Abstract
Background
Malnutrition impacts the clinical course of Crohn's disease; however, there is little evidence of its influence on perioperative adverse events. We assessed whether nutritional indicators are associated with postoperative complications in surgical treatment of Crohn's disease.
Methods
137 patients with Crohn's disease who underwent surgical treatment between January 2011 and December 2020 were included. Skeletal muscle index was calculated by a single CT slice. We analyzed the risk factors for adverse events.
Results
37 % of patients had postoperative complications. Adverse events occurred more frequently in patients with high serum C-reactive protein, low serum albumin, prognostic nutritional index <38.3, skeletal muscle index <38.9 cm2/m2, abdominoperineal resection, long surgical duration, and mass hemorrhage. Among patients with skeletal muscle index <38.9 cm2/m2, patients who experienced adverse events had higher visceral fat index compared with those who did not (0.85 vs. 0.45, P = 0.04). Multivariate analysis revealed that skeletal muscle index <38.9 cm2/m2 and low serum albumin were the independent risk factors for postoperative complications (Odds ratio, 2.85; 95 % confidence interval, 1.13–7.16; P = 0.03, 2.62; 1.09–6.26; P = 0.03, respectively). Separated by sex, low serum albumin (<3.5 and <2.8 g/dL, male and female, respectively) and skeletal muscle index (<38.9 and <36.6 cm2/m2, male and female, respectively) were statistically related to postoperative complications.
Conclusions
Skeletal muscle index is the most useful nutritional predictor of postoperative complications in Crohn's disease patients among other nutritional indices. We believe that these patients are at high risk of postoperative complications and need appropriate nutritional support in the perioperative period.
Keywords: Crohn's disease, Postoperative complications, Sarcopenia, Skeletal muscle mass index, Nutritional status
Introduction
Crohn's disease (CD) is an inflammatory bowel disorder and is associated with malnutrition. Several factors, including malabsorption, chronic inflammation, decrease of oral intake, and medication, may cause impairment of nutritional status in CD patients. As body composition reflects nutritional status, it has been studied in subjects with malignant disease and systematic disease and it can predict clinical outcomes in these diseases [[1], [2], [3]]. Recently, it has been indicated that malnutrition is a factor in the poor outcomes observed in inflammatory bowel disease (IBD) [4]. Sarcopenia, which is defined as a loss of skeletal muscle mass accompanied by both decreased muscle strength and physical weakness, has also been correlated with malnutritional status [5,6]. These definitions are useful to assess the nutritional status in aged patients who have malignant or systemic disease. However, IBD is more common in younger patients, and it is caused by various mechanisms including inflammation. Therefore, more adequate criteria are necessary for the nutritional assessment of CD patients.
The development of medical treatment of CD has achieved and maintained long-term clinical remission. However, surgical intervention is still often necessary in the lifetime of CD patients [7]. CD patients generally have an increased risk of postoperative adverse events due to the characteristics of the disease compared with patients without IBD [8]. Many studies have been conducted to identify risk factors for surgical treatment in CD patients. Several clinical factors including low serum albumin level, preoperative steroid use, previous surgical history, and the presence of preoperative abscesses, were detected as correlated risk factors for adverse events among surgical patients [9]. The European Crohn's and Colitis Organization has emphasized the importance of assessing the perioperative nutritional status of IBD patients [10]. Some recent reports have indicated that preoperative body composition has the potential to be a predictive indicator for the occurrence of adverse events in CD patients. Although a few reports have indicated that low skeletal muscle mass, or sarcopenia, is associated with postoperative complications in surgical treatment of CD [11,12], there are almost no studies comparing the various indicators of nutritional status. Therefore, we focused on nutritional factors collected from preoperative data as follows: serum albumin [9], body mass index (BMI) [13,14], Prognostic nutritional index (PNI) [15], visceral fat area (VFA) [16], subcutaneous fat area (SFA) [16] and skeletal muscle mass [11,12]. The aim of our study was to investigate which factors directly reflect the risk for postoperative complication in surgical treatment of CD patients. This study is a retrospective study, which reports our experience in the treatment of 137 cases. We analyzed the surgical outcomes to assess and investigate which nutritional indices are risk factors for postoperative complications.
Methods
Patients and data collection
Patients diagnosed with CD who underwent surgical treatment at the Department of Surgery and Oncology at Kyushu University between January 2011 and December 2020 were included (N = 143). Out of all the patients, 6 patients were excluded because of missing clinical data or imaging data that was unavailable for analysis, and 137 patients were included in the study. The clinical characteristics of the patients were determined from the clinical and histopathological reports. Patient demographics and clinical features collected at the time of surgery included sex, age, smoking status, disease period, preoperative treatment (5-aminosalicylic acid (5-ASA), biologics and corticosteroids), preoperative physical examination, BMI, surgical history, and laboratory findings. Penetrating lesions were defined as the occurrence of intra-abdominal fistulas, inflammatory masses, and/or abscesses at any time in the course of the disease, except with perianal fistulas or ulcers. PNI was calculated according to the following formula: 10 × albumin (g/dL) + 0.005 × total lymphocyte count (per mL). Onodera et al. reported that a PNI of <40 suggested a high degree of malnutrition in the perioperative period of gastrointestinal surgery [15]. All patients underwent imaging studies for diagnosis including colonoscopy, enteroclysis, barium enema and abdominal CT scans within two months before surgery. Postoperative complications were evaluated according to the Clavien–Dindo grading system for the classification of surgical complications, version 2.0 [17]. Operative findings such as duration of operation and hemorrhage, conversion, postoperative morbidity and mortality, postoperative hospital stay and reoperation due to the disease exacerbation were extracted from clinical records. The median follow-up period of these patients was 67.4 months (range 1–140). This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Kyushu University Hospital Human Research Ethics Committee (No. 2022–97). Written informed consent was waived because of the retrospective design.
Measurement of body components
BMI was calculated by dividing weight (kg) by height (m2). CT imaging was used to calculate VFA, SFA and skeletal muscle mass. A single-slice CT scan at the L3 level with the patient in the supine position was elected to perform the measurements of VFA, SFA and skeletal muscle mass [[18], [19], [20], [21]]. Visceral and subcutaneous fat and skeletal muscle were identified and quantified by use of Hounsfield unit thresholds (−50 to −150 and −29 to +150, respectively). VFA, SFA and skeletal muscle area (SMA) were outlined and measured in square centimeter based on the pixel count (Fig. 1). Cross-sectional area, which was automatically calculated by summing the pixilation, was then divided by the square of the height to generate visceral fat index (VFI) and skeletal muscle index (SMI) (cm2/m2) as previously described [[18], [19], [20], [21]]. Imaging analysis was performed by use of SYNAPS VINCENT software, version 5.5 (Fujifilm, Tokyo, Japan). A researcher blinded to patient information carried out these measurements.
Fig. 1.
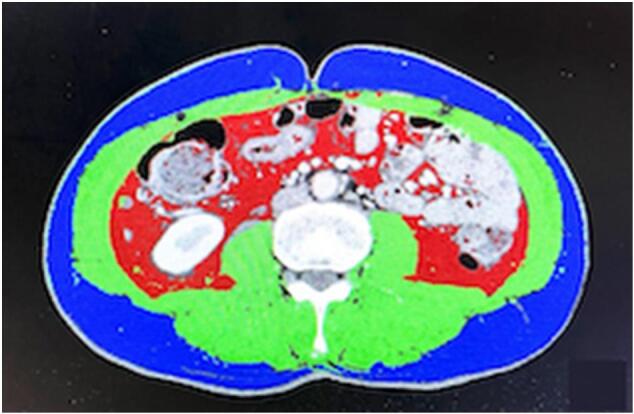
Evaluation of the body composition using a third lumbar computed tomography scan slice. Red: visceral adipose tissue, Blue: subcutaneous adipose tissue, Green: skeletal muscle. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Statistics analysis
Univariate analyses were conducted to compare patients with or without postoperative complications. All statistical analyses were performed using JMP version 15.1.0 software (SAS Institute, Cary, NC, USA). Clinical and demographic characteristics were analyzed using the χ2 test for categorical variables. Differences in continuous variables were compared using the Mann–Whitney U test. One-way analysis of variance test was used to compare the other continuous variables. Receiver operating characteristic curve was used to detect the cut-off values of continuous variables. Logistic regression analysis was used to determine independent risk factors for conversion and postoperative complications. In the multivariate analysis of all patients, all variables were included in a backward stepwise multiple logistic regression to identify significant risk factors for postoperative complications. The final model included preoperative treatment of 5-ASA, preoperative serum C-reactive protein (CRP), preoperative serum albumin, SMI, and surgical duration in all patients, and SMI and preoperative serum albumin in male and female patients. The fitness of the model was evaluated by the likelihood ratio test that showed P < 0.001. Values of P < 0.05 were considered to indicate statistical significance.
Results
Characteristics of Crohn's disease patients with or without postoperative complications
Table 1 shows the results of comparison of patient clinical features separated based on the occurrence of postoperative complications. Of 137 patients, 51 patients (37.2 %) had any grade of postoperative complications, and 86 patients had no complications. Grade 3 or 4 postoperative complications occurred in 17 patients (12.4 %) and infectious complications occurred in 26 patients (19.0 %). Sex, age, smoking status, preoperative disease period, immunosuppressive preoperative treatment, including corticosteroids, immunomodulators, and biologics, surgical history, and disease behavior were not significantly different between the two groups (defined by the presence or absence of postoperative complications). The patients without postoperative complications were more likely to have had 5-ASA treatment before surgery. Table 2 shows the preoperative parameters related to inflammation or nutrition status in patients with or without postoperative complications. Preoperative CRP was significantly higher in the patients who had postoperative complications compared with those who did not (0.53 mg/L vs. 0.19 mg/L, P < 0.01). The patients who had postoperative complications had significantly lower PNI (38.6 vs. 42.6, P < 0.01) associated with lower preoperative serum albumin (3.5 mg/dL vs. 3.8 mg/dL, P < 0.01) than those who did not. Although BMI, VFA, SFA, VFI, and skeletal muscle mass did not significantly differ between the two groups, SMI was significantly lower in the patients who had postoperative complications compared to those who did not (40.4 vs. 44.5 cm2/m2, P = 0.01).
Table 1.
Characteristics of Crohn's disease patients with or without postoperative complications.
| Present |
Absent |
P value | ||
|---|---|---|---|---|
| (n = 51) | (n = 86) | |||
| Age | Mean, years (range) | 43 (22–72) | 38 (18–71) | 0.06 |
| Sex | Male | 39 (76.5 %) | 57 (66.3 %) | 0.57 |
| Female | 12 (23.5 %) | 29 (33.7 %) | ||
| Smoking | Present | 10 (19.6 %) | 24 (27.9 %) | 0.27 |
| Disease period | Mean, years (range) | 15 (0–41) | 13 (0–49) | 0.22 |
| Preoperative treatment $ | 5-ASA | 20 (39.2 %) | 48 (55.8 %) | 0.06 |
| Corticosteroid | 4 (7.8 %) | 7 (8.1 %) | 0.95 | |
| Immunomodulator | 8 (15.7 %) | 16 (18.6 %) | 0.66 | |
| Anti-TNF alpha antibody | 28 (54.9 %) | 33 (38.4 %) | 0.23 | |
| Surgical history | Present | 26 (51.0 %) | 49 (57.0 %) | 0.50 |
| Behavior | Nonpenetrating | 21 (41.2 %) | 46 (53.5 %) | 0.16 |
| Penetrating | 30 (58.8 %) | 40 (46.5 %) | ||
CD; Crohn's disease, OS; Open surgery, LS; Laparoscopic surgery.
Duplicative data.
Table 2.
Clinical markers of disease activity and nutritional status.
| Present |
Absent |
P value | ||
|---|---|---|---|---|
| (n = 51) | (n = 86) | |||
| Preoperative serum CRP | mg/L, median, (range) | 0.53 (0.02–20.5) | 0.19 (0.01–10.7) | 0.006⁎ |
| Preoperative serum albumin | mg/dL, median, (range) | 3.5 (1.6–4.3) | 3.8 (2.3–4.9) | 0.002⁎ |
| PNI | Median, (range) | 38.6 (16.3–58) | 42.6 (25.1–58) | 0.009⁎ |
| BMI | Median, (range) | 18.8 (12.9–26.5) | 19.2 (11.1–26.5) | 0.37 |
| Visceral fat area | cm2, median, (range) | 43.4 (2.9–165.5) | 35.8 (2.9–259) | 0.43 |
| Subcutaneous fat area | cm2, median, (range) | 66.8 (2.0–224.8) | 65.9 (2.0–203.5) | 0.96 |
| VFI | Median, (range) | 0.78 (0.04–8.67) | 0.67 (0.06–10.0) | 0.42 |
| Skeletal muscle mass | cm2, median, (range) | 117.7 (65.8–180.2) | 126.0 (63.0–188.6) | 0.15 |
| SMI | Median, (range) | 40.4 (29.3–65.4) | 44.5 (28.7–69.5) | 0.01⁎ |
BMI; body mass index, CRP; C-reactive protein, PNI; prognostic nutritional index, VFI; visceral fat index, SMI; skeletal muscle index.
Indicates statistical significance (P < 0.05).
Table 3 shows the comparison of surgical outcomes between the two groups. The patients who underwent laparotomy had postoperative complications more frequently compared with those who underwent laparoscopic surgery (58.8 % vs. 24.4 %, P < 0.01). Postoperative complications occurred in 8 patients (72.7 %) of 11 patients who had abdominoperineal resection. The median surgical duration was significantly longer (384 min vs. 297 min, P < 0.01), and the median hemorrhage amount was larger in the patients who had postoperative complications than in the patients who did not (300 g vs. 119 g, P < 0.01). The patients who had postoperative complications needed a significantly longer postoperative hospital stay (20.5 days vs. 9 days, P < 0.01), and they required longer time prior to first administration of biologics after surgery than those who did not (33.5 d vs. 22 d, P < 0.01). There were no deaths after surgery in either group.
Table 3.
Surgical outcomes of Crohn's disease patients with or without postoperative complications.
| Present |
Absent |
P value | ||
|---|---|---|---|---|
| (n = 51) | (n = 86) | |||
| Approach | Open surgery | 30 (58.8 %) | 21 (24.4 %) | <0.001⁎ |
| Laparoscopic surgery | 21 (41.2 %) | 65 (75.6 %) | ||
| Procedure | Ileocecal resection | 9 (17.7 %) | 26 (30.2 %) | 0.1 |
| Resection of the small intestine | 21 (41.2 %) | 46 (53.5 %) | 0.16 | |
| Resection of the colorectum | 18 (35.3 %) | 21 (24.4 %) | 0.17 | |
| Abdominoperineal resection | 8 (15.7 %) | 3 (3.5 %) | 0.01⁎ | |
| Others | 7 (15.9 %) | 5 (7.4 %) | 0.16 | |
| Operative duration | min, median, (range) | 384 (114–811) | 297 (101–729) | 0.008⁎ |
| Hemorrhage | g, median, (range) | 300 (0–1548) | 119 (0–4270) | 0.002⁎ |
| Postoperative mortality | 0 (0.0 %) | 0 (0.0 %) | – | |
| Postoperative hospital stays | Mean, days (range) | 20.5 (6–79) | 9 (6–29) | <0.001⁎ |
| Days before restarting biologics | Mean, days (range) | 33.5 (18–90) | 22 (10–64) | <0.001⁎ |
CD; Crohn's disease, OS; Open surgery, LS; Laparoscopic surgery.
Indicates statistical significance (P < 0.05).
Risk factors of postoperative complications for Crohn's disease patients
Among the preoperative and operative factors, preoperative serum CRP levels and albumin levels, low PNI, decreased SMI, open surgery, abdominoperineal resection, long surgical duration, and amount of hemorrhage were significantly related to postoperative complications in CD patients by univariate analysis. The results of multivariate analysis for independent risk factors of postoperative complications are included in Table 4. SMI <38.9 cm2/m2 (Odds ratio (OR), 2.85; 95 % confidence interval (CI), 1.13–7.16; P = 0.03) and low preoperative serum albumin (OR, 2.62; 95 % CI, 1.09–6.26; P = 0.03) were the independent risk factors associated with postoperative complications in all of the CD patients by multivariate logistic regression analysis. Although VFI, which is a reliable marker of visceral obesity, did not have significant correlation with postoperative complications by itself, patients who experienced adverse events had higher visceral fat index compared with those who did not among patients with skeletal muscle index <38.9 cm2/m2, (0.85 vs. 0.45, P = 0.04).
Table 4.
Multivariate analysis of the risk factors for postoperative complications.
| Postoperative complication | Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95 % C.I. | P value | Odds ratio | 95 % C.I. | P value | ||
| Preoperative CRP | >0.48/<0.48 mg/dL | 2.97 | 1.44–6.11 | 0.003⁎ | 1.79 | 0.75 - 4.26 | 0.19 |
| Preoperative albumin | <3.5/>3.5 g/dL | 4.14 | 1.98–8.65 | <0.001⁎ | 2.62 | 1.09 - 6.26 | 0.03⁎ |
| PNI | <38.3/>38.3 | 3.34 | 1.58–7.16 | 0.002⁎ | |||
| SMI | <38.9/>38.9 | 4.08 | 1.61–8.01 | <0.001⁎ | 2.85 | 1.13 - 7.16 | 0.03⁎ |
| Surgical approach | Open/Laparoscopy | 2.5 | 1.19–5.28 | 0.02⁎ | |||
| Procedure | APR/Others | 5.15 | 1.30–20.40 | 0.02⁎ | 1.82 | 0.36 - 8.94 | 0.47 |
| Surgical duration | >383/<383 min | 3.43 | 1.63–7.21 | 0.001⁎ | 2.42 | 0.99 - 5.89 | 0.05 |
| Hemorrhage | >299/<299 g | 3.97 | 1.87–8.39 | <0.001⁎ | |||
CRP; C-reactive protein, PNI; prognostic nutritional index, SMI; skeletal muscle index, APR; abdominoperineal resection.
Indicates statistical significance (P < 0.05).
In general, SMI significantly differs between the sexes as seen in our data (median SMI: 37.8 vs. 46.7 cm2/m2, male and female, respectively. P < 0.001). Because of this sex difference, we conducted a multivariable analysis divided by sex and the results are shown in Table 5. Univariate analysis showed that disease duration over 18 years, preoperative serum albumin <2.8 g/dL, and SMI <36.6 cm2/m2 were significantly related to postoperative complications in female patients, while preoperative serum albumin <3.5 g/dL, PNI <38.6, SMI <38.9 cm2/m2, open surgery, surgical duration >405 min, and > 433 g of intraoperative bleeding in male patients were associated with higher risk for postoperative complications. Multivariate logistic regression analysis revealed that preoperative serum albumin <2.8 g/dL and SMI <36.6 cm2/m2 were the independent risk factors related to postoperative complications for female patients, and preoperative serum albumin <3.5 g/dL and SMI <38.9 cm2/m2 were the independent risk factors for male patients.
Table 5.
Multivariate analysis of the risk factors for postoperative complications according to sex.
| Postoperative complication | Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95 % C.I. | P value | Odds ratio | 95 % C.I. | P value | |||
| Female | Disease period | >18/<18 | 4.44 | 1.06–18.67 | 0.04⁎ | |||
| Preoperative albumin | <2.8/>2.8 g/dL | 18.9 | 3.01–118.82 | 0.002⁎ | 17.84 | 2.38–133.53 | 0.005⁎ | |
| SMI | <36.6/>36.6 | 5.00 | 1.21–20.61 | 0.03⁎ | 6.23 | 1.01–38.31 | 0.05⁎ | |
| Male | Preoperative albumin | <3.5/>3.5 g/dL | 7.62 | 3.04–19.10 | <0.001⁎ | 4.82 | 1.67–13.93 | 0.003⁎ |
| PNI | <38.6/>38.6 | 5.61 | 2.17–14.51 | <0.001⁎ | ||||
| SMI | <38.9/>38.9 | 24.9 | 3.08–201.43 | 0.003⁎ | 17.42 | 1.78–170.4 | 0.01⁎ | |
| Surgical approach | Open/Laparoscopy | 2.62 | 1.08–6.34 | 0.03⁎ | ||||
| Duration | >405/<405 min | 5.07 | 1.96–13.09 | <0.001⁎ | ||||
| Hemorrhage | >433/<433 mg | 13.9 | 3.70–52.26 | 0.001⁎ | ||||
PNI; prognostic nutritional index, SMI; skeletal muscle index.
Indicates statistical significance (P < 0.05).
Discussion
In the present study, we demonstrated that body composition-based nutritional status parameters are reliable predicative indicators for the occurrence of postoperative adverse events from the analysis of the clinical results of surgical treatment in CD patients. Our data revealed that preoperative inflammation indicated by high CRP and low serum albumin, malnutrition indicated by PNI and SMI, and highly invasive procedures with massive hemorrhage and long duration significantly correlated with the occurrence of adverse events after surgery in CD patients. We also showed that SMI is the most precise indicator for postoperative adverse events in CD patients among the nutritional factors measured, including BMI, PNI, VFA, SFA and SMA.
Invasive surgical intervention can cause inflammation and metabolic stress that result in involvement of immune responses and release of inflammatory mediators. Most patients with CD are in a malnourished state with chronic inflammation preoperatively, thus they often experience prolonged inflammatory response and have adverse events resulting in delayed recovery from surgery. The assessment of severe malnutrition in IBD patients indicated by the European Society for Clinical Nutrition and Metabolism guidance 2020 includes both chronological and subjective criteria, for example, weight loss within the past 6 months, subjective global assessment, and serum albumin < 3 g/dL [22]. However, it is controversial to use these criteria in the perioperative management of CD patients because they often require urgent surgery. Plasma measurements such as albumin or transferrin are affected by surgery and since they are expected to decrease in the presence of infection, inflammation, or trauma, serum albumin should not be used as the nutritional marker in active diseases, as recommended by the European Crohn's and Colitis Organization [10]. Therefore, we think that a nutritional indicator suitable for surgical CD cases should be chosen from measurements determined by body composition that can be objectively and simply measured.
BMI is one body composition parameter used in a daily practice to assess nutritional status. Weight loss and low BMI are common in patients with CD. A low BMI is also considered to increase the risk of postoperative infectious complications in patients with CD [13]. Meanwhile, other studies report that CD patients with a high BMI tend to experience postoperative infectious complications [14]. As we showed no correlation between BMI and postoperative complications, it is controversial to include BMI as a variable for the risk of postoperative complications in CD patients. Visceral obesity has also been focused on for its adverse effects in the disease course of CD [16]. Recent studies suggest that visceral adipose tissue, particularly mesenteric adipose tissue, plays an important role in the development of intestinal inflammation [23]. Massive visceral fat deposition leads adipocytes to release various proinflammatory factors, which is followed by the infiltration of macrophages that may be associated with disease onset and have a significant contribution to severity and complications in CD patients [24,25]. The report from USA showed that patients with CD who had a VFA >130 cm2 tend to experience adverse events postoperatively [16], a correlation between VFA and postoperative complications in this study. The different distribution of visceral obesity in Asian populations may cause insufficient power of VFA alone as a risk factor.
Recently, it has been indicated that skeletal muscle wasting could be caused by the local and systemic inflammation following release of proinflammatory cytokines from visceral adipose tissue in CD patients [27,28]. Sarcopenia is a well-known factor for poor clinical outcomes, including surgical outcomes, in IBD. Ryan et al. reported that sarcopenia defined by SMI can be a predictor of surgical intervention and that it correlates with an increased risk of major postoperative complications for patients with IBD [4]. In a study by Galata et al., CD patients with low SMI were more likely to experience surgical site infections [11]. Although there are only a small number of studies focused on the relationship between SMI and surgical morbidity, our results support these previous reports. In our study, it was also suggested that sarcopenic obese patients, who had both higher VFI and lower SMI, experienced postoperative adverse events more frequently. Sarcopenia is more common in malnourished people; however, it needs to be considered that a sarcopenic state in IBD patients can also possibly be accompanied by visceral obesity. In CD patients, mesenteric fat deposit induces intestinal inflammation, which may lead to skeletal muscle wasting, resulting in more severe disease. Thus, it makes sense that, in cases of CD requiring surgical treatment (more severe cases), both sarcopenia and visceral obesity are often observed [29,30]. Although we did not show a significant relationship between VFI and postoperative complications, visceral fat volume should be assessed in combination with skeletal muscle volume to verify whether it impacts disease progression.
Sarcopenia is defined according to the criteria of the International Consensus for healthy young adults (SMI <55 cm2/m2 for males and <39 cm2/m2 for females), but a clear definition of sarcopenia for IBD patients does not exist. Additionally, skeletal muscle mass volume is highly influenced by sex, especially in young people [31]. Thus, we need to make allowances for sex differences in the cut-off value of SMI as a risk factor, especially in CD patients. Galata et al. obtained a sarcopenia prevalence of 70.4 % in 162 CD patients and proposed that the cut-off value for risk of postoperative adverse events should be an SMI of <41.5 cm2/m2 in males and SMI of <31.8 cm2/m2 in female patients as calculated by abdominal CT or MRI [11]. Zhang et al. reported that the prevalence of sarcopenia was 27.4 % in 124 CD patients according to the cut-off previously reported by Martin et al. [32], which is SMI <41 cm2/m2 in females, SMI <43 cm2/m2 in males with BMI <25 kg/m2, and SMI <53 cm2/m2 in males with BMI ≥25 kg/m2 [33]. These cut-off values of sarcopenia were useful to detect CD patients at higher risk of postoperative complication, but the data was mostly derived from patients of a Western genetic background. Other special considerations are required for assessment of SMI as a marker of sarcopenia in Asian populations including Japanese patients because of the differences in genetic backgrounds and body composition compared with non-Asian populations [6]. Lau et al. reported that the relative total skeletal muscle of Hong Kong Chinese was 17 % lower in young Chinese men compared with American men, but the relative total skeletal muscle of young Chinese women was similar to that of American women [34]. Asian populations generally have lower BMIs than non-Asian populations, although their visceral fat volume is suggested to be greater than in non-Asian populations [35]. A previous report showed that CD patients have increased amounts of mesenteric fat compared with controls, even in an Asian population [36]. From the perspective of these differences, we should assess the correlation of SMI and VFI with postoperative adverse events, and a suitable cut-off for SMI as a risk factor in specific Asian populations needs to be defined. Thus, we propose that the perioperative sarcopenic status of Japanese CD patients be defined as follows: Sarcopenic status for males should be defined as SMI <38.9 cm2/m2, and in female patients it should be SMI <36.6 cm2/m2.
There are some limitations in this study. Our study was a retrospective single-institution study and so the patient population is too small to draw any firm conclusions. Although the proportion of males is generally larger than that of females among CD patients, we should consider that our study had a smaller number of female patients compared with male patients, making sex differences more difficult to observe. Therefore, additional large-scale prospective controlled studies are warranted to validate the definition of SMI in Japanese CD patients, including sex differences. Other nutritional factors such as serum prealbumin were not available due to lack of data. There were also data lacking on interventions such as perioperative nutritional support and rehabilitation, so the present study did not address whether these factors may also contribute to improving nutritional status.
Despite these limitations, this study revealed that sarcopenia as assessed by SMI can be used as an independent risk factor to predict the incidence of postoperative complications in surgical patients with CD. This is the largest study evaluating whether SMI and other body composition and nutritional indicators are risk factors for postoperative morbidity in patients with CD. CD itself has many factors that predispose to be sarcopenic state. We believe that active intestinal inflammation, which reflects visceral fat deposits, may contribute to developing sarcopenia, and sarcopenic state identified by SMI could help us to identify cases with a potentially high risk of complications. To improve surgical outcomes of CD patients, it is recommended to implement targeted risk reduction using body measurements before surgery by providing enteral or parenteral nutritional intervention. This can potentially reverse sarcopenia, making it a crucial aspect of preoperative care.
Funding information
The authors have no funding support.
Ethics statements
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Kyushu University Hospital Human Research Ethics Committee (No. 2022-97). Written informed consent was waived because of the retrospective design.
CRediT authorship contribution statement
Kinuko Nagayoshi: Conceptualization, Methodology, Data curation, Writing- Original draft preparation. Yusuke Mizuuchi, Koji Tamura and Masafumi Sada: Data curation, Investigation, Resources, Writing- Reviewing and Editing. Jinghui Zhang, Kyoko Hisano, Kohei Nakata, Kenoki Ohuchida: Writing- Reviewing and Editing. Masafumi Nakamura: Supervision.
Declaration of competing interest
The authors have declared no conflicts of interest.
Footnotes
Key message: Our retrospective study of 137 patients that underwent surgery for Crohn's disease revealed that decreased skeletal muscle index which was calculated by a single computed tomography slice was a significant risk factor for postoperative complications in Crohn's disease patients. SMI can be easily measured before surgery, and it may be useful in perioperative treatment as a nutritional indicator.
References
- 1.Bossi P., Delrio P., Mascheroni A., Zanetti M. The spectrum of malnutrition/cachexia/sarcopenia in oncology according to different cancer types and settings: a narrative review. Nutrients. 2021:13. doi: 10.3390/nu13061980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An H.J., Tizaoui K., Terrazzino S., Cargnin S., Lee K.H., Nam S.W., et al. Sarcopenia in autoimmune and rheumatic diseases: a comprehensive review. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21165678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhanji R.A., Montano-Loza A.J., Watt K.D. Sarcopenia in cirrhosis: looking beyond the skeletal muscle loss to see the systemic disease. Hepatology. 2019;70:2193–2203. doi: 10.1002/hep.30686. [DOI] [PubMed] [Google Scholar]
- 4.Ryan E., McNicholas D., Creavin B., Kelly M.E., Walsh T., Beddy D. Sarcopenia and inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2019;25:67–73. doi: 10.1093/ibd/izy212. [DOI] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyere O., Cederholm T., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L.K., Woo J., Assantachai P., Auyeung T.W., Chou M.Y., Iijima K., et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(300–7) doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Lin C.C., Lin H.H., Chen H.C., Chen N.C., Shih I.L., Hung J.S., et al. Perioperative optimization of Crohn’s disease. Ann Gastroent Surg. 2023;7:10–26. doi: 10.1002/ags3.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto T., Allan R.N., Keighley M.R. Risk factors for intra-abdominal sepsis after surgery in Crohn’s disease. Dis Colon Rectum. 2000;43:1141–1145. doi: 10.1007/BF02236563. [DOI] [PubMed] [Google Scholar]
- 9.Huang W., Tang Y., Nong L., Sun Y. Risk factors for postoperative intra-abdominal septic complications after surgery in Crohn’s disease: a meta-analysis of observational studies. J Crohns Colitis. 2015;9:293–301. doi: 10.1093/ecco-jcc/jju028. [DOI] [PubMed] [Google Scholar]
- 10.Adamina M., Gerasimidis K., Sigall-Boneh R., Zmora O., van Overstraeten A.D., Campmans-Kuijpers M., et al. Perioperative Dietary Therapy in inflammatory bowel disease. J Crohns Colitis. 2020;14:S044–S. doi: 10.1093/ecco-jcc/jjz160. [DOI] [PubMed] [Google Scholar]
- 11.Galata C., Hodapp J., Weiss C., Karampinis I., Vassilev G., Reissfelder C., et al. Skeletal muscle mass index predicts postoperative complications in intestinal surgery for Crohn’s disease. JPEN J Parenter Enteral Nutr. 2020;44:714–721. doi: 10.1002/jpen.1696. [DOI] [PubMed] [Google Scholar]
- 12.Zhang T., Cao L., Cao T., Yang J., Gong J., Zhu W., et al. Prevalence of sarcopenia and its impact on postoperative outcome in patients with Crohn’s disease undergoing bowel resection. JPEN J Parenter Enteral Nutr. 2017;41:592–600. doi: 10.1177/0148607115612054. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y., Zhou W., Qi W., Liu W., Chen M., Zhu H., et al. Body mass index is a practical preoperative nutritional index for postoperative infectious complications after intestinal resection in patients with Crohn’s disease. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000007113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenna N.P., Habermann E.B., Zielinski M.D., Lightner A.L., Mathis K.L. Body mass index: implications on disease severity and postoperative complications in patients with Crohn’s disease undergoing abdominal surgery. Surgery. 2019;166:703–708. doi: 10.1016/j.surg.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 15.Onodera T., Goseki N., Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–1005. [PubMed] [Google Scholar]
- 16.Ding Z., Wu X.R., Remer E.M., Lian L., Stocchi L., Li Y., et al. Association between high visceral fat area and postoperative complications in patients with Crohn’s disease following primary surgery. Color Dis. 2016;18:163–172. doi: 10.1111/codi.13128. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshizumi T., Nakamura T., Yamane M., Islam A.H., Menju M., Yamasaki K., et al. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–286. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 19.Shen W., Punyanitya M., Wang Z., Gallagher D., St-Onge M.P., Albu J., et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 1985;2004(97):2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 20.Prado C.M., Lieffers J.R., McCargar L.J., Reiman T., Sawyer M.B., Martin L., et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 21.Demerath E.W., Shen W., Lee M., Choh A.C., Czerwinski S.A., Siervogel R.M., et al. Approximation of total visceral adipose tissue with a single magnetic resonance image. Am J Clin Nutr. 2007;85:362–368. doi: 10.1093/ajcn/85.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weimann A., Braga M., Carli F., Higashiguchi T., Hubner M., Klek S., et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36:623–650. doi: 10.1016/j.clnu.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Bilski J., Mazur-Bialy A., Wojcik D., Surmiak M., Magierowski M., Sliwowski Z., et al. Role of obesity, mesenteric adipose tissue, and adipokines in inflammatory bowel diseases. Biomolecules. 2019:9. doi: 10.3390/biom9120780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peyrin-Biroulet L., Chamaillard M., Gonzalez F., Beclin E., Decourcelle C., Antunes L., et al. Mesenteric fat in Crohn’s disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56:577–583. doi: 10.1136/gut.2005.082925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fink C., Karagiannides I., Bakirtzi K., Pothoulakis C. Adipose tissue and inflammatory bowel disease pathogenesis. Inflamm Bowel Dis. 2012;18:1550–1557. doi: 10.1002/ibd.22893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuoco L., Vescovo G., Castaman R., Ravara B., Cammarota G., Angelini A., et al. Skeletal muscle wastage in Crohn’s disease: a pathway shared with heart failure? Int J Cardiol. 2008;127:219–227. doi: 10.1016/j.ijcard.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Bilski J., Mazur-Bialy A.I., Wierdak M., Brzozowski T. The impact of physical activity and nutrition on inflammatory bowel disease: the potential role of cross talk between adipose tissue and skeletal muscle. J Physiol Pharmacol. 2013;64:143–155. [PubMed] [Google Scholar]
- 29.Adams D.W., Gurwara S., Silver H.J., Horst S.N., Beaulieu D.B., Schwartz D.A., et al. Sarcopenia is common in overweight patients with inflammatory bowel disease and may predict need for surgery. Inflamm Bowel Dis. 2017;23:1182–1186. doi: 10.1097/MIB.0000000000001128. [DOI] [PubMed] [Google Scholar]
- 30.Grillot J., D’Engremont C., Parmentier A.L., Lakkis Z., Piton G., Cazaux D., et al. Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn’s disease. Clin Nutr. 2020;39:3024–3030. doi: 10.1016/j.clnu.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Auyeung T.W., Lee J.S., Leung J., Kwok T., Woo J. Adiposity to muscle ratio predicts incident physical limitation in a cohort of 3,153 older adults--an alternative measurement of sarcopenia and sarcopenic obesity. Age (Dordr) 2013;35:1377–1385. doi: 10.1007/s11357-012-9423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin L., Birdsell L., Macdonald N., Reiman T., Clandinin M.T., McCargar L.J., et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 33.Zhang C., Yu D., Hong L., Zhang T., Liu H., Fan R., et al. Prevalence of sarcopenia and its effect on postoperative complications in patients with Crohn’s disease. Gastroenterol Res Pract. 2021;2021:3267201. doi: 10.1155/2021/3267201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau E.M., Lynn H.S., Woo J.W., Kwok T.C., Melton L.J., 3rd. Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. J Gerontol A Biol Sci Med Sci. 2005;60:213–216. doi: 10.1093/gerona/60.2.213. [DOI] [PubMed] [Google Scholar]
- 35.Consultation W.H.O.E. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 36.Zhang T., Ding C., Xie T., Yang J., Dai X., Lv T., et al. Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy. Clin Nutr. 2017;36:1586–1592. doi: 10.1016/j.clnu.2016.10.004. [DOI] [PubMed] [Google Scholar]


