Abstract
Background
Prior work established a measure of tobacco dependence (TD) among adults that can be used to compare TD across different tobacco products. We extend this approach to develop a common, cross-product metric for TD among youth.
Methods
One thousand one hundred and forty-eight youth aged 12–17 who used a tobacco product in the past 30 days were identified from 13 651 youth respondents in Wave 1 of the Population Assessment of Tobacco and Health (PATH) Study.
Findings
Analyses confirmed a single primary latent construct underlying responses to TD indicators for all mutually exclusive tobacco product user groups. Differential Item Functioning analyses supported the use of 8 of 10 TD indicators for comparisons across groups. With TD levels anchored at 0.0 (standard deviation [SD] = 1.0) among cigarette only (n = 265) use group, mean TD scores were more than a full SD lower for e-cigarette only (n = 150) use group (mean = −1.09; SD = 0.64). Other single product use group (cigar, hookah, pipe, or smokeless; n = 262) on average had lower TD (mean = −0.60; SD = 0.84), and the group with the use of multiple tobacco products (n = 471) experienced similar levels of TD (mean = 0.14; SD = 0.78) as the cigarette only use group. Concurrent validity was established with product use frequency among all user groups. A subset of five TD items comprised a common metric permitting comparisons between youth and adults.
Conclusion
The PATH Study Youth Wave 1 Interview provided psychometrically valid measures of TD that enable future regulatory investigations of TD across tobacco products and comparisons between youth and adult tobacco product use group.
Implications
A measure of tobacco dependence (TD) has been established previously among adults to compare TD across tobacco products. This study established the validity of a similar, cross-product measure of TD among youth. Findings suggest a single latent TD construct underlying this measure, concurrent validity of the scale with product use frequency across different types of tobacco users, and a subset of common items that can be used to compare TD between youth and adults who use tobacco.
Introduction
Tobacco dependence (TD) in youth is associated with smoking quantity and frequency as well as difficulty quitting.1–3 A review of studies on adolescent TD found that at least two-thirds of adolescents who smoke report experiencing withdrawal symptoms (the most frequently studied component of dependence), and that craving desire to smoke are the most commonly reported withdrawal symptoms.4 Other studies have found TD symptoms even among non-daily youth who smoke, suggesting that even minimal exposure to smoking can lead to development of dependence.5,6 Most research has focused on TD among adolescents who smoke cigarettes, but as prevalence of cigarette smoking in this age group has declined and the use of other tobacco or nicotine products has increased,7 characteristics of dependence among these other product use groups should be explored. In addition, youth patterns of exposure to tobacco can be intermittent; youth may use multiple tobacco products concurrently or may switch from one product to another.8 Patterns of multiple products use may elicit different presentations of TD compared to those who only use one product.
Although some research has been conducted to assess the psychometric properties of nicotine or TD scales among youth,9–12 most evidence focuses on adults. Additional research could enhance current construction and discriminate among varying levels of measures of TD psychometrically among youth by emphasizing internal consistency of multiple-item indicators and establishing concurrent, discriminant, and predictive validity of these measures.13 Relatedly, it is important to explore the stability of features of dependence through different stages of development, so research is important to compare indicators of dependence between youth and adults to reveal possible thresholds throughout the developmental trajectory of dependence. Item Response Theory has been used to distinguish properties of individual items or scales to inform multiitem TD instrument dependence.9,14–16
In prior work, using Differential Item Functioning (DIF) analyses, a 16-item index to assess TD for adults (aged ≥18) using tobacco products was established and compared measurement of TD across adult cigarettes only, e-cigarette only, cigar only, hookah only, smokeless only, cigarette + e-cigarette only, and multiple product use groups. This index was concurrently valid, supported by positive associations between index scores and frequency of product usage.17 TD as measured by the index, predicted one-year quitting and switching behavior among adults in a nationally representative longitudinal cohort study, the Population Assessment of Tobacco and Health (PATH) Study.18 In addition, we provided evidence for the concurrent and predictive validity of the 16-item index using biomarkers of nicotine exposure and the quantity and frequency of product use for adults who smoke cigarettes and multiple tobacco products.19
In this study, we sought to examine indicators of TD among youth using the national PATH Study. We used methods based on Item Response Theory to identify measurement properties of the Wave 1 (W1) youth instrument to assess TD and evaluate comparability among youth with common patterns of multiple products, e-cigarettes, cigarettes, and other tobacco product use. We use W1 responses to evaluate the construct validity of TD among youth when compared to adults. Models comparing youth and adult TD responses will establish a common metric for levels of TD across youth and adults who use multiple products, e-cigarettes, cigarettes, and other tobacco products using previously evaluated W1 PATH Study data.17 This initial validation is designed to provide a foundation for a common metric that may facilitate longitudinal measurement of TD for youth and adults who use tobacco products.
Methods
Data Source
Data are from W1 of the PATH Study, conducted from mid-September 2013 to mid-December 2014. The PATH Study is a nationally representative, longitudinal cohort study. Audio-Computer Assisted Self-Interviews available in English and Spanish were used to collect information on tobacco-use patterns and associated health behaviors. Multi-stage, address-based, area-probability sampling with an in-person household screener served to establish the cohort of youths and adults at W1. Adults who used tobacco, young adults aged 18–24, and African Americans were oversampled relative to population proportions. This analysis includes 1148 youth aged 12–17 who used a tobacco product in the past 30 d who were identified from 13 651 youth respondents. For youth, current use is defined as using a product in the past 30 d. Missing responses for age, sex, race, or Hispanic ethnicity were logically assigned from other PATH Study data as described in the PATH Study Restricted Use File Users Guide.20 For age category, two cases were logically assigned from the household screener data. For sex, 38 were logically assigned from the household screener data and 1 was statistically imputed. For race-ethnicity, 582 were logically assigned from the household screener data and 173 were statistically imputed.
The PATH Study weighting procedures adjusted for oversampling and nonresponse; combined with the use of a probability sample, the weighted data allow the estimates produced by the PATH Study to be representative of the noninstitutionalized, civilian US population at the time of W1. Further details regarding the PATH Study design and methods are published elsewhere21 and can be found at https://doi.org/10.3886/ICPSR36231.v34. Westat’s Institutional Review Board approved the PATH Study design and protocol.
Symptoms of TD
The youth interview included seven Wisconsin Inventory of Smoking Dependence Motives (WISDM)22 items common to the adult instrument and additional items from the “Hooked on Nicotine Checklist”2 (HONC; two items). Time to First Tobacco Use (TTFU) was constructed by converting reported time into minutes and grouping responses into four categories, “<5,” “5–30,” “31–60,” and “>60” minutes. Current symptoms of TD in W1 (W1 current users of cigarettes, e-cigarettes, cigars, hookah, pipe, and smokeless tobacco products in the tobacco marketplace at the time, including traditional moist snuff, dip, spit, or chew and loose snus or snus pouch products; n = 1148 youth) from the PATH Study were also examined. Items enable evaluation of different levels of TD and represent multiple domains of TD including tolerance, craving, loss of control, cognitive enhancement, and negative reinforcement from tobacco use. Supplemental Table 1 shows the original W1 adult instrument and domain targeted by each examined symptom of TD.
Demographic Characteristics
We measured age (12–14 and 15–17 years), sex (male and female), and race and ethnicity (White, non-Hispanic; Black, non-Hispanic; Hispanic; and Other, non-Hispanic). Adult demographic characteristics are measured and reported elsewhere.17
Frequency of Tobacco Product Use
We measured frequency of days used of the following tobacco products in the past 30 d: cigarettes, e-cigarettes, cigars, hookah, pipe, and smokeless tobacco. W1 e-cigarette use assessed a broad category of products available at the time of the survey. For other single product users and multiple product users, frequency was assessed using the maximum days used for any product.
Comparison to Adults Who Use Tobacco
Sixteen TD symptoms, derived from three primary instruments, represent multiple domains of TD among adults: WISDM (11 items), the Nicotine Dependence Syndrome Scale (4 items), and the Diagnostic and Statistical Manual of Mental Disorders criteria (1 item). Of these items, only seven directly overlap with the youth instrument.
For adults who smoke cigarettes, a current established user was defined as an adult who had smoked at least 100 cigarettes in his or her lifetime and now smokes every day or some days. For adults using other tobacco products, a current established user was defined as an adult who had ever used the product “fairly regularly” and now uses it every day or some days. This analysis of 14 286 adult current established users of a tobacco product draws from the 32 320 Adult W1 Interviews (all participants aged 18 years and older). The W1 adult users in the current analysis were evaluated previously in the formative evaluation of the consistency of TD among adults who use multiple products, e-cigarettes, cigarettes, and other tobacco products.17 Youth and adults who use tobacco differ slightly in the products included. For example, adults who used a pipe only were excluded from the first validation study17 and again are excluded in this analysis. We included youth who used a pipe in the sample to be inclusive of examined tobacco product use, while youth use of dissolvable products, bidi, and kreteks was very infrequent and not considered.
Data Analyses
Youth exclusive use groups included cigarette only (n = 265), e-cigarette only (n = 150), any other single product use (ie, cigar, hookah, pipe, or smokeless; n = 262) and multiple product use (ie, more than one of following products: cigarette, e-cigarette, cigar, hookah, pipe, or smokeless; n = 471). TD responses from youth who used tobacco products in the past 30 d (n = 1148) and adults who reported current established tobacco use (n = 14 286) were used to assess the effectiveness and comparability of youth and adult TD measurement. Item response models (IRM) were used to describe the probability of endorsing symptoms23 and assessed the equivalence of these measures of TD across youth tobacco use groups. Levels of TD within each product user group were associated with corresponding product use. Confirmatory factor analytic models of polychoric correlations with complex sampling weights produced mean- and variance-adjusted X2 statistics that were used to evaluate unidimensional assumptions of IRM. All reported summaries of percentages and regression models used replicate weights and the Balanced Repeated Replication method with Fay adjustment (eg, Fay = 0.3) with the survey package in R.24,25
The graded response model was used to model sets of binary and multiple category items. Prior to fitting the graded response model, a non-parametric IRM was examined to (1) ensure that each increasing option reflected increasing levels of TD (monotonicity) and (2) collapse options that did not provide clear non-overlapping information. A series of parametric models isolated and compared each TD symptom from each tobacco use group in DIF analyses using a Benjamini–Hochberg26 adjustment to assign significance in the context of multiple comparisons. While youth were expected to have different and significantly lower levels of TD than adults, simultaneous calibration27,28 using items administered to both youth and adults allowed DIF to be evaluated while adjusting for average differences in levels of TD. Items that evaluate the TD construct differently for youth than adults will demonstrate significant DIF. Support for the construct validity of the youth TD will be provided by a finding of minimal DIF among examined items. Non-overlapping items were considered missing at random for models comparing youth and adults.29 These analyses focus on the aggregate impact of measured DIF and use a Differential Test Functioning (DTF) statistic to properly account for sampling variability in item parameter estimates30 when quantifying the amount of any scoring bias between groups (Signed DTF: sDTF). When true differences in levels of TD are held constant across focal and reference groups and the relationship between levels of TD and the expected test score differs, evidence for significant sDTF would emerge. Substantial sDTF would challenge the validity of the TD scores in that particular subgroup (focal group). Negative values of sDTF indicate that tobacco users in the reference group (cigarette only or adults) on average scored lower than the focal group (other product groups or youth).
Results
Descriptive Analyses
Weighted demographic characteristics of the W1 current users of each tobacco user group are presented in Table 1.
Table 1.
Demographic Characteristics of Youth Past 30-d Tobacco User Groups in Wave 1 of the PATH Study
| Cigarette (n = 265) |
E-cigarette (n = 150) |
Other single product (n = 262) |
Multiple products (n = 471) |
|||||
|---|---|---|---|---|---|---|---|---|
| Demographic factor | N | SE, % | N | SE, % | N | SE, % | N | SE, % |
| Gender | ||||||||
| Male | 110 | 42.7 | 90 | 59.7 | 143 | 56.4 | 294 | 63.3 |
| 3.3 | 3.9 | 3.3 | 2.2 | |||||
| Female | 155 | 57.3 | 60 | 40.3 | 119 | 43.6 | 177 | 36.7 |
| 3.3 | 3.9 | 3.3 | 2.2 | |||||
| Age group | ||||||||
| 12–14 | 45 | 16.4 | 31 | 18.5 | 45 | 16.4 | 72 | 14.0 |
| 2.2 | 3.2 | 2.1 | 1.7 | |||||
| 15–17 | 220 | 83.6 | 119 | 81.5 | 217 | 83.6 | 399 | 86.0 |
| 2.2 | 3.2 | 2.1 | 1.7 | |||||
| Racial/Ethnic group | ||||||||
| Non-Hispanic White | 156 | 64.7 | 75 | 55.5 | 134 | 57.3 | 292 | 67.8 |
| 3.1 | 4.6 | 3.6 | 2.2 | |||||
| Non-Hispanic Black | 29 | 11.4 | 17 | 12.3 | 42 | 17.3 | 36 | 7.7 |
| 1.9 | 2.8 | 2.8 | 1.2 | |||||
| Hispanic | 60 | 18.1 | 41 | 21.9 | 68 | 20.5 | 93 | 16.4 |
| 2.2 | 3.3 | 2.8 | 1.7 | |||||
| Other non-Hispanic race | 20 | 5.8 | 17 | 10.3 | 18 | 4.9 | 50 | 8.1 |
| 1.2 | 2.6 | 1.4 | 1.1 | |||||
Values for numbers of cases (N) are unweighted. All percentages (%) are weighted estimates and include standard errors (SE).
Domains of TD Symptoms Among Youth Tobacco Use Groups
Confirmatory factor models supported assumptions of a single primary dimension of TD. Fit to the unidimensional model was acceptable across cigarette only (Comparative Fit Index [CFI] = 0.97; Tucker-Lewis Index [TLI] = 0.96; Root Mean Square Error of Approximation [RMSEA] = 0.10), e-cigarette only (CFI = 0.98; TLI = 0.97; RMSEA = 0.06), other single product (CFI = 0.98; TLI = 0.98; RMSEA = 0.07), and multiple product user groups (CFI = 0.97; TLI = 0.96; RMSEA = 0.09).
Two items (Supplemental Table 1, item 11, WISDM: “I would feel alone without my [product].” and item 1: WISDM: “I find myself reaching for [product] without thinking about it.”) were omitted from further analyses due to low frequency of endorsement (only three of e-cigarette only users) and due to contributing only 38% of the item variance when assessed in CFA of e-cigarette only users. Subsequent IRMs focused first on the eight items with strong loadings on a single primary dimension and loadings ranging from 0.73 to 0.94 for cigarette only, 0.68 to 0.97 for e-cigarette only users, 0.85 to 0.95 for other single product, and 0.72 to 0.95 for multiple tobacco product use groups. Reliability estimates for the 8-item scale were strong in each group with coefficient alpha of 0.9, H of 0.65, and omega-hierarchical of 0.82 for cigarette only; alpha of 0.84, H of 0.52, and omega of 0.66 for e-cigarette only; alpha of 0.90, H of 0.70, and omega of 0.78 for other single product; and alpha of 0.89, H of 0.63, and omega of 0.79 for multiple product user groups. Final IRMs were used with the retained items to estimate levels of the latent construct of TD across all tobacco user groups.
Symptom Expression Across Youth Tobacco Use Groups
Table 2 provides a summary of DIF results for the eight examined items in four separate tobacco use groups, the IRM estimated strength of relationship with other symptoms of estimated TD (a: discrimination) and levels of estimated TD where each symptom is likely to be observed (b: severity).31 With levels of estimated TD anchored among the cigarette only use group, mean estimated TD was more than a full SD lower for the e-cigarette only use group (mean = −1.09; SD = 0.64). Other single product use group on average reported lower estimated TD (mean = −0.60; SD = 0.84) and users of multiple tobacco products reported similar levels of estimated TD (mean = 0.14; SD = 0.78) as the cigarette only use group.
Table 2.
Differential Item Functioning for Measuring Tobacco Dependence Across Wave 1 Use Groups and Across Wave 1 Youth and Wave 1 Adult Surveys from the PATH Study
| Do you ever have strong cravings to use tobacco? | Have you ever felt like you really needed to use tobacco? | I frequently crave [product]. | My [product] use is out of control. | I usually want to use [product] right after I wake up. | Using [product] would really help me feel better if I’ve been feeling down. | Using [product] helps me think better. | How soon after you wake up do you want to use tobacco? | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | a | b1 | b2 | a | b1 | b2 | a | b1 | b2 | a | b1 | b2 | a | b1 | b2 | a | b1 | b2 | a | b1 | b2 | a | b1 | b2 |
| Cigarette only | 2.75 | −0.38 | 1.66 | −0.71 | 2.83 | −0.62 | 0.77 | 1.20 | 0.63 | 2.57 | 3.25 | −0.01 | 0.75 | 0.78 | −1.89 | 0.85 | 0.92 | −0.42 | 2.26 | 2.02 | −0.79 | |||
| E-cigarette only | 2.75 | −0.38 | 3.87 | −0.74 | 1.97 | −0.71 | 0.62 | 1.20 | 0.63 | 2.57 | 3.25 | −0.01 | 0.75 | 1.47 | −1.53 | 0.25 | 0.92 | −0.42 | 2.26 | 0.44 | 0.16 | |||
| Cigarette only | 2.65 | −0.35 | 1.66 | −0.70 | 2.84 | −0.60 | 0.78 | 1.55 | 0.59 | 2.27 | 2.95 | −0.05 | 0.76 | 0.78 | −1.88 | 0.86 | 0.90 | −0.35 | 2.33 | 1.99 | −0.78 | |||
| Other single product | 2.30 | −0.11 | 1.49 | −0.17 | 2.84 | −0.60 | 0.78 | 1.71 | 0.48 | 1.76 | 3.43 | 0.08 | 1.01 | 1.15 | −1.97 | 0.32 | 1.93 | −0.74 | 0.62 | 1.81 | −0.81 | |||
| Cigarette only | 3.97* | 0.17 | 3.02 | 0.00 | 5.38 | 0.00 | 0.94 | 2.24 | 0.83 | 1.98 | 3.76 | 0.37 | 0.96 | 1.93 | −0.26 | 0.85 | 1.81 | 0.29 | 1.70 | 3.21 | −0.08 | |||
| Multiple products | 3.01 | 0.23 | 2.91 | 0.14 | 5.38 | 0.00 | 0.94 | 2.93 | 0.63 | 1.75 | 3.97 | 0.40 | 0.97 | 1.97 | −0.28 | 0.88 | 2.05 | 0.32 | 1.44 | 3.80 | −0.04 | |||
| Adults | 3.14 | −1.08 | 0.27 | 3.01 | −0.22 | 0.78 | 2.52 | −0.56 | 0.23 | 1.52 | −0.73 | 0.72 | 1.62 | −0.09 | 1.25 | |||||||||
| Youth | 4.03 | −0.44 | 0.72 | 2.03 | 0.44 | 1.82 | 3.28 | 0.08 | 0.77 | 1.72 | −0.76 | 0.56 | 1.62 | −0.09 | 1.25 | |||||||||
a: slope; b1: first item threshold where option becomes more likely than the previous one; b2: second item threshold when there are more than two options; we only tested DIF on the italicized items. The unitalicized items were constrained to be equal for the reference and focal groups. All the items tested for DIF (ie, italicized) showed statistically significant differences in both slope and severity parameters between the two groups (p < 0.001 based on Wald test, indicated by *). DIF models enabled the zeroExtreme option in MIRT software when evaluating user groups (e-cigarette only and other single product users) with frequent patterns of no symptoms.
Differences in Symptom Reports Within Youth Tobacco Use Groups
DIF analyses evaluated parameter(s) within IRM designed to isolate group differences in the likelihood of symptom endorsement given similar levels of estimated TD with the reference group of cigarette only smokers. E-cigarette only use group had infrequent endorsements of items reflecting high levels of estimated TD, which prevented statistical comparison of items reflecting more severe levels of estimated TD. DIF analyses examined four items (WISDM 2 and 9; TTFU; HONC 2) in models constraining remaining items to be equal across e-cigarette only and cigarette only use groups. Each of the examined items (Table 2) had statistically significant DIF (p’s < .001). Supplemental Figure 1, a1 and a4 displays items with the largest DIF and impact of measured DIF on total scores reflecting levels of estimated TD. sDTF suggested a statistically significant (p < .01) and small impact of item bias in the expected count of item responses (sDIF = 0.03, 95%CI = 0.02 to 0.04) reflecting a total bias of 0.3 raw score points (or 0.15%) higher for e-cigarette only to obtain the same score as the cigarette only use group (Plot a in Figure 1).
Figure 1.

Differential test functioning for youth product groups and youth and adult instruments. Plot a presents expected raw count of youth item responses to tobacco dependence (TD) for e-cigarette only (darker blue line) and cigarette only (lighter blue line) users. Plot b presents expected total TD for multiple product (darker blue line) and cigarette only (lighter blue line) users. Plot c presents expected raw count of youth and adult item responses for youth (purple line) and adult (green line) respondents.
When compared to cigarette only, analyses assessing TD among youth in other single product and multiple product use groups anchored models using a single item (WISDM: “Frequently Crave”) constrained to be equal in each group comparison. Significant DIF was observed for all items (item effects size range = 0.01–0.09; p’s < .01). For example, a WISDM item assessing cognitive enhancement (“…helps me think better”) was more discriminating among other single product users and a HONC item assessing craving (“…ever…really needed to use”) was less likely to be endorsed among other single product and multiple product use groups given the same level of estimated TD as the cigarette only group (Table 2). sDTF suggested a statistically significant (p < .001) and small bias in the expected count of item responses of −0.18 (95% CI = −0.18 to 0.19) raw score points, an average of 0.09% higher scores for cigarette only use with the same level of estimated TD as the other single product use group. Among multiple product users, assessments of the level of estimated TD associated with each symptom response were also similar to the cigarette only use group with examined items differing slightly for several symptoms (p’s < .01). sDTF suggested a statistically significant (p < .001) and small bias in the expected count of item responses of −0.03 (95% CI = −0.028 to 0.033) raw score points, an average of 0.15% higher scores for cigarette only use group with the same level of estimated TD as multiple product use group (Plot b in Figure 1).
Despite some variability, a majority of symptoms sustained strong relationships with estimated TD and provided information about a broad range of estimated TD severity. Three craving symptoms, two tolerance symptoms, one loss of control, one affiliative attachment, one cognitive enhancement, and one negative reinforcement showed substantial relationships with levels of estimated TD. Findings revealed that reasonable comparisons of youth with different patterns of tobacco use can be made using the same assessments with eight identified symptoms (Table 2).
Concurrent Validity of Youth TD Scale
Daily tobacco use was reported among 19% (±2.2%) of cigarette only, 2% (±1.1%) of e-cigarette only, 10% (±1.9%) of other single product, and 25% (±2.1%) of multiple product user groups. Weighted-ordered logistic regression models (Supplemental Table 2) suggested significantly higher levels of TD scores (raw scores transformed to range 0–100) among the higher frequency of use quartiles for cigarette only use group. Figure 2a shows the mean of TD for frequency of use quartiles for each youth use group. Given differences in the frequency with which products are used, we also arranged summaries of TD scores for each product user group using the bottom (0–1 d), middle (2–15 d), and upper third quartiles (>15 d) of observed past 30-d use of any tobacco product. Figure 2b shows the relatively lower levels of TD scores observed among e-cigarette only and other single product user groups. The relatively high levels of TD scores among cigarette only and multiple product use groups were most apparent among more frequent patterns of use (upper tertile of >15 d). Youth respondent TD scores were labeled as High Scores when above 43.8 (SE = 1.6), the weighted upper tertile (>66th percentile) of responses on the TD scores.
Figure 2.
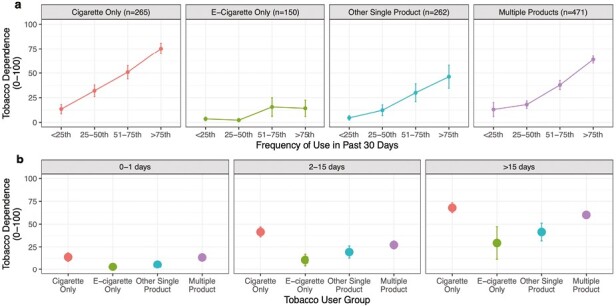
Association between increasing frequency of tobacco use and mean level of Tobacco Dependence (TD) among youth. Plot a presents mean TD for groups with typical ranges of frequency for each product user group. Increasing quartiles of frequency of use were computed separately within cigarette only, e-cigarette only, other single product, and multiple product users. Plot b presents mean TD for each user group across a simple range of days youth used their respective tobacco products within the past 30 d. All means and 95% confidence intervals are weighted.
Demographic Correlates of Youth TD
Among the cigarette only use groups, females (b = 7.6, p < .02) had higher TD scores than males after adjusting for current frequency of use (Supplemental Table 3). Among e-cigarette only use group and after adjusting for frequency of use, Black youth (b = −4.8, p < .06) exhibited lower levels of TD scores than White youth. Among the other single product use group and after adjusting for frequency of use, Black youth (b = −8.3, p < .03) had lower TD scores than White youth. Among the multiple product use group and after adjusting for frequency of use, youth 15–17 (b = 6.2, p < .04) had higher TD scores than youth aged 12–14.
Construct Validity of TD in Youth When Compared to Adult Respondents
DIF analyses examined the construct validity of TD among youth and adult responses to four common items. An anchor item with minimum differences in functioning in a non-parametric model was used to establish a common metric for DIF analyses (WISDM item 10: Supplemental Table 1). The anchor and non-overlapping items had slope and threshold estimates constrained to be the same across youth and adult groups when examining DIF. We observed statistically significant DIF in each item (p’s < .001). The largest DIF was observed for item 2 (effect size = 0.45) and item 3 (effect size = 0.40). Youth endorsed “…use is out of control” and “want to use right after I wake up” (item 2) at higher levels of estimated TD than adults (Supplemental Figure 1, b2 and b3). The smallest DIF was observed for reports of “Using would really help…if…feeling down” (effect size = 0.04). sDTF suggested a statistically significant (p < .001) and small bias in the expected count of item responses of 0.31 (95% CI = 0.31 to 0.32) raw score points, an average of 0.59% higher scores for adults with the same level of estimated TD as youth (Plot c in Figure 1). The small observed DIF between youth and adult responses to TD supports the construct validity of the youth TD instrument.
Simultaneous calibration of all item parameters in a combined sample placed youth and adults on a common metric for estimated TD. Estimates of the expected count of item responses32 for subsets of 8-item youth TD and 16-item adult TD across a range of estimated levels of TD are plotted (Supplemental Figure 2) to enable translation of each possible raw score from the youth to the adult raw scale value.
Discussion
Building upon prior psychometric work with adult tobacco product users, the results of this study of youth also supported a single dimension of TD to characterize item variability both within and across tobacco product users. Eight of the original 10 TD youth items were retained as effective for measuring TD across tobacco product users. Compared to exclusive cigarette smokers, the mean TD score was substantially lower for the W1 e-cigarette only use group. Although we provide evidence that the measurements of TD can be compared among a broad range of W1 e-cigarette product users (eg, refillable and disposable) and W1 cigarette only users, evolutions in the type and nicotine delivery of e-cigarette products will require continuing evaluation of TD measurement for these product use groups. The other W1 single product use group on average had lower TD, while the multiple tobacco product use group experienced levels similar to the cigarette only use group. These results accord with those observed in adults and implicate cigarette smoking, exclusively or in combination with other tobacco products, as well as concurrent use of more than one tobacco product, as the most potent drivers of TD at W1.
Across tobacco product user groups, intensity of TD was generally associated with increased frequency of use of respective products. Higher levels of TD were observed among the increasing quartiles of frequency of use for cigarette only, e-cigarette only, other single product, and multiple product use groups. The associations were most pronounced for the exclusive cigarette, single product, and multiple product use groups while, for exclusive the e-cigarette use group, there was a smaller increase in the average TD score which plateaued above the 50th frequency of use percentile. Similar findings were observed when frequency of use was defined as daily vs. nondaily. Thus, for the exclusive e-cigarette use group at W1, overall TD scores were low compared to other product user groups, and were less strongly linked to frequency of use. As frequency of use increases, the difference in dependence across products becomes more apparent. It is difficult to compare frequency and intensity of use across different classes of tobacco products because they can be used in very different ways subject to product characteristics and ease of use. For example, cigarettes generally are smoked completely in bouts of 8–10 puffs; e-cigarettes can be used for one or a few puffs at a time with episodes of use more easily interspersed throughout the day; and hookah usually is used in prescribed settings (hookah bars). More appropriate product-specific measures of use and intensity (eg, obtained via daily diary or ecological momentary assessment approaches) could yield a better understanding of the association of patterns of product use with TD symptoms.
Further analyses permitted simultaneous calibration of items, which placed youth and adult PATH Study respondents on a common metric for TD. Model-derived estimates of the TD scores for the 8-item youth and 16-item adult metrics exhibit an almost linear relationship. One feature of the PATH Study is that youth respondents are eligible to “age-in” to the adult sample. The item mapping will therefore facilitate longitudinal evaluation of youth respondents as they age into adulthood. More generally, the mapping process can be used to compare youth and adult levels of product-specific TD.
One limitation of this study is that, by seeking to find elements of TD that are common across products, product-specific indicators of TD were not selected or considered. Additional studies should seek to understand similarities and differences between different tobacco products and their users that contribute to both common and unique dimensions of TD. Additionally, more items could be added to a youth interview that focus on completing coverage of domains (eg, withdrawal relief, difficulty quitting) that differentiate severity of TD along its entire dimension. In addition, because of sample size limitations, it is difficult to discern whether there exist important differences in TD within a product class (eg, different refillable and disposable e-cigarette products) or across all product combinations. The e-cigarette products available at the time of the W1 survey may differ from newer products and thus average levels of TD associated with similar patterns of use may change as products change. Understanding the stability of TD measurement for users of evolving tobacco products will be important to maintaining comparable TD scores across different product use groups.
Demographic associations with TD highlighted higher scores among female youth who smoke cigarettes compared to male youth who smoke cigarettes after adjusting for differences in their frequency of cigarette use. The effect was consistent with evaluations of adults in the PATH Study17 and has been observed in some population surveys of youth.33,34 While previous studies34 have found non-White participants to have lower levels of TD compared to White participants, we only observed this among Black youth e-cigarette only and other single product use groups and frequency of use did not account for the differences in TD observed in this study.
At W1, the youth e-cigarette only use group had the lowest levels of TD among the concurrently examined use groups. Low levels of endorsement of TD symptoms in this sample affected the ability to examine DIF. A mismatch between higher severity of TD items and lower levels of TD can result in less precise ability to rank those in the e-cigarette only use group for detecting DIF within this lower range of TD. Examined items, however, did not suggest sufficient DIF that would bias scores for the e-cigarette only use group when compared to the cigarette only use group. Nevertheless, future research among youth tobacco users will benefit from developing items assessing the lower range of TD.
Common items enabled both const evaluation of the similarity of relationships between items and overall levels of TD for youth and adults as well as a translation of TD scores across these instruments. Graphical examinations were provided for assessment of the accumulated impact on test-score differences across each examined group of tobacco users.30 Significant DIF at the item level did not generate substantially biased total scores supporting construct validity for youth TD. Item DIF provided meaningful insight by quantifying how youth were less likely to perceive frequent craving, loss of control, or wanting to use upon waking given the same level of TD as adults. Less clear relationships between use behaviors (eg, time to first use, frequency of use) and TD suggest more work is important to better understand and comprehensively assess individual differences in TD among youth who use e-cigarettes only.
A strength of the current study is that we were able to construct a measure of TD that includes a range of indicators that are common across a nationally representative sample of youth using different tobacco products. As we learn more about the motives for the use of different tobacco products and as new products (eg, nicotine salt, JUUL-era e-cigarettes) continue to be introduced, additional items should be developed that reflect overall TD and product-specific TD across a wider range of severity. Recent work on development of measures of dependence on e-cigarettes could prove useful in this regard.35
Several questions remain that can be addressed in further research studies as products and tobacco product use patterns continue to evolve. One is how to define TD for multiple product users, where individuals may be more or less dependent on one product (eg, cigarettes) compared to others. Longitudinal progression of TD, in relation to patterns of product use, remains to be elucidated. Ensuring consistent measurement over time and quantifying the measurement precision of assessed changes will enhance support for assessment of TD in the population. In addition, further validation of TD in relation to biomarkers of exposure (eg, nicotine, nicotine equivalents) may be useful, as well as exploring their predictive validity for potentially important outcomes such as escalation of product use and inability to reduce use or quit.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr
Contributor Information
David R Strong, Cancer Prevention and Control Program, Moores Cancer Center University of California, San Diego, CA, USA; Department of Family Medicine and Public Health, University of California, San Diego, CA, USA.
Allison M Glasser, School of Global Public Health, New York University, New York, NY, USA.
Eric C Leas, Department of Family Medicine and Public Health, University of California, San Diego, CA, USA.
John P Pierce, Department of Family Medicine and Public Health, University of California, San Diego, CA, USA.
David B Abrams, School of Global Public Health, New York University, New York, NY, USA.
Mary Hrywna, Department of Health Behavior, Society and Policy, Rutgers School of Public Health, New Brunswick, NJ, USA.
Andrew Hyland, Roswell Park Cancer Institute, Buffalo, NY, USA.
K Michael Cummings, Medical University of South Carolina, Charleston, SC, USA.
Dorothy K Hatsukami, Department of Psychiatry and Behavioral Sciences, University of Minnesota, Minneapolis, MN, USA.
Geoffrey T Fong, School of Public Health Sciences, University of Waterloo, Waterloo, ON, Canada; Ontario Institute for Cancer Research, Toronto, ON, Canada.
Tara Elton-Marshall, School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada.
Eva Sharma, Westat, Rockville, MD, USA.
Kathryn C Edwards, Westat, Rockville, MD, USA.
Cassandra A Stanton, Westat, Rockville, MD, USA.
Michael D Sawdey, Center for Tobacco Products, Food and Drug Administration, Silver Spring, MD, USA.
Carolina P Ramôa, Center for Tobacco Products, Food and Drug Administration, Silver Spring, MD, USA.
Marushka L Silveira, Kelly Government Solutions, Rockville, MD, USA; National Institute of Dental and Craniofacial Research (NIDCR/NIH), Bethesda, MD, USA.
Heather L Kimmel, National Institute on Drug Abuse (NIDA/NIH), Bethesda, MD, USA.
Raymond S Niaura, School of Global Public Health, New York University, New York, NY, USA.
Declaration of Interests
RN receives funding from the Food and Drug Administration Center for Tobacco Products via contractual mechanisms with Westat and the National Institutes of Health. Within the past three years, he has served as a paid consultant to the Government of Canada via a contract with Industrial Economics Inc, and has received an honorarium for a virtual meeting from Pfizer Inc. RN was an unpaid grant reviewer for the Foundation for a Smoke Free World. KMC provides expert testimony on the health effects of smoking and tobacco industry tactics in lawsuits filed against the tobacco industry. He has also received payment as a consultant to Pfizer, Inc, for services on an external advisory panel to assess ways to improve smoking cessation delivery in health care settings. GTF has a Senior Investigator Award from the Ontario Institute for Cancer Research (IA-004). Over the past two years, he has received grant funding from the U.S. National Cancer Institute (5P01CA200512), Canadian Institutes of Health Research (FDN-148477), Health Research Council of New Zealand, Health Canada, Korea Health Promotion Institute, National Cancer Center Japan, Santé Publique France, Bill and Melinda Gates Foundation, British Heart Foundation, Mayo Foundation for Medical Education and Research, Cancer Research UK, and the Dutch Lung Foundation. He has been an expert witness/consultant for the governments of Uruguay and Australia defending their country’s policies/regulations in litigation, and an expert consultant for the government of Singapore regarding policies and regulations on tobacco products. He has served as a member of the Expert Group on Articles 9/10 of the WHO Framework Convention on Tobacco Control, the WHO Expert Group on COVID-19 and Tobacco Use, and Health Canada’s Scientific Advisory Board on Vaping Products. Tara Elton-Marshall acknowledges funding from Canadian Institutes of Health Research (CIHR) for the Ontario CRISM Node Team (grant #SMN-139150) and the Team Grant: Partnerships for Cannabis Policy Evaluation.
Funding
This manuscript is supported with Federal funds from the National Institute on Drug Abuse, National Institutes of Health, and the Center for Tobacco Products, Food and Drug Administration, Department of Health and Human Services, under contract to Westat (Contract Nos. HHSN271201100027C and HHSN271201600001C). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies. HL Kimmel was substantially involved in the scientific management of and provided scientific expertise for contract no. HHSN27101100027C. This work was completed while ML Silveira was employed at the National Institute on Drug Abuse, National Institutes of Health via Kelly Government Solutions.
Data Availability Statement
Data are available in a public, open-access repository, the National Addiction and HIV Data Archive: https://www.icpsr.umich.edu/web/NAHDAP/studies/36498.
References
- 1. Nonnemaker J, Mowery P, Hersey J, et al. Measurement properties of a nicotine dependence scale for adolescents. Nicotine Tob Res. 2004;6(2):295–301. [DOI] [PubMed] [Google Scholar]
- 2. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156(4):397–403. [DOI] [PubMed] [Google Scholar]
- 3. O’Loughlin J, DiFranza J, Tyndale RF, et al. Nicotine-dependence symptoms are associated with smoking frequency in adolescents. Am J Prev Med. 2003;25(3):219–225. [DOI] [PubMed] [Google Scholar]
- 4. Colby SM, Tiffany ST, Shiffman S, Niaura RS.. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug Alcohol Depend. 2000;59(suppl 1):S83–S95. [DOI] [PubMed] [Google Scholar]
- 5. DiFranza JR, Savageau JA, Rigotti NA, et al. Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tob Control. 2002;11(3):228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rose JS, Dierker LC, Donny E.. Nicotine dependence symptoms among recent onset adolescent smokers. Drug Alcohol Depend. 2010;106(2–3):126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang TW, Gentzke AS, Creamer MR, et al. Tobacco product use and associated factors among middle and high school students - United States, 2019. MMWR Surveill Summ. 2019;68(12):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson AL, Collins LK, Villanti AC, Pearson JL, Niaura RS.. Patterns of nicotine and tobacco product use in youth and young adults in the United States, 2011-2015. Nicotine Tob Res. 2018;20(suppl 1):S48–S54. doi: 10.1093/ntr/nty018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacPherson L, Strong DR, Myers MG.. Using an item response model to examine the nicotine dependence construct as characterized by the HONC and the mFTQ among adolescent smokers. Addict Behav. 2008;33(7):880–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Loughlin J, DiFranza J, Tarasuk J, et al. Assessment of nicotine dependence symptoms in adolescents: a comparison of five indicators. Tob Control. 2002;11(4):354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Loughlin J, Tarasuk J, Difranza J, Paradis G.. Reliability of selected measures of nicotine dependence among adolescents. Ann Epidemiol. 2002;12(5):353–362. [DOI] [PubMed] [Google Scholar]
- 12. Carpenter MJ, Baker NL, Gray KM, Upadhyaya HP.. Assessment of nicotine dependence among adolescent and young adult smokers: a comparison of measures. Addict Behav. 2010;35(11):977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colby SM, Tiffany ST, Shiffman S, Niaura RS.. Measuring nicotine dependence among youth: a review of available approaches and instruments. Drug Alcohol Depend. 2000;59(Suppl 1):S23–S39. [DOI] [PubMed] [Google Scholar]
- 14. Liu LC, Hedeker D, Mermelstein RJ.. Modeling nicotine dependence: an application of a longitudinal IRT model for the analysis of adolescent nicotine dependence syndrome scale. Nicotine Tob Res. 2013;15(2):326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Strong DR, Brown RA, Ramsey SE, Myers MG.. Nicotine dependence measures among adolescents with psychiatric disorders: evaluating symptom expression as a function of dependence severity. Nicotine Tob Res. 2003;5(5):735–746. [DOI] [PubMed] [Google Scholar]
- 16. Strong DR, Messer K, Hartman SJ, et al. Measurement of multiple nicotine dependence domains among cigarette, non-cigarette and poly-tobacco users: insights from item response theory. Drug Alcohol Depend. 2015;152:185–193. doi: 10.1016/j.drugalcdep.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Strong DR, Pearson J, Ehlke S, et al. Indicators of dependence for different types of tobacco product users: descriptive findings from Wave 1 (2013-2014) of the Population Assessment of Tobacco and Health (PATH) study. Drug Alcohol Depend. 2017;178:257–266. doi: 10.1016/j.drugalcdep.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 18. Strong DR, Leas E, Noble M, et al. Predictive validity of the adult tobacco dependence index: findings from waves 1 and 2 of the Population Assessment of Tobacco and Health (PATH) study. Drug Alcohol Depend. 2020;214:108134. doi: 10.1016/j.drugalcdep.2020.108134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strong DR, Leas E, Noble M, et al. Validation of the Wave 1 and Wave 2 Population Assessment of Tobacco and Health (PATH) Study Indicators of tobacco dependence using biomarkers of nicotine exposure across tobacco products. Nicotine Tob Res. 2022;24(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. United States Department of Health and Human Services. Population Assessment of Tobacco and Health (PATH) Study [United States] Restricted-Use Files. 2019. [Google Scholar]
- 21. Hyland A, Ambrose BK, Conway KP, et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob Control. 2017;26(4):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Piper ME, Piasecki TM, Federman EB, et al. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68). J Consult Clin Psychol. 2004;72(2):139–154. [DOI] [PubMed] [Google Scholar]
- 23. Hays RD, Morales LS, Reise SP.. Item response theory and health outcomes measurement in the 21st century. Med Care. 2000;38(suppl 9):II28–II42. doi: 10.1097/00005650-200009002-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lumley T. Survey: Analysis of Complex Survey Samples R Package Version 3.30. 2014. [Google Scholar]
- 25. R Core Team. R: Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- 26. Glickman ME, Rao SR, Schultz MR.. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67(8):850–857. [DOI] [PubMed] [Google Scholar]
- 27. Rosa K, A SK, Nelson L, Thissen D.. Item response theory applied to combinations of multiple-choice and constructed-response items — scale scores for patterns of summed scores. In: Thissen D, Wainer H, eds. Test Scoring. New York, NY: Lawrence Erlbaum Associates Publishers; 2001:253–292. [Google Scholar]
- 28. Wainer H, Thissen D.. On examinee choice in educational testing. Rev Educ Res. 1994;64(1):159–195. [Google Scholar]
- 29. Holland PW, Wainer H.. Differential Item Functioning. Hillsdale, New Jersey: Lawrence Erlbaum Associates Publishers; 1993. [Google Scholar]
- 30. Chalmers RP, Counsell A, Flora DB.. It might not make a big DIF: improved differential test functioning statistics that account for sampling variability. Educ Psychol Meas. 2016;76(1):114–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steinberg L, Thissen D.. Using effect sizes for research reporting: examples using item response theory to analyze differential item functioning. Psychol Methods. 2006;11(4):402–415. [DOI] [PubMed] [Google Scholar]
- 32. Chalmers RP. MIRT: A multidimensional item response theory package for the R environment. J Stat Softw. 2012;4(6):1–29. [Google Scholar]
- 33. Caraballo RS, Novak SP, Asman K.. Linking quantity and frequency profiles of cigarette smoking to the presence of nicotine dependence symptoms among adolescent smokers: findings from the 2004 National Youth Tobacco Survey. Nicotine Tob Res. 2009;11(1):49–57. [DOI] [PubMed] [Google Scholar]
- 34. Kandel DB, Griesler PC, Hu MC.. Intergenerational patterns of smoking and nicotine dependence among US adolescents. Am J Public Health. 2015;105(11):e63–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bold KW, Sussman S, O’Malley SS, et al. Measuring e-cigarette dependence: initial guidance. Addict Behav. 2018;79:213–218. doi: 10.1016/j.addbeh.2017.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a public, open-access repository, the National Addiction and HIV Data Archive: https://www.icpsr.umich.edu/web/NAHDAP/studies/36498.


