Abstract
Paracetamol is one of the most commonly used analgesic and antipyretic agents worldwide, attributed in part to its excellent safety profile when administered at recommended doses. Paracetamol allergy is not common, and the majority of the reactions are related to the pharmacological action of cyclooxygenase 1 inhibition. Selective and Immunoglobulin E (IgE)-mediated hypersensitivity reactions are rare. In this article, the authors report two cases of paracetamol allergy in which the mechanism of IgE-mediated hypersensitivity was demonstrated by positive skin tests and basophil activation tests. We highlight the relevance of identifying the mechanism underlying the reaction since patients with IgE-mediated paracetamol allergies will be able to tolerate non-steroidal anti-inflammatory drugs.
Keywords: skin test, paracetamol, ige, basophil activation test, allergy
Introduction
Paracetamol is one of the most commonly used analgesic and antipyretic agents worldwide, in part due to the paucity of its adverse effects when administered at recommended doses [1]. At therapeutic doses, paracetamol is a weak cyclooxygenase (COX) 1, COX-2, and COX-3 inhibitor, but it is not a non-steroidal anti-inflammatory drug (NSAID) [2-4]. Paracetamol allergy is not common, and most of the reported reactions are related to the pharmacological action of COX-1 inhibition [5]. Most reactions are documented by oral challenge tests [6-8], which do not elucidate the underlying pathophysiological mechanism that, in rare cases, has been shown to be IgE-mediated, with positive skin tests and/or detectable serum-specific IgE [9-10].
In this article, we present two rare cases of paracetamol allergy in which the mechanism of IgE-mediated hypersensitivity has been demonstrated and supported by in vivo and in vitro tests.
This data has been presented as a poster at the 40th annual meeting of the Sociedade Portuguesa de Alergologia e Imunologia Clínica in October 2019.
Case presentation
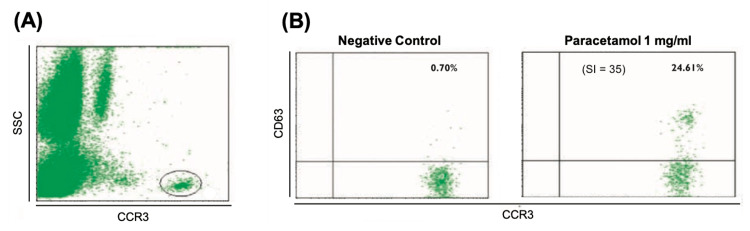
The first case is a 30-year-old female with no history of drug reactions who presented her first episode of generalized urticaria and oropharyngeal tightness starting 10-20 minutes after oral administration of 1000mg of paracetamol (Ben-u-ron®) as a symptomatic treatment for headaches. A few months later, she reported a second and third episode of generalized urticaria within 10-15 minutes after oral administration of 250 mg of paracetamol, 20 mg of pyrilamine maleate, and 30 mg of caffeine (Antigrippine®) and 1000 mg of paracetamol (Ben-u-ron®), respectively, for symptomatic relief of headaches and coryza. There were no gastrointestinal, pulmonary, or cardiovascular symptoms in any episode. After the third episode, the patient was referred to an Immunoallergology appointment with no instructions to avoid any particular drug. The patient described prior tolerance to acetylsalicylic acid (ASA), ibuprofen, and nimesulide, which she maintained after the three episodes. The investigation of suspected immediate hypersensitivity to paracetamol was performed with skin prick tests (SPT) with injectable paracetamol solution (10 mg/ml) [11], with the appearance of a 4 mm mean diameter wheal (6.5 mm mean diameter wheal for histamine) and erythema with a larger diameter of 15 mm. A serum-specific IgE measurement for paracetamol using the ImmunoCap® method (Phadia, Thermo Fisher Scientific, Sweden) was specially requested and was negative. A basophil activation test (BAT) with a paracetamol solution (1 mg/mL) was also performed. This was positive, with a basophil activation rate of 24.61% (proportion of CD63+ cells) corrected for the negative control (0.70%) and a stimulation index (SI) of 35 (ratio of CD63% of activated cells and negative control) (Figure 1). This concentration did not induce any significant basophilic activation in four healthy individuals. The patient was advised to avoid paracetamol to avoid the recurrence of similar episodes.
Figure 1. Basophil activation test for paracetamol: Case 1.
SI: stimulation index (ratio of CD63% of activated cells to negative control).
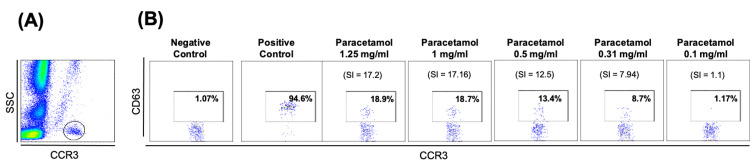
The second case is of a 26-year-old female who described her first episode of generalized urticaria after oral intake of 1000 mg of paracetamol (Doliprane®) and a second episode characterized by urticaria, and eyelid angioedema 10 minutes after oral administration of 1000 mg of paracetamol (Ben-u-ron®). A third episode of palmar and plantar itching, urticaria, and conjunctivitis started within 10 minutes after oral administration of 500 mg of paracetamol and 65 mg of caffeine (Ben-u-ron Caff®) for headaches. There were no signs or symptoms suggestive of pulmonary, gastrointestinal, or cardiovascular involvement in any episode. During the third episode, she was observed in the emergency department and treated with antihistamines and corticosteroids. She was referred to an outpatient Immunoallergology clinic with an indication to avoid paracetamol. After these three episodes, she tolerated ibuprofen, metamizole, and ASA. The immunoallergic research included SPT and intradermal tests with an intravenous paracetamol solution. The intradermal test at a concentration of 0.1 mg/ml (a non-irritant concentration as recommended by the European Network on Drug Allergy [11]) showed an immediate positive result, with an increase in the papule diameter of 3 mm and surrounding erythema of a greater diameter of 14 mm, at 15 minutes. According to the activation rate and the SI, Figure 2 shows a positive BAT for four paracetamol concentrations (0.31, 0.5, 1.0, and 1.25 mg/mL). The serum-specific IgE measurement for paracetamol was not performed.
Figure 2. Basophil activation test for paracetamol: Case 2.
(A) Identification of the basophil population as CCR3+ cells. (B) Flow cytometry dot plots of basophil activation expressed as a proportion of CD63+ on basophils corrected for the negative control. SI: stimulation index (ratio of CD63% of activated cells to negative control).
Written informed consent was obtained from both patients, and they were treated according to the ethical standards established in the Declaration of Helsinki.
Discussion
Hypersensitivity reactions to paracetamol are rare. Paracetamol is a weak COX inhibitor, and, therefore, inhibition of prostaglandin synthesis may be a mechanism to explain paracetamol reactions, similar to NSAIDs mechanisms [2-4]. However, cases of selective paracetamol reactions in NSAID-tolerant patients have been described [5]. In these cases, an IgE-mediated mechanism is possible.
In the few cases described of selective hypersensitivity to paracetamol, the diagnosis is mostly based on the oral challenge test, which does not elucidate the underlying pathophysiological mechanism [6-8]. The IgE-mediated mechanism was demonstrated by positive skin tests in only 10 reported cases [5,12-17] (Table 1).
Table 1. Reported cases of IgE mediated hypersensitivity to paracetamol.
ND: not done; NS: not stated; F: feminine.
| Article | Patient | Symptoms/signs | Skin prick test | Intradermal test | Oral challenge or accidental re-exposure |
| Martin JA et al., 1993[12] | F, Adult | Generalized urticaria, angioedema | Positive | ND | Positive |
| Sabbah A et al., 1997[13] | F, 39 years | Urticaria | Positive | NS | Positive |
| Galindo PA et al., 1998[14] | F, 20 years | Generalized urticaria, respiratory distress, wheezing | Negative | Positive | ND |
| Paramo B et al., 2000[15] | F, 21 years | Generalized urticaria, angioedema | Positive | ND | Positive |
| F, 24 years | Angiedema, hypotension | Positive | ND | Positive | |
| Rutkowski et al., 2012[5] | NS | Urticaria, facial angioedema, conjunctivitis, cough | Positive | ND | Positive |
| NS | Urticaria, facial angioedema, conjunctivitis, cough | Positive | ND | Positive | |
| NS | Generalized urticaria, dyspnea | Negative | Positive | ND | |
| Numata et al., 2016[16] | F, 20 years | Urticaria, dyspnea | Positive | ND | ND |
| Teles et al., 2021[17] | F, 9 years | Generalized urticaria, facial angioedema | Negative | Positive | Positive |
The authors describe two clinical cases of IgE-mediated hypersensitivity to paracetamol. Both are clinically characterized by the occurrence of several immediate-onset reactions 10-15 minutes after paracetamol administration, reproducible with repeated exposure, with no involvement of other drugs or allergen ingestion.
Both patients tolerate several NSAIDs, including ASA, demonstrating that COX-1 inhibition is not involved in these reactions. Although it is a limitation of the study, we considered that the benefit/risk ratio did not justify performing oral challenge tests to paracetamol in these patients because despite being the diagnostic gold standard of drug allergy, they are time- and resource-intensive and expose the patient to the risk of severe reactions. NSAIDs are effective antipyretic and analgesic drugs, making them a valid alternative to paracetamol.
The underlying specific IgE-mediated mechanism was demonstrated in both patients using paracetamol-positive skin tests. We also performed a BAT, which was positive in both patients. This basophil activation pattern confirmed the significant in vitro basophil degranulation specifically induced by paracetamol, which in these patients suggested an IgE-mediated mechanism [18-20]. Serum-specific IgE for paracetamol measurement was negative in the first case and was not performed in the second case. This analysis is not used in routine clinical practice, and some authors have reported negative results in patients with a proven IgE-mediated mechanism, which indicates that it has very low sensitivity [14,15].
Conclusions
The authors describe two cases of immediate hypersensitivity reactions to oral paracetamol, reproducible with repeated exposure, and tolerance to several NSAIDs, including strong COX-1 inhibitors. The presence of positive skin tests allowed us to presume an IgE-mediated mechanism. Additionally, it was possible to observe in vitro a significant basophilic degranulation specifically induced by paracetamol, which in these patients supports the assumed mechanism.
We propose that skin tests should be performed before oral challenge as paracetamol-specific IgE may play a role in this type of allergy, and those patients with an IgE-mediated paracetamol allergy will be able to tolerate NSAIDs.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Comparative safety evaluation of non-narcotic analgesics. Andrade S, Martinez C, Walker A. J Clin Epidemiol. 1998;51:1357–1365. doi: 10.1016/s0895-4356(98)00076-6. [DOI] [PubMed] [Google Scholar]
- 2.COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. Proc Natl Acad Sci U S A. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hypersensitivity to nonsteroidal anti-inflammatory drugs: from pathogenesis to clinical practice. Mota I, Gaspar A, Morais-Almeida M. https://www.spaic.pt/client_files/rpia_artigos/hipersensibilidade-a-anti-inflamatorios-nao-esteroides-da-patogenese-a-pratica-clinica.pdf Rev Port Imunoalergologia. 2018;26:207–220. [Google Scholar]
- 4.Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti-inflammatory drugs. Kowalski ML, Asero R, Bavbek S, et al. Allergy. 2013;68:1219–1232. doi: 10.1111/all.12260. [DOI] [PubMed] [Google Scholar]
- 5.Paracetamol hypersensitivity: clinical features, mechanism and role of specific IgE. Rutkowski K, Nasser SM, Ewan PW. Int Arch Allergy Immunol. 2012;159:60–64. doi: 10.1159/000335213. [DOI] [PubMed] [Google Scholar]
- 6.The accuracy of the diagnosis of suspected paracetamol (acetaminophen) hypersensitivity: results of a single-blinded trial. Kvedariene V, Bencherioua AM, Messaad D, Godard P, Bousquet J, Demoly P. Clin Exp Allergy. 2002;32:1366–1369. doi: 10.1046/j.1365-2222.2002.01476.x. [DOI] [PubMed] [Google Scholar]
- 7.Selective hypersensitivity reactions to acetaminophen: a 13-case series. Rojas-Pérez-Ezquerra P, Sánchez-Morillas L, Gómez-Traseira C, Gonzalez-Mendiola R, Alcorta Valle AR, Laguna-Martinez J. J Allergy Clin Immunol Pract. 2014;2:343–345. doi: 10.1016/j.jaip.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Paracetamol hypersensitivity: a 5 year review. Malheiro D, Sousa M, Moreira A, Cadinha S, Silva JM. Clin Transl Allergy. 2014;4:0. [Google Scholar]
- 9.Selective anaphylaxis to paracetamol in a child. Couto M, Gaspar A, Morais-Almeida M. https://pubmed.ncbi.nlm.nih.gov/23092003/ Eur Ann Allergy Clin Immunol. 2012;44:163–166. [PubMed] [Google Scholar]
- 10.Paracetamol allergy in clinical practice. Thompson G, Bundell C, Lucas M. Aust J Gen Pract. 2019;48:216–219. doi: 10.31128/AJGP-08-18-4689. [DOI] [PubMed] [Google Scholar]
- 11.Skin test concentrations for systemically administered drugs -- an ENDA/EAACI Drug Allergy Interest Group position paper. Brockow K, Garvey LH, Aberer W, et al. Allergy. 2013;68:702–712. doi: 10.1111/all.12142. [DOI] [PubMed] [Google Scholar]
- 12.Paracetamol anaphylaxis. Martin JA, Lazaro M, Cuevas M, Alvarez-Cuesta E. Clin Exp Allergy. 1993;23:534. doi: 10.1111/j.1365-2222.1993.tb03245.x. [DOI] [PubMed] [Google Scholar]
- 13.[Anaphylactic or anaphylactoid reactions to paracetamol. Report of 3 cases] Sabbah A, Sainte-Laudy J, Drouet M, Lauret MG, Loiry M. https://pubmed.ncbi.nlm.nih.gov/9221011/ Allerg Immunol (Paris) 1997;29:60–62. [PubMed] [Google Scholar]
- 14.Anaphylaxis to paracetamol. Galindo PA, Borja J, Mur P, Feo F, Gómez E, García R. https://pubmed.ncbi.nlm.nih.gov/9816409/ Allergol Immunopathol (Madr) 1998;26:199–200. [PubMed] [Google Scholar]
- 15.Paracetamol (acetaminophen) hypersensitivity. Paramo B, Gancedo Q, Cuevas M, Camo I, Martin J, Cosmes E. Ann Allergy Asthma Immunol. 2000;85:508–511. doi: 10.1016/s1081-1206(10)62580-x. [DOI] [PubMed] [Google Scholar]
- 16.Acetaminophen anaphylaxis diagnosed by skin prick test. Numata T, Fukushi R, Ito T, Tsuboi R, Harada K. Allergol Int. 2016;65:490–491. doi: 10.1016/j.alit.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Paracetamol allergy: a case of a 9-year-old female with history of atopy. Teles A, Ribeiro-Mourão F, Branco M, Araújo AR, Vieira T. Pediatr Allergy Immunol Pulmonol. 2021;34:80–82. doi: 10.1089/ped.2021.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The flow-cytometric determination of basophil activation induced by aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) is useful for in vitro diagnosis of the NSAID hypersensitivity syndrome. Gamboa P, Sanz ML, Caballero MR, Urrutia I, Antépara I, Esparza R, de Weck AL. Clin Exp Allergy. 2004;34:1448–1457. doi: 10.1111/j.1365-2222.2004.02050.x. [DOI] [PubMed] [Google Scholar]
- 19.In vitro diagnosis of immediate drug hypersensitivity: should we go with the flow. Mangodt EA, Van Gasse AL, Decuyper I, et al. Int Arch Allergy Immunol. 2015;168:3–12. doi: 10.1159/000440663. [DOI] [PubMed] [Google Scholar]
- 20.Recent applications of basophil activation tests in the diagnosis of drug hypersensitivity. Song WJ, Chang YS. Asia Pac Allergy. 2013;3:266–280. doi: 10.5415/apallergy.2013.3.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]