Abstract
Zoonotic diseases (zoonoses) originating from domestic animals pose a significant risk to people's health and livelihoods, in addition to jeopardizing animal health and production. Effective surveillance of endemic zoonoses at the animal level is crucial to assessing the disease burden and risk, and providing early warning to prevent epidemics in animals and spillover to humans. Here we aimed to prioritize and characterize zoonoses for which surveillance in domestic animals is important to prevent human infections at a global scale. A multi-criteria qualitative approach was used, where disease-specific information was obtained across literature of the leading international health organizations. Thirty-two zoonoses were prioritized, all of which have multi-regional spread, cause unexceptional human infections and have domestic animal hosts as important sources or sentinels of zoonotic infections. Most diseases involve multiple animal hosts and/or modes of zoonotic transmission, where a lack of specific clinical signs in animals further complicates surveillance. We discuss the challenges of animal health surveillance in endemic and resource-limited settings, as well as potential avenues for improvement such as the multi-disease, multi-sectoral and digital surveillance approaches. Our study will support global capacity-building efforts to strengthen the surveillance and control of endemic zoonoses at their animal sources.
This article is part of the theme issue ‘Challenges and opportunities in the fight against neglected tropical diseases: a decade from the London Declaration on NTDs’.
Keywords: zoonoses, disease prioritization, surveillance, domestic animals, public health
1. Introduction
It is estimated that at least 60% of known infectious diseases and 75% of emerging infectious diseases in humans are zoonotic in origin [1,2], with zoonotic disease outbreaks increasing globally in both total number and richness [3]. Zoonoses are largely classified as either emerging/re-emerging or endemic zoonoses [4–7]. Most emerging infectious diseases of humans are believed to originate from wildlife [8]. They often have small-scale direct health impacts, but a small minority can lead to epidemics or even pandemics in humans [1,9], such as the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) epidemics and the 2009 H1N1 influenza virus and SARS-CoV-2 pandemics. If not properly controlled, emerging and re-emerging infectious diseases can become endemic and persistent. Endemic zoonoses, such as rabies, brucellosis, cysticercosis and bovine tuberculosis, cause constant and regular outbreaks in areas where conditions favour their maintenance and spread [10]. They would be considered as emerging zoonoses if expanded to a new territory or host species or evolved new traits. Endemic zoonoses mostly affect people in low- and middle-income countries (LMICs) who live in close proximity to their animals, resulting in billions of illnesses and millions of deaths in humans every year [7,11]. In addition to the public health significance, endemic zoonoses impact livestock production and trade, further jeopardizing human livelihoods and food security [10,12]. Livestock production is one of the fastest-growing agricultural sectors in LMICs, as driven by population and economic growth and the associated increasing demand for livestock products [13,14]. While LMICs tend to bear a higher burden of zoonoses along with the expanding livestock production and high human–livestock interactions, they are also at a disadvantage in terms of existing capacity to tackle the disease risk [7,11,15]. Inadequate surveillance in animals can lead to delayed detection and response to disease outbreaks, increasing the risk of onward spread and zoonotic transmission.
Driven by the epidemics or pandemics caused by zoonotic influenza viruses, SARS-CoV, MERS-CoV, and more recently SARS-CoV-2, strengthening the capacity for the surveillance of and response to emerging zoonoses with pandemic potential has become the focus of public health interests [16,17]. Compared to their emerging zoonoses counterparts, endemic zoonoses often receive much less policy and research support and are rarely targeted by formal surveillance systems, so their frequencies and burdens are largely unknown and underestimated [10,11]. This underestimation in turn results in ‘neglect’ owing to a lack of evidence for decision-makers on the significance of these diseases and eventually serious consequences in terms of investments for control initiatives [10,12]. The persistence of these endemic zoonoses thereby plays an important role in perpetuating poverty and hindering progress towards the United Nations Sustainable Development Goals (UN SDGs).
Combating endemic zoonoses requires a holistic approach with close collaboration between human and animal health sectors, but this is often challenged by the divergences in the sectoral mandates and priorities [18–20]. While the World Health Organization (WHO)'s new neglected tropical diseases (NTDs) Roadmap sets a global strategy to tackle 20 NTDs by 2030, including a named subset of zoonoses, the listed targets and approaches mostly concern human infections [21]. The public health risk of endemic zoonoses is substantial, while the most effective and economic approach is often to control them at the animal sources [7,22]. However, veterinary authorities tend to prioritize diseases having more serious impacts on animal production and trade, especially in the face of limited resources, and subsequently endemic zoonoses often fall into the gap between public health needs and veterinary responsibilities [18,23]. Increased awareness, commitment and alignment with the One Health vision to tackle zoonoses are urgently needed to achieve the dual benefits of protecting both animal and human health. This includes improved upstream disease surveillance in the animal hosts and strengthened early warning and response systems at the animal–human interface. To boost international attention and global efforts to tackle endemic zoonoses at the animal level, the present study aimed to: (1) prioritize endemic zoonoses at a global scale for conducting disease surveillance in domestic animals to prevent human infections; and (2) characterize the prioritized diseases to inform cost-effective approaches to improve surveillance in endemic and resource-limited settings. This study is part of the Food and Agriculture Organization (FAO) project on the development of animal health surveillance guidelines for endemic zoonoses, relevant to the One Health Joint Plan of Action (2022–2026) Actions 3.1, 3.2 and 3.3 [6].
2. Methods
(a) . Initial list of diseases
We aimed to start with a broad list of diseases to encompass as many zoonoses of international importance as possible. The listed diseases by the World Organisation for Animal Health (WOAH, founded as OIE) are animal diseases that have the potential for very serious and rapid cross-border spread, cause particularly serious socio-economic or public health consequences, and are of major importance in the international trade of animals and animal products [24]. In addition, the WOAH Manual of diagnostic tests and vaccines for terrestrial animals covers WOAH-listed diseases and some other important animal diseases including zoonoses [25]. Moreover, the WHO One Health Companion Document to the WHO NTDs Roadmap 2021–2030 includes a subset of zoonotic NTDs that can impose a devastating health, social and economic burden [26]. Hence, an initial list was created by compiling diseases from the following three international publicly accessible sources:
-
1)
90 WOAH-listed terrestrial animal diseases as of May 2022 [27];
-
2)
111 diseases/disease groups from the WOAH Manual of diagnostic tests and vaccines for terrestrial animals as of May 2022 [25]; and
-
3)
11 zoonotic NTDs/groups of NTDs from the WHO One Health Companion Document to the WHO NTDs Roadmap 2021–2030 [26].
Bee diseases from the WOAH List and Manual were excluded from the assessment. For diseases that overlapped between different lists (e.g. leishmaniasis and rabies, etc.), only one was included. For diseases or infections that are caused by distinct agents and exhibit distinguishable epidemiological features in different animal species, they were separated by the animal host (e.g. bovine brucellosis, porcine brucellosis, and caprine and ovine brucellosis). Likewise, for diseases that have been grouped together within the original listing such as foodborne trematodiases, they were separated by individual disease for the assessment (e.g. fascioliasis, paragonimiasis, clonorchiasis and opisthorchiasis).
(b) . Algorithm and criteria for disease prioritization
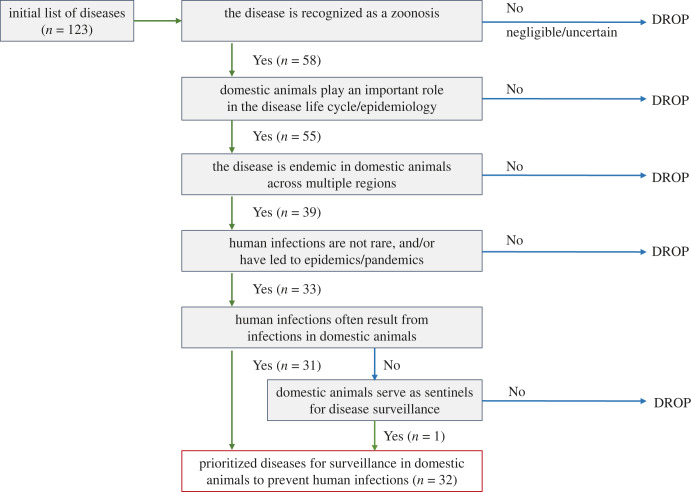
We aimed for a rapid assessment to narrow down the initial list of diseases to meet the requirements of this study. Our initial consideration was given to the application of quantitative prioritization criteria such as the disease frequency (prevalence or incidence), the disease burden (mortality and morbidity) and the magnitude of economic losses. However, an initial appraisal of literature and data sources excluded this option given the scarcity of data available for the majority of diseases. Thus, a qualitative algorithm was used with the aim of minimizing the exclusion of specific diseases due to a lack of available data [28]. The algorithm (figure 1) comprises the following sequential selection criteria to prioritize diseases for which surveillance in domestic animals is important to prevent human infections at a global scale:
-
1)
the disease is recognized as a zoonosis by the WOAH Terrestrial Animal Health Code [29], Manual [25] or the WHO One Health Companion Document to the WHO NTDs Roadmap 2021–2030 [26];
-
2)
domestic animals play an important role in the disease life cycle (e.g. serve as a reservoir, amplifier host, intermediate host or definitive host) or epidemiology (e.g. surveillance and control);
-
3)
the disease is endemic in domestic animals across multiple regions, as opposed to geographically restricted to a specific region/agroecosystem (e.g. Sub-Saharan Africa);
-
4)
human infections are not rare, and/or have led to epidemics or pandemics; and
-
5)
a. human infections often result from infections in domestic animals (i.e. zoonotic transmission), as opposed to being predominantly due to non-zoonotic sources such as infected humans or environmental reservoirs; or b. domestic animals serve as sentinels for disease surveillance.
Figure 1.
Qualitative algorithm for the prioritization of endemic zoonoses for conducting surveillance in domestic animals to protect public health.
(c) . Literature search and disease assessment
Literature searches were conducted in English by four authors (I.M., J.G., J.P.W. and Y.Q.) to collect information against the above-mentioned criteria for all the diseases included in the initial list. Different sources, including the WOAH Terrestrial Animal Health Code [29] and Manual [25], technical disease cards from WOAH (http://rr-middleeast.woah.org/en/technical-disease-cards/) and Center for Food Safety & Public Health (CFSPH) of Iowa State University (https://www.cfsph.iastate.edu/diseaseinfo/), publications from FAO (https://www.fao.org/publications/en) and WHO (https://www.who.int/publications), and the United States Centers for Disease Control and Prevention (US CDC) website (https://www.cdc.gov/) were searched. A Microsoft Excel spreadsheet (electronic supplementary material [30]) was developed to select/drop diseases based on yes/no choices. ‘Negligible’ was used to indicate that the zoonotic risk of a disease is negligible as infections in humans are extremely rare, and ‘uncertain’ was used to indicate where information was insufficient to make a conclusion. Any discordance in the judgement was resolved through the authors' internal discussions. For each prioritized disease, a more in-depth literature review was conducted by one author (Y.Q.), and information about the causative agent, domestic animal hosts, geographical distribution, clinical signs and modes of zoonotic transmission was collected.
3. Results
(a) . Prioritized zoonoses
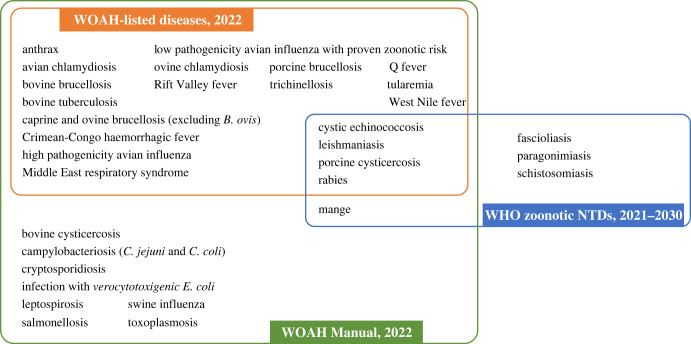
The initial list included 123 diseases, with 58 known to be zoonotic, and ultimately 32 were prioritized following the application of the algorithm (figure 1). Of the prioritized diseases, 29 are included in the WOAH Terrestrial Manual, 20 are WOAH-listed diseases, and 8 are WHO zoonotic NTDs (figure 2).
Figure 2.
Prioritized zoonoses and their inclusion in the World Organisation for Animal Health (WOAH) List of notifiable diseases [27] and Terrestrial Manual [25] and the World Health Organization (WHO) zoonotic neglected tropical diseases (NTDs) [26].
(b) . Characterization of the prioritized zoonoses
For each prioritized disease, the causative agent, domestic animal hosts, geographical distribution, clinical signs and modes of zoonotic transmission are summarized in table 1. Of the 32 prioritized zoonoses, 13 are bacterial, followed by parasitic (helminthic, protozoal or ectoparasitic) (n = 11) and viral (n = 8) diseases. Most prioritized zoonoses have broad host ranges, with ruminants (cattle, buffalo, sheep or goat) being the most common. Twenty-nine diseases are reported to be able to affect animal hosts without obvious clinical illness. More than half can be transmitted to humans by more than one mode, most commonly through the direct mode (i.e. close contact with infected animals or their products), followed by ingestion of contaminated food, and through water, fomites, vectors or aerosols.
Table 1.
Prioritized 32 zoonoses and factors influencing their surveillance.
| disease | disease agent |
key domestic animal hosts for disease surveillancea |
primary endemic areasb |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| virus | bacterium | protozoan | helminth | ectoparasite | cattle and buffalo | goat and sheep | pig | camel | equid | domestic bird | rabbit | dog | cat | high-income countries | LMICs | |
| anthrax | X | X | X | X | X | X | ||||||||||
| avian chlamydiosis | X | X | X | X | ||||||||||||
| bovine brucellosis (B. abortus) | X | X | X | X | X | |||||||||||
| bovine cysticercosis | X | X | X | |||||||||||||
| bovine tuberculosis | X | X | X | X | X | |||||||||||
| campylobacteriosis (C. jejuni and C. coli) | X | X | X | X | X | X | X | X | X | |||||||
| caprine and ovine brucellosis (B. melitensis) | X | X | X | X | X | |||||||||||
| Crimean-Congo haemorrhagic fever | X | X | X | X | X | |||||||||||
| cryptosporidiosis (C. parvum) | X | X | X | X | X | |||||||||||
| cystic echinococcosis | X | X | X | X | X | X | X | X | ||||||||
| fascioliasis | X | X | X | X | X | |||||||||||
| high pathogenicity avian influenza | X | X | X | |||||||||||||
| infection with verocytotoxigenic E. coli | X | X | X | X | X | |||||||||||
| leishmaniasis | X | X | X | |||||||||||||
| leptospirosis | X | X | X | X | X | X | X | |||||||||
| low pathogenicity avian influenza | X | X | X | |||||||||||||
| mange | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Middle East respiratory syndrome | X | X | X | |||||||||||||
| ovine chlamydiosis | X | X | X | X | ||||||||||||
| paragonimiasis | X | X | X | X | X | |||||||||||
| porcine brucellosis (B. suis) | X | X | X | |||||||||||||
| porcine cysticercosis | X | X | X | |||||||||||||
| Q fever | X | X | X | X | X | |||||||||||
| rabies | X | X | X | X | X | X | X | X | ||||||||
| Rift Valley fever | X | X | X | X | X | |||||||||||
| salmonellosis | X | X | X | X | X | X | X | |||||||||
| schistosomiasis | X | X | X | X | X | X | X | X | ||||||||
| swine influenza | X | X | X | X | ||||||||||||
| toxoplasmosis | X | X | X | X | X | X | X | X | X | |||||||
| trichinellosis | X | X | X | |||||||||||||
| Tularemia | X | X | X | X | X | X | ||||||||||
| West Nile fever | X | X | X | X | X | |||||||||||
| disease | common clinical signs in animalsc |
common modes of zoonotic transmissiond |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| indirect mode |
||||||||||||||
| no clinical signs | systemic | respiratory | reproductive | gastrointestinal | cutaneous | neurological | sudden death | direct mode | fomite | air-borne | food-borne | water-borne | vector-borne | |
| anthrax | X | X | X | X | ||||||||||
| avian chlamydiosis | X | X | X | X | X | X | ||||||||
| bovine brucellosis (B. abortus) | X | X | X | X | ||||||||||
| bovine cysticercosis | X | X | ||||||||||||
| bovine tuberculosis | X | X | X | X | X | |||||||||
| campylobacteriosis (C. jejuni and C. coli) | X | X | X | X | X | |||||||||
| caprine and ovine brucellosis (B. melitensis) | X | X | X | X | ||||||||||
| Crimean-Congo haemorrhagic fever | X | X | X | |||||||||||
| cryptosporidiosis (C. parvum) | X | X | X | X | X | X | ||||||||
| cystic echinococcosis | X | X | X | X | ||||||||||
| fascioliasis | X | X | X | X | X | |||||||||
| high pathogenicity avian influenza | X | X | X | X | X | X | X | X | ||||||
| infection with verocytotoxigenic E. coli | X | X | X | X | X | |||||||||
| leishmaniasis | X | X | X | X | ||||||||||
| leptospirosis | X | X | X | X | X | X | X | |||||||
| low pathogenicity avian influenza | X | X | X | X | X | |||||||||
| mange | X | X | X | X | ||||||||||
| Middle East respiratory syndrome | X | X | X | |||||||||||
| ovine chlamydiosis | X | X | X | X | ||||||||||
| paragonimiasis | X | X | X | |||||||||||
| porcine brucellosis (B. suis) | X | X | X | X | ||||||||||
| porcine cysticercosis | X | X | ||||||||||||
| Q fever | X | X | X | X | ||||||||||
| rabies | X | X | ||||||||||||
| Rift Valley fever | X | X | X | X | X | X | ||||||||
| salmonellosis | X | X | X | X | X | X | X | |||||||
| schistosomiasis | X | X | X | |||||||||||
| swine influenza | X | X | X | |||||||||||
| toxoplasmosis | X | X | X | X | X | X | ||||||||
| trichinellosis | X | X | ||||||||||||
| Tularemia | X | X | X | X | X | X | X | X | X | |||||
| West Nile fever | X | X | X | X | X | |||||||||
LMICs: low- and middle-income countries, including tropical and subtropical regions.
aKey domestic animal hosts can vary by the geographical region and the production system.
bPrimary areas where the disease is endemic in domestic animals.
cClinical signs can vary by agent factors (e.g. species/serotype/strain, infection dose, route of infection, etc.), host factors (e.g. species, age, immune status, etc.) and environmental factors (e.g. production system, husbandry procedures, stress, etc.).
dThe role of certain transmission mode may be underestimated for some diseases due to the paucity of evidence.
In terms of geographical distribution, certain enteric diseases (campylobacteriosis, cryptosporidiosis, salmonellosis and infections with verocytotoxigenic E. coli) and foodborne trematodiases (fascioliasis and paragonimiasis), avian and ovine chlamydiosis, mange, Q fever, swine influenza, toxoplasmosis, tularemia and West Nile fever (WNF) widely affect both high- and lower-income countries. Certain diseases, namely anthrax, three livestock brucellosis, bovine and porcine cysticercosis, bovine tuberculosis, cystic echinococcosis, high and low pathogenicity avian influenza (HPAI and LPAI), trichinellosis and rabies, have been successfully controlled or even eliminated in domestic animals in many high-income countries and they present a burden disproportionately to LMICs. Certain diseases are highly dependent on environmental factors, such as temperature, rainfall and presence of wildlife reservoirs or competent vectors, for their maintenance and transmission and they are primarily found in tropical and subtropical regions. These include vector-borne diseases Crimean-Congo haemorrhagic fever (CCHF), leishmaniasis and Rift Valley fever (RVF), as well as water-borne diseases leptospirosis and schistosomiasis. Certain diseases are closely associated with the pattern of animal husbandry. These include MERS that is circulating in dromedary camels in hot arid areas across the Middle East and its neighbours, swine influenza and salmonellosis that are most prevalent in areas of intensive animal farming, porcine cysticercosis and trichinellosis that are common in areas with poor hygiene and free-scavenging or backyard pig production, and cystic echinococcosis that is usually found in communities where grazing animals are reared together with dogs.
4. Discussion
Our study prioritized 32 zoonoses for conducting disease surveillance in domestic animals to prevent human infections. This broad list was used to inform the FAO project on the candidate diseases to be considered for developing animal health surveillance guidelines on endemic zoonoses, and it can have wider implications such as raising global awareness and commitment to tackle these diseases through a One Health approach. We started from an initial list of diseases compiled from WOAH and WHO, which are of significance in terms of animal and/or public health. It is noteworthy that some zoonoses of wildlife origin, such as SARS, Lyme disease, plague and hantavirus infection, were not included in the WOAH List and Manual that mostly concentrate on domestic animals. Hence, these diseases were excluded from our prioritization exercise. Some zoonoses from regional or national databases such as hepatitis E and yersiniosis also have important public health implications, but they were not included in our assessment, presenting a limitation of this study. Nonetheless, our priority diseases list includes all the ‘top 13 zoonoses’ important for poor livestock keepers in LMICs as identified by the International Livestock Research Institute (ILRI) [11], except for hepatitis E and listeriosis. For the latter, food processing environments rather than livestock reservoirs present the major sources of human infections [31]. Our priority list is also broader than the WHO Lists of neglected zoonotic diseases (NZDs) [22] and zoonotic NTDs [21,26], as it includes some relatively high-profile, epidemic-prone diseases such as zoonotic influenza and MERS and some diseases that have worldwide distribution such as salmonellosis and toxoplasmosis. Of note, some regionally important zoonoses such as human African trypanosomiasis and Chagas disease were not prioritized in this study, as they are geographically restricted to a specific region only. Some zoonoses such as HPAI and LPAI, RVF, CCHF, MERS, WNF and leishmaniasis are endemic in some countries but regarded as emerging or re-emerging diseases by others, given their substantial potential of transboundary spread. The endemicity of the prioritized diseases in domestic animals poses a continuous risk to humans living in the local communities, while the public health burden tends to be lower in high-income countries due to better application of sanitary measures and access to health care. Although not assessed by this study, the severity of infection in humans is also relevant when judging the overall risk of a zoonotic pathogen. For example, although human infections caused by Nipah virus are rare, the case fatality rate can exceed 70%, and thus the disease is considered as a priority in Bangladesh where human infections have been reported [32,33].
An important finding but also a challenge of this study was the significant data gaps that exist for most zoonoses in terms of frequencies and adverse impacts in both humans and animals. Many endemic zoonoses are not notifiable and subsequently not recorded in official statistics. Even for a notifiable disease such as rabies, both human and animal cases are considerably underreported in endemic countries, and the global burden of canine rabies could only be estimated through modelling studies [34]. In another example, brucellosis is assumed to be one of the most widespread zoonoses in the world. Yet, two WHO-commissioned studies published in 2012 concluded that it was not possible to accurately determine the global frequency of human brucellosis due to significant data gaps [35,36]. Likewise, studies of brucellosis in ruminants showed that the predicted annual cases based on seroprevalence studies may be 103–106 times higher than the numbers reported to WOAH [11]. This data scarcity largely precludes disease prioritization based on ‘hard’ figures of the disease frequency as we initially attempted. Similarly, we did not include disease impact evaluations as the one conducted by ILRI, given this could have strongly biased the results towards diseases for which such data exist, often because they are of importance in settings where resources have been made available for their study. Ultimately, we applied a qualitative prioritization approach, which is usually preferred to quantitative methods when evidence is highly scarce or of high uncertainty [37]. The criteria used in this study are simple, qualitative and inclusive, enabling diseases to be rapidly and realistically judged with limited available data.
Disease prioritization exercises were mostly performed at a national level and to a lesser extent at a regional level, but rarely at a global scale [28,38]. Prioritization exercises typically take a broadly similar approach, which includes formulating a list of candidate diseases, selecting and weighting the criteria, scoring diseases against the criteria, and creating a ranking based on the scores [37]. Here we used a multi-criteria qualitative approach specifically adapted to meet the purpose of this study and accommodate severe data gaps, which differs from some other commonly used processes in several aspects. Firstly, we did not give weight to the criteria or score the diseases, as the ultimate aim of our study was not to conduct a comprehensive risk ranking but to identify an inclusive list of zoonoses where surveillance should be targeted in domestic animals to prevent human infections. The limited number of criteria and the simplified process are easy to understand, improving the transparency and reproducibility of the study. Secondly, our prioritization exercise did not involve external expertise consultation due to time and resource constraints, and instead, we relied on literature of the leading international health organizations to mitigate biases related to the authors' opinions. Still, our study had a component of subjectivity especially in relation to the assessments of ‘human infections are not rare, and/or have led to epidemics/pandemics’ and ‘human infections often result from infections in domestic animals', for which evidence was sometimes highly scarce and may vary greatly between regions and over time. It is anticipated that more evidence will emerge through enhanced surveillance and research, and the disease prioritization will need to be updated. Thirdly, our prioritization process did not invite direct input from different One Health sectors through a participatory approach, as done by the US CDC One Health Zoonotic Disease Prioritization (OHZDP) process [39]. While the latter has additional benefits of strengthening the results’ ownership of each sector and facilitating multi-sectoral collaboration, it is primarily applied to identify priorities at the subnational, national [40,41] or small-regional level [42].
For all the prioritized 32 diseases, efficient surveillance in domestic animals is critical for understanding the burden of the diseases, for timely detection and control of the disease before further spread in animal populations, and for providing sentinel warnings to humans. For instance, experience from Kenya showed that enhanced syndromic surveillance of RVF in livestock can serve as an effective early warning for epidemics in livestock and spillover to humans [43]. Surveillance data of good quality are also essential to informing effective disease control programmes in animals. In the example of brucellosis control in livestock, when the disease prevalence is high, control relies on vaccination. As prevalence decreases, test-and-removal of seropositive adults can be considered [44,45]. In this respect, the detection of infected herds or flocks and assessment of the disease prevalence are crucial. However, several important challenges exist for the surveillance in LMICs, in addition to the infrastructure constraints. Firstly, most of the prioritized zoonoses are associated with asymptomatic or non-specific clinical presentations in animal hosts, making their identification difficult without laboratory confirmatory testing. This often presents a significant challenge to LMICs where diagnostic tools are not always accessible [46]. Secondly, for zoonoses that do not cause obvious clinical signs in animals, it can be difficult to engage agricultural stakeholders in animal health surveillance and interventions solely to benefit public health. Thirdly, the majority of prioritized diseases involve multiple animal hosts (including wildlife) and/or modes of zoonotic transmission, presenting additional challenges to quantifying the contribution of each host or mode to public health risk and prioritizing control activities [47]. Lastly, surveillance programmes mostly operate separately in human and animal health sectors, and surveillance in animals is under-resourced even more than surveillance in humans, especially in LMICs [48]. As a consequence, zoonotic agents are often not diagnosed until human outbreaks have been observed, which subsequently leads to disease investigation and detection in animals rather than the reverse [48,49].
To address the above challenges, practical recommendations to improve the efficiency and cost-effectiveness of surveillance are highly desirable. However, in contrast to high-profile diseases, literature on the surveillance of endemic zoonoses is limited [50]; and when available, such literature is frequently developed for resource-rich contexts or considers animal health surveillance in silos. This presents a significant gap that could be addressed by surveillance guidelines adapted to LMIC settings, where multiple endemic zoonoses are co-circulating and often share common factors relating to surveillance. As such, surveillance systems that are programmed to detect multiple pathogens would be a promising approach. For example, surveillance based on clinical indicators (i.e. syndromic surveillance) such as stormy abortion, excess mortalities in young animals or neurological signs can increase the sensitivity and timeliness of disease detection [50]. For diseases that cause subclinical infections in livestock as in the case of bovine tuberculosis, cysticercosis, echinococcosis, fascioliasis and trichinellosis, abattoir surveillance can be a highly cost-effective way to collect data on multiple diseases concurrently while preventing zoonotic transmissions through the food chain [51]. Facilitated by the advancement in molecular technologies and diagnostics, laboratory services can be equipped and expanded for multi-disease testing, bringing opportunities to improve cost-saving and the detection of poorly-funded diseases [52]. Moreover, the One Health surveillance integrates data collection, analysis and sharing across multiple sectors, which can improve the early detection of and response to zoonotic disease outbreaks, provide more accurate estimates of the disease burden, and reduce duplicated efforts and investments [53]. As an example, the Integrated Bite Case Management (IBCM) approach directly links animal health and public health sectors in the reporting and investigation of animal bite cases, and it has been demonstrated to significantly improve rabies case detection and the administration and cost-effectiveness of post-exposure prophylaxis [54,55]. The FAO-WOAH-WHO Tripartite Zoonoses Guide (TZG) and related operational tools are designed to support countries to build capacities for implementing the multi-sectoral, One Health approach to tackle zoonoses [56]. Thanks to modern information technologies and the penetration of mobile phones, rural communities where most endemic zoonoses occur could be empowered to actively participate in disease surveillance and reporting [57,58]. Digitalization of surveillance data further provides ground for data integration and interoperability across different sectors and databases, enabling more efficient data management and utilization [53]. The aforementioned approaches and developments are recommended to be considered by the surveillance guidelines for endemic zoonoses in LMICs.
The coronavirus disease 2019 (COVID-19) pandemic has further spurred global interests and investments in the surveillance of emerging zoonoses with pandemic potential. Hotspots for emerging zoonoses are predicted to be mostly concentrated in tropical and subtropical regions, which largely coincide with areas of weakest disease surveillance capacity [8,59]. Surveillance of newly emerged or yet unknown zoonoses is normally challenged by the lack of a clear case definition and laboratory diagnostic tools, which implies the need for approaches that are more comprehensive than those for endemic zoonoses [5,48]. In addition, it does not bring tangible benefits for the immediate health and development concerns in LMICs, which usually face a greater threat from endemic and neglected diseases and are under-resourced for disease surveillance and response [4,7]. By comparison, surveillance of endemic zoonoses can be regarded as low-hanging fruit given tools for disease detection are often available for both humans and animals [5,25]. It can also generate immediate benefits to the local communities and thus is more likely to be sustainable [4]. Capacity building for the surveillance and control of endemic zoonoses would not only mitigate the risk of endemic zoonoses in its own right, but meanwhile also strengthen the core capacity to detect and respond to emerging or exotic disease threats and future pandemics.
5. Conclusion
We prioritized 32 endemic zoonoses at a global scale for which disease surveillance in domestic animals is important to protect public health. This broad list would contribute to increasing awareness and commitment to tackle these diseases through a One Health approach. The severe data gaps about the disease frequency and burden encountered in this prioritization exercise once again highlight the need for improved surveillance. Given the characteristics of endemic zoonoses, the multi-disease, multi-sectoral and digital surveillance approaches are recommended to improve the timeliness, accuracy, depth and cost-effectiveness of data collection. Our study will support the overall capacity building for zoonoses surveillance and response, protect livestock production and farmers' livelihoods, and contribute to poverty alleviation, the global health security agenda and the UN SDGs.
Glossary
- Zoonosis (plural zoonoses)
a disease, infection or infestation naturally transmissible from vertebrate animals to humans.
- Endemic zoonosis
a zoonosis that is present constantly in a given geographical area or population where conditions for their maintenance or spread exist. Examples include brucellosis (Brucella abortus, B. melitensis and B. suis), leptospirosis (Leptospira spp.) and bovine tuberculosis (Mycobacterium bovis) in some parts of the world.
- Emerging zoonosis
a zoonosis that is either newly recognized, newly introduced or newly evolved, or has existed previously but rapidly increased in incidence or expanded in the geographical, host or vector range. Examples include Middle East respiratory syndrome (MERS), avian influenza (H5N1 and H7N9), Nipah virus infection and Ebola virus disease.
- Re-emerging zoonosis
a zoonosis that was previously under control or even nearing elimination or eradication but has a resurgence. Examples include the re-emergence of trichinellosis in southeastern Europe in the 1990s as a result of political and economic changes, and the re-emergence of schistosomiasis in Sichuan, China in the early 2000s as a result of environmental and socioeconomic changes.
- Epidemic
the occurrence of disease in a population with a frequency that clearly exceeds the normally expected level for a given area and season. Examples include periodic Rift Valley fever (RVF) epidemics in African countries associated with flooding, and seasonal West Nile fever (WNF) epidemics in North America and Europe associated with increased numbers of mosquitoes.
- Pandemic
an epidemic that occurs across international boundaries or worldwide, and affects large populations. Examples include the 2009 H1N1 influenza virus pandemic and the coronavirus disease 2019 (COVID-19) pandemic.
- Neglected tropical diseases (NTDs)
are, as defined by the World Health Organization (WHO), a diverse group of 20 conditions that are mainly prevalent in tropical and subtropical areas, where they mostly affect impoverished communities and generate significant health burdens and losses. Of these, 11 diseases are recognized as zoonotic NTDs by the WHO One Health Companion Document to the WHO NTDs Roadmap 2021–2030, including Chagas disease, dracunculiasis (Guinea-worm disease), echinococcosis, foodborne trematodiases, human African trypanosomiasis (sleeping sickness), zoonotic leishmaniasis, rabies, scabies and other ectoparasitoses, schistosomiasis, snakebite envenoming, and taeniasis/cysticercosis.
- Domestic animals
are animals that have been selectively bred and genetically adapted over generations to live alongside humans, including food-producing animals (cattle, buffalo, sheep, goat, pig, poultry, rabbit, etc.), companion animals (dog, cat, etc.) and working animals (equid, camel, etc.).
- Reservoir
the host or habitat in which an infectious agent normally lives and multiplies and from which it can be transmitted. The reservoir can be single or multiple species of living organisms or inanimate matter (soil, water, etc.). For example, wild aquatic birds are the natural reservoirs of avian influenza viruses and soil is the natural reservoir of anthrax spores.
- Amplifier host
a host in which infectious agents multiply rapidly to high levels, providing an important source of infection to other susceptible hosts. For example, pigs serve as the amplifier hosts for Japanese encephalitis virus.
- Intermediate host
a host that harbours the pathogen before transmitting it to the final hosts. In parasitology, it is the host that harbours asexual forms of a parasite. For example, pigs act as the intermediate hosts for Taenia solium and cattle are the intermediate hosts for Taenia saginata.
- Definitive host
a host in which the sexual maturation of a parasite occurs. For example, humans are the definitive hosts for T. solium and T. saginata.
- Vector
an invertebrate carrier that transports an infectious agent from an infected individual or its wastes to a susceptible individual or its food or immediate surroundings. The organism may or may not pass through a developmental cycle within the vector. For example, mosquitoes are the vectors for the transmission of RVF virus and West Nile virus.
Acknowledgements
We thank Ahmed ElIdrissi, Fairouz Larfaoui, Orr Rozov, Sean Shadomy, Jeffrey Lejeune, Ihab ElMasry, Madhur Dhingra, Melissa Mclaws, Emma Gardner, Giuliano Cecchi, Mo Salman, Usman Zaheer, Zelalem Tadesse and Keith Sumption for the valuable discussions on this topic and/or comments to this manuscript. We also acknowledge Chang Cai for her assistance in the figure design.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
The data are provided in the electronic supplementary material [30].
Authors' contributions
Y.Q.: conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, validation, writing—original draft; J.G.: conceptualization, data curation, formal analysis, methodology, validation, writing—review and editing; J.P.W.: conceptualization, data curation, formal analysis, methodology, validation, writing—review and editing; I.M.: data curation, formal analysis, methodology, validation, writing—review and editing; N.H.: validation, writing—review and editing; J.A.D.: validation, writing—review and editing; J.S.: conceptualization, funding acquisition, project administration, validation, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was supported by the FAO regular fund for the Priority Programme Area on One Health (Grant Number GF.CJWZD.RA50201080000).
References
- 1.Woolhouse ME, Gowtage-Sequeria S. 2005. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 11, 1842-1847. ( 10.3201/eid1112.050997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor LH, Latham SM, Woolhouse ME. 2001. Risk factors for human disease emergence. Phil. Trans. R. Soc. B 356, 983-989. ( 10.1098/rstb.2001.0888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith KF, Goldberg M, Rosenthal S, Carlson L, Chen J, Chen C, Ramachandran S. 2014. Global rise in human infectious disease outbreaks. J. R. Soc. Interface 11, 20140950. ( 10.1098/rsif.2014.0950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halliday J, et al. 2012. Bringing together emerging and endemic zoonoses surveillance: shared challenges and a common solution. Phil. Trans. R. Soc. B 367, 2872-2880. ( 10.1098/rstb.2011.0362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevenson M, Halpin K, Heuer C. 2021. Emerging and endemic zoonotic diseases: surveillance and diagnostics. Rev. Sci. Tech. 40, 119-129. ( 10.20506/rst.40.1.3212) [DOI] [PubMed] [Google Scholar]
- 6.FAO, UNEP, WHO, WOAH. 2022. One Health Joint Plan of Action (2022–2026). Working together for the health of humans, animals, plants and the environment. ( 10.4060/cc2289en) [DOI]
- 7.Cleaveland S, et al. 2017. One Health contributions towards more effective and equitable approaches to health in low- and middle-income countries. Phil. Trans. R. Soc. B 372, 20160168. ( 10.1098/rstb.2016.0168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990-993. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meslin FX, Stohr K, Heymann D. 2000. Public health implications of emerging zoonoses. Rev. Sci. Tech. 19, 310-317. ( 10.20506/rst.19.1.1214) [DOI] [PubMed] [Google Scholar]
- 10.Maudlin I, Eisler MC, Welburn SC. 2009. Neglected and endemic zoonoses. Phil. Trans. R. Soc. B 364, 2777-2787. ( 10.1098/rstb.2009.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grace D, et al. 2012. Mapping of poverty and likely zoonoses hotspots. (See https://cgspace.cgiar.org/bitstream/handle/10568/21161/ZooMap_July2012_final.pdf.)
- 12.Molyneux D, et al. 2011. Zoonoses and marginalised infectious diseases of poverty: where do we stand? Parasite Vectors 4, 106. ( 10.1186/1756-3305-4-106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornton PK. 2010. Livestock production: recent trends, future prospects. Phil. Trans. R. Soc. B 365, 2853-2867. ( 10.1098/rstb.2010.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FAO. 2018. World livestock: transforming the livestock sector through the Sustainable Development Goals. Rome, Italy: Food and Agriculture Organization of the United Nations. (See https://www.fao.org/3/CA1201EN/ca1201en.pdf.) [Google Scholar]
- 15.Worsley-Tonks KEL, et al. 2022. Strengthening global health security by improving disease surveillance in remote rural areas of low-income and middle-income countries. Lancet Glob. Health 10, e579-e584. ( 10.1016/S2214-109X(22)00031-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gostin LO, Halabi SF, Klock KA. 2021. An international agreement on pandemic prevention and preparedness. JAMA 326, 1257-1258. ( 10.1001/jama.2021.16104) [DOI] [PubMed] [Google Scholar]
- 17.Boyce MR, Sorrell EM, Standley CJ. 2023. An early analysis of the World Bank's Pandemic Fund: a new fund for pandemic prevention, preparedness and response. BMJ Glob. Health 8, e011172. ( 10.1136/bmjgh-2022-011172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okello A, Welburn S, Smith J. 2014. Crossing institutional boundaries: mapping the policy process for improved control of endemic and neglected zoonoses in sub-Saharan Africa. Health Policy Plan 30, 804-812. ( 10.1093/heapol/czu059) [DOI] [PubMed] [Google Scholar]
- 19.Johnson I, Hansen A, Bi P. 2018. The challenges of implementing an integrated One Health surveillance system in Australia. Zoonoses Public Health 65, e229-e236. ( 10.1111/zph.12433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K, Brumme ZL. 2012. Operationalizing the One Health approach: the global governance challenges. Health Policy Plan 28, 778-785. ( 10.1093/heapol/czs127) [DOI] [PubMed] [Google Scholar]
- 21.WHO. 2020. Ending the neglect to attain the Sustainable Development Goals: A road map for neglected tropical diseases 2021–2030. Geneva, Switzerland: World Health Organization. (See https://www.who.int/publications/i/item/9789240010352.) [Google Scholar]
- 22.WHO, ICONZ - Integrated control of neglected zoonotic diseases & United Kingdom. Dept for International Development Research in Use. 2011. The control of neglected zoonotic diseases: community based interventions for NZDs prevention and control: report of the third conference organized with ICONZ, DFID-RiU, SOS, EU, TDR and FAO with the participation of ILRI and OIE: 23–24 November 2010, WHO Heaquarters, Geneva, Switzerland. World Health Organization. (See https://apps.who.int/iris/handle/10665/44746.)
- 23.WHO. 2009. Integrated control of neglected zoonotic diseases in Africa: applying the one health concept, report of a joint WHO/EU/ILRI/DBL/FAO/OIE/AU meeting, ILRI headquarters, Nairobi, 13–15 November 2007. World Health Organization. (See https://apps.who.int/iris/handle/10665/69952.)
- 24.WOAH. 2022. Terrestrial Animal Health Code. Chapter 1.2. Criteria for the inclusion of diseases, infections and infestations in the OIE list. (See https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/?id=169&L=1&htmfile=chapitre_criteria_diseases.htm.)
- 25.WOAH. 2022. Manual of diagnostic tests and vaccines for terrestrial animals. (See https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/.)
- 26.WHO. 2021. One Health companion document to the neglected tropical diseases road map 2021–2030. (See https://www.who.int/publications/m/item/one-health-companion-document-to-the-neglected-tropical-diseases-road-map-2021–2030.)
- 27.WOAH. 2022. Terrestrial Animal Health Code. Chapter 1.3. Diseases, infections and infestations listed by the OIE. (See https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/?id=169&L=1&htmfile=chapitre_oie_listed_disease.htm.)
- 28.Ferroglio E, et al. 2022. Literature review on disease ranking tools, their characterisation, and recommendations for the method to be used by EFSA. EFSA Supporting Publications 19, 7578E. ( 10.2903/sp.efsa.2022.EN-7578) [DOI] [Google Scholar]
- 29.WOAH. 2022. Terrestrial Animal Health Code. (See https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/.)
- 30.Qiu Y, Guitian J, Webster JP, Musallam I, Haider N, Drewe JA, Song J. 2023. Global prioritization of endemic zoonotic diseases for conducting surveillance in domestic animals to protect public health. Figshare. ( 10.6084/m9.figshare.c.6753782) [DOI] [PMC free article] [PubMed]
- 31.Ferreira V, Wiedmann M, Teixeira P, Stasiewicz MJ. 2014. Listeria monocytogenes persistence in food-associated environments: epidemiology, strain characteristics, and implications for public health. J. Food Prot. 77, 150-170. ( 10.4315/0362-028X.JFP-13-150) [DOI] [PubMed] [Google Scholar]
- 32.Rahman M, Chakraborty A. 2012. Nipah virus outbreaks in Bangladesh: a deadly infectious disease. WHO South East Asia J. Public Health 1, 208-212. ( 10.4103/2224-3151.206933) [DOI] [PubMed] [Google Scholar]
- 33.CDC. 2017. One Health zoonotic disease prioritization for multisectoral engagement in Bangladesh. (See https://www.cdc.gov/onehealth/pdfs/bangladesh-508.pdf.)
- 34.Taylor LH, Hampson K, Fahrion A, Abela-Ridder B, Nel LH. 2017. Difficulties in estimating the human burden of canine rabies. Acta Trop. 165, 133-140. ( 10.1016/j.actatropica.2015.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dean AS, Crump L, Greter H, Schelling E, Zinsstag J. 2012. Global burden of human brucellosis: a systematic review of disease frequency. Plos. Negl. Trop. Dis. 6, e1865. ( 10.1371/journal.pntd.0001865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dean AS, Crump L, Greter H, Hattendorf J, Schelling E, Zinsstag J. 2012. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. Plos. Negl. Trop. Dis. 6, e1929. ( 10.1371/journal.pntd.0001929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Brien EC, Taft R, Geary K, Ciotti M, Suk JE. 2016. Best practices in ranking communicable disease threats: a literature review, 2015. Euro Surveill. 21, 30212. ( 10.2807/1560-7917.ES.2016.21.17.30212) [DOI] [PubMed] [Google Scholar]
- 38.Mehand MS, Millett P, Al-Shorbaji F, Roth C, Kieny MP, Murgue B. 2018. World Health Organization methodology to prioritize emerging infectious diseases in need of research and development. Emerg. Infect. Dis. 24, e171427. ( 10.3201/eid2409.171427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rist CL, Arriola CS, Rubin C. 2014. Prioritizing zoonoses: a proposed one health tool for collaborative decision-making. PLoS ONE 9, e109986. ( 10.1371/journal.pone.0109986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salyer SJ, Silver R, Simone K, Barton Behravesh C. 2017. Prioritizing zoonoses for global health capacity building—themes from One Health zoonotic disease workshops in 7 countries, 2014–2016. Emerg. Infect. Dis. 23, S55. ( 10.3201/eid2313.170418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CDC. 2022. Completed OHZDP workshops. (See https://www.cdc.gov/onehealth/what-we-do/zoonotic-disease-prioritization/completed-workshops.html.)
- 42.Goryoka GW, Lokossou VK, Varela K, Oussayef N, Kofi B, Iwar V, Behravesh CB. 2021. Prioritizing zoonotic diseases using a multisectoral, One Health approach for the Economic Community of West African States (ECOWAS). One Health Outlook 3, 24. ( 10.1186/s42522-021-00055-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oyas H, et al. 2018. Enhanced surveillance for Rift Valley Fever in livestock during El Nino rains and threat of RVF outbreak, Kenya, 2015–2016. PLoS Negl. Trop. Dis. 12, e0006353. ( 10.1371/journal.pntd.0006353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blasco JM, Moreno E, Moriyon I. 2021. Brucellosis vaccines and vaccine candidates. In Veterinary vaccines: principles and applications (eds Metwally S, Viljoen G, Idrissi AE), pp. 295-316. FAO, Rome: Wiley Blackwell. (See https://www.fao.org/documents/card/en?details=cc2031en%2f.) [Google Scholar]
- 45.Blasco JM, Molina-Flores B. 2011. Control and eradication of Brucella melitensis infection in sheep and goats. Vet. Clin. North Am. Food Anim. Pract. 27, 95-104. ( 10.1016/j.cvfa.2010.10.003) [DOI] [PubMed] [Google Scholar]
- 46.Halliday JE, Allan KJ, Ekwem D, Cleaveland S, Kazwala RR, Crump JA. 2015. Endemic zoonoses in the tropics: a public health problem hiding in plain sight. Vet. Rec. 176, 220-225. ( 10.1136/vr.h798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Webster JP, Borlase A, Rudge JW. 2017. Who acquires infection from whom and how? Disentangling multi-host and multi-mode transmission dynamics in the ‘elimination’ era. Phil. Trans. R. Soc. B 372, 20160091. ( 10.1098/rstb.2016.0091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keusch GT, Pappaioanou M, Gonzalez MC, Scott KA, Tsai P. 2009. National Research Council (US) Committee on achieving sustainable global capacity for surveillance and response to emerging diseases of zoonotic origin. In Sustaining global surveillance and response to emerging zoonotic diseases, pp. 545-551. Washington, DC: National Academies Press. ( 10.17226/12625) [DOI] [PubMed] [Google Scholar]
- 49.Bisson IA, Ssebide BJ, Marra PP. 2015. Early detection of emerging zoonotic diseases with animal morbidity and mortality monitoring. Ecohealth 12, 98-103. ( 10.1007/s10393-014-0988-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hattendorf J, Bardosh KL, Zinsstag J. 2017. One Health and its practical implications for surveillance of endemic zoonotic diseases in resource limited settings. Acta Trop 165, 268-273. ( 10.1016/j.actatropica.2016.10.009) [DOI] [PubMed] [Google Scholar]
- 51.Falzon LC, Ogola JG, Odinga CO, Naboyshchikov L, Fèvre EM, Berezowski J. 2021. Electronic data collection to enhance disease surveillance at the slaughterhouse in a smallholder production system. Sci Rep 11, 19447. ( 10.1038/s41598-021-98495-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunsperger E, et al. 2019. Building laboratory capacity to detect and characterize pathogens of public and global health security concern in Kenya. BMC Public Health 19, 477. ( 10.1186/s12889-019-6770-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mremi IR, George J, Rumisha SF, Sindato C, Kimera SI, Mboera LEG. 2021. Twenty years of integrated disease surveillance and response in Sub-Saharan Africa: challenges and opportunities for effective management of infectious disease epidemics. One Health Outlook 3, 22. ( 10.1186/s42522-021-00052-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lushasi K, et al. 2020. One Health in practice: using integrated bite case management to increase detection of rabid animals in Tanzania. Front Public Health 8, 13. ( 10.3389/fpubh.2020.00013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Undurraga EA, Meltzer MI, Tran CH, Atkins CY, Etheart MD, Millien MF, Adrien P, Wallace RM. 2017. Cost-effectiveness evaluation of a novel integrated bite case management program for the control of human rabies, Haiti 2014–2015. Am. J. Trop. Med. Hyg. 96, 1307-1317. ( 10.4269/ajtmh.16-0785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.WHO, FAO, WOAH. 2019. Taking a multisectoral, One Health approach: A Tripartite guide to addressing zoonotic diseases in countries. (See https://www.fao.org/documents/card/fr/c/CA2942EN/.)
- 57.Thumbi SM, Njenga MK, Otiang E, Otieno L, Munyua P, Eichler S, Widdowson M-A, McElwain TF, Palmer GH. 2019. Mobile phone-based surveillance for animal disease in rural communities: implications for detection of zoonoses spillover. Phil. Trans. R. Soc. B 374, 20190020. ( 10.1098/rstb.2019.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yano T, et al. 2018. A participatory system for preventing pandemics of animal origins: pilot study of the participatory One Health disease detection (PODD) system. JMIR Public Health Surveill. 4, e25. ( 10.2196/publichealth.7375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allen T, Murray KA, Zambrana-Torrelio C, Morse SS, Rondinini C, Di Marco M, Breit N, Olival KJ, Daszak P. 2017. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 8, 1124. ( 10.1038/s41467-017-00923-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Qiu Y, Guitian J, Webster JP, Musallam I, Haider N, Drewe JA, Song J. 2023. Global prioritization of endemic zoonotic diseases for conducting surveillance in domestic animals to protect public health. Figshare. ( 10.6084/m9.figshare.c.6753782) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data are provided in the electronic supplementary material [30].