Abstract
Introduction
The association between ultra-processed food (UPF) consumption and body composition, and potential variation by sociodemographic factors, is unclear. This study aims to examine the cross-sectional associations of UPF consumption with imaging markers of body fat distribution in a nationally representative sample of US adults, overall and by sociodemographic strata.
Methods
A total of 9,640 men and non-pregnant women aged 20-59 years were included from 4 cycles (2011-2012, 2013-2014, 2015-2016, 2017-2018) of the National Health and Nutrition Examination Survey (NHANES) with valid 24-hour dietary recalls and available whole-body DXA scans. UPFs were identified using the NOVA classification, with % energy from UPF assessed in quintiles. Primary outcomes were absolute % fat (total, android, gynoid), and secondary ones were % fat (head, arm, leg, trunk), total abdominal fat (area, mass, volume), subcutaneous adipose tissue (area, mass, volume), and visceral adipose tissue (area, mass, volume). Multivariable-adjusted generalized linear regressions estimated independent relationships of UPF intake with body composition overall and by sociodemographic subgroups. Analyses were conducted in September 2022 and January 2023
Results
UPF consumption accounted for more than half (55.5%) of daily energy consumption in this sample. Compared with the lowest quintile of UPF consumption (<39.4 %energy), adults in the highest quintile (>72.1 %energy) had 1.60 higher total %fat (95% CI, 0.94, 2.26), 2.08 higher android %fat (95% CI, 1.26, 2.89), and 1.32 higher gynoid %fat (95% CI, 0.71, 1.93) (all P-trend<0.001). Consistent findings were observed for secondary outcomes. Associations of UPF intake with total %fat, android %fat, and gynoid %fat varied by age, sex, race and ethnicity, education, and income. Among those in the highest quintile of UPF consumption compared to the lowest quintile counterpart, total %fat was 1.85 (95% CI, 0.86, 2.84) higher for non-Hispanic white adults and 1.57 (95% CI, 0.68, 2.46) higher for Hispanic adults (P-trends<0.001) while no difference was observed among non-Hispanic black adults (−0.22; 95% CI, −0.93, 1.36) (P-trend=0.47) and non-Hispanic Asian adults (0.93; 95% CI, −0.57, 2.42) (P-trend=0.04) (P-interaction=0.001). Associational patterns were similar for android %fat and gynoid %fat.
Conclusions
In a national US sample, higher intake of UPF was associated with greater body fat, in particular android fat, and this relationship was most prominent in certain population subgroups. These cross-sectional findings call for prospective and interventional studies to assess the impact of UPF on body composition in different populations.
Introduction
Ultra-processed foods (UPFs) are the most widely consumed foods in the United States (US), accounting for more than half of daily calorie intake.1 UPFs are defined as industrial formulations manufactured from substances derived from foods that undergo a series of physical, chemical, and biological processes.2 Typically, such foods have higher contents of refined starch, sugars, and salt and lower nutritional value, lack intact healthy food components, and include added flavors, artificial colors, and other additives.3
The rise in consumption of UPFs has paralleled the increasing prevalence of obesity,4,5 and a well-controlled metabolic trial demonstrated higher energy consumption and weight gain with an UPF diet, compared to a minimally processed diet.6 These findings suggest UPF may be one driver of the obesity epidemic and related diseases.7 In observational studies, UPFs are associated with obesity,8 type 2 diabetes,9 cardiovascular diseases (CVD),10 and certain types of cancer.11 Most prior studies of UPFs and obesity have focused on body mass index (BMI), and followed by waist circumference as a measure of central obesity.12,13 However, these measures are influenced not only by adiposity but also by muscle mass and body size, as well as associated with socioeconomic status and race/ethnicity.14,15 Other aspects of body composition – in particular the amounts and locations of body fat – appear to more specifically predict the risk of disease.16–18 A few studies have examined how UPFs relate to body fat distribution, including one on UPFs and fat mass, lean mass, and whole-body fat percent among youth and young adults,19 and two on UPFs and supine sagittal abdominal diameter in adolescents20 and adults.21 No prior studies have focused comprehensively on the association of UPFs with body fat distribution.
Prior studies have also demonstrated sociodemographic disparities in UPF consumption and, separately, prevalence of obesity.22–24 These disparities may impose significant cardiometabolic burdens on vulnerable populations such as those with low educational attainment or from traditionally marginalized racial or ethnic backgrounds. However, potential differences across sociodemographic strata in the association of UPFs with body fat distribution remain unknown.
To address those gaps, this study examined the associations of UPF consumption with imaging markers of body fat distribution among US adults, overall and across sociodemographic strata.
Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES) is a series of nationally representative, stratified, multistage probability cross-sectional surveys in two-year cycles to assess the health and nutritional status of Americans. Details on design, study protocol, and data collection have been reported, and all participants provided written informed consent.25 The study protocol was approved by the National Center for Health Statistics Research Ethics Review Board. The current analysis included the most recent four NHANES cycles (2011-2012, 2013-2014, 2015-2016, 2017-2018) with available measures on body composition. Dietary intakes were assessed using 1-2 24-hr dietary recalls per person (see Appendix Text for detailed methods). Body composition was measured using dual-energy x-ray absorptiometry (DXA) among participants aged 8 to 59 years only. This analysis focused on 9,640 men and non-pregnant women aged 20-59 years with both valid records of dietary intake and whole-body DXA scans (Appendix Figure 1). In this age range, characteristics for participants included and excluded in this analysis are shown in Appendix Table 1.
Classification of Ultraprocessed Food
The NOVA framework was used to classify all food items reported by participants into 4 categories based on the extent and purpose of food processing.5 In brief, the NOVA classification is mainly based on the underlying ingredients in particular food substances not commonly used in culinary preparations such as modified starches, hydrogenated oils, protein isolates and additives like colorants, flavorings, and emulsifiers. The processing categories were mutually exclusive and included unprocessed/minimally processed foods, processed culinary ingredients, processed foods, and UPFs – the latter characterized by various industrial processing techniques and ingredients. When food items were judged to be a homemade recipe, the classification process was applied to the underlying ingredients, to enable a more precise classification. The procedures for classifying UPFs are detailed in Appendix Text and elsewhere.26,27 This study focused on the % daily energy intake from UPFs (i.e., UPF consumption adjusted for total energy using the nutrient density approach),28 based on the mean of both recall days when available and one day otherwise.
Body Composition Measurements
Whole body DXA scans were administered in NHANES mobile examination centers by certified radiology technologists. The scans provide bone and soft tissue measurements for the total body and body regions. Detailed information regarding the DXA scans is documented.29 The primary outcomes were total percent (%) fat, android % fat, and gynoid %fat. The secondary outcomes were %fat (head, arm, leg, and trunk), total abdominal fat (TAF), subcutaneous adipose tissue (SAT), and visceral adipose tissue (VAT). Hologic APEX software was used to estimate the area, mass, and volume of subcutaneous and visceral adipose tissue. SAT is measured at the approximate location of the interspace between the L5 and L4 vertebrae outside the abdominal cavity. VAT is measured at the same location in the abdominal cavity. TAF refers to the accumulation of fat in the abdominal region, including SAT and VAT. In the population subgroups, primary outcomes are presented.
Assessment of Covariates
Sociodemographic and lifestyle factors including biological sex, age, race and ethnicity, educational level, income, smoking, and physical activity were self-reported via standardized questionnaires. Race and ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Non-Hispanic Asian, and other) was self-reported according to prespecified categories in the NHANES. Educational levels were categorized into < high school graduate, high school graduate or equivalent, some college, and ≥ college graduate. Household income was classified as the ratio of family income to poverty into <1.30, 1.30-3.49, and ≥3.50 according to Supplemental Nutrition Assistance Program eligibility.30 Nonsmokers were defined as those had smoked < 100 cigarettes during their lifetime. Former smokers were defined as those had previously smoked > 100 cigarettes during their lifetime but were not currently smoking. Current smokers were grouped into smokers smoking for some days and smokers smoking daily < 20 cigarettes per day and ≥ 20 cigarettes per day. Physical activity was calculated as a continuous variable in metabolic equivalent task-minutes (MET-min) per week using duration of self-reported moderate and vigorous work and recreational activities and MET score for each activity.31
Statistical Analysis
Participants were categorized according to quintiles of UPF contribution to total energy intake (% daily energy intake) in the diet. Multivariable-adjusted linear regression models were used to assess the associations between UPF in quintiles and each of primary and secondary outcomes, with predicted means standardized to the distribution of the model covariates within the analytical sample. All analyses incorporated NHANES sample weights, accounting for differential probabilities of selection and the complex survey design to produce nationally representative estimates of the noninstitutionalized US population. To minimize confounding, covariates included age, sex, race and ethnicity, education, income, smoking, and physical activity, selected prior to analysis based on their known or suspected role with consumption of UPF and body composition.
This study further explored stratified analyses for associations between UPF consumption and primary outcomes in key population subgroups, including by age, sex, race/ethnicity, education, and income. For potential effect modification by these subgroups, the survey-weighted Wald F statistic was used to test for an interaction between the quintiles of UPF consumption and subgroup variable. Because these subgroup analyses were exploratory, P-values for interaction were Bonferroni-corrected to 2-sided alpha=0.003 (0.05/ 5 [subgroups]×3[exposures]). Statistical analyses were performed using Stata, version 16.1, with 2-sided alpha=0.05.
Results
A total of 9,640 adults (mean [SD] age, 38.8 [11.9] years; 48.8% female) were included in the analysis, with UPF accounting for more than half (55.5%) of daily energy consumption. In crude analyses comparing higher to lower UPF consumption, individuals were on average younger, non-Hispanic White or non-Hispanic Black adults compared to other races/ethnicities, less educated, lower income, and current smokers (Table 1). UPF intake had a U-shaped relation with physical activity.
Table 1.
Characteristics of Study Participants Aged 20 -59 Years and Older by Quintiles of the Contribution of Ultraprocessed Foods to Total Energy Intake, NHANES 2011-2018
| Characteristics | N (Survey-weighted %)a | |||||
|---|---|---|---|---|---|---|
| Overall (n=9,640) | Q1 (n=2,221) | Q2 (n=1,965) | Q3 (n=1,854) | Q4 (n=1,765) | Q5 (n=1,835) | |
| % Total calorie intake | 55.5 | <39.4 | 39.4-51.1 | 51.1-61.2 | 61.2-72.1 | >72.1 |
| Age (SD), years | 39.0 (10.2) | 40.5 (10.8) | 39.4 (10.3) | 39.2 (9.75) | 39.0 (9.47) | 36.8 (10.2) |
| 20-39 | 51.6 | 47.5 | 50.7 | 51.5 | 50.1 | 58.5 |
| 40-59 | 48.4 | 52.5 | 49.3 | 48.5 | 49.9 | 41.5 |
| Sex | ||||||
| Male | 51.5 | 51.3 | 48.9 | 50.7 | 53.2 | 53.2 |
| Female | 48.5 | 48.7 | 51.1 | 49.3 | 46.8 | 46.8 |
| Race/Ethnicity | ||||||
| Non-Hispanic White | 60.0 | 49.3 | 58.7 | 61.4 | 63.8 | 66.5 |
| Non-Hispanic Black | 11.2 | 8.64 | 9.44 | 11.0 | 12.2 | 15.0 |
| Hispanic | 18.4 | 21.7 | 21.3 | 20.3 | 16.7 | 12.1 |
| Non-Hispanic Asian | 6.72 | 17.0 | 7.18 | 4.65 | 3.06 | 1.71 |
| Other | 3.66 | 3.34 | 3.45 | 2.61 | 4.22 | 4.68 |
| Education level | ||||||
| Less than high school graduate | 12.9 | 11.9 | 13.2 | 13.0 | 13.0 | 13.3 |
| High school graduate or GED | 22.0 | 18.1 | 19.2 | 20.2 | 22.8 | 29.8 |
| Some college | 33.3 | 28.4 | 30.7 | 33.6 | 35.6 | 38.4 |
| College graduate or above | 31.7 | 44.5 | 36.9 | 33.2 | 28.5 | 18.5 |
| Ratio of family income to poverty level | ||||||
| <1.30 | 22.8 | 20.5 | 22.6 | 20.5 | 22.1 | 28.4 |
| 1.30 to 3.49 | 32.2 | 29.7 | 32.1 | 30.8 | 33.1 | 35.2 |
| >=3.50 | 38.7 | 42.3 | 39.8 | 42.1 | 38.4 | 30.8 |
| Smoking status | ||||||
| Never | 58.9 | 60.6 | 61.2 | 60.0 | 57.1 | 55.6 |
| Former | 19.6 | 21.1 | 19.8 | 21.2 | 19.1 | 16.9 |
| Current smoker | ||||||
| Some days | 4.77 | 4.39 | 5.63 | 4.19 | 5.58 | 4.05 |
| <20 cigarettes/d | 11.3 | 9.16 | 8.56 | 11.1 | 12.6 | 14.9 |
| ≥20 cigarettes/d | 5.40 | 4.73 | 4.64 | 3.53 | 5.58 | 8.49 |
| Physical activity, METs-min | ||||||
| Q1 | 27.2 | 23.9 | 26.7 | 26.4 | 29.9 | 29.3 |
| Q2 | 23.1 | 24.5 | 23.8 | 24.3 | 22.5 | 20.4 |
| Q3 | 24.7 | 28.5 | 24.8 | 26.2 | 21.9 | 22.2 |
| Q4 | 25.0 | 23.1 | 24.7 | 23.2 | 25.8 | 28.1 |
Abbreviations: NHANES, National Health and Examination Survey; SD, standard deviation; METs-Min, metabolic equivalent Q1=<39.4%; Q2=39.4%-51.1%; Q3=51.1%-61.2%; Q4=61.2%-72.1%; Q5=>72.1%.
Data were weighted to be nationally representative.
The relationships of UPF consumption with body composition are shown in Table 2. Compared to adults in the lowest quintile of UPF consumption (<39.4 %energy), adults in the highest quintile (>72.1 %energy) had significantly higher percentage points of total %fat by 1.60 (95% CI, 0.94, 2.26), of android %fat by 2.08 (95% CI, 1.26, 2.89), and of gynoid %fat by 1.32 (95% CI, 0.71, 1.93) (all P-trend<0.001). Similar associational patterns with UPF consumption were observed for secondary outcomes. For example, those in the highest quintile of UPF consumption had higher percentage points of arm %fat by 1.73 (95% CI, 0.93, 2.52), of leg %fat by 1.32 (95% CI, 0.61, 2.03), and of trunk %fat by 1.87 (95% CI, 1.14, 2.59). Adults in the highest quintile of UPF consumption had 53.7 cm2 higher TAF area (95% CI, 35.7, 71.6), 259 g higher TAF mass (95% CI, 172, 345), 280 cm3 higher TAF volume (95% CI, 186, 373), 43.9 cm2 higher SAT area (95% CI, 29.7, 58), 212 g higher SAT mass (95% CI, 143, 280), 229 cm3 higher SAT volume (95% CI, 155, 302), 9.77 cm2 higher VAT area (95% CI, 5.14, 14.4), 47.1 g higher VAT mass (95% CI, 24.8, 69.4), and 50.9 cm3 higher VAT volume (95% CI, 26.8, 75.1) (all P-trend<0.001).
Table 2.
Associations of Ultraprocessed Food Consumption with Body Composition Among US Adults Aged 20 -59 Years, NHANES 2011-2018 (N=9,640). *
| Body composition measures | Differences across Quintiles of UPF Consumption (%E) | Mean Values across Quintiles of UPF Consumption (%E) | P for trend | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (<39.4) | Q2 (39.4-51.1) | Q3 (51.1-61.2) | Q4 (61.2-72.1) | Q5 (>72.1) | Q1 (<39.4) | Q2 (39.4-51.1) | Q3 (51.1-61.2) | Q4 (61.2-72.1) | Q5 (>72.1) | ||
| Primary outcomes | |||||||||||
| Total body %fat | 0.0 (Ref) | 0.47 (−0.15, 1.09) | 1.21 (0.69, 1.74) | 1.42 (0.78, 2.06) | 1.60 (0.94, 2.26) | 31.7 (31.2-32.2) | 32.1 (31.6-32.6) | 32.9 (32.5-33.3) | 33.1 (32.6-33.6) | 33.3 (32.8-33.8) | <0.001 |
| Android %fat | 0.0 (Ref) | 0.32 (−0.45, 1.09) | 1.57 (0.90, 2.24) | 1.83 (1.07, 2.59) | 2.08 (1.26, 2.89) | 33.6 (33-34.2) | 33.9 (33.3-34.6) | 35.2 (34.7-35.7) | 35.4 (34.8-36.0) | 35.7 (35.1-36.3) | <0.001 |
| Gynoid %fat | 0.0 (Ref) | 0.64 (0.08, 1.21) | 1.14 (0.67, 1.60) | 1.25 (0.69, 1.80) | 1.32 (0.71, 1.93) | 34.3 (33.9-34.7) | 34.9 (34.5-35.4) | 35.4 (35.1-35.8) | 35.6 (35.1-36.0) | 35.6 (35.1-36.1) | <0.001 |
| Secondary outcomes | |||||||||||
| Head %fat | 0.0 (Ref) | 0.005 (−0.06, 0.07) | 0.05 (−0.005, 0.10) | 0.09 (0.02, 0.15) | 0.10 (0.04, 0.15) | 24.0 (24-24.1) | 24.0 (24-24.1) | 24.1 (24-24.1) | 24.1 (24.1-24.2) | 24.1 (24.1-24.2) | <0.001 |
| Arm %fat | 0.0 (Ref) | 0.51 (−0.22, 1.25) | 1.25 (0.61, 1.90) | 1.29 (0.47, 2.11) | 1.73 (0.93, 2.52) | 32.4 (31.8-32.9) | 32.9 (32.3-33.5) | 33.6 (33.1-34.1) | 33.7 (33.0-34.3) | 34.1 (33.5-34.7) | <0.001 |
| Leg %fat | 0.0 (Ref) | 0.61 (−0.08, 1.30) | 1.13 (0.58, 1.68) | 1.36 (0.71, 2.0) | 1.32 (0.61, 2.03) | 34 (33.5-34.5) | 34.6 (34.1-35.1) | 35.1 (34.6-35.6) | 35.3 (34.8-35.9) | 35.3 (34.8-35.8) | <0.001 |
| Trunk %fat | 0.0 (Ref) | 0.39 (−0.31, 1.09) | 1.35 (0.74, 1.96) | 1.63 (0.93, 2.33) | 1.87 (1.14, 2.59) | 30.6 (30-31.2) | 31 (30.4-31.6) | 32.0 (31.5-32.4) | 32.2 (31.7-32.8) | 32.5 (31.9-33) | <0.001 |
| Total abdominal fat area (cm2) | 0.0 (Ref) | 13.6 (−4.0, 31.2) | 37.3 (21.0, 53.6) | 47.4 (30.9, 63.8) | 53.7 (35.7, 71.6) | 402 (390-415) | 416 (401-430) | 440 (427-452) | 450 (436-463) | 456 (441-471) | <0.001 |
| Total abdominal fat mass (gm) | 0.0 (Ref) | 65.5 (−19.0, 150) | 180 (101, 259) | 228 (149, 308) | 259 (172, 345) | 1939 (1879-1999) | 2005 (1934-2076) | 2119 (2059-2179) | 2168 (2101-2234) | 2198 (2127-2269) | <0.001 |
| Total abdominal fat volume (cm3) | 0.0 (Ref) | 70.9 (−21.0, 162) | 194 (109, 280) | 247 (161, 333) | 280 (186, 373) | 2097 (2032-2161) | 2167 (2091-2244) | 2291 (2226-2356) | 2344 (2271-2416) | 2376 (2300-2453) | <0.001 |
| SAT area (cm2) | 0.0 (Ref) | 12.3 (−1.70, 26.2) | 29.0 (16.5, 41.5) | 38.4 (24.9, 51.8) | 43.9 (29.7, 58) | 306 (296-315) | 318 (307-330) | 335 (325-344) | 344 (333-356) | 350 (338-361) | <0.001 |
| SAT mass (gm) | 0.0 (Ref) | 59.1 (−8.30, 127) | 140 (79.7, 200) | 185 (120, 250) | 212 (143, 280) | 1474 (1429-1520) | 1533 (1478-1589) | 1614 (1568-1661) | 1659 (1605-1714) | 1686 (1631-1741) | <0.001 |
| SAT volume (cm3) | 0.0 (Ref) | 63.9 (−9.0, 137) | 151 (86.1, 216) | 200 (130, 270) | 229 (155, 302) | 1594 (1545-1643) | 1658 (1598-1717) | 1745 (1695-1795) | 1794 (1735-1853) | 1823 (1763-1882) | <0.001 |
| VAT area (cm2) | 0.0 (Ref) | 1.34 (−3.10, 5.8) | 8.28 (3.91, 12.7) | 9.01 (4.73, 13.3) | 9.77 (5.14, 14.4) | 96.5 (92.8-100) | 97.8 (93.9-102) | 105 (101-108) | 105 (102-109) | 106 (102-110) | <0.001 |
| VAT mass (gm) | 0.0 (Ref) | 6.45 (−15.0, 27.9) | 39.9 (18.9, 61) | 43.4 (22.8, 64.1) | 47.1 (24.8, 69.4) | 465 (448-483) | 472 (453-491) | 505 (487-523) | 509 (491-526) | 512 (493-531) | <0.001 |
| VAT volume (cm3) | 0.0 (Ref) | 6.97 (−16.0, 30.2) | 43.2 (20.4, 66) | 47 (24.7, 69.3) | 50.9 (26.8, 75.1) | 503 (484-522) | 510 (489-530) | 546 (527-565) | 550 (531-569) | 554 (533-574) | <0.001 |
Abbreviation: NHANES, National Health and Nutrition Examination Survey; %fat, percent fat; SAT, subcutaneous adipose tissue; UPF, ultra-processed foods; VAT, visceral adipose tissue.
Values are multivariable-adjusted differences compared to the lowest quintile as the reference (left columns) or multivariable-adjusted mean values in each quintile (right columns). Analyses were weighted to be nationally representative and adjusted for age (years), sex (male, female), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and others), education (less than high school, high school graduate or GED, some college, or college graduate or above), and the ratio of family income to poverty (<1.30, 1.30–1.849, 1.85–2.99, and ≥3.0), smoking status (never smoker, former smoker, and smokers smoking for some days and smokers smoking daily less than 20 cigarettes per day and equal or larger than 20 cigarettes per day), and physical activity (METs-Min). Individuals with missing data on education (n=2), income (n=727), physical activity (n=1), and smoking (n=9) were created as a special category.
Abbreviations: SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
In stratified analysis, the associations of UPF consumption and primary outcomes were similar across most subgroups including by age, sex, education, and income (Figures 1–2, Appendix Tables 2–4). After adjusting for multiple comparison, the relationship between UPF consumption and total %fat and gynoid %fat appeared to be modified by race and ethnicity (P-interaction<0.003 each). Comparing the highest to lowest quintile of UPF consumption, the multivariable adjusted difference in percentage points of total %fat was 1.85 (95% CI, 0.86, 2.84) for non-Hispanic White adults, 1.57 (95% CI, 0.68, 2.46) for Hispanic adults, 0.22 (−0.93, 1.36) for non-Hispanic Black adults, and 0.93 (95% CI, −0.57, 2.42) for non-Hispanic Asian adults (P-interaction=0.001). The corresponding multivariable adjusted difference in gynoid %fat was 1.68 (95% CI, 0.80, 2.58) for non-Hispanic White adults, 1.12 (95% CI, 0.16, 2.09) for Hispanic adults, 0.18 (−0.77, 1.13) for non-Hispanic Black adults, and −0.16 (95% CI, −1.6, 1.25) for non-Hispanic Asian adults (P-interaction=0.0027). These associations also appeared visually less prominent among adults with lowest household income, but this difference was not statistically significant (P-interaction=0.04 for total, 0.03 for android, and 0.06 for gynoid %fat).
Figure 1.
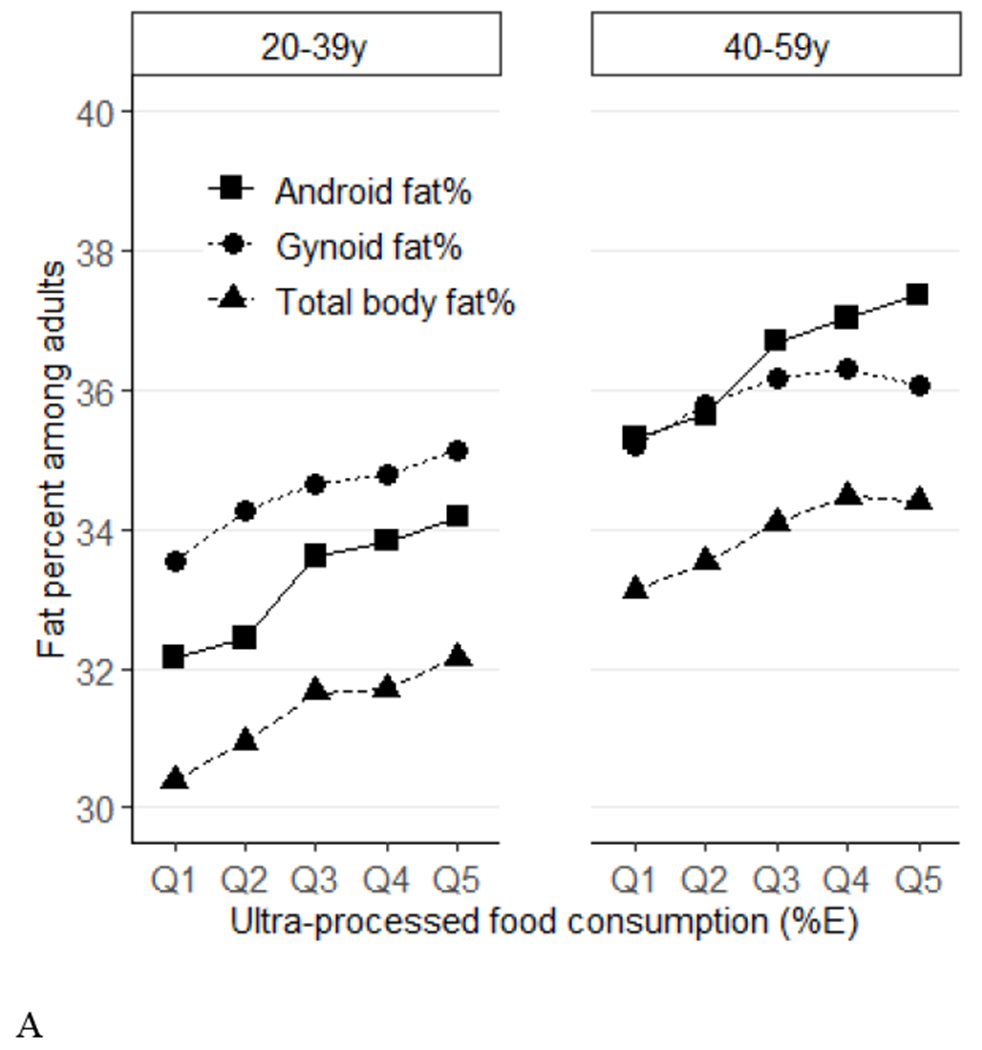
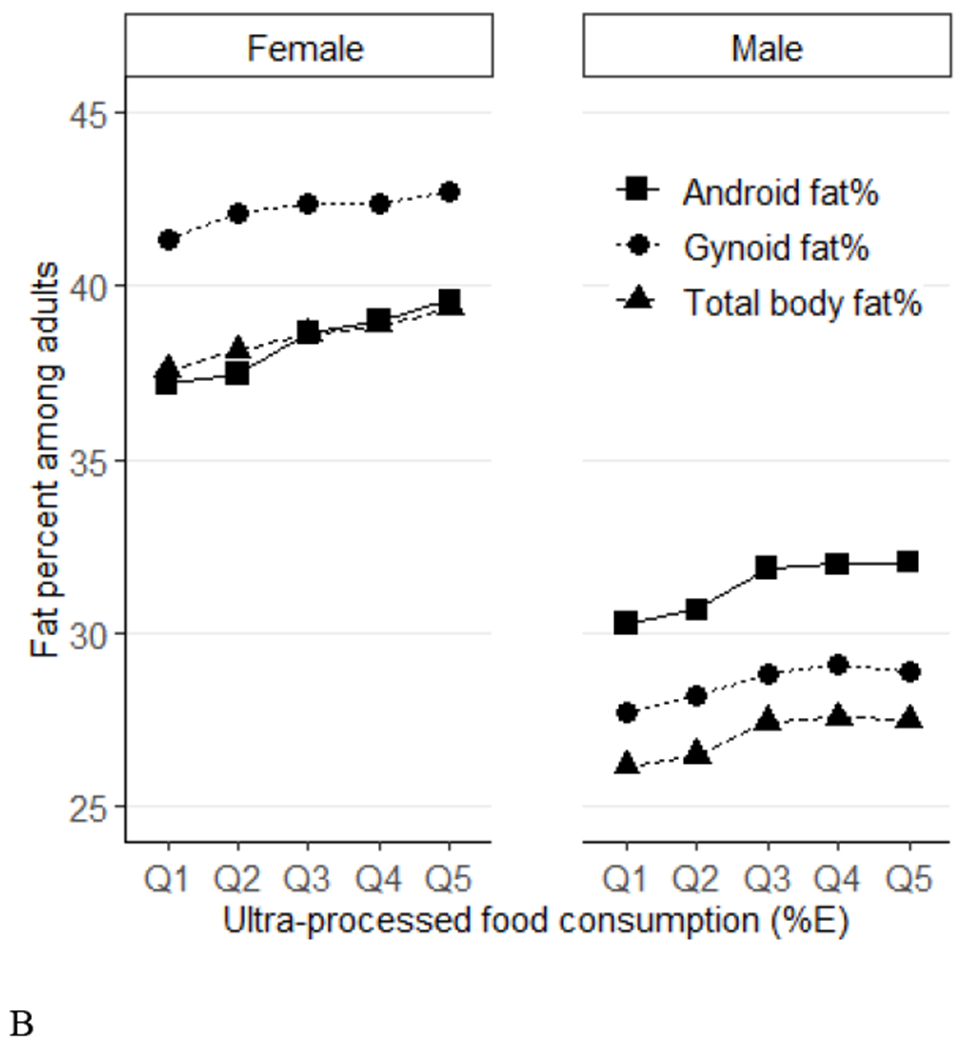
Predicted means of percent fat (total, android, gynoid) across quintiles of ultraprocessed food consumption (%E) among US adults (aged 20-59 years) by age groups (Panel A) and sex (Panel B), NHANES 2011–2018. Data were adjusted for NHANES survey weights to be nationally representative. %E, % energy; NHANES, National Health and Nutrition Examination Survey. By age, P-trend<0.001 for all. P-interactions are 0.88 for total %fat, 0.42 for android %fat, and 0.86 for gynoid. By sex, P-trend <0.001 for all. P-interactions are 0.19 for total %fat, 0.03 for android %fat, and 0.67 for gynoid.
Figure 2.
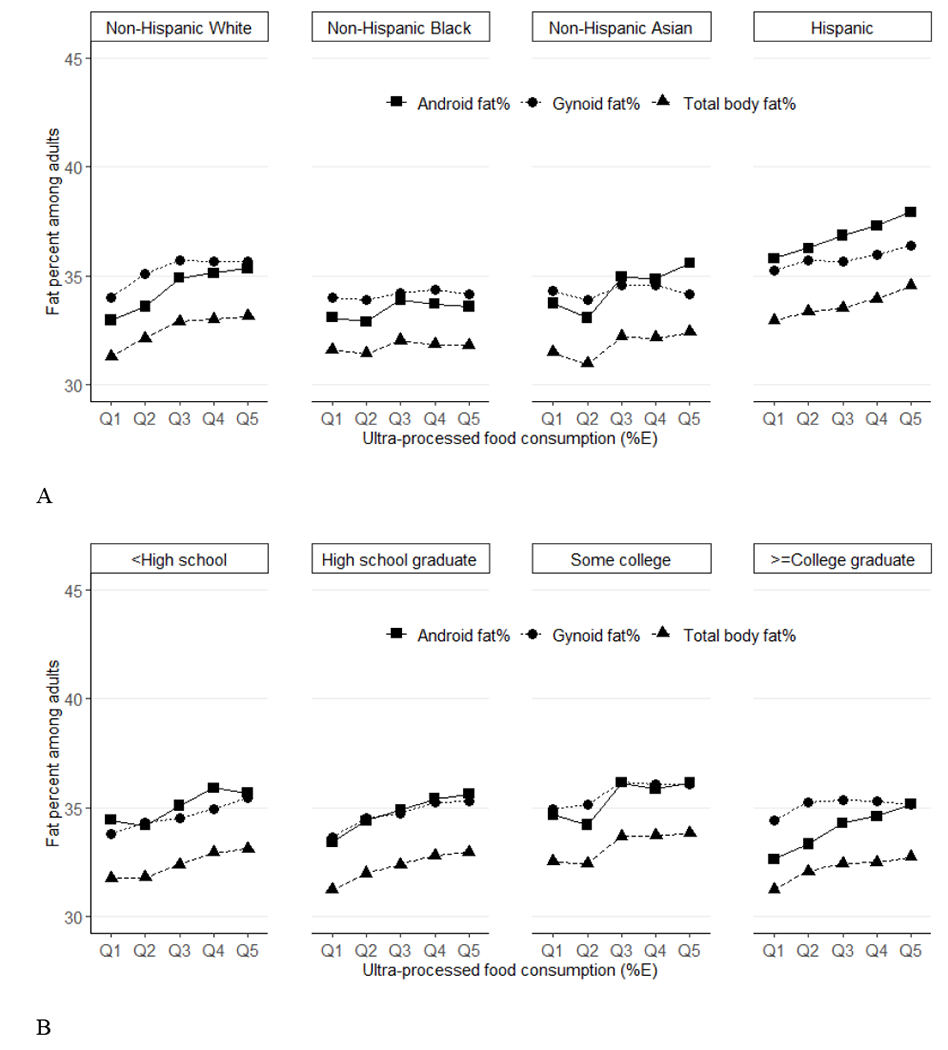
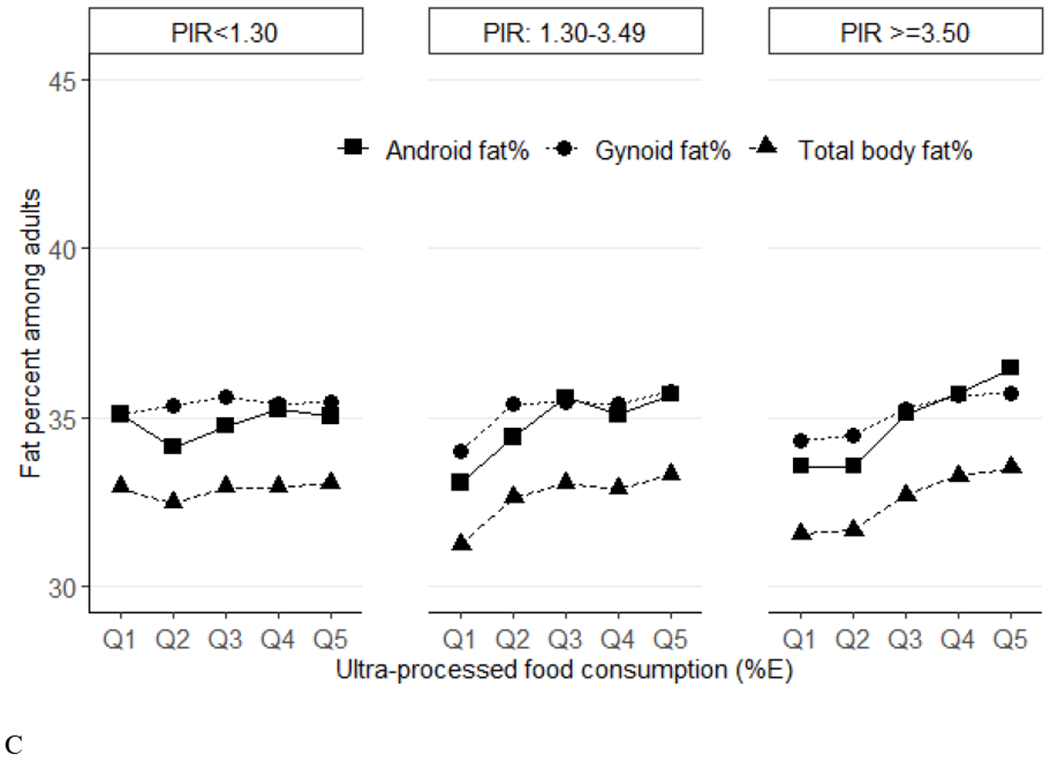
Predicted means of percent fat (total, android, gynoid) across quintiles of ultraprocessed food consumption (%E) among US adults (aged 20-59 years) by race/ethnicity (Panel A), education (Panel B) and income (Panel C), NHANES 2011–2018. Data were adjusted for NHANES survey weights to be nationally representative. %E, % energy; NHANES, National Health and Nutrition Examination Survey. By race/ethnicity, for total %fat, P-trend<0.001 for non-Hispanic White and Hispanic, P-trend=0.47 for non-Hispanic Black, P-trend=0.04 for non-Hispanic Asian. For android %fat, P-trend<0.001 for non-Hispanic White and Hispanic, P-trend=0.17 for non-Hispanic Black, P-trend=0.007 for non-Hispanic Asian. For gynoid, P-trend<0.001 for non-Hispanic White, P-trend=0.009 for Hispanic, P-trend=0.42 for non-Hispanic Black, P-trend=0.72 for non-Hispanic Asian. P-trend P-interactions are 0.001 for total %fat, 0.006 for android %fat, and 0.0027 for gynoid. By education, for total %fat, P-trend=0.004 for less than high school, P-trend=0.006 for high school graduate, P-trend=0.002 for some college and P-trend=0.003 for college graduate or above. For android %fat, P-trend=0.01 for less than high school, P-trend=0.004 for high school graduate, P-trend=0.006 for some college and P-trend<0.001 for college graduate or above. For gynoid %fat, P-trend<0.001 for less than high school, P-trend=0.003 for high school graduate, P-trend=0.01 for some college and P-trend=0.04 for college graduate or above. P-interactions are 0.87 for total %fat, 0.96 for android %fat, and 0.49 for gynoid. By income, for total %fat, P-trend=0.51 for ratio of family income to poverty level (PIR)<1.30, P-trend<0.001 for PIR: 1.30-3.49 and PIR>=3.50. For android, P-trend=0.50 for PIR<1.30, P-trend<0.001 for PIR: 1.30-3.49 and PIR>=3.50. For gynoid, P-trend=0.44 for PIR<1.30, P-trend=0.005 for PIR: 1.30-3.49, P-trend<0.001 for PIR>=3.50. P-interactions are 0.04 for total %fat, 0.03 for android %fat, and 0.06 for gynoid.
Discussion
In this nationally representative study, the cross-sectional relationships of UPF consumption with body fat composition were assessed among US men and non-pregnant women aged 20-59 years old. This investigation found a positive relationship of UPF consumption with total %fat, android %fat and gynoid %fat, as well as other secondary measures of body fat. In general, these patterns were consistent across subgroups including by age, sex, education, and income. In exploratory analyses, UPF consumption was less associated with body fat composition among non-Hispanic Black and non-Hispanic Asian adults than among non-Hispanic White and Hispanic adults.
This investigation extends prior studies assessing UPFs and adiposity by assessing objective measures of total and regional body fat. The role of body fat distribution, beyond body weight, in the onset of and clinical complications from diabetes and CVD can be dated back to 1947 and has been confirmed by a large body of subsequent research.32 In 1947, a French physician reported different features of android fat accumulation vs gynoid fat accumulation with diabetes or clinical signs of CVD.33 In 1980s, researchers from Sweden and US showed that the ratio of wait-to-hip circumference as a simple index of regional body fat distribution was more correlated with metabolic complications and CVD outcomes as compared to BMI.34–38 With advances in technology, more precise measures of body fat distribution are available and enable differentiation between subcutaneous and visceral fat. Several studies have shown positive associations of body fat measures with disease outcomes.39–42 For example, childhood fat mass rather than weight may be a more precise marker of long-term type 2 diabetes risk in adulthood.42 High volumes of VAT and SAT are associated with incident metabolic risk factors that could not be accounted for by overall adiposity.43 Specific body fat measures (whole-body fat, trunk fat, and leg fat) are also linked with risk of breast cancer as well as higher levels of insulin, triglycerides, leptin and C-reactive protein, and interleukin 6. 41
These findings are broadly consistent with the three prior studies on ultraprocessed food consumption and body fat distribution19,21,44 but extend and expand these prior findings in several ways. First, two of these prior studies were in relatively select populations, including those aged from 7 to 24 years from the Avon Country, southwest England or Spanish men and women aged 55-75 years with overweight or obesity and metabolic syndrome. This study extends previous findings by providing the most up-to-date estimates in a nationally representative US sample, examining previously unreported DXA scanned adiposity measures 45,46 and characterizing potential subgroup differences across sociodemographic characteristics. Second, UPF intake was quantified based on repeated 24-hr dietary recalls with detailed information on specific foods and beverages, including brand names, places of purchase and nutrition composition. Third, outcomes include diverse regional fat measures as well as measures of subcutaneous and visceral fat. Fourth, this study had reasonable statistical power to explore effect modification of the association of UPF consumption with body fat composition by important sociodemographic subgroups.
A key limitation of this and prior large studies is the cross-sectional nature of the analysis, which cannot establish temporality of the associations. However, in a 2-week randomized and controlled c+ross-over feeding trial, researchers found that even after matching on dietary energy density, fat, protein, carbohydrate, sugar, sodium and fiber for overall available meals, participants eating UPF had increased energy intake by ~500 kcal/d and increased weight gain of ~2 kg, compared to when they ate unprocessed or minimally processed foods.6 This study also found that consumption of UPFs increased body fat mass by 0.4 kg. Mechanisms for these effects are not established, but could include a faster consumption level of UPF; the generally higher glycemic index of UPF;47 the more complete digestion of UPF in the stomach and small intestine, resulting in less nutrition for the large gut microbiome and a higher proportion of energy consumed by the host vs. microbiota (causing differential host-microbiome energy partitioning);48,49 the potential influence of artificial additives in UPF on the microbiome, glycemic responses, or hunger and satiety;50 and the loss of specific prebiotics, phenolics, and other bioactives in UPF that may protect against adiposity.51 Moreover, obesogens present in food packaging and processing, which can disrupt production, release, transport, and action of hormones involved in regulating metabolism and appetite, may influence an increase in body fat.52 The new findings from the present study support the need for additional interventional and mechanistic studies of how UPF may influence body composition and adiposity.10,53
In exploratory analyses, this study identified statistically stronger associations of UPF consumption with body fat measures among non-Hispanic White and Hispanic adults than non-Hispanic Black or Asian adults. The reasons for these differences are unclear. Asian American adults sampled in NHANES are predominantly English-speaking, who tend to have higher income and education and healthier behaviors and lifestyles than non-sampled Asian adults; and the observed finding in this racial subgroup may not be generalizable to other Asian American adults. Although the models included major potential confounders, observed differences in relationships by race and ethnicity could also relate to how the composite measures of UPF was constructed, leading to differential misclassification across this subgroup; to reverse causation (individuals in these racial/ethnic categories with higher body fat choosing to eat less UPF); or to other unexplained factors. Although the impact of multiple comparisons was incorporated, these findings could also be due to chance. This study supports the need for future research to further evaluate these findings, including longitudinal studies using objective imaging techniques such as DXA scan, computed tomography, and magnetic resonance imaging among diverse population.
Since the emergence of NOVA classification system proposed by Monteiro et al. in 2010,5 associations of UPF consumption with many adverse health outcomes have been reported. Several meta-analysis and systematic reviews have synthesized the evidence from prospective cohort studies and reported that highest consumption of UPF was associated with higher risk of all-cause mortality, CVD, depression, and type 2 diabetes.7,54 In addition, a positive association of UPFs with risk of cancer has been seen.55,56 The findings from this study extend prior reports by evaluating the association of UPF consumption with a range of objectively measured body fat.
Limitations
Potential limitations should be noted. As described above, results are cross-sectional, precluding assessment of temporality. Dietary information was collected through self-report, which is subject to measurement errors. However, the dietary 24-hr recalls were conducted by trained dietary interviewers using the computer-assisted personal interview system and results were further adjusted for total energy, both of which reduce measurement error. While 24-hr diet recalls provide unbiased population average estimates, they may not accurately measure the habitual (long-term) diets of specific individuals. Some food items may have been misclassified due to the methods used for identifying and categorizing UPFs. However, in NHANES dietary data, detailed brand names, places of purchase, and nutritional composition for each food item are available to reasonably classify UPF. Inaccuracies may arise from using the Food and Nutrient Database for Dietary Studies to disaggregate potential handmade mixed dishes into underlying ingredients, because this database uses standard recipes based on assumptions about types and quantities of ingredients. To reduce potential classification errors, all food items were classified independently by 2 researchers. Absence of data or discrepancies regarding degree of processing were generally solved opting for the lesser degree of processing which may have underestimated UPF consumption. Known and suspected confounders were adjusted in the model; however, residual confounding or confounding from unknown or unmeasured factors may bias the results. Only participants aged 8-59 years were eligible for DXA scans, so adults aged 60 years and older were not included in this analysis.
Conclusion
In a national US sample, higher UPF consumption was positively associated with greater body fat—a direct measure of adiposity. These cross-sectional findings call for prospective, interventional, and mechanistic studies to assess the impact of UPF on body composition in different populations.
Supplementary Material
ACKNOWLEDGMENTS
We thank the reviewers and editors for their valuable comments. This research was supported by the S~ao Paulo Research Foundation (Processo 2018/17972-9-EMS; Processo 2015/14900-9-CAM), R01HL141427 (PI YL), R01HL115189-02 (PI DM), and 2R01HL115189 (PI DM). DM reports research funding from the NIH, Gates Foundation, Rockefeller Foundation, and Vail Institute for Global Research; consulting fees from Acasti Pharma, Barilla, Danone, and Motif FoodWorks; participating on scientific advisory boards of start-up companies focused on innovations for health including Beren Therapeutics Brightseed, Calibrate, DayTwo, Elysium Health, Filtricine, Foodome, HumanCo, January Inc., Perfect Day, Season, and Tiny Organics; and chapter royalties from UpToDate. All is outside the submitted work. Otherwise, no financial disclosures have been reported by the authors of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data described in the manuscript, code book, and analytic code will be made available pending e-mail request to the corresponding author.
Credit Author Statement
Junxiu Liu & Dariush Mozaffarian: Conceptualization; Junxiu Liu, Eurídice Martinez Steele, Carlos A. Monteiro & Dariush Mozaffarian: Methodology; Data curation: Junxiu Liu & Eurídice Martinez Steele; Junxiu Liu: Writing- Original draft preparation; Junxiu Liu: Visualization; All authors: Writing- Reviewing and Editing; Dariush Mozaffarian: Supervision.
References
- 1.Liu J, Steele EM, Li Y, et al. Consumption of Ultraprocessed Foods and Diet Quality Among U.S. Children and Adults. Am J Prev Med 2022;62:252–64. 10.1016/j.amepre.2021.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monteiro CA, Cannon G, Levy RB, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr 2019;22:936–41. 10.1017/S1368980018003762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteiro CA, Cannon G, Lawrence M, Costa Louzada ML and Pereira Machado P 2019. Ultra-processed foods, diet quality, and health using the NOVA classification system. Rome, FAO. [Google Scholar]
- 4.Cohen E, Cragg M, deFonseka J, Hite A, Rosenberg M, Zhou B. Statistical review of US macronutrient consumption data, 1965-2011: Americans have been following dietary guidelines, coincident with the rise in obesity. Nutrition 2015;31:727–32. 10.1016/j.nut.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 5.Monteiro CA, Levy RB, Claro RM, Castro IR, Cannon G. A new classification of foods based on the extent and purpose of their processing. Cad Saude Publica 2010;26:2039–49. 10.1590/S0102-311X2010001100005 [DOI] [PubMed] [Google Scholar]
- 6.Hall KD, Ayuketah A, Brychta R, et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab 2019;30:67–77 e3. 10.1016/j.cmet.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagliai G, Dinu M, Madarena MP, Bonaccio M, Iacoviello L, Sofi F. Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. Br J Nutr 2021;125:308–18. 10.1017/S0007114520002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Askari M, Heshmati J, Shahinfar H, Tripathi N, Daneshzad E. Ultra-processed food and the risk of overweight and obesity: a systematic review and meta-analysis of observational studies. Int J Obes (Lond) 2020;44:2080–91. 10.1038/s41366-020-00650-z [DOI] [PubMed] [Google Scholar]
- 9.Delpino FM, Figueiredo LM, Bielemann RM, et al. Ultra-processed food and risk of type 2 diabetes: a systematic review and meta-analysis of longitudinal studies. Int J Epidemiol 2021. 10.1093/ije/dyab247 [DOI] [PubMed] [Google Scholar]
- 10.Juul F, Vaidean G, Parekh N. Ultra-processed Foods and Cardiovascular Diseases: Potential Mechanisms of Action. Adv Nutr 2021;12:1673–80. 10.1093/advances/nmab049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kliemann N, Al Nahas A, Vamos EP, et al. Ultra-processed foods and cancer risk: from global food systems to individual exposures and mechanisms. Br J Cancer 2022. 10.1038/s41416-022-01749-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canhada SL, Luft VC, Giatti L, et al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Public Health Nutr 2020;23:1076–86. 10.1017/S1368980019002854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moradi S, Entezari MH, Mohammadi H, et al. Ultra-processed food consumption and adult obesity risk: a systematic review and dose-response meta-analysis. Crit Rev Food Sci Nutr 2021:1–12. 10.1080/10408398.2021.1946005 [DOI] [PubMed] [Google Scholar]
- 14.Wagner DR, Heyward VH. Measures of body composition in blacks and whites: a comparative review. Am J Clin Nutr 2000;71:1392–402. 10.1093/ajcn/71.6.1392 [DOI] [PubMed] [Google Scholar]
- 15.Nevill AM, Stewart AD, Olds T, Holder R. Relationship between adiposity and body size reveals limitations of BMI. Am J Phys Anthropol 2006;129:151–6. 10.1002/ajpa.20262 [DOI] [PubMed] [Google Scholar]
- 16.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol 2013;62:921–5. 10.1016/j.jacc.2013.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol 2006;35:83–92. 10.1093/ije/dyi253 [DOI] [PubMed] [Google Scholar]
- 18.Luscher TF. Novel insights into body fat distribution and cardiometabolic risk. Eur Heart J 2019;40:2833–6. 10.1093/eurheartj/ehz634 [DOI] [PubMed] [Google Scholar]
- 19.Chang K, Khandpur N, Neri D, et al. Association Between Childhood Consumption of Ultraprocessed Food and Adiposity Trajectories in the Avon Longitudinal Study of Parents and Children Birth Cohort. JAMA Pediatr 2021;175:e211573. doi: 10.1001/jamapediatrics.2021.1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neri D, Martinez-Steele E, Khandpur N, Levy R. Associations Between Ultra-processed Foods Consumption and Indicators of Adiposity in US Adolescents: Cross-Sectional Analysis of the 2011-2016 National Health and Nutrition Examination Survey. J Acad Nutr Diet 2022;122:1474–87 e2. 10.1016/j.jand.2022.01.005 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Kahn HS, Jackson SL, Steele EM, Gillespie C, Yang Q. Associations between ultra- or minimally processed food intake and three adiposity indicators among US adults: NHANES 2011 to 2016. Obesity (Silver Spring) 2022;30:1887–97. 10.1002/oby.23507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juul F, Parekh N, Martinez-Steele E, Monteiro CA, Chang VW. Ultra-processed food consumption among US adults from 2001 to 2018. Am J Clin Nutr 2022;115:211–21. 10.1093/ajcn/nqab305 [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Martinez Steele E, Du M, et al. Trends in Consumption of Ultraprocessed Foods Among US Youths Aged 2-19 Years, 1999-2018. JAMA 2021;326:519–30. doi: 10.1001/jama.2021.10238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B, Du Y, Wu Y, Snetselaar LG, Wallace RB, Bao W. Trends in obesity and adiposity measures by race or ethnicity among adults in the United States 2011-18: population based study. BMJ 2021;372:n365. doi: 10.1136/bmj.n365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. NHANES Survey Methods and Analytic Guidelines. Available at, https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx. Accessed on May 25, 2022.
- 26.Martinez Steele E, Baraldi LG, Louzada ML, Moubarac JC, Mozaffarian D, Monteiro CA. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open 2016;6:e009892. doi: 10.1136/bmjopen-2015-009892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juul F, Martinez-Steele E, Parekh N, Monteiro CA, Chang VW. Ultra-processed food consumption and excess weight among US adults. Br J Nutr 2018;120:90–100. 10.1017/S0007114518001046 [DOI] [PubMed] [Google Scholar]
- 28.Willett W Nutritional epidemiology: Oxford university press; 2012. [Google Scholar]
- 29.Centers for Disease Control and Prevention. Body Composition Procedures Manual. Available at, https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/Body_Composition_Procedures_Manual_2018.pdf. Accessed on July 6th 2022.
- 30.U.S. Department of Health and Human Services. National Health and Nutrition Examination Survey: Analytic Guidelines, 1999-2010. Available at, https://www.cdc.gov/nchs/data/series/sr_02/sr02_161.pdf. Accessed on Febuary 12, 2023.
- 31.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:S498–504. DOI: 10.1097/00005768-200009001-00009 [DOI] [PubMed] [Google Scholar]
- 32.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012;126:1301–13. DOI: 10.1161/CIRCULATIONAHA.111.067264 [DOI] [PubMed] [Google Scholar]
- 33.Vague J Sexual differentiation; Factor determining forms of obesity. Presse Med (1893) 1947;55:339. DOI: 10.1002/j.1550-8528.1996.tb00535.x [DOI] [PubMed] [Google Scholar]
- 34.Kissebah AH, Vydelingum N, Murray R, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 1982;54:254–60. DOI: 10.1210/jcem-54-2-254 [DOI] [PubMed] [Google Scholar]
- 35.Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest 1983;72:1150–62. DOI: 10.1172/JCI111040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohlson LO, Larsson B, Svardsudd K, et al. The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes 1985;34:1055–8. DOI: 10.2337/diab.34.10.1055 [DOI] [PubMed] [Google Scholar]
- 37.Larsson B, Svardsudd K, Welin L, Wilhelmsen L, Bjorntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 1984;288:1401–4. doi: 10.1136/bmj.288.6428.1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 1984;289:1257–61. doi: 10.1136/bmj.289.6454.1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neeland IJ, Turer AT, Ayers CR, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 2012;308:1150–9. DOI: 10.1001/2012.jama.11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 2005;165:777–83. DOI: 10.1001/archinte.165.7.777 [DOI] [PubMed] [Google Scholar]
- 41.Iyengar NM, Arthur R, Manson JE, et al. Association of Body Fat and Risk of Breast Cancer in Postmenopausal Women With Normal Body Mass Index: A Secondary Analysis of a Randomized Clinical Trial and Observational Study. JAMA Oncol 2019;5:155–63. DOI: 10.1001/jamaoncol.2018.5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hudda MT, Aarestrup J, Owen CG, et al. Association of Childhood Fat Mass and Weight With Adult-Onset Type 2 Diabetes in Denmark. JAMA Netw Open 2021;4:e218524. doi: 10.1001/jamanetworkopen.2021.8524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation 2015;132:1639–47. DOI: 10.1161/CIRCULATIONAHA.114.015000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konieczna J, Morey M, Abete I, et al. Contribution of ultra-processed foods in visceral fat deposition and other adiposity indicators: Prospective analysis nested in the PREDIMED-Plus trial. Clin Nutr 2021;40:4290–300. DOI: 10.1016/j.clnu.2021.01.019 [DOI] [PubMed] [Google Scholar]
- 45.National Health and Nutrition Examination Survey. Body composition procedures manual. Available at https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/Body_Composition_Procedures_Manual_2018.pdf Accessed on February 12, 2023.
- 46.Rhodes DG, Murayi T, Clemens JC, Baer DJ, Sebastian RS, Moshfegh AJ. The USDA Automated Multiple-Pass Method accurately assesses population sodium intakes. Am J Clin Nutr 2013;97:958–64. DOI: 10.3945/ajcn.112.044982 [DOI] [PubMed] [Google Scholar]
- 47.Basile A, Ruiz-Tejada A, Mohr A, et al. Ultra-Processed Foods Have a Lower Glycemic Index and Load Compared to Minimally Processed Foods. Current Developments in Nutrition 2022;6:504-. 10.1093/cdn/nzac077.007 [DOI] [Google Scholar]
- 48.Cuevas-Sierra A, Milagro FI, Aranaz P, Martinez JA, Riezu-Boj JI. Gut Microbiota Differences According to Ultra-Processed Food Consumption in a Spanish Population. Nutrients 2021;13. DOI: 10.3390/nu13082710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atzeni A, Martinez MA, Babio N, et al. Association between ultra-processed food consumption and gut microbiota in senior subjects with overweight/obesity and metabolic syndrome. Front Nutr 2022;9:976547. DOI: 10.3389/fnut.2022.976547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flemish Institute of Healthy Living (2020) Implications of food processing: the role of ultra-processed foods in a healthy and sustainable diet. Laken (Brussels), Online: gezondleven.be [Google Scholar]
- 51.Srour B, Kordahi MC, Bonazzi E, Deschasaux-Tanguy M, Touvier M, Chassaing B. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol Hepatol 2022. DOI: 10.1016/S2468-1253(22)00169-8 [DOI] [PubMed] [Google Scholar]
- 52.Heindel JJ, Blumberg B. Environmental Obesogens: Mechanisms and Controversies. Annu Rev Pharmacol Toxicol 2019;59:89–106. DOI: 10.1146/annurev-pharmtox-010818-021304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cordova R, Kliemann N, Huybrechts I, et al. Consumption of ultra-processed foods associated with weight gain and obesity in adults: A multi-national cohort study. Clin Nutr 2021;40:5079–88. 10.1016/j.clnu.2021.08.009 [DOI] [PubMed] [Google Scholar]
- 54.Taneri PE, Wehrli F, Roa Diaz ZM, et al. Association Between Ultra-Processed Food Intake and All-Cause Mortality: A Systematic Review and Meta-Analysis. Am J Epidemiol 2022. DOI: 10.1093/aje/kwac039 [DOI] [PubMed] [Google Scholar]
- 55.Fiolet T, Srour B, Sellem L, et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Sante prospective cohort. BMJ 2018;360:k322. doi: 10.1136/bmj.k322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romaguera D, Fernandez-Barres S, Gracia-Lavedan E, et al. Consumption of ultra-processed foods and drinks and colorectal, breast, and prostate cancer. Clin Nutr 2021;40:1537–45. DOI: 10.1016/j.clnu.2021.02.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


