Abstract
Background
Recent research has suggested that sarcopenia may have an impact on postoperative outcomes. The number of hepatocellular carcinoma (HCC) patients with metabolic dysfunction-associated fatty liver disease (MAFLD) has increased significantly over time. The main objective of this study was to investigate the impact of sarcopenia on the prognosis of HCC patients with MAFLD after hepatectomy.
Methods
A multivariate Cox proportional hazards model and a propensity score matching (PSM) analysis were conducted to ensure that the baseline characteristics were similar. Kaplan‒Meier survival curves were used to compare the prognosis of the two groups.
Results
This study involved 112 HCC patients with MAFLD undergoing hepatectomy. Sarcopenia was indicated as a risk factor for both recurrence-free survival (RFS) and overall survival (OS) in HCC patients with MAFLD after multivariate analysis (p=0.002 and 0.022, respectively). After conducting PSM analysis, Kaplan‒Meier survival curve analysis revealed significant differences in both the RFS and OS between the two groups (p=0.0002 and p=0.0047, respectively). All results showed that sarcopenia had a poor prognosis for HCC patients with MAFLD undergoing hepatectomy.
Conclusion
In summary, our study suggests that sarcopenia might be a risk factor for OS and RFS in HCC patients with MAFLD who underwent hepatectomy through multivariate analysis and PSM analysis. Sarcopenia imperils postoperative survival rates and this finding can guide clinical decision-making. For postoperative patients, preventing or treating sarcopenia can potentially improve survival outcomes for patients with HCC and MAFLD.
Keywords: sarcopenia, metabolic dysfunction-associated fatty liver disease, hepatocellular carcinoma, hepatectomy
Introduction
The prevalence of nonalcoholic fatty liver disease (NAFLD) is on the rise globally, and this has led to a growing number of individuals with the condition developing liver cancer.1–5 Recently, a consensus has been reached among experts that NAFLD is an inadequate term to describe the pathogenesis of fatty liver disease. Instead, they suggest using “MAFLD” as a more suitable term for metabolic dysfunction-associated fatty liver disease.6,7 A growing number of studies have reported that the occurrence of HCC linked to MAFLD has increased significantly over time, particularly among females.8,9
Low muscle mass and function are widely acknowledged as having a considerable negative impact on an individual’s quality of life, overall health, and even their survival.10,11 As one of the primary characteristics of cancer cachexia, the loss of skeletal muscle tissue is frequently associated with adverse prognosis.12,13 In patients, sarcopenia often indicates that protein degradation is significantly greater than protein synthesis. Thus, sarcopenia affects the metabolism and function of the body and influences the prognosis of patients.14,15 Especially for patients undergoing surgery, sarcopenia is a common occurrence and has a significant influence on prognosis. Recent studies have indicated that sarcopenia has a negative effect on postoperative outcomes including those for lung cancer,16 esophageal cancer,17 liver malignant tumors,18 and colorectal liver metastases.19
Due to the relatively recent proposal of the MAFLD definition, our current understanding of the influence of sarcopenia on the prognosis of hepatocellular carcinoma accompanied by metabolic dysfunction-associated fatty liver disease (MAFLD-HCC) patients is limited. Therefore, this study aimed to investigate whether sarcopenia has an impact on the prognosis of MAFLD-HCC patients.
Patients and Methods
Study Design and Patients
This study was designed as a retrospective study and was reviewed by the Ethics Committee of West China Hospital of Sichuan University (No. 2022–1774). To ensure a sufficiently long follow-up period, the study included patients undergoing liver resection who were diagnosed with MAFLD-HCC at West China Hospital of Sichuan University between January 2011 and December 2017.
The inclusion criteria for this study were patients undergoing liver resection with pathological histological diagnosis of hepatocellular carcinoma and who met the diagnostic criteria for metabolic dysfunction-associated fatty liver disease.20 The exclusion criteria were as follows: (1) any nonsurgical intervention, including transcatheter arterial chemoembolization, immunotherapy, and chemotherapy; (2) pathology after surgery confirmed nonhepatocellular carcinoma or other pathological types of tumors; (3) underwent liver resection but did not participate in postoperative follow-up; (4) clinical data or imaging data were missing; and (5) partial tumor resection or palliative surgery.
Data Collection and Study Outcomes
At the time of surgery, data were collected retrospectively; these data included age, gender, body mass index (BMI), type 2 diabetes mellitus (T2D), hypertension, ascites, extrahepatic metastasis, computed tomography (CT) imaging and other laboratory tests. Additionally, we recorded the histopathological characteristics of the tumor excised after liver resection, including satellite nodules, cirrhosis, microvascular invasion, tumor thrombus, lymph node metastasis, and degree of differentiation.
The main outcome of the study was recurrence-free survival (RFS), which was defined as the period between the surgery and the date when the first HCC recurrence was diagnosed. The secondary endpoint was overall survival (OS), which was defined as the period between the date of surgery and either death, liver transplantation, or the last follow-up.
Measurement of Muscle Mass
The skeletal muscle index (SMI) reflected whether a patient had sarcopenia intuitively by normalizing the muscle area to patient height.21 The total cross-sectional area (cm2) of skeletal muscle in the abdomen at the third lumbar vertebra (L3) was measured using SliceOmatic version 5.0 (Tomovision, Montreal, QC, Canada) image analysis software by applying Hounsfield unit (HU) thresholds.21,22 The specific HU range used for tissue demarcation was between −29 to 150 HU. It encompassed the L3 muscles, specifically the psoas, paraspinal, and abdominal wall muscles, which consist of the rectus abdominis, transverse abdominis, and internal and external oblique muscles.23 In this study, patients in the sarcopenia group were diagnosed with an L3 SMI value of ≤29.0 cm²/m² for women and ≤36.0 cm²/m² for men.24 Images of individual examples representing the two distinct groups were those of a man with an SMI value of 28.89 cm²/m² who was categorized as having sarcopenia and a woman with an SMI value of 66.48 cm²/m² who was classified as nonsarcopenic (Figure 1). In the subgroup analysis, patients in the overweight group were categorized as having a body mass index (BMI) of more than 23 kg/m².20
Figure 1.
The red shaded part in the CT image represents the area of skeletal muscle. L3 SMI= 28.89 cm²/m². L3 SMI= 66.48 cm²/m².
Statistical Analysis
In this study, statistical analyses were performed using IBM SPSS Version 23.0, and GraphPad Prism 8 was used to generate the graphical representations. Continuous variables are presented as the means and standard deviations, and categorical variables are reported as percentages and frequencies. The prognosis of the two groups was evaluated using Kaplan‒Meier survival curves, and differences between the survival curves were compared using the log-rank (Mantel‒Cox) test. To analyze the hazard ratio (HR) of RFS and OS, univariate and multivariate analyses were conducted using the Cox proportional hazards regression model. A p value less than 0.05 was considered statistically significant.25
Propensity score matching (PSM) analysis is commonly used in retrospective research analysis since it enables the adjustment of various baseline parameters post-hoc, thereby simulating the outcomes of a hypothetical randomized study.26 To enhance the credibility and validity of the findings, a propensity score matching (PSM) analysis was conducted using a 1:1 nearest-neighbor matching scheme with a caliper width of 0.1. This can also minimize the effects of potential confounding factors and reduce selection bias between the sarcopenia and nonsarcopenia groups.
Results
Patient Characteristics
To explore the impact of sarcopenia on the long-term prognosis of HCC patients with MAFLD undergoing hepatectomy, a total of 1525 patients diagnosed with HCC from West China Hospital of Sichuan University between January 2011 and December 2017 were enrolled. Among all HCC patients who underwent surgical treatment, 325 patients met the diagnostic criteria for MAFLD.6 Due to incomplete clinical and imaging data as well as patient loss to follow-up, our study preliminarily excluded 97 and 60 patients, respectively. In addition, to reduce the heterogeneity of enrolled patients and ensure the authenticity of the study, we excluded 60 patients who underwent nonsurgical treatments such as chemotherapy, transcatheter chemoembolization, and immunotherapy. Eventually, based on the value of the L3 skeletal muscle index,19,24 our study included a total of 112 patients (Figure S1). Of these, 38 MAFLD-HCC patients with sarcopenia comprised the sarcopenia group, and 74 MAFLD-HCC patients without sarcopenia comprised the nonsarcopenia group. The characteristics of the study group at baseline are displayed in Table 1. The mean age of the study population was 56.88 years, and the majority of patients were male (n=91, 81.30%). Compared to that of the sarcopenia group, the average SMI value of the nonsarcopenia group was significantly higher, as the values were 30.18 cm²/m² and 48.67 cm²/m², respectively.
Table 1.
Baseline Characteristics of Patients of the Whole Study Population
| All Patients (n=112) | Sarcopenia (n=38) | Non-Sarcopenia (n=74) | P value | |
|---|---|---|---|---|
| Age (years) | 56.88±11.51 | 59.55±12.34 | 55.50±10.90 | 0.055 |
| L3 SMI (cm²/ m²) | 42.40±13.65 | 30.18±3.53 | 48.67±12.63 | <0.001 |
| BMI (kg/m²) | 24.62±3.49 | 24.35±3.97 | 24.75±3.24 | 0.468 |
| Tumor size (cm) | 4.00(3.00–6.00) | 4.00(2.80–6.00) | 5.00(3.00–6.00) | 0.467 |
| Tumor number | 1.00(1.00–1.00) | 1.00(1.00–1.00) | 1.00(1.00–1.00) | 0.964 |
| Total bilirubin (µmol/L) | 14.00(11.30–19.10) | 13.85(11.48–17.53) | 14.05(10.78–20.98) | 0.949 |
| GGT (IU/L) | 62.50(36.00–132.00) | 56.50(35.50–115.00) | 62.50(36.00–132.50) | 0.842 |
| ALT (IU/L) | 37.00(24.25–54.50) | 36.00(26.75–60.50) | 38.00(22.75–52.25) | 0.743 |
| AST (IU/L) | 36.00(24.00–59.00) | 36.50(28.00–56.00) | 35.00(23.00–67.25) | 0.836 |
| ALB (g/L) | 41.02±5.22 | 41.27±5.94 | 40.90±4.84 | 0.376 |
| HDL (mmol/L) | 1.12(0.97–1.35) | 1.15(0.98–1.31) | 1.10(0.96–1.36) | 0.788 |
| LDL (mmol/L) | 2.36(1.97–2.77) | 2.32(1.98–2.78) | 2.41(1.93–2.80) | 0.549 |
| TG (mmol/L) | 1.31(0.87–1.71) | 1.04(0.74–1.67) | 1.35(0.94–1.72) | 0.162 |
| AFP (ng/mL) | 14.38(3.83–258.58) | 22.65(3.89–591.78) | 13.08(3.77–228.38) | 0.842 |
| Gender, [n (%)] | 0.655 | |||
| Female | 21(18.8%) | 8(21.1%) | 13(17.6%) | |
| Male | 91(81.3%) | 30(78.9%) | 61(82.4%) | |
| BCLC stage | 0.357 | |||
| 0 | 64(57.1%) | 24(63.2%) | 40(54.1%) | |
| A | 48(42.9%) | 14(36.8%) | 34(45.9%) | |
| Child Pugh grade | 0.095 | |||
| A | 77(68.8%) | 30(78.9%) | 47(63.5%) | |
| B | 35(31.2%) | 8(21.1%) | 27(36.5%) | |
| Hypertension, [n (%)] | 0.923 | |||
| NO | 73(65.2%) | 25(65.8%) | 48(64.9%) | |
| YES | 39(34.8%) | 13(34.2%) | 26(35.1%) | |
| Diabetes, [n (%)] | 0.249 | |||
| NO | 84(75.0%) | 26(68.4%) | 58(78.4%) | |
| YES | 28(25.0%) | 12(31.6%) | 16(21.6%) | |
| Cirrhosis, [n (%)] | 0.964 | |||
| NO | 74(66.1%) | 25(65.8%) | 49(66.2%) | |
| YES | 38(33.9%) | 13(34.2%) | 25(33.8%) | |
| Ascites, [n (%)] | 0.628 | |||
| NO | 110(98.2%) | 37(97.4%) | 73(98.6%) | |
| YES | 2(1.8%) | 1(2.6%) | 1(1.4%) | |
| Satellite nodule, [n (%)] | 0.798 | |||
| NO | 99(88.4%) | 34(89.5%) | 65(87.8%) | |
| YES | 13(11.6%) | 4(10.5%) | 9(12.2%) | |
| Tumor thrombus, [n (%)] | 0.701 | |||
| NO | 108(96.4%) | 37(97.4%) | 71(95.9%) | |
| YES | 4(3.6%) | 1(2.6%) | 3(4.1%) | |
| Lymph node metastasis, [n (%)] | 0.489 | |||
| NO | 108(96.4%) | 36(94.7%) | 72(97.3%) | |
| YES | 4(3.6%) | 2(5.3%) | 2(2.7%) | |
| Extrahepatic metastasis, [n (%)] | 0.701 | |||
| NO | 108(96.4%) | 37(97.4%) | 71(95.9%) | |
| YES | 4(3.6%) | 1(2.6%) | 3(4.1%) | |
| HBV status, [n (%)] | 0.580 | |||
| Negative | 49(43.8%) | 18(47.4%) | 31(41.9%) | |
| Positive | 63(56.3%) | 20(52.6%) | 43(58.1%) | |
| HCV status, [n (%)] | 0.208 | |||
| Negative | 107(95.5%) | 35(92.1%) | 72(97.3%) | |
| Positive | 5(4.5%) | 3(7.9%) | 2(2.7%) | |
| MVI, [n (%)] | 0.322 | |||
| Negative | 99(88.4%) | 32(84.2%) | 67(90.5%) | |
| Positive | 13(11.6%) | 6(15.8%) | 7(9.5%) | |
| High differentiation, [n (%)] | 0.257 | |||
| NO | 105(93.8%) | 37(97.4%) | 68(91.9%) | |
| YES | 7(6.3%) | 1(2.6%) | 6(8.1%) |
Abbreviations: L3 SMI, L3 skeletal muscle index; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; MAFLD, metabolic dysfunction-associated fatty liver disease; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ- glutamyl transpeptidase; TG, triglycerides; AFP, alpha-fetoprotein; BCLC, Barcelona clinic liver cancer; HDL, High-density lipoprotein; LDL, low-density lipoprotein; MVI, microvascular invasion.
Survival Outcomes Before Propensity Score Matching
The 1-, 3-, and 5-year RFS rates of the nonsarcopenia group were 66.2%, 47.3%, and 39.2%, respectively. These rates were higher than those of the sarcopenia group, which were 42.1%, 26.3%, and 18.4%, respectively (P < 0.0001). Moreover, the 1-, 3-, and 5-year OS rates of the nonsarcopenia group were 70.3%, 62.2%, and 58.1%, respectively. These rates were higher than those observed in the sarcopenia group, which were 55.3%, 39.5%, and 34.2%, respectively (P = 0.0076). In summary, the patients without sarcopenia in our study demonstrated significantly longer recurrence-free survival and overall survival than those with sarcopenia using Kaplan–Meier analysis (Figure 2).
Figure 2.
RFS (A) and OS (B) after hepatectomy in MAFLD-HCC patients with or without sarcopenia before PSM.
Sarcopenia was observed to be a risk factor for both RFS and OS in MAFLD-HCC patients after univariate analysis (p<0.001 and 0.013, respectively) (Table 2). According to multivariate analysis, body mass index, satellite nodule, HCV status, value of alpha-fetoprotein (AFP), microvascular invasion, and sarcopenia were all important factors that affect the RFS. Tumor thrombus and sarcopenia were identified as risk factors for OS in MAFLD-HCC patients who underwent hepatectomy (Table 2). The multivariate analysis results from the Cox proportional hazards model indicated that sarcopenia could be a significant predictor of both poor RFS and poor OS in MAFLD-HCC patients (p=0.001 and 0.022, respectively).
Table 2.
Univariate and Multivariate Analyses of Each Factor’s Value in Predicting RFS and OS of the Whole Study Population
| RFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analyses | Multivariate Analysis | Univariate Analyses | Multivariate Analysis | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age. y | ||||||||
| >60 vs ≤60 | 1.10(0.64–1.89) | 0.745 | 2.52(0.91–6.95) | 0.074 | ||||
| Gender | ||||||||
| Male vs female | 1.01(0.47–2.14) | 0.986 | 0.36(0.13–0.99) | 0.049 | 0.35(0.12–1.03) | 0.056 | ||
| BMI. kg/m² | ||||||||
| ≥23 vs <23 | 0.55(0.32–0.85) | 0.032 | 0.57(0.33–0.99) | 0.049 | 0.63(0.23–1.75) | 0.372 | ||
| Hypertension | ||||||||
| With vs without | 1.28(0.74–2.21) | 0.375 | 1.66(0.62–4.48) | 0.315 | ||||
| Diabetes | ||||||||
| With vs without | 1.27(0.71–2.28) | 0.422 | 1.81(0.66–4.99) | 0.250 | ||||
| Cirrhosis | ||||||||
| With vs without | 0.95(0.54–1.65) | 0.845 | 2.07(0.77–5.59) | 0.151 | ||||
| Ascites | ||||||||
| With vs without | 1.22(0.30–5.01) | 0.786 | 0.05(0.00–41,181) | 0.663 | ||||
| Satellite nodule | ||||||||
| With vs without | 3.55(1.70–7.39) | 0.001 | 0.67(0.16–2.88) | 0.589 | 3.45(0.97–12.29) | 0.056 | ||
| Tumor thrombus | ||||||||
| With vs without | 2.44(0.76–7.85) | 0.135 | 7.21(1.61–32.31) | 0.010 | 19.98(3.55–112.47) | 0.001 | ||
| Lymph node metastasis | ||||||||
| With vs without | 1.25(0.30–5.14) | 0.762 | 0.05(0.00–22,287) | 0.647 | ||||
| Extrahepatic metastasis | ||||||||
| With vs without | 2.65(0.82–8.56) | 0.102 | 0.05(0.00–46,467) | 0.665 | ||||
| HBV status | ||||||||
| Positive vs negative | 1.19(0.69–2.04) | 0.539 | 0.83(0.31–2.22) | 0.711 | ||||
| HCV status | ||||||||
| Positive vs negative | 3.07(1.18–8.00) | 0.021 | 3.20(1.17–8.75) | 0.023 | 1.53(0.20–11.72) | 0.683 | ||
| Tumor size. cm | ||||||||
| >5 vs ≤5 | 1.02(0.57–1.83) | 0.950 | 0.92(0.30–2.88) | 0.888 | ||||
| Tumor number | ||||||||
| Multiple vs single | 1.36(0.66–2.79) | 0.400 | 0.43(0.06–3.26) | 0.414 | ||||
| Sarcopenia | ||||||||
| With vs without | 3.19(1.84–5.53) | <0.001 | 2.52(1.42–4.49) | 0.002 | 3.74(1.32–10.56) | 0.013 | 4.09(1.23–13.60) | 0.022 |
| BCLC stage | ||||||||
| A vs 0 | 1.12(0.65–1.93) | 0.689 | 0.77(0.27–2.21) | 0.624 | ||||
| Child Pugh grade | ||||||||
| B vs A | 1.12(0.63–1.99) | 0.701 | 1.47(0.53–4.04) | 0.459 | ||||
| ALB. g/L | ||||||||
| ≤40 vs >40 | 0.69(0.40–1.19) | 0.183 | 1.06(0.37–3.07) | 0.909 | ||||
| Total bilirubin. µmol/L | ||||||||
| >20.5 vs ≤20.5 | 1.34(0.74–2.40) | 0.335 | 0.69(0.20–2.42) | 0.558 | ||||
| ALT. IU/L | ||||||||
| >50 vs ≤50 | 1.34(0.78–2.31) | 0.291 | 0.78(0.27–2.24) | 0.641 | ||||
| AST. IU/L | ||||||||
| >40 vs ≤40 | 1.40(0.82–2.38) | 0.221 | 0.76(0.28–2.10) | 0.599 | ||||
| GGT. IU/L | ||||||||
| >60 vs ≤60 | 1.52(0.89–2.60) | 0.127 | 1.46(0.54–3.92) | 0.455 | ||||
| TG. mmol/L | ||||||||
| ≥1.7 vs <1.7 | 1.06(0.58–1.93) | 0.847 | 0.42(0.10–1.86) | 0.253 | ||||
| AFP. ng/ml | ||||||||
| ≥400 vs <400 | 2.22(1.21–4.05) | 0.01 | 2.30(1.24–4.25) | 0.008 | 2.79(0.95–8.19) | 0.063 | ||
| HDL. mmol/L | ||||||||
| <1.0 vs ≥1.0 | 1.26(0.72–2.21) | 0.415 | 0.52(0.15–1.83) | 0.309 | ||||
| LDL. mmol/L | ||||||||
| ≥3.4 vs <3.4 | 0.99(0.36–2.74) | 0.984 | 0.80(0.11–6.05) | 0.828 | ||||
| MVI | ||||||||
| Positive vs negative | 8.13(3.65–18.13) | <0.001 | 10.76(2.31–50.04) | 0.002 | 6.23(1.72–22.58) | 0.005 | 2.82(0.71–11.28) | 0.142 |
| High differentiation | ||||||||
| NO vs YES | 1.49(0.46–4.83) | 0.51 | 1.86(0.24–14.32) | 0.552 | ||||
Note: A p-value less than 0.05 is displayed in bold black font, indicating a statistical difference between the two groups.
Abbreviations: BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; BCLC, Barcelona clinic liver cancer; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ- glutamyl transpeptidase; TG, triglycerides; AFP, alpha-fetoprotein; BCLC, Barcelona clinic liver cancer; HDL, High-density lipoprotein; LDL, low-density lipoprotein; CI, confidence interval; HR, hazard ratio; OS, overall survival from surgery to death; RFS, recurrence‐free survival after surgery; MVI, microvascular invasion.
Survival Outcomes After Propensity Score Matching
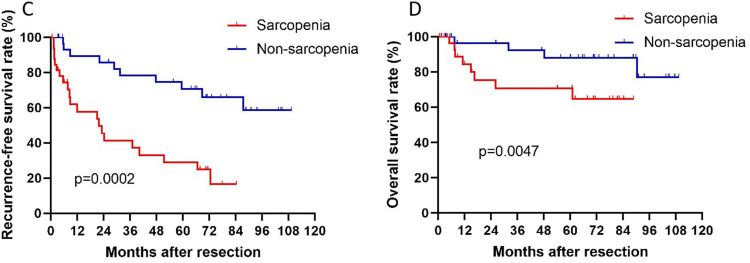
Additionally, we also conducted a PSM analysis of our data (Table 3). The main purpose of PSM analysis was to reduce random and systematic errors. After PSM analysis, the 1-, 3-, and 5-year OS rates of the sarcopenia group were 57.6%, 45.5%, and 39.4%, respectively. In the nonsarcopenia group, the 1-, 3-, and 5-year OS rates were 75.8%, 69.7%, and 60.6%, respectively. Moreover, the 1-, 3-, and 5-year RFS rates were higher in the nonsarcopenia group (75.8%, 63.6%, and 54.5%, respectively) than in the sarcopenia group (57.6%, 45.5%, and 39.4%, respectively). Therefore, sarcopenia imperils 1-year, 3-year, and 5-year OS and RFS rates of patients with MAFLD-HCC who had undergone hepatectomy. Additionally, we found that the two groups had significant differences in both RFS and OS based on the Kaplan–Meier survival curve analysis (p=0.0002 and p=0.0047, respectively) (Figure 3). These results were consistent with the results before PSM analysis. Thus, sarcopenia might be related to the poor prognosis of MAFLD-HCC patients undergoing liver resection.
Table 3.
Baseline Characteristics of Patients in the Propensity Score Matching Cohort
| Patients After PSM (n=66) | Sarcopenia (n=33) | Non-Sarcopenia (n=33) | P value | |
|---|---|---|---|---|
| Age (years) | 56.14±11.66 | 58.30±12.57 | 53.97±10.42 | 0.132 |
| L3 SMI (cm²/ m²) | 40.02±13.08 | 30.46±3.39 | 49.57±12.14 | <0.001 |
| BMI (kg/m²) | 24.68±3.42 | 24.73±4.00 | 24.62±2.77 | 0.900 |
| Tumor size (cm) | 4.00(3.00–6.00) | 4.00(2.90–6.00) | 5.00(3.00–5.05) | 0.781 |
| Tumor number | 1.00(1.00–1.00) | 1.00(1.00–1.00) | 1.00(1.00–1.00) | 1.000 |
| Total bilirubin (µmol/L) | 13.85(11.65–18.20) | 14.00(11.65–17.95) | 13.60(11.45–19.00) | 0.768 |
| GGT (IU/L) | 52.00(34.75–107.75) | 49.00(34.00–146.50) | 54.00(34.50–80.00) | 0.476 |
| ALT (IU/L) | 35.50(21.75–52.25) | 36.00(25.50–54.00) | 32.00(19.50–47.50) | 0.336 |
| AST (IU/L) | 33.00(23.75–49.75) | 36.00(25.00–55.50) | 27.00(21.50–45.50) | 0.084 |
| ALB (g/L) | 42.81±5.18 | 41.59±6.24 | 44.03±3.55 | 0.055 |
| HDL (mmol/L) | 1.14(0.97–1.32) | 1.14(0.96–1.29) | 1.14(0.98–1.52) | 0.478 |
| LDL (mmol/L) | 2.51(2.12–2.78) | 2.33(2.10–2.78) | 2.62(2.21–2.90) | 0.551 |
| TG (mmol/L) | 1.31(0.91–1.71) | 1.12(0.80–1.71) | 1.33(0.94–1.77) | 0.305 |
| AFP (ng/mL) | 7.49(3.58–289.40) | 10.45(3.87–390.00) | 5.21(2.76–201.93) | 0.307 |
| Gender, [n (%)] | 0.769 | |||
| Female | 15(22.7%) | 7(21.2%) | 8(24.2%) | |
| Male | 51(77.3%) | 26(78.8%) | 25(75.8%) | |
| BCLC stage | 0.800 | |||
| 0 | 41(62.1%) | 21(63.6%) | 20(60.6%) | |
| A | 25(37.9%) | 12(36.4%) | 13(39.4%) | |
| Child Pugh grade | 0.778 | |||
| A | 49(74.2%) | 25(75.8%) | 24(72.7%) | |
| B | 17(25.8%) | 8(24.2%) | 9(27.3%) | |
| Hypertension, [n (%)] | 0.602 | |||
| NO | 44(66.7%) | 23(69.7%) | 21(63.6%) | |
| YES | 22(33.3%) | 10(30.3%) | 12(36.4%) | |
| Diabetes, [n (%)] | 0.142 | |||
| NO | 51(77.3%) | 23(69.7%) | 28(84.8%) | |
| YES | 15(22.7%) | 10(30.3%) | 5(15.2%) | |
| Cirrhosis, [n (%)] | 0.447 | |||
| NO | 41(62.1%) | 22(66.7%) | 19(57.6%) | |
| YES | 25(37.9%) | 11(33.3%) | 14(42.4%) | |
| Ascites, [n (%)] | 0.314 | |||
| NO | 65(98.5%) | 32(97%) | 33(100.0%) | |
| YES | 1(1.5%) | 1(3%) | 0(0%) | |
| Satellite nodule, [n (%)] | 1.000 | |||
| NO | 60(90.9%) | 30(90.9%) | 30(90.9%) | |
| YES | 6(9.1%) | 3(9.1%) | 3(9.1%) | |
| Tumor thrombus, [n (%)] | 1.000 | |||
| NO | 64(97.0%) | 32(97.0%) | 32(97.0%) | |
| YES | 2(3.0%) | 1(3.0%) | 1(3.0%) | |
| Lymph node metastasis, [n (%)] | 0.314 | |||
| NO | 65(98.5%) | 32(97.0%) | 33(100.0%) | |
| YES | 1(1.5%) | 1(3.0%) | 0(0.0%) | |
| Extrahepatic metastasis, [n (%)] | 0.314 | |||
| NO | 65(98.5%) | 32(97.0%) | 33(100.00%) | |
| YES | 1(1.5%) | 1(3.0%) | 0(0.00%) | |
| HBV status, [n (%)] | 0.453 | |||
| Negative | 27(40.9%) | 15(45.5%) | 12(36.4%) | |
| Positive | 39(59.1%) | 18(54.5%) | 21(63.6%) | |
| HCV status, [n (%)] | 1.000 | |||
| Negative | 66(100.0%) | 33(100.0%) | 33(100.0%) | |
| Positive | 0(0.0%) | 0(0.0%) | 0(0.0%) | |
| MVI, [n (%)] | 1.000 | |||
| Negative | 56(84.8%) | 28(84.8%) | 28(84.8%) | |
| Positive | 10(15.2%) | 5(15.2%) | 5(15.2%) | |
| High differentiation, [n (%)] | 0.555 | |||
| NO | 63(95.5%) | 32(97.0%) | 31(93.9%) | |
| YES | 3(4.5%) | 1(3.0%) | 2(6.1%) |
Abbreviations: L3 SMI, L3 skeletal muscle index; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; MAFLD, metabolic dysfunction-associated fatty liver disease; BCLC, Barcelona clinic liver cancer; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ- glutamyl transpeptidase; TG, triglycerides; AFP, alpha-fetoprotein; HDL, High-density lipoprotein; LDL, low-density lipoprotein; MVI, microvascular invasion.
Figure 3.
RFS (C) and OS (D) after hepatectomy in MAFLD-HCC patients with or without sarcopenia after PSM.
Subgroup Analysis Based on the Definition of MAFLD and HBV Status
According to the diagnostic criteria for MAFLD,6,20 we divided the sarcopenia group into two groups for subgroup analysis based on BMI values: the lean/normal weight group (BMI <23 kg/m2) and the overweight group (BMI ≥23 kg/m2). In the subgroup analysis, there were no significant differences between the two groups in terms of RFS and OS (p=0.856 and p=0.554) (Figure S2). Similarly, based on the diabetes criteria in the MAFLD diagnostic standard, we divided the sarcopenia group into a type 2 diabetes group (T2D) and a nontype 2 diabetes group (non-T2D). We discovered that there was no statistical difference in either RFS or OS between the two groups (p=0.397 and p=0.056, respectively) (Figure S3).
MAFLD is a recently proposed concept that focuses more on the patient’s fat deposition and metabolic dysfunction than the concept of nonalcoholic fatty liver disease (NAFLD), without the need to exclude liver damage caused by factors such as HBV. We performed subgroup analysis on all patients with MAFLD-HCC who also had concurrent HBV infection that were included (Figure S4). Sarcopenia could be a poor predictor of RFS in MAFLD-HCC patients with HBV infection (p=0.0054). According to the Kaplan–Meier survival curve, it was evident that the 1-year, 3-year, and 5-year survival rates of the sarcopenia group were significantly lower than those of the nonsarcopenia group, although there was no statistically significant difference in overall survival rate (p=0.1009).
Discussion
The results of the Kaplan‒Meier survival curves and multivariate and PSM analyses in this study consistently suggested that sarcopenia might imperil both the RFS rate and the OS rate in MAFLD-HCC patients undergoing liver resection. The possible reason for our results could be that sarcopenia, a geriatric condition, is characterized by a progressive decline in muscle mass and function, and is linked to various negative health consequences.13,27 Moreover, cachexia is primarily characterized by skeletal muscle atrophy, and patients with sarcopenia may exhibit a propensity to develop cachexia, which could be the underlying reason for our results.12,14 Recently, there have been several studies on the relationship between sarcopenia and the prognosis of HCC. Voron et al discovered that sarcopenia was an independent predictor of both poor overall survival and disease-free survival in HCC patients undergoing hepatectomy.28 Yabusaki et al found that a low skeletal muscle index was an independent adverse prognostic factor for the cumulative recurrence rate in HCC patients with BMI ≥22 after hepatic resection.29 Yang et al and Wu et al stated that sarcopenia influenced short-term postoperative outcomes after hepatectomy in HCC patients.23,30 Iritani et al reported that sarcopenic HCC patients showed a significantly lower OS than those without sarcopenia.24 According to Kobayashi et al, preoperative sarcopenic obesity is an independent risk factor for both death and HCC recurrence after hepatectomy for HCC.31
Although the number of patients with MAFLD-HCC is increasing annually, there is currently no research on the prognosis of MAFLD-HCC patients after surgery in relation to sarcopenia. Compared to that in subjects having only MAFLD, the prevalence of significant liver fibrosis was higher in sarcopenic subjects with MAFLD.32 This indicated that we could not simply consider the relationship between HCC and sarcopenia when studying the association between MAFLD-HCC and sarcopenia. MAFLD might synergize with sarcopenia to promote liver fibrosis and thus impact the prognosis of HCC patients.
Some intervention measures might be taken to reduce the survival risks associated with sarcopenia.
A home-based exercise program focusing on fitness and physical function was found to increase exercise capacity in both arms of a previous study.11 Preoperative and postoperative nutritional support can also reduce the incidence of sarcopenia.33 To improve the poor prognosis of sarcopenia, it is necessary to explore more intervention measures. Prospective studies, multicenter studies, systematic reviews, and meta-analyses are also needed.
This research had certain limitations. There is evidence to suggest that sarcopenia is uncommon in patients with MAFLD.34 Thus, the number of samples was small. Further research, including larger sample sizes and more in-depth mechanistic studies, may be needed to validate these results. Due to limited resources, we can only diagnose sarcopenia by calculating the SMI value through CT scans. We cannot collect data from other diagnostic methods such as measuring grip strength.
Conclusion
Sarcopenia might decrease the overall survival (OS) and recurrence-free survival (RFS) of MAFLD-HCC patients. Preventing and treating sarcopenia can potentially lead to a better prognosis for MAFLD-HCC patients.
Funding Statement
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Abbreviations
HCC, hepatocellular carcinoma; NAFLD, nonalcoholic fatty liver disease; MAFLD, metabolic dysfunction-associated fatty liver disease; L3, the third lumbar vertebra; SMI, skeletal muscle index; CT, computed tomography; HU, Hounsfield unit; OS, overall survival; RFS, recurrence-free survival; BMI, body mass index; T2D, type 2 diabetes mellitus; non-T2D, without type 2 diabetes group; HBV, hepatitis B virus; HCV, hepatitis C virus; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ- glutamyl transpeptidase; TG, triglyceride; AFP, alpha-fetoprotein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MVI, microvascular invasion; MAFLD-HCC hepatocellular carcinoma accompanied by metabolic dysfunction-associated fatty liver disease.
Statements and Declarations
The authors declare that they have no conflicts of interest. We confirm that our research has been approved by the Ethics Committee of West China Hospital of Sichuan University. The approval number is No. 2022-1774. All the patients at the West China Hospital of Sichuan University had signed an informed consent form, which includes a provision stating: “Your personal information and data could potentially be utilized for scientific research purposes. If you sign this informed consent form, it means you allow your medical records to be reviewed”. All patients signed an informed consent form, and the use of patient data is confidential and complies with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Younossi ZM, Stepanova M, Younossi Y, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020;69(3):564–568. doi: 10.1136/gutjnl-2019-318813 [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–1586. doi: 10.1002/hep.28785 [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62(6):1723–1730. doi: 10.1002/hep.28123 [DOI] [PubMed] [Google Scholar]
- 4.Xue R, Yang RX, Fan JG. Epidemiological trends and clinical characteristic of NAFLD/MAFLD in Asia. J Dig Dis. 2022;23(7):354–357. doi: 10.1111/1751-2980.13117 [DOI] [PubMed] [Google Scholar]
- 5.Vitale A, Svegliati-Baroni G, Ortolani A, et al. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002–2033: the ITA.LI.CA database. Gut. 2023;72(1):141–152. doi: 10.1136/gutjnl-2021-324915 [DOI] [PubMed] [Google Scholar]
- 6.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 7.Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312 [DOI] [PubMed] [Google Scholar]
- 8.Myers S, Neyroud-Caspar I, Spahr L, et al. NAFLD and MAFLD as emerging causes of HCC: a populational study. JHEP Rep. 2021;3(2):100231. doi: 10.1016/j.jhepr.2021.100231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3(12):1683–1691. doi: 10.1001/jamaoncol.2017.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barazzoni R, Cederholm T, Zanetti M, Cappellari GG. Defining and diagnosing sarcopenia: is the glass now half full? Metabolism. 2023;155558. doi: 10.1016/j.metabol.2023.155558 [DOI] [PubMed] [Google Scholar]
- 11.Ngo-Huang AT, Parker NH, Xiao LC, et al. Effects of a pragmatic home-based exercise program concurrent with neoadjuvant therapy on physical function of patients with pancreatic cancer: the pancfit randomized clinical trial. Ann Surg. 2023. doi: 10.1097/sla.0000000000005878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peixoto da Silva S, Santos JMO, Costa ESMP, Gil da Costa RM, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle. 2020;11(3):619–635. doi: 10.1002/jcsm.12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan S, Larsson SC. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism. 2023;144:155533. doi: 10.1016/j.metabol.2023.155533 [DOI] [PubMed] [Google Scholar]
- 14.Schmidt SF, Rohm M, Herzig S, Berriel Diaz M. Cancer cachexia: more than skeletal muscle wasting. Trends Cancer. 2018;4(12):849–860. doi: 10.1016/j.trecan.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 15.Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5(2):e200. doi: 10.1038/oncsis.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura R, Inage Y, Tobita R, et al. Sarcopenia in resected NSCLC: effect on postoperative outcomes. J Thorac Oncol. 2018;13(7):895–903. doi: 10.1016/j.jtho.2018.04.035 [DOI] [PubMed] [Google Scholar]
- 17.Jogiat UM, Sasewich H, Turner SR, et al. Sarcopenia determined by skeletal muscle index predicts overall survival, disease-free survival, and postoperative complications in resectable esophageal cancer: a systematic review and meta-analysis. Ann Surg. 2022;276(5):e311–e318. doi: 10.1097/sla.0000000000005452 [DOI] [PubMed] [Google Scholar]
- 18.Diao YK, Liang L, Yang T. Association of sarcopenia and body composition with postoperative 90-day morbidity after liver resection for malignant tumors. JAMA Surg. 2021;156(6):590. doi: 10.1001/jamasurg.2021.0231 [DOI] [PubMed] [Google Scholar]
- 19.van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 2012;99(4):550–557. doi: 10.1002/bjs.7823 [DOI] [PubMed] [Google Scholar]
- 20.Eslam M, El-Serag HB, Francque S, et al. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat Rev Gastroenterol Hepatol. 2022;19(10):638–651. doi: 10.1038/s41575-022-00635-5 [DOI] [PubMed] [Google Scholar]
- 21.Carey EJ, Lai JC, Wang CW, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017;23(5):625–633. doi: 10.1002/lt.24750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai K, Takai K, Hanai T, et al. Skeletal muscle depletion predicts the prognosis of patients with hepatocellular carcinoma treated with sorafenib. Int J Mol Sci. 2015;16(5):9612–9624. doi: 10.3390/ijms16059612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Chen K, Zheng C, et al. Impact of sarcopenia on outcomes of patients undergoing liver resection for hepatocellular carcinoma. J Cachexia Sarcopenia Muscle. 2022;13(5):2383–2392. doi: 10.1002/jcsm.13040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iritani S, Imai K, Takai K, et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol. 2015;50(3):323–332. doi: 10.1007/s00535-014-0964-9 [DOI] [PubMed] [Google Scholar]
- 25.Liang G, Fu W, Wang K. Analysis of t-test misuses and SPSS operations in medical research papers. Burns Trauma. 2019;7:31. doi: 10.1186/s41038-019-0170-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiffel JA. Propensity score matching: the “Devil is in the detail” where more may be hidden than you know. Am J Med. 2020;133(2):178–181. doi: 10.1016/j.amjmed.2019.08.055 [DOI] [PubMed] [Google Scholar]
- 27.Chen LK, Woo J, Assantachai P, et al. Asian Working Group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. doi: 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 28.Voron T, Tselikas L, Pietrasz D, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. 2015;261(6):1173–1183. doi: 10.1097/SLA.0000000000000743 [DOI] [PubMed] [Google Scholar]
- 29.Yabusaki N, Fujii T, Yamada S, et al. Adverse impact of low skeletal muscle index on the prognosis of hepatocellular carcinoma after hepatic resection. Int J Surg. 2016;30:136–142. doi: 10.1016/j.ijsu.2016.04.049 [DOI] [PubMed] [Google Scholar]
- 30.Wu DH, Liao CY, Wang DF, et al. Textbook outcomes of hepatocellular carcinoma patients with sarcopenia: a multicenter analysis. Eur J Surg Oncol. 2023;49(4):802–810. doi: 10.1016/j.ejso.2022.12.009 [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi A, Kaido T, Hamaguchi Y, et al. Impact of sarcopenic obesity on outcomes in patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg. 2019;269(5):924–931. doi: 10.1097/SLA.0000000000002555 [DOI] [PubMed] [Google Scholar]
- 32.Chun HS, Kim MN, Lee JS, et al. Risk stratification using sarcopenia status among subjects with metabolic dysfunction-associated fatty liver disease. J Cachexia Sarcopenia Muscle. 2021;12(5):1168–1178. doi: 10.1002/jcsm.12754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funk Debleds P, Chambrier C, Slim K. Postoperative nutrition in the setting of enhanced recovery programmes. Eur J Surg Oncol. 2023. doi: 10.1016/j.ejso.2023.03.006 [DOI] [PubMed] [Google Scholar]
- 34.Nachit M, Lanthier N, Rodriguez J, et al. A dynamic association between myosteatosis and liver stiffness: results from a prospective interventional study in obese patients. JHEP Rep. 2021;3(4):100323. doi: 10.1016/j.jhepr.2021.100323 [DOI] [PMC free article] [PubMed] [Google Scholar]