Abstract
Objective(s):
This study examined the effects of melatonin treatment on steroidogenesis dysfunction and testosterone impairment, following CoCl2-induced hypoxia in TM3 Leydig cells.
Materials and Methods:
The TM3 cells were divided into four groups. The first group received no treatment. The MLT group was treated with a concentration of 1 mM melatonin. In the CoCl2 group, 0.2 mM CoCl2 was added to the medium to induce Hif1α overexpression. The MLT+CoCl2 group received 0.2 mM CoCl2 and 1 mM melatonin. After 24 hr treatment, the cells and supernatants were collected and used for further determination. The MTT assay was performed to estimate the decrease in cell viability throughout the CoCl2 and melatonin treatment. The mRNA and the protein levels were evaluated using Real-time PCR and Western blot analysis. The ELISA assay kit was used to detect the testosterone content.
Results:
CoCl2 treatment caused Hif1α overexpression in TM3 Leydig cells. Moreover, CoCl2 treatment of these cells led to considerable downregulation of Star, Hsd3b1, and Gata4 well as Mtnr1a and Mtnr1b mRNA/protein expression coupled with testosterone content repression in the cell culture medium. Melatonin administration in cells treated with CoCl2, decreased Hif1α mRNA/protein expression, but had no significant effect on Star, Hsd3b1, Gata4, Mtnr1a mRNA/protein expression, and the testosterone level in the cell culture medium. Melatonin caused recovery of decrease in the Mtnr1b gene and protein expression.
Conclusion:
There was no significant effect on steroidogenesis-related genes, proteins, and testosterone synthesis in the absence of gonadotropin treatment plus melatonin following CoCl2-induced hypoxia in TM3 Leydig cells.
Key Words: Hypoxia-inducible factor 1α, Leydig cells, Melatonin, Steroids, Testosterone
Introduction
It is estimated that infertility affects 8%-12% of couples of reproductive age globally (1). Reproductive disorders in men are responsible for 20% to 30% of infertility cases (2). Male fertility can be influenced by structural disorders, immunological defects, molecular and chromosomal abnormality, environmental and lifestyle factors, idiopathic factors, and endocrinological deficiency (3). Low O2 pressure (hypoxia) can cause steroidogenesis inhibition through enzymatic steroid production impairment (4, 5). Environmental exposure to cobalt chloride (CoCl2) can significantly affect male fertility in humans. Many animal models have confirmed the influence of acute and chronic exposure to CoCl2 on male reproduction (6, 7). CoCl2 is a hypoxia-mimicking agent in vitro and is a chemical inducer of hypoxia-inducible factor 1α (Hif1α), a subunit of Hif1(8). Hif1 consists of two subunits, Hif1α and Hif1β. Hif1α is rapidly hydroxylated and degraded in normoxia. Under hypoxic conditions, Hif1α stabilizes and, after its transfer from the cytoplasm to the nucleus and its association with Hif1β, the active Hif1 transcription complex is formed. This activated complex then attaches to the hypoxia response element in the regulatory regions of the target genes (9-12). It mediates the primary transcriptional responses to hypoxia and confers an adaptive role in proliferation, differentiation, energy metabolism, angiogenesis, metastasis, apoptosis, and steroidogenesis (13, 14). Studies have shown that steroidogenesis is affected by Hif1α protein, and disruption of Hif1α protein signaling can be linked to steroidogenesis disorders and reduced male reproductive function (5, 15).
Testicular Leydig cells are located in the interstitial space of the seminiferous tubules in the testis. They are responsible for the synthesis and release of testosterone in mammals (16). The steroidogenic acute regulatory (Star) protein transports cholesterol from the outer mitochondrial membrane to the inner mitochondrial membrane. The enzyme Cyp11a1 converts cholesterol to pregnenolone in the inner membrane of the mitochondria. Pregnenolone is converted to testosterone through catalysis of the hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1 (Hsd3b1) enzyme in the smooth endoplasmic reticulum (17).
Melatonin (N-acetyl-5-methoxytryptamine) is an indoleamine hormone composed of the amino acid tryptophan. It has been traditionally considered to be derived from the pineal gland and to interact with circadian rhythms (18-22). Sources of melatonin include the testes, retina, cornea, thymus, respiratory epithelium, cerebellum, bone marrow, gastrointestinal tract, and skin. Although melatonin can be found in many animal and plant foods, including fish, eggs, hazelnuts, coffee, and corn, melatonin supplements are usually made of synthetic melatonin (23).
Recent evidence supports the role of melatonin in male reproduction. It plays a key role in mediating the influence of photoperiod on seasonal breeding in different animal species (24). Melatonin acts as an inhibitor of hypothalamic gonadotropin-releasing hormone (GnRH) production via the suprachiasmatic nucleus of the hypothalamus. GnRH influences the secretion of pituitary gonadotropin hormones, including follicle-stimulating hormone and luteinizing hormone (LH), which directly controls the function of Leydig cells. In addition, melatonin can cross the blood-testis barrier and enter Leydig cells. This hormone is also synthesized in the testis and interacts with its receptor on Leydig cells to confer a direct regulatory effect on the function of these cells independent of the hypothalamic-pituitary-gonadal axis. Melatonin affects male reproductive functions through a complex signaling pathway mediated by melatonin receptor 1a (Mtnr1a) and melatonin receptor 1b (Mtnr1b), that is consistent with cell-specific reproductive action (25, 26). Studies on rodents indicate that melatonin decreased the expression of Star and other key steroidogenic enzymes (27-31). Melatonin treatment has been shown to upregulate the expression levels of Star, Hsd3b1, and other key steroidogenic enzymes in the Leydig cells of mice fed a high-fat diet and H2O2-treated TM3 cells (32). Melatonin has also proved to modulate reproductive functions and upregulate the expression of the Star and GATA binding protein 4 (Gata4) transcription factors in seasonally breeding mammals (33). In vitro promotor analysis has revealed that the GATA4 protein is involved in the regulation of target genes in the Leydig cells. GATA4 is a novel downstream regulator of the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling pathway in steroidogenic cells. Further, GATA4 protein deficiency affects the steroidogenesis metabolic pathways. The StAR gene includes a regulatory sequence recognized by the GATA4 protein in the testes (16). Studies reported that long-term melatonin supplementation promotes StAR and GATA4 expression levels in goats and rams as well as enhances testosterone production, which resulted in improved sperm generation (34, 35). The current study used the TM3 mouse Leydig cell line model to investigate the antagonistic or synergistic effects of combining melatonin plus CoCl2 treatments on steroidogenesis-related genes and proteins expression as well as testosterone synthesis in TM3 Leydig cells.
Materials and Methods
Cell culture
The TM3 cell line was purchased from the Pasteur Institute of Iran and was maintained in DMEM/F-12 medium (Bio-IDEA; Iran) supplemented with 10% fetal bovine serum (FBS; Gibco; USA) in an incubator at 37°C and 5% CO2. Cells attaining 70% to 80% confluency were divided into four groups in cell culture plates containing about 106 cells in 3 ml of medium. The first group received no treatment. The MLT group was treated with a concentration of 1 mM melatonin (Sigma–Aldrich; USA). In the CoCl2 group, 0.2 mM CoCl2 (Merck; Germany) was added to the medium to induce Hif1α overexpression. The MLT+CoCl2 group received 0.2 mM CoCl2 and 1 mM melatonin. After 24 hr treatment, the cells and supernatants were collected and used for further determination.
MTT assay
About 5000 TM3 Leydig cells were seeded in each well of the 96-well plate. After 24 hr of treatment, a final concentration of 5 mg/ml of 3-(4, 5-dimethylthiazolyl-2)-2, 5 diphenyltetrazolium bromide (MTT; Sigma–Aldrich; USA) solution in phosphate-buffered saline (PBS) was added to each well. After incubation at 37 °C for 4 hr, the supernatant was removed and the formazan crystals produced in the cells were dissolved in 100 μl of dimethyl sulfoxide (DMSO; Merck; Germany), after which the cells were incubated with DMSO for 15 min. The absorbance of the plate was measured at a wavelength of 570 nm using a ELISA plate reader (Space fax 2100, Awareness, USA, reference 660 nm ). Each experiment was performed in triplicate.
Real-time PCR analysis
Purified total RNA (1 μg) was extracted using Trizol following the manufacturer’s protocol and was converted to cDNA using the Stem Gene cDNA synthesis kit (Stem Gene; Iran). Real-time PCR was performed using the SYBR Green qPCR master mix (Stem Gene; Iran) on a Runmei-q 200 system (Runmei; China). PCR primers (Table 1) were designed for the Mtnr1a, Mtnr1b, Gata4, Star, Hif1α, and Hsd3b1 genes. Gapdh was used as an internal control. The fold-change in mRNA was determined using the 2−ΔΔCt method. Each experiment was performed in triplicate.
Table 1.
Primers used in this study for Real-time PCR analysis
| Gene | Primer sequence | Size (bp) | GeneBank ACC |
|---|---|---|---|
| Gapdh -forward | CATCACTGCCACCCAGAAGACTG | 152 | NM-001289726.1 |
| Gapdh -reverse | ATGCCAGTGAGCTTCCCGTTCAG | ||
| Gata4 -forward | GCCTCTATCACAAGATGAACGGC | 148 | NM-001310610.1 |
| Gata4 -reverse | TACAGGCTCACCCTCGGCATTA | ||
| Mtnr1a -forward | AACCTGCTGGTCATCCTGTCTG | 113 | NM-008639.3 |
| Mtnr1a -reverse | GGGATAAGGGTAAACAGCCACC | ||
| Mtnr1b -forward | TCTCAGTGCTCAGGAACCGCAA | 136 | AB377276.1 |
| Mtnr1b -reverse | AAGGACCCAACCGTCACGGATA | ||
| Hsd3b1 -forward | AGAACTGCAGGAGGTCAGAGCT | 117 | NM-008293.4 |
| Hsd3b1 -reverse | GGCATCCAGAATGTCTCCTTCC | ||
| Star -forward | GTGCTTCATCCACTGGCTGGAA | 112 | NM-011485.5 |
| Star -reverse | GTCTGCGATAGGACCTGGTTGA | ||
| Hif1α -forward | CCTGCACTGAATCAAGAGGTTGC | 109 | NM-001313919.1 |
| Hif1α -reverse | CCATCAGAAGGACTTGCTGGCT |
Western blot analysis
The total protein concentration was evaluated using the Bradford method. The protein was separated by 12% SDS-PAGE, then transferred to a nitrocellulose membrane. The membrane was blocked with 5% skim milk and incubated overnight at 4 °C with mild shaking using the anti-Hif1α (MAB1536, biotechne), anti-Hsd3b1 (ABIN2855488, antibodies), anti-Star (sc-166821, Santa Cruz), anti-Gata4 (ABIN6256227, antibodies), anti-Mtnr1a (ABIN361193, antibodies), anti-Mtnr1b (ABIN730318, antibodies), and anti-Gapdh (sc-365062, Santa Cruz) primary antibodies. The membrane was washed using PBS buffer, followed by incubation with the mouse anti-rabbit IgG-HRP (sc-2357, Santa Cruz) secondary antibody for 60 min at room temperature. The membrane was washed using PBS buffer. Immunocomplexes were detected using ECL Western blot detection reagents according to the manufacturer’s instructions in an X-ray processor (LD-14; China). The band density was determined using the JS2000 system (Bonnin Tech; China). Each experiment was performed in triplicate.
Testosterone determination
The cultured cells and supernatants were collected and an ELISA assay kit was used to detect the testosterone content according to the manufacturer’s instructions (AccuBind; USA). Each experiment was performed in triplicate.
Statistical analysis
Statistical analysis was performed in Graph Pad Prism 8 software (version 8.0; USA). Statistical significance was calculated using one-way ANOVA followed by Tukey and Dennett’s tests, being presented as mean ± Standard Deviation (mean ± S.D). Each experiment was performed in triplicate, with the sample size (n = 3) in each experiment, and P<0.05 was considered significant. The ranges of P-values were *P<0.05, **P< 0.01 and ***P<0.001.
Results
Cell viability of CoCl 2 and melatonin in mouse TM3 leydig cells
The MTT assay was performed to estimate the decrease in cell viability throughout the CoCl2 and melatonin treatment on TM3 Leydig cells. The cells were incubated with elevating melatonin concentrations (10-6 to 1 mM) for 24 hr. The results of the MTT assay indicated that the treatment of TM3 Leydig cells with different concentrations of melatonin did not decreased cell viability compared to the control group (Figure 1(a)); thus, the 1 mM melatonin concentration was selected for subsequent experiments. The cells were then incubated with elevating CoCl2 concentrations (0.1 to 2 mM) for 24 hr. It was determined that a 0.4 mM concentration of CoCl2 induced a significant decrease in cell viability compared to the control group (Figure 1(b)). The 0.2 mM concentration of CoCl2 was then selected for subsequent experiments.
Figure 1.
Cell viability evaluation performed using MTT assay. (a) TM3 cells treated with different concentrations of melatonin; (b) TM3 cells treated with different concentrations of CoCl2. The data are expressed as mean ± S.D (n=3, *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 versus the CTRL group)
Upregulation of Hif1α by CoCl 2 reduced steroidogenesis-related genes and proteins levels in TM3 leydig cells
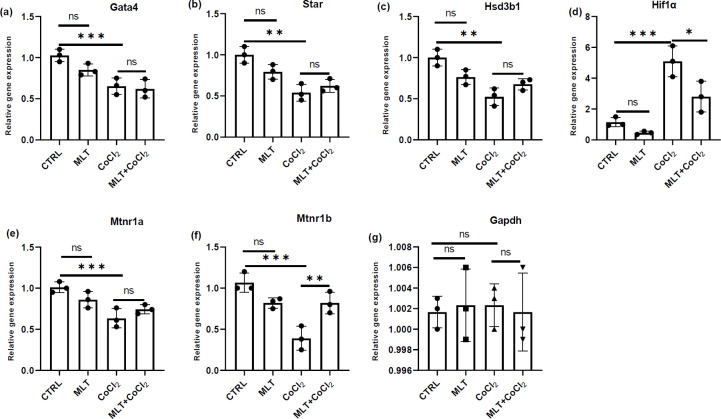
The mRNA and protein expression of Hif1α was upregulated after CoCl2 treatment compared to the control group in TM3 Leydig cells (Figures 2(d), 3(a), and 3(e)). The protein and mRNA levels of Gata4, Star, and Hsd3b1 decreased significantly compared to the control group after CoCl2 treatment (Figures 2(a-c), 3(a-d)). Melatonin treatment showed no significant effect on the expression of Hif1α, Gata4, Star, and Hsd3b1 genes compared to the control group (Figure 2(a-d)). Similar results were obtained in the Western blot test results for the Hif1α, Gata4, Star, and Hsd3b1 protein levels (Figures 3(a-e)). Melatonin treatment caused recovery of Hif1α mRNA and protein expression accumulation following CoCl2 treatment (Figures 2(d), 3(a), and 3(e)). The melatonin treatment revealed no significant effect on the downregulation of Star, Gata4, and Hsd3b1 mRNA and protein expression following CoCl2 treatment (Figures 2(a-c), 3(a-d)).
Figure 2.
Real-Time PCR analysis of Gata4 (a), Star (b), Hsd3b1 (c), Hif1α (d), Mtnr1a (e), and Mtnr1b (f) gene expression where Gapdh was used as an internal control. The data are expressed as mean ± S.D (n=3, *P<0.05, **P<0.01 and ***P<0.001)
Figure 3.
Western blot analysis of Gata 4 (a), (b); Star (a), (c); Hsd3b1 (a), (d); Hif1α (a), (e); Mtnr1a (a), (f); Mtnr1b (a), (g); protein expression where Gapdh was used as an internal control and testosterone concentration in the culture media (i). The data are expressed as mean ± S.D (n=3, *P<0.05, **P<0.01)
CoCl 2 decreased testosterone production in TM3 leydig cells
CoCl2 treatment significantly decreased the testosterone content in the culture medium compared to the control group. In addition, melatonin showed no significant effect on the testosterone content following the CoCl2 treatment (Figure 3(i)).
Decrease of melatonin receptor expression in CoCl 2 -treated mouse TM3 Leydig cells recovered by melatonin
Real-time PCR and Western blot analysis revealed that membrane receptors Mtnr1a and Mtnr1b were expressed excessively in the control group. CoCl2 treatment decreased the mRNA and protein expression of Mtnr1b compared to the control group (Figures. 2(e), 2(f), 3(a), 3(f), 3(g)). Melatonin treatment caused recovery of Mtnr1b mRNA and protein expression deficiencies of the CoCl2-treated cells (Figs. 2(f), 3(a), 3(g)).
Discussion
The results of this investigation confirmed a correlation between CoCl2-induced hypoxia and Hif1α accumulation with steroidogenesis-related genes and proteins downregulation as well as testosterone deficiency in TM3 Leydig cells. These results are in agreement with those of previous reports where Hif1α protein was involved in the regulation of Hsd3b1 and Star protein expression plus testosterone production in mouse TM3 Leydig cell line (15). It has been documented that overexpression of HIF1α protein in granulosa cells and canine lutein cells treated with CoCl2 exhibited similar effects on steroid synthesis (14, 36). Other signaling proteins and nuclear transcription factors also are involved in cell cycle arrest following HIF1α protein overexpression (14). Downregulation of basal and cAMP-induced Star protein expression as well as steroidogenesis mediated by Hif1α protein in murine granulosa cells under hypoxia has been demonstrated (37). Recent evidence has suggested that Hif1α protein stabilization under severe hypoxia produces detrimental effects on steroidogenesis. Possible regulatory mechanisms for the Hif1α protein involve cAMP production, regulating PKA activity, and phosphorylation of target transcription factors (38). It has been reported that HIF1α protein stabilization in a hypoxic environment may contribute to high intracellular reactive oxygen species (ROS) levels in Leydig cells. ROS is known to impair steroidogenesis by inducing oxidative stress, which leads to a reduction in the Bcl2/Bax ratio, p53 gene upregulation, and downregulation of the Bcl2 gene. Alterations in the ratio of Bcl2/Bax have been shown to lead to cytochrome C release, which promotes caspase-9 and activates the caspase-3 signaling cascades, leading to apoptosis (39). Further, the Hif1α protein is capable of regulating the transcription of the mouse Hsd3b1 gene by influencing its promotor activity (40).
Melatonin is a neuroendocrine molecule that modulates endogenous patterns with photoperiod changes (26). Melatonin signaling pathways in target cells lead to activation of MTNR1A and MTNR1B, resulting in inhibition of cAMP activity through coupling with Gi protein. Inhibition of cAMP can regulate PKA activity and reduce phosphorylation of cAMP response element-binding protein, its downstream effector, to decrease the expression of genes required for steroidogenesis, including StAR, HSD3B1, and GATA4 (29, 30, 35).
The present study revealed that melatonin treatment had no significant effect on the expression of Gata4, Star, and Hsd3b1 genes and proteins in TM3 Leydig cells compared to the control group. In addition, the testosterone concentration in the cell culture medium remained unchanged in these cells. These observations are in contrast with evidence that MA-10 Leydig cell line treatment with human chorionic gonadotropin (hCG)/cAMP analogue for stimulation of testosterone synthesis alone or with the hCG/cAMP analogue plus melatonin at different dosages resulted in downregulation of the Star protein expression and steroidogenesis. Melatonin attenuated Star protein expression and testosterone synthesis was cAMP pathway-dependent and stimulated by the hCG or cAMP analogue (28). This contrast may be due to the absence of gonadotropin treatment plus melatonin. The mechanism of testosterone production is regulated by multiple factors, where hCG or LH is widely used for the stimulation of testosterone production in cells (17). Another study has reported that melatonin treatment caused a significant decline in the expression of Gata4 and SF-1, crucial steroidogenic enzymes, and testosterone production in the TM3 Leydig cell line under LH treatment (29). The results of the present study demonstrated that melatonin suppressed the adverse effects of CoCl2 treatment in Hif1α mRNA and protein expression. Recent evidence has demonstrated that melatonin exerts its effect on metabolic pathways and cancer treatment by reducing the HIF1α protein (41). Administration of melatonin has been shown to decrease the HIF1α protein level inside the tumor mass and prevented the growth of tumors in mice (42). In another study, HIF1α protein was decreased in HCT116 cells using melatonin under hypoxia. HIF1α protein stabilization under hypoxic conditions has been shown to cause ROS production. The anti-oxidant properties of melatonin have been shown to remove intracellular ROS and destabilize the HIF1α protein (43).
The data revealed that mRNA and protein expression of Mtnr1b decreased following CoCl2-induced hypoxia in TM3 Leydig cells and that melatonin treatment caused recovery of the Mtnr1b gene and protein deficiencies. Similar to the present study, it has been reported that the Mtnr1a gene and protein deficiencies in hypoxic-ischemic mice with brain injuries were reduced by applying melatonin in vivo. Melatonin receptor antagonist was used to demonstrate the neuroprotective effect of melatonin in neonatal hypoxic-ischemic mice with brain injuries (44). Another study speculated that melatonin exerts its protective effect in hypoxia-induced retinopathy through preserving melatonin receptors (45).
Conclusion
In summary, the current study results indicated that CoCl2 reduced TM3 cell line steroidogenesis-related genes, proteins, and testosterone synthesis. Melatonin destabilized the Hif1α gene and protein as well as caused recovery of the Mtnr1b gene and protein deficiencies following CoCl2 treatment. There was no significant effect on steroidogenesis-related genes, proteins, and testosterone synthesis in the absence of gonadotropin treatment plus melatonin.
Authors’ Contributions
S K and M G designed the experiments; S K performed experiments and collected the data; S K and M G discussed the results and strategy; M G supervised, directed, and managed the study; S K, C J, KM, F B, and M G approved the final version to be published.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgment
The results described in this paper were part of student thesis. This research was funded by Kermanshah University of Medical Sciences (grant no.990675).
References
- 1.Agarwal A, Baskaran S, Parekh N, Cho C-L, Henkel R, Vij S, et al. Male infertility. The Lancet. 2021;397:319–333. doi: 10.1016/S0140-6736(20)32667-2. [DOI] [PubMed] [Google Scholar]
- 2.Cilio S, Rienzo M, Villano G, Mirto BF, Giampaglia G, Capone F, et al. Beneficial effects of anti-oxidants in male infertility management: A narrative review. Oxygen. 2022;2:1–11. [Google Scholar]
- 3.Marzouni ET, Ilkhani H, Harchegani AB, Shafaghatian H, Layali I, Shahriary A. Epigenetic modifications, a new approach to male infertility etiology: A review. Int J Fertil Steril. 2022;16:1–9. doi: 10.22074/IJFS.2021.138499.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu RMK, Chu DLH, Tan T-f, Li VWT, Chan AKY, Giesy JP, et al. Leptin-mediated modulation of steroidogenic gene expression in hypoxic zebrafish embryos: Implications for the disruption of sex steroids. Environ Sci Technol. 2012;46:9112–9119. doi: 10.1021/es301758c. [DOI] [PubMed] [Google Scholar]
- 5.Yu RMK, Chaturvedi G, Tong SKH, Nusrin S, Giesy JP, Wu RSS, et al. Evidence for microRNA-mediated regulation of steroidogenesis by hypoxia. Environ Sci Technol. 2015;49:1138–1147. doi: 10.1021/es504676s. [DOI] [PubMed] [Google Scholar]
- 6.Maciejewski R, Radzikowska-Büchner E, Flieger W, Kulczycka K, Baj J, Forma A, et al. An overview of essential microelements and common metallic nanoparticles and their effects on male fertility. Int J Environ Res Public Health. 2022;19:11066. doi: 10.3390/ijerph191711066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghafouri-Fard S, Shoorei H, Mohaqiq M, Tahmasebi M, Seify M, Taheri M. Counteracting effects of heavy metals and anti-oxidants on male fertility. Biometals. 2021;34:439–491. doi: 10.1007/s10534-021-00297-x. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz-Sánchez J, Chánez-Cárdenas ME. The use of cobalt chloride as a chemical hypoxia model. J Appl Toxicol. 2019;39:556–570. doi: 10.1002/jat.3749. [DOI] [PubMed] [Google Scholar]
- 9.Jahani M, Modaressi MH, Mansouri K. Hypoxia inducible factor: It’s role in angiogenesis and tumor. Tehran Univ Med J. 2016;73:757–766. [Google Scholar]
- 10.Jahani M, Shahlaei M, Norooznezhad F, Miraghaee SS, Hosseinzadeh L, Moasefi N, et al. TSGA10 over expression decreases Metastasic and metabolic activity by inhibiting HIF-1 in breast Cancer cells. Arch Med Res. 2020;51:41–53. doi: 10.1016/j.arcmed.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Amoorahim M, Valipour E, Hoseinkhani Z, Mahnam A, Rezazadeh D, Ansari M, et al. TSGA10 overexpression inhibits angiogenesis of HUVECs: A HIF-2α biased perspective. Microvasc Res. 2020;128:103952. doi: 10.1016/j.mvr.2019.103952. [DOI] [PubMed] [Google Scholar]
- 12.Hoseinkhani Z, Rastegari-Pouyani M, Oubari F, Mozafari H, Rahimzadeh AB, Maleki A, et al. Contribution and prognostic value of TSGA10 gene expression in patients with acute myeloid leukemia (AML) Pathol Res Pract. 2019;215:506–511. doi: 10.1016/j.prp.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Watts D, Stein J, Meneses A, Bechmann N, Neuwirth A, Kaden D, et al. HIF1α is a direct regulator of steroidogenesis in the adrenal gland. Cell Mol Life Sci. 2021;78:3577–3590. doi: 10.1007/s00018-020-03750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baddela VS, Sharma A, Michaelis M, Vanselow J. HIF1 driven transcriptional activity regulates steroidogenesis and proliferation of bovine granulosa cells. Sci Rep. 2020;10:1–12. doi: 10.1038/s41598-020-60935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Zou Z, Yang Z, Jiang S, Lu Y, Wang D, et al. HIF 1 inhibits StAR transcription and testosterone synthesis in murine Leydig cells. J Mol Endocrinol. 2019;62:1–13. doi: 10.1530/JME-18-0148. [DOI] [PubMed] [Google Scholar]
- 16.de Mattos K, Viger RS, Tremblay JJ. Transcription factors in the regulation of leydig cell gene expression and function. Front Endocrinol. 2022;13:881309. doi: 10.3389/fendo.2022.881309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zirkin BR, Papadopoulos V. Leydig cells: formation, function, and regulation. Biol Reprod. 2018;99:101–111. doi: 10.1093/biolre/ioy059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cipolla-Neto J, Amaral FG, Soares Jr JM, Gallo CC, Furtado A, Cavaco JE, et al. The crosstalk between melatonin and sex steroid hormones. Neuroendocrinology. 2022;112:115–129. doi: 10.1159/000516148. [DOI] [PubMed] [Google Scholar]
- 19.Gholami M, Ahmadi SAY, Abaszadeh A, Khaki A. Protective effects of melatonin and ghrelin on spermatogenesis: A narrative review of the literature. Int J Reprod Biomed. 2017;15:265. [PMC free article] [PubMed] [Google Scholar]
- 20.Gholami M, Saki G, Hemadi M, Khodadadi A. Effect of melatonin on the expression of apoptotic genes in vitrified-thawed spermatogonia stem cells type A of 6-day-old mice. Iran J Basic Med Sci. 2013;16:906. [PMC free article] [PubMed] [Google Scholar]
- 21.Gholami M, Saki G, Hemadi M, Khodadadi A, Mohammadiasl J. Melatonin effect on immature mouse testicular tissues, vitrified-thawed with different cryoprotectant media. Jentashapir J Health Res. 2015;6:e28704. [Google Scholar]
- 22.Gholami M, Saki G, Hemadi M, Khodadadi A, Mohammadi-Asl J. Melatonin improves spermatogonial stem cells transplantation efficiency in azoospermic mice. Iran j basic med sci. 2014;17:93. [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu C-N, Huang L-T, Tain Y-L. Perinatal use of melatonin for offspring health: Focus on cardiovascular and neurological diseases. Int J Mol Sci. 2019;20:5681. doi: 10.3390/ijms20225681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munley KM, Han Y, Lansing MX, Demas GE. Winter madness: Melatonin as a neuroendocrine regulator of seasonal aggression. J Exp Zool. 2022:337–889. doi: 10.1002/jez.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Y, Zhao S, Zhang Y, Zhang Q. Melatonin receptors: A key mediator in animal reproduction. Vet Sci. 2022;9:309. doi: 10.3390/vetsci9070309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozioł K, Broda D, Romerowicz-Misielak M, Nowak S, Koziorowski M. Melatonin concentration in peripheral blood and melatonin receptors (MT1 and MT2) in the testis and epididymis of male roe deer during active spermatogenesis. Theriogenology. 2020;149:25–37. doi: 10.1016/j.theriogenology.2020.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Q, Guo L, An W, Huang Z, Liu H, Zhao J, et al. Melatonin inhibits testosterone synthesis in Roosters Leydig cells by regulating lipolysis of lipid droplets. Theriogenology. 2022;189:118–126. doi: 10.1016/j.theriogenology.2022.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Wu CS, Leu SF, Yang HY, Huang BM. Melatonin inhibits the expression of steroidogenic acute regulatory protein and steroidogenesis in MA-10 cells. J Androl. 2001;22:245–254. [PubMed] [Google Scholar]
- 29.Qin F, Zhang J, Zan L, Guo W, Wang J, Chen L, et al. Inhibitory effect of melatonin on testosterone synthesis is mediated via GATA-4/SF-1 transcription factors. Reprod Biomed Online. 2015;31:638–646. doi: 10.1016/j.rbmo.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Yu K, Deng S-L, Sun T-C, Li Y-Y, Liu Y-X. Melatonin regulates the synthesis of steroid hormones on male reproduction: A review. Molecules. 2018;23:447. doi: 10.3390/molecules23020447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frungieri MB, Mayerhofer A, Zitta K, Pignataro OP, Calandra RS, Gonzalez-Calvar SI. Direct effect of melatonin on Syrian hamster testes: melatonin subtype 1a receptors, inhibition of androgen production, and interaction with the local corticotropin-releasing hormone system. Endocrinology. 2005;146:1541–1552. doi: 10.1210/en.2004-0990. [DOI] [PubMed] [Google Scholar]
- 32.Chen C, Ling My, Lin Fh, Xu L, Lv ZM. Melatonin appears to protect against steroidogenic collapse in both mice fed with high-fat diet and H2O2-treated TM3 cells. Andrologia. 2019;51:e13323. doi: 10.1111/and.13323. [DOI] [PubMed] [Google Scholar]
- 33.Yang M, Guan S, Tao J, Zhu K, Lv D, Wang J, et al. Melatonin promotes male reproductive performance and increases testosterone synthesis in mammalian Leydig cells. Biol Reprod. 2021;104:1322–1336. doi: 10.1093/biolre/ioab046. [DOI] [PubMed] [Google Scholar]
- 34.Deng SL, Chen SR, Wang ZP, Zhang Y, Tang JX, Li J, et al. Melatonin promotes development of haploid germ cells from early developing spermatogenic cells of Suffolk sheep under in vitro condition. J Pineal Res. 2016;60:435–447. doi: 10.1111/jpi.12327. [DOI] [PubMed] [Google Scholar]
- 35.Deng S-L, Zhang Y, Yu K, Wang X-X, Chen S-R, Han D-P, et al. Melatonin up-regulates the expression of the GATA-4 transcription factor and increases testosterone secretion from Leydig cells through RORα signaling in an in vitro goat spermatogonial stem cell differentiation culture system. Oncotarget. 2017;8:110592. doi: 10.18632/oncotarget.22855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kowalewski MP, Gram A, Boos A. The role of hypoxia and HIF1α in the regulation of STAR-mediated steroidogenesis in granulosa cells. Mol cell endocrinol. 2015;401:35–44. doi: 10.1016/j.mce.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 37.Gysin T, Kowalewski MP, Martin G. The involvement of hypoxia-inducible factor 1α (HIF1α)-stabilising factors in steroidogenic acute regulatory (STAR) protein-dependent steroidogenesis in murine KK1 granulosa cells in vitro. Reprod, Fertil Dev. 2021;33:865–880. doi: 10.1071/RD21170. [DOI] [PubMed] [Google Scholar]
- 38.Lanfranchi B, Rubia RF, Gassmann M, Schuler G, Kowalewski MP. Transcriptional regulation of HIF1α-mediated STAR expression in murine KK1 granulosa cell line involves cJUN, CREB and CBP-dependent pathways. Gen Comp Endocrinol. 2022;315:113923. doi: 10.1016/j.ygcen.2021.113923. [DOI] [PubMed] [Google Scholar]
- 39.Tripathi VK, Subramaniyan SA, Hwang I. Molecular and cellular response of co-cultured cells toward cobalt chloride (CoCl2)-induced hypoxia. ACS omega. 2019;4:20882–20893. doi: 10.1021/acsomega.9b01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lysiak JJ, Kirby JL, Tremblay JJ, Woodson RI, Reardon MA, Palmer LA, et al. Hypoxia-inducible factor-1α is constitutively expressed in murine Leydig cells and regulates 3β-hydroxysteroid dehydrogenase type 1 promoter activity. J Androl. 2009;30:146–156. doi: 10.2164/jandrol.108.006155. [DOI] [PubMed] [Google Scholar]
- 41.de Lima Mota A, Jardim-Perassi BV, de Castro TB, Colombo J, Sonehara NM, Nishiyama VKG, et al. Melatonin modifies tumor hypoxia and metabolism by inhibiting HIF-1α and energy metabolic pathway in the in vitro and in vivo models of breast cancer. Melatonin Res. 2019;2:83–98. [Google Scholar]
- 42.Kim KJ, Choi JS, Kang I, Kim KW, Jeong CH, Jeong JW. Melatonin suppresses tumor progression by reducing angiogenesis stimulated by HIF-1 in a mouse tumor model. J Pineal Res. 2013;54:264–270. doi: 10.1111/j.1600-079X.2012.01030.x. [DOI] [PubMed] [Google Scholar]
- 43.Jardim-Perassi BV, Lourenço MR, Doho GM, Grígolo IH, Gelaleti GB, Ferreira LC, et al. Melatonin regulates angiogenic factors under hypoxia in breast cancer cell lines. Anti-Cancer Agents Med Chem. 2016;16:347–358. doi: 10.2174/1871520615666150511094201. [DOI] [PubMed] [Google Scholar]
- 44.Sinha B, Wu Q, Li W, Tu Y, Sirianni AC, Chen Y, et al. Protection of melatonin in experimental models of newborn hypoxic-ischemic brain injury through MT 1 receptor. J Pineal Res. 2018;64:e12443. doi: 10.1111/jpi.12443. [DOI] [PubMed] [Google Scholar]
- 45.Huang R, Xu Y, Lu X, Tang X, Lin J, Cui K, et al. Melatonin protects inner retinal neurons of newborn mice after hypoxia-ischemia. J Pineal Res. 2021;71:e12716. doi: 10.1111/jpi.12716. [DOI] [PubMed] [Google Scholar]