Abstract
The substituted 1,2,3-triazole core is prevalent in numerous commercially available drugs utilized for a wide range of clinical applications. Simultaneously, chalcone represents a privileged framework discovered in natural products exhibiting intriguing bioactivities. In this study, we synthesized triazole-bonded chalcone compounds (4ax-4by), starting from a simple aromatic ketone, acetophenone, which underwent aldol condensation to give hydroxychalcone intermediate. In the second step, the hydroxyl group of chalcone compound was adducted with propargyl moiety through propargylation reaction. Then, the propargylated products underwent smooth copper-mediated azide-alkyne cyclization to give the triazole-bonded chalcones as the final products. They were characterized by IR, NMR and HRMS, and evaluated their radical scavenging activity against 2,2-diphenyl-1-picrylhydrazyl (DPPH). Among the tested products, compound 4by was denoted as the most potent derivative which can inhibit DPPH radical in 91.62 ± 0.10% at 500 ppm.
-
•
Acetophenone as a simple ketone was modified to triazole-bonded chalcones.
-
•
Modification was performed through three steps reaction.
-
•
Final products exhibited free radical scavenging activity.
Keywords: 1,2,3-triazole; Chalcone; Copper-mediated azide-alkyne cyclization; Radical scavenging
Method name: Synthesis of triazole-bonded chalcone derivatives via three steps reaction: aldol condensation, propargylation, and then copper-mediated azide-alkyne cyclization
Graphical abstract
Specifications Table
| Subject area: | Chemistry |
| More specific subject area: | Organic Chemistry |
| Name of your method: | Synthesis of triazole-bonded chalcone derivatives via three steps reaction: aldol condensation, propargylation, and then copper-mediated azide-alkyne cyclization |
| Name and reference of original method: | Synthesis of triazole derivatives Bioorg. Med. Chem. Lett., 2020, 30, 127434. |
| Resource availability: | The investigation was conducted in the Laboratory of Organic and Biochemistry, Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Indonesia, Depok. The reagents and chemicals used were purchased from commercial suppliers such as Merck, Sigma-Aldrich, and PT. Smart Lab Indonesia. The instruments used were FTIR, 1H and 13C NMR, and HRMS. |
Method details
Chalcones are excellent compounds having α, β-unsaturated ketone functionality between the two aromatic rings. They are flavonoids precursors and have been extensively researched as essential structural elements in medication development [1]. Many pharmacological properties of chalcone and its derivatives have been reported in recent studies, including anticonvulsant, anti-inflammatory, antimalarial, anticancer, antioxidant, antibacterial, anti-proliferative, and xanthine oxidase inhibitory effects [2,3]. Heterocycles have been crucial in drug discovery. The structural motif 1,2,3-triazole is a neoclassical bioisostere of amide, which is a frequently used functional group in approved medications [4]. In general, 1,2,3-triazole has a number of advantages in drug development [5]. For example, triazoles have two H-bond acceptors and can interact with biomolecular targets through H-bonds, π-π stacking, and dipole interactions. In recent studies, organic compounds bearing 1,2,3-triazole have shown good activity as antioxidant [6,7], and other biological properties like anticancer [8] and antidiabetic [9]. With thie above advantages, we synthesized the triazole-bonded chalcone compounds as described as follows.
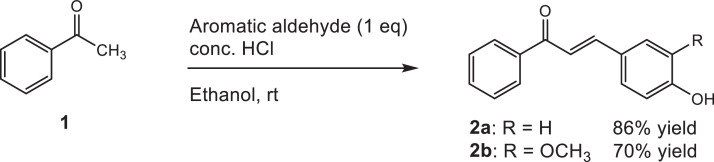
Our method is aimed to synthesize 1,2,3-triazole-bonded chalcones from simple aromatic ketone. To do it, acetophenone (1) was used as a starting material. Acetophenone (20 mmol, 2.33 mL), aromatic aldehyde (20 mmol), and ethanol (2 mL) were placed in a 50 mL round bottom flask. The reaction mixture was cooled down to 0°C and stirred for 5 min, then concentrated hydrochloric acid (5 mL) was added. The resulting mixture was stirred for 24 h at room temperature. After that, cold water (15 mL) and glacial acetic acid (15 mL) were added to the flask. The formed solid was filtered, washed with cold water, and then dried to give crude material, which was purified by silica gel column chromatography (hexane / ethyl acetate = 10 / 1 to 1 / 1) to give the corresponding hydroxychalcone 2a ((E)-3-(4-hydroxyphenyl)-1-phenylprop-2-en-1-one) or 2b ((E)-3-(4-hydroxy-3-methoxyphenyl)-1-phenylprop-2-en-1-one), respectively. Aldol condensation for the synthesis of chalcones (similar compounds to our research) were already performed by Sharma et al. (2014) in basic media using sodium hydroxide methanolic-aqueous solution [10]. However, in our experiments, performing aldol condensation in such condition was difficult when the substrate contains phenolic group, the product was obtained in low yield. Fortunately, under acidic condition, compounds 2a and 2b were obtained in good yield (Scheme 1).
Scheme 1.
Aldol condensation to produce hydroxychalcone.
The products from the aldol condensation were used in the second step. Propargylation reaction is a process to introduce propargyl moiety to a chemical structure [11]. For propargylation of phenolic compounds, potassium carbonate is often used as deprotonating agent [12]. Propargylation reaction of hydroxchalcone was performed by the following conditions (Scheme 2). In a 30 mL round bottom flask containing chalcone (5.0 mmol) in dimethylformamide (10 mL), potassium carbonate (10.0 mmol, 1.39 g) was added, and the mixture was stirred at room temperature for 1 h. A solution of propargyl bromide 80 wt.% in toluene (10.0 mmol, 1.12 mL) was added to the mixture, and the resulting solution was stirred at room temperature for 24 h. After that, the mixture was poured into cold water. The obtained solid was filtered and washed with cold water to afford propargylated chalcone 3a ((E)-1-phenyl-3-(4-(prop-2-yn-1-yloxy)phenyl)prop-2-en-1-one) or 3b ((E)-3-(3-methoxy-4-(prop-2-yn-1-yloxy)phenyl)-1-phenylprop-2-en-1-one), respectively.
Scheme 2.
Propargylation of hydroxychalcone.
Final step in our work is copper-mediated azide-alkyne [3+2] cyclization (Scheme 3). The typical reactions involve alkyne (propargylated compound, for example) and organic azide via 1,3-dipolar cycloaddition. Heating those substrates in the absence of catalyst will give a mixture of 1,4- and 1,5-disubstituted triazoles [13]. By using copper(I), the cycloaddition proceeds regioselectively to form 1,4-regioisomer [14,15]. In this experiment, we utilized in situ prepared Cu(I) from the redox reaction between copper(II) sulfate pentahydrate and ascorbic acid. In a 10 mL round bottom flask, a mixture containing propargylated chalcone (0.5 mmol), aromatic azide (0.5 mmol), ascorbic acid (0.20 mmol, 35.2 mg), and copper(II) sulfate pentahydrate (0.10 mmol, 25.0 mg) in dimethylformamide/water (1:1, 2.5 mL DMF and 2.5 mL water) was stirred at room temperature. After the completion of the reaction, the mixture was poured into ice cold water. The obtained solid was filtered, washed with excess water, and dried to afford triazole-bonded chalcone derivatives (4ax-4by).
Scheme 3.
Copper-mediated azide-alkyne [3+2] cyclization reaction.
The synthesized derivatives of 1,2,3-triazole-bonded chalcone derivatives were confirmed by Infrared (IR), Nuclear Magnetic Resonance (1H- and 13C-NMR), and High Resolution Mass Spectrometry (HRMS). The presence of conjugated carbonyl stretch in all compounds was confirmed at 1651–1654 cm−1. For acetyl derivatives of triazole-chalcone (compound 4ay and 4by), additional carbonyl stretches which are conjugated with phenyl ring was observed at 1679–1681 cm−1. From 1H-NMR, singlet signal at around 9.12–9.19 ppm indicated the presence of vinylic proton in triazole ring. The methoxy group in compound 4bx and 4by was indicated at peak 3.86 and 3.88 ppm, respectively. Meanwhile, methyl ketone proton in compound 4ay and 4by was confirmed at 2.65 ppm. In 13C-NMR, carbonyl of enone moiety was confirmed at 189 ppm. Meanwhile, carbonyl of acetyl group was observed at 197 ppm (compound 4ay and 4by) Methylene carbon next to triazole ring was indicated by peak at range 55–61 ppm. Finally, the findings of HRMS of synthesized derivatives were found to be very close to the theoretical values. Mass spectra of the synthesized derivatives reflected the characteristic [M+H]+ ion peaks.
In compared to other approaches, DPPH radical scavenging is a prominent methodology for measuring antioxidant activity in a very short period of time [16]. Using an ethanolic solution of the stable free radical DPPH, the antioxidant ability of the synthetic compounds (4ax–4by) was evaluated (Table 1). The sample solution (500 ppm, 2 mL) was added to the DPPH solution (100 ppm, 1 mL) in 2 mL of methanol. After homogenization, the solution was incubated for 30 min in dark condition. Absorption was read at 517 nm by UV-Vis spectrophotometer and compared with the blank. Based on evaluation, all final products, including compound 2a and 2b as their starting materials, showed radical scavenging activity. Compound 4by which contain methoxy and acetyl group was denoted as the most potent agent with inhibition capacity of 91.62 ± 0.10%. It may be attributed from the electronic effect of methoxy and acetyl group that can enhance the scavenging capacity.
Table 1.
Inhibition of DPPH by the synthesized products at 500 ppm.
| Compound | % Inhibition |
|---|---|
| 2a | 82.37 ± 0.10 |
| 2b | 89.01 ± 0.10 |
| 4ax | 43.40 ± 1.25 |
| 4ay | 73.59 ± 0.25 |
| 4bx | 81.33 ± 0.33 |
| 4by | 91.62 ± 0.10 |
| Ascorbic Acid | 98.66 ± 0.06 (at 25 ppm) |
CRediT authorship contribution statement
Atta Ullah: Investigation, Validation, Data curation. Nur Rohman: Investigation, Validation, Data curation. Bayu Ardiansah: Conceptualization, Methodology, Supervision, Writing – original draft. Antonius Herry Cahyana: Methodology, Supervision. Abdulrahman A. Almehizia: Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was fully supported by the Directorate of Research and Development, Universitas Indonesia through Hibah PUTI Q2 Batch 3 (Matching Fund) 2022 with contract No. NKB-1453/UN2.RST/HKP.05.00/2022. The authors would like to express our sincere gratitude to Assoc. Prof. Mohammad Abrar Alam (Department of Chemistry and Physics, Arkansas State University) for helpful discussion. We also thank Mr. Azhar Darlan (Puslabfor, POLRI) and Ms. Pratiwi Puji Lestari, M.Si. (ILRC Laboratory, Universitas Indonesia) for their kind help in recording HRMS and NMR spectra, respectively.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2023.102322.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- 1.Karthikeyan C., Moorthy N.S.H.N., Ramasamy S., Vanam U., Manivannan E., Karunagaran D., Trivedi P. Advances in chalcones with anticancer activities. Recent Pat. Anti-Cancer Drug Discov. 2015;10:97–115. doi: 10.2174/1574892809666140819153902. [DOI] [PubMed] [Google Scholar]
- 2.Nelson G., Alam M.A., Atkinson T., Gurrapu S., Kumar J.S., Bicknese C., Johnson J.L., Williams M. Synthesis and evaluation of p-N,N-dialkyl substituted chalcones as anti-cancer agents. Med. Chem. Res. 2013;22:4610–4614. [Google Scholar]
- 3.Patel S., Challagundla N., Rajput R.A., Mishra S. Design, synthesis, characterization and anticancer activity evaluation of deoxycholic acid-chalcone conjugates. Bioorg. Chem. 2022;127 doi: 10.1016/j.bioorg.2022.106036. [DOI] [PubMed] [Google Scholar]
- 4.Agalave S.G., Maujan S.R., Pore V.S. Click chemistry: 1,2,3-triazoles as pharmacophores. Chem.: Asian J. 2011;6:2696–2718. doi: 10.1002/asia.201100432. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Huang B., Liu X., Zhang P. Discovery of bioactive molecules from CuAAC click chemistry-based combinatorial libraries. Drug Discov. Today. 2016;21:118–132. doi: 10.1016/j.drudis.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Harej A., Macan A.M., Stepanić V., Klobučar M., Pavelić K., Pavelić S.K., Raić-Malić S. The antioxidant and antiproliferative activities of 1,2,3-triazolyl-L-ascorbic acid derivatives. Int. J. Mol. Sci. 2019;20:4735. doi: 10.3390/ijms20194735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ardiansah B., Hardhani M.R., Putera D.D.S.R., Wukirsari T., Cahyana A.H., Jia J., Khan M.M. Design, synthesis, and antioxidant evaluation of monocarbonyl curcumin analogues tethered 1,2,3-triazole scaffold. Case Stud. Chem. Environ. Eng. 2023;8 [Google Scholar]
- 8.Liang T., Sun X., Li W., Hou G., Gao F. 1,2,3-triazole-containing compounds as anti–lung cancer agents: current developments, mechanisms of action, and structure–activity relationship. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.661173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iraji A., Shareghi-Brojeni D., Mojtabavi S., Faramarzi M.A., Akbarzadeh T., Saeedi M. Cyanoacetohydrazide linked to 1,2,3-triazole derivatives: a new class of α-glucosidase inhibitors. Sci. Rep. 2022;12:8647. doi: 10.1038/s41598-022-11771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma N., Mohanakrishnan D., Sharma U.K., Kumar R., Richa A.K.Sinha, Sahal D. Design, economical synthesis and antiplasmodial evaluation of vanillin derived allylated chalcones and their marked synergism with artemisinin against chloroquine resistant strains of Plasmodium falciparum. Eur. J. Med. Chem. 2014;79:350–368. doi: 10.1016/j.ejmech.2014.03.079. [DOI] [PubMed] [Google Scholar]
- 11.Dwivedi P., Mishra K.B., Pritika B.B.Mishra, Tiwari V.K. Click inspired synthesis of triazole-linked vanillin glycoconjugates. Glycoconjugate J. 2017;34:61–70. doi: 10.1007/s10719-016-9729-4. [DOI] [PubMed] [Google Scholar]
- 12.Goswami L., Gupta L., Paul S., Vermani M., Vijayaraghavan P., Bhattacharya A.K. Design and synthesis of eugenol/isoeugenol glycoconjugates and other analogues as antifungal agents against Aspergilus fumigatus. RSC Med. Chem. 2022;13:955–962. doi: 10.1039/d2md00138a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes R.S., Jardim G.A.M., de Carvalho R.L., Araujo M.H., da Silva Junior E.N. Beyond copper-catalyzed azide-alkyne 1,3-dipolar cycloaddition: Synthesis and mechanism insight. Tetrahedron. 2019;75:3697–3712. [Google Scholar]
- 14.Worrell B.T., Malik J.A., Fokin V.V. Direct evidence of a dinuclear copper intermediate in Cu(I)-catalyzed azide-alkyne cycloadditions. Science. 2013;340:457–460. doi: 10.1126/science.1229506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patil P.S., Kasare S.L., Haval N.B., Khedkar V.M., Dixit P.P., Rekha E.M., Sriram D., Haval K.P. Novel isoniazid embedded triazole derivatives: synthesis, antitubercular and antimicrobial activity evaluation. Bioorg. Med. Chem. Lett. 2020;30 doi: 10.1016/j.bmcl.2020.127434. [DOI] [PubMed] [Google Scholar]
- 16.Cetin A, Gecibesler I.H. Evaluation as antioxidant agents of 1,2,4-triazole derivatives: effects of essential functional groups. J. Appl. Pharmaceut. Sci. 2015;5:120–126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.