Abstract
Chronic diseases are among the top causes of global death, disability, and healthcare expenditure. Digital health interventions (e.g., patient support delivered via technologies such as smartphones, wearables, videoconferencing, social media, virtual reality) may prevent and mitigate chronic disease by facilitating accessible, personalized care. While these tools have promise to reach historically marginalized groups, who are disproportionately affected by chronic disease, evidence suggests digital health interventions could unintentionally exacerbate health inequities. This commentary outlines opportunities to harness recent advancements in technology and research design to drive equitable digital health intervention development and implementation. We apply “calls to action” from the World Health Organization (WHO) Commission on Social Determinants of Health (CSDH) conceptual framework to the development of new, and refinement of existing, digital health interventions that aim to prevent or treat chronic disease by targeting intermediary, social, and/or structural determinants of health. Three mirrored “calls to action” are thus proposed for digital health research: 1) Develop, implement, and evaluate multi-level, context-specific digital health interventions; 2) Engage in intersectoral partnerships to advance digital health equity and social equity more broadly; and 3) Include and empower historically marginalized groups to develop, implement, and access digital health interventions. Using these “action items”, we review several technological and methodological innovations for designing, evaluating, and implementing digital health interventions that have greater potential to reduce health inequities. We also enumerate possible challenges to conducting this work, including leading interdisciplinary collaborations, diversifying the scientific workforce, building trustworthy community relationships, and evolving healthcare and digital infrastructures.
Keywords: digital health, health equity, technology, chronic disease, health disparities
Background
Over 70% of all global deaths can be attributed to chronic diseases, with close to one third of adults reporting multiple chronic conditions (1, 2). These chronic diseases contribute to extraordinary health care costs, loss of productivity and poorer quality of life (3–5). Furthermore, there are clear disparities in chronic disease observed across race, ethnicity, gender, sexual orientation, age, disability status, socioeconomic status, and geographic location (hereafter referred to as “historically marginalized groups”) (2, 6). There is thus a widely-recognized need to promote health equity and reduce health inequities in chronic disease prevention and treatment (7, 8). In this regard, digital health interventions (e.g., patient support delivered via technologies such as smartphones, wearables, videoconferencing, social media, virtual reality) are exciting tools with potential for increasing scalability and access to evidence-based support for health behavior change (9, 10). Unfortunately, evidence suggests that digital health interventions may be less effective for historically marginalized groups, and may unintentionally exacerbate inequities (11–13). This phenomenon is likely attributable to numerous inequities inherent in digital innovation (14); innovations developed based on the majority are often unsuccessfully translated to historically marginalized groups due to poor representation and inclusion in research throughout the “bench to bedside” pipeline, as well as inattention to important structural and social factors impacting these groups (15).
Experts have therefore highlighted the need for using action-oriented health equity frameworks to guide digital health intervention work (11). These frameworks act as scaffolds for helping researchers deeply understand the ecosystems perpetuating social disadvantage among the communities they wish to serve, thus resulting in digital health interventions that are more likely to promote health equity and less likely to maintain inequities. Previous commentaries have focused on developing novel integrated health equity, digital health, and behavioral science frameworks that provide high-level recommendations for future research (10, 16). For example, Alcaraz and colleagues proposed the ConNECT framework, which integrates principles from behavioral medicine and health equity fields to posit five broad guidelines for prevention/intervention research (from basic science to dissemination, inclusive of digital health) (10). This work has inherent value in breaking down siloes, however few existing commentaries focus on how to apply prevailing social determinants of health frameworks to galvanize equitable digital health intervention. This commentary seeks to add to prior literature by using an already-established social determinants of health framework with extant action-oriented principles (rather than developing another integrated model with new guidelines) as a basis for applying recent technological and methodological advances to develop, refine, and implement new or existing digital health interventions that reduce health inequities. Additionally, we seek to illustrate how such a framework can, in turn, inform novel research directions for continuing to advance the technologies and methods discussed herein.
First, we describe how digital health innovation can be embedded within the World Health Organization (WHO) Commission on Social Determinants of Health (CSDH) conceptual framework, which illustrates the complexity of social, economic, and political mechanisms that drive health inequities. We selected the CSDH framework because it was developed with the intent to guide intervention and policies to reduce inequities. Second, we posit how the CSDH “calls to action” could be used to refine existing digital health interventions and drive future digital health research. Third, we enumerate challenges to conducting this work.
Embedding Digital Health Innovation within a Social Determinants of Health Framework
The CSDH conceptual framework for alleviating inequities in health and well-being is shown in Figure 1 (17). Building upon previous social determinants of health frameworks (18–20), the CSDH framework highlights levels of causal impact on health inequities by distinguishing between social, economic, and political context as key drivers of social hierarchy and, in turn, daily living conditions (21). This framework shows Socioeconomic and Political Context (e.g., Policy, Societal Values) as key structural determinants but also demonstrates how other Structural and Social Determinants of Equity in Society (e.g., gender, racism, income) shape Intermediary Determinants of Health, and ultimately perpetuate health inequities. The Intermediary level includes material (e.g., physical environment), psychosocial (e.g., stressors) and behavioral and biological factors (e.g., nutrition, genetics).
Figure 1.
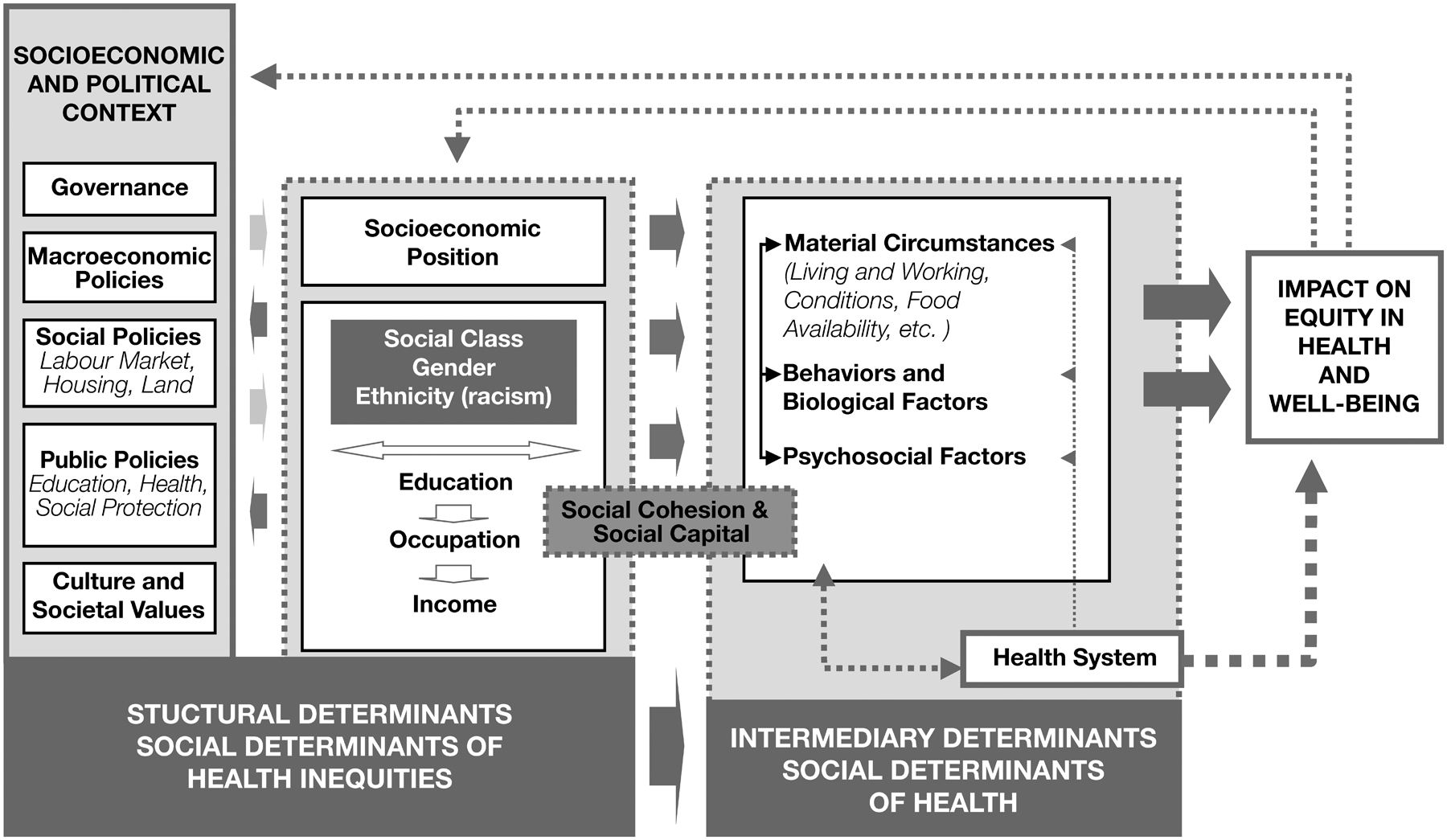
World Health Organization (WHO) Commission on Social Determinants of Health (CSDH) Conceptual Framework to guide action aimed towards reducing inequities in health and quality of life (17). Reproduced with permission from: Solar O, Irwin A. A conceptual framework for action on the social determinants of health. Social Determinants of Health Discussion Paper 2 (Policy and Practice), p. 48, Copyright 2010 (21).
Many digital interventions to prevent and treat chronic disease target intermediary level determinants, namely the biological, psychological, and/or behavioral factors that influence health (22, 23). Research has shown that health-related behaviors (e.g., cigarette smoking, physical activity, diet), as well as biological processes (e.g., gene variants, inflammatory reactions, cortisol production) and psychological factors (e.g., positive and negative affect, depression, optimism), are related to socioeconomic position and health outcomes (24–29). However, biological, psychological, and behavioral factors often do not completely explain the association between social context and health, indicating that continuing to target only intermediary determinants of health will be insufficient for promoting health equity (17, 26, 27, 30, 31).
Acknowledging the importance of considering social context and varied socioeconomic positions in future efforts to advance health equity, the CSDH framework posits three “calls to action”: (1) the importance of context-specific strategies and tackling structural as well as intermediary determinants; (2) intersectoral action; and (3) social participation and empowerment (17). Below, we propose three mirrored “calls to action” in the field of digital health that align with the action items from the CSDH framework and discuss how technological and methodological innovation can both support, and be driven by, these efforts.
“Calls to Action” to Develop and Implement Equitable Digital Health Interventions
Our CSDH-consistent “calls for action” apply to the development of new, and refinement of existing, digital health interventions that aim to prevent or treat chronic disease by targeting intermediary, social, and/or structural determinants of health. These calls to action (not in order of priority) are: 1) Develop, implement, and evaluate multi-level, context-specific digital health interventions, 2) Engage in intersectoral partnerships to advance digital health equity and social equity more broadly; and 3) Include and empower historically marginalized groups to develop, implement, and access digital health interventions. In the context of each call, we discuss how recent technological and methodological advancements can result in more equitable digital health interventions. Table 1 provides examples, along with priority areas for researchers who intend to harness these approaches for reducing health inequities. Table 2 (and Table S1, Supplemental Digital Content) contains practical recommendations and training resources for those who would like to incorporate the below-described technologies and/or methods into their work.
Table 1.
Examples and Suggested Research Priorities for Technologies and Methods that Can Support Equitable Digital Health Interventions
| Proposed Call to Action based on WHO CSDH | Supporting Technologies & Methods | Example(s)* *denotes hypothetical example |
Research Priorities |
|---|---|---|---|
| Develop, implement, and evaluate multi-level, context-specific digital health interventions | Optimization methods (Multiphase Optimization Strategy [MOST], Sequential Multiple Assignment Randomized Trials [SMART], Micro-randomized Trial [MRT]) | Factorial experiment (MOST) to optimize engagement in HIV care among Black & Hispanic people living with HIV by evaluating 5 culturally-based treatment additions to usual care (e.g., support group, peer mentors, patient navigation) (94) SMART evaluates how, when, and in what combinations to use EHR-based interventions, text messaging, and phone counseling to increase reach and feasibility of a smoking cessation treatment in low-income Community Health Centers (95) An MRT targeting physical activity considers a user’s geospatial location and concerns about neighborhood safety to suggest appropriate exercise options* |
1) Leverage optimization designs to intervene directly on social or structural determinants of health; 2) Integrate optimization designs with community-engaged research methods; 3) Address questions related to burden (what is the ideal dose of assessments/interventions) and cost-effectiveness |
|
Advanced analytics
(e.g., machine learning, network analysis) |
Study evaluating different ways to train machine learning models to improve prediction of cancer prognosis among African Americans in a large cohort dataset (96) An N-of-1 study explores how stress interventions can impact diet and physical activity among ethnically minoritized mothers over 6 months (97)* |
1) Solutions to prevent/address algorithm bias; 2) Understand social/structural impacts on algorithm performance and perceptions of precision medicine; 3) Use N-of-1 study designs to bolster research while improving patient care and outcomes. | |
| Combining digital assessment tools | Using transdermal alcohol concentration readings to validate ecological momentary assessment ratings of alcohol use among adults experiencing homelessness (98) Pairing heart rate variability with geolocation of neighborhood safety, food sources, & walkability contextualizes stress among individuals in blighted neighborhoods * |
1) Continue to validate tools and methods across multiple settings & individuals from historically marginalized communities; 2) Develop best practices on how to triangulate data from multiple assessments | |
| Implementation science | Series of five implementation studies of digital health tools in low-resource settings–gathered formative qualitative input from end users in groups experiencing structural healthcare barriers & designed to address specific community barriers to care (99) Use the Double Diamond model (iterative user-centered design with ongoing evaluation) to develop a digital COVID-19 vaccination promotion platform to address disparities in information access & improve uptake (100) |
1) Utilize established conceptual frameworks to guide engaging with stakeholders; 2) Adapt existing interventions to align with a given healthcare context (e.g., community health centers, primary care, daily life app use) | |
| Engage in intersectoral partnerships to advance digital health equity and social equity more broadly | Innovative partnerships to improve access & address digital divide | An electronic health record provider offers an application programming interface (API) to securely read in data from a smartwatch app that passively monitors activity, heart rate, & sleep.* A healthcare provider partners with educators and researchers to develop a digital health literacy program.* |
1) Generate evidence on the process of successful intersectoral collaboration; 2) Develop common digital platforms to promote shared space across sectors; 3) Improve interoperability and clarify centralization of technology in healthcare; 4) Conduct studies that allow technologies to meet regulation and accreditation standards |
| Engage in intersectoral partnerships to advance digital health equity and social equity more broadly | Combining digital assessments & Advanced analytics | A researcher partners with a local community health center to collect data on substance use patterns, location, and stress using a wearable devices & uses the findings to argue for a state-run safe injection site.* | 1) Provide mechanisms to support funding for integrating digital innovations, particularly among financially struggling organizations; 2) Use ‘information officers’ within organizations to improve trust and enhance willingness; 3) Conduct feasibility studies to quantify the potential labor new innovations requires of staff |
|
Community-engaged research methodologies
(e.g., CBPR) |
Food distribution stakeholders, digital health developers, community organizations, and community members partner to develop an ‘app’ that supports individuals in accessing food assistance resources in their local community, providing feedback on how they are using food assistance, and identifying areas of greater need for food assistance.* | 1) Use CBPR to fully appreciate macro-, meso-, and micro- barriers to uptake of digital innovations; 2) Adopt CBPR practices and priorities to garner trust and understanding between stakeholders | |
| Include and empower historically marginalized groups to develop, implement, and access digital health interventions |
Community-engaged research methodologies (e.g., CBPR) |
Work with community members from historically marginalized communities to communicate research results utilizing digital health platforms (101) The Family Listening Program used digital stories from community members to translate and disseminate research findings into the broader community (102) |
1) Combine CBPR with mixed methods approaches to understand determinants of health at multiple levels prior to developing digital health interventions; 2) Formally integrate CBPR methods into optimization frameworks; 3) Leverage existing partnerships to empower community stakeholders and bolster cross-sector collaboration |
| Social media | Exposure to a Facebook page with sexually transmitted infection prevention messages improves condom use over 2 months (103) A trial finds that a Facebook smoking cessation intervention tailored to sexual and gender minority smokers reduces smoking over 3 months (104) |
1) Conduct subgroup analyses to understand how social context impacts efficacy & engagement w/social media interventions; 2) Develop social media interventions via CBPR; 3) Develop digital literacy interventions that target productive engagement with social media. |
Table 2.
Practical Recommendations and Training Resources for Working with Technologies and Methods to Equitable Digital Health Interventions
| Supporting Technologies & Methods | Practical Recommendations (What to know when getting started) |
Training Resources (How to learn more about technologies/methods) |
|---|---|---|
| Optimization methods | 1) Designs require expertise to plan & execute randomization; 2) Pilot studies are necessary to determine intervention components & optimization parameters; 3) Aiming to maximize cost-effectiveness is critical for equity work |
|
| Advanced analytics | 1) Use publicly-available datasets or secondary analysis of your own data to test initial models and feasibility, but collecting your own sample may be desired; 2) Run descriptives prior to model development so you know who the models are learning from; 3) Model development is an iterative and collaborative process |
|
| Combining digital assessments | 1) Be sure that the tool has been validated in your population; 2) If funding is a concern – consider doing this work on a subset of participants or structuring data collection so you can re-use devices; 3) Consider how privacy concerns may differ across populations | |
| Implementation science | 1) Address multiple steps along a patient’s journey to receiving a digital health tool; implementation strategies can be deployed at all levels of a system (clinic, doctor, payer, health network); 2) Allow room for flexibility to adapt existing interventions to meet needs of diverse populations |
|
| Innovative partnerships to target access & address digital divide | 1) Use structured frameworks to guide collaborative decision-making; 2) Address power dynamics via hypothetical scenarios, continuous assessment, and accountability benchmarks; 3) Mutual training across sectors contributes to success; 4) Clearly differentiating between goals of intervention or policy implementation and policy design will be helpful. |
|
| Community-engaged research methodologies | 1) Focus on building on strengths and assets of a community vs. taking a deficit-focused approach; 2) Allow for additional time and funds - although the process may take more time and money, the results will be more relevant and sustainable |
|
| Social media | 1) Directly address mistrust and privacy concerns specific to the target population during consent and in the context of the intervention itself; 2) Social media technologies evolve rapidly – be mindful of potential for updating technology in future implementation & sustainment efforts; 3) Consider needs for combating misinformation & promoting safe Internet practices |
Note: URLs to all listed resources provided can be found in the Supplemental Digital Content, Table S1
First call to action: Develop, implement, and evaluate multi-level, context-specific digital health interventions
“Context” is defined within the CSDH framework as ‘all social and political mechanisms that generate, configure, and maintain social hierarchies’(17). A common criticism of many digital health interventions is that they primarily focus on intermediary determinants, and often overlook relevant ‘social contexts’ (i.e., structural and social determinants of health) (32, 33). We discuss how novel research methods, advanced analytics, and assessment approaches could support the development, implementation, and evaluation of digital health interventions targeting determinants of health at multiple levels.
Apply optimization methods to incorporate social and structural determinants of health.
The optimization framework was derived from engineering principles and involves three phases: preparation (e.g., finalizing conceptual model, piloting), optimization (e.g., developing an optimal intervention), and evaluation (e.g., confirming effectiveness/efficacy of the optimized intervention) (34). We focus on the research methods used in the optimization phase (hereafter referred to as optimization methods). These approaches aim to “engineer an intervention” by identifying the ideal intervention component(s) and/or the best timing or sequencing of intervention delivery that leads to optimal treatment outcomes (35). Optimization primarily occurs by considering practical constraints (e.g., time, cost, scalability) and individual needs (e.g., based on individual characteristics and/or response to interventions). Optimization methods can therefore be used to tailor digital health interventions to individual and contextual needs prior to conducting efficacy or pragmatic trials (36, 37).
Optimization methods encompass three main approaches (examples provided in Table 1). The Multiphase Optimization Strategy (MOST) framework allows for simultaneous testing of multiple components at once to identify the most effective combination of interventions, while optimizing relevant practical elements (e.g., cost-effectiveness) (34, 36, 38). Sequential Multiple Assignment Randomized Trials (SMARTs) can optimize stepped-care approaches, which increases efficiency and cost-effectiveness of digital health implementation (36). Micro-Randomized Trials (MRTs) can empirically evaluate how to tailor in-the-moment digital health interventions to someone’s immediate internal state and relevant social determinants of health (37).
Optimization methods are commonly being applied to ensure that our best available interventions targeting intermediary determinants also address social and structural contexts (e.g., tailoring an evidence-based behavioral intervention to a particular culture or treatment setting). To ensure cost-effective interventions that can be widely disseminated, these designs should focus on optimal interventions that are more feasible for implementation within extant structures, like healthcare or education systems. Furthermore, these powerful research designs could lead to interventions that directly target social and structural determinants of health. For example, a study could seek to optimize operations in structures that promote health equity (e.g., early education, community prevention programs) and the empirical data on cost savings and improved health outcomes could catalyze widespread programmatic change.
Incorporate advanced analytics to inform context- and person-specific digital health interventions, and enhance digital infrastructure.
Personalized medicine typically uses advanced analytic approaches (e.g., machine learning, network analysis) to glean salient drivers of health behavior change, and can thus be used to create digital health interventions that are tailored to these factors. For example, “big” datasets from hundreds of thousands of individuals can be used to develop algorithms for detecting a disease state, predicting behavior, and/or selecting an optimal treatment (39). There are also “small” data paradigms, or N-of-1 methods, in which algorithms can be built for each individual based on their own data (rather than using data from a examples of how these methods can improve digital health interventions for historically marginalized groups.
One clear path for advanced analytics to reduce health inequities is by providing support that is tailored to social or material circumstances (41–43). Ideally, this support is more accessible than standard care because it facilitates automated, personalized intervention sent directly to the individual. However, this form of personalized medicine often requires that participants possess certain technologies (e.g., recent smartphone models, wireless internet, wearable devices), as well as levels of digital literacy, that are less prevalent in some historically marginalized groups (44). Creating tools that provide precision care regardless of device quality (e.g., intervention delivery via text message, e-mail, or mail) and improving digital literacy are therefore important areas for future work. Advanced analytics could also be applied to improve the quality of healthcare, education, or other community resources. For example, machine learning could be integrated within digitized record systems to better understand and anticipate the needs of a community or an individual (e.g., improve clinical care via automated clinical decision support, identify families in need of services via housing records). While promising, this work is time- and resource-intensive, including requiring expertise to maintain novel digital infrastructures. Additionally, there is an urgent need to understand and address algorithm bias to ensure positive impact among historically marginalized groups (see Parikh et al for details (45)).
Combine digital assessment tools to achieve context-specific evaluation of digital and non-digital interventions.
Digital tools (e.g., wearable devices, smartphones, remote sensing) support detailed data collection on health outcomes, as well as relevant intermediary, social, and structural determinants of health, over long periods of time. They can provide insight into the various structural and social contexts that shape an individual’s health (and vice versa) before, during, and after digital and non-digital interventions. Measuring a single outcome of interest via two types of digital tools (i.e., using both smartphone-based subjective reports of stress and heart rate variability) is commonly referred to as multimodal assessment. Multimodal assessment is especially important among historically marginalized groups because some digital tools (developed and validated on the majority) can be less accurate and reliable when translated to the minority (e.g., FitBits may produce less accurate estimates of heart rate, oxygen saturation, and energy expenditure for people with darker skin tones (46, 47)). Multimodal assessment should be employed to validate new technologies and measurement approaches among historically marginalized groups (see Table 1 for an example), which ultimately improves the quality of outcomes assessment for (non)digital interventions.
Digitally measuring multiple outcomes of interest (e.g., a health outcome and its contextual correlates) could also be employed in a study or program evaluation. For example, a study could pair a measure of heart rate variability with location-based data to contextualize the experiences of stress among individuals living in blighted neighborhoods. Results from such assessment protocols can improve our understanding of how our (non)digital interventions are working within historically marginalized groups and therefore inform the refinement of these interventions to be more impactful. Moreover, the data could be combined to improve the measurement quality of digital tools for those in historically marginalized groups. For example, global position systems (GPS) have been integrated with accelerometry to ascertain physical activity type and setting (vs. standard step counts or physical activity minutes) (48).
Use implementation science to inform the dissemination of digital health interventions through explicit attention to structural contexts.
After initial development, digital health interventions that are designed to prioritize health equity must be thoughtfully integrated into the ecosystems in which individuals receive care. Digital health intervention uptake is catalyzed by provider referral, and engagement is often enhanced by clinician support and coaching (49, 50); however, many barriers exist to getting these interventions into the hands of users who may benefit (51). Implementation science can maximize reach and impact of digital health interventions by using established, equity-focused frameworks to answer questions about how best to engage with stakeholders and adapt interventions (and their implementation) to align with a given healthcare context. For example, the Health Equity Implementation Framework conceptualizes implementation determinants at the level of the organization/healthcare delivery system, recipients, and the technology itself, with explicit attention devoted to healthcare inequities manifested during implementation (e.g., details of the clinical encounter and understanding societal-level influences on implementation outcomes) (52, 53). Guided by such models, researchers can employ a variety of established implementation strategies to promote sustainable, real-world delivery of novel digital health interventions (54).
Second call to action: Engage in intersectoral partnerships to advance digital health equity and social equity more broadly
Intersectoral partnerships across key players in the healthcare ecosystem and outside of health sector (e.g., education, governmental agencies, housing authorities, civil rights organizations, policymakers) is required to achieve and sustain digital health equity (17). We outline two overarching areas in which intersectoral collaboration can facilitate equitable digital health intervention: 1) reducing structural barriers to digital access and supporting digital infrastructure, and 2) developing novel intervention approaches.
Efforts to reduce structural barriers and support digital infrastructure are particularly critical for ensuring equitable access to digital innovations.
Such efforts can include policy-level change (e.g., broadband access), but also systems- or program-level change (e.g., digital literacy education). Suggestions include (but are not limited to): identifying and serving areas of highest need for broadband expansion; reimbursing expenses for, or providing affordable, digital devices to those without access; establishing incentives and cost offsets for use of digital health applications (‘apps’); improving digital literacy and access to technical support (for both staff and patients); addressing issues of data security and privacy; digitization of record systems, and providing intervention content in different languages. Intersectoral efforts can focus on supporting the necessary infrastructure to ensure widespread implementation of novel digital health interventions. For example, private-sector companies (e.g., electronic health record providers) could offer more transparent and accessible options for interoperability between existing healthcare platforms and newly-developed digital health tools to ensure implementation of these tools into usual care (55). While these suggestions can narrow the future digital divide, the importance of considering the current status of the digital divide when designing and studying digital health interventions is discussed in the ‘Potential Challenges and Considerations’ section.
Intersectoral partnerships can be harnessed to inform the development of novel intervention approaches.
Exciting partnerships between researchers, healthcare decision-makers, policymakers, and community members can result in utilizing the above-mentioned technologies and methods (e.g., advanced analytics, combining digital assessments) to inform data-based intervention at the community or policy levels (56, 57). Intersectoral collaboration can also be used to design digital health interventions that directly support practical implementation of equity-focused health policy—regardless of the policy’s initial digital emphasis. See Table 1 for examples of these types of intersectoral actions.
In Table 2, we list practical recommendations for building strong and lasting partnerships needed to advance digital health equity in the main areas described above. However, a notable barrier to intersectoral work is that partners may have competing motivations and priorities. Two plausible solutions are to clearly outline the increasingly recognized benefits of intersectoral and multi-sector collaboration and, subsequently, foster partnerships around shared goals and collective impact (58, 59). There are several published frameworks that serve to guide individuals from different sectors to develop shared agendas to address a specific health need or social problem, including but not limited to the collective impact framework (60) and the Health in All Policies (HiAP) framework (61–63). For detailed recommendations on intersectoral collaboration, see Chircop et al. (59) and Bryson et al. (64).
Third call to action: Include and empower historically marginalized groups to develop, implement, and access digital health interventions.
The goal of social participation and empowerment is to re-distribute power that allows a community to influence decision-making geared towards improving their own health and quality of life (17). Working in close ongoing partnerships with historically marginalized communities is consistent with human rights and justice-oriented right to health perspectives, which can improve perceptions and relationships in communities (7). These community partnerships can lead to more accessible, relevant, engaging, and sustainable digital health interventions. We discuss two methods for engaging and empowering communities through development and implementation of digital health interventions: community based participatory research and social media.
Use a community engaged participatory research approach to empower communities and make digital health interventions more acceptable.
Community engaged research can be defined as “the process of working collaboratively with groups of people affiliated by geographic proximity, special interests, or similar situations with respect to issues affecting their well-being” (65). By working “with” communities instead of “on” communities, research questions and interventions will be more appropriate and meaningful for target populations. Meaningfully engaging with the community can ensure that digital innovation “meets people where they are at” by being mindful of levels of empowerment and access to flexible resources (66). Community engaged research can range from consultations with community partners to using a community based participatory research (CBPR) approach (67, 68). While the approach may vary across projects, it is critical that the shared community engaged principles (e.g., trust, bidirectional influence, equitable financing, shared governance) guide research to ensure meaningful community engagement and health equity outcomes (69).
A recent narrative review posed six best practices that should guide future community engaged research to develop digital health interventions targeted for historically marginalized communities (11). We elaborate on three ways in which recent and future innovations can emulate these best practices (examples provided in Table 1). First, researchers should begin efforts with a deep understanding of the structural factors and social determinants of health and action-oriented frameworks for advancing health equity (11). CBPR methods can be uniquely leveraged, especially within the context of the CSDH framework, to support a nuanced understanding of the determinants of health at multiple levels and at all stages of the research process. Second, community participation should occur throughout intervention development, implementation, evaluation, and dissemination (11). While authors cited integration of CBPR with traditional technology-based design methods (e.g., human-computer interaction, participatory design), we will further posit that research would benefit from formally integrating CBPR methods within the context of newer optimization frameworks described above (e.g., 70). Such innovation would support interdisciplinary collaborations and rigorous programs of research that have direct benefit to historically marginalized groups. Third, community-based research can tap into existing social and community networks while supporting marginalized communities. Partnerships forged by community engaged research can empower community and bolster cross-sector collaboration to design and implement interventions that leverage community assets, leadership, expertise, cultural norms, and systems infrastructure while achieving desired health outcomes (71). By quantifying the ways in which partnerships between communities and researchers impact individuals beyond the resulting digital health intervention, studies could elucidate methods by which we can continue to empower historically marginalized communities.
Use social media as a digital health intervention tool to facilitate social participation.
Participation in social media is a meaningful predictor of social capital and participatory behaviors (both online and offline) (72, 73). Developing digital health interventions that harness social media can engage communities and thereby reduce health inequities. Meta-analytic reviews have revealed that social media interventions for health behavior change were effective for populations demonstrating health inequities, however there was a substantial amount of variability in outcomes (74–76). Given pervasive feelings of mistrust and skepticism of social media that are prevalent among historically marginalized communities, social media interventions for historically marginalized groups could benefit from integrating CBPR methods, as described above (75, 77). Working with communities can lead to social media interventions that are more appealing and safer to the target population. Addressing privacy concerns and the spreading of misinformation is critical to the success of social media interventions, and researchers should remain flexible to considering alternative intervention modalities as appropriate (78).
Another novel research direction could be to develop interventions that specifically target productive engagement with social media more broadly. Effectively engaging with social media has the potential to promote awareness surrounding health inequities and facilitates access to health information. Moreover, raised awareness and increased political discourse on topics related to health and healthcare have the potential to influence policy (79, 80). Interventions to promote engagement with social media platforms could therefore reduce health inequities by empowering individuals to effect change, both directly and indirectly.
Potential Challenges and Considerations
Guided by three “calls to action” from the CSDH framework, we have enumerated several innovations that have the potential to drive the future of equitable digital health interventions for all. However, this work is not without challenges, and we therefore close by discussing logistical and practical barriers to actualizing our proposed research and policy goals.
Interdisciplinary collaboration & training
A significant challenge to the development of equitable digital health interventions is the effort and time required to successfully engage in interdisciplinary collaborations. Bringing researchers from different disciplines together ensures that teams have well-rounded expertise in content areas (e.g., chronic diseases, marginalized populations), but also various empirical methodologies (e.g., qualitative research, mixed methods designs, quantitative research) that could be leveraged in combination to enrich this work. Interdisciplinary teams of researchers with formal training and expertise in health equity and social determinants of health are thus critical. Extrapolating from CDSH’s calls to action, in the context of developing and disseminating equitable digital health interventions, the role of researchers involves growing the evidence base on the underlying causal processes of health inequities and developing or enhancing interventions to effectively alleviate them (81).
In addition to bringing teams from different research disciplines together, these projects must bring in experts from multiple levels—individual (e.g., members of the target population, family members, caregivers), structural (e.g., case workers, providers, healthcare administrators), and policy (e.g., political advocates) (82). Creation of these teams must be carefully considered from the outset, with time allotted to identify experts and forge appropriate partnerships. Moreover, project leaders would benefit from expertise in team organization and communication across disciplines and educational backgrounds (83). Team members would benefit from mutual cross-disciplinary and cross-sector training (59).
Lack of diversity and inclusion on our research teams
Diversity and inclusion in the scientific workforce drives innovation, and is critical for teams seeking to tackle health inequities (84). Along with continued efforts to recruit and retain individuals from diverse backgrounds in higher education, technological innovation can be harnessed to support teams in prioritizing diversity. For example, researchers have made electronic health record databases accessible to scientists across the world who can pose novel research questions and develop new methodologies to test them (85, 86).
Another way to promote diversity and inclusion is to create opportunities for individuals from representative communities to join research teams or conduct research independently. In this regard, there is a rapidly growing field of ‘citizen science’ through which nonscientists engage in scientific research (for detail, see (87)). While applying principles of citizen science and community engaged research can improve digital health equity, several barriers exist, including higher levels of mistrust toward the healthcare system, mistrust of research due to historical events, differences in priorities between community members and other partners, and the lack of time and resources to fully engage with community members (88). Distrust can be heightened with regards to digital health technologies, where human rights issues, such as privacy and confidentiality, are particularly salient (89). There is thus a need to increase research infrastructure in the community, prioritize genuine community engagement in academic research institutions, and continue to evaluate effective ways to engage in partnerships.
Persistent structural, socio-economic, and political challenges
The ever-evolving socio-economic and political contexts that historically marginalized groups face can impact the progress and relevance of digital health interventions (90). Digital health tools for historically marginalized groups must be considerate of, and operate alongside, ongoing structural changes that will disproportionately affect different populations. One such factor is the digital divide. While we make several suggestions for narrowing the digital divide in the long-term, this barrier will unfortunately persist over foreseeable future. Researchers should conduct an in-depth analysis, in partnership with all relevant partners, of how the digital divide impacts the digital infrastructure, digital inclusivity, institutional solutions, and digital proficiency of their target population prior to developing digital health interventions (91, 92). Such information should also be used in planning a study or program evaluation, e.g., reducing participant burden, developing appropriate study timelines, capitalizing on existing institutional/community resources, budgeting study staff and resources. Taking steps to account for the digital divide bolsters more inclusive research and ensures adequate evaluations of efficacy among historically marginalized groups. However, structural and policy-level changes are essential for ensuring that historically marginalized groups can benefit from digital health innovations outside of the context of research studies (90).
There must also be more dedicated public and private funding opportunities that prioritize the necessary work to develop more equitable digital health interventions. These efforts may be geared towards prioritizing: multidisciplinary trials in which at least one of the leads is not an MD/PhD, studies that validate tools or tailor existing treatments for new populations, digital health approaches that tackle social/political determinants of health at multiple levels, simplifying or streamlining tools for populations with low digital health literacy and access, and develop institutional infrastructures necessary to support this work (e.g., community advisory boards).
Lastly, digital interventions, methods, and tools may not fit certain social issues, health conditions, and/or historically marginalized groups. Researchers should remain aware of the overall impacts of technology on societal inequities (93). The decision to use a digital approach should be empirically, conceptually, and theoretically informed. There will be many instances in which non-digital interventions are more appropriate for targeting a particular chronic illness, social issue, and/or historically marginalized group. Importantly, many principles discussed herein, such as the importance of optimization designs, combining digital assessment tools, implementation designs, intersectoral action, and community-based participatory research can also be applied to non-digital interventions to prevent/treat chronic disease in historically marginalized groups.
Conclusions
Digital health interventions demonstrate both potential benefit and harm for addressing health inequities. For digital health interventions to truly revolutionize healthcare for all, efforts must be made to directly address the research and practice gaps that perpetuate this dialectic. The goal of this paper was to illustrate how an established social determinants of health framework can be applied to generate innovative research directions in the equitable development, evaluation, and implementation of novel digital health interventions. In doing so, we illustrated that digital health innovation for addressing health inequities can take many different forms. Innovation can be the technology itself but also the methods to develop, validate, deploy, sustain, and/or integrate technology into the everyday lives of marginalized communities.
Supplementary Material
Source of Funding:
The time to complete this work was supported by the National Institutes of Health. Grant numbers: R01 HL153543 awarded to SPG and K23 HL155733 awarded to HEH Espel-Huynh.
Abbreviations
- WHO
World Health Organization
- CSDH
Commission on Social Determinants of Health
- MOST
Multiphase Optimization Strategy
- SMART
Sequential Multiple Assignment Randomized Trials
- MRT
Micro-Randomized Trial
- GPS
Global Positioning Systems
- HiAP
Health in All Policies
- CBPR
Community based participatory research
- API
Application Programming Interface
Footnotes
Conflicts of Interest: All authors have no conflicts of interest to declare.
References
- 1.Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, Meinow B, Fratiglioni L. Aging with multimorbidity: a systematic review of the literature. Ageing research reviews. 2011;10:430–9. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Noncommunicable Diseases Fact Sheet. 2021. [cited 2022 August 27]; Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases.
- 3.Martin AB, Hartman M, Lassman D, Catlin A, Team NHEA. National Health Care Spending In 2019: Steady Growth For The Fourth Consecutive Year: Study examines national health care spending for 2019. Health Affairs. 2021;40:14–24. [DOI] [PubMed] [Google Scholar]
- 4.Thorpe KE, Chin K, Cruz Y, Innocent M, Singh L. The United States can reduce socioeconomic disparities by focusing on chronic diseases. Health Affairs Blog https://wwwhealthaffairsorg/do/101377/hblog20170817. 2019;61561. [Google Scholar]
- 5.Hajat C, Stein E. The global burden of multiple chronic conditions: a narrative review. Preventive medicine reports. 2018;12:284–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray CJ, Lopez AD. Measuring the global burden of disease. New England Journal of Medicine. 2013;369:448–57. [DOI] [PubMed] [Google Scholar]
- 7.Braveman PA, Kumanyika S, Fielding J, LaVeist T, Borrell LN, Manderscheid R, Troutman A. Health disparities and health equity: the issue is justice. American journal of public health. 2011;101:S149–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braveman P What are health disparities and health equity? We need to be clear. Public health reports. 2014;129:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakken S, Marden S, Arteaga SS, Grossman L, Keselman A, Le P-T, Creber RM, Powell-Wiley TM, Schnall R, Tabor D. Behavioral interventions using consumer information technology as tools to advance health equity. American journal of public health. 2019;109:S79–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alcaraz KI, Sly J, Ashing K, Fleisher L, Gil-Rivas V, Ford S, Yi JC, Lu Q, Meade CD, Menon U. The ConNECT Framework: a model for advancing behavioral medicine science and practice to foster health equity. Journal of behavioral medicine. 2017;40:23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brewer LC, Fortuna KL, Jones C, Walker R, Hayes SN, Patten CA, Cooper LA. Back to the future: achieving health equity through health informatics and digital health. JMIR mHealth and uHealth. 2020;8:e14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arcaya MC, Figueroa JF. Emerging trends could exacerbate health inequities in the United States. Health Affairs. 2017;36:992–8. [DOI] [PubMed] [Google Scholar]
- 13.Gilliard C, Culik H. Digital redlining, access, and privacy. 2016. [cited 2022 03/20/2022]; Available from: https://www.commonsense.org/education/articles/digital-redlining-access-and-privacy.
- 14.Sieck CJ, Sheon A, Ancker JS, Castek J, Callahan B, Siefer A. Digital inclusion as a social determinant of health. NPJ Digital Medicine. 2021;4:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez JA, Clark CR, Bates DW. Digital health equity as a necessity in the 21st century cures act era. Jama. 2020;323:2381–2. [DOI] [PubMed] [Google Scholar]
- 16.Lyles CR, Wachter RM, Sarkar U. Focusing on digital health equity. JAMA. 2021;326:1795–6. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization Commission on Social Determinants of Health. Closing the gap in a generation: health equity through action on the social determinants of health: Commission on Social Determinants of Health final report: World Health Organization; 2008.
- 18.West P Rethinking the health selection explanation for health inequalities. Social science & medicine. 1991;32:373–84. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Oxford University Press; 2002. p. 285–93. [PubMed] [Google Scholar]
- 20.Krieger N Theories for social epidemiology in the 21st century: an ecosocial perspective. International journal of epidemiology. 2001;30:668–77. [DOI] [PubMed] [Google Scholar]
- 21.Solar O, Irwin A. A Conceptual Framework for Action on the Social Determinants of Health [Internet]. Social Determinants of Health Discussion Paper 2 (Policy and Practice), 2010. [Google Scholar]
- 22.Fouad MN, Oates GR, Scarinci IC, Demark-Wahnefried W, Hamby BW, Bateman LB, Estrada JJ, Payton M, Sims M, Miele L. Advancing the science of health disparities through research on the social determinants of health. American journal of preventive medicine. 2017;52:S1–S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C-Q, Leeming E, Smith P, Chung P-K, Hagger MS, Hayes SC. Acceptance and commitment therapy for health behavior change: a contextually-driven approach. Frontiers in psychology. 2018:2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagby SP, Martin D, Chung ST, Rajapakse N. From the outside in: biological mechanisms linking social and environmental exposures to chronic disease and to health disparities. American journal of public health. 2019;109:S56–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowry R, Kann L, Collins JL, Kolbe LJ. The effect of socioeconomic status on chronic disease risk behaviors among US adolescents. Jama. 1996;276:792–7. [PubMed] [Google Scholar]
- 26.Lantz PM, Lynch JW, House JS, Lepkowski JM, Mero RP, Musick MA, Williams DR. Socioeconomic disparities in health change in a longitudinal study of US adults: the role of health-risk behaviors. Social science & medicine. 2001;53:29–40. [DOI] [PubMed] [Google Scholar]
- 27.Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annual review of psychology. 2011;62:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pressman SD, Jenkins BN, Moskowitz JT. Positive affect and health: what do we know and where next should we go? Annual review of psychology. 2019;70:627–50. [DOI] [PubMed] [Google Scholar]
- 29.Trudel-Fitzgerald C, Millstein RA, von Hippel C, Howe CJ, Tomasso LP, Wagner GR, VanderWeele TJ. Psychological well-being as part of the public health debate? Insight into dimensions, interventions, and policy. BMC public health. 2019;19:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marmot MG, Shipley MJ, Rose G. Inequalities in death—specific explanations of a general pattern? The Lancet. 1984;323:1003–6. [DOI] [PubMed] [Google Scholar]
- 31.Brown TM, Cueto M, Fee E. The World Health Organization and the transition from “international” to “global” public health. American journal of public health. 2006;96:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lupton D Health promotion in the digital era: a critical commentary. Health promotion international. 2014;30:174–83. [DOI] [PubMed] [Google Scholar]
- 33.JacksonJames S, WongDavid W, WoodFrederick B, DennyJoshua C, BournePhilip E. Translational health disparities research in a data-rich world. Health Equity. 2019. [Google Scholar]
- 34.Collins LM, Kugler KC. Optimization of behavioral, biobehavioral, and biomedical interventions. Cham: Springer International Publishing. 2018;10:978–3. [Google Scholar]
- 35.Guastaferro K, Shenk CE, Collins LM. The multiphase optimization strategy for developing and evaluating behavioral interventions. 2020.
- 36.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. American journal of preventive medicine. 2007;32:S112–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klasnja P, Hekler EB, Shiffman S, Boruvka A, Almirall D, Tewari A, Murphy SA. Microrandomized trials: An experimental design for developing just-in-time adaptive interventions. Health Psychology. 2015;34:1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guastaferro K, Collins LM. Achieving the goals of translational science in public health intervention research: The multiphase optimization strategy (MOST). American Public Health Association; 2019. p. S128–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beam AL, Kohane IS. Big data and machine learning in health care. Jama. 2018;319:1317–8. [DOI] [PubMed] [Google Scholar]
- 40.Hekler EB, Klasnja P, Chevance G, Golaszewski NM, Lewis D, Sim I. Why we need a small data paradigm. BMC medicine. 2019;17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins FS, Varmus H. A new initiative on precision medicine. New England journal of medicine. 2015;372:793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratcliff CL, Kaphingst KA, Jensen JD. When personal feels invasive: foreseeing challenges in precision medicine communication. Journal of health communication. 2018;23:144–52. [DOI] [PubMed] [Google Scholar]
- 43.Wongvibulsin S, Martin SS, Saria S, Zeger SL, Murphy SA. An individualized, data-driven digital approach for precision behavior change. American Journal of Lifestyle Medicine. 2020;14:289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwon I-WG, Kim S-H, Martin D. Integrating social determinants of health to precision medicine through digital transformation: an exploratory roadmap. International Journal of Environmental Research and Public Health. 2021;18:5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parikh RB, Teeple S, Navathe AS. Addressing bias in artificial intelligence in health care. Jama. 2019;322:2377–8. [DOI] [PubMed] [Google Scholar]
- 46.Shcherbina A, Mattsson CM, Waggott D, Salisbury H, Christle JW, Hastie T, Wheeler MT, Ashley EA. Accuracy in wrist-worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. Journal of personalized medicine. 2017;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feiner JR, Severinghaus JW, Bickler PE. Dark skin decreases the accuracy of pulse oximeters at low oxygen saturation: the effects of oximeter probe type and gender. Anesthesia & Analgesia. 2007;105:S18–S23. [DOI] [PubMed] [Google Scholar]
- 48.Maddison R, Ni Mhurchu C. Global positioning system: a new opportunity in physical activity measurement. International Journal of Behavioral Nutrition and Physical Activity. 2009;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gous N, Boeras DI, Cheng B, Takle J, Cunningham B, Peeling RW. The impact of digital technologies on point-of-care diagnostics in resource-limited settings. Expert review of molecular diagnostics. 2018;18:385–97. [DOI] [PubMed] [Google Scholar]
- 50.Sadasivam RS, Hogan TP, Volkman JE, Smith BM, Coley HL, Williams JH, DeLaughter K, Ray MN, Gilbert GH, Ford DE. Implementing point of care “e-referrals” in 137 clinics to increase access to a quit smoking internet system: the Quit-Primo and National Dental PBRN HI-QUIT Studies. Translational behavioral medicine. 2013;3:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palacholla RS, Fischer N, Coleman A, Agboola S, Kirley K, Felsted J, Katz C, Lloyd S, Jethwani K. Provider-and patient-related barriers to and facilitators of digital health technology adoption for hypertension management: scoping review. JMIR cardio. 2019;3:e11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harvey G, Kitson A. PARIHS revisited: from heuristic to integrated framework for the successful implementation of knowledge into practice. Implementation science. 2015;11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodward EN, Matthieu MM, Uchendu US, Rogal S, Kirchner JE. The health equity implementation framework: proposal and preliminary study of hepatitis C virus treatment. Implementation Science. 2019;14:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, Proctor EK, Kirchner JE. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implementation Science. 2015;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bates DW, Landman A, Levine DM. Health apps and health policy: what is needed? Jama. 2018;320:1975–6. [DOI] [PubMed] [Google Scholar]
- 56.Veinot TC, Ancker JS, Cole-Lewis H, Mynatt ED, Parker AG, Siek KA, Mamykina L. Leveling up: on the potential of upstream health informatics interventions to enhance health equity. Medical care. 2019;57:S108–S14. [DOI] [PubMed] [Google Scholar]
- 57.Jankowski P Towards participatory geographic information systems for community-based environmental decision making. Journal of environmental management. 2009;90:1966–71. [DOI] [PubMed] [Google Scholar]
- 58.Auerbach J Social determinants of health can only be addressed by a multisector spectrum of activities. Journal of Public Health Management and Practice. 2019;25:525–8. [DOI] [PubMed] [Google Scholar]
- 59.Chircop A, Bassett R, Taylor E. Evidence on how to practice intersectoral collaboration for health equity: a scoping review. Critical Public Health. 2015;25:178–91. [Google Scholar]
- 60.Kania J, Kramer M. Collective impact: FSG Beijing, China; 2011. [Google Scholar]
- 61.Baum F, Laris P. Improving health equity: action on social determinants of health through Health in All Policies. Implementing Health in All Policies: Adelaide. 2010:25–38. [Google Scholar]
- 62.Hall RL, Jacobson PD. Examining whether the health-in-all-policies approach promotes health equity. Health Affairs. 2018;37:364–70. [DOI] [PubMed] [Google Scholar]
- 63.Rudolph L, Caplan J, Ben-Moshe K, Dillon L. Health in all policies. A guide for state and local governments Washington, DC and Oakland, CA. 2013. [Google Scholar]
- 64.Bryson JM, Crosby BC, Stone MM. The design and implementation of Cross‐Sector collaborations: Propositions from the literature. Public administration review. 2006;66:44–55. [Google Scholar]
- 65.Lloyd Michener M, Cook J, Ahmed SM, Yonas MA, Coyne-Beasley T, Aguilar-Gaxiola S. Aligning the goals of community-engaged research: why and how academic health centers can successfully engage with communities to improve health. Academic Medicine. 2012;87:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Longwe SH. Gender awareness: The missing element in the Third World development project. 1991.
- 67.Key KD, Furr-Holden D, Lewis EY, Cunningham R, Zimmerman MA, Johnson-Lawrence V, Selig S. The continuum of community engagement in research: A roadmap for understanding and assessing progress. Progress in Community Health Partnerships: Research, Education, and Action. 2019;13:427–34. [DOI] [PubMed] [Google Scholar]
- 68.Israel BA, Eng E, Schulz AJ, Parker EA. Introduction to methods in community-based participatory research for health. Methods in community-based participatory research for health. 2005;3:26. [Google Scholar]
- 69.Ogilvie J Assessing Meaningful Community Engagement: A Conceptual Model to Advance Health Equity through Transformed Systems for Health. NAM Perspectives. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Windsor LC, Benoit E, Pinto RM, Gwadz M, Thompson W. Enhancing behavioral intervention science: using community-based participatory research principles with the multiphase optimization strategy. Translational behavioral medicine. 2021;11:1596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Korn AR, Hennessy E, Tovar A, Finn C, Hammond RA, Economos CD. Engaging coalitions in community-based childhood obesity prevention interventions: a mixed methods assessment. Childhood Obesity. 2018;14:537–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamamoto M, Kushin MJ, Dalisay F. Social media and mobiles as political mobilization forces for young adults: Examining the moderating role of online political expression in political participation. New Media & Society. 2015;17:880–98. [Google Scholar]
- 73.Gil de Zúñiga H, Jung N, Valenzuela S. Social media use for news and individuals’ social capital, civic engagement and political participation. Journal of computer-mediated communication. 2012;17:319–36. [Google Scholar]
- 74.Escobar-Viera CG, Melcher EM, Miller RS, Whitfield DL, Jacobson-López D, Gordon JD, Ballard AJ, Rollman BL, Pagoto S. A systematic review of the engagement with social media–delivered interventions for improving health outcomes among sexual and gender minorities. Internet interventions. 2021;25:100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petkovic J, Duench S, Trawin J, Dewidar O, Pardo JP, Simeon R, DesMeules M, Gagnon D, Roberts JH, Hossain A. Behavioural interventions delivered through interactive social media for health behaviour change, health outcomes, and health equity in the adult population. Cochrane Database of Systematic Reviews. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vereen RN, Kurtzman R, Noar SM. Are Social Media Interventions for Health Behavior Change Efficacious among Populations with Health Disparities?: A Meta-Analytic Review. Health Communication. 2021:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu P, Astudillo K, Velez D, Kelley L, Cobbs-Lomax D, Spatz ES. Use of mobile health applications in low-income populations: a prospective study of facilitators and barriers. Circulation: Cardiovascular Quality and Outcomes. 2020;13:e007031. [DOI] [PubMed] [Google Scholar]
- 78.Househ M, Borycki E, Kushniruk A. Empowering patients through social media: the benefits and challenges. Health informatics journal. 2014;20:50–8. [DOI] [PubMed] [Google Scholar]
- 79.Niederdeppe J, Bigman CA, Gonzales AL, Gollust SE. Communication about health disparities in the mass media. Journal of communication. 2013;63:8–30. [Google Scholar]
- 80.Moorhead SA, Hazlett DE, Harrison L, Carroll JK, Irwin A, Hoving C. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. Journal of medical Internet research. 2013;15:e1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solar O, Irwin A. A conceptual framework for action on the social determinants of health. WHO Document Production Services, 2010. [Google Scholar]
- 82.Nordgreen T, Rabbi F, Torresen J, Skar YS, Guribye F, Inal Y, Flobakk E, Wake JD, Mukhiya SK, Aminifar A. Challenges and possible solutions in cross-disciplinary and cross-sectorial research teams within the domain of e-mental health. Journal of Enabling Technologies. 2021. [Google Scholar]
- 83.Agurs-Collins T, Persky S, Paskett ED, Barkin SL, Meissner HI, Nansel TR, Arteaga SS, Zhang X, Das R, Farhat T. Designing and assessing multilevel interventions to improve minority health and reduce health disparities. American journal of public health. 2019;109:S86–S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swartz TH, Palermo A-GS, Masur SK, Aberg JA. The science and value of diversity: closing the gaps in our understanding of inclusion and diversity. The Journal of infectious diseases. 2019;220:S33–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnson AE, Pollard TJ, Shen L, Lehman L-wH, Feng M, Ghassemi M, Moody B, Szolovits P, Anthony Celi L, Mark RG. MIMIC-III, a freely accessible critical care database. Scientific data. 2016;3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pollard TJ, Johnson AE, Raffa JD, Celi LA, Mark RG, Badawi O. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Scientific data. 2018;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.National Academies of Sciences E, Medicine, . Learning through citizen science: Enhancing opportunities by design 2018. [PubMed]
- 88.Martinez LS, Carolan K, O’Donnell A, Diaz Y, Freeman ER. Community engagement in patient-centered outcomes research: benefits, barriers, and measurement. Journal of clinical and translational science. 2018;2:371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun N, Esom K, Dhaliwal M, Amon JJ. Human rights and digital health technologies. Health and Human Rights. 2020;22:21. [PMC free article] [PubMed] [Google Scholar]
- 90.Asthana S, Jones R, Sheaff R. Why does the NHS struggle to adopt eHealth innovations? A review of macro, meso and micro factors. BMC health services research. 2019;19:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chakravorti B How to Close the Digital Divide in the US. Harvard Business Review. 2021. [Google Scholar]
- 92.Warschauer M Technology and social inclusion: Rethinking the digital divide: MIT press; 2004. [Google Scholar]
- 93.Timmermans S, Kaufman R. Technologies and health inequities. Annual Review of Sociology. 2020;46:583–602. [Google Scholar]
- 94.Gwadz MV, Collins LM, Cleland CM, Leonard NR, Wilton L, Gandhi M, Scott Braithwaite R, Perlman DC, Kutnick A, Ritchie AS. Using the multiphase optimization strategy (MOST) to optimize an HIV care continuum intervention for vulnerable populations: a study protocol. BMC public health. 2017;17:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fernandez ME, Schlechter CR, Del Fiol G, Gibson B, Kawamoto K, Siaperas T, Pruhs A, Greene T, Nahum-Shani I, Schulthies S. QuitSMART Utah: an implementation study protocol for a cluster-randomized, multi-level Sequential Multiple Assignment Randomized Trial to increase Reach and Impact of tobacco cessation treatment in Community Health Centers. Implementation Science. 2020;15:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao Y, Cui Y. Deep transfer learning for reducing health care disparities arising from biomedical data inequality. Nature communications. 2020;11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Comulada WS, Swendeman D, Rezai R, Ramanathan N. Time Series Visualizations of Mobile Phone-Based Daily Diary Reports of Stress, Physical Activity, and Diet Quality in Mostly Ethnic Minority Mothers: Feasibility Study. JMIR formative research. 2018;2:e11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mun E-Y, Li X, Businelle MS, Hébert ET, Tan Z, Barnett NP, Walters ST. Ecological momentary assessment of alcohol consumption and its concordance with transdermal alcohol detection and timeline follow-back self-report among adults experiencing homelessness. Alcoholism: Clinical and Experimental Research. 2021;45:864–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCool J, Dobson R, Muinga N, Paton C, Pagliari C, Agawal S, Labrique A, Tanielu H, Whittaker R. Factors influencing the sustainability of digital health interventions in low-resource settings: lessons from five countries. Journal of global health. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ford KL, West AB, Bucher A, Osborn CY. Personalized Digital Health Communications to Increase COVID-19 Vaccination in Underserved Populations: A Double Diamond Approach to Behavioral Design. Frontiers in Digital Health. 2022;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vaughn NA, Jacoby SF, Williams T, Guerra T, Thomas NA, Richmond TS. Digital animation as a method to disseminate research findings to the community using a community‐based participatory approach. American Journal of Community Psychology. 2013;51:30–42. [DOI] [PubMed] [Google Scholar]
- 102.Belone L, Rae R, Hirchak KA, Cohoe-Belone B, Orosco A, Shendo K, Wallerstein N. Dissemination of an American Indian culturally centered community-based participatory research family listening program: implications for global indigenous well-being. Genealogy. 2020;4:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bull SS, Levine DK, Black SR, Schmiege SJ, Santelli J. Social media–delivered sexual health intervention: a cluster randomized controlled trial. American journal of preventive medicine. 2012;43:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vogel EA, Ramo DE, Meacham MC, Prochaska JJ, Delucchi KL, Humfleet GL. The Put It Out Project (POP) Facebook intervention for young sexual and gender minority smokers: outcomes of a pilot, randomized, controlled trial. Nicotine and Tobacco Research. 2020;22:1614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


