Abstract
Purpose
We aimed to explore the expressions of peptidoglycan recognition protein 1 (PGLYRP-1), neuron towards axon guidance factor-1 (Netrin-1) and miR-142-3p and their correlations in patients with rheumatoid arthritis.
Patients and Methods
Sixty patients with rheumatoid arthritis treated from January 2022 to January 2023 were enrolled as a rheumatoid group, 30 patients with osteoarthritis were selected as an osteoarthritis group, and 30 healthy volunteers were recruited as a control group. The enzyme-linked immunosorbent assay, Western blotting and reverse transcriptase-polymerase chain reaction were employed to measure the expressions of PGLYRP-1, Netrin-1 and miR-142-3p, respectively. The correlations among PGLYRP-1, Netrin-1 and miR-142-3p expressions in patients with rheumatoid arthritis were analyzed.
Results
In patients with rheumatoid arthritis, PGLYRP-1 expression was negatively correlated with Netrin-1 expression (r=−0.570, P=0.001) but positively correlated with miR-142-3p expression (r=0.599, P=0.001), and a negative correlation was found between Netrin-1 and miR-142-3p expressions (r=−0.468, P=0.001). The combined detection of PGLYRP-1, Netrin-1 and miR-142-3p was more sensitive and less specific for predicting the prognosis of patients with rheumatoid arthritis than the measurement of a single marker (P<0.05).
Conclusion
The combined measurement of PGLYRP-1, Netrin-1 and miR-142-3p has a predictive value for the prognosis of patients with rheumatoid arthritis.
Keywords: correlation, neuron towards axon guidance factor-1, peptidoglycan recognition protein 1, rheumatoid arthritis
Introduction
Rheumatoid arthritis, as a common immune disease, is mainly characterized by morning stiffness, joint damage, joint pain and even joint deformity, posing a severe threat to the health and quality of life of patients.1–3 Middle-aged females are highly susceptible groups for rheumatoid arthritis. In China, the prevalence rate of rheumatoid arthritis is 0.2–0.37%.4 Rheumatoid arthritis may further develop into aggressive arthritis or even result in the loss of joint function if not timely treated.5,6 The pathogenesis of rheumatoid arthritis has been extensively studied, but despite these enormous efforts, we do not understand it fully. The relapse rate of rheumatoid arthritis is high in clinical practice. Hence, researching the pathogenesis of rheumatoid arthritis and discovering new treatment targets are of importance for the diagnosis, treatment and prognosis of patients.7,8
Peptidoglycan recognition protein 1 (PGLYRP-1) is a circulating protein, and its expression has a close association with inflammatory responses.9 It activates the innate immune system and binds peptidoglycan to facilitate the development of inflammatory responses. PGLYRP-1 is abundant in eosinophils and neutrophils. Neuron towards axon guidance factor-1 (Netrin-1), as a main member of the laminin family, can suppress the migration of immune cells to inflammation sites, thus impeding the progression of inflammatory responses and mitigating tissue damage.10
Micro ribonucleic acids (MiRNAs) are abundant in most organisms in the form of non-coding single-strands and closely correlated with inflammatory responses and immune system regulation.11 The miRNA family has tissue specificity and stability, and participates in the development and progression of inflammatory responses and immune system diseases.12 As one of the main members of the miRNA family, miR-142-3p shows a close association with inflammatory responses and immune status.13
In this study, the expressions of PGLYRP-1, Netrin-1 and miR-142-3p in patients with rheumatoid arthritis were measured, and their correlations in rheumatoid arthritis and their predictive value for the prognosis were analyzed, aiming to provide a valuable basis for future treatment.
Materials and Methods
Subjects
A total of 60 patients with rheumatoid arthritis treated in our hospital (The First Affiliated Hospital of Guangxi Medical University) from January 2022 to January 2023 were enrolled as a rheumatoid group. All patients were diagnosed by CT scan in our hospital. Meanwhile, 30 patients with osteoarthritis treated in our hospital in the same period were selected as an osteoarthritis group. Moreover, 30 healthy volunteers receiving physical examination in our hospital in the same period were recruited as a control group. All the subjects were inquired regarding smoking history, drinking history, hypertension and coronary heart disease.
The subjects with comparable baseline clinical data were randomly enrolled without knowing the grouping results before this study. This study has been approved by the ethics committee of our hospital, and all subjects have signed the informed consent.
X-ray staging criteria for patients with rheumatoid arthritis:14 Stage I–II: normal or osteoporosis at the joint end, and fusiform swelling of soft tissues; narrow joint space, accompanied by subchondral cystic changes of the articular surface; stage III: destructive changes of the articular surface, obvious subchondral cystic destruction of the articular surface, and joint subluxation.
Scoring of disease activity in patients with rheumatoid arthritis: Comprehensive evaluation was performed based on erythrocyte sedimentation rate, CRP level, and number of swollen or tender joints. The disease activity was scored according to the DAS28 criteria.15 The total score was 10 points, and a higher score meant a higher activity. When the score was <2.6 points, the disease was in the remission stage, otherwise it was in the active stage.
The inclusion criteria were as follows: 1) The patients with rheumatoid arthritis met the diagnostic criteria in the Chinese Guideline for the Diagnosis and Treatment of Rheumatoid Arthritis (2018 Edition)16 and diagnosed by CT scan; 2) the patients with osteoarthritis met the diagnostic criteria in the Chinese Guideline for the Diagnosis and Treatment of Osteoarthritis (2021 Edition);17 3) patients with complete medical records; 4) those aged <70 years old; 5) those without receiving relevant treatment recently.
The exclusion criteria involved: 1) patients with incomplete medical records, 2) those with congenital joint deformities, 3) those with diseases of the immune system or coagulation system, 4) those who had received relevant treatment recently, 5) those with communication problems, or 6) those with mental diseases.
Sample Collection
Fasting venous blood (8 mL) was collected from the three groups on the day of enrollment, centrifuged (centrifugal radius: 10 cm, speed 3000 rpm, centrifugal time: 15 min) and stored at −70°C.
Measurement of PGLYRP-1 Level by Enzyme-Linked Immunosorbent Assay
PGLYRP-1 level was measured with a commercial enzyme-linked immunosorbent assay kit (Shanghai Future Industrial Limited by Share Ltd., China). After the enzyme plate was labeled and the standards were diluted, serum samples and 100 μL of standard solution were added to the enzyme plate, and a micro-plate reader was used to measure the PGLYRP-1 level. In brief, blank well 1, blank well 2 and experimental well were set up, followed by the addition of termination solution and color developer to blank well 1, diluted standards to blank well 2, and serum samples and antibodies to the experimental well. Then the membrane was blocked, shaken and incubated. Using blank well 1 as the reference, the absorbance value at 450 nm was read using a micro-plate reader (Shanghai Enzyme-Linked Biotechnology Co., Ltd., ML-dr3518) and the PGLYRP-1 level was calculated.
Determination of Netrin-1 Expression by Western Blotting
Samples were treated with lysis buffer. Next, 50 μg of protein was collected, denatured by boiling, subjected to SDS-PAGE, and transferred to a membrane. Afterwards, the membrane was blocked with Western blocking solution for 3 h, and incubated with primary antibodies (1:2000) at 4–6°C overnight. On the next day, the membrane was washed 3 times with Western solution and incubated with secondary antibodies (1:5000) for 2 h, followed by membrane washing with Western solution, color development, exposure and imaging. Finally, the grey value of the bands was detected and the relative expression of Netrin-1 was measured.
Detection of miR-142-3p Expression Through Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA extraction and elution were carried out according to the instructions. The following RT-PCR steps and settings were used. The SYBR GREEN staining method was selected for PCR, and a PCR system was prepared according to the instructions, with a total volume of 50 μL. There were 35 cycles of reaction at 95°C for 2 min, melting at 95°C for 5 s, extension at 72°C for 35s, and annealing at 56°C for 5 s. The miR-142-3p expression was calculated by 2−ΔΔCT, with U6 as the internal reference. The primer sequences of miR-142-3p were as follows: forward primer: 5’-GGGTGTAGTGTTTCCTACT-3’, and reverse primer: 5’-CAGTGCGTGTCGTGGAGT-3’.
Comparisons of Indicators
1) The expressions of PGLYRP-1, Netrin-1 and miR-142-3p in control, osteoarthritis and rheumatoid groups were compared. 2) The expressions of PGLYRP-1, Netrin-1 and miR-142-3p were compared among the rheumatoid arthritis patients with different clinical characteristics. 3) The correlations among PGLYRP-1, Netrin-1, miR-142-3p expressions in the patients with rheumatoid arthritis were analyzed. 4) The predictive values of PGLYRP-1, Netrin-1 and miR-142-3p for the prognosis of patients with rheumatoid arthritis were analyzed.
The main factors affecting the prognosis of patients with rheumatoid arthritis included: 1) General factors: gender, age, smoking and body weight; 2) disease factors: autoantibodies, disease activity, functional status, bone erosion, extra-articular manifestations and comorbidities; 3) treatment factors; 4) biomarkers: serological, imaging and genetic biomarkers. The scoring criteria for prognosis: Short-term control: the swelling and pain of the affected joints completely disappeared after treatment, and the joint function was improved or recovered to normal; significantly effective: the pain of the affected joints was alleviated significantly; effective: the pain was alleviated; ineffective: after treatment, the pain of the affected joints was not alleviated or even aggravated. The prognosis of patients was poor if the treatment was ineffective, whereas the prognosis was considered good in the case of the other three outcomes.
Statistical Analysis
IBM SPSS Statistics for Windows, Version 26.0 was employed for statistical analysis. The measurement data were expressed as (mean ± standard deviation) and compared among groups by the F-test and between groups by the independent sample t-test. The correlations were analyzed through Pearson’s analysis. As to predictive value analysis, the receiver operating characteristic (ROC) curve was plotted to obtain the area under the curve (AUC), confidence interval (CI), sensitivity and specificity. P<0.05 indicated that the difference was of statistical significance.
Results
In the rheumatoid group, there were 33 males and 27 females, with a mean age of (47.16±10.52) years old and a mean body mass index (BMI) of (23.26±2.25) kg/m2. In this group, 22 patients had joint deformity. There were 32 cases of stages I–II and 28 cases of stage III according to X-ray staging. In addition, 35 and 25 cases were in remission and active stages, respectively. The osteoarthritis group consisted of 17 males and 13 females aged (45.51±9.88) years old, with a mean BMI of (23.51±2.37) kg/m2. The control group was composed of 15 males and 15 females, with a mean age of (46.35±10.23) years old and a mean BMI of (23.05±2.16) kg/m2. The general data such as sex ratio, age, mean BMI, smoking history, drinking history, hypertension and coronary heart disease were of no statistically significant differences among the three groups (P>0.05) (Table 1).
Table 1.
General Data of the Three Groups
| General Data | Control Group | Osteoarthritis Group | Rheumatoid Group | F/x2 | P |
|---|---|---|---|---|---|
| Sex (M/F) | 15/15 | 17/13 | 33/27 | 0.298 | 0.903 |
| Age (year) | 46.35±10.23 | 45.51±9.88 | 47.16±10.52 | 1.132 | 0.260 |
| BMI (kg/m2) | 23.05±2.16 | 23.51±2.37 | 23.26±2.25 | 0.153 | 0.696 |
| Smoking history | 13/17 | 14/16 | 28/32 | 0.103 | 0.951 |
| Drinking history | 12/18 | 13/17 | 26/34 | 0.099 | 0.950 |
| Hypertension | 14/16 | 12/18 | 25/35 | 0.312 | 0.858 |
| Coronary heart disease | 11/19 | 10/20 | 21/39 | 0.073 | 0.964 |
Osteoarthritis and rheumatoid groups had higher expressions of PGLYRP-1 and miR-142-3p and a lower expression of Netrin-1 than those of the control group (P<0.05). The expressions of PGLYRP-1 and miR-142-3p were higher in the rheumatoid group than those in the osteoarthritis group, while the expression of Netrin-1 was lower in the rheumatoid group than that in the osteoarthritis group (P<0.05) (Table 2).
Table 2.
Expressions of PGLYRP-1, Netrin-1 and miR-142-3p in Different Subjects
| Subject | n | PGLYRP-1 (pg/mL) | Netrin-1 | miR-142-3p |
|---|---|---|---|---|
| Control group | 30 | 38.67±4.32 | 1.05±0.13 | 1.12±0.15 |
| Osteoarthritis group | 30 | 59.68±5.21* | 0.86±0.09* | 1.36±0.21* |
| Rheumatoid group | 60 | 137.52±12.66*# | 0.35±0.04*# | 2.15±0.34*# |
| F | 46.710 | 42.540 | 16.245 | |
| P | 0.001 | 0.001 | 0.001 |
Notes: *P<0.05 vs control group, #P<0.05 vs osteoarthritis group. Continuous variables are reported as mean ± SD.
The rheumatoid arthritis patients with X-ray stage III had higher expressions of PGLYRP-1 and miR-142-3p but a lower expression of Netrin-1 than those of the patients with stage I–II (P<0.05). PGLYRP-1 and miR-142-3p expressions were higher in the rheumatoid arthritis patients in the active stage than those of the patients in the remission stage, whereas the expression of Netrin-1 was lower in the patients in the active stage (P<0.05). Higher expressions of PGLYRP-1 and miR-142-3p and a lower Netrin-1 expression were found in the rheumatoid arthritis patients with joint deformity than those in the patients without joint deformity (P<0.05). Compared with the patients with good prognosis, the expressions of PGLYRP-1 and miR-142-3p increased, whereas the expression of Netrin-1 decreased in the patients with poor prognosis (P<0.05) (Table 3).
Table 3.
Correlations of PGLYRP-1, Netrin-1 and miR-142-3p Expressions with Clinical Characteristics of Patients with Rheumatoid Arthritis
| Clinical Characteristic | n | PGLYRP-1 (pg/mL) | Netrin-1 | miR-142-3p |
|---|---|---|---|---|
| X-Ray stage | ||||
| Stage I–II | 32 | 119.37±10.98 | 0.45±0.05 | 1.96±0.29 |
| Stage III | 28 | 151.63±14.19 | 0.23±0.03 | 2.32±0.41 |
| t | 9.912 | 20.290 | 3.963 | |
| P | 0.001 | 0.001 | 0.001 | |
| Disease activity | ||||
| Remission stage | 35 | 118.69±11.21 | 0.46±0.06 | 1.94±0.28 |
| Active stage | 25 | 153.28±15.06 | 0.24±0.04 | 2.36±0.42 |
| t | 10.210 | 15.960 | 4.650 | |
| P | 0.001 | 0.001 | 0.001 | |
| Joint deformity | ||||
| No | 38 | 117.29±10.75 | 0.47±0.05 | 1.95±0.31 |
| Yes | 22 | 154.40±14.68 | 0.22±0.02 | 2.34±0.39 |
| t | 11.240 | 22.370 | 4.267 | |
| P | 0.001 | 0.001 | 0.001 | |
| Prognosis | ||||
| Good | 40 | 114.31±10.90 | 0.48±0.04 | 1.93±0.27 |
| Poor | 20 | 156.32±14.72 | 0.21±0.02 | 2.35±0.40 |
| t | 12.490 | 28.380 | 4.815 | |
| P | 0.001 | 0.001 | 0.001 |
Notes: Continuous variables are reported as mean ± SD.
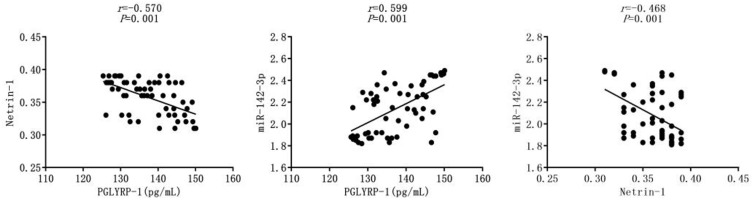
The correlation analysis revealed that PGLYRP-1 expression was negatively correlated with Netrin-1 expression in rheumatoid arthritis (r=−0.570, P=0.001). PGLYRP-1 and miR-142-3p expressions were positively correlated in rheumatoid arthritis (r=0.599, P=0.001). A negative correlation was found between Netrin-1 and miR-142-3p expressions in rheumatoid arthritis (r=−0.468, P=0.001) (Figure 1).
Figure 1.
Correlations among PGLYRP-1, Netrin-1 and miR-142-3p expressions in patients with rheumatoid arthritis.
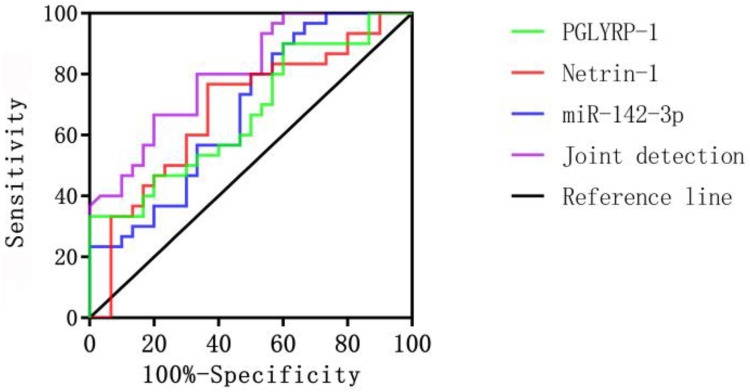
Compared with the measurement of a single marker, the combined measurement of PGLYRP-1, Netrin-1 and miR-142-3p had higher sensitivity and lower specificity in predicting the prognosis of patients with rheumatoid arthritis (P<0.05) (Table 4 and Figure 2).
Table 4.
Predictive Values of PGLYRP-1, Netrin-1 and miR-142-3p for the Prognosis of Patients with Rheumatoid Arthritis
| Indicator | AUC | P | Sensitivity (%) | Specificity (%) | 95% CI |
|---|---|---|---|---|---|
| PGLYRP-1 | 0.671 | 0.023 | 69.13 | 85.12 | 0.535–0.808 |
| Netrin-1 | 0.683 | 0.015 | 68.43 | 83.37 | 0.546–0.821 |
| miR-142-3p | 0.679 | 0.017 | 68.51 | 83.55 | 0.544–0.814 |
| Combination | 0.803 | 0.001 | 83.27 | 72.29 | 0.695–0.911 |
Figure 2.
ROC curves for predictive values of PGLYRP-1, Netrin-1, and miR-142-3p for the prognosis of patients with rheumatoid arthritis.
Discussion
As a common chronic inflammatory disease, rheumatoid arthritis is characterized by arthropathy and persistent inflammatory response of involved joints, or even skeletal deformity, joint dysfunction and other manifestations in severe cases.18,19 Currently, the patients with rheumatoid arthritis still have a high relapse rate and a poor prognosis after treatment. Therefore, researchers have endeavored to clarify the pathogenesis and treatment targets of rheumatoid arthritis using molecular biology and serum index tests.20,21
The level of PGLYRP-1 is strongly associated with the development and progression of atherosclerosis,22,23 but its correlation with the development and progression of rheumatoid arthritis has rarely been reported. In this study, the PGLYRP-1 level was higher in the patients with rheumatoid arthritis, so PGLYRP-1 may participate in the development and progression of rheumatoid arthritis. Additionally, the rheumatoid arthritis patients with higher X-ray stage, those in the active stage, those with joint deformity, and those with poor prognosis had higher PGLYRP-1 levels, indicating a correlation between PGLYRP-1 level and the severity of rheumatoid arthritis. Hence, PGLYRP-1 level is of diagnostic value for the severity of rheumatoid arthritis.
Netrin-1 can induce nerve regeneration and exert strong immunomodulatory effects,24 but studies on the expression and significance of Netrin-1 in rheumatoid arthritis are still lacking. The results of this study revealed that Netrin-1 expression was lower in the patients with rheumatoid arthritis. Besides, Netrin-1 expression was lower in the rheumatoid arthritis patients with higher X-ray stage, those in the active stage, those with joint deformity and those with poor prognosis, suggesting that Netrin-1 expression had a close relationship with the clinical characteristics of patients with rheumatoid arthritis. Probably, Netrin-1 modulated the immune system, and its abnormally low expression resulted in immune disorders, thus promoting the progression of rheumatoid arthritis. Hence, Netrin-1 may be a new target for the diagnosis and treatment of rheumatoid arthritis.
At present, most of the studies on miR-142-3p are related to malignant tumors,25,26 and there are fewer studies on miR-142-3p expression in rheumatoid arthritis. In this study, miR-142-3p expression was abnormally high in rheumatoid arthritis. Meanwhile, it was higher in the rheumatoid arthritis patients with higher X-ray stage, those in the active stage, those with joint deformity and those with poor prognosis, indicating that miR-142-3p expression was closely associated with the clinical characteristics of patients with rheumatoid arthritis. Therefore, the detection of miR-142-3p expression is of importance for the diagnosis and prognostic prediction of patients with rheumatoid arthritis.
Moreover, significant correlations were found among the expressions of PGLYRP-1, Netrin-1 and miR-142-3p in rheumatoid arthritis, and the combined detection of the three markers was more sensitive and less specific in predicting the prognosis of patients. Hence, PGLYRP-1, Netrin-1 and miR-142-3p may jointly participate in the development and progression of rheumatoid arthritis, and the combined measurement of the three markers has a higher predictive value for the prognosis of patients.
Conclusion
In conclusion, high expressions of PGLYRP-1 and miR-142-3p and a low Netrin-1 expression are found in patients with rheumatoid arthritis, and the expressions of PGLYRP-1, Netrin-1 and miR-142-3p are closely related to the clinical characteristics of these patients. The combined detection of PGLYRP-1, Netrin-1 and miR-142-3p is of predictive value for the prognosis of patients with rheumatoid arthritis. Nevertheless, this study is limited. First, this is a single-center study with a small sample size. Second, the correlations among PGLYRP-1, Netrin-1 and miR-142-3p in patients with osteoarthritis or the controls were not analyzed. Further studies with larger sample sizes will be conducted to verify our findings.
Abbreviations
PGLYRP-1, peptidoglycan recognition protein 1; Netrin-1, neuron towards axon guidance factor-1; miR-142-3p, micro ribonucleic acid-142-3p; RT-PCR, reverse transcriptase-polymerase chain reaction; ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Akiyama M, Kaneko Y. Pathogenesis, clinical features, and treatment strategy for rheumatoid arthritis-associated interstitial lung disease. Autoimmun Rev. 2022;21(5):103056. doi: 10.1016/j.autrev.2022.103056 [DOI] [PubMed] [Google Scholar]
- 2.Finckh A, Gilbert B, Hodkinson B, et al. Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol. 2022;18(10):591–602. doi: 10.1038/s41584-022-00827-y [DOI] [PubMed] [Google Scholar]
- 3.Littlejohn EA, Monrad SU. Early diagnosis and treatment of rheumatoid arthritis. Prim Care. 2018;45(2):237–255. doi: 10.1016/j.pop.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 4.Sun J, Dai S, Zhang L, Feng Y, Yu X, Zhang Z. Investigating the safety and compliance of using csDMARDs in rheumatoid arthritis treatment through face-to-face interviews: a cross-sectional study in China. Clin Rheumatol. 2021;40:1789–1798. doi: 10.1007/s10067-020-05458-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan Y, Buch MH. “Difficult to treat” rheumatoid arthritis: current position and considerations for next steps. RMD Open. 2022;8(2):e002387. doi: 10.1136/rmdopen-2022-002387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Guo R, Oduro PK, et al. The relationship between porphyromonas gingivalis and rheumatoid arthritis: a meta-analysis. Front Cell Infect Microbiol. 2022;12:956417. doi: 10.3389/fcimb.2022.956417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabi EM, Singh A, Althafar ZM, et al. Elucidating the role of hypoxia-inducible factor in rheumatoid arthritis. Inflammopharmacology. 2022;30(3):737–748. doi: 10.1007/s10787-022-00974-4 [DOI] [PubMed] [Google Scholar]
- 8.Gargano G, Oliva F, Oliviero A, Maffulli N. Small interfering RNAs in the management of human rheumatoid arthritis. Br Med Bull. 2022;142(1):34–43. doi: 10.1093/bmb/ldac012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortiz F, Nylund KM, Ruokonen H, et al. Salivary biomarkers of oral inflammation are associated with cardiovascular events and death among kidney transplant patients. Transplant Proc. 2020;52(10):3231–3235. doi: 10.1016/j.transproceed.2020.07.007 [DOI] [PubMed] [Google Scholar]
- 10.Ziegon L, Schlegel M. Netrin-1: a modulator of macrophage driven acute and chronic inflammation. Int J Mol Sci. 2021;23(1):275. doi: 10.3390/ijms23010275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Li Z, Wang L, et al. The role of non-coding RNAs (miRNA and lncRNA) in the clinical management of rheumatoid arthritis. Pharmacol Res. 2022;186:106549. doi: 10.1016/j.phrs.2022.106549 [DOI] [PubMed] [Google Scholar]
- 12.Chang C, Xu L, Zhang R, et al. MicroRNA-mediated epigenetic regulation of rheumatoid arthritis susceptibility and pathogenesis. Front Immunol. 2022;13:838884. doi: 10.3389/fimmu.2022.838884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Huang C, Yang Z, et al. Identification of potential biomarkers of gout through competitive endogenous RNA network analysis. Eur J Pharm Sci. 2022;173:106180. doi: 10.1016/j.ejps.2022.106180 [DOI] [PubMed] [Google Scholar]
- 14.Mupparapu M, Oak S, Chang YC, Alavi A. Conventional and functional imaging in the evaluation of temporomandibular joint rheumatoid arthritis: a systematic review. Quintessence Int. 2019;50(9):742–753. doi: 10.3290/j.qi.a43046 [DOI] [PubMed] [Google Scholar]
- 15.Takanashi S, Kaneko Y, Takeuchi T. CDAI and DAS28 in the management of rheumatoid arthritis in clinical practice. Ann Rheum Dis. 2020;79(5):671–674. doi: 10.1136/annrheumdis-2019-216607 [DOI] [PubMed] [Google Scholar]
- 16.Rheumatology Branch of Chinese Medical Association. 2018中国类风湿关节炎诊疗指南 [2018 Chinese guideline for the diagnosis and treatment of rheumatoid arthritis]. Chin J Intern Med. 2018;57(4):242–251. Chinese. doi: 10.3760/cma.j.issn.0578-1426.2018.04.004 [DOI] [Google Scholar]
- 17.Joint Surgery Branch of the Chinese Orthopaedic Association. 中国骨关节炎诊疗指南(2021年版) [Chinese guideline for diagnosis and treatment of osteoarthritis (2021 edition)]. Chin J Orthop. 2021;41(18):1291–1314. Chinese. doi: 10.3760/cma.j.cn121113-20210624-00424 [DOI] [Google Scholar]
- 18.Sugihara T. Treatment strategies for elderly-onset rheumatoid arthritis in the new era. Mod Rheumatol. 2022;32(3):493–499. doi: 10.1093/mr/roab087 [DOI] [PubMed] [Google Scholar]
- 19.Diesler R, Cottin V. Pulmonary fibrosis associated with rheumatoid arthritis: from pathophysiology to treatment strategies. Expert Rev Respir Med. 2022;16(5):541–553. doi: 10.1080/17476348.2022.2089116 [DOI] [PubMed] [Google Scholar]
- 20.Yuan S, Li X, Lin A, Larsson SC. Interleukins and rheumatoid arthritis: bi-directional Mendelian randomization investigation. Semin Arthritis Rheum. 2022;53:151958. doi: 10.1016/j.semarthrit.2022.151958 [DOI] [PubMed] [Google Scholar]
- 21.Mou X, Jin Y, Jin D, Guan J, Zhang Q. Serum HDAC4 level in rheumatoid arthritis: longitudinal change during treatment and correlation with clinical outcomes. J Clin Lab Anal. 2022;36(8):e24594. doi: 10.1002/jcla.24594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo Q, Li X, Zhang L, et al. Serum PGLYRP-1 is a highly discriminatory biomarker for the diagnosis of rheumatoid arthritis. Mol Med Rep. 2019;19(1):589–594. doi: 10.3892/mmr.2018.9632 [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Zhao B, Wu Y, et al. Impacts of diarrhea on the immune system, intestinal environment, and expression of PGRPs in New Zealand rabbits. PeerJ. 2017;5:e4100. doi: 10.7717/peerj.4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma P, Yadav P, Jain RP, Bera AK, Karunagaran D. miR-142-3p simultaneously targets HMGA1, HMGA2, HMGB1, and HMGB3 and inhibits tumorigenic properties and in-vivo metastatic potential of human cervical cancer cells. Life Sci. 2022;291:120268. doi: 10.1016/j.lfs.2021.120268 [DOI] [PubMed] [Google Scholar]
- 25.Barnault R, Verzeroli C, Fournier C, et al. Hepatic inflammation elicits production of proinflammatory netrin-1 through exclusive activation of translation. Hepatology. 2022;76(5):1345–1359. doi: 10.1002/hep.32446 [DOI] [PubMed] [Google Scholar]
- 26.Mediero A, Wilder T, Ramkhelawon B, Moore KJ, Cronstein BN. Netrin-1 and its receptor Unc5b are novel targets for the treatment of inflammatory arthritis. FASEB J. 2016;30(11):3835–3844. doi: 10.1096/fj.201600615R [DOI] [PMC free article] [PubMed] [Google Scholar]