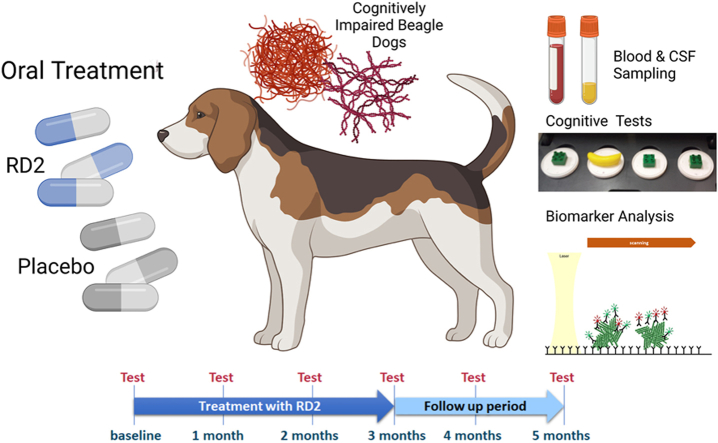
Abstract
Disease-modifying therapies to treat Alzheimer's disease (AD) are of fundamental interest for aging humans, societies, and health care systems. Predictable disease progression in transgenic AD models favors preclinical studies employing a preventive study design with an early pre-symptomatic treatment start, instead of assessing a truly curative approach with treatment starting after diagnosed disease onset. The aim of this study was to investigate the pharmacokinetic profile and efficacy of RD2 to enhance short-term memory and cognition in cognitively impaired aged Beagle dogs - a non-transgenic model of truly sporadic AD. RD2 has previously demonstrated pharmacodynamic efficacy in three different transgenic AD mouse models in three different laboratories. Here, we demonstrate that oral treatment with RD2 significantly reduced cognitive deficits in cognitively impaired aged Beagle dogs even beyond the treatment end, which suggests in combination with the treatment dependent CSF tau oligomer decrease a disease-modifying effect of RD2 treatment.
Keywords: Alzheimer's disease, Aβ oligomers, Tau oligomers, Cognitively impaired aged Beagle dogs, Sporadic AD animal model, Disease-modifying therapy, Pharmacokinetics, Pharmacodynamics
Graphical abstract
1. Introduction
Alzheimer's disease is the most common form of dementia and accounting for 60–70% of the more than 44 million people with dementia worldwide [1]. Cognitively impaired aged Beagle dogs are one of the very few non-transgenic AD animal models that may simulate the situation of spontaneous late onset AD, i.e. sporadic AD, which is the most common form of AD in humans. Pathophysiologically, the decline in cognitive proficiency, including memory and learning deficits, is attributed to structural and functional changes in the aging canine brain that reflects AD-like neurodegeneration [2]. Affected dogs exhibit, similar to human AD patients, progressive accumulation of amyloid beta (Aβ) deposits that manifest as cortical diffuse plaques and cerebral amyloid angiopathy (CAA) [2]. Additionally, reduced neurogenesis [3], increased neuronal death [4] and cerebral atrophy [5] have been described. In contrast to human AD patients, development of neurofibrillary tangle pathology has not been described in Beagle dogs; however, they exhibit hyperphosphorylated tau that possibly represents pre-tangle pathology [6].
Age-related cognitive impairment of the dogs parallels the cognitive symptomology of AD. The earliest deficits are most evident in performance on tasks involving working (short-term) memory and complex learning [7]. Affected dogs additionally develop deficits in discrimination learning as well as behavioral changes [8]. In conclusion, these characteristics suggest that aged Beagle dogs are an appropriate AD model for testing disease-modifying effects of new drug candidates for the treatment of AD.
The novel drug candidate RD2 is currently under clinical development for the treatment of AD [9]. RD2 is an all d-enantiomeric peptide, which was designed to directly destabilize, disassemble and ultimately eliminate toxic Aβ oligomers via direct disruption into native Aβ monomers, rather than by relying on the immune system for their degradation. This mechanism of action (MoA) was confirmed by successfully demonstrating target engagement in vitro, ex vivo and in vivo [[10], [11], [12], [13]]. RD2 has also proven pharmacodynamic activity in three different transgenic mouse models in independent studies conducted in three different laboratories. Treatment with orally applied RD2 enhanced the cognitive performance in two different AD mouse models (APPswe/PS1ΔE9 and APPSL) [10,11,14]. Efficacy was even demonstrated in aged AD mice with fully developed AD-associated pathology at the beginning of treatment resulting in cognition and behavior, which was indistinguishable from that of healthy wild type littermates at the end of treatment [10]. Furthermore, oral treatment with RD2 led to significant deceleration in the development of the motor neurodegenerative phenotype in transgenic TBA2.1 mice expressing human pyroglutamate-Aβ [15]. The aim of the current study was to investigate the pharmacokinetic profile and the efficacy of RD2 on cognition and biomarkers in a non-transgenic AD animal model - aged cognitively impaired Beagle dogs - that is not based on mutations of human early onset familial AD cases and thus is much closer to the situation of sporadic AD in humans. In addition, the cognitive tests were continued beyond the treatment end to be able to discriminate between acute and disease modifying effects.
2. Results
Two separate studies have been carried out. One study was designed as a pharmacokinetic (PK) study to obtain time dependent RD2 levels in blood and cerebrospinal fluid (CSF). The other study was designed as a placebo-controlled interventional study with a low and high once daily dose of RD2 to investigate pharmacodynamic parameters in aged dogs with three months of treatment and two more months after treatment end, “follow up phase”. In both studies study dogs were returned to the colony after completion of the study. Sample collection is therefore limited to blood and CSF samples.
2.1. Tolerability of treatment with RD2 and its pharmacokinetics
In the PK study, single intravenous (i.v.) doses of 3 mg/kg and oral (p.o.) doses of 20 mg/kg or 50 mg/kg of RD2 to each of six dogs were overall well tolerated. Intravenous administration of 3 mg/kg of RD2 resulted in hypersalivation in all dogs and trembling in a subset of dogs. After the 20 mg/kg oral dose, one dog demonstrated fasciculation of neck muscles. The events resolved without intervention. No remarkable findings were noted at the 50 mg/kg oral dose level.
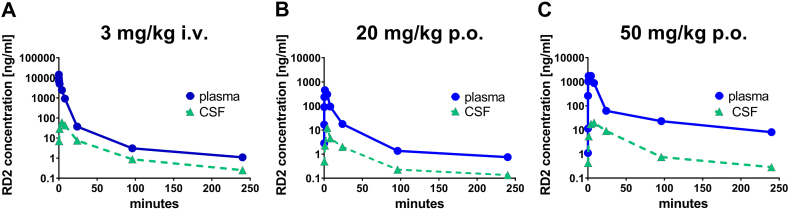
The PK study yielded time-dependent plasma and CSF levels of RD2 after single i.v. and p.o. dosing (Fig. 1A–C).
Fig. 1.
Concentration-time profiles of RD2 in plasma and CSF following single (A) 3 mg/kg i.v., (B) 20 mg/kg p.o. and (C) 50 mg/kg p.o. administration of RD2 to young dogs (1.7–1.8 years of age). Data are presented as mean; n = 6.
Following intravenous injection of 3 mg RD2 acetate/kg, a total clearance of 1.16 ml/min/kg and an apparent volume of distribution at steady-state of 0.66 l/kg was calculated from the mean AUC (area under the curve) of 28600 ng*h/ml. Plasma levels decreased rapidly from a Cmax (0.05 h post administration) of 32000 ng/ml to 37 ng/ml. Disposition was in three phases for which half-lives of 0.05, 2.9, and 71 h were estimated. 93% of total AUC was covered by the half-life of 2.9 h, whereas the long terminal phase only covered 3.4% of the total AUC. Following the oral administration of 20 mg RD2 acetate/kg (50 mg RD2 acetate/kg, respectively) maximum plasma levels of 567 ± 427 ng/ml (2380 ± 2280 ng/ml) were obtained at Tmax of 2.0 ± 1.6 h (2.7 ± 2.9 h). The AUC was calculated to 3340 ± 1370 ng*h/ml (19800 ± 19700 ng*h/ml) leading to an MRT of 40.0 ± 15 h (35 ± 10 h) and an absolute oral bioavailability of 2.0 ± 0.78% (5.5 ± 4.5%). The half-life of the disposition phase β was estimated from the mean plasma level to be 0.56 h (2.9 h). More than 75% of the total AUC is eliminated with these half-lives of 3 h or shorter and less than 25% of the dose is eliminated with the much longer half-life of the disposition phase γ of 62 h (77 h). There was a more than dose-proportional increase in peak concentrations and AUC from 20 mg/kg to 50 mg/kg oral dosing. The dogs dosed with 50 mg/kg exhibited 2.65 times higher oral bioavailability compared with the mean value of the dogs exposed to 20 mg/kg.
The obtained PK parameters are shown in Table 1 for plasma and in Table 2 for CSF.
Table 1.
Pharmacokinetic parameters for RD2 in plasma after single administration of RD2.
| Parameter | 3 mg/kg (i.v.) Mean ± SD |
20 mg/kg (p.o.) Mean ± SD |
50 mg/kg (p.o.) Mean ± SD |
|---|---|---|---|
| tmax (h) | 2.0 ± 1.6 | 2.7 ± 2.9 | |
| Cmax (ng/ml) | 32000 ± 18200 | 567 ± 427 | 2380 ± 2280 |
| t1/2 α (h) | 0.046 | n.c. | n.c. |
| 1% of AUC | 3.6 | n.c. | n.c. |
| t1/2 β (h) | 2.9 | 0.56 | 2.9 |
| 2% of AUC | 93 | 78 | 76 |
| t1/2 γ (h) | 71 | 62 | 77 |
| a% of AUC | 3.4 | 22 | 25 |
| AUC0-tlast (h·ng/ml) | 29900 ± 4230 | 3340 ± 1370 | 19800 ± 19700 |
| AUC0-inf (h·ng/ml) | 28600 ± 2540 | 3890 ± 1120 | 26000 ± 21900 |
| CLtotal (ml/min·kg) | 1.2 ± 0.11 | ||
| MRT (h) | 9.4 ± 1.0 | 40 ± 15 | 35 ± 10 |
| Vss (ml/kg) | 659 ± 140 | ||
| F (%) | 100 | 2.0 ± 0.78 | 5.5 ± 4.5 |
n.c. denotes not calculable, 1% of AUC covered by t1/2 α,2% of AUC covered by t1/2 β.
% of AUC covered by t1/2 γ.
Table 2.
Pharmacokinetic parameters for RD2 in CSF after single administration.
| Parameter | 3 mg/kg (i.v.) Mean ± SD |
20 mg/kg (p.o.) Mean ± SD |
50 mg/kg (p.o.) Mean ± SD |
|---|---|---|---|
| tmax (h) | 4.2 ± 2.2 | 8.0 ± 8.0 | 8.7 ± 7.8 |
| Cmax (ng/ml) | 71 ± 32 | 13 ± 15 | 21 ± 18 |
| Apparent t1/2 (h)a | 80 ± 5.6 | 190 ± 93 | 119 ± 37 |
| AUC0-tlast (h·ng/ml) | 961 ± 373 | 180 ± 77 | 614 ± 592 |
| AUC0-inf (h·ng/ml) | 990 ± 383 | 242 ± 47 | 659 ± 605 |
| MRT0-inf (h) | 39 ± 4.0 | 155 ± 131 | 97 ± 47 |
| CSF/Plasma AUC0-inf | 0.031 ± 0.011 | 0.066 ± 0.021 | 0.025 ± 0.020 |
half-life is estimated from the last 2 time points and thus may not be accurate.
The results of the pharmacokinetic evaluation (non-compartment analysis) for RD2 in CSF are given in Table 2. The mean peak CSF level of RD2 in animals treated intravenously with 3 mg RD2/kg was 71 ng/ml 0.05 h post administration. Mean AUC0-tlast value was 961 h ng/ml and mean residence time 39 h. The mean peak CSF level of RD2 in animals treated orally with 20 mg/kg was 13 ng/ml at 8.0 h post administration and with 50 mg/kg 21 ng/ml at 8.7 h post administration. Mean AUC0-tlast value were 180 h ng/ml for the 20 mg/kg treated animals and 614 h ng/ml for the 50 mg/kg treated animals. An AUC based CSF/plasma ratio of 0.031 (3 mg/kg iv) to 0.066 (20 mg/kg po) shows that RD2 is able to penetrate the brain.
Most importantly for the following treatment study is that the oral bioavailability of RD2 was between 2.0 and 5.5%. Elimination upon oral application followed a fast phase with a half-live between 0.6 and 2.9 h and a slow phase, which accounts for about 25% of the AUC, with a half-live of about 70 h. RD2 crossed the blood-brain-barrier with a ratio of AUCs in CSF and blood between 2.5 and 6.6%. High inter-individual differences in RD2 levels in the dogs of the PK study have been observed.
During the 3-month treatment phase of the interventional study (Fig. 3A), on day 80, one dog had to be euthanized. Unblinding revealed that this dog was in the placebo cohort. On day 123 (30 days after treatment end), another dog needed to be euthanized. Unblinding revealed that the dog was in the low dose (3 mg/kg/day) cohort. The causes of morbidity (forelimb paralysis and progressing vestibular disease, respectively) were considered consequences of underlying age-related background diseases in these old dogs. All other dogs survived the study and were returned to the colony at study conclusion.
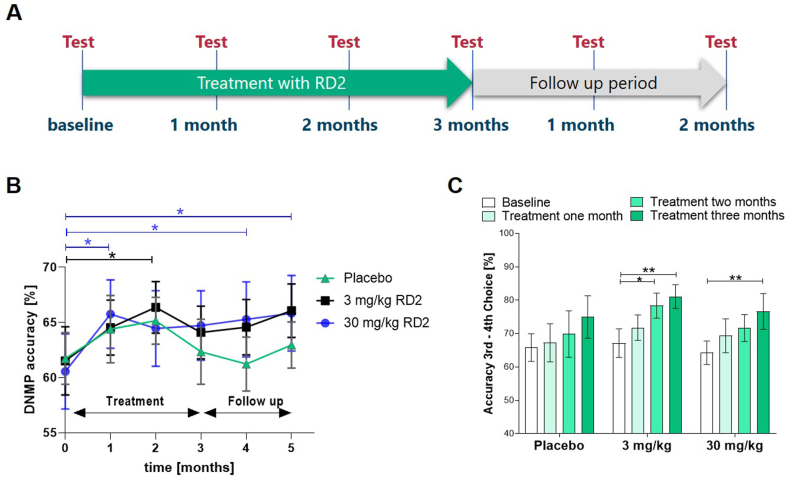
Fig. 3.
Study design and cognitive testing outcomes. A: Treatment and cognitive testing time scheme of the interventional study. B: Percent mean performance accuracy in the DNMP assay combined at 20 s and 90 s delay in placebo versus low (3 mg/kg daily) and high oral RD2 dosed (30 mg/kg daily) dogs. Two-way RM-ANOVA (followed by Fisher post hoc test) revealed significantly enhanced performance (p < 0.05) after one month of treatment compared to baseline in the high dose cohort and after two months treatment in the low dose cohort. The significant treatment effect (*p < 0.05) in the high dose group was maintained two months upon treatment end. Data is presented as mean ± SEM; n = 11 to 12. C: Percent mean performance accuracy on the selective attention - variable oddity performance combined across the 2 and 3 distractor conditions in placebo vs. low dose and high dose dogs treated with RD2. A two-way RM ANOVA (followed by Fisher post hoc test) revealed a significantly enhanced cognitive performance after three months of treatment of both treatment groups (**p < 0.01), and 3 mg/kg after two months of treatment (*p < 0.05). Data is presented as mean ± SEM; n = 11 to 12.
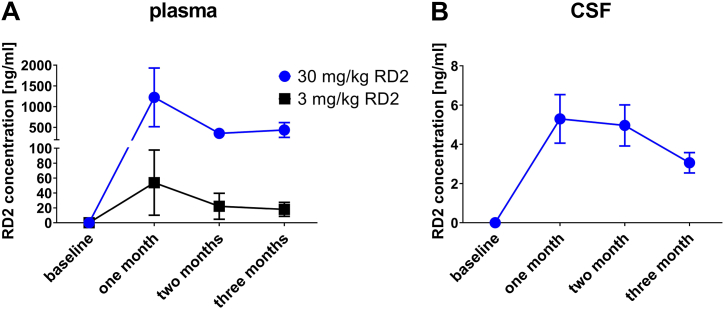
In the therapeutic study (Fig. 3A) blood samples were collected before and after three months of oral treatment for hematological (complete blood count) and clinical chemistry analyses including liver function tests (Tables S1, S2 and S3). In addition, RD2 levels in plasma (Fig. 2A) and CSF (Fig. 2B) were determined before and after one, two and three months of treatment.
Fig. 2.
Concentration-time profiles of RD2 in (A) plasma and (B) CSF following daily oral administration of 3 mg/kg or 30 mg/kg of RD2 for three months. CSF-values of 3 mg/kg treated animals were below the lower limit of quantification (LLOQ) of 0.5 ng/mg and were therefore excluded from the graph. Data is presented as mean±SEM; n = 11 to 12.
Increases of triglyceride and CK-MB concentrations after 3 mg/kg and 30 mg/kg RD2 p.o. compared to in-group baseline values, although statistically significant, were minor and comparable with the changes in the placebo group (Figs. S1 and S2). Another remarkable finding was the presence of Döhle bodies in neutrophils of three out of 12 dogs after three months treatment with 30 mg/kg (once daily). The occurrence of Döhle bodies is often interpreted as a marker for inflammatory processes in dogs [16]. In the absence of changes in neutrophil, total white blood cell and platelet counts, this occurrence was most probably not of toxicological relevance.
2.2. Efficacy parameters
The treatment study was designed to investigate RD2′s efficacy on cognitive outcome measures as the primary endpoint, as cognitive impairment is the predominant and most relevant symptom in human AD patients.
2.2.1. RD2 significantly decreased cognitive deficits compared to baseline even beyond the end of treatment
Dogs were tested on a variable-delay paradigm of the DNMP (delayed non-matching to position) test using delays of 20 s and 90 s. The task was performed in 5-day intervals to assess spatial working memory. Testing occurred during baseline and subsequently once a month - at three time points during the treatment phase and at two time points after treatment end (Fig. 3A). The mean performance accuracy across delays (20 s and 90 s combined) was analyzed using two-way repeated-measure ANOVA with testing time-point serving as a within-subject measure (Fig. 3B). The dogs in all three cohorts exhibited a practicing effect due to the repeated performance of the test. While this seemed to be a small tendency in the placebo group, dogs in the treatment cohorts showed a significant cognitive enhancement relative to their baseline level, which even extended beyond the end of the treatment period in the high dose group. Significantly enhanced cognitive performance was reached in dogs treated for only one month with high doses (30 mg/kg) of RD2, leading to RD2 levels of 5 ng/ml on average in CSF (Fig. 2B), or two months with low doses (3 mg/kg) of RD2. The results obtained in the DNMP test suggested a benefit of RD2 on working memory. After the follow up period of one and two months after treatment end, dogs previously treated with 30 mg/kg daily maintained significant increase in performance in DNMP accuracy compared to baseline.
As second cognitive task, variable discrimination testing with variable oddity was performed in order to assess selective attention performance of the dogs during treatment. As impairment in tasks involving complex learning develops earlier than in simple discrimination learning [8,[17], [18], [19]], the conditions with none or one distractor were excluded from analysis. RD2 enhanced learning on the more difficult conditions (two or three distractors) of the variable discrimination task in the low dose group at two months of treatment and in the high dose group at three months of treatment compared to baseline, but had no effect on the easier conditions. Percent accuracy was analyzed using a two-way repeated-measure ANOVA with test time-points serving as within subject measures. No significant learning effect of placebo treated Beagle dogs was found (Fig. 3C).
2.2.2. Analysis of neuropathological biomarkers in CSF and plasma
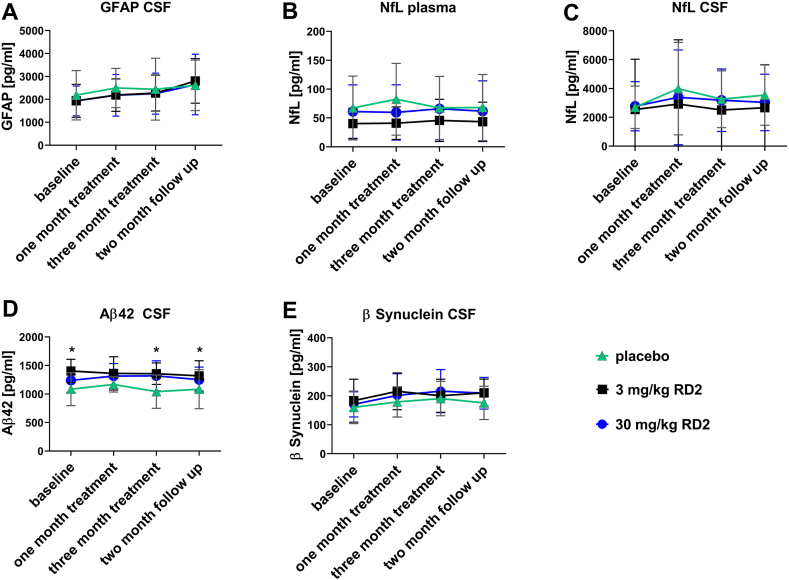
Neuropathological biomarkers were longitudinally assessed by analysis of Aβ42, total tau, GFAP, NfL and β-synuclein in CSF samples and for NfL in plasma (Fig. 4). Significant differences between the treatment groups were found only in total Aβ42 concentrations. Significantly lower concentrations were observed in placebo compared to 3 mg/kg RD2 treated animals at baseline, after 3 months of treatment and two months follow up and to 30 mg/kg RD2 treated animals after 3 months of treatment.
Fig. 4.
CSF and blood based biomarkers. (A) Concentrations of GFAP in CSF, (B) NfL in plasma, (C) NfL in CSF (D) Aβ42 in CSF, and (E) β-synuclein concentrations in CSF of placebo, 3 mg/kg and 30 mg/kg treated animals with RD2 were longitudinally determined. Statistical calculations were conducted using a two-way RM ANOVA (followed by Fisher post hoc test). A significant difference between placebo and 3 mg/kg RD2 treated animals (marked by *) was shown for Aβ42 levels at baseline (p = 0.004), after 3 months of treatment (p = 0.004) and two months after treatment end (p = 0.031) and between placebo and 30 mg/kg RD2 treated after 3 months of treatment (p = 0.009). Data is presented as mean ± SEM; n = 11 to 12; *p < 0.05; **p < 0.01.
2.2.3. Analysis of Aβ oligomers and tau oligomers in CSF
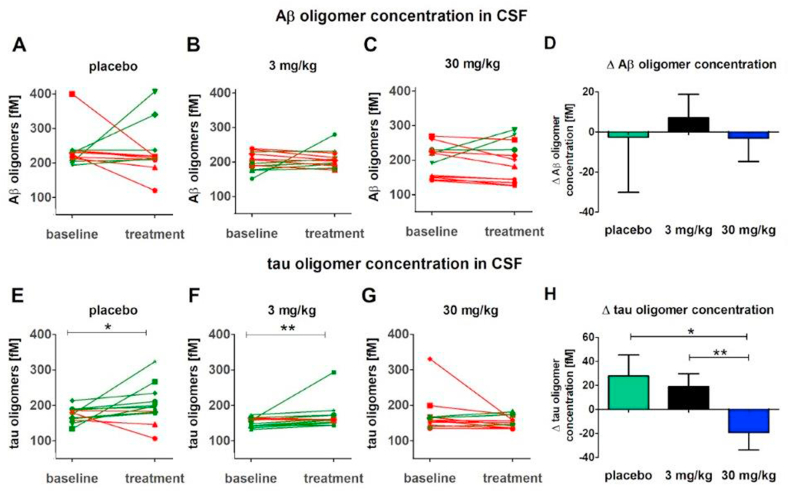
Aβ oligomer and tau oligomer levels were assessed in CSF before and after three-month treatment by means of the oligomer-specific sFIDA assay [13,[20], [21], [22], [23], [24]]. In the surface-based fluorescence intensity distribution analysis (sFIDA) assay, Aβ and tau oligomers were captured on a glass surface by a monoclonal anti‐Aβ antibody directed against the N-terminus of Aβ and a monoclonal anti-tau antibody directed against the proline rich region P2. After immobilization, the captured oligomers were detected by the same antibodies, which were fluorescence‐labeled. Therefore, fluorescence‐labelling of capture‐bound Aβ or tau monomers was excluded, thus rendering the assay insensitive for monomeric Aβ and tau. The assay surface was imaged by a multi-color total internal reflection fluorescence (TIRF) microscope. The number of pixels above a given background cutoff in each fluorescence channel directly correlates with the concentration of Aβ oligomers (Fig. 5A–C) or tau oligomers (Fig. 5D–F) in the sample.
Fig. 5.
Aβ oligomer and tau oligomer concentrations in CSF. Aβ oligomer (A–C) and tau oligomer concentrations (E–G) in CSF of placebo, 3 mg/kg and 30 mg/kg treated animals with RD2 were determined with sFIDA assay. A green connection line indicates a positive change and a red line indicates a negative change. Pairwise comparison with one-sided Wilcoxon signed-rank test showed no significant difference between baseline and three-month treatment in Aβ oligomer concentrations in any of the treatment groups (A–C), and in tau oligomer concentration in the 30 mg/kg RD2 group (G). In placebo (E) and 3 mg/kg RD2 (F) treated animals the tau oligomer concentration is significantly increased after three-month treatment compared to baseline. Changes (Δ) of Aβ oligomer (D) and tau oligomer (H) levels were calculated by subtracting baseline values from three-month treatment values. A negative change indicates a reduction of oligomer concentration, and a positive change indicates an increasing of oligomer concentration after treatment. A Mann-Whitney U test revealed a significant difference in Δ tau (H) between the 30 mg/kg RD2 group and both other treatment groups. *p < 0.05, **p < 0.01. Data is presented as mean ± SEM; n = 12. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
No significant changes in Aβ oligomer concentrations were found neither between baseline and the three-month treatment time point in the treatment groups (Fig. 5A–C) nor between the treatment groups (Fig. 5D). However, the treatment with 30 mg/kg RD2 led to a stagnation, if not a reduction, of the tau oligomer concentrations (p = 0.073) (Fig. 5G), while tau oligomer concentrations in 3 mg/kg RD2 (Fig. 5F) and placebo treated groups (Fig. 5E) increased significantly during the three months period (3 mg/kg p = 0.0043; placebo p = 0.027). In addition, the comparison between the treatment groups showed significant differences between 30 mg/kg RD2 and both other treatment groups (Fig. 5H, p = 0.016 for placebo vs. 30 mg/kg RD2, p = 0.0051 for 3 mg/kg vs. 30 mg/kg).
2.2.4. Correlation of RD2 plasma levels with CSF Aβ oligomer and tau oligomer concentrations
Because the treatment study was designed to investigate RD2′s efficacy on cognitive outcome measures as the primary endpoint, recruitment of the 36 dogs for the study was based on age and their cognitive status measured in three baseline cognitive tests (discrimination learning, delayed non-matching to position (DNMP) and attention as described in the methods). It was not based on typical human AD biomarker concentrations in CSF, like Aβ42 and tau, or brain imaging data. Thus, it must be expected that recruitment included not only dogs into the study that were cognitively impaired solely due to AD-like pathology, but also due to a reasonable fraction of other age- or non-age-related diseases or other constraints. To take this important background information into account, correlation analyses were carried out, which are able to reveal dose-response relationships based on individual drug plasma and exposure levels of the dogs.
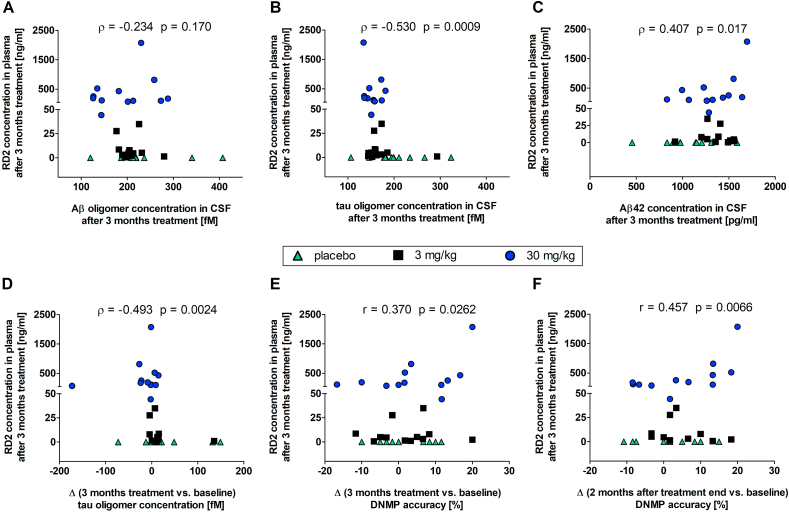
We examined the correlations of RD2 concentrations in plasma with Aβ oligomer (Fig. 6A) and tau oligomer (Fig. 6B) concentrations in CSF after three months of treatment by performing Spearman correlation analyses. Whereas both analyses yielded inverse correlations as expected, the RD2 plasma level correlation with Aβ oligomer concentrations did not become significant (Fig. 6A, Spearman −0.234, p-value 0.170). The correlation of RD2 plasma levels with tau oligomer concentrations after three months of treatment, however, was strong and significant (Fig. 6B, Spearman −0.530, p-value 0.00090). Thus, as expected from a successful treatment, also the correlation between RD2 plasma levels and the changes of tau oligomer concentrations (three months treatment vs. baseline) was inverse, strong and significant (Fig. 6D, Spearman −0.493, p-value 0.0024).
Fig. 6.
Dose-response relationships. Correlations of RD2 concentration in plasma with Aβ oligomer, tau oligomer and Aβ42 concentration and with DNMP accuracy. A–C: Correlations of RD2 concentration in plasma with Aβ oligomer (A), tau oligomer (B) and Aβ42 (C) concentrations in CSF after 3 months treatment. D: Correlation of RD2 concentration in plasma with changes of tau oligomer concentration. E, F: Correlation of RD2 concentration in plasma with treatment-dependent changes of DNMP accuracy. Changes (Δ) were calculated by subtracting baseline value from three-month treatment value (D, E) or from two-month after treatment end value (F). Correlations of RD2 in plasma with all parameters except Aβ oligomer concentration were statistically significant. Correlation was performed with Pearson (r) and Spearman (ρ) analysis at alpha level 0.05 with n = 11 to 12.
In humans, the most reliable biomarker for AD is the longitudinal decrease of total Aβ42 in CSF. In the here described treatment study, we observed a significant difference between the 30 mg/kg dose group and the placebo group after 3 months treatment, which was not significant at baseline (Fig. 4D). Because groups have not been randomized for Aβ42 levels in CSF, and the 3 mg/kg dose group had Aβ42 CSF levels significantly higher than placebo already at baseline, the overall significant and positive correlation between Aβ42 CSF levels and RD2 plasma levels may be interesting (Fig. 6C, Spearman 0.407, p-value 0.017).
Most remarkably, treatment-dependent changes of DNMP accuracy were found at three months of treatment as shown by the positive and significant correlation of RD2 plasma levels with the changes of DNMP accuracy at three months treatment versus baseline (Fig. 6E, Pearson 0.37, p-value 0.0262). This positive correlation did not disappear two months after treatment end, but became even stronger and more significant (Fig. 6F, Pearson 0.457, p-value 0.0066) suggesting a disease modification.
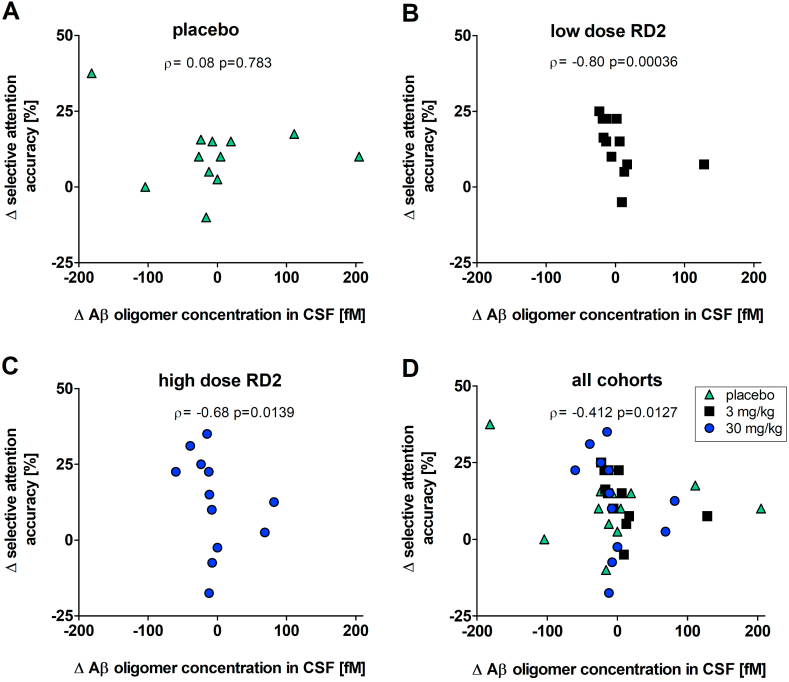
2.2.5. Correlation of CSF Aβ oligomer concentrations and selective attention
We examined the correlation of changes (after three months treatment versus baseline) of CSF Aβ oligomer concentration with changes of selective attention (Fig. 7). Changes of CSF Aβ oligomer concentrations inversely correlated with changes of selective attention for all animals (Fig. 7D; Spearman −0.412, p-value 0.0127). Separate analyses yielded significant and negative correlations between the individual changes in absolute Aβ oligomer concentrations and individual changes in selective attention for both RD2 treated cohorts (Fig. 7 B, C), but not for the placebo cohort (Fig. 7A). Thus, the more RD2-dependent reduction or stagnation of Aβ oligomer concentrations, the more the dogs did improve in the cognitive tests. The absence of this correlation in the placebo group may indicate that cognitive deficits indeed are not solely due to Aβ pathology, but some dogs had cognition deficits due to other reasons. Treatment effects with RD2, however, may drive cognition enhancements strong enough in the responsive fraction of dogs in the treated cohorts in order to outweigh the effects of non-responders. Such an effect is, of course, not present in the placebo group.
Fig. 7.
RD2 treatment dependent Aβ oligomer concentration change and its correlation with selective attention outcome. Correlation of changes of CSF Aβ oligomer concentrations with changes of selective attention accuracy. A: Correlation for placebo group. B: Correlation for low dose treated animals with 3 mg/ml RD2. C: Correlation for high dose treated animals with 30 mg/ml RD2. D: Correlation for all groups (placebo, low dose and high dose) together. Changes (Δ) were calculated by subtracting baseline value from three-month treatment value. Correlations of the low dose group, high dose group and all cohorts were significant. No significant correlation was found for the placebo group. Correlation was performed with Spearman (ρ) analysis at alpha level 0.05 with n = 11 to 12.
2.2.6. Correlation between CSF Aβ oligomer and tau oligomer concentrations
We found a significant and positive correlation between Aβ oligomer and tau oligomer concentrations, at baseline (Fig. 7A, Spearman 0.505, p-value 0.0018), after three months of treatment with RD2 or placebo (Fig. 7B, Spearman 0.306, p-value 0.029), and for the individual differences between both time points (Fig. 7C, Spearman 0.371, p-value 0.026). This indicates a robust correlation between Aβ oligomer and tau oligomer concentrations in CSF. To our knowledge, this is the first report of such a correlation in dogs.
3. Discussion
Among the numerous transgenic AD animal models, none is able to exert the full spectrum of human AD pathology together with typical cognition deficits observed in human AD patients. Our interventional study in cognitively impaired aged Beagle dogs is thus an unprecedented effort to show efficacy of a drug candidate, which was developed for disease modification and for beneficial effects on AD-relevant cognitive impairment, in a non-transgenic animal model of AD.
The pharmacokinetic study yielded an oral bioavailability of RD2 in blood between 2.0 and 5.5%. Elimination upon oral application was observed in two phases with a terminal half-live of about 70 h, very similar as was found for humans [9]. RD2 did cross the blood-brain-barrier with CSF-blood ratios between 2.5 and 6.6% indicating that the compound reaches its target organ, the brain. Orally administered RD2, at single doses of 20 mg/kg or 50 mg/kg in the PK study and at daily doses of 3 mg/kg and 30 mg/kg over three months in the interventional study, was well tolerated by the dogs. Also, the single intravenous dose of 3 mg/kg did not result in signs of intolerability. Laboratory investigations (hematology and blood clinical chemistry) as part of the 3-month efficacy study revealed no signs of severe adverse effects.
The interventional study was designed to investigate RD2′s efficacy on cognitive outcome measures as the primary endpoint, since cognitive decline is the major symptom in human AD patients. Recruitment of the study dogs was, therefore, based on age and the extent of cognitive deficits and not based on CSF biomarkers, like Aβ42 and tau, or brain imaging data. As a consequence, it must be expected that a reasonable fraction of dogs had their cognitive impairments due to other age- or non-age-related causes and not solely due to AD-like pathology. This is important to keep in mind, when analyzing the outcome the study. One way to deal with the unknown ratios of animals with or without AD-like pathology in the respective treatment cohorts would be to analyze only “responders” to the study drug for efficacy. For reasons of transparency, however, and because a responder analysis is not possible in the placebo group, we additionally carried out correlation analyses between several measures in order to account for potential non-responders within the treatment cohorts. Such correlation analyses can be used to explore expected dose-response relationships, because they consider the high inter-individual differences in drug exposure at a given oral dose. Thus, especially the correlations of any outcome measure with RD2 plasma levels can be expected to provide valuable results on dose-response relationships.
For assessment of deficits in working memory before and during the study, a delayed non-matching-to-position (DNMP) task was performed. To assess complex learning the variable discrimination learning task was conducted. Small positive practicing effects on the DNMP outcome during the first two months were observed in all groups but did not become significant in the placebo group. Significant increase of DNMP accuracy compared to baseline was observed in the high dose (30 mg/kg once daily) and the low dose (3 mg/kg once daily) group after one or two months, respectively. The significant increase in DNMP performance in the RD2 dosed groups suggests beneficial effects of RD2 on working memory. The effect was still present one- and two-months after treatment end in dogs treated with high dose RD2. In dogs treated with the low dose a prolonged impact on cognitive performance beyond treatment stop was also visible, but did not reach statistical significance (p = 0.06). This suggests a dose-dependent and disease-modifying effect on neuroprotection from Aβ oligomer induced toxicity (Fig. 3B). This is further supported by the positive and significant correlation of DNMP difference (three months treatment values versus baseline values) with RD2 plasma levels at month three with respect to a dose-response relationship (Fig. 6E).
RD2 treatment enhanced learning compared to baseline in the variable discrimination task in the low and the high dose group from two and three months of treatment onward, respectively, which implies a benefit of treatment on complex learning (Fig. 3C). Because reversal learning was assessed after the three months test, which required subjects to respond to their non-preferred object, the variable discrimination task was not useable to evaluate changes in selective attention during the follow up period. We found a significant and positive correlation between the results of the DNMP and the selective attention tests in the dogs of the placebo cohort at all time points available (Fig. S3F). This indicates that both tests are robustly measuring memory and cognition deficits in dogs.
Longitudinal monitoring of neuropathological biomarkers in the different treatment groups were assessed by ELISA analysis of CSF and blood samples (Fig. 4). None of the biomarkers was significantly different between the groups, except Aβ42 levels, which were different between groups at some time points, including baseline, reflecting the high inter-individual variability of the dogs.
RD2′s mode of action (MoA) is to stabilize Aβ monomers in their native intrinsically disordered protein (IDP)-like conformation, thereby destabilizing Aβ oligomers to ultimately disassemble them into Aβ monomers. This MoA was confirmed by respective successful target engagement in vitro [11] ex vivo [13] and in vivo in brain homogenates from RD2-treated versus placebo-treated transgenic mice using sFIDA analysis [10]. Therefore, also in the present study, Aβ oligomer, but also tau oligomer levels, were determined by sFIDA analysis in CSF from the dogs at baseline and after three months of treatment. No significant changes in Aβ oligomer levels, however, were observed in any of the groups during three months of treatment (Fig. 5A, B, C) or between the groups (Fig. 5D).
In contrast, tau oligomer levels in CSF increased significantly during the treatment duration in the placebo and low dose groups, while high dose treatment led to an overall stagnation of the tau oligomer levels (Fig. 5 E, F, G). Most individuals of this group showed even a reduction of tau oligomer levels in CSF upon treatment leading to statistical significance between the high dose and the other groups (Fig. 5H). There was a correlation between the Aβ and tau oligomer levels at baseline and at three months treatment (Figs. S3A and B). Why RD2 treatment in cognitively impaired Beagle dogs yielded significant reduction of tau oligomers but not of Aβ oligomers, at least under the described dosing and recruitment regime as well as the treatment duration, remains to be investigated. There are more and more data which suggest that the progression of AD may be driven by the synergistic interaction between tau and Aβ species [25,26]. Thus, targeting Aβ oligomers may possibly lead to effects on downstream targets like tau oligomers. The significant inverse correlation of RD2 plasma concentrations and tau oligomer levels after three months of treatment (Fig. 6B) supports this hypothesis.
Adding on the discussion above concerning the certainly suboptimal recruitment conditions, it is interesting to look again at the significant correlations between the individual changes in absolute Aβ oligomer concentrations (three-month treatment values minus baseline values) and individual changes in selective attention (three-month treatment values minus baseline values) for both RD2 treated cohorts (Fig. 7 B, C), but there was no such correlation for the placebo cohort (Fig. 7A). This is not only suggesting that RD2 treatment led to reduction or stagnation of CSF Aβ oligomer concentrations, but the absence of this correlation in the placebo group suggests that cognitive deficits in the recruited animals are not solely due to Aβ pathology but also due to other reasons. Treatment effects with RD2, however, may drive cognition enhancements strong enough in the responsive fraction of dogs to outweigh the effects of non-responders (Fig. 7D). Such an effect, of course, cannot be expected and was not found in the placebo group.
The here presented study, which investigated the pharmacokinetic profile and the efficacy of RD2 on cognition and AD-relevant biomarkers, especially Aβ oligomers and tau oligomers in CSF, using aged cognitively impaired Beagle dogs as a non-transgenic model of sporadic AD has certainly limitations concerning the recruitment strategy. Anyway, we demonstrate that RD2, with its unique MoA of disassembling Aβ oligomer complexes into Aβ monomers, crosses the blood-brain barrier in dogs and exerts beneficial effects on cognition and tau oligomer levels in CSF after oral administration. This has promising implications for the ongoing clinical development of RD2. Apart from the treatment results, the study shows that tau pathology is present in cognitively impaired old Beagle dogs, even if not in the form of tau tangles, but as tau oligomers in the CSF. The fact that their levels correlate with the levels of Aβ oligomers may be another relevant finding for dogs in general, and for this animal model for sporadic AD.
4. Methods
4.1. Study compound
The all-d-peptide RD2 (sequence: ptlhthnrrrrr, CBL Patras, Patras, Greece) consists of 12 d-enantiomeric amino acid residues with its C-terminus being amidated.
4.1.1. Test article and administration
Different batches of the acetate salt of RD2 were used as the test article in the canine studies. The compound was maintained at ultracold temperatures but thawed to room temperature for preparation of the dosing formulations.
Two separate studies have been carried out. One study was designed as a pharmacokinetic study to obtain time-dependent RD2 levels in blood plasma and CSF. The second study was designed as an interventional study to investigate pharmacodynamics parameters during three months of treatment and two more months after treatment end. The latter two months period is sometimes referred to as the “follow up phase”.
For oral application in the pharmacokinetic (PK) study, RD2 acetate was dissolved in sterile aqueous 0.9% NaCl, the pH was adjusted to 7.0 to 7.4 with NaOH. Solutions were prepared one day prior to dosing and kept refrigerated until 30 min prior to administration. The solutions contained 10 mg/ml or 25 mg/ml of RD2 acetate and were administered by oral gavage with a volume of 2 ml/kg (final dose 20 or 50 mg/kg). For the dosing procedure, the gavage tube was inserted into the stomach and 5 ml of tap water was flushed to ensure proper positioning. Then the test product was administered via syringe through the tube followed by 10 ml tap water.
For intravenous administration in the PK study, RD2 acetate was dissolved as described above. The solution contained 3 mg/ml and was administered by a single intravenous injection over 30 s with a dosing volume of 1 ml/kg. Injection was through a cephalic vein catheter that was flushed with 1 ml of sterile saline.
For oral application during the interventional study, RD2 acetate was administered as powder without excipients in hard gelatin/hypromellose (HPMC) capsules (Torpac, size 00). Empty capsules were used as placebo control. Amounts in the capsules were adjusted to the RD2 acetate dose of 3 or 30 mg/kg body weight (low or high dose). Prepared capsules were kept refrigerated until required for administration. On each assessment or collection day, dosing was staggered such that dogs were dosed 1 h (±15 min) prior to their cognitive assessment or 1-h (±10 min) prior to cerebrospinal fluid (CSF) collections. When none of these procedures were applied, the animals were dosed at approximately the same time daily.
4.2. Animals
Male and female Beagle dogs were obtained from the InterVivo Solutions Inc. colony. For the PK study 4 male and 4 female dogs and for the cognitive assessment study 12 male and 24 females were included. At study initiation, the age of the dogs for the PK study ranged from 1.7 to 1.8 years (1.8 ± 0.05). The age of the dogs for the interventional study ranged from 7 to 15 years (9.8 ± 2.5 years). Animals were included in the study after passing a general health evaluation.
4.2.1. Test site and approval
All animal experiments were performed at the site of InterVivo Solutions, Fergus, Canada, in accordance with principles of the Animal for Research Act of Ontario and the guidelines of Canadian Council on Animal Care (CCAC) and were approved by the Study Facility's Institutional Animal Care and Use Committee (IACUC) (approval number VRI108-17182-CE).
4.2.2. Pharmacokinetic (PK) study
The study was a randomized, non-blinded, preclinical study using a within-subject, incremental dose, design. The objective of the study was to evaluate the pharmacokinetic parameters of RD2, following a single intravenous or oral administration of RD2 acetate to Beagle dogs. Doses of 3 mg/kg (Day 0) were used for intravenous administration; 20 mg/kg (day 12) and 50 mg/kg (day 28 were used for oral administration. The concentration of RD2 was determined in serially collected blood plasma samples (3, 5, 10, and 20 min post dose, and 1, 4, 8, 24, 96, and 240 h post dose). Cerebrospinal fluid (CSF) samples were also serially collected (20 min post dose, and 1, 4, 8, 24, 96, and 240 h post dose) from the cisterna magna of the dogs while under isoflurane anesthesia.
A total of six (three male and three female) dogs were used to test each dose level. A washout period of at least 11 days was allowed before the same animal were treated with another dose. For serial sample collection procedures, animals were held in metabolic cages for observational purposes. Dogs were fed a standard commercial dry diet. Food consumption was not recorded. Water was provided ad libitum, except within 30 min prior to the start of anaesthetic procedures. Animal body weights were determined with a certified, verified scale prior to the beginning of each treatment arm. The recorded weights were used to determine individual treatment doses. Dosing sequence was started with 3 mg/kg intravenous (n = 6), followed by 20 mg/kg oral (n = 6) and 50 mg/kg oral (n = 6).
4.2.3. Study for evaluation of RD2 treatment-dependent cognitive parameters and CSF levels of Aβ and tau oligomers
This blinded preclinical study employed a controlled, parallel matched-group design. Following a baseline study phase including cognitive assessment, animals were allocated to one of three treatment groups (n = 12 dogs per group) balanced for initial cognitive performance to the extent possible. One group was assigned to receive a high oral dose of RD2 acetate (30 mg/kg once daily), while a second group received a low oral dose of RD2 acetate (3 mg/kg once daily). The third group was assigned to receive placebo. RD2 acetate or the placebo were administered orally once a day for three months. Test product and placebo were washed in for 17 days prior to initiation of treatment phase cognitive assessments. Following approximately three months of administration (93 days), cognitive function tests were repeated following two and six weeks of treatment washout.
For allocation of animals to treatment groups, the baseline phase cognitive status of the dogs was used to balance cognitive groups. Animals meeting the inclusion/exclusion criteria were ranked in descending order based on the three baseline cognitive tests (discrimination learning, delayed non-matching to position (DNMP) and attention), such that the best performing animals on each test received a rank of 1, and the poorest performing animals received a rank of 36. In the event that two or more animals had the same score, these subjects were ranked equally using the median rank according to the number of animals (i.e. if two animals had an equal score for ranks 4 and 5, then both were assigned a rank of 4.5 on the test; if three animals had an equal score for ranks 4, 5 and 6, then all three animals were assigned a rank of 5 on the test). The rank scores across the three tests were summed up for each animal and the rank sum was then used to rank dogs from highest performing to lowest performing using the procedures described above for animals with identical rank sums. Subsequently, subjects were then allocated to treatment groups based on rank sums such that rank sums 1, 2 and 3 were placed into treatment groups 1, 2 and 3 and rank sums 4, 5 and 6 were placed into treatment groups 3, 2 and 1, respectively. Dogs with identical rank sums were allocated to groups based on alphabetical order. Once all subjects had been allocated to groups in this manner, the allocation of individual subjects was adjusted to ensure that treatment groups were balanced to the extent possible across all applicable cognitive tests.
The study was blinded to all personnel in the investigation with the exception of the person(s) involved in administration of the experimental product and placebo, the person responsible for performing allocation and the Scientific Director. The treatment given to each animal was not revealed to the people collecting data.
Thirty-six dogs were screened on baseline cognitive tests, physical/neurological tests, hematology and blood biochemistry and that met the inclusion criteria were selected for the treatment phase. Dogs were maintained at the animal facility and group-housed in pens or rooms in compliance with the recommendations of the Canadian Council on Animal Care. Environmental management including lighting, ventilation, temperature, and humidity regulation were maintained and controlled according to standard operating procedures. All animals were fed according to the facilities standard operating procedures, using a standard commercial diet to maintain body condition (Purina ProPlan Savor Adult, Chicken and Rice Formula). Animals were fed at the end of each day following completion of any testing or sample collection procedures. Water was provided ad libitum.
During the 3-month treatment phase of the interventional study (Fig. 1A), on day 80, one dog needed to be euthanized. Unblinding revealed that this dog was in the placebo cohort. On day 123 (30 days after treatment end), another dog needed to be euthanized. Unblinding revealed that the dog was in the low dose (3 mg/kg/day) cohort. The causes of morbidity (forelimb paralysis and progressing vestibular disease, respectively) were considered consequences of underlying age-related background diseases in these old dogs. All other dogs survived the study and were returned to the colony at study conclusion.
4.3. Behavioral assessments
4.3.1. Discrimination Learning
Discrimination learning was performed over three weeks during baseline (days −38 to −17) in order to assess learning ability and executive function as well as to prepare the subjects for cognitive tasks that would be completed during the treatment phase. Initially, on the first day of testing, animals participated in a 10-trial preference test using two objects differing in size, shape and color (i.e. green block and yellow banana). Following this test, each dog's preferred object was used as the rewarded stimulus for subsequent learning sessions during which selection of the preferred object was required in order to obtain a reward. The subsequent daily sessions consisted of 20 trials and were carried out according to standard operating procedures. Animals were tested until they successfully passed a two-stage criterion as follows: Stage 1: 90% or greater on one session or a minimum of 80% over two consecutive sessions; Stage 2: 70% or greater over two consecutive sessions.
4.3.2. Variable delayed non-matching to position (DNMP)
The DNMP task was performed in 5-day intervals in order to assess spatial working memory. Testing occurred during baseline (days −16 to −12), at three time points during the treatment phase (days 23–27, 46 to 50 and 74 to 78) and at two time points during follow up (days 114–118 and 142 to 146). Testing was performed according to standard operating procedures. Briefly, subjects were initially presented with a single object (i.e. white block) on a sliding tray. The block was positioned over one of three possible food-well locations and the animal was required to displace the block with its nose in order to uncover a food reward. The tray was then removed from the dog's sight and a delay was initiated. Following the delay, the subject was presented with two white blocks - one in the original location and one in a new location. The dog was required to select the block in the new (non-matching) location in order to obtain a reward. On each designated testing day, animals participated in a single session, regardless of score, with delays of 20 and 90 s equally divided among 12 trials. During the treatment phase, testing was performed 1 h following dosing (±15 min).
4.3.3. Variable discrimination (selective attention task)
Variable discrimination testing was performed in 4-day intervals in order to assess selective attention. Testing occurred during baseline (days −11 to −8), at three time points during the treatment phase (days 17–20, 40 to 43 and 68 to 71) and at two time points during follow up (days 107–110 and 135 to 138). Animals were tested on the designated days regardless of score. During the treatment phase testing was performed 1 h following dosing (±15 min) according to standard operating procedures. This task was similar to discrimination learning; however, the preferred object was presented with either 0, 1, 2, or 3 negative stimuli (non-preferred objects) to serve as distractors. There were 20 trials per session.
4.3.4. Discrimination reversal
Discrimination reversal testing was carried over ten days in the treatment phase (days 81–90). This task occurred following all other treatment phase testing. The task was performed as described for Discrimination Learning; however, the non-preferred object from the baseline preference test was now rewarded. Animals were tested once daily regardless of score according to standard operating procedures.
4.4. Plasma and cerebrospinal fluid (CSF) collection
Whole blood collections were performed immediately following each CSF collection time point. Approximately 4 ml of blood were collected from a suitable vein according to standard operating procedures. Blood was placed into K3EDTA tubes and within 15 min of collection, plasma was isolated by centrifugation at 1500×g for 15 min at 4 °C.
CSF was collected at baseline (day −2), at three time points during the treatment phase (days 28, 51, and 93) and twice after treatment end (days 120 and 148) for determination of RD2 levels and biomarkers. Using sterile techniques, samples were obtained from the cisterna magna while the animals were anesthetized. Anesthesia was induced with Propofol (8 mg/kg, intravenous) to effect. Subjects were intubated and anesthesia was maintained with an isoflurane/oxygen mixture for the duration of the procedure. If a suitable vein could not be accessed for Propofol injection, masking the animal with the isoflurane/oxygen mixture was used as an alternative. In each treatment arm, CSF was collected 1 h following dosing (±10 min). Samples were collected and subsequently processed by centrifugation (within 15 min of collection) at 2000×g for 10 min at 4 °C to remove potential red blood cell contamination.
RD2 plasma and CSF concentrations were quantified using an ultra-high-pressure liquid chromatography method combined with mass spectrometric detection using a QTRAP6500 in positive MRM mode (TNO, Zeist, Netherlands). The method had been validated (in accordance with the EMA guidelines on bioanalytical method validation) in terms of reproducibility, specificity, robustness and precision. The lower limit of quantification (LLOQ) was 0.5 ng/ml. Plasma concentrations for each dose level following single i.v. or oral doses of RD2 were used to calculate the following pharmacokinetic parameters: maximum concentration (Cmax) and time to reach it (Tmax), area under the plasma concentration versus time curve between 0 and t, and from 0 to infinity (AUC0-tlast + AUC0-inf), terminal half-life (t1/2α,β,γ), and total plasma clearance (CLtotal) using TOPFIT 2.0 program [27].
4.5. Hematology and blood clinical chemistry
Whole blood collections were conducted on days −41 and −2 (baseline) as well as on day 93 of the treatment phase.
Complete blood count (CBC) and clinical chemistry analysis was performed by Antech Diagnostics (Mississauga, Ontario, Canada). CBC analysis included the blood parameters white blood cells (WBC), red blood cells (RBC), hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count, platelet estimate, neutrophils, lymphocytes, monocytes, eosinophils and basophils.
Clinical chemistry analysis included the blood parameters total protein, albumin, globulin, calculated ratio of albumin to globulin (A/G ratio), aspartate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGTP), total bilirubin, blood urea nitrogen (BUN), creatinine, BUN/creatinine ratio, phosphorus, glucose, calcium, magnesium, sodium, potassium, sodium/potassium ratio, chloride, cholesterol, triglycerides, amylase, lipase, creatine phosphokinase (CPK) and creatine kinase isozymes (CK-MM, CK-MB, and CK-BB).
4.6. Biomarker analysis
4.6.1. Aβ42 and total tau ELISA
Aβ42 and total tau ELISAs were purchased from Innotest (Fujirebio Germany GmbH, Hannover, Germany) and performed according to the manufacturer's protocol with CSF. Antibodies used for the specific detection of Aβ1-42 were 3D6, which recognizes amino acid residues 1 to 5 and 21F12, which recognizes amino acid residues 33 to 42 leading to a negligible cross reactivity for Aβ 1–40. Antibodies used for the detection of total tau were AT120, HT7 and BT2, all antibodies which are specific for the mid-domain region of the tau protein. All samples were measured in duplicates. Most of the tau concentration values were lower than the lower limit of quantification (50 pg/ml). Therefore, no further analysis of the data was performed.
4.6.2. Albumin ELISA
Albumin ELISA was purchased from Bethyl Laboratories (Montgomery, Alabama, USA) and performed according to the manufacturer's protocol with CSF and plasma. All samples were measured as duplicates.
4.6.3. GFAP and NfL single molecule array
GFAP and NfL single molecule arrays (Simoa) were purchased from Quanterix (Quanterix, Billerica, Massachusetts, USA) and performed according to the manufacturer's protocol with CSF on a Simoa HD-1 Analyzer (Quanterix, Billerica, Massachusetts, USA). All samples were measured as duplicates.
4.6.4. Surface-based fluorescence intensity distribution analysis (sFIDA) assay
Surface-based fluorescence intensity distribution analysis (sFIDA) assays were performed in 384 flat-bottom square well microplates with a glass bottom (ThermoFisher, Waltham, Germany) as previously described [19]. 2.5 μg/ml of Nab228 monoclonal antibody (mAB) (Sigma-Aldrich, Missouri, USA) and 2.5 μg/ml of Tau5 mAB (Biolegend, San Diego, USA) in NaHCO3, pH 8 were added directly to the wells and incubated overnight at 4 °C. The plate was washed five times each with TBS (Serva, Duisburg, Germany) + 0.1 % Tween 20 (AppliChem, Darmstadt, Germany) (TBST) and TBS. Each of the wells was blocked with 1% BSA (AppliChem, Darmstadt, Germany) in TBS for 1 h at room temperature (RT). After washing the wells five times each with TBST and TBS, CSF samples and oligomer standard were added to the plate and were incubated overnight at 4 °C. The next day, after washing the excessive sample away five times with TBS, 0.2 μg/ml Nab228, labeled with CF-488 dye and 2 μg/ml Tau5, labeled with CF-633 dye, (both: Sigma-Aldrich, Missouri, USA), both ultra-centrifuged (100,000×g, 1 h, 4 °C), were added to the wells and incubated for 1 h. After incubation, the excessive detection antibodies were washed away five times with TBS. All washing steps were carried out by an automated microplate washer (405 LS Microplate Washer, Agilent, Santa Clara, USA). For measurement, the buffer in the wells was changed against TBS with 0.03% ProClin (Sigma Aldrich, Missouri, USA) and the plate was sealed with a plastic foil and transferred to a Leica multi-color TIRF total internal reflection fluorescence system (AMTIRF MC, Leica Microsystems, Wetzlar, Germany). The TIRF system operated with an automated stage and a × 100 oil immersion objective (1.47 oil CORRTIRF Leica). Images were recorded consecutively with Ex/Em = 633/705 and 488/525 nm with a 1000 ms exposure time and a gain of 1000 for both color channels at a penetration depth of 200 nm. The microscope took 5 × 5 images per well in each channel, which corresponds to app. 3% of the well's surface. Each image consisted of 1000 × 1000 pixels with a lateral resolution of 116 nm (pixel to pixel) and an intensity resolution of 14 bit (gray scale). Image analysis was performed using sFIDAta, a custom-made software. The cutoff values were calculated for each channel based on the buffer blank. The software then applied the cutoff values to the sample results and counted the pixels that were higher than the cutoff for each channel (pixelcount). All samples were measured in triplicates.
The calibration standard for Aβ and tau oligomers were silica nanoparticles (SiNaPs) with a diameter of 20 nm with covalently attached epitopes for the detection antibodies, Aβ(1–15) and tau(210–230). These Aβ(1–15)-tau(210–230)-SiNaPs have been prepared as described previously [21], with the following modifications: Aβ and tau peptides, which were functionalized with cysteamine on the C-terminus (Peptides and Elephants, Henningsdorf, Germany) were crosslinked to the aminated surface of the SiNaPs by maleimido hexanoic acid (MIHA, abcr GmbH, Karlsruhe, Germany).
4.7. Statistical analysis
All statistical calculations were performed using SigmaPlot Version 11 (Systat Software, Germany) or OriginPro 2019 (OriginLab Corporation, USA). Normal distribution of data was tested using Kolmogorov-Smirnov-test. Statistical calculations were conducted using two-way RM ANOVA followed by Fisher post hoc test. Pairwise comparisons were performed with one-sided Wilcoxon signed-rank test for dependent data and Mann-Whitney U test for independent data. Correlations were first calculated with Pearson and then with Spearman analysis at alpha level 0.05 with n = 11 to 12.
Author contribution statement
Janine Kutzsche, Dieter Willbold: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sarah Schemmert, Wolfgang M. Rossberg, Michael Hümpel, Antje Willuweit: Analyzed and interpreted the data.
Tuyen Bujnicki: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Christian Zafiu, Steffen Halbgebauer, Markus Otto, Joseph A. Araujo: Conceived and designed the experiments; Performed the experiments.
Victoria Kraemer-Schulien: Performed the experiments.
Marlene Pils, Lara Blömeke, Julia Post, Andreas Kulawik: Contributed reagents, materials, analysis tools or data.
Dagmar Jürgens, Oliver Bannach: Conceived and designed the experiments.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Dieter Willbold reports a relationship with Priavoid GmbH that includes: board membership. Dieter Willbold has patent issued to Priavoid GmbH. Co-founder and co-owner of Priavoid GmbH.
Acknowledgments
D.W. was supported by the Technology Transfer Fund of the Forschungszentrum Jülich, Germany. D.W. was supported by “Portfolio Drug Research” of the “Impuls und Vernetzungs-Fonds der Helmholtzgemeinschaft”, Germany. Additional funding was received from Part the Cloud: Translational Research Funding for Alzheimer's Disease (PTC) Award Number: 18PTC-19-605853 from the Alzheimer's Association, US. We received funding from the European Union Seventh Framework Program (FP7/2007–2013), European Union, under grant agreement 602999 (SYMPATH project), the Federal Ministry of Education and Research, Germany, within the projects VIP (03V0641), KNDD (01GI1010A), JPND/BIOMARKAPD (01ED1203H), and NEUROALLIANZ (16GW0099). We were also supported by the programs “Biomarkers Across Neurodegenerative Diseases I + II” of The Alzheimer's Association, Alzheimer's Research UK and the Weston Brain Institute, Canada (11084 and BAND-19-614337). We are also grateful for support from The Michael J. Fox Foundation, US, for Parkinson's Research (14977), from the ALS Association, US, and from the Packard Center, US, (19-SI-476). We further received funding from the Deutsche Forschungsgemeinschaft, Germany (INST 208/616-1 FUGG, INST 208/794-1 FUGG) and the Helmholtz Association, Germany (HVF0079).
Footnotes
Appendix A
Supplementary data to this article can be found online at. https://doi.org/10.1016/j.heliyon.2023.e18443.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Li X., et al. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2019. Front. Aging Neurosci. 2022:14. doi: 10.3389/fnagi.2022.937486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Head E. A canine model of human aging and Alzheimer's disease. Biochim. Biophys. Acta. 2013;1832(9):1384–1389. doi: 10.1016/j.bbadis.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siwak-Tapp C.T., et al. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol. Learn. Mem. 2007;88(2):249–259. doi: 10.1016/j.nlm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siwak-Tapp C.T., et al. Region specific neuron loss in the aged canine hippocampus is reduced by enrichment. Neurobiol. Aging. 2008;29(1):39–50. doi: 10.1016/j.neurobiolaging.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tapp P.D., et al. Frontal lobe volume, function, and beta-amyloid pathology in a canine model of aging. J. Neurosci. 2004;24(38):8205–8213. doi: 10.1523/JNEUROSCI.1339-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pugliese M., et al. Diffuse beta-amyloid plaques and hyperphosphorylated tau are unrelated processes in aged dogs with behavioral deficits. Acta Neuropathol. 2006;112(2):175–183. doi: 10.1007/s00401-006-0087-3. [DOI] [PubMed] [Google Scholar]
- 7.Head E., et al. Spatial learning and memory as a function of age in the dog. Behav. Neurosci. 1995;109(5):851–858. doi: 10.1037//0735-7044.109.5.851. [DOI] [PubMed] [Google Scholar]
- 8.Milgram N.W., et al. Landmark discrimination learning in the dog. Learn. Mem. 1999;6(1):54–61. [PMC free article] [PubMed] [Google Scholar]
- 9.Kutzsche J., et al. Safety and pharmacokinetics of the orally available antiprionic compound PRI-002: a single and multiple ascending dose phase I study. Alzheimers Dement. (N Y) 2020;6(1) doi: 10.1002/trc2.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schemmert S., et al. Abeta oligomer elimination restores cognition in transgenic Alzheimer's mice with full-blown pathology. Mol. Neurobiol. 2019;56(3):2211–2223. doi: 10.1007/s12035-018-1209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Groen T., et al. The Abeta oligomer eliminating D-enantiomeric peptide RD2 improves cognition without changing plaque pathology. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-16565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang T., et al. Toward the mode of action of the clinical stage all-d-enantiomeric peptide RD2 on Aβ42 aggregation. ACS Chem. Neurosci. 2019;10(12):4800–4809. doi: 10.1021/acschemneuro.9b00458. [DOI] [PubMed] [Google Scholar]
- 13.Kass B., et al. Aβ oligomer concentration in mouse and human brain and its drug-induced reduction ex vivo. Cell Rep. Med. 2022;3(5) doi: 10.1016/j.xcrm.2022.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutzsche J., et al. Large-scale oral treatment study with the four most promising D3-derivatives for the treatment of Alzheimer's disease. Molecules. 2017;22(10) doi: 10.3390/molecules22101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schemmert S., et al. Deceleration of the neurodegenerative phenotype in pyroglutamate-Abeta accumulating transgenic mice by oral treatment with the Abeta oligomer eliminating compound RD2. Neurobiol. Dis. 2019;124:36–45. doi: 10.1016/j.nbd.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Bau-Gaudreault L., Grimes C.N. Effect of time and storage on toxic or pseudo-toxic change in canine neutrophils. Vet. Clin. Pathol. 2019;48(3):400–405. doi: 10.1111/vcp.12755. [DOI] [PubMed] [Google Scholar]
- 17.Milgram N.W., et al. Cognitive functions and aging in the dog: acquisition of nonspatial visual tasks. Behav. Neurosci. 1994;108(1):57–68. doi: 10.1037//0735-7044.108.1.57. [DOI] [PubMed] [Google Scholar]
- 18.Tapp P.D., et al. Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learn. Mem. 2003;10(1):64–73. doi: 10.1101/lm.54403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tapp P.D., et al. Effects of age on measures of complex working memory span in the beagle dog (Canis familiaris) using two versions of a spatial list learning paradigm. Learn. Mem. 2003;10(2):148–160. doi: 10.1101/lm.56503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrmann Y., et al. sFIDA automation yields sub-femtomolar limit of detection for Aβ aggregates in body fluids. Clin. Biochem. 2017;50(4–5):244–247. doi: 10.1016/j.clinbiochem.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Hülsemann M., et al. Biofunctionalized silica nanoparticles: standards in amyloid-β oligomer-based diagnosis of Alzheimer's disease. J. Alzheimers Dis. 2016;54(1):79–88. doi: 10.3233/JAD-160253. [DOI] [PubMed] [Google Scholar]
- 22.Kulawik A., et al. Advancements of the sFIDA method for oligomer-based diagnostics of neurodegenerative diseases. FEBS Lett. 2018;592(4):516–534. doi: 10.1002/1873-3468.12983. [DOI] [PubMed] [Google Scholar]
- 23.Wang-Dietrich L., et al. The amyloid-β oligomer count in cerebrospinal fluid is a biomarker for Alzheimer's disease. J. Alzheimers Dis. 2013;34(4):985–994. doi: 10.3233/JAD-122047. [DOI] [PubMed] [Google Scholar]
- 24.Blömeke L., et al. Quantitative detection of α-Synuclein and Tau oligomers and other aggregates by digital single particle counting. NPJ Parkinsons Dis. 2022;8(1):68. doi: 10.1038/s41531-022-00330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo J.P., et al. Abeta and tau form soluble complexes that may promote self aggregation of both into the insoluble forms observed in Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2006;103(6):1953–1958. doi: 10.1073/pnas.0509386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller Y., Ma B., Nussinov R. Synergistic interactions between repeats in tau protein and Aβ amyloids may Be responsible for accelerated aggregation via polymorphic states. Biochemistry. 2011;50(23):5172–5181. doi: 10.1021/bi200400u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinzel G., Woloszczak R., Thomann P. G. Fischer; Stuttgart: 1993. TopFit : Pharmacokinetic and Pharmacodynamic Data Analysis System for the PC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.