Abstract
Background:
This study aimed to evaluate the predicting factors affecting sperm retrieval. We prospectively assessed the relationship between sonographic and microdissection testicular sperm extraction (mTESE) findings in Klinefelter syndrome (KS).
Materials and Methods:
In this prospective study, 44 azoospermic men with 47, XXY karyotypes participated in this study. In order to evaluate the amount of blood supply in different parts of testicular tissue, a doppler ultra-sonographic was performed. Also, for the detection of sperm in this group mTESE technique was performed.
Results:
The age average of positive mTESE and negative mTESE groups was 29.4 and 33.6 years, respectively. By comparing the testicle volume (based on the data obtained from the clinical examinations conducted by the urologist) it was determined that there is no significant difference between mTESE positive and negative groups. Folliclestimulating hormone (FSH) levels in men with negative mTESE (P=0.03) and testosterone levels in men with positive mTESE significantly increased (P=0.017). The overall rate of testis vascularity was significantly higher in the positive mTESE group than in the negative mTESE group. The clinical pregnancy rate in positive mTESE men was 9% per cycle, 16.6% per embryos were transferred (ET), and 12.5% per cycle.
Conclusion:
Totally, our observation indicated that there is not a significant relationship between sonographic and mTESE results in KS patients. However, more investigations with bigger sample Size can be useful to validate our results.
Keywords: Azoospermic, Klinefelter, Testicular Sperm Extraction, Ultrasonography
Introduction
Infertility is a disease of the male or female reproductive system defined by the failure to achieve a pregnancy after 12 months or more of regular unprotected sexual intercourse. Causes of male infertility include disorder of spermatogenesis and sperm transfer, erectile dysfunction, impaired ejaculation, and intercourse (1). In many cases, there is no clear diagnosis of the cause of infertility. Although infertile men have normal masculine traits, the testicles are unable to produce sperm. About 90% of men’s infertility is associated with a lack of/oligospermia or absence/azoospermia of sperm in an ejaculation (2). Many factors can cause oligospermia or azoospermia such as Hereditary factors, infectious agents, environmental pollutants, age, and obesity (3).
Approximately, 11% of male infertilities have been detected to oligospermia with an unknown cause (4). The majority of infertility cases of unknown causes are due to genetic defects. Genetic diseases such as Klinefelter syndrome (KS), Y chromosome microdeletion and cystic fibrosis play an important role in male infertility. KS is a chromosomal disorder in which there is an extra X chromosome in a person's genome. Various genotypes are observed in this disease, such as 49, XXXXY, 48, XXXY, 48, XXYY, but the most common is 47, XXY (5). The prevalence rate of this syndrome is reported 1 to 500-1000 per person. People with KS usually have small testis that does not produce an adequate amount of testosterone hormone. Decreased levels of this hormone can cause delayed or incomplete puberty, enlarged breasts (gynecomastia), decreased facial/body hair, and infertility. Since there is no cure for this syndrome; so timely diagnosis and early treatment of KS are very important (6). Sperm freezing before azoospermia is considered a way to treat infertility. It can even be recommended for a group of patients in their early teens. Testicular biopsy is also one of the ways to treat infertility. Although the majority of people with KS are azoospermic, sperm-producing foci have been observed in the testicular tissue in some cases. Briefly, there is no definite indicator that can predict the success of mTESE in these groups of patients (7).
Doppler ultrasound is a well-known method for evaluating TESE in non‐obstructive azoospermia (NOA) men. It is based on the theory that testis structure in NOA men has several distinct points through which spermatogenesis takes place (8). On the other hand, there is a theory that relatively high blood flow is in these areas. Based on this evidence, it was concluded that the use of Doppler ultrasound is effective in predicting the presence of spermatogenesis in testis tissue (9). In the present study, we evaluated the relationship between sonographic and mTESE findings in KS patients.
Materials and Methods
This prospective study, was performed according to the guidelines of the Royan Institute Ethics Committee (IR. ACECR.ROYAN.REC.1398.166). Firstly, for infertile couples with male factor causes who were referred to the Royan institute, a complete history such as the history of treatment, used medications, and duration of infertility was taken. A spermogram test was requested for the patient, and the seminal fluid was examined for biochemical-cellular characteristics. The patients were examined for sexual secondary characteristics, testis, varicocele, and hernia. Then, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone hormones were monitored. In the absence of sperm in semen (Azoospermia) at least two semen analyses, increased levels of LH and FSH (two to three times) and decreased testosterone levels (less than 2.5-3 ng/ ml), as KS is possible. In addition to the tests above, the karyotype and Y-chromosome AZF region’s micro-deletions were requested by the physician. In this descriptive-analytical case-control study, 63 patients with 47, XXY karyotypes were included in this study, and 21 patients were excluded for various reasons. Finally, the current research was performed on 42 patients. After confirmation of KS, a Doppler ultrasound was requested to check the blood supply of testicular tissue. Doppler ultrasound was performed with Aloka ProSound Alpha and 10 MHz Linear probe. In this method, the testis was divided into 4 parts: lower medial (LM), lower lateral (LL), upper medial (UM) and upper lateral (UL).
Before performing the procedures, the informed consent form was completed by the patients.In mTESE method, the patient is under general anesthesia, and through scrotal incision testis delivered, the microtubule contents of the testis are examined under a microscope. A series of large, dilated, and opaque microtubules are biopsied for sperm. The sampling process continues to obtain 20-30 sperm. In the current study, diagnostic and therapeutic mTESE were performed. A diagnostic biopsy was performed on one or both testis based on the patient’s clinical condition. Therapeutic mTESE was simultaneous with the day of the oocyte puncture in the patient's spouse. The mTESE was carried out from the same testis (or performed in contralateral tesis) that diagnostic mTESE has performed previously. If sperm is observed, they are frozen for intracytoplasmic sperm injection (ICSI). Fresh sperm samples are used for the ICSI technique; otherwise, frozen samples were used. Tukey’s post hoc test was used to determine the differences between groups. The Pearson correlation coefficient was also used to evaluate the intensity of the relationship. Logistic regression analysis was used to control the effect of confounders on qualitative two-state variables and linear regression analysis was used on quantitative variables. A significance level of 5% (P<0.05) was considered.
Results
mTESE
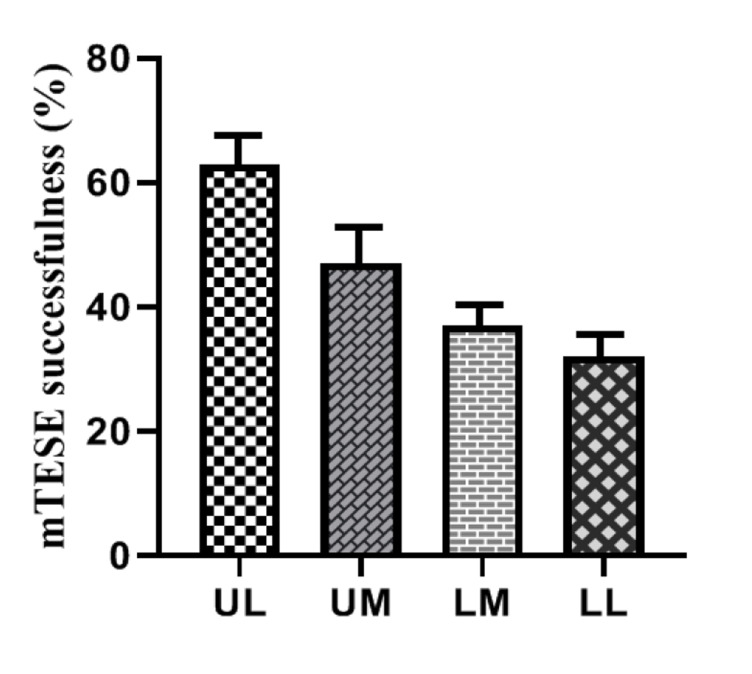
Among the 42 infertile men with KS included in this study, the mTESE results of 18 men were positive (42.8%), and 24 men (57.2%) were negative. Out of a total of 51 performed mTESE surgeries (some performed more than once), 24 (47%) had a successful recovery. The results of 27 (53%) mTESE surgery (diagnostic or therapeutic) were negative. Among them, 21 cases (77.7%) were from the right testis and 6 cases (22.3%) were from the left testis. The success rate of mTESE in terms of sperm recovery was 63% in UL regions and 32% in LL regions (Fig .1).
Fig.1.
Comparison of vascularity rates of different testis regions in positive and negative mTESE groups of the right and the left testis. mTESE; Microdissection testicular sperm extraction, UL; Upper lateral, UM; Upper medial, LM; Lower medial, and LL; Lower lateral.
Demographic information
The age average of positive mTESE and negative mTESE groups was 29.4 and 33.6 years, respectively. In this study, the age average of the positive group was significantly lower than the negative group (P=0.007). There was no significant difference between body mass index (BMI) in both groups (P=0.855, Table 1). By comparing the positive and negative mTESE groups, we observed that there is no significant difference between the levels of education between the two groups (Table 1).
Table 1.
Comparison of two groups with successful and unsuccessful mTESE in demographics
|
| |||
|---|---|---|---|
|
| |||
| Parameters | mTESE | P value | |
| + | - | ||
| Age (Y) | 29.47 ± 4.18 | 33.62 ± 5.21 | 0.007 |
| Age wife (Y) | 26.68 ± 11.17 | 31.17 ± 6.46 | 0.111 |
| BMI (kg/m2) | 25.91 ± 6.49 | 26.26 ± 5.79 | 0.8550.935 |
| Education | |||
| Under diploma | 9 (47.4) | 12 (50) | |
| Diploma | 6 (31.6) | 8 (33.3) | |
| Educated | 4 (21.1) | 4 (16.7) | |
|
| |||
Data are presented as mean ± SD or n (%). mTESE; Microdissection testicular sperm extraction and BMI; Body mass index.
Clinical information
At first, the normality test (Kolmogorov Smirnov) was performed, and since the distribution was not normal, non-parametric tests were used. By comparing the testicle volume (based on the data obtained from the clinical examinations conducted by the urologist) of the two mTESE positive and negative groups, it was determined that there is no significant difference between these two groups (P=0.855). The following graphs are a comparison between the average volume of the left and right testicles in two positive and negative groups (Table 2).
Table 2.
Comparison of testicular size in positive and negative mTESE groups
|
| |||
|---|---|---|---|
| mTESE | Number | Mean ± SD | P value |
|
| |||
| RT | |||
| Positive | 19 | 4.52 ± 2.29 | 0.158 |
| Negative | 24 | 3.70 ± 2.15 | 0.166 |
| LT | |||
| Positive | 19 | 5.00 ± 2.53 | 0.194 |
| Negative | 23 | 4.08 ± 2.29 | 0.195 |
|
| |||
mTESE; Microdissection testicular sperm extraction, RT; Right testis, and LT; Left testis.
Hormonal profiles
Regarding the hormonal profile of the patients, the Kolmogorov Smirnoff test was also performed first; The distribution was not normal and as a result, non-parametric tests were used. Our results showed that FSH in the negative group (P=0.03) and testosterone in the positive group were significantly higher (P=0.017). Although LH level did not show a significant difference between the two groups, it was higher in the negative group (P=0.06, Table 3). The logistic regression analysis on the microtesse variable status by the age of LH, FSH, testosterone and overall ultrasound confirmed the obtained results (Table 4).
Table 3.
Comparison of hormonal profiles in positive and negative mTESE groups
|
| ||||
|---|---|---|---|---|
| Hormone name | mTESE | Number | Mean ± SD | P value |
|
| ||||
| LH | Positive | 19 | 19.12 ± 9.96 | 0.060 |
| Negative | 23 | 25.20 ± 8.77 | ||
| FSH | Positive | 19 | 32.27 ± 16.07 | 0.030 |
| Negative | 24 | 40.57 ± 15.02 | ||
| Testosterone | Positive | 18 | 3.01 ± 1.76 | 0.017 |
| Negative | 23 | 1.81 ± 1.34 | ||
|
| ||||
mTESE; Microdissection testicular sperm extraction, LH; luteinizing hormone, FSH; Follicle-stimulating hormone.
Table 4.
Results of the logistic regression analysis on the mTESE variable status by the age of LH, FSH, testosterone and overall ultrasound
|
| |||
|---|---|---|---|
| Parameters | Variables | Exp (β) | P value |
|
| |||
| Demographic | Age (Y) | 0.836 | 0.022 |
| BMI (kg/m2) | 1.002 | 0.977 | |
| Testicular size | RT | 1.205 | 0.206 |
| LT | 1.198 | 0.188 | |
| Hormonal profiles | LH(ng/mL) | 0.922 | 0.044 |
| FSH(ng/mL) | 0.942 | 0.039 | |
| Testesterone(ng/mL) | 1.602 | 0.043 | |
| Ultrasonography | Total sono | 1.951 | 0.008 |
|
| |||
Logistic regression data by considering the microtese variable as a dependent variable, the age of LH, FSH, testosterone and overall ultrasound had a significant relationship. Age, LH, and FSH have a significant negative relationship (because the value of exp (β) is less than 1) and testosterone and ultrasonography overall had a positive relationship with microtese. BMI; Body mass index, RT; right testis, LT; Left testis, LH; Luteinizing hormone, and FSH; Follicle-stimulating hormone. Exp (β) shows the superiority ratios for each of the predictor variables.
Doppler ultrasonography
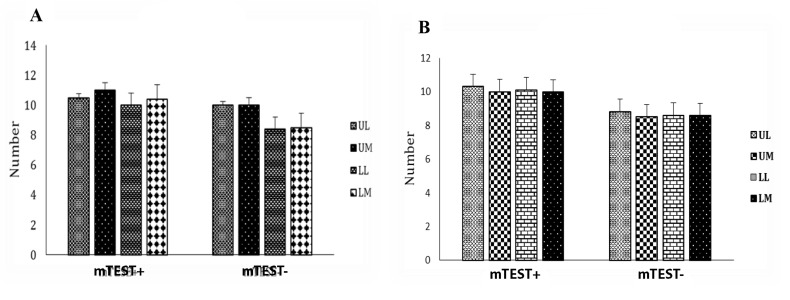
Comparison of Doppler ultrasonography with mTESE results showed no significant relationship in the left testis (Fig .1), while in the LL and LM areas of the right testis, a positive significant relationship was observed. Furthermore, in the UM area, a negative significant relationship was observed (Fig .2).
Fig.2.
Comparison of vascularity rates in different testis regions in positive and negative mTESE groups of right testis. A. Right testis and B. Left testis. mTESE; Microdissection testicular sperm extraction, UL; Upper lateral, UM; Upper medial, LM; Lower medial, and LL; Lower lateral.
Assisted reproductive technology cycle
Among 18 patients with positive mTESE, 10 (55.5%) patients entered the assisted reproductive technology (ART) cycle and a total of 11 cycles were performed. Sperm freezing was used for 7 patients and fresh sperm was used for 3 patients. For 5 patients no embryos were transferred (ET), however, for 5 other couples ET was performed (10 embryos in total) and finally, only one case was reported clinical pregnancy. The success rate of clinical pregnancy for this group of couples was estimated at 9% per cycle, 16.6% per ET, and 12.5% per embryo.
Discussion
KS is known as the most prevalent chromosomal abnormality among azoospermic patients, which can be along with some disorders such as extra gonadal germ cell tumours, cognition deficits, obesity, and gynecomastia (10, 11). It is stated that sonography outcomes can be used as a prognostic parameter in order to achievement in sperm recovery (10). Meanwhile, mTESE method has a capacity for sperm retrieval in NOA males (12). Hence, in the present study, we evaluated predicting factors affecting sperm parameters regarding these patients via finding a correlation between Doppler ultrasound and mTESE results. Regarding the effect of age on mTESE success rate, some studies have suggested that younger age has a positive effect on sperm recovery rate. Some researchers have observed that aging has a negative effect on sperm recovery rates (13, 14). The results of this study showed that age can be an influential factor in the success rate of sperm recovery in mTESE.
Studies on the effect of BMI on male fertility, semen parameters, and mTESE results are few and contradictory. The line with this, Pavan-Jukic et al. (15) by evaluating BMI effect on sperm recovery azoospermic subjects concluded that BMI cannot affect sperm recovery results in men with azoospermia. However, Kort et al. (16) revealed a dramatic and negative association between the whole number of normal sperm cells and BMI. This negative relationship is probably due to the imbalance between estradiol/testosterone hormones. Our results in this study did not show a significant difference in BMI between positive and negative mTESE groups.
Testis size is another effective criterion for sperm recovery rate, which was evaluated in this investigation. In this regard, Taha et al. (17) based on Doppler ultrasound findings provided a document that low sperm count in some fertility disorders may be significantly associated with reduced total testicular size. Here, the results of initial clinical examinations performed by a physician did not show a significant relationship between testis size and mTESE success rate. In accordance with our observation, Schill et al. (18) through the assessment of sonographic, clinical, and biochemical information before and after TESE in infertile men showed that TESE does not affect testis size in these patients. but our results by Doppler ultrasound showed a significant difference in testis size in positive and negative mTESE men. This emphasizes the importance of ultrasound examination of the testis in infertile men.
Hormone profile is another indicator used to predict mTESE results in KS men.
KS men possess LH and FSH higher than normal levels. According to the results of this study, FSH in the negative mTESE group and testosterone in the positive mTESE group are significantly higher. Although the LH level did not show a significant difference between the two groups, it was higher in the negative group. Also, our study showed that LH levels in positive mTESE men are lower than in controls. But according to evidence, mTESE in infertile males cannot change FSH, LH, and testosterone levels (18). On the contrary, Altinkilic et al. (9) reported significant differences in the levels of these hormones between negative and positive mTESE groups of patients with azoospermia.
Moreover, our results show that the echo-pattern of the right testis in mTESE group was negative. This parameter in about 71% of cases was heterogeneous, and in 29% of cases was homogeneous, which have significantly different from the positive group. These results are agreeing with the results previously reported by Rocher et al. (11), and testicular tissue in KS men showed a rough and knotted structure and in non-KS men showed a normal-striated pattern.
Having considered that the parameter of vascularity testicular has been reported as a predictor factor for spermatogenesis (19), the amount of vascularity in different areas of testicular tissue was examined by Doppler ultrasound. In the current study, vascularity was divided into three grades: normo-vascular, hypo-vascular, and hyper-vascular. Our study in terms of vascularity showed no significant difference between the positive and negative groups. The logistic regression analysis on the microtesse variable status by the age of LH, FSH, testosterone and overall ultrasound confirmed the obtained results.
The pathological examination of testicular can also be used to predict the outcome of mTESE. Based on this, it seems that there is a direct relationship between histopathological subtypes and mTESE success rate. But the point to note is that usually the sample sent to the embryologist and pathologist is not the same, and due to the heterogeneous nature of testicular tissue, it has often been observed that the results of embryological and histological examinations are inconsistent. In fact, Sousa's hypothesis can be used temporarily that there is no histological difference between the tissue sent to the pathologist and the embryologist. In this study, no significant difference was observed between the pathology results of positive and negativemTESEgroups. However, it is recommended that more studies on a larger population are needed to demonstrate our outcomes.
Conclusion
Totally, our observation indicated that there is not a significant relationship between sonographic and mTESE results in KS patients. However, more investigations with bigger sample Sizes can be useful to validate our results.
Acknowledgments
This study was financially supported by the Royan Institute-Academic Center for Education, Culture and Research. There is no conflict of interest in this study.
Author’s Contributions
F.A.; Participated in study design and writing the manuscript. N.T., F.R.-T., H.K, A.V.D.; Contributed to all experimental work, data collection, and evaluation. H.S., M.A.S.G.; Was responsible for project supervision, data analysis, and manuscript editing. M.M.; Contributed to data analysis and manuscript editing. All authors read and approved the final manuscript.
References
- 1.Durairajanayagam D. Lifestyle causes of male infertility. Arab J Urol. 2018;16(1):10–20. doi: 10.1016/j.aju.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyamoto T, Minase G, Shin T, Ueda H, Okada H, Sengoku K. Human male infertility and its genetic causes. Reprod Med Biol. 2017;16(2):81–88. doi: 10.1002/rmb2.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babakhanzadeh E, Nazari M, Ghasemifar S, Khodadadian A. Some of the factors involved in male infertility: a prospective review. Int J Gen Med. 2020;13:29–41. doi: 10.2147/IJGM.S241099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aliakbari F, Taghizabet N, Azizi F, Rezaei-Tazangi F, Gelehkolaee KS, Kharazinejad E. A review of methods for preserving male fertility. Zygote. 2022;30(3):289–97. doi: 10.1017/S0967199421000071. [DOI] [PubMed] [Google Scholar]
- 5.Corona G, Pizzocaro A, Lanfranco F, Garolla A, Pelliccione F, Vignozzi L, et al. Sperm recovery and ICSI outcomes in Klinefelter syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2017;23(3):265–275. doi: 10.1093/humupd/dmx008. [DOI] [PubMed] [Google Scholar]
- 6.Samango-Sprouse CA, Counts DR, Tran SL, Lasutschinkow PC, Porter GF, Gropman AL. Update on the clinical perspectives and care of the child with 47,XXY (klinefelter syndrome) Appl Clin Genet. 2019;12:191–202. doi: 10.2147/TACG.S180450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samango-Sprouse C, Lasutschinkow P, Powell S, Sadeghin T, Gropman A. The incidence of anxiety symptoms in boys with 47,XXY (Klinefelter syndrome) and the possible impact of timing of diagnosis and hormonal replacement therapy. Am J Med Genet A. 2019;179(3):423–428. doi: 10.1002/ajmg.a.61038. [DOI] [PubMed] [Google Scholar]
- 8.Nariyoshi S, Nakano K, Sukegawa G, Sho T, Tsuji Y. Ultrasonographically determined size of seminiferous tubules predicts sperm retrieval by microdissection testicular sperm extraction in men with nonobstructive azoospermia. Fertil Steril. 2020;113(1):97–104. doi: 10.1016/j.fertnstert.2019.08.061. e2. [DOI] [PubMed] [Google Scholar]
- 9.Altinkilic B, Pilatz A, Diemer T, Wolf J, Bergmann M, Schönbrunn S, et al. Prospective evaluation of scrotal ultrasound and intratesticular perfusion by color-coded duplex sonography (CCDS) in TESE patients with azoospermia. World J Urol. 2018;36(1):125–133. doi: 10.1007/s00345-017-2039-z. [DOI] [PubMed] [Google Scholar]
- 10.Ekerhovd E, Westlander G. Testicular sonography in men with Klinefelter syndrome shows irregular echogenicity and blood flow of high resistance. J Assist Reprod Genet. 2002;19(11):517–522. doi: 10.1023/A:1020959818687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocher L, Moya L, Correas JM, Mutuon P, Ferlicot S, Young J, et al. Testis ultrasound in Klinefelter syndrome infertile men: making the diagnosis and avoiding inappropriate management. Abdom Radiol (NY) 2016;41(8):1596–1603. doi: 10.1007/s00261-016-0713-z. [DOI] [PubMed] [Google Scholar]
- 12.Franco G, Scarselli F, Casciani V, De Nunzio C, Dente D, Leonardo C, et al. A novel stepwise micro-TESE approach in non obstructive azoospermia. BMC Urol. 2016;16(1):20–20. doi: 10.1186/s12894-016-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emre Bakircioglu M, Erden HF, Kaplancan T, Ciray N, Bener F, Bahceci M. Aging may adversely affect testicular sperm recovery in patients with Klinefelter syndrome. Urology. 2006;68(5):1082–1086. doi: 10.1016/j.urology.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 14.Okada H, Goda K, Yamamoto Y, Sofikitis N, Miyagawa I, Mio Y, et al. Age as a limiting factor for successful sperm retrieval in patients with nonmosaic Klinefelter's syndrome. Fertil Steril. 2005;84(6):1662–1664. doi: 10.1016/j.fertnstert.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 15.Pavan-Jukic D, Starc A, Stubljar D, Jukic T. Obesity with high body mass index does not influence sperm retrieval in males with azoospermia. Med Sci Monit. 2020;26:e923060–e923060. doi: 10.12659/MSM.923060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kort HI, Massey JB, Elsner CW, Mitchell-Leef D, Shapiro DB, Witt MA, Roudebush WE. Impact of body mass index values on sperm quantity and quality. J Androl. 2006;27(3):450–452. doi: 10.2164/jandrol.05124. [DOI] [PubMed] [Google Scholar]
- 17.Taha EA, Abd El-Wahed SR, Mostafa T. Relation of color doppler parameters with testicular size in oligoasthenoteratozoospermic men with a varicocele. Hum Androl. 2012;2(1):6–11. [Google Scholar]
- 18.Schill T, Bals-Pratsch M, Küpker W, Sandmann J, Johannisson R, Diedrich K. Clinical and endocrine follow-up of patients after testicular sperm extraction. Fertil Steril. 2003;79(2):281–286. doi: 10.1016/s0015-0282(02)04663-0. [DOI] [PubMed] [Google Scholar]
- 19.Har-Toov J, Eytan O, Hauser R, Yavetz H, Elad D, Jaffa AJ. A new power doppler ultrasound guiding technique for improved testicular sperm extraction. Fertil Steril. 2004;81(2):430–434. doi: 10.1016/j.fertnstert.2003.07.020. [DOI] [PubMed] [Google Scholar]