Abstract
Objective(s):
Rosa × damascena Herrm. belonging to the Rosaceae family has demonstrated anti-inflammatory and anti-oxidant effects previously. Excessive production of free radicals and activation of tyrosinase enzyme caused by UV induces excessive concentration of melanin pigment and skin spots in the long term. Therefore, finding natural sources with anti-oxidant and antityrosinase effects helps to regulate the melanogenesis process. In the current research, we investigated the antimelanogenic, anti-oxidant, and anti-tyrosinase effects of its essential oil, methanol extract (MeOH), and different fractions including n-hexane, dichloromethane (CH2Cl2), n-butanol (BuOH), ethyl acetate (EtOAc), and H2O of R. × damascena in B16F10 cell line.
Materials and Methods:
For this purpose, impacts of extracts and essential oil of R. × damascena were investigated on cell viability, cellular tyrosinase, melanin content, mushroom tyrosinase, reactive oxygen species (ROS) production, as well as the amount of tyrosinase protein in the B16F10 murine melanoma cell line.
Results:
Essential oil, MeOH, and different fractions of R. × damascena were not cytotoxic on B16F10 cells. However, they had significant reducing effects on mushroom tyrosinase activity, melanin content, and ROS production. Also, there is a significant decrease in tyrosinase protein levels at 200 µg/ml but not at other concentrations.
Conclusion:
Therefore, the essential oil, MeOH, and different fractions of R. × damascena had promising antimelanogenic activity via repression of mushroom tyrosinase activity and ROS production.
Key Words: B16F10 cell line, Melanogenesis, Rosa × damascena, Reactive oxygen species, Tyrosinase
Introduction
R. × damascena which is also called Damask rose (1) holds some varieties which are very important for essential oil production (2). Several studies show that this plant has anti-human immunodeficiency viruses (HIV), anti-viral effects (3), antibacterial (4), anti-inflammatory, and anti-oxidant (5, 6), as well as hypnotic (7) and bronchodilatory properties (8). R. × damascena contains compounds such as carboxylic acids, linalool C10H180O, eugenol, citronellol C10H20, farnesol, nerol C10H18, terpenes, myrcene, quercetin, kaempferol, and vitamin C. The oil extract of this plant contains 16–35% citronellol, 8–30% geraniol, 4–10% nerol, 4–16% nonadecane, 3–8% heneicosane, and 1–3% linalool. Also, compounds such as isoquercitrin, afzelin, quercetin gentabiosid, and cyanidin-3-O-beta-glucoside have been identified in the rosebud (9. Its hydro-alcoholic extract of petals and essential oil have significant scavenging effects on oxidative agents compared with the standards (6). In general, the medicinal functions of the rosacea family which include anti-oxidant, free radical absorber, anticancer, anti-inflammatory, and anti-depressant activity are largely dependent on phenolic compounds, (10). Melanin pigmentation in the skin has protective effects against the rays of the sun (11) besides, it is responsible for thermoregulation in lower vertebrates, camouflage, and mimicry (12). The production of excess melanin pigments leads to some skin problems, including melasma, and sunspots (13). Melanin production occurs in a part of the melanocyte called melanosome by the amino acid tyrosine. Tyrosinase is involved not only in the melanogenesis process in humans and animals but also in food browning. Therefore, inhibition of tyrosine production has important medical use in the treatment of the production of excess melanin pigments and is widely used in the cosmetics industry in skin whitening and depigmentation products, as well as in food processing and agriculture (13). The leading cause of skin hyperpigmentation is oxidative stress mostly caused by ROS such as ONOO-, ·O2, and NO· (14, 15). As a result, compounds with anti-oxidative properties might be useful as melanin synthesis regulators and antimelanogenesis agents (16). The Rose belongs to the Rosacea family and has over 200 species around the world (17). Some literature has been published on the biological functions of R. × damascena, however, there are few studies on the inhibitory effects of R. × damascena on melanin production. It was shown that the ethanol extract of the Rosa gallica petal has anti-aging and anti-oxidant properties due to its high flavonoid content (18). Additionally, it has been demonstrated that the extracts and compounds isolated from the Rosa chinensis cv. ‘JinBian’ have anti-aging, anti-tyrosinase, and antibacterial properties, and these natural substances can be used in the production of anti-aging, skin lightening, and antibacterial products (19). Similarly rose petal extract has whitening and anti-wrinkle effects on the skin through activation of mitogen-activated protein kinase (MAPK) and inhibition of matrix metalloproteinase 1 (MMP-1) (20). On the other hand, the anti-tyrosinase activity of the methanol extract of Rosa indica has been reported (21). Thus, here we investigated the anti-oxidant and inhibitory activity of essential oil, MeOH, and different fractions of R. × damascena against melanin production in the B16F10 murine melanoma cell line.
Materials and Methods
Materials
The aerial parts of R. × damascena were collected in May 2014 from Kashan, Isfahan province, Iran. The plant material has been identified and is kept in the Herbarium of the Faculty of Pharmacy, Mashhad Medical University. Resazurin, 2’,7’- dichlorodihydrofluorescein diacetate (DCFH-DA), Roswell park memorial institute medium 1640 (RPMI medium), L-DOPA, Kojic acid, mushroom tyrosinase, and QuantiPro™ BCA Assay Kit were purchased from Sigma (Germany); rabbit anti-tyrosinase antibody, β-Actin (13E5) and anti-rabbit IgG HRP-linked antibody were purchased from Cell Signaling Technology (USA); Fetal bovine serum (FBS) and penicillin-streptomycin (PS) were purchased from Gibco (USA); methanol, n-hexane, CH2Cl2, BuOH, and EtOAc were from Merk (Germany); B16F10 melanoma cells were purchased from Pasteur Institute (Iran).
Preparation of plant material
The aerial parts of R. × damascena were air-dried and powdered. Then 100 g of this powder was extracted with 1.5-liter methanol (90%) at room temperature by percolation method. The process was continued until the obtained extract was colorless. The extract was then concentrated under reduced pressure (Rotary evaporator) and dried completely using a freeze dryer. The weight of the obtained dried extract was 27.5 g. In order to fractionate the extract and abstract chlorophyll and lipophilic components, it was suspended in 90% methanol (MeOH) and successively partitioned n-hexane, CH2Cl2, BuOH, EtOAc, and H2O. Different phases were separated according to polarity. Eventually, the obtained extracts were evaporated by a rotary evaporator and dried completely by freeze drying. The essential oil of rose was obtained by a specified hydro-distillation method and donated by Dr A Ebrahimabadi, Department of Essential Oils Research Center, University of Kashan, Kashan, Iran (22).
Gas-chromatography (GC) and GC-MS
The GC-FID analyses were performed using a Varian CP-3800 GC apparatus with a CP-Sil 8CB column (30 m x 0.25 mm i.d., 0.12 µm film thickness) and an FID detector. Oven temperature 50 °C (5 min), 50 °C–250 °C (3 °C /min), 250 °C (5 min); injector temperature 250 °C; volume injection, 1 µl; split ratio, 1:5; carrier gas N2 at 2.0 ml/min; detector temperature, 280 °C.
The GC-MS analyses were done using an Agilent 5975 apparatus with an HP-5ms column (30 m x 0.25 mm i.d., 0.25 µm film thickness) interfaced with a quadruple mass detector and a computer equipped with Wiley 7n.l library; oven temperature 50 °C (5.0 min), 50 °C–250 °C (3 °C/min), 250 °C (5.0 min); injector temperature 250 °C; volume injection, 0.1 µl; split ration, 1:50; carrier gas Helium at 1.1 ml/min; ionization potential,70 eV; ionization current, 150 µA; ion source temperature, 250 °C; mass range, 35–465 mui.
Identification of individual compounds was made by comparison of their mass spectra and retention indices (RI) on their Retention Kovats Index, calculated in relation to the retention time of a series of n-alkanes (C8-C20) and with those authentic samples and those given in the literature (23). Quantification of the relative amount of the individual components was performed according to the area percentage method in GC-FID spectra without consideration of the calibration factor.
Cells and cell culture
B16F10 melanoma cells were cultured in a 37 °C incubator with 5% CO2 and 90% humidity for 24 hr. RPMI 1640 plus 10% FBS and 1% PS was used as culture media. Kojic acid (2 and 4 mM) was used as a positive control [1).
Cell viability assay
The Resazurin method was done to measure the cell viability. 105 cells were treated with R. × damascena essential oil, MeOH, and different fractions (0.2–200 µg/ml) for 24 hr and the absorbance was compared after addition of resazurin to each well (24).
Melanin content assay
105 cells were treated with R. × damascena essential oil, MeOH, and different fractions (0.2–200 µg/ml). After 24 hr, the purified melanin was extracted and its content was determined by reading the absorbance at 405 nm (11).
Mushroom tyrosinase activity assay
R. × damascena essential oil, MeOH, and different fractions (0, 10, 100, and 1000 µg/ml) were added to each well of 96-well microplates. The next day, cells were treated with 160 µl L-DOPA and 20 µl of mushroom tyrosinase, 30 min before measurement of absorbance at 457 nm using enzyme-linked immunosorbent assay (ELISA) Reader (Awareness, USA) (11).
Cellular tyrosinase activity assay
105 cells were treated with R. × damascena essential oil, MeOH, and different fractions (0.2–200 µg/ml) for 24 hr. Afterward, cells were lysed with 50 µl of lysis buffer and mixed with 30 µl of L-DOPA. After 2 hr, the absorbance of samples was read at 457 nm with an ELISA Reader (11).
Cellular ROS level determination
104 cells were treated with R. × damascena essential oil, MeOH, and different fractions (0.2–200 µg/ml) for 24 hr. After adding 2’, 7’-dichlorofluorescein diacetate (DCFH-DA) (10 μl) to each well, fluorescent emission of samples were read at 525 nm with Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, USA) (11).
Western blotting
Cells were treated with R. × damascena essential oil (0.2–200 µg/ml) for 24 hr. The next day, they were lysed in a buffer, and 50 µg of proteins was transferred to a polyvinylidene difluoride membrane after separation in gel electrophoresis with 10% SDS-polyacrylamide. After, the membrane was exposed to primary antibodies: rabbit anti-tyrosinase antibody (1:300), β-actin (13E5), and then to anti-rabbit IgG (1:2000) as a secondary antibody. Finally, bands were detected by a Gel-pro Analyzer V. 6.0 image analysis software (Media Cybernetics, InG, Bethesda, MD, USA). β-Actin was used as internal control (25).
Statistical analysis
Mean±SD of all data was measured and significant differences (P<0.05) between groups were analyzed with Graph Pad Prism®: ANOVA and Dennett’s post hoc test
Results
Essential oil composition
The chemical composition (99.8%) of the essential oils of R. × damascena aerial parts identified 63 components as volatiles using GC-MS analysis (Table 1).
Table 1.
Rosa × damascena essential oil GC-MS analysis. Chemical composition (%) of essential oil of R. damascena aerial parts
| NO | Compound | RI 1 | Percentage % |
|---|---|---|---|
| 1 | n-heptanal | 901 | 0.1 |
| 2 | α-pinene | 936 | 0.6 |
| 3 | benzaldehyde | 962 | t |
| 4 | sabinene | 975 | 0.1 |
| 5 | β-pinene | 977 | 0.2 |
| 6 | β-myrcene | 993 | 0.2 |
| 7 | ortho-cymene | 1026 | t |
| 8 | limonene | 1030 | t |
| 9 | 1,8-cineol | 1032 | t |
| 10 | γ-terpinene | 1062 | t |
| 11 | linalool | 1101 | 1.1 |
| 12 | nonanal | 1105 | 0.1 |
| 13 | cis-rose oxide | 1112 | 0.4 |
| 14 | phenyl ethyl alcohol | 1114 | 1.4 |
| 15 | trans-rose oxide | 1129 | 0.2 |
| 16 | nerol oxide | 1158 | 0.1 |
| 17 | terpinen-4-ol | 1178 | 0.6 |
| 18 | α-terpineol | 1191 | 0.4 |
| 19 | citronellol | 1225 | 37.1 |
| 20 | unknown | 1228 | 0.2 |
| 21 | neral | 1232 | 0.5 |
| 22 | geraniol | 1255 | 12.7 |
| 23 | geranial | 1268 | 0.6 |
| 24 | methyl geranate | 1327 | 0.1 |
| 25 | citronelyl acetate | 1358 | 0.8 |
| 26 | eugenol | 1361 | 1.9 |
| 27 | neryl acetate | 1368 | 0.1 |
| 28 | geranyl acetate | 1387 | 1.4 |
| 29 | β-elemene | 1392 | 0.2 |
| 30 | methyl eugenol | 1408 | 5.0 |
| 31 | β-caryophyllene | 1419 | 0.6 |
| 32 | β-copaene | 1430 | 0.1 |
| 33 | α-guaiene | 1440 | 0.9 |
| 34 | α-humulene | 1454 | 0.6 |
| 35 | germacrene D | 1482 | 1.5 |
| 36 | phenylethyl 2- methyl butanoate | 1487 | 0.3 |
| 37 | pentadecane | 1500 | 0.6 |
| 38 | trans-β-guaiene | 1506 | 0.5 |
| 39 | (E,E)-α-farnesene | 1510 | 0.1 |
| 40 | δ-cadinene | 1525 | 0.1 |
| 41 | E-nerolidol | 1566 | 0.1 |
| 42 | 2-phenyl ethyl tiglate | 1587 | 0.1 |
| 43 | hexadecane | 1600 | 0.1 |
| 44 | γ-eudesmol | 1633 | 0.1 |
| 45 | tau-muurolol | 1644 | 0.1 |
| 46 | α-eudesmol | 1651 | 0.1 |
| 47 | α-cadinol | 1656 | 0.3 |
| 48 | 8-heptadecene | 1678 | 0.3 |
| 49 | n-heptadecane | 1700 | 2.3 |
| 50 | Z,E-farnesal | 1716 | 0.1 |
| 51 | Z,E-farnesol | 1724 | 1.2 |
| 52 | benzyl benzoate | 1765 | 0.1 |
| 53 | octadecane | 1800 | 0.3 |
| 54 | phenyl ethyl benzoate | 1854 | 0.3 |
| 55 | Z-5-Nonadecene | 1876 | 3.9 |
| 56 | nonadecane | 1901 | 11.8 |
| 57 | ethyl hexadecanoate | 1996 | t |
| 58 | eicosane | 2001 | 1.2 |
| 59 | n-heneicosane | 2101 | 5.1 |
| 60 | n-docosane | 2200 | 0.1 |
| 61 | n-tricosane | 2300 | 0.9 |
| 62 | n-Tetracosane | 2400 | 0.1 |
| 63 | n-pentacosane | 2500 | 0.2 |
| Major Grouped Compounds | |||
| Monoterpene hydrocarbons | 1.2 | ||
| Oxygenated monoterpenes | 56.0 | ||
| Sesquiterpene hydrocarbone | 4.5 | ||
| Oxygenated sesquiterpenes | 1.8 | ||
| Miscellaneous compounds | 36.3 | ||
1- RI: The Kovats retention indices relative to C8-C20 n-alkanes were determined on CP-Sil 8CB capillary column
t: trace > 0.05%
Effect of R. × damascena essential oil, MeOH, and different fractions on cell viability of B16F10 cells
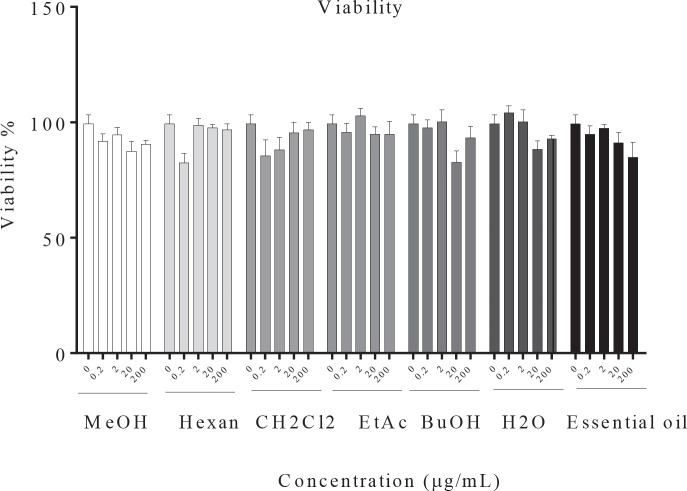
It was shown in the resazurin assay that essential oil, MeOH, and different fractions of R. × damascena have no significant cytotoxic effect (Figure 1). Hence, R. × damascena does not have cytotoxic effects on B16F10 melanoma cells.
Figure 1.
Effect of Rosa × damascena essential oil, MeOH, and different fractions on cell viability in B16F10 melanoma cells. The viability of B16F10 melanoma cells was evaluated after treatment with essential oil, MeOH, and different fractions of R. × damascena (0.2–200 µg/ml), Data are expressed as mean±SD. *P<0.05 compared with control
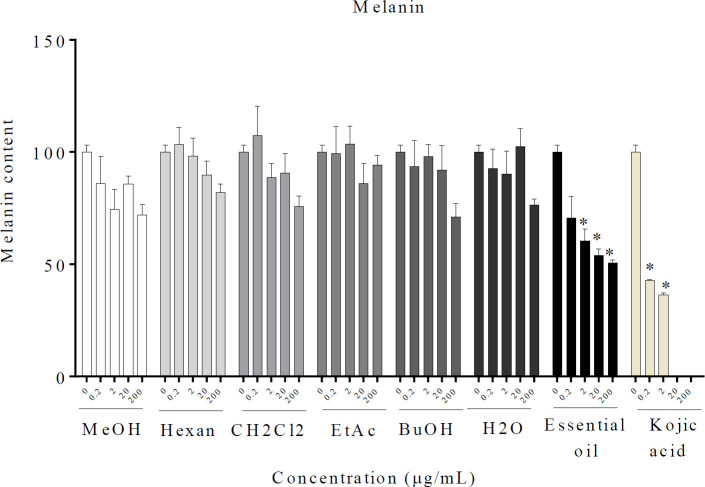
Effect of R. × damascena essential oil, MeOH, and different fractions on melanin production
It was seen that melanin levels were reduced by R. × damascena essential oil at concentrations of 2, 20, and 200 µg/ml. On the other hand, MeOH and fractions had no significant reducing effect on melanin content (Figure 2).
Figure 2.
Effect of Rosa × damascena essential oil, MeOH, and different fractions on melanin content in B16F10 melanoma cells. B16F10 cells were treated with essential oil, MeOH, and different fractions of R. × damascena (0.2–200 µg/ml). Data are expressed as mean±SD. *P<0.05 compared with control
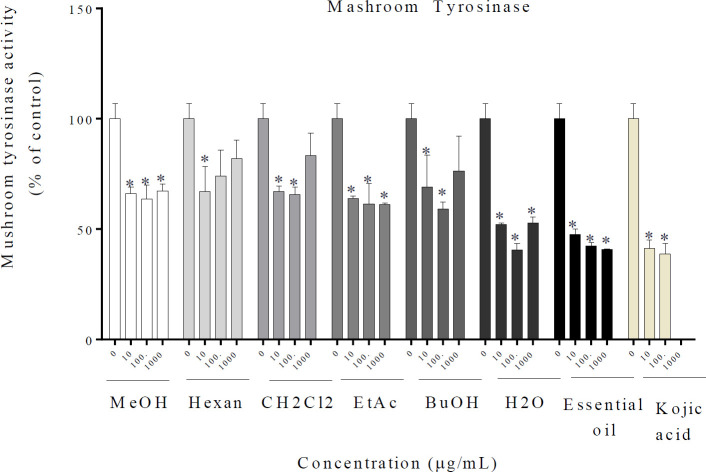
Effect of R. × damascena essential oil, MeOH, and different fractions on mushroom tyrosinase activity
The essential oil, MeOH, and different fractions significantly reduced the activity of mushroom tyrosinase. The obtained results are presented in Figure 3.
Figure 3.
Effect of Rosa × damascena essential oil, MeOH, and different fractions on mushroom tyrosinase in B16F10 melanoma cells. R. × damascena essential oil, MeOH, and different fractions and 2, 4 mM of Kojic acid were treated with mushroom tyrosinase and L-DOPA. Data are expressed as mean±SD. *P<0.05 compared with control
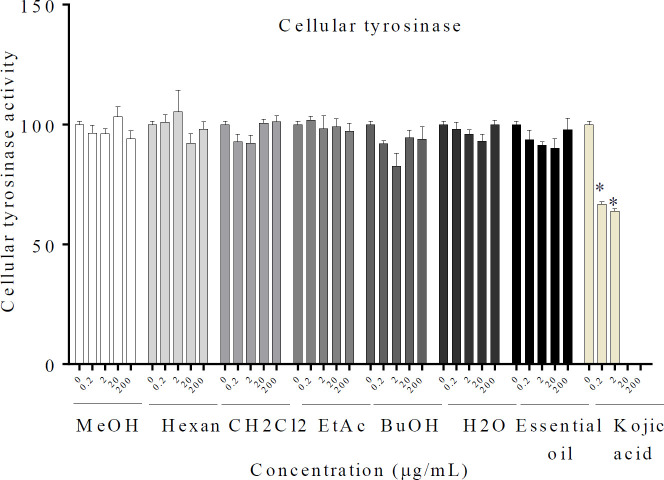
Effect of R. × damascena essential oil, MeOH, and different fractions on cellular tyrosinase activity of B16F10 cells
Looking at Figure 4, it is apparent that the essential oil, MeOH, and different fractions displayed no inhibitory effect. So, it has not directly affected cellular tyrosinase activity. Also, Kojic acid inhibited tyrosinase activity by 66.7 % and 63.8 % at concentrations of 2 and 4 mM.
Figure 4.
Effect of Rosa × damascena essential oil, MeOH, and different fractions on cellular tyrosinase in B16F10 melanoma cells. After treatment of B16F10 melanoma cells with essential oil, MeOH, and different fractions of R. × damascena (0.2–200 µg/ml), cellular tyrosinase activity was assessed. Data are expressed as mean±SD. *P<0.05 compared with control
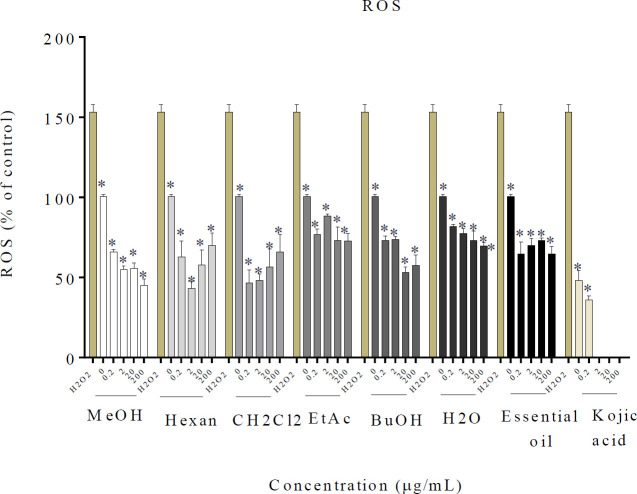
Effect of R. × damascena essential oil, MeOH, and different fractions on B16F10 cellular ROS level
As can be seen in Figure 5, all essential oils, MeOH, and the different fractions effectively suppressed the H2O2-induced oxidative stress, while the well containing only H2O2 increased ROS production.
Figure 5.
Anti-oxidant effect of Rosa × damascena essential oil, MeOH, and different fractions on cellular ROS level in B16F10 melanoma cells. The cells were treated with R. × damascena essential oil, MeOH, and different fractions (0.2–200 µg/ml) or Kojic acid (2.0–4.0 mM). Data are expressed as mean±SD. *P<0.05 compared with control
Figure 6.
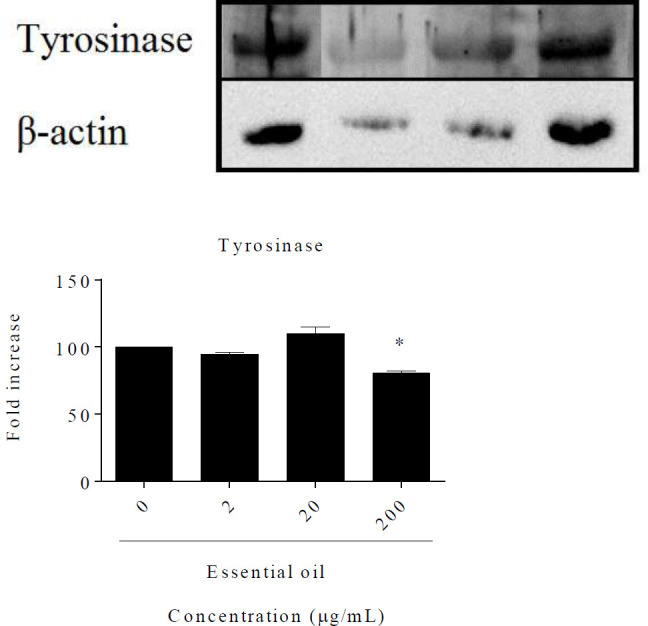
Effect of Rosa × damascena essential oil on tyrosinase protein level in B16F10 melanoma cells. Cells were treated with R. × damascena essential oil (2–200 µg/ml). The amount of tyrosinase proteins was normalized using their corresponding β-actin using Quantity One software (Bio-Rad, Hercules, CA, USA). Data are expressed as mean±SD. *P<0.05 compared with control
Discussion
Based on the available information, the present research is the first study on the effect of essential oil, MeOH, and different fractions of R. × damascena on B16F10 murine melanoma cell line and melanin production. We investigated the effect of essential oil, MeOH, and different fractions on cell viability, cellular and mushroom tyrosinase activity, melanin content, and ROS. Western blot was performed on total essential oil to determine the amount of tyrosinase antibody. Following UV exposure, free radicals (FR) and ROS play a major role in the production of lipid (L •) radicals, which induce the destruction of cell membranes and finally cells (26, 27). During skin contact with sunlight, especially its ultraviolet (UV) spectrum, the body’s melanocytes begin to synthesize melanin which has a protective effect on skin cells against mutations, DNA repair process errors, and skin cancers (28). Melanin is a biopolymer that is present in almost all living organisms. In humans, melanin is found mainly in the skin, eyes, and hair, and its distribution causes a variety of color patterns in external tissues. Its photochemical properties allow melanin to play a very important natural protective role in absorbing harmful ultraviolet rays (12). At the beginning of melanin synthesis, tyrosine is oxidized to dopaqinone which is catalyzed by the enzyme tyrosinase. The enzyme tyrosinase is responsible for catalyzing the oxidation of monophenols and diphenols to quinones (29) and its activity depends on the presence of copper (30). However, previous studies have shown that during the process of melanin production and skin care, the enzyme tyrosinase has a major role in the production of melanin and the transfer of produced melanin to adjacent cells (29). Due to the high importance of the tyrosinase enzyme in melanin production, the activity of the enzyme in the presence of essential oils, extracts, and different fractions in B16F10 cells was measured to indirectly decide on the effects of this plant on reducing melanin levels. Tyrosinase inhibitory activity was performed in two separate tests on mushroom tyrosinase also in melanoma cells. In general, the mushroom tyrosinase activity test had an acceptable answer in most concentrations used, but in the cellular tyrosinase activity test, no significant decrease in the amount of this enzyme was observed due to differences in the mechanism of these two tests. Kojic acid is a natural compound derived from the mushroom Aspergillus, Acetobacter, and Penicillium and is used in concentrations of 1 to 4% in formulations (31). Kojic acid could significantly reduce the activity of cellular and mushroom tyrosinase enzymes, melanin content, and ROS. Other studies have shown that kojic acid has anti-oxidant properties and scavenging free radicals, reducing the activity of mushroom tyrosinase enzymes and ROS which proves the results of our work (32). Hydroquinone and its derivatives have been associated with skin irritation, contact dermatitis, and skin depigmentation. Another complication of hydroquinone drugs is exogenous ochronosis, which usually occurs in black people after long-term use (33). According to the above, the initial toxicity of the essential oil, MeOH, and different fractions on the survival of B16F10 cells was measured. The essential oil, MeOH, and different fractions did not significantly reduce the percentage of viable cells up to concentrations of 200 μg/ml. Plant anti-oxidants reduce oxidative stress and have protective effects against oxidative stress and related diseases (32). Compounds with anti-oxidant properties reduce the oxidation of tyrosine to dopaqinone (34). Also, they reduce melanin synthesis in the melanogenesis pathway (35). Oxygen free radicals appear to have the main role in cardiovascular disease and the development of cancer. Scientists believe that the gradual accumulation of oxygen-free radicals accelerates the aging process throughout the body, including the skin. Also, the sun induces skin damage mainly due to the effect of oxygen-free radicals. Melanin absorbs free radicals produced by the cell cytoplasm and free radicals produced by UV light in the skin (36). Therefore, substances with anti-oxidant effects reduce the production of ROS and free radicals, also, they can decrease the amount of melanin. The existence of phenolic compounds in the ethanol extract of R. × damascena, as well as the high anti-oxidant activity of its extract were proven in the work of Chroho et al. (37). Using GC-MS, the oil component of the plant essential oil was determined from R. × damascene. As listed in Table 1 the essential oil contains 63 compounds, of which 12.7% geraniol and 37.1% citronellol were the chief components and the majority of compounds in essential oils were 56% oxygen monoterpenes (56%).
It is shown that geraniol reduces oxidative stress induced by cyclosporine A in rat kidneys. In this way, they decrease Wnt/β-catenin and ROS generation in the cell (38). Methyl-eugenol is identified as a specific anti-oxidant in some important aromatic herbs and spices (39). It is discovered that the great abundance of eugenol and methyl-eugenol in wild Laurus nobilis L. leaves is responsible for the higher level of anti-oxidant activities of this plant in comparison with the cultivated one (40). Additionally, it was shown that citronellol, one of the major monoterpenes in Artemisia scoporis essential oil, showed strong anti-oxidant and radical scavenging activity (41). In the work of Yasa et al., three flavonols were identified in the ethanol extract of rose with anti-oxidant properties (6). Another study showed that this plant inhibits fat oxidation and can prevent diseases caused by overproduction of free radicals (42). Furthermore, this plant contains vitamin C, which in itself has anti-inflammatory and anti-oxidant properties (43). Wang showed that roses have high amounts of polyphenols, especially flavonols, and possess anti-oxidant and free radical scavenging properties (44). In another study, the essential oil of rose showed an anti-oxidant effect and protected the sex cells exposed to ROS (45). The protective effect of R. × damascena on rat ovarian tissue in copper-induced poisoning was demonstrated to be via anti-oxidant effect (46). According to the results, 0.2, 20, and 200 µg/ml of essential oils had a significant effect on reducing melanin levels. As a result, it can be said that R. × damascena essential oil decreases the production of the total melanin content of B16F10 cells, mushroom tyrosinase activity, and ROS, which can ultimately reduce melanin production. In one study, the anti-oxidant and protective effects of compounds in citrus essential oils were investigated (47). Another study examined the compounds of 57 species of wild Sicilian rosemary. The predominant compounds identified were investigated and proven (48). GC-MASS analysis of Senecionudicaulis essential oil contains 1.13% citronellol and its anti-oxidant effects have been proven in one study (49). So, considering that the effect of the compounds in Rosa × damascena essential oil has been proven in the above studies, it can have a promising therapeutic effect in the treatment of inflammatory skin diseases, skin thickening, and skin brightening.
Conclusion
We have reported here the antimelanogenic and anti-tyrosinase effect of R. × damascena on the B16F10 murine melanoma cell line for the first time. The results indicate R. × damascena essential oil may be used as a promising agent in developing new treatments for hyperpigmentation in cosmetics and also in agriculture and food industries as an anti-browning agent.
Authors’ Contributions
E H and M RK were responsible for investigation, methodology, literature searches, and writing the original draft. SA E, JA, and Z B were responsible for investigation, methodology, literature searches, review, and editing, Z TN was responsible for conceiving, designing, review, and editing.
Ethics Issues
This work is carried out on B16F10 murine melanoma cells and there is no need for ethical clearance.
Conflicts of Interest
There are no conflicts of interest in this study.
Acknowledgment
This work was supported by Research Affairs of Mashhad University of Medical Sciences, Iran (Grant No. 930776).
References
- 1.Akhavan HR, Zarezadeh Mehrizi R. Effects of damask rose (Rosa damascena Mill ) extract on chemical, microbial, and sensory properties of sohan (an Iranian confection) during storage. J Food Qual Hazards Control. 2016:3:97–106. [Google Scholar]
- 2.Venkatesha KT, Gupta A, Rai AN, Jambhulkar SJ, Bisht R, Padalia RC. Recent developments, challenges, and opportunities in genetic improvement of essential oil-bearing rose (Rosa damascena): A review. Ind Crops Prod. 2022;184:114984. [Google Scholar]
- 3.Labban L, Thallaj N. The medicinal and pharmacological properties of Damascene Rose (Rosa damascena): A review. Int J Herb Med. 2020;8:33–37. [Google Scholar]
- 4.Shohayeb M, Abdel-Hameed ES, Bazaid SA, Maghrabi I. Antibacterial and antifungal activity of Rosa damascena MILL essential oil, different extracts of rose petals. Glob J Pharmacol. 2014;8:1–7. [Google Scholar]
- 5.Hamza RZ, Al-Yasi HM, Ali EF, Fawzy MA, Abdelkader TG, Galal TM. Chemical characterization of Taif rose (Rosa damascena Mill va trigentipetala) waste methanolic extract and its hepatoprotective and anti-oxidant effects against cadmium chloride (CdCl2)-induced hepatotoxicity and potential anticancer activities against liver cancer cells (HepG2) Crystals. 2022;12 [Google Scholar]
- 6.Yasa N, Masoumi F, Rouhani RS, Haji AA. Chemical composition and anti-oxidant activity of the extract and essential oil of Rosa damascena from Iran, population of Guilan. DARU J Pharm Sci. 2015;17:175–180. [Google Scholar]
- 7.Rakhshandah H, Hosseini M, Dolati K. Hypnotic effect of Rosa damascena in mice. Iran J Pharm Res. 2022;3:181–185. [Google Scholar]
- 8.Demirel S. Rosa damascena Miller essential oil relaxes rat trachea via KV channels, KATP channels, and BKCa channels. Prostaglandins Other Lipid Mediat. 2022;163:106673. doi: 10.1016/j.prostaglandins.2022.106673. [DOI] [PubMed] [Google Scholar]
- 9.Memariani Z, Amin G, Moghaddam G, Hajimahmoodi M. Comparative analysis of phenolic compounds in two samples of Rosa damascena by HPLC. Int J Biosci. 2015;7:112–118. [Google Scholar]
- 10.Działo M, Mierziak J, Korzun U, Preisner M, Szopa J, Kulma A. The potential of plant phenolics in prevention and therapy of skin disorders. Int J Mol Sci. 2016;17:160. doi: 10.3390/ijms17020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tayarani-Najaran Z, Akaberi M, Vatani M, Emami SA. Evaluation of anti-oxidant and antimelanogenic activities of different extracts from aerial parts of Nepeta binaludensis Jamzad in murine melanoma B16F10 cells. Iran J Basic Med Sci. 2016;19:662–669. [PMC free article] [PubMed] [Google Scholar]
- 12.Eghbali-Feriz S, Taleghani A, Al-Najjar H, Emami SA, Rahimi H, Asili J, Hasanzadeh S, Tayarani-Najaran Z. Antimelanogenesis and anti-tyrosinase properties of Pistacia atlantica subsp mutica extracts on B16F10 murine melanoma cells. Res Pharm Sci. 2018;13 doi: 10.4103/1735-5362.245965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MJ, Mohamed EA, Kim DS, Park MJ, Ahn BJ, Jeung EB, An BS. Inhibitory effects and underlying mechanisms of Artemisia capillaris essential oil on melanogenesis in the B16F10 cell line. Mol Med Rep. 2022;25:1–2. doi: 10.3892/mmr.2022.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamilijiang M, Zang D, Abudukelimu N, Aidarhan N, Liu G, Aisa HA. Antimelanogenesis effect of polysaccharide from saussurea involucrata on forskolin-induced melanogenesis in B16F10 melanoma cells. Nutrients. 2022;14:5044. doi: 10.3390/nu14235044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahimi VB, Askari VR, Emami SA, Tayarani-Najaran Z. Antimelanogenic activity of Viola odorata different extracts on B16F10 murine melanoma cells. Iran J Basic Med Sci. 2017;20 doi: 10.22038/ijbms.2017.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi MR, Lee H, Kim HK, Han J, Seol JE, Vasileva EA, Mishchenko NP, Fedoreyev SA, Stonik VA, Ju WS, Kim DJ. Echinochrome A inhibits melanogenesis in B16F10 cells by downregulating CREB signaling. Mar Drugs. 2022;20:555. doi: 10.3390/md20090555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalili Z, Taraghi Z, Ilali ES. The effect of damask rose and orange blossom on anxiety in older adults. J Complement Med Res. 2021;11:20–29. [Google Scholar]
- 18.Shin EJ, Han AR, Lee MH, Song YR, Lee KM, Nam TG, Lee P, Lee SY, Lim TG. Extraction conditions for Rosa gallica petal extracts with anti-skin aging activities. Food Sci Biotechnol. 2019;28:1439–1446. doi: 10.1007/s10068-019-00596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li MX, Xie J, Bai X, Du ZZ. Anti-aging potential, anti-tyrosinase and antibacterial activities of extracts and compounds isolated from Rosa chinensis cv. ‘JinBian’. Ind Crops Prod. 2021;159:113059. [Google Scholar]
- 20.Song YR, Lim WC, Han A, Lee MH, Shin EJ, Lee KM, Nam TG, Lim TG. Rose petal extract (Rosa gallica) exerts skin whitening and anti-skin wrinkle effects. J Med Food. 2020;23:870–878. doi: 10.1089/jmf.2020.4705. [DOI] [PubMed] [Google Scholar]
- 21.Vanitha M, Soundhari C. Isolation and characterisation of mushroom tyrosinase and screening of herbal extracts for anti-tyrosinase activity. Int J ChemTech Res. 2017;10:1156–1167. [Google Scholar]
- 22.Schmidt E. Handbook of Essential Oils Science, Technology, and Applications. 2nd ed. Taylor & Francis Group; 2016. [Google Scholar]
- 23.Fattahi A, Shakeri A, Tayarani-Najaran Z, Kharbach M, Segers K, Heyden YV, Taghizadeh SF, Rahmani H, Asili J. UPLC–PDA-ESI–QTOF–MS/MS and GC-MS analysis of Iranian Dracocephalum moldavica L. Food Sci Nutr. 2021;9:4278–4286. doi: 10.1002/fsn3.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamiloglu S, Sari G, Ozdal T, Capanoglu E. Guidelines for cell viability assays. Food Frontiers. 2020;1:332–349. [Google Scholar]
- 25.Rahiman N, Akaberi M, Sahebkar A, Emami SA, Tayarani-Najaran Z. Protective effects of saffron and its active components against oxidative stress and apoptosis in endothelial cells. Microvasc Res. 2018;118:82–89. doi: 10.1016/j.mvr.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Cleaver JE, Ortiz-Urda S, Arron S. Ultraviolet radiation carcinogenesis. Holland Frei Cancer Medicine. 2016;17:1–6. [Google Scholar]
- 27.De Jager TL, Cockrell AE, Du Plessis SS. Ultraviolet light induced generation of reactive oxygen species. Ultraviolet Light in Human Health, Diseases and Environment . 2017:15–23. doi: 10.1007/978-3-319-56017-5_2. [DOI] [PubMed] [Google Scholar]
- 28.Lee JS, Kim YR, Song IG, Ha SJ, Kim YE, Baek NI, Hong EK. Cyanidin-3-glucoside isolated from mulberry fruit protects pancreatic β-cells against oxidative stress-induced apoptosis. Int J Mol Med. 2015;35:405–412. doi: 10.3892/ijmm.2014.2013. [DOI] [PubMed] [Google Scholar]
- 29.Boghrati Z, Naseri M, Rezaie M, Pham N, Quinn RJ, Tayarani-Najaran Z, Iranshahi M. Tyrosinase inhibitory properties of phenylpropanoid glycosides and flavonoids from Teucrium polium L va gnaphalodes. Iran J Basic Med Sci. 2016;19:804–811. [PMC free article] [PubMed] [Google Scholar]
- 30.Largeron M. Aerobic catalytic systems inspired by copper amine oxidases: Recent developments and synthetic applications. Org Biomol Chem. 2017;15:4722–4730. doi: 10.1039/c7ob00507e. [DOI] [PubMed] [Google Scholar]
- 31.Xie W, Zhang H, He J, Zhang J, Yu Q, Luo C, Li S. Synthesis and biological evaluation of novel hydroxybenzaldehyde-based kojic acid analogues as inhibitors of mushroom tyrosinase. Bioorg Med Chem. 2017;27:530–532. doi: 10.1016/j.bmcl.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Baek SH, Nam IJ, Kwak HS, Kim KC, Lee SH. Cellular anti-melanogenic effects of a Euryale ferox seed extract ethyl acetate fraction via the lysosomal degradation machinery. Int J Mol Sci. 2015;16:9217–9235. doi: 10.3390/ijms16059217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishack S, Lipner SR. Exogenous ochronosis associated with hydroquinone: A systematic review. Int J Dermatol. 2022;61:675–684. doi: 10.1111/ijd.15878. [DOI] [PubMed] [Google Scholar]
- 34.Shamu TN, Lalitha J, Shinde M. Mushroom tyrosinase inhibition and antimelanogenesis activities of Bacopa monnieri ( L) methanol extract in B16F10 melanoma cells. J Biosci Med. 2020;8:189–202. [Google Scholar]
- 35.Kumari S, Tien Guan THNG S, Verma NK, Gautam HK. Melanogenesis inhibitors. Acta dermato-venereologica. 2018;98: 924–931. doi: 10.2340/00015555-3002. [DOI] [PubMed] [Google Scholar]
- 36.Tonolli PN, Baptista MS, Chiarelli-Neto O. Melanin, lipofuscin and the effects of visible light in the skin. J Photochem Photobiol. 2021;7:1–5. [Google Scholar]
- 37.Chroho M, Bouymajane A, Oulad El Majdoub Y, Cacciola F, Mondello L, Aazza M, Zair T, Bouissane L. Phenolic composition, anti-oxidant and antibacterial activities of extract from flowers of Rosa damascena from morocco. Separations. 2022;9:247. [Google Scholar]
- 38.Mahmoud NM, Elshazly SM, Rezq S. Geraniol protects against cyclosporine A-induced renal injury in rats: Role of Wnt/β-catenin and PPARγ signaling pathways. Life Sci. 2022;291:1–11. doi: 10.1016/j.lfs.2021.120259. [DOI] [PubMed] [Google Scholar]
- 39.Joshi A, Prakash O, Pant AK, Kumar R, Szczepaniak L, Kucharska-Ambrożej K. Methyl eugenol, 1, 8-cineole and nerolidol rich essential oils with their biological activities from three Melaleuca species growing in Tarai region of North India. Braz Arch Biol Technol. 2022:1–15. [Google Scholar]
- 40.Chahal KK, Kaur M, Bhardwaj U, Singla N, Kaur A. A review on chemistry and biological activities of Laurus nobilis L essential oil. J Pharmacogn Phytochem. 2017;6:1153–1161. [Google Scholar]
- 41.Ivănescu B, Burlec AF, Crivoi F, Roșu C, Corciovă A. Secondary metabolites from Artemisia genus as biopesticides and innovative nano-based application strategies. Molecules. 2021;26:1–42. doi: 10.3390/molecules26103061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rezvani-Kamran A, Salehi I, Shahidi S, Zarei M, Moradkhani S, Komaki A. Effects of the hydroalcoholic extract of Rosa damascena on learning and memory in male rats consuming a high-fat diet. Pharm Biol. 2017;55:2065–2073. doi: 10.1080/13880209.2017.1362010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akram M, Riaz M, Munir N, Akhter N, Zafar S, Jabeen F, Ali Shariati M, Akhtar N, Riaz Z, Altaf SH, Daniyal M. Chemical constituents, experimental and clinical pharmacology of Rosa damascena: A literature review. J Pharm Pharmacol. 2020;72:161–174. doi: 10.1111/jphp.13185. [DOI] [PubMed] [Google Scholar]
- 44.Verma A, Srivastava R, Sonar PK, Yadav R. Traditional, phytochemical, and biological aspects of Rosa alba L : A systematic review. Future J Pharm Sci. 2020;6:1–8. [Google Scholar]
- 45.Sakhaee E, Emadi L, Siahkouhi H. Supportive effects of Rosa damascene essential oil on epididymal sperm quality following long-term administration of copper sulfate in mice. Human Andrology. 2015;5:68–70. [Google Scholar]
- 46.Sakhaee E, Abshenas J, Emadi L, Azari O, Kheirandish R, Samaneh A. Effects of vitamin C on epididymal sperm quality following experimentally induced copper poisoning in mice. Comp Clin Path. 2014;23:181–186. [Google Scholar]
- 47.EM Mustafa N. Citrus essential oils: Current and prospective uses in the food industry. Recent Pat. Food Nutr Agric. 2015;7:115–127. doi: 10.2174/2212798407666150831144239. [DOI] [PubMed] [Google Scholar]
- 48.Napoli EM, Siracusa L, Saija A, Speciale A, Trombetta D, Tuttolomondo T, La Bella S, Virga G, Leone R, Leto C. Wild Sicilian rosemary: Phytochemical and morphological screening and anti-oxidant activity evaluation of extracts and essential oils. Chem Biodivers. 2015;12:1075–1094. doi: 10.1002/cbdv.201400274. [DOI] [PubMed] [Google Scholar]
- 49.Sharma P, Shah GC. Composition and anti-oxidant activity of Senecio nudicaulis Wall ex DC (Asteraceae): A medicinal plant growing wild in Himachal Pradesh, India. Nat Prod Res. 2015;29:883–886. doi: 10.1080/14786419.2014.990904. [DOI] [PubMed] [Google Scholar]