Abstract
Rationale and objectives
Intraductal papillary mucinous neoplasm of the bile ducts (IPMN-B) is a true pre-cancerous lesion, which shares common features with pancreatic IPMN (IPMN-P). While IPMN-P is a well described entity for which guidelines were formulated and revised, IPMN-B is a poorly described entity.
We carried out a systematic review to evaluate the existing literature, emphasizing the role of MRI in IPMN-B depiction.
Materials and methods
PubMed database was used to identify original studies and case series that reported MR Imaging features of IPMN-B. The search keywords were "IPMN OR intraductal papillary mucinous neoplasm OR IPNB OR intraductal papillary neoplasm of the bile duct AND Biliary OR biliary cancer OR hepatic cystic lesions”. Risk of bias and applicability were evaluated using the QUADAS-2 tool.
Results
884 Records were Identified through database searching. 12 studies satisfied the inclusion criteria, resulting in MR features of 288 patients. All the studies were retrospective. Classic features of IPMN-B are under-described. Few studies note worrisome features, concerning for an underlying malignancy. 50 % of the studies had a high risk of bias and concerns regarding applicability.
Conclusions
The MRI features of IPMN-B are not well elaborated and need to be further studied. Worrisome features and guidelines regarding reporting the imaging findings should be established and published. Radiologists should be aware of IPMN-B, since malignancy diagnosis in an early stage will yield improved prognosis
Keywords: Biliary tract, IPMN, IPNB, Cystic tumors, Mucinous tumors
Highlights
-
•
The entity of IPMN-B, including its MRI features are poorly known.
-
•
Distinguishing signs between IPMN-B and other hepatic or biliary cystic lesions or intraductal cholangiocarcinoma lack consensus.
-
•
Radiologists should recognize IPMN-B, as early detection of its malignant transformation improves prognosis.
1. Introduction
Intraductal papillary mucinous neoplasm of the bile ducts (IPMN-B) is a mucin producing cystic epithelial tumor. IPMN-B is characterized by intraluminal papillary ductal growth, communication with the biliary ducts, as well as intrahepatic and extrahepatic biliary ducts dilatation due to mucin hypersecretion [1], [2], [3]. The imaging appearance of IPMN-B varies, and depends on the equilibrium between the degree of intraductal proliferation and mucin hypersecretion. Predominant intraductal proliferation would appear on imaging as an intraductal mass, accompanied by a mostly upstream biliary dilatation. Substantial mucin production would manifest on imaging as diffuse dilatation of the bile ducts with no detectable mass [3].
On T1-weighted MRI, IPMN-B appears as hypointense or isointense, and on T2-weighted MR images as hyperintense. IPMN-B commonly presents as an intraductal mass, characterized by proximal and, at times, distal biliary dilatation [3]. At MRCP, in some cases, the intraluminal mucin appears as stringlike filling defects, known as the "Thread sign" [4], [5]. Usual enhancement pattern consists of isointensity or hyperintensity during the late arterial phase, with no remaining hyperintensity in the portal venous and late phases [6]. Persistent and progressive enhancement pattern in the portal venous and late phases is suspicious malignant intraductal cholangiocarcinoma [7].
Malignant IPMN-B typically present a clearly visible mass within the duct on imaging [8]. Approximately 28 % of cases exhibit evident invasion beyond the duct, while involvement of neighboring organs is observed in up to 8 % of cases [9]. Diffusion-weighted MRI enhances the visibility of tumors and has the potential to reveal their invasiveness, but it does not accurately depict the full extent of the tumor [10].
IPMN-B is considered to be analogous to the intraductal papillary mucinous neoplasm of the pancreas (IPMN-P); since both entities manifest with intraductal growth and excessive mucin production [3], [7], [11], [12]. IPMN-P is well described and diagnostic criteria and guidelines have been formulated and revised. Moreover, “high-risk stigmata” and “worrisome features" have been published and widely used in clinical practice to detect malignant transformation and guide treatment [13], [14].
IPMN-P is widely recognized while IPMN-B is still relatively an under-described entity [15], [16]. It has been argued that invasive carcinoma and malignancy are more frequently associated with IPMN-B than with IPMN-P [17]. Hence, it is important to diagnose IPMN-B early since it is a precursor lesion to a worse-prognosis biliary cholangiocarcinoma [16], [18], [19]. Unfortunately, currently, no guidelines exist regarding IPMN-B malignancy prediction or structured radiologic reporting.
In this study, we carried out a systematic review to evaluate the literature emphasizing the role of MRI in IPMN-B depiction and assessment.
2. Materials and methods
2.1. Literature search and eligibility criteria
The systematic review was conducted with adherence to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [20].
We searched the PubMed database for relevant studies. The search keywords were "IPMN OR intraductal papillary mucinous neoplasm OR IPNB OR intraductal papillary neoplasm of the bile duct AND Biliary OR biliary cancer OR hepatic cystic lesion”. Bibliographies of included studies were searched for additional relevant studies. The search included studies published from August 1975 up to September 23, 2022. Inclusion criteria were studies that (a) evaluated MR Imaging features of IPMN-B, (b) were published in English, and (c) were Original Articles or Case Series. Abstracts and case reports were excluded. We reviewed the bibliographies of the included studies for relevant studies as well.
The study was registered with PROSPERO, the prospective international register of systematic reviews (identification number CRD42022300610).
2.2. Data extraction and analysis
Data of all included studies were collected into a standardized data extraction sheet. The data sheet included: year of publication, journal name, journal's medical specialty, study design, size of the database.
Data from all included studies were collected into a standardized data extraction sheet as well. The data sheet included: Biliary Dilatation (intrahepatic/extrahepatic, downstream/upstream), Intraluminal mass, the presence of the “Thread sign”, cystic communication with the biliary tract, biliary stones, pancreatic duct dilatation, Intrahepatic cystic lesion, worrisome features, MRI diffusion series.
The Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) criteria were used to assess the risk of bias and applicability [21].
3. Results
3.1. Included studies and reported MR features
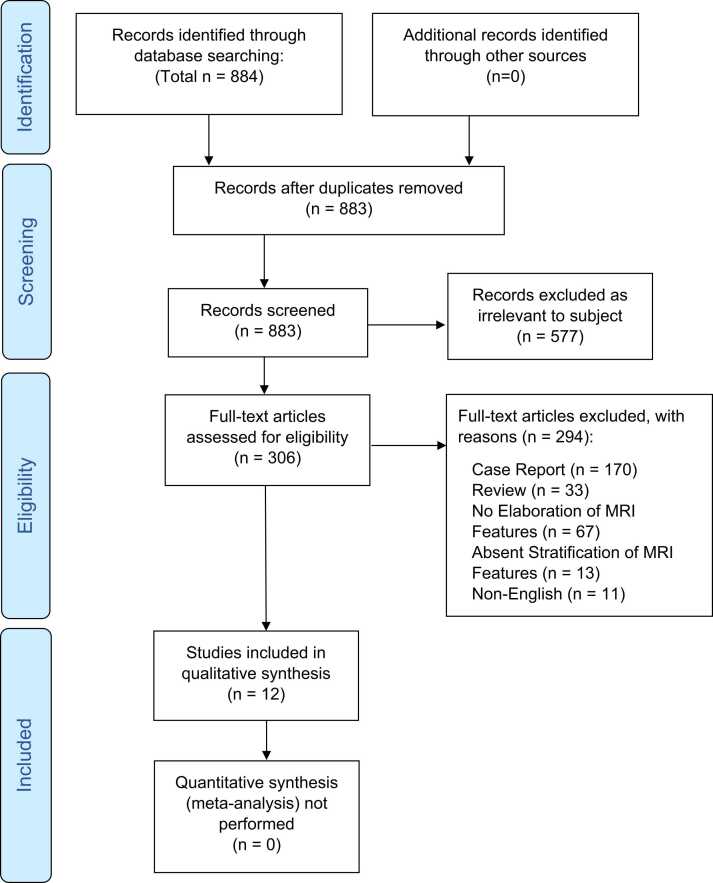
Of 884 retrieved studies, 12 studies [4], [5], [10], [22], [23], [24], [25], [26], [27], [28], [29], [30] satisfied the inclusion criteria, and included MR features of 288 patients. Flow diagram of the search and inclusion process is presented in Fig. 1.
Fig. 1.
Flow diagram of the search and inclusion process. The study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
The studies were all retrospective, and published between 2008 and 2020. The studies were published in journals with different affiliations, most prevalent was Radiology (8/12, 66.7 %), followed by Gastroenterology (3/12, 25.0 %) and Surgery (1/12, 8.3 %).
Characteristics of the included studies are summarized in Table 1.
Table 1.
Characteristics of the studies included in the qualitative synthesis.
| Study | Publication year | Journal's medical affiliation | Study design | Database size | Pathological correlation |
|---|---|---|---|---|---|
| Frezin et al. [22] | 2020 | Surgery | Retrospective | 4 | Yes |
| Siripongsakun et al. [23] | 2019 | Radiology | Retrospective | 8 | Yes |
| Lee et al. [24] | 2019 | Gastroenterology | Retrospective | 120 | Yes |
| Jin et al. [25] | 2019 | Radiology | Retrospective | 52 | Yes |
| Wu et al. [4] | 2017 | Radiology | Retrospective | 15 | Yes |
| Mondal et al. [26] | 2016 | Radiology | Retrospective | 6 | Yes |
| Hong et al. [5] | 2016 | Radiology | Retrospective | 38 | Yes |
| Ying et al. [27] | 2015 | Gastroenterology | Retrospective | 5 | Yes |
| Yoon et al. [10] | 2013 | Radiology | Retrospective | 23 | Yes |
| Lim et al. [28] | 2011 | Radiology | Retrospective | 3 | Yes |
| Li et al. [29] | 2009 | Gastroenterology | Retrospective | 10 | Yes |
| Lim et al. [30] | 2008 | Radiology | Retrospective | 4 | Yes |
Biliary dilatation was described in 8/12 (66.7 %) of the studies [23], [4], [26], [27], [10], [28], [29], [30], and only 2 studies [27], [28] distinguished between intrahepatic or extrahepatic biliary dilatation.
The presence or absence of an intra-ductal luminal mass was described in 8/12 (66.7 %) of the studies [23], [24], [25], [26], [27], [4], [5], [30]. The "Thread Sign" was described in only one study [5].
Differentiation of cyst morphology was described in 3/12 (25.0 %) of the studies [22], [24], [28].
Cystic communication with the biliary tract was mentioned in 3/12 (25.0 %) [22], [23], [26].
The presence of biliary stones or pancreatic dilatation were not mentioned.
The included studies report use of different field strengths and various MRI sequences.
4 Studies (33.3 %) [10], [23], [27], [30] used a 3.0-Tesla system. Yet, Lim et al. [30] reported that the MRI technique for 1 patient was unknown, being performed outside of the author's institution.
4 Studies (33.3 %) [4], [5], [25], [28] used a 1.5-Tesla system, and 1 study [24] reported that scans were made in either 1.5-Tesla or 3.0-Tesla system.
3 studies (25 %) [22], [26], [29] did not elaborate regarding the MRI field strength.
Description of using MR diffusion series in the scanning technique was described in 6 studies [5], [10], [23], [24], [25], [28].
4 studies [10], [23], [24], [25] reported signs differentiating IPMN-B from other hepatic or biliary cystic lesions or from intraductal cholangiocarcinoma.
One study [22] described that all (4/4, 100 %) IPMN-B cases were misdiagnosed prior to the pathology result. The other 11 studies did not mention if IPMN-B was diagnosed using imaging prior to the pathology result [4], [5], [10], [23], [24], [25], [26], [27], [28], [29], [30].
The radiological features are summarized in Table 2.
Table 2.
MR radiological features reported in the included studies.
| Study | Biliary dilatation |
Biliary dilatation |
Thread Sign | Intra-luminal | |||
|---|---|---|---|---|---|---|---|
| Yes | Intraheaptic | Extraheaptic | Upstream | Downstream | Mass | ||
| Frezin et al. [22] | NR | NR | NR | NR | NR | NR | NR |
| Siripongsakun et al. [23] | 87.5 % | NR | NR | NR | NR | NR | 62.5 % |
| Lee et al. [24] | NR | NR | NR | NR | NR | NR | 85.0 % |
| Jin et al. [25] | NR | NR | NR | NR | NR | NR | 26.8 % |
| Wu et al. [4] | NR | NR | NR | 100.0 % | 60.00 % | NR | 100.0 % |
| Mondal et al. [26] | 66.7 % | NR | NR | NR | NR | NR | 66.7 % |
| Hong et al. [5] | NR | NR | NR | NR | NR | 44.7–52.6 % | 50.0 % |
| Ying et al. [27] | 80.0 % | 80.0 % | 80.0 % | 100.0 % | 100.0 % | NR | 100.0 % |
| Yoon et al. [10] | 100.0 % | NR | NR | NR | NR | NR | NR |
| Lim et al. [28] | 100.0 % | 100.0 % | 66.7 % | NR | 66.7 % | NR | NR |
| Li et al. [29] | 70.0 % | NR | NR | NR | NR | NR | NR |
| Lim et al. [30] | 100.0 % | NR | NR | NR | NR | NR | 0.0 % |
| Study | Single cyst | Single cyst | Multiple cysts | Septations | Calcifications | Cystic communication |
|---|---|---|---|---|---|---|
| Unilocular | Multilocular | with the biliary tracts | ||||
| Frezin et al. [22] | 75.0 % | NR | 25.0 % | 50.0 % | 25.0 % | 50.0 % |
| Siripongsakun et al. [23] | NR | NR | NR | NR | NR | 75.0 % |
| Lee et al. [24] | NR | NR | 40.8 % | NR | NR | NR |
| Jin et al. [25] | NR | NR | NR | NR | NR | NR |
| Wu et al. [4] | NR | NR | NR | NR | 0.0 % | NR |
| Mondal et al. [26] | NR | NR | NR | NR | NR | 16.7 % |
| Hong et al. [5] | NR | NR | NR | NR | NR | NR |
| Ying et al. [27] | NR | NR | NR | NR | NR | NR |
| Yoon et al. [10] | NR | NR | NR | NR | NR | NR |
| Lim et al. [28] | NR | 33.3 % | 33.3 % | NR | NR | NR |
| Li et al. [29] | NR | NR | NR | NR | NR | NR |
| Lim et al. [30] | NR | NR | NR | NR | NR | NR |
NR – Not Reported.
3.2. Quality assessment
High risk of bias was ascribed to studies in which the interpreting radiologists were not blinded to the pathological diagnosis of IPMN-B. Studies that did not have MRI scans for all pathologically confirmed IPMN-B, therefore effectively excluding patients, were also deemed high risk of bias.
The QUADAS-2 tool identified high risk of bias and concerns regarding applicability 6/12 (50.0 %) of the studies, respectively.
Complete QUADAS-2 result are presented in Table 3.
Table 3.
Methodological analysis of the included studies based on the QUADAS-2 tool.
| Study | Risk of bias |
Applicability |
|||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Frezin et al. [22] | Low | Low | Unclear | Low | Low | High | Low |
| Siripongsakun et al. [23] | Low | Low | Unclear | Low | High | Low | Low |
| Lee et al. [24] | Low | Low | High | Low | Low | Low | Low |
| Jin et al. [25] | Low | Low | Low | Low | Low | Low | Low |
| Wu et al. [4] | Low | Low | Low | Low | Low | Unclear | Low |
| Mondal et al. [26] | High | Low | High | Low | High | High | Low |
| Hong et al. [5] | Low | Low | Low | Low | Low | Low | Low |
| Ying et al. [27] | Low | Low | Unclear | Low | Low | Low | Low |
| Yoon et al. [10] | Low | Low | High | Low | Low | Low | Low |
| Lim et al. [28] | High | Low | High | Low | High | Unclear | Low |
| Li et al. [29] | High | Low | Unclear | Low | Low | High | Low |
| Lim et al. [30] | High | Low | Unclear | Low | High | Unclear | Low |
4. Discussion
4.1. Classic MRI features
In this systematic review we have found that there are only several original articles or case series that inquired about the role of MR imaging in IPMN-B diagnosis and assessment. The available data lacks description of the classic MR findings of IPMN-B, including presence of an intra-luminal biliary mass, biliary dilatation or differentiation between intra-hepatic or extra-hepatic biliary duct involvement. Additionally, the "Thread-sign", which is almost pathognomonic to IPMN-B alone, is not mentioned in the majority of studies.
We speculate that the diversity in reporting derives from decreased familiarity, which in turn leads to a non-uniform report.
Signs differentiating IPMN-B from other hepatic or biliary cystic lesions or were not well elaborated. Alongside, the majority of the studies did not go into detail regarding the assumed diagnosis of the IPMN-B cases prior to the final pathology result. The only study that reported the assumed diagnosis based on imaging had a misdiagnosis rate of 100 % [22]. In our opinion, this further highlights the need to increase the awareness of radiologists to this important entity.
4.2. Invasiveness and worrisome features
Some studies did address the issue of IPMN-B which is suspicious for malignant transformation, or the presence of intraductal cholangiocarcinoma. Siripongsakun et al. [23] summarized that findings such as intraductal nodules and connection to the biliary tracts may help differentiate between IPMN-B and biliary intraepithelial neoplasia.
Lee et al. [24] described intraductal visible mass, tumor size ≥ 2.5 cm, multiplicity of the tumor, bile duct wall thickening, adjacent organ invasion, as significant MRI findings, suspicious for IPMN-B with an associated invasive carcinoma.
Jin et al. [25] suggested that using ADC histogram analysis provides a fair competence for prediction of IPMN-B invasiveness. Yoon et al. [10] reported that the diffusion series may be beneficial in improving tumor conspicuity and therefore helpful in defining IPMN-B invasiveness.
These features may serve as the basis for establishing the "high-risk stigmata" and "worrisome features" for IPMN-B.
IPMN-B is treated mainly by hepatic resection when possible, as this neoplasm commonly harbors high-grade dysplasia and invasive carcinoma components [31].
Also, the prognosis is better following complete resection [32].
Luvira et al. [33] examined the long-term outcomes and survival of IPMN-B patients that underwent curative-intent hepatic resection surgery. They found that lesser level of invasiveness was associated with better patient prognosis.
We believe this further validates the importance of IPMN-B early detection.
4.3. Limitations
Our review has several limitations. We focused on including case series with a minimum of 3 patients, although there were more individual case reports available. We think that case series offer a standardized approach to patient management and outcome assessment, which leads to data robustness and consistency. While individual case reports may exhibit greater variability in their approach and data presentation, despite their larger cumulative number.
We reviewed the bibliographies of the included studies, to ensure that we captured the most directly relevant sources of evidence.
Eventually, only a small cohort of studies were suitable to analysis, due to the scarce literature available and the exclusion criteria. Furthermore, all studies included were retrospective, and were accompanied by a high risk of suspected bias and concerns regarding applicability. These limitations may hazard both precision and pertinence of the results.
4.4. Conclusions
The existing literature describes MRI features of IPMN-B, but without adequate elaboration or consensus on a reporting template. Certain MRI characteristics could potentially define the "high-risk stigmata" and "worrisome features" for IPMN-B, yet their application remains limited. We advocate for more comprehensive studies on IPMN-B's MRI features. Concurrently, it's crucial to establish and disseminate definitive guidelines on reporting these imaging findings. Familiarity with IPMN-B among radiologists can facilitate early diagnosis and improved prognosis. We propose a structured report template for IPMN-B MRI findings, available in our Supplementary material.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical Statement
Not applicable.
CRediT authorship contribution statement
Sobeh Tamer: Writing – review & editing. Sara Apter: Writing – review & editing, Methodology, Formal analysis, Conceptualization. Yael Inbar: Writing – review & editing. Eli Konen: Writing – review & editing. Eyal Klang: Writing – review & editing, Methodology, Formal analysis, Conceptualization. Shelly Soffer: Writing – review & editing, Visualization, Validation, Formal analysis. Matan Kraus: Writing – original draft, Methodology, Investigation, Data curation, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Appendix A
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ejro.2023.100515.
Contributor Information
Matan Kraus, Email: Matan.Kraus@gmail.com, Matan.Kraus@sheba.health.gov.il.
Eyal Klang, Email: Eyal.Klang@sheba.health.gov.il.
Shelly Soffer, Email: Shellyso@assuta.co.il.
Yael Inbar, Email: Yael.Inbar@sheba.health.gov.il.
Eli Konen, Email: Eli.Konen@sheba.health.gov.il.
Tamer Sobeh, Email: Tamer.Sobeh@gmail.com.
Sara Apter, Email: Sara.Apter@sheba.health.gov.il.
Appendix A. Supplementary material
Supplementary material.
References
- 1.Chen T.C., Nakanuma Y., Zen Y., et al. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001;34(4 Pt 1):651–658. doi: 10.1053/jhep.2001.28199. [DOI] [PubMed] [Google Scholar]
- 2.Zen Y., Pedica F., Patcha V.R., et al. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod. Pathol. 2011;24:1079–1089. doi: 10.1038/modpathol.2011.71. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee A., Lopes Vendrami C., Nikolaidis P., et al. Uncommon intraluminal tumors of the gallbladder and biliary tract: spectrum of imaging appearances. Radiographics. 2019;39:388–412. doi: 10.1148/rg.2019180164. [DOI] [PubMed] [Google Scholar]
- 4.Wu C.-H., Yeh Y.-C., Tsuei Y.-C., et al. Comparative radiological pathological study of biliary intraductal tubulopapillary neoplasm and biliary intraductal papillary mucinous neoplasm. Abdom. Radiol. 2017;42:2460–2469. doi: 10.1007/s00261-017-1167-7. [DOI] [PubMed] [Google Scholar]
- 5.Hong G.-S., Byun J.H., Kim J.H., et al. Thread sign in biliary intraductal papillary mucinous neoplasm: a novel specific finding for MRI. Eur. Radiol. 2016;26:3112–3120. doi: 10.1007/s00330-015-4158-5. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa H., Itoh S., Nagasaka T., Suzuki K., Ota T., Naganawa S. CT findings of intraductal papillary neoplasm of the bile duct: assessment with multiphase contrast-enhanced examination using multi-detector CT. Clin. Radiol. 2012;67:224–231. doi: 10.1016/j.crad.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Wan X.-S., Xu Y.-Y., Qian J.-Y., et al. Intraductal papillary neoplasm of the bile duct. World J. Gastroenterol. 2013;19:8595–8604. doi: 10.3748/wjg.v19.i46.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takanami K., Yamada T., Tsuda M., et al. Intraductal papillary mucininous neoplasm of the bile ducts: multimodality assessment with pathologic correlation. Abdom. Imaging. 2011;36:447–456. doi: 10.1007/s00261-010-9649-x. [DOI] [PubMed] [Google Scholar]
- 9.Gordon-Weeks A.N., Jones K., Harriss E., Smith A., Silva M. Systematic review and meta-analysis of current experience in treating IPNB: clinical and pathological correlates. Ann. Surg. 2016;263:656–663. doi: 10.1097/SLA.0000000000001426. [DOI] [PubMed] [Google Scholar]
- 10.Yoon H.J., Kim Y.K., Jang K.-T., et al. Intraductal papillary neoplasm of the bile ducts: description of MRI and added value of diffusion-weighted MRI. Abdom. Imaging. 2013;38:1082–1090. doi: 10.1007/s00261-013-9989-4. [DOI] [PubMed] [Google Scholar]
- 11.Ainechi S., Lee H. Updates on precancerous lesions of the biliary tract: biliary precancerous lesion. Arch. Pathol. Lab. Med. 2016;140:1285–1289. doi: 10.5858/arpa.2015-0396-RS. [DOI] [PubMed] [Google Scholar]
- 12.Bennett S., Marginean E.C., Paquin-Gobeil M., et al. Clinical and pathological features of intraductal papillary neoplasm of the biliary tract and gallbladder. HPB. 2015;17:811–818. doi: 10.1111/hpb.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka M. International consensus on the management of intraductal papillary mucinous neoplasm of the pancreas. Ann. Transl. Med. 2015;3:286. doi: 10.3978/j.issn.2305-5839.2015.11.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka M., Fernández-Del Castillo C., Kamisawa T., et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–753. doi: 10.1016/j.pan.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Sakai Y., Ohtsuka M., Sugiyama H., et al. Current status of diagnosis and therapy for intraductal papillary neoplasm of the bile duct. World J. Gastroenterol. 2021;27:1569–1577. doi: 10.3748/wjg.v27.i15.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adibi A., Shabanikia N., Taheri A. Intraductal papillary mucinous neoplasm of biliary ducts: literature review and a case report with emphasis on radiological manifestation. J. Res. Med. Sci. 2020;25:114. doi: 10.4103/jrms.JRMS_119_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minagawa N., Sato N., Mori Y., Tamura T., Higure A., Yamaguchi K. A comparison between intraductal papillary neoplasms of the biliary tract (BT-IPMNs) and intraductal papillary mucinous neoplasms of the pancreas (P-IPMNs) reveals distinct clinical manifestations and outcomes. Eur. J. Surg. Oncol. 2013;39:554–558. doi: 10.1016/j.ejso.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Lee S.S., Kim M.-H., Lee S.K., et al. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004;100:783–793. doi: 10.1002/cncr.20031. [DOI] [PubMed] [Google Scholar]
- 19.Yeung Y.P., AhChong K., Chung C.K., Chun A.Y.W. Biliary papillomatosis: report of seven cases and review of English literature. J. Hepatobiliary Pancreat. Surg. 2003;10:390–395. doi: 10.1007/s00534-002-0837-0. [DOI] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 21.Whiting P.F., Rutjes A.W.S., Westwood M.E., et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 22.Frezin J., Komuta M., Zech F., et al. Mucin-producing hepatic cystic neoplasms: an uncommon but challenging disease often misdiagnosed and mismanaged. Acta Chir. Belg. 2020;120:6–15. doi: 10.1080/00015458.2018.1532706. [DOI] [PubMed] [Google Scholar]
- 23.Siripongsakun S., Sapthanakorn W., Mekraksakit P., et al. Premalignant lesions of cholangiocarcinoma: characteristics on ultrasonography and MRI. Abdom. Radiol. 2019;44:2133–2146. doi: 10.1007/s00261-019-01951-2. [DOI] [PubMed] [Google Scholar]
- 24.Lee S., Kim M.-J., Kim S., Choi D., Jang K.-T., Park Y.N. Intraductal papillary neoplasm of the bile duct: assessment of invasive carcinoma and long-term outcomes using MRI. J. Hepatol. 2019;70:692–699. doi: 10.1016/j.jhep.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Jin K.-P., Rao S.-X., Sheng R.-F., Zeng M.-S. Skewness of apparent diffusion coefficient (ADC) histogram helps predict the invasive potential of intraductal papillary neoplasms of the bile ducts (IPNBs) Abdom. Radiol. 2019;44:95–103. doi: 10.1007/s00261-018-1716-8. [DOI] [PubMed] [Google Scholar]
- 26.Mondal D., Silva M.A., Soonawalla Z., Wang L.M., Bungay H.K. Intraductal papillary neoplasm of the bile duct (IPN-B): also a disease of western Caucasian patients. A literature review and case series. Clin. Radiol. 2016;71:e79–e87. doi: 10.1016/j.crad.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Ying S.-H., Teng X.-D., Wang Z.-M., et al. Gd-EOB-DTPA-enhanced magnetic resonance imaging for bile duct intraductal papillary mucinous neoplasms. World J. Gastroenterol. 2015;21:7824–7833. doi: 10.3748/wjg.v21.i25.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim J.H., Zen Y., Jang K.T., Kim Y.K., Nakanuma Y. Cyst-forming intraductal papillary neoplasm of the bile ducts: description of imaging and pathologic aspects. AJR Am. J. Roentgenol. 2011;197:1111–1120. doi: 10.2214/AJR.10.6363. [DOI] [PubMed] [Google Scholar]
- 29.Li T., Ji Y., Zhi X.-T., et al. A comparison of hepatic mucinous cystic neoplasms with biliary intraductal papillary neoplasms. Clin. Gastroenterol. Hepatol. 2009;7:586–593. doi: 10.1016/j.cgh.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Lim J.H., Jang K.-T., Choi D. Biliary intraductal papillary-mucinous neoplasm manifesting only as dilatation of the hepatic lobar or segmental bile ducts: imaging features in six patients. AJR Am. J. Roentgenol. 2008;191:778–782. doi: 10.2214/AJR.07.2091. [DOI] [PubMed] [Google Scholar]
- 31.Ozcan K., Klimstra D.S. A review of mucinous cystic and intraductal neoplasms of the pancreatobiliary tract. Arch. Pathol. Lab. Med. 2022;146:298–311. doi: 10.5858/arpa.2021-0399-RA. [DOI] [PubMed] [Google Scholar]
- 32.Rocha F.G., Lee H., Katabi N., et al. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012;56:1352–1360. doi: 10.1002/hep.25786. [DOI] [PubMed] [Google Scholar]
- 33.Luvira V., Pugkhem A., Bhudhisawasdi V., et al. Long-term outcome of surgical resection for intraductal papillary neoplasm of the bile duct. J. Gastroenterol. Hepatol. 2017;32:527–533. doi: 10.1111/jgh.13481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.