Abstract
The adenovirus E1B 19,000-molecular-weight (19K) protein is a potent inhibitor of apoptosis and cooperates with E1A to transform primary rodent cells. E1B 19K shows sequence and functional homology to the mammalian antiapoptotic gene product, Bcl-2. Like Bcl-2, the biochemical mechanism of E1B 19K function includes binding to and antagonization of cellular proapoptotic proteins such as Bax, Bak, and Nbk/Bik. In addition, there is evidence that E1B 19K can affect gene expression, but whether this contributes to its antiapoptotic function has not been determined. In an effort to further understand the functions of E1B 19K, we screened for 19K-associated proteins by the yeast two-hybrid system. A novel protein, Btf (Bcl-2-associated transcription factor), that interacts with E1B 19K as well as with the antiapoptotic family members Bcl-2 and Bcl-xL but not with the proapoptotic protein Bax was identified. btf is a widely expressed gene that encodes a protein with homology to the basic zipper (bZip) and Myb DNA binding domains. Btf binds DNA in vitro and represses transcription in reporter assays. E1B 19K, Bcl-2, and Bcl-xL sequester Btf in the cytoplasm and block its transcriptional repression activity. Expression of Btf also inhibited transformation by E1A with either E1B 19K or mutant p53, suggesting a role in either promotion of apoptosis or cell cycle arrest. Indeed, the sustained overexpression of Btf in HeLa cells induced apoptosis, which was inhibited by E1B 19K. Furthermore, the chromosomal localization of btf (6q22-23) maps to a region that is deleted in some cancers, consistent with a role for Btf in tumor suppression. Thus, btf may represent a novel tumor suppressor gene residing in a unique pathway by which the Bcl-2 family can regulate apoptosis.
Apoptosis is a genetically controlled process of cell suicide that plays a critical role in maintaining homeostasis and preventing disease (reviewed in references 35 and 90). Disruption of apoptosis leads to impaired development, cancer, neurodegenerative and autoimmune diseases, and sustained viral infection. The regulation of apoptosis is a precarious balance between factors that promote survival and those responsible for initiating and executing cell death. One major advance toward the understanding of apoptosis regulation has been the characterization of the Bcl-2 family (90). This family consists of highly conserved proteins with opposing biological functions. Antiapoptotic Bcl-2 family members, such as Bcl-2 and Bcl-xL, inhibit apoptosis triggered by many circumstances, including tumor necrosis factor alpha (TNF-α), Fas, UV radiation, chemotherapeutic drugs, and growth factor or hormone withdrawal. In contrast, proapoptotic Bcl-2 family members, such as Bax, Bak, and Nbk/Bik, induce cell death in numerous model systems. The adenovirus E1B 19,000-molecular-weight (19K) protein cooperates with E1A in transformation assays and is a viral homologue of mammalian Bcl-2. Expression of E1B 19K, or Bcl-2, inhibits E1A-induced, p53-mediated apoptosis (13, 17). Like Bcl-2, E1B 19K interacts with and antagonizes several proapoptotic family members, including Bax (26), Nbk/Bik (8, 27), and Bak (19). However, it is still unclear how these proteins effect cell survival or death and whether binding to proteins unrelated to the Bcl-2 family contributes to apoptosis or other cellular processes.
While there has been some debate about the biochemical function of E1B 19K and other Bcl-2 family members in the regulation of apoptosis, recent studies have led to several possible mechanisms. Clearly, interactions between Bcl-2 family members play an integral part in the regulation of apoptosis and transformation. A common feature of the Bcl-2 family is the occurrence of protein-protein interactions between the anti- and proapoptotic proteins, the ratio of which controls the fate of the cell (5, 58). Structural and biochemical studies of Bcl-2 family members point to the possibility that these proteins form ion channels (1, 46, 55, 71). In addition, Bcl-2 family members bind to several unrelated proteins, including R-ras (20), Nip-1, Nip-2, and Nip-3 (8), Ced-4-like proteins (12, 28, 32, 61, 79, 98, 104), Bag-1 (80), lamin A/C (65), and p28Bap31 (57). Although the functional significances of some of these interactions are not yet known, these findings suggest that Bcl-2 regulates multiple signaling pathways that influence apoptosis.
There is growing evidence from several independent studies that Bcl-2-related proteins can trigger changes in gene expression (39, 50, 66, 74, 76, 103) which may or may not be related to their role in apoptosis regulation. Initial studies of E1B 19K mutant viruses raised the possibility that 19K functions to dampen transcriptional activation by E1A (92, 95). Subsequently, it has been shown that E1B 19K and Bcl-2 alleviate the trans-repressive activity of E1A and p53 (66, 76, 103). This derepression of transcription has been correlated with an up-regulation of one transcriptional target of p53, Mdm-2 (82). Since Mdm-2 inhibits p53 transcriptional activity and apoptosis (53), this model provides an alternative mechanism by which E1B 19K and Bcl-2 can regulate apoptosis. Furthermore, Bcl-2 family members may actually control several additional transcription factors, including NF-κB (23, 34, 72), NFAT (nuclear factor of activated T cells) (39), c-Jun (74), and the glucocorticoid receptor (50), indicating that Bcl-2 and its related proteins can have multiple effects on gene expression that may contribute to apoptosis.
Compared to apoptosis regulation, relatively little is known about the gene-regulatory function of the Bcl-2 family. In this paper, we describe a novel transcriptional repressor, Btf, that may contribute to the modulation of transcription by Bcl-2- related proteins. Btf was identified in a yeast two-hybrid screen against E1B 19K and subsequently shown to also interact with Bcl-2 and Bcl-xL. These Bcl-2 family members inhibit the translocation of Btf to the nucleus and abrogate its transcriptional activity. We also present evidence that sustained overexpression of Btf induces apoptosis and suppresses transformation by E1A and E1B 19K or mutant p53. Thus, the interaction with Btf provides a novel pathway by which the Bcl-2 family can regulate transcription and control apoptosis.
MATERIALS AND METHODS
Yeast two-hybrid system.
Procedures for using the two-hybrid system to identify E1B 19K binding proteins were described previously (26). A HeLa cDNA library was constructed in the pGAD-GH vector and screened against pGBT9-E1B 19K. Plasmids were transformed into Saccharomyces cerevisiae YGH1 cells, and positive clones were selected based on growth in the absence of histidine and production of β-galactosidase. False-positive clones were eliminated by testing for interactions with an irrelevant hydrophobic protein (Apc-2) and the empty pGBT9 vector. Missense and deletion mutants of E1B 19K tested for interaction with BP-1 were described previously (26). pGBT8–Bcl-2 (20) and pGBT9-Bax (26) were also described previously. pGBT8–Bcl-xL was kindly provided by G. Nuñez (University of Michigan, Ann Arbor, Mich.).
The full-length transcript btfS was identified by screening a λ cDNA library prepared from HeLa cells (Stratagene, La Jolla, Calif.) by using conventional techniques. The cDNA sequence of bp-1 and the subsequent full-length btf were analyzed with Sequenase 2.0 (U.S. Biochemical, Cleveland, Ohio) as specified by the manufacturer and later confirmed by fluorescent terminator cycle sequencing with an automated model 377 DNA sequencer (Perkin-Elmer, Applied Biosystems, Foster City, Calif.).
Plasmid construction.
A PCR product of btfS from the λ screen was digested with XmaI and NotI (blunt) and ligated into both pGAD-GH cut with XmaI-XhoI (blunt) and pGBT9 cut with XmaI-SalI (blunt). To prepare pcDNA3-Myc-BtfS for mammalian expression and in vitro translation, oligonucleotides encoding a Myc epitope with flanking KpnI and XmaI sites were annealed with a PCR-cloned fragment of btfS from Bluescript digested with XmaI and NotI and from pcDNA3 (Invitrogen, San Diego, Calif.) digested with KpnI and NotI. The resulting plasmid encodes a Myc epitope at the N-terminal end of BtfS. BtfS and two deletion mutants were fused in frame with the GAL4 DNA binding domain in the pm1 vector (kindly provided by C. Abate-Shen, Center for Advanced Biotechnology and Medicine, Piscataway, N.J.) for use in transcriptional reporter assays. The full-length btfS construct was prepared by digesting pGBT9-BtfS with XmaI and PstI and ligating it into the same sites in the pm1 vector. The ΔN552 and ΔC210 mutants were generated by digesting pGBT9-BtfS with EcoRI-PstI and XmaI-BamHI, respectively, and ligating it into the same restriction sites within pm1. btfS was also cloned into pIRES-EGFP (Clontech, San Francisco, Calif.) for cell cycle analysis of BtfS-expressing cells. The construct was prepared by digesting pcDNA3-Myc-BtfS with SmaI and NotI and ligating it to pIRES-EGFP cut with EcoRV and NotI. The pcDNA3–Bcl-2 plasmid was prepared by ligating an EcoRI-XhoI fragment from pSFFV–Bcl-2 (31) (provided by S. Korsmeyer, Washington University School of Medicine, St. Louis, Mo.) into pcDNA3. The C-terminally V5/His-tagged E1B 19K was prepared by TA cloning into pcDNA3.1/V5/His-TOPO (Invitrogen) as specified by the manufacturer.
Cell lines.
The HeLa cells were maintained in culture in Dulbecco modified Eagle medium–10% fetal bovine serum at 37°C under 5% CO2. Stable HeLa cell lines containing Bcl-XL were prepared by electroporating 1 μg of pcDNA3-Flag–Bcl-xL (45), provided by G. Nuñez, into HeLa cells and selecting with 1.2 mg of Geneticin per ml. Expression of Bcl-xL was verified by immunofluorescence.
Northern blotting.
Northern blot analyses were performed with commercially available blots of multiple tissues or cancer lines (Clontech). Each lane contained 2 μg of the indicated poly(A)+ RNA. The btfS probe was prepared by random priming with a purified XmaI-XhoI fragment from pGAD-GH-BtfS. Human β-actin cDNA supplied with the blots was used as a control probe to confirm equal mRNA loading. Hybridization was performed with ExpressHyb solution (Clontech) as specified by the manufacturer. Bands were visualized by autoradiography and with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
In vitro binding assay.
Binding reactions were performed by combining [35S]methionine-labeled, in vitro-translated Myc-BtfS or Myc-Bax (26) with V5/His-E1B 19K, Bcl-2, Flag–Bcl-xL, or luciferase (Promega Corp., Madison, Wis.) prepared by using the TnT T7 reticulocyte lysate system (Promega) as specified by the manufacturer. Samples were incubated with anti-Myc monoclonal antibody (Oncogene Research Products, Cambridge, Mass.) in 500 μl of NETN buffer (20 mM Tris [pH 8.0], 100 mM NaCl, 1 mM EDTA, 0.2% Nonidet P-40) for 1.5 h at 4°C followed by protein A-Sepharose for 30 min. All samples were then washed three times in NETN buffer, resuspended in 2× Laemmli buffer (12.5 mM Tris [pH 6.8], 20% glycerol, 4% sodium dodecyl sulfate [SDS], 0.5% bromophenol blue, 0.5% β-mercaptoethanol), boiled for 5 min, and analyzed by SDS-polyacrylamide gel electrophoresis (PAGE). The gels were fixed in 50% methanol–10% acetic acid for 1 h, dried, and then visualized by autoradiography.
DNA binding assay.
[35S]methionine-labeled, in vitro-translated Myc-BtfS and E1B 19K were prepared from 1 μg of pcDNA3-Myc-BtfS and pcDNA3-E1B 19K, respectively (26), using the TnT T7 reticulocyte lysate system. The proteins were incubated with 200 μl of native DNA-cellulose (Pharmacia Biotech, Piscataway, N.J.) in 500 μl of NETN buffer for 2 h at 4°C. The relative levels of BtfS and E1B 19K production were determined by immunoprecipitation with anti-Myc (Oncogene Research Products, Cambridge, Mass.) or anti-E1B 19K (94) in NETN buffer followed by protein A-Sepharose and then washing in NETN. Samples were resolved by SDS-PAGE and examined by autoradiography as described above.
Indirect immunofluorescence.
HeLa cells were electroporated with 15 μg of pcDNA3-Myc-BtfS along with 15 μg of either pCMV-E1B 19K (94) or pcDNA3–Bcl-2. The total amount of DNA was kept constant by using empty pcDNA3 vector. Cells grown on glass coverslips were stained 24 h posttransfection as described previously (63). Briefly, the cells were fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS) and then permeabilized with 0.5% Triton X-100 in PBS. The cells were double labeled with an anti-Myc mouse monoclonal antibody along with either an anti-E1B 19K rabbit polyclonal antibody (94) or an anti-Bcl-2 hamster monoclonal antibody (PharMingen, San Diego, Calif.). Antibody complexes were visualized with tetramethylrhodamine isothiocyanate-conjugated goat anti-mouse antibody along with the fluorescein isothiocyanate-conjugated goat anti-rabbit or goat anti-hamster antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.). Immunocytochemistry was also performed with HeLa–Bcl-xL cells electroporated with 15 μg of pcDNA3-Myc-BtfS. These cells were stained for the Myc epitope as described above. Expression of Flag-tagged Bcl-xL was confirmed by staining separate coverslips with anti-Flag M5 monoclonal antibody (Scientific Imaging Systems, New Haven, Conn.) and rhodamine-conjugated goat anti-mouse antibody. The cells were visualized by epifluorescence with an FXA microscope (Nikon Inc., Garden City, N.Y.).
Transcription assays.
Transcriptional reporter assays were performed as described previously (11). HeLa cells were plated onto 60-mm tissue culture dishes and grown to 50 to 75% confluency. The cells were then transfected with 2.5 μg of a GAL4 luciferase reporter construct (kindly provided by C. Abate-Shen) along with 2.5 μg each of a pm1 vector (BtfS, BtfS-ΔN552, or BtfS-ΔC210) and a bcl-2 family gene (pCMV-E1B 19K, pcDNA3–Bcl-2, or pcDNA3-Flag–Bcl-xL). The DNA concentrations were kept constant by using an appropriate empty vector, either pm1 or pcDNA3. The cells were transfected with SuperFect (Qiagen, Valencia, Calif.) as specified by the manufacturer and harvested 24 h posttransfection. Expression of mutant and wild-type BtfS proteins was verified by immunofluorescence with an antibody against the GAL4 DNA binding domain (Clontech). Luciferase activity was determined with the luciferase assay system (Promega) in a scintillation counter and then normalized for protein concentrations measured by the Bradford assay. Values were graphed as a percentage of the negative control (empty pm1, empty pcDNA3, and GAL-4 luciferase).
Transformation assay.
Transformation assays of baby rat kidney (BRK) cells were performed as described previously (96). Briefly, BRK cells prepared from 6-day-old Fisher rats were electroporated with carrier DNA along with linearized test DNA (15 μg of pCMV-E1A [91], 15 μg of pCMV-E1B 19K or pCMV-p53DD [75], and 45 μg of pcDNA3-Myc-BtfS). DNA concentrations were kept constant by using appropriate empty vectors. The cells were cultured for 3 to 4 weeks at 38.5°C in Dulbecco modified Eagle medium supplemented with 5% fetal bovine serum and then stained with Giemsa. Foci were counted from four dishes per condition.
Cell cycle analysis.
HeLa cells were electroporated with 20 μg of pIRES-EGFP-BtfS or empty pIRES-EGFP vector in combination with 10 μg of pCMV-E1B 19K or empty pcDNA3 vector. The cells were harvested 48 and 72 h posttransfection and fixed in 2% paraformaldehyde in PBS for 30 min at 4°C. They were washed and then stained for at least 30 min at room temperature with PBS containing 1 μg of propidium iodide per ml, 250 μg of RNase A per ml, and 0.1% Tween 20. The fluorescence intensities for enhanced green fluorescent protein (EGFP) and propidium iodide were analyzed by fluorescence-activated cell sorting (FACS) (EPICS PROFILE-II; Coulter, Miami, Fla.). In addition, the live transfected cells were stained with Hoechst dye to visualize the DNA and photographed with an FXA microscope.
RESULTS
Yeast two-hybrid assay.
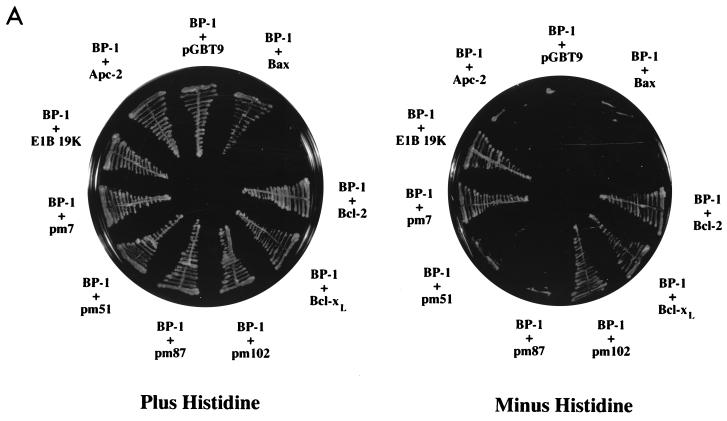
To identify novel cellular proteins that interact with E1B 19K, we screened a HeLa cDNA library by using the yeast two-hybrid assay. The library was constructed in the pGAD-GH plasmid in which the cDNA sequences were fused to the GAL4 activation domain, and the bait gene, E1B 19K, was fused to the GAL4 DNA binding domain in the pGBT9 vector (26). The plasmids were transformed into the YGH1 yeast strain and screened for GAL4-inducible phenotypes, namely, growth in the absence of histidine and production of β-galactosidase. Three million transformants were screened, yielding seven clones (BP-1 to BP-7) that specifically interacted with 19K and not with the pGBT9 vector alone or with an irrelevant protein, Apc-2. BP-2, BP-3, and BP-4 were previously reported as lamin A/C (65), Bax (26), and Nbk/Bik (27), respectively. Here, we describe one of the novel E1B 19K-associated proteins, BP-1. A 1.5-kb cDNA containing bp-1 was isolated seven times during the two-hybrid screen. Since Bcl-2 family members are highly homologous and frequently interact with the same cellular proteins, we tested BP-1 for interaction with Bcl-2-related apoptosis regulators. In addition to binding E1B 19K, BP-1 interacted with other related proteins, Bcl-2 and Bcl-xL, but not with the proapoptotic family member Bax (Fig. 1A).
FIG. 1.
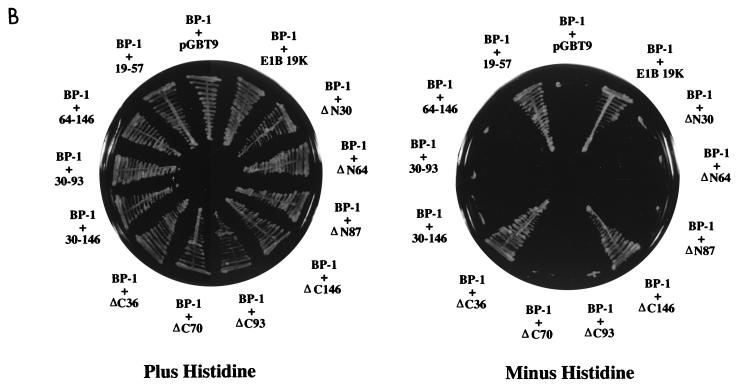
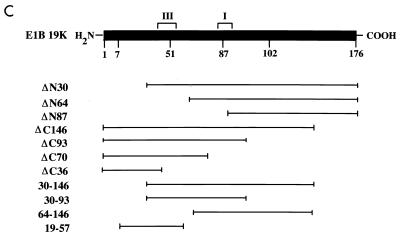
BP-1 interacts with E1B 19K in yeast. (A) The yeast two-hybrid assay was used to demonstrate binding between BP-1 and E1B 19K. Growth in the presence of histidine indicates that both plasmids can be expressed in yeast, and growth in the absence of histidine demonstrates an interaction between the two proteins. Apc-2 represents an irrelevant hydrophobic protein used as a negative control. The specificity of the interaction between BP-1 and E1B 19K was tested by using related proteins (Bcl-2, Bcl-xL, and Bax) as well as missense mutants of E1B 19K (pm7, pm51, pm87, and pm102). (B) The minimal regions of E1B 19K required for interaction with BP-1 were mapped by using a series of deletion mutants. (C) Schematic representation of E1B 19K showing the missense and deletion mutants tested in the two-hybrid assay. Regions I and III indicate the locations of the Bcl-2 homology domains BH1 and BH3, respectively. E1B 19K does not contain recognizable BH2 or BH4 domains.
The interaction between anti- and proapoptotic Bcl-2 family members generally occurs via their conserved domains, designated Bcl-2 homologous regions 1, 2, 3, and 4 (BH1, BH2, BH3, and BH4) (14, 15, 26, 33, 102). BH1 to BH3 are in close proximity, forming a hydrophobic cleft that is required for dimerization (55, 70). To determine the regions of E1B 19K that are required for interaction with BP-1, we tested its ability to bind a series of E1B 19K missense and deletion mutants (Fig. 1). These mutants have been characterized previously for binding to other E1B 19K binding proteins, including Bax, Nbk/Bik, lamin A/C, and Ced-4 (26–28, 65). Thus, these experiments allow us to compare the binding requirements within the E1B 19K protein with those of other 19K-associated proteins.
The E1B 19K protein may be divided into three regions: a moderately conserved N terminus which includes BH3, a highly conserved central region containing BH1, and a poorly conserved C terminus (14, 27, 96). It is important to note that most of the deletion mutants tested (ΔN30, ΔN64, ΔN87, ΔC93, ΔC70, 30–146, 30–93, and 64–136) failed to interact with BP-1 or with Bax, Nbk/Bik, and lamin A/C and that this may be due to abnormal protein folding or masking of the binding site(s) (Fig. 1B) (26, 27, 65). Like Bax, Nbk/Bik, and lamin A/C, binding of BP-1 was retained in ΔC146, which contains both BH1 and BH3 (26, 27, 65). The small E1B 19K fragment 19–57, containing only BH3, was also able to bind BP-1 (Fig. 1B). This fragment is also sufficient for binding to Bax as well as to Ced-4 (28). Surprisingly, we found that another small region of E1B 19K, ΔC36, also retained binding to BP-1 (Fig. 1B). This region did not bind to other E1B 19K-associated proteins, Bax, Nbk/Bik, lamin A/C, or Ced-4 (26–28, 65). The binding of BP-1 to the E1B 19K mutants ΔC36 and 19–57 suggests that the region of 19K immediately adjacent to BH3 (i.e., 19–36) may be sufficient for the interaction. This would represent a unique domain that contributes to protein-protein interactions and may correspond to the E1B 19K BH4, although the homology is weak.
We further addressed the binding specifications for BP-1 by using several point mutants (pm7, pm51, pm87, and pm102) that have previously been analyzed for interaction with E1B 19K-associated proteins as well as for their ability to inhibit apoptosis (14, 26–28, 96). While pm7 and pm102 retained the ability to bind to BP-1, replacement of either phenylalanine with serine at position 51 (pm51) or glycine with alanine at position 87 (pm87) resulted in a loss of binding (Fig. 1A). The lack of binding with pm51 is consistent with a role for BH3 in the interaction between E1B 19K and BP-1. Although the loss of binding with pm87 might suggest a role for BH1, it should be noted that the glycine residue at position 87 is absolutely conserved within the Bcl-2 family and is located in an integral region adjacent to the hydrophobic cleft which serves as the BH3 binding pocket (55, 70). Therefore, the pm87 mutant may interfere with the BH3 region and/or result in a highly misfolded protein. Indeed, pm87 is generally defective in binding and at inhibiting apoptosis. Thus, taken together, the mutational analyses indicate that the BH3 and the adjacent N-terminal sequences (possibly BH4) may play a more critical role in the interaction with BP-1. This binding profile overlaps but is distinct from that for Bax and Nbk/Bik and corresponds to a region of E1B 19K that is required for inhibition of apoptosis (26–28).
Characterization of BP-1.
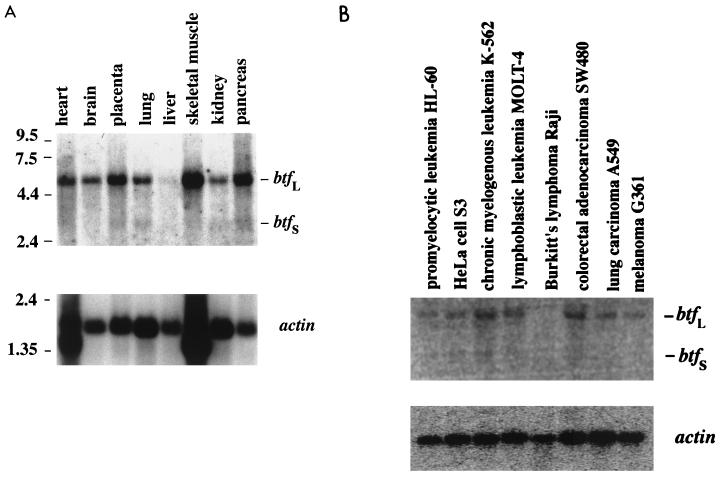
To determine the size and distribution of bp-1, we performed a Northern blot analysis with poly(A)+ RNA prepared from various tissues. Two transcripts which appeared to be ubiquitously expressed were detected at 5 and 3 kb, although only low levels were detected in the liver (Fig. 2A). The larger transcript appeared to be expressed more abundantly than the shorter form. Since the largest fragment of bp-1 obtained in the two-hybrid screen was only 1.5 kb, we sought to recover full-length cDNAs corresponding to bp-1. By using conventional library screening techniques along with database searches, we were able to identify both full-length transcripts, which were named btf (for Bcl-2-associated transcription factor). The 3-kb transcript, btfS, was isolated by screening a HeLa λ-cDNA library. The 3′ end of btfS was identical to bp-1 except that 147 bp were missing within the predicted coding sequence. While the 5-kb transcript, btfL, could not be recovered from the λ screen, it was identified as a full-length expressed sequence tag (EST) within GenBank (accession no. D79986) that was isolated from the human KG-1 cell line. Unlike btfS, btfL did contain the 147-bp region present in bp-1. Based on comparisons of sequences from GenBank with btfS, we found that the remainder of the coding sequences between btfS and btfL were the same and that the large size difference between the transcripts resulted from different 3′ untranslated regions.
FIG. 2.
Northern blotting was performed to determine btf expression in various tissues (A) and cancer cell lines (B). Two transcripts were observed, at 5 kb (btfL) and 3 kb (btfS). These transcripts appeared to be ubiquitously expressed, except that btf was not detected in Raji cells derived from Burkitt’s lymphoma. β-Actin expression was examined to assess the quality of the RNA and to control for loading efficiency.
The EST encoding BtfL was used to identify the subchromosomal location of btf. However, it remains to be determined whether btfL and btfS are formed from separate genes or are generated by alternative splicing of the same gene. Using the UniGene collection accessed through the National Center for Biotechnology Information, we found a 137-bp PCR fragment within the 3′ untranslated region of the EST (dbSTS entry G20483) that mapped to chromosome 6 between markers D6S292 and D6S1699. These markers correspond to 6q22-23, a locus with a high frequency of deletions in tumors, particularly lymphomas and leukemias (47). Thus, the locus of btf correlates with a chromosomal region that may contain a tumor suppressor gene. To test whether btf is actually deleted in tumors, we performed a Northern blot analysis with mRNA prepared from several human cancer cell lines. While both transcripts of btf were observed in most cell lines tested, they appeared to be less abundant than in normal cells, and btf was not detected in Raji cells, which were derived from Burkitt’s lymphoma (Fig. 2B). Indeed, Raji was the one cell line tested with known 6q deletions (62). The Northern blot data was therefore consistent with the chromosomal mapping of the gene. It will certainly be of interest to determine whether the loss of btf in Raji cells and possibly in other tumor cell lines actually contributes to tumor formation.
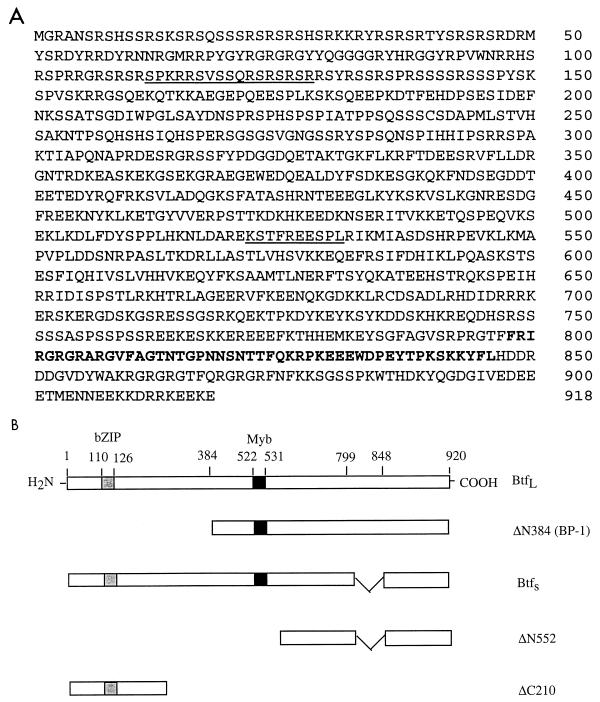
The primary amino acid sequences encoded by btfL and btfS are compared in Fig. 3. BtfL is 918 amino acids long and has a predicted molecular mass of 106 kDa, whereas BtfS is missing 49 amino acids near the carboxyl terminus (amino acids 797 to 846 of BtfL) and has a predicted size of 101 kDa (Fig. 3). The original bp-1 clone encodes amino acids 384 to 918 of BtfL (Btf ΔN384), which is slightly more than half of the full-length protein (Fig. 3B). In general, the 49-amino-acid region specific to BtfL and ΔN384 contains highly charged residues (48%), but the significance of this region is not known. Both BtfS and BtfL were able to bind E1B 19K in the yeast two-hybrid assay (data not shown). While the overall protein sequence of Btf is not significantly homologous to those of other known proteins, searches for conserved motifs in the PROSITE database revealed two nuclear localization signals (Fig. 3A) as well as putative DNA binding domains (Fig. 3B). There was 88% homology to the basic zipper (bZIP) DNA binding domain between amino acids 110 and 126 and 80% homology to the Myb DNA binding domain within amino acids 522 and 531 of Btf (Fig. 3B).
FIG. 3.
(A) Amino acid sequence of Btf. BtfL, identified in the GenBank database (accession no. D79986), is predicted to encode a 918-amino-acid protein, shown here. BtfS, which was obtained in the λ screen, contains the complete BtfL sequence except that it is missing 49 amino acids between residues 797 and 846 (bold) present in BtfL and BP-1. The underlined regions represent the positions of nuclear localization sequences. (B) A schematic representation of the Btf variants and deletion mutants used to characterize Btf function. The shaded and solid boxes represent the locations of putative bZIP and Myb-DNA binding domains, respectively. BP-1 (ΔN384), identified in the yeast two-hybrid screen for E1B 19K binding proteins, contains residues 384 to 918 of BtfL. Deletion mutants of BtfS, ΔN552, and ΔC210 were used in transcriptional reporter assays to determine the regions of BtfS that contribute to transcriptional repression.
BtfS is a nuclear DNA binding protein that is sequestered by the Bcl-2 family.
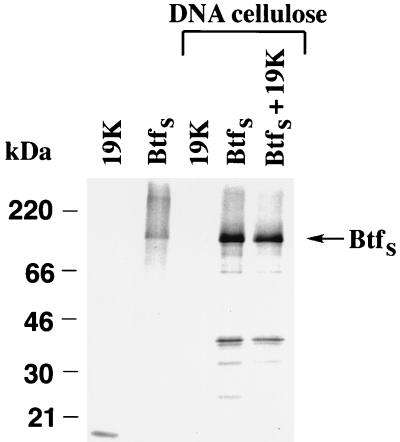
Since btfS, but not btfL, was isolated from the HeLa library, we concentrated our functional assays on the shorter variant. BtfS was cloned into the pcDNA3 vector with a Myc epitope for in vitro translation and mammalian expression. The protein sequence of BtfS suggested that it may bind to DNA. To test this hypothesis, [35S]methionine-labeled, in vitro-translated Myc-BtfS was incubated with native DNA-cellulose. DNA binding was detected with BtfS but not with E1B 19K, which was used as a negative control (Fig. 4). The strength of the interaction was comparable to that of a known transcription factor, Msx-1 (10), and was not competed by RNA (data not shown). In vitro-translated E1B 19K (Fig. 4) and Bcl-2 (data not shown) did not inhibit the ability of BtfS to bind to DNA, suggesting that the binding sites for DNA and for E1B 19K and Bcl-2 are in distinct domains within BtfS. Indeed, comparison of the putative DNA binding domains and the BP-1 (Btf ΔN384) protein which is sufficient for binding to the Bcl-2 family members is consistent with there being two separate domains.
FIG. 4.
BtfS binds to DNA-cellulose in vitro. [35S]methione-labeled, in vitro-translated Myc-BtfS and E1B 19K were prepared by using the TnT T7 reticulocyte lysate system and incubated with native DNA-cellulose in NETN buffer for 2 h. Samples were washed five times in NETN, and the proteins were resolved by SDS-PAGE (17% polyacrylamide). Immunoprecipitated proteins (lanes 1 and 2) were analyzed to confirm the presence of the in vitro-translated products.
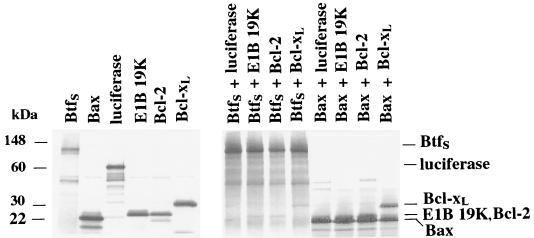
To confirm the association between BtfS and Bcl-2 family members, we performed an in vitro binding assay. [35S]methionine-labeled, in vitro-translated Myc-BtfS or Myc-Bax, used as a positive control, was combined with E1B 19K, Bcl-2, and Bcl-xL. Immunoprecipitation with an antibody against Myc revealed that BtfS bound to all three of the antiapoptotic Bcl-2 family members but not to luciferase, used a negative control (Fig. 5). However, these associations were weaker than those with Bax (Fig. 5). These results appear to support the data from the yeast two-hybrid assay.
FIG. 5.
In vitro interactions between BtfS and antiapoptotic Bcl-2 family members. [35S]methionine-labeled, in vitro-translated Myc-BtfS or Myc-Bax was combined with V5/His-E1B 19K, Bcl-2, Flag–Bcl-xL, or luciferase prepared by using the TnT T7 reticulocyte lysate system. The proteins were immunoprecipitated with anti-Myc monoclonal antibody in NETN buffer for 1.5 h followed by protein A-Sepharose for 0.5 h. Samples were washed in NETN buffer, resolved by SDS-PAGE (14% polyacrylamide), and visualized by autoradiography. In addition, 1 μl of each of translation reaction mixture was analyzed to verify that equal amounts of proteins were used in the binding assay.
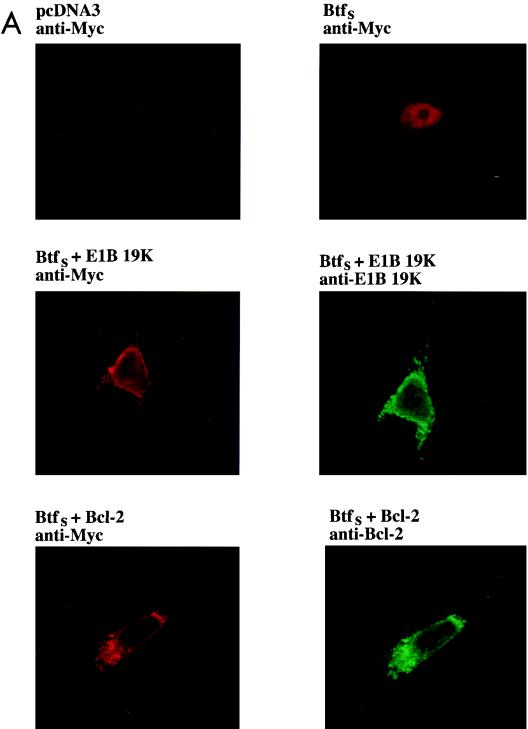
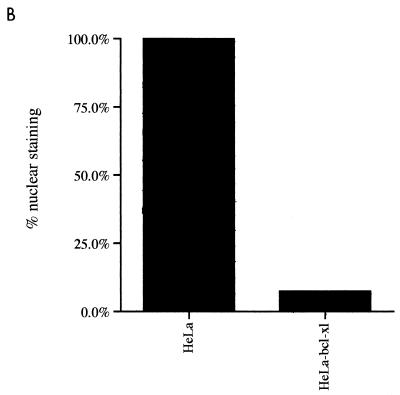
To examine the biological role of BtfS in vivo, we attempted to express the tagged protein in HeLa cells. While were unable to obtain stable expression of BtfS, even with an inducible promoter, we found that transient transfection did produce low levels of BtfS. Although the levels of BtfS were not high enough to be detected by Western blot analysis of whole-cell extracts (data not shown), which would allow us to examine whether BtfS could coimmunoprecipitate with Bcl-2 family members, we were able to visualize BtfS by immunofluorescence. Its expression was confined to the nucleus, and it was present in roughly 4% of the cells (Fig. 6A). This was consistent with the DNA binding activity but not with the established localization of Bcl-2-like proteins, which are generally found associated with membrane structures, particularly the mitochondria, endoplasmic reticulum, and nuclear envelope (22, 31, 54). Unlike other Bcl-2 family members, E1B 19K is not normally present in the mitochondria but, rather, is localized predominantly to the nuclear envelope (93). However, since the Bcl-2 family can act by sequestering proteins (25, 28, 63, 88), we checked whether E1B 19K, Bcl-2, and Bcl-xL could alter the subcellular localization of BtfS. First, E1B 19K and Bcl-2 were cotransfected with Myc-BtfS. The cells were fixed 24 h posttransfection and then double stained for the Myc epitope present on BtfS (tetramethylrhodamine isothiocyanate) and either E1B 19K or Bcl-2 (fluorescein isothiocyanate). While the percentage of BtfS-positive cells was similar to that when it was transfected by itself, the subcellular localization was altered. BtfS colocalized with E1B 19K and Bcl-2 within the cytoplasm and nuclear periphery in almost all coexpressing cells (Fig. 6A). Subsequent experiments were performed to determine if Bcl-xL could also sequester BtfS, since these proteins also bound in the yeast two-hybrid assay and in vitro. We could not costain for BtfS and Bcl-xL with the available antibodies, so we developed a stable HeLa cell line expressing Bcl-xL and stained for BtfS with the antibody against the Myc epitope. While control HeLa cells contained 100% nuclear BtfS expression, only 7% of the BtfS-positive cells in the HeLa–Bcl-xL cell line displayed nuclear BtfS staining and almost all of the BtfS staining was in the cytoplasm in a pattern similar to Bcl-xL (Fig. 6B). The few cells expressing nuclear BtfS may be accounted for by the variable levels of Bcl-xL observed in this cell line and/or by an incomplete ability of Bcl-xL to sequester BtfS. Nevertheless, this is in clear contrast to the parental HeLa cells, where the expression of BtfS was completely nuclear. These results suggest that E1B 19K, Bcl-2, and Bcl-xL can alter the localization, and therefore perhaps the function, of BtfS.
FIG. 6.
Nuclear subcellular localization of BtfS is altered by E1B 19K, Bcl-2, and BtfL. (A) HeLa cells were transfected with expression plasmid pcDNA3-Myc-BtfS alone or with pCMV E1B 19K or pcDNA3–Bcl-2 as indicated. The cells were fixed 24 h posttransfection and double stained against Myc and E1B 19K or Bcl-2. Magnification, ×1,000. (B) HeLa cells expressing Bcl-xL were transfected with pcDNA3-Myc-BtfS and stained against Myc 24 h posttransfection. Values represent the percentages of nuclear BtfS expression (BtfS/total BtfS) in these cells.
BtfS represses transcription which is inhibited by the Bcl-2 family.
Since BtfS was shown to bind DNA, we checked whether it could modulate transcription. We first performed a one-hybrid assay in yeast by generating a fusion protein of BtfS with the GAL4 DNA binding domain in the pGBT9 vector. If BtfS contained a trans-activation domain, one would expect that the adjacent activation and DNA binding domains would lead to GAL4-inducible phenotypes in yeast. Growth was not detected in the absence of histidine, suggesting that BtfS does not contain a transcriptional activation domain (data not shown). However, it remains possible that BtfS requires mammalian cofactors to activate transcription or that BtfS represses, rather than activates, transcription.
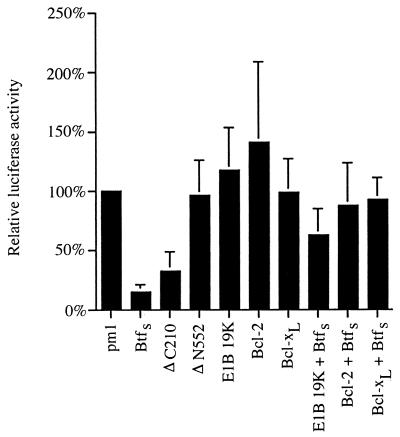
To address these issues, BtfS was cloned into a mammalian expression vector (pm1) containing the GAL4 DNA binding domain. Transcriptional activity was monitored with a reporter construct containing GAL4 promoter sites. Transient expression of pm1-BtfS with the luciferase reporter led to nearly a 10-fold decrease in transcription compared to that for an empty pm1 vector control (Fig. 7). This effect is comparable to the trans-repressive activity of other proteins, such as p53 and Msx-1 (10, 66). Two deletion mutants of BtfS, ΔN522 (amino acids 522 to 918 without residues 797 to 846) and ΔC210 (amino acids 1 to 210), were also cloned into pm1 to determine the general regions that may contribute to transcriptional repression (Fig. 3B). Both deletion mutants of BtfS were detectably expressed in HeLa cells to levels comparable to wild-type BtfS (data not shown). While BtfS ΔN552 was not able to repress transcription, the ΔC210 mutant was sufficient for repression and nearly as potent as full-length BtfS (Fig. 7). Interestingly, this fragment, which contains the bZIP homology segment, is also rich in serine and glycine residues, a feature that is present in other transcriptional repressors (11, 41, 59, 101).
FIG. 7.
Reporter assay demonstrating that BtfS is a transcription repressor and is inhibited by Bcl-2-like proteins. HeLa cells were transfected with 2.5 μg of the reporter construct (luciferase construct containing GAL4 DNA binding sites within its promoter), 2.5 μg of GAL4 DNA binding domain fusion genes (pm1-BtfS, pm1-ΔN552, and pm1-ΔC210 or empty pm1 vector control), and 2.5 μg of bcl-2 family gene (pCMV-E1B 19K, pcDNA3–Bcl-2, pcDNA3-Flag–Bcl-xL, or empty pcDNA3 vector control). The cells were harvested 24 hours posttransfection, and the luciferase activity was measured in a scintillation counter with the luciferase substrate luciferin. Values were normalized for protein concentrations measured by the Bradford assay and graphed as a percentage of the result for the negative control (empty pm1 vector). The experiment was performed six times, and the high and low values for each sample were dropped. Bars indicate standard deviations (n = 4).
Since the Bcl-2-like proteins were capable of sequestering BtfS in the cytoplasm, we hypothesized that they may also block the ability of BtfS to repress transcription. To test this possibility, we cotransfected E1B 19K, Bcl-2, and Bcl-xL with pm1-BtfS and the luciferase reporter construct. In the control samples, none of the members of the Bcl-2 family of proteins had a significant effect on transcription of the luciferase reporter (Fig. 7). However, transfection of any of the three Bcl-2 family members (E1B 19K, Bcl-2, and Bcl-xL), but not the empty pcDNA3 vector, abrogated BtfS-mediated transcriptional repression. These data suggest that E1B 19K, Bcl-2, and Bcl-xL inhibit BtfS trans-repression, probably by binding to and sequestering BtfS in the cytoplasm.
Sustained expression of BtfS inhibits transformation.
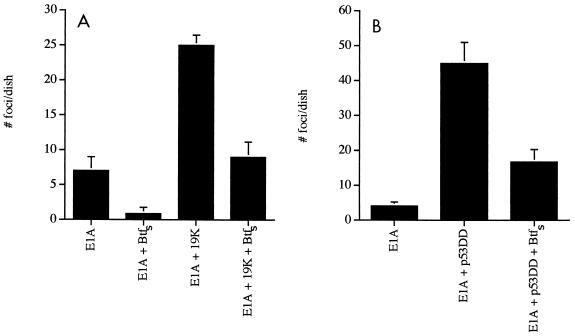
Transfection of the gene for adenovirus E1A along with the gene for E1B 19K or bcl-2 in primary BRK cells induces transformation as a consequence of dual proliferative and antiapoptotic signaling (17, 64). Expression of E1B 19K/Bcl-2 binding proteins, such as Bax and Nbk/Bik, antagonizes the antiapoptotic signal and causes a reduction in focus formation (26, 27). To determine if BtfS could also antagonize the ability of E1B 19K to transform primary cells, we transfected E1A and E1B 19K, with and without Myc-BtfS, into primary BRK cells. BtfS caused a 64% reduction in focus formation mediated by E1A and E1B 19K (Fig. 8A). This suggests that interaction between BtfS and E1B 19K inhibits 19K function in vivo and/or that BtfS is capable of suppressing transformation independently of 19K. The reduction in focus formation by BtfS and E1A compared to E1A alone suggested that the latter scenario is possible (Fig. 8A). However, we tested whether BtfS could inhibit another transforming signal, E1A with p53DD. p53DD is a p53 deletion mutant that contains the C-terminal oligomerization domain and functions in a dominant negative fashion to inhibit p53-mediated apoptosis and growth arrest (75). Since p53DD blocks both of these processes, it produces a very potent transforming signal (67). Here we show that expression of BtfS was capable of repressing focus formation by E1A and p53DD by about 60%, suggesting that btfS may act as a general suppressor of transformation (Fig. 8B).
FIG. 8.
BtfS inhibits transformation by E1A and E1B 19K (A) or p53DD (B). Primary BRK cells were transfected with carrier DNA along with a linearized test DNA (15 μg of pCMV-E1A, 15 μg of pCMV-E1B 19K or pCMV-p53DD, and 45 μg of pcDNA3-Myc-BtfS). DNA concentrations were kept constant by using appropriate empty vectors. The cells were cultured for 3 to 4 weeks and then stained with Giemsa. Foci were counted from four dishes per condition. Bars indicate standard deviations (n = 4).
BtfS functions to induce apoptosis.
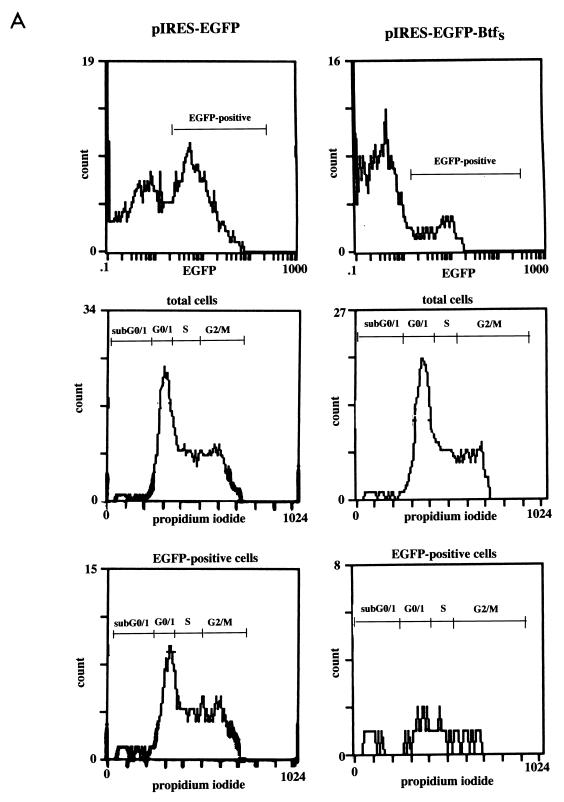
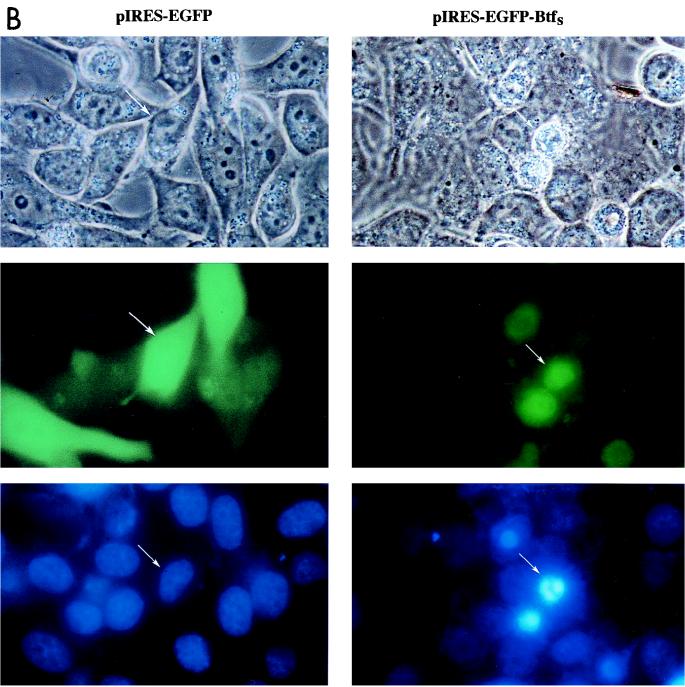
To further characterize the transformation-suppressing activity of BtfS, we tested the effect of its expression on apoptosis and cell cycle progression. Attempts to generate stable BRK or HeLa cell lines expressing BtfS failed, indicating that sustained BtfS expression is incompatible with either cell proliferation or viability. To monitor BtfS expression in transient-transfection assays, the cDNA was cloned into the pIRES-EGFP vector, which provides coexpression of BtfS and the EGFP marker. HeLa cells were transiently transfected and then harvested 48 and 72 h posttransfection. The cell cycle kinetics were analyzed by FACS analysis following propidium iodide staining. Since EGFP staining with BtfS was not detectable until 48 h posttransfection, we were unable to characterize the cell cycle characteristics at the earlier time points. The levels of EGFP expression and the cell cycle kinetics at 48 and 72 h are shown in Table 1. At 48 h posttransfection, there were only 19.5% EGFP-positive cells in the presence of BtfS whereas there were 61.2% positive cells in the control pIRES-EGFP empty vector. The FACS analysis demonstrated that BtfS led to an increase in the percentage of sub-G0/1 cells from 3.4 to 15.8%, indicating an increase in cell death. No other obvious changes in cell cycle parameters were observed at this time point. To test whether E1B 19K could inhibit this cell death, we cotransfected pIRES-EGFP-BtfS with pCMV-E1B 19K. E1B 19K inhibited BtfS-mediated cell death at 48 h as indicated by an increase in the percentage of EGFP-expressing cells from 19.5 to 34.3% and a decrease in the percentage of sub-G0/1 cells from 15.8 to 10.1% (Table 1). Thus, E1B 19K expression abrogated cell death induced by BtfS. At 72 h posttransfection, the degree of cell death triggered by BtfS increased and could no longer be inhibited by E1B 19K. In the presence of BtfS, there were only 13.2% EGFP- positive cells, 29.1% of which were represented in the sub-G0/1 peak (Fig. 9A). The 72-h time point also revealed a decrease in the G2/M peak in the presence of BtfS (16.7%) compared to the control vector (40.6%). The change in the G2/M peak as a result of BtfS may be an indication that cells are exiting from these phases of the cell cycle to go into apoptosis.
TABLE 1.
Overexpression of BtfS induces cell deatha
| Vectors | 48 h posttransfection
|
72 h posttransfection
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % EGFP positive | % Sub-G0/1 | % G0/1 | % S | % G2/M | % EGFP positive | % Sub-G0/1 | % G0/1 | % S | % G2/M | |
| pIRES-EGFP + pcDNA3 | 61.2 | 3.4 | 42.3 | 20.0 | 33.7 | 45.7 | 5.6 | 32.5 | 20.9 | 40.6 |
| pIRES-EGFP + pCMV-E1B 19K | 72.0 | 1.5 | 54.2 | 21.0 | 23.6 | 59.0 | 8.8 | 55.4 | 20.8 | 14.8 |
| pIRES-EGFP-BtfS + pcDNA3 | 19.5 | 15.8 | 36.7 | 21.7 | 25.6 | 13.2 | 29.1 | 33.2 | 20.6 | 16.7 |
| pIRES-EGFP-BtfS + pCMV-E1B 19K | 34.3 | 10.1 | 41.8 | 17.8 | 30.0 | 15.4 | 36.9 | 36.0 | 13.3 | 13.6 |
HeLa cells were electroporated with 20 μg of pIRES-EGFP-BtfS or empty vector in combination with 10 μg of pCMV-E1B 19K or empty pcDNA3 vector. The cells were harvested 48 and 72 h posttransfection, fixed in 2% paraformaldehyde, and stained with PBS containing 1 μg of propidium iodide per ml, 250 μg of RNase A per ml, and 0.1% Tween 20. The cells were incubated for at least 30 min at room temperature and then analyzed by FACS. The results show the percentage of EGFP-positive cells and the cell cycle kinetics for the EGFP-positive population.
FIG. 9.
BtfS-mediated cell death occurs by apoptosis. (A) Representative FACS scan from Table 1 at 72 h after transfection of pIRES-EGFP-BtfS and the empty pIRES-EGFP vector into HeLa cells. Cell cycle kinetics from propidium iodide staining are shown for the total cell population as well as the EGFP-positive cells. (B) Transfected cells were incubated with Hoechst dye 72 h posttransfection to visualize the DNA. Cells transfected with pIRES-EGFP-BtfS (right) were compared to those transfected with control pIRES-EGFP (left). Arrows for each set correspond to the same cell visualized with bright field (top), EGFP (green; middle), and Hoechst dye (blue; bottom). The presence of condensed chromatin in the presence of BtfS indicates cell death by apoptosis. Original magnification, ×1,000.
Interestingly, we also observed a decrease in the G2/M peak with E1B 19K in the absence of BtfS. At 72 h posttransfection, there was a change in the number of cells in G2/M from 40.6% with the control vector to 14.8% in E1B 19K-transfected cells. Changes in cell cycle progression due to the antiapoptotic Bcl-2 family members have been observed previously; i.e., they generally produce a decrease in cell cycle progression (6, 33, 39, 83, 92). While others have shown that Bcl-2 causes an increase in the percentage of G0/1 cells (33, 44, 83), we show here that E1B 19K causes a decrease in the G2/M peak. Thus, these results may reflect a difference in the mechanism of cell cycle regulation by E1B 19K and that by other, related proteins.
Cell death has been classified as either necrosis or apoptosis, where necrosis is considered a passive process of cell death associated with trauma and apoptosis is a genetically programmed active response leading to cell suicide (100). Unlike necrosis, apoptosis involves morphological changes such as chromatin condensation, DNA fragmentation, and cytoplasmic blebbing. To test whether the increase in the percentage of sub-G0/1 cells as a result of BtfS was due to apoptosis, we examined the nuclei of EGFP-positive cells by staining with Hoechst dye 72 h posttransfection. Chromatin condensation observed by this approach would be one indication of apoptosis. HeLa cells transfected with the empty pIRES-EGFP vector showed EGFP-stained cells with normal nuclei. However, in cells transfected with pIRES-EGFP-BtfS, the EGFP-positive cells had condensed chromatin staining, indicating that they were dying by apoptosis (Fig. 9B). Taken together, these reults suggest that BtfS can function to promote apoptosis, which may account for its ability to suppress transformation. E1B 19K can inhibit BtfS-induced apoptosis, albeit incompletely in some assays. Nonetheless, this suggests that the Bcl-2 family can affect apoptosis through modulation of transcription.
DISCUSSION
The Bcl-2 family regulates apoptosis through multiple mechanisms. For example, these proteins may function at the mitochondria by forming channels that regulate mitochondrial membrane potential and cytochrome c release. Alternatively, the Bcl-2 family may directly regulate caspase activation through interactions with Ced-4-like proteins. We describe a novel E1B 19K-interacting protein, Btf, isolated through a two-hybrid screen, that regulates an alternative pathway to control apoptosis. Two transcripts corresponding to btf, btfS and btfL, were identified. Both appeared to be widely expressed but were deleted in some tumors. In this paper, we describe the function and biological significance of the protein product generated from the shorter form, BtfS, which differs from BtfL in just 49 amino acids in the C-terminal region.
Double-staining experiments were able to show the colocalization of BtfS with E1B 19K, Bcl-2, and Bcl-xL, supporting the interactions observed in the yeast two-hybrid assay as well as by in vitro coimmunoprecipitation. While transiently expressed BtfS was nuclear and could be sequestered into the cytoplasm by the antiapoptotic Bcl-2 family members, the localization of endogenous BtfS remains to be determined. It is entirely possible that in normal, nonapoptotic cells BtfS is expressed predominantly in the cytoplasm. In contrast, in dying cells, where there is a higher percentage of proapoptotic than of antiapoptotic Bcl-2 family members, BtfS may translocate to the nucleus and potentiate apoptosis.
BtfS was able to bind DNA in vitro, and it repressed transcription in reporter assays. The trans-repressive activity was inhibited by E1B 19K, Bcl-2, and Bcl-xL, correlating with their cytoplasmic sequestration potentials. While it remains possible that part of this phenomenon is a result of BtfS-induced apoptosis, the degree of cell death observed at 24 h would not account for the 10-fold reduction in transcriptional activity. The evidence that BtfS is a transcription repressor was further supported by the identification of the ΔC210 deletion mutant that was sufficient for repressing transcription. The N-terminal fragment of BtfS is rich in glycine and serine, a feature that is common to transcriptional repressors (11, 41, 59, 101). Taken together, these data provide evidence for a novel trans-repressive protein that triggers apoptosis and is sequestered and inhibited by Bcl-2 family members.
One of the features of the Bcl-2 family exemplified by the BtfS interaction is their ability to sequester other cellular proteins from their normal subcellular localization. This process enables E1B 19K and Bcl-2 to inhibit apoptosis by using more than one cellular pathway. The best-characterized example concerns the association between proapoptotic and antiapoptotic Bcl-2 family members. While these interactions have been known for several years, recent studies of E1B 19K and Bax have demonstrated that these family members can alter each other’s subcellular localization (25). Bax is normally stimulated to go to the mitochondria during apoptosis and causes a loss of mitochondrial membrane potential (97). However, overexpression of E1B 19K causes Bax to be sequestered to the nuclear periphery, where E1B 19K is localized (25). This process most probably blocks the ability of Bax to disrupt mitochondrial function. The Bcl-2 family also associates with several unrelated proteins that may contribute to apoptosis regulation. For example, Bcl-2 associates with and targets the serine/threonine kinase Raf-1 to the mitochondria (88). Overexpression of Bcl-2 and that of Raf-1 cooperate to inhibit apoptosis. Another example that is now emerging is the association between the antiapoptotic Bcl-2 family members with Caenorhabditis elegans Ced-4 (12, 28, 79, 98) and with its mammalian homologue Apaf-1 (32, 61, 104). These interactions directly block the activation of downstream caspases. Thus far, the C. elegans Bcl-2 homologue, Ced-9, and Bcl-xL and E1B 19K have been shown to bind to Ced-4, and at least Ced-9 and E1B 19K can redistribute Ced-4 from the cytosol to the cytoplasmic membranes (28, 99). Bcl-xL also interacts with Apaf-1, and therefore it will be interesting to determine whether Bcl-xL can alter the localization of Apaf-1 (32, 61). In what is perhaps an analogous scenario, E1B 19K also sequesters the death-promoting protein FADD, an upstream component of the Fas- and TNF-α-mediated death signaling pathway (63). Overexpressed FADD becomes multimerized and produces filaments throughout the cell (63, 78). E1B 19K disrupts the FADD filaments, causing FADD to relocalize in regions normally associated with 19K, and inhibits FADD-dependent apoptosis (63). Here, we show that the Bcl-2 family members also sequester a nuclear transcription factor and that this may also play a role in apoptosis. The minimal region for E1B 19K required for interaction with Btf at the amino terminus (amino acids 1 to 36) appears to be distinct from the interaction regions involved in binding to other proapoptotic proteins (Bax, Ced-4, and Nbk/Bik). This amino-terminal region of E1B 19K may correspond to BH4 of Bcl-2 and Bcl-xL, although this remains to be tested directly. Together, these studies suggest that E1B 19K and other Bcl-2 family members act as binding proteins for a number of apoptosis regulators, which could contribute to their widespread role as apoptosis inhibitors.
Transcriptional regulation often plays a critical role during apoptosis by either activating or repressing genes encoding basic apoptotic components. Indeed, inhibition of RNA and protein synthesis blocks apoptosis induced by a number of circumstances, including growth factor deprivation (42, 73) and treatment with some chemotherapeutic drugs (2, 51, 86). In contrast, others have shown that these inhibitors can actually promote cell death, suggesting that the loss of a short-lived survival factor can also lead to apoptosis (3, 43, 66, 85). A number of transcription factors that may serve as positive or negative regulators of apoptosis have been identified. For example, the NF-κB transcription factor plays an important role in blocking apoptosis triggered by TNF-α (4, 84, 87). However, in other situations, NF-κB activation has been associated with induction of apoptosis (23). Furthermore, Bcl-2 and E1B 19K repress NF-κB activity, providing another mechanism for Bcl-2 family members to control apoptosis (23, 34, 72).
Another transcription factor that may be regulated by the Bcl-2 family during apoptosis is p53 (reviewed in reference 38). The p53 tumor suppressor protein is required for apoptosis during administration of ionizing radiation and chemotherapeutic drugs, as well as by transforming oncogenes such as c-myc and the E1A gene (16, 17, 30, 40). While p53 may have multiple functions, its transcriptional activity is clearly critical for the regulation of cell death in some (68) but not all (9, 29) situations. However, it is still unclear whether p53-mediated apoptosis requires its transcriptional activation or repression properties or perhaps both. On the one hand, p53 trans-activates both bax (26, 49) and fas (60), both of which are bona fide inducers of apoptosis. In contrast, several p53-repressible genes which may potentially contribute to apoptosis, including bcl-2 (48), MAP4 (56), the interleukin-6 gene (69), c-fos (37), and c-myc (52), have been identified. Expression of Bcl-2 and of E1B 19K alleviates the transcriptional repression activity of p53, providing a mechanism for Bcl-2 family regulation of apoptosis (66, 76). Thus, Bcl-2 family members can influence apoptosis by modulating the transcriptional activity of both NF-κB and p53. Inhibition of BtfS-mediated transcriptional repression and apoptosis by the Bcl-2 family establishes another connection between these apoptosis regulators and modulation of transcription. It will certainly be interesting to determine whether BtfS can influence the activities of other transcription factors related to apoptosis, such as NF-κB and p53.
We would predict that BtfS may repress the transcription of survival genes. Based on previous observations of regulation of gene expression by E1B 19K, one possible target may be the p53 inhibitor, Mdm-2. We have previously observed that stable expression of E1B 19K or Bcl-2 leads to an increase in Mdm-2 mRNA and protein levels (82). Such a mechanism could also account for the ability of E1B 19K to derepress the transcriptional repression mediated by p53 and E1A (56, 66, 76, 103). While it will be worthwhile to determine whether BtfS could affect the p53-mediated modulation of these targets, the fact that BtfS was capable of inhibiting transformation by p53DD suggests that another target, which is independent of p53, also exists. We also have not been able to detect binding between BtfS and p53 (data not shown), although it is still plausible that BtfS associates with p53-dependent coactivators such as p300. Since the putative DNA binding sites within BtfS contain homology to bZIP and Myb, it is conceivable that they have similar targets. Interestingly, correlations with apoptotic regulation exist for both of these transcription factor families (reviewed in references 36 and 89). For example, application of functional blocking antibodies against the AP-1 proteins, Fos and Jun, or transfection with dominant-interfering Jun inhibits apoptosis in neuronal cells following growth factor withdrawal (18, 24). However, relevant target genes that contribute to AP-1-mediated apoptosis have yet to be identified. The Myb family of transcriptional regulators inhibits apoptosis and regulates the transcriptional activation of bcl-2 (21, 81). This may therefore provide a feedback loop between BtfS and Bcl-2. Future studies will be performed to determine whether BtfS regulates bcl-2 or other genes involved in apoptosis. The link between BtfS and the Bcl-2 family may provide an alternative mechanism by which the Bcl-2 family is able to regulate cell survival.
ACKNOWLEDGMENTS
We thank Gabriel Nuñez for bcl-xL plasmids, S. Korsmeyer for the pSFFV–Bcl-2 plasmid, and Cory Abate-Shen for luciferase assay plasmids and reagents. In addition, we thank Christina DeCoste and Edward Yurkow for their technical assistance with the FACS analysis. We also thank Marjorie Moore for generating the HeLa–Bcl-xL cell lines, Arun Gaur for constructing the pcDNA3.1/V5/His-TOPO-E1B 19K plasmid, and Denise Perez and Kurt Degenhardt for their advice and critical reading of the manuscript.
This work was supported by grants from the National Institutes of Health (CA64807 and CA53370) to E. White and by the Howard Hughes Medical Institute.
REFERENCES
- 1.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J-J, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou J-C. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 2.Barry M A, Behnke C A, Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990;40:2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- 3.Bazer L S, Deeg H J. Ultraviolet B-induced DNA fragmentation (apoptosis) in activated T-lymphocytes and Jurkat cells is augmented by inhibition of RNA and protein synthesis. Exp Hematol. 1992;20:80–86. [PubMed] [Google Scholar]
- 4.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 5.Boise L H, Gonzalez-Garcia M, Postema C E, Ding L, Lindsten T, Turka L A, Mao X, Nuñez G, Thompson C. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 6.Borner C. Diminished cell proliferation associated with the death-protective activity of Bcl-2. J Biol Chem. 1996;271:12695–12698. doi: 10.1074/jbc.271.22.12695. [DOI] [PubMed] [Google Scholar]
- 7.Boyd J, Malstrom S, Subramanian T, Venkatesh L, Schaeper U, Elangovan B, D’Sa-Eipper C, Chinnadurai G. Adenovirus E1B 19kDa and bcl-2 proteins interact with a common set of cellular proteins. Cell. 1994;79:341–351. doi: 10.1016/0092-8674(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 8.Boyd J M, Gallo G J, Elangovan B, Houghton A B, Malstrom S, Avery B J, Ebb R G, Subramanian T, Chittenden T, Lutz R J, Chinnadurai G. Bik1, a novel death-inducing protein, shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11:1921–1928. [PubMed] [Google Scholar]
- 9.Caelles C, Helmberg A, Karin M. p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature (London) 1994;370:220–223. doi: 10.1038/370220a0. [DOI] [PubMed] [Google Scholar]
- 10.Catron K M, Wang H, Hu G, Shen M M, Abate-Shen C. Comparison of MSX-1 and MSX-2 suggests a molecular basis for functional redundancy. Mech Dev. 1996;55:185–199. doi: 10.1016/0925-4773(96)00503-5. [DOI] [PubMed] [Google Scholar]
- 11.Catron K M, Zhang H, Marshall S C, Inostroza J A, Wilson J M, Abate C. Transcriptional repression by Msx-1 does not require homeodomain DNA-binding sites. Mol Cell Biol. 1995;15:861–871. doi: 10.1128/mcb.15.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinnaiyan A M, O’Rourke K, Lane B R, Dixit V M. Interaction of CED-4 with CED-3 and CED-9: a molecular framework for cell death. Science. 1997;275:1122–1126. doi: 10.1126/science.275.5303.1122. [DOI] [PubMed] [Google Scholar]
- 13.Chiou S-K, Rao L, White E. Bcl-2 blocks p53-dependent apoptosis. Mol Cell Biol. 1994;14:2556–2563. doi: 10.1128/mcb.14.4.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiou S-K, Tseng C C, Rao L, White E. Functional complementation of the adenovirus E1B 19K protein with Bcl-2 in the inhibition of apoptosis in infected cells. J Virol. 1994;68:6553–6566. doi: 10.1128/jvi.68.10.6553-6566.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chittenden T, Flemmington C, Houghton A B, Ebb R G, Gallo G J, Elangovan B, Chinnadurai G, Lutz R J. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke A R, Purdie C A, Harrison D J, Morris R G, Bird C C, Hooper M L, Wyllie A H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature (London) 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 17.Debbas M, White E. Wild-type p53 mediates apoptosis by E1A which is inhibited by E1B. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 18.Estus S, Zaks W J, Freeman R S, Gruda M, Bravo R, Johnson E M., Jr Altered gene expression in neurons during programmed cell death: identification of c-jun as necessary for neuronal apoptosis. J Cell Biol. 1994;127:1717–1727. doi: 10.1083/jcb.127.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrow S N, White J H M, Martinou I, Raven T, Pun K-T, Grinham C J, Martinou J-C, Brown R. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature (London) 1995;374:731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Sarabia M J, Bischoff J R. Bcl-2 associates with the ras-related protein R-ras p23. Nature (London) 1994;366:274–275. doi: 10.1038/366274a0. [DOI] [PubMed] [Google Scholar]
- 21.Frampton J, Ramqvist T, Graf T. v-Myb of E26 leukemia virus up-regulates bcl-2 and suppresses apoptosis in myeloid cells. Genes Dev. 1996;10:2720–2731. doi: 10.1101/gad.10.21.2720. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Garcia M, Perez-Ballestero R, Ding L, Duan L, Boise L H, Thompson C B, Nuñez G. bcl-XL is the major bcl-x mRNA form expressed during murine development and its product localizes to mitochondria. Development. 1994;120:3033–3042. doi: 10.1242/dev.120.10.3033. [DOI] [PubMed] [Google Scholar]
- 23.Grimm S, Bauer M K A, Baeuerle P A, Schulze-Osthoff K. Bcl-2 down-regulates the activity of transcription factor NF-κB induced upon apoptosis. J Cell Biol. 1996;134:13–23. doi: 10.1083/jcb.134.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ham J, Babij C, Whitfield J, Pfarr C M, Lallemand D, Yaniv M, Rubin L L. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 25.Han J, Modha D, White E. Interaction of E1B 19K with Bax is required to block Bax-induced loss of mitochondrial membrane potential and apoptosis. Oncogene. 1998;17:2993–3005. doi: 10.1038/sj.onc.1202215. [DOI] [PubMed] [Google Scholar]
- 26.Han J, Sabbatini P, Perez D, Rao L, Modha D, White E. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev. 1996;10:461–477. doi: 10.1101/gad.10.4.461. [DOI] [PubMed] [Google Scholar]
- 27.Han J, Sabbatini P, White E. Induction of apoptosis by human Nbk/Bik, a BH3-containing protein that interacts with E1B 19K. Mol Cell Biol. 1996;16:5857–5864. doi: 10.1128/mcb.16.10.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han J, Wallen H D, Nuñez G, White E. E1B 19,000-molecular-weight protein interacts with and inhibits Ced-4-dependent, FLICE-mediated apoptosis. Mol Cell Biol. 1998;18:6052–6062. doi: 10.1128/mcb.18.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haupt Y, Rowan S, Shaulian E, Vousden K, Oren M. Induction of apoptosis in HeLa cells by transactivation-deficient p53. Genes Dev. 1995;9:2170–2183. doi: 10.1101/gad.9.17.2170. [DOI] [PubMed] [Google Scholar]
- 30.Hermeking H, Eick D. Mediation of c-Myc-induced apoptosis by p53. Science. 1994;265:2091–2093. doi: 10.1126/science.8091232. [DOI] [PubMed] [Google Scholar]
- 31.Hockenbery D, Nuñez G, Milliman C, Schreiber R D, Korsmeyer S. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature (London) 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y, Benedict M A, Wu D, Inohara N, Nuñez G. Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc Natl Acad Sci USA. 1998;95:4386–4391. doi: 10.1073/pnas.95.8.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang D C, O’Reilly L A, Strasser A, Cory S. The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO J. 1997;16:4628–4638. doi: 10.1093/emboj/16.15.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivanov V N, Deng G, Podack E R, Malek T R. Pleiotropic effects of Bcl-2 on transcription factors in T cells: potential role of NF-κB p50-p50 for the anti-apoptotic function of Bcl-2. Int Immunol. 1995;7:1709–1720. doi: 10.1093/intimm/7.11.1709. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson M D, Weil M, Raff M C. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 36.Karin M, Zg L, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 37.Kley N, Chung R Y, Fay S, Loeffler J P, Seizinger B R. Repression of the basal c-fos promoter by wild-type p53. Nucleic Acids Res. 1992;20:4083–4087. doi: 10.1093/nar/20.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 39.Linette G P, Li Y, Roth K, Korsmeyer S J. Cross talk between cell death and cell cycle progression: BCL-2 regulates NFAT-mediated activation. Proc Natl Acad Sci USA. 1996;93:9545–9552. doi: 10.1073/pnas.93.18.9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowe S W, Schmitt E M, Smith S W, Osborne B A, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature (London) 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 41.Madden S L, Cook D M, Rauscher F J D. A structure-function analysis of transcriptional repression mediated by the WT1, Wilm’s tumor suppressor protein. Oncogene. 1993;8:1713–1720. [PubMed] [Google Scholar]
- 42.Martin D P, Schmidt R E, DiStefano P S, Lowry O H, Carter J G, Johnson E M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988;106:829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin S J, Lennon S V, Bonham A M, Cotter T G. Induction of apoptosis (programmed cell death) in human HL-60 cells by inhibition of RNA or protein synthesis. J Immunol. 1990;145:1859–1867. [PubMed] [Google Scholar]
- 44.Mazel S, Burtrum D, Petrie H T. Regulation of cell division cycle progression by bcl-2 expression: a potential mechanism for inhibition of programmed cell death. J Exp Med. 1996;183:2219–2226. doi: 10.1084/jem.183.5.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merino R, Grillot D A, Simonian P L, Muthukkumar S, Fanslow W C, Bondada S, Nuñez G. Modulation of anti-IgM-induced B cell apoptosis by Bcl-xL and CD40 in WEHI-231 cells. Dissociation from cell cycle arrest and dependence on the avidity of the antibody-IgM receptor interaction. J Immunol. 1995;155:3830–3838. [PubMed] [Google Scholar]
- 46.Minn A J, Vélez P, Schendel S L, Liang H, Muchmore S W, Fesik S W, Fill M, Thompson C B. Bcl-XL forms an ion channel in synthetic lipid membranes. Nature (London) 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 47.Mitelman F. Catalog of chromosome aberrations in cancer. New York, N.Y: Wiley-Liss; 1995. [Google Scholar]
- 48.Miyashita T, Krajewski S, Krajewska M, Wang H G, Lin H K, Liebermann D A, Hoffman B, Reed J C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–1805. [PubMed] [Google Scholar]
- 49.Miyashita T, Reed J C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 50.Miyashita T, Inoue M U T, Reed J C, Yamada M. Bcl-2 relieves the trans-repressive function of the glucocorticoid receptor and inhibits the activation of CPP32-like cysteine proteases. Biochem Biophys Res Commun. 1997;233:781–787. doi: 10.1006/bbrc.1997.6559. [DOI] [PubMed] [Google Scholar]
- 51.Mizumoto K, Rothman R J, Farber J L. Programmed cell death (apoptosis) of mouse fibroblasts is induced by the topoisomerase II inhibitor etoposide. Mol Pharmacol. 1994;46:890–895. [PubMed] [Google Scholar]
- 52.Moberg K H, Tyndall W A, Hall D J. Wild-type murine p53 represses transcription from the murine c-myc promoter in a human glial cell line. J Cell Biochem. 1992;49:208–215. doi: 10.1002/jcb.240490213. [DOI] [PubMed] [Google Scholar]
- 53.Momand J, Zambetti G P, Olson D C, George D, Levine A J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 54.Monagan P, Robertson D, Amos T A, Dyer M J, Mason D Y, Greaves M F. Ultrastructural localization of Bcl-2 protein. J Histochem Cytochem. 1992;40:1819–1825. doi: 10.1177/40.12.1453000. [DOI] [PubMed] [Google Scholar]
- 55.Muchmore S W, Sattler M, Liang H, Meadows R P, Harlan J E, Yoon H S, Nettesheim D, Chang B S, Thompson C B, Wong S-L, Ng S-C, Fesik S W. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature (London) 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 56.Murphy M, Hinman A, Levine A J. Wild-type p53 negatively regulates the expression of a microtubule-associated protein. Genes Dev. 1996;10:2971–2980. doi: 10.1101/gad.10.23.2971. [DOI] [PubMed] [Google Scholar]
- 57.Ng F W H, Nguyen M, Kwan T, Branton P E, Nicholson D W, Cromlish J A, Shore G C. p28 Bap31, a Bcl-2/Bcl-XL- and procaspase-8-associated protein in the endoplasmic reticulum. J Cell Biol. 1997;139:327–338. doi: 10.1083/jcb.139.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oltvai Z N, Millman C L, Korsmeyer S J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 59.Osada S, Ikeda T, Xu M, Nishihara T, Imagawa M. Identification of the transcriptional repression domain of nuclear factor 1-A. Biochem Biophys Res Commun. 1997;283:744–747. doi: 10.1006/bbrc.1997.7382. [DOI] [PubMed] [Google Scholar]
- 60.Owen-Schaub L, Zhang W, Cusack J C, Angelo L S, Santee S M, Fujiwara T, Roth J A, Deisseroth A B, Zhang W-W, Kruzel E, Radinsky R. Wild-type human p53 and a temperature-sensitive mutant induce Fas/Apo-1 expression. Mol Cell Biol. 1995;15:3032–3040. doi: 10.1128/mcb.15.6.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan G, O’Rourke K, Dixit V M. Caspase-9, Bcl-XL, and Apaf-1 form a ternary complex. J Biol Chem. 1998;273:5841–5845. doi: 10.1074/jbc.273.10.5841. [DOI] [PubMed] [Google Scholar]
- 62.Parsa N Z, Gaidano G, Mukherjee A B, Hauptschein R S, Lenoir G, Dalla-Favera R, Chaganti R S. Cytogenetic and molecular analysis of 6q deletions in Burkitt’s lymphoma cell lines. Genes Chromosomes Cancer. 1994;9:13–18. doi: 10.1002/gcc.2870090104. [DOI] [PubMed] [Google Scholar]
- 63.Perez D, White E. E1B 19K inhibits Fas-mediated apoptosis through FADD-dependent sequestration of FLICE. J Cell Biol. 1998;141:1255–1266. doi: 10.1083/jcb.141.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rao L, Debbas M, Sabbatini P, Hockenbery D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci USA. 1992;89:7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rao L, Modha D, White E. The E1B 19K protein associates with lamins in vivo and its proper localization is required for inhibition of apoptosis. Oncogene. 1997;15:1587–1597. doi: 10.1038/sj.onc.1201323. [DOI] [PubMed] [Google Scholar]
- 66.Sabbatini P, Chiou S-K, Rao L, White E. Modulation of p53-mediated transcriptional repression and apoptosis by the adenovirus E1B 19K protein. Mol Cell Biol. 1995;15:1060–1070. doi: 10.1128/mcb.15.2.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sabbatini P, Han J H, Chiou S-K, Nicholson D, White E. Interleukin 1β converting enzyme-like proteases are essential for p53-mediated transcriptionally dependent apoptosis. Cell Growth Differ. 1997;8:643–653. [PubMed] [Google Scholar]
- 68.Sabbatini P, Lin J, Levine A J, White E. Essential role for p53-mediated transcription in E1A-induced apoptosis but not growth suppression. Genes Dev. 1995;9:2184–2192. doi: 10.1101/gad.9.17.2184. [DOI] [PubMed] [Google Scholar]
- 69.Santhanam U, Ray A, Sehgal P B. Repression of the interleukin 6 gene promoter by p53 and the retinoblastoma susceptibility gene product. Proc Natl Acad Sci USA. 1991;88:7605–7609. doi: 10.1073/pnas.88.17.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sattler M, Liang H, Nettesheim D, Meadows R P, Harlan J E, Eberstadt M, Yoon H S, Shuker S B, Chang B S, Minn A J, Thompson C B, Fesik S W. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 71.Schendel S L, Xie Z, Montal M O, Matsuyama S, Montal M. Channel formation by antiapoptotic protein Bcl-2. Proc Natl Acad Sci USA. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmitz M L, Indorf A, Limbourg F P, Städtler H, Traenckner E B-M, Baeuerle P A. The dual effect of adenovirus type 5 E1A 13S protein on NF-κB activation is antagonized by E1B 19K. Mol Cell Biol. 1996;16:4052–4063. doi: 10.1128/mcb.16.8.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scott S A, Davies A M. Inhibition of protein synthesis prevents cell death in sensory and parasympathetic neurons deprived of neurotrophic in vitro. J Neurobiol. 1990;21:630–638. doi: 10.1002/neu.480210410. [DOI] [PubMed] [Google Scholar]
- 74.See R H, Shi Y. Adenovirus E1B 19,000-molecular-weight protein activates c-Jun N-terminal kinase and c-Jun-mediated transcription. Mol Cell Biol. 1998;18:4012–4022. doi: 10.1128/mcb.18.7.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shaulian E, Zauberman A, Ginsberg D, Oren M. Identification of a minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol Cell Biol. 1992;12:5581–5592. doi: 10.1128/mcb.12.12.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen Y, Shenk T. Relief of p53 mediated transcriptional repression by the adenovirus E1B 19-kDa protein or the cellular Bcl-2 protein. Proc Natl Acad Sci USA. 1994;91:8940–8944. doi: 10.1073/pnas.91.19.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shibasaki F, Kondo E, Akagi T, McKeon F. Suppression of signalling through transcription factor NF-AT by interactions between calcineurin and Bcl-2. Nature (London) 1997;386:728–731. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]
- 78.Siegel R M, Martin D A, Zheng L, Ng S Y, Bertin J, Cohen J, Lenardo M J. Death-effector filaments: novel cytoplasmic structures that recruit caspases and trigger apoptosis. J Cell Biol. 1998;141:1243–1253. doi: 10.1083/jcb.141.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spector M S, Desnoyers S, Hoeppner D J, Hengartner M O. Interaction between the C. elegans cell-death regulators CED-9 and CED-4. Nature (London) 1997;385:653–656. doi: 10.1038/385653a0. [DOI] [PubMed] [Google Scholar]
- 80.Takayama S, Sato T, Krajewski S, Kochel K, Irie S, Millan J A, Reed J C. Cloning and functional analysis of Bag-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell. 1995;80:279–284. doi: 10.1016/0092-8674(95)90410-7. [DOI] [PubMed] [Google Scholar]
- 81.Taylor D, Badiani P, Weston K. A dominant interfering Myb mutant causes apoptosis in T cells. Genes Dev. 1996;10:2732–2744. doi: 10.1101/gad.10.21.2732. [DOI] [PubMed] [Google Scholar]
- 82.Thomas A, White E. Suppression of the p300-dependent mdm2 negative-feedback loop induces the p53 apoptotic function. Genes Dev. 1998;12:1975–1985. doi: 10.1101/gad.12.13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vairo G, Innes K M, Adams J M. Bcl-2 has a cell cycle inhibitory function separable from its enhancement of cell survival. Oncogene. 1996;13:1511–1519. [PubMed] [Google Scholar]
- 84.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 85.Vaux D L, Weissman I L. Neither macromolecular synthesis nor myc is required for cell death via the mechanism that can be controlled by Bcl-2. Mol Cell Biol. 1993;13:7000–7005. doi: 10.1128/mcb.13.11.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walker P R, Smith C, Youdale T, Leblanc J, Whitfield J F, Sikorska M. Topoisomerase II-reactive chemotherapeutic drugs induce apoptosis in thymocytes. Cancer Res. 1991;51:1078–1085. [PubMed] [Google Scholar]
- 87.Wang C-Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 88.Wang H G, Rapp U R, Reed J C. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 89.Weston K. Myb proteins in life, death and differentiation. Curr Opin Genet Dev. 1998;8:76–81. doi: 10.1016/s0959-437x(98)80065-8. [DOI] [PubMed] [Google Scholar]
- 90.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 91.White E, Cipriani R, Sabbatini P, Denton A. The adenovirus E1B 19-kilodalton protein overcomes the cytotoxicity of E1A proteins. J Virol. 1991;65:2968–2978. doi: 10.1128/jvi.65.6.2968-2978.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.White E, Denton A, Stillman B. Role of the adenovirus E1B 19,000-dalton tumor antigen in regulating early gene expression. J Virol. 1988;62:3445–3454. doi: 10.1128/jvi.62.9.3445-3454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.White E, Blose S H, Stillman B. Nuclear envelope localization of an adenovirus tumor antigen maintains the integrity of cellular DNA. Mol Cell Biol. 1984;4:2865–2875. doi: 10.1128/mcb.4.12.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.White E, Cipriani R. Specific disruption of intermediate filaments and the nuclear lamina by the 19-kDa product of the adenovirus E1B oncogene. Proc Natl Acad Sci USA. 1989;86:9886–9890. doi: 10.1073/pnas.86.24.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.White E, Faha B, Stillman B. Regulation of adenovirus gene expression in human WI38 cells by an E1B-encoded tumor antigen. Mol Cell Biol. 1986;6:3763–3773. doi: 10.1128/mcb.6.11.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.White E, Sabbatini P, Debbas M, Wold W S M, Kusher D I, Gooding L. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha. Mol Cell Biol. 1992;12:2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wolter K G, Hsu Y-T, Smith C L, Nechushtan A, Xi X-G. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu D, Wallen H D, Inohara N, Nuñez G. Interaction and regulation of the Caenorhabditis elegans death protease CED-3 by CED-4 and CED-9. J Biol Chem. 1997;272:21449–21454. doi: 10.1074/jbc.272.34.21449. [DOI] [PubMed] [Google Scholar]
- 99.Wu D, Wallen H D, Nuñez G. Interaction and regulation of subcellular localization of CED-4 by CED-9. Science. 1997;275:1126–1128. doi: 10.1126/science.275.5303.1126. [DOI] [PubMed] [Google Scholar]
- 100.Wyllie A H. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 101.Yang W M, Inouye C, Zeng Y, Bearss D, Seto E. Transcriptional repression by YY1 is mediated by interaction with a mammalian homolog of the yeast global regulator RPD3. Proc Natl Acad Sci USA. 1996;93:12845–12850. doi: 10.1073/pnas.93.23.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yin X-M, Oltvai Z, Korsmeyer S. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature (London) 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 103.Yoshida K, Venkatesh L, Kuppuswamy M, Chinnadurai G. Adenovirus transforming 19-kD T antigen has an enhancer dependent trans-activation function and relieves enhancer repression mediated by viral and cellular genes. Genes Dev. 1987;1:645–658. doi: 10.1101/gad.1.7.645. [DOI] [PubMed] [Google Scholar]
- 104.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]