Abstract
Aims
The article investigates whether chronic hyperglycaemia in Type 1 diabetes (T1D) is associated with a proinflammatory immune signature and with arterial wall inflammation, driving the development of atherosclerosis.
Methods and results
Patients with T1D (n = 41), and healthy age-, sex-, and body mass index–matched controls (n = 20) were recruited. Arterial wall inflammation and haematopoietic activity were measured with 2′-deoxy-2′-(18F)-fluoro-D-glucose (18F-FDG) positron emission tomography/computed tomography. In addition, flow cytometry of circulating leucocytes was performed as well as targeted proteomics to measure circulating inflammatory markers. 18F-FDG uptake in the wall of the abdominal aorta, carotid arteries, and iliac arteries was higher in T1D compared with that in the healthy controls. Also, 18F-FDG uptake in the bone marrow and spleen was higher in patients with T1D. CCR2 and CD36 expressions on circulating monocytes were higher in patients with T1D, as well as several circulating inflammatory proteins. In addition, several circulating inflammatory markers (osteoprotegerin, transforming growth factor-alpha, CX3CL1, and colony-stimulating factor-1) displayed a positive correlation with FDG uptake. Within T1D, no differences were found between people with a high and low HbA1c.
Conclusion
These findings strengthen the concept that chronic hyperglycaemia in T1D induces inflammatory changes that fuel arterial wall inflammation leading to atherosclerosis. The degree of hyperglycaemia appears to play a minor role in driving this inflammatory response in patients with T1D.
Keywords: 18F-FDG-PET/CT, Diabetes, Vascular inflammation, Atherosclerosis, Type 1 diabetes
Time of primary review: 51 days See the editorial comment for this article ‘Positron emission tomography imaging of inflammation in diabetes mellitus’, by N.J. Craig and M.R. Dweck, https://doi.org/10.1093/cvr/cvad109.
1. Introduction
Diabetes mellitus profoundly increases the risk for atherosclerotic cardiovascular diseases (CVDs). A large meta-analysis of over 100 prospective studies showed that diabetes confers a two-fold excess risk for the development of CVD, independent of other risk factors.1 This holds true for both Type 1 (T1D) and Type 2 diabetes (T2D). Recent studies have confirmed the increased prevalence of CVD in T1D, which was particularly high in patients with young-onset disease, accounting for ∼15 years loss of life.2 It is likely that this increased risk is related to the presence of chronic hyperglycaemia. Prospective studies have indeed confirmed that an increased risk of coronary artery disease starts at glucose levels below the cut-off point for diabetes (<7 mmol/L) and further increases with higher glucose levels.3
Atherosclerosis is a chronic, low-grade inflammatory disorder of the arterial wall, in which monocyte-derived macrophages are the most abundant immune cells.4 2′-Deoxy-2′-(18F)-fluoro-D-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) can detect vascular wall inflammation in the setting of atherosclerosis:5,6 arterial wall 18F-FDG uptake is associated with macrophage content and levels of inflammatory gene expression in atherosclerotic plaques.7,8 Moreover, 18F-FDG-PET/CT uptake in atherosclerotic plaques strongly predicts future cardiovascular events in patients with atherosclerosis.9 Previous research has revealed increased 18F-FDG uptake in the arterial walls of patients with T2D and patients with impaired glucose tolerance.10,11 In patients with T2D, arterial wall 18F-FDG uptake also correlates with arterial stiffness.12 Interestingly, 18F-FDG-PET/CT also allows assessment of haematopoietic activity in the bone marrow. Bone marrow 18F-FDG uptake is higher in patients with atherosclerosis, and predicts future cardiovascular events, suggesting that activation of the immune system in the bone marrow is a critical mechanism in atherosclerosis.13,14 Similarly, in subjects without diabetes, bone marrow FDG uptake is associated with metabolic syndrome and with high arterial metabolic activity.15 We recently showed reprogramming of bone marrow myeloid progenitor cells in patients with coronary artery disease.16 Finally, in mice, transient glucose peaks can induce prolonged immune cell activation by promoting myelopoiesis in the bone marrow.17
Vascular wall inflammation has not been investigated in patients with T1D, despite accumulating evidence for systemic inflammation and activation of the innate immune system in this condition. Moreover, isolated monocytes from patients with poorly controlled T1D show enhanced epigenetic activation of inflammatory pathways compared with better controlled patients.18 Recently, we demonstrated functional and metabolic changes in monocytes from patients with T1D, which were related to the glycaemic burden.19 Together, these findings suggest that chronic hyperglycaemia in T1D induces changes in the innate immune system and drives systemic inflammation, accelerating vascular wall inflammation.
We hypothesized that in patients with T1D, chronic hyperglycaemia induces activation of circulating innate immune cells and their bone marrow progenitors and increased circulating inflammatory proteins that translate into arterial wall inflammation. To test this hypothesis, we performed 18F-FDG-PET/CT imaging in patients with T1D over a range of glycaemic control and in non-diabetic-matched control subjects and also determined circulating immune cell phenotypes and inflammatory markers.
2. Methods
2.1. Participants and experimental design:
Between January 2018 and January 2019, 61 subjects were enrolled in a case–control study: 41 subjects with T1D and 20 healthy, age-, sex-, and body mass index (BMI)-matched, non-diabetic, control subjects (HC). All study subjects were between 20 and 60 years old, non-smoking, and non-obese (BMI <30 kg/m2). Subjects were rescheduled if they had an infection or vaccination within 3 months before inclusion or had a severe hypoglycaemia (hypoglycaemia requiring third-party assistance to recover) within 1 week before the inclusion day. The subjects with T1D had diabetes for at least 10 years. Subjects were not allowed to have important comorbidities [auto-inflammatory or auto-immune disease, chronic kidney disease (modification of diet in renal disease < 45 mL/min/1.73 m2), or a history of cardiovascular events (ischaemic stroke/TIA (Transient Ischemic Attack)), myocardial infarction, or peripheral arterial disease)]. They were not allowed to use immunosuppressive or immunomodulating medication or acetylsalicylic acid. Statins, if used, had to be discontinued for at least 2 weeks before inclusion. Cessation of statin use was confirmed by higher LDL cholesterol levels that were measured on the day of the PET/CT scan compared with the previous measurement. Also, all participants confirmed that they had stopped statin use 2 weeks prior to the PET scan. Additional exclusion criteria were pregnancy or claustrophobia.
Healthy subjects were recruited via advertisements, and patients with diabetes were recruited by their treating physician. Participants were screened for suitability by a telephonic questionnaire. Additionally, all healthy subjects were screened—including physical examination and blood tests—at the outpatient clinic of the Radboud University Medical Center, Nijmegen, The Netherlands. From people with diabetes, we also collected information about disease history from the medical records.
18F-FDG-PET/CT imaging was performed at the department of Radiology and Nuclear Medicine of the Radboud University Medical Center, Nijmegen, The Netherlands. All subjects arrived at the study day between 7 Am and 11.30 Am and fasted for at least 6 h. Details on this procedure and the analysis are provided below.
Ethical approval for this study was obtained from the Institutional Review Board of the Radboud University Medical Center (NL62200.091.17; 2017-3555) and registered at ClinicalTrials.gov (NCT03441919). Participant inclusion and experiments were conducted according to the principles expressed in the Declaration of Helsinki. All participants gave written informed consent before participation.
Venous blood was collected in 10 mL ethylenediaminetetraacetic acid (EDTA), 3.5 mL serum, 3 mL lithium heparin, and 2 mL sodium fluor EDTA tubes (Vacutainer system; Becton Dickinson (Franklin Lakes, New Jersey, Verenigde Staten)). Total cholesterol, triglycerides, HDL-cholesterol, high-sensitive C-reactive protein (hs-CRP), glucose, HbA1c, and creatinine were measured using commercially available enzymatic methods. LDL-cholesterol levels were calculated using the Friedewald equation. Chronic glycaemic control was determined by collecting the last 10 HbA1c levels from the medical records and calculating the mean.
2.2. 18F-FDG-PET/CT imaging and analyses
The 18F-FDG-PET/CT imaging procedures have been described elsewhere.20
In short (Figure 1), 18F-FDG-PET/CT scans were performed after >6 h of fasting following the European Association of Nuclear Medicine guidelines.21 Fasting glucose was measured at the presentation. Subjects with a fasting glucose ≥8.3 mmol/L received a correction bolus of short-acting intravenous insulin [mean = 2.35, standard deviation (SD) = 2.02] to reach a glucose level <8.3 mmol/L before 18F-FDG administration. Time between insulin and 18F-FDG administration was 60 min. Subjects underwent PET imaging and a low-dose non-contrast CT 2 h after the intravenous administration of 18F-FDG (2 MBq/kg), according to European guidelines.2118F-FDG uptake was determined in seven regions of interest (ROIs) by a single investigator (A.W.M.J.): the carotid arteries, the wall of the ascending, descending, and abdominal aorta, the iliac arteries, the bone marrow (vertebrae L2–L3), and spleen (Figure 1). Mean and maximal standardized uptake values (SUVs) were measured for each ROI. An average of the SUVs of both regions was used for the left and right carotid arteries, vertebrae L2 and L3, and the left and right iliac arteries. SUVs were corrected for blood glucose levels as previously described.21,22 The following formula was used for normalizing the measured glucose content for an overall population average of 90 mg/dL (5.0 mmol/L): SUVgluc × glucose patient in mg/dL (mmol/L)/90 mg/dL (5.0 mmol/L). The arterial target to background ratio (TBR) was calculated by normalizing the arterial SUV for blood-pool activity by dividing the arterial wall SUV values by the average blood-pool mean SUV (SUVmean) estimated from the thoracic aorta. For the non-vascular ROIs, the SUVmean was depicted.
Figure 1.
18F-FDG-PET/CT imaging and analyses: experimental set up and ROI. (A) Timeline of experimental day; at time point 0, blood was drawn and blood glucose was measured. If necessary to reach the target glucose level, a small amount of insulin (mean = 2.35, SD = 2.02) was administered intravenously. One hour after insulin administration, 18F-FDG was administered. A total of 120 min after 18F-FDG administration, a PET/CT was performed. (B) Vascular and haematopoietic ROI. (C) A representative PET/CT image; the arrow shows the ascending aorta, as an example of a vascular region of interest.
2.3. Flow cytometry
Total blood counts, leucocyte, and monocyte counts were measured with a Sysmex XN-450 Hematology Analyzer (Sysmex Corporation, Kobe, Japan). Undiluted blood (50 µL) was incubated for 15 min in the dark at room temperature with the following antibodies: CD3-APC-750 (mouse, dilution: 1:25; Beckman Coulter, Woerden, The Netherlands), CD56-APC (mouse, 1:25; Beckman Coulter), CD16-FITC (mouse, 1:25; eBioscience Thermo Fisher Scientific, Breda, The Netherlands), CD14-PC7 (mouse, 1:25; eBioscience Thermo Fisher Scientific), CCR2-BV421 (mouse, 1:50 and 1:25; BD Biosciences), CD36-APC-700 (mouse, 1:10; Beckman Coulter), CD41-PC5.5 (mouse, 1:50; Biolegend, San Diego, CA, USA), CD11b-BV785 (mouse, 1:50 and 1:25; Biolegend). Followed by the addition of 1 mL 10× diluted lysis buffer (BD Pharm Lyse; BD Biosciences), samples were mixed and incubated for 10 min in the dark at room temperature. Samples were then measured on a Beckman Coulter FC500 flow cytometer, each sample was measured both stained and unstained. Flow cytometry data were analysed using Kaluza software (Beckman Coulter); for gating strategy, see Supplementary material online, Figure S1. Besides determining the different monocyte subsets (classical, intermediate, or non-classical). Atherogenic markers CCR2, CD36, CD41, and CD11b were determined per monocyte subset.
2.4. Plasma analysis
Plasma was stored in −80°C until measurement. All samples were measured in one batch. Plasma E-selectin and vascular cell adhesion molecule (VCAM)-1 were measured by enzyme-linked immunosorbent assays using commercially available kits (R&D Systems; Bio-Techne, Minneapolis, MN, USA), following the manufacturer’s instructions. Circulating plasma inflammatory proteins were measured using the commercially available Olink Proteomics AB Inflammation Panel (92 inflammatory proteins; Uppsala, Sweden). Proteins are recognized by antibody pairs coupled to cDNA strands that bind in close proximity and extend by a polymerase reaction.23 Proteins were excluded for analysis when the detection level of 75% was not met. Quality control was performed by Olink Proteomics with two samples not passing the quality control and subsequently excluded from the analysis. Overall, 73 of the 92 (79%) proteins were detected in at least 75% of the plasma samples and included in the analysis.
2.5. Statistical analyses
Sample size calculation: to detect a difference of at least 0.2 in the mean TBR of the carotid artery (the primary objective of the study) between the groups with 80% power at a two-sided significance level of 0.05, at least 15 subjects would be needed per group. Given the uncertainty of TBR in people with T1D and the differences in glucose levels, the group size was increased to 20.
Differences of TBRmax and TBRmean in all ROIs were compared between the groups with the Mann–Whitney U test. Differences in immune cell populations were tested using an unpaired t-test. If these suggested differences, a Wilcoxon rank-sum test was performed, resulting in unadjusted and FDR-adjusted P-values. Additionally, Spearman’s correlation tests were used to correlate protein levels with TBR values for all ROIs in the complete cohort. Differences at probability values <0.05 were considered statistically significant. Statistical analyses were performed using SPSS 25.0 or RStudio 1.3.959.
3. Results
3.1. Baseline characteristics
Table 1 shows the baseline characteristics of the two groups. Patients with T1D and healthy controls were well matched.
Table 1.
Participant characteristics
| HC | DM | |
|---|---|---|
| Number (n) | 20 | 41 |
| Sex (males) | 50% (10) | 48.7% (20) |
| Age (years) | 43.5 (23) | 48 (26) |
| BMI (kg/m2) | 24.4 (6.1) | 24.7 (3.8) |
| Systolic blood pressure (mmHg) | 121 (18) | 126 (21) |
| Diastolic blood pressure (mmHg) | 78 (11) | 76 (12) |
| Duration diabetes (years) | – | 28 (21) |
| Mean history HbA1c (mmol/mol) | 33 (7)* | 64 (14)* |
| Macrovascular complications | 0 | 0 |
| Microvascular complications | – | 48.7% (20) |
| Retinopathy | – | 46.3% (19) |
| Nephropathy | – | 9.7% (4) |
| Neuropathy | – | 22.0% (9) |
| Current smoking | 0 | 0 |
| Past smoking | 25% (5) | 24.4% (10) |
| Insulin use | 0 | 100% (41) |
| Statin use | 0 | 19.5% (8) |
| Ezetimibe use | 0 | 2.4%(1) |
| ACE inhibitor/ARB use | 0** | 31.7% (13) |
| Calcium antagonist use | 0 | 7.3% (3) |
| Diuretic use | 0 | 7.3% (3) |
| β-Blocker use | 0 | 0 |
| Thyroid suppletion | 0 | 14.6% (6) |
| Creatinine (µmol/L) | 77 (18) | 74 (19) |
| MDRD-GFR (mL/min/1.73 m2) | 82 (20) | 88 (14) |
| hs-CRP (mg/L) | 0.9 (1.3) | 0.9 (2.2) |
| Total cholesterol (mmol/L) | 4.5 (1.6) | 4.4 (1.3) |
| LDL-cholesterol | 2.71 (1.08) | 2.52 (1.2) |
| HDL-cholesterol | 1.35 (0.43)*** | 1.65 (0.52) |
| Triglycerides | 0.90 (0.47) | 0.8 (0.44) |
| Non-HDL cholesterol | 3.1 (1.3) | 2.9 (1.3) |
| Glucose (mmol/L) | 5.1 (0.7)**** | 9 (4.1) |
| HbA1c at inclusion (mmol/mol) | 35 (6)* | 62 (18)* |
| Haemoglobin (mmol/L) | 9.1 (1.5) | 8.7 (1.2) |
| WBC 109/L | 5.7 (2.7) | 5.2 (2.2) |
| Platelets 109/L | 229 (91) | 234 (73) |
| Neutrophils 109/L | 3.0 (1.4) | 2.9 (1.8) |
| Lymphocytes 109/L | 1.6 (0.8) | 1.4 (0.7) |
| Monocytes 109/L | 0.4 (0.2) | 0.4 (0.3) |
| Eosinophils 109/L | 0.1 (0.1) | 0.1 (0.1) |
| Basophils 109/L | 0.03 (0.02) | 0.03 (0.02) |
Data are presented as median and interquartile range (age, BMI, systolic and diastolic blood pressure, duration diabetes, HbA1c, creatinine, MDRD-GFR, hs-CRP, LDL-cholesterol, HDL-cholesterol, non-HDL-cholesterol, glucose, haemoglobin, WBC, platelets, neutrophils, lymphocytes, monocytes, eosinophils, basophils), relative and absolute numbers (all other parameters except number). ACE, angiotensin-converting-enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; DM, Type 1 diabetes subjects; HC, healthy subject; hs-CRP, high-sensitive C-reactive protein; MDRD-GFR, modification of diet in renal disease glomerular filtration rate; WBC, white blood cell count.
Significant difference between all groups (P < 0.001).
Significant difference between healthy subjects and both diabetes groups (P < 0.05).
Significant difference between healthy subjects and DM ≤ 64 (P < 0.05).
Significant difference between healthy subjects and both diabetes groups (P < 0.001).
3.2. Patients with T1D show enhanced 18F-FDG uptake in vascular and haematopoietic regions
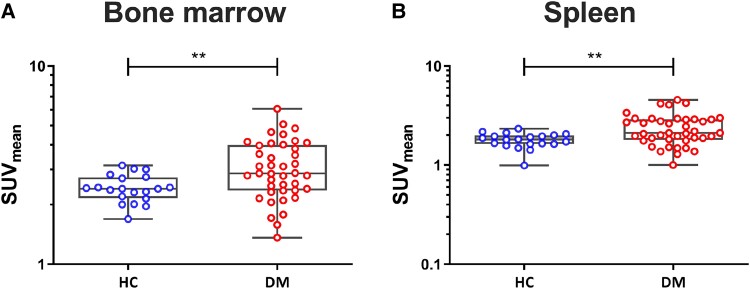
18F-FDG uptake was higher in all vascular regions (aorta, carotid arteries, and iliac arteries) in patients with T1D compared with controls (Figure 2, Supplementary material online, Figure S3). No differences in 18F-FDG uptake were seen between patients with T1D with an HbA1c ≤ 64 and an HbA1c > 64 mmol/mol (see Supplementary material online, Figure S2). Again no effect of HbA1c was seen in the additional sensitivity analysis, comparing the 10 participants with the highest HbA1c levels and the 10 participants with the lowest HbA1c levels (Figure 3A–C), nor was there any significant correlation between HbA1c and FDG uptake within the group of patients with T1D (Figure 3D–F). Results did not differ between men and women (data not shown). In patients with T1D, 18F-FDG uptake was also higher in the bone marrow and the spleen compared with healthy controls (Figure 4, Supplementary material online, Figure S3).
Figure 2.
Patients with T1D show enhanced 18F-FDG uptake in vascular regions. TBR in vascular regions. TBR, expressed as TBRmean of abdominal aorta (A), carotid arteries (B), and iliac arteries (C). DM, participants with T1D (n = 41); HC, healthy controls (n = 20); TBR, target to background ratio. Data are presented as median and interquartile range. ****P < 0.0001, Mann–Whitney U test.
Figure 3.
18F-FDG-PET/C of 10 lowest and 10 highest HbA1c levels. TBR, expressed as TBRmean of abdominal aorta (A), carotid arteries (B), and iliac arteries (C). Scatterplot of correlation between HbA1c and abdominal aorta (D), carotid arteries (E), and iliac arteries (F). TBR, target to background ratio. Data are presented as median and interquartile range, n = 10 per group. Mann–Whitney U test.
Figure 4.
Patients with T1D show enhanced 18F-FDG uptake in haematopoietic regions. 18F-FDG uptake in haematopoietic regions. SUV, expressed as SUVmean of bone marrow of the spine (A) and spleen (B). DM, participants with T1D (n = 41); HC, healthy controls (n = 20); SUV, standardized uptake value. Data are presented as median and interquartile range. **P < 0.01, Mann–Whitney U test.
3.3. The percentage of non-classical monocytes is lower in patients with T1D
Overall, no differences in white blood cell counts were found between healthy controls and participants with T1D (Table 1) but the percentage of non-classical monocytes was lower in T1D compared with healthy controls (Figure 5A). Monocyte activation markers CCR2 and CD36 were higher expressed in T1D compared with healthy controls, and no differences were found between groups in levels of CD41 and CD11b (Figure 5B). Additional cell surface markers did not differ between the groups, except for non-classical CD36+ monocytes, which were higher in healthy controls (see Supplementary material online, Figure S4).
Figure 5.
The percentage of non-classical monocytes is lower in patients with T1D. Monocytes subsets in whole blood were determined using flow cytometry. Graphs show different monocyte subsets (A) and atherogenic monocyte markers (B). DM, participants with T1D (n = 41); HC, healthy controls (n = 20). Data are presented as median and interquartile range. *P < 0.05, unpaired T-test.
3.4. Patients with T1D have higher levels of circulating inflammatory markers
Using a targeted proteomics approach, we measured >90 inflammatory circulating proteins. As shown in Figure 6A, 11 circulating inflammatory markers were higher (FDR corrected) in patients with T1D compared with HCs: Leukaemia inhibitory factor receptor (LIF-R; also Figure 6B, top), C-C motif chemokine 25 (CCL25; also Figure 6B, bottom), CUB domain-containing protein 1 (CDCP1), tumour necrosis factor receptor superfamily member 9 (TNFRSF9), adenosine deaminase (ADA), C-C motif chemokine 28 (CCL28), Delta and Notch-like epidermal growth factor-related receptor (DNER), interleukin-15 receptor subunit alpha (IL-15RA), interleukin-10 (IL-10), signalling lymphocytic activation molecule (SLAMF1), and interleukin-18 receptor 1 (IL-18R1).
Figure 6.
Patients with T1D have higher levels of circulating inflammatory markers. (A) Volcano plot showing circulating inflammatory proteins. Named proteins are significantly higher in patients with T1D (FDR-adjusted P-value < 0.05, Wilcoxon test). (B) Two representative examples of circulating inflammatory factors are shown. Top panel shows the protein LIF-R; bottom panel shows protein CCL25. Boxplot data are presented as median and interquartile range. DM, participants with T1D (n = 41); HC, healthy controls (n = 20); FDR, false discovery rate; LIF-R, leukaemia inhibitory factor receptor; CCL25, chemokine (C-C motif) ligand 25. * indicates FDR <0.05, Wilcoxon rank-sum test.
3.5. Circulating inflammatory proteins are correlated with 18F-FDG uptake in vascular and haematopoietic regions in patients with T1D
To determine whether the level of vascular wall inflammation in patients with T1D is associated with levels of circulating inflammatory proteins, 18F-FDG uptake in both vascular and haematopoietic regions was correlated with levels of circulating inflammatory proteins (Figure7A and B,Supplemental material online, Figure S5 and Table S1). A total of four inflammatory proteins showed a positive correlation with at least one vascular region. Additionally, three proteins correlated positively with at least one non-vascular region, while three proteins correlated negatively. Although not significant, noticeably, various proteins displaying a positive correlation with vascular inflammation showed the opposite in haematopoietic regions.
Figure 7.
Circulating inflammatory proteins are correlated with 18F-FDG uptake in vascular and haematopoietic regions in patients with T1D. (A) The correlation between inflammatory proteins and 18F-FDG uptake in participants with T1D (n = 41; only proteins that showed a significant correlation with at least one ROI were included in the graph). * indicates a Spearman correlation P-value <0.05. (B) Two representative correlation graphs of OPG. Top panel shows the positive Spearman correlation between TBRmax abdominal aorta and protein OPG. Bottom panel shows the negative correlation between SUVmax of the bone marrow and protein OPG. ROI, region of interest; OPG, osteoprotegerin; TBR, target to background ratio; SUV, standardized uptake value.
4. Discussion
Previous studies investigating arterial wall inflammation using 18F-FDG-PET/CT in diabetes were all performed in patients with T2D and reported higher arterial wall inflammation in diabetes patients, and patients with impaired glucose tolerance.11,12,21 However, the complexity of T2D makes it difficult to determine the direct effect of hyperglycaemia on 18F-FDG uptake and arterial wall inflammation, as often other cardiovascular risk factors and overweight/obesity co-exist. We therefore determined the relation between chronic hyperglycaemia and arterial wall inflammation in patients with T1D, lacking the cardiovascular risk factors often observed in T2D.
In this matched case–control study, we report that vascular wall inflammation, as measured by 18F-FDG-PET/CT, is higher in patients with T1D compared with non-diabetic controls. Haematopoietic activity, assessed by 18F-FDG uptake in the bone marrow and spleen, was also higher. The higher level of arterial wall inflammation was accompanied by increased monocyte expression of activation markers CCR2 and CD36, and by systemic inflammation as measured by several circulating inflammatory markers. Finally, the levels of vascular wall inflammation correlated significantly with several circulating inflammatory proteins, suggesting a direct link between specific circulating proteins and the severity of vascular wall inflammation.
Chronic hyperglycaemia during diabetes is suggested to alter the innate immune system, resulting in a more proinflammatory phenotype, which, in turn is associated with accelerated atherosclerosis.24 Previous research showed that hyperglycaemia enhances myelopoiesis in the bone marrow leading to monocytosis and increased entry of monocytes into atherosclerotic lesions in a mouse model of T1D.25 Additionally, the formation of coronary artery plaques, measured by ultrasound, was found to be related to mean HbA1c in T1D.26 In our study, we observed increased 18F-FDG uptake in the vascular wall of patients with T1D compared with healthy controls, but there was no effect of HbA1c. This suggests that the presence of diabetes increases vascular inflammation independently of HbA1c levels, and that factors beyond the average glucose concentration determine the elevated CVD risk. It is important to emphasize that elevated HbA1c levels may still be important, yet once the levels go beyond a certain threshold during diabetes, a further increase, even to levels seen in poorly controlled individuals, does not contribute to the worsening of vascular inflammation. Alternatively, glucometrics beyond mean glucose levels, such as glucose variability or glucose peaks, may be more important than HbA1c.17 In animal models, transient glucose peaks can induce long-term immune cell activation by their effect on bone marrow progenitors.17
Increased bone marrow 18F-FDG uptake has been reported in patients with atherosclerotic CVD,7 and is associated with an increased risk of future cardiovascular events.13,27,28 Bone marrow FDG uptake is associated with an increased ex vivo proliferation of progenitor cells in patients with atherosclerosis.27 We recently reported functional reprogramming of bone marrow progenitor cells in patients with coronary artery disease.16 In line with these findings, we found increased 18F-FDG uptake in the bone marrow and spleen in patients with T1D compared with controls, and we propose that this results in proinflammatory circulating monocytes that facilitate atherosclerotic plaque development.
An increase in 18F-FDG uptake reflects an elevated cellular glucose usage that is known to characterize inflammatory macrophages. Yet macrophages are not the only cells that use glucose, so theoretically other cell types might contribute to the increased 18F-FDG uptake in the vessel wall, including endothelial cells. However, 18F-FDG uptake is a well-established method for the detection of vascular wall inflammation in the setting of atherosclerosis and has been associated with macrophage content and levels of inflammatory gene expression in atherosclerotic plaques.7,8,20 Furthermore, a correlation between 18F-FDG uptake and macrophage density in both animal and human atheromas has been shown in previous research.29 These results strengthen our conclusion that hyperglycaemia in patients with T1D increases the level of vascular wall inflammation driven by macrophages. It is important to note that 18F-FDG uptake was used to measure vascular wall inflammation and reflects an increase in local net glucose uptake. In previous literature, 18F-FDG uptake was normalized to the blood pool SUVs, to reduce the effect of differences in blood glucose levels between participants.30 Due to the nature of T1D, glucose levels of participants differed upon arrival before the PET-CT scan. Although we corrected for blood glucose levels, the potential effects of the basal glucose concentration should be kept in mind since endogenous glucose can affect 18F-FDG uptake. In addition, statins use had to be discontinued for at least 2 weeks before inclusion. It is known that statins can lower arterial FDG uptake as described before,31 and we wanted to prevent that statin use in the patients would mask a higher arterial wall FDG uptake. However, we cannot conclude that this short interruption could have influenced also bone marrow FDG uptake since it has been described that in patients with T2D, 5 days of statin interruption increased circulating bone marrow-derived endothelial progenitor cells.32
A large part of plaque macrophages is derived from circulating monocytes. To determine whether arterial wall inflammation was associated with circulating immune cell populations, we measured both number and phenotype. Overall, no differences in circulating immune cell numbers were observed between patients with T1D and controls, except for a lower percentage of non-classical monocytes in T1D. A small, non-significant increase in classical and intermediate monocytes was found which would be in line with the reduction of non-classical monocytes and may indicate a shift in monocyte phenotype. Flow cytometry revealed a higher expression of CCR2 and CD36 on monocytes in patients with T1D. CCR2 is a monocyte chemokine receptor essential for transmigration, which is associated with arterial FDG uptake in patients at increased cardiovascular risk.33 CD36 is a scavenger receptor located on monocytes which is involved in foam cell transformation, and its expression has previously been shown to be increased by hyperglycaemia.34
In addition to circulating cells, we measured inflammatory proteins using a targeted proteomic approach. Our results revealed an up-regulation of systemic inflammation reflected by an increased concentration of 11 inflammatory proteins in patients with T1D. T1D has been associated with elevated levels of a broad spectrum of circulating inflammatory factors. Inflammatory markers E-selectin, hs-CRP, white blood cell count (WBC) and erythrocyte sedimentation rate (ESR) were found to be increased in adolescents with T1D and independently associated with diabetic complications.35 Several markers including E-selectin and VCAM are considered as biomarkers for CVD and atherosclerosis,36,37 but they were not different in patients with T1D compared with healthy controls in our study (data not shown). However, our targeted proteomic approach resulted in the identification of new potential candidates defining the enhanced inflammatory status of patients with T1D, yet also uncovered several proteins correlating with the level of vascular inflammation. Eleven inflammatory proteins (LIF-R, CCL25, CDCP1, TNFRSF9, ADA, DNER, CCL28, IL-15Ra, IL-10, SLAMF1, and IL-18R1) were increased in patients with T1D compared with healthy controls. Three (TNFRSF9, IL-18R1 and IL15-RA) of these proteins have previously been associated with diabetes-related complications. TNFRSF9 has been linked to CVD events in patients with coronary atherosclerosis,38 IL-18R1 has been linked to the development of diabetic nephropathy39 and the IL15-RA gene is linked to obesity and T2D and affects body composition and insulin sensitivity.40 All other identified proteins with higher levels in the patients with T1D compared with the HCs are known for their proinflammatory effect but have not been linked previously to T1D. Hypothetically, these inflammatory proteins could affect immune cell phenotype at an early stage in the bone marrow or at the side of an atherogenic plaque or impact on the migration of immune cells to the arterial wall. Alternatively, the increased levels are not causally involved in the development of vascular wall inflammation and merely reflect an adaptive response after the development of vascular wall inflammation. Interestingly, we also identified several circulating inflammatory markers that were significantly associated with the 18F-FDG uptake in patients with T1D, suggesting a direct link between these specific circulating inflammatory markers and the level of vascular wall inflammation. For example, the levels of osteoprotegerin (OPG), DNER, and ADA demonstrated a significant correlation with arterial wall inflammation, suggesting a role for these proteins in the development of arterial wall inflammation in T1D. These proteins have previously been linked to CVD.41–44 OPG is up-regulated by proinflammatory cytokines and contributes to vascular wall inflammation by inhibiting the activation and promoting the apoptosis in osteoclasts. High OPG expression levels have been found in atherosclerotic plaques.43 DNER is part of the Notch signalling pathway, which is important in the link between inflammation and the development of CVD.41 ADA is an enzyme of purine metabolism and converts adenosine to inosine or 2′-deoxyadenosine to 2′-deoxyinosine. Endogenous adenosine is regarded as a ‘retaliatory’ metabolite that can inhibit atherosclerosis formation in a negative feedback loop.42 Based on our findings, DNER and ADA might also serve as interesting biomarkers for the presence of vascular inflammation and ultimately CVD in patients with T1D. Furthermore, their pathways could indicate potential targets for new drugs to prevent CVD in T1D. Overall, our results suggest an association with circulating proinflammatory proteins and arterial wall inflammation. Further research is needed to determine the exact role of these proteins regarding the development of vascular wall inflammation and its underlying mechanism.
In summary, our results show that arterial wall inflammation, measured by 18F-FDG-PET/CT, is increased in patients with T1D with hyperglycaemia, but is not dependent on the level of glycaemic control. Arterial wall inflammation is associated with increased levels of several circulating inflammatory markers, suggesting that these proteins are directly involved in driving this process and yet may serve as future biomarkers to identify patients with T1D at risk for the development of CVD. Overall, our findings confirm the concept that chronic hyperglycaemia in T1D promotes systemic inflammatory changes that fuel arterial wall inflammation, ultimately leading to CVD.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
A.W.M.J., J.I.P.v.H., R.S., and N.P.R. wrote the first drafts of the manuscript. A.W.M.J. performed the study. A.W.M.J., J.I.P.v.H. R.S., and C.J.T. performed the experiments and analysed the data. A.W.M.J., J.A.v.D., N.P.R., and C.J.T. conceived and planned the study. All authors provided critical feedback and helped in design and conception of the study and final drafting of the manuscript. C.J.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary Material
Acknowledgements
The authors thank all participants for their contribution. Michel de Groot and Judith Thijssen were of great help in planning the PET/CT scans. The authors thank Cor Jacobs, Anneke Hijmans, and Kathrin Thiem for the technical assistance. They thank Valerie Koeken for her advice regarding the proteomics analysis. The figures are derived from Servier Medical Art by Servier (https://smart.servier.com/) and licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
Contributor Information
Anna W M Janssen, Department of Internal Medicine (463), Radboud University Medical Center, PO Box 9101, Geert Grooteplein 8, Nijmegen 6500 HB, The Netherlands.
Julia I P van Heck, Department of Internal Medicine (463), Radboud University Medical Center, PO Box 9101, Geert Grooteplein 8, Nijmegen 6500 HB, The Netherlands.
Rinke Stienstra, Department of Internal Medicine (463), Radboud University Medical Center, PO Box 9101, Geert Grooteplein 8, Nijmegen 6500 HB, The Netherlands; Division of Human Nutrition and Health, Wageningen University and Research Division of Human Nutrition and Health (Bode 62), P.O. Box 176700 AA, Wageningen, The Netherlands.
Erik H J G Aarntzen, Department of Radiology and Nuclear Medicine, Radboud University Medical Center, Geert Grooteplein Zuid 32, 6525 GA Nijmegen, The Netherlands.
Janna A van Diepen, Department of Internal Medicine (463), Radboud University Medical Center, PO Box 9101, Geert Grooteplein 8, Nijmegen 6500 HB, The Netherlands.
Niels P Riksen, Department of Internal Medicine (463), Radboud University Medical Center, PO Box 9101, Geert Grooteplein 8, Nijmegen 6500 HB, The Netherlands.
Cees J Tack, Department of Internal Medicine (463), Radboud University Medical Center, PO Box 9101, Geert Grooteplein 8, Nijmegen 6500 HB, The Netherlands.
Funding
This work was funded by the European Foundation for the Study of Diabetes (EFSD/AZ Macrovascular Programme 2015) and the Dutch Diabetes Foundation (2015.82.1824). N.P.R. received an IN-CONTROL CVON grant from the Dutch Heart Foundation (CVON2018-27) and a grant from the ERA-CVD Joint Transnational Call 2018, which is supported by the Dutch Heart Foundation (JTC2018, project MEMORY, 2018T093). C.J.T. is supported by the European research project Hypo-RESOLVE (Hypoglycaemia—Redefining SOLutions for better liVEs), which has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement no. 777460.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Translational Perspective.
Arterial wall inflammation is associated with increased levels of several circulating inflammatory markers, suggesting that these proteins are directly involved in driving this process and yet may serve as future biomarkers to identify patients with Type 1 diabetes (T1D) at risk for the development of cardiovascular disease (CVD). Additionally, these inflammatory markers could potentially be future treatment targets in reducing the risk of CVD in people with T1D.
References
- 1. Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J.. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rawshani A, Sattar N, Franzen S, Rawshani A, Hattersley AT, Svensson AM, Eliasson B, Gudbjornsdottir S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 2018;392:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group . Risk factors for cardiovascular disease in type 1 diabetes. Diabetes 2016;65:1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Viola J, Soehnlein O. Atherosclerosis—a matter of unresolved inflammation. Semin Immunol 2015;27:184–193. [DOI] [PubMed] [Google Scholar]
- 5. Abohashem S, Osborne MT, Dar T, Naddaf N, Abbasi T, Ghoneem A, Radfar A, Patrich T, Oberfeld B, Tung B, Fayad ZA, Rajagopalan S, Tawakol A.. A leucopoietic-arterial axis underlying the link between ambient air pollution and cardiovascular disease in humans. Eur Heart J 2021;42:761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V, Tang CY, Mulder WJ, Murrough JW, Hoffmann U, Nahrendorf M, Shin LM, Fayad ZA, Pitman RK.. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 2017;389:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ.. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006;48:1818–1824. [DOI] [PubMed] [Google Scholar]
- 8. Pedersen SF, Graebe M, Fisker Hag AM, Hojgaard L, Sillesen H, Kjaer A. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun 2010;31:423–429. [DOI] [PubMed] [Google Scholar]
- 9. Joseph P, Tawakol A. Imaging atherosclerosis with positron emission tomography. Eur Heart J 2016;37:2974–2980. [DOI] [PubMed] [Google Scholar]
- 10. Bucerius J, Mani V, Moncrieff C, Rudd JH, Machac J, Fuster V, Farkouh ME, Fayad ZA. Impact of noninsulin-dependent type 2 diabetes on carotid wall 18F-fluorodeoxyglucose positron emission tomography uptake. J Am Coll Cardiol 2012;59:2080–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim TN, Kim S, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: analysis with 18F-fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging 2010;3:142–148. [DOI] [PubMed] [Google Scholar]
- 12. de Boer SA, Hovinga-de Boer MC, Heerspink HJ, Lefrandt JD, van Roon AM, Lutgers HL, Glaudemans AW, Kamphuisen PW, Slart RH, Mulder DJ. Arterial stiffness is positively associated with 18F-fluorodeoxyglucose positron emission tomography-assessed subclinical vascular inflammation in people with early type 2 diabetes. Diabetes Care 2016;39:1440–1447. [DOI] [PubMed] [Google Scholar]
- 13. Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JH, Fayad ZA, Lehrer-Graiwer J, Korsgren M, Figueroa AL, Fredrickson J, Rubin B, Hoffmann U, Truong QA, Min JK, Baruch A, Nasir K, Nahrendorf M, Tawakol A.. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging 2015;8:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tarkin JM, Joshi FR, Rudd JH. PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol 2014;11:443–457. [DOI] [PubMed] [Google Scholar]
- 15. Devesa A, Lobo-Gonzalez M, Martinez-Milla J, Oliva B, Garcia-Lunar I, Mastrangelo A, Espana S, Sanz J, Mendiguren JM, Bueno H, Fuster JJ, Andrés V, Fernández-Ortiz A, Sancho D, Fernández-Friera L, Sanchez-Gonzalez J, Rossello X, Ibanez B, Fuster V.. Bone marrow activation in response to metabolic syndrome and early atherosclerosis. Eur Heart J 2022;43:1809–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noz MP, Bekkering S, Groh L, Nielen TM, Lamfers EJ, Schlitzer A, El Messaoudi S, van Royen N, Huys EH, Preijers FW, Smeets EM, Aarntzen EH, Zhang B, Li Y, Bremmers ME, van der Velden WJ, Dolstra H, Joosten LA, Gomes ME, Netea MG, Riksen NP.. Reprogramming of bone marrow myeloid progenitor cells in patients with severe coronary artery disease. Elife 2020;9:e60939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flynn MC, Kraakman MJ, Tikellis C, Lee MKS, Hanssen NMJ, Kammoun HL, Pickering RJ, Dragoljevic D, Al-Sharea A, Barrett TJ, et al. Transient intermittent hyperglycemia accelerates atherosclerosis by promoting myelopoiesis. Circ Res 2020;127:877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miao F, Chen Z, Genuth S, Paterson A, Zhang L, Wu X, Li SM, Cleary P, Riggs A, Harlan DM, Lorenzi G, Kolterman O, Sun W, Lachin JM, Natarajan R; DCCT/EDIC Research Group.. Evaluating the role of epigenetic histone modifications in the metabolic memory of type 1 diabetes. Diabetes 2014;63:1748–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thiem K, van Dierendonck X, Janssen AWM, Boogaard JP, Riksen NP, Tack CJ, Stienstra R. A high glycemic burden relates to functional and metabolic alterations of human monocytes in patients with type 1 diabetes. Diabetes 2020;69:2735–2746. [DOI] [PubMed] [Google Scholar]
- 20. van der Heijden CDCC, Smeets EMM, Aarntzen EHJG, Noz MP, Monajemi H, Kersten S, Kaffa C, Hoischen A, Deinum J, Joosten LAB, Netea MG, Riksen NP. Arterial wall inflammation and increased hematopoietic activity in patients with primary aldosteronism. J Clin Endocrinol Metab 2020;105:e1967–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bucerius J, Hyafil F, Verberne HJ, Slart RH, Lindner O, Sciagra R, Agostini D, Ubleis C, Gimelli A, Hacker M, Cardiovascular Committee of the European Association of Nuclear Medicine (EANM).. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur J Nucl Med Mol Imaging 2016;43:780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, Stroobants S, Delbeke D, Donohoe KJ, Holbrook S, Graham MM, Testanera G, Hoekstra OS, Zijlstra J, Visser E, Hoekstra CJ, Pruim J, Willemsen A, Arends B, Kotzerke J, Bockisch A, Beyer T, Chiti A, Krause BJ; European Association of Nuclear Medicine (EANM).. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42:328–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Assarsson E, Lundberg M, Holmquist G, Björkesten J, Thorsen SB, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt G, Andersson AC, Lindstedt P, Stenvang J, Gullberg M, Fredriksson S.. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Diepen JA, Thiem K, Stienstra R, Riksen NP, Tack CJ, Netea MG. Diabetes propels the risk for cardiovascular disease: sweet monocytes becoming aggressive? Cell Mol Life Sci 2016;73:4675–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, Schmidt AM, Orchard TJ, Fisher EA, Tall AR, Goldberg IJ.. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab 2013;17:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Larsen J, Brekke M, Sandvik L, Arnesen H, Hanssen KF, Dahl-Jorgensen K. Silent coronary atheromatosis in type 1 diabetic patients and its relation to long-term glycemic control. Diabetes 2002;51:2637–2641. [DOI] [PubMed] [Google Scholar]
- 27. van der Valk FM, Kuijk C, Verweij SL, Stiekema LCA, Kaiser Y, Zeerleder S, Nahrendorf M, Voermans C, Stroes ESG. Increased haematopoietic activity in patients with atherosclerosis. Eur Heart J 2017;38:425–432. [DOI] [PubMed] [Google Scholar]
- 28. Moens SJ B, van der Valk FM, Strang AC, Kroon J, Smits LP, Kneepkens EL, Verberne HJ, van Buul JD, Nurmohamed MT, Stroes ES. Unexpected arterial wall and cellular inflammation in patients with rheumatoid arthritis in remission using biological therapy: a cross-sectional study. Arthritis Res Ther 2016;18:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Evans NR, Tarkin JM, Chowdhury MM, Warburton EA, Rudd JH. PET imaging of atherosclerotic disease: advancing plaque assessment from anatomy to pathophysiology. Curr Atheroscler Rep 2016;18:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boellaard R, O'Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, Oyen WJ, Kotzerke J, Hoekstra OS, Pruim J, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging 2010;37:181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pirro M, Simental-Mendía LE, Bianconi V, Watts GF, Banach M, Sahebkar A. Effect of statin therapy on arterial wall inflammation based on 18F-FDG PET/CT: a systematic review and meta-analysis of interventional studies. J Clin Med 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fadini GP, Rigato M, Boscari F, Cappellari R, Menegazzo L, Pilutti C, Iori E, Marescotti M, Plebani M, Albiero M, Avogaro A.. Short-term statin discontinuation increases endothelial progenitor cells without inflammatory rebound in type 2 diabetic patients. Vascul Pharmacol 2015; 67–69:21–29. [DOI] [PubMed] [Google Scholar]
- 33. Verweij SL, Duivenvoorden R, Stiekema LCA, Nurmohamed NS, van der Valk FM, Versloot M, Verberne HJ, Stroes ESG, Nahrendorf M, Bekkering S, Bernelot Moens SJ. CCR2 expression on circulating monocytes is associated with arterial wall inflammation assessed by 18F-FDG PET/CT in patients at risk for cardiovascular disease. Cardiovasc Res 2018;114:468–475. [DOI] [PubMed] [Google Scholar]
- 34. Lopez-Carmona MD, Plaza-Seron MC, Vargas-Candela A, Tinahones FJ, Gomez-Huelgas R, Bernal-Lopez MR. CD36 overexpression: a possible etiopathogenic mechanism of atherosclerosis in patients with prediabetes and diabetes. Diabetol Metab Syndr 2017;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aulich J, Cho YH, Januszewski AS, Craig ME, Selvadurai H, Wiegand S, Jenkins AJ, Donaghue KC. Associations between circulating inflammatory markers, diabetes type and complications in youth. Pediatr Diabetes 2019;20:1118–1127. [DOI] [PubMed] [Google Scholar]
- 36. Peter K, Weirich U, Nordt TK, Ruef J, Bode C. Soluble vascular cell adhesion molecule-1 (VCAM-1) as potential marker of atherosclerosis. Thromb Haemost 1999;82(Suppl 1):38–43. [PubMed] [Google Scholar]
- 37. Tsoref O, Tyomkin D, Amit U, Landa N, Cohen-Rosenboim O, Kain D, Golan M, Naftali-Shani N, David A, Leor J. E-selectin-targeted copolymer reduces atherosclerotic lesions, adverse cardiac remodeling, and dysfunction. J Control Release 2018;288:136–147. [DOI] [PubMed] [Google Scholar]
- 38. Dregoesc MI, Tigu AB, Bekkering S, van der Heijden C, Bolboaca SD, Joosten LAB, Visseren FLJ, Netea MG, Riksen NP, Iancu AC. Relation between plasma proteomics analysis and major adverse cardiovascular events in patients with stable coronary artery disease. Front Cardiovasc Med 2022;9:731325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ni WJ, Tang LQ, Wei W. Research progress in signalling pathway in diabetic nephropathy. Diabetes Metab Res Rev 2015;31:221–233. [DOI] [PubMed] [Google Scholar]
- 40. Quinn LS, Anderson BG. Interleukin-15, IL-15 receptor-alpha, and obesity: concordance of laboratory animal and human genetic studies. J Obes 2011;2011:456347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Quillard T, Charreau B. Impact of notch signaling on inflammatory responses in cardiovascular disorders. Int J Mol Sci 2013;14:6863–6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kutryb-Zajac B, Mierzejewska P, Slominska EM, Smolenski RT. Therapeutic perspectives of adenosine deaminase inhibition in cardiovascular diseases. Molecules 2020;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Montagnana M, Lippi G, Danese E, Guidi GC. The role of osteoprotegerin in cardiovascular disease. Ann Med 2013;45:254–264. [DOI] [PubMed] [Google Scholar]
- 44. Toffoli B, Fabris B, Bartelloni G, Bossi F, Bernardi S. Dyslipidemia and diabetes increase the OPG/TRAIL ratio in the cardiovascular system. Mediators Inflamm. 2016;2016:6529728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.