Abstract
Background:
Controlling of Aedes aegypti and Ae. albopictus, vectors of five important mosquito-borne diseases, is known as the most effective method to prevent the transmission of arboviruses to humans, but the emergence of insecticide resistance is threat for control and prevention of vector borne diseases. A better understanding of mosquito resistance to insecticides will help to develop more effective methods to control insecticide resistance in mosquito vectors.
Methods:
Worldwide geographical distribution of insecticide resistance in Ae. aegypti and Ae. albopictus by the available papers and map of the data for carbamates, organochlorines, organophosphates, pyrethroids, microbial and insect growth regulator insecticides were reviewed. Article data published up to December 2022 were investigated by searching the following databases: “Google Scholar”, “PubMed”, “Scopus”, “SID” and “Web of Knowledge”.
Results:
The results showed that the susceptibility and resistance status of Ae. aegypti and Ae. albopictus to insecticides in the world is very diverse.
Conclusion:
Due to the importance of Ae. aegypti and Ae. albopictus in the transmission of mosquito-borne arboviruses, resistance management should be given more attention worldwide to prevent insecticide resistance in the arbovirus vector and replace the new approach for vector control.
Keywords: Insecticide, Resistant, Aedes, Arboviruses, World
Introduction
Among arboviruses that are widespread worldwide, chikungunya, dengue, Zika and yellow fever are widely transmitted by Aedes spp. (1, 2). Aedes aegypti mosquitoes originated from Africa, but they are also found in tropical, subtropical, and temperate parts of the world (3, 4). Aedes albopictus has adapted to urban, suburban, and rural regions. This species is a forest species that has spread from Asia to Africa, America, and Europe through the trade of used tires (5). Dengue is the fastest mosquito transmitted disease, it has increased more than 15-fold since 2000 and has affected more than 129 countries. Because vaccines or drug treatments exist for only a small number of vector-borne pathogens, the primary method for controlling many vector-borne diseases is direct vector control (6).
Chemical control by organic or inorganic insecticides is one of the methods of mosquito control and as part of integrated management. Insecticides used for mosquitoes, that are neurotoxic: organochlorines, organophosphates, carbamates, and pyrethroids (7). Insecticide resistance is a growing topic around the world that limits the effectiveness of control methods against vector mosquitoes. The data on insecticide resistance in Aedes is varying. Insecticides have an important role in the control of dengue fever, but the resistance of mosquitoes reduces the effectiveness of these interventions in some countries (8). According to the World Health Organization (WHO), resistance to insecticides is a major risk in interventions to control vector-borne diseases. It is essential to identify the geographical regions where vector resistance to insecticides exists and can make it difficult to control the vector, and to improve the induction of innovative tools for vector control. A better identifying of resistance of insecticides helps to formulate a global measure to control insecticide resistance in disease vectors.
Materials and methods
Published articles were searched by terms “resistance”, “Aedes aegypti”, “Aedes albopictus”, “pyrethroid”, “organochlorines”, “organophosphate”, “carbamate”, “IGR”, and “kdr”, “P450”, “monooxygenase”, “glutathione”, or “esterase”. Data were extracted from articles published up to December 2022. The terms in the following databases: PubMed, Web of Knowledge, Scopus, Google Scholar, and SID were reviewed. The WHO guideline was considered for insecticide resistant level.
Results
Resistant status of Aedes aegypti and Ae. albopictus to Organochlorines insecticides
In Africa
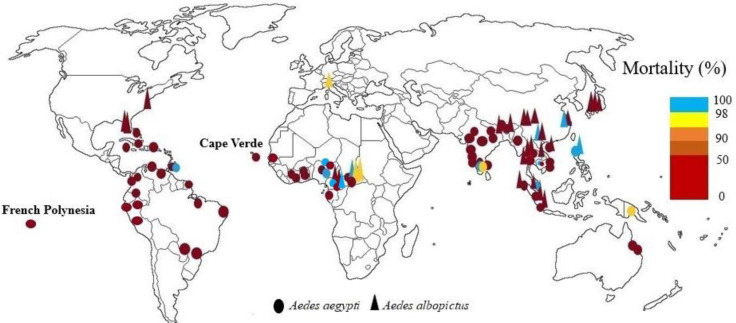
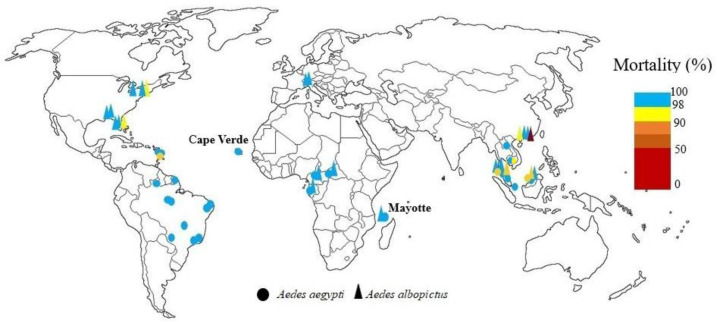
Resistance of two Aedes species to dichlorodiphenyltrichloroethane (DDT) has been reported from each country where susceptibility testing has been performed. In Ghana (9, 10), Cape Verde, and Senegal (11) high Ae. aegypti resistance to DDT was reported. Experiments showed one Ae. aegypti population from Gabon and two populations of Ae. albopictus from Cameroon were resistant to DDT (12, 13). Also, in another study adult bioassays of three field populations of Ae. albopictus and Ae. aegypti from Cameroon showed different susceptibility levels from 68.75% to 100% against 4% DDT (14). In Central African Republic Ae. aegypti was resistant to DDT and Ae. albopictus had a sensitive population and the rest of the populations were tolerant (15). In Lagos State of Nigeria all populations of Ae. aegypti showed resistance to DDT (16), while in Kwara state, Nigeria, Ae. aegypti was completely susceptible to this insecticide (17). Highest resistance to 4% of DDT was observed in populations of Ae. aegypti from northern Nigeria, while the knockdown rate in dieldrin was very high (18). Two population types of Ae. aegypti (white and brown populations) examined in Cote D'ivoire, both populations were resistant to DDT (19, 20). Nine populations tested of Ae. aegypti from Senegal were resistant to DDT (21) (Fig. 1).
Fig. 1.
World mapping of organochlorine insecticide resistance in Aedes aegypti and Aedes albopictus up to December 2022
In Asia
In Thailand, resistance, or increased tolerance to dieldrin has been reported, and there is resistance to DDT, although DDT is not used in Aedes control programs. DDT has been used for agriculture in Thailand since 1934 was banned in agriculture in 1983. In health and for indoor spraying, it has been used against malaria vectors since 1949, and since 2000, its use in health has been ceased due to its destructive effects on the environment and the development of physiological resistance in other mosquitoes (22–27). In Malaysia, Ae. aegypti and Ae. albopictus showed resistance to DDT. Aedes aegypti was susceptible to dieldrin except in one area. Dieldrin resistance was reported in Ae. albopictus except in Kuala Lumpur and two other areas. The higher resistance of Ae. albopictus to dieldrin was due to the ecology of this species and its breeding sites, which was close to plants and in agricultural areas, and therefore exposed to dieldrin because it was still used in agriculture to control soil pests (28, 29). The adult Ae. aegypti from Jharia, Bihar were the first case of DDT resistance in India (30). In India DDT resistance in field collected Ae. aegypti has reported from Goa (31) and Jharkhand (32). DDT and dieldrin resistance have been reported in all Indian Ae. aegypti. Aedes albopictus from western Bengal has shown resistance to DDT. Aedes aegypti females' exposure with 0.4% dieldrin resulted in 48% and 100% mortality, while 4% dieldrin for same exposure period showed 88% and 100% mortality (33–38). In China all Ae. albopictus adults tested populations showed resistance to DDT. Urban Ae. albopictus had a higher level of resistance to DDT than strains collected from rural areas (39–42). In Laos Ae. albopictus was resistant to DDT (43). There was high DDT resistance in adult of Ae. aegypti in Myanmar (44). In Philippines Ae. aegypti and Ae. albopictus larvae were sensitive to lindane, dieldrin, and DDT (45). Also, there was DDT resistance in Vietnam (46–48). In Japan all strains of Ae. albopictus were highly resistant to DDT (49). In Phnom Penh, Cambodia papers impregnated with DDT at 4% and dieldrin at 0.4 and 4% were used for the bioassays of adult Ae. aegypti. The mortality rates were reported 0% for 4% DDT and 87.6± 2.1% and 97.8±4.3, for 0.4% and 4% dieldrin%, respectively (50) (Fig. 1).
In America
The development of resistance to DDT in Mexico and many parts of the Americas was due to the widespread use of this insecticide during the 1950s and 1960s (51). In the Americas Ae. aegypti resistance to DDT was initially found in Trinidad in 1955, and it was susceptible to dieldrin. Now, all parts of the Caribbean have Ae. aegypti populations that are resistant to DDT and dieldrin. From French Guiana to the Bahamas, the situation is uniform regarding this resistance (52). DDT resistance has been confirmed in Ae. aegypti Caribbean populations (53) and in two Florida populations and one New Jersey population (54). There were 45 dengue epidemics in Cuba in 1977, 1981, 1997 and 2002 and Ae. aegypti resistance to DDT has been seen in this country (55, 56). In Colombia resistance to DDT was seen in all mosquito populations tested, although this insecticide did not use for vector control in any of the study areas (57–59). In Venezuela low mortality in larvae Ae. aegypti was exhibited by field strains toward DDT and confirmed the presence of high resistance to this insecticide (60). After exposure of adults of Ae. aegypti from Santo Domingo, Dominican Republic to discriminating concentrations of DDT, it was found that wild populations were resistant to this insecticide (61). In Brazil eradication programs from 1950 to 1970 might have led to spread of DDT resistance (62). All of populations of Ae. aegypti from Peru were resistant to DDT (4%), after 1 hour of exposure with papers treated (63) (Fig. 1).
In Australia and Oceania
Adult bioassays detected significant resistance to dieldrin and DDT in Ae. aegypti population from Townsville City, despite DDT has not been used for adult control in this city for minimum 12 years, and dieldrin is not an adulticide (64). Susceptibility to DDT of Ae. aegypti and Ae. albopictus populations seems to have decreased in Papua New Guinea (65) (Fig. 1).
Resistant status of Aedes aegypti and Ae. albopictus to Organophosphates insecticides
In Africa
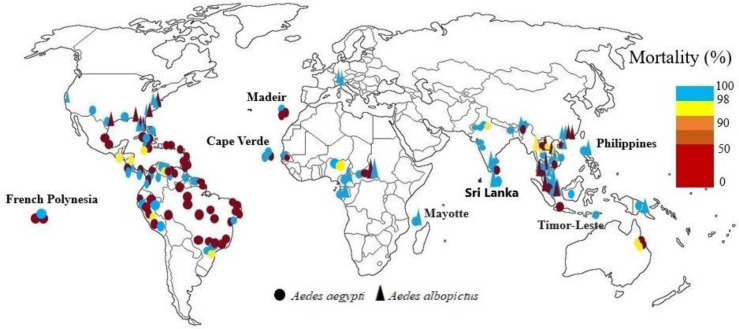
Complete susceptibility to temephos, fenitrothion and malathion has been reported from both vectors in Cameroun, Gabon (12, 13), and Mayotte (66). In Central African Republic larvae of both species were sensitive to temephos and fenitrothion and only one population of resistant to fenitrothion was reported (15). In Cape Verde Ae. aegypti was sensitive to fenitrothion (11), but it shows resistant to temephos and sensitivity to malathion (67). In Dakar, Senegal also its sensibility to fenitrothion was reported (11). Organophosphates (0.8% and 5% malathion, 0.05% pirimiphos-methyl and 1% fenitrothion) were used for bioassays of Ae. aegypti from Senegal. All the Ae. aegypti populations tested were susceptible to 5% malathion, while only two populations showed susceptibility to 0.8% malathion. Resistance in all populations were reported for 0.05% pirimiphos-methyl. Louga, Mbour and Barkédji strains showed resistance to 1% fenitrothion (21). The Ae. aegypti populations in northern Nigeria were susceptible to fenitrothion in 2018 and 2019, with mortality of 98.6% and 100%, respectively. But, in 2020, mortalities of 93% observed and moderate resistance were registered (18) (Fig. 2).
Fig. 2.
World mapping of organophosphates insecticide resistance in Aedes aegypti and Aedes albopictus up to December 2022
In Asia
In Thailand Ae. aegypti and Ae. albopictus resistance to temephos was demonstrated in some areas. Aedes aegypti showed susceptibility to malathion. All strains of Ae. aegypti were resistant to temephos except strains of Nakhon Ratchasima. Aedes albopictus was susceptible to both insecticides (68). In other study the larvae of both species were resistant to temephos and resistance or tolerance to Malathion and fenitrothion has been reported in different parts of north and south of Thailand, while in central and eastern parts of Thailand, were still susceptible to fenitrothion (24). Temephos has been used against Ae. aegypti larvae in Thailand since 1950, and malathion, fenitrothion, and pirimiphos methyl have been used for IRS and fogging (22–24, 26, 68, 69). In Sri Lanka Ae. aegypti and Ae. albopictus were sensitive to malathion. Although this insecticide has been widely used in the control of malaria and filariasis in endemic areas (70). Also, in 2020 reported that adult Ae. aegypti mosquitoes from Sri Lanka were susceptible to malathion 5%. For temephos the resistance ratios (RR50) varied between 0.69 and 3.93 (71). In one study in Malaysia, Ae. aegypti was sensitive to malathion and fenitrothion (29), in 2015 was sensitive to this insecticide, except in Kuala Lumpur and Ae. albopictus was resistant to malathion except in Kuala Lumpur. No resistance was observed in larvae to temephos, and various populations were susceptible or tolerant, although it has been widely used since the 1970s and during the 1998 global pandemic (28). Chen et al. reported greater resistance of Ae. aegypti to temephos than Ae. albopictus (72). In India the susceptibility of two species was reported to malathion in 1993 (73). In other studies, Ae. aegypti adults were tolerant to fenitrothion and sensitive to malathion, also the larvae were sensitive to three tested insecticides including temephos, fenitrothion and malathion (32–34). Resistance of this species to dichlorvos was also reported (74). Results from the studies in 2015 showed that only one Ae. aegypti population had high levels of resistance to temephos and the rest also Ae. albopictus were susceptible (35, 74). In 2017 Ae. aegypti was reported sensitive to temephos except one population. In this study, exposure of Ae. aegypti with 1% fenitrothion for 30 and 60 minutes caused 40% and 100% mortality, respectively (36). In 2018, larvae of Ae. aegypti was susceptible to temephos (37). In a recent study in West Bengal all populations Ae. aegypti were reported sensitive to malathion (75). In China, resistance to malathion was clear in Ae. aegypti in Hainan Province but Ae. albopictus was susceptible (76). Aedes albopictus larvae were resistant to temephos and adult was sensitive to dichlorvos, with 100% mortality (41), also adult populations of Ae. albopictus in the four districts were all sensitive to malathion, but high resistance to temephos was registered (42). In Cambodia there is larval resistance to temephos in endemic areas. In the first study on the sensitivity of two Ae. aegypti populations to temephos, in one population, the larvae showed some degree of resistance to temephos and the other population was sensitive (77). In 2022, moderate Ae. aegypti resistance was observed for temephos in Phnom Penh, Cambodia. Also, papers impregnated with fenitrothion at 1%, malathion at 0.8% and pirimiphos-methyl at 0.2%, were used for the bioassays of adult Ae. aegypti. Organophosphate insecticides showed high mortality rates (50). Development of mild resistance of Ae. aegypti to malathion was found in Jakarta, Indonesia. South Denpasar mosquitoes had the highest resistance to malathion, which was significantly different from the resistance reported in North Denpasar (78–80) and populations of Ae. aegypti in Banjarmasin, Kalimantan, Indonesia were susceptible to malathion 5% (81). In Vientiane Laos, based on larval bioassay, the wild population of Ae. aegypti strain was relatively resistant to temphos (82). In other study in Laos all seven population of Ae. albopictus but one was resistant to malathion and three of populations showed resistance to temephos and the rest were tolerant (43). In Philippines Ae. aegypti and Ae. albopictus larvae were reported sensitive to malathion (45). In Singapore, both species were reported to be sensitive to pirimiphos methyl (83). In another study, all Ae. aegypti populations were sensitive to pirimiphos methyl, and all populations of Ae. albupictus were resistant to pirimiphos methyl but southeastern populations were tolerant (84, 85). In another study, Ae. aegypti larvae were reported to be sensitive to temephos, although resistance was possible (86). Aedes aegypti from Dili, Timor-Leste was sensitive to malathion (87), also in Vietnam was sensitive to malathion (46–48). In Bangladesh Ae. aegypti mosquitoes varied in susceptibility to malathion (50μg/bottle) (88) (Fig. 2).
In America
High levels of resistance to chlorpyrifos in Ae. albopictus has been found in Alabama and Florida (89). None of the eight populations of US Ae. albopictus larvae were resistant to temephos, also Ae. albopictus resistance to malathion was reported in Florida and New Jersey (54). Levels of malathion and temephos resistance were observed in Ae. aegypti Caribbean populations. Guyana, Jamaica, and Suriname Ae. aegypti populations showed only slight resistance to the larvicide. In most Caribbean countries, where malathion has been used sporadically for 20 to 30 years to control adults, there is little resistance to this insecticide (90) and low levels of temephos resistance were confirmed in Grand Cayman (53). In United States Ae. albopictus from Florida, California, three different North Carolina populations and Ae. aegypti from Texas were susceptible to malathion. Conversely, Ae. albopictus from North Carolina and Texas were resistant to the same malathion doses (91). Mortality of Ae. aegypti mosquitoes tested in Jamaica with malathion, was from 84 to 90% at 30 minutes of exposure and 100% with increasing exposure time to 45 minutes (92). In 2019, Ae. aegypti strains from New Mexico were susceptible to the chlorpyrifos (93). In Mexico, the Ae. aegypti populations showed less resistant to chlorpyrifos than to pyrethroids (94). Resistance to fenthion, fenitrothion, and temephos, has been found in Cuba (55, 56). On the Island of Martinique (Caribbean), Ae. aegypti populations were resistant to naled (organophosphate) that showed insecticide resistance reduced its effect when applied by ultra-low volume (ULV) thermal fogging (95). There is evident that the organophosphate insecticide resistance (malathion, fenthion and temephos) is prevalent in Trinidad and Tobago larval strains of Ae. aegypti (96). In Cuba, Venezuela, Costa Rica and Jamaica, Ae. aegypti was sensitive to malathion despite its widespread use in vector control programs in these countries (97). Resistance to organophosphates is found throughout Latin America due to its intense use to control larvae, including Colombia, Cuba, Martinique, Costa Rica, Havana, Brazil, Bolivia, French Guiana, Argentina, French Polynesia, and the Caribbean. But resistance to temephos is lower in West Africa. The susceptibility of adults and larvae of Ae. aegypti to insecticides from Santo Domingo, Dominican Republic, was evaluated. Hatched larvae from eggs collected from ovitraps were resistant to temephos. Adults were resistant to malathion (61). larvae of Ae. aegypti from Cuba and other Latin-American countries were investigated for organophosphate insecticide resistance, including malathion, pirimiphos methyl, temephos, fenthion, fenitrothion and chlorpyriphos. All the strains showed resistance to temephos except Nicaragua strain with moderate resistance to temephos. The highest resistant ratio (RR) value to temephos was in the Havana city strain followed by Panama, Costa Rica, Peru, Jamaica, and Venezuela. Aedes aegypti larvae were susceptible to malathion in all of the strains, and the same results obtained for fenthion and fenitrothion, but Peru strain showed moderate resistance to fenthion, and the Havana city strain had high resistance to fenthion and moderate to fenitrothion. High resistance to pirimiphos methyl has been reported in Panama, Costa Rica and Venezuela; however, Santiago de Cuba, Jamaica and Peru showed moderate resistance to this chemical. The Santiago de Cuba strain had the highest chlorpyriphos, resistant ration of 50 followed by Costa Rica and Jamaica, with Havana city, panama, Nicaragua, and Peru showing susceptibility to chlorpyriphos. The Venezuela strain showed moderate resistance to chlorpyriphos (98). Aedes aegypti mosquitoes from Peru were exposed for 1 hour with malathion (5%) and pirimiphos-methyl (0.25%) papers, and for 2 hours with fenitrothion (1%) papers. The Chosica population showed resistance to fenitrothion and pirimiphos-methyl and developing resistance to malathion. While the Punchana and Piura populations were susceptible to malathion and showed resistance to other evaluated insecticides (63). In southern Ecuador Ae. aegypti was resistant to malathion and had a mortality rate below 80% (99). In Brazil from 1999–2000 until 2010–2011, the effectiveness of temephos on Ae. aegypti was high, resulting in larval mortality of more than 80% throughout Brazil. The frequency of temephos-resistant individuals in the insect population increased steadily during each biennial survey, indicating a significant reduction in the effectiveness of temephos. This trend reached high levels (less than 50% mortality) in about half of the country in early 2004–2005 (100). In Colombia mosquitoes were susceptible to malathion. A high frequency of fenitrothion resistance was reported in all Ae. aegypti mosquitoes (58), also in 2019 no evidence of malathion resistance was found in Ae. aegypti (59). In Venezuela high mortality was obtained with fenitrothion and fenthion against adults and larvae Ae. aegypti in field strains, which suggested the absence of any appreciable amount of resistance to these insecticides, but resistance to malathion, temephos, chlorpyrifos, and pirimiphos-methyl was observed (60). Larvae of Ae. aegypti mosquito from the Guadeloupe and Saint Martin islands were resistant to temephos and malathion compared with the susceptible Bora Bora strain. For the first time in Guadeloupe, mosquito populations of Guadeloupe and Saint Martin were resistant (weakly) to malathion (101). Aedes aegypti resistance to temephos has been observed in Colombia (102), and the Caribbean (90). Result of resistance of Ae. aegypti in Brazil show resistance to at least one of the organophosphates (temephos, fenitrothion, malathion) tested in all populations in the states of Rio de Janeiro and Espírito Santo (103). A study in the city of Curitiba, State of Paraná in Brazil reported that temephos could be used for control larvae of Ae. aegypti (104). In Sao Paulo, Ae. aegypti was resistant to temephos and susceptible to malathion. It was suggested that malathion be used in ULV instead of cypermethrin in the dengue fever control program in Sao Peteo, and fenitrothion be used in residual spraying (105). The larvae of all studied Ae. aegypti populations from different Brazilian regions were resistant to temephos (106, 107). Aedes aegypti larvae from northeastern Brazil showed the highest levels of resistance to temephos, also adults from northeastern showed the lowest levels of susceptibility to malathion (108), and adults from Recife Brazil were susceptible to malathion (109). In Tocantins state in Brazil all evaluated populations of Ae. aegypti were resistant to temephos (110). Resistance to fenitrothion for the four populations of Ae. aegypti distributed along the French Guiana exhibited resistance to fenitrothion (111). A high level of resistance to temephos was reported from Ae. aegypti populations in Martinique (French West Indies) (112). In this study the resistance level of Juazeiro do Norte population was much less (RR=7.2), which is only slightly lower than the 2003 level (RR=10.2), because temephos has not been used for at least seven years to control Aedes and has been replaced by Bacillus thuriginensis (Bti). The increased resistance levels to temephos in Crato and Barbalha populations, indicate that the resistance management programs in Juazeiro do Norte should be done in neighboring cities. These results also show that even if temephos was replaced in Juazeiro do Norte, the recovery of susceptibility was slow (113). The results of bioassays with temephos, that has been used for decades against Ae. aegypti larvae in Brazil caused all the evaluated populations to be resistant to temephos (110) (Fig. 2).
In Europe
Exposure of Ae. aegypti from Paúl do Mar (Madeira) to fenitrothion given 100% mortality that indicate mosquito susceptibility to this insecticide but Ae. aegypti from Funchal (Madeira) was resistant to malathion and fenitrothion (114). Aedes albopictus adults from the Swiss-Italian border region were susceptible malathion (109) (Fig. 2).
In Australia and Oceania
Investigating the state of sensitivity to insecticides in Ae. aegypti from Townsville showed that mosquitoes were resistant to malathion and fenthion and susceptible to other organophosphates, temephos, fenitrothion and chlorpyrifos (64). Aedes aegypti and Ae. albopictus were susceptible to malathion in Papua New Guinea (65) (Fig. 2).
Resistant status of Aedes aegypti and Ae. albopictus to Carbamates insecticides
In Africa
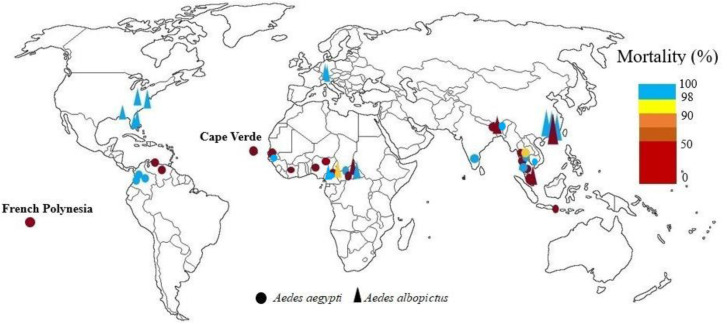
In Cameroon, resistance of Ae. aegypti to bandiocarb and tolerance of Ae. albopictus to this insecticide were reported (13). In 2021 it was reported this species was susceptible to bendiocarb (14). In Central African Republic both vectors were sensitive to propoxur and only one resistant population to propoxur was reported (15). In Kwara state, Nigeria, mortalities from exposure of Ae. aegypti to bendiocarb showed that all the mosquito samples tested were resistant (17). In northern Nigeria, Ae. aegypti mosquitoes were resistant to 0.1% propoxur (18). In Cape Verde low sensitivity Ae. aegypti to propoxur was reported and in Dakar, Senegal mortality of Ae. aegypti with propoxur was 87.2% (11). In Cote D'ivoire white and brown populations of Ae. aegypti were resistant to propoxur (19, 20). In Senegal, apart from the Matam populations of Ae. aegypti were susceptible to 0.1% bendiocarb, and all other populations were resistant to bendiocarb and propoxur (21) (Fig. 3).
Fig. 3.
World mapping of carbamates insecticide resistance in Aedes aegypti and Aedes albopictus up to December 2022
In Asia
Various levels of tolerance/resistance to propoxur and resistant to bendiocarb and propoxur in Ae. aegypti has been reported in parts of central and southern in Thailand (24, 26, 69). Aedes aegypti resistance to bendiocarb and propoxur was also reported from Malaysia as well as Ae. albopictus resistance (28, 29). In India, Ae. aegypti females were exposed to 0.1% propoxur for 30 and 60min and mortality after 24h was 96% and 100%, but in other study Ae. aegypti was resistant to propoxur, although this insecticide has not been used to control vectors in India. Resistance has been due to accidental exposure or to intersection resistance (36, 37), also Ae. albopictus from sub-Himalayan areas of West Bengal exhibit severe resistance against propoxur (38). In China, Ae. albopictus adult was sensitive to propoxur (41), and resistant to bendiocarb (42). In Indonesia Ae. aegypti was resistant to bendiocarb. Developed resistance, with about 80–90% mortality, was observed against bendiocarb 0.1% (80, 81). Papers impregnated with bendiocarb at 0.1% and propoxur at 0.1% were used for the bioassays of adult Ae. aegypt in Phnom Penh, Cambodia, results showed high mortality rates with carbamate (50). Complete susceptibility to bendiocarb was reported in populations of Ae. aegypti from Bangladesh (88) (Fig. 3).
In America
In the states of Alabama and Florida, propoxur cross-resistance levels were evaluated in Ae. albopictus. Propoxur showed the least toxicity to Ae. albopictus. The results showed that propoxur tolerance levels were absent or very low in all strains (89). In New Jersey, Pennsylvania, and Florida Ae. albopictus was sensitive to propoxur (54). In Colombia all of Ae. aegypti field-collected strains were susceptible to propoxur (59). In Venezuela Ae. aegypti resistance to propoxur was reported (60). Aedes aegypti adults from Santo Domingo, Dominican Republic, were resistant to propoxur (61) (Fig. 3).
In Europe
Aedes aegypti from two localities in Madeira (Funchal and Paúl do Mar) was resistant to bendiocarb (114). After 1h exposure and 24 h holding period to bendiocarb, Ae. albopictus field populations from the Swiss-Italian border region were susceptible (109) (Fig. 3).
In Australia and Oceania
In Australian 1995 strain of Ae. aegypti was significantly less susceptible to bendiocarb than the 1989 strain, and both strains showed high resistance to propoxur (64). Aedes aegypti from the Madang population, Papua New Guinea was susceptible to bendiocarb and Ae. albopictus also were found to be susceptible to bendiocarb (65) (Fig. 3).
Resistant status of Aedes aegypti and Ae. albopictus to Pyrethroids insecticides
In Africa
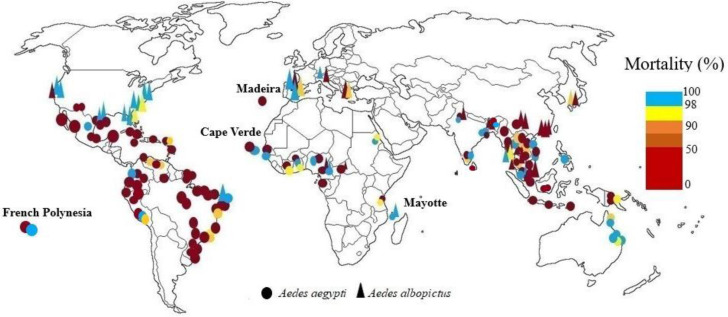
Both the Aedes species are present in Central Africa, but Ae. albopictus is more influential and was first reported in 2009. A study in this area found that both vectors were resistant to deltamethrin, and their mortality was less than 90% (15). In Ghana, Ae. aegypti was resistant to deltamethrin, and lambda-cyhalothrin but was sensitive to permethrin (9). In other study some populations were resistant to permethrin and some tolerant (10). In Mayotte both species had highly sensitive to deltamethrin. No resistance to deltamethrin has been recorded on the island, although deltamethrin has been used on the island since 1984 (66). In Cameroun complete susceptibility of both species was reported to deltamethrin except in Yaounde, where Ae. albopictus populations showed a mortality rate of about 80% (12), but in 2017 both species were resistant to deltamethrin and decreased sensitivity to permethrin was observed (13). In 2021 Ae. aegypti and Ae. albopictus mosquitoes from Cameroun were exposed to 0.05%, and 0.75% permethrin. Aedes albopictus populations showed full susceptibility to permethrin and deltamethrin insecticides, but Ae. aegypti was resistant to these insecticides (14). In a study conducted in agricultural and non-agricultural areas of Lagos State, Nigeria, all mosquitoes from both sites were susceptible to permethrin, but about deltamethrin the most populations in non-agricultural areas were resistant and mosquitoes collected from agricultural areas were reported sensitive (16). In another study, Ae. aegypti was completely sensitive to permethrin (17). Adult bioassays of Ae. aegypti in northern Nigeria were conducted by pyrethroids: 0.25% and 0.75% permethrin, 0.03% and 0.05% of deltamethrin, Lambda-cyhalothrin, and α-cypermethrin, and 0.15% cyfluthrin. Populations were susceptible to cyfluthrin but highly resistant to other type I and II pyrethroids (18). In Dakar, Senegal, Ae. aegypti populations was susceptible permethrin. About deltamethrin mortality was 94.5% and for lambda-cyhalothrin 81.6%. In Cape Verde Ae. aegypti was sensitive to deltamethrin, permethrin, and lambda-cyhalothrin (11). In another study, Ae. aegypti was resistant to deltamethrin, and cypermethrin (67). Bioassays of pyrethroids (0.25% and 0.75% permethrin), 0.05% deltamethrin, 0.03% and 0.5% lambda-cyhalothrin, 0.05% alpha-cypermethrin), by standard WHO test kits for adults of Ae. aegypti from Senegal showed that susceptibility to 0.75% permethrin was observed in all populations except in three, and one population had suspected resistance to 0.25% permethrin. Only populations of the southern regions were susceptible to type II pyrethroids (21). In Dar es Salaam, Tanzania all sites showed lower susceptibility to deltamethrin, permethrin and resistance to lambda-cyhalothrin (115). In Cote D'ivoire white populations of Ae. aegypti were resistant to deltamethrin and brown populations were susceptible (19, 20) (Fig. 4).
Fig. 4.
World mapping of pyrethroids insecticide resistance in Aedes aegypti and Aedes albopictus up to December 2022
In Asia
In Japan Ae. albopictus in Nagasaki showed high tolerance to pyrethroids. The cause for this may be due to the widespread use of DDT in the 1950s (116). Most studies on pyrethroid resistance in Ae. aegypti in Southeast Asia are from Thailand. The first outbreak of dengue fever in Thailand was in 1958 (117). Since 1950, carbamates, organophosphates, and organochlorines have been used for control. Synthetic pyrethroids have been used since 1992, low price of pyrethroids, their quick knockdown effect and relative safety for humans due to low toxicity to mammals has increased their use (22). Increased tolerance or resistance to pyrethroids has been reported from Ae. aegypti and Ae. albopictus in Thailand, such as deltamethrin and permethrin, which are widespread throughout Thailand, resistance to cypermethrin in parts of northern and western Thailand, and resistance to bioallethrin, bioresmethrin, and alpha-cypermethrin in larvae and adults. Mortality of Ae. aegypti with lambda cyhalothrin was 100% and had tolerated to mosquito coils. Aedes aegypti has shown behavioral resistance and avoidance to alpha-cypermethrin, deltamethrin, permethrin, tetramethrin, cyphenothrin in Thailand (26, 68, 118–120). In Malaysia, many studies have been conducted on the resistance of the two species to insecticides. The first study on pyrethroid resistance was in 2001. The Ae. aegypti urban strain had the highest resistance to permethrin, cyphenothrin, lambda-cyhalothrin, and deltamethrin. Aedes albopictus was sensitive to permethrin and deltamethrin (121–123). The first study on mosquito coils against Ae. aegypti was in 1996, that mortality was minimal (124). In 2017, in a study was reported that mosquito coils had low insecticidal activity against Ae. aegypti and therefore may have little protection for mosquito bites (125). In Malaysia was reported Ae. aegypti resistance to permethrin (29). In the other study across Malaysia, Ae. aegypti was resistant to permethrin and deltamethrin (28). In India, Ae. aegypti and Ae. albopictus resistance to pyrethroids such as permethrin has been reported in Delhi and Kerala (73). In 2001 Ae. aegypti adults was sensitive to deltamethrin, permethrin, and lambda cyhalothrin in Delhi (33). In Another study a total of five different pyrethroid compounds were tested against Ae. aegypti females with exposure period of 5 and 15min in Madurai West city in Tamil Nadu State, they were found invariably susceptible to all tested pyrethroid compounds. Based on the results, the efficacy in descending order of pyrethroids was cyfluthrin > permethrin > deltamethrin > lambda cyhalothrin and > etofenprox (36). In recent studies in West Bengal, all populations Ae. aegypti except one site were sensitive to deltamethrin and alpha-cypermethrin (75), and two Ae. albopictus populations, from sub-Himalayan districts were severely resistant to permethrin (38). In China, Ae. albopictus is resistant to deltamethrin, permethrin, cypermethrin, and beta-cypermethrin, and resistance to pyrethroids is greater than resistance to other common insecticide groups (40–42). In Cambodia, there is Ae. aegypti adult resistance to the two main insecticides including permethrin (nets) and deltamethrin (fumigation) in all regions (126). The pyrethroids alpha-cypermethrin at 0.03%, bifenthrin at 0.2%, cyfluthrin at 0.15%, deltamethrin at 0.03%, etofenprox at 0.5%, lambda-cyhalothrin at 0.03% and permethrin at 0.25% were used for the bioassays of adult Ae. aegypti. The adult mortalities were 0 % for etofenprox, 1±1.9% for permethrin, 3.1± 4.1% for bifenthrin, 4.2±8.3% for lambda-cyhalothrin, 10.2±7.1% for deltamethrin, 11± 4.5% for alpha-cypermethrin and 35±11% for cyfluthrin and there were low mortality rates with all the tested pyrethroid insecticides (50). In Indonesia, Ae. aegypti larvae were sensitive to pyrethroids (78, 127). Aedes aegypti adult resistance was reported to more than one pyrethroids of cypermethrin, permethrin and D-allethrin (78). In other study, adult Ae. aegypti resistance to permethrin and deltamethrin and high levels of resistance to alpha-cypermethrin and lambda cyhalothrin has been reported (128). In 2017, all Ae. aegypti adults tested in Jakarta were resistant to deltamethrin, permethrin, and lambda cyhalothrin, with a mortality rate of less than 90%. Resistance to permethrin was higher than the others (79). Also, resistance of Ae. aegypti to deltamethrin, permethrin, lambda cyhalothrin, cyfluthrin in Denpasar, Bali has been reported (80). In Banjarmasin, Kalimantan, Indonesia, all Ae. aegypti populations showed different degree of resistance to 0.15% cyfluthrin 0.05% deltamethrin, 0.05% Lambda-cyhalothrin, and 0.75% permethrin, with mortalities less than 90% (81). In Laos high level of resistance against permethrin, and high susceptibility to deltamethrin was reported in Ae. aegypti (129). In the other study seven populations of Ae. albopictus were sensitive to permethrin and deltamethrin (43). In Myanmar high pyrethroid resistance (permethrin and deltamethrin) has been reported in the larvae and adult of Ae. aegypti (44). In Singapore, Ae. aegypti is the primary vector of Dengue Virus. The first case was reported in the 1960s (130). Aedes aegypti resistance to permethrin, cypermethrin, and deltamethrin has been demonstrated in Singapore (83–85). In other two studies, Ae. aegypti larvae were resistant to permethrin and etofenprox (86), and Ae. aegypti adult with PermaNet® had less than 80 % mortality (131). Aedes aegypti from Dili, Timor-Leste, has become resistant to permethrin, lambda-cyhalothrin and resmethrin (87). In the first insecticide resistance investigation of Ae. aegypti in several locations of Central Highlands and Nam Bo in Vietnam, the mosquitoes were susceptible to pyrethroids such as deltamethrin in the central and northern regions. In the southern and central mountainous regions Ae. aegypti was resistant to deltamethrin, permethrin, lambda-cyhalothrin and alpha-cypermethrin. This is due to the long use of pyrethroids in the control of malaria and dengue fever and its use in agriculture, especially in the southern and central regions (46). In the next study, Ae. aegypti's sensitivity to permethrin, lambda-cyhalothrin, deltamethrin, and alpha-cypermethrin was reported in many parts of the north and center but was resistant to these insecticides in southern and central Vietnam (47). In 2009, it was reported that, Ae. aegypti sensitivity to D-allethrin has decreased. Mosquitoes were sensitive to pyrethroids in northern and central Vietnam but were resistant in southern and central Vietnam (132). In 2016 and 2003 also, Ae. aegypti resistance was reported to permethrin and lambda-cyhalothrin (48, 133). Also, populations of Ae. albopictus tested from Vietnam exhibited high-level resistance to permethrin (134). In 2010, study of pyrethroid susceptibility of larvae Ae. albopictus collected from Nagasaki City, Japan, indicated populations of Ae. albopictus tolerant to pyrethroids spread widely in Nagasaki (116). In other study in this city insecticide susceptibility tests were performed on Ae. albopictus adults and larvae of F1 colonies collected from Nagasaki City, Japan. The results were compared with those of several such colonies collected from other locations in Japan. The larvae collected from Nagasaki City, also from other locations in Japan were resistant to d-T80-allethrin and more than half of the adults of the Nagasaki and Fukuoka colonies showed resistance to permethrin (135). Susceptibility of adult Ae. aegypti mosquitoes from Sri Lanka to deltamethrin 0.05%, permethrin 0.75% were evaluated by the standard WHO mosquito bioassay protocol. Resalts revealed resistance in all Ae. aegypti populations for both permethrin (10–89%) and deltamethrin (40–92%) (71). In Bangladesh Ae. aegypti mosquitoes were exposed to diagnostic dose of 15μg/bottle for permethrin, and 10μg/bottle for deltamethrin. High levels of resistance to permethrin were reported in Ae. aegypti, while sensitivity to deltamethrin was different between populations (88) (Fig. 4).
In America
High resistance to pyrethroids were observed in Ae. aegypti. All Grand Cayman Ae. aegypti populations survived after one hour of exposure to pyrethroid-impregnated papers (53). In the USA adult mortality after a 24h exposure to the pyrethroid insecticides (deltamethrin, prallethrin, and phenothrin) at discriminating doses showed that, all the field populations tested were susceptible (99–100% mortality) (54). In Florida, California, North Carolina, and Texas, vectors were resistant to low doses of etofenprox. Ae. aegypti was also resistant to high doses in Texas. Six populations of Ae. albopictus showed susceptibility to bifenthrin and two populations Ae. aegypti and Ae. albopictus were resistant and possible resistance was shown in one Ae. albopictus population. Seven Aedes spp. populations were susceptible (100%) to permethrin and one population was resistant. Possible resistance was shown in one Ae. albopictus population. All tested Ae. albopictus were susceptible (100%) to phenothrin and one population of Ae. aegypti was resistant. Six Ae. albopictus populations were susceptible to the low dose of deltamethrin. At the high deltamethrin dose, seven Ae. albopictus populations were susceptible and two Ae. albopictus and Ae. aegypti populations showed possibility of resistance (91). Aedes aegypti from New Mexico showed high pyrethroid resistance and kdr mutation F1534C in the para gene (93). In Texas/Mexico border cities all populations of Ae. aegypti showed resistance to permethrin, deltamethrin and sumithrin, although none of these insecticides are commonly used for vector control activities in this region (136). All Ae. aegypti mosquitoes tested in Jamaica were resistant to permethrin (92). Laboratory bioassay on the Island of Martinique (Caribbean) showed that Ae. aegypti populations were strongly resistant to pyrethrins and deltamethrin therefore insecticide resistance decreased the efficacy of space sprays for adult mosquito and dengue control (95, 137). There is evidence of resistance to permethrin in populations in Baja California North, South and in Quintana Roo, south of Mexico and some states of northeast Mexico due to use for more than 10 years in Mexico for control of Ae. aegypti (138–140). The results obtained from Veracruz State Mexico showed that the field strains of Ae. aegypti were resistant to most of the pyrethroids analyzed including cypermethrin, deltamethrin, δ-phenothrin, α-cypermethrin, d-phenothrin, z-cypermethrin, λ-cyhalothrin, bifenthrin, and permethrin and suggested that populations in the state of Veracruz were under the strong selection pressure, causing from the continuous use of permethrin for more than a decade (141). In Cuba, Ae. aegypti resistance was reported to deltamethrin, lambda cyhalothrin, beta-cypermethrin, cypermethrin, and cyfluthrin (55, 56). Aedes aegypti from Jamaica was resistant to permethrin with types of the mode of mechanism (92). One study on mosquitoes collected from eight Latin American countries showed that larvae from the Havana city strain were resistant to all the pyrethroids, the highest RR50 was to deltamethrin, as well as lambda cyhalothrin, betacypermethrin and cyfluthrin showed high resistance. Larvae from Santiago De Cuba, Peru and Venezuela showed high deltamethrin RR50 values as well as Havana City (98). Populations of Ae. aegypti from Peru were exposed for 1h to papers treated with alpha-cypermethrin (0.05%), cypermethrin (0.05%), deltamethrin (0.05%), etofenprox (0.5 %), lambda-cyhalothrin (0.05%), and permethrin (0.75%). Results showed Ae. aegypti from Chosica was resistant to alpha-cypermethrin, cypermethrin, etofenprox, also developing resistance to lambda-cyhalothrin, and susceptibility to deltamethrin were reported. But the Punchana and Piura populations were resistant to all insecticides (63). In Tocantins state in Brazil the data showed different resistance to deltamethrin among the samples of Ae. aegypti (110). In southern Ecuador Ae. aegypti was resistant to deltamethrin and alpha-cypermethrin (99). In study of insecticide resistance status of Ae. aegypti from Colombia, susceptibility to deltamethrin and cyfluthrin was reported in all mosquito populations studied. Six populations were resistant to lambda-cyhalothrin. Four field populations showed resistance to permethrin, two populations were also resistant to lambda-cyhalothrin. Pyrethroid resistance was not found in populations from the state of Antioquia. Resistance to etofenprox was reported in all mosquito populations, however, there is no record of using this insecticide for dengue control programs at any of the study sites (58). In other study the most of field populations were resistant to permethrin. The resistance to deltamethrin was observed only in one site and lambda cyhalothrin resistance in three sites (59). The lambda cyhalothrin resistance has been commonly detected in Colombia (142). In Venezuela Ae. aegypti moderate levels of resistance to permethrin and lambda cyhalothrin was reported (60). Aedes aegypti in different Brazilian regions were resistant to deltamethrin, cypermethrin, permethrin (104–108, 113, 143). In Brazil Ae. albopictus adult mortality rates with permethrin and λ-cyhalothrin were close to 100% (109). Evaluation of insecticide resistance in Ae. aegypti populations the case of Tocantins state in Brazil showed different state of resistance to deltamethrin among the samples (110). In one study, severe resistance to deltamethrin with weak knockdown effect and low mortality was observed in French Guiana Ae. aegypti populations (111). Aedes aegypti adults from Santo Domingo, Dominican Republic, were reported resistant to permethrin and deltamethrin (61). Tests done with adult mosquitoes of Ae. aegypti reported moderate resistance status to deltamethrin from Guadeloupe and Saint Martin islands (101) (Fig. 4).
In Europe
In Italy resistance to α-cypermethrin and permethrin in adult Ae. albopictus has been reported. Resistance to permethrin was seen from Ferrara Province in Emilia-Romagna and from Bari Province in Puglia while the field-population from Athens (Greece) showed possibility of resistance. Resistance to α-cypermethrin was reported for the mosquitoes in Ferrara Province Venezia Province and Rome but four Italian populations were susceptible. Eight Italian populations, and the Albanian one, showed susceptibility to deltamethrin but the Greek laboratory colony was resistant (144). In the Swiss-Italian border region Ae. albopictus adult mortality rates with permethrin and λ-cyhalothrin were close to 100% (109). In Spain, 5 populations of Ae. albopictus were studied, four populations were sensitive to deltamethrin and permethrin. In the case of cypermethrin, two populations of Ae. albopictus were sensitive to this toxin and two populations were tolerant and one population was reported to be resistant (145). Aedes aegypti from Funchal was reported to be resistant to cyfluthrin and permethrin. Resistance to pyrethroids was also seen in the Paúl do Mar population. Pyrethroids mortality rates were between 2% (permethrin), and 88% (cyfluthrin) (114). Populations of Ae. albopictus tested from Italy exhibited high-level resistance to permethrin (134) (Fig. 4).
In Australia and Oceania
Results a study in 1999 showed that there was no evidence of resistance to synthetic pyrethroids (deltamethrin and permethrin) (64). Experiments on Queensland mosquitoes with pyrethroids (bifenthrin, deltamethrin and lambda-cyhalothrin) did not show Ae. aegypti resistant, and bioassays showed only weak tolerance. There was no evidence of kdr mutations in Queensland Ae. aegypti. In Cairns South, a low resistance to lambda-cyhalothrin was reported in a sample of Ae. aegypti at 24h after exposure. Obvious resistance to bifenthrin has not been detected in field populations of Ae. aegypti from northern Queensland (146). Study of resistance of Ae. aegypti and Ae. albopictus in Papua New Guinea clearly show high pyrethroid resistance: deltamethrin and lambda cyhalothrin in both Madang and Port Moresby Ae. aegypti populations. The Ae. aegypti population in Port Moresby show a higher level of deltamethrin resistance than Madang where susceptibility to lambda-cyhalothrin has been greatly reduced (65) (Fig. 4).
Resistant status of Aedes aegypti and Ae. albopictus to Insect growth regulator (IGRs) and microbial insecticides
In Africa
In Cameroon, Gabon (12) and Central African Republic (15) larvae of both species were susceptible to Bacillus thuringiensis israelensis (Bti). In Mayotte complete susceptibility to Bti, and IGRs including spinosad, diflubenzuron, pyriproxyfen, and methoprene was reported (66). In Cape Verde also was observed sensitivity to diflubenzuron and Bti (67) (Fig. 5).
Fig. 5.
World mapping of Insect growth regulator (IGRs) and microbial insecticides insecticide resistance in Aedes aegypti and Aedes albopictus up to December 2022
In Asia
In China, Ae. albopictus larvae from all studied areas were sensitive to Bti and hexafluoromoron, but in one population showed high resistance to pyriproxyfen and moderate resistance to pyriproxyfen (42). In Singapore, larval mortality due to Bti was 100% (86). In Malaysia susceptibility of field Ae. aegypti larvae to Bti was reported (147), but Ae. aegypti and Ae. albopictus populations from 12 states in Malaysia showed a different state of resistance to five IGRs (pyriproxyfen, methoprene, cyromazine, novaluron and diflubenzuron). Aedes albopictus was susceptible against cyromazine, pyriproxyfen, and methoprene. Field-collected Ae. aegypti showed high susceptibility to diflubenzuron, cyromazine, and novaluron (148). Susceptibility of a wild larval population of Ae. aegypti against Bti, diflubenzuron, pyriproxyfen and spinosad was tested in Vientiane Laos. With bioassays of larvae was found that the wild Ae. aegypti strain was moderately resistant to spinosad and susceptible to the other insecticides. Based on field bioassays, all tested insecticides remained above the WHO acceptable larvicidal threshold after 28 weeks (82). In Cambodia for larvae, moderate Ae. aegypti resistance was reported for spinosad (RR90 < 5.6) but, there was no resistance against Bti (RR90 < 1.6) (50) (Fig. 5).
In America
The susceptibility Ae. albopitus to imidacloprid, spinosad, and Bti was investigated in Alabama and Florida. These results reported that mosquitoes collected from field were much more susceptible to spinosad than to the other insecticides tested in the study (89). Of the eight USA populations of Ae. albopictus, none were resistant to the larvicides tested (Bti, spinosad, methoprene, and pyriproxyfen) but reduced susceptibility Ae. albopictus to the IGRs pyriproxyfen and methoprene was observed in Florida and New Jersey (54). The larvae of all studied Ae. aegypti populations from different Brazilian regions were susceptible to diflubenzuron and Bti (106, 107, 109). A tolerance to IGRs and full susceptibility to spinosad and Bti was reported in Martinique. In experiments pyriproxyfen and Bti had 28- and 37-weeks activities in permanent breeding containers, whereas under field conditions they could not control Ae. Aegypti populations after four weeks. But diflubenzuron and spinosad were effective for 16 weeks, therefore these chemicals can be alternatives to Bti and temephos to control resistant Ae. aegypti (112, 149). In French Guiana, ULV spraying with BT was caused 100% mortality in Ae. aegypti populations (111) (Fig. 5).
In Europe
The Ae. albopictus populations from the Canton of Ticino in southern Switzerland, and the Como area in northern Italy were all susceptible to Bti. The efficacy of Lysinibacillus sphaericus, another entomopathogenic bacterium, was tested against Ae. albopictus and showed good activity. The activity a mixture of Bti and L. sphaericus crystals “Vectomax®”, was also studied to determine if these insecticidal components are effective for controlling Ae. albopictus. In this study Ae. albopictus was also susceptible to diflubenzuron (109) (Fig. 5).
Mechanisms of insecticide resistance in Aedes aegypti and Ae. albopictus
The mode of action of insecticides is mostly on the nervous system of insects. Target-site mutations and metabolic resistance are two main resistance mechanisms in Ae. aegypti and Ae. albopictus. In the mutation at the target site, which is one of the worst resistance mechanisms in insects, the insect makes the insecticides ineffective by changing the structure of the targets of the insecticides. Pyrethroids and DDT act on voltage-gated sodium channels, and the mutation in the amino acid sequence of this gene causes the sensitivity of the channels to prevent the binding of pyrethroids and DDT to decrease. The use of pyrethroids with cross-resistance mechanisms (especially kdr mutations) existing in DDT caused to the rapid rise of pyrethroid resistance. Also, resistance to acetylcholinesterase (AChE) is current in Aedes and is related to organophosphate and carbamate insecticides. Mutations in the gene coding for the neurotransmitter acetylcholinesterase number one (ace-1) decrease the inhibition effect of the insecticide on the enzyme. Metabolic resistance is very current in mosquitoes and has been observed against all insecticides used for vector control. Mosquitoes have enzyme systems that protect them from foreign compounds, and some of these enzymes can break down insecticides before they reach their site of action. In metabolic resistance, the enzymes that detoxify the insecticide are overexpressed or the enzyme's affinity for the insecticide is altered by amino acid substitution. Three of the most important of these enzymes are cytochrome P450 monooxygenases, glutathione S-transferases (GSTs) and esterases (62, 150, 151). Bti bacterial larvicide due to the presence of an intact endotoxin complex and synergy among individual toxins, especially the presence of Cyt1A, it has the lowest risk of developing resistance. Bti plays an important role in reducing the development of resistance and maintaining sensitivity in other biolarvicids. Binary toxins from Bacillus sphaericus have many advantages in controlling mosquito larvae. Laboratory data and field studies worldwide showed that a combination of Bti with B. sphaericus, the best way to increase larvicidal activity is to prevent resistance and restore susceptibility to B. sphaericus. In the case of IGR insecticides, the risk of developing resistance is low. Late 4th instar larvae and pupae with lower internal juvenile hormone titers are more susceptible to juvenile hormone analogs such as methoprene and pyriproxyfen in the transfer from late 4th instar larvae to pupae and adults. In aquatic habitats, different ages of mosquito larvae coexist, with very different levels of internal rejuvenation hormones. This phenomenon leads to sublethal exposures, tolerance and even the development of resistance (152).
Conclusions
WHO recommended bendiocarb, propoxur, DDT, fenitrothion, malathion, pirimiphos-methyl, á-cypermethrin, bifenthrin, cyfluthrin, deltamethrin, etofenprox, lambda cyhalothrin has been recommend as indoor residual spraying for mosquito control. Alpha-cypermethrin, cyfluthrin, deltamethrin, deltamethrin, etofenprox, lambda cyhalothrin, permethrin are being used for impregnation of bednets. Fenitrothion, malathion, pirimiphos-methyl, bioresmethrin, cyfluthrin, cypermethrin, cyphenothrin, d,d-trans-cyphenothrin, deltamethrin, d-phenothrin, etofenprox, lambda cyhalothrin, permethrin, resmethrin for thermal fog and space spraying. Fuel oil, B. thurigiensis, chlorpyrifos, diflubenzuron, fenthion, methoprene, novaluron, pyriproxyfen, pirimiphos-methyl, and temephos are recommended as larvicides.
Because of importance of Ae. aegypti and Ae. albopictus in transmission mosquito-borne arboviruses, resistance management programs and strategies should be further considered in worldwide to prevention from insecticide resistance in arbovirus vectors.
Acknowledgements
This research is supported by Ministry of Health and Medical Education under code number of NIMAD 982984.
Footnotes
Conflict of interest statement
The authors declare there is no conflict of interests.
References
- 1.Vega-Rúa A, Lourenço-de-Oliveira R, Mousson L, Vazeille M, Fuchs S, Yébakima A. (2015) Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Negl Trop Dis. 9(5): e0003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreira-de-Brito A, Ribeiro IP, Miranda RMd, Fernandes RS, Campos SS, Silva KABd. (2016) First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Mem Inst Oswaldo Cruz. 111(10): 655–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochedez P, Jaureguiberry S, Debruyne M, Bossi P, Hausfater P, Brucker G. (2006) Chikungunya infection in travelers. Emerg Infect Dis. 12(10): 1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong P-SJ, Li M-zI, Chong CS, Ng LC, Tan CH. (2013) Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis. 7(8): e2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF. (2013) The global distribution and burden of dengue. Nature. 496(7446): 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) (2020) Ethics and vector-borne diseases: WHO guidance, available at: https://www.who.int/. License: CC BY-NC-SA 3.0 IGO/ (access 20 March 2020) [Google Scholar]
- 7.Rose RI. (2001) Pesticides and public health: integrated methods of mosquito management. Emerg Infect Dis. 7(1): 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO) (2014) Management of insecticide resistance in vectors of public health importance. Report of the ninth meeting of the Global Collaboration for Development of Pesticides for Public Health (GCDPP), available at: https://apps.who.int/iris/handle/10665/145673/ (access 10 September 2014) [Google Scholar]
- 9.Suzuki T, Osei JH, Sasaki S, Adimazoya M, Appawu M, Boakye D. (2016) Risk of transmission of viral haemorrhagic fevers and the insecticide susceptibility status of Aedes aegypti (Linnaeus) in some sites in Accra, Ghana. Ghana Med J. 50 (3): 136–141. [PMC free article] [PubMed] [Google Scholar]
- 10.Kawada H, Higa Y, Futami K, Muranami Y, Kawashima E, Osei JH. (2016) Discovery of point mutations in the voltage-gated sodium channel from African Aedes aegypti populations: potential phylogenetic reasons for gene introgression. PLoS Negl Trop Dis. 10(6): e0004780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dia I, Diagne CT, Ba Y, Diallo D, Konate L, Diallo M. (2012) Insecticide susceptibility of Aedes aegypti populations from Senegal and Cape Verde Archipelago. Parasit Vectors. 5(1): 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamgang B, Marcombe S, Chandre F, Nchoutpouen E, Nwane P, Etang J. (2011) Insecticide susceptibility of Aedes aegypti and Aedes albopictus in Central Africa. Parasit Vectors. 4(1): 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamgang B, Yougang AP, Tchoupo M, Riveron JM, Wondji C. (2017) Temporal distribution and insecticide resistance profile of two major arbovirus vectors Aedes aegypti and Aedes albopictus in Yaoundé, the capital city of Cameroon. Parasit Vectors. 10(1): 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djiappi-Tchamen B, Nana-Ndjangwo MS, Mavridis K, Talipouo A, Nchoutpouen E, Makoudjou I. (2021) Analyses of insecticide resistance genes in Aedes aegypti and Aedes albopictus mosquito populations from cameroon. Genes (Basel). 12(6): 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngoagouni C, Kamgang B, Brengues C, Yahouedo G, Paupy C, Nakouné E. (2016) Susceptibility profile and metabolic mechanisms involved in Aedes aegypti and Aedes albopictus resistant to DDT and deltamethrin in the Central African Republic. Parasit Vectors. 9(1): 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayorinde A, Oboh B, Oduola A, Otubanjo O. (2015) The insecticide susceptibility status of Aedes aegypti (Diptera: Culicidae) in farm and nonfarm sites of Lagos State, Nigeria. J Insect Sci. 15(1): 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oduola AO, Obembe A, Adelaja OJ, Ande AT. (2016) Surveillance and insecticide susceptibility status of culicine mosquitoes in selected communities utilizing long-lasting insecticidal nets in Kwara State, Nigeria. Anim Res Int. 13(3): 2483–2491. [Google Scholar]
- 18.Mukhtar MM, Ibrahim SS. (2022) Temporal Evaluation of Insecticide Resistance in Populations of the Major Arboviral Vector Aedes aegypti from Northern Nigeria. Insects. 13(2): 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guindo-Coulibaly N, Adja AM, Koffi AA, Diakité NR, Ahoua Alou P, Bassa KF. (2014) Insecticides susceptibility of two distinct morphologies at larval stage of Aedes aegypti (Diptera: Culicidae) from Abidjan (Côte d'Ivoire). Eur J Sci Res. 126: 434–443. [Google Scholar]
- 20.Konan LY, Coulibaly IZ, Kone BA, Ziogba J-CT, Diallo A, Ekra DK. (2012) Aedes aegypti susceptibility to insecticide from Abidjan City, Cote D'ivoire. Vector Borne Zoonotic Dis. 12(4): 325–329. [DOI] [PubMed] [Google Scholar]
- 21.Sene NM, Mavridis K, Ndiaye EH, Diagne CT, Gaye A, Ngom EHM. (2021) Insecticide resistance status and mechanisms in Aedes aegypti populations from Senegal. PLoS Negl Trop Dis. 15(5): e0009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chareonviriyaphap T, Aum-Aung B, Ratanatham S. (1999) Current insecticide resistance patterns in mosquito vectors in Thailand. Southeast Asian J Trop Med Public Health. 30: 184–194. [PubMed] [Google Scholar]
- 23.Somboon P, Prapanthadara L-a, Suwonkerd W. (2003) Insecticide susceptibility tests of Anopheles minimus sl, Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus in northern Thailand. Southeast Asian J Trop Med Public Health. 34(1): 87–93. [PubMed] [Google Scholar]
- 24.Jirakanjanakit N, Rongnoparut P, Saengtharatip S, Chareonviriyaphap T, Duchon S, Bellec C. (2014) Insecticide susceptible/resistance status in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Thailand during 2003–2005. J Econ Entomol. 100(2): 545–550. [DOI] [PubMed] [Google Scholar]
- 25.Yaicharoen R, Kiatfuengfoo R, Chareonviriyaphap T, Rongnoparut P. (2005) Characterization of deltamethrin resistance in field populations of Aedes aegypti in Thailand. J Vector Ecol. 30(1): 144–150. [PubMed] [Google Scholar]
- 26.Thanispong K, Sathantriphop S, Chareonviriyaphap T. (2008) Insecticide resistance of Aedes aegypti and Culex quinquefasciatus in Thailand. Pestic Sci. 33(4): 351–356. [Google Scholar]
- 27.Prapanthadara L-a, Promtet N, Koottathep S, Somboon P, Suwonkerd W, McCarroll L, (2002) Mechanisms of DDT and permethrin resistance in Aedes aegypti from Chiang Mai, Thailand. Dengue Bull. 26: 185–189. [Google Scholar]
- 28.Ishak IH, Jaal Z, Ranson H, Wondji CS. (2015) Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasit Vectors. 8: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loke S, Wei Ann A, Ahmad NW, L HL, Azirun MS. (2012) Insecticide susceptibility status of field-collected Aedes (Stegomyia) aegypti (L.) from a dengue endemic site in Shah Alam, Selangor, Malaysia. Southeast Asian J Trop Med Public Health. 43(1): 34–40. [PubMed] [Google Scholar]
- 30.Azeez S. (1967) A note on the prevalence and susceptibility status of Aedes (Stegomyia) aegypti (Linn.) in Jharia, Dhanbad District (Bihar). J Commun Dis. 4 (1): 59–62. [Google Scholar]
- 31.Thavaselvam D, KUMAR A, Sumodan P. (1993) Insecticide susceptibility status of Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti in Panaji, Goa. Indian J Malariol. l30: 75–79. [PubMed] [Google Scholar]
- 32.Singh R, Dhiman R, Mittal P, Dua V. (2011) Susceptibility status of dengue vectors against various insecticides in Koderma (Jharkhand), India. J Vector Borne Dis. 48(2): 116–121. [PubMed] [Google Scholar]
- 33.Katyal R, Tewari P, Rahman S, Pajni H, Kumar K, Gill K. (2001) Susceptibility status OF immature and Adult Stages of Aedes aegypti against conventional insecticides in Delhi, India. Dengue Bull. 25: 84–87. [Google Scholar]
- 34.Tikar S, Mendki M, Chandel K, Parashar B, Prakash S. (2008) Susceptibility of immature stages of Aedes (Stegomyia) aegypti; vector of dengue and chikungunya to insecticides from India. Parasitol. Res. 102(5): 907–1002. [DOI] [PubMed] [Google Scholar]
- 35.Yadav K, Rabha B, Dhiman S, Veer V. (2015) Multi-insecticide susceptibility evaluation of dengue vectors Stegomyia albopicta and St. aegypti in Assam, India. Parasit Vectors. 8(1): 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mariappan T, Selvam A, Rajamannar V, Arunachalam N. (2017) Susceptibility of Dengue/Chikungunya vector, Aedes aegypti against carbamate, organochlorine, organophosphate and pyrethroid insecticides. J Environ Biol. 38(2): 251–260. [Google Scholar]
- 37.Bharati M, Saha D. (2018) Multiple insecticide resistance mechanisms in primary dengue vector, Aedes aegypti (Linn.) from dengue endemic districts of sub-Himalayan West Bengal, India. PloS One. 13 (9): e0203207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bharati M, Rai P, Saha D. (2019) Insecticide resistance in Aedes albopictus Skuse from sub-Himalayan districts of West Bengal, India. Acta Trop. 192: 104–111. [DOI] [PubMed] [Google Scholar]
- 39.Neng W, Yan X, Fuming H, Dazong C. (1992) Susceptibility of Aedes albopictus from China to insecticides, and mechanism of DDT resistance. J Am Mosq Control Assoc. 8(4): 394–397. [PubMed] [Google Scholar]
- 40.Cui F, Raymond M, Qiao CL. (2006) Insecticide resistance in vector mosquitoes in China. Pest Manag Sci. 62(11): 1013–1022. [DOI] [PubMed] [Google Scholar]
- 41.Yiguan W, Xin L, Chengling L, Su T, Jianchao J, Yuhong G. (2017) A survey of insecticide resistance in Aedes albopictus (Diptera: Culicidae) during a 2014 dengue fever outbreak in Guangzhou, China. J Econ Entomol. 110(1): 239–244. [DOI] [PubMed] [Google Scholar]
- 42.Su X, Guo Y, Deng J, Xu J, Zhou G, Zhou T. (2019) Fast emerging insecticide resistance in Aedes albopictus in Guangzhou, China: Alarm to the dengue epidemic. PLOS Negl Trop Dis. 13(9): e0007665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tangena J-AA, Marcombe S, Thammavong P, Chonephetsarath S, Somphong B, Sayteng K. (2018) Bionomics and insecticide resistance of the arboviral vector Aedes albopictus in northern Lao PDR. PloS One. 13(10): e0206387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawada H, Oo SZM, Thaung S, Kawashima E, Maung YNM, Thu HM. (2014) Cooccurrence of point mutations in the voltage-gated sodium channel of pyrethroid-resistant Aedes aegypti populations in Myanmar. PLoS Negl Trop Dis. 8(7): e3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnsen R. (1967) Preliminary studies of mosquito resistance to insecticides in the Philippines. Mosq News. 27(1): 22–26. [Google Scholar]
- 46.Huong VD, Thi Bach Ngoc N. (1999) Susceptibility of Aedes aegypti to Insecticides in South Vietnam. Dengue Bull. 23: 85–88. [Google Scholar]
- 47.Huong VD, Bach Ngoc NT, Hien DT, Bich Lien NT. (2004) Susceptibility of Aedes aegypti to insecticides in Viet Nam. Dengue Bull. 28: 179–183. [Google Scholar]
- 48.Thi K, Viet H, Nguyen H. (2016) Major resistant mechanism to insecticides of Aedes aegypti mosquito: a vector of dengue and Zika virus in Vietnam. J Trop Med. 1(2): 1010–1016. [Google Scholar]
- 49.Kawada H. (2017) DDT and Pyrethroid Resistance in Aedes aegypti (L.) and Aedes albopictus (skuse): Past, Present, and Future. In: Sanders K. (Ed): DDT. Vol. 1. Nova Science Publishers, Inc, pp. 33–83. [Google Scholar]
- 50.Boyer S, Maquart P-O, Chhuoy K, Suor K, Chhum M, Heng K. (2022) Monitoring insecticide resistance of adult and larval Aedes aegypti (Diptera: Culicidae) in Phnom Penh, Cambodia. Parasit Vectors. 15(1): 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown AWA, Pal R. (1971) Insecticide resistance in arthropods. 2nd ed. World Health Organization. Available at: https://apps.who.int/iris/handle/10665/41685. [PubMed] [Google Scholar]
- 52.Camargo S. (1967) History of Aedes aegypti eradication in the Americas. Bull World Health Organ. 36(4): 602–603. [PMC free article] [PubMed] [Google Scholar]
- 53.Harris AF, Rajatileka S, Ranson H. (2010) Pyrethroid resistance in Aedes aegypti from Grand Cayman. Am J Trop Med Hyg . 83(2): 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marcombe S, Farajollahi A, Healy SP, Clark GG, Fonseca DM. (2014) Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PloS One. 9(7): e101992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodríguez MM, Bisset J, Ruiz M, Soca A. (2002) Cross-resistance to pyrethroid and organophosphorus insecticides induced by selection with temephos in Aedes aegypti (Diptera: Culicidae) from Cuba. J Med Entomol. 39(6): 882–888. [DOI] [PubMed] [Google Scholar]
- 56.Rodríguez MM, Bisset JA, De Armas Y, Ramos F. (2005) Pyrethroid insecticide-resistant strain of Aedes aegypti from Cuba induced by deltamethrin selection. J Am Mosq Control Assoc. 21(4): 437–445. [DOI] [PubMed] [Google Scholar]
- 57.Varón LS, Córdoba BC, Brochero HL. (2010) Susceptibilidad de Aedes aegypti a DDT, deltametrina y lambdacialotrina en Colombia. Rev Panam Salud Publica. 27(1): 67–73. [DOI] [PubMed] [Google Scholar]
- 58.Fonseca González I, Quiñones ML, Lenhart A, Brogdon WG. (2011) Insecticide resistance status of Aedes aegypti (L.) from Colombia. Pest Manag Sci. 67(4): 430–437. [DOI] [PubMed] [Google Scholar]
- 59.Aponte A, Penilla RP, Rodríguez AD, Ocampo CB. (2019) Mechanisms of pyrethroid resistance in Aedes (Stegomyia) aegypti from Colombia. Acta Trop. 191: 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mazzarri MB, Georghiou G. (1995) Characterization of resistance to organophosphate, carbamate, and pyrethroid insecticides in field populations of Aedes aegypti from Venezuela. J Am Mosq Control Assoc. 11(3): 315–322. [PubMed] [Google Scholar]
- 61.Mekuria Y, Gwinn T, Williams D, Tidwell M. (1991) Insecticide susceptibility of Aedes aegypti from santo Domingo, Dominican Republic. J Am Mosq Control Assoc. 7(1): 69–72. [PubMed] [Google Scholar]
- 62.Corbel V, Fonseca DM, Weetman D, Pinto J, Achee NL, Chandre F. (2017) International workshop on insecticide resistance in vectors of arboviruses, December Rio de Janeiro, Brazil. Bio Med Central. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pinto J, Palomino M, Mendoza-Uribe L, Sinti C, Liebman KA, Lenhart A. (2019) Susceptibility to insecticides and resistance mechanisms in three populations of Aedes aegypti from Peru. Parasit Vectors. 12(1): 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Canyon D, Hii J. (1999) Insecticide susceptibility status of Aedes aegypti (Diptera: Culicidae) from Townsville. Aust J Entomol. 38(1): 40–43. [Google Scholar]
- 65.Demok S, Endersby-Harshman N, Vinit R, Timinao L, Robinson LJ, Susapu M. (2019) Insecticide resistance status of Aedes aegypti and Aedes albopictus mosquitoes in Papua New Guinea. Parasit Vectors. 12(1): 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pocquet N, Darriet F, Zumbo B, Milesi P, Thiria J, Bernard V. (2014) Insecticide resistance in disease vectors from Mayotte: an opportunity for integrated vector management. Parasit Vectors. 7(1): 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rocha HDR, Paiva MHS, Silva NM, de Araújo AP, da Moura AJF, Gómez LF. (2015) Susceptibility profile of Aedes aegypti from Santiago Island, Cabo Verde, to insecticides. Acta Trop. 152: 66–73. [DOI] [PubMed] [Google Scholar]
- 68.Ponlawat A, Scott JG, Harrington LC. (2005) Insecticide susceptibility of Aedes aegypti and Aedes albopictus across Thailand. J Med Entomol. 42(5): 821–825. [DOI] [PubMed] [Google Scholar]
- 69.Pethuan S, Jirakanjanakit N, Saengtharatip S, Chareonviriyaphap T, Kaewpa D, Rongnoparut P. (2007) Biochemical studies of insecticide resistance in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Thailand. Trop Biomed. 24(1): 7–15. [PubMed] [Google Scholar]
- 70.Karunaratne SH, Hemingway J. (2001) Malathion resistance and prevalence of the malathion carboxylesterase mechanism in populations of mosquito vectors of disease in Sri Lanka. Bull World Health Organ. 79: 1060–1064. [PMC free article] [PubMed] [Google Scholar]
- 71.Fernando HSD, Saavedra-Rodriguez K, Perera R, Black WC, De Silva B. (2020) Resistance to commonly used insecticides and underlying mechanisms of resistance in Aedes aegypti (L.) from Sri Lanka. Parasit Vectors. 13(1): 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen C, Nazni W, Lee H, Sofian-Azirun M. (2005) Susceptibility of Aedes aegypti and Aedes albopictus to temephos in four study sites in Kuala Lumpur City Center and Selangor State, Malaysia. Trop Biomed. 22(2): 207–216. [PubMed] [Google Scholar]
- 73.Montada D, Vasuki V, Rajavel A. (1993) Evaluation of organophosphorus and synthetic pyrethroid insecticides against six vector mosquitoe species. Rev Saude Publica. 27: 391–397. [DOI] [PubMed] [Google Scholar]
- 74.Muthusamy R, Shivakumar M. (2015) Susceptibility status of Aedes aegypti (L.) (Diptera: Culicidae) to temephos from three districts of Tamil Nadu, India. J Vector Borne Dis. 52(2): 159–165. [PubMed] [Google Scholar]
- 75.Saha P, Chatterjee M, Ballav S, Chowdhury A, Basu N, Maji AK. (2019) Prevalence of kdr mutations and insecticide susceptibility among natural population of Aedes aegypti in West Bengal. Plos One. 14(4): e0215541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li M, Li S, Yang X, Liang Q, Lan X, Cao L. (1994) Monitoring report of insecticide resistance of vector mosquitoes in Hainan Province (in Chinese). Hainan Med J. 5: 206–208. [Google Scholar]
- 77.Polson KA, Curtis C, Seng CM, Olson JG, Chantha N, Rawlins SC. (2001) Susceptibility of two canbodian population of Aedes aegypti mosquito larvae to temephos during 2001. Dengue Bull. 25: 79–84. [Google Scholar]
- 78.Astari S, Ahmad I. (2005) Insecticide resistance and effect of piperonyl butoxide as a synergist in three strains of Aedes aegypti (Linn.) (Diptera: Culicidae) against insecticides permethrin, cypermethrin, and d-allethrin. Indonesian Bull Health Res. 33(2): 20271. [Google Scholar]
- 79.Hamid PH, Prastowo J, Widyasari A, Taubert A, Hermosilla C. (2017) Knockdown resistance (kdr) of the voltage-gated sodium channel gene of Aedes aegypti population in Denpasar, Bali, Indonesia. Parasit Vectors. 10(1): 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamid PH, Prastowo J, Ghiffari A, Taubert A, Hermosilla C. (2018) Aedes aegypti resistance development to commonly used insecticides in Jakarta, Indonesia. PLoS One. 12(12): e0189680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hamid P, Ninditya V, Prastowo J, Haryanto A, Taubert A, Hermosilla C. (2018) Current status of Aedes aegypti insecticide resistance development from Banjarmasin, Kalimantan, Indonesia. Biomed Res Int. ID 1735358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marcombe S, Chonephetsarath S, Thammavong P, Brey PT. (2018) Alternative insecticides for larval control of the dengue vector Aedes aegypti in Lao PDR: insecticide resistance and semi-field trial study. Parasit Vectors. 11(1): 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ping LT, Yatiman R, Gek L. (2001) Susceptibility of adult field strains of Aedes aegypti and Aedes albopictus in Singapore to pirimiphos-methyl and permethrin. J Am Mosq Control Assoc. 17(2): 144–146. [PubMed] [Google Scholar]
- 84.Lee R, Choong C, Goh B, Ng L, Lam-Phua S. (2014) Bioassay and biochemical studies of the status of pirimiphos-methyl and cypermethrin resistance in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Singapore. Trop Biomed. 31(4): 670–679. [PubMed] [Google Scholar]
- 85.Koou SY, Chong CS, Vythilingam I, Lee CY, Ng LC. (2014) Insecticide resistance and its underlying mechanisms in field populations of Aedes aegypti adults (Diptera: Culicidae) in Singapore. Parasit Vectors. 7(1): 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koou SY, Chong CS, Vythilingam I, Ng LC, Lee CY. (2014) Pyrethroid resistance in Aedes aegypti larvae (Diptera: Culicidae) from Singapore. J Med Entomol. 51(1): 170–181. [DOI] [PubMed] [Google Scholar]
- 87.Frances SP, Morton CJ, Pettit WJ. (2016) Studies of the susceptibility of Aedes aegypti (Diptera: Culicidae) from Timor-Leste to pyrethroid and organophosphate insecticides. Aust J Entomol. 55(3): 303–307. [Google Scholar]
- 88.Al-Amin HM, Johora FT, Irish SR, Hossainey MRH, Vizcaino L, Paul KK. (2020) Insecticide resistance status of Aedes aegypti in Bangladesh. Parasit Vectors. 13 (1): 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu H, Cupp EW, Guo A, Liu N. (2004) Insecticide resistance in Alabama and Florida mosquito strains of Aedes albopictus. J Med Entomol. 41(5): 946–952. [DOI] [PubMed] [Google Scholar]
- 90.Rawlins SC. (1998) Spatial distribution of insecticide resistance in Caribbean populations of Aedes aegypti and its significance. Rev Panam Salud Publica. 4: 243–251. [DOI] [PubMed] [Google Scholar]
- 91.Richards SL, Balanay JAG, White AV, Hope J, Vandock K, Byrd BD. (2018) Insecticide susceptibility screening against Culex and Aedes (Diptera: Culicidae) mosquitoes from the United States. J Med Entomol. 55(2): 398–407. [DOI] [PubMed] [Google Scholar]
- 92.Francis S, Saavedra-Rodriguez K, Perera R, Paine M, Black WC, IV, Delgoda R. (2017) Insecticide resistance to permethrin and malathion and associated mechanisms in Aedes aegypti mosquitoes from St. Andrew Jamaica. PloS One. 12(6): e0179673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kandel Y, Vulcan J, Rodriguez SD, Moore E, Chung H-N, Mitra S. (2019) Widespread insecticide resistance in Aedes aegypti L. from New Mexico, USA. PloS One. 14(2): e0212693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lopez B, Ponce G, Gonzalez JA, Gutierrez SM, Villanueva OK, Gonzalez G. (2014) Susceptibility to chlorpyrifos in pyrethroid-resistant populations of Aedes aegypti (Diptera: Culicidae) from Mexico. J Med Entomol. 51(3): 644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marcombe S, Carron A, Darriet F, Etienne M, Agnew P, Tolosa M. (2009) Reduced efficacy of pyrethroid space sprays for dengue control in an area of Martinique with pyrethroid resistance. Am J Trop Med Hyg. 80(5): 745–751. [PubMed] [Google Scholar]
- 96.Polson KA, Rawlins SC, Brogdon WG, Chadee DD. (2010) Organophosphate resistance in Trinidad and Tobago strains of Aedes aegypti. J Am Mosq Control Assoc. 26(4): 403–410. [DOI] [PubMed] [Google Scholar]
- 97.Rodríguez Coto MM, Bisset Lazcano JA, de Fernandez DM, Soca A. (2000) Malathion resistance in Aedes aegypti and Culex quinquefasciatus after its use in Aedes aegypti control programs. J Am Mosq Control Assoc. 16(4): 324–330. [PubMed] [Google Scholar]
- 98.Rodríguez MM, Bisset JA, Fernández D. (2007) Levels of insecticide resistance and resistance mechanisms in Aedes aegypti from some Latin American countries. J Am Mosq Control Assoc. 23(4): 420–429. [DOI] [PubMed] [Google Scholar]
- 99.Ryan S, Mundis S, Aguirre A, Lippi C, Beltrán E, Heras F. (2018) Phenotypic and genotypic resistance to commonly used insecticides in Aedes aegypti among four cities in southern Ecuador. Bio Rxiv. 1: 441360. [Google Scholar]
- 100.Chediak M, Pimenta F G, Jr, Coelho GE, Braga IA, Lima JBP, Cavalcante KRL. (2016) Spatial and temporal country-wide survey of temephos resistance in Brazilian populations of Aedes aegypti. Mem Inst Oswaldo Cruz. 111(5): 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goindin D, Delannay C, Gelasse A, Ramdini C, Gaude T, Faucon F. (2017) Levels of insecticide resistance to deltamethrin, malathion, and temephos, and associated mechanisms in Aedes aegypti mosquitoes from the Guadeloupe and Saint Martin islands (French West Indies). Infect Dis Poverty. 6(1): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grisales N, Poupardin R, Gomez S, Fonseca-Gonzalez I, Ranson H, Lenhart A. (2013) Temephos resistance in Aedes aegypti in Colombia compromises dengue vector control. PLoS Negl Trop Dis. 7 (9): e2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.LIma JBP, Da-Cunha MP, JÚNIOR RCDS, Galardo AKR, Soares SdS, Braga IA. (2003) Resistance of Aedes aegypti to organophosphates in several municipalities in the State of Rio de Janeiro and Espirito Santo, Brazil. Am J Trop Med Hyg. 68(3): 329–333. [PubMed] [Google Scholar]
- 104.Luna JED, Martins MF, Anjos AFd, Kuwabara EF, Navarro-Silva MA. (2004) Susceptibility of Aedes aegypti to temephos and cypermethrin insecticides, Brazil. Rev Saude Publica. 38: 842–843. [DOI] [PubMed] [Google Scholar]
- 105.Macoris MdLdG, Andrighetti MTM, Wanderley DMV, Ribolla PEM. (2014) Impact of insecticide resistance on the field control of Aedes aegypti in the State of São Paulo. Rev Soc Bras Med Trop. 47 (5): 573–578. [DOI] [PubMed] [Google Scholar]
- 106.Bellinato DF, Viana-Medeiros PF, Araújo SC, Martins AJ, Lima JBP, Valle D. (2016) Resistance status to the insecticides temephos, deltamethrin, and diflubenzuron in Brazilian Aedes aegypti populations. Biomed Res Int. 2016: 8603263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garcia GdA, David MR, Martins AdJ, Maciel-de-Freitas R, Linss JGB, Araújo SC. (2018) The impact of insecticide applications on the dynamics of resistance: The case of four Aedes aegypti populations from different Brazilian regions. PLOS Negl Trop Dis. 12(2): e0006227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Macoris MdLdG, Andrighetti MTM, Otrera VCG, Carvalho LRd, Caldas Júnior AL, Brogdon WG. (2007) Association of insecticide use and alteration on Aedes aegypti susceptibility status. Mem Inst Oswaldo Cruz. 102(8): 895–900. [DOI] [PubMed] [Google Scholar]
- 109.Suter T, Crespo MM, de Oliveira MF, de Oliveira TSA, de Melo-Santos MAV, de Oliveira CMF. (2017) Insecticide susceptibility of Aedes albopictus and Ae. aegypti from Brazil and the Swiss-Italian border region. Parasit Vectors. 10(1): 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sá ELRd, Rodovalho CdM, Sousa NPRd, Sá ILRd, Bellinato DF, Dias LdS. (2019) Evaluation of insecticide resistance in Aedes aegypti populations connected by roads and rivers: the case of Tocantins state in Brazil. Mem Inst Oswaldo Cruz. 114: e180318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dusfour I, Thalmensy V, Gaborit P, Issaly J, Carinci R, Girod R. (2011) Multiple insecticide resistance in Aedes aegypti (Diptera: Culicidae) populations compromises the effectiveness of dengue vector control in French Guiana. Mem Inst Oswaldo Cruz. 106(3): 346–352. [DOI] [PubMed] [Google Scholar]
- 112.Marcombe S, Mathieu RB, Pocquet N, Riaz M-A, Poupardin R, Sélior S. (2012) Insecticide resistance in the dengue vector Aedes aegypti from Martinique: distribution, mechanisms and relations with environmental factors. PloS One. 7(2): e30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lima EP, Paiva MHS, de Araújo AP, da Silva ÉVG, da Silva UM, de Oliveira LN. (2011) Insecticide resistance in Aedes aegypti populations from Ceará, Brazil. Parasit Vectors. 4(1): 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seixas G, Grigoraki L, Weetman D, Vicente JL, Silva AC, Pinto J. (2017) Insecticide resistance is mediated by multiple mechanisms in recently introduced Aedes aegypti from Madeira Island (Portugal). PLOS Negl Trop Dis. 11(7): e0005799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mathias L, Baraka V, Philbert A, Innocent E, Francis F, Nkwengulila G. (2017) Habitat productivity and pyrethroid susceptibility status of Aedes aegypti mosquitoes in Dar es Salaam, Tanzania. Infect Dis Poverty. 6(1): 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kawada H, Maekawa Y, Abe M, Ohashi K, Ohba S-y, Takagi M. (2010) Spatial distribution and pyrethroid susceptibility of mosquito larvae collected from catch basins in parks in Nagasaki City, Nagasaki, Japan. Jpn J Infect Dis. 63(1): 19–24. [PubMed] [Google Scholar]
- 117.Sheppard P, Macdonald W, Tonn R, Grab B. (1969) The dynamics of an adult population of Aedes aegypti in relation to dengue haemorrhagic fever in Bangkok. J Anim Ecol. pp. 661–702. [PMC free article] [PubMed] [Google Scholar]
- 118.Kongmee M, Prabaripai A, Akratanakul P, Bangs MJ, Chareonviriyaphap T. (2004) Behavioral responses of Aedes aegypti (Diptera: Culicidae) exposed to deltamethrin and possible implications for disease control. J Med Entomol. 41(6): 1055–1063. [DOI] [PubMed] [Google Scholar]
- 119.Paeporn P, Supaphathom K, Sathantriphop S, Chareonviritaphap T, Yaicharoen R. (2007) Behavioural responses of deltamethrin-and permethrin-resistant strains of Aedes aegypti when exposed to permethrin in an excito-repellency test system. WHO Regional Office for South-East Asia, available at: https://apps.who.int/iris/handle/10665/170365/ (access 31 December 2007) [Google Scholar]
- 120.Thanispong K, Achee NL, Grieco JP, Bangs MJ, Suwonkerd W, Prabaripai A. (2010) A high throughput screening system for determining the three actions of insecticides against Aedes aegypti (Diptera: Culicidae) populations in Thailand. J Med Entomol. 47(5): 833–841. [DOI] [PubMed] [Google Scholar]
- 121.Rohani A, Chu W, Saadiyah I, Lee H, Phang S. (2001) Insecticide resistance status of Aedes albopictus and Aedes aegypti collected from urban and rural areas in major towns of Malaysia. Trop Biomed. 18(1): 29–39. [Google Scholar]
- 122.Wan-Norafikah O, Nazni WA, Lee HL, Zainol-Ariffin P, Sofian-Azirun M. (2010) Permethrin resistance in Aedes aegypti (Linnaeus) collected from Kuala Lumpur, Malaysia. J Asia Pac Entomol. 3: 175–182. [Google Scholar]
- 123.Rosilawati R, Lee H, Nazni W, Nurulhusna A, Roziah A, MY MF. (2017) Pyrethroid resistance status of Aedes (Stegomyia) aegypti (Linneaus) from dengue endemic areas in Peninsular Malaysia. Int Med J Malays. 16(2): 40–46. [Google Scholar]
- 124.Yap H, Lim M, Chong N, Lee C. (1996) Efficacy and sublethal effects of mosquito coils on Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Proceedings of the 2nd International Conference on Insect pests in the urban environment; Edinburgh, Scotland: United Kingdom: Heriot-Watt University. [Google Scholar]
- 125.Chin A, Chen C, Low V, Lee H, Azidah A, Lau K. (2017) Comparative efficacy of commercial mosquito coils against Aedes aegypti (Diptera: Culicidae) in Malaysia: a nationwide report. J Econ Entomol. 110(5): 2247–2251. [DOI] [PubMed] [Google Scholar]
- 126.Boyer S, Lopes S, editors (2016) WHOPES methods to test insecticide susceptibility of 4 Aedes aegypti field populations in Cambodia. Dengue integrated vector management: dissemination and policy up-take workshop, 2016. December 1, Phnom Penh Hotel. [Google Scholar]
- 127.Astuti EP, Ipa M, Pradani FY. (2014) Resistance detection of Aedes aegypti larvae to cypermethrin from endemic area in Cimahi City West Java. Aspirator J Vector Borne Dis. 6(1): 7–12. [Google Scholar]
- 128.Sayono S, Hidayati APN, Fahri S, Sumanto D, Dharmana E, Hadisaputro S. (2016) Distribution of voltage-gated sodium channel (Nav) alleles among the Aedes aegypti populations in central Java Province and its association with resistance to pyrethroid insecticides. PLoS One. 11(3): e0150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marcombe S, Brey P. (2016) Vector mapping, characterization of insecticide resistance of Aedes populations, and entomology capacity development in the Lao PDR. Ministry of Health of Laos, Available at: https://www.pasteur.la/project-carried-on-in-the-lab-14/arbovec-plus/?print=print.
- 130.Lim K, Rudnick A, Chan Y. (1961) Recent studies of haemorrhagic fevers in Singapore. Singapore Med J. 2: 158–161. [PubMed] [Google Scholar]
- 131.Pang S, Chiang L, Tan C, Vythilingam I, Lam-Phua S, Ng L. (2015) Low efficacy of delthamethrin-treated net against Singapore Aedes aegypti is associated with kdr-type resistance. Trop Biomed. 32 (1): 140–150. [PubMed] [Google Scholar]
- 132.Kawada H, Higa Y, Nguyen YT, Tran SH, Nguyen HT, Takagi M. (2009) Nationwide investigation of the pyrethroid susceptibility of mosquito larvae collected from used tires in Vietnam. PLoS Negl Trop Dis. 3(3): e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huber K, Le Loan L, Hoang TH, Tien TK, Rodhain F, Failloux AB. (2003) Aedes aegypti in south Vietnam: ecology, genetic structure, vectorial competence and resistance to insecticides. Southeast Asian J Trop Med Public Health. 34: 81–86. [PubMed] [Google Scholar]
- 134.Kasai S, Caputo B, Tsunoda T, Cuong TC, Maekawa Y, Lam-Phua SG. (2016) First detection of a Vssc allele V1016G conferring a high level of insecticide resistance in Aedes albopictus collected from Europe (Italy) and Asia (Vietnam), 2016: a new emerging threat to controlling arboviral diseases. Euro Surveill. 24 (5): 1700847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pujiyati E, Kawada H, Sunahara T, Kasai S, Minakawa N. (2013) Pyrethroid resistance status of Aedes albopictus (Skuse) collected in Nagasaki City, Japan. Jpn J Appl Entomol Zool. 24(4): 143–153. [Google Scholar]
- 136.Hernandez HM, Martinez FA, Vitek CJ. (2022) Insecticide Resistance in Aedes aegypti Varies Seasonally and Geographically in Texas/Mexico Border Cities. J Am Mosq Control Assoc. 38(1): 59–69. [DOI] [PubMed] [Google Scholar]
- 137.Marcombe S, Darriet F, Tolosa M, Agnew P, Duchon S, Etienne M. (2011) Pyrethroid resistance reduces the efficacy of space sprays for dengue control on the island of Martinique (Caribbean). PLoS Negl Trop Dis. 5(6): e1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Flores AE, Albeldaño-Vázquez W, Salas IF, Badii MH, Becerra HL, Garcia GP. (2005) Elevated α-esterase levels associated with permethrin tolerance in Aedes aegypti (L.) from Baja California, Mexico. Pestic Biochem Physiol. 82(1): 66–78. [Google Scholar]
- 139.Flores AE, Grajales JS, Salas IF, Garcia GP, Becerra MHL, Lozano S. (2006) Mechanisms of insecticide resistance in field populations of Aedes aegypti (L.) from Quintana Roo, Southern Mexico. J Am Mosq Control Assoc. 22(4): 672–677. [DOI] [PubMed] [Google Scholar]
- 140.Flores AE, Solis GR, Salas IF, Ramos FJS, Garcia GP. (2009) Resistance to permethrin in Aedes aegypti (L.) in northern Mexico. Southwest Entomol. 34(2): 167–177. [Google Scholar]
- 141.Flores AE, Ponce G, Silva BG, Gutierrez SM, Bobadilla C, Lopez B. (2013) Wide spread cross resistance to pyrethroids in Aedes aegypti (Diptera: Culicidae) from Veracruz state Mexico. J Econ Entomol. 106(2): 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Granada Y, Mejía-Jaramillo AM, Strode C, Triana-Chavez O. (2018) A point mutation V419L in the sodium channel gene from natural populations of Aedes aegypti is involved in resistance to λ-cyhalothrin in Colombia. Insects. 9(1): 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Da-Cunha MP, Lima JBP, Brogdon WG, Moya GE, Valle D. (2005) Monitoring of resistance to the pyrethroid cypermethrin in Brazilian Aedes aegypti (Diptera: Culicidae) populations collected between 2001 and 2003. Mem Inst Oswaldo Cruz. 100(4): 441–444. [DOI] [PubMed] [Google Scholar]
- 144.Pichler V, Bellini R, Veronesi R, Arnoldi D, Rizzoli A, Lia RP. (2018) First evidence of resistance to pyrethroid insecticides in Italian Aedes albopictus populations 26 years after invasion. Pest Manag Sci. 74(6): 1319–1327. [DOI] [PubMed] [Google Scholar]
- 145.Bengoa M, Eritja R, Delacour S, Miranda MÁ, Sureda A, Lucientes J. (2017) First data on resistance to pyrethroids in wild populations of Aedes albopictus from Spain. J Am Mosq Control Assoc. 33 (3): 246–249. [DOI] [PubMed] [Google Scholar]
- 146.Endersby-Harshman NM, Wuliandari JR, Harshman LG, Frohn V, Johnson BJ, Ritchie SA. (2017) Pyrethroid susceptibility has been maintained in the dengue vector, Aedes aegypti (Diptera: Culicidae), in Queensland, Australia. J Med Entomol. 54(6): 1649–1658. [DOI] [PubMed] [Google Scholar]
- 147.Loke SR, Andy-Tan W, Benjamin S, Lee H, Sofian-Azirun M. (2010) Susceptibility of field-collected Aedes aegypti (L.) (Diptera: Culicidae) to Bacillus thuringiensis israelensis and temephos. Trop Biomed. 27(3): 493–503. [PubMed] [Google Scholar]
- 148.Lau KW, Chen CD, Lee HL, Norma-Rashid Y, Sofian-Azirun M. (2015) Evaluation of insect growth regulators against field-collected Aedes aegypti and Aedes albopictus (Diptera: Culicidae) from Malaysia. J Med Entomol. 52(2): 199–206. [DOI] [PubMed] [Google Scholar]
- 149.Marcombe S, Darriet F, Agnew P, Etienne M, Yp-Tcha M-M, Yébakima A. (2011) Field efficacy of new larvicide products for control of multi-resistant Aedes aegypti populations in Martinique (French West Indies). Am J Trop Med Hyg. 84 (1): 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li X, Schuler MA, Berenbaum MR. (2007) Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol. 52: 231–253. [DOI] [PubMed] [Google Scholar]
- 151.Liu N. (2015) Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol. 60: 537–559. [DOI] [PubMed] [Google Scholar]
- 152.Su T. (2016) Resistance and its management to microbial and insect growth regulator larvicides in mosquitoes. In: Trdan S. (Ed): Insecticides resistance. InTech Europe, Rijeka, Croatia, pp. 135–154. [Google Scholar]