Abstract
Purpose
To determine what policies exist regarding age and provision of fertility treatment in United States fertility clinics.
Methods
Medical directors of the Society for Assisted Reproductive Technology (SART) member clinics were surveyed regarding clinic demographics and current policies pertaining to age and provision of fertility treatment. Univariate comparisons were performed using Chi-square and Fisher exact tests as appropriate, with significance set at P ≤ 0.05.
Results
Of the 366 clinics surveyed, 18.9% (69/366) responded. A majority of clinics who responded 88.4% (61/69) reported having a policy regarding patient age and provision of fertility treatment. Responding clinics with an age policy did not differ from those without a policy on the basis of geographical location, (p = 0.5), insurance mandate status (p = 0.9), practice type (p = 0.4), or annual number of ART cycles (p = 0.7). Of all clinics who responded, 73.9% (51/69) had a maximum maternal age for autologous IVF, with a median of 45 years (range 42–54). Similarly, 79.7% (55/69) of responding clinics had a maximum maternal age for donor oocyte IVF, with a median of 52 years (range 48–56). Slightly under half, 43.4% (30/69) of responding clinics had a maximum maternal age for fertility treatment other than IVF (including ovulation induction or ovarian stimulation with or without IUI) with a median of 46 years (range 42–55). Of note, only 4.3% (3/69) of responding clinics had a policy with respect to maximum paternal age, with a median of 55 years (range 55–70).
The most commonly cited reasons for having an age-limit policy were maternal risks of pregnancy, lower ART success rates, fetal/neonatal risks, and concerns about patients’ ability to parent at an older age.
More than half 56.5% (39/69) of responding clinics reported making exceptions to these policies, most commonly for patients who have pre-existing embryos. The majority of medical directors who responded to the survey believed there should be an ASRM guideline regarding maximum maternal age for autologous IVF 71% (49/69), donor oocyte IVF 78% (54/69) and other fertility treatments 62% (43/69).
Conclusions
Most fertility clinics who responded to this national survey reported having a policy regarding maternal age (but not paternal age) and provision of fertility treatment. Policies were based on risk of maternal/fetal complications, lower success rates at older age, and concerns about patients’ ability to parent at an older age. The majority of medical directors of responding clinics believed there should be an ASRM guideline regarding age and provision of fertility treatment.
Keywords: Advanced reproductive age, Age policy, Fertility treatment
Introduction
With the advent of assisted reproductive technology (ART), patients can pursue family-building at increasingly older ages. The National Center for Health Statistics reports that the birth rate increased by 227% for United States women ages 45–49 and 630% for women ages 50–54 between 1998 and 2020 [1]. The use of donor oocytes has made pregnancy possible for women in their 50 s and 60 s [2, 3]. The live birth rate per embryo transfer for women using donor oocytes exceeds 50% in most clinical settings and only decreases minimally for recipients over the age of 45 [4, 5].
However, having children at a later age increases the risk of both maternal and fetal complications. While maternal risks of pregnancy increase in the late 30 s, women over the age of 45 have even more significantly increased risks of complications such as hypertensive disorders of pregnancy and diabetes [6]. In a series of 45 pregnancies in women over the age of 50, 20% were diagnosed with gestational diabetes, 35% developed preeclampsia, and 78% underwent cesarean section [7]. The fetal risks associated with advanced reproductive age (ARA) are often due to the increased risk of multiple gestation seen in the setting of fertility treatment [8]. However, even singleton pregnancies carry elevated risks of low birth weight and fetal mortality in women over 50 [9].
While the risk of chromosomal abnormalities is frequently mitigated by the use of donor oocytes, the use of sperm from an individual of advanced paternal age (APA) has been associated with bipolar disorder, attention deficit hyperactivity disorder, autism, schizophrenia and mood disorders [10, 11]. A growing body of evidence has also identified that these risks associated with APA impact not only to the children, but also the grandchildren, of older men [12].
In the past, ASRM recommended that non-identified sperm donors be less than age 40 [13], however in its most recent guidance, ASRM states that “men should ideally be young enough so that the risks to the offspring associated with an increased paternal age, such as autism, are minimized.” [14]. While a uniform definition of APA has not been developed, a 2015 review compared definitions of APA and found that most researchers use aged 40 and beyond as a threshold [15]. Offspring risks start to emerge after paternal age 40 and increase with paternal age over 50.[12].
Beyond the medical risks, parents of ARA and their children face several additional challenges. Life expectancy in the United States began declining in 2014 and has declined further since the COVID-19 pandemic [16]. Children born to older parents face parental health decline, increased caregiving responsibility, and parental bereavement earlier in life than their peers with younger parents [12]. Actuarial data suggests that 18% of children whose fathers were 50 at the time of their birth will experience parental death by the age of 18; 31% of these children will experience parental death by the age of 25 [17]. Children who experience death of a parent are more likely to suffer from psychiatric disorders [18], have lower academic achievement [19, 20], and face the collateral losses of moving from the family home, lowered family income, and decreased emotional support from the surviving parent who is now also bereaved [21]. MacDougall et al. surveyed individuals who became parents through IVF at age 40 and beyond [22]. They reported feeling more mature and emotionally prepared for parenting, but 90% of them concluded that individuals should pursue parenthood before age 40, effectively discouraging others from following in their footsteps [22]. As greater numbers of increasingly older prospective parents present for fertility treatment, reproductive endocrinologists face an ethical dilemma.
In 2016, the American Society for Reproductive Medicine (ASRM) Ethics Committee issued an opinion regarding oocyte/embryo donation to ARA patients. In this opinion, ASRM recognizes the potential medical and psychosocial complications of parenting at ARA, encourages discussion of these risks with ARA parents, discourages oocyte donation to women greater than age 55, and recognizes that it is ethically permissible for programs to decline to provide treatment to women of ARA based on concerns over the health and well-being of the parents and offspring [23]. Furthermore, the committee recommended psychosocial evaluation to determine if adequate supports exist to raise the future child to adulthood. Of note, this committee opinion did not address age policies for men. Despite these recommendations, ASRM does not currently provide clear guidance on whether there should be a maximum age for providing fertility treatment. It is not well established how individual fertility clinics vary in their specific age policies. Therefore, the objective of this study was to determine what policies, if any, have been established regarding age and provision of fertility treatment in United States fertility clinics.
Material/Methods
Following approval from the Duke Institutional Review Board (IRB) and SART Research Committee, a survey invitation was sent via email to the medical directors of the 366 SART member clinics. Two additional reminder emails were sent at 2 and 4 week intervals after the initial email. An invitation to complete the survey was sent electronically as a link that could be answered through the SART website portal. The survey questions assessed clinic demographics including geographic location, state-mandated insurance status, type of practice, and IVF cycle volume. The survey also assessed policies regarding age of patients pursuing autologous IVF, donor oocyte IVF, and other fertility treatment. Survey respondents who reported having a policy were surveyed regarding their reasons for developing policies, how they communicated these policies to patients, and if exceptions to age policies were granted. Finally, respondents were asked if they believe the ASRM should have national guidelines regarding age and provision of fertility treatment. Please see Appendix 1 for the survey. The survey was developed by the research team, tested internally with members of the research team, but was not validated or correlated with any other published measure.
Univariate comparisons were performed using Chi square or Fisher-exact tests as appropriate with significance at p ≤ 0.05. Analysis was performed using Graphpad Prism (version 9).
Results
A total of 69 anonymous responses were received, representing a 18.9% (69/366) response rate. The majority of clinics 96% (66/69) responded to all relevant questions in the survey. Of the respondents, 23.1% (16/69) were located in the Northeast, 17.4% (12/69) in the Midwest, 33.3% (23/69) in the South, and 26.1% (18/69) in the West. The majority of respondents 70.5% (49/69) had no state-mandated insurance coverage for fertility treatment, while 23.5% (16/69) had state-mandated coverage for some fertility treatment, and 5.8% (4/69) had state-mandated coverage for fertility treatment including IUI and IVF. More than half of respondents 56.5% (39/69) categorized their practice as private practice, with 31.8% (22/69) academic, 5.7% (4/69) hospital-based and 5.8% (4/69) a combination practice (a combination of private, academic or hospital-based) practice. The number of annual fresh ART cycles reported ranged from 30–4000. Of the respondents, 5.8% (4/69) reported fewer than 100 cycles per year, 58.8% (40/69) between 100–499 cycles, 20.5% (14/69) between 500–999 cycles, 4.4% (3/69) from 1000–1499 cycles, and 10.2% (7/69) greater than 1500 cycles. The number of annual frozen embryo transfer cycles reported ranged from 50–4000. The number of annual donor oocyte IVF cycles reported ranged from 0–400. Of the respondents, only one 1.4% (1/69) performed 0 donor IVF cycles, 13.2% (9/69) performed between 1–10 donor IVF cycles, 38.2% (26/69) performed between 11–25 donor IVF cycles, 27.9% (19/69) performed between 26–50 donor IVF cycles and 19.1% (14/69) performed greater than 50 donor IVF cycles. The majority of responding clinics 88.4% (61/69) reported having at least one policy regarding age and provision of fertility treatment. The presence of a policy regarding age and provision of fertility treatment for responding clinics did not vary on the basis of geographic region (p = 0.5), state-mandated insurance coverage (p = 0.9), practice type (p = 0.4), or ART cycle volume (p = 0.7) (Table 1).
Table 1.
Demographics of respondent centers by age policy
| Variable | Has Age Policy (n = 61) | Does Not Have Age Policy (n = 8) | P-Value | Total (n = 69) |
|---|---|---|---|---|
| Region | ||||
| Northeast | 14 (87.5) | 2 (12.5) | p = .5 | 16 (23.2) |
| Midwest | 12 (100) | 0 (0) | 12 (17.4) | |
| South | 19 (82.6) | 4 (17.4) | 23 (33.3) | |
| West | 16 (88.9) | 2 (11.1) | 18 (26.1) | |
| Mandated coverage | ||||
| No | 42 (87.5) | 6 (12.5) | p = .9 | 48 (70.6) |
| Yes | 18 (90) | 2 (10) | 20 (29.4) | |
| Type of Practice | ||||
| Academic | 21 (95.4) | 1 (4.6) | p = .4 | 22 (31.8) |
| Private | 33 (84.6) | 6 (15.4) | 39 (56.6) | |
| Combination | 4 (100) | 0 (0) | 4 (5.8) | |
| Hospital -based | 3 (75) | 1 (25) | 4 (5.8) | |
| ART cycle volume (annual number of fresh cycles) | ||||
| 0–99 | 4 (100) | 0 (0) | p = .7 | 4 (5.8) |
| 100–499 | 34 (85) | 6 (15) | 40 (58.8) | |
| 500–999 | 12 (85.7) | 2 (14.3) | 14 (20.6) | |
| 1000–1499 | 3 (100) | 0 (0) | 3 (4.4) | |
| 1500 and above | 7 (100) | 0 (0) | 7 (10.4) | |
Data are n (%). Totals may not add to 100 due to response omitted by respondent
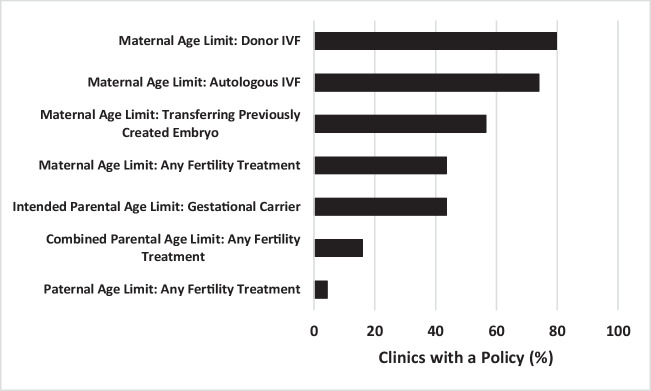
The types of policies regarding age and provision of fertility treatment varied (Fig. 1). Of the clinics who responded to the survey, 73.9% (51/69; 73.9%) reported a maximum maternal age to undergo autologous IVF, with a median of 45 years (range 42–54). A slightly higher percentage of responding clinics 79.7% (55/69) reported a maximum maternal age to undergo donor oocyte IVF, with a median of 52 years (range 48–56). 56.5% (39/69) of responding clinics had a different policy regarding maximum maternal age for transferring an embryo that was previously created, with a median of 52 years (range 45–56). Only 43.4% (30/69) of responding clinics had a maximum maternal age for offering fertility treatment other than IVF (ovulation induction and intrauterine insemination (IUI)), with a median of 46 (range 42–55). Of note, only 4.3% (3/69) of responding clinics reported a policy with respect to maximum paternal age for offering fertility treatment, with a median of 55 years (range 55–70). Finally, 15.9% (11/69) of responding clinics reported a policy for maximum combined parental age for offering any fertility treatment, with a median of 110 years (range 100–110).
Fig. 1.
Age Policies at United States Fertility Clinics
The most common methods that responding clinics used to communicate age policies to patients were via the physician at the initial consultation 87% (58/67) or the front desk staff/administrative team members when scheduling initial consultations 55% (37/67). Less often, this information was conveyed by nurses 30% (20/67) or the clinic website 12% (8/67).
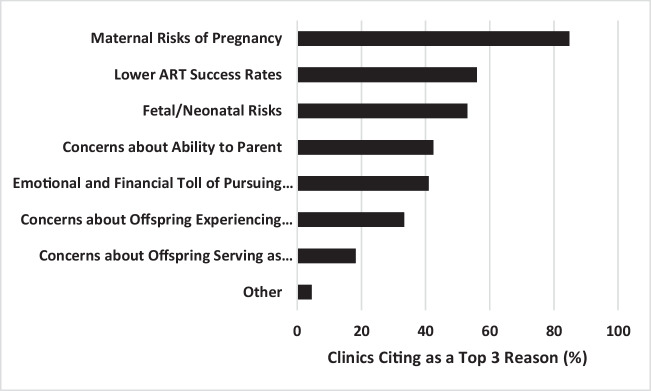
Survey respondents were asked to choose their top three reasons for developing policies regarding age and provision of fertility treatment (Fig. 2). The most common reasons given were the maternal risks of pregnancy 84% (56/66), lower success rates 56% (37/66), and fetal/neonatal risks 53% (35/66). Other reasons included concerns about ability to parent at an older age 42% (28/66), the emotional and financial toll of pursuing futile treatment 41% (27/66), concerns about offspring experiencing parental death at a young age 33% (22/66), and concerns about offspring serving as parental caregivers at a young age 18% (12/66).
Fig. 2.
Commonly Cited Reasons for Development of Age Policies
Of all responding clinics, 55% (38/69) reported granting exceptions, most commonly due to the presence of pre-existing embryos 69% (26/38). Additional reasons for granting exceptions included the absence of other risk factors for complications in pregnancy 55% (21/38) and a prior uncomplicated pregnancy 40% (15/38). Less common but still cited reasons for granting exceptions included the patient’s ability to comprehend the risks of pregnancy at ARA, the presence of a younger partner, a robust psychosocial support system, and financial resources (e.g., ability to afford a gestational carrier).
As part of the survey, respondents were asked how often they recommend Maternal Fetal Medicine (MFM) consultation for ARA patients. For older patients planning to carry a pregnancy, the majority of responding clinics 88.4% (61/69) routinely recommended MFM consultation. The mean age for recommending this consultation was 44 years (range 35–50). While often recommended, responding clinics reported that this consultation never 38% (26/69) or rarely 50% (34/69) resulted in a patient being denied care.
The majority of survey respondents indicated an interest in having formal ASRM guidelines regarding age and provision of fertility treatment. Most respondents 71% (49/69) believed there should be an ASRM policy regarding maximum maternal age for offering autologous IVF, with the median recommended age of 45 years (range 43–54). A slightly higher percentage of respondents 78% (54/69) favored development of an ASRM policy regarding maximum maternal age for offering donor oocyte IVF, with the median recommended age of 51 years (range 44–56). Additionally, there was broad support 62% (43/69) for an ASRM policy regarding maximum maternal age for offering fertility treatment other than IVF (ovulation induction and IUI), with the median recommended age being 47 years (range 43–55).
Discussion
The advent of ART and third-party reproduction has extended the reproductive lifespan for many, allowing family-building at older ages. The purpose of this study was to understand the variety of policies regarding age and provision of fertility treatment, the rationale behind the development of such policies, and opinions regarding ASRM guidance on age policies. Although our data demonstrate that the majority of United States fertility clinics who responded to the survey do have policies regarding age and provision of fertility treatment, these policies vary substantially in terms of maximum allowable age and treatment type to which they apply. This inconsistency can create confusion for patients, as it is possible for patients to be eligible for treatment at one center and barred from the same treatment at another. This inconsistency also puts pressure on providers to offer fertility care in situations about which they may have significant concerns. For example, if a patient can be seen at one fertility clinic with no age cut-off and they then go to another clinic expecting similar treatment, the fertility provider is likely to feel pressure to provide that care in order to placate the patient or prevent receiving a bad review. Given that many metropolitan areas have multiple fertility centers and fertility patients commonly compare clinics, the provider may feel pressured to provide care to an ARA patient even if they have ethical concerns about providing futile care, creating a high-risk pregnancy, or exposing the offspring to a high risk of parental bereavement. Consistency regarding age policies across clinics could help decrease patient confusion and increase provider comfort in administering or limiting fertility care with older patients.
The overwhelming majority of survey respondents indicated having age policies for autologous and donor IVF cycles, with the maximum age for pursuing donor IVF 7 years later than for autologous IVF (median 52 years vs. median 45 years). This distinction was likely based upon the fact that donor IVF is less likely to be futile than autologous IVF in older patients. The median age cut-off for provision of other types of fertility treatment (including ovulation induction or ovarian stimulation, with or without IUI) was approximately 1 year later than that for autologous IVF, likely due to the lower cost and medical risks involved [24]. Interestingly, slightly more than half of the responding clinics did not have a policy regarding age and provision of non-IVF fertility treatment.
Importantly, only a minority of respondents reported policies regarding paternal age and provision of fertility treatment. The concept of a “reproductive clock” is frequently applied to women, but rarely to men; in fact, older fatherhood is often celebrated in the public eye. However, increasing paternal age, especially above 50 years, is associated with lower IVF success rates [25] and paternal age above 40 years has been associated with offspring neurodevelopmental and psychosocial risks [12]. Furthermore, while a number of reporting clinics noted concerns about offspring experiencing parental death or serving as parental caregivers at a young age in their reasoning for developing age policies, there was a disproportionally small number of clinics with advanced paternal age cut-offs. Thus, the absence of paternal age policies may be based on provider biases or cultural mores rather than the current empirical data about the risks of older fatherhood and psychological impact of losing a father at a young age. Also, it is important to acknowledge that maternal age cutoffs were motivated by risks to the mother (i.e. risks of carrying a pregnancy at an older age) which are not present in the father. Nevertheless, further studies into the impact of paternal age on ART outcomes as well as increased patient and provider awareness of these risks should lead to a more balanced implementation of age policies for older fertility patients of any gender.
In addition to describing a variety of United States fertility clinic policies regarding age and provision of fertility treatment, we sought to explore the decisional and ethical frameworks underlying those policies. Responding clinics with age policies were primarily focused on the immediate medical risks that older mothers face in pregnancy as well as decreased cycle success rates for older patients. However, some responding clinics also reported concerns about the long-term implications of older parenthood such as impaired ability to parent at an older age, parental health decline and death, and offspring caregiving burden guided their development of age policies for fertility care. Although not the primary reason for developing policies, this consideration of downstream psychosocial implications of older parenthood for children was notable.
Furthermore, while a majority of responding clinics did have age policies, a substantial percentage of them had made exceptions to these policies, especially in cases where embryos have already been created. Once providers have formed a relationship with the patient and embryos are already in existence, enforcing age policies is likely to be more difficult. Equitable adherence to established age policies is likely to hinge on communication of such policies at the outset of treatment and a team approach including doctors, nursing, and the laboratory to have a standard method when considering exemptions.
These survey results highlighted the wide variability in policies regarding age and provision of fertility treatment across United States fertility clinics who responded to a national SART survey. It is therefore unsurprising that a majority of respondents expressed the need for ASRM guidance regarding this topic. While ASRM has released an Ethics Committee opinion recognizing the ethical permissibility for clinics to decline treatment to women of ARA based on health concerns for patients and offspring, it does not currently offer any specific guidelines regarding age and provision of fertility treatment nor does it address age policies for men [23]. The results of this study suggest that more comprehensive guidance is needed.
With regard to strengths, this is the first survey study to assess individual clinic policies regarding age and provision of fertility treatment, therefore addressing a significant knowledge gap. We surveyed SART member clinics from across the United States, gathering data not just on current policies, but also the rationale used to develop those policies.
With regard to study limitations, the survey questionnaire was created exclusively for this study and, thus, was not previously validated. More importantly, our study had a small sample size and relatively low survey response rate of 18.9%, despite sending multiple contacts through SART. As a result, our data may reflect response bias, as those clinics who responded to the survey may differ in important ways from those who did not respond. Specifically, respondents may be more likely to have policies regarding age and provision of fertility treatment. However, our response rate was not significantly different from another survey of U.S. fertility clinics that contributed significantly to our understanding of clinical practice patterns. Specifically, Kaye and colleagues surveyed U.S. fertility centers regarding their BMI limits for fertility treatment and had a response rate of 19.5% [26]. Additionally, given the growing numbers of older patients presenting for care, providing fertility clinics with possible policy models and decision-making rationales is critical.
In summary, in this SART survey, we found interesting data on the kinds of age policies that exist across the United States and the rationales that these clinics have for developing age policies. These policies were most often guided by provider concerns about the medical risks of pregnancy for older patients, but also revealed longer-term concerns about the ability to parent at an older age, parental health decline and death, and offspring caregiving burden. Furthermore, most respondents indicated a need for ASRM guidance on this topic. As older prospective patients present for care in greater numbers, elucidating frameworks for policies regarding age and provision of fertility treatment will offer providers more scaffolding for making these difficult ethical decisions and result in more consistent, safe, and equitable patient care.
Appendix 1 Survey regarding patient age and provision of fertility treatment in the United States
Data availability
The data generated and/or reviewed during the above publication are not publicly available but available from the corresponding author by reasonable request.
Code availability
Coding was not utilized in the completion of this project.
Declarations
Ethics approval
This study was approved as exempt by the Duke Institutional Review Board.
Consent to participate
Informed consent was obtained by all individual participants included in the survey.
Conflicts of interests/Competing interests
The authors have no competing or conflicting interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Center for Health Statistics. US birth rates by age. www.cdc.gov/nchs. Accessed 28 Oct 2022.
- 2.Sauer MV, Paulson RJ, Lobo RA. Pregnancy after age 50: application of oocyte donation to women after natural menopause. Lancet. 1993;341:321–323. doi: 10.1016/0140-6736(93)90132-Z. [DOI] [PubMed] [Google Scholar]
- 3.Paulson RJ, Thornton MH, Francis MM, Salvador HS. Successful pregnancy in a 63-year-old woman. Fertil Steril. 1997;67:949–951. doi: 10.1016/S0015-0282(97)81413-6. [DOI] [PubMed] [Google Scholar]
- 4.Yeh JS, Steward RG, Dude AM, Shah AA, Goldfarb JM, Muasher SJ. Pregnancy rates in donor oocyte cycles compared to similar autologous in vitro fertilization cycles: An analysis of 26,457 fresh cycles from the Society for Assisted Reproductive Technology. Fertil Steril Elsevier Inc. 2014;102:399–404. doi: 10.1016/j.fertnstert.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 5.Yeh JS, Steward RG, Dude AM, Shah AA, Goldfarb JM, Muasher SJ. Pregnancy outcomes decline in recipients over age 44: An analysis of 27,959 fresh donor oocyte in vitro fertilization cycles from the Society for Assisted Reproductive Technology. Fertil Steril. Elsevier Inc. 2014;101:1331–1336.e1. doi: 10.1016/j.fertnstert.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 6.Grotegut CA, Chisholm CA, Johnson LNC, Brown HL, Heine RP, James AH. Medical and obstetric complications among pregnant women aged 45 and older. PLoS One. 2014;9(4):e96237. [DOI] [PMC free article] [PubMed]
- 7.Paulson RJ, Boostanfar R, Saadat P, Mor E, Tourgeman DE, Slater CC, et al. Pregnancy in the sixth decade of life obstetric outcomes in women of advanced reproductive age. JAMA. 2002;288(18):2320–3. doi: 10.1001/jama.288.18.2320. [DOI] [PubMed] [Google Scholar]
- 8.Sauer MV, Paulson RJ, Lobo RA. Oocyte donation to women of advanced reproductive age: Pregnancy results and obstetrical outcomes in patients 45 years and older. Hum Reprod. 1996;11:2540–2543. doi: 10.1093/oxfordjournals.humrep.a019155. [DOI] [PubMed] [Google Scholar]
- 9.Salihu HM, Shumpert MN, Slay M, Kirby RS, Alexander G. Childbearing beyond maternal age 50 and fetal outcomes in the United States. Obstet Gynecol. 2003;102:1006–14. doi: 10.1016/s0029-7844(03)00739-7. [DOI] [PubMed] [Google Scholar]
- 10.Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, et al. Advancing paternal age and autism. Arch Gen Psychiatry. 2006;63(9):1026–32. doi: 10.1001/archpsyc.63.9.1026. [DOI] [PubMed] [Google Scholar]
- 11.Sipos A, Rasmussen F, Harrison G, Lewis G, Leon DA, Gunnell D. Paternal age and schizophrenia: a population based cohort study. BMJ. 2004;329(7474):1070. doi: 10.1136/bmj.38243.672396.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zweifel JE, Woodward JT. The risky business of advanced paternal age: neurodevelopmental and psychosocial implications for children of older fathers. Fertil Steril. 2022;118(6):1013–21. doi: 10.1016/j.fertnstert.2022.10.029. [DOI] [PubMed] [Google Scholar]
- 13.ASRM Practice Committee Guidelines for gamete and embryo donation. Fertil Steril. 2006;2006:86. doi: 10.1016/j.fertnstert.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 14.ASRM Practice Committee Guidance regarding gamete and embryo donation. Fertil Steril. Elsevier Inc. 2021;115:1395–410. doi: 10.1016/j.fertnstert.2021.01.045. [DOI] [PubMed] [Google Scholar]
- 15.Ramasamy R, Chiba K, Butler P, Lamb DJ. Male biological clock: A critical analysis of advanced paternal age. Fertil Steril. Elsevier Inc. 2015;103:1402–6. doi: 10.1016/j.fertnstert.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woolf SH, Schoomaker H. Life Expectancy and Mortality Rates in the United States, 1959–2017. JAMA. 2019;322(20):1996–2016. doi: 10.1001/jama.2019.16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arias E, Heron M, Xu J. Unites States life tables, 2014. National Vital Statistics Reports; vol 66, no 4. Hyattsville, MD: National Center for Health Statistics; 2017. Available at: https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_04.pdf. Accessed 1 Nov 2022. [PubMed]
- 18.McKay MT, Cannon M, Healy C, Syer S, O’Donnell L, Clarke MC. A meta-analysis of the relationship between parental death in childhood and subsequent psychiatric disorder. Acta Psychiatr Scand. 2021;143:472–486. doi: 10.1111/acps.13289. [DOI] [PubMed] [Google Scholar]
- 19.Kailaheimo-Lönnqvist S, Erola J. Child’s age at parental death and university education. Eur Soc. 2020;22(4):433–455. doi: 10.1080/14616696.2020.1719179. [DOI] [Google Scholar]
- 20.Liu C, Grotta A, Hiyoshi A, Berg L, Rostila M. School outcomes among children following death of a parent. JAMA Open Network. 2022;5(4):e223842. doi: 10.1001/jamanetworkopen.2022.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biank NM, Werner-Lin A. Growing up with grief: revisiting the death of a parent over the life course. Omega. 2011;63(3):271–290. doi: 10.2190/OM.63.3.e. [DOI] [PubMed] [Google Scholar]
- 22.MacDougall K, Beyene Y, Nachtigall RD. ‘Inconvenient biology:’ advantages and disadvantages of first-time parenting after age 40 using in vitro fertilization. Hum Reprod. 2012;27(4):1058–1065. doi: 10.1093/humrep/des007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daar J, Benward J, Collins L, Davis J, Francis L, Gates E, et al. Oocyte or embryo donation to women of advanced reproductive age: an Ethics Committee opinion. Fertil Steril. 2016;106:e3–7. doi: 10.1016/j.fertnstert.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Liu K, Case A, Cheung AP, Sierra S, AlAsiri S, Carranza-Mamane B, et al. Advanced Reproductive Age and Fertility. J Obstet Gynaecol Canada Elsevier Masson SAS. 2011;33:1165–75. doi: 10.1016/S1701-2163(16)35087-3. [DOI] [PubMed] [Google Scholar]
- 25.Morris G, Mavrelos D, Odia R, Viñals Gonzalez X, Cawood S, Yasmin E, et al. Paternal age over 50 years decreases assisted reproductive technology (ART) success: a single UK center retrospective analysis. Acta Obstet Gynecol Scand. 2021;100(10):1858–67. doi: 10.1111/aogs.14221. [DOI] [PubMed] [Google Scholar]
- 26.Kaye L, Sueldo C, Engmann L, Nulsen J, Benadiva C. Survey assessing obesity policies for assisted reproductive technology in the United States. Fertil Steril. Elsevier Inc. 2016;105:703–706.e2. doi: 10.1016/j.fertnstert.2015.11.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated and/or reviewed during the above publication are not publicly available but available from the corresponding author by reasonable request.
Coding was not utilized in the completion of this project.