Abstract
Multimodal hypersensitivity (MMH)—greater sensitivity across multiple sensory modalities (e.g., light, sound, temperature, pressure)—is associated with the development of chronic pain. However, previous MMH studies are restricted given their reliance on self-report questionnaires, narrow use of multimodal sensory testing, or limited follow-up. We conducted multimodal sensory testing on an observational cohort of 200 reproductive-age women including those at elevated risk for chronic pelvic pain conditions and pain-free controls. Multimodal sensory testing included visual, auditory, bodily pressure, pelvic pressure, thermal, and bladder pain testing. Self-reported pelvic pain was examined over four years. A principal component analysis of sensory testing measures resulted in three orthogonal factors that explained 43% of the variance: MMH, pressure pain stimulus-response, and bladder hypersensitivity. The MMH and bladder hypersensitivity factors correlated with baseline self-reported menstrual pain, genitourinary symptoms, depression, anxiety, and health. Over time, MMH increasingly predicted pelvic pain and was the only component to predict outcome four years later, even when adjusted for baseline pelvic pain. MMH was a better predictor of pelvic pain outcome than a questionnaire-based assessment of generalized sensory sensitivity. These results suggest that MMH’s overarching neural mechanisms convey more substantial long-term risk for pelvic pain than variation in individual sensory modalities. Further research on the modifiability of MMH could inform future treatment developments in chronic pain.
Keywords: Multimodal hypersensitivity, sensory testing, pelvic pain
Multimodal hypersensitivity (MMH), a hallmark feature of chronic pain conditions [23,29,34,43,53,78,95], is associated with the development of chronic pain [8,38] and serves as a determinant of treatment response [35,45]. MMH is increased sensitivity across multiple sensory modalities (e.g., light, sound, temperature, pressure) common in functional or chronic pain conditions [5,29,85]. Individuals with chronic pelvic pain conditions, like irritable bowel syndrome and bladder pain syndrome, similarly exhibit degrees of MMH despite not having any outward pelvic pathology (e.g., infection, endometriosis, etc.) [58,85]. Previous studies have attempted to quantify MMH [7,17,39,63,92], its stability over time [85], and its relationship to chronic pain severity and outcome [46,66,67,86]. Our understanding of MMH is limited given these investigations often relied on subjective self-report questionnaires (e.g., generalized sensory sensitivity [85]), inadequately assessed MMH by using unidimensional quantitative sensory testing (QST), or lacked long-term follow-up. Despite the ubiquitous use of QST to study pain conditions [89], we lack a full picture of how different QST methods relate to each other and predict changes in pelvic pain symptomatology [41,66]. A synergistic approach encompassing multiple modalities of QST with longitudinal symptom assessment could improve our understanding of MMH and further quantify an individual’s susceptibility to developing chronic pain.
Therefore, we performed secondary analyses on a four-year longitudinal cohort of young reproductive age women that had multimodal sensory testing performed at baseline (CRAMPP: Chronic Pain Risk Associated with Menstrual Pelvic Pain; NCT02214550). Because menstrual pain is among the leading risk factors for chronic pelvic pain [62,100], we focused on recruitment of women with significant menstrual pain. We also included pain-free controls and a subset of women with chronic pelvic pain to provide a full range of sensory profiles for analysis. CRAMPP’s multimodal sensory testing battery included provocation with pressure, cold, and audio/visual stimuli, temporal summation (a measure of spinal wind-up), conditioned pain modulation (a metric of descending inhibition), and bladder distension (a measure of visceral sensitivity). Analysis of CRAMPP’s baseline data revealed that individuals with dysmenorrhea and bladder pain hypersensitivity have impaired conditioned pain modulation, increased sensitivity to pressure and thermal stimuli (e.g., cold) [51], and visual provocation [59], even without any formal chronic pain diagnoses. However, we have not explored whether these sensory sensitivities were unified (i.e., MMH) and predicted long-term pelvic pain outcome. Therefore, we analyzed the measures obtained from sensory testing in two ways. We combined our multimodal sensory testing panel measures into three a priori defined sensory testing composites: traditional QST, non-invasive bladder distension (i.e., provoked visceral sensitivity), and supraspinal audio/visual sensitivity. Their ability to predict longitudinal pelvic pain outcome was compared to sensory testing components that were instead derived from principal component analysis (PCA). We hypothesized that the PCA would produce an MMH component that would predict long-term pelvic pain outcome and outperform our a priori composites of sensory testing.
Methods
Participants
A total of 354 participants were enrolled in CRAMPP between August 2014 and December 2018 (see Figure 1). Participants were recruited by public advertising and flyers in Evanston, Illinois and the surrounding communities. The severity of menstrual pain was confirmed with internet-based prospective symptom diaries for 1–2 months prior to enrollment [51].
Figure 1.
Participant Recruitment and Enrollment Flowchart for CRAMPP.
Enrolled participants included women with low menstrual pain (<3 on a 0–10 Numerical Rating Scale [NRS]; 0 = no pain; 10 = worst pain imaginable) or moderate to severe menstrual pain (≥5 on a 0–10 NRS) but no other chronic pain. We also included participants diagnosed with bladder pain syndrome (BPS), and participants diagnosed with a non-pelvic chronic pain condition (general pain ≥5 on a 0–10 NRS for more than three consecutive months). BPS participants were required to meet American Urological Association diagnostic criteria, and report bladder pain ≥3 on a 0–10 NRS for more than 3 consecutive months [42].
Participants were excluded for the presence of active pelvic or abdominal malignancies, absence of regular menses (except the chronic pain without BPS group), active genitourinary infection in the last four weeks, inability to read or comprehend the informed consent in English, refusal to undergo pelvic examination/testing, hypertension, or refusal to withdraw from oral contraceptives for two months prior to the study visit. All participants provided informed consent and followed protocols approved by NorthShore University HealthSystem’s Institutional Review Board (EH13–094). Participants were monetarily compensated for their time.
From the 354 participants, n=154 were excluded for the following reasons: 52 participants were missing one or more data points from QST (e.g., declined participation in task(s), equipment malfunction, migraine sensitivity precluded participation in visual stimulation, etc.) and one participant was under the influence of recreational or illicit substances during the testing appointment. Additionally, 25 participants were lost to follow-up, 16 participants withdrew from the study, and 60 participants were disqualified (e.g., over-recruited dysmenorrhea without bladder pain, started oral contraceptives, or were unable to complete protocol). Ultimately, a total of 200 participants had complete QST data from their baseline assessment visit.
Participants completed annual questionnaires following their baseline visit for up to five years. Annual questionnaires were a reduced version of what was asked at their screen and baseline assessment visits and were used to assess pelvic pain outcome. Because the collection of year five annual questionnaires was incomplete at the time of analysis, we included here completed annual questionnaires until year four. Demographic variables of interest are presented in Table 1.
Table 1.
Participant Baseline Demographics.
| Measure | n (%) | Mean (SD) | Measure | n (%) |
|---|---|---|---|---|
| Race | Pre-Existing conditions | |||
| White | 121 (60.5%) | Ovarian Cysts | 27 (14%) | |
| Black or African American | 32 (16%) | Lower Back Pain | 21 (11%) | |
| Asian | 30 (15%) | Chronic Pelvic Pain | 18 (9%) | |
| Multiple | 15 (7.5%) | Migraine Headaches | 18 (9%) | |
| Native American | 1 (.5%) | Irritable Bowel Syndrome | 16 (8%) | |
| No Response | 1 (.5%) | Bladder Pain Syndrome | 13 (7%) | |
| Ethnicity | Endometriosis | 13 (7%) | ||
| Not Hispanic or Latino | 176 (88%) | Chronic Constipation | 6 (3%) | |
| Hispanic or Latino | 24 (12%) | Fibroids | 5 (3%) | |
| Groups | Inflammatory Bowel Disease | 5 (3%) | ||
| Dysmenorrhea | 132 (66%) | Kidney Stones | 4 (2%) | |
| Pain-Free Controls | 30 (15%) | Fibromyalgia | 3 (2%) | |
| Chronic Pain | 22 (11%) | Pelvic Inflammatory Disease | 1 (1%) | |
| Bladder Pain Syndrome | 16 (8%) | Chronic Diarrhea | 1 (1%) | |
| Age (years) | 200 | 24.9 (6.4) | Arthritis | 1 (1%) |
| Height (inches) | 196 | 64.6 (2.8) | Parous | |
| Weight (lbs.) | 195 | 143 (30.1) | Ever Pregnant | 29 (15%) |
| Body Mass Index (BMI) | 195 | 24 (4.63) | 1 or more deliveries | 14 (7%) |
Note. Percentages were calculated from the total sample size (n=200). Some participants had multiple diagnoses.
From the 200 participants with complete QST data, 22 were randomized into a 12-month clinical trial that evaluated the efficacy of cyclical (n=4) and continuous (n=12) oral contraceptive pills (OCPs) for treating menstrual and bladder pain compared to a control group that did not take OCPs (n=6). QST was administered by research staff unaware of participant group assignment or history. Because of the low adherence to OCP use and small comparative sample sizes, we did not consider participants’ enrollment in the clinical trial as exclusionary from our analyses of subsequent annual questionnaires. In addition, multimodal sensory testing was performed on all participants before beginning the use of OCPs, so analyses on internal relationships of MMH on longitudinal outcome were not expected to be confounded by OCP use.
Procedure
Eligible participants that were enrolled in the study first participated in a screen visit and then a second baseline assessment visit at Evanston Hospital (Evanston, IL). During the baseline visit, performed in the midluteal (pain-free) phase of the menstrual cycle, participants completed a panel of medical history and psychosocial questionnaires. Participants next underwent a multimodal sensory testing panel that included mechanosensation, cold pressor, visceral provocation, conditioned pain modulation (CPM), temporal summation (TS), and auditory/visual stimulation. All sensory testing measures and self-report questionnaires are detailed below.
Bladder Filling Test
We have developed a non-invasive bladder filling task [90] to characterize visceral hypersensitivity observed across CPP conditions like bladder pain syndrome and irritable bowel syndrome [6,30,57]. This task has been validated in participants with bladder pain syndrome and chronic pelvic pain [91]. Notably, even in participants without bladder pain conditions, elevated pain during this task was associated with more frequent report of daily bladder symptoms [49]. After voiding their bladder, participants ingested 20 fluid ounces of water. They rated their bladder pain and urgency on a 0–100 VAS across four time points: baseline (BL), first sensation (FS) of bladder filling, first urge (FU) which is the usual desire to void their bladder, and maximum tolerance (MT) of bladder filling (corresponding to widely used cystometric sensory thresholds) [48]. After reaching maximum tolerance (or 2 hours) and voiding their bladder, participants rated their perceived bladder pain on 0–100 VAS according to four McGill pain questionnaire descriptors to potentially differentiate Aδ from C fiber pain components: sharp, pressing, dull, and prickling [10]. In sum, 12 measures from the bladder task were included in this analysis (i.e., four provoked bladder pain ratings and four bladder urgency ratings across the time points, and four McGill descriptor ratings at completion).
Pressure Pain Thresholds (PPTs)
We examined PPTs transvaginally and externally because local alterations in myofascial pelvic sensitivity [50] and widespread alterations in bodily sensitivity are thought to underlie centralized pain [37]. We determined participants’ PPTs using a digital algometer (Wagner Instruments, Greenwich, CT) with a 1-cm2 rubber tip driven at a ramp rate of 4 Newtons (N)/sec at three fibromyalgia tender point sites [97]—right trapezius, the right medial knee fat pad, and the right greater trochanter (hip)—and the forehead. Vaginal PPTs were measured using a finger mounted 1 cm2 diameter force-sensing resistor (Trossen Robotics, Downers Grove, IL) at a ramp rate of 0.5 N/sec at four vaginal sites: right (5 o’clock position) and left iliococcygeus (7 o’clock), anteriorly against the bladder (12 o’clock), and posteriorly against the anorectal raphe (6 o’clock). Body and vaginal PPT procedures utilized software that guided stimulus application and resulted in high (α > .89) inter- and intra-examiner reliability [51]. After each set of PPTs, participants were asked to rate their pain on a 0–10 NRS at each site. These after-pain ratings were adjusted for baseline pain ratings recorded before PPT procedures. We previously established that PPTs and after-pain represent two different components of sensation contributing to MMH [50]. PPTs represent the average force from two independent trials separated by a 2 minute break period. These averaged PPTs were multiplied by −1 so that a greater value indicates increased sensitivity. In total, 16 PPT measures were included in the planned PCA: eight PPTs (four body and four vaginal sites) and eight after-pain ratings (four body and four vaginal sites).
Conditioned Pain Modulation (CPM)
We included CPM in our QST panel because prior studies have demonstrated that CPM predicts pain outcome [98,99]. CPM efficiency is thought to be a metric of descending inhibition as tested by “pain inhibits pain” paradigms [70]. We assessed participants’ CPM by repeat PPT testing of the left medial knee fat pad before and after ice water immersion of the contralateral hand. After an initial PPT measurement, participants waited two minutes before submerging their right hand up to their wrist into a circulating water bath maintained at 0–6°C. After 10 seconds of submersion, participants rated their hand/cold pain on a 0–10 NRS. After 20 seconds of submersion, a repeat PPT measure was taken from the left medial knee fat pad, after which participants were allowed to remove their hand from the cold-water bath. CPM was calculated by subtracting the PPT force (in Newtons) taken before the water bath from the PPT taken after the water bath (i.e., CPM =After-Before). CPM values were then multiplied by −1 so that a greater number denoted reduced/inefficient CPM (i.e., increased impairment) before including results in the PCA. Additionally, cold pain ratings were adjusted for the water temperature by extracting the residuals from a linear model predicting cold pain as a function of water temperature. In total, two measures from CPM testing were included into the PCA: one CPM score and one cold pain rating adjusted for water temperature.
Temporal Summation (TS)
Increased response to repeated application of noxious stimuli (i.e., wind-up pain) is thought to reflect a unique component of spinally mediated sensitization that may be associated with increased risk of chronic pain [19,71,88]. Therefore, we measured TS using a commonly used strategy: 10 pressure pulses delivered to the right medial knee fat pad using the same body PPT algometer as described above [19]. Each pulse was delivered at a ramp rate of 4 N/sec with 1 second breaks between pulses using a software-based metronome to guide application. Each pulse was applied until the initial threshold for a pain rating of one was reached. Participants rated their baseline pain at the application site on a 0–10 NRS following each pulse. The TS task ended when participants reached a pain rating ≥ six or after the tenth trial. Each participant’s baseline pain was subtracted from their pain ratings collected after each pulse. A total of three TS measures were entered into the PCA: the average pain experienced during TS, the rate of change in pain ratings as a function of trial (i.e., slope), and the maximum TS trial experienced. The maximum TS trial was multiplied by −1 so that greater values indicated increased sensitivity (i.e., fewer trials allowed due to reaching a pain rating of six).
Visual Stimulation
Investigations of MMH are well served to include additional sensory modalities, such as vision, given that light hypersensitivity is commonly reported in conditions with generalized sensory hypersensitivity, like fibromyalgia and migraine [32,45,65]. We assessed participants’ visual unpleasantness sensitivity by presenting a periodic pattern-reversal blue/yellow checkerboard stimulus alternating at 25 Hz for 20 seconds across five blocks. Each block contained a single maximal brightness intensity (1, 30, 60, 90, or 120 lux), and block order was randomized across participants. After each block, participants rated stimulus unpleasantness using the Gracely Box Scale which lists the numbers 0 to 20 next to a set of verbal anchors [36]. A total of two visual sensitivity measures were entered into the PCA: the average visual unpleasantness rating across the blocks, and the rate of change in visual unpleasantness as a function of brightness intensity [59].
Auditory Stimulation
Auditory stimulation assessed an additional sensory modality with reported hypersensitivities in functional pain syndromes [53,64,95]. Prior to measuring the participants’ auditory unpleasantness sensitivity, a program first verified that participants maintained less than 20 dB hearing loss (250–8000 Hz) [75] to equate hearing ability. Next, we presented a series of auditory steady state 80Hz [26] volume modulated tones (1200 and 1350Hz) in random order of intensity (15, 30 45, or 60 dB) delivered via ground-isolated optimally flat frequency response pneumatic insert earphones (Etymotic, Elk Grove Village, IL). After each 20 second stimulus, the participant rated her perceived unpleasantness on the Gracely Box Scale as described above. A total of two auditory sensitivity measures were entered into the PCA: the average auditory unpleasantness rating across the blocks, and the rate of change in auditory unpleasantness as a function of loudness intensity (i.e., slope).
We recorded participants’ scalp electroencephalography (EEG) during visual and auditory sensitivity tasks. Given the behavioral focus of this investigation, these EEG data were out of scope and not included in the present investigation. EEG data from the visual task are published elsewhere [59].
Self-Report Questionnaires
In the health history profile, we administered several validated self-report questionnaires that assessed various aspects of pelvic pain and associated symptoms. Bladder symptom severity was assessed via the Interstitial Cystitis Symptom Index (ICSI) and Problem Index (ICPI) [72]. The Genitourinary Pain Index (GUPI) provided a complementary assessment of these urogenital symptoms [20]. The Complex Medical Symptoms Inventory (CMSI) was used to assess functional symptom burdens that occurred for at least three months in the past year and at any time in the participants’ lifetime [96]. Somatic symptoms were assessed using the Brief Symptom Inventory (BSI) [25]. We assessed several health domains from the NIH Patient Reported Outcomes Measurement System (PROMIS) [16], including anxiety (short form 8a), depression (8b), pain interference (6), pain behavior (7), global physical health (4), and global mental health (4). Specifically, PROMIS raw short form scores were analyzed. Menstrual pain is associated with non-cyclic pelvic pain [93] and non-pelvic pain hypersensitivity [51,54,73,74], suggesting that menstrual pain may be a risk factor for developing chronic pain [54,73]. Therefore, we assessed participants’ menstrual pain on a 0–100 VAS on the worst day of their period over the past three months in the absence of pain relievers (e.g., non-steroidal anti-inflammatory drugs [NSAIDs], acetaminophen, etc.)
Pelvic Pain Outcome
Participants completed annual questionnaires virtually by email in REDCap for up to four years. As part of this questionnaire, participants rated their average feeling of 1) menstrual and non-menstrual pelvic pain, 2) pain with urination, and 3) pain with bowel movements during the past week using a 0–100 VAS (0=no pain; 100=worst pain imaginable). VAS scales are more sensitive to changes than descriptive word-based scales [87] and have linear properties amenable to averaging [68]. Also, these three questions were highly collinear at the baseline assessment: 1 vs. 2, r (198) = .61 95% confidence interval (CI) [.51, .69], p < .001; 1 vs. 3, r (198) = .56 [.45, .65], p < .001; and 2 vs. 3, r (198) = .66 [.57, .73], p < .001. Therefore, we averaged these three questions to create a composite pelvic pain outcome variable. Similar composite pain recall variables formed by averaging have demonstrated high validity and reliability comparable to daily diary pain ratings [56].
QST, Bladder Test, and Audio/Visual Predictor Composite Variables
To evaluate the ability of different sensory tests to predict pelvic pain outcome, we combined the 40 measures from the multimodal sensory testing panel into three composite variables using summed Z-scores: traditional QST measures, bladder test measures, and audio/visual stimulation. The QST composite comprised 25 measures including PPTs (i.e., thresholds, after pain, and descriptors), TS, CPM, and cold pain. The bladder test composite comprised 11 measures from the bladder test, including pain, urgency and descriptors. The audio/visual sensitivity composite comprised 4 measures, including mean unpleasantness and slope of the stimulus-response function from the auditory and visual tests. Prior to calculating Z-scores, all measures maintained the same directionality such that greater values denoted increased pain/impairment/sensitivity. Final composites were mean centered for regression analyses.
Generalized Sensory Sensitivity Brief Scale
To examine the predictive ability of self-reported somatic symptoms, we utilized the Generalized Sensory Sensitivity Brief Scale (GSS Brief) [85]. The GSS Brief approximates GSS (developed using confirmatory factor analysis) that captures comorbid sensory hypersensitivity often present in chronic overlapping pain conditions. Participants indicate regions on a body map where they have experienced pain during the last week and endorse whether they had any of the following symptoms for at least three months in the past year: 1) dry mouth, 2) rapid heart rate, 3) problems with balance, 4) sensitivity to certain chemicals, such as perfumes, laundry detergents, gasoline, and others, 5) sensitivity to sound, and 6) frequent sensitivity to bright lights. The GSS Brief is a good approximation of GSS, and the GSS factor structure was recently replicated in the cohort used in this study [84].
Statistical Analyses
A formal power analysis was used to plan the broader clinical trial (NCT02214550). Because the present investigation was a secondary analysis, all participants’ data were included if complete.
To assess how well baseline QST, bladder test, and audio/visual measures independently predicted future pelvic pain outcome, we performed four multiple regressions using self-report data from annual follow-up questionnaires. Pelvic pain outcome served as the dependent variable. The independent variables included the summed Z-scores (QST, bladder test, audio/visual sensitivity) defined above. We also included baseline pelvic pain outcome as a covariate. All independent variables were measures collected at the participants’ baseline visit.
Additionally, we reduced the dimensionality of our sensory testing panel (40 measures/columns) using principal components analysis (PCA) [2]. All measures maintained the same directionality such that greater values denoted increased pain/impairment/sensitivity/etc. Each column was then Z-scored prior to decomposing the matrix via singular value decomposition [1].
Inferential statistics were performed using data resampling techniques [2,9]. Permutation testing for the PCA was conducted by creating null distributions for each principal component (PC) by shuffling each column’s values without replacement and repeating the PCA for 2,000 iterations. Probability values for each PC were calculated by comparing our fixed-effects eigenvalues to their respective null distributions. Contributions were calculated by dividing each measure’s squared factor score by the component eigenvalue [9]. Bootstrap samples were formed by selecting participants at random with replacement. Bootstrap distributions were formed by supplementary projecting the bootstrap samples onto the eigenspace generated from the fixed-effects analysis [9]. This procedure was repeated 2,000 times. Bootstrapping quantified each measure’s loading stability/contribution importance using bootstrap ratios (BSRs). A BSR is the ratio between a measure’s fixed-effect factor score (i.e., loading) and the standard deviation of its bootstrapped distribution. BSRs are interpreted like Student’s t value. Therefore, significantly contributing measures have |BSRs| > 1.96 (p < .05).
To compare PCs with measures not included in their initial formulation, we calculated bootstrapped correlations between the row-wise (i.e., participant) factor scores and validated self-report questionnaires. To assess how well QST-based PCs predicted future pelvic pain outcome, we repeated the multiple regression procedure as described above except that pertinent PCs served as the independent variables instead of the three Z-scored measures.
A sensitivity analysis was performed to examine whether adjusting for prevalence rates of dysmenorrhea, bladder pain syndrome, and other types of chronic pain in the general population altered regression results. Keeping our sample size constant (n=200), participants were sampled with replacement according to the following prevalence rates [12,24,82]: 50% pain-free healthy controls (n=100), 40% moderate-severe dysmenorrhea (n=80), 5% bladder pain syndrome (n=10), 5% other chronic pain (n=10). Regression analyses were recomputed using these bootstrapped data samples. This procedure was repeated for 2,000 iterations. Mean regression estimates and effect sizes were then compared to original unadjusted values.
We conducted post hoc model comparison analyses to assess the relative performance of PCs over a priori defined sensory testing composites (summed Z-scores) in predicting future pelvic pain. Given the multicollinearity between PCs and the composites, we compared the Akaike and Bayesian information criterion (AIC and BIC, respectively) values between two models estimated separately at each year of follow-up in accordance with previously published guidelines [18]. Evidence ratios for the best model versus a comparator model were calculated as where Δ is the change in AIC between models [see 18].
Analyses were performed in R (4.1.0) within RStudio (1.4.1106) using the following packages: fixed- and random-effects PCA were performed using ExPosition [9], bootstrapped correlations were computed using psych [76], effect sizes were calculated using effectsize [11], data processing and figures were generated using tidyverse packages [94], color palettes were inspired by RColorBrewer [69] and ghibli [52]. All code used to process, analyze, and visualize the data in this manuscript is available on GitHub (https://github.com/mkmiecik14/mmh), and data are available on Open Science Framework (https://doi.org/10.17605/OSF.IO/27KY9).
Results
QST and Visceral Sensitivity Modestly Predict Pelvic Pain Outcome
Distributions of predictor variables are visualized in Figure 2A (see Table 2 and Table 3 for complete sensory testing data). Median pelvic pain outcome across the four-year follow up was stable and many participants reported moderate pelvic pain (see Figure 2B left panel). Cross-sectional relationships between sensory tests and baseline pelvic pain demonstrated that sensitivities across the QST, bladder test, or audio/visual composites were associated with worse baseline pelvic pain (see Figure 2B right panel).
Figure 2. MMH provides better prediction of future pelvic pain.
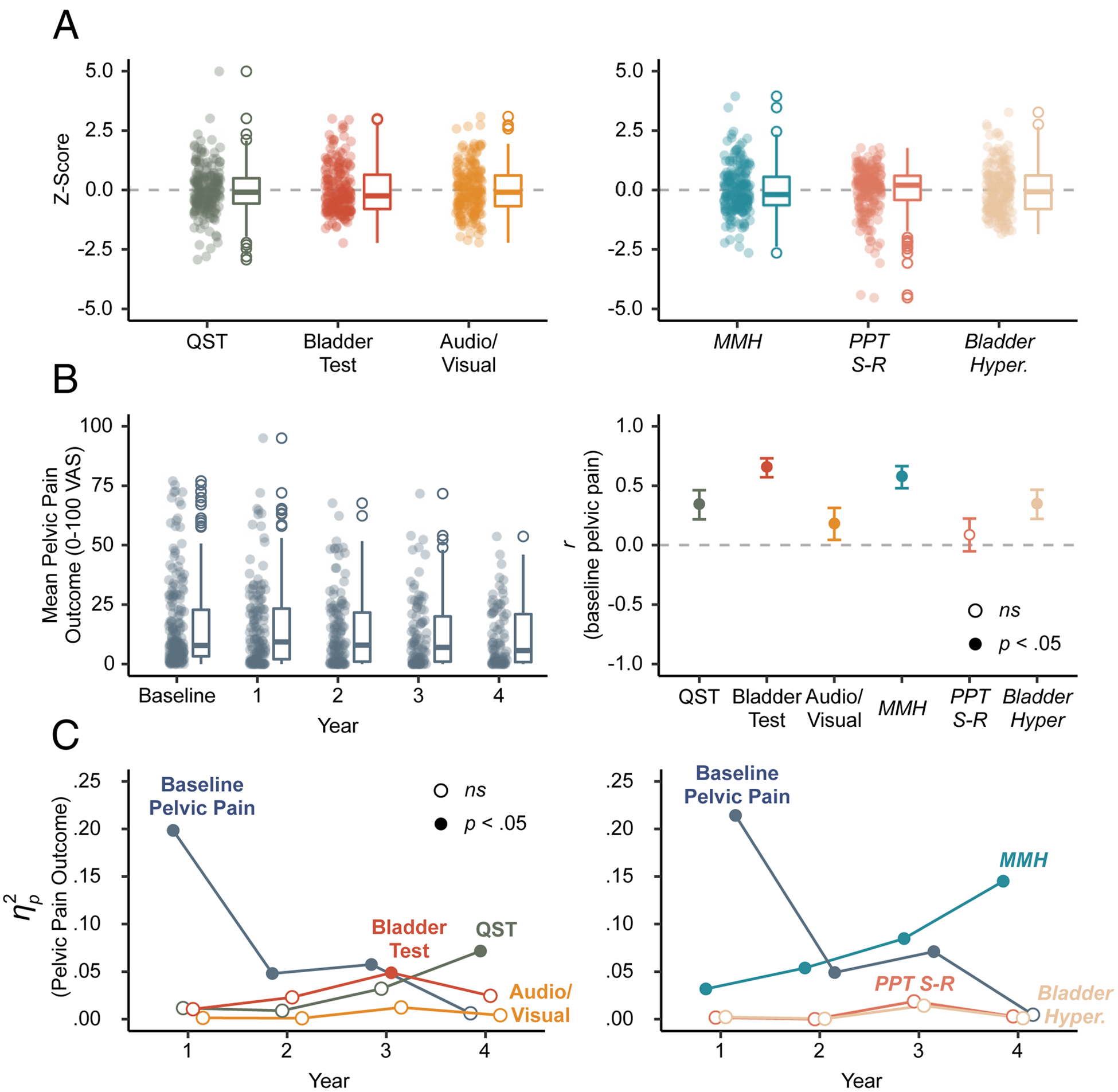
A) Box plots of Z-scores for each sensory testing composite variable (left) and principal component (PC) used for regression modeling (right). B) Box plots of pelvic pain outcome across the baseline visit and annual questionnaires (left); Correlations between baseline pelvic pain and predictors (i.e., sensory testing composites and PCs). Error bars are 95% confidence intervals and all correlations were significant (filled circles) except PPT S-R (right). C) Explained variance () of pelvic pain outcome for each sensory testing composite (left) and PC (right) accounting for baseline pelvic pain. Significant predictors (filled circles) include baseline pelvic pain, bladder test, QST, and MMH. Italicized text labels refer to PCs. QST=quantitative sensory testing; MMH=multimodal hypersensitivity; PPT-SR=pressure pain threshold stimulus-response (function); Hyper.=hypersensitivity; VAS=visual analog scale.
Table 2.
Descriptive Statistics for PPTs and Bladder Task of CRAMPP Participants.
| QST Measure | Mean | SD | Min | Max |
|---|---|---|---|---|
| PPT (Newtons) | ||||
| Vaginal 12 o’clock | 9.7 | 6.8 | 0.9 | 30.7 |
| Vaginal 5 o’clock | 9.0 | 5.6 | 0.4 | 31.0 |
| Vaginal 6 o’clock | 8.6 | 5.6 | 0.6 | 29.4 |
| Vaginal 7 o’clock | 7.2 | 4.9 | 0.8 | 30.1 |
| Forehead | 18.1 | 8.9 | 3.5 | 42.5 |
| Hip | 25.5 | 12.0 | 4.5 | 73.7 |
| Knee | 21.8 | 9.6 | 2.1 | 65.3 |
| Shoulder | 20.4 | 9.8 | 4.4 | 57.2 |
| After-pain (baseline adjusted 0–10 NRS) | ||||
| Vaginal 12 o’clock | 1.4 | 1.0 | −4.0 | 6.0 |
| Vaginal 5 o’clock | 1.5 | 1.1 | −3.0 | 6.5 |
| Vaginal 6 o’clock | 1.4 | 1.0 | −3.0 | 7.0 |
| Vaginal 7 o’clock | 1.5 | 1.1 | −3.5 | 7.0 |
| Forehead | 1.2 | 0.6 | 0.0 | 5.0 |
| Hip | 1.3 | 0.7 | −0.5 | 5.5 |
| Knee | 1.2 | 0.6 | −0.5 | 3.5 |
| Shoulder | 1.2 | 0.6 | −3.0 | 4.5 |
| Bladder Task (0–100 VAS) | ||||
| BL Pain | 5.1 | 10.7 | 0 | 57 |
| FS Pain | 8.4 | 13.4 | 0 | 66 |
| FU Pain | 16.2 | 19.9 | 0 | 100 |
| MT Pain | 30.7 | 29.5 | 0 | 93 |
| FS Urgency | 21.7 | 14.1 | 0 | 67 |
| FU Urgency | 48.8 | 16.2 | 2 | 100 |
| MT Urgency | 83.4 | 12.9 | 6 | 100 |
| Bladder Descriptors (0–100 VAS) | ||||
| Dull | 27.3 | 28.1 | 0 | 90 |
| Pressing | 48.2 | 32.0 | 0 | 100 |
| Prickling | 13.9 | 20.6 | 0 | 87 |
| Sharp | 20.3 | 25.6 | 0 | 98 |
| Pelvic Descriptors (0–10 NRS) | ||||
| Dull | 1.4 | 1.7 | 0 | 9 |
| Pressing | 3.3 | 2.3 | 0 | 10 |
| Prickling | 0.6 | 1.5 | 0 | 8 |
| Sharp | 2.0 | 1.9 | 0 | 8 |
Note. After-pain ratings were adjusted by subtracting the baseline pain ratings prior to PPT testing. Therefore, negative values are possible when PPT reduced after-pain ratings below the baseline. PPT=Pressure Pain Threshold; BL=Baseline; FS=First Sensation; FU=First Urge; MT=Maximum Tolerance; NRS=numeric rating scale; VAS=visual analog scale.
Table 3.
Additional Multimodal Sensory Testing Descriptive Statistics (Cold Pain, CPM, TS, and Visual/Auditory Stimulation) of CRAMPP Participants.
| Measure | Mean | SD | Min | Max |
|---|---|---|---|---|
| Cold Pain (0–10 NRS) | ||||
| Rating | 5.49 | 2.34 | 0 | 10 |
| Residuals | −0.1 | 2.3 | −5.7 | 4.5 |
| CPM | ||||
| Left Knee (Newtons) | 6.7 | 9.1 | −10.8 | 47.2 |
| Temporal Summation | ||||
| Max Trial | 9.5 | 1.6 | 2 | 10 |
| Mean Pain (0–10 NRS) | 2.2 | 1.3 | −0.5 | 5.3 |
| Slope | 0.2 | 0.4 | −0.3 | 3.0 |
| Visual Unpleasantness | ||||
| Mean (0–20 GBS) | 8.1 | 3.9 | 0.0 | 19.6 |
| Slope | 0.6 | 0.7 | −1.5 | 3.0 |
| Auditory Unpleasantness | ||||
| Mean (0–20 GBS) | 6.4 | 2.5 | 1.2 | 13.6 |
| Slope | 1.9 | 0.8 | −1.3 | 4.0 |
Note. Cold pain residuals, not ratings, were used in the principal component analysis. CPM=conditioned pain modulation; NRS=numeric rating scale; GBS=Gracely Box Scale.
Multiple regressions were used to assess how well QST, bladder test, and audio/visual testing predicted pelvic pain outcome on annual questionnaires administered up to four years following the baseline visit (see Table 4). We accounted for baseline pelvic pain by including it as a covariate (see Table S1 for descriptive statistics). Baseline pelvic pain was the strongest predictor of year 1 pelvic pain, but steadily declined over time (see Figure 2C left panel). The bladder test and QST predicted pelvic pain at years 3 and 4, respectively. Audio/visual testing did not predict outcome at any year. After adjusting for population-based prevalence rates of patient groups, baseline pelvic pain was a stronger predictor of year 4 pelvic pain outcome (see Figure S1). Also, QST explained little to no variance in pelvic pain outcome at any year. Thus, overall baseline pelvic pain was a better predictor of future pelvic pain than QST, bladder test, and audio/visual composites during the first 3 years, but was not significant at year 4.
Table 4.
Regression Models of Pelvic Pain Outcome for Summed Z-Score Sensory Testing Composites.
| Year | Parameter | b | SE | β | SS | MSE | F | p | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Intercept | 16.3 | 1.2 | 0.58 | 37298 | 194 | 191.9 | < .001 | |
| Baseline Pelvic Pain | 0.5 | 0.1 | 0.51 | 0.20 | 6641 | 34.2 | < .001 | ||
| QST | 0.1 | 0.1 | 0.10 | 0.01 | 318 | 1.6 | 0.20 | ||
| Bladder Test | 0.3 | 0.2 | 0.11 | 0.01 | 286 | 1.5 | 0.23 | ||
| Audio/Visual | 0.2 | 0.5 | 0.03 | 0.00 | 37 | 0.2 | 0.66 | ||
| 2 | Intercept | 14.2 | 1.2 | 0.52 | 25407 | 186 | 135.8 | < .001 | |
| Baseline Pelvic Pain | 0.2 | 0.1 | 0.26 | 0.05 | 1171 | 6.3 | 0.01 | ||
| QST | 0.1 | 0.1 | 0.10 | 0.01 | 209 | 1.1 | 0.29 | ||
| Bladder Test | 0.4 | 0.2 | 0.19 | 0.02 | 546 | 2.9 | 0.09 | ||
| Audio/Visual | 0.2 | 0.5 | 0.03 | 0.00 | 23 | 0.1 | 0.72 | ||
| 3 | Intercept | 12.8 | 1.3 | 0.51 | 15380 | 160 | 95.2 | < .001 | |
| Baseline Pelvic Pain | 0.2 | 0.1 | 0.27 | 0.06 | 907 | 5.6 | 0.02 | ||
| QST | 0.2 | 0.1 | 0.17 | 0.03 | 493 | 3.0 | 0.08 | ||
| Bladder Test | 0.5 | 0.2 | 0.26 | 0.05 | 762 | 4.7 | 0.03 | ||
| Audio/Visual | −0.6 | 0.5 | −0.10 | 0.01 | 187 | 1.2 | 0.29 | ||
| 4 | Intercept | 11.7 | 1.2 | 0.52 | 11317 | 127 | 89.4 | < .001 | |
| Baseline Pelvic Pain | 0.1 | 0.1 | 0.10 | 0.01 | 65 | 0.5 | 0.48 | ||
| QST | 0.3 | 0.1 | 0.27 | 0.07 | 801 | 6.3 | 0.01 | ||
| Bladder Test | 0.4 | 0.3 | 0.21 | 0.02 | 262 | 2.1 | 0.15 | ||
| Audio/Visual | 0.3 | 0.5 | 0.06 | 0.00 | 43 | 0.3 | 0.56 |
Note. The df for each model were: Year 1 (1, 138), Year 2 (1, 124), Year 3 (1, 92), and Year 4 (1, 82).
PCA Identifies Three Components Explaining Variability in Multimodal Sensory Testing
We determined the number of PCs underlying QST variability across the cohort by examining the scree plot (see Figure S2 and Table S2), permutation testing results, and loadings using geometrically plotted factor scores of QST measures [2]. Accordingly, we identified three components as interpretable. The first PC (PC1) explained 20.6% of the variance (p = .0005), the second (PC2) 12.4% (p = .0005), and the third (PC3) 446 9.5% (p = .0005).
Factor score plots for the first three PCs are shown in Figure 3 (see Figure S3 for contributions and Figure S4 for bootstrapped significance of factor loadings). Given that all measures loaded positively on PC1, we interpret PC1 to represent MMH (i.e., increased sensitivity on one measure was associated with an increased sensitivity on another). Forehead, hip, knee, shoulder, and vaginal PPTs positively loaded on PC2, while their respective after-pain ratings were opposed on PC2. This factor is representative of a stimulus-response function of pressure and after-pain resulting from PPT testing, hereafter referred to as PPT S-R (PPT stimulus-response). In other words, participants with lower PPTs (i.e., less force, greater sensitivity) reported less after-pain ratings. PC3 depicted an opposing relationship between bladder task measures and PPTs (thresholds and after-pain ratings). Given that additional measures (e.g., visual mean) had weak loadings on PC2, PPT-SR may represent another complex integratory mechanism. However, the contribution of these additional measures steeply drops off (Figure S3). Given the orthogonality of PCs, PC3 captured bladder pain hypersensitivity that was distinct from PC1 (MMH). Hereafter, we distinguish our interpretations of the observed PCs from theoretical constructs by using italics for PC interpretations, e.g., MMH = PC1, and unitalicized text for theoretical constructs (e.g., MMH).
Figure 3. Factor Score Plots of Sensory Testing Variables.
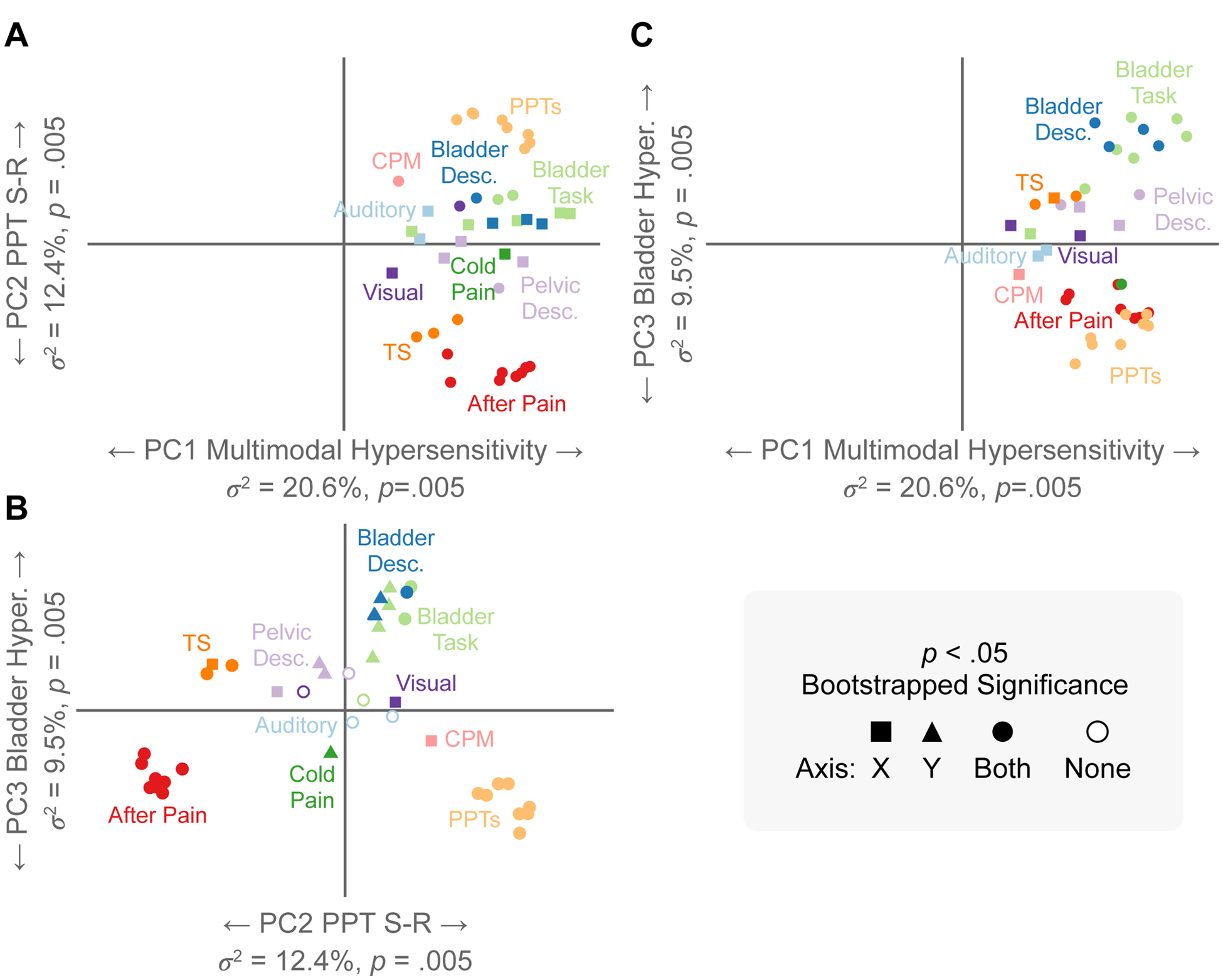
Each measure’s position denotes its relative loading/correlation with the orthogonal principal components (PCs) plotted across the x-y coordinate plane. Measures in close proximity within an axis plane depict positive relationships (concomitant sensitivity across proximal measures). Measures that are distant appear on opposite sides of the origin and depict negative relationships within an axis plane. A bootstrapping procedure quantified loading significance for each measure on each PC depicted with shapes. A) Within the x-axis plane (PC1), all measures load positively onto PC1 and are proximal, indicating positive relationships across all measures with PC1. B) Within the x-axis plane (PC2), PPTs and after-pain measures are distant from each other and are located on opposite sides of the origin. This indicates a negative relationship between PPTs and after-pain measures (this can also be seen across the y-axis in A). Within the y-axis plane (PC3), measures from the bladder task positively load onto PC3 and are distant/oppose the PPTs and after-pain measures. C) Loading patterns of PC1 (x-axis) and PC3 (y-axis). Bladder Task = pain and urgency measurements; Bladder Desc.= Bladder descriptors of Dull/Pressing/Prickling/Sharp pain; PC=Principal Component; PPTs=Pressure Pain Thresholds; CPM=Conditioned Pain Modulation; TS=Temporal Summation.
PCs Correlate with Baseline Self-Report Measures
Figure 4 depicts the bootstrapped correlations between row-wise factor scores (i.e., participants) of PCs and validated questionnaires of self-reported menstrual pain, genitourinary symptoms, depression, anxiety, and health (see Table S3 for descriptive statistics of self-report measures). MMH and bladder hypersensitivity correlated strongly with every measure included, while PPT S-R only weakly correlated with two standardized clinical questionnaires for bladder pain: the ICSI and GUPI. These widespread correlations observed across PCs 1 and 3, but not PC2, demonstrate that these two dimensions (MMH and bladder pain hypersensitivity) explain variability in participants’ current pain- and health-related quality of life. Also, given the orthogonality of PCs, these results suggest the contribution of two mechanisms to explain patients’ current pelvic pain health-related quality of life: 1) MMH and 2) bladder hypersensitivity.
Figure 4. Correlations Between Principal Components (PCs) and Self-Report Questionnaires.
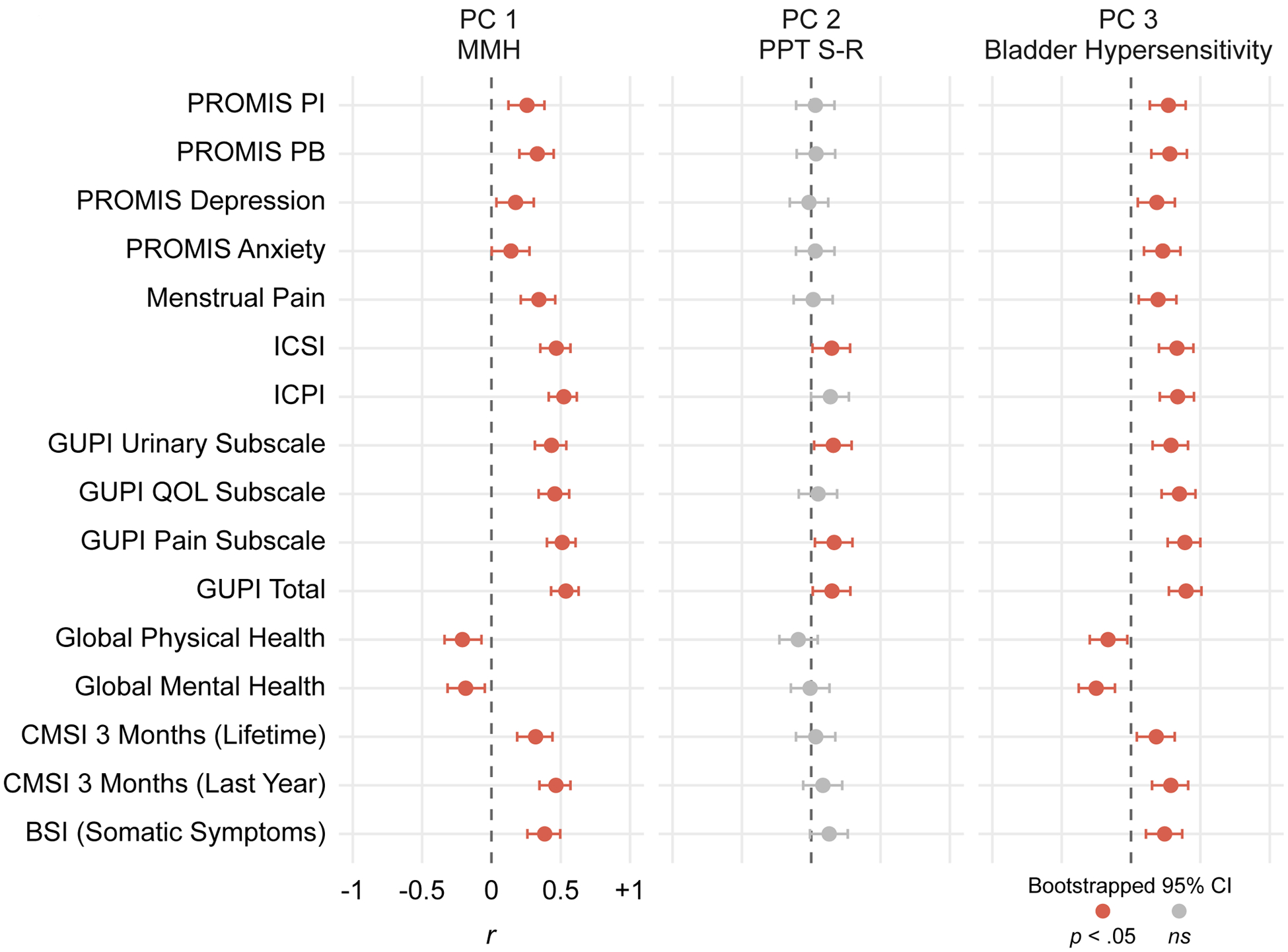
Each point depicts a Pearson’s pairwise correlation between the participants’ factor scores across the three PCs of interest and their responses to a self-report questionnaire. Error bars denote bootstrapped 95% confidence intervals; therefore, intervals crossing zero indicate non-significant correlations and are colored grey. Except for global mental/physical health, greater scores on self-report questionnaires denote worse symptoms/outcome. All MMH and bladder hypersensitivity correlations were significant and in the expected direction: greater hypersensitivity was associated with worse affective symptoms, somatic symptoms, genitourinary pain, menstrual pain, and overall pain and health. PROMIS=Patient Reported Outcomes Measurement Information System; PI=Pain Interference; PB=Pain Behavior; ICSI=Interstitial Cystitis Symptom Index; ICPI= Interstitial Cystitis Problem Index; GUPI=Genitourinary Pain Index; QOL=Quality of Life; CMSI=Complex Medical Symptoms Inventory; BSI=Brief Symptom Inventory.
PC1 (MMH) Predicts Longitudinal Pelvic Pain Outcome Four Years Later
Multiple regressions were used to assess how well the three obtained PCs (i.e., MMH, PPT S-R, and bladder hypersensitivity) predicted pelvic pain outcome on annual questionnaires administered up to four years following the baseline visit (see Table 5 for regression results). Distributions of the PCs are presented in Figure 2A. Similar to the self-report measures, baseline pelvic pain correlated with both MMH and bladder hypersensitivity, but not with PPT-SR (see Figure 2B right panel). PCs were orthogonal to each other (r=0).
Table 5.
Regression Models of Pelvic Pain Outcome for PCA-based Construct Models.
| Year | Parameter | b | SE | β | SS | MSE | F | p | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Intercept | 16.3 | 1.2 | 0.59 | 37791 | 194 | 195.1 | < .001 | |
| Baseline Pelvic Pain | 0.6 | 0.1 | 0.54 | 0.21 | 7287 | 37.6 | < .001 | ||
| PC 1 - MMH | 1.1 | 0.5 | 0.17 | 0.03 | 878 | 4.5 | 0.04 | ||
| PC 2 - PPT S-R | −0.3 | 0.6 | −0.03 | < .01 | 46 | 0.2 | 0.63 | ||
| PC 3 - Bladder Hyper. | −0.4 | 0.7 | −0.04 | < .01 | 61 | 0.3 | 0.57 | ||
| 2 | Intercept | 14.3 | 1.2 | 0.53 | 25600 | 186 | 137.4 | < .001 | |
| Baseline Pelvic Pain | 0.3 | 0.1 | 0.27 | 0.05 | 1192 | 6.4 | 0.01 | ||
| PC 1 - MMH | 1.4 | 0.5 | 0.25 | 0.05 | 1315 | 7.1 | 0.01 | ||
| PC 2 - PPT S-R | 0.1 | 0.6 | 0.01 | < .01 | 1 | 0.0 | 0.93 | ||
| PC 3 - Bladder Hyper. | 0.2 | 0.7 | 0.02 | < .01 | 10 | 0.1 | 0.82 | ||
| 3 | Intercept | 12.6 | 1.3 | 0.50 | 14737 | 160 | 91.9 | < .001 | |
| Baseline Pelvic Pain | 0.3 | 0.1 | 0.31 | 0.07 | 1128 | 7.0 | 0.01 | ||
| PC 1 - MMH | 1.6 | 0.6 | 0.31 | 0.08 | 1366 | 8.5 | 0.004 | ||
| PC 2 - PPT S-R | −0.7 | 0.6 | −0.12 | 0.02 | 282 | 1.8 | 0.19 | ||
| PC 3 - Bladder Hyper. | 0.8 | 0.7 | 0.11 | 0.01 | 213 | 1.3 | 0.25 | ||
| 4 | Intercept | 11.8 | 1.2 | 0.53 | 11506 | 127 | 90.8 | < .001 | |
| Baseline Pelvic Pain | 0.1 | 0.1 | 0.09 | < .01 | 49 | 0.4 | 0.54 | ||
| PC 1 - MMH | 2.0 | 0.5 | 0.44 | 0.15 | 1763 | 13.9 | < .001 | ||
| PC 2 - PPT S-R | −0.3 | 0.6 | −0.05 | < .01 | 31 | 0.2 | 0.62 | ||
| PC 3 - Bladder Hyper. | 0.2 | 0.7 | 0.03 | < .01 | 11 | 0.1 | 0.77 |
Note. The df for each model were: Year 1 (1, 138), Year 2 (1, 124), Year 3 (1, 92), and Year 4 (1, 82). MMH=multimodal hypersensitivity; PPT S-R=pressure pain threshold stimulus-response; CI=confidence interval
Baseline pelvic pain was the strongest predictor of year 1 pelvic pain, but that association steadily decreased over time. In contrast, MMH increased in its predictability of pelvic pain outcome over time and predicted worse pelvic pain outcome continuously up to four years later (see Figure 2C right panel). A 1SD increase in MMH at baseline predicted a .44SD increase, or nearly 6 VAS points, in pelvic pain ratings four years later. PPT S-R and bladder pain hypersensitivity did not predict outcome at any year. Adjusting for population-based prevalence rates of included diagnostic groups replicated the observed sample-wise regression results (see Figure S1). Thus, the positive association between MMH and pelvic pain was robust and differentiated participant four-year trajectories (see Figure 5). Additional analyses examining the effect of annual questionnaire attrition determined that longitudinal data were missing at random and did not selectively depend on recruited participant groups, pelvic pain outcome, nor predictor variables of interest (see the Supplementary Material section on attrition for details).
Figure 5. MMH (PC1) Outer-Quartile Participants Demonstrate Different Pelvic Pain Trajectories.
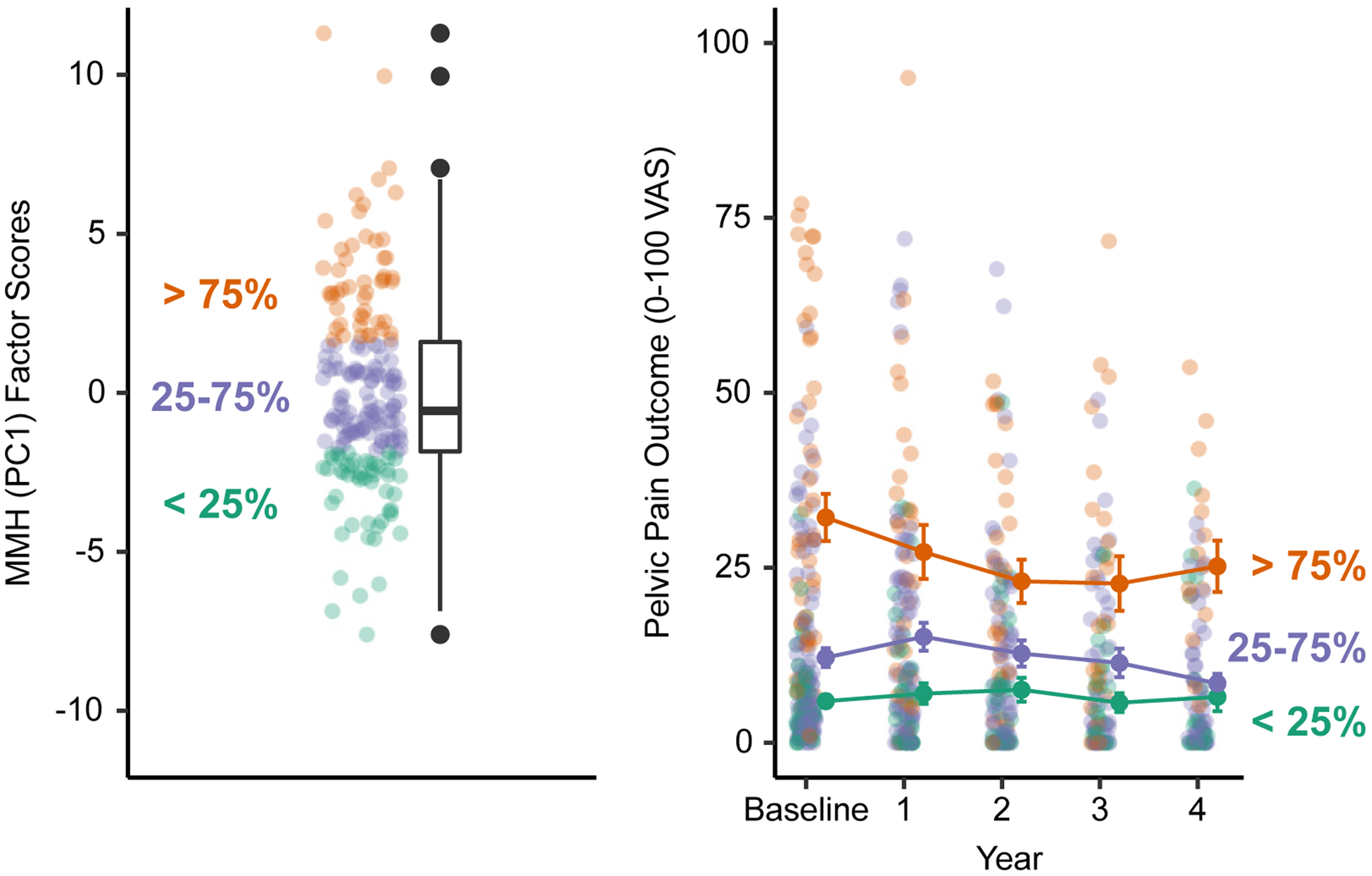
Participants were first differentiated based on whether they are inside or outside the inter-quartile range for baseline MMH (PC1) factor loadings (left). Participants with MMH (PC1) factor scores > 75% of the sample demonstrated worse pelvic pain outcome at baseline that persisted across the four-year follow-up period (right). Error bars are the standard error of the mean.
PC1 (MMH) Outperforms Composite and Questionnaire-based Measures of Sensory Sensitivity
We compared the added predictive value of MMH over sensory testing composites by comparing the AIC and BIC values between two models estimated separately at each year of follow-up: 1) the MMH model regressed pelvic pain outcome on MMH and 2) the composite model regressed pelvic pain outcome on the QST, bladder test, and audio/visual summated Z-scored composite variables. Baseline pelvic pain served as a covariate in both models. Across all four follow-up years, the MMH model was the best model with the lowest AIC and BIC values. The strength of the evidence (i.e., evidence ratio) for the MMH model over the composite model (i.e., nx denotes evidence is n times stronger for the MMH than composite model) using the more conservative AIC was 7.28x at year 1 (ΔAIC = 3.97, ΔBIC = 9.90), 9.50x at year 2 (ΔAIC = 4.50, 508 ΔBIC = 10.2), 1.73x at year 3 (ΔAIC = 1.10, ΔBIC = 6.25), and 5.58x at year 4 (ΔAIC = 3.44, ΔBIC = 8.37) [18]. These results suggest a preference for the simple and more parsimonious MMH model in predicting pelvic pain outcome over the composite model.
To compare MMH to the GSS Brief—a questionnaire-based measurement of increased sensory and diffuse pain sensitivity—we examined their correlations and compared their performance in predicting pelvic pain using multiple regression. Zero-order Pearson correlations demonstrated a strong positive association between baseline pelvic pain and the GSS Brief, r = .56 95% [.45, .64], p < .001, but a weaker positive relationship between GSS Brief and MMH, r = .41 [.28, .52], p < .001. Despite these correlations, all predictors demonstrated low multicollinearity (variance inflation factor <2) across all four years in regression models predicting pelvic pain outcome as a function of MMH, the GSS Brief, and baseline pelvic pain serving a covariate. Baseline pelvic pain decreased in predictive strength over time, while MMH increased in predictive strength. However, the GSS Brief did not predict pelvic pain outcome at any year, suggesting that MMH is a better predictor of outcome than questionnaire-based methods (see Table S4).
Discussion
Inadequate understanding of mechanisms underlying pain sensitivity, and how sensitivity conveys risk for developing chronic pain, remains a barrier to treating and preventing chronic pain conditions. QST is the most widely used method for systematically measuring pain sensitivity [21,22,77]; however, traditional assays that measure single nociceptive modalities (e.g., thermal, pressure) have demonstrated inconsistent predictive power for pain outcome [15,31,80,89]. Likewise, the ability of dynamic QST paradigms assessing pain modulation, such as CPM and TS, to predict pain outcome have also been mixed [27,66,70,71]. Our results demonstrate that the utility of QST can be improved by measuring sensory hypersensitivity more broadly (i.e., including several disparate sensory modalities to evaluate MMH). This approach is aligned with the repeated observation that many functional pain syndromes additionally report increased sensitivity to environmental stimuli (e.g., lights, sounds, odors). Thus, dysfunction in global sensory processing may be more crucial to chronic pain risk than any nociceptive mechanism underlying hyperalgesia.
In a cohort of women with a range of chronic pelvic pain risk, we found that including both nociceptive and non-nociceptive (e.g., visual and auditory) assays identified that PC1, MMH, was a robust common denominator underlying sensory testing variability. Notably, baseline MMH predicted pelvic pain annually for four years even when accounting for baseline pelvic pain. MMH outperformed sensory sensitivity composite models and was a better predictor of outcome than a questionnaire-based method of assessing generalized sensory sensitivity. MMH, rather than solitary dysfunction in specific nociceptive modalities or pain modulation, appears to underlie evolution of pain vulnerability and likely plays a substantial role in the development of chronic pain conditions.
MMH: Nociceptive and Non-Nociceptive QST
QST studies often infer centralized or generalized mechanisms of pain sensitivity to explain observed hypersensitivity in chronic pain conditions [30,34,40,41,45,46,51,53,59,65]. Relationships between and within QST modalities supports the hypothesis that QST assays measure distinct mechanisms of pain sensitivity [39,47,55,61]. However, given that most studies relied on singular noxious modalities of QST, or combinatory approaches of select modalities (e.g., heat and pressure), they provide only a limited ability to evaluate generalized mechanisms of hypersensitivity in centralized pain conditions [13].
The current investigation improves upon these previous attempts by administering a broader panel of nociceptive sensory tests (PPTs, CPM, TS, cold pressor, bladder provocation) and non-nociceptive supraspinal tests (visual and auditory sensitivity). Like previous QST studies [39,47], a PCA of these 40 QST measures resulted in modality-specific components: PPT S-R [79] and bladder hypersensitivity [91]. In contrast, the largest source of underlying QST variability was MMH, a component that was not modality specific. Although previous studies have conjectured that centralized hypersensitivity (e.g., generalized sensory sensitivity, somatization, somatic symptoms disorders, sensory modulation disorder, etc.) [8,14,23,85] underlies chronic pain conditions, our results provide evidence of MMH being a broad construct that correlates with affective symptoms, somatic symptoms, genitourinary pain, menstrual pain, and overall pain and health. Given the consistent and robust correlation between MMH and these other factors crucially affecting chronic pain, we hypothesize neural regions underlying MMH, such as the insula and cingulate cortex [e.g., 4,45,64,83], contain critical circuits in functional pain syndromes.
PC1 (MMH) Predicts Pelvic Pain Outcome
Studies that have used various QST measures to predict long-term outcome have not generated uniform conclusions [e.g., 27,35,38,46,66,67,86,98,99]. In contrast, current pain intensity has consistently been associated with future pain experience [3,33,60,98]. This investigation may explain the equivocal findings for QST on future pain outcomes: risk for worse pain outcome is possibly not due to alterations in normal interpretation of specific nociceptive modalities, but rather MMH. When testing whether current pain (baseline pelvic pain), MMH, or nociceptive modality-specific components (PCs 2 and 3) best predicted future pelvic pain outcome, we found that current pain provided a diminishing degree of predictive power annually, while MMH provided an increasing degree of predictive power over time. Additionally, MMH was the only significant predictor of year four pelvic pain intensity. This pattern of results held consistent even when adjusting for population prevalence rates of dysmenorrhea, bladder pain syndrome, and other types of chronic pain (see Figure S1).
In contrast, sensory testing composites sporadically predicted pelvic pain outcome: a more sensitive bladder test and QST assessment predicted worse pelvic pain at years three and four, respectively, with baseline pelvic pain decreasing in predictive power annually. A similar pattern of results was observed when adjusting for population prevalence rates; however, baseline pelvic pain became an important predictor of year four pelvic pain (see Figure S1). When sensory testing was split into composite measures, current (baseline) pelvic pain was the best predictor of future pelvic pain, but longitudinal estimates became unstable.
Therefore, the modality-specific QST differences often observed in cross-sectional studies comparing pain patients to controls [e.g., 40,46] could reflect dynamic states of hypersensitivity. In contrast, the increased MMH in participants that develop worse pelvic pain suggest that a pervasive sensory processing mechanism is stable across time.
Strengths and Limitations
The multimodal sensory testing panel used here is one of the largest efforts to characterize MMH in individuals harboring variable degrees of risk for CPP and includes one of the longest follow-up periods of any previous QST pelvic pain study. The current investigation leveraged PCA that facilitated dimensionality reduction (from 40 to 3 variables) in a racially diverse sample enriched with at-risk individuals. However, some limitations may affect the generalizability of our results. The sample included a large number of college students. As a result, the sample was young (M=25, SD=6 years), and the vast majority were nulliparous (85% without a prior pregnancy). A strength of our study is the long-term follow-up, although there was some attrition. Prior studies of long-term pain had comparable attrition rates (50–80%), and attrition had a marginal impact on outcome [28,81]. Together, our analyses suggest that longitudinal data were missing at random and did not selectively depend on recruited participant groups, pelvic pain outcome, nor predictor variables of interest calculated either via sensory testing composites or PCA.
Given that comprehensive multimodal sensory testing is onerous on patients and staff, it remains challenging to validate these findings within large clinical populations. Although the longitudinal stability of MMH is unknown, it is encouraging that MMH derived from a one-day sensory testing panel predicted a single week of average pelvic pain four years later. Future work would be well served to establish the minimum battery of sensory tests needed to evaluate MMH. Thus, we envision that with further refinements of MMH testing, such as automated methods [e.g., 46], it may possible to validate a clinically oriented short protocol. Developing early predictive methods for long-term chronic pain risk are essential for preventing its deleterious effects. Because the goal of CRAMPP was to evaluate the role of menstrual pain and other nociceptive mechanisms in the development of chronic pelvic pain in an at-risk cohort, the follow-up questionnaires were specifically focused towards interrogating pelvic pain. Future studies should investigate the role of MMH on non-pelvic pain risk.
Conclusion
This analysis provided crucial evidence supporting MMH, a hypothesized construct underlying “centralized” mechanisms of pain sensitivity, and its predictive ability of worse pelvic pain outcome. Our study demonstrates that multimodal sensory testing improves the prediction of pain status or outcome over parsimonious unimodal approaches or questionnaire-based strategies. MMH exists on a continuum, and individuals that report increased sensory sensitivity or demonstrate hypersensitive QST responses are more vulnerable to worse future pain [8,38,66,89]. Neuroimaging paradigms have implicated the anterior insula and cingulate cortex as important for multimodal sensory integration and nociceptive appraisal [44,45,64]. Therefore, future work to abrogate the course of chronic pain would be well served to understand and target the neural mechanisms that underlie MMH.
Supplementary Material
Acknowledgments
The authors thank Dr. GF Gebhart for advice on study design, interpretation, and editorial assistance. The authors are also grateful for our lab staff performing the sensory testing assessments and CRAMPP study participants’ contributions. This analysis was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100368) and the National Institute of Child and Human Development (R01HD098193). All data and code are available on Open Science Framework (DOI: 10.17605/OSF.IO/27KY9). FFT reports personal fees from Myovant, Tremeau Pharmaceuticals, and UroShape, royalties from Wolters Kluwer, and grant support from Dot Laboratories and Eximis outside the submitted work. The remaining authors report no additional conflicts of interest.
Footnotes
An earlier version of this manuscript is available as a preprint on medRxiv: https://www.medrxiv.org/content/10.1101/2022.04.01.22272964v1
References
- [1].Abdi H Singular Value Decomposition (SVD) and Generalized Singular Value Decomposition (GSVD). Thousand Oaks, CA: Sage, 2007. [Google Scholar]
- [2].Abdi H, Williams LJ. Principal component analysis. WIREs Comp Stat 2010;2:433–459. [Google Scholar]
- [3].Andersson HI. The course of non-malignant chronic pain: a 12-year follow-up of a cohort from the general population. European Journal of Pain 2004;8:47–53. [DOI] [PubMed] [Google Scholar]
- [4].Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain 2005;9:463–463. [DOI] [PubMed] [Google Scholar]
- [5].Arendt-Nielsen L Mechanistic similarities between fibromyalgia and other chronic pain conditions. PAIN Reports 2017;2:3. [Google Scholar]
- [6].Aslam N, Harrison G, Khan K, Patwardhan S. Visceral hyperalgesia in chronic pelvic pain. BJOG: An International Journal of Obstetrics & Gynaecology 2009;116:1551–1555. [DOI] [PubMed] [Google Scholar]
- [7].Aykan S, Vatansever G, Doğanay-Erdoğan B, Kalaycıoğlu C. Development of Sensory Sensitivity Scales (SeSS): Reliability and validity analyses. Research in Developmental Disabilities 2020;100:103612. [DOI] [PubMed] [Google Scholar]
- [8].Bar-Shalita T, Granovsky Y, Parush S, Weissman-Fogel I. Sensory Modulation Disorder (SMD) and Pain: A New Perspective. Front Integr Neurosci 2019;13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Beaton D, Chin Fatt CR, Abdi H. An ExPosition of multivariate analysis with the singular value decomposition in R. Computational Statistics & Data Analysis 2014;72:176–189. [Google Scholar]
- [10].Beissner F, Brandau A, Henke C, Felden L, Baumgärtner U, Treede R-D, Oertel BG, Lötsch J. Quick Discrimination of Adelta and C Fiber Mediated Pain Based on Three Verbal Descriptors. PLoS ONE 2010;5:e12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ben-Shachar MS, Makowski D, Lüdecke D. Compute and interpret indices of effect size. 2020. Available: https://github.com/easystats/effectsize.
- [12].Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of Symptoms of Bladder Pain Syndrome/Interstitial Cystitis Among Adult Females in the United States. Journal of Urology 2011;186:540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bhalang K, Sigurdsson A, Slade GD, Maixner W. Associations Among Four Modalities of Experimental Pain in Women. The Journal of Pain 2005;6:604–611. [DOI] [PubMed] [Google Scholar]
- [14].Birket-Smith M. Somatization and chronic pain. Acta Anaesthesiologica Scandinavica 2001;45:1114–1120. [DOI] [PubMed] [Google Scholar]
- [15].Bordeleau M, Barron D, Léonard G, Backonja M. Realigning the role of quantitative sensory testing in sensory profiling of patients with and without neuropathic pain. PAIN 2021;162:2780. [DOI] [PubMed] [Google Scholar]
- [16].Broderick J, DeWit EM, Rothrock N, Crane P, Forrest CB. Advances in Patient Reported Outcomes: The NIH PROMIS Measures. eGEMs (Generating Evidence & Methods to improve patient outcomes) 2013;1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brown C, Tollefson N, Dunn W, Cromwell R, Filion D. The Adult Sensory Profile: Measuring Patterns of Sensory Processing. The American Journal of Occupational Therapy 2001;55:75–82. [DOI] [PubMed] [Google Scholar]
- [18].Burnham KP, Anderson DR, Huyvaert KP. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 2011;65:23–35. [Google Scholar]
- [19].Cathcart S, Winefield AH, Rolan P, Lushington K. Reliability of Temporal Summation and Diffuse Noxious Inhibitory Control. Pain Research and Management 2009;14:433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Clemens JQ, Calhoun EA, Litwin MS, McNaughton-Collins M, Kusek JW, Crowley EM, Landis JR. Validation of a Modified National Institutes of Health Chronic Prostatitis Symptom Index to Assess Genitourinary Pain in Both Men and Women. Urology 2009;74:983–987.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cruz-Almeida Y, Fillingim RB. Can Quantitative Sensory Testing Move Us Closer to Mechanism-Based Pain Management? Pain Med 2014;15:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Curatolo M Diagnosis of Altered Central Pain Processing. Spine 2011;36:S200. [DOI] [PubMed] [Google Scholar]
- [23].Curatolo M, Arendt-Nielsen L, Petersen-Felix S. Central Hypersensitivity in Chronic Pain: Mechanisms and Clinical Implications. Physical Medicine and Rehabilitation Clinics of North America 2006;17:287–302. [DOI] [PubMed] [Google Scholar]
- [24].Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults — United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med 1983;13:595–605. [PubMed] [Google Scholar]
- [26].D’haenens W, Dhooge I, Maes L, Bockstael A, Keppler H, Philips B, Swinnen F, Vinck BM. The Clinical Value of the Multiple-Frequency 80-Hz Auditory Steady-State Response in Adults With Normal Hearing and Hearing Loss. Archives of Otolaryngology–Head & Neck Surgery 2009;135:496–506. [DOI] [PubMed] [Google Scholar]
- [27].Fernandes C, Pidal-Miranda M, Samartin-Veiga N, Carrillo-de-la-Peña MT. Conditioned pain modulation as a biomarker of chronic pain: a systematic review of its concurrent validity. PAIN 2019;160:2679–2690. [DOI] [PubMed] [Google Scholar]
- [28].Fillingim RB, Slade GD, Greenspan JD, Dubner R, Maixner W, Bair E, Ohrbach R. Long-term changes in biopsychosocial characteristics related to temporomandibular disorder: findings from the OPPERA study. Pain 2018;159:2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fitzcharles M-A, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. The Lancet 2021;397:2098–2110. [DOI] [PubMed] [Google Scholar]
- [30].FitzGerald MP, Koch D, Senka J. Visceral and cutaneous sensory testing in patients with painful bladder syndrome. Neurourology and Urodynamics 2005;24:627–632. [DOI] [PubMed] [Google Scholar]
- [31].Forstenpointner J, Ruscheweyh R, Attal N, Baron R, Bouhassira D, Enax-Krumova EK, Finnerup NB, Freynhagen R, Gierthmühlen J, Hansson P, Jensen TS, Maier C, Rice ASC, Segerdahl M, Tölle T, Treede R-D, Vollert J. No pain, still gain (of function): the relation between sensory profiles and the presence or absence of self-reported pain in a large multicenter cohort of patients with neuropathy. PAIN 2021;162:718–727. [DOI] [PubMed] [Google Scholar]
- [32].Friedman DI, De Ver Dye T. Migraine and the Environment. Headache: The Journal of Head and Face Pain 2009;49:941–952. [DOI] [PubMed] [Google Scholar]
- [33].Gandhi W, Pomares FB, Naso L, Asenjo J-F, Schweinhardt P. Neuropathic pain after thoracotomy: Tracking signs and symptoms before and at monthly intervals following surgery. European Journal of Pain 2020;24:1269–1289. [DOI] [PubMed] [Google Scholar]
- [34].Geisser ME, Glass JM, Rajcevska LD, Clauw DJ, Williams DA, Kileny PR, Gracely RH. A Psychophysical Study of Auditory and Pressure Sensitivity in Patients With Fibromyalgia and Healthy Controls. The Journal of Pain 2008;9:417–422. [DOI] [PubMed] [Google Scholar]
- [35].Georgopoulos V, Akin-Akinyosoye K, Zhang W, McWilliams DF, Hendrick P, Walsh DA. Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: a systematic review and meta-analysis. Pain 2019;160:1920–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gracely RH, Kwilosz DM. The Descriptor Differential Scale: applying psychophysical principles to clinical pain assessment: Pain 1988;35:279–288. [DOI] [PubMed] [Google Scholar]
- [37].Granges G, Littlejohn G. Pressure pain threshold in pain-free subjects, in patients with chronic regional pain syndromes, and in patients with fibromyalgia syndrome. Arthritis & Rheumatism 1993;36:642–646. [DOI] [PubMed] [Google Scholar]
- [38].Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Diatchenko L, Liu Q, Maixner W. Pain Sensitivity and Autonomic Factors Associated with Development of TMD: the OPPERA Prospective Cohort Study. J Pain 2013;14:T63–74.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Mulkey F, Rothwell R, Maixner W. Pain Sensitivity Risk Factors for Chronic TMD: Descriptive Data and Empirically Identified Domains from the OPPERA Case Control Study. The Journal of Pain 2011;12:T61–T74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Greenspan JD, Slade GD, Rathnayaka N, Fillingim RB, Ohrbach R, Maixner W. Experimental Pain Sensitivity in Subjects with Temporomandibular Disorders and Multiple Other Chronic Pain Conditions: The OPPERA Prospective Cohort Study. J Oral Facial Pain Headache 2020;34:s43–s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Grundström H, Larsson B, Arendt-Nielsen L, Gerdle B, Kjølhede P. Associations between pain thresholds for heat, cold and pressure, and Pain Sensitivity Questionnaire scores in healthy women and in women with persistent pelvic pain. European Journal of Pain 2019;23:1631–1639. [DOI] [PubMed] [Google Scholar]
- [42].Hanno PM, Burks DA, Clemens JQ, Dmochowski RR, Erickson D, FitzGerald MP, Forrest JB, Gordon B, Gray M, Mayer RD, Newman D, Nyberg L, Payne CK, Wesselmann U, Faraday MM. AUA Guideline for the Diagnosis and Treatment of Interstitial Cystitis/Bladder Pain Syndrome. Journal of Urology 2011;185:2162–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Harper DE, Schrepf A, Clauw DJ. Pain Mechanisms and Centralized Pain in Temporomandibular Disorders. J Dent Res 2016;95:1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Harte SE, Harris RE, Clauw DJ. The neurobiology of central sensitization. J Appl Behav Res 2018;23:e12137. [Google Scholar]
- [45].Harte SE, Ichesco E, Hampson JP, Peltier SJ, Schmidt-Wilcke T, Clauw DJ, Harris RE. Pharmacologic attenuation of cross-modal sensory augmentation within the chronic pain insula: PAIN 2016;157:1933–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Harte SE, Schrepf A, Gallop R, Kruger GH, Lai HHH, Sutcliffe S, Halvorson M, Ichesco E, Naliboff BD, Afari N, Harris RE, Farrar JT, Tu F, Landis JR, Clauw DJ, for the MAPP Research Network. Quantitative assessment of nonpelvic pressure pain sensitivity in urologic chronic pelvic pain syndrome: a MAPP Research Network study. Pain 2019;160:1270–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hastie BA, Riley JL, Robinson ME, Glover T, Campbell CM, Staud R, Fillingim RB. Cluster analysis of multiple experimental pain modalities. Pain 2005;116:227–237. [DOI] [PubMed] [Google Scholar]
- [48].Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand PK, Schaer GN. An international urogynecological association (IUGA)/international continence society (ICS) joint report on the terminology for female pelvic floor dysfunction: Terminology for Female Pelvic Floor Dysfunction. Neurourol Urodyn 2010;29:4–20. [DOI] [PubMed] [Google Scholar]
- [49].Hellman KM, Datta A, Steiner ND, Kane Morlock JN, Garrison EF, Clauw DJ, Tu FF. Identification of experimental bladder sensitivity among dysmenorrhea sufferers. American Journal of Obstetrics and Gynecology 2018;219:84.e1–84.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hellman KM, Patanwala IY, Pozolo KE, Tu FF. Multimodal nociceptive mechanisms underlying chronic pelvic pain. American Journal of Obstetrics and Gynecology 2015;213:827.e1–827.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hellman KM, Roth GE, Dillane KE, Garrison EF, Oladosu FA, Clauw DJ, Tu FF. Dysmenorrhea subtypes exhibit differential quantitative sensory assessment profiles. PAIN 2020;161:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Henderson E ghibli: Studio Ghibli Colour Palettes. 2020. Available: https://CRAN.R-project.org/package=ghibli.
- [53].Hollins M, Harper D, Gallagher S, Owings EW, Lim PF, Miller V, Siddiqi MQ, Maixner W. Perceived intensity and unpleasantness of cutaneous and auditory stimuli: An evaluation of the generalized hypervigilance hypothesis: Pain 2009;141:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update 2015;21:762–778. [DOI] [PubMed] [Google Scholar]
- [55].Janal MN, Glusman M, Kuhl JP, Clark WC. On the absence of correlation between responses to noxious heat, cold, electrical and ischemie stimulation. Pain 1994;58:403–411. [DOI] [PubMed] [Google Scholar]
- [56].Jensen MP, Wang W, Potts SL, Gould EM. Reliability and Validity of Individual and Composite Recall Pain Measures in Patients with Cancer. Pain Medicine 2012;13:1284–1291. [DOI] [PubMed] [Google Scholar]
- [57].Kanazawa M, Hongo M, Fukudo S. Visceral hypersensitivity in irritable bowel syndrome. Journal of Gastroenterology and Hepatology 2011;26:119–121. [DOI] [PubMed] [Google Scholar]
- [58].Kaya S, Hermans L, Willems T, Roussel N, Meeus M. Central sensitization in urogynecological chronic pelvic pain : a systematic literature review. PAIN PHYSICIAN 2013;16:291–308. [PubMed] [Google Scholar]
- [59].Kmiecik MJ, Tu FF, Silton RL, Dillane KE, Roth GE, Harte SE, Hellman KM. Cortical mechanisms of visual hypersensitivity in women at risk for chronic pelvic pain. Pain 2022;163:1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Landmark T, Romundstad P, Butler S, Kaasa S, Borchgrevink P. Development and course of chronic widespread pain: the role of time and pain characteristics (the HUNT pain study). PAIN 2019;160:1976–1981. [DOI] [PubMed] [Google Scholar]
- [61].Lautenbacher S, Rollman GB. Sex differences in responsiveness to painful and non-painful stimuli are dependent upon the stimulation method. Pain 1993;53:255–264. [DOI] [PubMed] [Google Scholar]
- [62].Li R, Li B, Kreher DA, Benjamin AR, Gubbels A, Smith SM. Association between dysmenorrhea and chronic pain: a systematic review and meta-analysis of population-based studies. American Journal of Obstetrics and Gynecology 2020;223:350–371. [DOI] [PubMed] [Google Scholar]
- [63].Lionetti F, Aron A, Aron EN, Burns GL, Jagiellowicz J, Pluess M. Dandelions, tulips and orchids: evidence for the existence of low-sensitive, medium-sensitive and high-sensitive individuals. Transl Psychiatry 2018;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].López-Solà M, Pujol J, Wager TD, Garcia-Fontanals A, Blanco-Hinojo L, Garcia-Blanco S, Poca-Dias V, Harrison BJ, Contreras-Rodríguez O, Monfort J, Garcia-Fructuoso F, Deus J. Altered Functional Magnetic Resonance Imaging Responses to Nonpainful Sensory Stimulation in Fibromyalgia Patients: Brain Response to Nonpainful Multisensory Stimulation in Fibromyalgia. Arthritis & Rheumatology 2014;66:3200–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Martenson ME, Halawa OI, Tonsfeldt KJ, Maxwell CA, Hammack N, Mist SD, Pennesi ME, Bennett RM, Mauer KM, Jones KD, Heinricher MM. A possible neural mechanism for photosensitivity in chronic pain. PAIN 2016;157:868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Morris MC, Bruehl S, Stone AL, Garber J, Smith C, Palermo TM, Walker LS. Does Quantitative Sensory Testing Improve Prediction of Chronic Pain Trajectories? A Longitudinal Study of Youth With Functional Abdominal Pain Participating in a Randomized Controlled Trial of Cognitive Behavioral Treatment. The Clinical Journal of Pain 2021;37:648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Müller M, Bütikofer L, Andersen OK, Heini P, Arendt-Nielsen L, Jüni P, Curatolo M. Cold pain hypersensitivity predicts trajectories of pain and disability after low back surgery: a prospective cohort study. Pain 2021;162:184–194. [DOI] [PubMed] [Google Scholar]
- [68].Myles PS, Troedel S, Boquest M, Reeves M. The Pain Visual Analog Scale: Is It Linear or Nonlinear? Anesthesia & Analgesia 1999;89:1517. [DOI] [PubMed] [Google Scholar]
- [69].Neuwirth E RColorBrewer: ColorBrewer Palettes. 2014. Available: https://CRAN.R-project.org/package=RColorBrewer.
- [70].Nir R-R, Yarnitsky D Conditioned pain modulation. Current Opinion in Supportive and Palliative Care 2015;9:131–137. [DOI] [PubMed] [Google Scholar]
- [71].O’Brien AT, Deitos A, Triñanes Pego Y, Fregni F, Carrillo-de-la-Peña MT. Defective Endogenous Pain Modulation in Fibromyalgia: A Meta-Analysis of Temporal Summation and Conditioned Pain Modulation Paradigms. The Journal of Pain 2018;19:819–836. [DOI] [PubMed] [Google Scholar]
- [72].O’Leary MP, Sant GR, Fowler FJ, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology 1997;49:58–63. [DOI] [PubMed] [Google Scholar]
- [73].Payne LA, Rapkin A, Seidman L, Zeltzer L, Tsao J. Experimental and procedural pain responses in primary dysmenorrhea: a systematic review. JPR 2017;Volume 10:2233–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Payne LA, Seidman LC, Sim M-S, Rapkin AJ, Naliboff BD, Zeltzer LK. Experimental evaluation of central pain processes in young women with primary dysmenorrhea: PAIN 2019;160:1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Picou EM, Ricketts TA, Hornsby BWY. How Hearing Aids, Background Noise, and Visual Cues Influence Objective Listening Effort: Ear and Hearing 2013;34:e52–e64. [DOI] [PubMed] [Google Scholar]
- [76].Revelle W psych: Procedures for Personality and Psychological Research. 2020. Available: https://CRAN.R-project.org/package=psych. [Google Scholar]
- [77].Rolke R, Baron R, Maier C, Tölle TR, Treede R-D, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihöfner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. PAIN 2006;123:231–243. [DOI] [PubMed] [Google Scholar]
- [78].Sandri A, Cecchini MP, Riello M, Zanini A, Nocini R, Fiorio M, Tinazzi M. Pain, Smell, and Taste in Adults: A Narrative Review of Multisensory Perception and Interaction. Pain Ther 2021;10:245–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Santana AN, de Santana CN, Montoya P. Chronic Pain Diagnosis Using Machine Learning, Questionnaires, and QST: A Sensitivity Experiment. Diagnostics 2020;10:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Schmelz M What can we learn from the failure of quantitative sensory testing? PAIN 2020;162:663–664. [DOI] [PubMed] [Google Scholar]
- [81].Schmidt CO, Raspe H, Pfingsten M, Hasenbring M, Basler HD, Eich W, Kohlmann T. Does attrition bias longitudinal population-based studies on back pain? European Journal of Pain 2011;15:84–91. [DOI] [PubMed] [Google Scholar]
- [82].Schoep ME, Nieboer TE, van der Zanden M, Braat DDM, Nap AW. The impact of menstrual symptoms on everyday life: a survey among 42,879 women. American Journal of Obstetrics and Gynecology 2019;220:569.e1–569.e7. [DOI] [PubMed] [Google Scholar]
- [83].Schrepf A, Harper DE, Harte SE, Wang H, Ichesco E, Hampson JP, Zubieta J-K, Clauw DJ, Harris RE. Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain 2016;157:2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Schrepf A, Hellman KM, Bohnert AM, Williams DA, Tu FF. Generalized sensory sensitivity is associated with comorbid pain symptoms: a replication study in women with dysmenorrhea. PAIN 2022: 10.1097/j.pain.0000000000002676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Schrepf A, Williams DA, Gallop R, Naliboff BD, Basu N, Kaplan C, Harper DE, Landis JR, Clemens JQ, Strachan E, Griffith JW, Afari N, Hassett A, Pontari MA, Clauw DJ, Harte SE. Sensory sensitivity and symptom severity represent unique dimensions of chronic pain: a MAPP Research Network study. PAIN 2018;159:2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Slade GD, Sanders AE, Ohrbach R, Fillingim RB, Dubner R, Gracely RH, Bair E, Maixner W, Greenspan JD. Pressure pain thresholds fluctuate with - but do not usefully predict - the clinical course of painful temporomandibular disorder. Pain 2014;155:2134–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sriwatanakul K, Kelvie W, Lasagna L, Calimlim JF, Weis OF, Mehta G. Studies with different types of visual analog scales for measurement of pain. Clinical Pharmacology & Therapeutics 1983;34:234–239. [DOI] [PubMed] [Google Scholar]
- [88].Thompson HD, Tang S, Jarrell JF. Temporal Summation in Chronic Pelvic Pain. Journal of Obstetrics and Gynaecology Canada 2020;42:556–560. [DOI] [PubMed] [Google Scholar]
- [89].Treede R-D. The role of quantitative sensory testing in the prediction of chronic pain. Pain 2019;160:S66–S69. [DOI] [PubMed] [Google Scholar]
- [90].Tu FF, Epstein AE, Pozolo KE, Sexton DL, Melnyk AI, Hellman KM. A Noninvasive Bladder Sensory Test Supports a Role for Dysmenorrhea Increasing Bladder Noxious Mechanosensitivity. The Clinical Journal of Pain 2013;29:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Tu FF, Kane JN, Hellman KM. Noninvasive experimental bladder pain assessment in painful bladder syndrome. BJOG: Int J Obstet Gy 2017;124:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang D, Merkle SL, Lee JE, Sluka KA, Rakel B, Graven-Nielsen T, Frey-Law LA. Multisensory Sensitivity is Related to Deep-Tissue but Not Cutaneous Pain Sensitivity in Healthy Individuals. JPR 2020;Volume 13:2493–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Westling AM, Tu FF, Griffith JW, Hellman KM. The association of dysmenorrhea with noncyclic pelvic pain accounting for psychological factors. American Journal of Obstetrics and Gynecology 2013;209:422.e1–422.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, Grolemund G, Hayes A, Henry L, Hester J, Kuhn M, Pedersen TL, Miller E, Bache SM, Müller K, Ooms J, Robinson D, Seidel DP, Spinu V, Takahashi K, Vaughan D, Wilke C, Woo K, Yutani H. Welcome to the Tidyverse. Journal of Open Source Software 2019;4:1686. [Google Scholar]
- [95].Wilbarger JL, Cook DB. Multisensory Hypersensitivity in Women With Fibromyalgia: Implications for Well Being and Intervention. Archives of Physical Medicine and Rehabilitation 2011;92:653–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Williams DA, Schilling S. Advances in the Assessment of Fibromyalgia. Rheumatic Disease Clinics of North America 2009;35:339–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Michael Franklin C, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, Mccain GA, John Reynolds W, Romano TJ, Jon Russell I, Sheon RP. The american college of rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis & Rheumatism 1990;33:160–172. [DOI] [PubMed] [Google Scholar]
- [98].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best L-A, Granot M. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain 2008;138:22–28. [DOI] [PubMed] [Google Scholar]
- [99].Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 2012;153:1193–1198. [DOI] [PubMed] [Google Scholar]
- [100].Zondervan KT, Yudkin PL, Vessey MP, Jenkinson CP, Dawes MG, Barlow DH, Kennedy SH. Chronic pelvic pain in the community—Symptoms, investigations, and diagnoses. American Journal of Obstetrics and Gynecology 2001;184:1149–1155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


