Abstract
Background
In a demonstration project, long-acting, injectable cabotegravir-rilpivirine (CAB-RPV) achieved viral suppression in a high proportion of people with HIV (PWH) who were virologically nonsuppressed with adherence barriers. We projected the long-term impact of CAB-RPV for nonsuppressed PWH experiencing adherence barriers.
Methods
Using the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model, we compared 3 strategies: (1) standard of care oral integrase inhibitor–based ART (INSTI); (2) INSTI-based ART with supportive social services (“wraparound services” [WS]) (INSTI/WS); and (3) CAB-RPV with WS (CAB-RPV/WS). Model outcomes included viral suppression (%) and engagement in care (%) at 3 years, and life expectancy (life-years [LYs]). Base case cohort characteristics included mean age of 47y (standard deviation [SD], 10y), 90% male at birth, and baseline mean CD4 count 150/µL (SD, 75/µL). Viral suppression at 3 months was 13% (INSTI), 28% (INSTI/WS), and 60% (CAB-RPV/WS). Mean loss to follow-up was 28/100 person-years (PY) (SD, 2/100 PY) without WS and 16/100 PY (SD, 1/100 PY) with WS.
Results
Projected viral suppression at 3 years would vary widely: 16% (INSTI), 38% (INSTI/WS), and 44% (CAB-RPV/WS). Life expectancy would be 7.4 LY (INSTI), 9.0 LY (INSTI/WS), and 9.4 LY (CAB-RPV/WS). Projected benefits over oral ART would be greater for PWH initiating CAB-RPV/WS at lower CD4 counts. Across plausible key parameter ranges, CAB-RPV/WS would improve viral suppression and life expectancy compared with oral INSTI strategies.
Conclusions
These model-based results support that long-acting injectable CAB-RPV with extensive support services for nonsuppressed PWH experiencing adherence barriers is likely to increase viral suppression and improve survival. A prospective study to provide further evidence is needed.
Keywords: adherence, HIV, long-acting antiretrovirals, resistance, simulation modeling
Options are limited for people with HIV and persistent viremia due to adherence barriers. This model-based analysis supports that long-acting, injectable antiretroviral therapy (ART) with effective support services is likely to improve viral suppression and survival outcomes substantially compared with oral ART.
Introduction
Most people with human immunodeficiency virus (HIV) (PWH) achieve durable viral suppression using currently available oral antiretroviral therapy (ART) regimens [1, 2]. Challenges remain, however, with ART adherence and engagement in care for some PWH due to structural, behavioral, and social barriers [3, 4]. Given the multifaceted nature of adherence barriers, programs that provide intensive psychosocial assistance, care coordination, and outreach can improve engagement in care and ART adherence for some, but others continue to experience persistent viremia due to the severity of challenges such as housing instability, serious mental illness, and substance use disorders [3–7]. A long-acting ART (LA-ART) strategy designed for infrequent, observed dosing would eliminate the need for strict daily adherence to oral ART [8, 9], substantially lowering the barriers to ART adherence.
Recent randomized trials showed that an injectable formulation of cabotegravir (CAB), an integrase strand transfer inhibitor (INSTI), with rilpivirine (RPV), a nonnucleoside reverse transcriptase inhibitor (NNRTI), dosed intramuscularly every 4 or 8 weeks was noninferior to oral ART among PWH already virologically suppressed on oral ART and with no history of treatment failure or resistance [10, 11]. Based on the results of these trials, the United States (US) Food and Drug Administration (FDA) approved CAB-RPV in 2021 for both ART-naive and ART-experienced PWH in the US who have sustained virologic suppression [12, 13].
No clinical trials have evaluated CAB-RPV in PWH with viremia, and current treatment guidelines in the US advise against using this regimen in nonsuppressed PWH [13, 14]. A demonstration project in San Francisco, however, reported 96.4% (55/57) viral suppression with CAB-RPV coupled with extensive social and clinical supports in 57 nonsuppressed PWH experiencing barriers to adherence (median follow-up, 26 weeks) [15]. With limited data on the effectiveness of CAB-RPV in nonsuppressed PWH, simulation modeling allows for an examination of the impact of uncertainty in available data, as well as projecting long-term outcomes beyond the time horizon of empirical studies. Our objective was to use an HIV simulation model to project the long-term clinical outcomes of CAB-RPV in nonsuppressed PWH experiencing barriers to adherence to oral antiretrovirals.
METHODS
Analytic Overview
We used the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model, a validated microsimulation model of HIV disease and treatment [16–18], to investigate the clinical impact of three treatment strategies for nonsuppressed PWH experiencing adherence barriers: (1) current US standard of care with INSTI-based oral ART, consistent with US Department of Health and Human Services guidelines [13] (INSTI); (2) INSTI coupled with additional social services including community-based supports (eg, case managers, nursing services), hereafter referred to as wraparound services, or “WS” (INSTI/WS); and (3) long-acting injectable CAB-RPV with the same wraparound services (CAB-RPV/WS). This approach allowed us to examine the independent potential benefits of LA-ART and wraparound services.
Management of Observed Viremia
A major concern about prescribing CAB-RPV to nonsuppressed PWH is the development of acquired drug resistance to two critical classes of ART: INSTIs and NNRTIs [19]. To bias the analysis against CAB-RPV, we assumed that people who develop viremia while using injectable CAB-RPV could develop INSTI and NNRTI resistance and would need to switch to a protease inhibitor (PI)–based oral ART regimen. If PWH are detected to have viremia while prescribed an oral INSTI-based or PI-based regimen, we assume that they restart the same regimen, given the infrequent development of acquired resistance and the availability of genotypic resistance testing in the US to inform ART selection [20, 21].
Model Structure
CEPAC is an individual-level microsimulation model of HIV disease and treatment that projects long-term clinical outcomes and survival [16–18]. Simulated PWH enter the model with age, sex at birth, CD4 count, HIV RNA level, and adherence to care drawn from user-specified distributions (Supplementary Methods). For simulated PWH taking oral ART, PWH with lower adherence have a lower probability of achieving initial suppression and are more likely to develop subsequent viremia. For LA-ART, the probability of initial suppression and subsequent viremia do not depend on the adherence of simulated people because the long-acting formulation eliminates the need for adherence to daily pills. All simulated PWH with lower adherence are also at increased risk of disengaging from care, which results in discontinuing oral or LA-ART. In the absence of any ART, PWH experience a monthly decline in CD4 cell count, an increase in HIV RNA level, and increased HIV-related morbidity and mortality [16–18]. Wraparound services promote better virologic suppression through strengthening engagement in care. Additional details about the CEPAC model are available online: https://mpec.massgeneral.org/cepac-model/.
Model Outcomes
For each strategy, model outcomes included viral suppression, engagement in care, and survival at 3 years, as well as life expectancy. For the CAB-RPV/WS strategy, we also projected the number of people who develop viremia while treated with CAB-RPV. We use the comparison between CAB-RPV/WS with INSTI to examine the maximum potential benefit of using CAB-RPV with added supportive services for nonsuppressed PWH. The comparison between CAB-RPV/WS with INSTI/WS allows the evaluation of additional benefit, if any, of using CAB-RPV in place of oral INSTI with the same wraparound services in place.
Input Parameters
Cohort Characteristics
We simulated a cohort with demographic and clinical characteristics similar to those in the demonstration project in San Francisco among nonsuppressed PWH experiencing barriers to adherence [15]. Mean initial age is 47 years, 90% of the cohort is male sex at birth, and mean initial CD4 count is 150/µL (standard deviation [SD], 75/µL) (Table 1). No simulated PWH have INSTI or NNRTI resistance mutations at model start.
Table 1.
Selected Base Case Input Parameters in an Analysis of Long-Acting Cabotegravir-Rilpivirine With Wraparound Services Compared With Oral Integrase Inhibitor–Based Antiretroviral Therapy With or Without Wraparound Services for Nonsuppressed People With HIV Experiencing Adherence Barriers
| Parameter | Value | Source | |
|---|---|---|---|
| Cohort characteristics | Derived from [15] | ||
| Age, y, mean (SD) | 47 (10) | ||
| Sex at birth, male/female % | 90/10 | ||
| CD4 count, cells/µL, mean (SD) | 150 (75) | ||
| Adherence distribution on oral INSTI-based ART | Without WS | With WS | Derived from [7] |
| Mean, % | 65 | 67 | |
| Proportion with MPR 50%–66% | 63 | 32 | |
| Proportion with MPR 66%–70% | 35 | 57 | |
| Proportion with MPR 70%–95% | 2 | 10 | |
| HIV RNA suppression at 3 mo after ART initiation | |||
| INSTI, INSTI/WS | 13% | 28% | [7, 21–25] |
| CAB-RPV/WS | NA | 60% | [15] |
| Subsequent rates of viremia, per 100 PY | |||
| INSTI, INSTI/WS | 7.37 | 7.19 | [7, 21–25] |
| CAB-RPV/WS | NA | 3.29 | [26] |
| Engagement in care | |||
| Loss to follow-up rate, per 100 PY, mean (SD) | 28 (2) | 16 (1) | Derived from [7, 27] |
| Return to care rate, per 100 PY | 18 | 18 | [28] |
| RMR of non-HIV-related mortality vs the general population | 9 | 9 | [29] |
Abbreviations: ART, antiretroviral therapy; CAB-RPV/WS, long-acting injectable cabotegravir-rilpivirine with wraparound services; HIV, human immunodeficiency virus; INSTI, oral integrase inhibitor–based antiretroviral therapy; INSTI/WS, oral integrase inhibitor–based antiretroviral therapy with wraparound services; MPR, medication possession ratio; NA, not applicable; PY, person-years; RMR, relative mortality ratio; SD, standard deviation; WS, wraparound services.
Adherence, Virologic Suppression, and Engagement in Care
We derived probabilities of achieving initial virologic suppression at 3 months and rates of subsequent viremia among PWH with high adherence from randomized controlled trials of oral and injectable ART (Supplementary Methods) [21–25, 30]. To account for lower adherence among a population facing barriers to adherence, we calibrated the model to achieve initial virologic suppression reported from a population before and after wraparound services were available: 13% at 3 months with INSTI versus 28% when wraparound services are provided (INSTI/WS) (Supplementary Methods) [7]. Similarly, we derived the rates of subsequent viremia among people achieving initial suppression on INSTI: 7.37/100 person-years (PY) (INSTI) and 7.19/100 PY (INSTI/WS). We selected the lowest reported estimate of virologic suppression at 3 months with CAB-RPV/WS (60%), which is substantially lower than that reported in the demonstration project (96.4%) [15], making the analysis conservative with respect to the potential benefits of CAB-RPV. The rate of subsequent viremia on CAB-RPV was 3.29/100 PY for people who received an on-time injection [26]. We estimated mean loss to follow-up (LTFU) rates to be: 28/100 PY (SD, 2/100 PY) without wraparound services and 16/100 PY (SD, 1/100 PY) with wraparound services [7].
Non-HIV-Related Mortality
In CEPAC, mortality is stratified by HIV-related causes (eg, opportunistic infections) and non-HIV-related causes (ie, age-stratified and sex-specific mortality). In this modeling analysis, non-HIV-related mortality is adjusted for disadvantageous social determinants of health that are more common among this nonsuppressed population with persistent adherence barriers due to injection drug use, serious mental illness, and unstable housing (Supplementary Table A1) [31, 32].
Sensitivity Analysis
To investigate the impact of uncertainty in parameter estimates on model projections, we performed one-way sensitivity analyses by varying key parameters individually across plausible ranges while holding all other parameters at their baseline values. We examined a range of (1) cohort characteristics (eg, CD4 distribution at model start and percentage of male at birth); (2) ART effectiveness (eg, initial virologic suppression at 3 months, hereafter referred to as efficacy, and subsequent rates of viremia on oral INSTI-based ART; CAB-RPV; and PI-based ART after viremia on CAB-RPV); (3) LTFU rates (eg, during wraparound services, which captures the impact of wraparound services in strengthening engagement in care, and while on CAB-RPV, which captures changes in engagement in care for people taking CAB-RPV and is distinct from the impact of wraparound services); and (4) non-HIV-related mortality (which captures differences in various HIV risk groups among nonsuppressed PWH). Key uncertain parameters including the efficacy of CAB-RPV, the efficacy of PI-based oral ART after viremia on CAB-RPV, and LTFU rates on CAB-RPV were varied widely so that we could examine the impact on model-projected outcomes, if CAB-RPV performs less well than oral ART.
In two multiway sensitivity analyses, we examined the interplay among the most influential parameters from one-way sensitivity analyses. We first compared CAB-RPV/WS with INSTI and examined the efficacy of CAB-RPV, LTFU rates with wraparound services, and the impact of non-HIV-related mortality. Then, we compared CAB-RPV/WS with INSTI/WS and assessed the efficacy of CAB-RPV, the efficacy of PI-based ART after viremia on CAB-RPV, and LTFU rates while on CAB-RPV.
RESULTS
Base Case
For a cohort of nonsuppressed PWH experiencing adherence barriers with mean baseline CD4 count of 150/µL (SD, 75/µL), the projected viral suppression at 3 years would be 16% (INSTI), 38% (INSTI/WS), and 44% (CAB-RPV/WS) (Table 2). Engagement in care at 3 years would range from 45% (INSTI) to 57% (INSTI/WS) to 58% (CAB-RPV/WS). The projected survival at 3 years would be 73% (INSTI), 77% (INSTI/WS), and 79% (CAB-RPV/WS), with life expectancy of 7.4 years (INSTI), 9.0 years (INSTI/WS), and 9.4 years (CAB-RPV/WS). Among all PWH who initiated CAB-RPV, 28% would have confirmed viremia on CAB-RPV by 3 years; PWH in care but not suppressed would be transitioned to a PI-based regimen due to potential development of INSTI and/or NNRTI resistance, with 18% of the cohort suppressed when treated with a PI-based regimen. Supplementary Figure A1 displays the projected changes in viral suppression, engagement in care, and survival over time for all three base case strategies.
Table 2.
Projected Clinical Impact of Long-Acting Cabotegravir-Rilpivirine With Wraparound Services Compared With Oral Integrase Inhibitor–Based Antiretroviral Therapy With or Without Wraparound Services for Nonsuppressed People With HIV Experiencing Adherence Barriers
| At 3 Years | ||||
|---|---|---|---|---|
| Strategies | Viral Suppression, %a | Engagement in Care, %a | Survival, %a | Life Expectancy, y |
| CD4 count = 150 (SD, 75) cells/µL (base case) | ||||
| INSTI | 16 | 45 | 73 | 7.4 |
| INSTI/WS | 38 | 57 | 77 | 9.0 |
| CAB-RPV/WS | 44 | 58 | 79 | 9.4 |
| One-way sensitivity analyses for cohorts of PWH with different baseline CD4 counts | ||||
| CD4 count = 50 (SD, 25) cells/µL | ||||
| INSTI | 16 | 40 | 62 | 6.3 |
| INSTI/WS | 37 | 54 | 71 | 8.3 |
| CAB-RPV/WS | 43 | 56 | 74 | 8.7 |
| CD4 count = 350 (SD, 75) cells/µL | ||||
| INSTI | 16 | 46 | 81 | 9.0 |
| INSTI/WS | 38 | 59 | 83 | 10.2 |
| CAB-RPV/WS | 44 | 59 | 83 | 10.4 |
| CD4 count = 500 (SD, 75) cells/µL | ||||
| INSTI | 16 | 46 | 83 | 9.7 |
| INSTI/WS | 38 | 59 | 84 | 10.7 |
| CAB-RPV/WS | 44 | 59 | 84 | 10.8 |
Abbreviations: CAB-RPV/WS, long-acting injectable cabotegravir-rilpivirine with wraparound services; INSTI, oral integrase inhibitor–based antiretroviral therapy; INSTI/WS, oral integrase inhibitor–based antiretroviral therapy with wraparound services; PWH, people with human immunodeficiency virus; SD, standard deviation.
These percentages were calculated by dividing the number of simulated PWH who would be virologically suppressed, engaged in care, or alive at 3 years by the total number of simulated PWH at model start.
Sensitivity Analysis
One-Way Sensitivity Analyses
Improvements in short-term outcomes would be greatest among people with lower CD4 counts for CAB-RPV/WS compared with INSTI or INSTI/WS strategies, given their high risk of AIDS-related complications (Table 2). For a cohort with mean baseline CD4 count 50/µL, 3-year survival with CAB-RPV/WS would increase by 12 percentage points compared with INSTI and by 3 percentage points compared with INSTI/WS. In a cohort with higher CD4 count (mean, 500/µL), 3-year survival would increase by only 1 percentage point with CAB-RPV/WS compared with INSTI and would be the same with INSTI/WS.
Similarly, the life expectancy benefit for CAB-RPV/WS compared with INSTI or INSTI/WS would be greatest among PWH with lower CD4 counts. Among a cohort with mean baseline CD4 count 50/µL treated with CAB-RPV/WS, projected life expectancy would increase by 2.4 years compared with INSTI and 0.4 years compared with INSTI/WS. Life expectancy gains with CAB-RPV/WS would be smaller in magnitude for a cohort with mean baseline CD4 count 500/µL: 1.1 year compared with INSTI and 0.1 year compared with INSTI/WS.
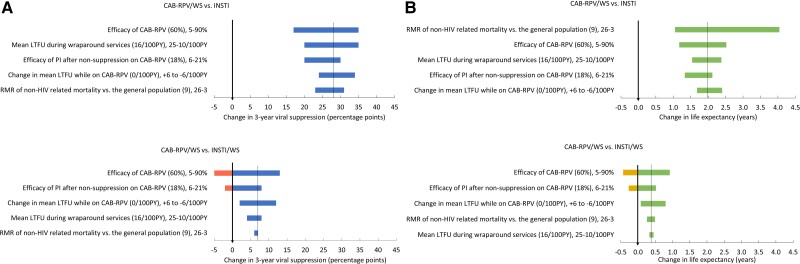
When compared with INSTI, CAB-RPV/WS would result in substantial gains in 3-year viral suppression (17–35 percentage points) and 1.17–4.04 additional years of life gained (YLG) across all parameter ranges considered, even when the parameter estimates were biased against CAB-RPV (Figure 1A and 1B, top panels). The most influential parameters were LTFU rates with wraparound services, the efficacy of CAB-RPV, and non-HIV-related mortality rates. If the efficacy of CAB-RPV at 3 months was 90% (base case: 60%), CAB-RPV/WS would increase 3-year viral suppression by 35 percentage points and lead to 2.5 YLG compared with INSTI; if CAB-RPV efficacy were lower than INSTI efficacy, then CAB-RPV/WS would still increase 3-year viral suppression compared with INSTI because of the benefits of wraparound services. When non-HIV-related mortality varied between 3-fold and 26-fold (base case: 9-fold) greater than in the general population (corresponding to 95% to 85% survival at 1 year with INSTI [29, 33], capturing a wide range of HIV risk groups among nonsuppressed PWH, CAB-RPV/WS would still result in 1.1–4.0 additional YLG compared with INSTI.
Figure 1.
One-way sensitivity analyses for strategies that use long-acting cabotegravir-rilpivirine with wraparound services (CAB-RPV/WS) compared with integrase inhibitor–based antiretroviral therapy (ART) without wraparound services (INSTI) or INSTI-based ART with wraparound services (INSTI/WS) for the outcome of change in 3-year viral suppression (A) and life expectancy (B). The tornado diagrams in each panel display the impact of varying individual parameters on the change in 3-year viral suppression (A) and life expectancy (B) comparing CAB-RPV/WS with INSTI (top) and INSTI/WS (bottom). Each horizontal bar displays the range of outcomes that result from varying a single parameter. The base case value is in parentheses, followed by the range varied; the left-hand value would result in a decrease in the benefit of CAB-RPV/WS, and the right-hand value would result in an increase in the benefit of CAB-RPV/WS compared with INSTI (top) or INSTI/WS (bottom). A longer bar reflects a greater change in outcome as the parameter was varied. The thin vertical lines mark the outcomes from base case estimates: 3-year viral suppression (A, top: 28 percentage points gain with CAB-RPV/WS compared with INSTI; bottom: 7 percentage points gain with CAB-RPV/WS compared with INSTI/WS) and life expectancy (B, top: 2.0 years of life gained [YLG] with CAB-RPV/WS compared with INSTI; bottom: 0.4 YLG with CAB-RPV/WS compared with INSTI/WS). The thick vertical lines show where outcomes would be the same with the strategies. The bars that extend to the right of thick vertical lines (A, blue; B, green) show where CAB/RPV/WS would result in improved outcomes; bars that extend to the left of thick vertical lines (A, pink; B, yellow) show where INSTI (top) or INSTI/WS (bottom) would be preferred. Abbreviations: CAB-RPV/WS, long-acting injectable cabotegravir-rilpivirine with wraparound services; HIV, human immunodeficiency virus; INSTI, oral integrase inhibitor–based antiretroviral therapy; INSTI/WS, oral integrase inhibitor–based antiretroviral therapy with wraparound services; LTFU, loss to follow-up; PI, oral protease inhibitor–based antiretroviral therapy; PY, person-years; RMR, relative mortality ratio.
When comparing both strategies with wraparound services (CAB-RPV/WS vs INSTI-WS), the most influential parameters were the efficacy of CAB-RPV, the efficacy of PI-based regimens after viremia with CAB-RPV, and LTFU rates while on CAB-RPV (Figures 1A and 1B, bottom panels). When the efficacy of CAB-RPV at 3 months is extremely low, 5% (base case: 60%), CAB-RPV/WS would lead to 3-year viral suppression that is 5 percentage points less than with INSTI/WS, which would result in 0.5 years of life lost; at higher estimates of CAB-RPV efficacy (90%), CAB-RPV/WS would lead to 3-year viral suppression that is 13 percentage points greater than with INSTI/WS, resulting in 0.9 YLG. When the efficacy of the PI-based regimen at 3 months is varied between 6% and 21% (base case: 18%), CAB-RPV/WS would result in change in 3-year viral suppression that ranged from −2 to 8 percentage points and 0.3 years of life lost to 0.5 YLG compared with INSTI/WS. When the LTFU rate while on CAB-RPV was increased or decreased by 6/100 PY from the base case (16/100 PY), CAB-RPV/WS would result in 3-year viral suppression between 2 and 12 percentage points greater than with INSTI/WS and 0.1 to 0.8 YLG. All other parameters, including subsequent rates of viremia on CAB-RPV, LTFU rates during wraparound services, and percentage of male at birth were not found to be influential. Appendix Figure A2 shows the one-way sensitivity analyses results for the outcome of change in 3-year engagement in care for comparisons between INSTI, INSTI/WS, and CAB-RPV/WS.
Multiway Sensitivity Analyses
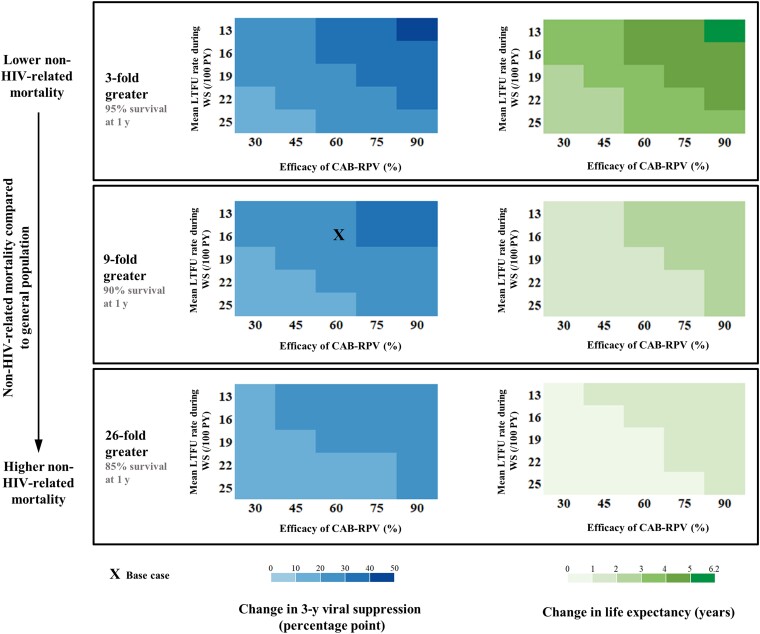
In multiway sensitivity analyses, comparing CAB-RPV/WS with INSTI, we varied LTFU rates with wraparound services, the efficacy of CAB-RPV, and non-HIV-related mortality (Figure 2). Across wide ranges, CAB-RPV/WS would improve 3-year viral suppression (base case: 28 percentage points [range, 12–44 percentage points]) and life expectancy (base case, 2.0 years [range, 0.6–5.5 years]) compared with INSTI. Benefits would be greatest in a population with lower non-HIV-related mortality.
Figure 2.
Multiway sensitivity analyses comparing the strategies that use long-acting cabotegravir-rilpivirine with wraparound services (CAB-RPV/WS) and integrase inhibitor–based antiretroviral therapy without wraparound services (INSTI). Comparing CAB-RPV/WS with INSTI, we examined virologic suppression at 3 years (left) and life expectancy (right) when varying 3 parameters: non-HIV-related mortality (rows), mean loss to follow-up rate during wraparound services (y-axis), and efficacy of CAB-RPV at 3 months (x-axis). The base case is marked with an X. In all scenarios, CAB-RPV/WS would result in improved virologic suppression at 3 years (blue) and improved life expectancy (green) compared with INSTI. Abbreviations: CAB-RPV, long-acting injectable cabotegravir-rilpivirine; HIV, human immunodeficiency virus; INSTI, oral integrase inhibitor–based antiretroviral therapy; LTFU, loss to follow-up; PY-person-years; WS, wraparound services.
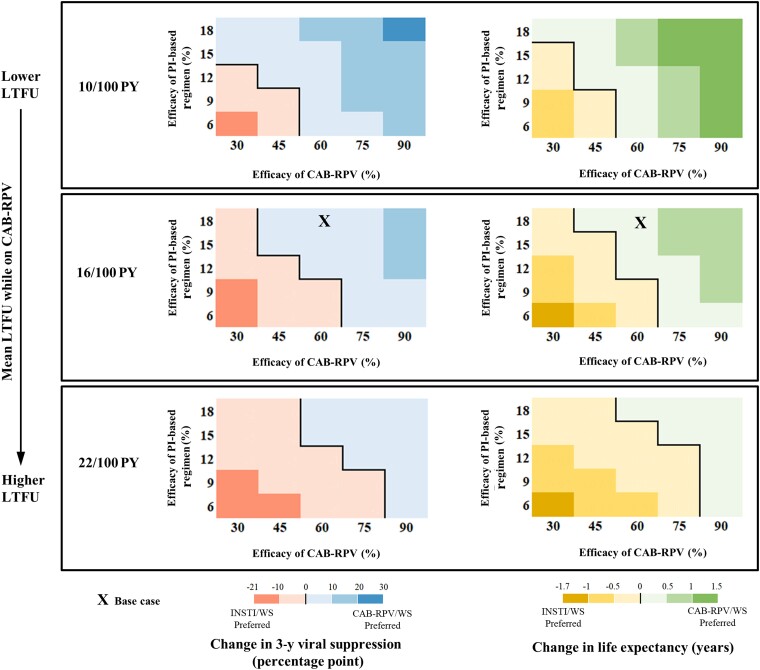
Compared with INSTI/WS, CAB-RPV/WS would improve 3-year viral suppression and life expectancy except at extremely low CAB-RPV efficacy (Figure 3, left of each shaded rectangle) or low efficacy of PI-based regimen (Figure 3, bottom of each shaded rectangle). When the mean LTFU rate on CAB-RPV was higher or the same as on oral ART, CAB-RPV/WS would still improve 3-year viral suppression and life expectancy compared with INSTI/WS, as long as CAB-RPV efficacy was greater than 75% or 60%, respectively (Figure 3, middle and bottom shaded rectangles), even at extremely low PI efficacy of 6%. When considering that LTFU rates could improve during treatment with CAB-RPV (Figure 3, top shaded rectangle), CAB-RPV/WS would improve 3-year viral suppression and life expectancy compared with INSTI/WS, even at CAB-RPV efficacy as low as 45%, as long as PI-based regimen efficacy was 12% or greater after viremia on CAB-RPV. Appendix Figures A3 and A4 show the multiway sensitivity analyses results for the outcome of change in 3-year engagement in care for comparisons between INSTI, INSTI/WS, and CAB-RPV/WS.
Figure 3.
Multiway sensitivity analyses comparing the strategies that use long-acting cabotegravir-rilpivirine with wraparound services (CAB-RPV/WS) and integrase inhibitor–based antiretroviral therapy with wraparound services (INSTI/WS). Comparing CAB-RPV/WS with INSTI/WS, we examined virologic suppression at 3 years (left) and life expectancy (right) when varying 3 parameters: the mean loss to follow-up rate while on CAB-RPV (rows), efficacy at 3 months of protease inhibitor–based oral regimen after viremia on CAB-RPV (y-axis), and efficacy of CAB-RPV at 3 months (x-axis). The base case is marked with an X. The black line inside each panel distinguishes scenarios in which CAB-RPV/WS (blue) or INSTI/WS (pink) would result in improved virologic suppression at 3 years. On the right side, the black line inside each panel distinguishes scenarios in which CAB-RPV/WS (green) or INSTI/WS (yellow) would result in improved life expectancy. Abbreviations: CAB-RPV, long-acting injectable cabotegravir-rilpivirine; CAB-RPV/WS, long-acting injectable cabotegravir-rilpivirine with wraparound services; INSTI/WS, oral integrase inhibitor–based antiretroviral therapy with wraparound services; LTFU, loss to follow-up; PI, oral protease inhibitor–based antiretroviral therapy; PY, person-years; WS, wraparound services.
DISCUSSION
While most people with HIV in the US achieve and maintain virologic suppression on oral ART regimens, a substantial minority have ongoing viremia given barriers to adherence to oral therapy. In the absence of suppressive therapy, this population remains at substantial risk of HIV disease progression and death [34, 35]. Compared with standard-of-care oral INSTI-based therapy, we found that initiation of long-acting, injectable CAB-RPV with added outreach and social supports for this group of people would substantially improve viral suppression, engagement in care, and survival over 3 years and beyond. Benefits would be greatest in people with lower CD4 counts at CAB-RPV initiation, reflecting their very high risk of HIV disease progression.
Across wide ranges, a strategy of CAB-RPV with added social services would improve projected viral suppression at 3 years (12–44 percentage points gain) and would lead to substantial gains in life expectancy (0.6–5.5 years) compared with INSTI-based oral ART without wraparound services, even when the efficacy of CAB-RPV was lower than that of INSTI-based oral ART, given the projected benefits of wraparound services. Survival benefits would further increase in a population with lower non-HIV-related mortality, when additional supportive services focused on substance use disorder or poverty are incorporated [36, 37], or with additional time on suppressive ART [38]. This model-based analysis provides further evidence regarding the benefits of effective and sustainable case management, psychosocial, and nursing support for any strategy focused on improving outcomes for nonsuppressed people with HIV experiencing barriers to adherence. Adapting such intensive support to the needs of specific populations remains a key component of the US End the HIV Epidemic strategy [39]; ensuring sustainable funding for these essential services is critical.
Even with social supports in place, however, a strategy of injectable CAP-RPV in place of oral ART would likely result in further improved viral suppression and survival, if CAB-RPV retains effectiveness long-term in this population. Indeed, worse outcomes with CAB-RPV compared with oral ART would be projected only at implausibly low estimates of initial virologic suppression with CAB-RPV or if LTFU rates were higher among people prescribed CAB-RPV compared with oral ART regimens. In fact, LTFU may be lower with injectable CAB-RPV than with oral ART.
Despite the success of CAB-RPV in the San Francisco demonstration project, an important concern remains the selection of multiclass drug resistance for people experiencing viremia after CAB-RPV. Of particular importance is the selection of INSTI resistance because this drug class is part of all recommended first-line ART regimens [13, 14]. Underscoring this concern is that 1%–2% of people in the licensing trials of CAB-RPV developed resistance despite having virologic suppression at baseline and adhering to the recommended dosing schedule [10, 11, 26, 40]. Given the favorable characteristics of the study population in these trials, viremia with acquired drug resistance is likely to be higher among nonsuppressed PWH experiencing barriers to adherence and other psychosocial challenges, especially because inconsistent adherence to the scheduled injections could lead to a prolonged duration of subinhibitory drug concentrations [8]. Follow-up data from the initial demonstration project in San Francisco demonstrated that 2 of 57 (3.5%) participants developed early virologic failure with resistance [15]. However, when we incorporated substantially higher rates of breakthrough viremia and acquired drug resistance in this modeling analysis, we still found the CAB-RPV/WS strategy to be better at achieving viral suppression than oral ART, leading to improved survival.
Management of people with INSTI and NNRTI resistance after CAB-RPV failure would likely require PI-based ART, which is less effective and more poorly tolerated than INSTI-based ART [23, 24]. However, if substantially higher viral suppression could be achieved with CAB-RPV compared with oral ART, prescribing long-acting, injectable ART to people experiencing adherence challenges would likely outweigh the negative consequences of needing to use a PI-based regimen for the small number of PWH with integrase resistance acquired during CAB-RPV use.
There are several limitations to this study. First, the effectiveness of CAB-RPV compared with oral ART among people with viremia is uncertain and could vary in different populations and settings. We, therefore, selected the lowest reported value in the base case for CAB-RPV efficacy among people with viremia (3-month viral suppression of 60%) [41], which is substantially lower than the 96.4% reported from the demonstration project at 1 year [15]. In sensitivity analyses, we found that a strategy of CAB-RPV/WS would improve clinical outcomes, even at much lower rates of viral suppression. Second, we assumed that viral suppression with CAB-RPV would depend primarily on engagement in care because infrequent dosing would eliminate the need for daily medication adherence. This approach does not capture the complexities of differential patterns of missed injections (eg, length of treatment interruptions, time with sufficient drug concentration) or the association between these patterns and virologic suppression with CAB-RPV [42, 43]. We therefore examined a broad range for LTFU rates on CAB-RPV, including the scenario where LTFU was more frequent on CAB-RPV than on oral ART. Third, we did not try to estimate the likelihood of a more effective injectable agent becoming an available treatment option in the future. Due to the high 3-year mortality in PWH with persistent viremia and advanced immunosuppression, waiting for the potential availability of a more effective regimen is unlikely to be an effective strategy. Fourth, we did not capture either the benefits of reducing transmission to others, or the potential harms of transmitted drug resistance. Finally, we did not conduct a probabilistic sensitivity analysis because the findings from extensive deterministic sensitivity analyses suggest that there would be minimal decision uncertainty [44].
In conclusion, in this model-based analysis, we found that long-acting injectable CAB-RPV coupled with extensive support services in nonsuppressed PWH experiencing adherence barriers in the US would result in substantial improvements in viral suppression and survival. For people engaged in care but not taking oral ART, this approach could have a major and immediate impact in improving clinical outcomes, especially for people with advanced HIV disease. A prospective study of this important strategy conducted in diverse clinical sites and involving a larger number of participants is needed.
Supplementary Material
Contributor Information
Wanyi Chen, Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, Massachusetts, USA.
Monica Gandhi, Division of HIV, Infectious Diseases, and Global Medicine, University of California, San Francisco, San Francisco, California, USA.
Paul E Sax, Harvard Medical School, Boston, Massachusetts, USA; Division of Infectious Diseases, Brigham and Women's Hospital, Boston, Massachusetts, USA.
Anne M Neilan, Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA; Division of Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts, USA; Division of General Academic Pediatrics, Massachusetts General Hospital, Boston, Massachusetts, USA.
Wendy H Garland, Division of HIV and STD Programs, Los Angeles County Department of Public Health, Los Angeles, California, USA.
Timothy Wilkin, Division of Infectious Diseases, Weill Cornell Medicine, New York, New York, USA.
Rebecca Cohen, Division of HIV and STD Programs, Los Angeles County Department of Public Health, Los Angeles, California, USA.
Andrea L Ciaranello, Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA; Division of Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts, USA; Center for AIDS Research, Harvard University, Cambridge, Massachusetts, USA.
Sonali P Kulkarni, Division of HIV and STD Programs, Los Angeles County Department of Public Health, Los Angeles, California, USA.
Joseph Eron, Division of General Academic Pediatrics, Massachusetts General Hospital, Boston, Massachusetts, USA; Department of Medicine, Division of Infectious Diseases, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Kenneth A Freedberg, Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA; Division of Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts, USA; Center for AIDS Research, Harvard University, Cambridge, Massachusetts, USA; Division of General Internal Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA; Department of Health Policy and Management, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Emily P Hyle, Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA; Division of Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts, USA; Center for AIDS Research, Harvard University, Cambridge, Massachusetts, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Study design: W. C., K. A. F., and E. P. H. Data analysis: W. C. and E. P. H. Interpretation of results: W. C., M. G., T. W., J. E., P. E. S., K. A. F., and E. P. H. Drafting the manuscript: W. C. and E. P. H. Critical revision of the manuscript and final approval of submitted version: All authors.
Acknowledgments. We acknowledge Mr Kyu Young Kevin Chi for his assistance with the analysis and manuscript, and Ms Lotanna Dike for her project management.
Patient consent. This study did not include factors necessitating patient consent or institutional review board approval. Research projects utilizing the CEPAC model are approved by the Mass General Brigham Human Research Committee (protocol 2014P002708).
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the AIDS Clinical Trial Group (UM1AI068636 to K. A. F.), which is co-funded by the National Institute on Drug Abuse, National Institute of Neurological Disorders and Stroke, and the National Institute of Allergy and Infectious Diseases (NIAID). The research received additional support from the NIAID (grant number R01AI042006 to K. A. F.) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant numbers R01HD079214 to A. L. C. and R01 HD111355-01 to A. M. N.) of the NIH, and the Steve and Deborah Gorlin Massachusetts General Hospital (MGH) Research Scholars Award (to K. A. F.), the MGH Jerome and Celia Reich Endowed Scholar Award (to E. P. H.), the MGH Research Scholars Award in Population and Health Care Research (to A. L. C.), and the MGH Department of Medicine Transformative Scholars Award (to A. M. N.) of the MGH Executive Committee on Research. Research reported in this publication was supported by the NIAID/NIH (award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701).
References
- 1. Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. HIV-CAUSAL Collaboration; Ray M, Logan R, Sterne JAC, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS 2010; 24:123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shubber Z, Mills EJ, Nachega JB, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med 2016; 13:e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalichman S, Kalichman MO, Cherry C. Medication beliefs and structural barriers to treatment adherence among people living with HIV infection. Psychol Health 2016; 31:383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. New York City Department of Health, Bureau of HIV/AIDS Prevention . HIV care coordination tools. 2023. Available at: https://www1.nyc.gov/site/doh/health/health-topics/aids-hiv-care-coord-tools.page. Accessed 2 May 2023.
- 6. Family Service of Greater Baton Rouge . HIV care coordination. Available at: https://www.fsgbr.org/index.php?option=com_content&view=article&id=10&Itemid=10. Accessed 2 May 2023.
- 7. Flash MJE, Garland WH, Martey EB, et al. Cost-effectiveness of a medical care coordination program for people with HIV in Los Angeles County. Open Forum Infect Dis 2019; 6:ofz537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scarsi KK, Swindells S. The promise of improved adherence with long-acting antiretroviral therapy: what are the data? J Int Assoc Provid AIDS Care 2021; 20:23259582211009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swindells S, Flexner C, Fletcher CV, Jacobson JM. The critical need for alternative antiretroviral formulations, and obstacles to their development. J Infect Dis 2011; 204:669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Overton ET, Richmond G, Rizzardini G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet 2020; 396:1994–2005. [DOI] [PubMed] [Google Scholar]
- 11. Swindells S, Andrade-Villanueva J-F, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382:1112–23. [DOI] [PubMed] [Google Scholar]
- 12. ViiV Healthcare . Dosing and drug interactions CABENUVA (cabotegravir; rilpivirine). Available at: https://cabenuvahcp.com/dosing/?utm_source=bing&utm_medium=cpc&utm_term=cabotegravir%20rilpivirine&utm_campaign=BS%20-%20Branded_Cabenuva_Generic%20Alone%20EX&gclid=37f23979ae401dfd8a405d5c797778b3&gclsrc=3p.ds&. Accessed 2 May 2023.
- 13. Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Available at: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv. Accessed 2 May 2023.
- 14. Gandhi RT, Bedimo R, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society–USA panel. JAMA 2023; 329:63–84. [DOI] [PubMed] [Google Scholar]
- 15. Gandhi M, Salazar J, Hickey MD, et al. High virologic suppression rates on long-acting ART in a safety-net clinic population. 2023. Available at: https://www.croiconference.org/abstract/high-virologic-suppression-rates-on-long-acting-art-in-a-safety-net-clinic-population/. Accessed 2 May 2023.
- 16. Walensky RP, Sax PE, Nakamura YM, et al. Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States. Ann Intern Med 2013; 158:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ross EL, Weinstein MC, Schackman BR, et al. The clinical role and cost-effectiveness of long-acting antiretroviral therapy. Clin Infect Dis 2015; 60:1102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med 2001; 344:824–31. [DOI] [PubMed] [Google Scholar]
- 19. Charpentier C, Storto A, Soulié C, et al. Prevalence of genotypic baseline risk factors for cabotegravir + rilpivirine failure among ARV-naive patients. J Antimicrob Chemother 2021; 76:2983–7. [DOI] [PubMed] [Google Scholar]
- 20. Stellbrink H-J, Lazzarin A, Woolley I, Llibre JM. The potential role of bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) single-tablet regimen in the expanding spectrum of fixed-dose combination therapy for HIV. HIV Med 2020; 21(Suppl 1):3–16. [DOI] [PubMed] [Google Scholar]
- 21. Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380–1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet 2017; 390:2073–82. [DOI] [PubMed] [Google Scholar]
- 22. Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2013; 13:927–35. [DOI] [PubMed] [Google Scholar]
- 23. Clotet B, Feinberg J, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–31. [DOI] [PubMed] [Google Scholar]
- 24. Orrell C, Hagins DP, Belonosova E, et al. Fixed-dose combination dolutegravir, abacavir, and lamivudine versus ritonavir-boosted atazanavir plus tenofovir disoproxil fumarate and emtricitabine in previously untreated women with HIV-1 infection (ARIA): week 48 results from a randomised, open-label, non-inferiority, phase 3b study. Lancet HIV 2017; 4:e536–46. [DOI] [PubMed] [Google Scholar]
- 25. Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380–1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet 2017; 390:2063–72. [DOI] [PubMed] [Google Scholar]
- 26. Margolis DA, Gonzalez-Garcia J, Stellbrink H-J, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017; 390:1499–510. [DOI] [PubMed] [Google Scholar]
- 27. Fleishman JA, Yehia BR, Moore RD, Korthuis PT, Gebo KA; HIV Research Network . Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr 2012; 60:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Helleberg M, Engsig FN, Kronborg G, et al. Retention in a public healthcare system with free access to treatment: a Danish nationwide HIV cohort study. AIDS 2012; 26:741–48. [DOI] [PubMed] [Google Scholar]
- 29. Shebl FM, Foote JH, Reddy KP, et al. Increased mortality among people at high risk for HIV in the United States. 2020. Available at: https://www.croiconference.org/abstract/increased-mortality-among-people-at-high-risk-for-hiv-in-the-united-states/. Accessed 2 May 2023.
- 30. Madruga JV, Berger D, McMurchie M, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet 2007; 370:49–58. [DOI] [PubMed] [Google Scholar]
- 31. Singh K, Chander G, Lau B, Edwards JK, Moore RD, Lesko CR. Association of history of injection drug use with external cause-related mortality among persons linked to HIV care in an urban clinic, 2001–2015. AIDS Behav 2019; 23:3286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Melo JS, Hessol NA, Pipkin S, Buchbinder SP, Hsu LC. Effect of social determinants of health on uncontrolled human immunodeficiency virus (HIV) infection among persons with HIV in San Francisco, California. Open Forum Infect Dis 2022; 9:ofac312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hickey MD, Imbert E, Appa A, et al. HIV treatment outcomes in POP-UP: drop-in HIV primary care model for people experiencing homelessness. J Infect Dis 2022; 226:S353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Horberg MA, Hurley LB, Silverberg MJ, Klein DB, Quesenberry CP, Mugavero MJ. Missed office visits and risk of mortality among HIV-infected subjects in a large healthcare system in the United States. AIDS Patient Care STDS 2013; 27:442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mugavero MJ, Lin H-Y, Willig JH, et al. Missed visits and mortality in patients establishing initial outpatient HIV treatment. Clin Infect Dis 2009; 48:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jawa R, Tin Y, Nall S, et al. Estimated clinical outcomes and cost-effectiveness associated with provision of addiction treatment in US primary care clinics. JAMA Netw Open 2023; 6:e237888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lazar R, Kersanske L, Xia Q, Daskalakis D, Braunstein SL. Hospitalization rates among people with HIV/AIDS in New York City, 2013. Clin Infect Dis 2017; 65:469–76. [DOI] [PubMed] [Google Scholar]
- 38. Morton ZP, Christina Mehta C, Wang T, et al. Cumulative human immunodeficiency virus (HIV)-1 viremia is associated with increased risk of multimorbidity among US women with HIV, 1997–2019. Open Forum Infect Dis 2022; 10:ofac702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–45. [DOI] [PubMed] [Google Scholar]
- 40. Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382:1124–35. [DOI] [PubMed] [Google Scholar]
- 41. D’Amico R, Cenoz Gomis S, Moodley R, et al. Compassionate use of long-acting cabotegravir plus rilpivirine for people living with HIV-1 in need of parenteral antiretroviral therapy. HIV Med 2023; 24:202–11. [DOI] [PubMed] [Google Scholar]
- 42. Parienti J-J, Das-Douglas M, Massari V, et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One 2008; 3:e2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Genberg BL, Wilson IB, Bangsberg DR, et al. Patterns of antiretroviral therapy adherence and impact on HIV RNA among patients in North America. AIDS 2012; 26:1415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM modeling good research practices task force-6. Value Health 2012; 15:835–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.