/311,735 (20.41%) | <.001 |
| Chronic cardiac disease | Yes | 69,992/154,485 (45.31%) | 93,459/311,498 (30%) | <.001 |
| Chronic kidney disease | Yes | 28,060/181,053 (15.5%) | 6183/311,734 (1.98%) | <.001 |
| Chronic pulmonary disease | Yes | 26,885/175,568 (15.31%) | 460/19,737 (2.33%) | <.001 |
| Chronic neurologic disease | Yes | 20,369/180,524 (11.28%) | 394/21,138 (1.86%) | <.001 |
| Malignant neoplasm | Yes | 17,275/180,539 (9.57%) | 168/21,138 (0.79%) | <.001 |
| Chronic hematologic disease | Yes | 7111/175,932 (4.04%) | 42/19,737 (0.21%) | <.001 |
| Chronic liver disease | Yes | 186/4593 (4.05%) | 19/1401 (1.36%) | <.001 |
| Oral steroid use on admission | Yes | 900/14,759 (6.1%) | 100/11,474 (0.9%) | <.001 |
3.1. Trends in coagulopathy complications and mortality over time
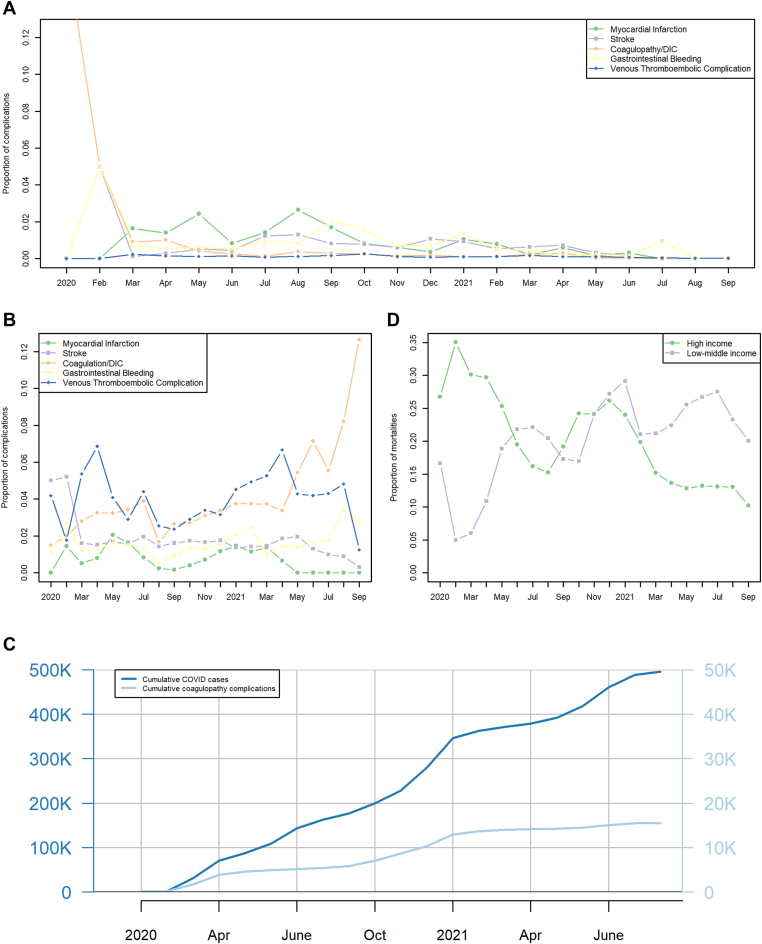
Over the 21 months of the study period, there were minor fluctuations in various coagulopathy complications, with a substantially lower frequency in LMICs than in HICs (Figure 2A, B). A plot of the cumulative prevalence of all coagulopathy complications and case counts showed steady accumulation over time (Figure 2C). For HICs, the mortality decreased over time, whereas for LMICs, the mortality increased over the study period (Figure 2D). Using a logistic interaction model, the OR for mortality over time was 0.588 (95% CI, 0.572-0.604; P < .001) in HICs; the OR for mortality over time was 1.321 (95% CI, 1.295-1.348; P < .001) in LMICs, indicating that there was a significant reduction in mortality over the study period in HICs but a significant increase in mortality over the study period in LMICs (Multimedia Content 4, Logistic Regression for Mortality over Time, Supplementary Tables for Figure 2D).
Figure 2.
(A) Trends in coagulopathy complications over time for LMICs. Months specified along x-axis; "coagulation" stands for "coagulopathy/DIC"; "venous thromboembolic complication" is the combination of deep venous thrombosis and pulmonary embolism. (B) Trends in coagulopathy complications over time for HICs. Months specified along x-axis. "Coagulation/DIC" stands for "Coagulopathy/Disseminated intravascular coagulopathy"; Venous thromboembolic complication is the combination of both deep venous thrombosis and pulmonary embolism. (C) Cumulative Number of COVID-19 Cases and Complications: The x-axis displays the study period, dark blue line: cumulative number of COVID-cases in the registry over time; light blue line: cumulative number of coagulopathy complications over time. (D) Mortality over time by Income Cohort. The x-axis specifies month of study period, the monthly mortality rate is green for HICs and lavender for LMICs.
3.2. Prevalence of coagulopathy complications
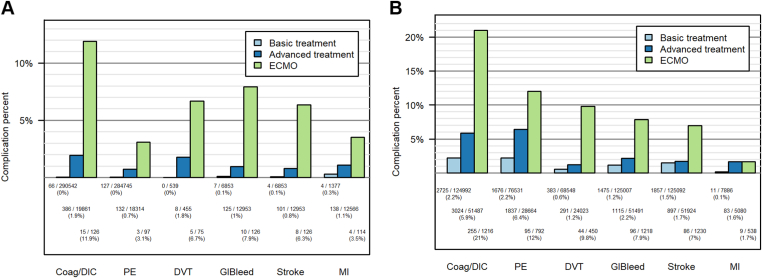
In LMICs, the prevalence of coagulopathy complications was <1% for stroke, coagulation disorder/disseminated intravascular coagulation (DIC), PE, and GIB and just >1% for MI and DVT (Table 2). In HICs, the complications with the highest prevalence were PE (3.4% [3608/105,987]) and coagulation disorder/DIC (3.4% [6004/177,695]). There was a significantly higher prevalence of 4 complication types in HICs: stroke, coagulation disorder/DIC, PE, and GIB (Table 2, P < .001 for each comparison). The prevalence of MI and DVT was higher in LMICs and was significantly higher for MI (P = .016) (Table 2). For both economic cohorts, the prevalence of coagulopathy complications was higher among patients receiving advanced treatment than the prevalence among patients receiving basic treatment (Figure 3A, B). The prevalence of all complication types was higher among patients undergoing advanced treatment in HICs than the prevalence of complications among patients in the advanced-treatment cohort in LMICs, except for DVT. Notably, the ECMO cohort had a substantially increased prevalence of all complication types compared with the prevalence in both the advanced-treatment and basic-treatment cohorts.
Table 2.
Coagulopathy complications stratified by the economic classification of country.
| Coagulopathy complication | Low- and middle-income countries | High-income countries | P value |
|---|---|---|---|
| Myocardial infarction | 146/14,057 (1.04%) | 103/13,504 (0.76%) | .016 |
| Stroke | 113/19,932 (0.57%) | 2840/178,246 (1.59%) | <.001 |
| Coagulation disorder/disseminated intravascular coagulation | 467/310,529 (0.15%) | 6004/177,695 (3.38%) | <.001 |
| Pulmonary embolism | 262/303,156 (0.09%) | 3608/105,987 (3.4%) | <.001 |
| Gastrointestinal bleeding | 142/19,932 (0.71%) | 2686/177,716 (1.51%) | <.001 |
| Deep venous thrombosis | 13/1069 (1.22%) | 718/93,021 (0.77%) | .10 |
Figure 3.
(A) Frequency of complications in low- and middle-income countries. Light blue bars: patients receiving basic treatment, dark blue bars: patients receiving advanced treatments; green bars: patients treated with extracorporeal membrane oxygenation. (B) Frequency of complications in high-income countries. Light blue bars: patients receiving basic treatment, dark blue bars: patients receiving advanced treatments; green bars: patients treated with extracorporeal membrane oxygenation. Coag/DIC, coagulopathy/disseminated intravascular coagulation; DVT, deep venous thrombosis; ECMO, extracorporeal membrane oxygenation; GI, gastrointestinal; MI, myocardial infarction; PE, pulmonary embolism.
3.3. Characteristics of patients with vs without coagulopathy complications
The characteristics of patients with coagulopathy complications were compared with those of patients free from complications, without adjustment (Table 3). In addition, 61.9% of patients with coagulopathy complications were men. The comorbidities with a significantly higher prevalence in the cohort with coagulopathy complications were chronic cardiac disease and chronic kidney disease (each with an SMD of >0.2). Current smoking was reported for 26.9% (4009/14,888) of patients with complications compared with 10.2% (48,082/473,315) of patients without complications (SMD, 0.41). The average age of patients with coagulopathy complications was 67.2 years (SD, 16.3 years) compared with 58.9 years (SD, 18.2 years) in those without (SMD, 0.48).
Table 3.
Comparison of demographics and comorbidities of patients with vs without coagulopathy complications.
| Characteristic | No coagulopathy complication N = 480,176 (96.9%) |
Coagulopathy complication present N = 15,506 (3.1%) |
Standardized mean difference (absolute value) | P value |
|---|---|---|---|---|
| Age (y), mean (SD) | 58.89 (18.15) | 67.22 (16.25) | 0.48 | <.001 |
| Median (IQR) | 59 (46-73) | 69 (56-80) | ||
| Female | 241,627/480,133 (50.3%) | 5893/15,505 (38%) | 0.25 | <.001 |
| Male | 238,256/480,133 (49.6%) | 9603/15,505 (61.9%) | 0.25 | |
| Pregnant patients | 10,904/480,176 (2.3%) | 79/15,506 (0.5%) | 0.15 | <.001 |
| Diabetes | 112,038/477,447 (23.5%) | 4443/15,158 (29.3%) | 0.13 | <.001 |
| Chronic cardiac disease | 156,611/452,250 (34.6%) | 6831/13,733 (49.7%) | 0.31 | <.001 |
| Chronic kidney disease | 31,839/477,613 (6.7%) | 2404/15,174 (15.8%) | 0.29 | <.001 |
| Chronic pulmonary disease | 25,234/180,473 (14%) | 2111/14,832 (14.2%) | 0.02 | <.001 |
| Chronic neurologic disease | 18,908/186,797 (10.1%) | 1855/14,865 (12.5%) | 0.07 | <.001 |
| Malignant neoplasm | 15,877/186,811 (8.5%) | 1566/14,866 (10.5%) | 0.07 | <.001 |
| Chronic hematologic disease | 6311/180,802 (3.5%) | 842/14,867 (5.7%) | 0.10 | <.001 |
| Smoking | 48,082/473,315 (10.2%) | 4009/14,888 (26.9%) | 0.41 | <.001 |
| Oral steroid use on admission | 893/24,636 (3.6%) | 107/1597 (6.7%) | 0.14 | <.001 |
3.4. Mortality associated with coagulopathy complications
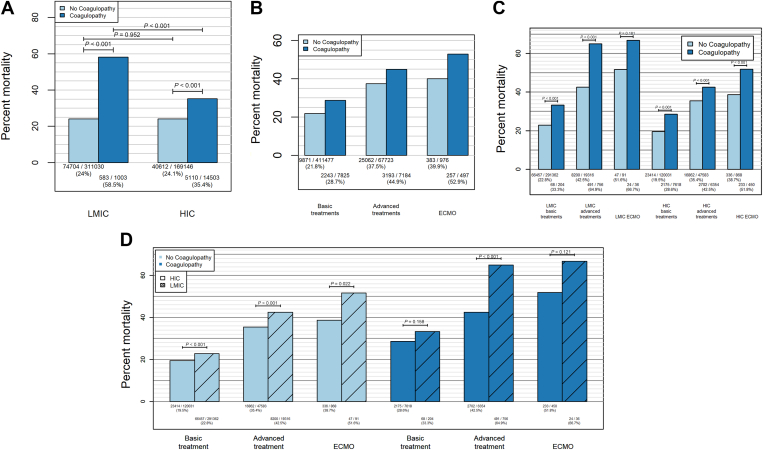
Patients with coagulopathy complications had higher in-hospital mortality than patients without coagulopathy complications (Figure 4A, P < .001). The mortality was almost identical across the economic stratification among patients without coagulopathy complications (24.0% [74,704/311,030] vs 24.1% [40,162/169,146]; P = .952), but the mortality among patients with coagulopathy complications in LMICs was 58.5% (583/1003) compared with 35.4% (5110/14,503) among patients in HICs (P < .001). Across treatment intensity categories, coagulopathy complications were associated with increased mortality; the relative increase was greatest for the ECMO cohort (Figure 4B). When stratified by treatment intensity and economic classification, the mortality was higher in LMICs, both for those with and without coagulopathy complications (Figure 4C). The relative increase in mortality associated with coagulopathy complications in HICs was largest for the basic-treatment cohort (46%). For patients in LMICs, the largest relative increase in mortality associated with coagulopathy complications was for the advanced-treatment cohort (52%). When the mortality across economic strata and disease severity was compared, patients in LMICs without coagulopathy complications had significantly higher mortality in each severity category than patients in HICs (P < .001 for basic and advanced cohorts, P = .02 for the ECMO cohort; Figure 4D). In contrast, the mortality was significantly higher among patients in LMICs with coagulopathy complications only in the advanced-treatment cohort (P < .001 for advanced treatment, P = .16 for basic treatment, and P = .12 for ECMO) (Figure 4D). The mortality among patients in LMICs with advanced treatment and coagulopathy complications was similar to the mortality among patients in LMICs who received ECMO (64.9% [491/756] vs 66.7% [24/36], respectively) (Figure 4D).
Figure 4.
In-hospital mortality by the presence of coagulopathy complications (dark blue) vs no such complications (light blue), for low- and middle-income countries (LMICs) and high-income countries (HICs). (B) Mortality by treatment intensity, light blue for cohorts without coagulopathy complications, dark blue for cohorts with coagulopathy complications, patient fractions specified along x-axis. (C) Mortality by income category (LMIC on left and HIC on right), coagulopathy free (light blue), coagulopathy complications (dark blue). (D) Mortality by treatment intensity, economic stratification (HIC, open rectangles; LMIC, cross-hatched rectangles), and treatment intensity.
3.5. Risk factors for coagulopathy complications
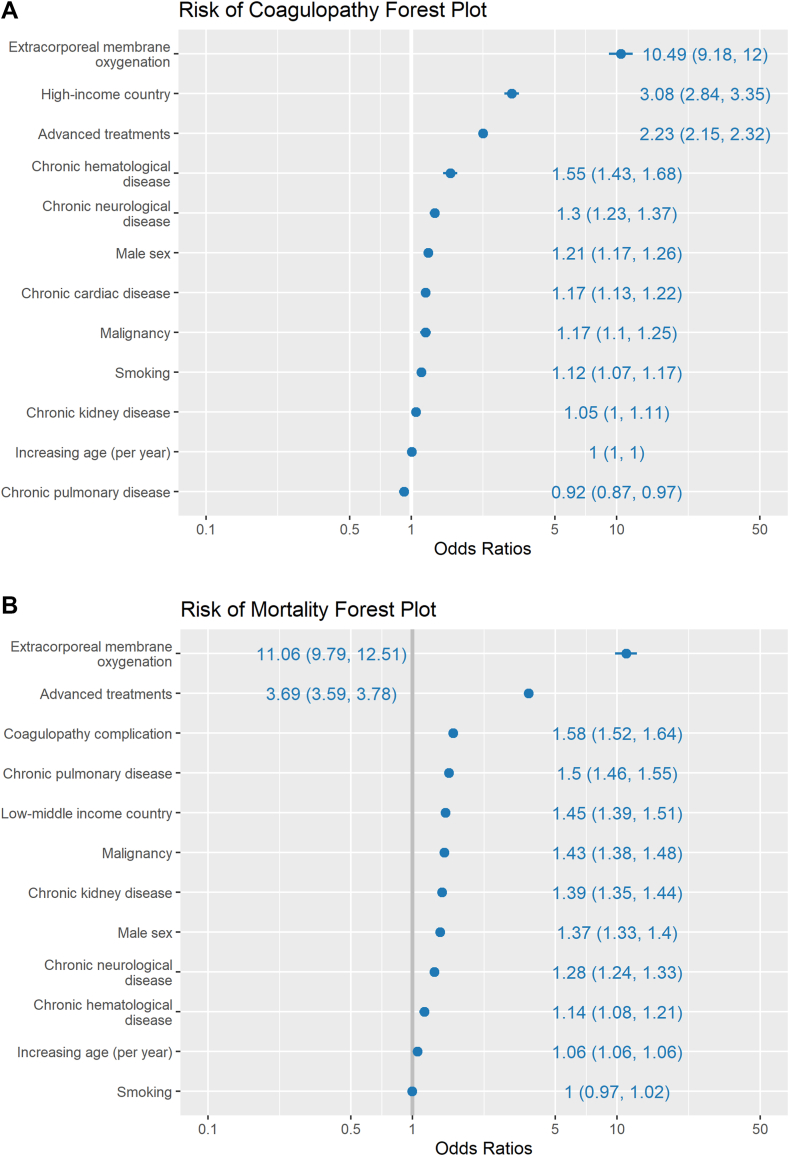
Using multivariable logistic regression, selected risk factors for any coagulopathy complication were compared (Figure 5A). The covariates associated with the largest increased odds of coagulopathy complications were HIC economic classification (OR, 3.08; 95% CI, 2.84-3.35), advanced treatment (OR, 2.23; 95% CI, 2.15-2.32), and ECMO (OR, 10.49; 95% CI, 9.18-12). The comorbidities associated with an increased risk of coagulopathy complications were hematologic disease, chronic neurologic disease, cancer, and chronic cardiac disease. Other risk factors included smoking and male sex. When other risk factors were controlled for, increasing age, chronic kidney disease, and chronic pulmonary disease were not associated with an increased risk of coagulopathy complications (Figure 5A).
Figure 5.
(A) Multivariable analysis of risk factors for coagulopathy complications. Odds ratios and 95% CIs are indicated along the margin. (B) Multivariable analysis of risk factors for mortality. Odds ratios and 95% CIs are indicated along the margin.
3.6. Risk factors for in-hospital mortality
A multivariable analysis was performed to determine whether there was an independent association between coagulopathy complications and mortality (Figure 5B). After adjustment for economic cohort, advanced treatment, ECMO, multiple chronic diseases, male sex, and age, coagulopathy complications were associated with an increased risk of mortality (OR, 1.58; 95% CI, 1.52-1.64). Compared with patients in HICs, patients in LMICs had an increased risk of mortality (OR, 1.45; 95% CI, 1.39-1.51).
4. Discussion
In this multinational, prospective, observational study, we found that the frequency of coagulopathy complications associated with COVID-19 was lower than that reported in several smaller case series. Moreover, we found that coagulopathy complications were detected more frequently in HICs than in LMICs. Finally, we found that across all stages of treatment intensity (which would be expected to correlate with disease severity) and for both economic strata, the presence of coagulopathy complications was associated with increased mortality.
The frequency of venous thromboembolic complications (PE + DVT) in our study was substantially lower than the prevalence of venous thromboembolism in early reports from China (25%) and Europe (27%) but was comparable with the prevalence reported in 1 study from the United States (4.8%) (Table 2) [10,11,13].
The potential reasons for the low prevalence of these complications in our study include differences in the intensity of screening (ie, routine screening vs testing only for symptoms suggesting thromboembolism) and differences in anticoagulation practices as experience with treating COVID-19 evolved. The thrombotic complications reported in other studies included some outcomes, ie, central and arterial line thrombosis, clotting of dialysis circuits, and systemic arterial embolism, which were not included in the complication questions of the ISARIC case report forms [11,13]. In the early stages of characterization of this new disease, laboratory and radiology assessments may have been performed exhaustively in institutions engaged in clinical research, leading to the detection of asymptomatic coagulopathy complications. Alternatively, early COVID-19 variants may in fact have had higher thrombogenicity than later variants. Further studies are needed to clarify these potential explanations.
There are many potential reasons for the higher frequency of coagulopathy complications observed in HICs, including more diagnostic resources such as computed tomography and laboratory tests of coagulation disorders, and resources for higher-intensity routine surveillance. In a survey of diagnostic resources available in LMICs, computed tomography was one of the most limited resources [27]. A markedly lower incidence of venous thromboembolism in LMICs than that in HICs has been reported in other international studies [28]. Early in the pandemic, intensive surveillance, including universal screening for thromboembolic disease in well-funded hospitals, may have led to reports that a majority of patients with COVID-19 had manifestations of a hypercoagulable state, leading to a working hypothesis that thromboembolic complications were central to the pathophysiology of many fatal cases. Based on this perspective, many clinicians rapidly adopted a practice of routinely using full or partial anticoagulation (rather than standard-dose prophylactic anticoagulation) for hospitalized patients. Subsequently, randomized controlled trials demonstrated that full anticoagulation of patients with COVID-19 did not improve outcomes across the severity spectrum [[29], [30], [31]]. Extensive use of imaging and laboratory testing was mainly a feature of HICs, based on the depth and breadth of diagnostic resources not available in LMICs. We thus hypothesize that the availability of diagnostic resources to learn about a new disease in HICs is the most likely explanation for a higher frequency of coagulopathy complications of COVID-19 in HICs than that in LMICs. Many of these diagnoses in HICs may have been mild or asymptomatic disorders, which would not be expected to lead to increased mortality.
The increased mortality among those with coagulopathy complications was substantial in not only the advanced-treatment cohort but also the basic treatment cohort: an absolute mortality difference of 10.5% among patients in LMICs receiving basic treatment and 9.1% among the patients in HICs receiving basic treatment. This contradicts the concept that inflammation, coagulopathy, and the severity of COVID-19 pulmonary disease could be modeled as a pyramid of increasing illness, with parallel increases in cardiopulmonary failure and coagulopathy [32]. An alternative paradigm is that a distinct subset of patients with COVID-19 who are free from severe respiratory failure or shock and thus do not require advanced treatment may still have significant coagulopathy derangements, with clinical consequences that may be life threatening. This illustrates the importance of taking an individualized approach to coagulopathy risk stratification and prophylaxis [33].
For patients with coagulopathy complications, the difference in mortality between LMIC and HIC cohorts was significant in the advanced-treatment cohort (Figure 4D). The potential explanations for the high mortality in this LMIC cohort (64.9%) are that many diagnoses may have been made when the complication was clinically severe or at an advanced stage when treatment was not likely to be effective.
In the multivariable analysis, both coagulopathy complications and living in a LMIC were independently associated with increased mortality after adjustment for treatment intensity and multiple comorbidities (Figure 5B). The mortality was higher across the spectrum of treatment intensity among patients in LMICs, and the increase in mortality associated with coagulopathy complications was greater among patients in LMICs than among patients in HICs. The potential reasons for this include delayed presentation to the hospital for patients in rural settings and lack of resources for escalating care for patients not improving with basic care. Limited resources for advanced care are a potential explanation for the observation that only 6.5% of patients in LMICs received advanced care compared with 29.7% of patients in HICs. A finding that calls for additional investigation is that mortality improved over the study period for HICs but worsened for LMICs. This finding highlights the profound need for distributing specific antiviral therapies (such as vaccines and novel antiviral medications) to developing countries during a pandemic.
Although COVID-19 is no longer classified as a global public health crisis, this study has important implications for future novel diseases. Multicenter registries are important for gathering information representative of a broad sample of clinical presentations, which may differ substantially from initial reports from single centers. Another key insight is that case report forms for new diseases should contain a wide variety of symptoms and complications; this will make it more likely that unexpected manifestations of the disease can be captured earlier and in a larger number of patients than if case report forms must be modified. Finally, this work highlights the imperative to develop evaluation and intervention strategies that can be used in low-resource settings to narrow the stark disparities in outcomes in LMICs vs HICs during the conditions of a pandemic crisis.
4.1. Limitations
This study has some limitations and strengths that are important to acknowledge. Each site participated in the registry voluntarily, which constitutes selection bias, resulting in unknown external validity of the observations. There were no protocols specifying the criteria for testing patients for coagulopathy complications; testing was based on clinical judgment or local policies for managing patients with COVID-19. In addition, the advanced-treatment cohort was based on treatments such as mechanical ventilation, administration of vasoactive medications, and admission to intensive care, with treatment decisions at the discretion of attending medical staff. While a similar method of defining a cohort with advanced care needs, as a surrogate for severe COVID-19, has been used in another ISARIC study, we cannot determine to what extent resource limitations and variability of treatment thresholds, eg, criteria for admission to the intensive care unit across sites, might lead to important differences in the treatment intensity cohorts between LMICs and HICs. Unfortunately, information such as blood gas values was missing in too many patients to use more objective criteria for disease severity [26]. Another important limitation to consider is that the type of laboratory assay to confirm SARS-CoV-2 infection was not specified in the case report form instrument; thus, it is not possible to determine, for example, the number of tests based on polymerase chain reaction vs based on antigen detection using colorimetry. Data collection was performed across a wide range of resource settings and during periods of surges, necessitating the accommodation of centers with limited data sets, and patients without data on complications had to be excluded. This resulted in the largest limitation of the study: the number of patients in LMICs with case report forms including the complications MI, stroke, GIB, and DVT was relatively small. This subset of patients in LMICs with details regarding complications may have differed in important ways from patients who did not have complication data available for analysis. The insight that coagulopathy and thrombosis were the hallmarks of severe COVID-19 led to addition of specific fields for DVT and PE to the complications section of case report forms after the initial enrollment period. Because of missing data, medication use, including the use of anticoagulants and steroids, as well as coagulation laboratory values could not be incorporated into the analysis. At the time of data extraction, vaccination status was not included in the registry; a future study is focused on this important aspect. The strengths of the study include the number of patients involved and inclusion of patients who received both advanced and basic treatments as well as recruitment from both HICs and LMICs. Additionally, 80% of the patients were enrolled in countries with full national recruitment of patients hospitalized for COVID-19, and all sites were required to adhere to a strategy to prevent selection bias (Supplementary File).
5. Conclusions
In this large, international registry, we found that the prevalence of coagulopathy complications (defined as any of the following 6 outcomes: PE, DVT, stroke/cerebrovascular accident, MI, GIB, and coagulation disorder/DIC) in adult patients hospitalized for COVID-19 was substantially lower than that reported in smaller case series, particularly from reports published early during the pandemic. Coagulopathy complications were more frequently observed in HICs than in LMICs, and the prevalence was higher among patients who received advanced treatment. In-hospital mortality was higher among patients with coagulopathy complications, and the increase in mortality associated with coagulopathy complications was greater in LMICs than in HICs, particularly for patients in the advanced-treatment cohort. Coagulopathy complications were associated with increased mortality across economic settings and among patients receiving both basic and advanced treatment; therefore, future efforts should be directed toward early diagnosis and intervention, particularly in lower-resource settings.
Acknowledgments
The investigators acknowledge the support of the COVID clinical management team, All India Institute of Medical Sciences, Rishikesh, India; the dedication and hard work of the Groote Schuur Hospital Covid Intensive Care Unit Team, supported by the Groote Schuur nursing and University of Cape Town registrar bodies coordinated by the Division of Critical Care at the University of Cape Town; the dedication and hard work of the Norwegian SARS-CoV-2 study team; endorsement of the Irish Critical Care- Clinical Trials Group, co-ordination in Ireland by the Irish Critical Care- Clinical Trials Network at University College Dublin; Cambridge National Institute for Health and Care Research Biomedical Research Centre; Liverpool School of Tropical Medicine and the University of Oxford; Imperial National Institute for Health and Care Research Biomedical Research Centre; and preparedness work conducted by the Short Period Incidence Study of Severe Acute Respiratory Infection. This work uses data provided by patients and collected by the National Health Service as part of their care and support #DataSavesLives. The data used for this research were obtained from ISARIC Comprehensive Clinical Characterisation Collaboration. We are extremely grateful to the 2648 frontline National Health Service clinical and research staff and volunteer medical students who collected these data in challenging circumstances as well as the generosity of the patients and their families for their individual contributions in difficult times. COVID-Clinical Information Network data were collated by ISARIC Comprehensive Clinical Characterisation Collaboration Investigators. We also acknowledge the support of Jeremy J. Farrar and Nahoko Shindo.
Funding
This work was made possible by the UK Foreign, Commonwealth and Development Office and Wellcome (215091/Z/18/Z, 222410/Z/21/Z, 225288/Z/22/Z and 220757/Z/20/Z); the Bill & Melinda Gates Foundation (OPP1209135); philanthropic support of the donors to the University of Oxford’s COVID-19 Research Response Fund (0009109); CIHR Coronavirus Rapid Research Funding Opportunity OV2170359 and coordination in Canada by Sunnybrook Research Institute; funding by the Health Research Board of Ireland (CTN-2014-12); Rapid European COVID-19 Emergency Response research (H2020 project 101003589) and European Clinical Research Alliance on Infectious Diseases (965313); the Research Council of Norway grant no 312780, and a philanthropic donation from Vivaldi Invest A/S owned by Jon Stephenson von Tetzchner; the Comprehensive Local Research Networks, of which PJMO is an NIHR Senior Investigator (NIHR201385); Innovative Medicines Initiative Joint Undertaking under Grant Agreement No. 115523 Combating Bacterial Resistance in Europe, the resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007- 2013) and European Federation of Pharmaceutical Industries and Associations companies, in-kind contribution; the French COVID cohort (NCT04262921) is sponsored by INSERM and is funded by the REACTing (REsearch & ACtion emergING infectious diseases) consortium and by a grant of the French Ministry of Health (PHRC n 20-0424); Stiftungsfonds zur Förderung der Bekämpfung der Tuberkulose und anderer Lungenkrankheiten of the City of Vienna, Project Number: APCOV22BGM; Italian Ministry of Health “Fondi Ricerca corrente–L1P6” to IRCCS Ospedale Sacro Cuore–Don Calabria; Australian Department of Health grant (3273191); Gender Equity Strategic Fund at University of Queensland, Artificial Intelligence for Pandemics (A14PAN) at University of Queensland, the Australian Research Council Centre of Excellence for Engineered Quantum Systems (CE170100009), the Prince Charles Hospital Foundation, Australia; Brazil, National Council for Scientific and Technological Development Scholarship number 303953/2018- 7; the Firland Foundation, Shoreline, Washington, USA; a grant from foundation Bevordering Onderzoek Franciscus; the South Eastern Norway Health Authority and the Research Council of Norway; Institute for Clinical Research, National Institutes of Health supported by the Ministry of Health Malaysia; grants from the National Institute for Health Research (NIHR; award CO-CIN-01), the Medical Research Council (grant MC_PC_19059), and by the NIHR Health Protection Research Unit in Emerging and Zoonotic Infections at University of Liverpool in partnership with Public Health England (award 200907), NIHR Health Protection Research Unit in Respiratory Infections at Imperial College London with Public Health England (award 200927), Liverpool Experimental Cancer Medicine Centre (grant C18616/A25153), NIHR Biomedical Research Centre at Imperial College London (award IS-BRC-1215-20013), and NIHR Clinical Research Network providing infrastructure support. M.J.G. received support through the Faculty Small Grant Program from the Vice President for Research of the University of Utah to fund statistical analysis. This project was supported by the University Research Committee and the Study Design and Biostatistics Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, Grant 8UL1TR000105 (formerly UL1RR025764)) at the University of Utah and by Universidad de La Sabana (MED-309-2021). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the University Research Committee, Vice President for Research Office, or University of Utah.
Ethics statement
Ethics approval and informed consent were obtained at each site by the site investigators, according to local regulations, which included a waiver of consent to collect de-identified data at several sites due to the burden on front-line workers and the data protection framework in place. The WHO-ISARIC Clinical Characterization Protocol was approved by the WHO Ethics Committee (RPC571 and RPC572) (Reference [22], pages 2-3).
Author contributions
M.J.G. was involved in study conception and design, acquisition of data, analysis and interpretation, drafting and revision of the article, as well as conception and revision of figures. P.T.B., S.-M.C., and L.F.R. were involved in study design, analysis and interpretation of data, revision of the article. D.P.E. was involved in statistical analysis, data cleaning, analysis of data, composition and formatting of figures, and drafting of the manuscript. D.R. was involved in study conception and design, analysis and interpretation of data, and manuscript drafting and revising. L.M. was involved in study design, data acquisition, interpretation of results, revision of data analysis, and proofreading. B.W.C. was involved in study design, data acquisition, and revision of the manuscript. J.F. and P.M.A.A. were involved in study design and interpretation of results. J.F. was involved in concept and design of database and contribution of data. H.D. was involved in concept and design of the study. All authors read and approved the final version of the paper.
Relationship disclosure
There are no competing interests to disclose.
Data availability
The data that underpin this analysis are highly detailed clinical data on individuals hospitalized for COVID-19. Due to the sensitive nature of these data and the associated privacy concerns, they are available via a governed data access mechanism following review of a data access committee. Data can be requested via the Infectious Disease Data Observatory COVID-19 Data Sharing Platform (http://www.iddo.org/covid-19). The Data Access Application, Terms of Access, and details of the Data Access Committee are available on the website. Briefly, the requirements for access are a request from a qualified researcher working with a legal entity who has a health and/or research remit; a scientifically valid reason for data access that adheres to appropriate ethical principles. The full terms are at https://www.iddo.org/document/covid-19-data-access-guidelines. A small subset of sites that contributed data to this analysis have not agreed to pooled data sharing as above. In the case of requiring access to these data, please contact the corresponding author in the first instance, who will look to facilitate access.
Footnotes
Handling Editor: Pantep Angchaisuksiri
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2023.102142
Contributor Information
Matthew J. Griffee, Email: Matthew.griffee@hsc.utah.edu.
ISARIC Clinical Characterisation Group:
Sheryl Ann Abdukahil, Nurul Najmee Abdulkadir, Ryuzo Abe, Laurent Abel, Amal Abrous, Lara Absil, Andrew Acker, Elisabeth Adam, Diana Adrião, Saleh Al Ageel, Shakeel Ahmed, Kate Ainscough, Tharwat Aisa, Ali Ait Hssain, Younes Ait Tamlihat, Takako Akimoto, Ernita Akmal, Chika Akwani, Eman Al Qasim, Razi Alalqam, Angela Alberti, Tala Al-dabbous, Senthilkumar Alegesan, Marta Alessi, Beatrice Alex, Kévin Alexandre, Abdulrahman Al-Fares, Huda Alfoudri, Imran Ali, Kazali Enagnon Alidjnou, Jeffrey Aliudin, Qabas Alkhafajee, Clotilde Allavena, Nathalie Allou, João Alves, Rita Alves, João Melo Alves, Joana Alves Cabrita, Maria Amaral, Nur Amira, Phoebe Ampaw, Roberto Andini, Claire Andréjak, Andrea Angheben, François Angoulvant, Séverine Ansart, Sivanesen Anthonidass, Massimo Antonelli, Carlos Alexandre Antunes de Brito, Ardiyan Apriyana, Yaseen Arabi, Irene Aragao, Francisco Arancibia, Carolline Araujo, Antonio Arcadipane, Patrick Archambault, Lukas Arenz, Jean-Benoît Arlet, Christel Arnold-Day, Lovkesh Arora, Rakesh Arora, Elise Artaud-Macari, Diptesh Aryal, Angel Asensio, Muhammad Ashraf, Jean Baptiste Assie, Amirul Asyraf, Minahel Atif, Anika Atique, Johann Auchabie, Hugues Aumaitre, Adrien Auvet, Laurène Azemar, Cecile Azoulay, Benjamin Bach, Delphine Bachelet, Claudine Badr, Nadia Baig, J. Kenneth Baillie, Erica Bak, Agamemnon Bakakos, Nazreen Abu Bakar, Andriy Bal, Mohanaprasanth Balakrishnan, Valeria Balan, Firouzé Bani-Sadr, Renata Barbalho, Nicholas Yuri Barbosa, Wendy S. Barclay, Saef Umar Barnett, Michaela Barnikel, Helena Barrasa, Audrey Barrelet, Cleide Barrigoto, Marie Bartoli, Joaquín Baruch, Romain Basmaci, Muhammad Fadhli Hassin Basri, Denise Battaglini, Jules Bauer, Diego Fernando Bautista Rincon, Abigail Beane, Alexandra Bedossa, Ker Hong Bee, Husna Begum, Sylvie Behilill, Albertus Beishuizen, Aleksandr Beljantsev, David Bellemare, Anna Beltrame, Beatriz Amorim Beltrão, Marine Beluze, Nicolas Benech, Lionel Eric Benjiman, Dehbia Benkerrou, Suzanne Bennett, Binny Benny, Luís Bento, Jan-Erik Berdal, Delphine Bergeaud, Hazel Bergin, José Luis Bernal Sobrino, Giulia Bertoli, Lorenzo Bertolino, Simon Bessis, Sybille Bevilcaqua, Karine Bezulier, Amar Bhatt, Krishna Bhavsar, Claudia Bianco, Farah Nadiah Bidin, Moirangthem Bikram Singh, Felwa Bin Humaid, Mohd Nazlin Bin Kamarudin, Zeno Bisoffi, François Bissuel, Laurent Bitker, Jonathan Bitton, Pablo Blanco-Schweizer, Catherine Blier, Frank Bloos, Mathieu Blot, Lucille Blumberg, Filomena Boccia, Laetitia Bodenes, Debby Bogaert, Anne-Hélène Boivin, Isabela Bolaños, Pierre-Adrien Bolze, François Bompart, Diogo Borges, Raphaël Borie, Hans Martin Bosse, Elisabeth Botelho-Nevers, Lila Bouadma, Olivier Bouchaud, Sabelline Bouchez, Dounia Bouhmani, Damien Bouhour, Kévin Bouiller, Laurence Bouillet, Camile Bouisse, Anne-Sophie Boureau, John Bourke, Maude Bouscambert, Aurore Bousquet, Jason Bouziotis, Bianca Boxma, Marielle Boyer-Besseyre, Maria Boylan, Fernando Augusto Bozza, Axelle Braconnier, Cynthia Braga, Timo Brandenburger, Filipa Brás Monteiro, Luca Brazzi, Patrick Breen, Dorothy Breen, David Brewster, Kathy Brickell, Shaunagh Browne, Alex Browne, Nicolas Brozzi, Marjolein Brusse-Keizer, Petra Bryda, Nina Buchtele, Marielle Buisson, Erlina Burhan, Aidan Burrell, Ingrid G. Bustos, André Cabie, Susana Cabral, Eder Caceres, Cyril Cadoz, Rui Caetano Garcês, Kate Calligy, Jose Andres Calvache, João Camões, Valentine Campana, Paul Campbell, Josie Campisi, Cecilia Canepa, Mireia Cantero, Pauline Caraux-Paz, Sheila Cárcel, Chiara Simona Cardellino, Sofia Cardoso, Filipe Cardoso, Filipa Cardoso, Nelson Cardoso, Simone Carelli, Nicolas Carlier, Thierry Carmoi, Gayle Carney, Inês Carqueja, Marie-Christine Carret, François Martin Carrier, Ida Carroll, Gail Carson, Maire-Laure Casanova, Mariana Cascão, Siobhan Casey, José Casimiro, Bailey Cassandra, Silvia Castañeda, Nidyanara Castanheira, Guylaine Castor-Alexandre, Ivo Castro, Ana Catarino, François-Xavier Catherine, Paolo Cattaneo, Roberta Cavalin, Alexandros Cavayas, Minerva Cervantes-Gonzalez, Anissa Chair, Catherine Chakveatze, Adrienne Chan, Meera Chand, Christelle Chantalat Auger, Jean-Marc Chapplain, Charlotte Charpentier, Julie Chas, Jonathan Samuel Chávez Iñiguez, Anjellica Chen, Yih-Sharng Chen, Léo Chenard, Matthew Pellan Cheng, Antoine Cheret, Thibault Chiarabini, Julian Chica, Suresh Kumar Chidambaram, Leong Chin Tho, Catherine Chirouze, Davide Chiumello, Sung-Min Cho, Bernard Cholley, Marie-Charlotte Chopin, Ting Soo Chow, Hiu Jian Chua, Jonathan Chua, Jose Pedro Cidade, José Miguel Cisneros Herreros, Barbara Wanjiru Citarella, Anna Ciullo, Jennifer Clarke, Emma Clarke, Rolando Claure-Del Granado, Sara Clohisey, Cassidy Codan, Caitriona Cody, Alexandra Coelho, Megan Coles, Jennifer Coles, Gwenhaël Colin, Michael Collins, Sebastiano Maria Colombo, Pamela Combs, Jennifer Connolly, Marie Connor, Anne Conrad, Elaine Conway, Graham S. Cooke, Mary Copland, Hugues Cordel, Amanda Corley, Sabine Cornelis, Alexander Daniel Cornet, Arianne Joy Corpuz, Andrea Cortegiani, Grégory Corvaisier, Emma Costigan, Camille Couffignal, Sandrine Couffin-Cadiergues, Roxane Courtois, Stéphanie Cousse, Rachel Cregan, Cosimo Cristella, Sabine Croonen, Gloria Crowl, Jonathan Crump, Claudina Cruz, Juan Luis Cruz Bermúdez, Jaime Cruz Rojo, Marc Csete, Ailbhe Cullen, Matthew Cummings, Gerard Curley, Elodie Curlier, Colleen Curran, Paula Custodio, Ana da Silva Filipe, Charlene Da Silveira, Al-Awwab Dabaliz, Andrew Dagens, Darren Dahly, Heidi Dalton, Jo Dalton, Seamus Daly, Juliana Damas, Nick Daneman, Corinne Daniel, Emmanuelle A. Dankwa, Jorge Dantas, Frédérick D'Aragon, Menno de Jong, Gillian de Loughry, Etienne De Montmollin, Rafael Freitas de Oliveira França, Ana Isabel de Pinho Oliveira, Rosanna De Rosa, Thushan de Silva, Peter de Vries, David Dean, Alexa Debard, Bianca DeBenedictis, Marie-Pierre Debray, Nathalie DeCastro, William Dechert, Lauren Deconninck, Romain Decours, Eve Defous, Isabelle Delacroix, Eric Delaveuve, Karen Delavigne, Nathalie M. Delfos, Ionna Deligiannis, Andrea Dell'Amore, Christelle Delmas, Pierre Delobel, Corine Delsing, Elisa Demonchy, Emmanuelle Denis, Dominique Deplanque, Pieter Depuydt, Mehul Desai, Diane Descamps, Mathilde Desvallées, Santi Dewayanti, Pathik Dhanger, Alpha Diallo, Sylvain Diamantis, André Dias, Fernanda Dias Da Silva, Juan Jose Diaz, Rodrigo Diaz, Priscila Diaz, Kévin Didier, Jean-Luc Diehl, Wim Dieperink, Jérôme Dimet, Vincent Dinot, Fara Diop, Alphonsine Diouf, Yael Dishon, Félix Djossou, Annemarie B. Docherty, Helen Doherty, Maria Donnelly, Christl A. Donnelly, Sean Donohue, Yoann Donohue, Chloe Donohue, Peter Doran, Céline Dorival, Eric D'Ortenzio, James Joshua Douglas, Nathalie Dournon, Triona Downer, Joanne Downey, Mark Downing, Tom Drake, Aoife Driscoll, Murray Dryden, Claudio Duarte Fonseca, Vincent Dubee, François Dubos, Alexandre Ducancelle, Toni Duculan, Susanne Dudman, Abhijit Duggal, Paul Dunand, Jake Dunning, Mathilde Duplaix, Emanuele Durante-Mangoni, Lucian Durham, III, Bertrand Dussol, Juliette Duthoit, Xavier Duval, Anne Margarita Dyrhol-Riise, Sim Choon Ean, Marco Echeverria-Villalobos, Siobhan Egan, Carla Eira, Mohammed El Sanharawi, Subbarao Elapavaluru, Brigitte Elharrar, Jacobien Ellerbroek, Philippine Eloy, Tarek Elshazly, Isabelle Enderle, Tomoyuki Endo, Chan Chee Eng, Ilka Engelmann, Vincent Enouf, Olivier Epaulard, Martina Escher, Mariano Esperatti, Hélène Esperou, Catarina Espírito Santo, Marina Esposito-Farese, João Estevão, Manuel Etienne, Nadia Ettalhaoui, Anna Greti Everding, Mirjam Evers, Isabelle Fabre, Marc Fabre, Amna Faheem, Arabella Fahy, Cameron J. Fairfield, Pedro Faria, Hanan Fateena, Arie Zainul Fatoni, Karine Faure, Raphaël Favory, Mohamed Fayed, Niamh Feely, Laura Feeney, Jorge Fernandes, Marília Andreia Fernandes, Susana Fernandes, François-Xavier Ferrand, Eglantine Ferrand Devouge, Joana Ferrão, Mário Ferraz, Sílvia Ferreira, Bernardo Ferreira, Benigno Ferreira, Isabel Ferreira, Nicolas Ferriere, Céline Ficko, Claudia Figueiredo-Mello, Juan Fiorda, Thomas Flament, Clara Flateau, Tom Fletcher, Aline-Marie Florence, Letizia Lucia Florio, Deirdre Flynn, Claire Foley, Jean Foley, Tatiana Fonseca, Simon Forsyth, Denise Foster, Giuseppe Foti, Erwan Fourn, Robert A. Fowler, Marianne Fraher, Diego Franch-Llasat, John F. Fraser, Christophe Fraser, Marcela Vieira Freire, Ana Freitas Ribeiro, Ricardo Fritz, Stéphanie Fry, Nora Fuentes, Masahiro Fukuda, Valérie Gaborieau, Rostane Gaci, Massimo Gagliardi, Jean-Charles Gagnard, Amandine Gagneux-Brunon, Sérgio Gaião, Linda Gail Skeie, Phil Gallagher, Carrol Gamble, Yasmin Gani, Arthur Garan, Rebekha Garcia, Noelia García Barrio, Esteban Garcia-Gallo, Denis Garot, Valérie Garrait, Nathalie Gault, Aisling Gavin, Anatoliy Gavrylov, Alexandre Gaymard, Johannes Gebauer, Eva Geraud, Louis Gerbaud Morlaes, Nuno Germano, Jade Ghosn, Marco Giani, Jess Gibson, Tristan Gigante, Morgane Gilg, Elaine Gilroy, Guillermo Giordano, Michelle Girvan, Valérie Gissot, Daniel Glikman, Eric Gnall, François Goehringer, Siri Goepel, Jean-Christophe Goffard, Jin Yi Goh, Jonathan Golob, Joan Gómez-Junyent, Marie Gominet, Alicia Gonzalez, Patricia Gordon, Isabelle Gorenne, Conor Gormley, Laure Goubert, Cécile Goujard, Tiphaine Goulenok, Margarite Grable, Jeronimo Graf, Edward Wilson Grandin, Pascal Granier, Giacomo Grasselli, Christopher A. Green, Courtney Greene, William Greenhalf, Segolène Greffe, Domenico Luca Grieco, Matthew Griffee, Fiona Griffiths, Ioana Grigoras, Albert Groenendijk, Heidi Gruner, Yusing Gu, Jérémie Guedj, Martin Guego, Dewi Guellec, Daniela Guerreiro, Romain Guery, Anne Guillaumot, Laurent Guilleminault, Maisa Guimarães de Castro, Thomas Guimard, Marieke Haalboom, Daniel Haber, Hannah Habraken, Ali Hachemi, Nadir Hadri, Sheeba Hakak, Adam Hall, Matthew Hall, Sophie Halpin, Ansley Hamer, Rebecca Hamidfar, Terese Hammond, Naomi Hammond, Lim Yuen Han, Rashan Haniffa, Kok Wei Hao, Hayley Hardwick, Ewen M. Harrison, Janet Harrison, Samuel Bernard Ekow Harrison, Alan Hartman, Junaid Hashmi, Ailbhe Hayes, Leanne Hays, Jan Heerman, Lars Heggelund, Ross Hendry, Martina Hennessy, Aquiles Henriquez-Trujillo, Maxime Hentzien, Diana Hernandez, Jaime Hernandez-Montfort, Andrew Hershey, Liv Hesstvedt, Eibhlin Higgins, Dawn Higgins, Rupert Higgins, Rita Hinchion, Samuel Hinton, Hiroaki Hiraiwa, Hikombo Hitoto, Antonia Ho, Yi Bin Ho, Alexandre Hoctin, Isabelle Hoffmann, Wei Han Hoh, Oscar Hoiting, Rebecca Holt, Jan Cato Holter, Peter Horby, Juan Pablo Horcajada, Koji Hoshino, Ikram Houas, Catherine L. Hough, Stuart Houltham, Jimmy Ming-Yang Hsu, Jean-Sébastien Hulot, Abby Hurd, Samreen Ijaz, M. Arfan Ikram, Hajnal-Gabriela Illes, Patrick Imbert, Hugo Inácio, Carmen Infante Dominguez, Yun Sii Ing, Elias Iosifidis, Mariachiara Ippolito, Sarah Isgett, Tiago Isidoro, Nadiah Ismail, Margaux Isnard, Junji Itai, Daniel Ivulich, Danielle Jaafar, Salma Jaafoura, Julien Jabot, Clare Jackson, Victoria Janes, Pierre Jaquet, Waasila Jassat, Coline Jaud-Fischer, Stéphane Jaureguiberry, Denise Jaworsky, Florence Jego, Anilawati Mat Jelani, Synne Jenum, Ruth Jimbo-Sotomayor, Ong Yiaw Joe, Ruth N. Jorge García, Cédric Joseph, Mark Joseph, Mercé Jourdain, Philippe Jouvet, Anna Jung, Hanna Jung, Dafsah Juzar, Ouifiya Kafif, Florentia Kaguelidou, Neerusha Kaisbain, Thavamany Kaleesvran, Sabina Kali, Smaragdi Kalomoiri, Muhammad Aisar Ayadi Kamaluddin, Zul Amali Che Kamaruddin, Nadiah Kamarudin, Darshana Hewa Kandamby, Chris Kandel, Kong Yeow Kang, Pratap Karpayah, Christiana Kartsonaki, Daisuke Kasugai, Anant Kataria, Kevin Katz, Aasmine Kaur, Christy Kay, Lamees Kayyali, Hannah Keane, Seán Keating, Claire Kelly, Yvelynne Kelly, Andrea Kelly, Niamh Kelly, Aoife Kelly, Sadie Kelly, Maeve Kelsey, Ryan Kennedy, Kalynn Kennon, Maeve Kernan, Younes Kerroumi, Sharma Keshav, Imrana Khalid, Antoine Khalil, Coralie Khan, Irfan Khan, Michelle E. Kho, Saye Khoo, Ryan Khoo, Denisa Khoo, Khor How Kiat, Yuri Kida, Peter Kiiza, Beathe Kiland Granerud, Anders Benjamin Kildal, Antoine Kimmoun, Detlef Kindgen-Milles, Alexander King, Nobuya Kitamura, Paul Klenerman, Rob Klont, Gry Kloumann Bekken, Stephen R. Knight, Malte Kohns Vasconcelos, Mamoru Komatsu, Caroline Kosgei, Arsène Kpangon, Karolina Krawczyk, Sudhir Krishnan, Vinothini Krishnan, Oksana Kruglova, Deepali Kumar, Ganesh Kumar, Pavan Kumar Vecham, Dinesh Kuriakose, Ethan Kurtzman, Demetrios Kutsogiannis, Galyna Kutsyna, Konstantinos Kyriakoulis, Marie Lachatre, Marie Lacoste, John G. Laffey, Nadhem Lafhej, Marie Lagrange, Fabrice Laine, Olivier Lairez, Antonio Lalueza, Marc Lambert, François Lamontagne, Marie Langelot-Richard, Vincent Langlois, Eka Yudha Lantang, Marina Lanza, Cédric Laouénan, Samira Laribi, Delphine Lariviere, Stéphane Lasry, Odile Launay, Didier Laureillard, Yoan Lavie-Badie, Andy Law, Teresa Lawrence, Cassie Lawrence, Minh Le, Clément Le Bihan, Cyril Le Bris, Georges Le Falher, Lucie Le Fevre, Quentin Le Hingrat, Marion Le Maréchal, Soizic Le Mestre, Gwenaël Le Moal, Vincent Le Moing, Hervé Le Nagard, Paul LeTurnier, Ema Leal, Marta Leal Santos, Todd C. Lee, Su Hwan Lee, James Lee, Jennifer Lee, Heng Gee Lee, Biing Horng Lee, Yi Lin Lee, Gary Leeming, Bénédicte Lefebvre, Laurent Lefebvre, Benjamin Lefèvre, Sylvie LeGac, Jean-Daniel Lelievre, François Lellouche, Adrien Lemaignen, Véronique Lemee, Anthony Lemeur, Gretchen Lemmink, Ha Sha Lene, Jenny Lennon, Rafael León, Marc Leone, Michela Leone, Quentin Lepiller, François-Xavier Lescure, Olivier Lesens, Mathieu Lesouhaitier, Amy Lester-Grant, Sophie Letrou, Bruno Levy, Yves Levy, Claire Levy-Marchal, Katarzyna Lewandowska, Erwan L'Her, Gianluigi Li Bassi, Geoffrey Liegeon, Wei Shen Lim, Kah Chuan Lim, Chantre Lima, Bruno Lina, Lim Lina, Andreas Lind, Guillaume Lingas, Sylvie Lion-Daolio, Samantha Lissauer, Keibun Liu, Marine Livrozet, Patricia Lizotte, Navy Lolong, Leong Chee Loon, Diogo Lopes, Dalia Lopez-Colon, Anthony L. Loschner, Paul Loubet, Bouchra Loufti, Guillame Louis, Silvia Lourenco, Lee Lee Low, Marije Lowik, Jia Shyi Loy, Jean-Christophe Lucet, Carlos Lumbreras Bermejo, Carlos M. Luna, Olguta Lungu, Liem Luong, Nestor Luque, Dominique Luton, Nilar Lwin, Ruth Lyons, Olavi Maasikas, Oryane Mabiala, Moïse Machado, Sara Machado, Gabriel Macheda, Hashmi Madiha, Guillermo Maestro de la Calle, Rafael Mahieu, Sophie Mahy, Ana Raquel Maia, Lars S. Maier, Mylène Maillet, Thomas Maitre, Maximilian Malfertheiner, Nadia Malik, Paddy Mallon, Fernando Maltez, Denis Malvy, Victoria Manda, Laurent Mandelbrot, Frank Manetta, Julie Mankikian, Edmund Manning, Aldric Manuel, Ceila Maria Sant`Ana Malaque, Flávio Marino, Samuel Markowicz, Ana Marques, Catherine Marquis, Brian Marsh, Laura Marsh, Megan Marshal, John Marshall, Celina Turchi Martelli, Emily Martin, Guillaume Martin-Blondel, F. Eduardo Martinez, Ignacio Martin-Loeches, Martin Martinot, João Martins, Ana Martins, Nuno Martins, Caroline Martins Rego, Gennaro Martucci, Olga Martynenko, Eva Miranda Marwali, Marsilla Marzukie, David Maslove, Sabina Mason, Mohd Basri Mat Nor, Moshe Matan, Daniel Mathieu, Mathieu Mattei, Laurence Maulin, Michael Maxwell, Javier Maynar, Thierry Mazzoni, Lisa Mc Sweeney, Colin McArthur, Peter McCanny, Anne McCarthy, Aine McCarthy, Colin McCloskey, Rachael McConnochie, Sherry McDermott, Sarah E. McDonald, Aine McElroy, Samuel McElwee, Victoria McEneany, Natalie McEvoy, Allison McGeer, Chris McKay, Johnny McKeown, Kenneth A. McLean, Paul McNally, Bairbre McNicholas, Elaine McPartlan, Edel Meaney, Cécile Mear-Passard, Maggie Mechlin, Omar Mehkri, Ferruccio Mele, Luis Melo, Joao Joao Mendes, Kusum Menon, France Mentré, Alexander J. Mentzer, Emmanuelle Mercier, Noémie Mercier, Antoine Merckx, Mayka Mergeay-Fabre, Blake Mergler, Laura Merson, António Mesquita, Osama Metwally, Agnès Meybeck, Dan Meyer, Alison M. Meynert, Vanina Meysonnier, Amina Meziane, Mehdi Mezidi, Céline Michelanglei, Isabelle Michelet, Efstathia Mihelis, Vladislav Mihnovit, Jennene Miller, Hugo Miranda-Maldonado, Nor Arisah Misnan, Tahira Jamal Mohamed, Nik Nur Eliza Mohamed, Asma Moin, Elena Molinos, Brenda Molloy, Sinead Monahan, Mary Mone, Agostinho Monteiro, Claudia Montes, Giorgia Montrucchio, Shona C. Moore, Sarah Moore, Lina Morales Cely, Lucia Moro, Ben Morton, Catherine Motherway, Ana Motos, Hugo Mouquet, Clara Mouton Perrot, Julien Moyet, Caroline Mudara, Ng Yong Muh, Dzawani Muhamad, Jimmy Mullaert, Fredrik Müller, Karl Erik Müller, Aisling Murphy, Lorna Murphy, Patrick Murray, Marlène Murris, Srinivas Murthy, Himed Musaab, Gugapriyaa Muyandy, Dimitra Melia Myrodia, Dave Nagpal, Alex Nagrebetsky, Mangala Narasimhan, Nageswaran Narayanan, Alasdair Nazerali-Maitland, Nadège Neant, Holger Neb, Raul Neto, Emily Neumann, Pauline Yeung Ng, Wing Yiu Ng, Anthony Nghi, Duc Nguyen, Orna Ni Choileain, Niamh Ni Leathlobhair, Alistair D. Nichol, Prompak Nitayavardhana, Stephanie Nonas, Nurul Amani Mohd Noordin, Marion Noret, Nurul Faten Izzati Norharizam, Lisa Norman, Mahdad Noursadeghi, Adam Nowinski, Saad Nseir, Jose I. Nunez, Elsa Nyamankolly, Fionnuala O. Brien, Annmarie O. Callaghan, Annmarie O'Callaghan, Giovanna Occhipinti, Derbrenn OConnor, Max O'Donnell, Tawnya Ogston, Takayuki Ogura, Tak-Hyuk Oh, Sophie O'Halloran, Katie O'Hearn, Shinichiro Ohshimo, João Oliveira, Larissa Oliveira, Piero L. Olliaro, David S.Y. Ong, Jee Yan Ong, Wilna Oosthuyzen, Anne Opavsky, Peter Openshaw, Claudia Milena Orozco-Chamorro, Jamel Ortoleva, Javier Osatnik, Linda O'Shea, Miriam O'Sullivan, Siti Zubaidah Othman, Nadia Ouamara, Rachida Ouissa, Eric Oziol, Maïder Pagadoy, Justine Pages, Amanda Palacios, Massimo Palmarini, Giovanna Panarello, Prasan Kumar Panda, Lai Hui Pang, Mauro Panigada, Nathalie Pansu, Aurélie Papadopoulos, Rachael Parke, Jérémie Pasquier, Bruno Pastene, Fabian Patauner, Mohan Dass Pathmanathan, Luís Patrão, Patricia Patricio, Juliette Patrier, Lisa Patterson, Christelle Paul, Mical Paul, Jorge Paulos, William A. Paxton, Jean-François Payen, Sandra L. Peake, Kalaiarasu Peariasamy, Miguel Pedrera Jiménez, Giles J. Peek, Florent Peelman, Nathan Peiffer-Smadja, Vincent Peigne, Mare Pejkovska, Paolo Pelosi, Ithan D. Peltan, Rui Pereira, Daniel Perez, Luis Periel, Thomas Perpoint, Antonio Pesenti, Vincent Pestre, Lenka Petrou, Michele Petrovic, Ventzislava Petrov-Sanchez, Frank Olav Pettersen, Gilles Peytavin, Scott Pharand, Walter Picard, Olivier Picone, Maria de Piero, Carola Pierobon, Djura Piersma, Carlos Pimentel, Raquel Pinto, Valentine Piquard, Catarina Pires, Isabelle Pironneau, Lionel Piroth, Chiara Piubelli, Riinu Pius, Laurent Plantier, Hon Shen Png, Julien Poissy, Ryadh Pokeerbux, Sergio Poli, Georgios Pollakis, Diane Ponscarme, Diego Bastos Porto, Andra-Maris Post, Douwe F. Postma, Pedro Povoa, Diana Póvoas, Jeff Powis, Sofia Prapa, Sébastien Preau, Christian Prebensen, Jean-Charles Preiser, Anton Prinssen, Mark G. Pritchard, Lucia Proença, Sravya Pudota, Oriane Puéchal, Bambang Pujo Semedi, Gregory Purcell, Luisa Quesada, Víctor Quirós González, Else Quist-Paulsen, Mohammed Quraishi, Fadi Qutishat, Christian Rabaud, Aldo Rafael, Marie Rafiq, Rozanah Abd Rahman, Ahmad Kashfi Haji Ab Rahman, Fernando Rainieri, Giri Shan Rajahram, Nagarajan Ramakrishnan, José Ramalho, Ahmad Afiq Ramli, Blandine Rammaert, Grazielle Viana Ramos, Ritika Ranjan, Christophe Rapp, Menaldi Rasmin, Indrek Rätsep, Tharmini Ravi, Andre Real, Stanislas Rebaudet, Sarah Redl, Brenda Reeve, Liadain Reid, Dag Henrik Reikvam, Renato Reis, Jordi Rello, Jonathan Remppis, Martine Remy, Hongru Ren, Hanna Renk, Anne-Sophie Resseguier, Matthieu Revest, Oleksa Rewa, Tiago Reyes, Luis Felipe Reyes, Maria Ines Ribeiro, David Richardson, Denise Richardson, Laurent Richier, Siti Nurul Atikah Ahmad Ridzuan, Ana L. Rios, Asgar Rishu, Patrick Rispal, Karine Risso, Nicholas Rizer, Chiara Robba, André Roberto, Stephanie Roberts, David L. Robertson, Olivier Robineau, Ferran Roche-Campo, Paola Rodari, Simão Rodeia, Bernhard Roessler, Pierre-Marie Roger, Claire Roger, Emmanuel Roilides, Amanda Rojek, Juliette Romaru, Roberto Roncon-Albuquerque, Jr., Mélanie Roriz, Manuel Rosa-Calatrava, Michael Rose, Dorothea Rosenberger, Andrea Rossanese, Matteo Rossetti, Bénédicte Rossignol, Patrick Rossignol, Stella Rousset, Carine Roy, Benoît Roze, Clark D. Russell, Maria Ryan, Maeve Ryan, Steffi Ryckaert, Aleksander Rygh Holten, Isabela Saba, Musharaf Sadat, Valla Sahraei, Maximilien Saint-Gilles, Pranya Sakiyalak, Leonardo Salazar, Gabriele Sales, Stéphane Sallaberry, Charlotte Salmon Gandonniere, Hélène Salvator, Olivier Sanchez, Emely Sanchez, Angel Sanchez-Miralles, Vanessa Sancho-Shimizu, Gyan Sandhu, Zulfiqar Sandhu, Pierre-François Sandrine, Oana Sandulescu, Marlene Santos, Shirley Sarfo-Mensah, Bruno Sarmento Banheiro, Iam Claire E. Sarmiento, Benjamin Sarton, Sree Satyapriya, Rumaisah Satyawati, Egle Saviciute, Parthena Savvidou, Yen Tsen Saw, Justin Schaffer, Tjard Schermer, Arnaud Scherpereel, Marion Schneider, Stephan Schroll, Michael Schwameis, Brendan Scicluna, Janet T. Scott, James Scott-Brown, Nicholas Sedillot, Tamara Seitz, Mageswari Selvarajoo, Caroline Semaille, Malcolm G. Semple, Rasidah Bt Senian, Eric Senneville, Claudia Sepulveda, Filipa Sequeira, Tânia Sequeira, Ary Serpa Neto, Pablo Serrano Balazote, Ellen Shadowitz, Syamin Asyraf Shahidan, Mohammad Shamsah, Shaikh Sharjeel, Pratima Sharma, Catherine A. Shaw, Victoria Shaw, John Robert Sheenan, Haixia Shi, Nobuaki Shime, Keiki Shimizu, Sally Shrapnel, Hoi Ping Shum, Nassima Si Mohammed, Ng Yong Siang, Jeanne Sibiude, Atif Siddiqui, Louise Sigfrid, Piret Sillaots, Catarina Silva, Rogério Silva, Maria Joao Silva, Benedict Sim Lim Heng, Wai Ching Sin, Punam Singh, Budha Charan Singh, Pompini Agustina Sitompul, Karisha Sivam, Vegard Skogen, Sue Smith, Benjamin Smood, Coilin Smyth, Michelle Smyth, Morgane Snacken, Dominic So, Tze Vee Soh, Joshua Solomon, Tom Solomon, Emily Somers, Agnès Sommet, Rima Song, Myung Jin Song, Tae Song, Jack Song Chia, Michael Sonntagbauer, Azlan Mat Soom, Albert Sotto, Edouard Soum, Marta Sousa, Ana Chora Sousa, Maria Sousa Uva, Vicente Souza-Dantas, Alexandra Sperry, Elisabetta Spinuzza, Shiranee Sriskandan, Sarah Stabler, Thomas Staudinger, Stephanie-Susanne Stecher, Trude Steinsvik, Ymkje Stienstra, Birgitte Stiksrud, Eva Stolz, Amy Stone, Adrian Streinu-Cercel, Anca Streinu-Cercel, Ami Stuart, David Stuart, Richa Su, Jacky Y. Suen, Gabriel Suen, Prasanth Sukumar, Asfia Sultana, Charlotte Summers, Dubravka Supic, Deepashankari Suppiah, Magdalena Surovcová, Sarah Syahrin, Konstantinos Syrigos, Jaques Sztajnbok, Konstanty Szuldrzynski, Shirin Tabrizi, Fabio S. Taccone, Lysa Tagherset, Shahdattul Mawarni Taib, Sara Taleb, Jelmer Talsma, Maria Lawrensia Tampubolon, Kim Keat Tan, Yan Chyi Tan, Hiroyuki Tanaka, Taku Tanaka, Hayato Taniguchi, Coralie Tardivon, Pierre Tattevin, M. Azhari Taufik, Hassan Tawfik, Richard S. Tedder, Tze Yuan Tee, João Teixeira, Sofia Tejada, Marie-Capucine Tellier, Sze Kye Teoh, Vanessa Teotonio, François Téoulé, Pleun Terpstra, Olivier Terrier, Nicolas Terzi, Hubert Tessier-Grenier, Adrian Tey, Alif Adlan Mohd Thabit, Zhang Duan Tham, Suvintheran Thangavelu, Elmi Theron, Vincent Thibault, Simon-Djamel Thiberville, Benoît Thill, Jananee Thirumanickam, Shaun Thompson, David Thomson, Emma C. Thomson, Mathew Thorpe, Surain Raaj Thanga Thurai, Ryan S. Thwaites, Paul Tierney, Vadim Tieroshyn, Jean-François Timsit, Noémie Tissot, Fiona Toal, Jordan Zhien Yang Toh, Maria Toki, Kristian Tonby, Sia Loong Tonnii, Marta Torre, Margarida Torres, Antoni Torres, Hernando Torres-Zevallos, Michael Towers, Tony Trapani, Théo Trioux, Cécile Tromeur, Ioannis Trontzas, Tiffany Trouillon, Jeanne Truong, Christelle Tual, Sarah Tubiana, Helen Tuite, Alexis F. Turgeon, Jean-Marie Turmel, Lance C.W. Turtle, Anders Tveita, Pawel Twardowski, Makoto Uchiyama, Andrew Udy, Roman Ullrich, Alberto Uribe, Asad Usman, Timothy M. Uyeki, Cristinava Vajdovics, Luís Val-Flores, Amélie Valran, Stijn Van de Velde, Marcel van den Berge, Machteld Van der Feltz, Job van der Palen, Paul van der Valk, Nicky Van Der Vekens, Peter Van der Voort, Sylvie Van Der Werf, Laura van Gulik, Jarne Van Hattem, Carolien van Netten, Frank van Someren Gréve, Ilonka van Veen, Hugo Van Willigen, Noémie Vanel, Henk Vanoverschelde, Pooja Varghese, Michael Varrone, Shoban Raj Vasudayan, Charline Vauchy, Shaminee Veeran, Aurélie Veislinger, Sebastian Vencken, Sara Ventura, Annelies Verbon, José Ernesto Vidal, César Vieira, Joy Ann Villanueva, Judit Villar, Pierre-Marc Villeneuve, Andrea Villoldo, Benoit Visseaux, Hannah Visser, Chiara Vitiello, Harald Vonkeman, Fanny Vuotto, Noor Hidayu Wahab, Suhaila Abdul Wahab, Nadirah Abdul Wahid, Marina Wainstein, Laura Walsh, Chih-Hsien Wang, Steve Webb, Jia Wei, Katharina Weil, Tan Pei Wen, Sanne Wesselius, Murray Wham, Bryan Whelan, Nicole White, Paul Henri Wicky, Aurélie Wiedemann, Surya Otto Wijaya, Keith Wille, Sue Willems, Bailey Williams, Virginie Williams, Patricia J. Williams, Evert-Jan Wils, Jessica Wittman, Calvin Wong, Xin Ci Wong, Yew Sing Wong, Teck Fung Wong, Gan Ee Xian, Lim Saio Xian, Pei Xuan Kuan, Ioannis Xynogalas, Siti Rohani Binti Mohd Yakop, Masaki Yamazaki, Elizabeth Yarad, Yazdan Yazdanpanah, Nicholas Yee Liang Hing, Cécile Yelnik, Chian Hui Yeoh, Stephanie Yerkovich, Toshiki Yokoyama, Hodane Yonis, Obada Yousif, Akram Zaaqoq, Marion Zabbe, Kai Zacharowski, Masliza Zahid, Maram Zahran, Nor Zaila Binti Zaidan, Maria Zambon, Miguel Zambrano, Alberto Zanella, Nurul Zaynah, Hiba Zayyad, Alexander Zoufaly, and David Zucman
Appendix
The ISARIC Clinical Characterisation Group includes the following participants: Sheryl Ann Abdukahil; Nurul Najmee Abdulkadir; Ryuzo Abe; Laurent Abel; Amal Abrous; Lara Absil; Andrew Acker; Elisabeth Adam; Diana Adrião; Saleh Al Ageel; Shakeel Ahmed; Kate Ainscough; Tharwat Aisa; Ali Ait Hssain; Younes Ait Tamlihat; Takako Akimoto; Ernita Akmal; Chika Akwani; Eman Al Qasim; Razi Alalqam; Angela Alberti; Tala Al-dabbous; Senthilkumar Alegesan; Marta Alessi; Beatrice Alex; Kévin Alexandre; Abdulrahman Al-Fares; Huda Alfoudri; Imran Ali; Kazali Enagnon Alidjnou; Jeffrey Aliudin; Qabas Alkhafajee; Clotilde Allavena; Nathalie Allou; João Alves; Rita Alves; João Melo Alves; Joana Alves Cabrita; Maria Amaral; Nur Amira; Phoebe Ampaw; Roberto Andini; Claire Andréjak; Andrea Angheben; François Angoulvant; Séverine Ansart; Sivanesen Anthonidass; Massimo Antonelli; Carlos Alexandre Antunes de Brito; Ardiyan Apriyana; Yaseen Arabi; Irene Aragao; Francisco Arancibia; Carolline Araujo; Antonio Arcadipane; Patrick Archambault; Lukas Arenz; Jean-Benoît Arlet; Christel Arnold-Day; Lovkesh Arora; Rakesh Arora; Elise Artaud-Macari; Diptesh Aryal; Angel Asensio; Muhammad Ashraf; Jean Baptiste Assie; Amirul Asyraf; Minahel Atif; Anika Atique; Johann Auchabie; Hugues Aumaitre; Adrien Auvet; Laurène Azemar; Cecile Azoulay; Benjamin Bach; Delphine Bachelet; Claudine Badr; Nadia Baig; J. Kenneth Baillie; Erica Bak; Agamemnon Bakakos; Nazreen Abu Bakar; Andriy Bal; Mohanaprasanth Balakrishnan; Valeria Balan; Firouzé Bani-Sadr; Renata Barbalho; Nicholas Yuri Barbosa; Wendy S. Barclay; Saef Umar Barnett; Michaela Barnikel; Helena Barrasa; Audrey Barrelet; Cleide Barrigoto; Marie Bartoli; Joaquín Baruch; Romain Basmaci; Muhammad Fadhli Hassin Basri; Denise Battaglini; Jules Bauer; Diego Fernando Bautista Rincon; Abigail Beane; Alexandra Bedossa; Ker Hong Bee; Husna Begum; Sylvie Behilill; Albertus Beishuizen; Aleksandr Beljantsev; David Bellemare; Anna Beltrame; Beatriz Amorim Beltrão; Marine Beluze; Nicolas Benech; Lionel Eric Benjiman; Dehbia Benkerrou; Suzanne Bennett; Binny Benny; Luís Bento; Jan-Erik Berdal; Delphine Bergeaud; Hazel Bergin; José Luis Bernal Sobrino; Giulia Bertoli; Lorenzo Bertolino; Simon Bessis; Sybille Bevilcaqua; Karine Bezulier; Amar Bhatt; Krishna Bhavsar; Claudia Bianco; Farah Nadiah Bidin; Moirangthem Bikram Singh; Felwa Bin Humaid; Mohd Nazlin Bin Kamarudin; Zeno Bisoffi;François Bissuel; Laurent Bitker; Jonathan Bitton; Pablo Blanco-Schweizer; Catherine Blier; Frank Bloos; Mathieu Blot; Lucille Blumberg; Filomena Boccia; Laetitia Bodenes; Debby Bogaert; Anne-Hélène Boivin; Isabela Bolaños; Pierre-Adrien Bolze; François Bompart; Diogo Borges; Raphaël Borie; Hans Martin Bosse; Elisabeth Botelho-Nevers; Lila Bouadma; Olivier Bouchaud; Sabelline Bouchez; Dounia Bouhmani; Damien Bouhour; Kévin Bouiller; Laurence Bouillet; Camile Bouisse; Anne-Sophie Boureau; John Bourke; Maude Bouscambert; Aurore Bousquet; Jason Bouziotis; Bianca Boxma; Marielle Boyer-Besseyre; Maria Boylan; Fernando Augusto Bozza; Axelle Braconnier; Cynthia Braga; Timo Brandenburger; Filipa Brás Monteiro; Luca Brazzi; Patrick Breen; Dorothy Breen; David Brewster; Kathy Brickell; Shaunagh Browne; Alex Browne; Nicolas Brozzi; Marjolein Brusse-Keizer; Petra Bryda; Nina Buchtele; Marielle Buisson; Erlina Burhan; Aidan Burrell; Ingrid G. Bustos; André Cabie; Susana Cabral; Eder Caceres; Cyril Cadoz; Rui Caetano Garcês; Kate Calligy; Jose Andres Calvache; João Camões; Valentine Campana; Paul Campbell; Josie Campisi; Cecilia Canepa; Mireia Cantero; Pauline Caraux-Paz; Sheila Cárcel; Chiara Simona Cardellino; Sofia Cardoso; Filipe Cardoso; Filipa Cardoso; Nelson Cardoso; Simone Carelli; Nicolas Carlier; Thierry Carmoi; Gayle Carney; Inês Carqueja; Marie-Christine Carret; François Martin Carrier; Ida Carroll; Gail Carson; Maire-Laure Casanova; Mariana Cascão; Siobhan Casey; José Casimiro; Bailey Cassandra; Silvia Castañeda; Nidyanara Castanheira; Guylaine Castor-Alexandre; Ivo Castro; Ana Catarino; François-Xavier Catherine; Paolo Cattaneo; Roberta Cavalin; Alexandros Cavayas; Minerva Cervantes-Gonzalez; Anissa Chair; Catherine Chakveatze; Adrienne Chan; Meera Chand; Christelle Chantalat Auger; Jean-Marc Chapplain; Charlotte Charpentier; Julie Chas; Jonathan Samuel Chávez Iñiguez; Anjellica Chen; Yih-Sharng Chen; Léo Chenard; Matthew Pellan Cheng; Antoine Cheret; Thibault Chiarabini; Julian Chica; Suresh Kumar Chidambaram; Leong Chin Tho; Catherine Chirouze; Davide Chiumello; Sung-Min Cho; Bernard Cholley; Marie-Charlotte Chopin; Ting Soo Chow; Hiu Jian Chua; Jonathan Chua; Jose Pedro Cidade; José Miguel Cisneros Herreros; Barbara Wanjiru Citarella; Anna Ciullo; Jennifer Clarke; Emma Clarke; Rolando Claure-Del Granado; Sara Clohisey; Cassidy Codan; Caitriona Cody; Alexandra Coelho; Megan Coles; Jennifer Coles; Gwenhaël Colin; Michael Collins; Sebastiano Maria Colombo; Pamela Combs; Jennifer Connolly; Marie Connor; Anne Conrad; Elaine Conway; Graham S. Cooke; Mary Copland; Hugues Cordel; Amanda Corley; Sabine Cornelis; Alexander Daniel Cornet; Arianne Joy Corpuz; Andrea Cortegiani; Grégory Corvaisier; Emma Costigan; Camille Couffignal; Sandrine Couffin-Cadiergues; Roxane Courtois; Stéphanie Cousse; Rachel Cregan; Cosimo Cristella; Sabine Croonen; Gloria Crowl; Jonathan Crump; Claudina Cruz; Juan Luis Cruz Bermúdez; Jaime Cruz Rojo; Marc Csete; Ailbhe Cullen; Matthew Cummings; Gerard Curley; Elodie Curlier; Colleen Curran; Paula Custodio; Ana da Silva Filipe; Charlene Da Silveira; Al-Awwab Dabaliz; Andrew Dagens; Darren Dahly; Heidi Dalton; Jo Dalton; Seamus Daly; Juliana Damas; Nick Daneman; Corinne Daniel; Emmanuelle A Dankwa; Jorge Dantas; Frédérick D'Aragon; Menno de Jong; Gillian de Loughry; Etienne De Montmollin; Rafael Freitas de Oliveira França; Ana Isabel de Pinho Oliveira; Rosanna De Rosa; Thushan de Silva; Peter de Vries; David Dean; Alexa Debard; Bianca DeBenedictis; Marie-Pierre Debray; Nathalie DeCastro; William Dechert; Lauren Deconninck; Romain Decours; Eve Defous; Isabelle Delacroix; Eric Delaveuve; Karen Delavigne; Nathalie M. Delfos; Ionna Deligiannis; Andrea Dell'Amore; Christelle Delmas; Pierre Delobel; Corine Delsing; Elisa Demonchy; Emmanuelle Denis; Dominique Deplanque; Pieter Depuydt; Mehul Desai; Diane Descamps; Mathilde Desvallées; Santi Dewayanti; Pathik Dhanger; Alpha Diallo; Sylvain Diamantis; André Dias; Fernanda Dias Da Silva; Juan Jose Diaz; Rodrigo Diaz; Priscila Diaz; Kévin Didier; Jean-Luc Diehl; Wim Dieperink; Jérôme Dimet; Vincent Dinot; Fara Diop; Alphonsine Diouf; Yael Dishon; Félix Djossou; Annemarie B. Docherty; Helen Doherty; Maria Donnelly; Christl A. Donnelly; Sean Donohue; Yoann Donohue; Chloe Donohue; Peter Doran; Céline Dorival; Eric D'Ortenzio; James Joshua Douglas; Nathalie Dournon; Triona Downer; Joanne Downey; Mark Downing; Tom Drake; Aoife Driscoll; Murray Dryden; Claudio Duarte Fonseca; Vincent Dubee; François Dubos; Alexandre Ducancelle; Toni Duculan; Susanne Dudman; Abhijit Duggal; Paul Dunand; Jake Dunning; Mathilde Duplaix; Emanuele Durante-Mangoni; Lucian Durham III; Bertrand Dussol; Juliette Duthoit; Xavier Duval; Anne Margarita Dyrhol-Riise; Sim Choon Ean; Marco Echeverria-Villalobos; Siobhan Egan; Carla Eira; Mohammed El Sanharawi; Subbarao Elapavaluru; Brigitte Elharrar; Jacobien Ellerbroek; Philippine Eloy; Tarek Elshazly; Isabelle Enderle; Tomoyuki Endo; Chan Chee Eng; Ilka Engelmann; Vincent Enouf; Olivier Epaulard; Martina Escher; Mariano Esperatti; Hélène Esperou; Catarina Espírito Santo; Marina Esposito-Farese; João Estevão; Manuel Etienne; Nadia Ettalhaoui; Anna Greti Everding; Mirjam Evers; Isabelle Fabre; Marc Fabre; Amna Faheem; Arabella Fahy; Cameron J. Fairfield; Pedro Faria; Hanan Fateena; Arie Zainul Fatoni; Karine Faure; Raphaël Favory; Mohamed Fayed; Niamh Feely; Laura Feeney; Jorge Fernandes; Marília Andreia Fernandes; Susana Fernandes; François-Xavier Ferrand; Eglantine Ferrand Devouge; Joana Ferrão; Mário Ferraz; Sílvia Ferreira; Bernardo Ferreira; Benigno Ferreira; Isabel Ferreira; Nicolas Ferriere; Céline Ficko; Claudia Figueiredo-Mello; Juan Fiorda; Thomas Flament; Clara Flateau; Tom Fletcher; Aline-Marie Florence; Letizia Lucia Florio; Deirdre Flynn; Claire Foley; Jean Foley; Tatiana Fonseca; Simon Forsyth; Denise Foster; Giuseppe Foti; Erwan Fourn; Robert A. Fowler; Marianne Fraher; Diego Franch-Llasat; John F Fraser; Christophe Fraser; Marcela Vieira Freire; Ana Freitas Ribeiro; Ricardo Fritz; Stéphanie Fry; Nora Fuentes; Masahiro Fukuda; Valérie Gaborieau; Rostane Gaci; Massimo Gagliardi; Jean-Charles Gagnard; Amandine Gagneux-Brunon; Sérgio Gaião; Linda Gail Skeie; Phil Gallagher; Carrol Gamble; Yasmin Gani; Arthur Garan; Rebekha Garcia; Noelia García Barrio; Esteban Garcia-Gallo; Denis Garot; Valérie Garrait; Nathalie Gault; Aisling Gavin; Anatoliy Gavrylov; Alexandre Gaymard; Johannes Gebauer; Eva Geraud; Louis Gerbaud Morlaes; Nuno Germano; Jade Ghosn; Marco Giani; Jess Gibson; Tristan Gigante; Morgane Gilg; Elaine Gilroy; Guillermo Giordano; Michelle Girvan; Valérie Gissot; Daniel Glikman; Eric Gnall; François Goehringer; Siri Goepel; Jean-Christophe Goffard; Jin Yi Goh; Jonathan Golob; Joan Gómez-Junyent; Marie Gominet; Alicia Gonzalez; Patricia Gordon; Isabelle Gorenne; Conor Gormley; Laure Goubert; Cécile Goujard; Tiphaine Goulenok; Margarite Grable; Jeronimo Graf; Edward Wilson Grandin; Pascal Granier; Giacomo Grasselli; Christopher A. Green; Courtney Greene; William Greenhalf; Segolène Greffe; Domenico Luca Grieco; Matthew Griffee; Fiona Griffiths; Ioana Grigoras; Albert Groenendijk; Heidi Gruner; Yusing Gu; Jérémie Guedj; Martin Guego; Dewi Guellec; Daniela Guerreiro; Romain Guery; Anne Guillaumot; Laurent Guilleminault; Maisa Guimarães de Castro;Thomas Guimard; Marieke Haalboom; Daniel Haber; Hannah Habraken; Ali Hachemi; Nadir Hadri; Sheeba Hakak; Adam Hall; Matthew Hall; Sophie Halpin; Ansley Hamer; Rebecca Hamidfar; Terese Hammond; Naomi Hammond; Lim Yuen Han; Rashan Haniffa; Kok Wei Hao; Hayley Hardwick; Ewen M. Harrison; Janet Harrison; Samuel Bernard Ekow Harrison; Alan Hartman; Junaid Hashmi; Ailbhe Hayes; Leanne Hays; Jan Heerman; Lars Heggelund; Ross Hendry; Martina Hennessy; Aquiles Henriquez-Trujillo; Maxime Hentzien; Diana Hernandez; Jaime Hernandez-Montfort; Andrew Hershey; Liv Hesstvedt; Eibhlin Higgins; Dawn Higgins;Rupert Higgins; Rita Hinchion; Samuel Hinton; Hiroaki Hiraiwa; Hikombo Hitoto; Antonia Ho; Yi Bin Ho; Alexandre Hoctin; Isabelle Hoffmann; Wei Han Hoh; Oscar Hoiting; Rebecca Holt; Jan Cato Holter; Peter Horby; Juan Pablo Horcajada; Koji Hoshino; Ikram Houas; Catherine L. Hough; Stuart Houltham; Jimmy Ming-Yang Hsu; Jean-Sébastien Hulot; Abby Hurd; Samreen Ijaz; M. Arfan Ikram; Hajnal-Gabriela Illes; Patrick Imbert; Hugo Inácio; Carmen Infante Dominguez; Yun Sii Ing; Elias Iosifidis; Mariachiara Ippolito; Sarah Isgett; Tiago Isidoro; Nadiah Ismail; Margaux Isnard; Junji Itai; Daniel Ivulich; Danielle Jaafar; Salma Jaafoura; Julien Jabot; Clare Jackson; Victoria Janes; Pierre Jaquet; Waasila Jassat; Coline Jaud-Fischer; Stéphane Jaureguiberry; Denise Jaworsky; Florence Jego; Anilawati Mat Jelani; Synne Jenum; Ruth Jimbo-Sotomayor; Ong Yiaw Joe; Ruth N. Jorge García; Cédric Joseph; Mark Joseph; Mercé Jourdain; Philippe Jouvet; Anna Jung; Hanna Jung; Dafsah Juzar; Ouifiya Kafif; Florentia Kaguelidou; Neerusha Kaisbain; Thavamany Kaleesvran; Sabina Kali; Smaragdi Kalomoiri; Muhammad Aisar Ayadi Kamaluddin; Zul Amali Che Kamaruddin; Nadiah Kamarudin; Darshana Hewa Kandamby; Chris Kandel; Kong Yeow Kang; Pratap Karpayah; Christiana Kartsonaki; Daisuke Kasugai; Anant Kataria; Kevin Katz; Aasmine Kaur; Christy Kay; Lamees Kayyali; Hannah Keane; Seán Keating; Claire Kelly; Yvelynne Kelly; Andrea Kelly; Niamh Kelly; Aoife Kelly; Sadie Kelly; Maeve Kelsey; Ryan Kennedy; Kalynn Kennon; Maeve Kernan; Younes Kerroumi; Sharma Keshav; Imrana Khalid; Antoine Khalil; Coralie Khan; Irfan Khan; Michelle E Kho; Saye Khoo; Ryan Khoo; Denisa Khoo; Khor How Kiat; Yuri Kida; Peter Kiiza; Beathe Kiland Granerud; Anders Benjamin Kildal; Antoine Kimmoun; Detlef Kindgen-Milles; Alexander King; Nobuya Kitamura; Paul Klenerman; Rob Klont; Gry Kloumann Bekken; Stephen R Knight; Malte Kohns Vasconcelos; Mamoru Komatsu; Caroline Kosgei; Arsène Kpangon; Karolina Krawczyk; Sudhir Krishnan; Vinothini Krishnan; Oksana Kruglova; Deepali Kumar; Ganesh Kumar; Pavan Kumar Vecham; Dinesh Kuriakose; Ethan Kurtzman; Demetrios Kutsogiannis; Galyna Kutsyna; Konstantinos Kyriakoulis; Marie Lachatre; Marie Lacoste; John G. Laffey; Nadhem Lafhej; Marie Lagrange; Fabrice Laine; Olivier Lairez; Antonio Lalueza; Marc Lambert; François Lamontagne; Marie Langelot-Richard; Vincent Langlois; Eka Yudha Lantang; Marina Lanza; Cédric Laouénan; Samira Laribi; Delphine Lariviere; Stéphane Lasry; Odile Launay; Didier Laureillard; Yoan Lavie-Badie; Andy Law; Teresa Lawrence; Cassie Lawrence; Minh Le; Clément Le Bihan; Cyril Le Bris; Georges Le Falher; Lucie Le Fevre; Quentin Le Hingrat; Marion Le Maréchal; Soizic Le Mestre; Gwenaël Le Moal; Vincent Le Moing; Hervé Le Nagard; Paul LeTurnier; Ema Leal; Marta Leal Santos; Todd C. Lee; Su Hwan Lee; James Lee; Jennifer Lee; Heng Gee Lee; Biing Horng Lee; Yi Lin Lee; Gary Leeming; Bénédicte Lefebvre; Laurent Lefebvre; Benjamin Lefèvre; Sylvie LeGac; Jean-Daniel Lelievre; François Lellouche; Adrien Lemaignen; Véronique Lemee; Anthony Lemeur; Gretchen Lemmink; Ha Sha Lene; Jenny Lennon; Rafael León; Marc Leone; Michela Leone; Quentin Lepiller; François-Xavier Lescure; Olivier Lesens; Mathieu Lesouhaitier; Amy Lester-Grant; Sophie Letrou; Bruno Levy; Yves Levy; Claire Levy-Marchal; Katarzyna Lewandowska; Erwan L'Her; Gianluigi Li Bassi; Geoffrey Liegeon; Wei Shen Lim; Kah Chuan Lim; Chantre Lima; Bruno Lina; Lim Lina; Andreas Lind; Guillaume Lingas; Sylvie Lion-Daolio; Samantha Lissauer; Keibun Liu; Marine Livrozet; Patricia Lizotte; Navy Lolong; Leong Chee Loon; Diogo Lopes; Dalia Lopez-Colon; Anthony L. Loschner; Paul Loubet; Bouchra Loufti; Guillame Louis; Silvia Lourenco; Lee Lee Low; Marije Lowik; Jia Shyi Loy; Jean-Christophe Lucet; Carlos Lumbreras Bermejo; Carlos M. Luna; Olguta Lungu; Liem Luong; Nestor Luque; Dominique Luton; Nilar Lwin; Ruth Lyons; Olavi Maasikas; Oryane Mabiala; Moïse Machado; Sara Machado; Gabriel Macheda; Hashmi Madiha; Guillermo Maestro de la Calle; Rafael Mahieu; Sophie Mahy; Ana Raquel Maia; Lars S. Maier; Mylène Maillet; Thomas Maitre; Maximilian Malfertheiner; Nadia Malik; Paddy Mallon; Fernando Maltez; Denis Malvy; Victoria Manda; Laurent Mandelbrot; Frank Manetta; Julie Mankikian; Edmund Manning; Aldric Manuel; Ceila Maria Sant`Ana Malaque; Flávio Marino; Samuel Markowicz; Ana Marques; Catherine Marquis; Brian Marsh; Laura Marsh; Megan Marshal; John Marshall; Celina Turchi Martelli; Emily Martin; Guillaume Martin-Blondel; F. Eduardo Martinez; Ignacio Martin-Loeches; Martin Martinot; João Martins; Ana Martins; Nuno Martins; Caroline Martins Rego; Gennaro Martucci; Olga Martynenko; Eva Miranda Marwali; Marsilla Marzukie; David Maslove; Sabina Mason; Mohd Basri Mat Nor; Moshe Matan; Daniel Mathieu; Mathieu Mattei; Laurence Maulin; Michael Maxwell; Javier Maynar; Thierry Mazzoni; Lisa Mc Sweeney; Colin McArthur; Peter McCanny; Anne McCarthy; Aine McCarthy; Colin McCloskey; Rachael McConnochie; Sherry McDermott; Sarah E. McDonald; Aine McElroy; Samuel McElwee; Victoria McEneany; Natalie McEvoy; Allison McGeer; Chris McKay; Johnny McKeown; Kenneth A. McLean; Paul McNally; Bairbre McNicholas; Elaine McPartlan; Edel Meaney; Cécile Mear-Passard; Maggie Mechlin; Omar Mehkri; Ferruccio Mele; Luis Melo; Joao Joao Mendes; Kusum Menon; France Mentré; Alexander J. Mentzer; Emmanuelle Mercier; Noémie Mercier; Antoine Merckx; Mayka Mergeay-Fabre; Blake Mergler; Laura Merson; António Mesquita;Osama Metwally; Agnès Meybeck; Dan Meyer; Alison M. Meynert; Vanina Meysonnier; Amina Meziane; Mehdi Mezidi; Céline Michelanglei; Isabelle Michelet; Efstathia Mihelis; Vladislav Mihnovit; Jennene Miller; Hugo Miranda-Maldonado; Nor Arisah Misnan; Tahira Jamal Mohamed; Nik Nur Eliza Mohamed; Asma Moin; Elena Molinos; Brenda Molloy; Sinead Monahan; Mary Mone; Agostinho Monteiro; Claudia Montes; Giorgia Montrucchio; Shona C. Moore; Sarah Moore; Lina Morales Cely; Lucia Moro; Ben Morton; Catherine Motherway; Ana Motos; Hugo Mouquet; Clara Mouton Perrot; Julien Moyet; Caroline Mudara; Ng Yong Muh; Dzawani Muhamad; Jimmy Mullaert; Fredrik Müller; Karl Erik Müller; Aisling Murphy; Lorna Murphy; Patrick Murray; Marlène Murris; Srinivas Murthy; Himed Musaab; Gugapriyaa Muyandy; Dimitra Melia Myrodia; Dave Nagpal; Alex Nagrebetsky; Mangala Narasimhan; Nageswaran Narayanan; Alasdair Nazerali-Maitland; Nadège Neant; Holger Neb; Raul Neto; Emily Neumann; Pauline Yeung Ng; Wing Yiu Ng; Anthony Nghi; Duc Nguyen; Orna Ni Choileain; Niamh Ni Leathlobhair; Alistair D Nichol; Prompak Nitayavardhana; Stephanie Nonas; Nurul Amani Mohd Noordin; Marion Noret; Nurul Faten Izzati Norharizam; Lisa Norman; Mahdad Noursadeghi; Adam Nowinski; Saad Nseir; Jose I Nunez; Elsa Nyamankolly; Fionnuala O Brien; Annmarie O Callaghan; Annmarie O'Callaghan; Giovanna Occhipinti; Derbrenn OConnor; Max O'Donnell; Tawnya Ogston; Takayuki Ogura; Tak-Hyuk Oh; Sophie O'Halloran; Katie O'Hearn; Shinichiro Ohshimo; João Oliveira; Larissa Oliveira; Piero L. Olliaro; David S.Y. Ong; Jee Yan Ong; Wilna Oosthuyzen; Anne Opavsky; Peter Openshaw; Claudia Milena Orozco-Chamorro; Jamel Ortoleva; Javier Osatnik; Linda O'Shea; Miriam O'Sullivan; Siti Zubaidah Othman; Nadia Ouamara; Rachida Ouissa; Eric Oziol; Maïder Pagadoy; Justine Pages; Amanda Palacios; Massimo Palmarini; Giovanna Panarello; Prasan Kumar Panda; Lai Hui Pang; Mauro Panigada; Nathalie Pansu; Aurélie Papadopoulos; Rachael Parke; Jérémie Pasquier; Bruno Pastene; Fabian Patauner; Mohan Dass Pathmanathan; Luís Patrão; Patricia Patricio; Juliette Patrier; Lisa Patterson; Christelle Paul; Mical Paul; Jorge Paulos; William A. Paxton; Jean-François Payen; Sandra L Peake; Kalaiarasu Peariasamy; Miguel Pedrera Jiménez; Giles J. Peek; Florent Peelman; Nathan Peiffer-Smadja; Vincent Peigne; Mare Pejkovska; Paolo Pelosi; Ithan D. Peltan; Rui Pereira; Daniel Perez; Luis Periel; Thomas Perpoint; Antonio Pesenti; Vincent Pestre; Lenka Petrou; Michele Petrovic; Ventzislava Petrov-Sanchez; Frank Olav Pettersen; Gilles Peytavin; Scott Pharand; Walter Picard; Olivier Picone; Maria de Piero; Carola Pierobon; Djura Piersma; Carlos Pimentel; Raquel Pinto; Valentine Piquard; Catarina Pires; Isabelle Pironneau; Lionel Piroth; Chiara Piubelli; Riinu Pius; Laurent Plantier; Hon Shen Png; Julien Poissy; Ryadh Pokeerbux; Sergio Poli; Georgios Pollakis; Diane Ponscarme; Diego Bastos Porto; Andra-Maris Post; Douwe F. Postma; Pedro Povoa; Diana Póvoas; Jeff Powis; Sofia Prapa; Sébastien Preau; Christian Prebensen; Jean-Charles Preiser; Anton Prinssen; Mark G. Pritchard; Lucia Proença; Sravya Pudota; Oriane Puéchal; Bambang Pujo Semedi; Gregory Purcell; Luisa Quesada; Víctor Quirós González; Else Quist-Paulsen; Mohammed Quraishi; Fadi Qutishat; Christian Rabaud; Aldo Rafael; Marie Rafiq; Rozanah Abd Rahman; Ahmad Kashfi Haji Ab Rahman; Fernando Rainieri; Giri Shan Rajahram; Nagarajan Ramakrishnan; José Ramalho; Ahmad Afiq Ramli; Blandine Rammaert; Grazielle Viana Ramos; Ritika Ranjan; Christophe Rapp; Menaldi Rasmin; Indrek Rätsep; Tharmini Ravi; Andre Real; Stanislas Rebaudet; Sarah Redl; Brenda Reeve; Liadain Reid; Dag Henrik Reikvam; Renato Reis; Jordi Rello; Jonathan Remppis; Martine Remy; Hongru Ren; Hanna Renk; Anne-Sophie Resseguier; Matthieu Revest; Oleksa Rewa; Tiago Reyes; Luis Felipe Reyes; Maria Ines Ribeiro; David Richardson; Denise Richardson; Laurent Richier; Siti Nurul Atikah Ahmad Ridzuan; Ana L Rios; Asgar Rishu; Patrick Rispal; Karine Risso; Nicholas Rizer; Chiara Robba; André Roberto; Stephanie Roberts; David L. Robertson; Olivier Robineau; Ferran Roche-Campo; Paola Rodari; Simão Rodeia; Bernhard Roessler; Pierre-Marie Roger; Claire Roger; Emmanuel Roilides; Amanda Rojek; Juliette Romaru; Roberto Roncon-Albuquerque Jr; Mélanie Roriz; Manuel Rosa-Calatrava; Michael Rose; Dorothea Rosenberger; Andrea Rossanese; Matteo Rossetti; Bénédicte Rossignol; Patrick Rossignol; Stella Rousset; Carine Roy; Benoît Roze; Clark D. Russell; Maria Ryan; Maeve Ryan; Steffi Ryckaert; Aleksander Rygh Holten; Isabela Saba; Musharaf Sadat; Valla Sahraei; Maximilien Saint-Gilles; Pranya Sakiyalak; Leonardo Salazar; Gabriele Sales; Stéphane Sallaberry; Charlotte Salmon Gandonniere; Hélène Salvator; Olivier Sanchez; Emely Sanchez; Angel Sanchez-Miralles; Vanessa Sancho-Shimizu; Gyan Sandhu; Zulfiqar Sandhu; Pierre-François Sandrine; Oana Sandulescu; Marlene Santos; Shirley Sarfo-Mensah; Bruno Sarmento Banheiro; Iam Claire E. Sarmiento; Benjamin Sarton; Sree Satyapriya; Rumaisah Satyawati; Egle Saviciute; Parthena Savvidou; Yen Tsen Saw; Justin Schaffer; Tjard Schermer; Arnaud Scherpereel; Marion Schneider; Stephan Schroll; Michael Schwameis; Brendan Scicluna; Janet T. Scott; James Scott-Brown; Nicholas Sedillot; Tamara Seitz; Mageswari Selvarajoo; Caroline Semaille; Malcolm G. Semple; Rasidah Bt Senian; Eric Senneville; Claudia Sepulveda; Filipa Sequeira; Tânia Sequeira; Ary Serpa Neto; Pablo Serrano Balazote; Ellen Shadowitz; Syamin Asyraf Shahidan; Mohammad Shamsah; Shaikh Sharjeel; Pratima Sharma; Catherine A. Shaw; Victoria Shaw; John Robert Sheenan; Haixia Shi; Nobuaki Shime; Keiki Shimizu; Sally Shrapnel; Hoi Ping Shum; Nassima Si Mohammed; Ng Yong Siang; Jeanne Sibiude; Atif Siddiqui; Louise Sigfrid; Piret Sillaots; Catarina Silva; Rogério Silva; Maria Joao Silva; Benedict Sim Lim Heng; Wai Ching Sin; Punam Singh; Budha Charan Singh; Pompini Agustina Sitompul; Karisha Sivam; Vegard Skogen; Sue Smith; Benjamin Smood; Coilin Smyth; Michelle Smyth; Morgane Snacken; Dominic So; Tze Vee Soh; Joshua Solomon; Tom Solomon; Emily Somers; Agnès Sommet; Rima Song; Myung Jin Song; Tae Song; Jack Song Chia; Michael Sonntagbauer; Azlan Mat Soom; Albert Sotto; Edouard Soum; Marta Sousa; Ana Chora Sousa; Maria Sousa Uva; Vicente Souza-Dantas; Alexandra Sperry; Elisabetta Spinuzza; Shiranee Sriskandan; Sarah Stabler; Thomas Staudinger; Stephanie-Susanne Stecher; Trude Steinsvik; Ymkje Stienstra; Birgitte Stiksrud; Eva Stolz; Amy Stone; Adrian Streinu-Cercel; Anca Streinu-Cercel; Ami Stuart; David Stuart; Richa Su; Jacky Y. Suen; Gabriel Suen; Prasanth Sukumar; Asfia Sultana; Charlotte Summers; Dubravka Supic; Deepashankari Suppiah; Magdalena Surovcová; Sarah Syahrin; Konstantinos Syrigos; Jaques Sztajnbok; Konstanty Szuldrzynski; Shirin Tabrizi; Fabio S. Taccone; Lysa Tagherset; Shahdattul Mawarni Taib; Sara Taleb; Jelmer Talsma; Maria Lawrensia Tampubolon; Kim Keat Tan; Yan Chyi Tan; Hiroyuki Tanaka; Taku Tanaka; Hayato Taniguchi; Coralie Tardivon; Pierre Tattevin; M Azhari Taufik; Hassan Tawfik; Richard S. Tedder; Tze Yuan Tee; João Teixeira; Sofia Tejada; Marie-Capucine Tellier; Sze Kye Teoh; Vanessa Teotonio; François Téoulé; Pleun Terpstra; Olivier Terrier; Nicolas Terzi; Hubert Tessier-Grenier; Adrian Tey; Alif Adlan Mohd Thabit; Zhang Duan Tham; Suvintheran Thangavelu; Elmi Theron; Vincent Thibault; Simon-Djamel Thiberville; Benoît Thill; Jananee Thirumanickam; Shaun Thompson; David Thomson; Emma C. Thomson; Mathew Thorpe; Surain Raaj Thanga Thurai; Ryan S. Thwaites; Paul Tierney; Vadim Tieroshyn; Jean-François Timsit; Noémie Tissot; Fiona Toal; Jordan Zhien Yang Toh; Maria Toki; Kristian Tonby; Sia Loong Tonnii; Marta Torre; Margarida Torres; Antoni Torres; Hernando Torres-Zevallos; Michael Towers; Tony Trapani; Théo Trioux; Cécile Tromeur; Ioannis Trontzas; Tiffany Trouillon; Jeanne Truong; Christelle Tual; Sarah Tubiana; Helen Tuite; Alexis F. Turgeon; Jean-Marie Turmel; Lance C.W. Turtle; Anders Tveita; Pawel Twardowski; Makoto Uchiyama; Andrew Udy; Roman Ullrich; Alberto Uribe; Asad Usman; Timothy M. Uyeki; Cristinava Vajdovics; Luís Val-Flores; Amélie Valran; Stijn Van de Velde; Marcel van den Berge; Machteld Van der Feltz; Job van der Palen; Paul van der Valk; Nicky Van Der Vekens; Peter Van der Voort; Sylvie Van Der Werf; Laura van Gulik; Jarne Van Hattem; Carolien van Netten; Frank van Someren Gréve; Ilonka van Veen; Hugo Van Willigen; Noémie Vanel; Henk Vanoverschelde; Pooja Varghese; Michael Varrone; Shoban Raj Vasudayan; Charline Vauchy; Shaminee Veeran; Aurélie Veislinger; Sebastian Vencken; Sara Ventura; Annelies Verbon; José Ernesto Vidal; César Vieira; Joy Ann Villanueva; Judit Villar; Pierre-Marc Villeneuve; Andrea Villoldo; Benoit Visseaux; Hannah Visser; Chiara Vitiello; Harald Vonkeman; Fanny Vuotto; Noor Hidayu Wahab; Suhaila Abdul Wahab; Nadirah Abdul Wahid; Marina Wainstein; Laura Walsh; Chih-Hsien Wang; Steve Webb; Jia Wei; Katharina Weil; Tan Pei Wen; Sanne Wesselius; Murray Wham; Bryan Whelan; Nicole White; Paul Henri Wicky; Aurélie Wiedemann; Surya Otto Wijaya; Keith Wille; Sue Willems; Bailey Williams; Virginie Williams; Patricia J Williams; Evert-Jan Wils; Jessica Wittman; Calvin Wong; Xin Ci Wong; Yew Sing Wong; Teck Fung Wong; Gan Ee Xian; Lim Saio Xian; Pei Xuan Kuan; Ioannis Xynogalas; Siti Rohani Binti Mohd Yakop; Masaki Yamazaki; Elizabeth Yarad; Yazdan Yazdanpanah; Nicholas Yee Liang Hing; Cécile Yelnik; Chian Hui Yeoh; Stephanie Yerkovich; Toshiki Yokoyama; Hodane Yonis; Obada Yousif; Akram Zaaqoq; Marion Zabbe; Kai Zacharowski; Masliza Zahid; Maram Zahran; Nor Zaila Binti Zaidan; Maria Zambon; Miguel Zambrano; Alberto Zanella; Nurul Zaynah; Hiba Zayyad; Alexander Zoufaly; David Zucman; Mazankowski Heart Institute; The Western Australian COVID-19 Research Response.
Supplementary material
Supplement Fig.
data
References
- 1.Conway E.M., Mackman N., Warren R.Q., Wolberg A.S., Mosnier L.O., Campbell R.A., et al. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol. 2022;22:639–649. doi: 10.1038/s41577-022-00762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iba T., Connors J.M., Levy J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020;69:1181–1189. doi: 10.1007/s00011-020-01401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura J., Tsujino I., Yachi S., Takeyama M., Nishimoto Y., Konno S., et al. Incidence, risk factors, and clinical impact of major bleeding in hospitalized patients with COVID-19: a sub-analysis of the CLOT-COVID Study. Thromb J. 2022;20:1–9. doi: 10.1186/s12959-022-00414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Six I., Guillaume N., Jacob V., Mentaverri R., Kamel S., Boullier A., et al. The endothelium and COVID-19: an increasingly clear link brief title: endotheliopathy in COVID-19. Int J Mol Sci. 2022;23:6196. doi: 10.3390/ijms23116196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Agnillo F., Walters K.A., Xiao Y., Sheng Z.M., Scherler K., Park J., et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abj7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasoppokakorn T., Kullavanijaya P., Pittayanon R. Risk factors of active upper gastrointestinal bleeding in patients with COVID-19 infection and the effectiveness of PPI prophylaxis. BMC Gastroenterol. 2022;22:465. doi: 10.1186/s12876-022-02568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modin D., Claggett B., Sindet-Pedersen C., Lassen M.C.H., Skaarup K.G., Jensen J.U.S., et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142:2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight R., Walker V., Ip S., Cooper J.A., Bolton T., Keene S., et al. Association of COVID-19 with major arterial and venous thrombotic diseases: a population-wide cohort study of 48 million adults in England and Wales. Circulation. 2022;146:892–906. doi: 10.1161/CIRCULATIONAHA.122.060785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kollias A., Kyriakoulis K.G., Lagou S., Kontopantelis E., Stergiou G.S., Syrigos K. Venous thromboembolism in COVID-19: a systematic review and meta-analysis. Vasc Med. 2021;26:415–425. doi: 10.1177/1358863X21995566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyriakoulis K.G., Dimakakos E., Kyriakoulis I.G., Catalano M., Spyropoulos A.C., Schulman S., et al. Practical recommendations for optimal thromboprophylaxis in patients with COVID-19: a consensus statement based on available clinical trials. J Clin Med. 2022;11:5997. doi: 10.3390/jcm11205997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulman S., Sholzberg M., Spyropoulos A.C., Zarychanski R., Resnick H.E., Bradbury C.A., et al. ISTH guidelines for antithrombotic treatment in COVID-19. J Thromb Haemost. 2022;20:2214–2225. doi: 10.1111/jth.15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reyes L.F., Murthy S., Garcia-Gallo E., Merson L., Ibáñez-Prada E.D., Rello J., et al. Respiratory support in patients with severe COVID-19 in the International Severe Acute Respiratory and Emerging Infection (ISARIC) COVID-19 study: a prospective, multinational, observational study. Crit Care. 2022;26:1–16. doi: 10.1186/s13054-022-04155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khedr A., Al Hennawi H., Rauf I., Khan M.K., Mushtaq H.A., Lodhi H.S., et al. Differential mortality with COVID-19 and invasive mechanical ventilation between high-income and low-and middle-income countries: a systematic review, meta-analysis, and meta-regression. Infez Med. 2022;30:51–58. doi: 10.53854/liim-3001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marwali E.M., Kekalih A., Yuliarto S., Wati D.K., Rayhan M., Valerie I.C., et al. Paediatric COVID-19 mortality: a database analysis of the impact of health resource disparity. BMJ Paediatr Open. 2022;6 doi: 10.1136/bmjpo-2022-001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mura M., Simon F., Pommier de Santi V., Tangy F., Tournier J.N. Role and limits of COVID-19 vaccines in the delicate transition from pandemic mitigation to endemic control. Vaccines. 2022;10:1555. doi: 10.3390/vaccines10091555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akkız H. The biological functions and clinical significance of SARS-CoV-2 variants of concern. Front Med. 2022;9 doi: 10.3389/fmed.2022.849217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ISARIC Clinical Characterisation Group COVID-19 symptoms at hospital admission vary with age and sex: results from the ISARIC prospective multinational observational study. Infection. 2021;49:889–905. doi: 10.1007/s15010-021-01599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ISARIC Clinical Characterization Group. Garcia-Gallo E., Merson L., Kennon K., Kelly S., Citarella B.W., et al. ISARIC-COVID-19 dataset: a prospective, standardized, global dataset of patients hospitalized with COVID-19. Sci Data. 2022;9:454. doi: 10.1038/s41597-022-01534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ISARIC Clinical Characterisation Group The value of open-source clinical science in pandemic response: lessons from ISARIC. Lancet Infect Dis. 2021;21:1623–1624. doi: 10.1016/S1473-3099(21)00565-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanning J.P., Weaver N., Fanning R.B. Hemorrhage, disseminated intravascular coagulopathy, and thrombosis (HECTOR) complications among critically ill patients with COVID-19: an international COVID-19 Critical Care Consortium Study. Critical Care Med. 2023;51:619–631. doi: 10.1097/CCM.0000000000005798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyes L.F., Murthy S., Garcia-Gallo E., Irvine M., Merson L., Martin-Loeches I., et al. Clinical characteristics, risk factors and outcomes in patients with severe COVID-19 registered in the International Severe Acute Respiratory and Emerging Infection Consortium WHO clinical characterisation protocol: a prospective, multinational, multicentre, observational study. ERJ Open Res. 2022;8:00552–2021. doi: 10.1183/23120541.00552-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav H., Shah D., Sayed S., Horton S., Schroeder L.F. Availability of essential diagnostics in ten low-income and middle-income countries: results from national health facility surveys. Lancet Glob Health. 2021;9:e1553–e1560. doi: 10.1016/S2214-109X(21)00442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegal D.M., Eikelboom J.W., Lee S.F., Rangarajan S., Bosch J., Zhu J., et al. Variations in incidence of venous thromboembolism in low-, middle-, and high-income countries. Cardiovasc Res. 2021;117:576–584. doi: 10.1093/cvr/cvaa044. [DOI] [PubMed] [Google Scholar]
- 29.INSPIRATION Investigators. Sadeghipour P., Talasaz A.H., Rashidi F., Sharif-Kashani B., Beigmohammadi M.T., et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spyropoulos A.C., Goldin M., Giannis D., Diab W., Wang J., Khanijo S., et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181:1612–1620. doi: 10.1001/jamainternmed.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators. Goligher E.C., Bradbury C.A., McVerry B.J., et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fish M., Rynne J., Jennings A., Lam C., Lamikanra A.A., Ratcliff J., et al. Coronavirus disease 2019 subphenotypes and differential treatment response to convalescent plasma in critically ill adults: secondary analyses of a randomized clinical trial. Intensive Care Med. 2022;48:1525–1538. doi: 10.1007/s00134-022-06869-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
data
Data Availability Statement
The data that underpin this analysis are highly detailed clinical data on individuals hospitalized for COVID-19. Due to the sensitive nature of these data and the associated privacy concerns, they are available via a governed data access mechanism following review of a data access committee. Data can be requested via the Infectious Disease Data Observatory COVID-19 Data Sharing Platform (http://www.iddo.org/covid-19). The Data Access Application, Terms of Access, and details of the Data Access Committee are available on the website. Briefly, the requirements for access are a request from a qualified researcher working with a legal entity who has a health and/or research remit; a scientifically valid reason for data access that adheres to appropriate ethical principles. The full terms are at https://www.iddo.org/document/covid-19-data-access-guidelines. A small subset of sites that contributed data to this analysis have not agreed to pooled data sharing as above. In the case of requiring access to these data, please contact the corresponding author in the first instance, who will look to facilitate access.