Abstract
Objective:
To evaluate associations between endometriosis and uterine leiomyomas with ovarian cancer risk by race and the impact of hysterectomy on these associations.
Methods:
We used data from 4 case-control studies and 2 case-control studies nested within prospective cohorts in the Ovarian Cancer in Women of African Ancestry (OCWAA) consortium. The study population included 3,124 Black participants and 5,458 White participants, of which 1,008 Black participants and 2,237 White participants had ovarian cancer. Logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for the associations of endometriosis and leiomyomas with ovarian cancer risk, by race, stratified by histotype and hysterectomy.
Results:
The prevalence of endometriosis and leiomyomas were 6.4% and 43.2% among Black participants and 7.0% and 21.5% among White participants, respectively. Endometriosis was associated with an increased risk of endometrioid and clear cell ovarian cancer in both racial groups (e.g., ORs for endometrioid tumors for Black and White participants of 7.06 [95% CI=3.86–12.91] and 2.17 [95% CI=1.36–3.45], respectively, phetereogeneity=0.003). The association between endometriosis and ovarian cancer risk in White participants was stronger in those without a hysterectomy but no difference was observed in Black participants (all pinteraction≥0.05). Leiomyomas were associated with an elevated risk of ovarian cancer only in those without a hysterectomy in both Black (OR=1.34; 95% CI=1.11–1.62) and White participants (OR=1.22; 95% CI=1.05–1.41)(all pinteraction≥0.05).
Conclusions:
Black and White participants with endometriosis had a higher risk of ovarian cancer, and hysterectomy modified this association among White participants. Leiomyomas were associated with an increased risk of ovarian cancer in both racial groups, with hysterectomy modifying the risk in both groups. Understanding how racial differences in access to care and treatment options (e.g., hysterectomy) may help guide future risk reduction strategies.
Précis
Black and White participants with endometriosis had a higher risk of ovarian cancer, and hysterectomy modified this association only among White participants.
Introduction
Endometriosis and uterine leiomyomas are common gynecologic conditions that have significant impact on reproductive age women.(1–12) At present, endometriosis is more likely to be diagnosed among White women than Black women,(13) however, racial differences in the diagnosis of endometriosis may reflect disparities in access to care as opposed to biological differences in incidence.(14) Conversely, leiomyomas are two to three times more common in Black women than White women, but reasons for this difference are not well understood.(15)
The incidence of ovarian cancer is approximately 30% higher in White women than Black women.(16) Reproductive conditions such as endometriosis and leiomyomas and/or treatments related to these conditions (e.g., hysterectomy, hormone use) could contribute to this difference, but research has been limited in this area.(17) The association between hysterectomy and ovarian cancer risk is conflicting, with studies examining the association prior to 2000 indicating a lower risk of ovarian cancer among those with a hysterectomy, while post 2000 studies suggest a higher risk.(18) For endometriosis, the association with ovarian cancer is well-established with epidemiologic data indicating that women with endometriosis have two- to three-fold greater risk of endometrioid and clear cell ovarian cancer,(19–21) but these estimates are from studies comprised predominantly of White women. A consistent association of leiomyomas with ovarian cancer risk has not been demonstrated. However, leiomyomas may indirectly impact ovarian cancer risk, as leiomyomas are the most common indication for hysterectomy in the U.S.,(22, 23) and hysterectomy has been associated with lower ovarian cancer risk in women with leiomyomas.(24) The objective of the present study was to evaluate associations of endometriosis and uterine leiomyomas with epithelial ovarian cancer risk among Black and White participants, taking into consideration the impact of hysterectomy, oral contraceptive use, and post-menopausal hormone therapy use, on these associations.
Methods
The Ovarian Cancer in Women of African Ancestry (OCWAA) consortium was established with the objective to understand racial differences as they relate to risk factors and outcomes in epithelial ovarian cancer.(25) In this analysis, we included individual-level data from 6 OCWAA studies that collected data on endometriosis and/or leiomyomas (4 case-control studies and 2 case-control studies nested within prospective cohorts). Across the six studies, 8,682 total participants (3,124 Black participants and 5,458 White participants) were included with 1,008 Black and 2,237 White participants with ovarian cancer, and 2,116 Black and 3,221 White controls.
Five of the 6 studies collected data on endometriosis (African American Cancer Epidemiology Study [AACES], Black Women’s Health Study [BWHS], Cook County Case Study [CCCS], North Carolina Ovarian Cancer Study [NCOCS], Los Angeles County Ovarian Cancer Study [LACOCS]) and 5 of the 6 studies collected data on leiomyomas (AACES, BWHS, NCOCS, LACOCS, Southern Community Cohort Study [SCCS]). Each study collected demographic, reproductive history, lifestyle, and medical history data via self-administered questionnaires and/or interviews. All OCWAA studies classified race based on self-report. Each study obtained informed consent from its participants; the individual studies and the OCWAA Consortium were approved by the relevant Institutional Review Boards (University of Virginia, Duke University, Boston University School of Medicine, University of Illinois at Chicago, University of Southern California, Vanderbilt University Medical Center, and Meharry Medical College).
Data on cancer diagnoses were abstracted from medical records and cancer registry reports. Only epithelial ovarian cancer cases defined using International Classification of Diseases for Oncology, Version 3, were eligible for inclusion. Tumor histotype was determined by combining morphology and grade information according to diagnostic guidelines for ovarian carcinomas in 2014 from the World Health Organization (WHO) Classification of Tumors of Female Reproductive Organs.(26) Each study collected data on the diagnosis of endometriosis and/or leiomyomas via standardized questionnaires that were either interviewer-administered or self-administered (Appendix 1, available online at http://links.lww.com/xxx).
Heterogeneity was quantified by calculating I2 statistics and corresponding 95% confidence intervals.(27–29) As no significant heterogeneity by study was detected, data was pooled for all analyses.(29, 30) Logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for the associations between endometriosis and leiomyomas with ovarian cancer risk, overall, and by race; polytomous models were used for analyses histotype (high-grade serous, low-grade serous, endometrioid, clear cell, mucinous). Wald joint χ2 tests of histotype-specific coefficients were used to calculate p-values for heterogeneity between histotypes. We also examined the endometrioid, clear cell, and low-grade serous histotypes combined as these histotypes have previously been the most strongly associated with ovarian cancer risk among women with endometriosis.(19)
We defined potential confounders as factors potentially associated with both ovarian cancer and endometriosis and/or leiomyomas risk, including education (high school graduate/GED or less, some college, college graduate, graduate/professional school), parity (no full-term pregnancies, 1–2 full-term pregnancies, ≥3 full-term pregnancies), duration of oral contraceptive use (never, <5 years, ≥5 years), age at menarche, body mass index (BMI; <25.0 kg/m2, 25.0–29.9 kg/m2, 30.0–34.9 kg/m2, ≥35.0 kg/m2), smoking status (ever smoker, never smoker), tubal ligation (yes, no), first-degree family history of breast or ovarian cancer (yes, no), menopausal status, postmenopausal hormone use duration (never, <5 years, ≥5 years), and premenopausal hysterectomy (yes/no) with categories created for missing covariate data. Participants were classified as having a premenopausal hysterectomy if their age at hysterectomy was prior to the onset of menopause; participants were classified as not having a premenopausal hysterectomy if their age at hysterectomy was after the onset of menopause, if the reason for the hysterectomy was due to ovarian cancer, if the hysterectomy was less than a year before diagnosis, or if they never received a hysterectomy.
As hysterectomy, oral contraceptive use, and postmenopausal hormone use have the potential to be confounders or mediators of the endometriosis and/or leiomyomas and ovarian cancer relations, we examined their influence in two ways. First, we adjusted for each variable to examine as a potential confounder (described above). Second, we applied a counterfactual method of mediation to assess the presence of mediation on the endometriosis or leiomyomas and ovarian cancer risk relation by each of these variables.(31) This method is much more flexible than the product method, allows for non-linearity, and is equivalent to the product method(32) when there are no exposure-mediator interactions. To fit logistic mediation models to this analysis based on both cohort and case-control studies in this consortia, adjustments were needed to satisfy the rare event assumption. Ovarian cancer rates were rare in the cohort studies, but were not in the case-control studies. To account for this, weights for participants in the case-control studies were calculated by a function of race-specific ovarian cancer prevalence in the general and study populations, and these weights were applied within the models of the mediator as the outcome.(33) To implement this, a SAS macro designed by Valeri and VanderWeele was slightly modified to include these weights in the appropriate regression models.(31)
Finally, we assessed effect modification by hysterectomy and postmenopausal hormone use as prior studies have observed differential associations between endometriosis and/or uterine leiomyomas and ovarian cancer risk by these factors.(24, 34, 35) The results from effect modification analysis include analyses stratified by the effect modifier of interest (i.e., hysterectomy and postmenopausal hormone use) and the estimation of ORs using a single reference category (e.g., women who did not report a history of endometriosis and who did not report a hysterectomy).(33, 36) Finally, we performed a sensitivity analysis using a variable that compared non-cancer related hysterectomies (including both pre- and postmenopausal hysterectomies) to no hysterectomy or cancer-related hysterectomies. This information was not available for BWHS or SCCS. All statistical analyses were performed using SAS 9.4 (Cary, NC).
Results
A total of 2,724 Black participants and 5,281 White participants were included in the endometriosis analysis. Among all participants, 6.4% of Black participants and 7.0% of White participants reported a history of endometriosis, with the corresponding values among cases of 81 (8.5%) for Black participants and 198 (9.0%) for White participants, and 92 (5.2%) and 173 (5.6%) for Black and White controls, respectively. Among Black and White participants with ovarian cancer, those reporting a history of endometriosis were more likely to have endometrioid and clear cell tumors compared to those not reporting a history of endometriosis. Both Black and White participants with endometriosis were more likely to report a premenopausal hysterectomy and to have ever used oral contraceptives than participants without endometriosis (Table 1). The mean age of endometriosis diagnosis was 35 years in both Black and White participants.
Table 1.
Characteristics of the study population by history of endometriosis
| N (%) or Mean (SD) | Black Participants | White Participants | ||
|---|---|---|---|---|
| N = 2724 | N = 5281 | |||
| Endometriosis = No | Endometriosis = Yes | Endometriosis = No | Endometriosis = Yes | |
| (N = 2551) | (N = 173) | (N = 4910) | (N = 371) | |
| Site | ||||
| AACES | 1232 (48.3) | 94 (54.3) | N/A | N/A |
| BWHS | 644 (25.3) | 54 (31.2) | N/A | N/A |
| CCCCS | 116 (4.6) | 7 (4.1) | 617 (12.6) | 36 (9.7) |
| LACOCS | 260 (10.2) | 11 (6.4) | 2790 (56.8) | 170 (45.8) |
| NCOCS | 299 (11.7) | 7 (4.1) | 1503 (30.6) | 165 (44.5) |
| Case-Control Status | ||||
| Cases | 870 (34.1) | 81 (46.8) | 1998 (40.7) | 198 (53.4) |
| Controls | 1681 (65.9) | 92 (53.2) | 2912 (59.3) | 173 (46.6) |
| Age at Ovarian Cancer Diagnosis or Interview | 55.05±11.46 | 53.48±9.23 | 56.83±11.52 | 54.99±10.16 |
| Histotype * | ||||
| High-Grade Serous | 571 (65.6) | 38 (46.9) | 1218 (61.0) | 97 (49.0) |
| Low-Grade Serous | 31 (3.6) | 1 (1.2) | 76 (3.8) | 5 (2.5) |
| Endometrioid | 62 (7.1) | 22 (27.2) | 159 (8.0) | 27 (13.6) |
| Clear Cell | 29 (3.3) | 6 (7.4) | 144 (7.2) | 33 (16.7) |
| Mucinous | 50 (5.8) | 2 (2.5) | 133 (6.7) | 11 (5.6) |
| Carcinosarcoma | 21 (2.4) | 3 (3.7) | 40 (2.0) | 2 (1.0) |
| Other epithelial | 93 (10.7) | 5 (6.2) | 228 (11.4) | 23 (11.6) |
| Synchronous | 13 (1.5) | 4 (4.9) | 0 (0.0) | 0 (0.0) |
| Stage * | ||||
| Localized | 163 (18.7) | 28 (34.6) | 388 (19.4) | 61 (30.8) |
| Regional | 75 (8.6) | 9 (11.1) | 193 (9.7) | 28 (14.1) |
| Distant | 577 (66.3) | 41 (50.6) | 1398 (70.0) | 104 (52.5) |
| Missing | 55 (6.3) | 3 (3.7) | 19 (1.0) | 5 (2.5) |
| Education | ||||
| HS or less | 945 (37.0) | 44 (25.4) | 934 (19.0) | 55 (14.8) |
| Some college | 664 (26.0) | 42 (24.3) | 1149 (23.4) | 97 (26.2) |
| College grad | 543 (21.3) | 45 (26.0) | 1312 (26.7) | 114 (30.7) |
| Grad/prof school | 395 (15.5) | 42 (24.3) | 1515 (30.9) | 105 (28.3) |
| Missing | 4 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Parity | ||||
| No pregnancies | 406 (15.9) | 50 (28.9) | 1044 (21.3) | 112 (30.2) |
| 1–2 pregnancies | 1131 (44.3) | 88 (50.9) | 2310 (47.1) | 182 (49.1) |
| ≥3 pregnancies | 1012 (39.7) | 34 (19.7) | 1556 (31.7) | 77 (20.8) |
| Missing | 2 (0.1) | 1 (0.6) | 0 (0.0) | 0 (0.0) |
| Oral Contraceptive Duration | ||||
| Never | 857 (33.6) | 42 (24.3) | 1833 (37.3) | 83 (22.4) |
| <5 years | 922 (36.1) | 54 (31.2) | 1647 (33.5) | 146 (39.4) |
| ≥5 years | 746 (29.2) | 75 (43.4) | 1373 (28.0) | 137 (36.9) |
| Missing | 26 (1.0) | 2 (1.2) | 57 (1.2) | 5 (1.4) |
| Body Mass Index, kg/m 2 | ||||
| <25 | 511 (20.0) | 39 (22.5) | 2631 (53.6) | 194 (52.3) |
| 25–29.9 | 763 (29.9) | 54 (31.2) | 1293 (26.3) | 96 (25.9) |
| 30–34.9 | 610 (23.9) | 45 (26.0) | 584 (11.9) | 46 (12.4) |
| ≥35 | 650 (25.5) | 35 (20.2) | 362 (7.4) | 34 (9.2) |
| Missing | 17 (0.7) | 0 (0.0) | 40 (0.8) | 1 (0.3) |
| Smoking Status | ||||
| Never smoker | 1398 (54.8) | 94 (54.3) | 2538 (51.7) | 189 (50.9) |
| Former smoker | 734 (28.8) | 46 (26.6) | 1708 (34.8) | 134 (36.1) |
| Current smoker | 417 (16.4) | 33 (19.1) | 642 (13.1) | 46 (12.4) |
| Missing | 2 (0.1) | 0 (0.0) | 22 (0.5) | 2 (0.5) |
| Tubal Ligation Ever | ||||
| No | 1567 (61.4) | 118 (68.2) | 4006 (81.6) | 304 (81.9) |
| Yes | 913 (35.8) | 51 (29.5) | 896 (18.3) | 67 (18.1) |
| Missing | 71 (2.8) | 4 (2.3) | 8 (0.2) | 0 (0.0) |
| First-Degree Family History of Breast or Ovarian Cancer | ||||
| No | 1952 (76.5) | 130 (75.1) | 3985 (81.2) | 294 (79.3) |
| Yes | 487 (19.1) | 35 (20.2) | 875 (17.8) | 69 (18.6) |
| Missing | 112 (4.4) | 8 (4.6) | 50 (1.0) | 8 (2.2) |
| Menopausal Status | ||||
| Premenopausal | 873 (34.2) | 62 (35.8) | 1547 (31.5) | 131 (35.3) |
| Postmenopausal | 1676 (65.7) | 111 (64.2) | 3355 (68.33) | 240 (64.7) |
| Missing | 2 (0.1) | 0 (0.0) | 8 (0.2) | 0 (0.0) |
| Hormone Therapy Use Duration | ||||
| Never | 2009 (78.8) | 123 (71.1) | 2879 (58.6) | 194 (52.3) |
| <5 years | 324 (12.7) | 27 (15.6) | 833 (17.0) | 68 (18.3) |
| ≥5 years | 206 (8.1) | 21 (12.1) | 1104 (22.5) | 97 (26.2) |
| Missing | 12 (0.5) | 2 (1.2) | 94 (1.9) | 12 (3.2) |
| Age at Menarche | ||||
| <11 years | 274 (10.7) | 20 (11.6) | 313 (6.4) | 33 (8.9) |
| 11–12 years | 1048 (41.1) | 81 (46.8) | 2002 (40.8) | 154 (41.5) |
| 13–14 years | 903 (35.4) | 52 (30.1) | 2049 (41.7) | 149 (40.2) |
| 15–16 years | 267 (10.5) | 14 (8.1) | 465 (9.5) | 28 (7.6) |
| ≥17 years | 53 (2.1) | 6 (3.5) | 73 (1.5) | 6 (1.6) |
| Missing | 6 (0.2) | 0 (0.0) | 8 (0.2) | 1 (0.3) |
| Premenopausal Hysterectomy | ||||
| No | 1961 (76.9) | 108 (62.4) | 4148 (84.5) | 246 (66.3) |
| Yes | 576 (22.6) | 65 (37.6) | 744 (15.2) | 123 (33.2) |
| Missing | 14 (0.6) | 0 (0.0) | 18 (0.4) | 2 (0.5) |
| History of Infertility † | ||||
| No | 1714 (74.8) | 99 (61.1) | 1674 (79.0) | 128 (63.7) |
| Yes | 573 (25.0) | 63 (38.9) | 443 (20.9) | 73 (36.3) |
| Missing | 4 (0.2) | 0 (0.0) | 3 (0.1) | 0 (0.0) |
Distributions of histotype and stage are only shown for cases
History of infertility was not collected for LACOCS: 260 Black women without endometriosis, 11 Black women with endometriosis, 2790 White women without endometriosis, 170 White women with endometriosis
A history of endometriosis was associated with a higher risk of ovarian cancer in both Black participants (OR=2.12; 95% CI=1.50–3.00) and White participants (OR=1.58; 95% CI=1.26–1.98) (phetereogeneity=0.22). Statistically significant differences in the effect estimates across histotypes were identified when both Black and White participants were combined (phetereogeneity<0.001) and when associations were examined by race (phetereogeneity=0.047 for difference across histotypes for Black participants and phetereogeneity=0.004 for White participants). When stratified by histotype, the association with endometriosis was strongest for the endometrioid histotype among Black participants (OR=7.06; 95% CI=3.86–12.91) compared to White participants (OR=2.17; 95% CI=1.36–3.45) with a significant difference in the effect estimates by race for only the endometrioid histotype (phetereogeneity=0.003). Black participants (OR=4.82; 95% CI=1.81–21.85) and White participants (OR=3.48; 95% CI=2.24–5.41) had a higher risk of clear cell ovarian cancer (phetereogeneity=0.64). A similar increased risk of high-grade serous ovarian cancer was observed in both Black and White participants (OR=1.63; 95% CI=1.06–1.64 and OR=1.31; 95% CI=1.00–1.72, respectively, phetereogeneity=0.56) (Table 2 and Figure 1).
Table 2.
Odds ratios* and 95% confidence intervals for the association between endometriosis, overall and by race, for all ovarian cancer and stratified by histotype
| All histotypes | High-Grade Serous | Low-Grade Serous | Endometrioid | Clear Cell | Mucinous | pheterogeneity | Low-Grade Serous, Endo, & Clear Cell | |
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | between histotypes | OR (95% CI) | |
| All participants (cases/controls) | 3147/4858 | 1924/4858 | 113/4858 | 270/4858 | 212/4858 | 196/4858 | 595/4858 | |
| Yes | 1.75 (1.45–2.11) | 1.40 (1.11–1.76) | 0.97 (0.41–2.30) | 3.30 (2.31–4.71) | 3.93 (2.64–5.83) | 1.31 (0.72–2.36) | <0.001 | 3.05 (2.31–4.02) |
| Black participants (cases/controls) | 951/1773 | 609/1773 | 32/1773 | 84/1773 | 35/1773 | 52/1773 | 151/1773 | |
| Yes | 2.12 (1.50–3.00) | 1.63 (1.06–2.51) | 1.00 (0.13–7.99) | 7.06 (3.86–12.91) | 4.82 (1.81–12.85) | 0.89 (0.21–3.82) | 0.047 | 4.91 (2.93–8.23) |
| White participants (cases/controls) | 2196/3085 | 1315/3085 | 81/3085 | 186/3085 | 177/3085 | 144/3085 | 444/3085 | |
| Yes | 1.58 (1.26–1.98) | 1.31 (1.00–1.72) | 0.98 (0.38–2.55) | 2.17 (1.36–3.45) | 3.48 (2.24–5.41) | 1.41 (0.73–2.70) | 0.004 | 2.39 (1.71–3.34) |
| pheterogeneity by race | 0.22 | 0.56 | 0.94 | 0.003 | 0.64 | 0.50 |
Odd ratios were adjusted for site, age at diagnosis, education, parity, oral contraceptive use, BMI, smoking status, tubal ligation, family history of breast or ovarian cancer, menopausal status, post-menopausal hormone duration, age at menarche, and premenopausal hysterectomy.
Figure 1.
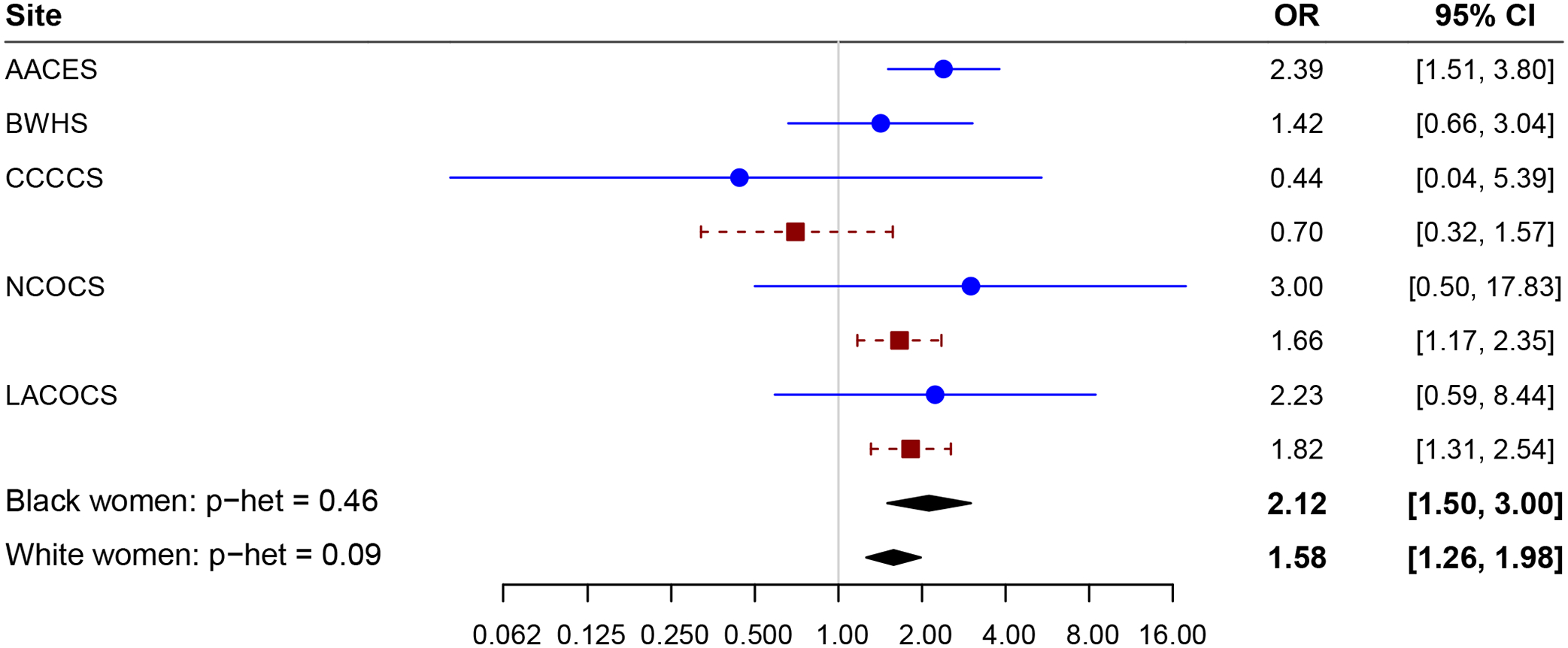
Forest plot of endometriosis by study and race. Adjusting for age at diagnosis, education, parity, oral contraceptive duration, body mass index, smoking status, tubal ligation, family history of breast or ovarian cancer, menopausal status, postmenopausal hormone duration, age at menarche, and premenopausal hysterectomy. AACES, African American Cancer Epidemiology Study; BWHS, Black Women’s Health Study; CCCCS, Cook County Case Study; NCOCS, North Carolina Ovarian Cancer Study; LACOCS, Los Angeles County Ovarian Cancer Study.
Mediation analysis of endometriosis and ovarian cancer by oral contraceptive use showed evidence for a suppressive indirect effect (IE) overall (Percent mediated −3.9%; IE p=0.02) and among White participants (Percent mediated −7.5%; Indirect Effect p=0.01) and a non-significant effect in Black participants (Percent mediated −0.3%; IE p=0.91)(Appendix 2, available online at http://links.lww.com/xxx). Mediation analyses suggested that part of the association between endometriosis and ovarian cancer is attributable to hysterectomy overall (Percent mediated 4.6%, IE p=0.08), with a larger percent mediated among White participants (8.1%, IE p=0.36) than Black participants (1.7%, IE p=0.55), although none reached statistical significance (Appendix 3, available online at http://links.lww.com/xxx). No mediation was observed by postmenopausal hormone use (Appendix 3, http://links.lww.com/xxx).
When stratified by premenopausal hysterectomy, the association between endometriosis and ovarian cancer in White participants was stronger in those who did not report a premenopausal hysterectomy (OR=1.85, 95% CI=1.41–2.43) than those who did report a hysterectomy (OR=1.09, 95% CI=0.73–1.64) while in Black participants the higher odds of ovarian cancer among those with endometriosis was observed in both those with and without hysterectomy (OR=2.32, 95% CI=1.51–3.59 for no hysterectomy and OR=1.91, 95% CI=1.05–3.48 for hysterectomy). However, these differences were not statistically significant (all p>0.05) (Table 3). When pre- and postmenopausal hysterectomies were examined, the associations were similar (data not shown).
Table 3.
Estimated Joint Effect* of Premenopausal Hysterectomy or Hormone Therapy and Endometriosis on the Odds of Ovarian Cancer
| Endometriosis | Endometriosis within Strata of effect modifier | RERIa | RERIm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||||||
| N Cases | N Controls | OR† | 95% CI | N Cases | N Controls | OR† | 95% CI | OR† | 95% CI | |||
| Premenopausal Hysterectomy | ||||||||||||
| All participants | ||||||||||||
| No | 2267 | 3842 | 1.00 | 184 | 170 | 1.97 | 1.57–2.48 | 1.99 | 1.58–2.50 | −0.55 (−1.22–0.12) p=0.10 | 0.69 (0.46–1.02) p=0.07 | |
| Yes | 582 | 738 | 1.17 | 1.02–1.33 | 93 | 95 | 1.59 | 1.16–2.16 | 1.32 | 0.94–1.84 | ||
| Black participants | ||||||||||||
| No | 649 | 1312 | 1.00 | 52 | 56 | 2.28 | 1.48–3.51 | 2.32 | 1.51–3.59 | −0.36 (−1.84–1.11) p=0.62 | 0.81 (0.39–1.65) p=0.56 | |
| Yes | 211 | 365 | 1.09 | 0.88–1.36 | 29 | 36 | 2.01 | 1.15–3.53 | 1.91 | 1.05–3.48 | ||
| White participants | ||||||||||||
| No | 1618 | 2530 | 1.00 | 132 | 114 | 1.83 | 1.39–2.39 | 1.85 | 1.41–2.43 | −0.65 (−1.68–0.09) p=0.09 | 0.63 (0.39–1.01) p=0.06 | |
| Yes | 371 | 373 | 1.24 | 1.05–1.47 | 64 | 59 | 1.42 | 0.98–2.07 | 1.09 | 0.73–1.64 | ||
| Hormone Therapy | ||||||||||||
| All participants | ||||||||||||
| No | 1773 | 3115 | 1.00 | 161 | 156 | 1.96 | 1.54–2.50 | 2.01 | 1.57–2.58 | −0.46 (−1.12–0.19) p=0.17 | 0.74 (0.51–1.07) p=0.11 | |
| Yes | 1094 | 1465 | 1.13 | 1.00–1.26 | 116 | 109 | 1.63 | 1.22–2.18 | 1.40 | 1.04–1.87 | ||
| Black participants | ||||||||||||
| No | 693 | 1316 | 1.00 | 56 | 67 | 2.03 | 1.35–3.05 | 1.99 | 1.32–3.01 | 0.34 (−1.45–2.13) p=0.71 | 1.10 (0.51, 2.41) p=0.80 | |
| Yes | 176 | 362 | 1.11 | 0.87–1.41 | 23 | 25 | 2.48 | 1.28–4.78 | 2.39 | 1.18–4.83 | ||
| White participants | ||||||||||||
| No | 1080 | 1799 | 1.00 | 105 | 89 | 1.89 | 1.39–2.57 | 1.97 | 1.44–2.70 | −0.57 (−1.30–0.15) p=0.12 | 0.68 (0.44–1.06) p=0.09 | |
| Yes | 918 | 1103 | 1.11 | 0.97–1.27 | 93 | 84 | 1.43 | 1.03–1.98 | 1.24 | 0.90–1.72 | ||
Measure of relative excess of interaction on the additive and multiplicative scales.
Odd ratios were adjusted for site, age at diagnosis, education, parity, OC duration, BMI, smoking status, tubal ligation, family history of cancer, menopausal status, hormone therapy duration or premenopausal hysterectomy, and age at menarche.
A total of 2,995 Black participants and 4,876 White participants were included in the leiomoyoma analysis. Among all participants, 43.2% of Black participants and 21.5% of White participants reported a history of leiomoyomas, with the corresponding values among cases of 444 (46.2%) for Black participants and 488 (24.5%) for White participants, and 851 (41.9%) and 559 (19.4%) for Black and White controls, respectively. Among Black and White participants with ovarian cancer, the distribution of histotypes was similar regardless of race or history of leiomyomas. Both Black and White participants with a history of leiomyomas were more likely to report a hysterectomy than those without a history of leiomyomas. While Black participants were more likely to report tubal ligation than White participants, the percentage reporting tubal ligation within each racial group did not vary materially by history of leiomyomas (Table 4). The mean age of leiomyoma diagnosis was 37 years in Black participants and 40 years in White participants.
Table 4.
Characteristics of the study population by history of uterine leiomyoma
| N (%) or Mean (SD) | Black Participants | White Participants | ||
|---|---|---|---|---|
| N = 2995 | N = 4876 | |||
| Leiomyoma=No | Leiomyoma=Yes | Leiomyoma=No | Leiomyoma=Yes | |
| N = 1700 | N = 1295 | N = 3829 | N = 1047 | |
| Site | ||||
| AACES | 760 (44.7) | 569 (43.9) | N/A | N/A |
| BWHS | 296 (17.4) | 402 (31.0) | N/A | N/A |
| LACOCS | 163 (9.6) | 106 (8.2) | 2283 (59.6) | 663 (63.3) |
| NCOCS | 194 (11.4) | 110 (8.5) | 1326 (34.6) | 340 (32.5) |
| SCCS | 287 (16.9) | 108 (8.3) | 220 (5.8) | 44 (4.2) |
| Case-Control Status | ||||
| Cases | 518 (30.5) | 444 (34.3) | 1504 (39.3) | 488 (46.6) |
| Controls | 1182 (69.5) | 851 (65.7) | 2325 (60.7) | 559 (53.4) |
| Age at Ovarian Cancer Diagnosis or Interview | 54.58±11.86 | 56.07±9.75 | 56.71±11.52 | 59.03±9.55 |
| Histotype * | ||||
| High-Grade Serous | 334 (64.5) | 280 (63.1) | 901 (59.9) | 299 (61.3) |
| Low-Grade Serous | 18 (3.5) | 12 (2.7) | 60 (4.0) | 11 (2.3) |
| Endometrioid | 35 (6.8) | 47 (10.6) | 119 (7.9) | 41 (8.4) |
| Clear Cell | 16 (3.1) | 18 (4.1) | 125 (8.3) | 36 (7.4) |
| Mucinous | 23 (4.4) | 25 (5.6) | 88 (5.9) | 27 (5.5) |
| Carcinosarcoma | 13 (2.5) | 15 (3.4) | 33 (2.2) | 6 (1.2) |
| Other epithelial | 70 (13.5) | 39 (8.8) | 178 (11.8) | 68 (13.9) |
| Synchronous | 9 (1.7) | 8 (1.8) | 0 (0.0) | 0 (0.0) |
| Stage * | ||||
| Localized | 95 (18.3) | 92 (20.7) | 289 (19.2) | 94 (19.3) |
| Regional | 51 (9.9) | 40 (9.0) | 153 (10.2) | 51 (10.5) |
| Distant | 339 (65.4) | 283 (63.7) | 1042 (69.3) | 333 (68.2) |
| Missing | 33 (6.4) | 29 (6.5) | 20 (1.3) | 10 (2.1) |
| Education | ||||
| HS or less | 794 (46.7) | 381 (29.4) | 707 (18.5) | 136 (13.0) |
| Some college | 407 (23.9) | 365 (28.2) | 918 (24.0) | 250 (23.9) |
| College grad | 317 (18.7) | 288 (22.2) | 1056 (27.6) | 288 (27.5) |
| Grad/prof school | 178 (10.5) | 260 (20.1) | 1148 (30.0) | 373 (35.6) |
| Missing | 4 (0.2) | 1 (0.1) | 0 (0.0) | 0 (0.0) |
| Parity | ||||
| No pregnancies | 253 (14.9) | 213 (16.5) | 831 (21.7) | 222 (21.2) |
| 1–2 pregnancies | 673 (39.6) | 643 (49.7) | 1812 (47.3) | 509 (48.6) |
| ≥3 pregnancies | 772 (45.4) | 436 (33.7) | 1186 (31.0) | 316 (30.2) |
| Missing | 2 (0.1) | 3 (0.2) | 0 (0.0) | 0 (0.0) |
| Oral Contraceptive Duration | ||||
| Never | 595 (35.0) | 380 (29.3) | 1309 (34.2) | 354 (33.8) |
| <5 years | 618 (36.4) | 460 (35.5) | 1287 (33.6) | 390 (37.3) |
| ≥5 years | 458 (26.9) | 445 (34.4) | 1186 (31.0) | 288 (27.5) |
| Missing | 29 (1.7) | 10 (0.8) | 47 (1.2) | 15 (1.4) |
| Body Mass Index, kg/m 2 | ||||
| <25 | 343 (20.2) | 207 (16.0) | 1942 (50.7) | 530 (50.6) |
| 25–29.9 | 492 (28.9) | 401 (31.0) | 1028 (26.9) | 283 (27.0) |
| 30–34.9 | 413 (24.3) | 337 (26.0) | 494 (12.9) | 135 (12.9) |
| ≥35 | 439 (25.8) | 343 (26.5) | 337 (8.8) | 86 (8.2) |
| Missing | 13 (0.8) | 7 (0.5) | 28 (0.7) | 13 (1.2) |
| Smoking Status | ||||
| Never smoker | 908 (53.4) | 743 (57.4) | 1977 (51.6) | 569 (54.4) |
| Former smoker | 460 (27.1) | 366 (28.3) | 1305 (34.1) | 389 (37.2) |
| Current smoker | 331 (19.5) | 185 (14.3) | 545 (14.2) | 89 (8.5) |
| Missing | 1 (0.1) | 1 (0.1) | 2 (0.1) | 0 (0.0) |
| Tubal Ligation Ever | ||||
| No | 1021 (60.1) | 778 (60.1) | 3059 (79.9) | 858 (82.0) |
| Yes | 645 (37.9) | 474 (36.6) | 764 (20.0) | 189 (18.1) |
| Missing | 34 (2.0) | 43 (3.3) | 6 (0.2) | 0 (0.0) |
| First-Degree Family History of Breast or Ovarian Cancer | ||||
| No | 1291 (75.9) | 1009 (77.9) | 3098 (80.9) | 818 (78.1) |
| Yes | 313 (18.4) | 237 (18.3) | 668 (17.5) | 216 (20.6) |
| Missing | 96 (5.7) | 49 (3.8) | 63 (1.7) | 13 (1.2) |
| Menopausal Status | ||||
| Premenopausal | 621 (36.5) | 381 (29.4) | 1196 (31.2) | 217 (20.7) |
| Postmenopausal | 1077 (63.4) | 914 (70.6) | 2633 (68.8) | 830 (79.3) |
| Missing | 2 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hormone Therapy Use Duration | ||||
| Never | 1426 (83.9) | 924 (71.4) | 2347 (61.3) | 480 (45.9) |
| <5 years | 159 (9.4) | 210 (16.2) | 602 (15.7) | 186 (17.8) |
| ≥5 years | 100 (5.9) | 156 (12.1) | 815 (21.3) | 359 (34.3) |
| Missing | 15 (0.9) | 5 (0.4) | 65 (1.7) | 22 (2.1) |
| Age at Menarche | ||||
| <11 years | 174 (10.2) | 152 (11.7) | 239 (6.2) | 78 (7.5) |
| 11–12 years | 637 (37.5) | 577 (44.6) | 1560 (40.7) | 447 (42.7) |
| 13–14 years | 596 (35.1) | 432 (33.4) | 1561 (40.8) | 421 (40.2) |
| 15–16 years | 228 (13.4) | 106 (8.2) | 399 (10.4) | 89 (8.5) |
| ≥17 years | 52 (3.1) | 24 (1.9) | 60 (1.6) | 12 (1.2) |
| Missing | 13 (0.8) | 4 (0.3) | 10 (0.3) | 0 (0.0) |
| Premenopausal Hysterectomy | ||||
| No | 1484 (87.3) | 779 (60.2) | 3322 (86.8) | 690 (65.9) |
| Yes | 207 (12.2) | 511 (39.5) | 497 (13.0) | 356 (34.0) |
| Missing | 9 (0.5) | 5 (0.4) | 10 (0.3) | 1 (0.1) |
| History of Infertility † | ||||
| No | 916 (73.3) | 790 (73.1) | 991 (74.7) | 265 (77.9) |
| Yes | 334 (26.7) | 287 (26.6) | 332 (25.0) | 75 (22.1) |
| Missing | 0 (0.0) | 4 (0.4) | 3 (0.2) | 0 (0.0) |
Distributions of histotype and stage are only shown for cases
History of infertility was not collected for SCCS & LACOCS: 450 Black participants without leiomyomas, 214 Black participants with leiomyomas, 2503 White participants without leiomyomas, 707 White women with leiomyomas.
A history of leiomyomas was associated with a slightly elevated risk of ovarian cancer overall (OR=1.26; 95% CI=1.12–1.41), and in Black (OR=1.34; 95% CI=1.11–1.62) and White participants (OR=1.22; 95% CI=1.05–1.41). Among Black participants, a history of leiomyomas was associated with a higher risk of all histotypes, with statistically significant associations observed for the endometrioid (OR=2.05; 95% CI=1.24–3.39) and high-grade serous (OR=1.32; 95% CI=1.06–1.64) histotypes (pheterogeneity for differences across histotypes=0.002). No statistically significant associations were observed between leiomyomas and specific histotypes in White participants, however, suggestions of higher risk were present in all histotypes except low grade serous and no significant differences in the associations across histotypes were observed (pheterogeneity=0.33) (Table 5 and Figure 2). No significant mediation of the association between leiomyomas and ovarian cancer was observed for hysterectomy, postmenopausal hormone use, or oral contraceptive use (all p>0.05).
Table 5.
Odds ratios* and 95% confidence intervals for the association between uterine leiomyoma, overall and by race, for all ovarian cancer and stratified by histotype
| Overall | High-Grade Serous | Low-Grade Serous | Endometrioid | Clear Cell | Mucinous | p-het | Low-Grade Serous, Endo, & Clear Cell | |
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | (between histotypes) | OR (95% CI) | |
| All participants (cases/controls) | 2954/4917 | 1814/4917 | 101/4917 | 242/4917 | 195/4917 | 163/4917 | 538/4917 | |
| Yes | 1.26 (1.12–1.41) | 1.25 (1.09–1.43) | 0.99 (0.59–1.64) | 1.59 (1.18–2.15) | 1.26 (0.89–1.78) | 1.54 (1.07–2.23) | 0.03 | 1.35 (1.09–1.68) |
| Black participants (cases/controls) | 962/2033 | 614/2033 | 30/2033 | 82/2033 | 34/2033 | 48/2033 | 146/2033 | |
| Yes | 1.34 (1.11–1.62) | 1.32 (1.06–1.64) | 2.05 (0.83–5.07) | 2.05 (1.24–3.39) | 1.64 (0.77–3.48) | 1.92 (0.99–3.69) | 0.002 | 1.92 (1.31–2.80) |
| White participants (cases/controls) | 1992/2884 | 1200/2884 | 71/2884 | 160/2884 | 161/2884 | 115/2884 | 392/2884 | |
| Yes | 1.22 (1.05–1.41) | 1.18 (0.99–1.41) | 0.77 (0.39–1.53) | 1.41 (0.95–2.10) | 1.27 (0.84–1.90) | 1.45 (0.91–2.32) | 0.33 | 1.21 (0.92–1.59) |
| pheterogeneity by race | 0.54 | 0.69 | 0.22 | 0.21 | 0.39 | 0.52 |
Odd ratios were adjusted for site, age at diagnosis, education, parity, oral contraceptive duration, BMI, smoking status, tubal ligation, family history of breast or ovarian cancer, menopausal status, post-menopausal hormone duration, age at menarche, and premenopausal hysterectomy.
Figure 2.
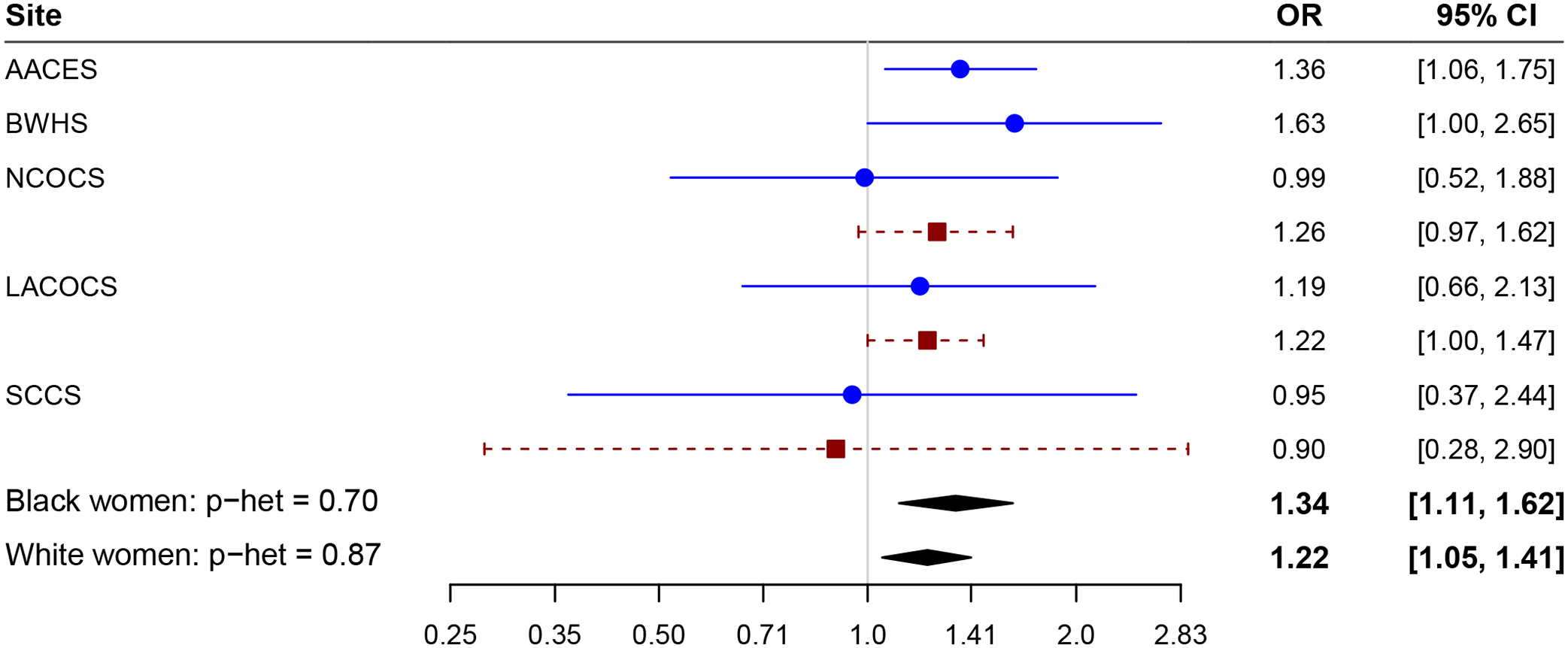
Forest plot of leiomyoma by study and race. Adjusting for age at diagnosis, education, parity, oral contraceptive duration, body mass index, smoking status, tubal ligation, family history of breast or ovarian cancer, menopausal status, postmenopausal hormone duration, age at menarche, and premenopausal hysterectomy. AACES, African American Cancer Epidemiology Study; BWHS, Black Women’s Health Study; NCOCS, North Carolina Ovarian Cancer Study; LACOCS, Los Angeles County Ovarian Cancer Study; SCCS, Southern Community Cohort Study.
When stratified by premenopausal hysterectomy, the association between leiomyomas and ovarian cancer was present only among those who did not report a premenopausal hysterectomy for both racial groups. Black participants who had a history of leiomyomas and no premenopausal hysterectomy had 53% greater odds of ovarian cancer (95% CI=1.23–1.89) compared to those without leiomyomas, while there was no association with leiomyomas in the premenopausal hysterectomy group (OR=0.92, 95% CI=0.62–1.36). This interaction was of borderline significance on both the multiplicative (p=0.05) and additive (p=0.08) scales. A similar pattern was observed among White participants (OR=1.31, 95% CI=1.10–1.55 among those with no premenopausal hysterectomy and OR=1.00, 95% CI=0.74–1.35 among those with a premenopausal hysterectomy), but the interaction was not significant on either the multiplicative (p=0.21) or the additive (p=0.28) scales (Table 6). When a common reference group was applied (those who did not have leiomyomas and did not have a hysterectomy), Black and White participants in all other exposure categories had a higher risk of ovarian cancer (Table 6). There were no significant interactions between hormone therapy and leiomyomas in any subset (Table 6). Associations were similar when pre- and postmenopausal hysterectomies were examined (data not shown).
Table 6.
Estimated joint effect* of premenopausal hysterectomy or hormone therapy and uterine leiomyoma on the odds of ovarian cancer
| Leiomyoma | Leiomyoma within Strata of effect modifier | RERIa | RERIm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||||||
| N Cases | N Controls | OR† | 95% CI | N Cases | N Controls | OR† | 95% CI | OR† | 95% CI | |||
| Premenopausal Hysterectomy | ||||||||||||
| All participants | ||||||||||||
| No | 1695 | 3111 | 1.00 | 577 | 892 | 1.38 | 1.21–1.57 | 1.40 | 1.22–1.60 | −0.41 (−0.74–−0.08) p=0.01 | 0.71 (0.55–0.91) p=0.01 | |
| Yes | 311 | 393 | 1.29 | 1.08–1.53 | 351 | 516 | 1.26 | 1.06–1.48 | 0.96 | 0.76–1.21 | ||
| Black participants | ||||||||||||
| No | 435 | 1049 | 1.00 | 268 | 511 | 1.48 | 1.20–1.83 | 1.53 | 1.23–1.89 | −0.52 (−1.10–0.06) p=0.08 | 0.66 (0.43–1.00) p=0.05 | |
| Yes | 75 | 132 | 1.33 | 0.95–1.87 | 173 | 338 | 1.29 | 1.01–1.66 | 0.92 | 0.62–1.36 | ||
| White participants | ||||||||||||
| No | 1260 | 2062 | 1.00 | 309 | 381 | 1.29 | 1.08–1.53 | 1.31 | 1.10–1.55 | −0.24 (−0.67–0.19) p=0.28 | 0.81 (0.58–1.13) p=0.21 | |
| Yes | 236 | 261 | 1.26 | 1.02–1.55 | 178 | 178 | 1.31 | 1.03–1.66 | 1.00 | 0.74–1.35 | ||
| Hormone Therapy | ||||||||||||
| All participants | ||||||||||||
| No | 1292 | 2448 | 1.00 | 529 | 864 | 1.30 | 1.13–1.50 | 1.33 | 1.15–1.54 | −0.08 (−0.35–0.19) p=0.57 | 0.93 (0.75–1.16) p=0.50 | |
| Yes | 729 | 1054 | 1.08 | 0.94–1.23 | 402 | 545 | 1.30 | 1.09–1.54 | 1.19 | 0.99–1.43 | ||
| Black participants | ||||||||||||
| No | 434 | 978 | 1.00 | 320 | 596 | 1.37 | 1.11–1.68 | 1.37 | 1.11–1.69 | −0.12 (−0.65–0.42) p=0.67 | 0.89 (0.59–1.35) p=0.58 | |
| Yes | 83 | 201 | 1.17 | 0.85–1.61 | 123 | 254 | 1.42 | 1.05–1.92 | 1.25 | 0.83–1.87 | ||
| White participants | ||||||||||||
| No | 858 | 1470 | 1.00 | 209 | 268 | 1.23 | 1.00–1.52 | 1.29 | 1.04–1.60 | −0.02 (−0.37–0.32) p=0.89 | 0.98 (0.73–1.31) p=0.89 | |
| Yes | 646 | 853 | 1.01 | 0.87–1.18 | 279 | 291 | 1.22 | 0.99–1.51 | 1.18 | 0.96–1.46 | ||
Measure of relative excess of interaction on the additive and multiplicative scales.
Odd ratios were adjusted for site, age at diagnosis, education, parity, OC duration, BMI, smoking status, tubal ligation, family history of cancer, menopausal status, hormone therapy duration or premenopausal hysterectomy, and age at menarche.
Discussion
In this consortium of 6 studies, both Black and White participants with a history of endometriosis had a higher risk of ovarian cancer, with the strongest associations among Black participants for the endometrioid and clear cell histotypes. Hysterectomy modified the association between endometriosis and ovarian cancer, but only among White participants. Leiomyomas were associated with a modestly increased risk of ovarian cancer among both Black and White participants. When stratified by hysterectomy, this association persisted only among those without a premenopausal hysterectomy, irrespective of race.
Observational studies have consistently found an association between endometriosis and epithelial ovarian cancer, specifically endometrioid and clear cell ovarian cancer histotypes.(19–21) However, prior studies were conducted in predominantly White populations and did not compare effect estimates by race. The largest prior study of Black participants to examine this association was a pooled analysis including data from AACES, NCOCS, LACOCS (which are also included in our analyses) and 9 other case-control studies in the Ovarian Cancer Association Consortium, which also found a similar stronger association between endometriosis and ovarian cancer risk among Black participants compared to non-Hispanic White participants, but did not report associations for endometriosis-associated histotypes.(17)
Few studies have examined the association between leiomyomas and ovarian cancer risk and to our knowledge none have examined the association among Black women. A Danish registry study found an association between leiomyomas and ovarian cancer risk (OR=1.36; 95% CI=1.16–1.60). However, the association was present only among those who had been diagnosed with leiomyomas within the year preceding the ovarian cancer diagnosis, suggesting that detection bias might explain the observed association.(37) Dixon-Suen et al., also examined leiomyomas and ovarian cancer risk, and reported positive associations that persisted across all histotypes.(24) This is consistent with our study in which elevated effect estimates were present for most histotypes, although not all reached statistical significance.
Consistent with results from recent studies, hysterectomy modified the association between endometriosis(24, 34, 38) and leiomyomas(24) and ovarian cancer risk. In our analyses, the positive association between both endometriosis and leiomyomas and ovarian cancer risk was strongest among participants who had not had a premenopausal hysterectomy. The exception to this was among Black participants with endometriosis, who had a higher risk of ovarian cancer regardless of hysterectomy status. Hysterectomy eliminates the risk of leiomyoma recurrence, but it may not eliminate risk of recurrent endometriosis. Among those with endometriosis and leiomyomas, hysterectomy may indicate the duration or severity of these conditions, since alternative treatments for both conditions are available. It is possible that removal of the fallopian tubes along with the uterus may explain part of the lower risk of ovarian cancer observed among those with a hysterectomy, but this would be expected to impact primarily high-grade serous ovarian cancers that arise from the fallopian tube.
Limitations of this study include potential misclassification of endometriosis and leiomyomas through self-report. For both conditions, the prevalence among controls was lower than current population estimates, which may reflect under-reporting by participants, cohort effects in incidence, and/or period effects in diagnosis. While recall bias is a possibility in the retrospective case-control studies, in the prospective BWHS and SCCS (included in these analyses), we observed similar reporting patterns to that of the case-control studies indicating that recall bias does not likely explain our results. Thus, across our included studies, it is most likely that misclassification of endometriosis and leiomyomas was non-differential with respect to the outcome of ovarian cancer. In addition, the percentage of participants reporting endometriosis and leiomyomas differed across the included studies, with generally higher prevalence reported in more recent studies (Appendix 1, http://links.lww.com/xxx), suggesting period effects in diagnosis. In individual studies that included both Black and White participants, Black participants were more likely than White participants to report leiomyomas and White women were more likely than Black participants to report endometriosis, which is consistent with current diagnostic patterns.(13, 15) Racial differences in endometriosis diagnosis may reflect disparities in access to appropriate diagnostic methods, as opposed to biological differences in incidence.(14)
In conclusion, both Black and White participants with endometriosis had a higher risk of ovarian cancer, and hysterectomy modified this association among White participants. Leiomyomas were associated with a modestly increased risk of ovarian cancer among Black and White participants, with hysterectomy modifying the risk in both groups. Further research is needed to understand how racial differences in access to care and treatment options (e.g., hysterectomy), impact or modify ovarian cancer risk.
Supplementary Material
Funding:
This study is supported by the National Institutes of Health (R01-CA207260 to Schildkraut and Rosenberg and K01-CA212056 to Bethea). AACES was funded by NCI (R01-CA142081 to Schildkraut); BWHS was funded by NCI (R01-CA058420,UM1-CA164974, and U01-CA164974 to Rosenberg); CCCCS was funded by NIH/NCI (R01-CA61093 to Rosenblatt); LACOCS was funded by NCI (R01-CA17054 to Pike, R01-CA58598 to Goodman and Wu, and Cancer Center Core Grant P30-CA014089 to Henderson and Wu) and by the California Cancer Research Program (2II0200 to A. Wu); and NCOCS was funded by NCI (R01-CA076016 to Schildkraut). SCCS is supported by the NCI (grants R01-CA092447 and U01-CA202979 to Blot and Zheng) and data collection is performed by the Survey and Biospecimen Shared Resource, which is supported by the Vanderbilt-Ingram Cancer Center (P30-CA68485 to Pietenpol). In addition, support to add SCCS to OCWAA was provided by a Pilot Award (to Beeghly) from the NIH Precision Medicine and Health Disparities Collaborative (NIMHD/NHGRI U54-MD010722 to Wilkins).
Footnotes
Presented at the 2022 American Association for Cancer Research (AACR) Conference on the Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved, September 16–19, 2022, Philadelphia, Pennsylvania.
Each author has confirmed compliance with the journal’s requirements for authorship.
Financial Disclosure
Elisa V. Bandera reports receiving payment from Pfizer, Inc. as an advisory board member of committee to enhance participation of minorities in clinical trials). The other authors did not report any potential conflicts of interest.
References
- 1.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. American journal of obstetrics and gynecology 2003;188(1):100–7 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 2.Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, et al. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril 2011. Aug;96(2):360–5 10.1016/j.fertnstert.2011.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buttram VC Jr., Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril 1981. Oct;36(4):433–45 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 4.Cramer SF, Patel A. The frequency of uterine leiomyomas. American journal of clinical pathology 1990. Oct;94(4):435–8 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 5.Flynn M, Jamison M, Datta S, Myers E. Health care resource use for uterine fibroid tumors in the United States. American journal of obstetrics and gynecology 2006. Oct;195(4):955–64 10.1016/j.ajog.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Kjerulff KH, Erickson BA, Langenberg PW. Chronic gynecological conditions reported by US women: findings from the National Health Interview Survey, 1984 to 1992. American journal of public health 1996. Feb;86(2):195–9 10.2105/ajph.86.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am 2003;30(1):1–19 10.1016/s0889-8545(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 8.Nnoaham KE, Hummelshoj L, Webster P, d’Hooghe T, de Cicco Nardone F, de Cicco Nardone C, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011;96(2):366–73.e8 10.1016/j.fertnstert.2011.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simoens S, Hummelshoj L, D’Hooghe T. Endometriosis: cost estimates and methodological perspective. Human reproduction update 2007. Jul-Aug;13(4):395–404 10.1093/humupd/dmm010. [DOI] [PubMed] [Google Scholar]
- 10.Simoens S, Meuleman C, D’Hooghe T. Non-health-care costs associated with endometriosis. Hum Reprod 2011. Sep;26(9):2363–7 10.1093/humrep/der215. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol 1994. Apr;83(4):549–55 10.1097/00006250-199404000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Winkel CA. Modeling of medical and surgical treatment costs of chronic pelvic pain: new paradigms for making clinical decisions. The American journal of managed care 1999. May;5(5 Suppl):S276–90 10.1097/00006250-199404000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Bougie O, Yap MI, Sikora L, Flaxman T, Singh S. Influence of race/ethnicity on prevalence and presentation of endometriosis: a systematic review and meta-analysis. Bjog 2019. Aug;126(9):1104–15 10.1111/1471-0528.15692. [DOI] [PubMed] [Google Scholar]
- 14.Farland LV, Horne AW. Disparity in endometriosis diagnoses between racial/ethnic groups. Bjog 2019. Aug;126(9):1115–6 10.1111/1471-0528.15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. Bjog 2017. Sep;124(10):1501–12 10.1111/1471-0528.14640. [DOI] [PubMed] [Google Scholar]
- 16.Park HK, Ruterbusch JJ, Cote ML. Recent Trends in Ovarian Cancer Incidence and Relative Survival in the United States by Race/Ethnicity and Histologic Subtypes. Cancer Epidemiol Biomarkers Prev 2017. Oct;26(10):1511–8 10.1158/1055-9965.Epi-17-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peres LC, Risch H, Terry KL, Webb PM, Goodman MT, Wu AH, et al. Racial/ethnic differences in the epidemiology of ovarian cancer: a pooled analysis of 12 case-control studies. Int J Epidemiol 2018. Apr 1;47(2):460–72 10.1093/ije/dyx252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan SJ, Nagle CM, Coory MD, Maresco D, Protani MM, Pandeya NA, et al. Has the association between hysterectomy and ovarian cancer changed over time? A systematic review and meta-analysis. Eur J Cancer 2013. Nov;49(17):3638–47 10.1016/j.ejca.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. The Lancet Oncology 2012. Apr;13(4):385–94 10.1016/s1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J Clin Oncol 2016. August 20, 2016;34(24):2888–98 10.1200/jco.2016.66.8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermens M, van Altena AM, Nieboer TE, Schoot BC, van Vliet H, Siebers AG, et al. Incidence of endometrioid and clear-cell ovarian cancer in histological proven endometriosis: the ENOCA population-based cohort study. American journal of obstetrics and gynecology 2020. Jul;223(1):107.e1–.e11 10.1016/j.ajog.2020.01.041. [DOI] [PubMed] [Google Scholar]
- 22.Velebil P, Wingo PA, Xia Z, Wilcox LS, Peterson HB. Rate of hospitalization for gynecologic disorders among reproductive-age women in the United States. Obstet Gynecol 1995. Nov;86(5):764–9 10.1016/0029-7844(95)00252-M. [DOI] [PubMed] [Google Scholar]
- 23.Morgan DM, Kamdar NS, Swenson CW, Kobernik EK, Sammarco AG, Nallamothu B. Nationwide trends in the utilization of and payments for hysterectomy in the United States among commercially insured women. American journal of obstetrics and gynecology 2018. Apr;218(4):425.e1–.e18 10.1016/j.ajog.2017.12.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon-Suen SC, Webb PM, Wilson LF, Tuesley K, Stewart LM, Jordan SJ. The Association Between Hysterectomy and Ovarian Cancer Risk: A Population-Based Record-Linkage Study. Journal of the National Cancer Institute 2019. Oct 1;111(10):1097–103 10.1093/jnci/djz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schildkraut JM, Peres LC, Bethea TN, Camacho F, Chyn D, Cloyd EK, et al. Ovarian Cancer in Women of African Ancestry (OCWAA) consortium: a resource of harmonized data from eight epidemiologic studies of African American and white women. Cancer causes & control : CCC 2019. Sep;30(9):967–78 10.1007/s10552-019-01199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurman R, Carcangiu M, Herrington C, Young R. WHO classification of tumours of female reproductive organs. Lyon; 2014. [Google Scholar]
- 27.Bangdiwala SI, Bhargava A, O’Connor DP, Robinson TN, Michie S, Murray DM, et al. Statistical methodologies to pool across multiple intervention studies. Transl Behav Med 2016. Jun;6(2):228–35 10.1007/s13142-016-0386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang BH, Hoaglin DC. Meta-Analysis of Odds Ratios: Current Good Practices. Med Care 2017. Apr;55(4):328–35 10.1097/mlr.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole SR, Chu H, Greenland S. Maximum likelihood, profile likelihood, and penalized likelihood: a primer. Am J Epidemiol 2014. Jan 15;179(2):252–60 10.1093/aje/kwt245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 2016. Mar;7(1):55–79 10.1002/jrsm.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013. Jun;18(2):137–50 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol 2007;58:593–614 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VanderWeele T, Knol M. A Tutorial on Interaction. Epidemiologic Methods 2014;3(1):33–72 10.1515/em-2013-0005. [DOI] [Google Scholar]
- 34.Khoja L, Weber RP, Webb PM, Jordan SJ, Muthukumar A, Chang-Claude J, et al. Endometriosis and menopausal hormone therapy impact the hysterectomy-ovarian cancer association. Gynecologic oncology 2022. Jan;164(1):195–201 10.1016/j.ygyno.2021.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peres LC, Alberg AJ, Bandera EV, Barnholtz-Sloan J, Bondy M, Cote ML, et al. Premenopausal Hysterectomy and Risk of Ovarian Cancer in African-American Women. Am J Epidemiol 2017. Jul 1;186(1):46–53 10.1093/aje/kwx055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol 2012. Apr;41(2):514–20 10.1093/ije/dyr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brinton LA, Sakoda LC, Sherman ME, Frederiksen K, Kjaer SK, Graubard BI, et al. Relationship of benign gynecologic diseases to subsequent risk of ovarian and uterine tumors. Cancer Epidemiol Biomarkers Prev 2005. Dec;14(12):2929–35 10.1158/1055-9965.Epi-05-0394. [DOI] [PubMed] [Google Scholar]
- 38.Modugno F, Ness RB, Allen GO, Schildkraut JM, Davis FG, Goodman MT. Oral contraceptive use, reproductive history, and risk of epithelial ovarian cancer in women with and without endometriosis. American journal of obstetrics and gynecology 2004. Sep;191(3):733–40 10.1016/j.ajog.2004.03.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


