Abstract
Pseudomonas aeruginosa (P. aeruginosa) is a serious zoonotic pathogen threaten the poultry industry causing severe economic losses therefor, this study aimed to isolation, phenotypic, molecular identification of P. aeruginosa from different avian sources (chickens, turkey, pigeons, table eggs, and dead in shell chicken embryos), from different Egyptian governorates (Giza, Qalubia, Beheira, El-Minya, and Al-Sharqia) with applying of antibiotic sensitivity test on all P. aeruginosa isolates. Highly resistant isolates (n = 49) were subjected to molecular identification of P. aeruginosa with detection of resistant genes including carbapenemase-encoding genes blaKPC, blaOXA-48, and blaNDM. On the base of molecular results, a highly resistant P. aeruginosa strain was tested for its pathogenicity on day old specific pathogen free (SPF) chicks. Also, in vitro experiment was adopted to evaluate the efficacy of silver nanoparticles (Ag-NPs) against highly antibiotic-resistant P. aeruginosa strains. The overall isolation percentage was from all examined samples were 36.2% (571/1,576) representing 45.2% (532/1,176) from different birds' tissues and 39/400 (9.7%) from total egg samples. Some of isolated strains showed multidrug resistance (MDR) against kanamycin, amoxicillin, amoxicillin-clavulanic acid, neomycin, chloramphenicol, vancomycin, cefotaxime clavulanic acid, lincomycin-spectinomycin, co-trimoxazole, cefoxitin, gentamycin, and doxycycline. These MDR strains were also molecularly positive for ESBL and carbapenemase-encoding genes. MDR strain showed high pathogenicity with histopathological alterations in different organs in challenged birds. Main histopathological lesions were necrosis of hepatocytes, renal tubular epithelium, and heart muscle bundles. The MDR strain showed in vitro sensitivity to Ag-NPs. In conclusion, MDR P. aeruginosa is a serious pathogen causing high morbidity, mortality, and pathological tissue alterations. Ag NPs revealed a promising in vitro antimicrobial sensitivity against MDR P. aeruginosa and further in vivo studies were recommended.
Key words: antibiotic resistance, histopathology, pathogenicity testing, resistant gene, silver nanoparticle
INTRODUCTION
Pseudomonas aeruginosa is a serious pathogen affecting most avian species at any age causing severe economic losses in the poultry sector, it is considered as a bacterial hazard that contaminated hatcheries and induces severe respiratory signs, enteritis, septicemia, keratitis, sinusitis, omphalitis, rapid morbidity, embryonic death, as well as mortalities in all ages (Walker et al., 2002; Shukla and Mishra, 2015; Algammal et al., 2023). In case of experimental infection, P. aeruginosa induced mortalities about 70% when inoculated via subcutaneous route in 3-days-old chicks. Mortalities of 90 and 100% were recorded after dipping or inoculation in yolk sac of 17-days-old embryonated chicken eggs, respectively (Bakheet and Torra, 2020). The haphazard treatments resulting in multiple drug resistance of most P. aeruginosa strains as they are highly susceptible to genetic modifications (Odoi et al., 2021). Besides, they can produce biofilm (Channa et al., 2019; Shahat et al., 2019). P. aeruginosa is a gram-negative, motile, nonspore-forming rods, produce watery soluble green pigment with a specific fruity odor (Dinev et al., 2013; Elsayed et al., 2016; Eman et al., 2017). P. aeruginosa has attracted attention as a dangerous pathogen for public health, causing a variety of infections in humans and food animals, and carrying multidrug resistance characteristics that can be passed on to other human and animal pathogens (Shahat et al., 2019). Antimicrobial resistance is currently one of the most prominent public health issues and it is on the rise in developing countries (Le Guern et al., 2023). Indeed, there is mounting evidence that animals serve as a reservoir for antimicrobial resistance (Abd El-Hack et al., 2022). In gram-negative bacteria, synthesis of ampC-lactamases, carbapenemases, and extended spectrum lactamases (ESBL) is the main mechanisms via which resistance is mediated (Schill et al., 2017). These enzymes' genes are frequently found on plasmids that also contain resistance genes to other commonly used antibiotics in clinical settings (Egorov et al., 2018). Infections with these multidrug-resistant organisms (MDROs) will thus pose therapeutic challenges, the antibiotic pipeline is drying up, and no new antimicrobial agents to treat infections caused by MDROs are expected in the near future (Bettiol and Harbarth, 2015; Dougnon et al., 2023). Antibiotics are commonly used in veterinary medicine for both therapeutic and prophylactic purposes and this is prohibited by the European Union (Jawher and Hasan, 2023). However, some countries especially in North America still use it as growth promoters (Economou and Gousia, 2015). In recent years, the prevalence of gram-negative bacteria producing ESBLs and carbapenemase has been widely reported in food-producing animals (Haenni et al., 2016). Therefore, it is critical to identify P. aeruginosa susceptibility pattern and detect it precisely and easily avoid antibiotics that are ineffective (Haleem et al., 2011; Hamisi et al., 2012). Nanotechnology applications in diagnosis and treatment of poultry diseases have been studied in the literature (Abd El-Ghany et al., 2021). The nanoparticles, which are small, engineered particles in the size of 1 to 100 nm, have emerged as a potential alternative to antibiotics especially in the cases of multidrug-resistant bacteria (Shaalan et al., 2016; Lang et al., 2021). With special regard to silver nanoparticles, potent antibacterial activity against gram-negative bacteria was recorded (Farouk et al., 2020; Elgendy et al., 2022). In this study, a trial to isolate P. aeruginosa from birds and eggs, performed the bacterial identification, antibacterial sensitivity test and molecular characterization of the bacteria with detection of resistant genes. Pathogenicity study will be conducted with monitoring of morbidity and mortality rates and histopathological lesions besides, a trail to synthesis and characterize silver nanoparticles and test them in vitro for antibacterial activity against the isolated bacteria.
MATERIALS AND METHODS
This work was conducted according to ARRIVE 2.0 guidelines and ethically approved by The Institutional Animal Care and Use Committee, Faculty of Veterinary Medicine Cairo University with code VETCU23052022444.
Isolation and Identification of Pseudomonas Aeruginosa
Sample's Collection
Samples (3 freshly dead birds/farm) were collected from broiler chicken, turkey, and pigeons’ farms suffered from high mortalities at first 3 wk of rearing as well as, 20 commercial table eggs were collected from each governorate (5 eggs/market), and 20 dead in shell chicken embryos (5 eggs/hatchery) were collected from each of the investigated Egyptian governorates as summarized in Table 1. Samples were collected, labeled, and transported on ice box for further investigations in the laboratory of Microbiology Department, Faculty of Veterinary Medicine, Cairo University.
Table 1.
Number of examined poultry samples in different Egyptian governorates.
| Governorate | Chicken farms (1–21-days old) | Turkey farms | Pigeon farms | Commercial table eggs | Dead in shell chicken embryos |
|---|---|---|---|---|---|
| Giza | 35 | 1 | 2 | 20 | 20 |
| Qalubia | 18 | 5 | 2 | 20 | 20 |
| Beheira | 8 | 1 | 0 | 20 | 20 |
| El- Minya | 6 | 3 | 0 | 20 | 20 |
| Al-Sharqia | 5 | 2 | 1 | 20 | 20 |
| Total | 72 | 12 | 5 | 100 | 100 |
Tissue Samples
Under complete hygienic conditions, heart, liver, kidney, air sac, unabsorbed yolk sac (if found), and subcutaneous swabs (in case of facial edema or cellulitis) were collected separately from each examined bird as well as, swabs were collected from external and internal content each egg separately as summarized in Table 2. Samples n = 1,576 representing (1,176 bird tissue sample and 400 egg samples) were inoculated in nutrient broth and incubated aerobically at 37°C for 24 h. A loopful from growth was streaked on Cetrimide agar, Pseudomonas agar and MacConkey agar then incubated under aerobic condition at 37°C for 24 h.
Table 2.
Number of examined tissue samples.
| Tissue source | Heart | Liver | Kidney | Air sac | Yolk sac | Subcutaneous tissue swabs | External shell swabs | Internal egg content |
|---|---|---|---|---|---|---|---|---|
| Chicken (72 × 3) = 216 | 216 | 216 | 216 | 216 | 80 | 20 | 0 | 0 |
| Turkey (12 × 3) = 36 | 36 | 36 | 36 | 36 | 0 | 8 | 0 | 0 |
| Pigeon (5 × 3) = 15 | 15 | 15 | 15 | 15 | 0 | 0 | 0 | 0 |
| Table eggs (100) | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 |
| Dead in shell embryos (100) | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 |
| Total | 267 | 267 | 267 | 267 | 80 | 28 | 200 | 200 |
Gram Staining
The isolated bacteria (n = 571) were characterized morphologically using Gram's staining method as described by (Merchant and Packer, 1967).
Biochemical Identification
The bacteria that showing characteristics colonial morphology of P. aeruginosa (n = 571) were subjected to biochemical test such as sugar fermentation (glucose and mannitol), oxidase, indole, catalase, MR, VP, motility test, citrate, urease, nitrate reduction, and gelatin hydrolysis according to Barrow and Feltham (2003), also all isolates were examined for growth pattern on MacConkey agar at 4°C and 42°C under aerobic condition.
Antibiotic Sensitivity Test
All isolated bacteria (n = 571) were tested for antibiotic susceptibility using Kirby Bauer disk diffusion method (Bauer et al., 1966), Muller Hinton broth, Muller Hinton agar, and antibiotic disks are used (kanamycin K30 µg, amoxicillin Aml10 µg, amoxicillin-clavulanic acid AX25 µg, neomycin N10 µg, chloramphenicol C30 µg, vancomycin VA30 µg, cefotaxime clavulanic acid CTC40 µg, lincomycin-spectinomycin LS109 µg, co-trimoxazole COT25 µg, cefoxitin CX30 µg, gentamycin GEN10 µg, and Doxycycline DO5 µg). The results were interpreted according to NCCLS (2002).
Molecular Characterization of P. Aeruginosa
Genomic DNA extraction was performed using a boiling method for 49 highly resistant P. aeruginosa isolates. Specific primers targeting the genes oprL (F: ATggAAATgCTgAAATTCggC and R: CTTCTTCAgCTCgACgCgACg) were used to amplify 504 bp products (Chand et al., 2021). Following optimization, the following reaction mixtures (25 µL) were set up: 5 µL of DNA template, 0.5 µL of each set of primers, 12.5 µL of 2× PCR Master Mix, and 6.5 µL DNase/RNase-free water. The reaction mixtures were treated to the following thermal cycling conditions: 94°C for 5 min, then 30 cycles lasting 1 min each at 94°C, 55°C, and 72°C, followed by a final extension lasting 10 min at 72°C. Using GeneRuler 100 bp DNA Ladder and a 1.5% agarose gel, electrophoresis was carried out.
Molecular Detection of Resistant Genes
A total of 49 highly resistant P. aeruginosa isolates on the base of antibiotic sensitivity test were chosen, and the carbapenemase-encoding genes blaKPC, blaOXA-48, and blaNDM were subsequently examined. The gene-specific PCR primers provided in Table 3 were used to amplify these gene fragments. One microliter of genomic DNA template, 12.5 μL of DNA polymerase master mix (Takara Bio Inc., Shiga, Japan), and 0.4 μM of each primer made up the PCR mixes, which had a total reaction volume of 25 μL. The following cycling parameters were used for the blaKPC, blaOXA-48, and blaNDM PCR amplifications: 30 cycles with a 1-min denaturation step at 94°C, a 1-min annealing step at 55°C, a 2-min extension step at 72°C, and a final single 10-min extension step at 72°C.
Table 3.
Primer sequences for carbapenemase-encoding genes (Elshafiee et al., 2019).
| Gene | Sequence | Size (bp) |
|---|---|---|
| blaKPC | F:ATGTCACTGTATCGCCGTCT R:TTTTCAGAGCCTTACTGCCC |
882 |
| blaOXA-48 | F:TTGGTGGCATCGATTATCGG R:GAGCACTTCTTTTGTGATGGC |
743 |
| blaNDM | F:GGTTTGGCGATCTGGTTTTC R:CGGAATGGCTCATCACGATC |
621 |
The genes for ESBLs were examined in all positive P. aeruginosa isolates. For the purpose of identifying the resistance determinant genes SHV, TEM, and CTX-M, multiplex polymerase chain reaction (PCR) was carried out utilizing particular oligonucleotide primers (Table 4). PCR mixtures of 25 μL total volume were prepared from 12.5 μL Emerald Amp GT PCR master mix (Takara Bio Inc., Shiga, Japan), 0.5 μL from each primer with concentration of 10 pmol, 3 μL template DNA from each isolate, and 5.5 μL water. The following conditions were used to amplify all reactions: initial denaturation at 95°C for 15 min; 30 cycles of 94°C for 30 s, 62°C for 90 s, and 72°C for 60 s; and finally, a final extension at 72°C for 10 min. The PCR products were electrophoresed on 1.5% agarose gel, a 100-bp DNA ladder.
Table 4.
Primer sequences for ESBL-encoding genes (Monstein et al., 2007).
| Gene | Sequence | Size (bp) |
|---|---|---|
| blaTEM | F: CGC CGC ATA CAC TAT TCT CAG AAT GA R: ACG CTC ACC GGC TCC AGA TTT AT |
445 |
| blaSHV | F: CTT TAT CGG CCC TCA CTCAA R: AGG TGC TCA TCA TGG GAA AG |
237 |
| blaCTXM | F: ATG TGC AGY ACC AGTAAR GTK ATG GC R: TGG GTR AAR TAR GTS ACC AGA AYC AGC GG |
593 |
Pathogenicity Testing
On the base of antibiotic sensitivity test against the selected antibiotics, the highly resistant strains were selected and then subjected to molecular detection of the resistant genes and the strain that showed highly genetic resistant against all the tested resistant genes was selected to pathogenicity testing.
Thirty, 1-day-old SPF chicks from the SPF Farm, Koum Oshein, El-Fayoum, Egypt were divided into 3 groups 10 chicks in each as follow; Group 1: each chick was injected S/C with 0.25 mL fresh nutrient broth containing 1.5 × 108 CFU/mL (0.5 McFarland tube which is confirmed by plate count) of molecularly identified MDR P. aeruginosa strain, Group 2: each chick was inoculated orally with 0.5 mL nutrient broth containing 1.5 × 108 CFU/mL (0.5 McFarland tube) of MDR P. aeruginosa strain by crop gavages for 2 successive days, and Group 3: kept as control negative noninfected group. Birds were kept in the laboratory units of Microbiology Department, Faculty of Veterinary Medicine, Cairo University, and birds were reared on 3 separate pens under controlled environmental conditions and birds supplied with sterile water and autoclaved starter commercial balanced ration ad libitum.
Morbidity and Mortality Rates
Daily observation of clinical signs and mortalities were adopted all over the experimental period (2 wk).
Sampling
Tissue samples (liver and heart) were collected from all freshly dead birds for reisolation. Also, at 5-days postinfection (7-days old), 3 chicks per group were ethically slaughtered to collect tissue samples form (liver and heart) for reisolation as well as from (kidneys, liver, and heart) for further histopathological examination.
Histopathological Examination
Samples from kidneys, liver, and heart were fixed in neutral buffered formalin (NBF) 10% for 24 h, then trimmed, processed, embedded, and sectioned by automated microtome at a thickness of 5 µm. Tissue sections were mounted and stained with H&E stain (Bancroft and Gamble, 2008). Images were taken by a digital camera attached to a light microscope.
In Vitro Assessment of Silver Nanoparticles Efficacy Against Resistant Strains of P. Aeruginosa
Inoculum Preparation
A molecularly identified P. aeruginosa highly antibiotic-resistant strain was selected. The selected strain was inoculated into nutrient broth and incubated under aerobic conditions at 37°C for 18 h and the resulted growth was adjusted at a concentration of 106 CFU/mL.
Silver Nanoparticles Preparation and Characterization
Silver nanoparticles were produced via chemical reduction of silver nitrate. The method utilized both sodium citrate and sodium borohydride as reducing agents with coating of the produced particles with polyvinylpyrrolidone as described by Shaalan et al. (2020). The produced nanoparticles were characterized by transmission electron microscopy imaging to define shape and size.
Minimal Inhibitory Concentration Assay
The synthesized silver nanoparticles were evaluated for the minimal inhibitory concentration (MIC) value. The assay was performed in triplicates according to Shaalan et al. (2017). Freshly prepared bacterial culture of P. aeruginosa (106 CFU/mL) was incubated with different silver nanoparticles concentrations (25, 12.5, 6.25, and 3.13 µg/mL). After 24 h incubation, 100 µL of each concentration was streaked on Müller Hinton agar plate and incubated overnight. MIC value was determined based on the concentration at which no bacterial growth was detected on the agar plate.
RESULTS
Isolation and Identification of P. Aeruginosa
As presented in Tables 5 and 6, the positive colonies appeared as green fluorescence and a few colonies appeared as brownish colonies on the surface of Cetrimide agar. The total isolation percentages from all examined samples were 36.2% (571/1,576). In chickens, 464 tissue samples out of 964 were positive for P. aeruginosa with isolation percentage (48.1%), followed by turkey 51 tissue samples were positive out of 152 (45%) and in pigeons, 17 samples were positive out of 45 (37.7%) tissue samples.
Table 5.
Isolation percentage from different examined tissue samples.
| No. of examined birds | Heart | Liver | Kidney | Air sac | Yolk sac | S/C swabs | Total positive |
|---|---|---|---|---|---|---|---|
| Isolation results | |||||||
| Chicken (n = 216) | 112/216 (51.8%) | 109/216 (50.4%) | 43/216 (20%) | 110/216 (51%) | 76/80 (95%) | 14/20 (70%) | 464/964 (48.13 %) |
| Turkey (n = 36) | 21/36 (58.3%) | 16/36 (44.4%) | 6/36 (16.6%) | 2/36 (5.5%) | - | 6/8 (75%) | 51/ 152 (45%) |
| Pigeon (n = 15) | 7/15 (46.6%) | 6/15 (40%) | 4/15 (26.75) | - | - | - | 17/ 45 (37.7%) |
| Total bird samples (n = 267) | 140/267 (52.4%) | 131/267 (49%) | 53/267 (19.8%) | 112/252 (44.4%) | 76/80 (95%) | 20/28 (71%) | 532 / 1176 (45.2%) |
Table 6.
Isolation percentage eggs.
| Samples | Isolation rate |
Total isolation rate from eggs | |
|---|---|---|---|
| External eggshell swabs | Internal egg contents | ||
| Table eggs (100) | - | 10/100 (10%) | 39/400 (9.7%) |
| Dead in shell embryos (100) | 4/100 (4%) | 25/100 (25%) | |
| Total egg samples | 4/200 (2%) | 35/200 (17.5%) | |
The total isolation rate of P. aeruginosa from eggs was 9.7% (39/400). In table eggs P. aeruginosa isolation percentage was 10% from internal egg contents while, in dead in shell embryos isolation percentage was 4% (4/100) from the external eggshell swabs and 25% (25/100) from internal egg contents.
The primary bacteriological isolation percentage from different tissues of total birds 267 (216 chicken, 36 turkey and 15 pigeons) was 52.4, 49, 19.8, 44.4, 95, and 71.4% from heart, liver, kidney, air sac, yolk sac, and S/C swabs, respectively.
Gram staining of the suspected colonies showed the presence of gram-negative rods. The suspected greenish colonies were further subjected to biochemical identification and positive isolates glucose fermentation (negative), mannitol fermentation (positive), oxidase (positive), indole (negative), catalase (positive), MR (negative), VP (negative), motility test (motile), citrate (positive), urease (negative), nitrate reduction (positive), gelatin hydrolysis (positive), and Cetrimide test (positive).
All positive isolates were further subjected to antibiotic sensitivity testing and the results of the antibiotic sensitivity of P. aeruginosa was tested by using disk diffusion method against different antimicrobials, showed that the high resistance (100%) was noticed against amoxicillin Aml10 µg, and amoxicillin-clavulanic acid AX25 µg, followed by neomycin N10 µg (60%), co-trimoxazole COT25 µg, cefoxitin CX30 µg (58%), lincomycin-spectinomycin LS109 µg (45%), cefotaxime clavulanic acid CTC40 µg (40%), vancomycin VA30 µg (36%), kanamycin K30 µg (25%), chloramphenicol C30 µg (18%), doxycycline DO5 µg (16%), gentamycin GEN10 µg (8%).
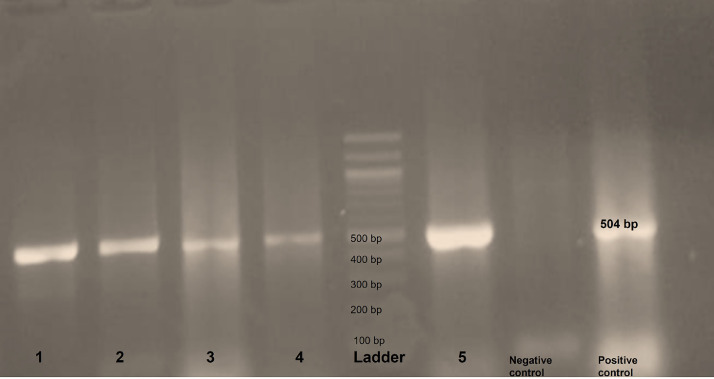
From the previous results, 49 of MDR strains for the almost tested antibiotics were selected and subjected to further molecular identification to detect the genes oprL of P. aeruginosa, ESBL-encoding genes, and carbapenemase-encoding genes (Figure 1).
Figure 1.
Photo of the agarose gel (1%) electrophoresis of PCR assay for the oprL gene, samples in lanes from 1 to 5 are samples showing positive bands at 504 bp.
The molecular detection of P. aeruginosa was higher in chicken (n = 34) following by Turkey (n = 14), whereas the lowest rate found in pigeon (n = 1). The molecular investigation of ESBLs producing isolates for (TEM, Ctx-M, and Shv) as presented in Table 7, revealed that the most infected organ in chicken was liver (12 out of 15), heart (3 out of 4), air sac (3 out of 6) whereas the isolates of yolk sac and table egg show 100%. The most prevalent site in turkey was heart and subcutaneous tissue (4 out of 4) followed by liver (2 out of 3). Most of the isolates were found to harbor one or more of the investigated genes as shown in Table 8 where TEM gene was the most prevalent gene with a percentage of 100% at the same time the isolates harbored at least 2 different ESBLs genes.
Table 7.
Molecular characterization of P. aeruginosa and resistant genes.
| Source tissue |
Pseudomonas aeruginosa |
ESBL-encoding genes |
Carbapenemase-encoding genes |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Chicken | Turkey | Pigeon | Chicken | Turkey | Pigeon | Chicken | Turkey | Pigeon | |
| Liver | 15 | 3 | 1 | 12 | 2 | 0 | 4 | 2 | 0 |
| Heart | 4 | 4 | 0 | 3 | 4 | 0 | 0 | 2 | 0 |
| Kidney | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Air sac | 6 | 2 | 0 | 3 | 0 | 0 | 1 | 0 | 0 |
| Yolk sac | 4 | 0 | 0 | 4 | 0 | 0 | 1 | 0 | 0 |
| Subcutaneous tissue swabs | 0 | 4 | 0 | 0 | 4 | 0 | 0 | 1 | 0 |
| Table egg | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Sinus | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 34 | 14 | 1 | 25 | 10 | 0 | 6 | 5 | 0 |
Table 8.
Molecular detection of ESBL and carbapenemase-encoding genes.
| Encoding genes | Resistant genes | Chicken |
Turkey |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver | Heart | Kidney | Air sac | Yolk sac | Table egg | Liver | Heart | Kidney | Air sac | S/C | ||
| ESBL-encoding genes | blaTEM | 12 | 3 | 1 | 3 | 3 | 2 | 2 | 4 | 0 | 0 | 4 |
| blaSHV | 4 | 0 | 1 | 0 | 2 | 0 | 2 | 4 | 0 | 0 | 4 | |
| blaCTXM | 9 | 1 | 1 | 3 | 2 | 0 | 2 | 4 | 0 | 0 | 4 | |
| Carbapenemase-encoding genes | blaKPC | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| blaOXA-48 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| blaNDM | 4 | 0 | 0 | 1 | 1 | 0 | 2 | 2 | 0 | 0 | 1 | |
Pathogenicity Testing of MDR P. Aeruginosa
The pathogenicity testing of molecularly identified highly resistant strain revealed 100% morbidity and 100% mortalities in G1 within 48 h post-S/C inoculation of P. aeruginosa and the birds in this group showed a septicemic picture in postmortem (PM) examination appeared in the shape of congestion of subcutaneous tissue and muscled, engorgement of subcutaneous blood vessels, and enlargement with congestion in the parenchymatous organs while G2 revealed 80% morbidity and 40% mortality within week postoral infection with highly resistant strain of P. aeruginosa and challenged birds in this group showed clinical signs of depression, off food, huddled together, ruffled feathers, brownish mixed with white color diarrhea, some birds showed hard respiration and the PM lesions in this group were nephritis with distended ureters with ureates, congestion with subcapsular hemorrhages in liver with distended gall bladder, air sacculitis, unabsorbed yolk sac, pericarditis, enteritis, and enlarged congested spleen. On the hand, G3 showed neither clinical sign nor mortalities during the observation period.
For reisolation, the bacteriological examination of the tissue samples (liver and heart) which were collected from freshly dead birds showed suspected colonies for P. aeruginosa on Cetrimide agar, Pseudomonas agar, and MacConkey agar. No organism was isolated from the control negative birds in Group 3.
Histopathological Examination
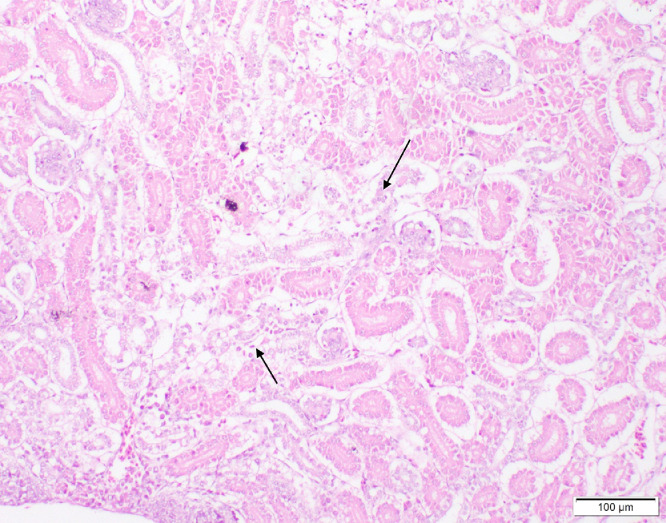
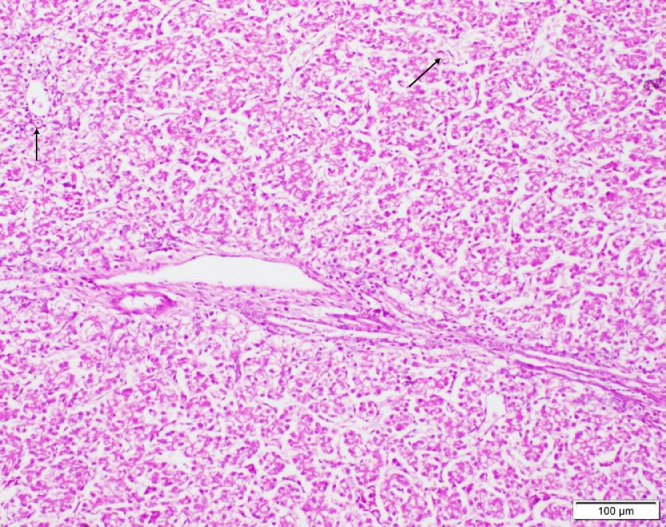
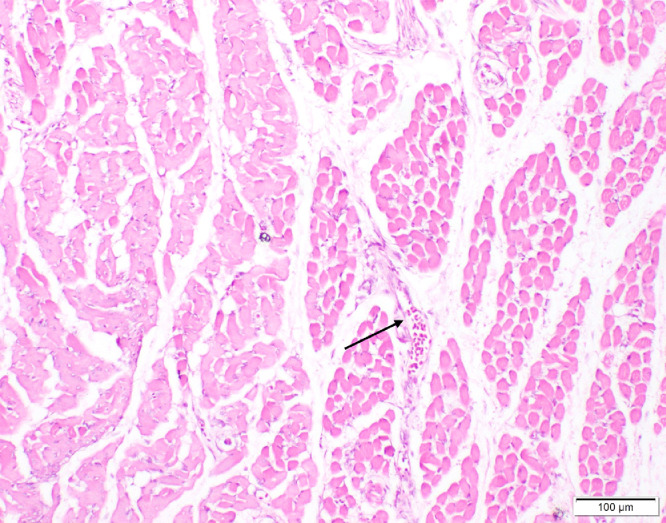
Freshly dead birds in both Groups 1 and 2 revealed different histopathological lesions in different organs, the lesions were mainly of degenerative nature. Necrosis of the epithelial lining of renal tubules in kidney was observed (Figure 2), we noted multiple foci of hepatocytic coagulative necrosis in liver (Figure 3) and degenerative changes of heart muscle bundles (Figure 4). The inflammatory reaction was noted and manifested by infiltration of inflammatory cells in the renal interstitial tissue (Figure 2), congestion of heart blood vessels and edema between cardiac muscle bundles were observed as well (Figure 4).
Figure 2.
Kidney of chicks showing necrobiotic changes of the epithelial lining of renal tubules with focal infiltrations of inflammatory cells (arrows) (H&E, scale bar = 100 µm).
Figure 3.
Liver of chick showing multifocal areas of coagulative necrosis (arrows) (H&E, scale bar = 100 µm).
Figure 4.
Heart of chick showing congestion of blood vessels (arrow), edema between cardiac muscles and degenerative changes of muscle bundles (H&E, scale bar = 100 µm).
Silver Nanoparticles Preparation and Characterization
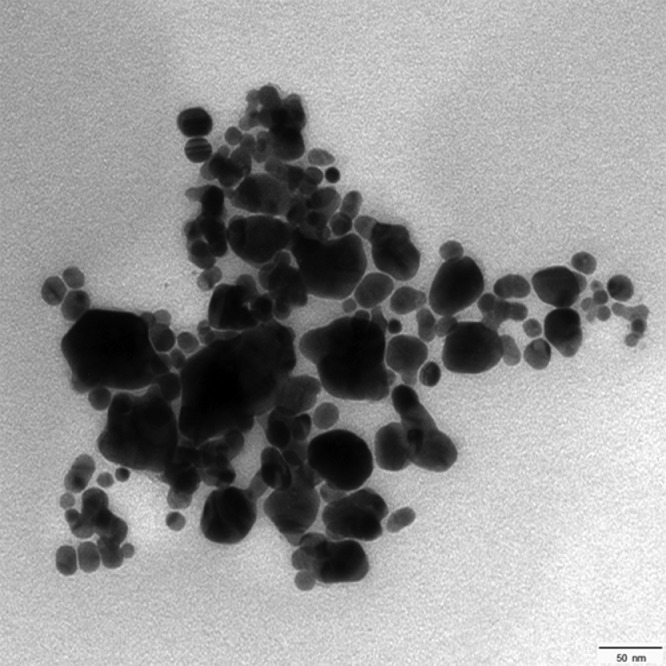
Transmission electron microscopy (TEM) imaging of the synthesized silver nanoparticles shows their spherical shape. The mean diameter of the nanoparticles was 33 nm (Figure 5).
Figure 5.
TEM image shows the morphology of silver nanoparticles which appear as spheres with a mean diameter of 33 nm (scale bar = 50 nm).
In Vitro Assessment of Silver Nanoparticles Efficacy Against Resistant Strains of P. Aeruginosa
Minimal inhibitory concentration assay: The bacterial growth of P. aeruginosa was completely inhibited after 24 h incubation with silver nanoparticles (25 µg/mL) and this was evidenced by the complete absence of bacterial growth on agar plates, but other concentrations (12.5, 6.25, and 3.13 μg/mL) showed incomplete inhibition of bacterial growth.
DISCUSSION
The hatchery is the primary source of disease transmission in the poultry industry, the issue often begins with infected eggs that have been incubated under suitable conditions for microbiological development, from dead in shell embryos, various bacterial pathogens that contaminate hatcheries have been isolated especially P. aeruginosa (Shahat et al., 2019; Abd El-Ghany, 2021; Algammal et al., 2023).
In this study, the overall isolation percentage from all examined samples was 36.2% (571/1,576) representing 45.2% (532/1,176) from different birds' tissues and 39/400 (9.7%) from total egg samples. In Egypt, lower isolation rates were reported by Mohamed (2004) who detected P. aeruginosa with incidence of 8.7%, and 17.6% from broiler chicken flocks in various governorates. Hassan (2013) recovered P. aeruginosa from diseased with isolation percentage of 25.3% and from dead broiler chicks with isolation percentage of 10% also, Eraky et al. (2020) isolated P. aeruginosa with incidence of (8%) from hatcheries and 8.25% from dead in shell embryos. Shukla and Mishra (2015) isolated P. aeruginosa with incidence of (20%) from 4-days-old sick chicks in Jabalpur. The variations in the isolation rate may be contributed to the differences in the collected samples, isolation media, isolation procedure, and the isolation conditions.
In this study, P. aeruginosa was isolated from different organs (heart, liver, kidney, air sac, yolk sac, subcutaneous tissue, external eggshell swabs, and internal egg content) also, Shukla and Mishra (2015) recovered P. aeruginosa from heart, lung, liver, and air sacs of 4-day-old broiler chickens.
Pseudomonas infection was considered an extensive economic problem in poultry farms, especially P. aeruginosa which causes a high mortality in poultry (Elsayed et al., 2016). The complications caused by P. aeruginosa in poultry have appeared in the form of respiratory signs, septicemia, keratitis, and sinusitis (Hai-ping, 2009).
In this study, the molecular detection of P. aeruginosa was higher in chicken (n = 34) following by Turkey (n = 14), whereas the lowest rate found in pigeon (n = 1). Our findings were consistent with those of Shahat et al. (2019), who isolated P. aeruginosa at a 42% incidence from dead broiler chicks. OPRL genes are thought to be a specific marker for molecular detection of P. aeruginosa and encode a protein in the inner and outer membranes that is necessary for the invasion of epithelial cells (De Vos et al., 1997). This reflects on the use of OPRL genes in molecular detection which confirmed the existence of P. aeruginosa.
However, the results of molecular identification of P. aeruginosa from turkey is dramatically different from Estepa et al. (2015) who detected only 5 isolates of P. aeruginosa from which one of them only isolated from turkey meat.
The results in Table 7 showed that the most isolated site of P. aeruginosa in chicken was liver followed by air sac, yolk sac then heart and kidney. On other hand, El sadda et al. (2021) found the most isolated site was yolk sac 15.5% (13 out of 84), liver 4.5% (9 out of 200); 1.5% (3 out of 200) from heart blood, and zero from kidney, while in turkey the most prevalent organ was heart and subcutaneous tissue; liver and air sac then the lowest isolated organ was sinus.
P. aeruginosa gains attention as a dangerous disease for consumer health that causes a variety of infections in people and food animals and possesses features that are transferrable to other pathogens that affect both people and animals (Wood et al., 2023). One of the biggest issues facing the globe today is antimicrobial resistance, which is getting worse in developing nations (El-Saadony et al., 2023). As a result, it is critical to diagnose P. aeruginosa accurately and rapidly and determine its susceptibility pattern to prevent unnecessary antibiotic use that could result in antibiotic-resistant organisms (Hamisi et al., 2012).
The molecular investigation of ESBLs producing isolates for (TEM, Ctx-M, and Shv) as shown in Table 7, revealed that the most infected organ in chicken was liver (12 out of 15), heart (3 out of 4), air sac (3 out of 6) whereas the isolates of yolk sac and table egg show 100%. On other hand, the most prevalent site in turkey was heart and subcutaneous tissue (4 out of 4) followed by liver (2 out of 3). Most of the isolates were found to harbor one or more of the investigated genes as shown in Table 8 where at least 2 different ESBL genes were present in the isolates, with 100% prevalence of the TEM gene being the most common gene. The genes for TEM-1, TEM-2, SHV-1, and other β-lactamases are the source of ESBLs due to mutations that change the amino acid structure surrounding their active sites. The range of β-lactam antibiotics susceptible to hydrolysis by these enzymes is thereby expanded (Paterson and Bonomo, 2005). Plasmids are routinely used to encode the ESBLs whereas genes encoding resistance to different drug classes are typically included in plasmids that produce ESBLs.
Our results are sharply different from Radwan et al. (2018) who confirmed ESBLs phenotypically in 10 (40%) out of 25 isolates of P. aeruginosa recovered from inflamed kidneys. From which Ctx-M gene was the most prevalent gene with a percentage of 50%.
Along with table egg, the high prevalence of ESBL-encoding genes (100%) and this can facilitate the spread of multidrug-resistant organisms (MDRO).This results can be is worrying, as these can contribute to not only local but global dissemination of antimicrobial resistance and this agrees by Dierikx et al. (2013). Studies have demonstrated that ESBL producers are persistently carried by chicken (Huijbers et al., 2016). According to Nilsson et al. (2014) and Reich et al. (2013), ESBL producers are separated from broiler chickens and grandparent breeding stock and this ease the transmission to the surrounding environment especially water and soil leading to the potential hazard to the human. At the same manner, eggs considered one of the main food chain, the presence of MDRO on it facilitate its spreading to human consequently being one of the causative agents for infections with limited therapeutic options (Bettiol and Harbarth, 2015).
Carbapenems are beta-lactam antibiotics that are frequently used as a last-resort antimicrobial against MDRO (Temkin et al., 2014). Carbapenems are effective against gram-negative bacilli that produce ESBL and AmpC. Because of the widespread spread of MDRO, these antimicrobials have recently become widely used in human medicine. As a result, carbapenem resistance has spread rapidly, and it is now a common occurrence in hospitals and, to a lesser extent, community settings.
The results of carbapenemase-encoding genes as shown in Table 5 illustrated that its rate in chicken is approximately like turkey but differ in the isolation sites where the most prevalent organ in chicken was liver (4 out of 15) then air sac (1 out of 6) and yolk sac (1 out of 4). However, liver and heart were the most prevalent organs in turkey with percentage rate 66% (2 out of 3) and 50% (2 out 4) relatively. Sinus of turkey shows 25% (1 out of 4) isolation rate of carbapenemase-encoding genes. All isolates were carrying NDM type as shown in Table 6 and this agree with Abdallah et al. (2015) who mentioned that the NDM group was found in all carbapenemase-producing Enterobacteriaceae isolates from retail chicken meat in Egypt. Hamza et al. (2016) detected 43% of their isolates from chicken were carbapenem-resistant and all of them were positive for blaNDM.
Since the usage of carbapenem “which are a class of beta-lactam antibiotics that are active against a wide range of organisms” is prohibited in veterinary medicine, its presence indicates the contamination of the chicken farms either from the infected human carrier or sewage effluent which may contaminate the drinking water inside poultry farms and subsequently infect the poultry and residues of resistant genes remain inside different organs causing human health risk in the public sphere.
From our results, the pathogenicity testing of MDR P. aeruginosa strain revealed 100% morbidity and 100% mortalities in G1 and the birds in this group showed a septicemic picture in PM examination while, G2 revealed 80% morbidity and 40% mortality within week postoral challenge and the birds showed a variety of clinical signs and PM lesions. Similar findings were reported by Shukla and Mishra (2015) who challenged chicks with P. aeruginosa with 2 different methods as intramuscularly (I/M) with 0.25 mL of the broth culture or via swapping the palatine cleft, and reported 100% mortalities in I/M infected birds within 24 h with the reisolation of the organism, while, they recorded 30% mortalities in those infected via the swapping of the palatine cleft within 72 h after challenge and they also, recorded similar signs and noticed lungs congestion, liver paleness with petechial hemorrhages, impacted gall bladder, enteritis and intestine filled with cheesy sticky substances, distended cloaca, and unabsorbed yolk in PM examination of freshly dead birds.
Bellanti et al. (1987) referred to that one of the adverse reactions of vaccination is the transmission of pathogens especially Pseudomonas spp. via contaminated inactivated vaccines. From the results of S/C injection of P. aeruginosa and the resulted 100% mortalities, call us to emphasize the need to ensure that inactivated vaccines are free from contamination with the P. aeruginosa, and to make sure that sterile syringes are used when dealing with birds to avoid the spread of the P. aeruginosa through contaminated inactivated vaccines and/or contaminated syringes, which may in turn lead to 100% mortality as a result of the transmission of P. aeruginosa to birds through injections.
Regarding histopathological lesions, the characteristic lesions of P. aeruginosa infection were mainly of degenerative and inflammatory nature. Focal hepatic necrosis found in this study was previously reported in P. aeruginosa infected chicks (Supartika et al., 2006). Edema found between heart muscle bundles are attributed to the effects of P. aeruginosa toxins on the permeability of endothelial junctions (Rejman et al., 2007).
Nanoparticle applications in veterinary medicine are surging (Shaalan et al., 2016; Abdel Ghany et al., 2021; Abu-Elala et al., 2021). Nanoparticles show potential activity against different bacterial species including multidrug-resistant ones (Lang et al., 2021; Elgendy et al., 2022). In fact, Ag-NPs have improved universal antibacterial, antiviral and antiparasitic activities (Rizzello and Pompa, 2014). Specifically, silver nanoparticles are one of the most potent nanoantibacterial agents studied in the literature, they are employed in a variety of avian production processes, including as detergents formulations in hatcheries (Banach et al., 2016), in addition, studies have been applied to show their efficacy against various pathogens (Farouk et al., 2020; El-Adawy et al., 2021).
From our in vitro results, Ag NPs revealed a potent antibacterial activity against MDR P. aeruginosa. Our results are parallel to those conducted by El-Adawy et al. (2021) who referred to its antibacterial activity against P. aeruginosa strain isolated from fish, Salem et al. (2021) who confirmed the efficacy of Ag-NPs against Clostridia perfringens in broiler chickens, Farouk et al. (2020) showed that it was effective against MDR Salmonella spp., Vadalasetty et al. (2018) studied its potency against Campylobacter jejuni in chickens and Attia et al. (2022) who confirmed the antiparasitic efficacy of chitosan-silver nanoparticles against Pseudolynchia canariensis in pigeons.
The antibacterial activity of Ag-NPs could be attributed to its ability to disrupt and penetrating the microorganism's cell membrane, resulting in damage to the cell wall and cytoplasm leakage, and through their intimate association with the thiol groups found in the primary respiratory enzymes, Ag NPs have been demonstrated to have antibacterial inhibitory activities (Gordon et al., 2010; Hassan et al., 2021).
CONCLUSIONS
Multidrug-resistant P. aeruginosa hazard threatens the poultry industry revealing high morbidity and mortalities at first week of age with a zoonotic hazard. In this study, some strains showed MDR against (kanamycin, amoxicillin, amoxicillin-clavulanic, neomycin, chloramphenicol, vancomycin, cefotaxime clavulanic acid, lincomycin-spectinomycin, co-trimoxazole, cefoxitin, gentamycin, and doxycycline) which were confirmed by the molecularly detected (ESBL and carbapenemase-encoding genes). The selected MDR strain showed severe pathogenicity with histopathological tissue change in both S/C and oral challenged birds inducing 100 and 40% mortalities, respectively. MDR P. aeruginosa strain sowed in vitro sensitivity to silver nanoparticles, and further studies are recommended for the in vivo evaluation of the effect of different concentration of silver nanoparticles against P. aeruginosa experimental infection with studying its residual effect in the edible part of chicken meats. The control of P. aeruginosa and overcoming MDR requires effective alternative treatments to antibiotics side by side with the application of hygienic measures in both hatcheries and poultry farms levels.
ACKNOWLEDGMENTS
The research was supported partially by the National Natural Science Foundation of China (NSFC, Nos. 81700973 and 81870770).
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in the present study.
REFERENCES
- Abd El-Hack M.E., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B., Swelum A.A. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022;101:101696. doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah H.M., Reuland E.A., Wintermans B.B., Al Naiemi N., Koek A., Abdelwahab A., Ammar A.M., Mohamed A.A., Vandenbroucke-Grauls C.M.J.E. Extended-spectrum b-lactamases and/or carbapenemases-producing Enterobacteriaceae isolated from retail chicken meat in Zagazig, Egypt. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Ghany W.A. Pseudomonas aeruginosa infection of avian origin: zoonosis and one health implications. Vet. World. 2021;14:2155. doi: 10.14202/vetworld.2021.2155-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Ghany W.A., Shaalan M., Salem H.M. Nanoparticles applications in poultry production: an updated review. WPSJ. 2021;77:1001–1025. [Google Scholar]
- Abu-Elala N.M., Shaalan M., Ali S.E., Younis N.A. Immune responses and protective efficacy of diet supplementation with selenium nanoparticles against cadmium toxicity in Oreochromis niloticus. Aquacult. Res. 2021;52:3677–3686. [Google Scholar]
- Algammal A.M., Eidaroos N.H., Alfifi K.J., Alatawy M., Al-Harbi A.I., Alanazi Y.F., Ghobashy M.O., Khafagy A.R., Esawy A.M., El-Sadda S.S., Hetta H.F. opr L gene sequencing, resistance patterns, virulence genes, quorum sensing and antibiotic resistance genes of XDR Pseudomonas aeruginosa isolated from broiler chickens. Infect. Drug Resist. 2023;16:853–867. doi: 10.2147/IDR.S401473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M.M., Yehia N., Soliman M.M., Shukry M., El-Saadony M.T., Salem H.M. Evaluation of the antiparasitic activity of the chitosan-silver nanocomposites in the treatment of experimentally infested pigeons with Pseudolynchia canariensis. Saudi J. Biol. Sci. 2022;29:1644–1652. doi: 10.1016/j.sjbs.2021.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakheet A.A., Torra D.E. Detection of Pseudomonas aeruginosa in dead chicken embryo with reference to pathological changes and virulence genes. Alexandria J. Vet. Sci. 2020;65:81–89. [Google Scholar]
- Banach M., Tymczyna L., Chmielowiec-Korzeniowska A., Pulit-Prociak J. Nanosilver biocidal properties and their application in disinfection of hatchers in poultry processing plants. Bioinorg. Chem. Appl. 2016;2016 doi: 10.1155/2016/5214783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft J.D., Gamble M. Theory and Practice of Histological Techniques. Elsevier Health Sciences; 2008. [Google Scholar]
- Barrow G.I., Feltham R.A. Cowan and Steel’s Manual for the Identification of Medical Bacteria. 3rd. Cambridge University Press; Cambridge, UK: 2003. [Google Scholar]
- Bauer A.W., Kirby W.M., Sherris J.C., Turuk M. Antibiotic susceptibility testing by a standardized single disc method. J. Clin. Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- Bellanti J.A., Fishman H.J., Wientzen R.L. Adverse reactions to vaccines. Immunol. Allergy Clin. North Am. 1987;7:423–445. [Google Scholar]
- Bettiol E., Harbarth S. Development of new antibiotics: taking off finally? Swiss Med. Wkly. 2015;145:w14167. doi: 10.4414/smw.2015.14167. [DOI] [PubMed] [Google Scholar]
- Chand Y., Khadka S., Sapkota S., Sharma S., Khanal S., Thapa A., Rayamajhee B., Khadka D.K., Panta O.P., Shrestha D., Poudel P. Clinical specimens are the pool of multidrug-resistant Pseudomonas aeruginosa harbouring oprL and toxA virulence genes: findings from a tertiary hospital of Nepal. Emerg. Med. Int. 2021;2021:1–8. doi: 10.1155/2021/4120697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channa I.A., Kalhoro N.H., Channa A.A., Korejo N.A., Sethar A., Soomro A.H., Munir U., Soomro H., Yaseen G. Prevalence, biochemical characteristics and antibiotic susceptibility of pathogenic bacteria in poultry litter. Sindh Univ. Res. J. (Surg.) 2019;51:533–540. [Google Scholar]
- De Vos D., Lim A.J., Pirnay J.P., Struelens M., Vandenvelde C., Duinslaeger L., Vanderkelen A., Cornelis P. Direct detection and identification of Pseudomonas aeruginosa in clinical samples such as skin biopsy specimens and expectorations by multiplex PCR based on two outer membrane lipoprotein genes, oprI and oprL. J. Clin. Microbiol. 1997;35:1295–1299. doi: 10.1128/jcm.35.6.1295-1299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierikx C.M., van der Goot J.A., Smith H.E., Kant A., Mevius D.J. Presence of ESBL/AmpC-producing Escherichia coli in the broiler production pyramid: a descriptive study. PLoS One. 2013;8:e79005. doi: 10.1371/journal.pone.0079005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinev I., Denev S., Beev G. Clinical and morphological studies on spontaneous cases of Pseudomonas aeruginosa infections in birds. Pak. Vet. J. 2013;33:398–400. [Google Scholar]
- Dougnon P., Dougnon V., Legba B., Fabiyi K., Soha A., Koudokpon H., Sintondji K., Deguenon E., Hounmanou G., Quenum C., Aminou T., Baba-moussa L. Antibiotic profiling of multidrug resistant pathogens in one-day-old chicks imported from Belgium to Benin. BMC Vet. Res. 2023;19:17. doi: 10.1186/s12917-023-03570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou V., Gousia P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015;8:49–61. doi: 10.2147/IDR.S55778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov A.M., Ulyashova M.M., Rubtsova M.Y. Bacterial enzymes and antibiotic resistance. Acta Nat. 2018;10:33–39. [PMC free article] [PubMed] [Google Scholar]
- El-Adawy M.M., Eissa A.E., Shaalan M., Ahmed A.A., Younis N.A., Ismail M.M., Abdelsalam M. Green synthesis and physical properties of Gum Arabic-silver nanoparticles and its antibacterial efficacy against fish bacterial pathogens. Aquac. Res. 2021;52:1247–1254. [Google Scholar]
- Elgendy M.Y., Shaalan M., Abdelsalam M., Eissa A.E., El-Adawy M.M., Seida A.A. Antibacterial activity of silver nanoparticles against antibiotic-resistant Aeromonas veronii infections in Nile tilapia, Oreochromis niloticus (L.), in vitro and in vivo assay. Aquacult Res. 2022;53:901–920. [Google Scholar]
- El-Saadony M.T., Saad A.M., Yang T., Salem H.M., Korma S.A., Ahmed A.E., Ibrahim S.A. Avian campylobacteriosis, prevalence, sources, hazards, antibiotic resistance, poultry meat contamination and control measures: a comprehensive review. Poult. Sci. 2023;102:102786. doi: 10.1016/j.psj.2023.102786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-sadda S., Esawy A., ELTarabili R.M., Khafagy A. Molecular characterization and genetic analysis of Pseudomonas aeruginosa recovered from broiler chickens. SCVMJ. 2021;26:61–77. [Google Scholar]
- Elsayed M.S.A., Ammar A.M., Al Shehri Z.S., Abd-El Rahman N.A. Virulence repertoire of Pseudomonas aeruginosa from some poultry farms with detection of resistance to various antimicrobials and plant extracts. Cell. Mol. Biol. 2016;62:124–132. [Google Scholar]
- Elshafiee E.A., Nader S.M., Dorgham S.M., Hamza D.A. Carbapenem-resistant Pseudomonas aeruginosa originating from farm animals and people in Egypt. J. Vet. Res. 2019;63:1–5. doi: 10.2478/jvetres-2019-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eman M.F., Heba R., Bakheet A.A., Abd El Hafez S.A., Heba B. Advanced studies on Pseudomonas aeruginosa infection in chicken. Anim. Health Res. J. 2017;5:207–217. [Google Scholar]
- Eraky R.D., Abd El-Ghany W.A., Soliman K.M. Studies on Pseudomonas aeruginosa infection in hatcheries and chicken. J. Hellenic Vet. Med. Soc. 2020;71:1953–1962. [Google Scholar]
- Estepa V., Rojo-Bezares B., Torres C., Sa´enz Y. Genetic lineages and antimicrobial resistance in Pseudomonas spp. isolates recovered from food samples. Foodborne Pathog. Dis. 2015;12:486–491. doi: 10.1089/fpd.2014.1928. [DOI] [PubMed] [Google Scholar]
- Farouk M.M., El-Molla A., Salib F.A., Soliman Y.A., Shaalan M. The role of silver nanoparticles in a treatment approach for multidrug-resistant Salmonella species isolates. Int. J. Nanomed. 2020;15:6993. doi: 10.2147/IJN.S270204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon O., Vig Slenters T., Brunetto P.S., Villaruz A.E., Sturdevant D.E., Otto M., Fromm K.M. Silver coordination polymers for prevention of implant infection: thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrob. Agents Chemother. 2010;54:4208–4218. doi: 10.1128/AAC.01830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenni M., Saras E., Ponsin C., Dahmen S., Petitjean M., Hocquet D., Madec J.Y. High prevalence of international ESBL CTX-M-15-producing Enterobacter cloacae ST114 clone in animals. J. Antimicrob. Chemother. 2016;71:1497–1500. doi: 10.1093/jac/dkw006. [DOI] [PubMed] [Google Scholar]
- Hai-ping H.E. Isolation and identify of Pseudomonas aeruginosa in chicken dead-embryos Chinese Qinghai. J. Anim. Vet. Sci. 2009;3:25–27. [Google Scholar]
- Haleem H., Kadhim J., Ilham T., Banyan A. Isolation of Pseudomonas aeruginosa from clinical cases and environmental samples, and analysis of its antibiotic resistant spectrum at Hilla Teaching Hospital. Med. J. Babyl. 2011;8:618–624. [Google Scholar]
- Hamisi Z., Tuntufye H., Shahada F. Antimicrobial resistance phenotypes of Escherichia coli isolated from tropical free-range chickens. Int. J. Sci. Res. 2012;3:34. [Google Scholar]
- Hamza E., Dorgham S.M., Hamza D.A. Carbapenemase-producing Klebsiella pneumoniae in broiler poultry farming in Egypt. J. Glob. Antimicrob. Resist. 2016;7:8–10. doi: 10.1016/j.jgar.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Hassan H.M. Faculty of Veterinary Medicine, Beni-Suef University; Egypt: 2013. Characterization of Pseudomonas Aeruginosa Isolated From Different Pathological Lesions in Chickens. MVSc Thesis. [Google Scholar]
- Hassan A., Yousif M.H., Abd-Elkhaliq H.M.M., Wahba A.K.A., El-Hamaky A.M.A. The antimicrobial potential of selenium nanoparticles singly and in combination with cinnamon oil against fungal and bacterial causes of diarrhea in buffaloes. Adv. Anim. Vet. Sci. 2021;9:1238–1248. [Google Scholar]
- Huijbers P.M., Graat E.A., van Hoek A.H., Veenman C., de Jong M.C., van Duijkeren E. Transmission dynamics of extended-spectrum beta-lactamase and AmpC beta-lactamase-producing Escherichia coli in a broiler flock without antibiotic use. Prev. Vet. Med. 2016;131:12–19. doi: 10.1016/j.prevetmed.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Jawher I.M., Hasan M.G. Antibiotics resistance patterns of Pseudomonas aeruginosa isolated from meat at Mosul city retails. Iraqi J. Vet. Sci. 2023;37:363–367. [Google Scholar]
- Lang C., Mission E.G., Fuaad A.A.H.A., Shaalan M. Nanoparticle tools to improve and advance precision practices in the Agrifoods Sector towards sustainability - a review. J. Clean. Prod. 2021;293 [Google Scholar]
- Le Guern R., Grandjean T., Stabler S., Bauduin M., Gosset P., Kipnis É., Dessein R. Gut colonisation with multidrug-resistant Klebsiella pneumoniae worsens Pseudomonas aeruginosa lung infection. Nat. Commun. 2023;14:78. doi: 10.1038/s41467-022-35767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant I.A., Packer R.A. 7th ed. The Iowa University Press; Ames, Iowa: 1967. Pages 286–301 in Veterinary Bacteriology and Virology. [Google Scholar]
- Mohamed H.A. Some studies on Pseudomonas species in chicken embryos and broiler in Assiut governorate. Ass. Univ. Bull. Environ. 2004;7:23–31. [Google Scholar]
- Monstein H.J., Stholm-Balkhedm A.O., Nilsson M.V., Dornbusch K., Nilsson L.E. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM, and blaCTX-M genes in Enterobacteriaceae. APMIS. 2007;115:1400–1408. doi: 10.1111/j.1600-0463.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- National Committee for Clinical Laboratory Standards (NCCLS) Performance Standards for Antimicrobial Disc Susceptibility Test. 7th ed., Approved Standards M2-A8. National Committee for Clinical Laboratory Standards; Wayne, PA: 2002. [Google Scholar]
- Nilsson O., Borjesson S., Landen A., Bengtsson B. Vertical transmission of Escherichia coli carrying plasmid-mediated AmpC (pAmpC) through the broiler production pyramid. J. Antimicrob. Chemother. 2014;69:1497–1500. doi: 10.1093/jac/dku030. [DOI] [PubMed] [Google Scholar]
- Odoi H., Boamah V.E., Boakye Y.D., Agyare C. Prevalence and phenotypic and genotypic resistance mechanisms of multidrug-resistant Pseudomonas aeruginosa strains isolated from clinical, environmental, and poultry litter samples from the Ashanti region of Ghana. J. Environ. Public Health. 2021;2021 doi: 10.1155/2021/9976064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson D.L., Bonomo R.A. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan I.A.H., Shehata A.H.E., Abd ELwahab S.H. Phenotypic and genotypic characterization of Pseudomonas aeruginosa recovered from kidney lesions of broiler chickens. Assiut Vet. Med. J. 2018;64:110–116. [Google Scholar]
- Reich F., Atanassova V., Klein G. Extended-spectrum beta-lactamase- and AmpC-producing enterobacteria in healthy broiler chickens, Germany. Emerg. Infect. Dis. 2013;19:1253–1259. doi: 10.3201/eid1908.120879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejman J., Di Gioia S., Bragonzi A., Conese M. Pseudomonas aeruginosa infection destroys the barrier function of lung epithelium and enhances polyplex-mediated transfection. Hum. Gene Ther. 2007;18:642–652. doi: 10.1089/hum.2006.192. [DOI] [PubMed] [Google Scholar]
- Rizzello L., Pompa P.P. Nanosilver-based antibacterial drugs and devices: mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 2014;43:1501–1518. doi: 10.1039/c3cs60218d. [DOI] [PubMed] [Google Scholar]
- Salem H.M., Ismael E., Shaalan M. Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int. J. Nanomed. 2021;16:6783. doi: 10.2147/IJN.S319708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schill F., Abdulmawjood A., Klein G., Reich F. Prevalence and characterization of extended-spectrum beta-lactamase (ESBL) and AmpC beta-lactamase producing Enterobacteriaceae in fresh pork meat at processing level in Germany. Int. J. Food Microbiol. 2017;257:58–66. doi: 10.1016/j.ijfoodmicro.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Shaalan M.I., El-Mahdy M.M., Theiner S., El-Matbouli M., Saleh M. In vitro assessment of the antimicrobial activity of silver and zinc oxide nanoparticles against fish pathogens. Acta Vet. Scand. 2017;59:1–11. doi: 10.1186/s13028-017-0317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaalan M., Saleh M., El-Mahdy M., El-Matbouli M. Recent progress in applications of nanoparticles in fish medicine: a review. Nanomed: NBM. 2016;12:701–710. doi: 10.1016/j.nano.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Shaalan M., Sellyei B., El-Matbouli M., Székely C. Efficacy of silver nanoparticles to control flavobacteriosis caused by Flavobacterium johnsoniae in common carp Cyprinus carpio. Dis. Aquat. Organ. 2020;137:175–183. doi: 10.3354/dao03439. [DOI] [PubMed] [Google Scholar]
- Shahat H.S., Mohamed H.M.A., Abd Al-Azeem M.W., Nasef S.A. Molecular detection of some virulence genes in Pseudomonas aeruginosa isolated from chicken embryos and broilers with regard to disinfectant resistance. SVU – Int. J. Vet. Sci. 2019;2:52–70. [Google Scholar]
- Shukla S., Mishra P. Pseudomonas aeruginosa infection in broiler chicks in Jabalpur. Int. J. Ext. Res. 2015;6:37–39. [Google Scholar]
- Supartika I.K., Toussaint M.J., Gruys E. Avian hepatic granuloma. A review. Vet. Q. 2006;28:82–89. doi: 10.1080/01652176.2006.9695213. [DOI] [PubMed] [Google Scholar]
- Temkin E., Adler A., Lerner A., Carmeli Y. Carbapenem‐resistant Enterobacteriaceae: biology, epidemiology, and management. Ann. N. Y. Acad. Sci. 2014;1323:22–42. doi: 10.1111/nyas.12537. [DOI] [PubMed] [Google Scholar]
- Vadalasetty K.P., Lauridsen C., Engberg R.M., Vadalasetty R., Kutwin M., Chwalibog A., Sawosz E. Influence of silver nanoparticles on growth and health of broiler chickens after infection with Campylobacter jejuni. BMC Vet. Res. 2018;14:1–11. doi: 10.1186/s12917-017-1323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S.E., Sander J.E., Cline J.L., Helton J.S. Characterization of Pseudomonas aeruginosa isolates associated with mortality in broiler chicks. Avian Dis. 2002;46:1045–1050. doi: 10.1637/0005-2086(2002)046[1045:COPAIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Wood S.J., Kuzel T.M., Shafikhani S.H. Pseudomonas aeruginosa: infections, animal modeling, and therapeutics. Cells. 2023;12:199. doi: 10.3390/cells12010199. [DOI] [PMC free article] [PubMed] [Google Scholar]