Abstract
This study examined the relationship between dysphagia and adverse outcomes across frailty conditions among surgical patients ≥ 50 years of age. A retrospective cohort analysis of surgical hospitalizations in the Healthcare Cost and Utilization Project’s National Inpatient Sample among patients ≥ 50 years of age undergoing intermediate/high risk surgery not involving the larynx, pharynx, or esophagus. Of 3,298,835 weighted surgical hospitalizations, dysphagia occurred in 1.2% of all hospitalizations and was higher in frail patients ranging from 5.4% to 11.7%. Dysphagia was associated with greater length of stay, higher total costs, increased non-routine discharges, and increased medical/surgical complications among both frail and non-frail patients. Dysphagia may be an independent risk factor for poor postoperative outcomes among surgical patients ≥ 50 years of age across frailty conditions and is an important consideration for providers seeking to reduce risk in vulnerable surgical populations.
Keywords: dysphagia, frailty, surgery, outcomes
Introduction:
The US surgical population will involve a greater number of older patients who present additional challenges for perioperative care.1 This cohort may have less physiologic reserve to respond to surgical stress with associated poor outcomes in addition to higher costs, longer length of stay, and increased morbidity, and mortality.2, 3 Thus, understanding the factors that influence postoperative outcomes is essential to optimize care for the aging surgical population.
Increased risk of poor surgical outcomes among older surgical patients can likely be attributed to many factors including chronic comorbidity burden, functional status, cognition and falls.4, 5 Additionally, adverse outcomes, loss of independence, disability, and mortality are especially common in surgical patients with sarcopenia and frailty, a clinical state of vulnerability with reduced ability to cope with health stressors and impaired patient resilience.6–8 Furthermore, frailty is not only a consideration for older adults but also has important clinical implications, such as mortality risk, among middle aged adults around age 50.9, 10
Dysphagia may be an important perioperative risk factor for poor inpatient outcomes. Dysphagia prevalence begins to increase in middle aged adults around age 50 and occurs in up to 30% of independently living adults ≥ 65 years of age.11, 12 Moreover, dysphagia is associated with greater cost, mortality, and morbidity including poor nutrient or fluid intake predisposing to malnutrition, aspiration, and is common in patients with frailty and sarcopenia.13–18
Whether dysphagia is independently associated with poor inpatient outcomes among middle aged and older surgical patients, or is simply a marker of frailty or comorbidity burden, is not fully known, as prior studies on dysphagia and outcomes have not routinely assessed frailty.219-21 Given the overlap between frailty and dysphagia among middle aged and older adults, understanding the link between dysphagia and patient outcomes during surgical hospitalizations, especially those without surgical procedures directly involving the swallowing mechanism (i.e. involving the larynx, pharynx, or esophagus) where dysphagia may be underappreciated, is an important step towards identifying patient vulnerability in the perioperative setting.
Using the Agency for Healthcare Research and Quality (AHRQ) sponsored Healthcare Cost and Utilization Project (HCUP) National Inpatient Sample (NIS) database, we examined the association of dysphagia, frailty, and their interaction with adverse inpatient outcomes among a surgical cohort of middle aged and older adults who did not have procedures directly involving the larynx, pharynx, or esophagus. We hypothesized that (1) dysphagia is more prevalent among frail surgical patients and that (2) dysphagic patients have longer length of stay (LOS), higher hospitalization cost, more non-routine discharge, and greater medical and surgical complications compared to non-dysphagic patients independent of frailty status.
Methods:
This study was considered exempt by the Duke University Medical Center Institutional Review Board. A retrospective analysis of surgical inpatient hospitalizations between 2014 and the first three quarters of 2015 was undertaken with discharge data from the NIS database, the largest publically available, all-payer inpatient health care database developed by the AHRQ-HCUP. It is a 20% stratified sample of discharges from all inpatient stays in US community hospitals, excluding short-term rehabilitation and long-term acute care hospitals and includes clinical and nonclinical data.22
This is a subset analysis of a previous general analysis across all medical and surgical inpatients regardless of surgical procedure.23 This subset analysis consists of specific surgical hospitalizations that do not directly involve surgery of the swallowing mechanism (i.e. larynx, pharynx, or esophagus), focusing on surgical patients where dysphagia may not be expected. Inclusion criteria included (a) inpatient surgical hospital stays of patients ≥ 50 years of age with (b) one of the following surgical procedures based on the presence of International Classification of Disease, Ninth Revision Clinical Modification (ICD-9-CM) volume 3 codes: peripheral arterial bypass surgery, cardiac surgery, carotid endarterectomy, abdominal aortic aneurysm repair, total/partial/revision hip replacement, total or revision knee replacement, anterior cervical spinal fusion, large bowel surgery, liver resection, pancreas resection, nephrectomy, cystectomy, pneumonectomy, lobectomy/segmental resection, or general surgery (open/laparoscopic cholecystectomy, inguinal hernia repair, anterior abdominal wall hernia repair) (eTable 1). These surgical procedures are common in middle aged and older adults and were a focus of studies examining links between frailty and patient outcomes.8, 24
Exposures
The primary exposure was dysphagia diagnosis defined by ICD-9-CM codes: 787.2, 787.20, 787.21, 787.22, 787.23, 787.24, 787.29 on discharge records (eTable 1).25 As previously mentioned, frailty is a clinical state or phenotype manifesting as vulnerability that impairs the response to health stressors resulting in poor outcomes.6–8 However, there is no consensus on frailty measurement, and frameworks include assessment of physical function; deficit accumulation models of various symptoms and diseases, disability, and cognitive status; and multi-dimensional assessments including physical function, social support, functional status, and cognition.26–28
To facilitate use in daily clinical practice, methods for frailty assessment in administrative data and based on readily available clinical data have been developed. First, the Johns Hopkins Adjusted Clinical Groups (ACG) employs frailty-defining diagnoses and is a binary yes/no variable based on the presence of at least 1 of 10 frailty-defining diagnoses (Table 1).29 This measure is associated with mortality, risk of hospitalization, intensive care stay, non-routine discharge, readmission, LOS, medical complications, and cost and has been validated in administrative databases, such as NIS in patients ≥ 18 years of age.7,8, 30 The Frailty Risk Score (FRS), a 19-item measure (Table 1) biopsychosocial measure developed to identify hospitalized frail persons ≥ 50 years of age in electronic health record data, is associated with mortality for specific LOS, readmission, and post-hospitalization institutional discharge.31, 32 Due to the lack of a gold standard for frailty measurement, we used the ACG as our primary measure and the FRS as secondary frailty measure as a sensitivity analysis to examine the consistency of our findings. Because the FRS included some clinical data such as low white blood cell count (not available in the NIS dataset), the FRS was operationalized for administrative data by including ICD-9-CM codes for low white blood cell count.
Table 1.
Frailty measures with associated conditions and related ICD-9-CM codes.
| 10-Item ACG* | 19-Item FRS† | |||
|---|---|---|---|---|
| Variable | Diagnoses | Codes | Diagnosis | Codes |
| Malnutrition | Nutritional marasmus Other severe protein-calorie malnutrition |
261, 262, 263.8, 263.9, V77.2 | Malnutrition | 261, 262, 263.0, 263.1, 263.2, 263.8, 263.9, V77.2 |
| Dementia | Senile dementia with delusional or depressive features Senile dementia with delirium |
290.20, 290.21, 290.3 | Cognition problems/Dementia | 290.0, 290.20, 290.2, 290.21, 290.3, 294.2, 294.20, 294.21, |
| Severe vision impairment | Profound impairment, both eyes Moderate or severe impairment, better eye/lesser eye profound |
369.0, 369.00, 369.01, 369.03, 369.04, 369.06, 369.07, 369.08, 369.1, 369.10, 369.16, 396.18, 396.12, 369.14 | Severe vision impairment | 369.0, 369.00, 369.01, 369.03, 369.04, 369.05, 369.06, 369.07, 369.08, 369.1, 369.10, 369.11, 369.13, 369.16, 369.15, 369.17, 396.18, 396.12, 369.14, 369.20, 369.21, 369.22,369.23, 369.24, 369.25, 369.4, 366.9, 366.8, 366.1, 366.10, 366.11, 366.13, 366.14, 366.15, 366.16, 366.17, 366.18, 366.19, 366.12, 366.18, 366.4, 366.46, 366.45, 366.41, 366.43, 366.42, 366.2,366.20, 366.21, 366.22, 366.23, 366.3, 366.30, 366.31, 366.32, 366.33, 366.34, 366.53, 365.10, 365.11, 365.12, 365.13, 365.15, 365.20, 365.21, 365.20, 365.23, 365.24, 365.31, 365.32, 365.51, 365.52, 365.9, 365.1, 365.13, 365.11, 365.10, 365.8, 365.89, 365.81, 365.82, 365.9, 365.7, 365.70, 365.71, 365.72, 365.73, 365.74, 365.73, 365.60, 365.61, 365.62, 365.63, 365.64, 365.65, 362.5, 362.50, 362.53, 362.51, 362.52, 362.54, 362.56, 362.7, 362.76, 362.75, 362.77, 362.70, 362.73, 362.1, 362.11, 362.12, 362.10, 362.0, 362.02, 362.03, 362.04, 362.05, 362.06, 362.01, 362.4, 362.41, 363.31 |
| Decubitus ulcer | Decubitus ulcer | 707.0, 707.00, 707.01, 707.02, 707.03, 707.04, 707.05, 707.06, 707.07, 707.09, 707.20, 707.21, 707.22, 707.23, 707.24, 707.25 | Decubitus ulcer | 707.0, 707.00, 707.01, 707.02, 707.03, 707.04, 707.05, 707.06, 707.07, 707.09, 707.20, 707.21, 707.22, 707.23, 707.24, 707.25 |
| Incontinence of urine | Incontinence without sensory awareness Continuous leakage |
788.34, 788.37 | Incontinence of urine | 788.34, 788.37, 788.39, 788.30, 788.38, 788.31, 788.32, 788.33, 788.91, 625.6 |
| Loss of weight | Abnormal loss of weight and underweight Feeding difficulties and mismanagement |
783.2, 783.21, 783.22, 783.3, CM_WGHTLOSS | Loss of weight | 783.2, 783.21, 783.22, 783.3, CM_WGHTLOSS |
| Fecal incontinence | Incontinence of feces | 787.6, 787.60 | Fecal incontinence | 787.6, 787.60 |
| Social support needs | Lack of housing Inadequate housing Inadequate material resources |
V60.0, V60.1, V60.2 | Social support needs | V60.0, V60.1, V60.2, V60.3, V60.4, V62.0, V62.3, V62.4 |
| Difficulty in walking | Difficulty in walking Abnormality of gait |
719.7, 781.2 | Difficulty in walking | 719.7, 781.2 |
| Fall | Fall on stairs or steps Fall from wheelchair |
E880, E880.0, E880.1, E880.9, E884.3 | Fall | E880, E880.0, E880.1, E880.9, E884.3, E884.2, E884.4, E884.5, E884.6 |
| Weakness | 728.87 | |||
| Fatigue | 780.79, 780.71, 780.7 | |||
| Dyspnea | 786.00, 786.01, 786.02, 786.03, 786.04, 786.05, 786.06, 786.07, 786.09 | |||
| Chronic pain | 338.21, 338.22, 338.28, 338.29 | |||
| Anemia | 280.0, 280.1, 280.8, 280.9, 285.2, 285.21, 285.22, 285.29, 285.9, 281.0, 281.1, 281.2, 281.3, 281.4, 281.8, 281.9 | |||
| Depression | 311, 296.2, 296.20, 296.21, 296.22, 296.23, 296.24, 296.25, 296.3, 296.30, 296.31, 296.32, 296.33, 296.34, 296.35 | |||
| High WBC | 288.6, 288.60 | |||
| Low WBC | 288.5, 288.59, 288.50 | |||
| Delirium | 293.0, 293.1, 780.97 |
The 10-item ACG is the Johns Hopkins Adjusted Clinical Groups (ACG) frailty-defining diagnosis indicators and is a binary frail yes/no variable.
The 19-item FRS is the Frailty Risk Score and can have a score of 0 to 19.
Covariates
Hospital characteristics (bed size), patient demographics (age, sex, race), geographic region (northeast, Midwest, south, west), insurance (Medicare, Medicaid, private, self-pay), smoking status (ICD-9-CM codes V15.82, 305.1), household income, and admission type (elective, non-elective) were recorded. Comorbid diseases were assessed using the 29 individual Elixhauser comorbidities coded at any time during the hospital stay (eTable 2).33 Weight loss, depression, and anemia were included in the FRS frailty measure and were not counted as comorbid conditions in analyses with the FRS. In analyses with the ACG frailty measure, depression and anemia were classified as comorbid disease; weight loss was part of the ACG measure.
Outcomes
LOS represents the number of days from admission to discharge. HCUP cost-to-charge ratio files were used to convert total hospital charges to costs, adjusting for inflation based on the US Bureau of Labor Statistics indices to 2015 USD.34 Discharge disposition was routine (home discharge) or non-routine (discharge to short-term hospital, facility, or home health). Medical and surgical complications were based on having at least one ICD-9-CM diagnosis codes consistent with prior publications using NIS (eTable 3).7
Statistical Analysis
To account for stratification, clustering, and unequal weighting of the NIS survey design, discharge weights, NIS hospital number, and NIS stratum used to sample discharges were used to generate nationally representative estimates. Statistical models for our outcomes used discharge trend weighted generalized linear models (GLMs). Since we combined multiple NIS databases, “year” was added as a stratification variable. Analytic procedures for subpopulation analyses (i.e. domain analyses), as recommended by AHRQ were conducted to yield correct standard errors.35 For LOS and total cost, we used generalized estimating equations (GEE) with a log link. For our binary outcomes, multivariable logistic regression was used, with the Taylor series linearization method to estimate the covariance matrix for the regression parameters, a common method for complex survey designs.36, 37
Categorical variables were reported as a weighted number (weighted percentage), and continuous variables were reported as weighted mean (standard error). Rao-Scott chi-square test and Wald F test for categorical and continuous variables, respectively, were used.38 Nonzero discharge counts with fewer than 10 observations were masked in accordance with the HCUP data-use agreement. The sample size may vary by outcome as observations with missing values for the outcomes and covariates were not included. Discharges with extreme LOS or cost less than 1% or greater than 99% were excluded. For analyses involving surgical complications, only surgical categories with > 1% complication rate were included (total knee revision, total/partial hip revision, anterior cervical spinal fusion were excluded).
For all models, possible explanatory variables included age category (50-64, 65-80, > 80), sex, race, insurance, hospital bed-size, hospital geographic region, median household income, admission type, smoking status, surgical category, and the individual Elixhauser comorbidities. Because smoking status capture may be incomplete in administrative data, analyses were run with and without smoking status with no change in results, and thus, smoking status remained in the models. Models including dysphagia, frailty, and their interaction were created for each frailty definition: (1) binary ACG and (2) non-frail, pre-frail, frail cut-offs using the 19-item FRS index score. While the frailty literature suggests that deficit accumulation frailty measures should have at least 30 items, we used published cut-points for non-frail (≤ 0.08), pre-frail (> 0.08 but < 0.25), frail (≥ 0.25) to assess dysphagia’s impact while controlling for the number of frailty deficits.39–41 The statistical analyses were conducted using GENMOD and SURVEYs procedures in SAS 9.4 (SAS Institute, Cary, NC, USA).
Results:
There were 659,767 raw discharges in adults ≥ 50 years of age with surgical hospitalizations involving our surgical procedures of interest in 2014 and first 3 quarters of 2015, giving a weighted estimate of 3,298,835 discharges. 52.5% were female and 47.5% male with a mean age of 67.2 years (SE: 0.03 years). The demographic characteristics of the study cohort are displayed in Table 2.
Table 2.
Characteristics of the study cohort. Weighted N = 3,298,835.
| ACG Frailty* and Dysphagia Group | Dysphagia Only | Frailty Only | Frailty + Dysphagia | Neither | Total | P value |
|---|---|---|---|---|---|---|
| Weighted N | N=31,440 | N=130,920 | N=7,465 | N=3,129,010 | N=3,298,835 | |
| Elective versus non-elective admission | <0.0011 | |||||
| Missing | 135 | 405 | 30 | 8,815 | 9,385 | |
| Elective admission | 20,120 (64.3%) | 61,455 (47.1%) | 2,790 (37.5%) | 2,449,725 (78.5%) | 2,534,090 (77.0%) | |
| Non-elective admission | 11,185 (35.7%) | 69,060 (52.9%) | 4,645 (62.5%) | 670,470 (21.5%) | 755,360 (23.0%) | |
| Age | <0.0011 | |||||
| 50 – 64 | 10,055 (32.0%) | 42,805 (32.7%) | 1,715 (23.0%) | 1,297,075 (41.5%) | 1,351,650 (41.0%) | |
| 65 – 80 | 15,440 (49.1%) | 63,265 (48.3%) | 3,695 (49.5%) | 1,539,160 (49.2%) | 1,621,560 (49.2%) | |
| > 80 | 5,945 (18.9%) | 24,850 (19.0%) | 2,055 (27.5%) | 292,775 (9.4%) | 325,625 (9.9%) | |
| Race | <0.0011 | |||||
| Missing | 2,025 | 7,285 | 475 | 197,580 | 207,365 | |
| White | 23,345 (79.4%) | 97,045 (78.5%) | 5,305 (75.9%) | 2,392,860 (81.6%) | 2,518,555 (81.5%) | |
| Black | 3,005 (10.2%) | 12,735 (10.3%) | 730 (10.4%) | 236,010 (8.1%) | 252,480 (8.2%) | |
| Other | 3,065 (10.4%) | 13,855 (11.2%) | 955 (13.7%) | 302,560 (10.3%) | 320,435 (10.4%) | |
| Sex | <0.0011 | |||||
| Missing | 10 | 25 | 0 | 310 | 345 | |
| Female | 14,615 (46.5%) | 66,235 (50.6%) | 2,985 (40.0%) | 1,646,970 (52.6%) | 1,730,805 (52.5%) | |
| Male | 16,815 (53.5%) | 64,660 (49.4%) | 4,480 (60.0%) | 1,481,730 (47.4%) | 1,567,685 (47.5%) | |
| Median household income for patient’s ZIP Code | <0.0011 | |||||
| Missing | 630 | 2,420 | 135 | 54,935 | 58,120 | |
| 0-25th percentile | 8,395 (27.2%) | 36,945 (28.8%) | 2,060 (28.1%) | 730,570 (23.8%) | 777,970 (24.0%) | |
| 26th to 50th percentile (median) | 8,170 (26.5%) | 35,425 (27.6%) | 1,995 (27.2%) | 832,255 (27.1%) | 877,845 (27.1%) | |
| 51st to 75th percentile | 7,625 (24.7%) | 30,250 (23.5%) | 1,750 (23.9%) | 795,430 (25.9%) | 835,055 (25.8%) | |
| 76th to 100th percentile | 6,620 (21.5%) | 25,880 (20.1%) | 1,525 (20.8%) | 715,820 (23.3%) | 749,845 (23.1%) | |
| Primary expected payer | <0.0011 | |||||
| Missing | 50 | 155 | 0 | 4,195 | 4,400 | |
| Medicare | 21,510 (68.5%) | 89,050 (68.1%) | 5,720 (76.6%) | 1,791,050 (57.3%) | 1,907,330 (57.9%) | |
| Medicaid | 1,620 (5.2%) | 9,155 (7.0%) | 450 (6.0%) | 155,140 (5.0%) | 166,365 (5.0%) | |
| Private insurance | 6,815 (21.7%) | 27,520 (21.0%) | 1,030 (13.8%) | 1,051,420 (33.6%) | 1,086,785 (33.0%) | |
| Self-pay/No charge/Other | 1,445 (4.6%) | 5,040 (3.9%) | 265 (3.5%) | 127,205 (4.1%) | 133,955 (4.1%) | |
| Bed size of hospital | <0.0011 | |||||
| Large | 17,690 (56.3%) | 72,625 (55.5%) | 4,500 (60.3%) | 1,545,565 (49.4%) | 1,640,380 (49.7%) | |
| Medium | 8,765 (27.9%) | 37,505 (28.6%) | 1,950 (26.1%) | 903,185 (28.9%) | 951,405 (28.8%) | |
| Small | 4,985 (15.9%) | 20,790 (15.9%) | 1,015 (13.6%) | 680,260 (21.7%) | 707,050 (21.4%) | |
| Geographic region | <0.0011 | |||||
| Northeast | 4,390 (14.0%) | 22,400 (17.1%) | 930 (12.5%) | 581,195 (18.6%) | 608,915 (18.5%) | |
| Midwest | 8,080 (25.7%) | 34,105 (26.1%) | 2,285 (30.6%) | 791,850 (25.3%) | 836,320 (25.4%) | |
| South | 12,970 (41.3%) | 54,250 (41.4%) | 2,965 (39.7%) | 1,205,175 (38.5%) | 1,275,360 (38.7%) | |
| West | 6,000 (19.1%) | 20,165 (15.4%) | 1,285 (17.2%) | 550,790 (17.6%) | 578,240 (17.5%) |
The 10-item ACG is the Johns Hopkins Adjusted Clinical Groups (ACG) frailty-defining diagnosis indicators and is a binary frail yes/no variable.
Overall, 38,905 (1.2%) of surgical inpatients ≥ 50 years of age had perioperative dysphagia. Based on the binary ACG, the prevalence of frailty was 138,385 (4.2%). Dysphagia presented in 31,440 (1.0%) and 7,465 (5.4%) of ACG non-frail and ACG frail surgical hospitalizations, respectively (p<0.001). Using the FRS, the prevalence of non-frail, pre-frail, and frail inpatient surgical stays was 2,981,035 (90.4%), 313,645 (9.5%), and 4,155 (0.1%), respectively. Dysphagia presented in 27,720 (0.9%), 10,700 (3.4%), and 485 (11.7%) of non-frail, pre-frail and frail surgical hospitalizations, respectively (p<0.001), based on the indexed FRS. The top 5 positive frailty categories were: weight loss (93,790; 2.8%), malnutrition (60,540; 1.8%), difficulty walking (29,050; 0.9%), decubitus ulcer (14,215; 0.4%), and severe vision impairment (2,580; 0.1%) for the ACG; anemia (415,300; 12.6%), depression (389,045; 11.8%), chronic pain (134,370; 4.1%), high white blood cell count 129,725; 3.9%), and severe vision impairment (119,415; 3.6%) for the FRS.
Dysphagia prevalence increased across age groups: 11,770 (0.9%) in 50 – 64 years of age, 19,135 (1.2%) in 65 – 80 years of age, and 8,000 (2.5%) in surgical patients > 80 years of age. As assessed by the ACG frailty definition, frailty alone and dysphagia + frailty prevalence increased across age groups: 42,805 (3.3%) and 1,715 (0.1%) in 50 – 64 years of age, 63,265 (3.9%) and 3,695 (0.2%) in 65-80 years of age, and 24,850 (7.6%) and 2,055 (0.6%) in > 80 years of age, respectively. Table 3 demonstrates a greater dysphagia prevalence among ACG frail versus non-frail patients for all surgical procedures with similar results among FRS frailty categories for all surgical procedures.
Table 3:
Prevalence of Dysphagia by ACG Frailty* and Surgical Procedure.
| ACG Frailty and Dysphagia Group | No Frailty | Frailty | Total |
|---|---|---|---|
| Weighted N | N=3,160,450 | N=138,385 | N=3,298,835 |
| Peripheral arterial bypass | |||
| N | 77,785 | 6,420 | 84,205 |
| Dysphagia | 660 (0.8%) | 240 (3.7%) | 900 (1.1%) |
| No Dysphagia | 77,125 (99.2%) | 6,180 (96.3%) | 83,305 (98.9%) |
| Cardiac surgery | |||
| N | 380,260 | 21,445 | 401,705 |
| Dysphagia | 7,035 (1.9%) | 2,040 (9.5%) | 9,075 (2.3%) |
| No Dysphagia | 373,225 (98.1%) | 19,405 (90.5%) | 392,630 (97.7%) |
| CEA | |||
| N | 132,175 | 2,945 | 135,120 |
| Dysphagia | 3,280 (2.5%) | 455 (15.4%) | 3,735 (2.8%) |
| No Dysphagia | 128,895 (97.5%) | 2,490 (84.6%) | 131,385 (97.2%) |
| AAA | |||
| N | 7,320 | 1,205 | 8,525 |
| Dysphagia | 165 (2.3%) | 115 (9.5%) | 280 (3.3%) |
| No Dysphagia | 7,155 (97.7%) | 1,090 (90.5%) | 8,245 (96.7%) |
| Total/partial/revision hip | |||
| N | 559,525 | 12,350 | 571,875 |
| Dysphagia | 1,745 (0.3%) | 345 (2.8%) | 2,090 (0.4%) |
| No Dysphagia | 557,780 (99.7%) | 12,005 (97.2%) | 569,785 (99.6%) |
| Total/revision knee | |||
| N | 1,104,925 | 15,850 | 1,120,775 |
| Dysphagia | 2,675 (0.2%) | 175 (1.1%) | 2,850 (0.3%) |
| No Dysphagia | 1,102,250 (99.8%) | 15,675 (98.9%) | 1,117,925 (99.7%) |
| Cervical fusion | |||
| N | 152,530 | 4,835 | 157,365 |
| Dysphagia | 9,055 (5.9%) | 970 (20.1%) | 10,025 (6.4%) |
| No Dysphagia | 143,475 (94.1%) | 3,865 (79.9%) | 147,340 (93.6%) |
| Large bowel surgery | |||
| N | 157,265 | 36,250 | 193,515 |
| Dysphagia | 1,700 (1.1%) | 1,480 (4.1%) | 3,180 (1.6%) |
| No Dysphagia | 155,565 (98.9%) | 34,770 (95.9%) | 190,335 (98.4%) |
| Liver resection | |||
| N | 11,490 | 950 | 12,440 |
| Dysphagia | 50 (0.4%) | 20 (2.1%) | 70 (0.6%) |
| No Dysphagia | 11,440 (99.6%) | 930 (97.9%) | 12,370 (99.4%) |
| Pancreas resection | |||
| N | 18,465 | 4,790 | 23,255 |
| Dysphagia | 115 (0.6%) | 80 (1.7%) | 195 (0.8%) |
| No Dysphagia | 18,350 (99.4%) | 4,710 (98.3%) | 23,060 (99.2%) |
| Nephrectomy | |||
| N | 79,725 | 2,760 | 82,485 |
| Dysphagia | 380 (0.5%) | 115 (4.2%) | 495 (0.6%) |
| No Dysphagia | 79,345 (99.5%) | 2,645 (95.8%) | 81,990 (99.4%) |
| Cystectomy | |||
| N | 16,350 | 1,855 | 18,205 |
| Dysphagia | 160 (1.0%) | 40 (2.2%) | 200 (1.1%) |
| No Dysphagia | 16,190 (99.0%) | 1,815 (97.8%) | 18,005 (98.9%) |
| General surgery | |||
| N | 406,785 | 23,470 | 430,255 |
| Dysphagia | 3,690 (0.9%) | 1,155 (4.9%) | 4,845 (1.1%) |
| No Dysphagia | 403,095 (99.1%) | 22,315 (95.1%) | 425,410 (98.9%) |
| Pneumonectomy | |||
| N | 2,400 | 305 | 2,705 |
| Dysphagia | 40 (1.7%) | 35 (11.5%) | 75 (2.8%) |
| No Dysphagia | 2,360 (98.3%) | 270 (88.5%) | 2,630 (97.2%) |
| Lobectomy/segmental resection | |||
| N | 53,450 | 2,955 | 56,405 |
| Dysphagia | 690 (1.3%) | 200 (6.8%) | 890 (1.6%) |
| No Dysphagia | 52,760 (98.7%) | 2,755 (93.2%) | 55,515 (98.4%) |
The 10-item ACG is the Johns Hopkins Adjusted Clinical Groups (ACG) frailty-defining diagnosis indicators and is a binary frail yes/no variable. Note similar findings of increasing dysphagia prevalence among frail vs prefrail vs non-frail patients based on the Frailty Risk Score were noted across all surgical categories.
After removing outliers, total hospital charges adjusted to 2015 inflation were a mean $19,907 (SE $93.9); median LOS was 2.6 days (interquartile range 1.6 to 4.7 days) and mean LOS was 4.3 days (SD 0.02 days). Excluding missing discharge dispositions, 1,515,255 (46.0%) discharges were routine, and 948,825 (28.8%) of the cohort had at least one medical complication: acute cardiac event (739,945; 22.4%), acute renal failure (229,825; 7.0%), urinary tract infection (126,680; 3.8%), acute pulmonary edema/failure (99,340; 3.0%), and pneumonia (48,385; 1.5%). 96,215 (6.6%) had at least one surgical complication: hemorrhage, hematoma, or seroma (40,145; 2.8%), postoperative infection (19,300; 1.33%), and shock (16,210; 1.12%).
Figure 1 and 2 summarize the univariate analysis of frailty, dysphagia, and their interaction on adverse health outcomes using the ACG and FRS frailty definitions, respectively. The exposures of dysphagia and frailty consistently corresponded to increased prevalence of adverse outcomes.
Figure 1.
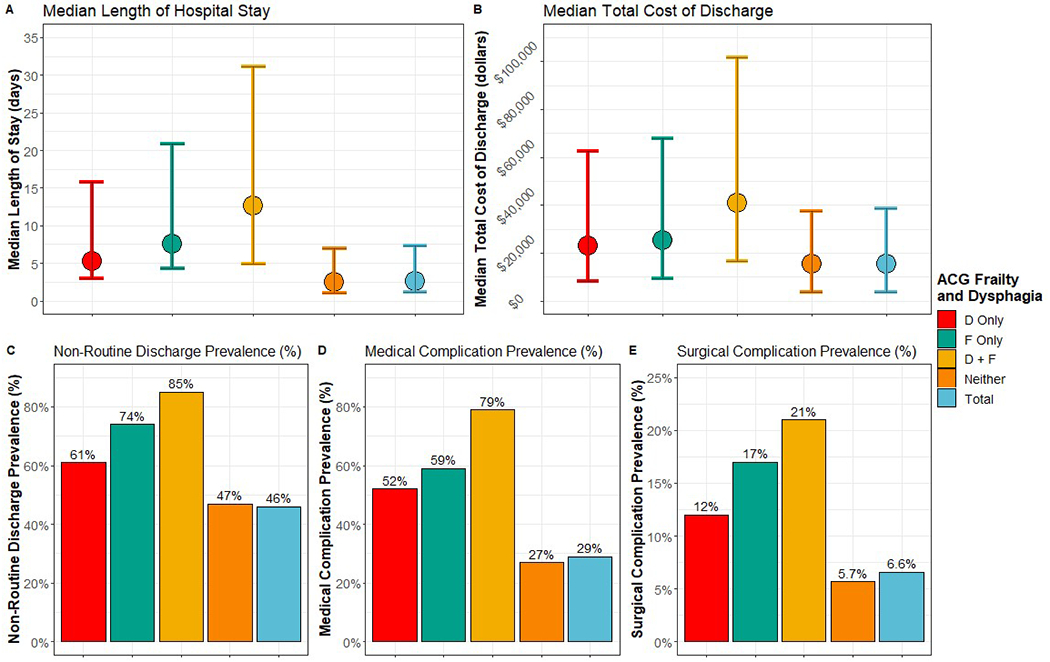
Distribution of outcomes by dysphagia and binary 10-item Johns Hopkins Adjusted Clinical Groups (ACG) frailty-defining diagnosis indicators frailty measure. D = dysphagia. F = frailty. For length of stay and total cost of discharge, the point represents the median and the bars represent the Q1 and Q3, respectively.
Figure 2.
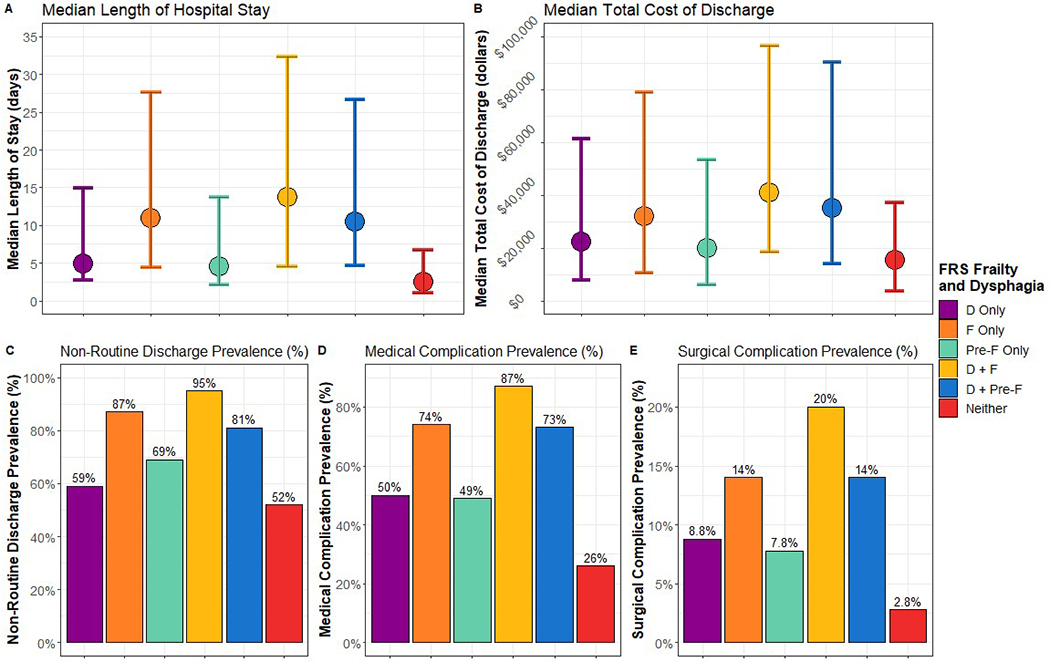
Distribution of outcomes by dysphagia and 19-item Frailty Risk Score indexed and categorized by non-frail, pre-frail, frail. The non-frail, pre-frail, frail cut-points were ≤ 0.08, > 0.08 but < 0.25, and ≥ 0.25, respectively. D = dysphagia. F = frailty. Pre-F = pre-frail. For length of stay and total cost of discharge, the point represents the median and the bars represent the Q1 and Q3, respectively.
Multiple multi-variable models were constructed to examine the relationship between dysphagia and adverse outcomes in patients with varied frailty status (Table 4). Dysphagia was associated with adverse outcomes (higher LOS, greater hospital costs, more non-routine discharges, medical complications, and surgical complications) across frailty level with odds ratios (ORs) above 1.2 except hospital costs and surgical complications in FRS frail index category (Table 4). Results were similar, regardless of how frailty was measured. For LOS, hospital costs, and surgical complications, the ORs tended to be higher in the non-frail population, compared to frail or pre-frail patients. Across all models (Table 4), significant interactions between dysphagia and frailty were also detected (p<0.001).
Table 4.
Multivariable analyses of adverse health outcomes by frailty (F), dysphagia (D), and their interaction.
| Model | Length of stay‡ odds ratio (95% CI) | Hospital cost odds ratio (95% CI) |
|---|---|---|
| Model 1: ACG binary * | ||
| D vs. No D for F | 1.51 (1.45, 1.58) | 1.27 (1.23, 1.31) |
| D vs. No D for non-F | 1.67 (1.63, 1.70) | 1.30 (1.28, 1.32) |
| Model 2: FRS index categories † | ||
| D vs. No D for F | 1.30 (1.11, 1.53) | 1.11 (0.98, 1.24) |
| D vs. No D for pre-F | 1.66 (1.60, 1.72) | 1.37 (1.33, 1.41) |
| D vs. No D for non-F | 1.66 (1.62, 1.70) | 1.29 (1.27, 1.31) |
| Model | Odds ratio of non-routine discharges (95% CI) | Odds ratio of medical complication (95% CI) | Odds ratio of surgical complication (95% CI) |
|---|---|---|---|
| Model 1: ACG binary * | |||
| D vs. No D for F | 2.36 (1.95, 2.85) | 1.85 (1.58, 2.17) | 1.24 (1.06, 1.45) |
| D vs. No D for non-F | 2.69 (2.49, 2.90) | 1.98 (1.85, 2.12) | 1.76 (1.57, 1.96) |
| Model 2: FRS index categories † | |||
| D vs. No D for F | 3.08 (1.20, 7.89) | 2.48 (1.20, 5.13) | 1.33 (0.67, 2.66) |
| D vs. No D for pre-F | 3.06 (2.64, 3.55) | 1.99 (1.76, 2.26) | 1.33 (1.15, 1.55) |
| D vs. No D for non-F | 2.61 (2.41, 2.82) | 1.99 (1.86, 2.13) | 1.86 (1.66, 2.09) |
The ACG binary is the Johns Hopkins Adjusted Clinical Groups (ACG) 10 frailty-defining diagnosis indicators and is a binary frail yes/no variable.
A FRS index was created from the FRS measure by number of positive categories divided by 19. The non-frail, pre-frail, frail cut-points were ≤ 0.08, > 0.08 but < 0.25, and ≥ 0.25, respectively.
N for LOS models = 2,979,475, after excluding outliers and observations with missing covariates. N for total hospital encounter cost models = 2,910,880, after excluding outliers and observations with missing covariates. N for discharge disposition models = 3,023,335, after excluding missing values for discharge disposition. N for medical complications models = 3,024,415, after excluding observations with missing covariates. Due to low prevalence, AIDS, Chronic blood loss anemia, Congestive heart failure, Drug abuse, Lymphoma, Paralysis, Pulmonary circulation disorders, Solid tumors without metastasis, Peptic ulcer disease excluding bleeding, and Psychoses were not considered as covariates in the models of medical complications. N for the surgical complications models = 1,332,180, after excluding observations with missing covariates.
In the surgical complications subset, due to low prevalence, AIDS, Lymphoma, Peptic ulcer disease excluding bleeding, and Psychoses were not considered as covariates in these models.
Otherwise, all models adjusted for: age, sex, race, primary payer, hospital bed-size, hospital geographic region, median household income, admission type, surgical procedure, and the Elixhauser Comorbidities.
Discussion:
The aim of this investigation was to measure the relationship between dysphagia, and inpatient outcomes across frailty conditions in a subset of patients ≥ 50 years of age having surgically-related hospitalizations without surgery directly involving the swallowing mechanism. While methods for diagnosing dysphagia may vary (self-report, instrumental swallow evaluations, nursing assessments), these data could not be ascertained in our study. However, our estimated dysphagia prevalence of 1.2% is comparable to estimates of 0.35% to 5.7% documented in administrative datasets.14, 16, 42 While dysphagia diagnoses are typically under-coded, making our dysphagia prevalence of 1.2% almost certainly an underestimate, a diagnosis of dysphagia has high specificity (i.e. low false positive rate) for physiologic swallowing impairment.25 Similarly, our ACG frailty prevalence of 4.2% may be an underestimate but is comparable to rates of 3.1% to 11.5% measured in administrative surgical cohort studies.7, 8, 43 Our data show increasing frequency of dysphagia, frailty, and combined dysphagia and frailty across age groups with important clinical implications. (Table 2).
Perioperative dysphagia was associated with adverse inpatient outcomes across frailty level. This association between dysphagia and poor inpatient outcomes across frailty level is consistent with a previous investigation among all medical and surgical admissions irrespective of surgical procedure. 23 As previously discussed, the current analysis extends this line of inquiry by focusing on the subset of patients without surgical procedures directly involving the swallowing mechanism where dysphagia may be underappreciated. In both frailty model analyses, dysphagia was significantly associated with higher LOS, more non-routine discharges, medical complications, and was significantly associated with greater hospital costs and more surgical complications, except among FRS frail patients (Table 4). These analyses adjusted for covariates including surgical category, as anterior cervical fusion and carotid patients might be expected to have more dysphagia due to the proximity of the surgical approach to the innervation of the larynx/pharynx. Furthermore, the significant interaction between frailty and dysphagia indicates excess likelihood for poor outcomes when patients had both conditions.
Perioperative dysphagia prevalence did vary by surgical category and was more prevalent in patients with coexisting frailty, indicating the dysphagia may be an important factor even in patients where dysphagia may not be anticipated, such as lower bowel surgery (Table 3). Mechanisms for the link between our adverse inpatient outcomes and dysphagia may be mediated by dysphagia-related complications, such as aspiration pneumonia, dehydration, and malnutrition, and potential interventions like gastrostomy tubes.13, 15, 42 Dysphagia may also inhibit the full potential of perioperative nutrition interventions, by limiting the use of oral nutrition supplements.44 Furthermore, dysphagia may result in a vicious cycle with frailty, sarcopenia, and malnutrition leading to worse outcomes.17, 18 Pre-frail patients who may be showing signs of functional decline, such as patients with fall risk, may be particular targets for dysphagia screening and treatment in an effort to reduce the synergy between dysphagia and frailty exacerbation. Further assessment of current, real-world perioperative dysphagia-related practice patterns and related patient outcomes are warranted.
Through a multidisciplinary assessment of dysphagia, including specialties such as otolaryngology, gastroenterology, speech-language pathology, registered dietician nutritionists, and respiratory therapy, a variety of interventions may improve swallowing function and related outcomes. Non-invasive treatments emphasize swallowing compensation strategies, exercise-based rehabilitation, and dietary modifications which improve swallowing function and reduce pneumonia risk.45, 46 With 24% of hospitalized older patients with dysphagia not receiving nutritional intervention, increased use of nutritional therapy may represent an opportunity to reduce dysphagia-related complications.47 Oral hygiene protocols may also improve dysphagia management. While frail dysphagic patients have higher oral bacterial colonization, perioperative oral care in lung cancer resection patients was associated with lower rates of postoperative pneumonia.48 Dysphagia may also benefit from surgical intervention or gastroenterology-related procedures, and thus, shared decision making and patient focused care considering patient age, prognosis, and functional status are necessary to determine the most appropriate steps.17 Given that dysphagia is a potentially modifiable risk factor for poor inpatient outcomes in middle aged and older surgical patients, perioperative care teams including nursing may have an important role in helping identify and treat perioperative dysphagic patients similar to stroke patient care protocols.49 How such protocols and their timing impact surgical outcomes among middle aged and older adults without surgery directly involving the swallowing mechanism is worthy of investigation.
Certain methodologic limitations are important to acknowledge. We were unable to differentiate between those with oropharyngeal and/or esophageal involvement which may be important. Due to the nature of the database, we also could not determine if patients were admitted from a nursing home or readmitted, whether dysphagia was present pre or postoperatively, and if medical complication diagnoses were pre-existing prior to hospitalization. Future investigations are needed to explore the temporal relationships between dysphagia and outcomes in surgical patients. Additionally, patients with increased LOS may have more debilitation which could affect swallowing function. Mechanical ventilation, including the length of mechanical ventilation, is associated with dysphagia. However, data about mechanical ventilation nor the length of intensive care unit stay were available. Further investigation is needed to assess the relationship between dysphagia, frailty, and mechanical ventilation in surgical patients.50, 51 Our study does not prove causation and further investigation is needed to examine the directionality of the relationship between perioperative dysphagia and complications and LOS. The selected frailty measures were used because one alternative includes comorbid disease and demographic variables preventing separate adjustment for the concepts of comorbidity and frailty, and another includes variables and claims for durable medical equipment not available in NIS.52, 53 Our dysphagia prevalence in frail and non-frail patients and positive frailty categories differed for the ACG and FRS and was likely related to different diagnostic categories and coding algorithms. Nonetheless, using these two frailty measures produced consistent findings of an independent relationship between perioperative dysphagia and adverse inpatient outcomes in surgical patients ≥ 50 years of age across frailty levels.
As previously discussed, dysphagia and frailty-related diagnoses are likely under-coded but would be a conservative bias. A selection bias may exist in which patients with more severe swallowing problems were coded for dysphagia. While such patients may be more likely to have adverse outcomes than patients with less swallowing impairment, our findings justify further study examining the relationship between dysphagia severity and adverse outcomes to identify at-risk middle aged and older surgical patients. Lastly, dysphagia undercoding might actually suggest under-diagnosis and under-recognition as prior studies have suggested that dysphagia is often not detected, evaluated, or managed despite being a treatable condition with serious and preventable potential complications.54–56
Conclusion:
Among surgical patients ≥ 50 years of age, dysphagia was more common in frail patients and associated with increased LOS, hospital cost, non-routine discharge, and medical and surgical complications, independent of frailty status, suggesting that dysphagia is not simply a surrogate for poor overall health status. Our findings support efforts aimed at improving dysphagia diagnosis and appropriate treatment of middle aged and older dysphagic surgical inpatients regardless of frailty level. Future studies are needed to corroborate our findings and explore mechanisms between dysphagia and adverse outcomes. Investigations are also needed to examine different methodology for identifying dysphagic patients during their perioperative care as there may be opportunities for prehabilitation or improved rehabilitation.
Supplementary Material
Take Home Points.
Dysphagia is common in frail patients with associated morbidity. Yet, whether dysphagia is simply a marker of disease severity or an independent risk factor for poor postoperative outcomes is not known.
The relationship between dysphagia and adverse outcomes was examined across frailty conditions in middle-aged and older surgical patients and identified that dysphagia was independently associated with adverse post-operative outcomes. An interaction between dysphagia and frailty was identified indicating that patients with both conditions had increased likelihood of adverse outcomes.
Identification and treatment of perioperative dysphagia is an important consideration for providers seeking to reduce risk in vulnerable surgical populations of middle-aged and older adults.
Acknowledgments
We assure this work is original and has not been submitted elsewhere.
Funding:
There is no study sponsor. Research reported in this publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002553. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement/Conflict of interest:
Seth Cohen is a consultant for Zsquare, Data Safety Monitoring Board Member for Syneos Health. Stephanie Misono has NIH K23DC016335 funding from the NIDCD. Kathryn Starr has funding from the U.S. Department of Veterans Affairs Rehabilitation Research and Development Service IK2RX002348. Sudha Raman has research support from Glaxo Smith Kline and the Federal Drug Administration. Thomas Risoli, Hui-Jie Lee, and Harrison Jones have no disclosures.
Footnotes
Informed consent: This study used de-identified data and informed consent from study participants was not possible nor required.
References
- 1.Etzioni DA, Liu JH, Maggard MA, Ko CY. The aging population and its impact on the surgery workforce. Ann Surg. 2003;238(2):170–7. DOI: 10.1097/01.SLA.0000081085.98792.3d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamel MB, Henderson WG, Khuri SF, Daley J. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc. 2005;53(3):424–9. DOI: 10.1111/j.1532-5415.2005.53159.x. [DOI] [PubMed] [Google Scholar]
- 3.Khan NA, Quan H, Bugar JM, Lemaire JB, Brant R, Ghali WA. Association of postoperative complications with hospital costs and length of stay in a tertiary care center. J Gen Intern Med. 2006;21(2):177–80. DOI: 10.1111/j.1525-1497.2006.00319.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones TS, Dunn CL, Wu DS, Cleveland JC Jr, Kile D, Robinson TN. Relationship between asking an older adult about falls and surgical outcomes. JAMA Surg 2013;148(12):1132–8. DOI: 10.1001/jamasurg.2013.2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whittle J, Wischmeyer PE, Grocott MPW, Miller TE. Surgical Prehabilitation: Nutrition and Exercise. Anesthesiol Clin. 2018;36(4):567–80. DOI: 10.1016/j.anclin.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 6.Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: a systematic literature review. J Am Geriatr Soc 2011:59(11):2129–38. DOI: 10.1111/j.1532-5415.2011.03597.x [DOI] [PubMed] [Google Scholar]
- 7.Nieman CL, Pitman KT, Tufaro AP, Eisele DW, Frick KD, Gourin CG. The effect of frailty on short-term outcomes after head and neck cancer surgery. Laryngoscope 2018;128(1):102–110. DOI: 10.1002/lary.26735 [DOI] [PubMed] [Google Scholar]
- 8.McIsaac DI, Bryson GL, Van Walraven C. Association of 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg 2016;151(6):538–45. DOI: 10.1001/jamasurg.2015.5085 [DOI] [PubMed] [Google Scholar]
- 9.Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci 2009;64(6):675–81. DOI: 10.1093/gerona/glp012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective anlaysis of 493.737 UK Biobank participants. Lancet Public Health 2018;e323–e332. DOI: 10.1016/S2468-2667(18)30091-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baijens LW, Clave P, Cras P, Ekberg O, Forster A, Kolb GF, Leners JC, Masiero S, Mateos-Nozal J, Ortega O, Smithard DG, Speyer R, Walshe M. European Society for Swallowing Disorders - European Union Geriatric Medicine Society white paper: oropharyngeal dysphagia as a geriatric syndrome. Clin Interv Aging 2016;11:1403–28. DOI: 10.2147/CIA.S107750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharyya N The prevalence of dysphagia among adults in the United States. Otolaryngol Head Neck Surg 2014;151(5):765–9. DOI: 10.1177/0194599814549156 [DOI] [PubMed] [Google Scholar]
- 13.van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Meta-analysis of dysphagia and aspiration pneumonia in frail elders. J Dent Res 2011;90(12):1398–404. DOI: 10.1177/0022034511422909 [DOI] [PubMed] [Google Scholar]
- 14.Patel DA, Krishnaswami S, Steger E, Conover E, Vaezi MF, Cuicci MR, Francis DO. Economic and survival burden of dysphagia among inpatients in the United States. Dis Esophagus 2018;31(1):1–7. DOI: 10.1093/dote/dox131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tagliaferri S, Lauretani F, Pela G, Meschi T, Maggio M. The risk of dysphagia is associated with malnutrition and poor functional outcomes in a large population of outpatient older individuals. Clin Nutr 2019;38(6):2684–9. DOI: 10.1016/j.clnu.2018.11.022 [DOI] [PubMed] [Google Scholar]
- 16.Altman KW, Yu GP, Schaefer SD. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg 2010;136(8):784–9. DOI: 10.1001/archoto.2010.129 [DOI] [PubMed] [Google Scholar]
- 17.Payne M, Morley JE. Editorial: Dysphagia, Dementia and Frailty. J Nutr Health Aging 2018;22(5):562–5. DOI: 10.1007/s12603-018-1033-5 [DOI] [PubMed] [Google Scholar]
- 18.Fujishima I, Fujiu-Kurachi M, Arai H, Hyodo M, Kagaya H, Maeda K, Mori T, Nishioka S, Oshima F, Ogawa S, Ueda K, Umezaki T, Wakabayashi H, Yamawaki M, Yoshimura Y. Sarcopenia and dysphagia: Position paper by four professional organizations. Geriatr Gerontol Int. 2019;19(2):91–7. DOI: 10.1111/ggi.13591 [DOI] [PubMed] [Google Scholar]
- 19.Starmer HM, Quon H, Simpson M, Webster K, Tippett D, Herbert RJ, Eisele DW, Gourin CG. Speech-language pathology care and short- and long-term outcomes of laryngeal cancer treatment in the elderly. Laryngoscope 2015;125(12):2756–63. DOI: 10.1002/lary.25454 [DOI] [PubMed] [Google Scholar]
- 20.Bock JM, Varadarajan V, Brawley MC, Blumin JH. Evaluation of the natural history of patients who aspirate. Laryngoscope 2017;127 Suppl 8:S1–S10. DOI: 10.1002/lary.26854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen S, Zhu A, Toppen W, Ashfaq A, Shemin R Mendelsohn AH, Benharash P. Dysphagia after cardiac operations is associated with increased length of stay and cost. Am Surg 2016;82(10):890–3. [DOI] [PubMed] [Google Scholar]
- 22.Introduction to the HCUP National Inpatient Sample (NIS) 2014. Healthcare Cost and Utilization Project (HCUP). 2016. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2014.jsp. Accessed August 6, 2019. [Google Scholar]
- 23.Cohen SM, Lekan D, Risoli T Jr, Lee HJ, Misono S, Whitson HE, Raman S. Association between dysphagia and inpatient outcomes across frailty level among patients ≥ 50 years of age. Dysphagia 2019; Dec 7 [Epub ahead of print]. DOI: 10.1007/s00455-019-10084-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald SR, Heflin MT, Whitson HE, Dalton TO, Lidsky ME, Liu P, Poer CM, Sloane R, Thacker JK, White HK, Yanamadala M, Lagoo-Deenadayalan SA. Association of Integrated Care Coordination With Postsurgical Outcomes in High-Risk Older Adults: The Perioperative Optimization of Senior Health (POSH) Initiative. JAMA Surg. 2018;153(5):454–62. DOI: 10.1001/jamasurg.2017.5513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González-Fernández M, Gardyn M, Wyckoff S, Ky PK, Palmer JB. Validation of ICD-9 Code 787.2 for identification of individuals with dysphagia from administrative databases. Dysphagia 2009;24(4):398–402. DOI: 10.1007/s00455-009-9216-1 [DOI] [PubMed] [Google Scholar]
- 26.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004. Mar;59(3):255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 27.Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013. Sep;61(9):1537–51. doi: 10.1111/jgs.12420. [DOI] [PubMed] [Google Scholar]
- 28.Gobbens RJ, van Assen MA, Luijkx KG, Schols JM. Testing an integral conceptual model of frailty. J Adv Nurs. 2012. Sep;68(9):2047–60. doi: 10.1111/j.1365-2648.2011.05896.x. [DOI] [PubMed] [Google Scholar]
- 29.The Johns Hopkins ACG System: Version 11.0 Technical Reference Guide. Chapter 2. Baltimore, MD: The Johns Hopkins University; 2015. [Google Scholar]
- 30.Sternberg SA, Bentur N, Abrams C, et al. Identifying frail old people using predictive modeling. Am J Manag Care 2012;18(10):e392–7. [PubMed] [Google Scholar]
- 31.Lekan DA, Wallace DC, McCoy TP, Hu J, Silva SG, Whitson HE. Frailty assessment in hospitalized older adults using electronic health record. Biol Res Nurs 2017;19(2):213–228. DOI: 10.1177/1099800416679730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lekan DA, McCoty TP, Jenkins M, Mohanty, S, Manda P. Comparison of a frailty risk score and comorbidity for early rehospitalization using electronic health record data. Innov Aging 2019. Nov;3(Suppl 1):S906. DOI: 10.1093/geroni/igz038.3307 [DOI] [PubMed] [Google Scholar]
- 33.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36(1):8–27. DOI: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 34.http://www.usinflationcalculator.com/inflation/consumer-price-index-and-annual-percent-changes-from-1913-to-2008/, accessed October, 2019.
- 35.Houchens R, Ross D, Elixhauser A. Final Report on Calculating National Inpatient Sample (NIS) Variances for Data Years 2012 and Later. 2015. HCUP Methods Series Report # 2015–09 ONLINE. December 14, 2015. U.S. Agency for Healthcare Research and Quality. Available: http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp. Accessed August 6, 2019. [Google Scholar]
- 36.Hardin JW, Hilbe JM. Generalized linear models and extensions, 2nd ed. College Station, TX: Stata Press; 2007, p. 89. [Google Scholar]
- 37.https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_surveylogistic_a0000000386.htm. Accessed August 6, 2019.
- 38.Rao JNK, Scott AJ. On Simple Adjustments to Chi-Square Tests with Survey Data. Annals Statistics 1987;15:385–397. [Google Scholar]
- 39.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 2007;62(7):738–743. DOI: 10.1093/gerona/62.7.738 [DOI] [PubMed] [Google Scholar]
- 40.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geritr Soc 2010;58(4):681–7. DOI: 10.1111/j.1532-5415.2010.02764.x [DOI] [PubMed] [Google Scholar]
- 41.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;Sep 30;8:24. DOI: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilmskoetter J, Simpson AN, Logan SL, Simpson KN, Bonilha HS. Impact of gastrostomy feeding tube placement on the 1-year trajectory of care in patients after stroke. Nutr Clin Pract 2018;33(4):553–66. DOI: 10.1002/ncp.10015 [DOI] [PubMed] [Google Scholar]
- 43.Segal JB, Huang J, Roth DL, Varadhan R. External validation of the claims-based frailty index in the national health and aging trends study cohort. Am J Epidemiol 2017;186(6):745–7. DOI: 10.1093/aje/kwx257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wischmeyer PE, Carli F, Evans DC, Guilbert S, Kozar R, Pryor A, Thiele RH, Everett S, Grocott M, Gan TJ, Shaw AD, Thacker JKM, Miller TE, Peroperative Quality Initiative 2 Workgroup. American society for enhanced recover and perioperative quality initiative joint consensus statement on nutrition screening and therapy within a surgical enhanced recovery pathway. Anesth Analg 2018;26(6):1883–95. DOI: 10.1213/ANE.0000000000002743 [DOI] [PubMed] [Google Scholar]
- 45.Rogus-Pulia N, Rusche N, Hind JA, Zielinski J, Gangnon R, Safdar N, Robbins J. Effects of device-facilitated isometric progressive resistance oropharyngeal therapy on swallowing and health-related outcomes in older adults with dysphagia. J Am Geriatr Soc 2016;64:417–424. DOI: 10.1111/jgs.13933 [DOI] [PubMed] [Google Scholar]
- 46.Hinchey JA, Shephard T, Furie K, et al. Stroke Practice Improvement Network I. Formal dysphagia screening protocols prevent pneumonia. Stroke 2005;36(9):1972–6. DOI: 10.1161/01.STR.0000177529.86868.8d. [DOI] [PubMed] [Google Scholar]
- 47.Speyer R, Baijens L, Heijnen M, Zwijnenberg I. Effects of therapy in oropharyngeal dysphagia by speech and language therapist: a systematic review. Dysphagia 2010;25(1):40–65. DOI: 10.1007/s00455-009-9239-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Egleseer D, Halfens RJG, Schols JMGA, Lohrmann C. Dysphagia in hospitalized in older patients : associated factors and nutritional interventions. J Nutr Health Aging 2018;22(1):103–10. DOI: 10.1007/s12603-017-0928-x [DOI] [PubMed] [Google Scholar]
- 49.Iwata E, Hasegawa T, Yamada SI, Kawashita Y, Yoshimatsu M, Mizutani T, Nakahara H, Mori K, Shibuya Y, Kurita H, Komori T. Effects of perioperative oral care on prevention of postoperative pneumonia after lung resection: multicenter retrospective study with propensity score matching analysis. Surgery 2019;165(5):1003–7. DOI: 10.1016/j.surg.2018.11.020 [DOI] [PubMed] [Google Scholar]
- 50.Skoretz SA, Flowers HL, Martino R. The incidence of dysphagia following endotracheal intubation: a systematic review. Chest. 2010;137(3):665–73. doi: 10.1378/chest.09-1823. [DOI] [PubMed] [Google Scholar]
- 51.Zuercher P, Schenk NV, Moret C, Berger D, Abegglen R, Schefold JC. Risk Factors for Dysphagia in ICU Patients After Invasive Mechanical Ventilation. Chest. 2020. Nov;158(5):1983–1991. doi: 10.1016/j.chest.2020.05.576. [DOI] [PubMed] [Google Scholar]
- 52.Segal JB, Chang H-Y, Walston JD, Carlson MC, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care 2017;55(7):716–22. DOI: 10.1097/MLR.0000000000000729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci 2018;73(7):980–7. DOI: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaspar K, Ekberg O. Identifying vulnerable patients: role of the EAT-10 and the multidisciplinary team for early intervention and comprehensive dysphagia care. Nestle Nutr Inst Workshop Ser. 2012;72:19–31. DOI: 10.1159/000339977 [DOI] [PubMed] [Google Scholar]
- 55.Takizawa C, Gemmell E, Kenworthy J, Speyer R. A Systematic Review of the Prevalence of Oropharyngeal Dysphagia in Stroke, Parkinson’s Disease, Alzheimer’s Disease, Head Injury, and Pneumonia. Dysphagia. 2016;31(3):434–441. DOI: 10.1007/s00455-016-9695-9. [DOI] [PubMed] [Google Scholar]
- 56.Wirth R, Dziewas R, Beck AM, et al. Oropharyngeal dysphagia in older persons - from pathophysiology to adequate intervention: a review and summary of an international expert meeting. Clinical Interv Aging. 2016;11:189–208. DOI: 10.2147/CIA.S97481 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


