Abstract
Approximately 20–30% of patients with acute ischemic stroke, caused by large intracranial vessel occlusion, have a tandem lesion, defined as simultaneous presence of high-grade stenosis or occlusion of the cervical internal carotid artery and thromboembolic occlusion of the intracranial terminal internal carotid artery or its branches, usually the middle cerebral artery. Patients with tandem lesions have usually worse outcomes than patients with single intracranial occlusions, and intravenous thrombolysis is less effective in these patients. Although endovascular thrombectomy is currently a cornerstone therapy in the management of acute ischemic stroke due to large vessel occlusion, the optimal management of extracranial carotid lesions in tandem occlusion remains controversial. Acute placement of a stent in the cervical carotid artery lesion is the most used therapeutic strategy compared with stented balloon angioplasty and thrombectomy alone without carotid artery revascularization; however, treatment strategies in these patients are often more complex than with single occlusion, so treatment decisions can change based on clinical and technical considerations. The aim of this review is to analyze the results of different studies and trials, investigating the periprocedural neurointerventional management of patients with tandem lesions and the safety, efficacy of the different technical strategies available as well as their impact on the clinical outcome in these patients, to strengthen current recommendations and thus optimize patient care.
Keywords: Acute Ischemic Stroke, Tandem lesion, Endovascular Thrombectomy, Tandem Occlusion, Stenting
1. Introduction
Tandem lesions are defined as simultaneous presence of high-grade stenosis or occlusion, such as atherosclerotic stenosis or artery dissection, of the cervical internal carotid artery and thromboembolic occlusion of the intracranial terminal internal carotid artery or its branches, usually the middle cerebral artery [1]. Acute ischemic stroke due to tandem lesions accounts for about 15–25% of all LVO strokes in clinical trials and published data in Tandem Occlusions registries [2], [3], [4]. Patients with TLs have usually worse outcomes than patients with isolated intracranial occlusions, and natural history of these lesions usually leads to high morbidity, up to 70% and mortality, up to 50% [5]. Intravenous thrombolysis (IVT) alone is associated with a poor functional outcome in up to 80% of patients [6], presumably related to a larger clot burden and low anterograde flow impeding thrombolytic drug access to the intracranial thrombus [7].
Endovascular thrombectomy (EVT), where clot is extracted mechanically through an endovascular access is currently a cornerstone for treatment of intracranial LVO, with higher recanalization rate compared to IVT alone and better functional outcomes for patients [8], [9]. The efficacy of endovascular revascularization for acute ischemic stroke due to large vessel occlusion is demonstrated, and this procedure has been rapidly implemented into national stroke guidelines and has been widely performed in various institutions [9], [10]. Currently, however, there are no guidelines or recommendations based on high- quality evidence for treatment of TL’s undergoing EVT, that’s because three of major EVT trials [11], [12], [13] excluded these patients; in the remaining major randomized controlled trials of EVT, relatively few patients with TL’s were included, representing 13–32% [15] of study population, therefore current evidence is based on metanalysis and smaller retrospective studies [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. Some of these studies suggests importance of acute stenting of cervical-ICA in tandem occlusions, to reduce risk of recurrent stroke [16], [17], but in absence of strong randomized trial data, no firm recommendations about optical management can be forwarded, as reflected in most recent American and European guidelines.
The aim of this review is to analyze not only the results of different studies and trials, but also to highlight the different pathophysiological mechanisms related to TS, which is essential for the diagnosis of the disease and then evaluate efficacy of the different technical strategies available and their impact on the clinical outcome of these patients.
2. Pathophysiological mechanism and diagnosis of tandem lesions
Tandem lesions are encountered in approximately 15% of cases undergoing endovascular treatment. As the proximal occlusion is usually due to complication preexisting atherosclerotic carotid artery stenosis, which is more common in men, the same predilection is seen in tandem lesions. Prevalence of extracranial artery disease also varies significantly by race. Native American and Caucasian individuals have the highest prevalence of carotid artery stenosis, whereas African American males and Asian females appear to have the lowest prevalence [18]. The predominant pathophysiologic process involved in acute occlusion of the extracranial ICA is ruptured atherosclerotic plaque and superimposed thrombus, with distal embolization of thrombus fragment (artery-to-artery embolization [19]) or embolization of the clot from and arterial dissection in the cervical ICA to the intracranial circulation [20]; about 60%− 70% of TL’s are usually related to atherosclerotic plaque, 20–30% to ICA dissection and less than 5% to carotid web and cardiac emboli [21]. Carotid web is a non-atheromatous and non-dissecting membrane-like strand that protrudes into the lumen of the carotid artery with aspects similar to the fibromuscular dysplasia and abnormalities mainly involving the intimal layer [22]. Carotid web is mostly located in the posterior wall of the carotid bulb and is currently considered as an underrecognised cause of recurrent stroke [23]. Cardioembolic occlusion of the proximal internal carotid artery have also been reported, presumably related to a large cardiac embolus in the c-ICA and embolization of fragments in the intracranial circulation [24].
Most of the tandem occlusion recent literature data reported a mix of atheromatous and dissection, pooled as a single anatomic description. From a nosologic point of view, it looks confusing to associate two different diseases in different populations of patients with a different physiopathologic origin in a unique strategic approach [25]. Patients with dissection are significantly younger and exhibit a lower prevalence of vascular risk factors. Arterial dissection is an acute process, hampering the opportunity to develop cerebral circulation collaterals (such as cardioembolic strokes), which is a well-known predictive factor of good outcome after reperfusion [26]; however, this group seems to be more prone to present with a very good circle of Willis to justify a simple intracranial treatment. Furthermore, while the recurrence of stroke is proved in ICA atheromatous stenosis, [27], [28] the stroke recurrence from ICA dissection after efficient medical therapy seems to be very low. [29] Diagnosis of ICA dissection usually doesn’t require conventional angiography, that’s because non-invasive imaging modalities, such as angio-MR and angio-CT, have been found to have similar sensitivity and specificity, compared to conventional angiography [30]. Non-contrast brain CT is insensitive for dissection but may demonstrate cerebral ischemia/infarct or an arterial wall hematoma in the upper portion of the ICA, more than 2 cm from ICA origin, as a spontaneous crescent-shaped hyperattenuating focus [31]. Angio-CT highlights a narrowed eccentric lumen, the so called “flame-shaped” aspect, surrounded by a crescent-shaped mural thrombus and thin annular enhancement, related to vasa-vasorum enhancement in the adventitia [32]. Double barrel lumen and intimal flap are rarely seen [33]. MRI can be useful to detect ICA dissection with T1 FS, T2 and SWI [34] sequences which allow for highlighting an high signal crescent sign indicating intramural hematoma and angio-MR, which usually detects absent flow void or abnormal vessel contour, considered an high sensitivity and specificity sign [34].
Atherosclerotic lesions are usually seen in the elderly and more commonly in males. Tandem occlusions in these patients are the results of vulnerable atherosclerotic plaque with artery-to-artery distal embolization of thrombus fragments. The situation for the atherosclerotic process is different, compared to arterial dissection, as the first evolves slowly, enhancing the possibility to develop good leptmeningeal collateral [35]; at last, atherosclerotic plaques are usually located at the carotid bulbar bifurcation, or in any case close to the origin of the ICA and are typically associated with adjacent calcified plaques [36]. Another factor that favors the etiological diagnosis of atherosclerotic pathology is the simultaneous presence of plaques in other cervical arterial segments [37].
Although in clinical practice the diagnosis of tandem lesions is considered simple and immediate, it is still important to keep in mind the differential diagnosis with pseudo-occlusion of the cervical internal carotid artery. Carotid pseudo-occlusion refers to apparent occlusion of the cervical internal carotid artery on CT angiography or digital subtraction angiography due to a stagnant column of unopacified blood proximal to terminal T-junction occlusion by thromboembolism. The finding of cervical ICA PO has the potential to impact care because of its misdiagnosis as a tandem extracranial–intracranial ICA occlusion or dissection and lead to the erroneous exclusion of isolated intracranial ICA patients from clinical trials. Delayed CTA, multiphase CTA, or carotid ultrasonography may improve diagnostic accuracy [38], but despite that PO remains a significant diagnostic issue even after long-acquisition DSA, with angiographic microcatheter exploration being often required for the proper diagnosis [39].
3. Treatment strategies and review of literature
3.1. Stenting vs Angioplasty alone
Two different main approaches can be performed in patients with TLs (TAB. 1): the first is based on the immediate release, during the mechanical thrombectomy procedure, of a carotid stent, in order to obtain an immediate recanalization of the cervical tract of the ICA (Fig. 1), while the second involves only the execution of the balloon angioplasty treatment, usually performed together with thromboaspiration through large-bore catheter, and crossing the stenotic tract of the cervical ICA, which will then be treated in the following days or weeks through CAS, CEA or medical treatment alone (Fig. 2). Both are common widely used and have advantages and risks to consider when facing a TL. Obviously the most evident advantage of the CAS is to treat in the acute phase the carotid lesion, atherosclerotic or dissecting, responsible for the stroke, thus reducing the risk of stroke recurrence. Furthermore, the treatment of the cervical carotid lesion not only acts by favoring the spontaneous intracranial recanalization, favoring the lysis of the clot, but also acts by determining a recovery of the haemodynamic alterations which usually occur in carotid stenoses. Obviously, these advantages are counterbalanced by the increased thrombotic risk that occurs in these patients, with a consequent increased risk of stent occlusion, hence the need to immediately start an antithrombotic therapy, with all the consequent difficulties, related to the need to set up an acute treatment. On the other hand, balloon angioplasty treatment alone with stent placement at a later stage, in an elective regimen and in the best possible conditions for the patient, does not require the execution of an antithrombotic therapy in the acute phase, it seems be associated with a lower risk of intracranial haemorrhagic bleeding, although on this last point the data in the literature appear discordant and finally it seems to be associated with a lower risk of distal embolization, with however an increased risk of reocclusion and stroke recurrence. Based on the currently available literature, there is no treatment that can be considered definitive (TAB.2).
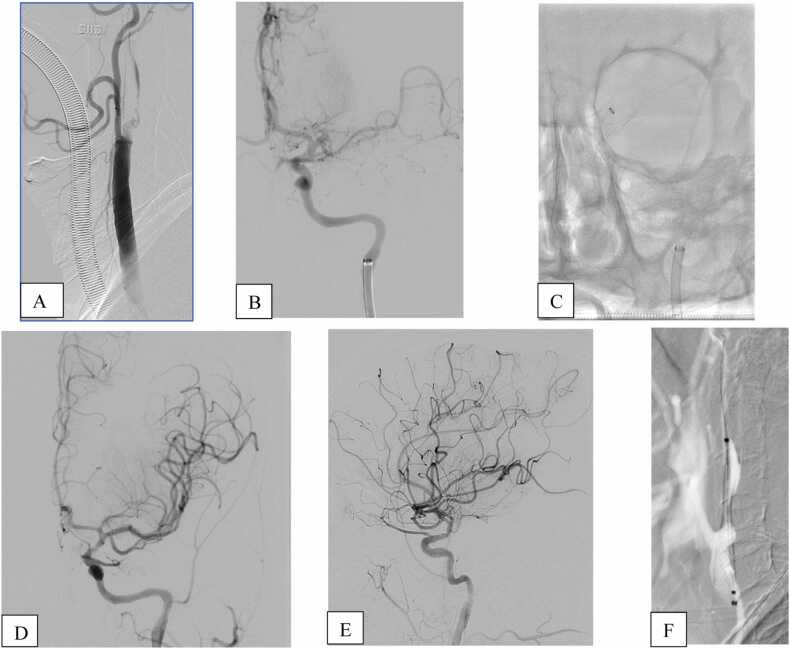
Fig. 1.
Angiographic data(A-B) showed tandem occlusion of left proximal internal carotid artery+occlusion of M1 tract of MCA. After crossing extracranial lesion, a first attempt of thromboaspiration was performed(C), with a complete recanalization, TICI 3, of the intracranial circulation (D-E). Carotid stent was then deployed with dilatation of angioplastic Balloon(F).
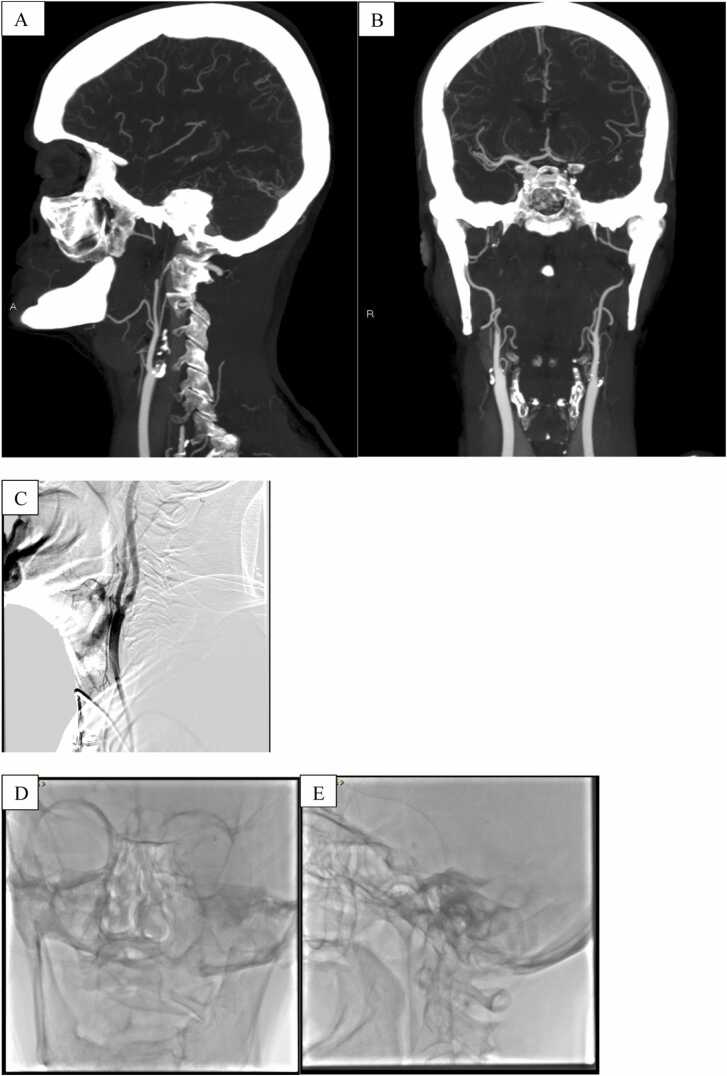
Fig. 2.
Angio-CT (A-B) showed tandem occlusion of left proximal internal carotid artery+occlusion of M1 tract of MCA. Dilatation with angioplastic balloon, without deployement of carotid stent, was performed (C), in order to cross extracranial lesion.After two attempts of thromboaspiration, complete recanalization, TICI 3, of the intracranial circulation was obtained (D-E).
Endovascular treatment of tandem occlusion remains poorly studied, that’s because of lack of high-quality evidence of major randomized controlled trials, where TL is usually considered as an exclusion criteria11–40–41–42. In the ESCAPE trial, only 54 patients presented with a tandem occlusion; of these 30 were in the endovascular treatment arm and 17 received stenting for the cervical ICA occlusion, 10 before and 7 after intracranial thrombectomy [43]. MR CLEAN and REVASCAT investigators reported a similar incidence of TOs, 32% and 19%, respectively [44], [45]; in the ESCAPE-NA1 trial, among 115/1105 patients (10.4%) with tandem occlusions, 62 (53.9%) received stenting for the cervical ICA occlusion. Of these, 46 (74.2%) were stented after and 16 (25.8%) before the intracranial thrombectomy and resulted that tandem occlusion in patients with acute large vessel stroke did not lower the odds of good functional outcome in our study and functional outcomes were similar irrespective of the management of the cervical ICA occlusion (stenting vs not stenting) [46], [47], although it’s important to say that ESCAPE-NA1 trial was not designed for evaluate efficacy and safety of endovascular treatment in TL’s but to assess efficacy and safety of nerinetide in ischaemia-reperfusion that occurs with rapid endovascular thrombectomy in patients who had an acute ischaemic stroke. Furthermore, in all these trials there was no subgroup analysis for patients with cervical dissection, because, as we say before, most of the tandem occlusion literature data reported a mix of atheromatous and dissection, pooled as a single anatomic description. A recent study analyzed all carotid artery dissection tandem occlusion strokes and isolated anterior circulation occlusions from RECOST study [25], stating that endovascular treatment of carotid artery dissection tandem occlusions could be a treatment safe and effective, in case of failure of a conservative approach [48]. Similar results also emerged from a recent meta-analysis, wherein both primary stenting and stenting of spontaneous CAD yielded unfavourable results with respect to stent thrombosis and stroke rates. Conversely, stenting following MT had acceptable mortality and complication rates corroborating the use of stenting in the setting of CAD as a second line treatment [49].
An international survey [50] of stroke experts highlighted uncertainty in treatment of the cervical ICA lesion in patients with TLs, with 75% of respondents admitting to having equipoise regarding therapy for these patients; same results also emerged in “The PICASSO survey”, which demonstrated multiple areas of uncertainty regarding the medical and endovascular management of TOs, with most practitioners (70%) agreed that there is equipoise regarding the optimal endovascular treatment of cervical lesions in TO [51]. This equipoise also emerged from several studies that were all observational and often only described stented patients. Several main meta-analyses of these smaller case series have been published; some of these [55] focused primarily on efficacy and safety of thrombectomy using stent-retrievers with emergency stenting of cervical ICA in acute anterior stroke patients with tandem occlusion ICA, without, however, defining best modality to treat proximal stenosis. A recent meta-analysis [57] tried to compare the anterograde and retrograde approaches to provide updated clinical evidence of strategy selection, stating that the retrograde approach seems to achieve a higher successful reperfusion rate and better functional outcome with a comparable safety profile when compared with an antegrade approach. Most studies, instead, focused primarily on safety and efficacy of carotid artery stenting in patients with tandem occlusion, however, with inconsistent results. Specifically, one meta-analysis [54] stated no benefit from emergency stenting in parameters such as successful revascularization (TICI≥2b), clinical outcome (mRS≤2) or 90-day mortality, unlike the others, in which, it was defined that acute stent placement seems to be associated with higher odds of successful reperfusion and good functional outcome than no-stenting in patients with acute ischemic stroke due to tandem occlusion52,53,55,56, with good safety outcomes, highlighted by the absence of statistically significant differences, between stenting and no-stenting group, in sICH rates, reported to range from 4% to 9%, as demonstrated in other studies, and mortality.
Most of the evidence related to tandem occlusions comes from the TITAN registry [58], a retrospective analysis of consecutive patients presenting to 18 comprehensive stroke centers with AIS due to tandem lesion of the anterior circulation who underwent MT. In this large multicenter retrospective cases-series a total of 395 patients were included. Successful reperfusion, expressed in terms of modified thrombolysis in cerebral infarction score 2b-3 was achieved in 79.4% while, at 90 days, 53.4% achieved a good outcome, expressed in terms of modified Rankin Scale score 0–2; intravenous thrombolysis and emergent carotid artery stenting were predictors of a successful reperfusion, as independently associated with the latter while, lower age, absence of hypercholesterolemia, lower NIHSS scores, ASPECT Score ≥ 7 and proximal middle cerebral artery occlusion independently predicted a good 90-day outcome [59], [60], suggesting that patients treated with acute antiplatelet medications and stenting have more favorable outcomes than patients treated with angioplasty alone or those with no acute ICA intervention [61].
Better results came from analysis of data of STRATIS registry [62], a prospective, nonrandomized study of patients undergoing mechanical thrombectomy with the Solitaire device. Of 147/984 (14.9%) patients with tandem lesions treated, stenting of the extracranial lesion during thrombectomy was performed in 80 patients and withheld in 67 patients. Good outcomes, expressed in terms of modified Rankin Scale, 0–2 at 90 days were higher in the stenting group with no difference in mortality or symptomatic hemorrhage [63]. However, patients in the stenting group had lower baseline NIHSS scores, shorter onset to arterial puncture time and lower rates of atrial fibrillation compared to the non-stenting group, although after adjustment for covariates, stenting continued to be associated with superior outcomes. Clearly, observational and non-randomized design from STRATIS studies has known methodological shortcomings and could have led to bias.
The Endovascular Acute Stroke Intervention (EASI ClinicalTrials.gov: Identifier NCT02157532) trial was the first single-center randomized care trial trying to randomly allocate patients with tandem lesions [64]. In this trial, performed before the MR CLEAN results, patients were randomized to BMT alone versus BMT with EVT, and patients allocated to EVT with TL underwent a second randomization, allocating them to acute stent placement or not. Randomized allocation was interrupted when other trials showed the benefits of endovascular therapy [65]. Despite this, the importance of the data obtained was still high, because it allowed the development of a further trial, the Endovascular Acute Stroke Intervention–Tandem Occlusion (EASI-TOC) trial (ClinicalTrials.gov: Identifier NCT04261478), a phase III multi-centre, prospective, randomized, open-label, blinded endpoint (PROBE) controlled trial, with 458 patients enrolled and randomized (1:1) to undergo acute ICA stenting during the thrombectomy procedure (either before or after intracranial thrombectomy, at the discretion of the treating physician) or to intracranial thrombectomy alone without ICA stenting. The trial will seek to determine if in patients undergoing acute intracranial thrombectomy for anterior circulation stroke with concurrent ipsilateral symptomatic high-grade (≥70%) atherosclerotic stenosis or occlusion of the extracranial ICA, endovascular ICA revascularization with stenting is superior to intracranial thrombectomy alone with regards to functional outcome at 90 days (measured using the Modified Rankin Scale).
Simultaneously to EASI-TOC, also Intracranial Thrombectomy and Extracranial Carotid Stenting Versus Intracranial Thrombectomy Alone In Acute Anterior Circulation Strokes With TANdem Occlusion: the Randomized Controlled TITAN Trial [66] (ClinicalTrials.gov: Identifier NCT03978988), an investigator-initiated, multicenter, prospective, randomized, open-label, blinded-endpoint (PROBE) study, with 432 patients enrolled and randomized after tandem occlusion confirmation on angiogram, will try to compare the two types of treatment in patients with acute ischemic stroke due to anterior circulation tandem occlusion, especially assessing the safety and efficacy of emergent internal carotid artery stenting associated with at least one antiplatelet therapy in the acute phase of stroke reperfusion.
In this context, only data of these two randomized-controlled trials will help to identify the best therapeutic strategy for patients with tandem occlusions. In absence of these results, at the date, only data from observational studies can suggest the optimal management of patients with AIS due to TLs, with all implications related to lack of consensus.
3.2. Anterograde vs Retrograde approach
Two endovascular approaches are commonly used in the management of patients with tandem occlusions [67], [68] (TAB. 3). According to the sequence of extracranial or intracranial lesions addressed, the treatment strategies are classified as the anterograde approach and retrograde approach. Tailored evidence for the optimal treatment order between antegrade and retrograde approaches remains inconsistent among studies, as both the approaches have innate advantages and disadvantages; in fact, some studies have shown comparable results between the two approaches [69], [70], [75], [77], while, in other studies a better outcome of the anterograde [74] or retrograde [68], [71], [72], [73], [76] approach have been shown. In patients from the TITAN cohort, the order of treatment of intracranial occlusion or cervical occlusion led to a similar rate of successful reperfusion [61], with faster time from puncture to reperfusion in the retrograde approach group but with similar clinical outcomes. Anterograde approach provides a proximal-to-distal recanalization, with angioplasty and/or stenting of the cervical lesion first, followed by intracranial thrombectomy. The main advantage of the anterograde approach is related to the restored blood-flow, with increased likelihood of successful distal recanalization due to the increase blood-flow in non-occluded arteries from collaterals, preventing possible recurrent occlusion of intracranial distal vessels due to slow-flow. Furthermore, another advantage of the anterograde technique is the improved accessibility of intracranial lesions for EVT, with presumable low risk of vessel dissection or perforation because of non-blind navigation of the ICA with the microwire and microcatheter; also, it is possible to work with “flow arrest” by simply advance the balloon guide catheter. The most important disadvantage of the antegrade approach is that, treating cervical-ICA first, might increase the time for intracranial recanalization, which might lead to an increased infarct volume [73]. Mechanical thrombectomy device can be entrapped within the carotid stent, a potential complication that can be prevented by advancing the occlusion balloon or sheath distal to the carotid stent.
The retrograde approach provides a distal-to-proximal recanalization, with treatment of the intracranial occlusion with mechanical thrombectomy first, followed by treatment of the cervical ICA occlusion. Advantages to the retrograde technique are clearly related to shorter intracranial recanalization time, in order to limit the size of the cerebral infarction [78], and avoidance of potential snagging of the stent-retriever in the struts of an already-deployed cervical-ICA stent [73]. The major disadvantage of the retrograde approach is that treatment may provoke distal embolization of the intracranial thrombus [79], an important complication that, however, can also be observed with the anterograde approach, as shown by results of some studies that highlight an higher rate of distal embolism in this latter, probably related to the restoration of blood-flow and the augmented pressure on the intracranial thrombus, which may lead to a clot fragmentation distally in new territories, worsening outcome of the patients [80].
Due to distal embolization risk related to both approaches, balloon angioplasty and carotid stent placement could be performed utilizing distal or proximal protection. Data concerning efficacy and safety of embolic protection devices in non-acute carotid artery stenting for primary and secondary stroke prevention proved that use of an embolic protection device is independently associated with lower in-hospital risk for stroke or death, major stroke or death, and stroke [81]; however, data concerning benefit of embolic protection devices during acute ICA intervention are scarce, regarding only single-center non-randomized studies [82], [83], with relatively small sample size and single case-reports. At the date, considering urgency of mechanical thrombectomy for stroke, embolic protection devices are rarely used.
Concerning the safety profile between anterograde and retrograde approaches, many studies have highlighted no significative statistical differences in terms of symptomatic hemorrhage, 90-day independence, or mortality [57], [68], [84], with a slight lower incidence of periprocedural complications with the retrograde approach.
The optimal technical approach still needs clarification in prospective studies with meticulous design, however it is unquestionable that in AIS due to tandem occlusions, distal vascular revascularization is more important than the proximal lesion treatment; early reperfusion shortens the ischemic duration and could bring more beneficial for poor leptomeningeal collateral circulation in the acute phase of stroke, leading to theoretical better clinical outcomes for patients with TLs. For these reasons, to date, the retrograde approach is the one of choice in many centers; however, must be kept in mind that in some cases the retrograde approach is not feasible, due to high-grade stenosis and the anterograde approach seems to be the only choice.
3.3. Deferred ICA intervention
Deferred ICA intervention refers to a staged approach, in which CAS or endarterectomy, for carotid recanalization, are usually performed a few days after intracranial EVT and represents an additional treatment option. During the acute stage, cervical-ICA lesion is treated with only balloon angioplasty, in order gain access to intracranial vessels. Few data are available regarding delayed ICA revascularization. In the ESCAPE trial [3], among the 30 intervention-arm subjects, 17 (57%) underwent emergency endovascular treatment of the extracranial disease. Of the remaining 13 subjects, only four required staged carotid revascularizations due to persistent severe carotid stenosis. Recent studies [85], [86] tried to assess the efficacy of balloon angioplasty-assisted mechanical thrombectomy without urgent stenting in the carotid artery, with promising results in terms of successful recanalization, good clinical outcomes and sICH. According to these results and these studies, staged approach could be considered a safer approach for the endovascular treatment of tandem occlusions, especially in patients with specifically clinical and radiological findings, such as dissection etiology, high bleeding risk, low ASPECT, and incomplete or absent intracranial recanalization (mTICI 0–1)avoiding futile stenting and occurrence of hemorrhagic complications [15]; however, due to the design of these studies, retrospective single-center studies, non-randomized and with a relatively small number of cases, none of the evidence can be considered definitive.
3.4. Medical treatment of tandem occlusion
The initial step in the treatment of tandem occlusions is often the administration of thrombolytic medications, such as tissue plasminogen activator (tPA), which help dissolve blood clots and restore blood flow. The benefit of intravenous thrombolysis (IVT) in tandem occlusion is debatable [87] because of the large clot burden and the potential need for periprocedural antiplatelet therapy in cases of acute carotid artery stenting, with only 20% of patients have a good clinical outcome after IVT [4]; also, intra-arterial treatment in TOs seems to be related to low efficacy due to length of thrombus [88]. A recent study found that among patients with tandem lesions, favorable outcomes observed in rt-PA-treated patients had no statistically significant difference to those observed in untreated patient [89]; however, it’s important to note that IVT is an added value in EVT-treated patients with tandem occlusion and improves early outcome, as highlighted by several studies. TITAN and ETIS investigators found that IVT+EVT group had higher odds of favorable outcome, excellent outcome (90-day mRS score 0–1), and successful reperfusion (express in terms of mTICI), compared to EVT group [90], with no statistical significative difference in the risk of significant hemorrhagic complications between groups [91]. IVT may serve as an adjunct for EVT by altering clot properties and thus aiding mechanical recanalization but also may dissolve harder-to-reach clots and additional microemboli.
The medical treatment of tandem occlusions tends to differ according to the placement or not of a carotid stent in the acute phase. Acute stent placement is usually associated to antithrombotic medication, to avoid stent thrombosis, which occurs in around 2% of patients and negatively impacts neurological outcomes [92]. The need to prevent intra-stent thrombosis collides with the need to prevent the symptomatic hemorrhagic transformation of the infarcted brain tissue, which occurs in about 10% of patients with TOs and significantly worsens the functional outcome [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92]. Antithrombotic treatment during stent acute placement should increase the risk of sICH, due to the coagulopathy caused by thrombolysis and to the disruption of the blood-brain barrier [93](TAB.3). In this context, also prior IVT may have a role in increasing risk of sICH. Recent literature data document how IVT in patients pretreated with Acetylsalicylic acid, who do not undergo EVT, is associated to a low but statically significantly elevated risk of sICH, with no effect on clinical outcome [94] and should not be given within 24 h after IVT. Similar results also emerged in patients with double antiplatelet therapy [95]. Despite this, things are different; indeed, as mentioned above, not only bridging therapy (IVT+EVT) in patients with acute ischemic stroke due to anterior tandem occlusion is safe and may improve functional outcome, but even in patients treated with acute carotid artery stenting, bridging therapy is associated with higher odds of favorable outcome and lower odds of mortality at 90 days [58], [59], without an increased incidence of sICH [20], although, on this last point, data in literature lack of consensus88–96–97.
Current data available from literature couldn’t give a clear answer as to what is antithrombotic treatment that appears to be superior for patients with TOs undergoing acute stent placement, with or without prior IVT. Several antithrombotic treatments have been proposed, including No antiplatelets, single antiplatelet treatment with administration of aspirin alone, with a stent occlusion rate within 7 days of 10.3% [71], dual antiplatelets therapy (DAPT) with a combination of aspirin and clopidogrel. DAPT is the most used antithrombotic treatment in many studies, albeit in different ways, with a marginal benefit on good functional outcome and carotid stent patency without a significant increase in risk of sICH [98]. Some studies have reported use of an IV loading dose of aspirin (250–500 mg) with a loading dose of clopidogrel (300 mg), which can be given immediately, without prior IVT [68], [69], [70], [71], [72], [73], [74], [75], [76], [77] or 24 h later, after a postprocedural follow-up CT scan excludes hemorrhage [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], followed by DAPT for 3 months. In other studies patients received aspirin (100 mg) and clopidogrel (75 mg) 24 h after carotid stent placement [100]. In some cases, a different approach has also been proposed, using more aggressive antithrombotic therapy, with aspirin clopidogrel and abciximab, basing on results of SAMPRISS trial [101], but off 13 patients receiving an intravenous loading dose of abciximab during the procedure, 4/13 had SICH (31%), compared with 1/10 (10%) of those who did not, with the incidence of SICH that seems to be associated with the use of abciximab and advanced patient age [102]. In this context, promising results regarding the use of eptifibatide have emerged from small case series, after that a retrospective analysis of the cohorts of the 16 comprehensive stroke centers documented that Glycoprotein IIb/IIIa inhibitor was significantly associated with stent patency but not with symptomatic intracranial hemorrhage [103]. Low dose of eptifibatide administered during treatment of tandem occlusions, overlapped with DAPT, seems not only to be safe in tandem occlusions [104] (symptomatic intracranial hemorrhage, 2%) but also feasible with good recanalization rates and low risk of stent occlusion [105]. Literature data relating to the use of new antiplatelet agents, such as ticagrelor and cangrelor, are scarce, consequently their use cannot be recommended [106], [107]; however, it is known that patients may demonstrate a so-called high on-treatment platelet reactivity with clopidogrel, also acknowledged as resistance or nonresponsiveness to therapy. In this context, DAPT with aspirin/ticragrelor could be a valid alternative. Further results about the use of ticagrelor in carotid artery stenting may emerge from the PRECISE-MRI trial (ClinicalTrials.gov Identifier: NCT02677545).
Some studies also tried to investigate the impact of heparin, used during endovascular therapy of tandem occlusions, on the functional and safety outcomes, with conflicting results. Analysis from TITAN Registry [108] found periprocedural heparin was not associated with better functional, angiographic or safety outcomes, while analysis of the TREVO 2 trial showed that the use of heparin was independently associated with good functional outcome [109]. In this context, the role of the MR-CLEAN MED [110] trial for evaluation of safety and efficacy of aspirin and unfractionated heparin appears decisive, stating that periprocedural intravenous aspirin and unfractionated heparin during endovascular stroke treatment is associated with an increased risk of symptomatic intracranial haemorrhage without evidence for a beneficial effect on functional outcome [111].
Medical treatment of non-acute ICA stent placement looks more uniform, given the results that have also emerged from various studies and trials [112]; because of this, DAPT is now considered to be the norm during the perioperative period for CAS, as associated with a lower ischemic complications and lower hemorrhagic complications than anticoagulant therapy [113]. DAPT should be orally administered for at least 4 days prior to the operation, and, postoperatively, clopidogrel should be continued for at least 30 days, and aspirin should be continued for at least 12 months, often the rest of the life, considering the patient's clinical condition.
3.5. Coil occlusion and other treatment options
Other treatment options have been performed infrequently in the past and, consequently, data on their use are sparse. The most relevant of these treatments is without any doubt permanent occlusion of cervical carotid artery with coils, performed in order to prevent early embolic recurrence in patients with cervical-ICA occlusion associated to an intraluminal thrombus, especially in patients with good collateral flow is provided via the circle of Willis. Labeyrie et al. [114] was the first to report this approach, together with others five approaches to manage the cervical occlusion in 49 patients with TL (14% of MT): medical treatment alone in 16/64 (25%), stenting/angioplasty in 16/64 (25%), occlusion with coils in 12/64 (19%), angioplasty alone in 9/64 (14%), stent retriever in 8/64 (12%), and/or thromboaspiration in 3/64 (5%). Occlusion with coils had a lower rate of radiological intracranial hemorrhage at 48-hour compared to other approaches with similar rates of favorable outcome and mortality at 90-days; however, duo to several limitiations of the study, first the small size of the sample, no high-grade recommendation can be made.
The same study also suggests, in patients with persistent cervical-ICA occlusion, to leave the latter untreated, with medical treatment which alone may be sufficient when no cervical intraluminal thrombus is present, the Willis polygon is effective, and the cervical occlusion can be crossed easily to perform the intracranial thrombectomy.
Emergent Carotid Endarterectomy following mechanical thrombectomy, a treatment option based on results of a recent comparative study between the latter and carotid artery stenting [115], has, to date, a marginal role in the treatment of tandem occlusions, but it could eventually have a rationale in the rare circumstance of being unable to cross the cervical occlusion during management of acute ischemic stroke with tandem occlusion.Table 1, Table 2, Table 3.
Table 1.
Diagram summarizing the main advantages and disadvantages of acute stent placement vs PTA alone.
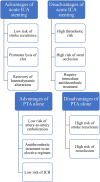 |
Table 2.
Suggested algorithm for investigation and management of Tandem lesions.
 |
Table 3.
Diagram summarizing the main advantages and disadvantages of antegrade and retrograde approach.
 |
4. Conclusion
Acute ischemic stroke due to tandem lesions accounts for about 15–25% of all LVO strokes. Despite the recent randomized controlled trials demonstrating effectiveness of MT in AIS, there is still a lack of consensus, due to research gap, about management of tandem occlusions. There are some evidences in favor of EVT with acute stent placement of the cervical ICA, while, other results suggest that balloon angioplasty technique without acute stenting could have a comparably favorable outcome rate. Furthermore, the optimal anticoagulation and antiplatelet regimen has not been established. Because of this, only results of randomized controlled trials, comparing the long-term outcome of a stenting cohort to a non-stenting cohort following successful reperfusion, will be the key to clarify the best treatment strategy for this group patients.
Ethical Standards and Patient Consent
We declare that all human and animal studies have been approved by the Ethics Committe of our Institution and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient and public involvement
There was no involvement from either patients or the public in this review.
Author Contribution
All authors have contributed equally in the submission to take public responsibility for its content.
Ethical statement
All procedures performed in this review were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Declaration of Competing Interest
There are no conflicts of interest in this review.
References
- 1.Jadhav A.P., Zaidat O.O., Liebeskind D.S., et al. Emergent management of tandem lesions in acute ischemic stroke. Stroke. 2019;50:428–433. doi: 10.1161/STROKEAHA.118.021893. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M., Menon B.K., van Zwam W.H., et al. HERMES collaborators Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from fiverandomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 3.Assis Z., Menon B.K., Goyal M., et al. ESCAPE Trialists Acute ischemic stroke with tandem lesions: technical endovascular management and clinical outcomes from the ESCAPE trial. J. Neurointerv. Surg. 2018;10:429–433. doi: 10.1136/neurintsurg-2017-013316. [DOI] [PubMed] [Google Scholar]
- 4.Rubiera M., Ribo M., Delgado-Mederos R., et al. Tandem internal carotid artery/middle cerebral artery occlusion: an independent predictor of poor outcome after systemic thrombolysis. Stroke. 2006;37(09):2301–2305. doi: 10.1161/01.STR.0000237070.80133.1d. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y.S., Garami Z., Mikulik R., et al. Early recanalization rates and clinical outcomes in patients with tandem internal carotid artery/ middle cerebral artery occlusion and isolated middle cerebral artery occlusion. [DOI] [PubMed]
- 6.Shi Z.S., Loh Y., Walker G., et al. Endovascular thrombectomy for acute ischemic stroke in failed intravenous tissue plasminogen activator versus non-intravenous tissue plasminogen activator patients: revascularization and outcomes stratified by the site of arterial occlusions. Stroke. 2010;41:1185–1192. doi: 10.1161/STROKEAHA.109.568451. [DOI] [PubMed] [Google Scholar]
- 7.Rubiera M., Ribo M., Delgado-Mederos R., et al. Tandem internal carotid artery/ middle cerebral artery occlusion: an independent predictor of poor outcome after systemic thrombolysis. Stroke. 2006;37:2301–2305. doi: 10.1161/01.STR.0000237070.80133.1d. [DOI] [PubMed] [Google Scholar]
- 8.Urra X., Abilleira S., Dorado L., et al. Mechanical thrombectomy in and outside the REVASCAT trial: insights from a concurrent population-based stroke registry. Stroke. 2015;46(12):3437–3442. doi: 10.1161/STROKEAHA.115.011050. [DOI] [PubMed] [Google Scholar]
- 9.Powers W.J., Rabinstein A.A., Ackerson T., et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2018;2018(49):e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 10.Turc G., Bhogal P., Fischer U., et al. European stroke organisation (ESO)–European society for minimally invasive neurological therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke endorsed by strokealliance for Europe (SAFE) Eur. Stroke J. 2019;4:6–12. doi: 10.1177/2396987319832140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saver J.L., Goyal M., Bonafe A., et al. SWIFT PRIME Investigators Stent- retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 12.Campbell B.C., Mitchell P.J., Kleinig T.J., et al. EXTEND-IA Investigators Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 13.Bracard S., Ducrocq X., Mas J.L., et al. THRACE investigators Mechanical thrombectomy after intravenous alteplase versus alte- plase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–1147. doi: 10.1016/S1474-4422(16)30177-6. [DOI] [PubMed] [Google Scholar]
- 14.Wilson M.P., Murad M.H., Krings T., et al. Management of tandem occlusions in acute ischemic stroke - intracranial versus extracranial first and extracranial stenting versus angioplasty alone: a systematic review and meta-analysis. J. Neurointerv. Surg. 2018;10:721–728. doi: 10.1136/neurintsurg-2017-013707. [DOI] [PubMed] [Google Scholar]
- 15.Poppe A.Y., Jacquin G., Roy D., et al. Tandem carotid lesions in acute ischemic stroke: mechanisms, therapeutic challenges, and future directions. AJNR Am. J. Neuroradiol. 2020;41(7):1142–1148. doi: 10.3174/ajnr.A6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steglich-Arnholm H., Holtmannspötter M., Kondziella D., et al. Thrombectomy assisted by carotid stenting in acute ischemic stroke management: benefits and harms. J. Neurol. 2015;262:2668–2675. doi: 10.1007/s00415-015-7895-0. [DOI] [PubMed] [Google Scholar]
- 17.Feil K., Herzberg M., Dorn F., et al. Tandem lesions in anterior circulation stroke: analysis of the German stroke registry-endovascular treatment. Stroke. 2021;52:1265–1275. doi: 10.1161/STROKEAHA.120.031797. [DOI] [PubMed] [Google Scholar]
- 18.Rockman C.B., Hoang H., Guo Y., et al. The prevalence of carotid artery stenosis varies significantly by race. J. Vasc. Surg. 2013;57(2):327–337. doi: 10.1016/j.jvs.2012.08.118. [DOI] [PubMed] [Google Scholar]
- 19.Cote R., Caron J.‐L. Management of carotid artery occlusion. Curr. Conc. Carebrovasc. Dis. Stroke. 1989;20:123–126. doi: 10.1161/01.str.20.1.123. [DOI] [PubMed] [Google Scholar]
- 20.Papanagiotou P., Haussen D.C., Turjman F., et al. TITAN Investigators Carotid stenting with antithrombotic agents and intracranial thrombectomy leads to the highest recanalization rate in patients with acute stroke with tandem lesions. JACC Cardiovasc Inter. 2018;11:1290–1299. doi: 10.1016/j.jcin.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 21.Gory B., Piotin M., Haussen D.C., et al. TITAN Investigators Thrombectomy in acute stroke with tandem occlusions from dissection versus atherosclerotic cause. Stroke. 2017;48:3145–3148. doi: 10.1161/STROKEAHA.117.018264. [DOI] [PubMed] [Google Scholar]
- 22.Kim S.J., Nogueira R.G., Haussen D.C. Current understanding and gaps in research of carotid webs in ischemic strokes. JAMA Neurol. 2019;76:355. doi: 10.1001/jamaneurol.2018.3366. [DOI] [PubMed] [Google Scholar]
- 23.Compagne K.C.J., et al. Prevalence of carotid web in patients with acute intracranial stroke due to intracranial large vessel occlusion. Radiology. 2018;286:1000–1007. doi: 10.1148/radiol.2017170094. [DOI] [PubMed] [Google Scholar]
- 24.Vavrova J., Koznar B., Peisker T., et al. Stroke etiology by occlusion site – data from the PRAGUE 16 study. Eur. Heart J. 2020 [Google Scholar]
- 25.Marnat G., Mourand I., Eker O., et al. Endovascular management of tandem occlusion stroke related to internal carotid artery dissection using a distal to proximal approach: insight from the RECOST study. AJNR Am. J. Neuroradiol. 2016;37(7):1281–1288. doi: 10.3174/ajnr.A4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkhemer O.A., Jansen I.G., Beumer D., et al. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke. 2016;47(3):768–776. doi: 10.1161/STROKEAHA.115.011788. [DOI] [PubMed] [Google Scholar]
- 27.Lovett J.K., Coull A.J., Rothwell P.M. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569–573. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- 28.Marnane M., Ni Chroinin D.N., Callaly E., et al. Stroke recurrence within the time window recommended for carotid endarterectomy. Neurology. 2011;77:738–743. doi: 10.1212/WNL.0b013e31822b00cf. [DOI] [PubMed] [Google Scholar]
- 29.Arauz A., Hoyos L., Espinoza C., et al. Dissection of cervical arteries: long-term follow-up study of 130 consecutive cases. Cereb. Dis. 2006;22:150–154. doi: 10.1159/000093244. [DOI] [PubMed] [Google Scholar]
- 30.Provenzale J.M., Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am. J. Roentgenol. 2009;193:1167–1174. doi: 10.2214/AJR.08.1688. [DOI] [PubMed] [Google Scholar]
- 31.Rodallec M.H., Marteau V., Gerber S., et al. Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. Radiographics. 2008;28(6):1711–1728. doi: 10.1148/rg.286085512. [DOI] [PubMed] [Google Scholar]
- 32.Leclerc X., Godefroy O., Salhi A., et al. Helical CT for the diagnosis of extracranial internal carotid artery dissection. Stroke. 1996;27(3):461–466. doi: 10.1161/01.str.27.3.461. [DOI] [PubMed] [Google Scholar]
- 33.Lee V.H., Brown R.D., Jr, Mandrekar J.N., et al. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67:1809–1812. doi: 10.1212/01.wnl.0000244486.30455.71. [DOI] [PubMed] [Google Scholar]
- 34.Hakimi R., Sivakumar S. Imaging of carotid dissection. Curr. Pain. Headache Rep. 2019 doi: 10.1007/s11916-019-0741-9. [DOI] [PubMed] [Google Scholar]
- 35.Rebello L.C., Bouslama M., Haussen D.C., et al. Stroke etiology and collaterals: atheroembolic strokes have greater collateral recruitment than cardioembolic strokes. Eur. J. Neurol. 2017;24(6):762–767. doi: 10.1111/ene.13287. [DOI] [PubMed] [Google Scholar]
- 36.Kim J.J., Dillon W.P., Glastonbury C.M. et al. Sixty-four-section multidetector CT angiography of carotid arteries: a systematic analysis of image quality and artifacts.AJNR Am J Neuroradiol. 2010. [DOI] [PMC free article] [PubMed]
- 37.Koelemay M.J., Nederkoorn P.J., Reitsma J.B., et al. Systematic review of computed tomographic angiography for assessment of carotid artery disease. Stroke. 2004;35(10):2306–2312. doi: 10.1161/01.STR.0000141426.63959.cc. [DOI] [PubMed] [Google Scholar]
- 38.Volders D., Shewchuk J.R., Marangoni M., et al. Beyond the collaterals: additional value of multiphase CTA in acute ischemic stroke evalu- ation. Neuroradiol. J. 2019;32:309. doi: 10.1177/1971400919845361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grossberg J.A., Haussen D.C., Cardoso F.B., et al. Cervical carotid pseudo-occlusions and false dissections: intracranial occlusions masquerading as extracranial occlusions. Stroke. 2017;48(3):774–777. doi: 10.1161/STROKEAHA.116.015427. [DOI] [PubMed] [Google Scholar]
- 40.Pereira V., Gralla J., Davalos A., et al. Prospective, multicentre, single-arm study of mechanical thrombectomy using solitaire flow restoration in acute ischemic stroke: STAR. Stroke. 2014;44:2802–2807. doi: 10.1161/STROKEAHA.113.001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saver J.L., Jahan R., Levy E.I., et al. Solitaire flow restoration device versus the Merci retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 42.Nogueira R.G., Lutsep H.L., Gupta R., et al. TREVO 2 Trialists Trevo versus merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goyal M., Demchuk A.M., Menon B.K., et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015;372(11):1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 44.Berkhemer O.A., Fransen P.S., Beumer D., et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015;372(01):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 45.Jovin T.G., Chamorro A., Cobo E., et al. Thrombectomy within 8 h after symptom onset in ischemic stroke. N. Engl. J. Med. 2015;372(24):2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 46.Hill M.D., Goyal M., Menon B.K., et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395(10227):878–887. doi: 10.1016/S0140-6736(20)30258-0. [DOI] [PubMed] [Google Scholar]
- 47.Marko M., Cimflova P., Poppe A.Y., et al. Management and outcome of patients with acute ischemic stroke and tandem carotid occlusion in the ESCAPE-NA1 trial. J. Neurointerv. Surg. 2022;14(5) doi: 10.1136/neurintsurg-2021-017474. neurintsurg-2021-017474. [DOI] [PubMed] [Google Scholar]
- 48.Costalat V., Lobotesis K., Machi P., et al. Prognostic factors related to clinical outcome following thrombectomy in ischemic stroke (RECOST study). 50 patients prospective study. Eur. J. Radiol. 2012;81(12):4075–4082. doi: 10.1016/j.ejrad.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 49.Bontinis V., Antonopoulos C.N., Bontinis A., et al. A systematic review and meta-analysis of carotid artery stenting for the treatment of cervical carotid artery dissection. Eur. J. Vasc. Endovasc. Surg. 2022;64(4):299–308. doi: 10.1016/j.ejvs.2022.07.048. [DOI] [PubMed] [Google Scholar]
- 50.Jacquin G., Poppe A.Y., Labrie M., et al. Lack of consensus among stroke experts on the optimal management of patients with acute tandem occlusion. Stroke. 2019;50:1254–1256. doi: 10.1161/STROKEAHA.118.023758. [DOI] [PubMed] [Google Scholar]
- 51.Zevallos C.B., Farooqui M., Quispe-Orozco D., et al. Proximal internal carotid artery acute stroke secondary to tandem occlusions (PICASSO) international survey. J. Neurointerv. Surg. 2021;13(12):1106–1110. doi: 10.1136/neurintsurg-2020-017025. [DOI] [PubMed] [Google Scholar]
- 52.Diana F., Romoli M., Toccaceli G., et al. Emergent carotid stenting versus no stenting for acute ischemic stroke due to tandem occlusion: a meta-analysis. J. Neurointerv. Surg. 2023;15(5):428–432. doi: 10.1136/neurintsurg-2022-018683. [DOI] [PubMed] [Google Scholar]
- 53.Zevallos C.B., Farooqui M., Quispe-Orozco D., et al. Acute carotid artery stenting versus balloon angioplasty for tandem occlusions: a systematic review and meta-analysis. J. Am. Heart Assoc. 2022;11:2. doi: 10.1161/JAHA.121.022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pires Coelho A., Lobo M., Gouveia R., et al. Overview of evidence on emergency carotid stenting in patients with acute ischemic stroke due to tandem occlusions: a systematic review and meta-analysis. J. Cardiovasc Surg. (Torino) 2019;60(6):693–702. doi: 10.23736/S0021-9509.18.10312-0. [DOI] [PubMed] [Google Scholar]
- 55.Sivan-Hoffmann R., Gory B., Armoiry X., et al. Stent-retriever thrombectomy for acute anterior ischemic stroke with tandem occlusion: a systematic review and meta-analysis. Eur. Radio. 2017;27(1):247–254. doi: 10.1007/s00330-016-4338-y. [DOI] [PubMed] [Google Scholar]
- 56.Sadeh-Gonik U., Tau N., Friehmann T., et al. Thrombectomy outcomes for acute stroke patients with anterior circulation tandem lesions: a clinical registry and an update of a systematic review with meta-analysis. Eur. J. Neurol. 2018;25(4):693–700. doi: 10.1111/ene.13577. [DOI] [PubMed] [Google Scholar]
- 57.Min X., Du J., Bai X., et al. Antegrade or retrograde approach for the management of tandem occlusions in acute ischemic stroke: a systematic review and meta-analysis. Front Neurol. 2022;12 doi: 10.3389/fneur.2021.757665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gory B., Haussen D.C., Piotin M., et al. Impact of intravenous thrombolysis and emergent carotid stenting on reperfusion and clinical outcomes in patients with acute stroke with tandem lesion treated with thrombectomy: a collaborative pooled analysis. Eur. J. Neurol. 2018;25(9):1115–1120. doi: 10.1111/ene.13633. [DOI] [PubMed] [Google Scholar]
- 59.Anadani M., Marnat G., Consoli A., et al. Endovascular therapy of anterior circulation tandem occlusions: pooled analysis from the TITAN and ETIS registries. Stroke. 2021;52(10):3097–3105. doi: 10.1161/STROKEAHA.120.033032. [DOI] [PubMed] [Google Scholar]
- 60.Anadani M., Spiotta A.M., Alawieh A., et al. Emergent carotid stenting plus thrombectomy after thrombolysis in tandem strokes: analysis of the TITAN registry. Stroke. 2019;50(8):2250–2252. doi: 10.1161/STROKEAHA.118.024733. [DOI] [PubMed] [Google Scholar]
- 61.Zhu F., Bracard S., Anxionnat R., et al. Impact of emergent cervical carotid stenting in tandem occlusion strokes treated by thrombectomy: a review of the TITAN collaboration. Front Neurol. 2019;10:206. doi: 10.3389/fneur.2019.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mueller-Kronast N.H., Zaidat O.O., Froehler M.T., et al. STRATIS Investigators Systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke: primary results of the STRATIS Registry. Stroke. 2017;48:2760–2768. doi: 10.1161/STROKEAHA.117.016456. [DOI] [PubMed] [Google Scholar]
- 63.Lee H., Qureshi A.M., Mueller-Kronast N.H., et al. Subarachnoid hemorrhage in mechanical thrombectomy for acute ischemic stroke: analysis of the STRATIS registry, systematic review, and meta-analysis. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.663058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khoury N.N., Darsaut T.E., Ghostine J., et al. Endovascular thrombectomy and medical therapy versus medical therapy alone in acute stroke: a randomized care trial [published correction appears in J Neuroradiol. 2017 Sep;44(5):351] J. Neuroradiol. 2017;44(3):198–202. doi: 10.1016/j.neurad.2017.01.126. [DOI] [PubMed] [Google Scholar]
- 65.Poppe A.Y., Jacquin G., Stapf C., et al. A randomized pilot study of patients with tandem carotid lesions undergoing thrombectomy. J. Neuroradiol. 2020;47(6):416–420. doi: 10.1016/j.neurad.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Zhu F., Hossu G., Soudant M., et al. Effect of emergent carotid stenting during endovascular therapy for acute anterior circulation stroke patients with tandem occlusion: a multicenter, randomized, clinical trial (TITAN) protocol. Int. J. Stroke. 2021;16(3):342–348. doi: 10.1177/1747493020929948. [DOI] [PubMed] [Google Scholar]
- 67.Maus V., Borggrefe J., Behme D., et al. Order of treatment matters in ischemic stroke: mechanical thrombectomy first, then carotid artery stenting for tandem lesions of the anterior circulation. Cereb. Dis. 2018;46(1–2):59–65. doi: 10.1159/000492158. [DOI] [PubMed] [Google Scholar]
- 68.Yang D., Shi Z., Lin M., et al. Endovascular retrograde approach may be a better option for acute tandem occlusions stroke. Inter. Neuroradiol. 2019;25(2):194–201. doi: 10.1177/1591019918805140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wallocha M., Chapot R., Nordmeyer H., et al. Treatment methods and early neurologic improvement after endovascular treatment of tandem occlusions in acute ischemic stroke. Front Neurol. 2019;10:127. doi: 10.3389/fneur.2019.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neuberger U., Moteva K., Vollherbst D.F., et al. Tandem occlusions in acute ischemic stroke - impact of antithrombotic medication and complementary heparin on clinical outcome and stent patency. J. Neurointerv. Surg. 2020;12:1088–1093. doi: 10.1136/neurintsurg-2019-015596. [DOI] [PubMed] [Google Scholar]
- 71.Eker O.F., Bühlmann M., Dargazanli C., et al. Endovascular treatment of atherosclerotic tandem occlusions in anterior circulation stroke: technical aspects and complications compared to isolated intracranial occlusions. Front Neurol. 2018;9:1046. doi: 10.3389/fneur.2018.01046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hellegering J., Uyttenboogaart M., Bokkers R.P.H., et al. Treatment of the extracranial carotid artery in tandem lesions during endovascular treatment of acute ischemic stroke: a systematic review and meta-analysis. Ann. Transl. Med. 2020;8(19):1278. doi: 10.21037/atm-2020-cass-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lockau H., Liebig T., Henning T., et al. Mechanical thrombectomy in tandem occlusion: procedural considerations and clinical results. Neuroradiology. 2015;57(06):589–598. doi: 10.1007/s00234-014-1465-5. [DOI] [PubMed] [Google Scholar]
- 74.Puri A.S., Kühn A.L., Kwon H.J., et al. Endovascular treatment of tandem vascular occlusions in acute ischemic stroke. J. Neurointerv. Surg. 2015;7(3):158–163. doi: 10.1136/neurintsurg-2013-011010. [DOI] [PubMed] [Google Scholar]
- 75.Mpotsaris A., Kabbasch C., Borggrefe J., et al. Stenting of the cervical internal carotid artery in acute stroke management: the Karolinska experience. Inter. Neuroradiol. 2017;23(2):159–165. doi: 10.1177/1591019916681983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vu-Dang L., Nguyen Q.A., Nguyen-Thi-Thu T., et al. Endovascular treatment for acute tandem occlusion stroke: results from case series of 17 patients. Ann. Indian Acad. Neurol. 2020;23(1):78–83. doi: 10.4103/aian.AIAN_464_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park J.S., Lee J.M., Kwak H.S., et al. Endovascular treatment of acute carotid atherosclerotic tandem occlusions: predictors of clinical outcomes as technical aspects and location of tandem occlusions. J. Stroke Cereb. Dis. 2020;29(9) doi: 10.1016/j.jstrokecerebrovasdis.2020.105090. [DOI] [PubMed] [Google Scholar]
- 78.Cohen J.E., Gomori M., Rajz G., et al. Emergent stent-assisted angioplasty of extracranial internal carotid artery and intracranial stent-based thrombectomy in acute tandem occlusive disease: technical considerations. J. Neurointerv. Surg. 2013;5:440–446. doi: 10.1136/neurintsurg-2012-010340. [DOI] [PubMed] [Google Scholar]
- 79.Mbabuike N., Gassie K., Brown B., et al. Revascularization of tandem occlusions in acute ischemic stroke: review of the literature and illustrative case. Neurosurg. Focus. 2017;42 doi: 10.3171/2017.1.FOCUS16521. [DOI] [PubMed] [Google Scholar]
- 80.Gratz P.P., Schroth G., Gralla J., et al. Whole-brain susceptibility-weighted thrombus imaging in stroke: fragmented thrombi predict worse outcome. AJNR Am. J. Neuroradiol. 2015;36(07):1277–1282. doi: 10.3174/ajnr.A4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knappich C., Kuehnl A., Tsantilas P., et al. The Use of Embolic Protection Devices Is Associated With a Lower Stroke and Death Rate After Carotid Stenting. JACC Cardiovasc Inter. 2017;10(12):1257–1265. doi: 10.1016/j.jcin.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 82.Chen W.H., Yi T.Y., Wu Y.M., et al. Endovascular Therapy Strategy for Acute Embolic Tandem Occlusion: The Pass-Thrombectomy-Protective Thrombectomy (Double PT) Technique. World Neurosurg. 2018;120:e421–e427. doi: 10.1016/j.wneu.2018.08.096. [DOI] [PubMed] [Google Scholar]
- 83.Yi T.Y., Chen W.H., Wu Y.M., et al. Another endovascular therapy strategy for acute tandem occlusion: protect-expand-aspiration-revascularization-stent (PEARS) technique. World Neurosurg. 2018;113:e431–e438. doi: 10.1016/j.wneu.2018.02.052. [DOI] [PubMed] [Google Scholar]
- 84.Haussen D.C., Turjman F., Piotin M., et al. Head or neck first? Speed and rates of reperfusion in thrombectomy for tandem large vessel occlusion strokes. Inter. Neurol. 2020;8(2–6):92–100. doi: 10.1159/000496292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akpinar C.K., Gürkaş E., Aytac E. Carotid angioplasty-assisted mechanical thrombectomy without urgent stenting may be a better option in acute tandem occlusions. Inter. Neuroradiol. 2017;23(4):405–411. doi: 10.1177/1591019917701113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blassiau A., Gawlitza M., Manceau P.F., et al. Mechanical thrombectomy for tandem occlusions of the internal carotid artery-results of a conservative approach for the extracranial lesion. Front Neurol. 2018;9:928. doi: 10.3389/fneur.2018.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsivgoulis G., Katsanos A.H., Schellinger P.D., et al. Successful reperfusion with intravenous thrombolysis preceding mechanical thrombectomy in large-vessel occlusions. Stroke. 2018;49(1):232–235. doi: 10.1161/STROKEAHA.117.019261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kappelhof M., Marquering H.A., Berkhemer O.A., Majoie C.B. Intra-arterial treatment of patients with acute ischemic stroke and internal carotid artery occlusion: a literature review. J. Neurointerv. Surg. 2015;7(1):8–15. doi: 10.1136/neurintsurg-2013-011004. (Jan) [DOI] [PubMed] [Google Scholar]
- 89.Wang D, Zhang L, Hu X, et al. Intravenous Thrombolysis Benefits Mild Stroke Patients with Large-Artery Atherosclerosis but No Tandem Steno-Occlusion. Front Neurol. 2020 May 5;11:340. [DOI] [PMC free article] [PubMed]
- 90.Anadani M., Marnat G., Consoli A., et al. TITAN (Thrombectomy In TANdem lesions) Investigators; Endovascular Treatment in Ischemic Stroke (ETIS) Investigators Endovascular therapy with or without intravenous thrombolysis in acute stroke with tandem occlusion. J. Neurointerv. Surg. 2022;14(4):314–320. doi: 10.1136/neurintsurg-2020-017202. (Apr) [DOI] [PubMed] [Google Scholar]
- 91.Pikija S., Magdic J., Sztriha L.K., et al. Endovascular therapy for tandem occlusion in acute ischemic stroke: intravenous thrombolysis improves outcomes. J. Clin. Med. 2019;8(2):228. doi: 10.3390/jcm8020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moulakakis K.G., Mylonas S.N., Lazaris A., et al. Acute carotid stent thrombosis: a comprehensive review. Vasc. Endovascula Surg. 2016;50(7):511–521. doi: 10.1177/1538574416665986. [DOI] [PubMed] [Google Scholar]
- 93.Cirio J.J., Ciardi C., Lopez M., et al. Endovascular management of tandem occlusions in stroke: treatment strategies in a real-world scenario. J. Neurosci. Neurol. Disord. 2021;5:055–060. [Google Scholar]
- 94.Yaghi S., Eisenberger A., Willey J.Z. Symptomatic intracerebral hemorrhage in acute ischemic stroke after thrombolysis with intravenous recombinant tissue plasminogen activator: a review of natural history and treatment. JAMA Neurol. 2014;71(9):1181–1185. doi: 10.1001/jamaneurol.2014.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Diener H.C., Foerch C., Riess H., et al. Treatment of acute ischaemic stroke with thrombolysis or thrombectomy in patients receiving anti-thrombotic treatment. Lancet Neurol. 2013;12(7):677–688. doi: 10.1016/S1474-4422(13)70101-7. [DOI] [PubMed] [Google Scholar]
- 96.Cucchiara B., Kasner S.E., Tanne D., et al. SAINT Investigators. Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke: pooled analysis of placebo data from the stroke-acute ischemic NXY treatment (SAINT) I and SAINT II trials. Stroke. 2009;40(9):3067–3072. doi: 10.1161/STROKEAHA.109.554386. [DOI] [PubMed] [Google Scholar]
- 97.Heck D.V., Brown M.D. Carotid stenting and intracranial thrombectomy for treatment of acute stroke due to tandem occlusions with aggressive antiplatelet therapy may be associated with a high incidence of intracranial hemorrhage. J. Neurointerv. Surg. 2015;7:170–175. doi: 10.1136/neurintsurg-2014-011224. [DOI] [PubMed] [Google Scholar]
- 98.Diana F., Abdalkader M., Behme D., et al. APT-eCAS collaboration Antithrombotic regimen in emergent carotid stenting for acute ischemic stroke due to tandem occlusion: a meta-analysis of aggregate data. J. Neurointerv. Surg. 2023 doi: 10.1136/jnis-2023-020204. jnis-2023-020204. [DOI] [PubMed] [Google Scholar]
- 99.Rodrigues M., Cunha A., Figueiredo S., et al. Emergent carotid artery stenting in atherosclerotic disease of the internal carotid artery with tandem intracranial occlusion. J. Neurol. Sci. 2018;387:196–198. doi: 10.1016/j.jns.2018.02.034. [DOI] [PubMed] [Google Scholar]
- 100.Li W., Chen Z., Dai Z., et al. Management of acute tandem occlusions: stent-retriever thrombectomy with emergency stenting or angioplasty. J. Int. Med. Res. 2018;46(7):2578–2586. doi: 10.1177/0300060518765310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Derdeyn C.P., Chimowitz M.I., Lynn M.J., et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014;383(9914):333–341. doi: 10.1016/S0140-6736(13)62038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heck D.V., Brown M.D. Carotid stenting and intracranial thrombectomy for treatment of acute stroke due to tandem occlusions with aggressive antiplatelet therapy may be associated with a high incidence of intracranial hemorrhage. J. Neurointerv Surg. 2015;7(3):170–175. doi: 10.1136/neurintsurg-2014-011224. [DOI] [PubMed] [Google Scholar]
- 103.Chang Y., Kim B.M., Bang O.Y., et al. Rescue stenting for failed mechanical thrombectomy in acute ischemic stroke: a multicenter experience. Stroke. 2018;49(4):958–964. doi: 10.1161/STROKEAHA.117.020072. [DOI] [PubMed] [Google Scholar]
- 104.Jost A., Roels C., Brown M., Janjua R., Heck D. Low-dose eptifibatide for tandem occlusion in stroke: safety and carotid artery patency. AJNR Am. J. Neuroradiol. 2021;42(4):738–742. doi: 10.3174/ajnr.A6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Osteraas N.D., Crowley R.W., Panos N., Dafer R.M. Eptifibatide use following emergent carotid stenting in acute anterior circulation ischemic stroke with tandem occlusion. J. Stroke Cereb. Dis. 2020;29(9) doi: 10.1016/j.jstrokecerebrovasdis.2020.105021. [DOI] [PubMed] [Google Scholar]
- 106.Cervo A., Ferrari F., Barchetti G., et al. Use of cangrelor in cervical and intracranial stenting for the treatment of acute ischemic stroke: a "real life" single-center experience. AJNR Am. J. Neuroradiol. 2020;41(11):2094–2099. doi: 10.3174/ajnr.A6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Linfante I., Ravipati K., Starosciak A.K., Reyes D., Dabus G. Intravenous cangrelor and oral ticagrelor as an alternative to clopidogrel in acute intervention. J. Neurointerv. Surg. 2021;13(1):30–32. doi: 10.1136/neurintsurg-2020-015841. [DOI] [PubMed] [Google Scholar]
- 108.Marcaccio C.L., Patel P.B., Liang P., et al. Efficacy and safety of perioperative dual antiplatelet therapy with ticagrelor versus clopidogrel in carotid artery stenting. J. Vasc. Surg. 2022;75(4):1293–1303. doi: 10.1016/j.jvs.2021.09.045. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu F., Piotin M., Steglich-Arnholm H., Labreuche J., et al. TITAN (thrombectomy in tandem lesions) investigators. Periprocedural heparin during endovascular treatment of tandem lesions in patients with acute ischemic stroke: a propensity score analysis from TITAN registry. Cardiovasc Interv. Radio. 2019;42(8):1160–1167. doi: 10.1007/s00270-019-02251-4. [DOI] [PubMed] [Google Scholar]
- 110.Winningham M.J., Haussen D.C., Nogueira R.G., et al. Periprocedural heparin use in acute ischemic stroke endovascular therapy: the TREVO 2 trial. J. Neurointerv. Surg. 2018;10(07):611–614. doi: 10.1136/neurintsurg-2017-013441. [DOI] [PubMed] [Google Scholar]
- 111.Chalos V., van de Graaf R.A., Roozenbeek B., et al. MR CLEAN-MED investigators Multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke. The effect of periprocedural medication: acetylsalicylic acid, unfractionated heparin, both, or neither (MR CLEAN-MED). Rationale and study design. Trials. 2020;21(1):644. doi: 10.1186/s13063-020-04514-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McKevitt F.M., Randall M.S., Cleveland T.J., Gaines P.A., Tan K.T., Venables G.S. The benefits of combined anti-platelet treatment in carotid artery stenting. Eur. J. Vasc. Endovasc. Surg. 2005;29(5):522–527. doi: 10.1016/j.ejvs.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 113.Dalainas I., Nano G., Bianchi P., et al. Dual antiplatelet regime versus acetyl-acetic acid for carotid artery stenting. Cardiovasc Interv. Radio. 2006;29:519–521. doi: 10.1007/s00270-005-5288-y. [DOI] [PubMed] [Google Scholar]
- 114.Labeyrie M.A., Ducroux C., Civelli V., et al. Endovascular management of extracranial occlusions at the hyperacute phase of stroke with tandem occlusions. J. Neuroradiol. 2018;45(3):196–201. doi: 10.1016/j.neurad.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 115.Slawski D.E., Jumaa M.A., Salahuddin H., et al. Emergent carotid endarterectomy versus stenting in acute stroke patients with tandem occlusion. J. Vasc. Surg. 2018;68(4):1047–1053. doi: 10.1016/j.jvs.2017.12.077. [DOI] [PubMed] [Google Scholar]