Abstract
INTRODUCTION:
High-performing biomarkers measuring the vascular contributions to cognitive impairment and dementia are lacking.
METHODS:
Using a multi-site observational cohort study design, we examined the diagnostic accuracy of plasma placental growth factor (PlGF) within the MarkVCID Consortium (n=335; CDR 0-1). Subjects underwent clinical evaluation, cognitive testing, MRI, and blood sampling as defined by Consortium protocols.
RESULTS:
In the prospective population of 335 subjects (72.2±7.8 years of age, 49.3% female), plasma PlGF (pg/mL) shows an ordinal OR of 1.16 [1.07-1.25; p=0.0003] for increasing Fazekas score and ordinal OR of 1.22 [1.14-1.32; p<0.0001] for functional cognitive impairment measured by the Clinical Dementia Rating Scale. We achieved the primary study outcome of a site-independent association of plasma PlGF (pg/mL) with white matter injury and cognitive impairment in two of three study cohorts. Secondary outcomes using the full MarkVCID cohort demonstrated plasma PlGF can significantly discriminate individuals with Fazekas ≥ 2 and CDR=0.5 (AUC = 0.74) and CDR=1 (AUC = 0.89) from individuals with CDR=0.
DISCUSSION:
Plasma PlGF measured by standardized immunoassay functions as a stable, reliable, diagnostic biomarker for cognitive impairment associated with substantial white matter burden.
Keywords: placental growth factor, diagnosis, biomarker, vascular cognitive impairment
Introduction
Cerebral small vessel disease is a common, progressive endotheliopathy that is now recognized to be a significant driver of cognitive impairment and dementia. Chronic injury to cerebral arterioles, capillaries, and venules ultimately results in a myriad cascade of blood-brain barrier leakage, cortical and subcortical microinfarction, and delayed neurodegeneration [1, 2]. These vascular contributions to cognitive impairment and dementia (VCID) develop in parallel with traditional pathologies associated with Alzheimer’s disease, confounding the ability to identify causative pathologies in individual cases. The identification of reproducible biomarkers that precisely characterize the presence and progression of VCID, particularly in its early stages, are crucial to: (1) risk stratify patients based on the predominant disease pathology, (2) demonstrate target engagement or mechanism of action for candidate interventions, and (3) monitor disease progression [1, 3].
Cerebral small vessel disease constitutes a major cerebrovascular pathology leading to VCID [4] and involves progressive dysfunction of brain endothelial cells and altered signaling in mural cells [5, 6]. Because chronic cerebral hypoperfusion is a robust stimulus for angiogenesis, failure of pro-angiogenic signaling may be a central feature of aging and thus VCID [7]. Placental growth factor (PlGF), a member of the VEGF (vascular endothelial growth factor) family that signals through the Flt-1 receptor, is known to drive increased tissue vascularization and regulate vascular permeability [8, 9]. While its action within the brain is not fully understood, it is required for proper development of the cerebral circulation, is responsive to cerebral ischemia [10], and therefore may serve as an excellent biomarker of endotheliopathy.
The MarkVCID Consortium was established in 2016 with the goal of developing and validating fluid- and imaging-based biomarkers for cerebral small vessel disease encapsulated by VCID [11, 12]. As such, the Consortium identified 11 candidate biomarker “kits” alongside a range of harmonized procedures and protocols that defined parameters for participant enrollment, clinical and cognitive evaluation, collecting and handling of fluid samples, acquisition of neuroimaging, and pre-specified statistical analyses in alignment with the STARDdem Initiative [13]. Of these biomarker kits, fluid-based biomarkers could be particularly useful for assessing VCID because of their relative ease of use both during the prodromal and progressive phases of disease [14]. The MarkVCID Plasma Endothelial Signaling Kit includes PlGF as well as other angiogenic signaling molecules and is proposed as a susceptibility/risk biomarker for VCID. While longitudinal data are needed to determine the ability of this biomarker to measure VCID risk continues to be collected, here we present initial findings focused on the diagnostic value of PlGF and demonstrate its performance in a multi-site study of reliability and validity as a robust diagnostic biomarker for VCID [15].
Methods
Design and Oversight
The Plasma Endothelial Signaling Kit was selected for biomarker validation by the MarkVCID Consortium Steering Committee. Pre-specified hypothesis, study design, target per-site enrollment, and analytic plan for the evaluation of the kit as a susceptibility biomarker were detailed and approved by the MarkVCID Fluid Biomarker Subcommittee. After data collection, the plan to examine plasma PlGF as a diagnostic biomarker was presented and approved by the Fluid Biomarker Subcommittee. The MarkVCID Coordinating Center provided oversight of the analytic performance of the Plasma Endothelial Signaling Kit [11].
Participants
The MarkVCID consortium was designed to validate candidate biomarkers for cerebral small vessel diseases across multiple sites and multiple types of participant cohorts ranging across cognitive statuses (normal, mild cognitive impairment, and dementia), vascular risk factor exposures, race/ethnic groups, and recruitment sources (clinic, community, or population-based). The inclusion and exclusion criteria for each subject cohort have been previously reported [11]. Across the five participating sites outlined in Figure 1, aggregate inclusion criteria were subjects >40 years old with varying degrees of cognitive impairment including cognitively normal to demented individuals with or without vascular risk factors. All participating site protocols, including the MarkVCID template consent language, were approved by site IRBs. Participants were enrolled between July 2016 and December 2020.
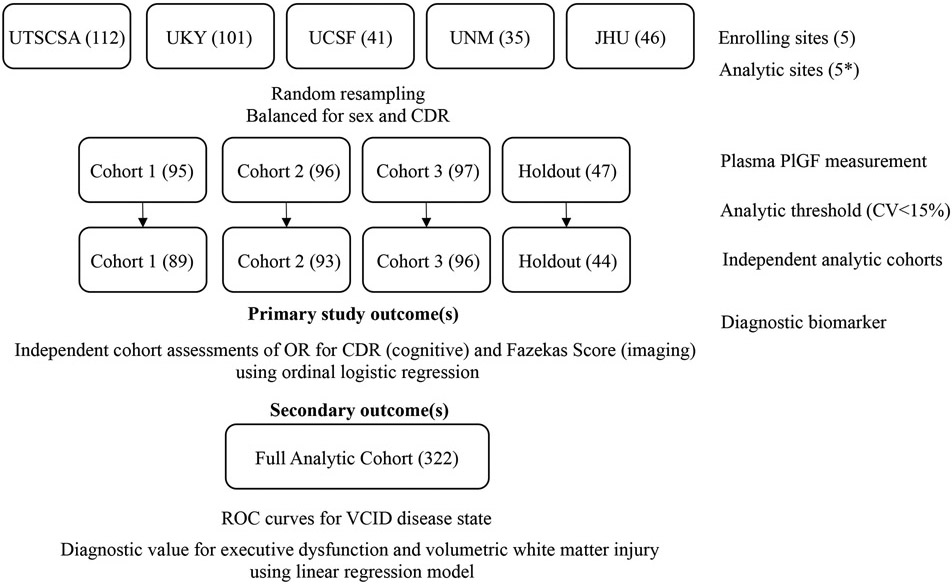
Figure 1. MarkVCID Endothelial Signaling Kit Study Design.
Consortium sites with enrolled subjects for endothelial signaling biomarker kit development. Random resampling resulted in three independent cohorts with a holdout cohort for validation. After measurement of plasma PlGF (pg/mL), intent to analyze cohorts were reduced if sample CoV>15%. Independent analytic cohorts were used to determine primary and secondary cognitive and imaging study outcomes. UTSCSA = University of Texas Health Science Center at San Antonio; UKY = University of Kentucky; UCSF = University of California San Francisco; UNM = University of New Mexico; JHU = Johns Hopkins University. * Analytic site information available in eTable 2. Parentheses = subject number.
Cognitive Evaluation and Executive Dysfunction Measurement
All subjects participated in completion of the Uniform Data Set version 3 (UDS3) [16]. Clinical Dementia Rating (CDR) Scale scores were used as the reference standard for cognitive impairment based on its utility as a validated functional measure of cognitive dysfunction [17]. CDR scores were determined using established informant-based assessment by trained raters at the time of the cognitive testing. A composite measure of executive function was generated from UDS3 responses using item-response theory as previously reported [18].
MRI Imaging
All participating subjects underwent a 3T MRI scan on either Siemens Prisma or Phillips Achieva scanners using previously reported acquisition protocols [19]. The inter-scanner reproducibility and inter-rater reliability of measurement have been previously reported [20]. Fazekas scoring [21] was performed on all subjects by trained raters and selected as the reference standard for white matter injury given its scoring validity. Volumetric measurement of white matter intensities was performed as described [22].
Blood Sample Collection and Analytic Sites
In all subjects, blood sample collection was performed within 3 months of cognitive and imaging assessments. Blood collection followed standardized consortium protocol available online. Briefly, plasma samples were collected in EDTA collection tubes (BD Biosciences #366643), processed for plasma collection within 2 hrs of collection, aliquoted into barcoded cryovials (DWK #W985874), and frozen. Frozen aliquots were shipped following consortium protocol and only thawed for analysis. Plasma sample analysis was performed at five independent analytic labs after completion of a 10 biosample protocol training validation step with a benchmark of 90% technical CoV<15%. Test-retest sampling was performed in a subset of subjects at three enrollment sites. Fasting and non-fasting samples were generated by the MarkVCID Coordinating Center.
Plasma PlGF Measurement
Plasma Placental Growth Factor (PlGF) levels were measured in technical triplicate using the MesoScale Discovery V-PLEX Angiogenesis Panel 1 Human Kit (K15190D). In this V-PLEX kit, the lower limit of detection of PlGF is 0.21 pg/mL. Manufacturer protocol was followed with two modifications including a pre-analytic centrifugation step at 2000 rpm for 3 min and an overnight incubation during the initial analyte-spot binding step. Mean signal values were measured on MESO Quickplex SQ 120 or MESO Sector 600 device and converted to pg/mL using a per-plate standard curve. During reliability testing, cross validation of plasma sample performance at different analytic sites was measured as well as stability over time and the effect of fasting. Additional protocol details are available at www.partners.markvcid.org and in the supplemental information.
Data Handling
During both the reliability and biologic validation phases of the biomarker kit testing, run dates, V-PLEX Kit lot numbers, protocol deviations, and raw data output were collected by the MarkVCID Coordinating Center using an electronic web interface. Raw data files were transferred to the central analytic lab (UCLA) for quality control review and batch analysis. Samples with a coefficient of variance (CoV) >15% were excluded from analysis. Demographic, imaging and cognitive variables for enrolled subjects were managed by the Coordinating Center and distributed for the final statistical analysis after the collection of all PlGF values. Subjects with missing data for volumetric white matter hyperintensity measurement or UDS3-EF scoring were excluded from analysis.
Statistical Analysis
To determine assay reliability across analytic labs, the intra-class correlation coefficient value was determined using the C-1 consistency method [23]. Power estimations suggested an n=40 would be sufficient to determine an ICC ≥0.82. Due to COVID-19, enrollment was below the pre-specified enrolling site cohort n of 96. Therefore, we assessed reliability through stratified random resampling of the full cohort into three equal cohorts balanced for sex and CDR with the residual subjects used as a holdout cohort. Association of unadjusted plasma PlGF values (pg/mL) with Fazekas and CDR in each cohort was determined using an age- and sex-adjusted ordinal logistic regression model. Association of unadjusted plasma PlGF (pg/mL) values with log-adjusted volumetric white matter hyperintensities and UDS3-EF scores were determined using an age- and sex-adjusted linear regression model. To determine diagnostic accuracy, ROC curves were generated using multiple logistic regression analysis after combining subject data into distinct disease categories: normal (CDR = 0, Fazekas <2), white matter injury only (CDR = 0, Fazekas ≥2), or vascular cognitive impairment (CDR > 0, Fazekas ≥2). Similar categories were generated using tertiles of UDS3-EF and log-adjusted volumetric white matter hyperintensity measurement. Resampling performed using pandas 1.3.4 and sci-kit learn 0.24.2 libraries in Python. Other analyses were performed using Matlab, Prism, and Stata software packages.
Results
Characteristics of Plasma PlGF as a VCID Biomarker
A reliable diagnostic biomarker for VCID will have several core features including analytic reliability, temporal stability, and minimal contribution of confounding effects such as fasting status. Analytic reliability of plasma PlGF was established using a 40 subject reliability cohort within MarkVCID that included 10 subjects derived from each of four analytic sites. Consistent with the intended enrollment demographics of VCID subjects, this cohort is enriched for white matter injury (median Fazekas = 3) and mild cognitive impairment (median CDRsum = 1.5) (eTable 1). Plasma PlGF values in four separate plasma specimen aliquots from each subject were measured at each analytic lab. Mean coefficient of variance (CoV) across sites was 6.63±3.99% (eTable 2). Within the reliability cohort, plasma PlGF values showed excellent concordance across sites (eFigure 1), confirmed using an intraclass correlation coefficient (ICC) analysis (r=0.83, p=0.002). Within subject, test-retest plasma PlGF values were stable over time (eFigure 2). ICC analysis of plasma PlGF values over time demonstrated stability across both 2 blood draws (r=0.93, p=4.3x10−6) and 3 blood draws (r=0.91, p=9.7x10−4). Similarly, no significant effect of fasting on plasma PlGF values was observed (fasting 2.99±1.05 vs. non-fasting 2.93±0.82, n=10, p=0.88) (eFigure 3). No subjects experienced any adverse events in performance of the blood sampling, cognitive testing, or imaging.
Plasma PlGF as a Candidate VCID Biomarker
To test the validity of plasma PlGF as a blood-based diagnostic biomarker for VCID, we established a prospective biologic validation cohort of 335 subjects across five independent MarkVCID enrolling sites (Figure 1). Plasma PlGF values were measured within five analytic laboratories and the data transferred to a central analytic lab (UCLA) for quality control analysis. To meet the pre-specified power analysis, random resampling was used to distribute these samples into three independent cohorts of the approximate pre-specified cohort size and confined the remainder to a holdout cohort for post-hoc validation. The demographics of each cohort are provided in Table 1 and are well-balanced as predicted by random resampling. The distribution of analytic measurement site within these cohorts was equally distributed (eFigure 4). Subjects with an unreliable plasma PlGF analytic measurement (CoV>15%) were excluded from subsequent analysis, reducing each cohort by an average of 4.4%. The mean time between plasma sampling and cognitive evaluation was 4.6±34.6 days.
Table 1.
Baseline demographics and features of each MarkVCID Cohort
| Cohort 1 (n=95) | Cohort 2 (n=96) | Cohort 3 (n=97) | Combined Cohort (n=335) | |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Age in years (SD) | 71.7 (8.2) | 72.5 (6.8) | 71.8 (8.5) | 72.2 (7.8) |
| Sex (% female) | 49.5% | 49.0% | 49.5% | 49.3% |
| Vascular Risk Factors | ||||
| Hypertension (%) | 47.4% | 46.9% | 52.6% | 48.7% |
| Diabetes (%) | 17.9% | 27.1% | 17.5% | 21.5% |
| Hyperlipidemia (%) | 49.5% | 53.1% | 63.9% | 53.7% |
| H/o stroke (%) | 8.4% | 8.3% | 7.2% | 7.8% |
| Plasma Biomarker | ||||
| Median plasma PlGF (pg/mL) [IQR] | 8.71 [6.61 – 10.0] | 8.06 [6.92 – 10.8] | 8.46 [7.10 – 10.1] | 8.31 [6.77 – 10.1] |
| Study Outcome Variables | ||||
| Median CDRg (IQR) | 0 (0 - 0.5) | 0 (0 – 0.5) | 0 (0 – 0.5) | 0 (0 – 0.5) |
| Median CDRsum (IQR) | 0.5 (0 – 1) | 0 (0 - 0.5) | 0 (0 – 1) | 0 (0 – 1) |
| Median Fazekas (IQR) | 3 (1 – 3) | 3 (2 – 3) | 2 (1 – 3) | 3 (2 – 3) |
| Mean LogWMH (IQR) | 0.71 (−0.21 - 1.61) | 1.38 (0.57 - 2.28) | 1.20 (0.08 - 2.29) | 1.12 (0.13 - 2.17) |
| Mean UDS3-EF (IQR) | −0.45 (−1.03 - 0.17) | −0.54 (−1.07 - 0.05) | −0.31 (−0.75 - 0.22) | −0.43 (−0.94 - 0.19) |
CDR – Clinical Dementia Rating scale; IQR – Interquartile range; LogWMH – log-adjusted volumetric white matter hyperintensity measurement; PlGF – placental growth factor; UDS3-EF – Uniform Data Set 3 – Executive Function score
The primary study hypothesis was that plasma PlGF could function as a diagnostic biomarker for the detection of cognitive impairment among those with cerebral small vessel disease as measured by white matter injury on MRI. To determine the diagnostic value of plasma PlGF for functional cognitive impairment, we used an age- and sex-adjusted ordinal logistic regression model with the Clinical Dementia Rating (CDR) scale score as the outcome variable. In two of the three primary cohorts after CoV correction, unadjusted plasma PlGF (pg/mL) was significantly associated with functional cognitive impairment as measured by CDR. The third cohort showed a similar directional effect with a marginal p-value (0.068). Similarly, in an age- and sex-adjusted ordinal logistic regression model, plasma PlGF was significantly associated with Fazekas scores in two of three independent analytic cohorts (Table 2).
Table 2.
Ordinal logistic regression models of plasma PlGF as a diagnostic VCID biomarker.
| Outcome Variable |
Cohort 1 (n=89) | Cohort 2 (n=93) | Cohort 3 (n=96) | Combined Cohort (n=322) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary | Beta | OR (CI) |
p | Beta | OR (CI) |
p | Beta | OR (CI) |
p | Beta | OR (CI) |
p |
| Imaging Outcome* (Fazekas) | 0.095 | 1.10 (0.93-1.30) | 0.27 | 0.249 | 1.28 (1.06-1.55) | 0.011 | 0.217 | 1.24 (1.05-1.47) | 0.011 | 0.147 | 1.16 (1.07-1.25) | 0.0003 |
| Cognitive Outcome* (CDR) | 0.162 | 1.18 (0.99-1.40) | 0.068 | 0.231 | 1.26 (1.09 - 1.45) | 0.001 | 0.463 | 1.59 (1.31-1.93) | <0.0001 | 0.202 | 1.22 (1.14-1.32) | <0.0001 |
| Secondary | ||||||||||||
| Imaging Outcome** (vWMH) | 0.048 | 1.78e-4 | 0.057 | 2.50e-3 | 0.172 | 5.93e-5 | 0.104 | 5.42e-15 | ||||
| Cognitive Outcome** (UDS3-EF) | −0.022 | 0.83 | −0.052 | 0.147 | −0.049 | 0.108 | −0.030 | 0.070 | ||||
Age- and sex-adjusted ordinal logistic regression model using unadjusted plasma PlGF values (pg/mL).
Age- and sex-adjusted linear regression model using unadjusted plasma PlGF values (pg/mL).
CDR – Cognitive Dementia Rating Scale; vWMH – volumetric white matter hyperintensity imaging; UDS3-EF – Uniform Data Set 3.0 – Executive Function score
Secondary Outcomes
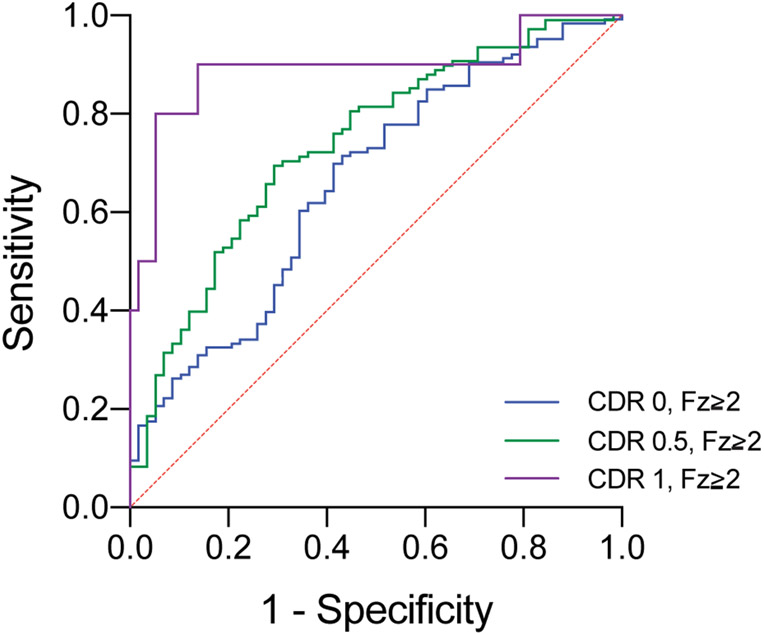
Several secondary study outcomes associated with the diagnostic value of plasma PlGF as a VCID biomarker were assessed. Within the full biologic validation cohort (n=322), an age- and sex-adjusted ordinal logistic regression model also demonstrated a significant association of plasma PlGF (pg/mL) with both CDR [OR = 1.22 (1.14-1.32)] and Fazekas score [OR = 1.16 (1.07-1.25)] (Table 2). To determine the sensitivity and specificity of plasma PlGF to identify relevant VCID disease states, we reclassified subjects within the full biologic cohort into four categories using the ordinal variables associated with our primary hypothesis: cognitively normal with low WMH (CDR=0, Fazekas <2, n=58), cognitively normal with WMH (CDR=0, Fazekas ≥2, n=126), mild cognitive impairment with WMH (CDR=0.5, Fazekas ≥2, n=108), and dementia with WMH (CDR>1, Fazekas≥2, n=10). In this analysis of the full MarkVCID cohort, plasma PlGF demonstrated increasing diagnostic accuracy for all three VCID disease states: WMH only (AUC=0.66±0.04, p=0.005), mild cognitive impairment with WMH (AUC=0.74±0.04, p<0.0001), and dementia with WMH (AUC=0.89±0.07, p<0.0001) (Figure 2).
Figure 2. Diagnostic value of plasma PlGF for VCID disease states.
ROC curves for VCID disease states including WMH only (blue, normal cognition with Fazekas ≥2), mild cognitive impairment with WMH (green, CDR=0.5 with Fazekas ≥2), or dementia with WMH (purple, CDR=1 with Fazekas ≥2).
We also determined the relationship between plasma PlGF and log-adjusted volumetric white matter hyperintensities (logWMH) within each of the three primary independent analytic MarkVCID biologic cohorts as a more quantitative measure of cerebral small vessel disease [24]. Using an age- and sex-adjusted linear regression model, logWMH were significantly associated with plasma PlGF in all three cohorts (Table 2).
The CDR scale is a well-validated clinical tool for staging the degree of cognitive impairment. Since the primary cognitive domains influenced by VCID involve executive dysfunction rather than amnestic predominance [25-28], we utilized the UDS3-EF as a continuous measure of cognitive function. The UDS3-EF score is a recently validated composite score of executive function that is proposed as an emerging cognitive endpoint for clinical trials [18]. We determined the relationship between plasma PlGF and the UDS3-EF within each of the three independent analytic MarkVCID biologic cohorts and the full biologic cohort using an age- and sex-adjusted linear regression model. Within each of the cohorts after CoV correction, plasma PlGF values were directionally associated with worse cognitive performance in the UDS3-EF, though these associations were modest and lacked statistical significance (Table 2).
To further test the diagnostic accuracy of PlGF using these quantitative measures, we reclassified the previously identified VCID disease states based on tertiles of logWMH and UDS3-EF as follows: normal (UDS3-EF 1st tertile, logWMH 1st tertile, n=34), WMH only (UDS3-EF 1st tertile, logWMH 2nd and 3rd tertiles, n=63), mild cognitive impairment with WMH (UDS3-EF 2nd tertile, logWMH 2nd and 3rd tertiles, n=66), dementia with WMH (UDS3-EF 3rd tertile, logWMH 3rd tertile, n=38), and suspected non-vascular cognitive impairment (UDS-EF 2nd and 3rd tertiles, logWMH 1st tertile, n=64) (eFigures 5-6). In this multiple logistic regression analysis, the diagnostic accuracy of plasma PlGF was retained or improved for the defined VCID disease states: WMH only measured by volumetric imaging (AUC=0.73±0.05, p=0.002), mild cognitive impairment with WMH (AUC=0.78±0.05, p<0.0001), and dementia with WMH (AUC=0.85±0.05, p<0.0001) (eFigure 7). In the suspected non-vascular CID group, plasma PlGF lacked diagnostic discriminatory ability when compared to normal (AUC=0.61±0.05, p=0.08). To establish its specificity for detecting cognitive impairment associated with vascular brain injury, we compared the diagnostic potential of plasma PlGF in detection of mild cognitive impairment and dementia with WMH compared to the non-vascular cognitive impairment group. In this analysis, plasma PlGF again demonstrated significant diagnostic discriminatory ability for both mild cognitive impairment with WMH (AUC=0.74±0.04, p<0.0001) and dementia with WMH (AUC=0.84±0.04, p<0.0001) among cognitively impaired individuals (eFigure 8).
These data demonstrate the diagnostic value of continuous measurement of plasma PlGF across the full sensitivity range of the MSD V-Plex assay. However, clinical diagnostic biomarkers are often utilized with a cutoff threshold. Using a plasma PlGF threshold value of 10.1 pg/mL, corresponding to the minimum value in the upper 4th quartile of the full MarkVCID cohort (eFigure 9), odds ratios for the detection of CDR>0 and Fazekas >2 were 3.32 (1.89-5.82, p<0.0001) and 2.45 (1.35-4.42, p=0.003), respectively.
Discussion
A central challenge in advancing therapeutics for vascular cognitive impairment is the lack of informative biomarkers associated with vascular contributions to cognitive impairment or dementia for an individual. The MarkVCID Consortium was established to develop reliable biomarkers specifically focused on the vascular component of cerebral pathologies that contribute to dementia. Using a structured study design and selection process, specific imaging and fluid-based biomarker kits were selected and subjected to reliability testing and validation using a multi-site design. In this framework, we identified plasma PlGF as a unique, single candidate biomarker for VCID and proposed that it may function both as an accurate cross-sectional diagnostic biomarker and using longitudinal outcome data, contribute as a susceptibility/risk biomarker. Here, employing both an enrollment and analytic site independent cohort approach, we demonstrate the diagnostic validity of plasma PlGF in identifying subjects at risk for VCID due to a high burden of WMH and show that this simple plasma measure can reliably detect subjects with both white matter brain injury and/or cognitive impairment. Using routine benchmark measures including the Fazekas score and CDR scale, we demonstrate a significant proportional relationship between plasma PlGF values and both imaging and cognitive outcome scales. While the primary study outcome was not achieved in one of three cohorts, site variation in subject characteristics that were not utilized to stratify the random resampling approach likely limited the ability to show a significant relationship in cohort 1. For example, the composite rates of vascular risk factor burden in cohort 1 are notably below those in cohorts 2 and 3, potentially limiting the amount of vascular pathology in this cohort. Nonetheless, this novel effort to identify a reliable VCID diagnostic biomarker was successful particularly in context of the secondary study outcomes.
The relationship between cross-sectional plasma PlGF values and cerebral small vessel pathology is strengthened when using the more advanced imaging technique of volumetric white matter hyperintensity measurement. Compared to the ordinal Fazekas score, volumetric white matter hyperintensity measures were consistently associated with plasma PlGF in all three cohorts. When considering clinically relevant VCID disease states that combine the imaging and cognitive outcomes obtained in the study, we find that plasma PlGF maintains or improves its diagnostic accuracy. Plasma PlGF was adequate at detecting individuals with imaging-only evidence of cerebral small vessel disease but even better at detecting MCI with a prominent contribution of cerebral small vessel disease by imaging. This VCID-disease state relationship was true when utilizing the routine benchmark measures (Fazekas, CDR) as well as the advanced measures (volumetric white matter injury, UDS3-EF). While the diagnostic accuracy of a single plasma molecule for a complex disease state like VCID can be questioned, when combined in context with other measures and additional emerging fluid and imaging biomarkers specific for VCID, it will carry significant diagnostic potential.
Potential role for angiogenesis signaling in VCID/CSVD
Failure of angiogenic signaling is a core feature of aging [29], a key risk factor for the development of VCID and cerebral small vessel disease. In the presence of significant cerebral small vessel disease pathology by MRI, collateral cerebral blood flow is compromised [30] further suggesting that impaired angiogenic signaling is a central contributor to the pathogenesis of VCID. Multiple other biomarkers have been suggested for VCID including those reflecting inflammatory cytokine signaling, prothrombotic cascades, neuro-axonal injury, and circulating microRNAs [31]. Many of these proposed biomarkers associate with MRI measures of CSVD including WMH and lacunar infarcts, comparatively few find strong cross-sectional associations with cognitive impairment. Fewer still have been studied in a rigorous study design as enabled by the structure of the MarkVCID Consortium. Our finding of high PlGF levels in individuals with cognitive impairment and imaging features of CSVD, suggests that cerebral small vessel disease is driving an increase in circulating angiogenic factors but a failure of downstream Flt-1 receptor signaling in cerebral small vessels, as has been suggested to drive age-related vascular changes. Notably, other angiogenic markers measured by the immunoassay, including soluble Flt-1, used in these studies did not show similar independent diagnostic relationships with VCID. Efforts to identify the precise role of PlGF signaling on the cerebral vasculature may prove this pathway to be of therapeutic importance.
Importance of a plasma biomarker for VCID
Vascular brain injury resulting from cerebral small vessel disease is commonly diagnosed by imaging which provides critical contextual clues to its contribution to VCID. The value of a surrogate diagnostic biomarker is its role in guiding appropriate diagnostic imaging and in provision of a quantitative, easily repeatable measure. Further, a plasma diagnostic biomarker for VCID can augment the interpretation of imaging, particularly when balanced against other emerging dementia biomarkers. The identification of plasma PlGF as a VCID biomarker can also function to accelerate therapeutic clinical trials targeting VCID by risk stratification of those individuals harboring a significant burden of VCID at enrollment. However, such a biomarker would need to be highly reliable, stable over time, and not effected by metabolic confounders such as fasting status or renal insufficiency. Here, we show that plasma PlGF demonstrates these biomarker characteristics including reliability of measurement across four independent analytic laboratories, within-subjects stability over three weeks, and no contribution of fasting to plasma measurement. Notably, this cohort lacks reliable measurement of renal function. Chronic kidney dysfunction may modestly increase the circulating level of PlGF [32], though this renal adjustment is not noted to appreciably affect the association of PlGF with cardiovascular outcomes [33]. While this is an important limitation of this study, a renally-adjusted measurement of PlGF using the MarkVCID protocol should become the default approach in future studies targeting VCID.
Implications for PlGF as a susceptibility and prognostic biomarker
In this study, we used prospective, cross-sectional MarkVCID data collected as part of the MarkVCID Plasma Endothelial Signaling Kit to establish the value of plasma PlGF as a diagnostic biomarker for VCID. In this context, plasma PlGF values may provide additive data to the evaluation of patients with cognitive impairment and a suspected vascular contribution. The strongest diagnostic value is provided in individuals with plasma PlGF levels above 10 pg/mL, though ordinal regression models indicates that those with plasma PlGF values lower than 10 but above 3 pg/mL also carry a proportional risk of milder states of VCID including cognitively normal or mildly impaired individuals with extensive WMH. It is this group with intermediate plasma PlGF values that will determine the true value of this biomarker in predicting future cognitive decline due to cerebral small vessel disease mechanisms. Longitudinal assessment of the predictive value of baseline plasma angiogenic signaling molecules to identify those with progressive cognitive impairment is the pre-specified hypothesis of the full MarkVCID Plasma Endothelial Signaling Kit that incorporates not only PlGF but also other markers of angiogenic signaling. This longitudinal assessment may allow this new tool to also function as a susceptibility biomarker to measure the risk of future cognitive decline. Serial cognitive testing and plasma PlGF measurement of MarkVCID subjects is ongoing.
Limitations
This cohort study was limited by low enrollment at several sites forcing aggregation of cohorts to achieve the pre-specified n. Random resampling into equal cohorts created an underpowered holdout cohort that may have reduced the true estimations of diagnostic accuracy. Measures of co-morbid Alzheimer’s disease pathology including plasma amyloid ratios and tau were not performed potentially limiting the ability of plasma PlGF to distinguish subtypes of VCID that may also have a significant Alzheimer’s-type neurodegenerative component.
Conclusions
In individuals at risk for cognitive impairment and dementia, plasma PlGF may function as a diagnostic biomarker measuring the degree of vascular injury contributing to baseline cognitive dysfunction.
Supplementary Material
Research In Context.
Systematic Review: The authors reviewed the literature using traditional sources including Pubmed and meeting abstracts. There are multiple suggested circulating biomarkers associated with cerebral small vessel disease and critical related studies are appropriately cited. Few diagnostic biomarkers are associated with vascular cognitive impairment using a rigorous study design and none have implicated angiogenesis as a contributor to vascular cognitive impairment.
Interpretation: Plasma PlGF can function as a stable, reliable, and accurate diagnostic tool to identify individuals with vascular contributions to cognitive impairment and dementia with increasing accuracy across the progressive stages of vascular brain injury.
Future Directions: Plasma PlGF may be considered a useful adjunct in evaluating subjects with suspected vascular cognitive impairment and/or dementia. Additional study is needed to determine the ability of plasma PlGF to prospectively predict future cognitive decline.
Funding & Acknowledgements:
The authors would like to thank all the members of the MarkVCID consortium including those serving on the Fluid Biomarker Subcommittee. MarkVCID is supported by the National Institutes of Health (grant numbers: U24NS100591, UH2NS100599, UH2/UH3NS100605, UH2NS100588, UH2NS100608, UH2NS100606, UH2NS100598, UH2NS100614, UF1NS125513). Drs. Satizabal and Seshadri are partly supported by P30 AG066546. Dr. Seshadri is also supported by the Bill and Rebecca Reed Endowment for Precision Therapies and Palliative Care and by an endowment from the Barker Foundation.
Footnotes
Disclosures: The authors report nothing to disclose.
References
- [1].Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, et al. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cell Mol Neurobiol. 2016;36:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pantoni L Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- [3].Corriveau RA, Koroshetz WJ, Gladman JT, Jeon S, Babcock D, Bennett DA, et al. Alzheimer's Disease-Related Dementias Summit 2016: National research priorities. Neurology. 2017;89:2381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zlokovic BV, Gottesman RF, Bernstein KE, Seshadri S, McKee A, Snyder H, et al. Vascular contributions to cognitive impairment and dementia (VCID): A report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimers Dement. 2020;16:1714–33. [DOI] [PubMed] [Google Scholar]
- [5].Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684–96. [DOI] [PubMed] [Google Scholar]
- [6].Quick S, Moss J, Rajani RM, Williams A. A Vessel for Change: Endothelial Dysfunction in Cerebral Small Vessel Disease. Trends Neurosci. 2021;44:289–305. [DOI] [PubMed] [Google Scholar]
- [7].Grunewald M, Kumar S, Sharife H, Volinsky E, Gileles-Hillel A, Licht T, et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science. 2021;373. [DOI] [PubMed] [Google Scholar]
- [8].Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–83. [DOI] [PubMed] [Google Scholar]
- [9].Odorisio T, Schietroma C, Zaccaria ML, Cianfarani F, Tiveron C, Tatangelo L, et al. Mice overexpressing placenta growth factor exhibit increased vascularization and vessel permeability. J Cell Sci. 2002;115:2559–67. [DOI] [PubMed] [Google Scholar]
- [10].Luna RL, Kay VR, Ratsep MT, Khalaj K, Bidarimath M, Peterson N, et al. Placental growth factor deficiency is associated with impaired cerebral vascular development in mice. Mol Hum Reprod. 2016;22:130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wilcock D, Jicha G, Blacker D, Albert MS, D'Orazio LM, Elahi FM, et al. MarkVCID cerebral small vessel consortium: I. Enrollment, clinical, fluid protocols. Alzheimers Dement. 2021;17:704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gladman JT, Corriveau RA, Debette S, Dichgans M, Greenberg SM, Sachdev PS, et al. Vascular contributions to cognitive impairment and dementia: Research consortia that focus on etiology and treatable targets to lessen the burden of dementia worldwide. Alzheimers Dement (N Y). 2019;5:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Noel-Storr AH, McCleery JM, Richard E, Ritchie CW, Flicker L, Cullum SJ, et al. Reporting standards for studies of diagnostic test accuracy in dementia: The STARDdem Initiative. Neurology. 2014;83:364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Altendahl M, Maillard P, Harvey D, Cotter D, Walters S, Wolf A, et al. An IL-18-centered inflammatory network as a biomarker for cerebral white matter injury. PLoS One. 2020;15:e0227835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Group. F-NBW. BEST (Biomarkers, EndpointS, and other Tools) Resource MD: Silver Spring: Food and Drug Administration (US) National Institutes of Health; 2016-. [PubMed] [Google Scholar]
- [16].Weintraub S, Besser L, Dodge HH, Teylan M, Ferris S, Goldstein FC, et al. Version 3 of the Alzheimer Disease Centers' Neuropsychological Test Battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord. 2018;32:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. [DOI] [PubMed] [Google Scholar]
- [18].Staffaroni AM, Asken BM, Casaletto KB, Fonseca C, You M, Rosen HJ, et al. Development and validation of the Uniform Data Set (v3.0) executive function composite score (UDS3-EF). Alzheimers Dement. 2021;17:574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lu H, Kashani AH, Arfanakis K, Caprihan A, DeCarli C, Gold BT, et al. MarkVCID cerebral small vessel consortium: II. Neuroimaging protocols. Alzheimers Dement. 2021;17:716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maillard P, Lu H, Arfanakis K, Gold BT, Bauer CE, Zachariou V, et al. Instrumental validation of free water, peak-width of skeletonized mean diffusivity, and white matter hyperintensities: MarkVCID neuroimaging kits. Alzheimers Dement (Amst). 2022;14:e12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–22. [DOI] [PubMed] [Google Scholar]
- [22].DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McGraw KO, Wong SP. Forming inferences about some intraclass correlations coefficients (vol 1, pg 30, 1996). Psychol Methods. 1996;1:390- [Google Scholar]
- [24].DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- [25].Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–41. [DOI] [PubMed] [Google Scholar]
- [26].Stephens S, Kenny RA, Rowan E, Allan L, Kalaria RN, Bradbury M, et al. Neuropsychological characteristics of mild vascular cognitive impairment and dementia after stroke. Int J Geriatr Psychiatry. 2004;19:1053–7. [DOI] [PubMed] [Google Scholar]
- [27].Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz L, Looi JC, Wen W, et al. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology. 2004;62:912–9. [DOI] [PubMed] [Google Scholar]
- [28].Dichgans M, Leys D. Vascular Cognitive Impairment. Circ Res. 2017;120:573–91. [DOI] [PubMed] [Google Scholar]
- [29].Le Couteur DG, Lakatta EG. A vascular theory of aging. J Gerontol A Biol Sci Med Sci. 2010;65:1025–7. [DOI] [PubMed] [Google Scholar]
- [30].Rost NS, Cougo P, Lorenzano S, Li H, Cloonan L, Bouts MJ, et al. Diffuse microvascular dysfunction and loss of white matter integrity predict poor outcomes in patients with acute ischemic stroke. J Cereb Blood Flow Metab. 2018;38:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cipollini V, Troili F, Giubilei F. Emerging Biomarkers in Vascular Cognitive Impairment and Dementia: From Pathophysiological Pathways to Clinical Application. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zakiyanov O, Kalousová M, Zima T, Tesař V. Placental growth factor in patients with decreased renal function. Ren Fail. 2011;33(3):291–7. [DOI] [PubMed] [Google Scholar]
- [33].Matsui M, Uemura S, Takeda Y, Samejima K, Matsumoto T, Hasegawa A, et al. Placental Growth Factor as a Predictor of Cardiovascular Events in Patients with CKD from the NARA-CKD Study. J Am Soc Nephrol. 2015. Nov;26(11):2871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.