Abstract
We isolated a Drosophila fickleP (ficP) mutant with a shortened copulatory duration and reduced adult-stage life span. The reduced copulatory duration is ascribable to incomplete fusion of the left and right halves of the apodeme that holds the penis during copulation. ficP is an intronic mutation occurring in the Btk gene, a gene which encodes two forms (type 1 and type 2) of a Bruton’s tyrosine kinase (Btk) family cytoplasmic tyrosine kinase as a result of alternative exon usage. The ficP mutation prevents the formation of the type 2 isoform but leaves expression of the type 1 transcript intact. Ubiquitous overexpression of the wild-type cDNA by using a heat shock 70 promoter during the late larval or pupal stages rescued the life span and genital defects in the mutant, respectively, establishing the causal relationship between the ficP phenotypes and the Btk gene mutation. The stage specificity of the rescuing ability suggests that the Btk gene is required for the development of male genitalia and substrates required for adult survival.
The Src superfamily of non-receptor-type tyrosine kinases is composed of four families, the Src family, the Csk family, the Abl family, and the Btk family, each of which is represented by multiple family members (37). These kinases have been suggested to play diverse roles in cell proliferation, differentiation, survival, and death (5, 8, 22, 32, 39, 41). Among these four families, the Bruton’s tyrosine kinase (Btk) family is unique in that the kinases in this family have extended N-terminal sequences that are called collectively the pleckstrin homology (PH) domain (17, 18). This domain is known to bind the βγ subunits of heterotrimeric G proteins as well as membrane lipids such as phosphatidylinositol-4,5-bisphosphate (16, 25, 27, 29). Another feature unique to the Btk family resides in the restricted localization of its members in mammalian tissues. For example, Btk is present only in B cells whereas the expression of Itk is confined strictly to T cells (2, 35). Similarly, one of the two forms of the Tec kinase is liver specific whereas the other is hepatocyte specific.
In Drosophila flies, Dsrc29A (11, 38, 43) is the sole kinase that represents the Btk family, and it is most similar to Btk itself in terms of overall homology. However, the reported N-terminal sequence of Dsrc29A has no similarity to the PH domain (37). This fact raises the possibility that Dsrc29A is not an ortholog of Btk but represents a distinct member of the Btk family. Deficits in Btk function are responsible for X chromosome-linked agammaglobulinemia in humans and X chromosome-linked immunodeficiency in mice, where B-cell maturation is blocked (24, 28, 31, 40, 42). The defense mechanism involving immunoglobulins secreted from B lymphocytes exists only in higher vertebrates, and the machinery for antibody production is expected to have its origin in an apparently unrelated cellular function in lower animals. Thus, functional analysis of Dsrc29A would provide insights into the evolution of Btk-like kinases.
We have isolated a genetic variant, fickleP (ficP), that removes the Btk homolog in Drosophila flies, which lack the B-cell-dependent immune system, in a screening of mutants with defects in mating behavior. The mating behavior of Drosophila melanogaster is made up of several discrete elementary steps (10, 13, 46). First, the male finds and tracks the female. While tracking, the male approaches the female to tap her abdomen with his forelegs. The male then performs courtship songs by using unilateral wing vibration. Provided that she is sexually receptive, the female frequently stops moving when exposed to the courtship songs, offering the male a chance to lick her genitalia and to attempt copulation. If the male is successful, he mounts on and copulates with the female for 10 to 17 min. Upon termination of copulation, the male releases his genitalia from the female’s and dismounts. These behavioral acts are considered to be fixed-action patterns, i.e., instinctive behavior (13).
There are mutations that are known to affect the unique aspects of mating behavior in D. melanogaster (46, 47). The fruitless (fru) and dissatisfaction (dsf) mutations alter the male’s sexual orientation from heterosexual to homosexual or bisexual (9, 19, 34, 45). dissonance (diss) (23), cacophony (cac) (44), and croaker (cro) (48) are mutations that produce aberrant courtship songs. Some mutant males court normally but fail to copulate. celibate (cel) is an example of the class of mutation causing this behavior (14).
Other type of mutations affect copulation and postcopulatory behavior. These include stuck (sk) and coitus interruptus (coi) (14). The sk males often fail to withdraw their genitalia after copulation, with the result that the male and female pair tug at each other, pulling in opposite directions. The coi mutation affects males, causing copulation to terminate prematurely, even before completion of the sperm transfer from the male to the female. Phenotypically, ficP seemed to belong to this class of mutations. The ficP flies exhibited an extremely variable copulatory duration ranging from 1 to 15 min, in sharp contrast to the wild-type flies (see above). Unlike wild-type flies, ficP mutant flies tended to mate repeatedly within a short period (minutes).
In this paper, we show that these behavioral phenotypes of ficP are probably a consequence of malformation of male genitalia. Another conspicuous phenotype of ficP is a reduced life span after adult emergence. The functional rescue experiments of the ficP mutant demonstrated that Drosophila Btk is required in the pupal stage for normal adult longevity and male genital formation. The longevity phenotype is believed to be linked to Btk expression in the developing brain, while the genital phenotype is associated with its expression in the developing genital disc.
Interestingly, the Btk homolog is generated by an alternative exon usage of the transcription unit for the Dsrc29A kinase (11), which was recently reported to function in ring canal growth during oogenesis (12, 33). Both the Btk-coding transcript (we refer to it as type 2) and the Dsrc29A kinase transcript (type 1) are expressed in the central nervous system (CNS) and imaginal discs; the domains and/or timing of expression in these tissues are distinct from each other. Complete loss of function of the gene (i.e., loss of both types 1 and 2) causes oocyte undergrowth and embryonic death accompanied by defective head involution, while selective loss of the type 2 transcript spares life but reduces the life span in the adult and leads to malformation of the male genitalia. Thus, the single Btk/Dsrc29A kinase gene exerts pleiotropic functions in different developmental contexts in different tissues through the generation of distinct forms of protein products by means of alternative splicing.
MATERIALS AND METHODS
The jump-start method was used for mutagenesis with the BmΔ-w element as a mutator and the P (ry+ Δ2-3) transposon as a jump starter. All flies subjected to mutagenesis had a white− (w−) background, whereas the BmΔ-w element carried a copy of w+, allowing us to recover chromosomes with BmΔ-w insertions by selecting flies with a nonwhite eye color. After establishment of fly lines with new insertions, homozygous virgin males and females were collected at eclosion, placed singly in food vials, and grown for 3 days. For behavior screening, single male-female pairs were introduced into disposable plastic syringes (volume, 1 cm3). At least 10 pairs per strain were observed with the naked eye for 1 h, and the time taken until copulation, the duration of copulation, and the percentage of pairs copulating were recorded (19). The observations were performed by experimenters ignorant of the genotypes of the flies. Through introduction of the P (ry+ Δ2-3) chromosome into the ficP line, the mutator element was remobilized, resulting in approximately 60 lines with white eyes. ficR and ficL1-3 are representatives of these lines. The ficP mutant used in this study had been outcrossed to the w1118 strain with the Canton-Special (CS) genetic background for five generations. The mutant phenotypes were unchanged by this treatment.
For scanning electron microscopy, the flies were prepared for critical-point drying and coated with a 2-nm layer of gold. Images were taken on a low-voltage prototype scanning electron microscope.
The plasmid-rescued DNA fragment (15) was used to initiate a genomic walk by using a λ EMBL3 CS genomic library (Clontech Laboratories, Inc.). Using Drosophila head and embryonic cDNA libraries (provided by T. L. Schwarz and K. Zinn), 106 phages were screened and several Btk/Dsrc29A cDNAs were isolated. The nucleotide sequences of the cDNAs were determined with a 377 DNA sequencer (Perkin Elmer). Total RNA was isolated with Trizol (Gibco BRL), and poly(A)+ RNA was prepared by oligo(dT)-cellulose affinity chromatography with an mRNA purification kit (Pharmacia). A 1-μg portion of poly(A)+ RNA was separated on a 1% agarose gel containing formaldehyde. Following transfer to Biodyne-Plus (Pall), the filter was hybridized with digoxigenin (DIG)-labelled probes and signals were detected with a DIG luminescence detection kit (Boehringer Mannheim).
For the reverse transcription-PCR (RT-PCR) experiment, cDNA was synthesized from 1 μg of total RNA with SuperScript II reverse transcriptase (Bethesda Research Laboratories) and random hexanucleotide primers (Pharmacia) and subjected to PCR with the Expand High-Fidelity PCR system (Boehringer Mannheim). The synthetic primers used for the PCR were UPS-1 (5′-CTGCGTGAGTTTGGCAGAAACG), UPS-2 (5′-CGCCCATTGGCGTGAGG), DWS-C (5′-GGTATACCGCCAGGCATATTGGC), and DWS-W (5′-AGAGCTCAAACAGCTCGGAAG). The RT-PCR products were separated by agarose gel electrophoresis, stained with SYBR Green I (FMC Corp.), and observed with a FluorImager 595 (Molecular Dynamics). The authenticity of the RT-PCR products was confirmed by sequencing. Rapid amplification of cDNA ends was performed with the Marathon cDNA amplification kit (Clontech).
For the generation of Btk/Dsrc29A transformant flies, the type 1 or 2 cDNA was introduced into the CaSpeR-hs vector (hs-cDNA). hs-cDNA was then injected into w1118 homozygous embryos with a phsπ helper plasmid. Five independent fly lines with hs-cDNA on the third chromosome were obtained, and fly lines doubly homozygous for the transgene and ficP mutation were constructed for examination of the activity of the transgene in rescuing the ficP phenotype. Individuals were exposed repeatedly to heat shock treatment at 33°C for 1 h at 3-h intervals throughout the pupal stage.
In situ hybridization of whole-mount tissues was performed with DIG-labeled single-stranded DNA probes by the method of Lehmann and Tautz (26).
Nucleotide sequence accession numbers.
The sequences reported in this paper have been deposited in the DDBJ/EMBL/GenBank database (accession no. AB009840 and AB009841).
RESULTS
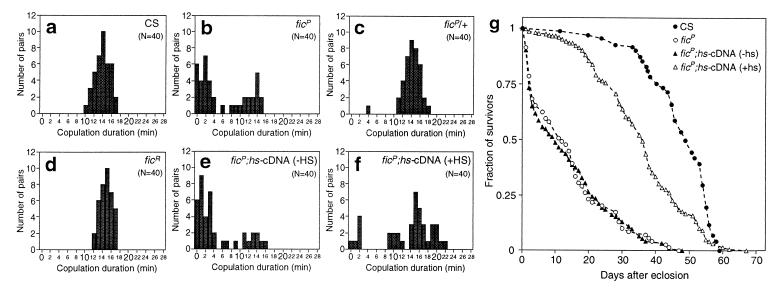
fickleP (ficP), a P-element-induced mutation in the locus encoding the Btk homolog, was isolated during our screening for mating-behavior mutants and found to be responsible for variable copulatory duration with occasional remating (46). The wild-type pair copulated for 10 to 17 min with a mean of 15 min. In contrast, copulation involving ficP mutant males often terminated shortly after initiation. Pairs composed of a wild-type male and a ficP female displayed a copulatory duration similar to that of wild-type pairs. Frequency histograms of copulatory durations were constructed to aid quantitative comparisons of the durations of copulatory events involving the wild-type and ficP males (Fig. 1a and b). The patterns of distribution of the copulatory durations are distinctly different in the two histograms. The wild-type pattern basically follows a normal distribution. Due to the significant contribution from very short copulatory events, the distribution pattern for ficP is seen to deviate grossly from the wild-type pattern. The distribution pattern of copulatory duration for heterozygous males is indistinguishable from that for wild-type males, indicating that the ficP phenotype is recessive (Fig. 1c). Besides the variable copulatory duration, some ficP males repeated copulation with the same partners after uncoupling. Such multiple copulation was observed in 8 of 105 pairs of wild-type females and ficP males and in none of the 115 wild-type female-male pairs.
FIG. 1.
Behavioral and longevity phenotypes of the fic mutant. (a to f) Frequency distributions of copulation duration. The duration of copulation was measured for each pair, and the copulatory events were sorted into groups defined by the duration of copulation. The number of copulatory events is plotted as a function of duration (abscissa) for wild-type (a), ficP (b), ficP/+ (c), ficR (d), and ficP;hs-cDNA (e and f) males. ficP;hs-cDNA flies were raised at 25°C with (f) or without (e) exposure to the heat shock regimen (see Materials and Methods). The females used were wild type in all cases. The distributions of the copulatory duration were compared between wild-type, ficP, and ficP;hs-cDNA males with heat shock and ficP;hs-cDNA males without heat shock by nonparametric analyses of variance. The null hypothesis that all four distributions are indistinguishable was rejected by using the Kruskal-Wallis test (in which the H variant was 64.285, where the 99.5% confidence interval was H < 12.838). Subsequently, another null hypothesis, that two arbitrarily chosen distributions are indistinguishable, was evaluated by the simultaneous-comparison procedure based on the Mann-Whitney U test. The distribution shown in panel f was considered to be different from that in panel b or e but not different from that in panel a (P < 0.05), and thus we conclude that overexpression of hs-cDNA rescued the behavioral phenotype. The type 1 cDNA was used in the illustrated experiment. A similar result was obtained with the type 2 cDNA. (g) Longevity of adult flies. Percent survival after eclosion is plotted for wild-type (CS), ficP, and ficP;hs-cDNA flies which developed from pupae which had (+hs) or had not (−hs) been subjected to heat shock. Heat shocks were given during the period between pupation and 48 h after pupation. The type 2 cDNA was used in the rescue experiment illustrated here.
The mutation was considered to be induced by a P-element insertion, because revertants were obtained at a high frequency by excision of the P element. Among the 59 excision lines established, 37 were phenotypically wild type (i.e., revertants), and the remaining 22 were nonviable. Southern blot analysis was used to define the molecular nature of these excision lines. ficR is a revertant in which precise excision took place in the genome and shows a normal distribution of copulatory durations (Fig. 1d). Two other fic alleles, l(2)k05610 and l(2)k00206, were identified in a lethal P-insertion collection by another research group (12). The fic locus thus represents an essential gene (33). In accord with this, we obtained several lethal alleles formed by imprecise excision of the P-element in ficP.
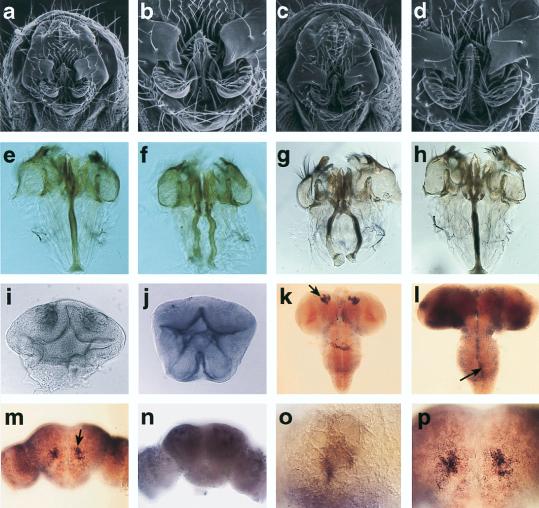
The abnormal copulation behavior observed in ficP flies is probably due to a defect in the genitalia. Although no overt abnormality was detected in the ficP male external genitalia from scanning electron microscopic observations (Fig. 2c and d), dissected ficP male genitalia revealed a marked difference from those of the wild type. Two apodemes were present bilaterally at the base of the penis apparatus in all ficP males (Fig. 2f), while in wild-type males a single apodeme was attached to the penis apparatus (Fig. 2e). The genitalia of ficP/ficL1-3 males were also noted to have two apodemes without exception (not illustrated). ficL1-3 is a putative null allele of the locus, since its lethality is not alleviated when it is placed over Df(2L)TE29Aa-11, a large deficiency which deletes the entire Btk/Dsrc29A transcription unit together with some flanking genes. The penetrance of the genital phenotype was lower in ficP/l(2)k05610 (65%) and ficP/l(2)k00206 (75%) mutants than in ficP and ficP/ficL1-3 mutants (100% in both cases). Interestingly, in a weaker hypomorph, ficEX1-52, obtained by imprecise excision of the P-element from the ficP chromosome, a single apodeme was often noted that split into left and right halves only in its distal portion. This configuration of the apodeme suggests that bilaterally symmetrical primordial apodemes failed to fuse during development in these ficP mutants. In addition to apodeme malformation, the ficP mutant males also exhibited prominent atrophy of the posterior ejaculatory duct (not illustrated). As a consequence of the behavioral and/or genital abnormalities, ficP males are infertile.
FIG. 2.
Morphological phenotype of the ficP mutants and tissue localization of fic mRNA. (a to d) Scanning electron micrographs of the male external genitalia of ficP (c and d) compared with wild-type (a and b) flies. (b and d) Enlarged views of panels a and c, respectively. (e to h) Dissected male genitalia from wild-type (e), ficP (f), ficP;hs-cDNA not exposed to heat shock (g), and ficP;hs-cDNA exposed to heat shock (h) flies. The type 2 cDNA was used for the rescue experiment. (i to p) fic mRNA expression detected by in situ hybridization. (i and j) Ventral views of early pupal genital discs hybridized with a probe specific for type 1 (i) or type 2 (j). (k to p) fic mRNA expression in the brain at 4 h after pupation (k), early-pupal (1-day-old) (l), and mid-pupal (3-day-old) (m and n) stages in wild-type flies. Striking accumulation of type 2 mRNA in the mushroom body was observed only in the prepupal stage (arrow in panel k; an enlarged view is illustrated in panel o) and in the late pupal stage (arrow in panel n). In the mid-pupal stage, expression of type 1 mRNA became widespread in the brain, with prominent expression in the midline glia in the ventral ganglion (arrow in panel l). In the late pupal stage, type 2 expression was restricted to the mid-frontal region of the brain near the antennal lobe (arrow in panel m; also shown at a higher magnification in panel p). A type 1-specific probe was used in panel l, and a type 2-specific probe was used in panels k, m, n, o, and p. Use of a sense probe in hybridization yielded no signal (not illustrated).
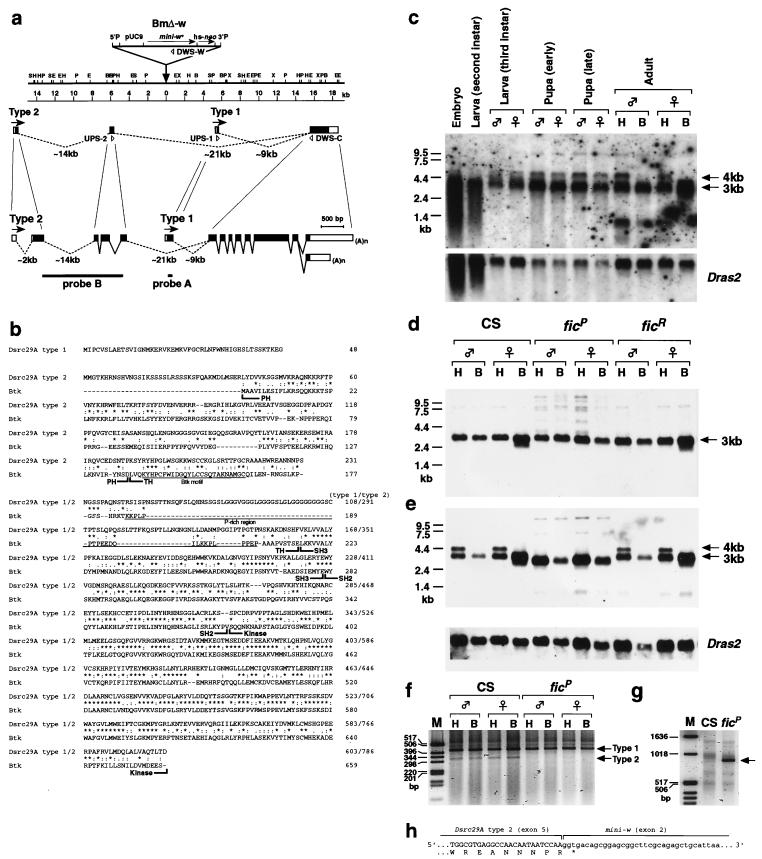
The P-element insertion was located at 29A, as determined by polytene chromosome in situ hybridization with a genomic fragment obtained by plasmid rescue. Subsequent screenings of a genomic DNA library allowed us to clone the 22-kb chromosomal region flanking the P-element insertion site (Fig. 3). Through a systematic search for transcripts from this region by Northern blot analysis (see below), we identified a single transcription unit that spanned the P-element insertion point (Fig. 3a).
FIG. 3.
Molecular analysis of the Btk/Dsrc29A locus. (a) Restriction map of the chromosomal region defined by the ficP P-element insertion (indicated by a triangle). The Btk/Dsrc29A transcription unit is shown below the map. The direction of transcription is indicated by arrows. The exon-intron organization is illustrated below the map at two different scales. Open boxes represent noncoding regions; solid boxes represent coding regions; and lines represent introns. The sequences used as probes in Northern blot analysis are indicated by dotted lines at the bottom. The upstream primers (UPS-1 and UPS-2) and the downstream primers (DWS-C) for the PCR experiments are also shown. S, SacI; H, HindIII; P, PstI; E, EcoRI; B, BamHI; X, XbaI. (b) Primary structures of the Btk/Dsrc29A products deduced from the cDNA nucleotide sequences, and alignment of the amino acid sequence with that of human Btk. Identical residues are marked with asterisks. Conservative substitutions are dotted. The PH, TH, SH3, SH2, and kinase domains are indicated. The Btk motif and the proline-rich region are underlined. (c) Northern blot analysis of the developmental profile of Btk/Dsrc29A transcripts. The 4- and 3-kb mRNA species are expressed in embryos, second-instar larvae, third-instar larvae, and pupae. The 4-kb transcript is abundant in the head (H) but is not detectable in the body (B) of the adult flies. The blots were also hybridized with the Dras2 probe as a reference for the loading amount. (d) Comparison of the transcripts expressed in the adult stage between wild-type (CS), ficP, and a revertant, ficR. Probe A (see panel a) was used. (e) Similar comparison of the transcripts in CS, ficP, and ficR adults, detected with probe B (see panel a), which selectively hybridizes with the transcript having the PH domain-encoding region (i.e., type 2). ficP lacks two kinds of type 2 transcripts (i.e., the 3- and 4-kb transcripts), both having the 5′ sequence corresponding to the PH domain. These two transcripts are present in ficR and in the wild type. The 4-kb type 2 transcript is detectable only in the head part in wild-type and ficR flies. (f) PCR amplification of the type 1 and 2 products with CS and ficP RNA. Molecular size markers are shown in lane M. The primers used are as illustrated in panel a. The type 1-specific primer and the type 2-specific primer are expected to yield products of 426 and 324 bp, respectively (arrows). ficP lacks the type 2 product. (g) PCR amplification of CS and ficP RNA with the type 2-specific upstream primer and the mini-w-specific downstream primer. The product is detectable only in ficP (arrow). The size of the product (approximately 900 bp) matches the expected size when the 5′ half of type 2 RNA is spliced to the second exon of the mini-w gene in the P-element. (h) Sequence of the chimeric transcript composed of 5′ exons of type 2 and mini-w mRNA.
We obtained two types of cDNA for this gene from subsequent library screenings. The type 1 clone was about 2.9 kb long and was isolated from an embryonic cDNA library (a gift from K. Zinn), whereas the type 2 clone was about 3.7 kb long and was isolated from an adult head cDNA library (a gift from T. L. Schwarz). Although the type 1 and 2 clones had identical 3′ coding sequences, they differed from each other in the 5′ half. This difference appears to have resulted from alternative exon usage (Fig. 3a).
Sequencing of the type 1 cDNA clone revealed a long open reading frame which encoded a protein of 603 amino acids (Fig. 3b), if the first ATG codon was chosen as the translation initiation site. A database search for similar sequences revealed that an amino acid sequence very similar to that in the aforementioned protein had been reported in Drosophila by Gregory et al. (11). The reported sequence was that of Dsrc29A. An important difference between the sequence found in this study and the reported Dsrc29A sequence was found in their N termini; the open reading frame for the cDNA clones isolated by us was open for an additional 45 bp upstream of the methionine start codon chosen by Gregory et al. (11). Furthermore, there were 34 amino acid differences in the deduced protein sequences. Dsrc29A belongs to the Src superfamily, having highly conserved sequence motifs including the SH2 (Src-homology 2), the SH3 (Src-homology 3), and the catalytic (kinase) domains (Fig. 3b). Dsrc29A differs from Src in that it has a long, basic N-terminal region upstream of the SH3 domain (Fig. 3b).
Analysis of the type 2 cDNA revealed that the protein encoded by the second form of the transcript has an amino acid sequence identical to that of the type 1 product in the C-terminal two-thirds, including the SH3, SH2, and kinase domains, but has a unique N-terminal stretch of 231 amino acids (Fig. 3b). This isoform has not been reported previously. Although the type 1 product does not contain any discernible conserved motifs in its N-terminal extension, the newly identified type 2 isoform bears typical PH and TH domains, which are regarded as hallmarks of the Btk family kinases, such as Atk, Itk, Tec, and Btk. A striking difference between the type 2 product and Btk is the presence of a polyglycine stretch insertion in the proline-rich region (Fig. 3b). Overall, the percent similarity between the Drosophila type 2 protein and mammalian Btk is 67.7%. When compared for each domain, the value is 56.9% (PH), 33.8% (TH), 78.8% (SH3), 80.4% (SH2), and 85.3% (kinase). The low value for the TH domain is ascribable to the polyglycine stretch present in the Drosophila sequence. The similarity increases to 76.9% if only the Btk motif in the TH domain is considered for comparison. Thus, the type 2 product is very likely to be the Drosophila ortholog of Btk.
Northern blot analysis with a sequence common to both type 1 and 2 clones as a probe revealed that a major 3-kb transcript and a minor 4-kb transcript are expressed at constant levels throughout development (Fig. 3c). In the adult stage, there are additional transcripts, of 0.3 to 1.35 kb. The 4-kb transcript was detectable in poly(A)+ mRNA extracted from the heads but not from the bodies of adult flies. This transcript may represent an isoform specific to neural tissue that occupies most of the head. To determine the relative contributions of the type 1 and 2 transcripts to the expressed mRNA, the membrane was probed with type 1-specific (probe A, Fig. 3a) and type 2-specific (probe B, Fig. 3a) sequences, respectively. In the wild-type Drosophila, a 4-kb transcript was detected in the adult head poly(A)+ RNA (Fig. 3e) when probed with the type 2-specific sequence. In addition, the type 2-specific probe hybridized with a transcript, of about 3 kb, in both the head and body parts (Fig. 3e). On the other hand, the type 1-specific probe detected a 3-kb transcript in the head and body poly(A)+ RNA. Thus, the type 1 cDNA corresponded to a 3-kb transcript whereas the type 2 cDNA corresponded to a different 3-kb transcript and a 4-kb transcript. The 3-kb type 1 and 3-kb type 2 transcripts are expressed in both the head and body, while the 4-kb type 2 transcript is head specific.
The difference between wild-type and ficP strains was evident when a type 2-specific probe was used for Northern blot analysis (Fig. 3e); neither of the 4-kb and 3-kb transcripts were detected in ficP flies. The absence of these transcripts is associated with the fic mutation, since ficR, a phenotypic revertant obtained by excision of the inserted P element (Fig. 1d), has both the transcripts (Fig. 3d). The sole transcript detectable in ficP flies with the type 2-specific probe was distinct from any of the transcripts found in the wild type. The mutant transcript was slightly shorter than the wild-type 3-kb type 2 transcript, implying that it was a truncated version of the type 2 transcript. An RT-PCR experiment supported this hypothesis: PCR was performed with the downstream primer specific to the common region in combination with the type 1-specific and type 2-specific upstream primers at each time. The RT-PCR products derived from both the type 1 and 2 transcripts were successfully amplified from wild-type total RNA. In ficP, in contrast, the type 2-specific primer did not yield any product but the type 1-specific primer successfully amplified a sequence (Fig. 3f). Another PCR experiment demonstrated that the 5′ half of the gene encoding the type 2 transcript was incorrectly spliced to an exon of the mini-white (w) gene carried by the P element (BmΔ-w) in ficP. In this experiment, the type 2-specific upstream primer and a downstream primer specific to exon 3 of the mini-w gene yielded an RT-PCR product from ficP total RNA. Sequencing of this PCR product demonstrated that the splice donor site at the 3′ end of exon 5 of the gene encoding the type 2 transcript conjoined the splice acceptor site of mini-w exon 2 (Fig. 3h). Therefore, we conclude that the functional type 2 transcript is lost in the ficP mutant. From these experiments, it is also clear that the 4- and 3-kb transcripts share the 5′ sequences. On the other hand, a 3′ rapid amplification of cDNA ends experiment yielded two products of different sizes. Since the size difference is ascribed exclusively to a noncoding sequence, the two type 2 transcripts must produce the same protein.
To examine whether the ficP phenotypes are causally linked to malfunction of the cloned gene, we generated ficP mutant lines carrying the full-length type 1 or 2 wild-type cDNA driven by the heat shock promoter (hs-cDNA). Although the phenotypes of ficP resulted from the selective loss of the type 2 transcript, ubiquitous overexpression of either type 1 or 2 similarly rescued these phenotypes. This fact suggests that the specific phenotype of the ficP mutant reflects disruption of specific temporal and/or spatial expression of the type 2 transcript rather than functional specificity of the type 2 product.
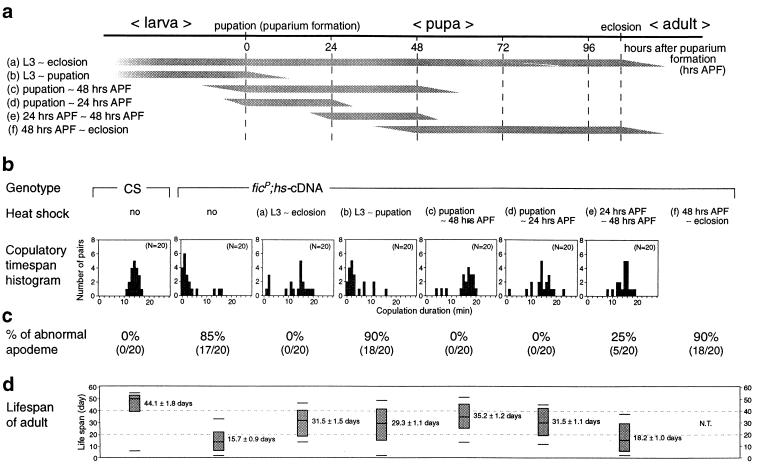
Mutant flies carrying hs-cDNA, reared at 25°C, retained the ficP phenotype: the apodeme of the male genitalia remained torn (Fig. 2g). Consistent with this observation, the histogram for copulatory duration revealed a pattern similar to that for ficP flies without hs-cDNA (Fig. 1e). Heat shock application upon ficP homozygotes without hs-cDNA did not alleviate the genital and behavioral phenotypes (data not shown). Subjecting the ficP;hs-cDNA males to heat shock throughout the pupal stage restored normal apodeme structure (Fig. 2h) and was accompanied by a dramatic alteration in mating behavior after emergence (Fig. 1f). The pattern of distribution of copulatory duration for the ficP;hs-cDNA flies after heat shock resembled that for wild-type flies (Fig. 1a and f). The results of nonparametric analyses of variance indicated that the distribution of copulatory duration for ficP;hs-cDNA flies which had not been subjected to heat shock was significantly different from that for wild-type flies but not from that for ficP flies. Conversely, the distribution for ficP;hs-cDNA flies after heat shock was significantly different from that for ficP flies but not from that for wild-type flies. Thus, expression of hs-cDNA in the pupal stage rescues the ficP defect in the genitalia and restores normal copulation behavior, establishing the causal relationship between the mutant phenotype and the gene. Heat shock induction of hs-cDNA on the first or second day of pupal life was sufficient for rescue of both the behavioral and genital phenotypes (Fig. 4). Repeated remating was not observed between pairs of ficP;hs-cDNA males and wild-type females, when the males had been exposed to heat shock after the third-instar larval stage until emergence (data not shown).
FIG. 4.
Time specificity in the rescuing effects of hs-cDNA (type 1) expression. (a) Schematic of the period when a heat shock was applied to induce transgene expression. Six regimens (a to f) were tested. L3, third-instar larval stage. (b) Frequency histograms of the duration of copulation. The genotypes of the flies tested are indicated above the histograms. The two histograms on the left are reproduced from Fig. 1, for comparison. Below the genotypes, the period of heat shock induction is indicated (regimens a to f; see panel a). APF, after puparium formation. (c) Percentage of male flies having abnormal apodemes. The actual number of flies is given below (aberrant/examined). (d) Distribution of the life span after eclosion for CS flies and ficP;hs-cDNA flies exposed to heat shock at various stages. Shown on the coordinate is survival (in days) after emergence. The uppermost line and the lowermost line indicate the days on which 90 and 10% of the observed flies died, respectively. The upper side and lower side of the box represent the days on which 75 and 25% of the observed flies died, respectively. The line illustrated in the box corresponds to the day on which 50% of the flies died, i.e., the median value. N.T., not tested.
Apart from the defects in the male genitalia and mating behavior, we noted that the life span after eclosion was decreased in ficP flies compared to wild-type flies (Fig. 1g). The longevity phenotype of ficP adult flies was also rescued by ubiquitous overexpression of either type 1 or 2 cDNA (Fig. 1g and 4), indicating that the type 1 and 2 transcripts are functionally equivalent to each other in terms of life span regulation. This implies that a specific site at which type 2 mRNA, but not type 1 mRNA, is expressed plays a critical role for adult survival. Heat shock application upon ficP homozygotes without hs-cDNA did not improve adult survival (data not shown).
The most effective period of hs-cDNA induction for rescue of the longevity phenotype was between the last day of the third larval instar stage and the first day of pupation (Fig. 4). Thus, the results of the heat shock experiments suggest a requirement for Btk/Dsrc29A in the prepupal to early pupal stage for normal copulation and genital formation and in the third larval instar to early pupal stage for normal adult life span.
To study the tissue localization of the Btk/Dsrc29A transcript during the “critical period,” whole-mount in situ RNA hybridization was performed (Fig. 2i to p). Both the type 1 and 2 transcripts were detected in the male genital disc (Fig. 2i and j), although their expression patterns were distinctly different from each other. The expression of the type 1 transcript is confined to the middle portion of the anterior bulbus (Fig. 2i), which is known to form the internal genitalia (4). On the other hand, the type 2 transcript is strongly expressed along the edge of the male genital primordium facing the lumen (Fig. 2j). Fate map studies (4) indicated that this part of the male genital primordium contributes to the main body of the male genitalia including the penis apparatus, to which the apodeme is attached. This finding supports the notion that the specific phenotype of the ficP mutant correlates with the spatial restriction of the type 2 transcript.
The other tissue that is rich in Btk/Dsrc29A mRNA is the CNS: particularly strong expression was detected in the cells of the inner optic anlage (not illustrated). Several hours after puparium formation, strong Btk/Dsrc29A mRNA expression was noted in the cells above the mushroom bodies (Fig. 2k). Hybridization with type-specific probes demonstrated that the transcript expressed in the mushroom body region consisted exclusively of the type 2 transcript. In other areas of the brain, type 1 transcript predominates. These cells are thought to contribute to the development of mushroom bodies (20). The expression was detected in many mushroom body ganglion cells but not in neuroblasts (Fig. 2m and o). Soon thereafter, weak expression of type 1 was noted in many scattered cells in the central brain and ventral ganglion while expression in the optic lobe ceased. In the ventral ganglia, weak Btk/Dsrc29A expression was detected in many scattered cells in a manner similar to that in the central brain. Strong expression was observed in the cells on the midline of the ventral ganglion. Since these were irregularly shaped cells enwrapping the anterior and posterior comissures in each segment (Fig. 2l), they are likely to be midline glia (1, 21, 30). The expression in the ventral ganglia consisted exclusively of the type 1 transcript. No expression of the type 2 transcript was detected in this region. At around 72 h after puparium formation, nearly half of the cells in the brain expressed type 1 mRNA. At this stage, expression of the type 2 mRNA was observed only in a cluster of cells just above each antennal lobe (Fig. 2m) and cells near the mushroom body. The level of type 2 mRNA expression in these cells was remarkably high. Most of the cells expressing this mRNA in the antennal lobe region appeared to be neurons because the cell bodies were round. The antennal lobe is known to consist of approximately 1,200 cells (36). Of these, only a very small portion exhibited strong Btk/Dsrc29A expression. Interestingly, there were other clusters of cells above the antennal lobes that did not express Btk/Dsrc29A (Fig. 2n and p). On the other hand, four clusters of cells expressing the mRNA per hemisphere were observed in the mushroom body region. Each cluster apparently corresponded to the progeny of one of four mushroom body neuroblasts. Thus, it is concluded that the type 2 transcript is selectively expressed in the cells of the mushroom body and the antennal lobe whereas the type 1 transcript is expressed in dispersed neurons and the midline glial cells in the CNS during the larval and pupal stages. The localizations of types 1 and 2 mRNA appear to be mutually exclusive in both the CNS and the genital disc.
DISCUSSION
In this study, we demonstrated that a hypomorphic mutation in the Drosophila gene encoding the Btk-like tyrosine kinase impairs genital development, leading to premature termination of copulation. The defects in genital structure and copulatory behavior were both rescued by overexpressing the wild-type product of Btk/Dsrc29A in the first 2 days of the pupal stage of the mutant flies. The coincidence of time specificity in the rescuing effect of wild-type Btk/Dsrc29A cDNA on these two phenotypes suggests that the malformation of the male genitalia during the pupal stage is responsible for the abnormal copulatory behavior in the adult. It should be noted, however, that the temporal correlation between the genital and behavioral phenotypes revealed by the rescue experiments with time-restricted Btk/Dsrc29A expression does not exclude the possibility of a neural origin of the behavioral phenotype. Besides the genital and copulatory phenotypes, ficP also affects the longevity of adult flies. The critical period for the rescue of the longevity phenotype by the wild-type transgene was found to be from late third instar to the early pupal stage, which is different from that for the genital and copulatory phenotypes.
Since Btk/Dsrc29A products were expressed at high levels in the CNS, this is a possible site of their action affecting longevity. Since the ficP mutant with reduced adult life span selectively eliminates the type 2 transcript, the site which normally expresses type 2 but not type 1 mRNA should be responsible for this phenotype. It is intriguing that the type 2 transcript transiently accumulates in the mushroom body and the antennal lobe, which are essential for higher-order behavior including olfactory learning and memory (6, 7) and sexual orientation (19). For determination of whether these sites are critical for the expression of the behavioral phenotypes as well as the longevity phenotype, further experimentation is required to identify the tissue in which the wild-type product of Btk/Dsrc29A must be expressed for the phenotype rescue to occur. A targeted expression system utilizing the yeast GAL4 and the upstream activation site sequence (3) would allow us to determine the site of Btk/Dsrc29A action relevant to longevity.
Even though a hypomorphic allele of ficP is associated with highly specific phenotypes, Btk/Dsrc29A is pleiotropic. The phenotypic specificity of ficP may be correlated with the observation that expression of only the type 2 transcript is affected in the mutant, leaving expression of the type 1 transcript intact. In fact, the Dsrc29A kinase, together with the Dsrc64 kinase, has recently been shown to be required for ring canal growth in the egg chamber. The widespread expression of the Btk/Dsrc29A product also suggests that this gene is indeed pleiotropic. This contrasts with the physiological functions of its mammalian counterparts, Btk and Itk, which play specific roles in the differentiation of B and T cells, the only cell types which express the respective kinases. It is speculated that the Btk/Dsrc29A-like kinases, which are widely expressed and are pleiotropic in function, represent ancestral members of the Btk kinase family, which have developed during mammalian evolution by gene duplication. It is probable that the descendants of the Btk kinase family, presumably arising from gene duplication, acquired specific roles only in the cell types to which their expression is limited. Btk and Itk are probably representatives of such kinases.
The Btk/Dsrc29A gene is distinctly different from other members of the Btk family genes in two respects. First, it is alternatively spliced so as to give rise to two different proteins, one of which has, at its N terminus, a unique domain without sequence homology to any other known protein, in place of the PH domain in the sibling protein. Second, the N-terminal segments in both isomers are separated from the main body of the protein by an intervening polyglycine stretch. This structural complexity may be necessary for the support of the pleiotropic functions of the Btk/Dsrc29A kinase in Drosophila, in which no other members of the Btk kinase family exist.
It is of interest that the PH domain is dispensable for normal copulation and survival, as evidenced by the observation that the hs-type 1 cDNA was effective in rescuing both phenotypes when expressed in ficP. It should be pointed out, however, that the adult life span of the rescued mutant flies was not identical to that of wild-type flies. Such partial rescue may indicate that the function of the type 1 product only partially overlaps that of the type 2 product. The type 1 product, when ubiquitously overexpressed by heat shock induction, can complement the loss of the type 2 product to a certain extent. Alternatively, ectopic expression, inappropriate temporal expression, or genomic insertion site-specific expression of the hs-cDNA transgene could be the cause of the incomplete rescue. The structural motifs common to type 1 and 2 isoforms may be involved in the molecular interactions required for normal genital development and survival.
The identification of unique associating proteins for Btk/Dsrc29A would provide insights into the molecular signals crucial for genital formation and for longevity. The yeast two-hybrid system would probably enable us to identify candidate proteins that potentially interact with the functional domains of Btk/Dsrc29A. Screening for dominant modifiers of the ficP phenotypes might be a feasible genetic approach to elucidate the molecular cascade of the action of Btk/Dsrc29A in vivo. Such experiments are currently in progress.
ACKNOWLEDGMENTS
We thank K. Ito for his help in determining the expressing cells; E. Roulier and S. K. Beckendorf for sharing their unpublished results on Btk/Dsrc29A; Y. N. Jan, T. Uemura, T. L. Schwarz, and K. Zinn for the cDNA libraries; Eric Nilsson for his guidance on statistics; the members of our laboratory for their helpful suggestions about the manuscript; and Y. Hotta for his encouragement. We also thank June Takahashi and Sachiko Kondo for their secretarial assistance.
REFERENCES
- 1.Awad T A, Truman J W. Postembryonic development of the midline glia in the CNS of Drosophila: proliferation, programmed cell death, and endocrine regulation. Dev Biol. 1997;187:283–297. doi: 10.1006/dbio.1997.8587. [DOI] [PubMed] [Google Scholar]
- 2.Belmont J W. Insights into lymphocyte development from X-linked immune deficiencies. Trends Genet. 1995;11:112–116. doi: 10.1016/S0168-9525(00)89012-5. [DOI] [PubMed] [Google Scholar]
- 3.Brand A H, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 4.Bryant P J, Hsei B W. Pattern formation in asymmetrical and symmetrical imaginal discs of Drosophila melanogaster. Am Zool. 1977;17:595–611. [Google Scholar]
- 5.Cooper J A, Howell B. The when and how of Src regulation. Cell. 1993;73:1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- 6.Davis R L. Physiology and biochemistry of learning mutants. Physiol Rev. 1996;76:299–317. doi: 10.1152/physrev.1996.76.2.299. [DOI] [PubMed] [Google Scholar]
- 7.de Belle J S, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- 8.Erpel T, Courtneidge S A. Src family protein tyrosine kinases and cellular signal transduction pathways. Curr Opin Cell Biol. 1995;7:176–182. doi: 10.1016/0955-0674(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 9.Finley K D, Taylor B J, Milstein M, McKeown M. Dissatisfaction, a gene involved in sex-specific behavior and neural development of Drosophila melanogaster. Proc Natl Acad Sci USA. 1997;94:913–918. doi: 10.1073/pnas.94.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenspan R J. Understanding the genetic construction of behavior. Sci Am. 1995;272:72–78. doi: 10.1038/scientificamerican0495-72. [DOI] [PubMed] [Google Scholar]
- 11.Gregory R J, Kammermeyer K L, Vincent W S, Wadsworth S G. Primary sequence and developmental expression of a novel Drosophila melanogaster src gene. Mol Cell Biol. 1987;7:2119–2127. doi: 10.1128/mcb.7.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guarnieri D J, Dodson G S, Simon M A. Src64 regulates the localization of a Tec-family kinase required for Drosophila ring canal growth. Mol Cell. 1998;1:831–840. doi: 10.1016/s1097-2765(00)80082-9. [DOI] [PubMed] [Google Scholar]
- 13.Hall J C. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 14.Hall J C, Siegel R W, Tomkins L, Kyriacou C P. Neurogenetics of courtship in Drosophila. Stadler Genet Symp. 1980;12:43–82. [Google Scholar]
- 15.Hanahan D, Lane D, Lipsich L, Wigler M, Botchan M. Characteristics of an SV-40 plasmid recombinant and its movement into and out of the genome of a murine cell. Cell. 1980;21:127–139. doi: 10.1016/0092-8674(80)90120-8. [DOI] [PubMed] [Google Scholar]
- 16.Harlan J E, Hajduk P J, Yoon H S, Fesik S W. Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature. 1994;371:168–170. doi: 10.1038/371168a0. [DOI] [PubMed] [Google Scholar]
- 17.Haslam R J, Koide H B, Hemmings B A. Pleckstrin domain homology. Nature. 1993;363:309–310. doi: 10.1038/363309b0. [DOI] [PubMed] [Google Scholar]
- 18.Ingley E, Hemmings B A. Pleckstrin homology (PH) domains in signal transduction. J Cell Biochem. 1994;56:436–443. doi: 10.1002/jcb.240560403. [DOI] [PubMed] [Google Scholar]
- 19.Ito H, Fujitani K, Usui K, Shimizu-Nishikawa K, Tanaka S, Yamamoto D. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc Natl Acad Sci USA. 1996;93:9687–9692. doi: 10.1073/pnas.93.18.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- 21.Ito K, Urban J, Technau G M. Distribution, classification, and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Roux’s Arch Dev Biol. 1995;204:284–307. doi: 10.1007/BF02179499. [DOI] [PubMed] [Google Scholar]
- 22.Kipreos E T, Wang J Y J. Cell cycle-regulated binding of c-Abl tyrosine kinase to DNA. Science. 1992;256:382–385. doi: 10.1126/science.256.5055.382. [DOI] [PubMed] [Google Scholar]
- 23.Kulkarni S J, Steinlauf A F, Hall J C. The dissonance mutant of courtship song in Drosophila melanogaster: isolation, behavior and cytogenetics. Genetics. 1988;118:267–285. doi: 10.1093/genetics/118.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurosaki T, Kurosaki M. Transphosphorylation of Bruton’s tyrosine kinase on tyrosine 551 is critical for B cell antigen receptor function. J Biol Chem. 1997;272:15595–15598. doi: 10.1074/jbc.272.25.15595. [DOI] [PubMed] [Google Scholar]
- 25.Langhans-Rajasekaran S A, Wan Y, Huang H-Y. Activation of Tsk and Btk tyrosine kinases by G protein βγ subunits. Proc Natl Acad Sci USA. 1995;92:8601–8605. doi: 10.1073/pnas.92.19.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehmann R, Tautz D. In situ hybridization to RNA. In: Goldstein L S, Fyrberg E A, editors. Drosophila melanogaster: practical uses in cell and molecular biology. London, United Kingdom: Academic Press, Ltd.; 1994. pp. 575–598. [DOI] [PubMed] [Google Scholar]
- 27.Maekawa M, Li S, Iwamatsu A, Morishita T, Yokota K, Imai Y, Kohsaka S, Nakamura S, Hattori S. A novel mammalian Ras GTPase-activating protein which has phospholipid-binding and Btk homology regions. Mol Cell Biol. 1994;14:6879–6885. doi: 10.1128/mcb.14.10.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattsson P T, Vihinen M, Smith C I E. X-linked agammaglobulinemia (XLA): a genetic tyrosine kinase (Btk) disease. Bioessays. 1996;18:825–834. doi: 10.1002/bies.950181009. [DOI] [PubMed] [Google Scholar]
- 29.Pitcher J A, Touhara K, Payne E S, Lefkowitz R J. Pleckstrin homology domain-mediated membrane association and activation of the β-adrenergic receptor kinase requires coordinate interaction with Gβγ subunits and lipid. J Biol Chem. 1995;270:11707–11710. doi: 10.1074/jbc.270.20.11707. [DOI] [PubMed] [Google Scholar]
- 30.Prokop A, Technau G M. Normal function of the mushroom body defect gene of Drosophila is required for the regulation of the number and proliferation of neuroblasts. Dev Biol. 1994;161:321–337. doi: 10.1006/dbio.1994.1034. [DOI] [PubMed] [Google Scholar]
- 31.Rawlings D J, Saffran D C, Tsukada S, Largaespada D A, Grimaldi J C, Cohen L, Mohr R N, Bazan J F, Howard M, Copeland N G, Jenkins N A, Witte O N. Mutation of unique region of Bruton’s tyrosine kinase in immunodeficient X1D mice. Science. 1993;216:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 32.Rawlings D J, Scharenberg A M, Park H, Wahl M I, Lin S, Kato R M, Fluckiger A-C, Witte O N, Kinet J-P. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 33.Roulier E M, Panzer S, Beckendorf S K. The Tec 29 tyrosine kinase is required during Drosophila embryogenesis and interacts with Src 64 in ring canal development. Mol Cell. 1998;1:819–829. doi: 10.1016/s1097-2765(00)80081-7. [DOI] [PubMed] [Google Scholar]
- 34.Ryner L C, Goodwin S F, Castrillon D H, Anand A, Villella A, Baker B S, Hall J C, Taylor B J, Wasserman S A. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/s0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- 35.Siliciano J D, Morrow T A, Desiderio S V. itk, a T-cell-specific tyrosine kinase gene inducible by interleukin 2. Proc Natl Acad Sci USA. 1992;89:11194–11198. doi: 10.1073/pnas.89.23.11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stocker R F, Lienhard M C, Borst A, Fischbach K F. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990;262:9–34. doi: 10.1007/BF00327741. [DOI] [PubMed] [Google Scholar]
- 37.Superti-Furga G, Courtneidge S A. Structure-function relationships in Src family and related protein tyrosine kinases. Bioessays. 1995;17:321–330. doi: 10.1002/bies.950170408. [DOI] [PubMed] [Google Scholar]
- 38.Theroux S J, Wadsworth S C. Protein-tyrosine kinase activity of alternate protein products of the Drosophila Dsrc28C locus. FEBS Lett. 1992;311:1–6. doi: 10.1016/0014-5793(92)81353-n. [DOI] [PubMed] [Google Scholar]
- 39.Thomas S M, Soriano P, Imamoto A. Specific and redundant roles of Src and Fyn in organizing the cytoskeleton. Nature. 1995;376:267–271. doi: 10.1038/376267a0. [DOI] [PubMed] [Google Scholar]
- 40.Tsukada S, Saffran D C, Rawlings D J, Parolini O, Allen R C, Klisak I, Sparkes R S, Kubagawa H, Mohandas T, Quan S, Belmont J W, Cooper M D, Conley M E, Witte O N. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 41.Uckun F M, Waddick K G, Mahajan S, Jun X, Takata M, Bolen J, Kurosaki T. BTK as a mediator of radiation-induced apoptosis in DT-40 lymphoma B cells. Science. 1996;273:1096–1100. doi: 10.1126/science.273.5278.1096. [DOI] [PubMed] [Google Scholar]
- 42.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarström L, Kinnon C, Levinsky R, Bobrow M, Smith C I E, Bentley D R. The gene involved in X-linked agammaglobulinemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 43.Vincent W S, Gregory R J, Wadsworth S C. Embryonic expression of a Drosophila src gene: alternative forms of the protein are expressed in segmental stripes and in the nervous system. Genes Dev. 1989;3:334–347. doi: 10.1101/gad.3.3.334. [DOI] [PubMed] [Google Scholar]
- 44.von Schilcher F. The behavior of cacophony, a courtship song mutant in Drosophila melanogaster. Behav Biol. 1976;17:187–196. doi: 10.1016/s0091-6773(76)90444-2. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto D, Ito H, Fujitani K. Genetic dissection of sexual orientation: behavioral, cellular, and molecular approaches in Drosophila melanogaster. Neurosci Res. 1996;26:95–107. doi: 10.1016/s0168-0102(96)01087-5. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto D, Jallon J-M, Komatsu A. Genetic dissection of sexual behavior in Drosophila melanogaster. Annu Rev Entomol. 1997;42:551–585. doi: 10.1146/annurev.ento.42.1.551. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto D, Nakano Y. Genes for sexual behavior. Biochem Biophys Res Commun. 1998;246:1–6. doi: 10.1006/bbrc.1998.8259. [DOI] [PubMed] [Google Scholar]
- 48.Yokokura T, Ueda R, Yamamoto D. Phenotypic and molecular characterization of croaker, a new mating behavior mutant of Drosophila melanogaster. Jpn J Genet. 1995;70:103–117. doi: 10.1266/jjg.70.103. [DOI] [PubMed] [Google Scholar]