Abstract
Objectives
Dietary fiber is recognized as an important nutrient for gut health. However, research on the relations of different types of fibers (soluble and insoluble) to the human microbiota health is limited. This study aimed to identify whether higher habitual intake of soluble and/or insoluble fiber have a different influence on the composition, diversity, and abundance of microbiota.
Methods
We examined the fecal microbial composition of 92 healthy females aged 18 and above using the novel shotgun metagenomics sequencing technique. The habitual fiber intake was determined using the Saudi food frequency questionnaire. Pearson’s correlation was used for the correlations between total, soluble, and insoluble fiber and gut microbiota. α- and β-diversities were applied to acquire the distinctions in the relative abundances of bacterial taxa.
Results
Our findings show that higher dietary fiber, particularly insoluble fiber, was significantly correlated with the abundances of Bacteroides_u_s, Bacteroides uniformis, and Lactobacillus acidophilus (r = 0.26, 0.29, 0.26, p-value < 0.05, respectively). Non-significant difference was noted in the microbial α-diversity and β-diversity in low and high soluble/insoluble dietary fiber.
Conclusions
Current findings suggest that insoluble dietary-fiber intake is favorably correlated with the health of the human gut microbiota. However, further investigations are necessary to identify the effect of types of fiber on the specific species identified in this study.
Keywords: Fiber, Soluble/insoluble, BMI, Obesity, Gut microbiota
1. Introduction
The gastrointestinal tract houses a complex microbial ecosystem that co-evolves with the host over time (Thursby and Juge 2017). Maintaining homeostasis between metabolically healthy microbiota and dysbacteriosis is crucial. It can be affected by several endogenous and exogenous factors such as the host’s genetics, medication intake, smoking habits, physical activity, and nutrient intake (Fan and Pedersen 2021). Recent studies have identified a complex relationship between dietary fiber, human health, and disease, with reports indicating that dietary fiber has a major influence on gut health (e.g., composition, richness, and diversity) (Waddell and Orfila 2022). For example, a cohort study of 1879 adults demonstrated that habitual high fiber intake from fruits (approximately 150 g/day) but not vegetables was significantly associated with a shift of α-diversity (diversity within a sample) and β-diversity (diversity between samples) (Jiang et al., 2020). Moreover, randomized controlled trials (RCTs) have reported that supplementation with fructo-oligosaccharides, galacto-oligosaccharides, whole grain, or wheat bran significantly enhances the richness of Bifidobacteria, Bifidobacterium, and Lactobacilli (Jiang et al., 2020).
Different types of dietary fibers, such as water-soluble fibers (which form a viscous gel that is fermented by gut microbiota, such as pectin or gums) and water-insoluble fibers (such as wheat bran or cellulose that have limited fermentation in the colon), have beneficial metabolic effects in the host body, such as improving insulin sensitivity and the gastrointestinal immune barrier (Waddell and Orfila 2022). However, research on the impact of various types of fibers on the human microbiota is scarce, specifically in the Saudi population, where the intake of fiber-rich diets was reportedly insufficient, especially in women, according to the most recent 2016 Saudi National survey (Moradi-Lakeh et al., 2017).
In recent decades, significant changes in food habits in Saudi Arabia, from fiber-rich traditional dietary habits to low-fiber Western diets, have played a vital role in the emergence of obesity chronic conditions (Tyrovolas et al., 2020). This is especially concerning for women of childbearing age given that non-communicable diseases risk in offspring is highly related to the nutritional status before conception (Barker et al., 2018), and the fact that habitual high fiber intake could lower the likelihood of developing gestational diabetes mellitus and other chronic conditions (Reynolds et al., 2020).
Few reports have investigated the relation between the intake of various types of fiber (soluble and insoluble) and gut microbiota among Saudi females. This study aimed to identify the correlation between habitual fiber intake among Saudi females and the composition, diversity, and abundance of gut microbiota.
2. Methods
2.1. Design
The current analysis was a secondary analysis of a case-control study investigating gut signatures in relation to adiposity, with details published (Aljazairy EA 2022). The focus of this study was to report on dietary nutrients, particularly fiber (soluble and insoluble), in relation to gut signatures. We excluded females who were pregnant, aged < 18 or greater than 25 years, diagnosed with chronic conditions (e.g., gastrointestinal diseases), on antibiotic therapy, or taking dietary supplements in the last six months (n = 193). After the participants who consented to participate in the study were gathered (n = 92), appointments were booked at the clinic for data collection, and containers were provided for fecal sample collection. Approval to conduct this study was granted from the ethical committee (IRB #E-19–3625) of King Khaled University Hospital.
2.2. Habitual dietary intake
During the clinic visit, dietary intake was estimated using a validated “Food Frequency Questionnaire” (FFQ) (Gosadi et al., 2017) developed by the Saudi Food and Drug Authority and administered via interviews by trained dietitians. The FFQ, developed in Arabic, consists of 133 foods and beverages. Habitual food intake was estimated based on the standard serving size of 9 consumption frequencies ranging from “never/< once a month” to “above 6 times/d.” Detailed questions regarding food items not previously listed were added to avoid underreporting or missing data. Visuals were used to help participants estimate the portion size during the interview. The quality of the collected dietary data was continuously checked using the standard protocol by the study site manager.
The FFQ was used to estimate the total daily macronutrient/micronutrient intake (total, soluble, and insoluble fiber) using ESHA, a Food Processor Nutrition Analysis Software (ESHA Research Inc., version 10.8, 2010, Salem, Oregon, USA), and data were entered by trained dietitians. The software includes more than 35,000 food and beverage items. In addition, other recipes for mixed dishes and traditional foods consumed in Saudi Arabia that were not available in the ESHA database were manually added from a traditional Saudi cookbook. When an exact match of a food item was not available in the ESHA database, a decision was made regarding the correct categorization of the item by the research team of trained dietitians. The amount of daily food intake was obtained using the product sum (Sirirat et al., 2019) and was calculated as follows: the summation of the average consumption frequency in times per day multiplied by the portion size consumed and multiplied by the standard serving size of the food item.
2.3. Stool sample (fecal microbiota composition)
Gut composition in participants’ stool samples was determined using whole-genome shotgun sequencing (WGS), a high-standard method for evaluating the impact of microbiota on human health (Liu et al., 2021). During the clinic visit, stool samples were obtained under strict sterile and anaerobic conditions in clean and dry containers and then stored at − 80 °C upon reception at the study laboratory. Afterward, extracted DNA samples were kept at − 20 °C for further sequencing. Multi-kingdom microbiome analysis was performed for microbial sequencing. CosmosID platform system for bioinformatics (CosmosID Inc., Maryland, USA) was used to compute the relative abundance. Each organism detected was presented in a tabular format with four variables: frequency, percentage of unique matches, percentage of total microbial community detected, and relative abundance.
2.4. Anthropometric measurements
A standardized method was applied to compute weight, height, and body mass index (BMI) during the clinic visits. Anthropometric measurements were repeated, and the means were calculated for data analyses. Weight was measured to the closest 0.1 kg, excluding heavy clothes, barefoot, on a standard scale (Digital Pearson Scale; ADAM Equipment Inc., Oxford, Connecticut, USA). Using the same scale, height was recorded to the closest 0.5 cm, also barefoot. Weight (kg) was divided by height (m2) to estimate the BMI. Body fat and muscle distribution (body-fat % and muscle weight) were recorded (BIA; 770; In Body, Seoul, South Korea). Waist/hip perimeter were determined to the closest 0.5 cm by applying standard measures: a non-stretchable tape in a standing position with closed legs. For abdominal obesity, waist-perimeter was recorded between the lower rib and the umbilicus, whereas hip-perimeter was measured over the greater trochanters. The waist/hip ratio was computed by dividing the average waist- by the average hip-perimeter.
2.5. Statistical analysis
Normality and skewness of the quantitative data were checked before analyses. Mean ± SD and frequencies were used for continuous and categorical variables, respectively. Data are presented by the median total fiber intake (low; [<9 g/1000 calories/day], high; [≥9 g/1000 calories/day]). Unpaired t-test was applied to compute differences (e.g., anthropometric data).
The correlations between total, soluble, and insoluble fiber and gut microbiota, as well as with anthropometric measurements was calculated using Pearson’s correlation coefficient (r). The online CosmosID™ bioinformatics program was used to assess the statistical difference in the microbial α- and β-diversity between low and high intake of total fiber, soluble fiber (low, <3 g per 1000 calories per day and, high, ≥3 g per 1000 calories per day), and insoluble fiber (low, <7 g per 1000 calories per day and high, ≥7 g per 1000 calories per day).
To acquire the distinctions in the relative abundances of bacterial taxa, the α- (Simpson’s Diversity Index), and β-diversity (Bray-Curtis test) were applied for differences in community composition. Significance was indicated by a p-value < 0.05. IBM SPSS (IBM 24, New York, USA) was used for all analyses.
3. Results
3.1. Descriptive data
The overall dietary fiber intake was 15 g/day (data not shown). Table 1 presents the descriptive data of study participants (n = 92) stratified by total fiber intake. Anthropometric measurements (BMI, body fat (%), and muscle mass [%]) and total energy intake were all higher in participants with lower vs. higher total fiber intake. Furthermore, specific species (Bacteroides_u_s and Lactobacillus acidophilus) were more abundant in those with higher vs. lower total fiber intake (mean [SD]: 0.00562 [0.00990] vs. 0.00279 [0.00368] and 0.000064 [0.000261] vs. 0.000011 [0.000274], respectively).
Table 1.
General Characteristics of Participants Stratified by Total Fiber Intake, n = 92†.
| Variables | Low total fiber (<9 g/1000 calories) | High total fiber (≥9 g/1000 calories) | p-value |
|---|---|---|---|
| N | 46 | 46 | |
| Median fiber (g/1000 calories) | 5.4 | 7.8 | |
| Age (y) | 21.3 (1.5) | 20.8 (1.5) | 0.11 |
| Anthropometric Measurements | |||
| BMI (kg/m2) | 30.4 (8.3) | 26.7 (7.2) | 0.02 |
| WHR (ratio) | 0.74 (0.15) | 0.73 (0.07) | 0.64 |
| Body fat (%) | 44.9 (8.8) | 40.2 (9.3) | 0.02 |
| Muscle mass (%) | 26.3 (7.0) | 30.4 (9.4) | 0.02 |
| Dietary data | |||
| Energy (Calories/d) | 4507 (1700) | 3306 (1440) | 0.05 |
| Dietary protein (%) | 16.3 (5.8) | 16.0 (2.3) | 0.17 |
| Dietary total fat (%) | 39.5 (10.8) | 37.5 (13.1) | 0.39 |
| Saturated fat (%) | 12.5 (4.8) | 11.6 (3.3) | 0.13 |
| Monounsaturated fat (%) | 11.9 (4.5) | 15.2 (5.4) | <0.01 |
| Polyunsaturated fat (%) | 10.9 (5.7) | 11.5 (5.0) | 0.59 |
| Dietary carbohydrate (%) | 44.2 (14.1) | 46.5 (11.4) | 0.74 |
| Total fiber (g/1000 calories) | 7.0 (2.0) | 11.1 (2.9) | <0.0001 |
| Soluble fiber (g/1000 calories) | 2.1 (0.6) | 3.6 (0.9) | <0.0001 |
| Insoluble fiber (g/1000 calories) | 4.9 (1.4) | 8.5 (2.0) | <0.0001 |
| Gut Microbiota | |||
| Firmicutes | 0.2368 (0.1028) | 0.2341 (0.1069) | 0.89 |
| Blautia Wexlerae | 0.00644 (0.00495) | 0.00808 (0.00613) | 0.16 |
| Flavonifractor Plautii | 0.00103 (0.000861) | 0.00113 (0.00185) | 0.74 |
| Fusobacteria | 0.000185 (0.00125) | 0.000011 (0.000077) | 0.35 |
| Clostridium Bolteae | 0.000956 (0.00160) | 0.00065 (0.00185) | 0.39 |
| Faecalibacterium Prausnitzii | 0.0217 (0.0124) | 0.0207 (0.0129) | 0.70 |
| Clostridioides Difficile§ | 0.000129 (0.000463) | 0.000092 (0.000488) | 0.71 |
| Bacteroidetes | 0.7039 (0.1242) | 0.7145 (1291) | 0.07 |
| Bacteroides Faecichinchillae§ | 0.00011 (0.00375) | 0.000028 (0.000193) | 0.19 |
| Bacteroides Thetaiotaomicron | 0.00817 (0.00808) | 0.00883 (0.00739) | 0.67 |
| Bacteria_u_p | 0.000734 (0.0011) | 0.000802 (0.00158) | 0.81 |
| Bacteroides_u_s | 0.00279 (0.00368) | 0.00562 (0.00990) | 0.04 |
| Bacteroides Uniformis | 0.0755 (0.0492) | 0.0627 (0.0350) | 0.15 |
| Bifidobacterium Adolescentis | 0.00753 (0.0105) | 0.0108 (0.0159) | 0.25 |
| Bifidobacterium Kashiwanohense | 0.000889 (0.00205) | 0.0011 (0.00190) | 0.62 |
| Bifidobacterium Longum | 0.00672 (0.00703) | 0.0068 (0.00657) | 0.96 |
| Bifidobacterium Merycicum | 0.000067 (0.000397) | 0.000054 (0.000330) | 0.87 |
| Lactobacillus Acidophilus | 0.000011 (0.000274) | 0.000064 (0.000261) | 0.05 |
| Actinobacteria | 0.0376 (0.0316) | 0.0400 (0.0340) | 0.72 |
| Bifidobacterium Pseudocatenulatum | 0.00321 (0.00674) | 0.00238 (0.00403) | 0.48 |
| Verrucomicrobia | 0.00459 (0.0143) | 0.00495 (0.00785) | 0.88 |
| Akkermansia Muciniphila | 0.00479 (0.0143) | 0.00496 (0.00784) | 0.94 |
| Proteobacteria | 0.0143 (0.0144) | 0.0152 (0.00941) | 0.73 |
| Fusobacteria | 0.000185 (0.00125) | 0.000011 (0.000077) | 0.35 |
| F/B (ratio) | 0.38 (0.26) | 0.40 (0.40) | 0.81 |
†Data presented as mean (SD) and %. p-value < 0.05 considered significant. Bacteria written in bold indicate phylum while in italic indicate species. Body Mass Index (BMI), Firmicutes/Bacteroidetes (F/B), Waist Hip Ratio (WHR).
3.2. Fiber intake and gut microbiota species, abundance, and diversity
Pearson’s correlation test revealed that BMI and body fat% were inversely correlated with total and insoluble fiber intake (Table 2). Bacteroides_u_s, Bacteroides uniformis, and Lactobacillus acidophilus were positively correlated with fiber intake, with stronger correlations observed for insoluble fiber (r = 0.26, 0.29, and 0.26, respectively) (Table 2).
Table 2.
Correlations of Total, Soluble, and Insoluble Fiber with Gut Microbiota and Other Variables, n = 92†.
| Dietary Energy | Total fiber | Insoluble fiber | ||
|---|---|---|---|---|
| BMI | 0.02 | −0.21* | −0.25* | |
| WHR | −0.08 | −0.07 | −0.08 | |
| Waist | −0.04 | −0.16 | −0.19 | |
| Body fat% | 0.06 | −0.22* | −0.24* | |
| Firmicutes | −0.01 | −0.05 | −0.08 | |
| Blautia Wexlerae | 0.16 | 0.01 | 0.02 | |
| Flavonifractor Plautii | 0.09 | −0.03 | −0.01 | |
| Fusobacteria | −0.03 | −0.11 | −0.10 | |
| Clostridium Bolteae | 0.08 | −0.08 | −0.07 | |
| Faecalibacterium Prausnitzii | 0.01 | 0.05 | 0.07 | |
| Clostridioides Difficile§ | −0.01 | −0.10 | −0.11 | |
| Bacteroidetes | 0.04 | 0.06 | 0.08 | |
| Bacteroides Faecichinchillae§ | −0.02 | 0.16 | 0.19 | |
| Bacteroides Thetaiotaomicron | −0.05 | 0.02 | 0.03 | |
| Bacteria_u_p | 0.01 | 0.03 | 0.03 | |
| Bacteroides_u_s | 0.03 | 0.23* | 0.26* | |
| Bacteroides Uniformis | 0.17 | 0.25* | 0.29* | |
| Bifidobacterium Adolescentis | 0.06 | 0.07 | 0.06 | |
| Bifidobacterium Kashiwanohense | 0.09 | 0.03 | 0.03 | |
| Bifidobacterium Longum | 0.11 | 0.13 | 0.15 | |
| Bifidobacterium Merycicum | 0.04 | 0.03 | 0.02 | |
| Lactobacillus Acidophilus | 0.02 | 0.21 | 0.26 | |
| Actinobacteria | −0.08 | 0.01 | 0.01 | |
| Bifidobacterium Pseudocatenulatum | −0.08 | −0.03 | −0.04 | |
| Verrucomicrobia | −0.05 | 0.06 | 0.01 | |
| Akkermansia Muciniphila | −0.05 | −0.02 | −0.01 | |
| Proteobacteria | −0.04 | 0.14 | 0.15 | |
| Fusobacteria | −0.03 | −0.10 | −0.12 | |
| F/B (ratio) | −0.04 | 0.07 | 0.08 |
†Asterisks indicate statistical significance (p < 0.05). Body Mass Index (BMI), Firmicutes/Bacteroidetes (F/B), Waist Hip Ratio (WHR).
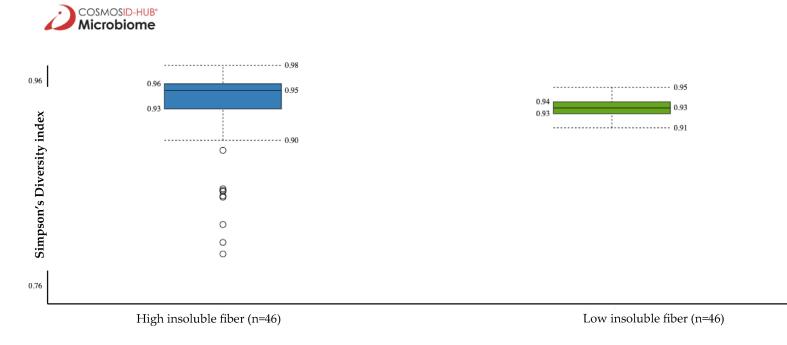
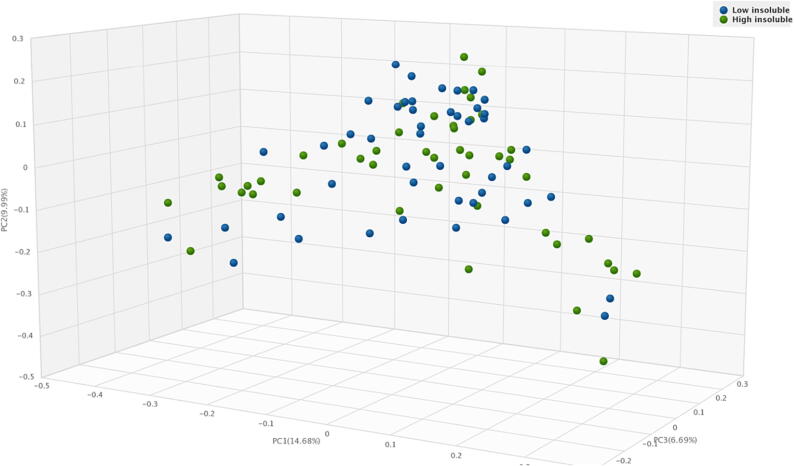
Non-significant difference in the microbiota α-diversity was noted between low vs. high total and soluble fiber intake. However, the Simpson’s Diversity Index revealed that participants with higher insoluble fiber intake had a higher α-diversity than those with lower insoluble fiber intake (0.95 vs. 0.93, p = 0.04) (Fig. 1). Non-significant difference was found in the microbiota β-diversity in low vs. high total, soluble, and insoluble fiber intake (Fig. 2).
Fig. 1.
Comparison of gut microbiota α-diversity by insoluble fiber intake using Simpson’s Diversity Index, p = 0.04.
Fig. 2.
Principle coordinate analysis (PCoA) of β- diversity of gut microbiota relative to low and high insoluble fiber intake.
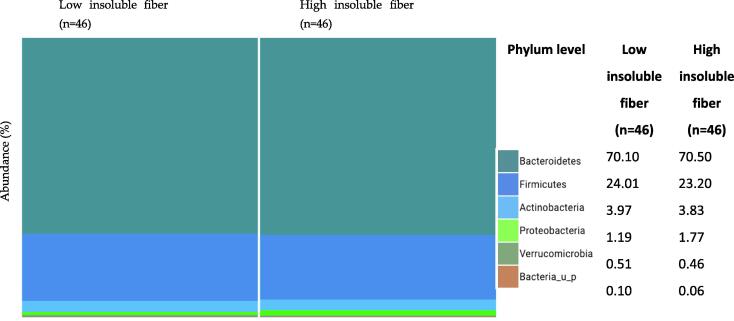
Relative abundance of the major phyla was similar in the low and high insoluble fiber intake groups, with the dominant phyla being Actinobacteria, Bacteroidetes, and Firmicutes. A slightly higher abundance of Proteobacteria was observed in those with a high insoluble fiber intake than in those with a low intake (1.19% vs. 1.77%, p < 0.05) (Fig. 3).
Fig. 3.
Phylum-level classification of gut microbiota relative to insoluble fiber.
4. Discussion
The current study tested the hypothesis that total, soluble and insoluble fiber are favorably related to the composition, diversity, and abundance of gastrointestinal microbiota in Saudi females. We demonstrated that more dietary fiber intake, especially insoluble fiber, is positively associated with the community structure of the microbiome, particularly in Bacteroidetes species. Our findings suggest that different types of fiber may impact bacterial richness differently.
Another important observation in this study was that overall fiber intake was generally low (15 g/day) compared with the recommended daily fiber intake for females (21–25 g/day) (Lupton et al., 2002). The present dietary changes from traditional Saudi food to westernized dietary patterns (low fiber content) (Moradi-Lakeh et al., 2017) may have contributed to the low diversity of human gut microbiota (Cronin et al., 2021). Such a result is also worrisome because dietary fiber has long been related to reducing the risk of chronic diseases, including coronary heart-disease (Bechthold et al., 2019) and atherosclerosis (Reynolds et al., 2019) and improving immunity by increasing short-chain fatty-acid (SCFA) (Beukema et al., 2020). Moreover, it helps in weight control by increasing gastric acids and satiety and decreasing hunger hormones (Hume et al., 2017).
We found that participants with more fiber intake demonstrated higher levels of Bacteroides_u_s and Lactobacillus acidophilus. It is worth noting that “Bacteroides_u_s” is an unidentified species, indicating that the organism is novel and has not yet been characterized. Furthermore, insoluble fiber intake was directly correlated with the abundance of Bacteroides_u_s and Bacteroides uniformis. This agrees with the findings of a previous study with a larger sample of healthy adults, in which high dietary fiber from fruits was related to the composition of Bacteroidetes phyla (Jiang et al., 2020). Most RCTs have investigated the effects of high-fiber dietary sources, but only a few have investigated the effects of fiber types, such as insoluble fibers, on the health of gastrointestinal bacteria (Aoe et al., 2018, Kaczmarek et al., 2019). For example, a 4-week RCT of 60 participants from Japan compared the impact of wheat bran alone and in combination with barley (a rich source of insoluble fiber) on the gut microbiota (Aoe et al., 2018). A significant interactive effect on the genus Bacteroides was associated with both groups, whereas the combination with barley increased the relative-abundance of butyrate-producing-bacteria owing to greater fecal concentrations of SCFAs, mainly butyrate (Aoe et al., 2018). In contrast, a crossover trial of 20 participants diagnosed with glucose intolerance and/or hypercholesterolemia aimed to identify the correlation of 6-week administration of whole-grain oat-granola on cardiovascular risk factors, in addition to its influence on the fecal microbiota of the host (Connolly et al., 2016). In their study, the intake of 45 g of whole-grain oats resulted in significant modulation of the abundance of Bifidobacteria, Lactobacilli, and the total bacterial count (p < 0.001), but not of Bacteroides (Connolly et al., 2016). Inconsistent results may be attributed to different study designs, specifically the sample age group, as other studies showed that the gut microbiota composition varies among different ages (Salazar et al., 2019).
Our results showed an higher α-diversity in those with higher insoluble fiber intake, which is consistent with the findings reporting that habitual high fiber intake was strongly correlated with the shift of α-diversity and β-diversity (Jiang et al., 2020). In a study in mice, kale positively modulated richness/β-diversity of Bacteroidetes microbiota and decreased the Firmicutes/Bacteroidetes ratio (Kaczmarek et al., 2019). The majority of fibers in kale are non-digestible plant polysaccharides involved in inflammatory mechanisms and are abundant in phytochemical metabolic products (e.g., polyphenols, glucosinolates, and sulfur-containing indolics) (Shahinozzaman et al., 2021), thus influencing the microbiota composition of the host. This contrasts with our findings, as we observed no association between β-diversity and total, soluble, or insoluble fiber intake.
Previous studies have concluded that variances in gut composition and diversity may be attributed to various lifestyle-factors, which may explain the inconsistent findings between local and global studies. Varying cultural and dietary traditions result in disparities in the gut composition (Yasir et al., 2015). Another explanation for the differences between studies may be attributed to sex, as evidence shows that sex-hormones play a vital role in regulating gut microbiota diversity (Markle et al., 2013, Yurkovetskiy et al., 2013). This may also explain why no significant difference was noted in bacterial abundance between high and low total, soluble, and insoluble fiber intake in our study, whereas other studies reported that the intake of broccoli lowered Firmicutes relative abundance and elevated that of Bacteroidetes and Bacteroides in adults in comparison to controls (Shahinozzaman et al., 2021). As for the identification of gut composition, we used WGS, whereas previous reports used 16S rRNA-sequencing with MiSeq technology (Yasir et al., 2015, Harakeh et al., 2020).
The impact of fiber intake on the gut may be due to high SCFA-production (Cronin et al., 2021). SCFAs, mainly acetate and butyrate, play major roles in boosting the mucosal layer of the gut, producing antimicrobial substances, reducing oxygen levels, and maintaining the overall immunity of the host (Makki et al., 2018). Furthermore, polyphenols in fruits, vegetables, and cereals can significantly enhance beneficial gut bacteria (e.g., Bifidobacterium), and prevent several pathogenic species in the host (Selma et al., 2009). Both in vivo and in vitro trials have demonstrated that polyphenol fermentation can positively modulate the composition/diversity of the microbiota ecosystem, in addition to enhancing the growth of several Bacteroidetes phyla by reducing the ratio of Firmicutes to Bacteroidetes (Etxeberria et al., 2015, Seo et al., 2015). In addition, the favorable effects of insoluble fibers found in apples, dried figs, pears, kale, broccoli, peanuts, walnuts, whole grains, cereals, and seeds may be attributed to the high fat-soluble vitamin content in some insoluble fiber-rich foods (2021, n.d., 2022, n.d.). For example, fortified cereals rich in vitamin D (2021) may positively influence the microbiota and increase SCFA production (Ciubotaru et al., 2015, Jeffery et al., 2015, Lv et al., 2016, Wang et al., 2017). Vitamin A supplementation may improve gut integrity and decrease the morbidity and mortality rates from gastrointestinal tract infectious diseases (Thornton et al., 2014) by elevating the richness of Lactobacillus sp. (Liu et al., 2017) and reducing the ratio of Firmicutes to Bacteroidetes (Liu et al., 2017). Additionally, vitamin E in nuts, such as almonds and peanuts, and in spinach and broccoli (2021) can positively influence the microbiota (Wang et al., 2017).
This study had several strengths. We used WGS, a highly standard and sensitive method for identifying taxonomies. In addition, several measures were used to ensure accurate data collection, including face-to-face interviews and repeated anthropometric measurements. To measure microbial diversity, we presented α-diversity (indicating gut richness) and β-diversity (indicating composition differences). However, the limitations of this study should also be acknowledged. Although the FFQ is considered a reliable tool for estimating habitual food intake, measurement errors may exist owing to recall bias. Furthermore, causality cannot be confirmed because this study was observational, and potential confounding factors cannot be ruled out. Finally, the study population included only female participants from one setting. Therefore, our results may not be generalizable to the entire study population.
5. Conclusions
Our findings suggest that higher habitual insoluble dietary fiber is correlated with favorable health of the human gut microbiota, favoring beneficial bacterial communities, particularly the relative abundance of Bacteroides_u_s and Bacteroides_uniformis. The majority of currently published trials focused on the short-term/acute impact of dietary fiber sources or prebiotics on gut microbiota composition (Connolly et al., 2016, Aoe et al., 2018, Kaczmarek et al., 2019, Shahinozzaman et al., 2021) but not on different types of fiber. Future studies that address the effects of fiber intake, especially insoluble fiber intake, on individuals with altered gut microbiota and suboptimal gastrointestinal function (e.g., constipation) are warranted.
6. Funding
We acknowledge the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project no. (IFKSUOR3-513).
7. Ethical consent
Informed consent was obtained from each subject, after receiving approval of the study protocol by the local the ethical committee.
8. Institutional review board statement
The study protocol was approved by the ethical committee (Institutional Review Board IRB #E-19–3625) of King Saud University (College of Medicine).
9. Informed consent statement
All participants were given a consent form prior to their inclusion in the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Ghadeer S. Aljuraiban, Email: galjuraiban@ksu.edu.sa.
Sarah S. Algabsani, Email: 442202850@student.ksu.edu.sa.
Shaun Sabico, Email: ssabico@ksu.edu.sa.
Salem AlShammari, Email: salshammari1@ksu.edu.sa.
Esra'a A. Aljazairy, Email: 438203213@student.ksu.edu.sa.
Sara AL-Musharaf, Email: salmosharruf@ksu.edu.sa.
References
- 2021. Office of Dietary Supplements - Vitamin D. Ods.od.nih.gov Retrieved 10-4-2022, 2022, from https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/.
- 2021. Office of Dietary Supplements - Vitamin E. Ods.od.nih.gov Retrieved 10-4-2022, 2022, from https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/.
- Aljazairy EA, A.-M. S., Abudawood M, Almaarik B, Hussain SD, Alnaami AM, Sabico S, Al-Daghri NM, Clerici M, Aljuraiban GS., 2022. Influence of Adiposity on the Gut Microbiota Composition of Arab Women: A Case-Control Study. Biology. 11 (11) 1586. https://doi.org/ https://doi.org/10.3390/biology11111586. [DOI] [PMC free article] [PubMed]
- Aoe S., Nakamura F., Fujiwara S. Effect of wheat bran on fecal butyrate-producing bacteria and wheat bran combined with barley on bacteroides abundance in Japanese healthy adults. Nutrients. 2018;10(12) doi: 10.3390/nu10121980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker M., Dombrowski S.U., Colbourn T., et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet. 2018;391(10132):1853–1864. doi: 10.1016/s0140-6736(18)30313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechthold A., Boeing H., Schwedhelm C., et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2019;59(7):1071–1090. doi: 10.1080/10408398.2017.1392288. [DOI] [PubMed] [Google Scholar]
- Beukema M., Faas M.M., de Vos P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020;52(9):1364–1376. doi: 10.1038/s12276-020-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciubotaru I., Green S.J., Kukreja S., et al. Significant differences in fecal microbiota are associated with various stages of glucose tolerance in African American male veterans. Transl. Res. 2015;166(5):401–411. doi: 10.1016/j.trsl.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly M.L., Tzounis X., Tuohy K.M., et al. Hypocholesterolemic and prebiotic effects of a whole-grain oat-based granola breakfast cereal in a cardio-metabolic “at risk” population. Front. Microbiol. 2016;7:1675. doi: 10.3389/fmicb.2016.01675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin P., Joyce S.A., O'Toole P.W., et al. Dietary Fibre Modulates the Gut Microbiota. Nutrients. 2021;13(5):1655. doi: 10.3390/nu13051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etxeberria U., Arias N., Boqué N., et al. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015;26(6):651–660. doi: 10.1016/j.jnutbio.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021;19(1):55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- Gosadi I.M., Alatar A.A., Otayf M.M., et al. Development of a Saudi Food Frequency Questionnaire and testing its reliability and validity. Saudi Med. J. 2017;38(6):636–641. doi: 10.15537/smj.2017.6.20055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harakeh S., Angelakis E., Karamitros T., et al. Impact of smoking cessation, coffee and bread consumption on the intestinal microbial composition among Saudis: A cross-sectional study. PLoS One. 2020;15(4):e0230895. doi: 10.1371/journal.pone.0230895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume M.P., Nicolucci A.C., Reimer R.A. Prebiotic supplementation improves appetite control in children with overweight and obesity: a randomized controlled trial. Am. J. Clin. Nutr. 2017;105(4):790–799. doi: 10.3945/ajcn.116.140947. [DOI] [PubMed] [Google Scholar]
- Jeffery L.E., Qureshi O.S., Gardner D., et al. Vitamin D antagonises the suppressive effect of inflammatory cytokines on CTLA-4 expression and regulatory function. PLoS One. 2015;10(7):e0131539. doi: 10.1371/journal.pone.0131539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Sun T.-Y., He Y., et al. Dietary fruit and vegetable intake, gut microbiota, and type 2 diabetes: results from two large human cohort studies. BMC Med. 2020;18(1):1–11. doi: 10.1186/s12916-020-01842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek J.L., Liu X., Charron C.S., et al. Broccoli consumption affects the human gastrointestinal microbiota. J. Nutr. Biochem. 2019;63:27–34. doi: 10.1016/j.jnutbio.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Liu X., Xiong X.-Q., et al. Effect of vitamin A supplementation on gut microbiota in children with autism spectrum disorders - a pilot study. BMC Microbiol. 2017;17(1):204. doi: 10.1186/s12866-017-1096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Zhou H., Xu T., et al. Multicenter assessment of shotgun metagenomics for pathogen detection. EBioMedicine. 2021;74 doi: 10.1016/j.ebiom.2021.103649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupton, J. R., J. Brooks, N. Butte, et al., 2002. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. National Academy Press: Washington, DC, USA. 5 589-768. [DOI] [PubMed]
- Lv Z., Wang Y., Yang T., et al. Vitamin A deficiency impacts the structural segregation of gut microbiota in children with persistent diarrhea. J. Clin. Biochem. Nutr. 2016;59(2):113–121. doi: 10.3164/jcbn.15-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki K., Deehan E.C., Walter J., et al. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Markle J.G., Frank D.N., Mortin-Toth S., et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- Moradi-Lakeh M., El Bcheraoui C., Afshin A., et al. Diet in Saudi Arabia: findings from a nationally representative survey. Public Health Nutr. 2017;20(6):1075–1081. doi: 10.1017/s1368980016003141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Dietary Supplements - Vitamin A and Carotenoids. Ods.od.nih.gov Retrieved 10-4-2022, 2022, from https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/.
- Reynolds A.N., Akerman A.P., Mann J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A., Mann J., Cummings J., et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet (London, England) 2019;393(10170):434–445. doi: 10.1016/s0140-6736(18)31809-9. [DOI] [PubMed] [Google Scholar]
- Salazar N., Arboleya S., Fernández-Navarro T., et al. Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: a cross-sectional study. Nutrients. 2019;11(8):1765. doi: 10.3390/nu11081765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selma M.V., Espín J.C., Tomás-Barberán F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009;57(15):6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- Seo D.B., Jeong H.W., Cho D., et al. Fermented green tea extract alleviates obesity and related complications and alters gut microbiota composition in diet-induced obese mice. J. Med. Food. 2015;18(5):549–556. doi: 10.1089/jmf.2014.3265. [DOI] [PubMed] [Google Scholar]
- Shahinozzaman M., Raychaudhuri S., Fan S., et al. Kale attenuates inflammation and modulates gut microbial composition and function in C57BL/6J mice with diet-induced obesity. Microorganisms. 2021;9(2):238. doi: 10.3390/microorganisms9020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirirat R., Heskey C., Haddad E., et al. Comparison of phytosterol intake from FFQ with repeated 24-h dietary recalls of the Adventist Health Study-2 calibration sub-study. Br. J. Nutr. 2019;121(12):1424–1430. doi: 10.1017/s0007114519000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton K.A., Mora-Plazas M., Marín C., et al. Vitamin A deficiency is associated with gastrointestinal and respiratory morbidity in school-age children. J. Nutr. 2014;144(4):496–503. doi: 10.3945/jn.113.185876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thursby E., Juge N. Introduction to the human gut microbiota. Biochem. J. 2017;474(11):1823–1836. doi: 10.1042/bcj20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrovolas S., El Bcheraoui C., Alghnam S.A., et al. The burden of disease in Saudi Arabia 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Planet. Health. 2020;4(5):e195–e208. doi: 10.1016/S2542-5196(20)30075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell I.S., Orfila C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Crit. Rev. Food Sci. Nutr. 2022;1–16 doi: 10.1080/10408398.2022.2061909. [DOI] [PubMed] [Google Scholar]
- Wang H., Chen W., Li D., et al. Vitamin D and chronic diseases. Aging Dis. 2017;8(3):346–353. doi: 10.14336/ad.2016.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasir M., Angelakis E., Bibi F., et al. Comparison of the gut microbiota of people in France and Saudi Arabia. Nutr. Diabetes. 2015;5:e153. doi: 10.1038/nutd.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkovetskiy L., Burrows M., Khan A.A., et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]