Abstract
Traditional Chinese herbs have been widely researched as a green, safe, and effective feed additive for poultry. The purpose of this study was to investigate the effects of traditional Chinese prescription (TCP) based on various herbs in a specific ratio on the growth performance, carcass traits, immunity, antioxidant level, and intestinal health of Ningdu yellow chickens. A total of 420 female Ningdu yellow chickens were randomly divided into 5 groups, with 6 replicates of 14 each. The chickens were fed with a basal diet supplemented with 0 (CON), 0.2, 0.4, 0.6, or 0.8% TCP from d 43 to 105. Body weight, feed intake, and serum biochemical indicators were recorded at d 70 and 105, intestinal morphology and microflora of the carcass were determined at d 105. Compared to the control group, chickens fed with TCP, particularly at the level of 0.6%, showed improved average daily gain and breast muscle percentage, as well as a lower feed-to-gain ratio with statistical significance (P < 0.05). Between 43 and 70 d of age, chickens fed with TCP exhibited higher levels of serum glutathione peroxidase activity, total antioxidant capacity, and superoxide dismutase, particularly in the group fed with the 0.6% level of TCP (P < 0.05). Between 43 and 105 d of age, feeding chickens with 0.4 and 0.6% TCP resulted in a decrease in serum IL-2 concentration, and increase in the IL-4 content (P < 0.05). Chickens fed with 0.4, 0.6, and 0.8% TCP had significantly higher jejunum villous height (P < 0.05), TCP supplementation also led to a marked increase in the relative abundance of Bacteroidota compared to the control group (P < 0.05). Collectively, the study suggests that TCP supplementation can enhance immune and antioxidant functions, improve jejunum morphology, and positively impact cecum microflora in chickens. Based on these results, a level of 0.6% TCP could be considered an optimum level as a feed supplement for Ningdu yellow chickens aged 43 to 105 d.
Key words: chicken, traditional Chinese prescription, growth performance, immunity and antioxidant, intestinal health
INTRODUCTION
Ningdu yellow chicken is a Chinese local breed that is widely recognized for its early maturation, excellent meat quality, high nutritional value, and overall genetic performance, and is highly favored by consumers (Hu et al., 2021). However in recent years, the growth performance, immunity, and disease resistance of Ningdu yellow chickens have declined due to the large-scale breeding practices and restricted use of antibiotics (Zhong et al., 2022). This resulted in an increase in morbidity and mortality rates among the chickens (Peng et al., 2022).
Maintaining intestinal health is crucial to optimize the health, welfare, and performance of poultry (Bedford and Apajalahti, 2022). The composition of the intestinal microbiota in chickens is subject to change based on factors such as age, genotype, and production system. Metabolites produced by the microbiota, such as vitamins, short-chain fatty acids, indole, and tryptamine, interact with the host-microbiota crosstalk and participate in maintaining gut barrier function and immunological homeostasis (Melaku et al., 2021). In-feed supplementation could be a potential and promising approach to enhancing host performance and colonization resistance to gut pathogens.
Traditional Chinese herbs have gained attention as a potential therapeutic tool due to its preventive and therapeutic effects, medicinal value, and nutritional benefits. Traditional Chinese herbal medicine formulations containing various herbs in a specific ratio has been shown to have synergistic effects by combining multiple herbs and reconciling the properties of different single herbs (Chen et al., 2018). The bioactivity of these formulations is attributed to their diverse array of chemical constituents, including alkaloids, flavonoids, terpenoids, and polysaccharides, among others. These compounds have demonstrated various pharmacological effects, such as anti-inflammatory, antioxidant, anticancer, antimicrobial, and immunomodulatory activities (Ding and Lian, 2015). The complex nature of traditional Chinese herbal formulations allows for synergistic interactions between different constituents, resulting in enhanced therapeutic effects (Zhang et al., 2020; Li et al., 2021).
Apart from their medicinal properties, traditional Chinese herbal medicine formulations are also rich in proteins, vitamins, minerals, and polysaccharides, contribute to several nutritional roles. These include stimulating the growth of beneficial gut bacteria, improving gut health, and regulating nutrient metabolism and endocrine function (Munsterman et al., 2019; Sharma et al., 2020; Mahmoud et al., 2021). Commonly used herbs in these TCPs, such as licorice, Codonopsis pilosula, Astragalus membranaceus, Atractylodes, Angelica sinensis, Rhizoma cimicifugae, Radix bupleuri, dried tangerine peel, ginger, and jujube, have been shown to possess various properties. These herbs are believed to enhance the immune system by stimulating the activity of various immune cells, such as T cells and natural killer cells (Gao et al., 2020). Studies have also indicated that these herbs can reduce animals' susceptibility to infections, improve overall health, and even enhance growth performance in some cases (Shao et al., 2004; Song et al., 2010). Therefore, As a natural immune enhancer, TCP may be a useful addition to animal feed, particularly in situations where animals are at risk of disease or stress.
In this study, we prepared the TCP using a specific mixture of multiple herbs. The TCP was formulated based on the principles of clearing heat and detoxifying, tonifying the middle jiao and qi (which refers to a traditional Chinese medicine concept aiming to strengthen the digestive system, improve energy levels, and enhance overall well-being), reinforcing the spleen and stomach, and improving gastrointestinal health according to Chinese herbal. Our hope is that this study could provide more options for promoting the health of poultry and also inspire further research and development of new alternatives to antibiotics.
MATERIALS AND METHODS
Birds and Experiment Design
Four hundred and twenty female Ningdu yellow chickens, aged 43 d and with similar body weights, were purchased from Huida Industrial Co., Ltd. in Ganzhou, Jiangxi, China, and reared on the chicken farm of the Institute of Animal Husbandry and Veterinary Medicine of Jiangxi Academy of Agricultural Sciences until they reached 105 d of age. All the chickens were kept in cages with dimensions of 70 cm deep × 100 cm wide × 50 cm high, at a stocking density of 7 chickens per cage. During the entire trial period, the room where the chickens were kept was naturally ventilated and maintained at a temperature range of 20°C to 26°C. The room was illuminated with an intensity of 3 lux, 24 h/d. The chickens were given ad libitum access to both feed and water throughout the trial period. A completely randomized design was used to allocate chickens to 5 treatments, each with 6 replicates of 14 chickens per replicate. The chickens were fed corn-soybean pellet diets, which were either supplemented with 0 (CON), 0.2, 0.4, 0.6, or 0.8% TCP, respectively. The duration of the treatment period was 63 d.
The Preparation of Experimental Diets
The main raw materials of TCP included per 100 g licorice root (scorched) 13.98 g, Codonopsis pilosula root 8.33 g, Astragalus membranaceus root (scorched) 27.78 g, Atractylodes root (stir-fried) 8.33 g, Angelica sinensis root 8.33 g, Rhizoma cimicifugae root 8.33 g, Radix bupleuri root 8.33 g, dried tangerine peel 8.33 g, ginger root 2.78 g, and jujube with Pit 5.56 g. After air-drying, the above herbs were powdered, sieved through an 80-mesh sieve, thoroughly mixed for later use. The nutritional composition of each stage's feed was formulated based on the established dietary requirements for yellow-feathered broilers (Shini et al., 2005). The basal diet composition and nutrient level are shown in Table 1.
Table 1.
Composition and nutrient levels of basal diets (air-dry basis).
| Items | 43–70 d of age | 71–105 d of age |
|---|---|---|
| Ingredients | ||
| Corn | 53.52 | 56.16 |
| Soybean meal | 28.92 | 24.59 |
| Wheat middling3 | 10.00 | 12.00 |
| Soybean oil | 3.54 | 3.65 |
| CaHPO4 | 1.69 | 1.44 |
| Mountain flour | 1.14 | 1.04 |
| Premix1 | 1.00 | 1.00 |
| Lys | 0.15 | 0.00 |
| Met | 0.04 | 0.12 |
| Total | 100.00 | 100.00 |
| Nutrient levels2 | ||
| ME (MJ/kg) | 12.60 | 12.81 |
| CP | 18.53 | 17.05 |
| Lys | 0.98 | 0.85 |
| Met | 0.42 | 0.37 |
| Cys | 0.72 | 0.65 |
| Ca | 0.93 | 0.82 |
| TP | 0.65 | 0.61 |
The premix provided the following amounts per kg of the diets: VA 12,500 IU, VD 2,500 IU, VE 25 mg, VK3 3 mg, VBl 3 mg, VB2 8 mg, VB6 7 mg, VBl2 0.03 mg, D-pantothenic acid 20 mg, niacin 50 mg, biotin 0.1 mg, folic acid 1.5 mg, Cu (as copper sulfate) 8 mg, Fe (as ferrous sulfate) 100 mg, Mn (as manganese sulfate) 100 mg, Zn (as zinc sulfate) 100 mg, I (as potassium iodide) 0.6 mg, Se (as sodium selenite) 0.16 mg.
ME was a calculated value, while the other nutrient levels were measured values.
Each treatment group added 0 g, 200 g, 400 g, 600 g, and 800 g of TCP, respectively, per 100 kg of daily feed to replace wheat middling. The nutritional composition of each group was determined to be consistent.
Growth Performance
During the rearing period, the chickens' health status and food intake were monitored on a daily basis. Feed supply was halted 12 h before the end of the experiment, while feed consumption was accurately measured. The following day, the experimental chickens' average daily gain (ADG), average daily feed intake (ADFI), and feed/gain ratio (F/G) were analyzed.
Serum Parameters
At the end of the experimental period, 12 birds per treatment (2 birds per replicate) were selected randomly. After a 12-h fast (with ad libitum access to water), blood samples were obtained from the broilers by puncturing their wing vein and transferring the samples into 1.5 mL Eppendorf tubes. These tubes were centrifuged at 3,000 × g for 15 min to separate the serum, which was subsequently stored at −80°C for analysis of serum parameters. The level of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), total antioxidant capacity (T-AOC), and malondialdehyde (MDA) content were measured with an automatic biochemical analyzer (Mindray BS-420, Shenzhen, Guangdong, China) and a colorimetric kit (Nanjing Jiancheng Bioengineering Institute Co., Ltd., Nanjing, Jiangsu, China). Total protein (TP), immunoglobulin A (IgA), immunoglobulin G (IgG), immunoglobulin M (IgM), interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-2 (IL-2), and interleukin-4 (IL-4) were detected using an ELISA kit (Nanjing Jiancheng Bioengineering Institute Co., Ltd., Nanjing, Jiangsu, China) and microplate absorbance reader (Huawei Delang DR-200BS, Wuxi, Jiangsu, China) according to the manufacturer's instructions.
Carcass Traits
The carcass traits were measured after the collection of blood. The birds in each replicate were sacrificed by exsanguination, slaughtered, and weighed to determine the dressing percentage. The trachea, esophagus, verbose sac, intestine, spleen, pancreas, gallbladder, reproductive organs, and gizzard contents were removed from the birds, and the weight of the carcasses was determined to calculate the half eviscerated yield percentage. After the half evisceration, the heart, liver, glandular stomach, gizzard, lungs, abdominal fat, heads, and feet were removed, and their weight was measured to determine the eviscerated yield percentage. The abdominal fat, breast muscles, and leg muscles were removed swiftly and weighed to determine their respective percentages.
Immune Organ Index
The immune organs, such as the spleen, bursa of Fabricius, and thymus, were subsequently peeled off from these birds, the excess fat, blood, and water were removed from the immune organs using filter paper. To record the immune organ indices, the following formula was used: immune organ index (mg/g) = immune organ weight (mg) divided by fasting body weight before slaughter (g). The resulting index was expressed relative to live weight.
Jejunal Villus Morphology
Approximately 3 cm of tissue from the jejunum was collected. The contents of the intestine were washed out using normal saline solution, and then the jejunum tissue was preserved by fixing it in 4% formaldehyde solution. The fixed jejunum tissue was subjected to hematoxylin and eosin (H&E) staining. The H&E stained jejunum tissue section was examined using a light microscope (Nikon Eclipse CI, Nikon Inc., Tokyo, Japan). Image-Pro Plus 6.0 software (Media Cybernetics Inc., Bethesda, MD) was used to measure the villus height (VH), crypt depth (CD), and villous height/crypt depth (V/C).
Cecum Microflora
The contents of the cecum were collected aseptically into sterile tubes for further processing. After being quenched with liquid nitrogen, the samples were stored at −80°C in refrigerators for preservation. The PowerSoil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA) was used to extract total bacterial DNA from fecal samples in accordance with the manufacturer's instructions. The quality and quantity of DNA were assessed based on the ratios of OD 260 nm/280 nm and OD 260 nm/230 nm. The DNA was stored at −80°Cuntil additional processing. To analyze the richness and diversity of cecal microbiota, 16S rDNA sequencing was conducted on the Illumina NovaSeq platform targeting the V3 to V4 hypervariable region. Novogene Co., Ltd. (Beijing, China) performed the sequencing.
Statistical Analysis
The data were analyzed using SPSS (IBM, SPSS Inc., Chicago, IL, version 24.0). For this experiment, the replicate was treated as the experimental unit. Statistical analysis was performed using the 1-way ANOVA test and linear and quadratic regression with Tukey's multiple-range test. Orthogonal polynomial contrasts were employed to assess the linear and quadratic effects of TCP levels. The results were presented as Mean and standard error of the mean (SEM). All the P values were 2-sided and the differences were considered statistically significant at P < 0.05.
RESULTS
Effect of TCP on the Growth Performance
As shown in Table 2 after supplementing TCP for 28 d, BW and ADG increased quadratically (P < 0.05), while F/G reduced linearly (P = 0.017) and quadratically (P = 0.011) in response to TCP. With an extension of supplementaion duration TCP showed a more remarkable effect during the 71- to 105-day period, resulting in linear and quadratic increases in BW at 105 d of age and ADG (P < 0.05), as well as linear (P = 0.016) and quadratic (P = 0.004) reductions in F/G. It is noteworthy to mention that the chickens fed with 0.6% TCP had the highest ADG and the lowest F/G. However, there were no significant differences in ADFI among groups.
Table 2.
Growth performance of Ningdu yellow chickens.
| Items | Additive amount of TCP |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0% | 0.2% | 0.4% | 0.6% | 0.8% | ANOVA | Linear | Quadratic | ||
| 43–70 d | |||||||||
| BW at 43 d of age/g | 417.11 | 417.67 | 417.39 | 417.38 | 416.60 | 0.18 | 0.358 | 0.386 | 0.203 |
| BW at 70 d of age/g | 856.55b | 862.50b | 877.38ab | 886.31a | 857.14b | 3.82 | 0.018 | 0.270 | 0.031 |
| ADG (g/d) | 15.69b | 15.89b | 16.43ab | 16.75a | 15.73b | 0.14 | 0.019 | 0.247 | 0.034 |
| ADFI (g/d) | 56.72 | 55.17 | 56.45 | 55.58 | 54.21 | 0.36 | 0.108 | 0.086 | 0.201 |
| F/G | 3.62a | 3.48ab | 3.44ab | 3.32b | 3.45ab | 0.03 | 0.035 | 0.017 | 0.011 |
| 71–105 d | |||||||||
| BW at 105 d of age/g | 1289.48c | 1341.58b | 1346.35b | 1395.44a | 1338.80b | 9.08 | 0.003 | 0.009 | 0.001 |
| ADG (g/d) | 12.37b | 13.69ab | 13.40ab | 14.55a | 13.76ab | 0.23 | 0.046 | 0.017 | 0.018 |
| ADFI (g/d) | 69.08 | 69.38 | 68.67 | 68.76 | 70.56 | 0.50 | 0.996 | 0.583 | 0.602 |
| F/G | 5.59a | 5.08b | 5.16b | 4.74b | 5.16b | 0.08 | 0.004 | 0.016 | 0.004 |
| 43–105 d | |||||||||
| ADG (g/d) | 13.85c | 14.67b | 14.75b | 15.52a | 14.64b | 0.14 | 0.003 | 0.008 | 0.001 |
| ADFI (g/d) | 63.59 | 63.06 | 63.24 | 62.90 | 63.29 | 0.32 | 0.890 | 0.727 | 0.846 |
| F/G | 4.59a | 4.31b | 4.29b | 4.05c | 4.33b | 0.04 | <0.001 | 0.005 | <0.001 |
Values are represented as the mean and SEM (n = 6).
Means within a row lacking a common superscript differ significantly (P < 0.05).
Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; BW, body weight; F/G, feed/gain; SEM, standard error of the mean; TCP, traditional Chinese prescription.
Effect of TCP on Carcass Traits
As shown in Table 3, compared to the control group, TCP intake showed a linear (P = 0.023) and quadratic (P = 0.008) increase in breast muscle percentage. However, there were no significant effects of diets on leg muscle percentage, abdominal fat percentage, dressing percentage, half-eviscerated yield percentage, and eviscerated yield percentage (P > 0.05).
Table 3.
Carcass traits of Ningdu yellow chickens.
| Items | Additive amount of TCP |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0% | 0.2% | 0.4% | 0.6% | 0.8% | ANOVA | Linear | Quadratic | ||
| Dressing percentage | 91.01 | 91.86 | 92.11 | 91.79 | 91.52 | 0.17 | 0.296 | 0.427 | 0.096 |
| Half eviscerated yield percentage | 79.87 | 79.65 | 80.38 | 80.86 | 80.83 | 0.36 | 0.491 | 0.218 | 0.387 |
| Eviscerated yield percentage | 70.48 | 69.46 | 70.20 | 71.32 | 71.08 | 0.36 | 0.775 | 0.223 | 0.478 |
| Breast muscle percentage | 14.50b | 15.62a | 15.83a | 16.17a | 15.71a | 0.21 | 0.044 | 0.023 | 0.008 |
| Leg muscle percentage | 20.99 | 21.59 | 21.34 | 22.30 | 21.29 | 0.17 | 0.154 | 0.330 | 0.239 |
| Abdominal fat percentage | 5.91 | 5.72 | 6.95 | 5.23 | 5.96 | 0.23 | 0.209 | 0.814 | 0.821 |
Values are the means and SEM of 12 broilers (2 broilers per replicate).
Means within a row lacking a common superscript differ significantly (P < 0.05).
Abbreviations: SEM, standard error of the mean; TCP, traditional Chinese prescription.
Effect of TCP on Immune Indicators
Supplemental TCP showed a tendency to increase thymus index linearly (P = 0.096), while the diet had no significant effects on spleen index and bursa of Fabricius index (P > 0.05), as shown in Table 4.
Table 4.
Immune organ index of Ningdu yellow chickens.
| Items | Additive amount of TCP |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0% | 0.2% | 0.4% | 0.6% | 0.8% | ANOVA | Linear | Quadratic | ||
| Spleen index | 1.54 | 1.47 | 1.58 | 1.69 | 1.72 | 0.05 | 0.476 | 0.096 | 0.225 |
| Bursa of Fabricius index | 0.51 | 0.35 | 0.42 | 0.58 | 0.51 | 0.03 | 0.251 | 0.341 | 0.427 |
| Thymus index | 1.24 | 1.23 | 1.25 | 1.37 | 1.13 | 0.08 | 0.934 | 0.897 | 0.867 |
Values are the means and SEM of 12 broilers (2 broilers per replicate).
Abbreviations: SEM, standard error of the mean; TCP, traditional Chinese prescription.
As shown in Table 5, during the period of 43 to 70 d of age, serum IgG and IgA concentration increased both linearly (P = 0.031, P < 0.001, respectively) and quadratically (P = 0.041, P < 0.001, respectively), serum IgM concentration increased quadratically in response to TCP (P < 0.001). However, it should be noted that this effect disappeared from d 71 to 105. As the chickens aged from 43 to 70 d, there was a linear (P = 0.011) and quadratic (P < 0.001) decrease in serum IL-2 concentrations, but a linear (P = 0.005) and quadratic (P < 0.001) increase in serum IL-4 concentrations (P < 0.05) was observed. During the 43 to 70 d of age, serum concentration of IL-2 and albumin decreased quadratically (P < 0.001), IL-4 increased linearly (P = 0.025) and quadratically (P < 0.001) in response to TCP. It is worth noting that extending the TCP supplementation duration did not result in a significant linear effect on serum IL-2 concentrations (P > 0.05). In addition, the addition of TCP to the chicken diets did not affect serum concentrations of TP, TNF-α, and IFN-γ (P > 0.05).
Table 5.
Serum immune index of Ningdu yellow chickens.
| Items | Additive amount of TCP |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0% | 0.2% | 0.4% | 0.6% | 0.8% | ANOVA | Linear | Quadratic | ||
| 43–70 d | |||||||||
| IgG (g/L) | 3.82b | 4.19a | 4.08ab | 4.14a | 4.18a | 0.05 | 0.049 | 0.031 | 0.041 |
| IgM (g/L) | 1.22b | 1.40a | 1.37a | 1.39a | 1.31a | 0.02 | 0.001 | 0.122 | <0.001 |
| IgA (g/L) | 2.30b | 2.44a | 2.47a | 2.54a | 2.54a | 0.02 | 0.001 | <0.001 | <0.001 |
| TP (g/L) | 19.62 | 21.75 | 19.65 | 20.85 | 21.32 | 0.90 | 0.931 | 0.740 | 0.947 |
| lL-2 (pg/mL) | 322.11a | 290.78a | 232.88b | 230.24b | 287.58a | 7.35 | <0.001 | 0.011 | <0.001 |
| lL-4 (pg/mL) | 7.36b | 8.17b | 10.64a | 10.73a | 8.47b | 0.25 | <0.001 | 0.005 | <0.001 |
| TNF-α (pg/mL) | 64.25 | 59.03 | 55.94 | 55.54 | 58.07 | 1.28 | 0.198 | 0.075 | 0.047 |
| IFN-γ (pg/mL) | 45.34 | 42.33 | 41.67 | 41.52 | 42.01 | 0.64 | 0.306 | 0.097 | 0.094 |
| 71–105 d | |||||||||
| IgG (g/L) | 5.14 | 5.29 | 5.18 | 5.39 | 5.23 | 0.12 | 0.975 | 0.756 | 0.911 |
| IgM (g/L) | 1.69 | 1.70 | 1.71 | 1.89 | 1.73 | 0.04 | 0.492 | 0.375 | 0.590 |
| IgA (g/L) | 2.91 | 2.96 | 3.00 | 3.12 | 3.03 | 0.06 | 0.861 | 0.357 | 0.604 |
| TP (g/L) | 23.45 | 23.55 | 28.44 | 31.20 | 30.41 | 1.75 | 0.471 | 0.079 | 0.209 |
| lL-2 (pg/mL) | 259.07a | 236.12ab | 221.25bc | 209.35c | 246.36ab | 4.39 | 0.001 | 0.099 | <0.001 |
| lL-4 (pg/mL) | 9.57b | 10.10b | 11.20a | 11.83a | 10.12b | 0.18 | <0.001 | 0.025 | <0.001 |
| TNF-α (pg/mL) | 46.57 | 45.54 | 45.15 | 41.10 | 39.42 | 1.20 | 0.252 | 0.026 | 0.073 |
| IFN-γ (pg/mL) | 37.73 | 35.53 | 36.92 | 34.53 | 36.25 | 0.82 | 0.780 | 0.500 | 0.672 |
Values are the means and SEM of 12 broilers (2 broilers per replicate).
Means within a row lacking a common superscript differ significantly (P < 0.05).
Abbreviations: IFN-γ, interferon-γ; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; IL-2, interleukin-2; IL-4, interleukin-4; SEM, standard error of the mean; TCP, traditional Chinese prescription; TNF-α, tumor necrosis factor-α; TP, total protein.
Effects of TCP on Serum Antioxidant Level
During the 43 to 70 d of age, dietary supplementation with the TCP quadratically increased serum GSH-Px (P = 0.002) and SOD (P < 0.001) activity. In response to TCP, the T-AOC ability increased linearly (P = 0.034) and quadratically (P < 0.001). No significant differences in MDA content among chicken groups were observed (P > 0.05). During the 70 to 105 d of age, there were no differences in serum GSH-Px, SOD activity, T-AOC ability, and MDA content among the 5 groups (P > 0.05) (Table 6).
Table 6.
Serum antioxidant level of Ningdu yellow chickens.
| Items | Additive amount of TCP |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0% | 0.2% | 0.4% | 0.6% | 0.8% | ANOVA | Linear | Quadratic | ||
| 43–70 d | |||||||||
| GSH-PX (U/mL) | 246.35c | 280.89ab | 284.96a | 283.88a | 254.36bc | 4.67 | 0.012 | 0.578 | 0.002 |
| T-AOC (U/mL) | 9.17d | 10.52bc | 11.38ab | 11.87a | 9.98cd | 0.20 | <0.001 | 0.034 | <0.001 |
| SOD (U/mL) | 57.86b | 69.27ab | 75.95a | 75.76a | 61.18b | 0.10 | 0.003 | 0.287 | <0.001 |
| MDA (nmol/mL) | 4.72 | 4.49 | 4.15 | 4.05 | 4.28 | 0.90 | 0.210 | 0.062 | 0.063 |
| 71–105 d | |||||||||
| GSH-PX (U/mL) | 309.40 | 311.12 | 318.07 | 321.50 | 311.76 | 3.47 | 0.776 | 0.528 | 0.532 |
| T-AOC (U/mL) | 11.69 | 11.76 | 11.74 | 12.56 | 11.94 | 0.20 | 0.636 | 0.354 | 0.618 |
| SOD (U/mL) | 82.88 | 84.44 | 83.92 | 83.74 | 83.52 | 1.01 | 0.994 | 0.964 | 0.937 |
| MDA (nmol/mL) | 4.09 | 3.91 | 3.76 | 3.79 | 4.01 | 0.08 | 0.698 | 0.648 | 0.338 |
Values are the means and SEM of 12 broilers (2 broilers per replicate).
Means within a row lacking a common superscript differ significantly (P < 0.05).
Abbreviations: GSH-Px, glutathione peroxide; MDA, malondialdehyde; SEM, standard error of the mean; SOD, superoxide dismutase; T-AOC, total antioxidant capacity; TCP, traditional Chinese prescription.
Effects of TCP on Intestinal Morphology
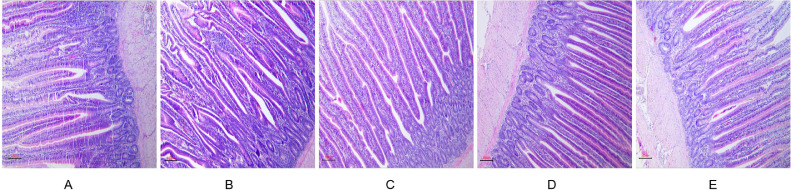
As represented in Figure 1, in comparison with the controls, the villi of the jejunum in TCP groups were closely and orderly arranged, and the number was increased. Jejunal VH increased linearly (P < 0.001) and quadratically (P < 0.001) in response to TCP. No significant differences were observed in jejunal CD and V/C among the 5 groups (P > 0.05) (Table 7).
Figure 1.
Jejunum villi morphology in different groups with addition of TCP. (A) 0.0% TCP addition group; (B) 0.2% TCP addition group; (C) 0.4% TCP addition group; (D) 0.6% TCP addition group; (E) 0.8% TCP addition group.
Table 7.
Histological morphology of jejunum mucosa of Ningdu yellow chickens.
| Items | Additive amount of TCP |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0% | 0.2% | 0.4% | 0.6% | 0.8% | ANOVA | Linear | Quadratic | ||
| VH (μm) | 868.62b | 982.33b | 1144.02a | 1148.00a | 1141.09a | 25.67 | <0.001 | <0.001 | <0.001 |
| CD (μm) | 89.83 | 100.13 | 96.80 | 102.05 | 102.05 | 2.00 | 0.282 | 0.069 | 0.152 |
| V/C | 9.79 | 10.10 | 11.79 | 11.21 | 11.49 | 0.28 | 0.098 | 0.021 | 0.046 |
Values are the means and SEM of 12 broilers (2 broilers per replicate).
Means within a row lacking a common superscript differ significantly (P < 0.05).
Abbreviations: CD, crypt depth; SEM, standard error of the mean; TCP, traditional Chinese prescription; V/C, villous height/crypt depth; VH, villous height.
Effects of TCP on Intestinal Microflora
The analysis of alpha diversity showed that the efficiency of sequences in each group was higher than 61.34%, and the degree of coverage in each group was above 0.997, which indicated that the detection rate of microorganisms in the samples was high and met the standard of sequencing and database analysis (Table 8). The TCP treatment had a positive impact on the diversity and richness of microbial species present, as indicated by the significant increases in observed species, Shannon index, Chao1 index, and ACE index (P < 0.05). However, there was no significant effect on evenness, as measured by the Simpson index (P > 0.05).
Table 8.
Alpha diversity of cecum microflora.
| Items | Additive amount of TCP |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0% | 0.2% | 0.4% | 0.6% | 0.8% | ANOVA | Linear | Quadratic | ||
| Effective/% | 62.96 | 62.82 | 61.92 | 61.34 | 62.53 | 0.350 | 0.606 | 0.356 | 0.400 |
| OTUs number | 942.00a | 773.00b | 758.50b | 677.50b | 653.50b | 28.658 | 0.002 | <0.001 | <0.001 |
| Shannon index | 6.93a | 6.38b | 6.10b | 6.13b | 6.09b | 0.093 | 0.004 | 0.001 | <0.001 |
| Simpson index | 0.97 | 0.97 | 0.96 | 0.96 | 0.97 | 0.003 | 0.298 | 0.281 | 0.089 |
| Chao1 index | 1017.05a | 837.37b | 821.33b | 746.49b | 719.84b | 29.708 | 0.002 | <0.001 | <0.001 |
| Ace index | 1024.83a | 842.06b | 838.97b | 756.45b | 740.57b | 29.550 | 0.004 | <0.001 | 0.001 |
| Coverage | 0.997 | 0.998 | 0.997 | 0.998 | 0.998 | 0.0001 | 0.905 | 0.544 | 0.836 |
Values are the means and SEM of 12 broilers (2 broilers per replicate).
Means within a row lacking a common superscript differ significantly (P < 0.05).
Abbreviations: OTUs, operational taxonomic units; SEM, standard error of the mean; TCP, traditional Chinese prescription.
A phylum-level analysis of the cecum microflora in different chicken groups was conducted to identify and compare the bacterial groups present. Results revealed that the dominant phyla in the cecum of chickens were Bacteroidota and Firmicutes. The relative abundance of Bacteroidota ranged from 58.23 to 73.20%, while Firmicutes accounted for 14.89 to 21.78% of the cecum microflora in Ningdu yellow chickens. The relative abundance of Bacteroidota was significantly increased by TCP in a linear (P < 0.05) and quadratic (P < 0.05) manner. Chickens fed with 0.8% TCP showed a significant decrease (P < 0.05) in the relative abundance of the unidentified phylum compared to the control group (P < 0.05). The relative abundance of other phyla among the groups showed no significant differences (P > 0.05), indicating that the cecal microflora varies among individuals (Table 9).
Table 9.
Relative abundances of intestinal bacteria at phylum level of Ningdu yellow chickens %.
| Items | Additive amount of TCP |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0% | 0.2% | 0.4% | 0.6% | 0.8% | ANOVA | Linear | Quadratic | ||
| Bacteroidota | 58.23b | 70.11a | 70.08a | 73.20a | 68.07a | 1.548 | 0.005 | 0.028 | 0.001 |
| Firmicutes | 21.78 | 17.66 | 18.06 | 14.89 | 20.15 | 0.955 | 0.167 | 0.374 | 0.082 |
| Fusobacteriota | 4.64 | 1.93 | 0.27 | 0.30 | 0.08 | 0.719 | 0.199 | 0.025 | 0.043 |
| Euryarchaeota | 3.27 | 1.25 | 0.98 | 3.46 | 2.40 | 0.530 | 0.517 | 0.899 | 0.652 |
| Proteobacteria | 2.08 | 1.36 | 1.42 | 2.09 | 2.68 | 0.327 | 0.741 | 0.406 | 0.377 |
| Desulfobacterota | 2.99 | 2.72 | 2.54 | 1.41 | 1.20 | 0.309 | 0.223 | 0.017 | 0.060 |
| Verrucomicrobiota | 0.63 | 0.24 | 0.69 | 0.22 | 1.25 | 0.261 | 0.749 | 0.517 | 0.528 |
| Campylobacterota | 1.10 | 0.62 | 0.45 | 1.22 | 0.64 | 0.173 | 0.613 | 0.795 | 0.898 |
| WPS-2 | 0.07 | 0.02 | 0.92 | 0.02 | 0.13 | 0.143 | 0.316 | 0.910 | 0.632 |
| Unidentified_Bacteria | 2.12a | 1.38ab | 1.37ab | 1.27ab | 0.96b | 0.131 | 0.036 | 0.004 | 0.011 |
Values are the means and SEM of 12 broilers (2 broilers per replicate).
Means within a row lacking a common superscript differ significantly (P < 0.05).
Abbreviations: SEM, standard error of the mean; TCP, traditional Chinese prescription.
Bacteroides was the most abundant phylum in all groups, comprising approximately 30% of the relative abundance. Although there was no statistically significant difference (P > 0.05) in the relative abundance of genera in the cecum among the groups, their distribution varied greatly. The relative abundance of Bacteroides and Rikenellaceae was the lowest but the relative abundance of Fusobacterium was the highest in the control groups, the relative abundance of Fusobacterium showed a nonsignificant reduction in response to TCP (Table 10).
Table 10.
Relative abundances of intestinal bacteria at genus level of Ningdu yellow chickens %.
| Items | Additive amount of TCP |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0% | 0.2% | 0.4% | 0.6% | 0.8% | ANOVA | Linear | Quadratic | ||
| Bacteroides | 23.14 | 32.64 | 33.27 | 34.22 | 28.05 | 1.643 | 0.152 | 0.328 | 0.033 |
| Rikenellaceae | 11.26 | 15.22 | 13.97 | 14.49 | 14.60 | 1.118 | 0.844 | 0.456 | 0.619 |
| UCG-001 PrevotellaceaeUCG-001 | 4.36 | 4.33 | 4.36 | 8.35 | 3.96 | 0.724 | 0.257 | 0.533 | 0.566 |
| Fusobacterium | 4.64 | 1.93 | 0.27 | 0.30 | 0.08 | 0.719 | 0.199 | 0.025 | 0.043 |
| UCG-004 | 0.71 | 0.02 | 3.37 | 0.97 | 0.06 | 0.473 | 0.222 | 0.917 | 0.433 |
| Methanobrevibacter | 3.27 | 1.25 | 0.98 | 3.46 | 2.40 | 0.530 | 0.517 | 0.899 | 0.652 |
| Phascolarctobacterium | 1.00 | 2.00 | 2.52 | 2.32 | 3.35 | 0.339 | 0.265 | 0.027 | 0.090 |
| Anaerobiospirillum | 0.03 | 0.11 | 0.05 | 0.26 | 1.30 | 0.272 | 0.562 | 0.154 | 0.235 |
| Desulfovibrio | 2.84 | 2.61 | 2.46 | 1.30 | 1.13 | 0.308 | 0.245 | 0.021 | 0.072 |
| Prevotellaceae_Ga6A1_group | 0.57 | 0.04 | 1.70 | 0.16 | 0.05 | 0.264 | 0.320 | 0.628 | 0.645 |
Values are the means and SEM of 12 broilers (2 broilers per replicate).
Abbreviations: SEM, standard error of the mean; TCP, traditional Chinese prescription.
DISCUSSION
Recent research strongly supported the positive impact of TCP supplementation on chicken production, highlighting its effectiveness in improving the physiological characteristics of chickens (Qiao et al., 2018; Chen et al., 2019; Huang et al., 2021). In our study, we observed a range of positive effects of TCP supplementation on Ningdu yellow chickens. These included increased weight gain and breast muscle percentage, as well as a reduction in the F/G. These findings indicated that TCP could be a valuable supplement for enhancing the growth performance and meat quality of Ningdu yellow chickens. Furthermore, biochemical indicators revealed that TCP supplementation elevated serum levels of IgG, IgM, and IgA, while also boosting the activities of GSH-Px and SOD enzymes, as well as the overall T-AOC in the chickens. These findings suggest potential benefits of TCP in terms of enhancing immune system function and mitigating oxidative stress in the chickens. Additionally, the study demonstrated significant improvements in the morphology of the jejunum mucosa, the inner lining of the small intestine, with TCP supplementation. In addition, TCP was found to increase the relative abundances of Bacteroidetes, a beneficial group of bacteria associated with gut health. These findings suggest that TCP may have positive effects on intestinal well-being in Ningdu yellow chickens.
Licorice, Codonopsis pilosula, Astragalus membranaceus, Atractylodes, Angelica sinensis, Rhizoma cimicifugae, Radix bupleuri, dried tangerine peel, ginger, and jujube had been studied for their potential health-regulating effects. These studies have reported that these herbs possess properties such as anti-inflammatory, immune-modulating, and antiviral effects (Xu et al., 2014; Liu et al., 2017; Karkanis and Bailly, 2021; Nai et al., 2021; Tang et al., 2021a,b; Hu et al., 2022; Hua et al., 2022). The combination of dried tangerine peel, ginger, jujube, and licorice is often used as a condiment to enhance appetite due to their unique aroma and health-regulating effects (Fakhri et al., 2021). Over all, TCP have shown potential as in-feed supplements, improving gastrointestinal health by enhancing anti-inflammatory and bactericidal properties, leading to better overall health and improved growth performance and carcass traits in animals (Bailly, 2021; Zhang et al., 2021). It is worth mentioning that while the individual bioactive effects of these herbs may have certain limitations in clinical practice, combining different Chinese herbal compounds can provide a more effective approach. This approach allows for the use of lower dosages and can lead to more favorable outcomes. Therefore, we conducted a study to investigate the preparation of TCP, hoping to enhance its efficiency and minimize potential side effects.
Our findings suggest that TCP has no significant effect on the feed intake of yellow chicken. However, it can significantly increase ADG and reduce F/G. It was reported that feeding pigs with Chinese herbal decoction improved their growth performance and intestinal glucose absorption (Song et al., 2010). Despite incorporating sweet ingredients like jujube and licorice into TCP to improve palatability, chicken feed intake remained unchanged due to their underdeveloped sense of taste, which indirectly suggests that TCP improved nutrient digestibility and utilization efficiency, thereby enhancing the growth performance of the subjects.
Muscle mass is associated with the balance between muscle protein synthesis and degradation (Smith et al., 2004). Active ingredients found in Chinese herbs, such as Glycyrrhiza flavone, have demonstrated the ability to inhibit muscle atrophy-related genes, promote protein synthesis, and increase muscle mass (Yoshioka et al., 2018). In this study, all groups of Ningdu yellow chickens exhibited a dressing percentage above 90%, with an eviscerated yield percentage of approximately 70%, indicating that all chickens exhibited good meat production performance. Our study indicated that the addition of 0.6% TCP significantly increased the percentage of breast muscle in chickens. We speculate that the observed increase in breast muscle percentage may be attributed to TCP's ability to enhance muscle protein synthesis and regulate lipid metabolism.
There were pieces of evidence to suggest that TCP has potent immune-modulating and antioxidant properties. TCP has been shown to exert its effects by regulating the activity of various immune cells such as T-cells, B-cells, NK cells, and macrophages, as well as modulating the expression of cytokines and chemokines involved in immune response (Liu et al., 2018). Additionally, TCP had been demonstrated to possess antioxidant compounds that scavenge free radicals and protect cells from oxidative damage (Mu et al., 2021). Studies suggest that herbal medicine can upregulate the expression levels of antioxidant enzyme-related genes, potentially contributing to its antioxidant effects. Furthermore, herbs have been shown to reduce the secretion of proinflammatory cytokines and chemokines by increasing the expression of apoptosis-related genes, Bcl-2 and p53, in lymphocytes, thus helping alleviate inflammation (Shan et al., 2018). Wu (2018) noted that feeding Astragalus polysaccharide can improve the growth performance of chicks, increase digestive enzyme activity, raise serum levels of IgG, IgM, and IgA, and decrease serum levels of MDA. Our results are consistent with previous studies, indicating that TCP supplementation can elevate the serum levels of IgA, IgG, and IgM in Ningdu yellow chickens, as well as enhance the activities of antioxidant enzymes SOD, GSH-Px, and T-AOC. Furthermore, our experimental results revealed that chickens fed with TCP exhibited a decrease in IL-2 levels and an increase in IL-4 levels. This suggests that TCP supplementation can enhance the immune and antioxidant capabilities of Ningdu yellow chickens. The effectiveness of TCP may be attributed, in part, to its antioxidant activity via the Nrf2 pathway and anti-inflammatory activity through the NF-κB pathway (Luo et al., 2021; Pang et al., 2021). We also noticed that The regulatory effects of TCP on immune and antioxidant functions exhibit dose-dependency and bidirectional modulation. Low doses of TCP can promote immune and antioxidant functions, while high doses may inhibit these responses. This is due to a phenomenon called biphasic dose-response relationship, where the effects of a substance vary depending on its concentration or dosage. This can also be explained by the Chinese concept of “yin-yang regulation,” which states that the actions of a substance can produce opposite effects based on the balance between its inhibitory (yin) and stimulatory (yang) properties. For TCP, low doses may have more yang effects in promoting immune and antioxidant functions, while high doses may have more yin effects in inhibiting these responses (Wang et al., 2021). Our results demonstrated that the influence of TCP supplementation on serum biochemical parameters shifted from a linear effect at 43 to 70 d of age to a quadratic effect at 71 to 105 d of age as the feeding period was extended. This suggested that high doses of TCP had certain impact on the growth of broilers, which may be related to the increase content of fiber and other substances in diets. Therefore, an appropriate dosage of herbal supplements may exist at a moderate level (0.6%) rather than the highest level (0.8%) set in the current study. However, it should be noted that factors such as feed composition, environmental conditions, and genetic factors may also influence the effectiveness of TCP supplementation.
TCP has been recognized as an important strategy to improve metabolic state by regulating the gut microbiota (Zhang et al., 2021). In broilers, the cecum is dominated by bacteria from the Firmicutes and Bacteroides phyla. The small intestine is responsible for nutrient absorption, and TCP supplementation has been reported to increase the length of the ileum in chickens, thus increasing the intestinal surface area and promoting intestinal digestion and absorption (Guo et al., 2019). In this study, the VH and CD of jejunum mucosa in Ningdu yellow chickens were analyzed as these indices reflect the nutrient absorption area of the intestine. The results showed that TCP significantly increased the jejunum VH, thereby increasing the nutrient absorption area and improving the growth performance of Ningdu yellow chickens. Furthermore, the study investigated the impact of TCP on the relative abundance of intestinal bacterial structure in different chicken groups. The study revealed that TCP increased the proportion of Bacteroidota in the cecum of Ningdu yellow chickens, while not causing significant changes in other types of intestinal bacteria. Bacteroidota, commonly found in the gut microbiome, exhibit various health benefits, including the breakdown and fermentation of complex carbohydrates such as sulfate polysaccharides. Additionally, they have been found to be involved in lipid catabolism and possess inhibitory effects on metabolic syndrome induced by a high-fat diet (Xu et al., 2020). These findings further support the important roles played by Bacteroidota in gut health, including the breakdown of complex carbohydrates and the regulation of the immune system. Notably, it has been well-established that Bacteroidota specifically promote the biochemical function of sulfate polysaccharides, which are known for their anti-inflammatory and antioxidant effects (Sun et al., 2021). Therefore, the increased abundance of Bacteroidota observed in this study may contribute to improved gut function, enhanced metabolism and utilization of sulfate polysaccharides, and overall animal health.
In conclusion, this study highlights the significant positive effects of TCP supplementation on Ningdu yellow chickens. The key findings demonstrate that TCP supplementation effectively improves weight gain, growth performance, carcass traits, antioxidant levels, immune function, intestinal morphology, and intestinal microflora balance. These results underscore the potential of TCP as a growth-promoting agent, enhancer of meat quality, and promoter of overall chicken health. The optimal supplemental level of TCP for chickens aged 43 to 105 d was determined to be 0.6%. However, further research is needed to explore TCP's potential in stress relief, disease prevention, and its impact on inflammation or oxidative stress models. Overall, TCP supplementation holds promise in enhancing production efficiency and animal health in poultry farming.
ACKNOWLEDGMENTS
This work was supported by the Key Research and Development Program of Jiangxi Province (20202BBFL63027), the Key Research and Development Program of Jiangxi Province (20212BBF63025), and the Technical System of the National Broiler Industry (CARS-42-Z10).
Ethical Statement: This study got approval from the Ethics Committee of the Institute of Animal Husbandry and Veterinary Science, Jiangxi Academy of Agricultural Sciences.
DISCLOSURES
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
REFERENCES
- Bailly C. Atractylenolides, essential components of atractylodes-based traditional herbal medicines: antioxidant, anti-inflammatory and anticancer properties. Eur. J. Pharmacol. 2021;891 doi: 10.1016/j.ejphar.2020.173735. [DOI] [PubMed] [Google Scholar]
- Bedford M.R., Apajalahti J.H. The role of feed enzymes in maintaining poultry intestinal health. J. Sci. Food Agric. 2022;102:1759–1770. doi: 10.1002/jsfa.11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Li A.P., Li K., Qin X.M. Research progress on chemical composition and analysis methods of fangji huangqi decoction and herbal medicines. Zhongcaoyao. 2018;49:1695–1702. [Google Scholar]
- Chen Y., Ni J., Li H. Effect of green tea and mulberry leaf powders on the gut microbiota of chicken. BMC Vet. Res. 2019;15:77. doi: 10.1186/s12917-019-1822-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z., Lian F. Traditional Chinese medical herbs staged therapy in infertile women with endometriosis: a clinical study. Int. J. Clin. Exp. Med. 2015;8:14085–14089. [PMC free article] [PubMed] [Google Scholar]
- Fakhri S., Patra J.K., Das S.K., Das G., Majnooni M.B., Farzaei M.H. Ginger and heart health: from mechanisms to therapeutics. Curr. Mol. Pharmacol. 2021;14:943–959. doi: 10.2174/1874467213666201209105005. [DOI] [PubMed] [Google Scholar]
- Gao Z., Zhang C., Jing L., Feng M., Li R., Yang Y. The structural characterization and immune modulation activitives comparison of Codonopsis pilosula polysaccharide (CPPS) and selenizing CPPS (sCPPS) on mouse in vitro and vivo. Int. J. Biol. Macromol. 2020;160:814–822. doi: 10.1016/j.ijbiomac.2020.05.149. [DOI] [PubMed] [Google Scholar]
- Guo X., Cheng L., Liu J., Zhang S., Sun X., Al-Marashdeh O. Effects of licorice extract supplementation on feed Intake, digestion, rumen function, blood indices and live weight gain of karakul sheep. Animals. 2019;9:279. doi: 10.3390/ani9050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Qi C., Feng F., Wang Y., Di T., Meng Y., Wang Y., Zhao N., Zhang X., Li P., Zhao J. Combining network pharmacology, RNA-seq, and metabolomics strategies to reveal the mechanism of cimicifugae rhizoma smilax glabra roxb herb pair for the treatment of psoriasis. Phytomedicine. 2022;105 doi: 10.1016/j.phymed.2022.154384. [DOI] [PubMed] [Google Scholar]
- Hu X., Wan L., Liu S., Chen B., Li W., Wu C., Xiong T., Xi S., Mao H., Liu S. Comparative analysis of meat quality and chemical composition among three weight groups of Chinese Ningdu yellow chicken: implications for customer choice. Anim. Sci. J. 2021;92:e13638. doi: 10.1111/asj.13638. [DOI] [PubMed] [Google Scholar]
- Hua Y., Xu X.X., Guo S., Xie H., Yan H., Ma X.F., Niu Y., Duan J.A. Wild jujube (Ziziphus jujuba var. spinosa): a review of its phytonutrients, health benefits, metabolism, and applications. J. Agric. Food Chem. 2022;70:7871–7886. doi: 10.1021/acs.jafc.2c01905. [DOI] [PubMed] [Google Scholar]
- Huang P., Wang P., Xu J., Sun M., Liu X., Lin Q., Liu W., Qing Z., Zeng J. Fermented traditional Chinese medicine alters the intestinal microbiota composition of broiler chickens. Res. Vet. Sci. 2021;135:8–14. doi: 10.1016/j.rvsc.2020.12.021. [DOI] [PubMed] [Google Scholar]
- Karkanis A., Bailly C.M. Atractylenolides, essential components of atractylodes-based traditional herbal medicines: antioxidant, anti-inflammatory and anticancer properties. Eur. J. Pharmacol. 2021;891 doi: 10.1016/j.ejphar.2020.173735. [DOI] [PubMed] [Google Scholar]
- Li C., Huang B., Zhang Y.W. Chinese herbal medicine for the treatment of depression: effects on the neuroendocrine-immune network. Pharmaceuticals (Basel, Switzerland) 2021;14 doi: 10.3390/ph14010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.Y., Li G., Zhang J., Huo X.W., Cao L. Anti-inflammatory and anti-oxidant effects of licorice flavonoids on ulcerative colitis in mouse model. Chin. Herb. Med. 2017;9:358–368. [Google Scholar]
- Liu L., Wang L.P., He S., Ma Y. Immune homeostasis: effects of Chinese herbal formulae and herb-derived compounds on allergic asthma in different experimental models. Chin. J. Integr. Med. 2018;24:390–398. doi: 10.1007/s11655-018-2836-2. [DOI] [PubMed] [Google Scholar]
- Luo L.F., Guan P., Qin L.Y., Wang J.X., Wang N., Ji E.S. Astragaloside IV inhibits adriamycin-induced cardiac ferroptosis by enhancing Nrf2 signaling. Mol. Cell. Biochem. 2021;476:2603–2611. doi: 10.1007/s11010-021-04112-6. [DOI] [PubMed] [Google Scholar]
- Mahmoud H.K., Reda F.M., Alagawany M., Farag M.R. Ameliorating deleterious effects of high stocking density on oreochromis niloticus using natural and biological feed additives. Aquaculture. 2021;531:1–9. [Google Scholar]
- Melaku M., Zhong R., Han H., Wan F., Yi B., Zhang H. Butyric and citric acids and their salts in poultry nutrition: effects on gut health and intestinal microbiota. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms221910392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu S., Yang W., Huang G. Antioxidant activities and mechanisms of polysaccharides. Curr. Opin. Chem. Biol. 2021;97:628–632. doi: 10.1111/cbdd.13798. [DOI] [PubMed] [Google Scholar]
- Munsterman A.S., Dias Moreira A.S., Marqués F.J. Evaluation of a Chinese herbal supplement on equine squamous gastric disease and gastric fluid pH in mares. J. Vet. Intern. Med. 2019;33:2280–2285. doi: 10.1111/jvim.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai J., Zhang C., Shao H., Li B., Li H., Gao L., Dai M., Zhu L., Sheng H. Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide. Int. J. Biol. Macromol. 2021;183:2337–2353. doi: 10.1016/j.ijbiomac.2021.05.213. [DOI] [PubMed] [Google Scholar]
- Pang J., Ma S., Xu X.Y., Zhang B.X., Cai Q. Effects of rhizome of atractylodes koreana (Nakai) kitam on intestinal flora and metabolites in rats with rheumatoid arthritis. J. Ethnopharmacol. 2021;281:114026. doi: 10.1016/j.jep.2021.114026. [DOI] [PubMed] [Google Scholar]
- Peng S., Zhang H., Song D., Chen H., Lin X., Wang Y., Ji L. Distribution of antibiotic, heavy metals and antibiotic resistance genes in livestock and poultry feces from different scale of farms in Ningxia. J. Hazard. Mater. 2022;440 doi: 10.1016/j.jhazmat.2022.129719. [DOI] [PubMed] [Google Scholar]
- Qiao H., Song Y., Shi H., Bian C. Fermented astragalus in diet altered the composition of fecal microbiota in broiler chickens. AMB Exp. 2018;8:151. doi: 10.1186/s13568-018-0682-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C.H., Guo J., Sun X., Li N., Yang X., Gao Y., Qiu D., Li X., Wang Y., Feng M., Wang C., Zhao J.J. Effects of fermented Chinese herbal medicines on milk performance and immune function in late-lactation cows under heat stress conditions. J. Anim. Sci. 2018;96:4444–4457. doi: 10.1093/jas/sky270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao B.-M., Xu W., Dai H., Tu P., Li Z., Gao X.-M. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem. Biophys. Res. Commun. 2004;320:1103–1111. doi: 10.1016/j.bbrc.2004.06.065. [DOI] [PubMed] [Google Scholar]
- Sharma M.K., Dinh T., Adhikari P.A. Production performance, egg quality, and small intestine histomorphology of the laying hens supplemented with phytogenic feed additive. J. Appl. Poult. Res. 2020;29:362–371. [Google Scholar]
- Shini S., Li X., Bryden W. Methionine requirement and cell-mediated immunity in chicks. Asia Pac. J. Clin. Nutr. 2005;14(Suppl):123. [Google Scholar]
- Smith H.J., Greenberg N.A., Tisdale M.J. Effect of eicosapentaenoic acid, protein and amino acids on protein synthesis and degradation in skeletal muscle of cachectic mice. Br. J. Cancer. 2004;91:408–412. doi: 10.1038/sj.bjc.6601981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Xu J., Wang T., Liu F. Traditional Chinese medicine decoction enhances growth performance and intestinal glucose absorption in heat stressed pigs by up-regulating the expressions of SGLT1 and GLUT2 mRNA. Livest. Sci. 2010;128:75–81. [Google Scholar]
- Sun X., Liu Y., Jiang P., Song S., Ai C. Interaction of sulfated polysaccharides with intestinal bacteroidales plays an important role in its biological activities. Int. J. Biol. Macromol. 2021;168:496–506. doi: 10.1016/j.ijbiomac.2020.12.024. [DOI] [PubMed] [Google Scholar]
- Tang S., Liu W., Zhao Q., Li K., Zhu J., Yao W., Gao X. Combination of polysaccharides from astragalus membranaceus and codonopsis pilosula ameliorated mice colitis and underlying mechanisms. J. Ethnopharmacol. 2021;264 doi: 10.1016/j.jep.2020.113280. [DOI] [PubMed] [Google Scholar]
- Tang L.P., Liu Y.L., Ding K.N., Hou X.J., He Y.M. Chai Hu oral liquid enhances the immune functions of both spleen and bursa of Fabricius in heat-stressed broilers through strengthening TLR4-TBK1 signaling pathway. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Du Y., Wu C., Xu M., Liu Y., Di X. UHPLC-MS/MS analysis of cAMP and cGMP in rat plasma as potential biomarkers of Yin-Yang disharmony in traditional Chinese medicine. J. Pharm. Anal. 2021;11:458–464. doi: 10.1016/j.jpha.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. Effect of dietary astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018;97:3489–3493. doi: 10.3382/ps/pey220. [DOI] [PubMed] [Google Scholar]
- Xu Y., Ai C., Jiang P., Sun X., Liu Y., Jiang G., Song S. Oligosaccharides from Gracilaria lemaneiformis better attenuated high fat diet-induced metabolic syndrome by promoting the bacteroidales proliferation. Food Funct. 2020;11:1049–1062. doi: 10.1039/c9fo01996k. [DOI] [PubMed] [Google Scholar]
- Xu J.J., Wu X., Li M.M., Li G.Q., Yang Y.T., Luo H.J., Huang W.H., Chung H.Y., Ye W.C., Wang G.C., Li Y.L. Antiviral activity of polymethoxylated flavones from “Guangchenpi”, the edible and medicinal pericarps of citrus reticulata ‘Chachi’. J. Agric. Food Chem. 2014;62:2182–2189. doi: 10.1021/jf404310y. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y., Yamashita Y., Kishida H., Nakagawa K., Ashida H. Licorice flavonoid oil enhances muscle mass in KK-A(y) mice. Life Sci. 2018;205:91–96. doi: 10.1016/j.lfs.2018.05.024. [DOI] [PubMed] [Google Scholar]
- Zhang H.T., Huang M.X., Liu X., Zheng X.C., Li X.H., Chen G.Q., Xia J.Y., Hong Z.S. Evaluation of the adjuvant efficacy of natural herbal medicine on COVID-19: a retrospective matched case-control study. Am. J. Chin. Med. 2020;48:779–792. doi: 10.1142/S0192415X20500391. [DOI] [PubMed] [Google Scholar]
- Zhang H.Y., Tian J.X., Lian F.M., Li M., Liu W.K., Zhen Z., Liao J.Q., Tong X.L. Therapeutic mechanisms of traditional Chinese medicine to improve metabolic diseases via the gut microbiota. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110857. [DOI] [PubMed] [Google Scholar]
- Zhong M., Li J., Qiu G., Zhong R., Cheng D., Hu Y., Guo X., Lin X., Liu R. Detection and analysis on pathogens of infectious diseases in Ningduhuang chicken during 2018 to 2020. China Poult. 2022;44:74–78. [Google Scholar]