Abstract
The N-terminal region of Dvl-1 (a mammalian Dishevelled homolog) shares 37% identity with the C-terminal region of Axin, and this related region is named the DIX domain. The functions of the DIX domains of Dvl-1 and Axin were investigated. By yeast two-hybrid screening, the DIX domain of Dvl-1 was found to interact with Dvl-3, a second mammalian Dishevelled relative. The DIX domains of Dvl-1 and Dvl-3 directly bound one another. Furthermore, Dvl-1 formed a homo-oligomer. Axin also formed a homo-oligomer, and its DIX domain was necessary. The N-terminal region of Dvl-1, including its DIX domain, bound to Axin directly. Dvl-1 inhibited Axin-promoted glycogen synthase kinase 3β-dependent phosphorylation of β-catenin, and the DIX domain of Dvl-1 was required for this inhibitory activity. Expression of Dvl-1 in L cells induced the nuclear accumulation of β-catenin, and deletion of the DIX domain abolished this activity. Although expression of Axin in SW480 cells caused the degradation of β-catenin and reduced the cell growth rate, expression of an Axin mutant that lacks the DIX domain did not affect the level of β-catenin or the growth rate. These results indicate that the DIX domains of Dvl-1 and Axin are important for protein-protein interactions and that they are necessary for the ability of Dvl-1 and Axin to regulate the stability of β-catenin.
Genetic and biochemical analyses have revealed that there are components which are structurally and functionally conserved in the Wnt signaling pathway among flies, frogs, and mammals (6, 9, 26). In mammals, these include Wnt, frizzled, Dvl (a Dishevelled [Dsh] homolog), glycogen synthase kinase 3β (GSK-3β), β-catenin, and Lef/Tcf, and there are multiple Wnt, frizzled, and Dvl families (6, 9, 26). In the absence of Wnt, GSK-3β phosphorylates β-catenin, resulting in the degradation of β-catenin. Wnt inactivates GSK-3β probably through Dvl, although the mechanism is not clear (7). This leads to the stabilization of β-catenin. The accumulated β-catenin translocates to the nucleus (48) and binds to transcription factors of the Lef/Tcf family (4, 27).
Several Wnt proteins are thought to be essential for the proper development of different parts of the brains and spinal cord (30) and have been implicated in the establishment of dorsoventral and anteroposterior axes in vertebrates (14, 32, 37, 38). Axin was originally identified as a product of the mouse Fused locus (49). The mouse mutant Fused is recessive lethal; mutants have a duplication of the embryonic anteroposterior axis (11, 31). Injection of Axin into Xenopus embryos causes strong axis defects, and coexpression of Axin inhibits the Wnt-dependent axis duplication (49). Thus, Axin is a negative regulator of the Wnt signaling pathway and inhibits axis formation. We have identified rat Axin (rAxin) and its homolog, Axil (for Axin like), as GSK-3β-interacting proteins (16, 45). Conductin has been identified as a β-catenin-binding protein (3) and is identical to Axil. Both Axin and Axil bind not only to GSK-3β but also to β-catenin (3, 13, 16, 17, 36, 45) and promote GSK-3β-dependent phosphorylation of β-catenin (16, 45). Furthermore, the regulators of G-protein signaling (RGS) domains of Axin and conductin directly interact with adenomatous polyposis coli protein (APC), and expression of rAxin or conductin in COS or SW480 cells stimulates the degradation of β-catenin (3, 13, 19, 20). Thus, Axin family members downregulate β-catenin. In addition to GSK-3β-, β-catenin-, and APC-binding sites, the C-terminal region of Axin has a domain that is homologous to the N-terminal region of Dvl (16, 49). This region is called the Dsh homologous domain or the DIX domain (6). However, the function of this domain is not known.
Dsh encodes a cytoplasmic protein of unknown biochemical function in flies (21, 42). Dsh mediates Wg signaling during embryogenesis and adult fly development, which in turn determine the ultimate cell polarity and fate in Drosophila (21, 42). Genetic evidence shows that Dsh acts upstream of shaggy, a GSK-3β homolog, and inhibits its activity (6, 9, 26). Overexpression of Dsh in the Drosophila imaginal disc cell line clone 8 causes the accumulation of Armadillo, a β-catenin homolog (46, 47). Dsh homologs are conserved in Xenopus frogs and mammals (22, 33, 38, 40). Overexpression of Xenopus Dsh (Xdsh) induces a secondary body axis in Xenopus embryos (38). In mammals, Dvl-1, -2, and -3 genes have been isolated (22, 33, 40). All Dsh protein family members contain three highly conserved domains: an N-terminal DIX domain; a central PDZ domain, which has been shown to be a protein-protein interaction surface; and a DEP domain, which can also be found in several other proteins (6, 9). Disruption of the PDZ domain abolishes its activity in the Wg-Armadillo pathway and in the Xenopus axis induction assay (38, 46, 47). Recently, it has been reported that the DEP domain is critical for rescue of the dsh planar polarity defect and in the activation of Jun N-terminal kinase (5, 24). However, the function of the DIX domain of Dvl is not clear.
To clarify the function of the DIX domain, we have tried to find a Dvl-binding protein by using the DIX domain of Dvl-1 as bait in the yeast two-hybrid method. Here we report that Dvl-1 and Dvl-3 bind one another through their DIX domains and that oligomerization of Axin requires its DIX domain. We also show that Dvl-1 directly binds to Axin and that Dvl-1 inhibits Axin-promoted GSK-3β-dependent phosphorylation of β-catenin and APC. Furthermore, we demonstrate that the deletion of the DIX domains of Dvl-1 and Axin destroys their abilities to accumulate and to degrade β-catenin, respectively. These results suggest that the DIX domain is a novel protein-protein interaction domain and that the DIX domains of Dvl and Axin are necessary for their functions.
MATERIALS AND METHODS
Materials and chemicals.
Saccharomyces cerevisiae L40, plasmid vectors for two-hybrid screening, and a pGAD-derived rat brain cDNA library were kindly supplied by Y. Takai and K. Tanaka (Osaka University, Suita, Japan). Human Dvl-1 and Dvl-3 cDNAs were provided by B. Dallapiccola and G. Novelli (Vergata University, Rome, Italy) (33). SW480 cells and L cells were provided by E. Tahara (Hiroshima University, Hiroshima, Japan) and A. Nagafuchi and S. Tsukita (Kyoto University, Kyoto, Japan), respectively. The anti-glutathione S-transferase (anti-GST) and anti-maltose-binding protein (anti-MBP) antibodies, anti-hemagglutinin 1 (anti-HA) antibody, and recombinant baculoviruses expressing GST–Dvl-1 (full length) and GST–APC-(1211-2075) were provided by M. Nakata (Sumitomo Electronics, Yokohama, Japan), Q. Hu (Chiron Corp., Emeryville, Calif.), and Y. Matsuura (National Institute of Infectious Diseases, Tokyo, Japan), respectively. GST fusion proteins, MBP fusion proteins, and six-histidine-tagged β-catenin (His6–β-catenin) were purified from Escherichia coli except that GST–Dvl-1 (full length) and GST–APC-(1211-2075) were purified from Spodoptera frugiperda sf9 cells. The anti-Myc antibody was prepared from 9E10 cells. Other materials and chemicals were purchased from commercial sources.
Plasmid construction.
pBSKS/rAxin, pEF-BOS-Myc/rAxin, pUC19/rAxin, pUC19/rAxin-(298-832), pBSKS/rAxin-(508-832), pBJ-Myc/rAxin, pBJ-Myc/rAxin-(1-229), pEF-BOS/Myc-rAxin-(298-506), pBJ-Myc/rAxin-(713-832), pEF-BOS-Myc/rAxin-(1-713), and pMAL-c2/rAxin were constructed as described elsewhere (16, 19, 20). To construct pUC19/Dvl-1, pBSKS/Dvl-1 was digested with XbaI, blunted with Klenow fragment, and digested with SalI. The 2.0-kb fragment encoding Dvl-1 was inserted into SalI- and SmaI-cut pUC19. pUC19/Dvl-1 was digested with SalI and EcoRI, and the 2.0-kb fragment encoding Dvl-1 was inserted into SalI- and EcoRI-cut pBTM116HA to generate pBTM116HA/Dvl-1. To construct pBTM116HA/Dvl-1-(1-82) and pBTM116HA/rAxin, the 0.25-kb fragment encoding Dvl-1-(1-82) and the 2.5-kb fragment encoding rAxin were inserted into pBTM116HA. To construct pVIKS/Dvl-1, pBSKS/Dvl-1 was digested with SacI, blunted with T4 DNA polymerase, and digested with XbaI. The 2.0-kb fragment encoding Dvl-1 was inserted into XbaI- and SmaI-cut pVIKS. To construct pRSETA/β-catenin, pGAD/β-catenin was digested with BamHI and inserted into BamHI-cut pRSETA. pVIKS/APC-(1211-2075) was constructed as follows. pMKITneo/APC was digested with NdeI, blunted with Klenow fragment, and digested with BglII. The 2.6-kb fragment encoding APC-(1211-2075) was inserted into pBluescript KS (pBSKS), which was digested with SpeI, blunted with Klenow fragment, and digested with BamHI to generate pBSKS/APC-(1211-2075). pBSKS/APC-(1211-2075) was digested with SpeI and HindIII and the 2.6-kb fragment encoding APC-(1211-2075) was inserted into XbaI- and HindIII-cut pMAL-c2 to generate pMAL-c2/APC-(1211-2075). To construct pVIKS/APC-(1211-2075), pMAL-c2/APC-(1211-2075) was digested with BamHI and SmaI and the 2.6-kb fragment encoding APC-(1211-2075) was inserted into BamHI- and SmaI-cut pVIKS. To express HA-tagged Dvl-1, rAxin, and their deletion mutants in COS and L cells, various deletion mutants of Dvl-1 and rAxin cDNAs were made and inserted into pCGN. To express Myc-tagged Dvl-1, Dvl-3, rAxin, and their deletion mutants in COS cells, various deletion mutants of Dvl-1, Dvl-3, and rAxin cDNAs were made and inserted into pBJ-Myc and pEF-BOS-Myc (16, 20, 45). To make GST and MBP fusion proteins, deletion mutants of Dvl-1, Dvl-3, and rAxin cDNAs were inserted into pGEX-2T, pGEX-KG, and pMAL-c2.
Yeast two-hybrid screening.
S. cerevisiae L40 was used as a host for the two-hybrid screening (15, 16, 43). L40 carrying pBTM116HA/Dvl-1-(1-82) was transformed with a rat brain cDNA library constructed in pGAD10. Approximately 3.5 × 106 transformants were screened. Plasmids harboring cDNAs were recovered from positive colonies, and the nucleotide sequences of plasmid DNAs which conferred the His+ and LacZ+ phenotype on L40 containing pBTM116HA/Dvl-1-(1-82) were determined. To examine the interaction of Dvl-1 with other proteins, plasmids expressing Dvl-3, rAxin, GSK-3β, β-catenin, and Ras were made by restriction enzyme digestion or PCR and tested for interaction in a β-galactosidase assay.
Kinase assay.
Ninety nanomolar MBP-rAxin (full length) and 90 nM GST–GSK-3β were incubated with 1.4 μM His6-β-catenin or 250 nM GST–APC-(1211-2075) in 30 μl of reaction mixture (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 1 mM dithiothreitol [DTT], 50 μM [γ-32P]ATP [500 to 1,500 cpm/pmol]) in the presence or absence of MBP–Dvl-1 or its deletion mutants for 15 min at 30°C. The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by autoradiography, and then the radioactivities of the phosphorylated β-catenin and APC were counted. The kinase activities of GSK-3β for GSK peptide 1 were measured as described elsewhere (16, 29, 45).
Interaction of Dvl-1, Dvl-3, and rAxin in intact cells and in vitro.
COS cells transfected with plasmids containing HA–Dvl-1, Myc–Dvl-1, Myc–Dvl-3, Myc-rAxin, HA-rAxin, and their deletion mutants were lysed as described previously (16, 20, 45). The lysates were immunoprecipitated with the anti-Myc or anti-HA antibody, and then the precipitates were probed with the anti-Myc and anti-HA antibodies (16, 20, 45). To examine the direct binding of Dvl-1, Dvl-3, and rAxin, GST fusion proteins (25 to 50 pmol) were incubated with MBP fusion proteins (10 to 30 pmol) immobilized on amylose resin in 100 μl of reaction mixture (20 mM Tris-HCl [pH 7.5], 1 mM DTT) for 1 h at 4°C. MBP fusion proteins were precipitated by centrifugation, and then the precipitates were probed with the anti-GST antibody.
Cell lines stably expressing Myc-rAxin.
Cotransfection of wild-type SW480 cells with pEF-BOS-Myc/rAxin-(1-713) or pBJ-Myc/rAxin-(298-832) and pNeo was carried out by using Transfast (Promega Corp., Madison, Wis.). Colonies of the cells resistant to G418 (Geneticin; GIBCO-BRL, Life Technologies, Inc., Rockville, Md.) were picked up, and the G418-resistant cells were further selected by immunoblot analysis using the anti-Myc antibody.
Microinjection, immunofluorescence, and confocal laser-scanning microscopy.
L cells were grown on glass coverslips and microinjected with various plasmids (0.2 to 0.8 mg/ml), using a 5171 micromanipulator and 5246 transjector (Eppendorf-Netheler-Hinz GmbH, Hamburg, Germany). The following procedures were performed at room temperature. At 4 h postmicroinjection, the cells were fixed for 20 min in phosphate-buffered saline (PBS) containing 4% paraformaldehyde. After being washed with PBS three times, the cells were permeabilized with PBS containing 0.2% Triton X-100 and 2 mg of bovine serum albumin per ml for 12 h. The cells were washed and incubated for 1 h with the anti-Myc, anti-HA, or anti-β-catenin antibody. After being washed with PBS, they were further incubated for 1 h with Cy3-labeled anti-mouse immunoglobulin G and Cy2-labeled anti-rabbit immunoglobulin G. Coverslips were washed with PBS, mounted on glass slides, and viewed with a confocal laser-scanning microscope (TCS-NT; Leica-laser-technik GmbH, Heidelberg, Germany).
Gel filtration column chromatography.
Purified MBP, MBP–Dvl-1, and MBP-rAxin (4 to 8 μg of each protein) were applied to Superdex 200HR 10/30 columns equilibrated with equilibration buffer (25 mM Tris-HCl [pH 8.0], 250 mM NaCl, 1 mM DTT, 0.1% Nonidet P-40) and eluted with the same buffer at a flow rate of 0.5 ml/min. Fractions of 0.5 ml each were collected. An aliquot (20 μl) of each fraction was probed with the anti-MBP antibody.
RESULTS
Identification of proteins which interact with Dvl.
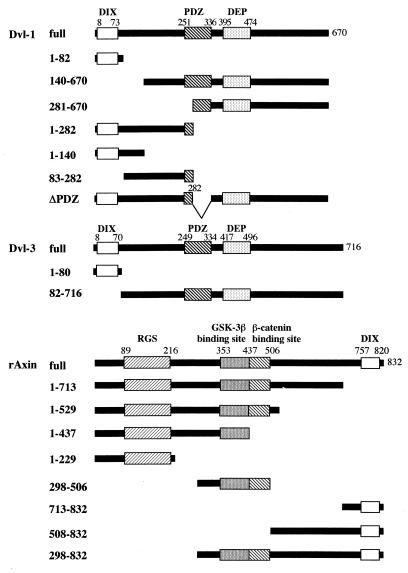
To identify proteins that physically interact with the DIX domains of rAxin [rAxin-(757-820)] and Dvl-1 [Dvl-1-(1-82)], we screened a rat brain cDNA library using the yeast two-hybrid method. We could not obtain the clones which interact with the DIX domain of rAxin, while several clones were found to confer both the His+ and LacZ+ phenotypes on L40 containing pBTM116HA/Dvl-1-(1-82). Among these, one clone was found to encode a sequence containing Dvl-3. As assessed by filter assays of β-galactosidase, Dvl-1-(1-82) indeed bound to Dvl-3 (full length) (Table 1). The association of Dvl-1-(1-82) with rAxin (full length), GSK-3β (full length), or β-catenin (full length) was not observed. When full-length Dvl-1 was used, it bound not only to Dvl-3 but also to rAxin, while it did not bind to GSK-3β or β-catenin. rAxin bound to Dvl-3, and interestingly rAxin interacted with rAxin itself. Ras, used as a negative control, did not bind to Dvl-1-(1-82), Dvl-1, or rAxin. These results suggest that Dvl-1 forms a complex with Dvl-3 through its DIX domain, that Dvl-1 and Dvl-3 associate with Axin, and that Axin forms a homo-oligomer. The structures of the mutants of Dvl-1, Dvl-3, and rAxin used in the following experiments are shown in Fig. 1.
TABLE 1.
Interaction of Dvl-1, Dvl-3, and Axin in the yeast two-hybrid systema
| Protein | β-Galactosidase activity
|
||
|---|---|---|---|
| Dvl-1-(1-82) (pBTM116HA) | Dvl-1 (pBTM116HA) | rAxin (pBTM116HA) | |
| Dvl-3 (pGAD) | + | + | + |
| rAxin (pGAD) | − | + | + |
| GSK-3β (pGAD) | − | − | + |
| β-Catenin (pGAD) | − | − | + |
| Ras (pGAD) | − | − | − |
L40 cells were cotransformed with pGAD- and pBTM116HA-derived plasmids, as indicated in parentheses. To assay for β-galactosidase activity, cells were streaked on permissive (histidine-containing) medium and incubated for 3 days at 30°C, and then β-galactosidase activity was measured as negative (−) or positive (+). All constructs except Dvl-1-(1-82) are full-length proteins.
FIG. 1.
Schematic representations of Dvl-1, Dvl-3, and rAxin constructs used in this study.
Interaction of Dvl-1 with Dvl-3.
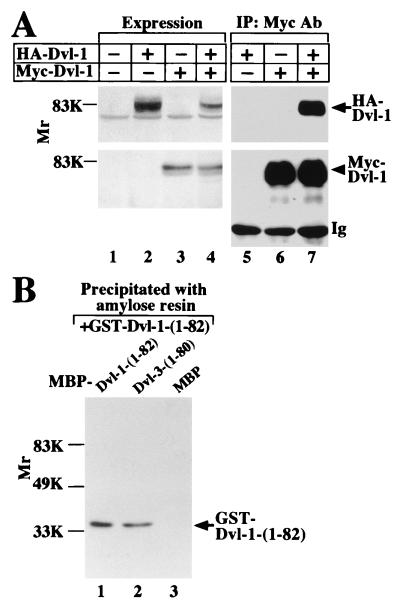
To examine whether Dvl-1 forms a complex with Dvl-3 in intact cells, we coexpressed HA–Dvl-1 with Myc–Dvl-3 in COS cells (Fig. 2A). When the lysates coexpressing HA–Dvl-1 and Myc–Dvl-3 were immunoprecipitated with the anti-Myc antibody, HA–Dvl-1 was detected in the Myc–Dvl-3 immune complex (Fig. 2A). HA–Dvl-1 was not immunoprecipitated when nonimmune immunoglobulin was used instead of the anti-Myc antibody (data not shown). To confirm that the DIX domain of Dvl-1 is necessary for its interaction with Dvl-3, HA–Dvl-1-(140-670) was coexpressed with Myc–Dvl-3. Unexpectedly, HA–Dvl-1-(140-670) was coprecipitated with Myc–Dvl-3 (Fig. 2A). These results suggest that both the DIX domain and the remaining region of Dvl-1 can form a complex with Dvl-3 in intact cells. However, the results obtained with COS cells do not exclude the possibility that the interaction of Dvl-1 with Dvl-3 is indirect.
FIG. 2.
Interaction of Dvl-1 with Dvl-3. (A) Intact cells. Lysates (20 μg of protein) of COS cells expressing Myc–Dvl-3 (lane 2), HA–Dvl-1 (lane 3), HA–Dvl-1-(140-670) (lane 4), Myc–Dvl-3 and HA–Dvl-1 (lane 5), or Myc–Dvl-3 and HA–Dvl-1-(140-670) (lane 6) were simultaneously probed with anti-Myc and anti-HA antibodies. The same lysates (200 μg of protein) were immunoprecipitated with the anti-Myc antibody, and the immunoprecipitates were probed with the anti-Myc and anti-HA antibodies (lanes 7 to 11). The lysates of COS cells transfected with empty vectors were used as a control (lane 1). IP, immunoprecipitation; Ab, antibody; Ig, immunoglobulin. The arrows and arrowhead indicate the positions of HA–Dvl-1 or HA–Dvl-1-(140-670) and Myc–Dvl-3, respectively. (B) Direct binding. After GST–Dvl-1-(1-82) (lanes 1 to 3) or GST–Dvl-1-(140-670) (lanes 4 to 6) (50 pmol of each) was incubated with MBP–Dvl-3 (full length) (lanes 1 and 4), MBP–Dvl-3-(1-80) (lanes 2 and 5), and MBP (lanes 3 and 6) (30 pmol of each) immobilized on amylose resin, MBP fusion proteins were precipitated by centrifugation. The precipitates were probed with the anti-GST antibody. The positions of GST–Dvl-1-(1-82) and GST–Dvl-1-(140-670) are shown in lanes 7 and 8, respectively. The results shown are representative of three independent experiments.
To examine the direct interaction of Dvl-1 with Dvl-3, deletion mutants of GST–Dvl-1 and MBP–Dvl-3 were purified from E. coli. GST–Dvl-1-(1-82) bound to both MBP–Dvl-3 (full length) and MBP–Dvl-3-(1-80) but not to MBP (Fig. 2B). However, GST–Dvl-1-(140-670) did not bind to MBP–Dvl-3 or MBP–Dvl-3-(1-80) (Fig. 2B). Furthermore, GST–Dvl-1-(1-82), GST–Dvl-1-(83-282), or GST–Dvl-1-(1-140) did not bind to MBP–Dvl-3-(82-716) (data not shown). Therefore, the DIX domains of Dvl-1 and Dvl-3 bind directly to one another, but regions other than the DIX domain do not. These results suggest that Dvl-1 and Dvl-3 directly interact with one another through their DIX domains and that they also form a complex via other proteins which bind to some region other than the DIX domains.
Self-association of Dvl-1.
To examine whether Dvl-1 forms a homo-oligomer, HA–Dvl-1 was coexpressed with Myc–Dvl-1 in COS cells (Fig. 3A). HA–Dvl-1 formed a complex with Myc–Dvl-1 in COS cells (Fig. 3A). An in vitro binding assay showed that GST–Dvl-1-(1-82) directly binds to MBP–Dvl-1-(1-82) but not to MBP alone (Fig. 3B). On gel filtration column chromatography, MBP–Dvl-1 purified from E. coli eluted as a single protein population with a peak Mr of around 340,000 under conditions in which MBP alone eluted with a peak Mr of about 50,000, indicating that MBP does not oligomerize (data not shown). Since the Mr of MBP–Dvl-1 on SDS-PAGE was about 125,000, Dvl-1 may form a trimer. These results indicate that Dvls form a homo- or hetero-oligomer.
FIG. 3.
Self-association of Dvl-1. (A) Intact cells. Lysates (20 μg of protein) of COS cells expressing HA–Dvl-1 (lane 2), Myc–Dvl-1 (lane 3), or HA–Dvl-1 and Myc-Dvl-1 (lane 4) were sequentially probed with anti-HA and anti-Myc antibodies. The same lysates (200 μg of protein) were immunoprecipitated with the anti-Myc antibody, and the immunoprecipitates were probed with the anti-HA and anti-Myc antibodies (lanes 5 to 7). The lysates of COS cells transfected with empty vectors were used as a control (lane 1). IP, immunoprecipitation; Ab, antibody; Ig, immunoglobulin. The arrow and arrowhead indicate the positions of HA–Dvl-1 and Myc–Dvl-1, respectively. (B) Direct binding. After GST–Dvl-1-(1-82) was incubated with MBP–Dvl-1-(1-82) (lane 1), MBP–Dvl-3-(1-80) (lane 2), or MBP (lane 3) immobilized on amylose resin, MBP fusion proteins were precipitated by centrifugation. The precipitates were probed with the anti-GST antibody. The results shown are representative of three independent experiments.
Self-association of rAxin.
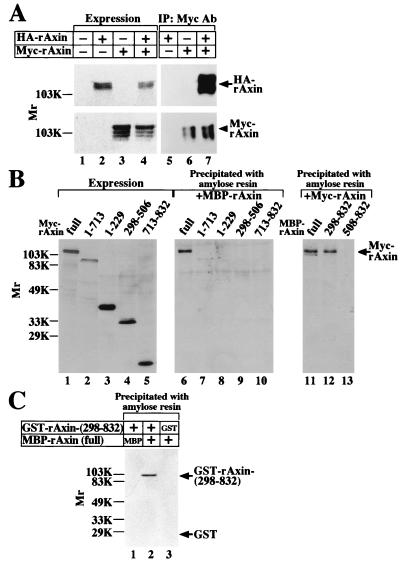
In two-hybrid experiments, rAxin interacted with rAxin (Table 1). We examined whether rAxin forms a homo-oligomer in intact cells. When HA-rAxin and Myc-rAxin were coexpressed in COS cells, HA-rAxin was found in the Myc-rAxin immune complex (Fig. 4A). To determine which region of rAxin is required for its self-association, the lysates of COS cells expressing deletion mutants of Myc-rAxin were incubated with MBP-rAxin (full length). Only Myc-rAxin (full length) was complexed with MBP-rAxin (full length), but Myc–rAxin-(1-713), Myc–rAxin-(1-229), Myc–rAxin-(298-506), or Myc–rAxin-(713-832) did not form a complex with MBP-rAxin (full length) (Fig. 4B). When the lysates of COS cells expressing Myc-rAxin (full length) were incubated with MBP–rAxin-(298-832) and MBP–rAxin-(508-832), Myc-rAxin formed a complex with MBP–rAxin-(298-832) but not with MBP–rAxin-(508-832) (Fig. 4B). These results suggest that the N-terminal region of rAxin including the RGS domain is not necessary for its self-association and that the remaining region, containing the GSK-3β- and β-catenin-binding sites and the DIX domain, is necessary, but that none of these individual sites is sufficient. However, the results observed with COS cells do not exclude the possibility that other cellular proteins are present.
FIG. 4.
Self-association of Axin. (A) Intact cells. Lysates (20 μg of protein) of COS cells expressing HA-rAxin (lane 2), Myc-rAxin (lane 3), or HA-rAxin and Myc-rAxin (lane 4) were probed with anti-HA and anti-Myc antibodies. The same lysates (200 μg of protein) were immunoprecipitated with the anti-Myc antibody, and the immunoprecipitates were probed with the anti-HA and anti-Myc antibodies (lanes 5 to 7). The lysates of COS cells transfected with empty vectors were used as a control (lane 1). IP, immunoprecipitation; Ab, antibody. The arrow and arrowhead indicate the positions of HA-rAxin and Myc-rAxin, respectively. (B) In vitro. The lysates of COS cells expressing Myc-rAxin (full length) (lanes 1 and 6), Myc–rAxin-(1-713) (lanes 2 and 7), Myc–rAxin-(1-229) (lanes 3 and 8), Myc–rAxin-(298-506) (lanes 4 and 9), or Myc–rAxin-(713-832) (lanes 5 and 10) were directly probed with the anti-Myc antibody (lanes 1 to 5) or incubated with MBP-rAxin (full length) (10 pmol) immobilized on amylose resin, and then MBP-rAxin was precipitated by centrifugation (lanes 6 to 10). The lysates of COS cells expressing Myc-rAxin (full length) were incubated with MBP-rAxin (full length) (lane 11), MBP–rAxin-(298-832) (lane 12), or MBP–rAxin-(508-832) (lane 13) (10 pmol of each) immobilized on amylose resin. MBP-rAxin and its deletion mutants were precipitated by centrifugation, and the precipitates were probed with the anti-Myc antibody. The arrow indicates the position of Myc-rAxin (full length). (C) Direct binding. GST–rAxin-(298-832) (lanes 1 and 2) or GST (lane 3) (50 pmol of each) was incubated with MBP (lane 1) or MBP-rAxin (full length) (lanes 2 and 3) (10 pmol of each) immobilized on amylose resin, and then MBP-rAxin and MBP were precipitated by centrifugation. The precipitates were probed with the anti-GST antibody. The arrows indicate the positions of GST–rAxin-(298-832) and GST. The results shown are representative of three independent experiments.
To examine the direct self-association of rAxin, MBP-rAxin was incubated with GST–rAxin-(298-832). GST–rAxin-(298-832) but not GST alone bound to MBP-rAxin (Fig. 4C). MBP-rAxin purified from E. coli eluted as a single protein population with a peak at an Mr of around 500,000 on gel filtration column chromatography (data not shown), and the Mr of MBP-rAxin on SDS-PAGE was about 140,000, suggesting that rAxin forms a trimer or tetramer.
Interaction of Dvl-1 with rAxin.
In two-hybrid experiments, Dvl-1 interacted with rAxin (Table 1). To examine whether Dvl-1 forms a complex with rAxin in intact cells, we coexpressed HA–Dvl-1 with Myc-rAxin in COS cells (Fig. 5A). When the lysates coexpressing Myc-rAxin with HA–Dvl-1 were immunoprecipitated with the anti-HA antibody, Myc-rAxin was detected in the HA–Dvl-1 immune complex (Fig. 5A). Next, we examined which region of rAxin interacts with Dvl-1 in intact cells. Various deletion mutants of Myc-rAxin were coexpressed with HA–Dvl-1 in COS cells, and the cell lysates were immunoprecipitated with the anti-Myc antibody (Fig. 5B). Myc-rAxin (full length) and Myc–rAxin-(1-713) but not Myc–rAxin-(1-437), Myc–rAxin-(1-229), or Myc–rAxin-(298-506) were coprecipitated with HA–Dvl-1 (Fig. 5B). HA–Dvl-1 was also present faintly in the Myc–rAxin-(713-832) immune complex (Fig. 5B). From these results obtained with COS cells, it could be concluded that Dvl-1 forms a complex with Axin and that the DIX domain of Axin is sufficient for the complex formation of Dvl-1, although weakly. However, their interaction may be indirect.
FIG. 5.
Interaction of Dvl-1 with Axin. (A) Intact cells. Lysates (20 μg of protein) of COS cells expressing Myc-rAxin (lane 2), HA–Dvl-1 (lane 3), or Myc-rAxin and HA–Dvl-1 (lane 4) were probed with anti-Myc and anti-HA antibodies. The lysates of COS cells transfected with empty vectors served as the control (lane 1). The same lysates (250 μg of protein) of COS cells prepared in lanes 2 to 4 were immunoprecipitated with the anti-HA antibody (lanes 5 to 7). The immunoprecipitates were probed with the anti-Myc and anti-HA antibodies. IP, immunoprecipitation; Ab, antibody; Ig, immunoglobulin. The arrow and arrowhead indicate the positions of Myc-rAxin and HA–Dvl-1, respectively. (B) Binding region. The lysates (250 to 500 μg of protein) of COS cells coexpressing HA–Dvl-1 and Myc-rAxin (full length) (lanes 1 and 7), Myc–rAxin-(1-713) (lanes 2 and 8), Myc–rAxin-(1-437) (lanes 3 and 9), Myc–rAxin-(1-229) (lanes 4 and 10), Myc–rAxin-(298-506) (lanes 5 and 11), or Myc–rAxin-(713-832) (lanes 6 and 12) were immunoprecipitated with the anti-Myc antibody, and the immunoprecipitates were probed with the anti-Myc (lanes 1 to 6) and anti-HA (lanes 7 to 12) antibodies, respectively. IB, immunoblotting. The arrow indicates the positions of HA–Dvl-1. (C) Direct binding. GST–Dvl-1-(1-282) (lane 1), GST–Dvl-1-(1-140) (lane 2), GST–Dvl-1-(1-82) (lane 3), GST–Dvl-1-(83-282) (lane 4), GST–Dvl-1-(281-670) (lane 5), or GST (lane 6) (25 pmol of each) was incubated with MBP-rAxin (10 pmol) immobilized on amylose resin, and then MBP-rAxin was precipitated by centrifugation. The precipitates were probed with the anti-GST antibody. After GST–Dvl-1-(1-282) (25 pmol) was incubated with MBP-rAxin (full length) (lane 7), MBP–rAxin-(1-529) (lane 8), MBP–rAxin-(508-832) (lane 9), MBP–rAxin-(713-832) (lane 10), or MBP alone (lane 11) (10 pmol each) immobilized on amylose resin, MBP fusion proteins were precipitated by centrifugation. The precipitates were probed with the anti-GST antibody. The arrows and arrowhead indicate the positions of GST–Dvl-1-(1-282) and GST–Dvl-1-(281-670), respectively. (D) Effects of Dvl-1 on the binding of GSK-3β, β-catenin, and APC to rAxin. MBP-rAxin (full length) (10 pmol) immobilized on amylose resin was incubated with 1 μM GST–GSK-3β, 1.4 μM GST–β-catenin, or 250 nM GST–APC-(1211-2075) in the presence of the indicated concentrations of GST–Dvl-1 (full length). MBP-rAxin was precipitated by centrifugation, and then the precipitates were probed with the anti-GSK-3β, anti-β-catenin, or anti-GST [for GST–APC-(1211-2075)] antibody. The positions of GST–APC-(1211-2075), GST–β-catenin, and GST–GSK-3β are indicated by the arrows. The results shown are representative of three independent experiments.
To confirm the direct binding of rAxin and Dvl-1 and to examine which region of Dvl-1 binds to rAxin, various deletion mutants of GST–Dvl-1 were incubated with MBP-rAxin (Fig. 5C). Both GST–Dvl-1-(1-282) and GST–Dvl-1-(281-670) bound to MBP-rAxin, but GST–Dvl-1-(1-82), GST–Dvl-1-(1-140), or GST–Dvl-1-(83-282) did not bind to MBP-rAxin. These results indicate that Dvl-1 has two binding sites to rAxin and that in the N-terminal binding site the DIX domain of Dvl-1 is necessary but not sufficient for its interaction with rAxin. GST–Dvl-1-(281-670) inhibited the binding of GST–Dvl-1-(1-282) to MBP–rAxin-(508-832), suggesting that both regions of Dvl-1 can bind to the same site on Axin (data not shown). Various deletion mutants of MBP-rAxin were incubated with GST–Dvl-1-(1-282) (Fig. 5C). GST–Dvl-1-(1-282) bound to MBP-rAxin (full length) and MBP–rAxin-(508-832) but not to MBP–rAxin-(1-529) or MBP–rAxin-(713-832) (Fig. 5C). GST–Dvl-1-(281-670) also bound to MBP-rAxin (full length) and MBP–rAxin-(508-832) but not to MBP–rAxin-(1-529) (data not shown). These results from in vitro experiments suggest that the region containing amino acids 530 to 712 of rAxin is important for its direct interaction with Dvl-1. Taken together with the experiments with COS cells, the DIX domain of rAxin may form a complex with Dvl-1 through other proteins. Thus, Axin has a Dvl-binding site in addition to the binding sites for GSK-3β, β-catenin, and APC. However, the binding of GST–Dvl-1 (full length) to MBP-rAxin (full length) did not affect the interaction of GST–GSK-3β or GST–β-catenin to MBP-rAxin (Fig. 5D). APC-(1211-2075) containing seven 20-amino-acid repeat motifs binds to Axin (20). Likewise, GST–Dvl-1 did not affect the binding of GST–APC-(1211-2075) to MBP-rAxin (Fig. 5D).
Inhibition of Axin-promoted GSK-3β-dependent phosphorylation of β-catenin by Dvl.
It has been shown that Axin-induced ventralization is rescued by Xdsh in Xenopus embryos, suggesting that Dvl antagonizes the functions of Axin (49). Therefore, we examined whether Dvl-1 affects Axin’s ability to promote GSK-3β-dependent phosphorylation of β-catenin. MBP–Dvl-1 itself was not phosphorylated by GST–GSK-3β (data not shown). GST–GSK-3β phosphorylated His6–β-catenin in the presence of MBP-rAxin in a time-dependent manner (16) (Fig. 6A). MBP–Dvl-1 inhibited this phosphorylation of His6–β-catenin (Fig. 6A). This inhibitory activity of MBP–Dvl-1 was dose dependent, and MBP alone did not inhibit the GSK-3β-dependent phosphorylation of β-catenin (Fig. 6B). Deletion of the N-terminal region containing the DIX domain [MBP–Dvl-1-(140-670)] reduced but did not eliminate the Dvl-1 activity (Fig. 6B). MBP–Dvl-1-(Δ283-336) (MBP–Dvl-1ΔPDZ), which lacks the PDZ domain, did not inhibit the phosphorylation of β-catenin (Fig. 6B). It has been demonstrated that GSK-3β phosphorylates APC directly and that Axin promotes GSK-3β-dependent phosphorylation of APC (13, 35). Consistent with these previous observations, GST–GSK-3β phosphorylated GST–APC-(1211-2075) and MBP-rAxin enhanced this phosphorylation (Fig. 6C). MBP–Dvl-1 inhibited the phosphorylation of GST–APC-(1211-2075) in the presence but not in the absence of MBP-rAxin (Fig. 6C). However, MBP-rAxin did not enhance the phosphorylation of synthetic peptide by GST–GSK-3β, and the phosphorylation was not affected by MBP–Dvl-1 in the presence or absence of MBP-rAxin (Fig. 6D). Taken together, these results suggest that Dvl-1 inhibits Axin-promoted GSK–3β-dependent phosphorylation of β-catenin and APC but not GSK-3β activity itself.
FIG. 6.
Inhibition of GSK-3β-dependent phosphorylation of β-catenin in the presence of Axin by Dvl. (A) Time course. Ninety nanomolar MBP-rAxin and 90 nM GST–GSK-3β were incubated with 1.4 μM His6–β-catenin in the presence of 1 μM MBP or MBP–Dvl-1 for the indicated periods. (B) Effects of the DIX and PDZ domains of Dvl on its activity. GST–GSK-3β was incubated with His6–β-catenin and MBP-rAxin in the presence of the indicated concentrations of MBP–Dvl-1 (●), MBP–Dvl-1-(140-670) (▴), MBP–Dvl-1ΔPDZ (■), or MBP (○) for 15 min. (C) Inhibition of GSK-3β-dependent phosphorylation of APC in the presence of Axin by Dvl. GST–GSK-3β was incubated with 250 nM GST–APC-(1211-2075) and the indicated concentrations of MBP–Dvl-1 in the presence (●) or absence (○) of MBP-rAxin for 15 min. (D) Phosphorylation of synthetic peptide. GST–GSK-3β was incubated with 10 μM GSK peptide 1 and the indicated concentrations of MBP–Dvl-1 in the presence (●) or absence (○) of MBP-rAxin for 15 min. The results shown are representative of five independent experiments.
Effect of Dvl-1 on β-catenin nuclear accumulation.
It has been shown that overexpression of Drosophila Dsh elevates Armadillo levels in Drosophila imaginal disc cell line clone 8 and that both the DIX and PDZ domains are essential for the action of Dsh (47). Therefore, we examined the effects of mammalian Dvl-1 on β-catenin. Microinjection of Dvl-1 into L cells induced the accumulation of β-catenin in the nucleus (Fig. 7A and B). Deletion of the N-terminal region including the DIX domain abolished the ability of Dvl-1 to cause nuclear accumulation of β-catenin (Fig. 7C and D). Furthermore, the PDZ domain deletion mutant also did not have this activity (Fig. 7E and F). These results were consistent with the observations that removal of the DIX or PDZ domain from Dvl-1 abolishes its ability to increase β-catenin in COS-7 cells (24) and indicate that mammalian Dvl accumulates β-catenin in the nucleus and that the DIX and PDZ domains are necessary for this ability.
FIG. 7.
Roles of the DIX domains of Dvl-1 and rAxin on the nuclear accumulation of β-catenin. L cells grown on coverslips were microinjected with pCGN/Dvl-1 (full length) (A and B), pCGN/Dvl-1-(140-670) (C and D), pCGN/Dvl-1ΔPDZ (E and F), pBJ-Myc/rAxin (full length) (G and H), pEF-BOS-Myc/rAxin-(1-713) (I and J), or pBJ-Myc/rAxin-(713-832) (K and L). Cells were fixed, permeabilized, and stained with anti-HA (A, C, and E), anti-Myc (G, I, and K), and anti-β-catenin (B, D, F, H, J, and L) antibodies. The arrows indicate injected cells. The results shown are representative of three independent experiments.
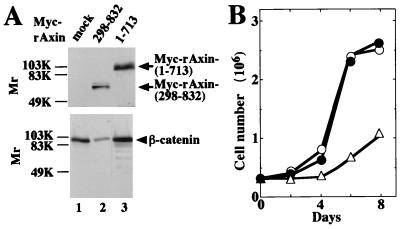
Requirement of the DIX domain of rAxin for β-catenin degradation activity.
To clarify the function of the DIX domain of rAxin, we made SW480 cells stably expressing Myc–rAxin-(298-832) or Myc–rAxin-(1-713). Consistent with the previous observations (3, 13), expression of Myc–rAxin-(298-832), which lacks the RGS domain, reduced the cellular level of β-catenin (Fig. 8A). However, expression of Myc–rAxin-(1-713) did not affect the level of β-catenin (Fig. 8A). Furthermore, expression of Myc–rAxin-(298-832) but not Myc–rAxin-(1-713) in SW480 cells reduced the cell growth rate (Fig. 8B). These results suggest that the DIX domain of Axin is necessary for its abilities to cause degradation of β-catenin and to suppress cellular proliferation. Since the expression of β-catenin in the cytosol of L cells was low, no significant reduction of β-catenin was observed after microinjection of rAxin (full length) into L cells (Fig. 7G and H). However, rAxin-(1-713) and rAxin-(713-832) stabilized cytoplasmic β-catenin in L cells (Fig. 7I to L). It appears that these rAxin fragments act through a dominant-negative mechanism to inhibit the endogenous Axin activity. It is notable that in contrast to Dvl-1, these rAxin mutants do not drive nuclear accumulation of β-catenin.
FIG. 8.
Effects of the DIX domain of rAxin on its function. (A) Effects of rAxin-(1-713) and rAxin-(298-832) on the degradation of β-catenin. Lysates (20 μg of each protein) of wild-type SW480 cells (lane 1) or SW480 stably expressing Myc–rAxin-(298-832) (lane 2) or Myc–rAxin-(1-713) (lane 3) were probed with anti-Myc and anti-β-catenin antibodies. The arrows and arrowhead indicate the positions of Myc–rAxin-(298-832) or Myc–rAxin-(1-713) and endogenous β-catenin, respectively. (B) Growth rates. The numbers (35-mm-diameter dish) of wild-type SW480 cells (○) and SW480 cells stably expressing Myc–rAxin-(1-713) (●) or Myc–rAxin-(298-832) (▵) were determined. The results shown are representative of three independent experiments.
DISCUSSION
A region of about 80 amino acids at the N terminus of the Dishevelled family is evolutionarily conserved and is referred to as the DIX domain. More than 60% of the amino acids of the DIX domains are identical among Drosophila Dsh, Xenopus Dsh, and mammalian Dvl. Expression of Dsh in Drosophila imaginal disc cell line clone 8 induces the accumulation of Armadillo, and deletion of the 166 N-terminal amino acids including the DIX domain abolishes the activity (47). Expression of the DIX domain of Dsh functions dominant negatively in fly cuticle formation (2). These results have suggested that the DIX domain of Dsh contacts other proteins to transmit the signal. We have found that the DIX domain of Dvl-1 directly binds to the DIX domains of Dvl-1 and Dvl-3. Furthermore, the apparent molecular weight of recombinant Dvl-1 is three times larger than that of the monomer on gel filtration column chromatography, suggesting that Dvl undergoes association to form a homo- or hetero-oligomer through the DIX domain in mammalian Dvl-1, -2, and -3. Taken together, these results suggest that the DIX domain of Dvl functions in protein-protein interactions and that oligomerization may be necessary for the activity of Dvl-1. We have also shown that expression of Dvl-1 in L cells induces the accumulation of β-catenin in the nucleus and that the deletion of the 139 N-terminal amino acids including the DIX domain abolishes this activity. However, the same Dvl-1 mutant reduces but does not eliminate the Dvl-1 activity to inhibit GSK-3β-dependent phosphorylation of β-catenin in in vitro assays. If the former assay is not sufficiently sensitive to determine whether any residual activity is present in the deletion mutant of Dvl-1, these results suggest that a domain other than the DIX domain in Dvl-1 confers some ability to promote β-catenin stabilization. Indeed, at least two domains in Dvl-1, one in the N-terminal region that contains the DIX domain and the other in the C-terminal region that contains the PDZ and DEP domains, are capable of binding to rAxin. Consistent with these observations, Dsh mutants lacking the DIX domain can still rescue the cuticle phenotype of dsh mutants in Drosophila, but the activity is reduced relative to that of wild-type Dsh (2). We have also shown that the PDZ domain of Dvl-1 is necessary for its ability to induce nuclear accumulation of β-catenin, consistent with the previous observations on Drosophila and Xenopus (2, 38, 47). These results suggest that both the DIX and PDZ domains are important for stabilizing β-catenin and promoting its nuclear import.
The DIX domain is also found in the C-terminal region of rAxin. Whereas 14 amino acids among 66 amino acids are different between the DIX domains of Dvl-1 and Dvl-3 (33), the identity between the DIX domains of Dvl-1 and rAxin is much lower, 37% (16, 49). The DIX domain of Dvl-1 does not bind to the DIX domain of rAxin, nor does the DIX domain of rAxin show self-association. Thus, the characteristics of the DIX domain of rAxin are different from those of Dvl-1. However, rAxin also undergoes a homo-oligomerization like Dvl. The DIX domain of rAxin is not sufficient but is necessary for its self-association. Stabilization of β-catenin due to its own mutation or the loss of APC has been shown to cause several human cancers (23, 28, 34), and expression of APC downregulates β-catenin and Tcf activity (23, 28). Therefore, it is possible that the inhibition of the cellular proliferation by Axin is due to less activation of Tcf through the degradation of β-catenin. Since deletion of the DIX domain from rAxin abolishes its ability to promote the degradation of β-catenin and to inhibit cellular proliferation in SW480 cells, the region containing the DIX domain of Axin is necessary for its oligomerization and oligomerization may be important for the Axin action. Alternatively, other proteins that are required for the degradation of β-catenin may bind to the DIX domain of Axin. Since rAxin-(1-713) enhances GSK-3β-dependent phosphorylation of β-catenin, our results showing that rAxin-(1-713) does not downregulate the levels of β-catenin also suggest that the phosphorylation of β-catenin is not sufficient for its degradation. Therefore, it is intriguing to speculate that the DIX domain of Axin may bind to βTrCP, a Slimb homolog, which is an F-box protein; Slimb is necessary for the degradation of β-catenin’s fly homolog, Armadillo (18). It is notable that in contrast to Dvl-1, rAxin-(1-713) and rAxin-(713-832) increase the cytosolic but not nuclear β-catenin. These results suggest that the cytosolic accumulation of β-catenin is not sufficient for its nuclear import and that another signal from Dvl-1 may be necessary.
The region containing amino acid residues 508 to 713 of rAxin is important for the binding of Axin to Dvl-1. The DIX domain of rAxin does not bind to Dvl-1 directly but is sufficient for its complex formation with Dvl-1. Dvl-1 has two binding sites to rAxin. In the N-terminal binding site, the DIX domain of Dvl-1 is necessary but not sufficient for the interaction with rAxin. These two regions interact with the overlapping and perhaps identical site on rAxin. Which region of Dvl-1 associates with rAxin in intact cells remains to be clarified. One intriguing possibility is that the mode of binding of Dvl-1 to rAxin is regulated by the Wnt signal. This Dvl-binding site on rAxin is distinct from the binding sites of APC, GSK-3β, and β-catenin (13, 16, 20), suggesting that these proteins form a pentamer. Since APC and β-catenin also form homo- or hetero-oligomers (34, 39), the Axin complex could be more complicated.
We have demonstrated that Dvl-1 directly inhibits GSK-3β-dependent phosphorylation of β-catenin and APC in the presence of Axin. These studies provide the first biochemical evidence in support of the proposed genetic model that Drosophila Dsh antagonizes shaggy activity (6, 9). It has been shown that serine phosphorylation of GSK-3 induced by p90rsk, protein kinase B (PKB), or PKC is important for the regulation of GSK-3 activity (8, 10, 12, 41). Since Dvl-1 is not a protein kinase, Dvl-1 could inactivate GSK-3β by different mechanisms. Dvl-1 does not inhibit kinase activity itself of GSK-3β for a synthetic peptide substrate, Dvl-1 inhibits only Axin-promoted phosphorylation of β-catenin and APC but does not affect their phosphorylation in the absence of Axin, and the binding of Dvl-1 to Axin does not affect the interaction of GSK-3β, β-catenin, or APC with rAxin. Therefore, it is possible that the binding of Dvl-1 to Axin induces the structural change of the Axin complex, and thus GSK-3β does not effectively phosphorylate β-catenin or APC. However, β-catenin is usually phosphorylated, ubiquitinated, and degraded in resting cells (1) even though Dvl-1 forms a complex with Axin. Since higher concentrations (micromolar order) of Dvl-1 are required to inhibit the action of GSK-3β in our in vitro experiments, modification of Dvl-1 such as phosphorylation may be necessary to act on the Axin complex in intact cells. It has been shown that soluble Wg protein inhibits GSK-3 activity in 10T1/2 fibroblasts and that phorbol ester-sensitive PKC may be involved in this signaling pathway (7). Although the relationship of Dvl-1 to PKC is unclear, Dvl-1 may lie downstream of PKC. Furthermore, in Drosophila imaginal disc cell line clone 8, a Dsh-associated kinase that phosphorylates Dsh has been identified as casein kinase 2 (44). Taken together with the observations that Dsh is a phosphoprotein localized predominantly in the cytoplasm of the cells and that Wg stimulation in the cells leads to hyperphosphorylation of Dsh (44, 46, 47), it is possible that the hyperphosphorylated form of Dvl-1 is an active form and that phosphorylated Dvl-1 transduces the signal onto the next signaling component, leading to the inhibition of the phosphorylation by GSK-3β. Studies to clarify the mechanism by which Wnt activates Dvl are under way.
Structures of Dvl family members are highly conserved and Dvl genes (Dvl-1, Dvl-2, and Dvl-3) are ubiquitously expressed in fetal and adult tissues, including brain, lung, skeletal muscle, and heart (22, 33, 40). Dvl-1-deficient mice are viable, fertile, and structurally normal but exhibit abnormal sensorimortor gating and reduced social interaction (25). These observations suggest redundancy of function among the Dvl genes in the Wnt signaling pathway and the participation of Wnt signaling components through Dvls in complex behavioral phenomena. Since whether Dvls have similar or distinct roles in the Wnt signaling pathway is not known, it remains to be elucidated whether Dvl-2 and -3 bind to Axin and suppress the GSK-3β-dependent phosphorylation of β-catenin.
ACKNOWLEDGMENTS
We are grateful to B. Dallapiccola, G. Novelli, Y. Takai, K. Tanaka, A. Nagafuchi, S. Tsukita, E. Tahara, Q. Hu, M. Nakata, and Y. Matsuura for donating plasmids, cells, antibodies, and viruses. We thank the Research Center for Molecular Medicine and Research Facilities for Laboratory Animal Sciences, Hiroshima University School of Medicine, for the use of their facilities.
This work was supported by Grants-in-Aid for Scientific Research and Exploratory Research from the Ministry of Education, Science, and Culture, Japan (1997, 1998), and by grants from the Yamanouchi Foundation for Research on Metabolic Disorders (1997, 1998), the Kato Memorial Bioscience Foundation (1997), and the Naito Foundation (1997).
REFERENCES
- 1.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axelrod J D, Miller J R, Shulman J M, Moon R T, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behrens J, Jerchow B-A, Würtele M, Grimm J, Asbrand C, Wirtz R, Kühl M, Wedlich D, Birchmeier W. Functional interaction of an Axin homolog, conductin, with β-catenin, APC, and GSK3β. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 4.Behrens J, von Kries J P, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 5.Boutros M, Paricio N, Strutt D I, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- 6.Cadigan K M, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 7.Cook D, Fry M J, Hughes K, Sumathipala R, Woodgett J R, Dale T C. Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a protein kinase C. EMBO J. 1996;15:4526–4536. [PMC free article] [PubMed] [Google Scholar]
- 8.Cross D A E, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 9.Dale T C. Signal transduction by the Wnt family ligands. Biochem J. 1998;329:209–223. doi: 10.1042/bj3290209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eldar-Finkelman H, Seger R, Vandenheede J R, Krebs E G. Inactivation of glycogen synthase kinase-3 by epidermal growth factor is mediated by mitogen-activated protein kinase/p90 ribosomal protein S6 kinase signaling pathway in NIH/3T3 cells. J Biol Chem. 1995;270:987–990. doi: 10.1074/jbc.270.3.987. [DOI] [PubMed] [Google Scholar]
- 11.Gluecksohn-Schoenheimer S. The effects of a lethal mutation responsible for duplications and twinning in mouse embryos. J Exp Zool. 1949;110:47–76. doi: 10.1002/jez.1401100105. [DOI] [PubMed] [Google Scholar]
- 12.Goode N, Hughes K, Woodgett J R, Parker P J. Differential regulation of glycogen synthase kinase-3 β by protein kinase C isotypes. J Biol Chem. 1992;267:16878–16882. [PubMed] [Google Scholar]
- 13.Hart M J, de los Santos R, Albert I N, Rubinfeld B, Polakis P. Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK-3β. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 14.He X, Saint-Jeannet J-P, Woodgett J R, Varmus H E, Dawid I B. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature. 1995;374:617–622. doi: 10.1038/374617a0. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda M, Ishida O, Hinoi T, Kishida S, Kikuchi A. Identification and characterization of a novel protein interacting with Ral-binding protein 1, a putative effector protein of Ral. J Biol Chem. 1998;273:814–821. doi: 10.1074/jbc.273.2.814. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh K, Krupnik V E, Sokol S Y. Axis determination in Xenopus involves biochemical interactions of Axin, glycogen synthase kinase 3 and β-catenin. Curr Biol. 1998;8:591–594. doi: 10.1016/s0960-9822(98)70229-5. [DOI] [PubMed] [Google Scholar]
- 18.Jiang J, Struhl G. Regulation of the hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- 19.Kishida M, Koyama S, Kishida S, Matsubara K, Nakashima S, Higano K, Takada R, Takada S, Kikuchi A. Axin prevents Wnt-3a-induced accumulation of β-catenin. Oncogene. 1999;18:979–985. doi: 10.1038/sj.onc.1202388. [DOI] [PubMed] [Google Scholar]
- 20.Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, directly interacts with Adenomatous Polyposis Coli and regulates the stabilization of β-catenin. J Biol Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 21.Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 1994;8:118–130. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- 22.Klingensmith J, Yang Y, Axelrod J D, Beier D R, Perrimon N, Sussman D J. Conservation of dishevelled structure and function between flies and mice: isolation and characterization of Dvl2. Mech Dev. 1996;58:15–26. doi: 10.1016/s0925-4773(96)00549-7. [DOI] [PubMed] [Google Scholar]
- 23.Korinek V, Barker N, Morin P J, van Wichen D, de Weger R, Kinzler K W, Vogelstein B, Clevers H. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Yuan H, Xie W, Mao J, Caruso A M, McMahon A, Sussman D J, Wu D. Dishevelled proteins lead to two signaling pathways. J Biol Chem. 1999;274:129–134. doi: 10.1074/jbc.274.1.129. [DOI] [PubMed] [Google Scholar]
- 25.Lijam N, Paylor R, McDonald M P, Crawley J N, Deng C X, Herrup K, Stevens K E, Maccaferri G, McBain C J, Sussman D J, Wynshaw-Boris A. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- 26.Miller J R, Moon R T. Signal transduction through β-catenin and specification of cell fate during embryogenesis. Genes Dev. 1996;10:2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- 27.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 28.Morin P J, Sparks A B, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K W. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 29.Murai H, Okazaki M, Kikuchi A. Tyrosine dephosphorylation of glycogen synthase kinase-3 is involved in its extracellular signal-dependent inactivation. FEBS Lett. 1996;392:153–160. doi: 10.1016/0014-5793(96)00806-x. [DOI] [PubMed] [Google Scholar]
- 30.Parr B A, McMahon A P. Wnt genes and vertebrate development. Curr Opin Genet Dev. 1994;4:523–528. doi: 10.1016/0959-437x(94)90067-d. [DOI] [PubMed] [Google Scholar]
- 31.Perry W L, III, Vasicek T J, Lee J J, Rossi J M, Zeng L, Zhang T, Tilghman S M, Costantini F. Phenotypic and molecular analysis of a transgenic insertional allele of the mouse Fused locus. Genetics. 1995;141:321–332. doi: 10.1093/genetics/141.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierce S B, Kimelman D. Regulation of Spemann organizer formation by the intracellular kinase Xgsk-3. Development. 1995;121:755–765. doi: 10.1242/dev.121.3.755. [DOI] [PubMed] [Google Scholar]
- 33.Pizzuti A, Amati F, Calabrese G, Mari A, Colosimo A, Silani V, Giardino L, Ratti A, Penso D, Calzà L, Palka G, Scarlato G, Novelli G, Dallapiccola B. cDNA characterization and chromosomal mapping of two human homologues of the Drosophila dishevelled polarity gene. Hum Mol Genet. 1996;5:953–958. doi: 10.1093/hmg/5.7.953. [DOI] [PubMed] [Google Scholar]
- 34.Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim Biophys Acta. 1997;1332:F127–F147. doi: 10.1016/s0304-419x(97)00008-5. [DOI] [PubMed] [Google Scholar]
- 35.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 36.Sakanaka C, Weiss J B, Williams L T. Bridging of β-catenin and glycogen synthase kinase-3β by axin and inhibition of β-catenin-mediated transcription. Proc Natl Acad Sci USA. 1998;95:3020–3023. doi: 10.1073/pnas.95.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sokol S, Christian J L, Moon R T, Melton D A. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991;67:741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- 38.Sokol S Y. Analysis of Dishevelled signaling pathways during Xenopus development. Curr Biol. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- 39.Stewart D B, Nelson W J. Identification of four distinct pools of catenins in mammalian cells and transformation-dependent changes in catenin distributions among these pools. J Biol Chem. 1997;272:29652–29662. doi: 10.1074/jbc.272.47.29652. [DOI] [PubMed] [Google Scholar]
- 40.Sussman D J, Klingensmith J, Salinas P, Adams P S, Nusse R, Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Dev Biol. 1994;166:73–86. doi: 10.1006/dbio.1994.1297. [DOI] [PubMed] [Google Scholar]
- 41.Sutherland C, Leighton I A, Cohen P. Inactivation of glycogen synthase kinase-3β by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296:15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theisen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh J. Dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120:347–360. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- 43.Vojtek A B, Hollenberg S M, Cooper J A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 44.Willert K, Brink M, Wodarz A, Varmus H, Nusse R. Casein kinase 2 associates with and phosphorylates Dishevelled. EMBO J. 1997;16:3089–3096. doi: 10.1093/emboj/16.11.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto H, Kishida S, Uochi T, Ikeda S, Koyama S, Asashima M, Kikuchi A. Axil, a member of the Axin family, interacts with both glycogen synthase kinase 3β and β-catenin and inhibits axis formation of Xenopus embryos. Mol Cell Biol. 1998;18:2867–2875. doi: 10.1128/mcb.18.5.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yanagawa S, Lee J, Haruna T, Oda H, Uemura T, Takeichi M, Ishimoto A. Accumulation of Armadillo induced by Wingless, Dishevelled, and dominant-negative Zeste-white 3 leads to elevated DE-cadherin in Drosophila clone 8 wing disc cells. J Biol Chem. 1997;272:25243–25251. doi: 10.1074/jbc.272.40.25243. [DOI] [PubMed] [Google Scholar]
- 47.Yanagawa S, van Leeuwen F, Wodarz A, Klingensmith J, Nusse R. The Dishevelled protein is modified by Wingless signaling in Drosophila. Genes Dev. 1995;9:1087–1097. doi: 10.1101/gad.9.9.1087. [DOI] [PubMed] [Google Scholar]
- 48.Yost C, Torres M, Miller J R, Huang E, Kimelman D, Moon R T. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 49.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek T J, Perry III W L, Lee J J, Tilghman S M, Gumbiner B M, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]