Abstract
Background
Difficult cannulation is a risk factor for post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP). It has been postulated that the pancreatic duct guidewire (PGW) technique may improve biliary cannulation success and reduce the risk of PEP in people with difficult cannulation.
Objectives
To systematically review evidence from randomised controlled trials (RCTs) assessing the effectiveness and safety of the PGW technique compared to persistent conventional cannulation (CC) (contrast‐ or guidewire‐assisted cannulation) or other advanced techniques in people with difficult biliary cannulation for the prevention of PEP.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and CINAHL databases, major conference proceedings, and for ongoing trials on the ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) up to March 2016, using the Cochrane Upper Gastrointestinal and Pancreatic Diseases model with no language restrictions.
Selection criteria
RCTs comparing the PGW technique versus persistent CC or other advanced techniques in people undergoing ERCP with difficult biliary cannulation.
Data collection and analysis
Two review authors independently conducted study selection, data extraction, and methodological quality assessment. Using intention‐to‐treat analysis with random‐effects models, we combined dichotomous data to obtain risk ratios (RR) with 95% confidence intervals (CI). We assessed heterogeneity using the Chi2 test (P < 0.15) and I2 test (> 25%). To explore sources of heterogeneity, we conducted a priori subgroup analyses according to trial design, use of pancreatic duct (PD) stent, involvement of trainees in cannulation, publication type, and risk of bias. To assess the robustness of our results, we carried out sensitivity analyses using different summary statistics (RR versus odds ratio (OR)) and meta‐analytic models (fixed‐effect versus random‐effects).
Main results
We included seven RCTs comprising 577 participants. There was no significant heterogeneity among trials for the outcome of PEP (P = 0.32; I2 = 15%). The PGW technique significantly increased PEP compared to other endoscopic techniques (RR 1.98, 95% CI 1.14 to 3.42; low‐quality evidence). The number needed to treat for an additional harmful outcome was 13 (95% CI 5 to 89). Among the three studies that compared the PGW technique with persistent CC, the incidence of PEP was 13.5% for the PGW technique and 8.7% for persistent CC (RR 1.58, 95% CI 0.83 to 3.01; low‐quality evidence). Among the two studies that compared the PGW technique with precut sphincterotomy, the incidence of PEP was 29.8% in the PGW group versus 10.3% in the precut group (RR 2.92, 95% CI 1.24 to 6.88; low‐quality evidence). Among the two studies that compared the PGW technique with PD stent placement, the incidence of PEP was 11.7% for the PGW technique and 5.0% for PD stent placement (RR 1.75, 95% CI 0.08 to 37.50; very low‐quality evidence). There was no significant difference in common bile duct (CBD) cannulation success with the randomised technique (RR 1.04, 95% CI 0.87 to 1.24; low‐quality evidence) or overall CBD cannulation success (RR 1.04, 95% CI 0.91 to 1.18; low‐quality evidence) between the PGW technique and other endoscopic techniques. There was also no statistically significant difference in the risk of other ERCP‐related complications (bleeding, perforation, cholangitis, and mortality). The results were robust in sensitivity analyses. The overall quality of evidence for the outcome of PEP was low or very low because of study limitations and imprecision.
Authors' conclusions
In people with difficult CBD cannulation, sole use of the PGW technique appears to be associated with an increased risk of PEP. Prophylactic PD stenting after use of the PGW technique may reduce the risk of PEP. However, the PGW technique is not superior to persistent attempts with CC, precut sphincterotomy, or PD stent in achieving CBD cannulation. The influence of co‐intervention in the form of rectal peri‐procedural nonsteroidal anti‐inflammatory drug administration is unclear.
Plain language summary
Accessing the bile duct by inserting a guidewire into the pancreatic duct to prevent inflammation of the pancreas after endoscopic retrograde cholangiopancreatography (ERCP)
Review question
To compare the effects of the pancreatic duct guidewire (PGW) technique with other endoscopic techniques for gaining access to the bile duct when access to the bile duct is considered to be difficult using traditional techniques.
Background
Endoscopic retrograde cholangiopancreatography (ERCP) combines endoscopy and X‐ray to diagnose and treat problems of the bile ducts and pancreatic ducts. An endoscope is passed down the oesophagus, through the stomach, and into the duodenum where the opening of the bile and pancreatic ducts (papilla) is located. A catheter is then inserted through the endoscope and through the papilla into the bile duct. Dye is injected into the bile duct, and X‐rays are taken to look for gallstones or blockage. The major risk of ERCP is the development of inflammation of the pancreas (pancreatitis) by the dye or catheter, which occurs in 5% to 10% of all procedures. There is also a small risk of bleeding or making a hole in the bowel wall.
There are two traditional techniques for gaining access to the bile duct during ERCP. The first technique involves inserting a catheter directly into the papilla and injecting dye to confirm access to the bile duct, and the second involves the use of a guidewire to probe the papilla to gain access to the bile duct. Once the guidewire is confirmed on X‐ray to be in the bile duct, dye is injected into the bile duct.
When accessing the bile duct using traditional techniques is difficult, the endoscopist can persist with the traditional techniques or use more advanced techniques such as blind incision into the papilla (precut sphincterotomy) or insertion of a stent into the pancreatic duct (PD) to facilitate access to the bile duct. The PGW placement technique is a new technique to gain access to the bile duct and to reduce the risk of postprocedure pancreatitis in people in whom traditional techniques fail to gain access to the bile duct. The PGW technique involves inserting a first guidewire deep into the PD. A second guidewire is then used to probe the papilla to gain access to the bile duct. The first guidewire facilitates access to the bile duct by blocking the PD opening.
Study characteristics
We conducted a search of the literature on 15 April 2016. We identified seven randomised controlled trials conducted in China, Japan, South Korea, Spain, Thailand, and the United States including a total of 577 participants. These trials compared the PGW technique versus persistent use of traditional techniques or other advanced techniques in people undergoing ERCP in whom access to the bile duct using traditional techniques was considered by the endoscopists to be difficult. As in clinical practice, the criteria used to define difficult access to the bile duct were highly variable among studies. We assessed outcomes of post‐ERCP pancreatitis (PEP), success rates in accessing the bile duct, and other post‐ERCP complications (bleeding, infection, hole in the bowel wall, death).
Key results
Contrary to popular belief, the PGW technique appears to increase the risk of PEP and does not improve the success rate of gaining access to the bile duct compared to other endoscopic techniques. The technique may increase the risk of mild PEP, but not moderate or severe PEP. There was no significant difference in success rates for accessing the bile duct. The risks for other complications such as bleeding, hole in the bowel wall, inflammation of the bile duct, and death appear to be low.
Quality of the evidence
Overall, we considered the quality of evidence for the outcome of PEP to be low. We considered none of the included studies to be at low risk of bias for all criteria. In most of the studies, both the participants and the medical staff were aware of which method was being used, therefore their judgments may not have been objective and the results should be interpreted cautiously.
Summary of findings
Summary of findings for the main comparison. Pancreatic duct guidewire (PGW) or double guidewire technique (DGT) compared to other endoscopic techniques for the prevention of post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis.
| Pancreatic duct guidewire (PGW) or double guidewire technique (DGT) compared to other endoscopic techniques for the prevention of post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis | |||||
| Patient or population: people with biliary cannulation for the prevention of post‐ERCP pancreatitis Settings: hospital Intervention: PGW or DGT Comparison: other endoscopic techniques | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Other endoscopic techniques | PGW or DGT | ||||
| Post‐ERCP pancreatitis | 80 per 1000 | 158 per 1000 (91 to 273) | RR 1.98 (1.14 to 3.42) | 577 (7 studies) | ⊕⊕⊝⊝ low1,2,3 |
| PGW vs persistent attempts with conventional cannulation technique | |||||
| 87 per 1000 | 137 per 1000 (72 to 261) | RR 1.58 (0.83 to 3.01) | 305 (3 studies) | ⊕⊕⊝⊝ low1,2,3 | |
| PGW vs precut sphincterotomy | |||||
| 103 per 1000 |
302 per 1000 (128 to 712) |
RR 2.92 (1.24 to 6.88) | 115 (2 studies) | ⊕⊕⊝⊝ low1,2,3 | |
| PGW vs PD stent placement | |||||
| 50 per 1000 | 88 per 1000 (4 to 1000) | RR 1.75 (0.08 to 37.5) | 157 (2 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | |
| CBD cannulation success with the randomised technique (before the use of rescue techniques) | 663 per 1000 | 690 per 1000 (577 to 822) | RR 1.04 (0.87 to 1.24) | 577 (7 studies) | ⊕⊕⊝⊝ low1,5 |
| Overall cannulation success | 816 per 1000 | 849 per 1000 (743 to 963) | RR 1.04 (0.91 to 1.18) | 577 (7 studies) | ⊕⊕⊝⊝ low1,6 |
| Post‐ERCP bleeding | 35 per 1000 | 17 per 1000 (5 to 63) | RR 0.48 (0.13 to 1.79) | 513 (6 studies) | ⊕⊕⊝⊝ low1,2 |
| Post‐ERCP perforation | 4 per 1000 | 4 per 1000 (0 to 58) | RR 0.94 (0.06 to 14.78) | 513 (6 studies) | ⊕⊕⊝⊝ low1,2 |
| Post‐ERCP cholangitis | 16 per 1000 | 44 per 1000 (13 to 152) | RR 2.71 (0.79 to 9.35) | 373 (4 studies) | ⊕⊕⊝⊝ low1,2 |
| Mortality | 8 per 1000 | 2 per 1000 (0 to 60) | RR 0.31 (0.01 to 7.58) | 258 (2 studies) | ⊕⊕⊝⊝ low1,2 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CBD: common bile duct; CI: confidence interval; PD: pancreatic duct; PEP: post‐ERCP pancreatitis;RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded one level due to limitations in the study design. Most information is obtained from studies with unclear risk of bias for blinding of participants and personnel (other than the endoscopists). Inability to blind the endoscopists may have an impact on the rates of PEP depending on the preference and expertise of the endoscopist performing the procedure. 2Downgraded one level due to imprecision. The results of the main analysis for the outcome of PEP appeared to be imprecise with wide confidence intervals. 3We could not assess publication bias because of less than 10 included studies. Given the complexity of intervention trials involving ERCP, there may not be many unpublished trials. However, publication bias could be present as negative studies may not be published.
4Downgraded one level due to significant unexplained heterogeneity (I2 = 76%).
5Downgraded one level due to significant heterogeneity (I2 = 63%).
6Downgraded one level due to significant heterogeneity (I2 = 66%).
*The assumed risk is based on the mean baseline risk from the studies in the control group in this meta‐analysis. This is obtained by dividing the total number of events in the control groups by the total number of participants in the control groups.
Background
A glossary of terms appears in Appendix 1.
Description of the condition
Endoscopic retrograde cholangiopancreatography (ERCP) is a commonly performed endoscopic procedure that has both diagnostic and therapeutic roles in various hepatobiliary and pancreatic disorders. Despite its potential benefits, ERCP is not without risks. Acute pancreatitis is one of the most common serious complications of ERCP (Cotton 1991). The incidence of post‐ERCP pancreatitis (PEP) varies between 5% and 10%, although it may exceed 25% in certain high‐risk patient populations (Freeman 2004). While most PEP manifests as minor illness with two to three days of additional hospitalisation and an expected full recovery, severe pancreatitis is a devastating illness with significant morbidity, such as pancreatic necrosis, multi‐organ failure, and mortality. Severe pancreatitis has been reported to occur in 0.1% to 0.5% of ERCPs in prospective series (Freeman 2004).
The pathophysiologic mechanisms of PEP are likely to be multifactorial and are incompletely understood (Freeman 2004; Pezzilli 2002). These may include:
mechanical injury to the papilla and pancreatic duct due to instrumental manipulation, resulting in obstruction or impairment of pancreatic flow;
chemical injury due to contrast injection into the pancreatic duct;
hydrostatic injury due to contrast injection into the pancreatic duct;
thermal injury due to the electrosurgical current used for biliary or pancreatic sphincterotomy;
enzymatic injury from the introduction of activated proteolytic enzymes into the pancreatic duct;
microbiological injury due to contamination or instillation of intestinal flora or bacteria into the pancreatic duct.
There have been considerable efforts to identify risk factors for PEP. Multivariate analyses of prospective studies have found a number of patient‐related risk factors for PEP, including young age, female gender, sphincter of Oddi dysfunction(SOD), recurrent pancreatitis, and a history of PEP (Cheng 2006; Freeman 2001). Procedure‐related risk factors include difficult cannulation, multiple injections of the pancreatic duct, precut sphincterotomy, pancreatic sphincterotomy, and biliary sphincter balloon dilation (Cheng 2006; Freeman 2001). Operator‐related risk factors considered to potentially influence the outcome of ERCP include the endoscopist's expertise, case volume, and trainee involvement in the procedure. Indeed, low case volumes have been found to be associated with higher ERCP failure and complication rates (Freeman 1996; Loperfido 1998). However, large prospective studies have provided conflicting evidence as to whether any of these operator‐related risk factors increases the risk of PEP (Cheng 2006; Colton 2009; Freeman 1996; Freeman 2001; Loperfido 1998; Testoni 2010; Vandervoort 2002; Wang 2009; Williams 2007b). This is likely due to the fact that any difference in the rates of PEP between low‐ and high‐volume centres or endoscopists is often blunted by a disparity in case mix. In contrast, trainee participation has been shown to be a significant risk factor for the development of PEP (Cheng 2006). This increased risk is possibly due to multiple cannulation attempts by trainees.
In clinical practice, as recommended by current guidelines including the most recently updated Atlanta Classification (Banks 2013; Forsmark 2007; Tenner 2013; Working Group IAP/APA Acute Pancreatitis 2013), acute pancreatitis is diagnosed by the presence of two of the following three features:
abdominal pain typical of acute pancreatitis;
greater than or equal to three‐fold elevation in amylase or lipase;
computed tomography (CT) evidence of pancreatitis.
However, much controversy remains about the definition of PEP. In an attempt to establish reliable criteria for defining PEP, a consensus definition was developed in 1991 based on data collected from more than 15,000 procedures (Cotton 1991). PEP was defined as a rise in serum amylase level to greater than or equal to three‐fold above the upper limit of normal 24 hours after ERCP, accompanied by abdominal pain characteristic of pancreatitis requiring an unplanned hospital stay or an extension of a planned hospital stay by at least two days (Cotton 1991). The severity of PEP (mild, moderate, severe) was graded according to the length of stay and local or systemic complications related to pancreatitis. However, this consensus definition has not been widely adopted, and varying definitions of PEP have been used in clinical trials. This likely reflects the ongoing controversy in defining PEP in the context of post‐ERCP complications. The consensus definition for PEP has also not been updated since 1991 and is arguably distinct from that used in clinical practice for diagnosing acute pancreatitis. Furthermore, neither the consensus definition nor the clinical definition has been shown to reliably diagnose PEP. This is due to the fact that asymptomatic transient elevations in amylase or lipase levels, or both, are often seen post‐ERCP (up to 70%) (Conn 1991; Skude 1976; Testoni 1999). Asymptomatic hyperamylasaemia with levels more than five times the upper limit of normal, lasting for 24 hours after ERCP, has been reported in about 27% of cases (Testoni 1999). Moreover, serum lipase is now considered to be more sensitive and specific than serum amylase in the diagnosis of acute pancreatitis (Yadav 2002). In addition, abdominal pain postprocedure could be due to a multitude of factors other than PEP (for example air insufflation). The duration of pain is therefore essential for defining PEP because pain that subsides within 24 hours is unlikely to indicate pancreatitis. Moreover, mild pain disappearing within 24 to 48 hours and not requiring analgesics, a prolonged hospital stay, or both, still does not fulfil the criteria for clinical pancreatitis. Taken together, these two common findings post‐ERCP (pain and elevation in amylase) may lead to overdiagnosis of PEP. Due to the lack of specificity of pain and hyperamylasaemia after ERCP, CT has been proposed as the most appropriate method to confirm the diagnosis of PEP (Badalov 2009; Kiriyama 2010). To add to the controversy, the need for diagnostic criteria for PEP distinct from those used for acute pancreatitis of other etiologies has been challenged by a recent study suggesting that the consensus definition in Cotton 1991, may underdiagnose PEP (Artifon 2010). On the other hand, the clinical definition may overdiagnose PEP without having any significant impact on clinical management or patient outcomes.
Most recently, the Atlanta Classification of acute pancreatitis was updated in 2012 (Banks 2013). This classification defines severity based on the presence or absence of organ failure and of local or systemic complications. Although this classification provides a uniform nomenclature including radiographic findings to classify acute pancreatitis, its limitations include the fact that it was not primarily developed to define PEP, but for all‐cause acute pancreatitis. The most recent European Society of Gastrointestinal Endoscopy guidelines on PEP, in Dumonceau 2014, suggest that both the consensus definition, in Cotton 1991, and the revised Atlanta definition and classification of acute pancreatitis, in Banks 2013, may be used.
Description of the intervention
ERCP involves passage of a side‐viewing endoscope into the duodenum and cannulation of the common bile duct (CBD) with a device (sphincterotome or catheter). Contrast can then be injected in a retrograde manner into the CBD. Selective deep cannulation of the CBD is a prerequisite to successful diagnostic and therapeutic ERCP.
The conventional techniques used to achieve primary deep biliary cannulation have been contrast‐ or guidewire‐assisted cannulation (Freeman 2005). Achieving deep cannulation of the CBD can be difficult, and success depends primarily on the skill and experience of the endoscopist, but also on anatomical variations and the cannulation device used (Cortas 1999; Laasch 2003). Even among experienced endoscopists, failure of biliary cannulation may occur in 15% to 35% of cases (Testoni 2011; Varadarajulu 2006; Williams 2007a).
In difficult biliary cannulation, when conventional techniques (contrast‐ or guidewire‐assisted cannulation) fail, advanced techniques (for example precut sphincterotomy, pancreatic duct guidewire placement, pancreatic duct stent placement, endoscopic ultrasound‐guided rendezvous technique) are often used to gain access to the CBD. Among the advanced techniques, precut sphincterotomy is most often used as a rescue technique to achieve selective biliary cannulation (Siegel 1989), with variable immediate success rates (35% to 96%) (Freeman 2005). However, the precut technique requires a steep learning curve and has been reported to be associated with an increased risk of complications (2% to 34%) including PEP, bleeding, and perforation (Cennamo 2010; Freeman 2001; Masci 2003). It remains controversial as to whether the increased risk is due to the precut itself or to the prolonged attempts at cannulation prior to the use of precut. However, both precut and difficult cannulation (with repeated attempts at cannulation of the papilla) have been reported as independent procedure‐related risk factors for PEP (Cheng 2006; Freeman 2001; Loperfido 1998; Masci 2003; Vandervoort 2002; Williams 2007b). Recently, pancreatic duct guidewire (PGW) placement or a double guidewire technique (DGT) have been used as an alternative to precut sphincterotomy in cases of difficult CBD cannulation, especially in people with distorted anatomy caused by neoplasia or surgery (Dumonceau 1998; Gotoh 2001; Gyokeres 2003; Maeda 2003).
Other options to facilitate difficult biliary cannulation (without resorting to advanced techniques) include persistent attempts with conventional cannulation techniques, changing the cannulation device or the endoscopist, or stopping and repeating the procedure on another day (Freeman 2005).
Conventional cannulation techniques
Contrast‐assisted cannulation
Conventional contrast‐assisted cannulation of the CBD is the direct injection of contrast through a catheter or sphincterotome into the papilla under fluoroscopy (Freeman 2005). With this technique, a catheter or a sphincterotome is first aligned with the CBD and advanced into the papilla. Contrast is then injected to determine if the CBD has been entered. Upon visualisation of the CBD, more contrast can be injected for optimal opacification, and the catheter or the sphincterotome is then advanced further into the CBD for deep cannulation. If contrast is noted to fill the pancreatic duct, the catheter or sphincterotome is then withdrawn and reoriented to the direction of the CBD and the above steps repeated until the CBD is accessed. However, inadvertent contrast injection of the pancreatic duct or the papilla itself (submucosal injection), as well as repeated cannulation attempts may increase the risk of PEP (Cheng 2006; Freeman 2001).
Guidewire‐assisted cannulation
With the guidewire‐assisted cannulation technique, a guidewire is protruded slightly beyond the catheter or sphincterotome within the papilla and passed in small increments under fluoroscopy into the CBD (Freeman 2005). Alternatively, the tip of the catheter or sphincterotome is first dipped within the papilla and oriented to the CBD followed by advancement of the guidewire to probe and gain access to the duct. The position of the guidewire indicates cannulation of the CBD without using contrast injection. If the guidewire inadvertently enters the pancreatic duct, it is withdrawn into the catheter or sphincterotome and repeated attempts are made to enter the CBD. Once the guidewire is noted to have entered the CBD, the catheter or sphincterotome can be advanced deeper into the CBD, and contrast is injected for optimal opacification. It has been postulated that the guidewire‐assisted cannulation technique may improve biliary cannulation success and prevent PEP by avoiding papillary trauma and inadvertent contrast injection of the pancreatic duct or the papilla itself.
Advanced techniques to facilitate difficult biliary cannulation
For people who fail conventional cannulation techniques, advanced techniques are often used to gain access to the CBD. These include precut sphincterotomy, the PGW technique or DGT, the use of a pancreatic duct stent, and the endoscopic ultrasound‐guided rendezvous technique. There are currently no accepted standards for deciding which advanced techniques to use in cases of difficult biliary cannulation (Testoni 2011).
Precut (access) sphincterotomy
Precut sphincterotomy refers to a variety of endoscopic techniques used to gain access to the CBD either with a needle‐knife or a sphincterotome after conventional methods have failed (Freeman 2005). Precut sphincterotomy is usually followed by conventional sphincterotomy, which permits completion of therapies (for example stone extraction). Several precut techniques have been described (Freeman 2005).
Needle‐knife precut sphincterotomy. Using a needle‐knife, a freehand incision can be made starting at the papillary orifice and extending upward for a variable distance.
Needle‐knife fistulotomy. A variation of the needle‐knife precut sphincterotomy technique that involves puncturing the papilla above the orifice and then cutting upward or downward towards the orifice.
Transpancreatic precut sphincterotomy. This is done by inserting the tip of a sphincterotome into the pancreatic duct and cutting through the septum in the direction of the CBD.
All of the precut techniques can be performed before or after a pancreatic duct stent has been placed to reduce the risk of PEP (Choudhary 2011; Kubota 2012; Mazaki 2010; Testoni 2011).
Pancreatic duct guidewire placement or double guidewire technique
With the PGW technique or DGT, a guidewire is first inserted into the pancreatic duct via a cannulation device (sphincterotome or catheter). The cannulation device is withdrawn, leaving the guidewire deep in the pancreatic duct. The cannulation device is then loaded with a second guidewire and reinserted through the working channel of the endoscope alongside the previously placed pancreatic guidewire. The tip of the cannulation device is positioned in the papilla, bending the pancreatic wire and targeting the direction of the CBD for cannulation with the second guidewire. Once the second guidewire is noted to enter the CBD, the catheter or sphincterotome can be advanced deeper into the CBD, and contrast is injected for optimal opacification.
Although there is substantial evidence supporting placement of pancreatic duct stents to reduce the risk of PEP in high‐risk patients (for example difficult cannulation or SOD), it remains uncertain whether prophylactic pancreatic duct stenting is necessary after the use of the PGW technique (Choudhary 2011; Freeman 2005; Mazaki 2010).
Pancreatic duct stent placement
In difficult biliary cannulation, placement of a pancreatic duct stent has been used to facilitate biliary cannulation (prior to the use of precut sphincterotomy, together with the PGW technique, or after repeated attempts with conventional cannulation techniques). This is based on the concept that the pancreatic duct stent occupies the pancreatic orifice and deflects a guidewire or a catheter into the CBD (Freeman 2005). In high‐risk patients (for example difficult cannulation, SOD, precut sphincterotomy, pancreatic sphincterotomy, biliary balloon dilatation of intact papilla for stone extraction, endoscopic ampullectomy, and pancreatic brush cytology), the placement of a prophylactic pancreatic duct stent after ERCP has been shown to reduce the risk of PEP (Choudhary 2011; Mazaki 2010). However, it remains uncertain whether prophylactic pancreatic duct stenting is necessary after the use of the PGW technique (Choudhary 2011; Freeman 2005; Mazaki 2010). Pancreatic duct stents can be technically difficult to place even for the most experienced endoscopists, with reported failure in up to 10% of cases (Freeman 2004). In high‐risk patients, pancreatic duct manipulation followed by failure to place the stent may be associated with a higher risk of PEP than no attempt at all (Freeman 2004).
Endoscopic ultrasound‐guided rendezvous for biliary access
Endoscopic ultrasound‐guided rendezvous is a relatively new technique that has emerged as a useful option to achieve biliary access when standard or advanced ERCP techniques (or both) for biliary access have failed (Dhir 2012; Iwashita 2012; Shah 2012). Endoscopic ultrasound rendezvous involves using endoscopic ultrasound technology to access the bile duct with a small needle and manipulate a wire across the biliary orifice and into the duodenum. This wire can then be retrieved endoscopically ('rendezvous') to complete the ERCP. Retrospective series have reported a higher success rate with the endoscopic ultrasound‐guided rendezvous compared to precut sphincterotomy, with no significant difference in the rate of procedural complications (Dhir 2012).
How the intervention might work
Cannulation techniques have been recognised as an important factor in causing PEP (Freeman 2001; Freeman 2004). Mechanical injury to the pancreatic orifice from repeated cannulation may lead to oedema and obstruction of pancreatic ductal flow. In addition, the inadvertent injection of contrast agent into the pancreatic duct may lead to both chemical and hydrostatic injuries of the pancreas. These factors are thought to play an important role in the development of PEP with conventional contrast‐ or guidewire‐assisted cannulation of the CBD.
In difficult cannulation cases when conventional cannulation techniques (contrast‐ or guidewire‐assisted cannulation) fail, advanced techniques such as precut sphincterotomy and the PGW technique are often used to facilitate biliary access and reduce the risk of PEP. However, precut sphincterotomy has been reported to be an independent risk factor for post‐ERCP complications, including PEP (Cennamo 2010; Freeman 2001; Masci 2003). It has been postulated that the PGW technique may improve biliary cannulation success and reduce the risk of PEP (Freeman 2004). The rationale is that placement of a guidewire deep into the main pancreatic duct may open a stenotic papillary orifice, stabilise the papilla, and straighten both the pancreatic duct and CBD while at the same time closing the pancreatic orifice, thus facilitating CBD cannulation and potentially minimising repeated injections or cannulation of the pancreatic duct, leading to PEP (Freeman 2005; Gotoh 2001; Gyokeres 2003). In difficult biliary cannulation, the PGW technique may therefore offer less traumatic biliary cannulation than precut sphincterotomy or persistent attempts with conventional cannulation techniques by protecting the pancreatic duct from unintentional cannulation or injection. However, there are concerns with the PGW technique, including perforation and pancreatic ductal injury, which may potentially trigger PEP. Also, deep placement of a guidewire into the main pancreatic duct for adequate positioning can be technically challenging even for the most experienced endoscopists, especially in people with a small or tortuous main pancreatic duct (or both), with reported failure in up to 10% of cases (Testoni 2011).
Why it is important to do this review
PEP is the most common serious complication of ERCP and carries significant morbidity and mortality. Prevention of PEP has been the 'holy grail' of ERCP. Investigators have long searched for a pharmacological agent that will prevent PEP, but nearly all agents evaluated (with the exception of rectal non‐steroidal anti‐inflammatory drugs) have failed to demonstrate efficacy in randomised controlled trials (RCTs) or logistic feasibility in real‐life settings (Elmunzer 2012; Testoni 2006). Similarly, numerous endoscopic interventions have been studied for the prevention of PEP (Freeman 2004). The findings of these studies have often provided conflicting results due to different study designs, definitions of outcomes, patient populations, and interventions used.
Cannulation technique is believed to be pivotal in the pathogenesis of PEP. In a recent Cochrane systematic review and meta‐analysis (Tse 2012), the guidewire‐assisted cannulation technique was found to increase the primary cannulation rate (risk ratio (RR) 1.07, 95% confidence interval (CI) 1.00 to 1.15) and reduce the use of precut sphincterotomy (RR 0.75, 95% CI 0.60 to 0.95) and the risk of PEP (RR 0.51, 95% CI 0.32 to 0.82) compared to the contrast‐assisted cannulation technique. In difficult cannulation cases, there is often a fine balance between facilitating biliary access and minimising the risk of PEP, and considerable controversy remains about the use of advanced techniques such as the PGW technique to facilitate biliary cannulation and prevent PEP. A comprehensive meta‐analysis of the safety and efficacy of the PGW technique will allow us to make recommendations for clinical practice and research.
This systematic review is part of a series of reviews examining endoscopic interventions for the prevention of PEP; it evaluates the role of the PGW technique in difficult cannulation cases for the prevention of PEP. The use of precut sphincterotomy (early versus delayed) and the use of pancreatic duct stents for the prevention of PEP will be examined in separate systematic reviews. In addition, we have plans to conduct a series of reviews examining pharmacological interventions for the prevention of PEP. The findings of this review are relevant to patients, clinicians, and healthcare systems.
Objectives
This project aimed to assess the clinical effectiveness of the PGW technique in difficult CBD cannulation for the prevention of PEP by systematic review and meta‐analysis of RCTs.
The objectives of this review were two‐fold:
-
to assess whether the PGW technique shows any overall benefit in reducing adverse clinical outcomes, including PEP and other ERCP‐related complications (bleeding, perforation, cholangitis, mortality) compared to:
persistent attempts with conventional cannulation techniques (contrast‐ or guidewire‐assisted cannulation) and/or
other advanced techniques (e.g. precut sphincterotomy, pancreatic duct stent placement, endoscopic ultrasound rendezvous technique) in difficult biliary cannulation; and
-
to assess whether the PGW technique can improve the technical success of CBD cannulation compared to:
persistent attempts with conventional cannulation techniques (contrast‐ or guidewire‐assisted cannulation) and/or
advanced techniques (e.g. precut sphincterotomy, pancreatic duct stent placement, endoscopic ultrasound rendezvous) in difficult biliary cannulation.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing the PGW technique versus persistent attempts with conventional cannulation techniques (contrast‐ or guidewire‐assisted cannulation) or other advanced techniques (for example precut sphincterotomy, pancreatic duct stent placement, endoscopic ultrasound rendezvous) in people undergoing ERCP with difficult biliary cannulation. Trials that permitted other concomitant therapies were eligible, as long as the therapies were administered to both the intervention and the control arms. We excluded trials that permitted other advanced techniques prior to the use of the PGW technique. We did not include trials that employed non‐random methods of allocation such as judgement of the clinician or preference of the participant, results of a laboratory test or series of tests, or availability of the intervention, as the allocation was not truly random. We considered published and unpublished studies, full articles and abstracts without language restriction for inclusion in this review.
Types of participants
Trials were eligible for inclusion in the review if they recruited men and women aged at least 18 years undergoing ERCP with difficult biliary cannulation using conventional cannulation techniques (contrast‐ or guidewire‐assisted cannulation).
However, difficult biliary cannulation can be difficult to define. There is currently no established time limit or limits to unsuccessful attempts before the cannulation is termed difficult (Udd 2010). For this review, we have defined difficult cannulation as a situation where the endoscopist, using conventional cannulation techniques (contrast‐ or guidewire‐assisted cannulation), fails within a certain time limit or after a certain number of unsuccessful attempts to achieve biliary access (Freeman 1996; Testoni 2011; Udd 2010). We accepted the definitions of difficult cannulation adopted by the primary studies.
Types of interventions
We analysed the following comparisons: PGW technique versus persistent attempts with conventional cannulation techniques (contrast‐ or guidewire‐assisted cannulation) or other advanced techniques (for example precut sphincterotomy, pancreatic duct stent placement, endoscopic ultrasound rendezvous), or both to facilitate difficult biliary cannulation.
Types of outcome measures
Primary outcomes
The primary outcome measure was post‐ERCP pancreatitis (PEP), as defined by the primary studies. If the same study provided different definitions of PEP, we used the consensus definition for assessment of this outcome (Cotton 1991).
Secondary outcomes
The secondary outcome measures were as follows:
Severity of PEP as defined by the primary studies. If the same study provided different definitions of severity of PEP, we used the consensus criteria for assessment of this outcome (Cotton 1991).
CBD cannulation success with the randomised technique.
Overall CBD cannulation success (during the index procedure). If the randomised technique fails to gain biliary access, trials may permit the use of rescue techniques (e.g. technique 'cross‐over' to the other comparison arm, precut sphincterotomy, insertion of pancreatic duct stent to facilitate cannulation) according to study protocol or at the discretion of the endoscopist. Successful CBD cannulation during repeat ERCP at a different endoscopic session was not counted towards overall CBD cannulation success.
Post‐sphincterotomy bleeding.
Perforation
Post‐ERCP cholangitis.
Mortality.
Search methods for identification of studies
We constructed the search strategies by using a combination of subject headings and text words relating to ERCP and acute pancreatitis. We applied the standard Cochrane search strategy filter for identifying RCTs to all searches. See also the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group search strategy.
Electronic searches
We conducted a comprehensive literature search to identify all published and unpublished RCTs with no language restriction. We searched the following electronic databases to identify potential studies:
Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 4, 2016) (Appendix 2);
Ovid MEDLINE and Ovid MEDLINE In‐Process & Other Non‐Indexed Citations (1946 to 15 April 2016) (Appendix 3);
EMBASE (1974 to 15 April 2016) (Appendix 4); and
CINAHL (1982 to 15 April 2016) (Appendix 5).
Searching other resources
Two review authors (YY, FT) handsearched the published abstracts from the conference proceedings in Digestive Disease Week (published in Gastroenterology andGastrointestinal Endoscopy), United European Gastroenterology Week (published in Gut), and the American College of Gastroenterology (published in American Journal of Gastroenterology) from 2004 to 2015. We handsearched references cited in studies found by the above search to identify further relevant trials. Two review authors also independently conducted a search for ongoing trials on the ClinicalTrials.gov (http://clinicaltrials.gov) and World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (http://apps.who.int/trialsearch/).
Data collection and analysis
Selection of studies
Two review authors (YY, FT) independently screened titles and abstracts identified by the search strategy for potential inclusion in the review using predefined inclusion and exclusion criteria. We assessed each trial for potential duplicate publication. We resolved differences by discussion and consensus. A third review author (AB) was consulted to resolve any disagreements. The same two review authors (YY, FT) retrieved and reviewed the complete report of all selected articles. We contacted authors of trial reports if they were published only as abstracts or if additional data were required for analyses. In case of duplicate publications, we retained only the most comprehensive report.
Data extraction and management
Two review authors (YY, FT) independently recorded the following study and participant characteristics:
setting (single centre or multicentre);
country of origin;
enrolment period;
year of publication, format (abstract or journal article);
study design (permission of technique 'cross‐over' versus non‐permission of technique 'cross‐over'; permission of the use of rescue technique versus non‐permission of rescue technique);
inclusion and exclusion criteria used;
indications for ERCP (stone, malignant biliary obstruction, suspected sphincter of Oddi dysfunction (SOD));
definition of difficult biliary cannulation;
diagnostic criteria of PEP;
endoscopists (number, experience, trainee involvement);
number of participants assigned per intervention;
participant demographics and characteristics, including gender, mean age, comorbidities, suspected SOD, previous history of PEP or recurrent pancreatitis;
endoscopic interventions evaluated;
specific endoscopic interventions (types of guidewire, types of sphincterotome/catheter, electrosurgical generator and current used for sphincterotomy and precut, use of pancreatic stent, precut sphincterotomy and technique);
pharmacological prophylaxis for PEP;
outcomes (PEP, severity of PEP, CBD cannulation success with the randomised technique, overall CBD cannulation success, precut, postsphincterotomy bleeding, postsphincterotomy cholangitis, perforation, mortality);
failure to place guidewire in the pancreatic duct (PGW technique);
dropouts or loss to follow‐up; and
study quality (generation of allocation sequence, allocation concealment, blinding, incomplete outcome data, selective reporting, other bias).
We summarised studies and, if appropriate, undertook meta‐analysis.
Assessment of risk of bias in included studies
Two review authors (YY, FT) independently assessed the methodological quality of the included studies based on the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). We assessed each included study regarding sequence generation, allocation sequence concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential sources of bias. We resolved disagreement by discussion and consensus. A third review author (AB) was consulted to resolve any disagreements.
Random sequence generation
Low risk, if the allocation sequence was generated by a computer or a random number table.
Unclear, if the trial was described as randomised, but the method used for generation of the allocation sequence was not described.
High risk, if a system involving dates, names, or hospital record numbers was used for the allocation of participants.
Allocation concealment
Low risk, if the allocation of participants involved central allocation or sequentially numbered, opaque, sealed envelopes.
Unclear, if there was insufficient information to permit a judgement of low risk or high risk.
High risk, if the allocation was based on using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards, alternation or rotation; date of birth; case record number; or any other explicitly unconcealed procedure.
Blinding of participants and personnel (post‐ERCP pancreatitis)
Low risk, if blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken.
Unclear risk, if there was insufficient information to permit a judgement of low risk or high risk.
High risk, if there was no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of study participants and personnel was attempted, but it is likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
Blinding of outcome assessment (post‐ERCP pancreatitis)
Low risk, if blinding of outcome assessment was ensured, and it is unlikely that the blinding could have been broken.
Unclear risk, if there was insufficient information to permit a judgement of low risk or high risk.
High risk, if there was no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; there was blinding of outcome assessment, but it is likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk, if there were no missing outcome data; reasons for missing outcome data are unlikely to be related to true outcome; missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups; the proportion of missing outcomes compared with the observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate; missing data have been imputed using appropriate methods.
Unclear, if there was insufficient reporting of attrition/exclusions to permit a judgement of low risk or high risk (e.g. number randomised not stated, no reasons for missing data provided).
High risk, if the reasons for missing outcome data are likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; the proportion of missing outcomes compared with the observed event risk is enough to induce clinically relevant bias in the intervention effect estimate; per‐protocol analysis was done with substantial departure of the intervention received from that assigned at randomisation; there was potentially inappropriate application of simple imputation.
Selective reporting
Low risk, if the published reports included all expected outcomes, including those that were prespecified.
Unclear, if there was insufficient information to permit a judgement of low risk or high risk.
High risk, if not all of the study's prespecified primary outcomes have been reported; if one or more primary outcomes was reported using measurements, analysis methods, or subsets of the data that were not prespecified; one or more reported primary outcomes were not prespecified; one or more outcomes of interest were reported incompletely; or the study report failed to include results for a key outcome that would be expected to have been reported for such a study.
Other risk of bias
We reported any other important concerns about bias identified in the studies.
Measures of treatment effect
Primary outcome
The primary outcome was PEP. We expected dichotomous data for PEP, which we expressed as risk ratio (RR) with 95% confidence interval (CI). We defined RR as the risk of PEP with the PGW technique compared to persistent attempts with conventional cannulation techniques (contrast‐ or guidewire‐assisted cannulation) or other advanced techniques (for example precut sphincterotomy, pancreatic duct stent placement, endoscopic ultrasound rendezvous).
Secondary outcome
We expressed dichotomous outcomes of severity of PEP, cannulation success with the randomised technique, overall cannulation success, and post‐ERCP complications (bleeding, cholangitis, perforation, mortality) as RR with 95% CI.
Unit of analysis issues
We included trials that permitted the use of rescue technique(s) (for example precut sphincterotomy, insertion of pancreatic duct stent to facilitate cannulation, technique 'cross‐over' in which participants were allowed to receive the alternative endoscopic technique if the randomised technique failed) according to a predefined study protocol or at the discretion of the endoscopist in this review. However, these trials are at risk for contamination due to carry‐over effects in the subgroup of participants who received the rescue technique(s) after failing the assigned technique. We therefore also performed subgroup analysis according to trial design (permission of rescue techniques versus non‐permission of rescue techniques).
Dealing with missing data
We contacted authors for any data missing from included studies. We performed analyses on an intention‐to‐treat (ITT) basis with inclusion of data from all participants randomised whenever possible. We otherwise adopted the 'complete‐case analysis'. We assumed that there should not be any missing data with respect to cannulation success, as this outcome is assessed during the procedure and is not dependent on follow‐up of participants. We assumed most participants with PEP would require admission to the hospital for treatment. However, participants may not be admitted to the same hospital where the study was conducted. Nevertheless, it is unlikely that there are systematic differences between comparison groups in the likelihood of being admitted to other hospitals for PEP. Given that the risk of PEP may be high in the patient populations included (up to 30% in SOD patients), we planned to conduct two analyses: an available‐case analysis and then a 'worst‐case scenario' analysis (PEP) for trials with missing data. We considered all participants who were lost to follow‐up in the PGW group to have PEP, whereas we considered those who were lost to follow‐up in the other comparison groups to have a favourable outcome (no PEP). We intended to conduct this sensitivity analysis by imputing the missing data to determine whether the overall results were sensitive to this assumption.
Assessment of heterogeneity
We assessed heterogeneity with the Chi2 test (P < 0.15 equals significant heterogeneity) and I2 statistic (> 25% equals heterogeneity) using a random‐effects model along with visual inspection of the forest plots. When we found significant heterogeneity, we investigated possible explanations by subgroup analyses and sensitivity analyses to test the robustness of the overall results. We hypothesised the following potential sources of heterogeneity a priori:
trial design (permission of rescue techniques versus non‐permission of rescue techniques);
use of pancreatic duct stent (yes versus no versus unclear);
involvement of trainees in cannulation (yes versus no versus unclear);
publication type (abstracts versus full text);
risk of bias (low versus unclear and high).
Assessment of reporting biases
We designed this review to include published and unpublished studies with no language restriction. We assessed publication bias by examining the relationship between the treatment effects and the standard error of the estimate using a funnel plot.
Data synthesis
We conducted a meta‐analysis for the comparisons of the PGW technique versus persistent attempts with conventional contrast‐ or guidewire‐assisted biliary cannulation or other advanced techniques (for example precut sphincterotomy, pancreatic duct stent placement, endoscopic ultrasound rendezvous technique), or both. We performed meta‐analysis only when we found two or more trials with similar comparisons and outcome measures. Where appropriate, we combined data using a random‐effects model (the Mantel‐Haenszel method) to determine a summary estimate of the RR and the 95% CI. We calculated the RR of the incidence of PEP as the primary outcome. We calculated the RRs of dichotomous secondary outcomes including severity of PEP, CBD cannulation success with the randomised technique, overall CBD cannulation success, postsphincterotomy bleeding, postsphincterotomy cholangitis, perforation, and mortality. We obtained number needed to treat for an additional beneficial outcome (NNTB) with CI by using the formula NNTB = (1/(ACR x (1 ‐ RR)); ACR (assumed control risk) was based on the pooled control event rate from the eligible studies. We used the Cochrane Review Manager 5 software to carry out the analysis based on the ITT principle (RevMan 2014). We presented results on forest plots, using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We decided to perform the following subgroup analyses for the incidence of PEP a priori.
Trial design (permission of rescue techniques versus non‐permission of rescue techniques). Trials that permitted the use of rescue techniques (e.g. precut sphincterotomy, insertion of pancreatic duct stent to facilitate cannulation, technique 'cross‐over' to the other comparison arm) were at risk of contamination due to carry‐over effects in the subgroup of participants who received other techniques after failing the assigned technique.
Use of pancreatic duct stent (yes versus no versus unclear).
Involvement of trainees (yes versus no versus unclear).
Risk of bias (high versus low versus unclear).
Publication type (abstracts versus full text).
We performed tests for subgroup differences based on the fixed‐effect inverse‐variance method (implemented in RevMan 5) for the above outcomes with P < 0.05 considered statistically significant.
Sensitivity analysis
Sensitivity analyses were as follows:
Summary statistic (risk ratios versus odds ratios).
Meta‐analysis modelling (fixed‐effect versus random‐effects).
Summary of findings tables
We employed the GRADE approach to interpret findings (Langendam 2013), and the GRADEprofiler allowed us to import data from Review Manager 5.2 to create 'Summary of findings' tables (GRADE 2008; RevMan 2014). These tables provide outcome‐specific information concerning the overall quality of evidence from studies included in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes we considered.
We assessed the quality of evidence for the following primary and secondary outcomes, which we included in the 'Summary of findings' tables:
Post‐ERCP pancreatitis
Severity of post‐ERCP pancreatitis
CBD cannulation success (before the use of rescue techniques)
Overall cannulation success
Post‐ERCP bleeding
Post‐ERCP perforation
Post‐ERCP cholangitis
Mortality
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search strategy used for CENTRAL, MEDLINE, EMBASE, and CINAHL identified 443 articles (Figure 1). A recursive search of the reference lists of these articles and the handsearching of conference proceedings from Digestive Disease Week (published in Gastroenterology and Gastrointestinal Endoscopy) and United European Gastroenterology Week (published in Gut) from 2004 to 2015 identified 14 further articles. After reviewing the abstracts of the above articles, we excluded 290 articles that were clearly not relevant. We retrieved the full articles for the remaining 35 trials. Of these, 24 did not meet the eligibility criteria and were excluded for the following reasons: non‐randomised trial design (Balderas 2011; Chandran 2012; Grönroos 2011; Huang 2015; Ito 2008; Ito 2010b; Ito 2012; Kim 2012; Kim 2014; Kim 2015a; Kim 2015b; Miao 2015; Nagano 2010; Nakahara 2014; Patel 2009; Song 2013; Suzuki 2012; Tanaka 2013; Yang 2015), inappropriate patient population (Cha 2012; Sasahira 2015), and inappropriate intervention (Kim 2013; Ozaslan 2014; Zang 2014). Four articles were preliminary or duplicate data (Angsuwatcharakon 2010; Cha 2011; Coté 2010; Herreros de Tejada 2007). We included seven RCTs comprising 577 participants (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013; Zheng 2010). A detailed summary of all included and excluded studies can be found in Characteristics of included studies and Characteristics of excluded studies.
1.
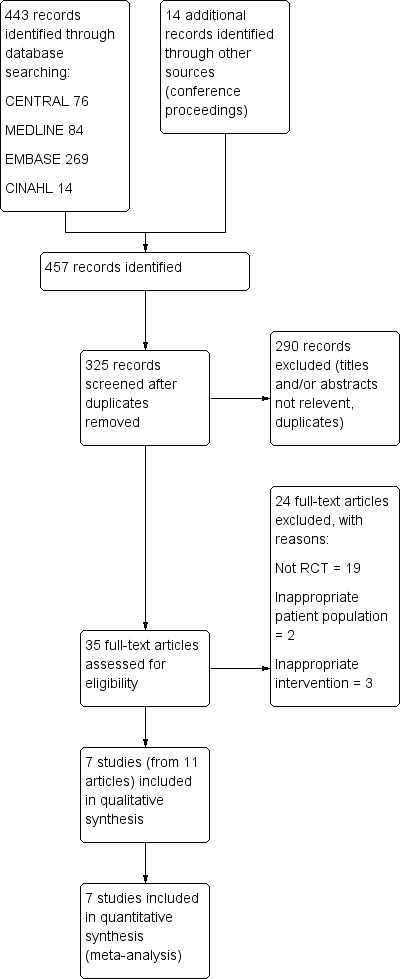
Study flow diagram.
Included studies
See: study characteristics (Table 2).
1. Study characteristics.
| Study | Inclusion criteria | Exclusion criteria | Definition of difficult cannulation (prior to randomisation) | Primary cannulation success (prior to randomisation) | Patients with difficult cannulation enrolled/patients screened (%) | Techniques used in control group | Failure of PD guidewire placement in the PGW group, % | CBD cannulation limit with the randomised technique | Rescue techniques used after the randomised technique failed |
|
Angsuwatcharakon 2012 Single centre Full text Thailand |
Consecutive patients aged > 15 yrs undergoing ERCP in whom cannulation of the CBD failed | Altered anatomy of the stomach/papilla, obstructive PD, recent pancreatitis | Inability to cannulate the CBD within 5 min by trainees, followed by another 10 min by an expert endoscopist with conventional contrast‐assisted technique | 91.8% | 44/534 (8.2%) | Precut with freehand fistulotomy technique without placement of a PD stent | 13.0% | 10 min | The other technique (cross‐over to precut in PGW group, cross‐over to PGW in precut group) or repeat ERCP |
|
Coté 2012 Multicentre Full text USA |
People undergoing ERCP in whom cannulation of the CBD had failed | Prior biliary or pancreatic sphincterotomy, suspected SOD, endoscopic pancreatic therapeutics, postsurgical anatomy | Inability to cannulate the CBD within 6 min (additional 6 min if trainees were involved) or 3 inadvertent PD cannulations by expert endoscopists using conventional guidewire‐ (preferably) or contrast‐assisted techniques | 81.3% | 87/442 (19.7%) | PD stent placement followed by cannulation of the CBD with guidewire‐assisted technique without pancreatic sphincterotomy | 19.0% | 6 min | Persist with the same technique with or without precut in the PD stent group. Cross‐over to PD stent with or without precut in the PGW group |
|
Herreros de Tejada 2009 Multicentre Full text Spain |
Consecutive patients undergoing ERCP and admitted for ≥ 24 hours in whom cannulation of the CBD had failed | Prior endoscopic sphincterotomy or papillary balloon dilatation, prior surgical biliary‐intestinal operations, pancreas divisum, prophylactic drug for PEP, pancreatic/biliary stenting within 6 mos, pregnancy/breastfeeding | Inability to cannulate the CBD after 5 attempts with conventional guidewire‐assisted technique by an expert endoscopist or trainees | 73.0% | 188/845 (22.2%) | Persistent conventional guidewire‐assisted technique | 25.0% | 10 attempts | Abort the ERCP or continue with backup technique (cross‐over, precut, PD stent) |
|
Ito 2010a Single centre Full text Japan |
People aged > 18 yrs undergoing ERCP in whom cannulation of the CBD had failed and successful guidewire insertion into the PD was achieved | Inability to insert a guidewire into the PD, prior endoscopic sphincterotomy or papillary balloon dilatation, pancreas divisum, pregnancy/breastfeeding | Inability to cannulate the CBD after 5 attempts with contrast‐assisted technique by expert endoscopists | 92.8% | 70/1451 (4.8%) | PGW technique followed by PD stent placement | Only patients with deep PD guidewire cannulation were enrolled. 8.3% in all patients with PGW attempted | No limit | Precut, second ERCP, PTBD, or a “substitute modality” such as CT/MRI/EUS |
|
Maeda 2003 Single centre Full text Japan |
Consecutive patients with hepatobiliary disease undergoing ERCP in whom deep cannulation of the CBD had failed | Prior endoscopic sphincterotomy or papillary balloon dilatation | Inability to cannulate the CBD within 10 min using conventional contrast‐assisted technique | 50.5% | 53/107 (49.5%) | Persistent conventional contrast‐assisted technique | 7.4% | No limit | None |
|
Yoo 2013 Single centre Full text Korea |
Consecutive patients undergoing ERCP in whom free cannulation of the CBD had failed and successful guidewire insertion into the PD was achieved | Age < 18 years, prior biliary or pancreatic sphincterotomy or dilatation or stenting of either duct, acute pancreatitis, pregnancy | Inability to cannulate the CBD after 10 min or 10 attempts with conventional guidewire‐assisted technique by an expert endoscopist | 92.6% | 71/1394 (5.1%) |
Transpancreatic precut sphincterotomy | Only patients with deep PD guidewire cannulation were enrolled. 31.1% in all patients with PGW attempted | 10 attempts | ERCP was repeated in 2 to 5 days using the same cannulation technique |
|
Zheng 2010 Single centre Abstract China |
People with biliary complications after liver transplantation in whom cannulation of the CBD had failed | NA | Inability to cannulate the CBD within 10 min using conventional guidewire‐assisted technique | NA | NA | Persistent conventional guidewire‐assisted technique | NA | 20 min | None |
CBD: common bile duct CT: computed tomography ERCP: endoscopic retrograde cholangiopancreatography EUS: endoscopic ultrasonography MRI: magnetic resonance imaging NA: not applicable PD: pancreatic duct PEP: post‐ERCP pancreatitis PGW: pancreatic duct guidewire placement PTBD: percutaneous transhepatic biliary drainage SOD: sphincter of Oddi dysfunction
Design
All seven included studies were RCTs. Of these, six were published in full text (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013), and one in abstract format (Zheng 2010).
All studies included people in whom cannulation of the CBD had failed with conventional contrast‐ or guidewire‐assisted cannulation techniques. However, the criteria used to define difficult cannulation (or failure to achieve deep biliary cannulation) were highly variable among studies. Difficult biliary cannulation was defined as inability to cannulate the bile duct within 10 minutes by two studies (Maeda 2003; Zheng 2010); within 15 minutes (of which the first 5 minutes were by trainees) by one study (Angsuwatcharakon 2012); after five unsuccessful attempts by two studies (Herreros de Tejada 2009; Ito 2010a); within 10 minutes or after 10 unsuccessful attempts by one study (Yoo 2013); and within 6 minutes (and additional 6 minutes if trainees were involved); or after three inadvertent pancreatic duct (PD) cannulations by one study (Coté 2012). As a result, the proportions of participants with difficult cannulation fulfilling the inclusion criteria were highly variable among studies: 4.8% (Ito 2010a), 5.1% (Yoo 2013), 8.2% (Angsuwatcharakon 2012), 19.7% (Coté 2012), 22.2% (Herreros de Tejada 2009), and 49.5% (Maeda 2003).
Successful placement of a guidewire into the PD was a requirement for enrolment in only two studies (Ito 2010a; Yoo 2013). After randomisation, most studies imposed a cannulation limit with the randomised technique, although the limits were highly variable among the studies, ranging from 6 to 20 minutes or up to 10 cannulation attempts (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Yoo 2013; Zheng 2010). Two studies did not impose a cannulation limit with the randomised technique on the endoscopists (Ito 2010a; Maeda 2003). When the randomised technique failed, three studies did not permit the use of rescue techniques (Maeda 2003; Yoo 2013; Zheng 2010). Other studies permitted the use of rescue techniques including "crossover" to the alternative endoscopic technique (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009), precut sphincterotomy (Coté 2012; Herreros de Tejada 2009; Ito 2010a), or insertion of PD stent to facilitate biliary cannulation (Herreros de Tejada 2009). Some studies also allowed repeat ERCP, in Angsuwatcharakon 2012, Ito 2010a, and Yoo 2013, percutaneous transhepatic biliary drainage, in Ito 2010a, or alternative imaging techniques such as computed tomography or magnetic resonance imaging, in Ito 2010a, in cases of unsuccessful biliary cannulation with the randomised technique. However, the options of which rescue technique to use or to abort the ERCP were often left to the discretion of the endoscopists.
Sample sizes
The number of participants per trial ranged from 44, in Angsuwatcharakon 2012, to 188, in Herreros de Tejada 2009. One study excluded eight participants (four in each group) after randomisation because of protocol violations and 17 cases of unintentional CBD cannulation in the PGW group without having placed a guidewire in the PD (and therefore not meeting the criteria for the PGW technique) (Herreros de Tejada 2009).
According to the ITT principle, we included all randomised participants for the main analyses (N = 577).
Setting
Five of the studies were conducted in a single centre (Angsuwatcharakon 2012; Ito 2010a; Maeda 2003; Yoo 2013; Zheng 2010). Two were multicentre studies (Coté 2012; Herreros de Tejada 2009). In three studies, the procedures were performed by one experienced endoscopist, in Angsuwatcharakon 2012 and Yoo 2013, or two experienced endoscopists, in Zheng 2010. In three other studies, the procedures were performed by multiple experienced endoscopists at a single centre, in Ito 2010a, or multiple centres, in Coté 2012 and Herreros de Tejada 2009. One study did not report on the experience or the number of endoscopists who performed the procedures (Maeda 2003). Trainees were allowed to participate in cannulation prior to randomisation in three studies (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009). After randomisation, trainees were involved in cannulation in only one study (Herreros de Tejada 2009); in this study, trainees with "enough experience" were allowed to continue after randomisation in "selected cases". Three studies did not involve trainees in the procedures before or after randomisation (Ito 2010a; Yoo 2013; Zheng 2010). One study did not provide information as to whether trainees were involved in cannulation (Maeda 2003).
Participants
The seven studies included in the main analyses comprised a total of 577 participants undergoing ERCP with difficult biliary cannulation (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013; Zheng 2010). Of these, 289 were randomised to the PGW placement technique and 288 to other cannulation techniques, including persistent attempts with conventional cannulation techniques (N = 150) and other advanced techniques such as precut sphincterotomy (N = 58) and PD stent placement (N = 80) to facilitate difficult biliary cannulation.
The included studies were heterogeneous in their participant selection criteria. We have outlined the specific criteria for each study in the Characteristics of included studies section. In general, studies included participants with intact papilla who required ERCP for pancreaticobiliary diseases. Participants were excluded if they had had previous endoscopic sphincterotomy (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013), endoscopic papillary balloon dilatation (Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013), prior pancreatic or biliary stent placement (Angsuwatcharakon 2012; Herreros de Tejada 2009; Yoo 2013), altered anatomy (Billroth II or Roux‐en‐Y anastomosis) (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009), ampullary mass (Angsuwatcharakon 2012), recent or acute pancreatitis (Angsuwatcharakon 2012; Yoo 2013), suspected SOD (Coté 2012), indication for endoscopic pancreatic therapeutics (Coté 2012), obstructive PD (Angsuwatcharakon 2012), pancreas divisum (Herreros de Tejada 2009; Ito 2010a), prophylactic drug use for PEP (Herreros de Tejada 2009), and pregnancy or breastfeeding (Herreros de Tejada 2009; Ito 2010a; Yoo 2013). Two studies excluded people in whom insertion of a guidewire into the PD could not be achieved (Ito 2010a; Yoo 2013). One study (Zheng 2010), in abstract format, included people with biliary complications after liver transplantation, but did not provide details with regards to the specific inclusion and exclusion criteria.
Indications for the procedure were provided by all studies (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013), except for one (Zheng 2010): CBD stones (203/513, 39.6%), pancreaticobiliary malignancy (102/426, 23.9%), and SOD (9/345, 2.6%). In addition, peri‐ampullary diverticulum was reported to be present in 83/416, 20.0% of cases.
Six studies reported the mean age of participants: 65.1 (Angsuwatcharakon 2012), 57.8 (Coté 2012), 67.7 (Herreros de Tejada 2009), 69.0 (Ito 2010a), 64.0 (Maeda 2003), and 65.3 years (Yoo 2013). Six studies reported the gender of the participants (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013). Overall, there were similar proportions of males and females (240/273): 23/21 (Angsuwatcharakon 2012), 38/49 (Coté 2012), 76/112 (Herreros de Tejada 2009), 39/31 (Ito 2010a), 23/30 (Maeda 2003), and 41/30 (Yoo 2013).
See: participant characteristics (Table 3).
2. Participant characteristics.
| Study | PGW/Control | ||||||
| Sample size | Mean age | Female | CBD stone | Pancreaticobiliary malignancy | SOD | History of pancreatitis (acute/chronic) | |
| Angsuwatcharakon 2012 | 23/21 | 66/64 | 43/52 | 57/48 | 26/24 | 0 | Excluded |
| Coté 2012 | 42/45 | 58/57 | NA | 24/30 | NA | Excluded | NA |
| Herreros de Tejada 2009 | 97/91 | 70/66 | 61/58 | 54/53 | 21/20 | 4/3 | 18/13 |
| Ito 2010a | 35/35 | 70/68 | 43/46 | 29/34 | 43/31 | 0/6 | 9/0 |
| Maeda 2003 | 27/26 | 64/64 | 59/54 | 7/0 | 11/23 | NA | NA |
| Yoo 2013 | 34/37 | 67/64 | 47/38 | 41/43 | 26/24 | 0 | NA |
| Zheng 2010 | 31/33 | NA | NA | NA | NA | NA | NA |
CBD: common bile duct NA: not applicable PGW: pancreatic duct guidewire placement SOD: sphincter of Oddi dysfunction
Interventions
In total, we identified seven studies that assessed the clinical effectiveness of the PGW technique in difficult CBD cannulation: three studies compared the PGW technique versus persistent attempts with conventional cannulation techniques (contrast‐ or guidewire‐assisted cannulation) (Herreros de Tejada 2009; Maeda 2003; Zheng 2010); two studies compared the PGW technique versus precut sphincterotomy (Angsuwatcharakon 2012; Yoo 2013); one study compared the PGW technique versus PD stent placement (Coté 2012); and one study compared PD stent placement versus no PD stent placement in people who had undergone the PGW technique (Ito 2010a).
Conventional cannulation techniques (contrast‐ or guidewire‐assisted cannulation) prior to randomisation
Prior to randomisation, three studies attempted initial CBD cannulation using conventional contrast‐assisted technique with a standard catheter, in Maeda 2003, or with either a standard catheter or a sphincterotome, in Angsuwatcharakon 2012 and Ito 2010a. Three other studies used conventional guidewire‐assisted cannulation technique with a sphincterotome, in Zheng 2010, or with either a standard catheter or a sphincterotome, in Herreros de Tejada 2009 and Yoo 2013. In one study (Coté 2012), a specific cannulation technique was not mandated, but guidewire‐assisted technique using a sphincterotome was usually the preferred primary approach.
Pancreatic duct guidewire placement or double guidewire technique
In the PGW technique group, four studies used either a standard catheter or a sphincterotome preloaded with a (0.025‐inch or 0.035‐inch) hydrophilic guidewire to facilitate pancreatic duct cannulation (Angsuwatcharakon 2012; Herreros de Tejada 2009; Ito 2010a; Yoo 2013). Two studies used a sphincterotome preloaded with a (0.025‐inch or 0.035‐inch) guidewire, but did not specify whether or not the guidewire was hydrophilic (Coté 2012; Zheng 2010). One study used a standard catheter preloaded with a (0.025‐inch, 0.032‐inch, or 0.035‐inch) hydrophilic guidewire (Maeda 2003). The PGW technique was performed by first inserting the guidewire into the PD. However, only one study prespecified the depth of wire insertion to at least half of the presumed total length of the PD (Herreros de Tejada 2009). In two studies (Angsuwatcharakon 2012; Herreros de Tejada 2009), contrast was not injected into the PD and fluoroscopy was used to confirm the position of the pancreatic guidewire. Three studies used both contrast and fluoroscopy to position the guidewire in the PD (Ito 2010a; Maeda 2003; Yoo 2013). Two studies did not indicate whether contrast or fluoroscopy was used to confirm the position of the pancreatic guidewire (Coté 2012; Zheng 2010). After placement of the pancreatic guidewire, the cannulation device (a standard catheter or a sphincterotome) was withdrawn from the endoscope and reinserted into the working channel of the scope alongside the pancreatic guidewire. Biliary cannulation was attempted with a cannulation device either alone with contrast‐assisted cannulation technique, in Ito 2010a and Maeda 2003, or with a second guidewire using the guidewire‐assisted cannulation technique (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Yoo 2013; Zheng 2010).
Persistent attempts with conventional cannulation techniques (contrast‐ or guidewire‐assisted cannulation) after randomisation
In people with difficult cannulation, three studies compared the PGW technique versus persistent attempts with conventional contrast‐assisted, in Maeda 2003, or guidewire‐assisted, in Herreros de Tejada 2009 and Zheng 2010, cannulation techniques. In one study (Maeda 2003), persistent attempts with conventional contrast‐assisted technique were carried out using a standard catheter, the direction of the catheter adjusted by moving the duodenoscope and the scope channel. In the two studies that evaluated persistent attempts with guidewire‐assisted cannulation techniques, one study, Herreros de Tejada 2009, used either a standard catheter or a sphincterotome, and the other study, Zheng 2010, used a sphincterotome exclusively for cannulation.
Precut (access) sphincterotomy
In people with difficult cannulation, two studies compared the PGW technique versus precut sphincterotomy (Angsuwatcharakon 2012; Yoo 2013). In one study (Angsuwatcharakon 2012), the precut sphincterotomy technique was carried out by using a needle‐knife in a freehand fistulotomy fashion without placement of a PD stent. However, it was unclear which cannulation techniques (contrast‐ versus guidewire‐assisted) and what cannulation devices were used to achieve biliary cannulation after the precut sphincterotomy (Angsuwatcharakon 2012). In the other study (Yoo 2013), transpancreatic precut sphincterotomy (TPS) was performed by first placing a guidewire deep into the PD, then wedging the tip of a sphincterotome into the pancreatic orifice and incising through the septum between the pancreatic and biliary duct with the aim of exposing the biliary ductal orifice. After TPS, the guidewire placed in the PD was removed. Biliary cannulation was then attempted using a standard catheter or a sphincterotome, either with contrast‐ or guidewire‐assisted cannulation technique (Yoo 2013). PD stent was not placed after the TPS (Yoo 2013).
Pancreatic duct stent placement
In people with difficult cannulation, one study compared the PGW technique versus PD stent placement (Coté 2012). In this study (Coté 2012), a guidewire (0.025 inch or 0.035 inch) was first placed in the mid‐body of the pancreas to facilitate PD stent placement. The type of stent was left to the discretion of the endoscopists: either a 4‐ or 5‐Fr stent (2 to 9 cm long) with an external pigtail and single internal flange, or a 5‐Fr stent with a double external and single internal flange (Coté 2012). A pancreatic sphincterotomy was not performed (Coté 2012). After PD stent placement, the pancreatic guidewire was removed, and biliary cannulation was attempted by using a sphincterotome with guidewire‐assisted cannulation technique (Coté 2012). In the study by Ito et al (Ito 2010a), people with difficult cannulation who underwent PGW placement technique were randomised to PD stent placement or no PD stent placement. In the PD stent placement group, a 5‐Fr (4 cm long) stent with a single pigtail was used (Ito 2010a). The endoscopists determined the timing of the PD stenting during the procedure (Ito 2010a). Hence, the PD stent could be used to maintain PD drainage postprocedure or to facilitate biliary cannulation, or both (Ito 2010a). Although the designs of these two studies appeared to be different (Coté 2012; Ito 2010a), we decided to combine them for analyses due to the fact that placement of a PD stent would require deep PD guidewire placement (whether to facilitate biliary cannulation or to maintain PD drainage postprocedure, or both).
Of the five studies that did not use PD stent as a comparative arm (Angsuwatcharakon 2012; Herreros de Tejada 2009; Maeda 2003; Yoo 2013; Zheng 2010), one study, Herreros de Tejada 2009, permitted the use of PD stent (12/97 in the PGW group versus 9/91 in the persistent conventional cannulation group). However, it was unclear from the report whether the PD stent was used for prophylaxis of PEP or to facilitate cannulation of the CBD as a 'backup technique' when the randomised technique failed. Two studies explicitly stated that PD stent was not used (Angsuwatcharakon 2012; Yoo 2013). One study (Zheng 2010), in abstract format, did not provide information about the use of PD stent. We contacted the authors of the primary study, Zheng 2010, and confirmed that PD stent was not used in the study. One study did not report the use of PD stent (Maeda 2003).
Endoscopic ultrasound rendezvous
We did not identify any RCTs that compared the PGW technique with endoscopic ultrasound rendezvous technique.
Outcomes
Commonly reported outcomes included PEP, overall cannulation success rates, and cannulation success rates with the randomised technique. All seven studies, Angsuwatcharakon 2012, Coté 2012, Herreros de Tejada 2009, Ito 2010a, Maeda 2003, Yoo 2013, and Zheng 2010, defined PEP as a rise in serum amylase level to greater than or equal to three‐fold above the upper limit of normal 24 hours after ERCP accompanied by abdominal pain characteristic of pancreatitis according to the consensus definition (Cotton 1991). In addition to the consensus definition (Cotton 1991), one study defined PEP as abdominal pain with computed tomography or magnetic resonance imaging evidence of acute pancreatitis (Angsuwatcharakon 2012). One study regarded only people graded above "moderate" according to the consensus definition as having PEP (Maeda 2003). One study provided the rates of PEP based on per‐protocol (PP) data (Herreros de Tejada 2009). We contacted the authors (Herreros de Tejada 2009), who provided the rates of PEP based on ITT data.
All seven studies, Angsuwatcharakon 2012, Coté 2012, Herreros de Tejada 2009, Ito 2010a, Maeda 2003, Yoo 2013, and Zheng 2010, graded severity of PEP using the consensus criteria (Colton 2009). In the original report by Yoo et al (Yoo 2013), data regarding moderate to severe PEP were pooled together. We contacted the authors (Yoo 2013), and obtained further information regarding the severity of pancreatitis (moderate versus severe in each group). One study reported the severity of PEP based on PP data (Herreros de Tejada 2009). We contacted the authors (Herreros de Tejada 2009), who provided information on the severity of PEP based on ITT data. One study (Zheng 2010), in abstract format, reported that "the majority of the cases were mild and had recovered by conservative treatment", but did not provide the incidences of PEP stratified by severity.
All but one study, Herreros de Tejada 2009, reported overall cannulation success rates based on ITT analyses. We obtained additional data regarding overall cannulation success rates after the use of 'backup technique' from the authors of this primary study (Herreros de Tejada 2009). All studies provided outcome data regarding cannulation success with the randomised technique prior to the use of rescue techniques or technique 'cross‐over'.
Six studies reported post‐ERCP complications including bleeding (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013), cholangitis (Angsuwatcharakon 2012; Herreros de Tejada 2009; Ito 2010a; Yoo 2013), perforation (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013), and mortality (Herreros de Tejada 2009; Ito 2010a). One study (Zheng 2010), in abstract format, reported that "the ratios of other complications were low and were no different between two groups".
Excluded studies
Twenty‐four studies did not meet the eligibility criteria and were excluded for the following reasons: non‐randomised trial design (Balderas 2011; Chandran 2012; Grönroos 2011; Ito 2008; Ito 2010b; Ito 2012; Kim 2012; Kim 2014; Kim 2015a; Kim 2015b; Miao 2015; Nagano 2010; Patel 2009; Song 2013; Suzuki 2012; Tanaka 2013; Yang 2015), inappropriate patient population (Cha 2012; Sasahira 2015), and inappropriate intervention (Kim 2013; Ozaslan 2014; Zang 2014). Specifically, we excluded two studies as they evaluated the early use of the PGW technique by including people who had guidewire placed in the pancreatic duct by chance and not in people with difficult cannulation (Cha 2012; Sasahira 2015). Four articles were preliminary or duplicate data of included studies (Angsuwatcharakon 2010; Cha 2011; Coté 2010; Herreros de Tejada 2007).
See: Characteristics of excluded studies and Results of the search.
Risk of bias in included studies
The methodological quality of the included studies is summarised in Characteristics of included studies and shown in Figure 2 and Figure 3.
2.
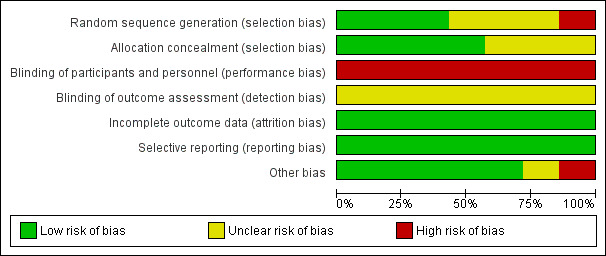
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We considered three studies to be at low risk of bias for random sequence generation as they reported the use of computer‐generated numbers (Coté 2012; Herreros de Tejada 2009; Yoo 2013). We considered three studies to be at unclear risk of bias for random sequence generation as no specific information was provided regarding the randomisation process (Angsuwatcharakon 2012; Ito 2010a; Maeda 2003). We considered one study (Zheng 2010), in abstract format, to be at high risk of bias for random sequence generation. The conference proceeding stated that "patients were randomly assigned to" (Zheng 2010). We contacted the authors of the original report and received further information about the randomisation process (Zheng 2010): "Randomization was performed by the method of ballot of odd and even numbers into two groups." In addition, six participants who had had pancreatic guidewire inserted into the PD prior to randomisation were automatically assigned to the PGW group (Zheng 2010). We therefore considered the allocation of participants to treatment groups in this study to be not truly randomised (Zheng 2010).
Allocation concealment
We considered four studies to be at low risk of bias for allocation concealment (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a). Three studies used envelopes that were concealed (Coté 2012), sealed (Ito 2010a), or sealed and opaque (Herreros de Tejada 2009). One study stated that the ERCP team did not know the sequence of randomisation until they declared such people to be eligible, then the enveloped code was broken (Angsuwatcharakon 2012). Three studies had uncertain concealment (Maeda 2003; Yoo 2013; Zheng 2010).
Blinding
In all of the included studies, the endoscopists performing the procedure could not be blinded. This may have had an impact on cannulation success and the rates of PEP depending on the expertise, preference, and perseverance of the endoscopists performing the procedure. Blinding of participants, healthcare providers, data collectors, and outcome assessors should be possible, but may be less important when an outcome can be objectively defined (for example death). In the case of PEP, there is some degree of subjectivity in the interpretation of pancreatic pain. Blinding of these groups is therefore essential for reducing performance and detection bias. Only one study reported blinding of participants (Coté 2012). None of the included studies reported blinding of personnel (other than the endoscopists) and outcome assessors. In two studies (Angsuwatcharakon 2012; Ito 2010a), all participants were admitted to the hospital for at least 24 hours to observe for post‐ERCP complications. As a result, participants may be more likely to undergo clinical, laboratory, and radiological evaluation as opposed to being discharged home following ERCP. If outcome assessors were not blinded, this could lead to differential detection bias. We therefore considered all studies to be at high risk of bias for blinding of participants and personnel (the endoscopists), and unclear risk of bias for outcome assessment (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013; Zheng 2010).
Incomplete outcome data
We considered all studies to be at low risk of bias for incomplete outcome data on PEP (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013; Zheng 2010). Six studies either reported PEP in both the ITT and PP sample (Ito 2010a), or in the ITT sample (Angsuwatcharakon 2012; Coté 2012; Maeda 2003; Yoo 2013; Zheng 2010). One study excluded a total of 25 participants after randomisation due to protocol violations, 17 of which were in the PGW group because of unintentional CBD cannulation without having placed a guidewire into the PD and therefore without meeting the criteria for the PGW technique (Herreros de Tejada 2009). The original report provided only PP data for PEP (Herreros de Tejada 2009). We contacted the authors (Herreros de Tejada 2009), and obtained ITT data on PEP. There was no loss to follow‐up in any of the included studies.
Selective reporting
All studies reported all important outcomes, and were therefore considered to be at low risk of bias for selective reporting (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013; Zheng 2010).
Other potential sources of bias
Interim analysis
One study, Coté 2012, planned for a sample size of 108 cases (n = 40 in the PGW group; n = 68 in the PD stent group, including 'cross‐overs' from the PGW group), to achieve 80% statistical power to detect a difference in the primary outcome of cannulation success within 6 minutes, with a two‐sided alpha error of 5%. However, participant recruitment was terminated after the targeted sample size was achieved in the PGW group (n = 42) but not the PD stent group (n = 45) due to a lower than anticipated rate of 'cross‐over' from the PGW group to the PD stent group (Coté 2012). An interim analysis revealed "marginal differences for the primary outcome between the study groups" (Coté 2012). It was therefore concluded that completing enrolment to the target sample size would not have impacted interpretation of the results (Coté 2012). The observed difference in efficacy (13.8%) for the primary endpoint (CBD cannulation within 6 minutes) would have required greater than 400 participants to have adequate statistical power to detect a significant difference (Coté 2012). However, the decision to perform the interim analysis was not based on predetermined futility‐stopping rules. This can lead to three potential problems: 1) underestimating the treatment difference by committing a type II error; 2) increasing the risk of imbalance in prognostic factors; and 3) jeopardising the analyses of secondary outcomes such as PEP in this particular study (Lachin 2009; Pocock 2006). Furthermore, the ability to perform a comprehensive assessment of treatment impact of an intervention (risk‐benefit ratio) is often limited by early stopping of a trial (Briel 2012). Indeed, there was a higher prevalence of "anticipated difficult cannulation" in the PGW group versus the PD stent group (48.1% versus 38.0%) based on the endoscopist's visual inspection of the papilla (Coté 2012). Arguably, the interobserver and intraobserver variability of visual inspection of the papilla is unknown, but if a greater number of participants with "more difficult" papilla were randomised to the PGW group, this may introduce a bias favouring the PD stent technique. The study suggested that the primary outcome of cannulation success within 6 minutes was similar between the PGW technique and the PD stent technique (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.42 to 1.04). However, the 95% CI of the RR is consistent with a clinically important benefit or a negligible risk with the PD stent technique compared to the PGW technique with regards to the primary outcome.
Effects of interventions
See: Table 1
The primary objective of the main analysis (Analysis 1) was to determine if the PGW technique or double guidewire technique (DGT) compared to other endoscopic techniques including (a) persistent attempts with conventional cannulation techniques (contrast‐ or guidewire‐assisted cannulation) or (b) other advanced techniques (for example precut sphincterotomy, pancreatic duct stent placement), or both was beneficial in reducing the risk of PEP in people with difficult biliary cannulation. We included seven RCTs in the main analysis (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013; Zheng 2010). The secondary objectives of this review were to determine if the PGW technique compared to other endoscopic techniques had any effect on the severity of PEP; CBD cannulation success with the randomised technique; overall CBD cannulation success (during the index procedure); and ERCP‐related complications including bleeding, post‐ERCP cholangitis, perforation, and mortality.
To explore sources of heterogeneity, we then performed prespecified subgroup analyses according to trial design (permission of rescue techniques versus non‐permission of rescue techniques) (Analysis 2), use of a PD stent (in trials that evaluated PD stent as a rescue technique or for prophylaxis of PEP, and not as a main comparison technique) (Analysis 3), involvement of trainees in cannulation (Analysis 4), risk of bias (Analysis 5), and publication type (Analysis 6) for the outcomes of PEP and overall cannulation success.
We calculated unweighted pooled rates and RRs with 95% CIs for each of the outcomes using a random‐effects model for the PGW technique compared to the other endoscopic techniques. We analysed data on an ITT basis.
To assess the robustness of our results, we carried out sensitivity analyses using different summary statistics (RR versus odds ratio (OR)) and meta‐analytic models (fixed‐effect versus random‐effects). As no trials had missing data, we did not conduct an available‐case analysis versus 'worst‐case scenario' analysis.
Analysis 1: PGW or DGT compared to other endoscopic techniques
Post‐ERCP pancreatitis
PGW technique versus other endoscopic techniques
All seven studies included in the main analysis reported PEP rates and comprised a total of 289 participants in the PGW technique group and 288 in the other endoscopic techniques group (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013; Zheng 2010). There was no significant heterogeneity among the studies (P = 0.32; I2 = 15%). Unweighted pooled rates of PEP were 16.3% for the PGW technique and 8.0% for the other endoscopic techniques. The PGW technique significantly increased PEP compared to the other endoscopic techniques based on ITT analysis (RR 1.98, 95% CI 1.14 to 3.42; P = 0.01; Analysis 1.1). The number needed to treat for an additional harmful outcome (NNTH) was 13 (95% CI 5 to 89). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model (Analysis 1.1). In a post‐hoc sensitivity analysis with removal of the one study that was considered to be at high risk of bias for random sequence generation (Zheng 2010), the results remained robust (RR 2.14, 95% CI 1.06 to 4.33; P = 0.03).
1.1. Analysis.
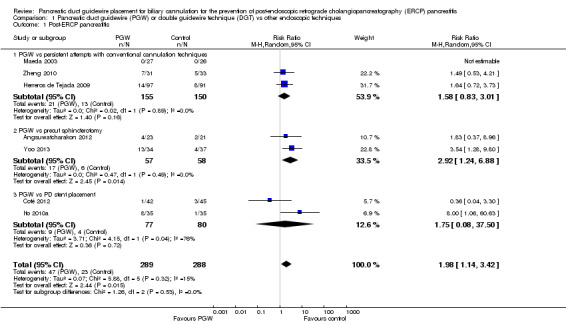
Comparison 1 Pancreatic duct guidewire (PGW) or double guidewire technique (DGT) vs other endoscopic techniques, Outcome 1 Post‐ERCP pancreatitis.
PGW technique versus persistent attempts with conventional cannulation techniques
Among the seven studies, three studies with a total of 305 participants compared the PGW technique (n = 155) versus persistent attempts with conventional contrast‐assisted, in Maeda 2003, or guidewire‐assisted, in Herreros de Tejada 2009 and Zheng 2010, cannulation techniques (n = 150). There was no significant heterogeneity among the three studies (P = 0.89; I2 = 0%). Unweighted pooled rates of PEP were 13.5% for the PGW technique and 8.7% for persistent attempts with conventional cannulation techniques. There was no statistically significant difference in the rates of PEP between the PGW technique and persistent attempts with conventional cannulation techniques (RR 1.58, 95% CI 0.83 to 3.01; P = 0.16; Analysis 1.1). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model.
PGW technique versus precut sphincterotomy
Two studies with a total of 115 participants compared the PGW technique (n = 57) versus precut sphincterotomy (n = 58) (Angsuwatcharakon 2012; Yoo 2013). There was no significant heterogeneity among the two studies (P = 0.49; I2 = 0%). Unweighted pooled rates of PEP were 29.8% for the PGW technique and 10.3% for precut sphincterotomy. The PGW technique significantly increased PEP compared to precut sphincterotomy based on ITT analysis (RR 2.92, 95% CI 1.24 to 6.88; P = 0.01; Analysis 1.1). The NNTH was 5 (95% CI 2 to 40). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model.
PGW technique versus PD stent placement
Two studies with a total of 157 participants compared the PGW technique (n = 77) versus PD stent placement (n = 80) (Coté 2012; Ito 2010a). There was significant heterogeneity among the two studies (P = 0.04; I2 = 76%). Unweighted pooled rates of PEP were 11.7% for the PGW technique and 5.0% for PD stent placement. There was no statistically significant difference in the rates of PEP between the PGW technique and PD stent placement (RR 1.75, 95% CI 0.08 to 37.50; P = 0.72; Analysis 1.1). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model.
The test for subgroup differences indicated no statistically significant differences between the three subgroups (P = 0.53).
Severity of post‐ERCP pancreatitis
Six studies provided data regarding the severity of PEP for all randomised participants, comprising a total of 258 participants in the PGW technique group and 255 in the other endoscopic techniques group (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013). There was no significant heterogeneity among the studies for the outcomes of both mild (P = 0.32; I2 = 14%) and moderate (P = 0.38; I2 = 0%) PEP. Heterogeneity was not estimable for the outcome of severe PEP because only one study contributed to the event rates (Herreros de Tejada 2009). Unweighted pooled rates of mild PEP were 12.8% for the PGW technique and 4.7% for the other endoscopic techniques. The PGW technique significantly increased mild PEP compared to the other endoscopic techniques based on ITT analysis (RR 2.70, 95% CI 1.27 to 5.76; P = 0.01; Analysis 1.2). The results remained robust with OR or a fixed‐effect model. Unweighted pooled rates of moderate PEP were 1.9% for the PGW technique and 2.0% for the other endoscopic techniques. There was no statistically significant difference in the rates of moderate PEP between the PGW technique and the other endoscopic techniques (RR 0.95, 95% CI 0.27 to 3.38; P = 0.94; Analysis 1.2). The results remained non‐significant with OR or a fixed‐effect model. Unweighted pooled rates of severe PEP were 0.8% for the PGW technique and 0.4% for the other endoscopic techniques. There was no statistically significant difference in the rates of severe PEP between the PGW technique and the other endoscopic techniques (RR 1.88, 95% CI 0.17 to 20.34; P = 0.60; Analysis 1.2). The results remained non‐significant with OR or a fixed‐effect model.
1.2. Analysis.
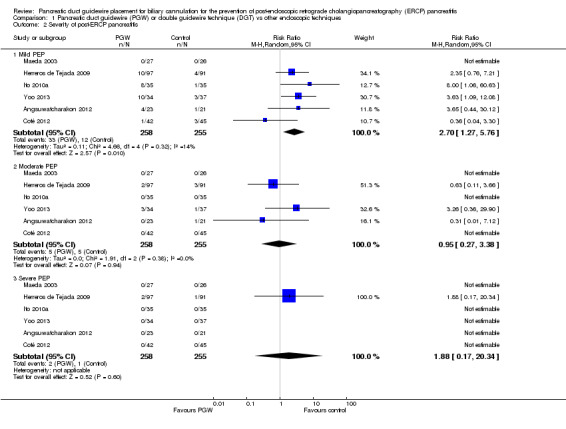
Comparison 1 Pancreatic duct guidewire (PGW) or double guidewire technique (DGT) vs other endoscopic techniques, Outcome 2 Severity of post‐ERCP pancreatitis.
CBD cannulation success with the randomised technique (before the use of rescue techniques)
All seven studies reported CBD cannulation success rates with the randomised technique and comprised a total of 289 participants in the PGW technique group and 288 in the other endoscopic techniques group (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013; Zheng 2010). There was significant heterogeneity among the studies (P = 0.01; I2 = 63%). Unweighted pooled cannulation success rates with the randomised technique were 66.1% for the PGW technique and 66.3% for the other endoscopic techniques. There was no statistically significant difference in the cannulation success rates with the randomised technique between the PGW technique and the other endoscopic techniques (RR 1.04, 95% CI 0.87 to 1.24; P = 0.68; Analysis 1.3). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model (Analysis 1.3).
1.3. Analysis.
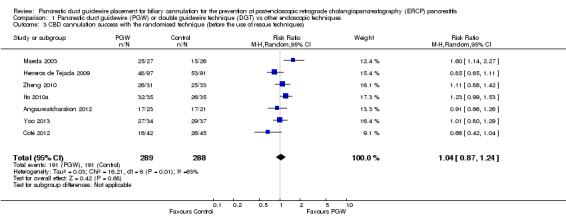
Comparison 1 Pancreatic duct guidewire (PGW) or double guidewire technique (DGT) vs other endoscopic techniques, Outcome 3 CBD cannulation success with the randomised technique (before the use of rescue techniques).
Overall CBD cannulation success (during the index procedure)
All seven studies reported overall CBD cannulation success rates and comprised a total of 289 participants in the PGW technique group and 288 in the other endoscopic techniques group (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013; Zheng 2010). There was significant heterogeneity among the studies (P = 0.007; I2 = 66%). Unweighted pooled overall cannulation success rates were 82.4% for the PGW technique and 81.6% for the other endoscopic techniques. There was no statistically significant difference in the overall cannulation success rates between the PGW technique and the other endoscopic techniques (RR 1.04, 95% CI 0.91 to 1.18; P = 0.59; Analysis 1.4). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model.
1.4. Analysis.
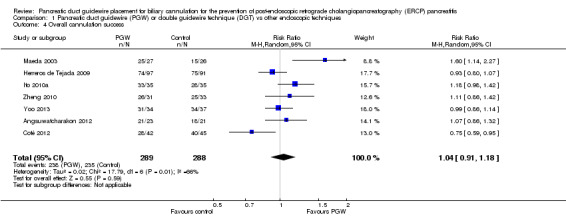
Comparison 1 Pancreatic duct guidewire (PGW) or double guidewire technique (DGT) vs other endoscopic techniques, Outcome 4 Overall cannulation success.
ERCP‐related complications
Bleeding
Six studies with a total of 513 participants reported postsphincterotomy bleeding (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013). All bleeding episodes were described as mild and were controlled by endoscopic therapies in one study (Angsuwatcharakon 2012). Other studies reported either no bleeding episodes, in Coté 2012, Ito 2010a, and Maeda 2003, or provided no further information, in Herreros de Tejada 2009 and Yoo 2013, regarding the severity of the bleeding. There was no significant heterogeneity among the studies (P = 0.36; I2 = 2%). Unweighted pooled rates of postsphincterotomy bleeding were 1.2% for the PGW technique and 3.5% for the other endoscopic techniques. There was no statistically significant difference in the rates of postsphincterotomy bleeding between the PGW technique and the other endoscopic techniques (RR 0.48, 95% CI 0.13 to 1.79; P = 0.27; Analysis 1.5). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model.
1.5. Analysis.
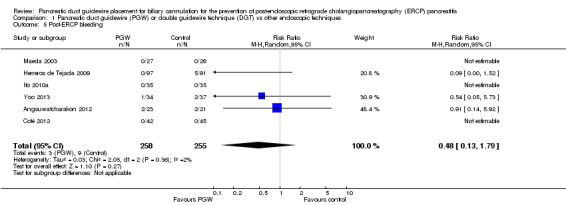
Comparison 1 Pancreatic duct guidewire (PGW) or double guidewire technique (DGT) vs other endoscopic techniques, Outcome 5 Post‐ERCP bleeding.
Perforation
Six studies with a total of 513 participants reported perforation (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013). However, only one study reported the actual occurrence of perforation (Herreros de Tejada 2009). Heterogeneity was not estimable for this outcome. Unweighted pooled rates of perforation were 0.4% for the PGW technique and 0.4% for the other endoscopic techniques. There was no statistically significant difference in the rates of perforation between the PGW technique and the other endoscopic techniques (RR 0.94, 95% CI 0.06 to 14.78; P = 0.96; Analysis 1.6).
1.6. Analysis.
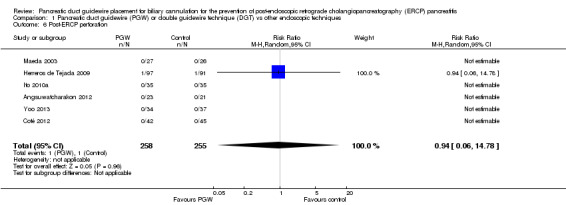
Comparison 1 Pancreatic duct guidewire (PGW) or double guidewire technique (DGT) vs other endoscopic techniques, Outcome 6 Post‐ERCP perforation.
Cholangitis
Four studies with a total of 373 participants reported post‐ERCP cholangitis (Angsuwatcharakon 2012; Herreros de Tejada 2009; Ito 2010a; Yoo 2013). There was no significant heterogeneity among the studies (P = 0.37; I2 = 0%). Unweighted pooled rates of cholangitis were 4.8% for the PGW technique and 1.6% for the other endoscopic techniques. There was no statistically significant difference in the rates of cholangitis between the PGW technique and the other endoscopic techniques (RR 2.71, 95% CI 0.79 to 9.35; P = 0.11; Analysis 1.7). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model.
1.7. Analysis.

Comparison 1 Pancreatic duct guidewire (PGW) or double guidewire technique (DGT) vs other endoscopic techniques, Outcome 7 Post‐ERCP cholangitis.
Mortality
Only two studies with a total of 258 participants reported mortality (Herreros de Tejada 2009; Ito 2010a), and only one procedure‐related death due to aspiration pneumonia occurred in the persistent cannulation technique group in one study (RR 0.31, 95% CI 0.01 to 7.58; Analysis 1.8) (Herreros de Tejada 2009).
1.8. Analysis.
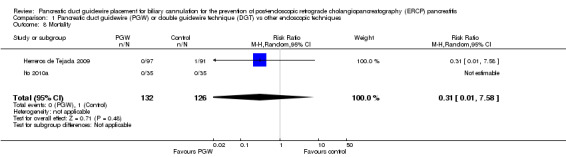
Comparison 1 Pancreatic duct guidewire (PGW) or double guidewire technique (DGT) vs other endoscopic techniques, Outcome 8 Mortality.
Analysis 2: PGW or DGT compared to other endoscopic techniques according to trial design (permission of rescue techniques versus non‐permission of rescue techniques)
Post‐ERCP pancreatitis
All four studies that permitted the use of rescue techniques (for example precut sphincterotomy, insertion of PD stent to facilitate cannulation, technique 'cross‐over' to the other comparison arm) when the randomised technique failed reported PEP for all randomised participants, comprising a total of 197 participants in the PGW technique group and 192 in the other endoscopic techniques group (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a). There was significant heterogeneity among the studies (P = 0.24; I2 = 28%). Unweighted pooled rates of PEP were 13.7% for the PGW technique and 7.3% for the other endoscopic techniques. Among this subgroup of studies, there was no statistically significant difference in the rates of PEP between the PGW technique and the other endoscopic techniques (RR 1.76, 95% CI 0.72 to 4.26; P = 0.21; Analysis 2.1). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model.
2.1. Analysis.
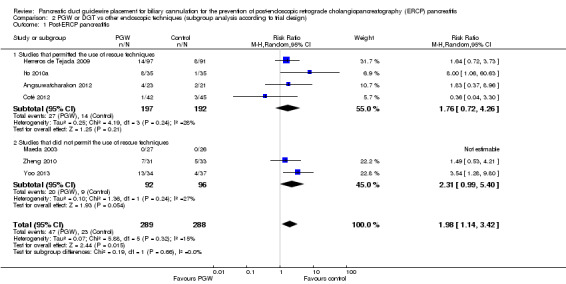
Comparison 2 PGW or DGT vs other endoscopic techniques (subgroup analysis according to trial design), Outcome 1 Post‐ERCP pancreatitis.
All three studies that did not permit the use of rescue techniques when the randomised technique failed reported PEP for all randomised participants, comprising a total of 92 participants in the PGW technique group and 96 in the other endoscopic techniques group (Maeda 2003; Yoo 2013; Zheng 2010). There was significant heterogeneity among the studies (P = 0.24; I2 = 27%). Unweighted pooled rates of PEP were 21.7% for the PGW technique and 9.4% for the other endoscopic techniques. Among this subgroup of studies, there was a non‐significant trend for increased rates of PEP with the PGW technique compared to the other endoscopic techniques (RR 2.31, 95% CI 0.99 to 5.40; P = 0.05; Analysis 2.1). In sensitivity analyses, the results remained non‐significant with OR, but became statistically significant with a fixed‐effect model (RR 2.39, 95% CI 1.17 to 4.88; P = 0.02) favouring the use of the other endoscopic techniques.
Nevertheless, the test for subgroup differences indicated no statistically significant differences between the two subgroups (trials that permitted the use of rescue techniques versus trials that did not permit the use of rescue techniques) for the outcome of PEP (P = 0.66) (Analysis 2.1).
Overall CBD cannulation success (during the index procedure)
All four studies that permitted the use of rescue techniques when the randomised technique failed reported overall CBD cannulation success, comprising a total of 197 participants in the PGW technique group and 192 in the other endoscopic techniques group (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a). There was significant heterogeneity among the studies (P = 0.02; I2 = 71%). Unweighted pooled overall cannulation success rates were 79.2% for the PGW technique and 83.9% for the other endoscopic techniques. In this subgroup of studies, there was no statistically significant difference in the overall cannulation success rates between the PGW technique and the other endoscopic techniques (RR 0.97, 95% CI 0.81 to 1.16; P = 0.76; Analysis 2.2). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model.
2.2. Analysis.
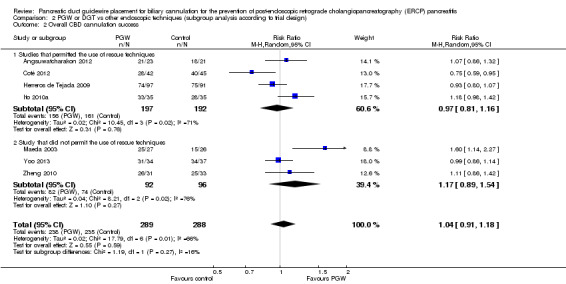
Comparison 2 PGW or DGT vs other endoscopic techniques (subgroup analysis according to trial design), Outcome 2 Overall CBD cannulation success.
All three studies that did not permit the use of rescue techniques when the randomised technique failed reported overall CBD cannulation success for all randomised participants, comprising a total of 92 participants in the PGW technique group and 96 in the other endoscopic techniques group (Maeda 2003; Yoo 2013; Zheng 2010). There was significant heterogeneity among the studies (P = 0.02; I2 = 76%). Unweighted pooled overall cannulation success rates were 89.1% for the PGW technique and 77.1% for the other endoscopic techniques. In this subgroup of studies, there was no statistically significant difference in the overall cannulation success rates between the PGW technique and the other endoscopic techniques (RR 1.17, 95% CI 0.89 to 1.54; P = 0.27; Analysis 2.2). In sensitivity analyses, the results remained non‐significant with OR, but became statistically significant with a fixed‐effect model (RR 1.16, 95% CI 1.02 to 1.33; P = 0.03) favouring the use of the PGW technique.
The test for subgroup differences indicated no statistically significant differences between the two subgroups (trials that permitted the use of rescue techniques versus trials that did not permit the use of rescue techniques) for the outcome of overall CBD cannulation success (P = 0.27) (Analysis 2.2).
Analysis 3: PGW or DGT compared to other endoscopic techniques according to the use of a PD stent (in trials that permitted PD stent as a rescue technique or for prophylaxis of PEP, and not as a main comparison technique)
Post‐ERCP pancreatitis
All four studies that did not permit the use of PD stents provided data regarding the rates of PEP (Angsuwatcharakon 2012; Maeda 2003; Yoo 2013; Zheng 2010), comprising a total of 114 participants in the PGW technique group and 114 participants in the other endoscopic techniques group. There was no significant heterogeneity among the studies (P = 0.53; I2 = 0%). Unweighted pooled rates of PEP were 16.7% for the PGW technique and 7.9% for the other endoscopic techniques. Among this subgroup of studies, there was a non‐significant trend for increased rates of PEP with the PGW technique compared to the other endoscopic techniques (RR 2.03, 95% CI 0.96 to 4.29; P = 0.06; Analysis 3.1). In sensitivity analyses, the results remained non‐significant with OR, but became statistically significant with a fixed‐effect model (RR 2.15, 95% CI 1.03 to 4.49; P = 0.04), suggesting an increased risk of PEP with the PGW technique compared to other endoscopic techniques when PD stent was not used.
3.1. Analysis.
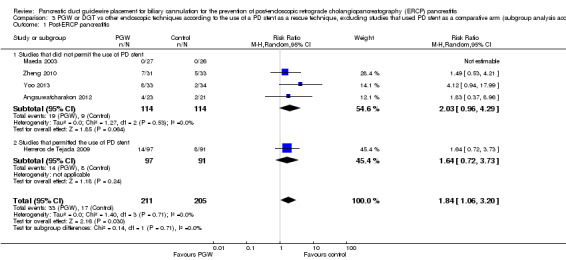
Comparison 3 PGW or DGT vs other endoscopic techniques according to the use of a PD stent as a rescue technique, excluding studies that used PD stent as a comparative arm (subgroup analysis according to the use of PD stent), Outcome 1 Post‐ERCP pancreatitis.
Only one study permitted the use of PD stents (Herreros de Tejada 2009). The rates of PEP were 14.4% in the PGW technique group and 8.8% for the other endoscopic technique (persistent attempts with conventional cannulation). There was no statistically significant difference in the rates of PEP between the two groups (RR 1.64, 95% CI 0.72 to 3.73; P = 0.24; Analysis 3.1).
Nevertheless, the test for subgroup differences indicated no statistically significant differences between the two subgroups (trials that did not permit the use of PD stents versus trials that permitted the use of PD stents) for the outcome of PEP (P = 0.71) (Analysis 3.1).
Analysis 4: PGW or DGT compared to other endoscopic techniques according to involvement of trainees in cannulation
Post‐ERCP pancreatitis
Three studies had involvement of trainees and reported the rates of PEP (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009), comprising a total of 162 participants in the PGW technique group and 157 participants in the other endoscopic techniques group. There was no significant heterogeneity among the studies (P = 0.43; I2 = 0%). Unweighted pooled rates of PEP were 11.7% for the PGW technique and 8.3% for the other endoscopic techniques. In this subgroup of studies, there was no statistically significant difference in the rates of PEP between the PGW technique and the other endoscopic techniques (RR 1.44, 95% CI 0.72 to 2.89; P = 0.30; Analysis 4.1). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model.
4.1. Analysis.
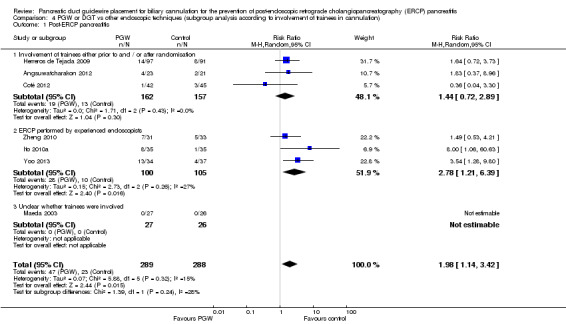
Comparison 4 PGW or DGT vs other endoscopic techniques (subgroup analysis according to involvement of trainees in cannulation), Outcome 1 Post‐ERCP pancreatitis.
Three studies with involvement of only experienced endoscopists reported the rates of PEP for all randomised participants (Ito 2010a; Yoo 2013; Zheng 2010), comprising a total of 100 participants in the PGW technique group and 105 participants in the other endoscopic techniques group. There was significant heterogeneity among the studies (P = 0.26; I2 = 27%). Unweighted pooled rates of PEP were 28.0% for the PGW technique and 9.5% for the other endoscopic techniques. In this subgroup of studies, the PGW technique significantly increased the risk of PEP compared to the other endoscopic techniques (RR 2.78, 95% CI 1.21 to 6.39; P = 0.02; Analysis 4.1). The NNTH was 6 (95% CI 2 to 50). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model.
One study did not provide information as to whether trainees were involved in the procedures (Maeda 2003). The rates of PEP were 0% in the PGW technique group and 0% for the other endoscopic technique (persistent attempts with conventional cannulation).
The test for subgroup differences indicated no statistically significant differences between the subgroups (studies with versus without trainee involvement) for the outcome of PEP (P = 0.24) (Analysis 4.1)
Overall CBD cannulation success (during the index procedure)
Three studies had involvement of trainees and reported the overall CBD cannulation success for all randomised participants (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009), comprising a total of 162 participants in the PGW technique group and 157 participants in the other endoscopic techniques group. There was significant heterogeneity among the studies (P = 0.09; I2 = 59%). Unweighted pooled rates of PEP were 75.9% for the PGW technique and 84.7% for the other endoscopic techniques. In this subgroup of studies, there was no statistically significant difference in the overall cannulation success rates between the PGW technique and the other endoscopic techniques (RR 0.91, 95% CI 0.76 to 1.09; P = 0.30; Analysis 4.2). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model.
4.2. Analysis.

Comparison 4 PGW or DGT vs other endoscopic techniques (subgroup analysis according to involvement of trainees in cannulation), Outcome 2 Overall cannulation success.
Three studies with involvement of only experienced endoscopists reported the overall CBD cannulation success for all randomised participants (Ito 2010a; Yoo 2013; Zheng 2010), comprising a total of 100 participants in the PGW technique group and 105 participants in the other endoscopic techniques group. There was no significant heterogeneity among the studies (P = 0.31; I2 = 15%). Unweighted pooled rates of PEP were 90.0% for the PGW technique and 82.9% for the other endoscopic techniques. In this subgroup of studies, there was no statistically significant difference in the overall cannulation success rates between the PGW technique and the other endoscopic techniques (RR 1.07, 95% CI 0.96 to 1.20; P = 0.24; Analysis 4.2). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model.
One study did not provide information as to whether trainees were involved in the procedures (Maeda 2003). The overall CBD cannulation success rates were 92.6% in the PGW technique group and 57.7% in the other endoscopic technique (persistent attempts with conventional cannulation) group.
The test for subgroup differences indicated no statistically significant differences between the two subgroups (studies with versus without trainee involvement) (P = 0.13), but significant subgroup differences were found with inclusion of the one study that did not provide information regarding involvement of trainees, Maeda 2003, for the outcome of overall CBD cannulation success (P = 0.02) (Analysis 4.2).
Analysis 5: PGW or DGT compared to other endoscopic techniques according to risk of bias
We considered all included studies to be at low risk of bias for incomplete outcome assessment and selective reporting, high risk of bias for blinding of participants and personnel (the endoscopists), and unclear risk of bias for outcome assessment. We therefore performed subgroup analyses according to risk of bias for random sequence generation and allocation concealment.
Random sequence generation
We considered three studies to be at low risk, Coté 2012, Herreros de Tejada 2009, and Yoo 2013, three at unclear risk, Angsuwatcharakon 2012, Ito 2010a, and Maeda 2003), and one study at high risk, Zheng 2010, of bias for random sequence generation. There was significant heterogeneity among the studies considered to be at low risk of bias for random sequence generation (P = 0.16; I2 = 46%). Among the studies considered to be at unclear risk of bias for random sequence generation, there was no significant heterogeneity (P = 0.25; I2 = 25%). Among the studies considered to be at low risk of bias for random sequence generation, there was no statistically significant difference in the rates of PEP between the PGW technique and the other endoscopic techniques (RR 1.78, 95% CI 0.70 to 4.53; P = 0.22; Analysis 5.1). In sensitivity analyses, the results remained non‐significant with OR, but became statistically significant with a fixed‐effect model (RR 1.88, 95% CI 1.05 to 3.37; P = 0.03) favouring the use of the other endoscopic techniques. In studies considered to be at unclear risk of bias for random sequence generation, there was no statistically significant difference in the rates of PEP between the PGW technique and the other endoscopic techniques (RR 3.35, 95% CI 0.77 to 14.54; P = 0.11; Analysis 5.1). In sensitivity analyses, the results remained non‐significant with OR, but became statistically significant with a fixed‐effect model (RR 3.82, 95% CI 1.15 to 12.76; P = 0.03) favouring the use of the other endoscopic techniques. In the study considered to be at high risk of bias for random sequence generation (Zheng 2010), there was no statistically significant difference in the rates of PEP between the PGW technique and the other endoscopic technique (persistent attempts with conventional cannulation) (RR 1.49, 95% CI 0.53 to 4.21; P = 0.45; Analysis 5.1).
5.1. Analysis.
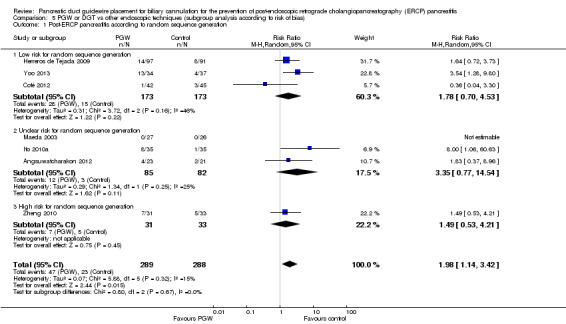
Comparison 5 PGW or DGT vs other endoscopic techniques (subgroup analysis according to risk of bias), Outcome 1 Post‐ERCP pancreatitis according to random sequence generation.
Most importantly, the test for subgroup differences indicated no statistically significant differences between the subgroups (according to risk of bias for random sequence generation) for the outcome of PEP (P = 0.48) (Analysis 5.1).
Allocation concealment
We considered four studies to be at low risk, Angsuwatcharakon 2012, Coté 2012, Herreros de Tejada 2009, and Ito 2010a, and three at unclear risk, Maeda 2003, Yoo 2013, and Zheng 2010, of bias for allocation concealment. There was significant heterogeneity among the studies considered to be at low risk of bias for allocation concealment (P = 0.24; I2 = 28%). In studies considered to be at low risk of bias for allocation concealment, there was no statistically significant difference in the rates of PEP between the PGW technique and the other endoscopic techniques (RR 1.76, 95% CI 0.72 to 4.26; P = 21; Analysis 5.2). In sensitivity analyses, the results remained non‐significant with OR or a fixed‐effect model. In studies considered to be at unclear risk of bias for allocation concealment, there was no statistically significant difference in the rates of PEP between the PGW technique and the other endoscopic techniques (RR 2.31, 95% CI 0.99 to 5.40; P = 0.05; Analysis 5.2). In sensitivity analyses, the results remained non‐significant with OR, but became statistically significant with a fixed‐effect model (RR 2.39, 95% CI 1.17 to 4.88; P = 0.02) favouring the use of the other endoscopic techniques.
5.2. Analysis.
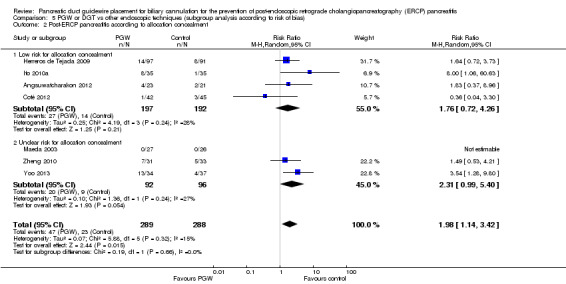
Comparison 5 PGW or DGT vs other endoscopic techniques (subgroup analysis according to risk of bias), Outcome 2 Post‐ERCP pancreatitis according to allocation concealment.
Most importantly, the test for subgroup differences indicated no statistically significant differences between the subgroups (according to risk of bias for allocation concealment) for the outcome of PEP (P = 0.66) (Analysis 5.2).
Analysis 6: PGW or DGT compared to other endoscopic techniques according to publication type
All six studies published in full text reported PEP for all randomised participants (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013), comprising a total of 258 participants in the PGW technique group and 255 in the other endoscopic techniques group. There was significant heterogeneity among the studies (P = 0.24; I2 = 27%). Unweighted pooled rates of PEP were 15.5% for the PGW technique and 7.1% for the other endoscopic techniques. The PGW technique significantly increased PEP compared to the other endoscopic techniques (RR 2.14, 95% CI 1.06 to 4.33; P = 0.03; Analysis 6.1). The NNTH was 12 (95% CI 4 to 236). In sensitivity analyses, the results remained robust with OR or a fixed‐effect model. In the study that was published in abstract format (Zheng 2010), there was no statistically significant difference in the rates of PEP between the PGW technique and the other endoscopic technique (persistent attempts with conventional cannulation) (RR 1.49, 95% CI 0.53 to 4.21; P = 0.45; Analysis 6.1).
6.1. Analysis.

Comparison 6 PGW or DGT vs other endoscopic techniques (subgroup analysis according to publication type), Outcome 1 Post‐ERCP pancreatitis.
Most importantly, the test for subgroup differences indicated no statistically significant differences between the subgroups (according to publication type) for the outcome of PEP (P = 0.57) (Analysis 6.1).
Discussion
Difficult cannulation has been recognised as an independent risk factor for PEP (Cheng 2006; Freeman 2001). When deep biliary cannulation fails with conventional cannulation techniques (contrast‐ or guidewire‐assisted cannulation), advanced techniques such as precut sphincterotomy, the PGW technique or DGT, and the insertion of PD stent are often used to facilitate biliary access. Among the advanced techniques, precut sphincterotomy is most often used as a rescue technique to achieve selective biliary cannulation (Freeman 2005; Testoni 2011). However, the precut technique has been reported to be associated with an increased risk of complications including PEP (Cennamo 2010; Freeman 2001; Masci 2003). Recently, the PGW technique or DGT has been proposed as an alternative to precut sphincterotomy in cases of difficult CBD cannulation to facilitate selective bile duct cannulation (Testoni 2011). However, it remains controversial whether the PGW technique can reduce the risk of PEP and improve biliary cannulation success compared to persistent attempts with conventional cannulation or other advanced techniques. The primary objective of this systematic review and meta‐analysis was to assess the clinical effectiveness and safety of the PGW technique or DGT compared to persistent attempts with conventional cannulation techniques (contrast‐ or guidewire‐assisted) or other advanced techniques in people with difficult cannulation.
Summary of main results
We included seven RCTs in this meta‐analysis (Angsuwatcharakon 2012; Coté 2012; Herreros de Tejada 2009; Ito 2010a; Maeda 2003; Yoo 2013; Zheng 2010): three comparing the PGW technique with persistent attempts with conventional cannulation techniques (Herreros de Tejada 2009; Maeda 2003; Zheng 2010); two comparing the PGW technique with precut sphincterotomy (Angsuwatcharakon 2012; Yoo 2013); and two comparing the PGW technique with insertion of a PD stent (Coté 2012; Ito 2010a). We found the quality of evidence for all outcomes to be low, primarily due to the risk of bias and imprecise results due to few events.
Post‐ERCP pancreatitis
PEP is the primary outcome of this systematic review. Overall, we found that the PGW technique significantly increased the risk of PEP compared to other endoscopic techniques (persistent attempts with conventional cannulation, precut sphincterotomy, PD stent insertion) in people with difficult cannulation (NNTH = 13). This finding was robust in all sensitivity analyses. We found no statistically significant subgroup differences related to the type of endoscopic technique used (persistent attempts with conventional cannulation, precut sphincterotomy, PD stent insertion) with the PGW technique as the common comparator technique. Due to the observational nature of subgroup analyses, small sample sizes of individual studies, and differences in study designs, the results of the subgroup analysis should be interpreted cautiously. The lack of significant heterogeneity in this analysis should also be interpreted with caution. With only a few included studies, the I2 test provides little power to reject the null hypothesis of homogeneity (I2 = 0%) even if substantial heterogeneity is present (Ioannidis 2007a). Indeed, the 95% confidence interval for the I2 estimate of this analysis extends from 0% (no heterogeneity) to 66.5% (moderate heterogeneity). Additionally, all included studies had small sample sizes with wide overlapping confidence intervals of their effect estimates. Considerable heterogeneity between studies cannot therefore be excluded with confidence for this analysis. Nevertheless, the current evidence from randomised trials does not support the use of the PGW technique for the prevention of PEP in people with difficult cannulation.
Severity of post‐ERCP pancreatitis
The severity of PEP is an important clinical outcome as it correlates with mortality, complications, and length of hospital stay. We found that the PGW technique significantly increased the risk of mild PEP compared to other endoscopic techniques (persistent attempts with conventional cannulation, precut sphincterotomy, PD stent insertion) in people with difficult cannulation. In contrast, we found no statistically significant difference in the rates of moderate or severe PEP between the PGW technique and other endoscopic techniques. However, low event rates for moderate and severe PEP may have led to inadequate power to detect clinically important differences between the PGW technique and other endoscopic techniques.
CBD cannulation success with the randomised technique
CBD cannulation success with the randomised technique (before the use of rescue techniques) is an important indicator of the effectiveness of the technique in gaining biliary access. A high CBD cannulation success with the randomised technique reduces the risk of repeated cannulation attempts and further trauma to the papillary orifice/pancreatic duct. We found no significant difference in the rates of CBD cannulation success with the randomised technique between the PGW technique and other endoscopic techniques (persistent attempts with conventional cannulation, precut sphincterotomy, PD stent insertion) in people with difficult cannulation.
Overall CBD cannulation success
Overall cannulation success is an important outcome as failed procedures usually necessitate repeat ERCP, or a radiological or surgical procedure, which carry additional costs and risks (Perdue 2004). We found no statistically significant difference in the overall cannulation success rates between the PGW technique and other endoscopic techniques (persistent attempts with conventional cannulation, precut sphincterotomy, PD stent insertion) in people with difficult cannulation.
ERCP‐related complications
With regard to safety endpoints, there was no statistically significant difference in the risk of postsphincterotomy bleeding, perforation, or cholangitis between the PGW technique and other endoscopic techniques (persistent attempts with conventional cannulation, precut sphincterotomy, PD stent insertion) in people with difficult cannulation. Mortality appeared to be very low.
Summary of findings on subgroup analyses
All subgroup analyses indicated no statistically significant differences in the outcomes of PEP or overall CBD cannulation success, or both between the PGW technique and other endoscopic techniques. However, the results of the subgroup analyses should not be interpreted as definitive conclusions since they are observational by nature and are not based on randomised comparisons. Furthermore, the number of studies or sample size, or both was small, which may have limited our power to detect important differences.
Overall completeness and applicability of evidence
This systematic review and meta‐analysis was designed to include trials from around the world comparing the PGW technique or DGT with all other endoscopic techniques regardless of publication status or language of publication. All studies identified by the search could be retrieved in full. Moreover, we were able to obtain unpublished data from authors of the primary studies (Coté 2012; Herreros de Tejada 2009; Yoo 2013; Zheng 2010). Hence, we believe this review is comprehensive, and the results reflect the best available evidence for the use of the PGW technique or DGT for the prevention of PEP in people with difficult cannulation.
It is important to note that all studies defined PEP as a rise in serum amylase level to greater than or equal to three‐fold above the upper limit of normal 24 hours after ERCP accompanied by abdominal pain characteristic of pancreatitis according to the consensus definition (Cotton 1991).
The participants included in this meta‐analysis had intact papilla and underwent ERCP for a variety of pancreaticobiliary diseases, most commonly CBD stones and pancreaticobiliary malignancies. Only a small proportion of participants had sphincter of Oddi dysfunction (2.6%) or a history of acute or chronic pancreatitis (10.6%). Additionally, all included participants were considered to have "difficult" biliary cannulation by the primary studies. In general, difficult cannulation is a situation where the endoscopist, using conventional cannulation techniques (contrast‐ or guidewire‐assisted cannulation), fails within a certain time limit or after a certain number of unsuccessful attempts to achieve biliary access (Freeman 1996; Testoni 2011; Udd 2010). Yet, difficult biliary cannulation is a subjective term, and can be difficult to define. There is currently no established time limit or limits to unsuccessful attempts before the cannulation is termed difficult (Udd 2010). As in real‐world practice, studies have used highly variable definitions of difficult cannulation. Consequently, the proportions of people with difficult cannulation were highly variable among the included studies, ranging from 4.8%, in Ito 2010a, to 49.5%, in Maeda 2003. In an attempt to standardise the definition of difficult biliary cannulation, the recent European Society of Gastrointestinal Endoscopy (ESGE) guideline proposed that future studies should define difficult biliary cannulation in an intact papilla as any of the following: cannulation attempts of a duration of more than 5 minutes, more than five attempts, or two pancreatic guidewire passages (Dumonceau 2010; Dumonceau 2014). However, this definition has not been widely adopted. In keeping with real life and generalisability, we accepted any definition of difficult cannulation adopted by the primary studies.
All studies were conducted in high‐volume tertiary‐care settings. Procedures were performed by either a single or multiple experienced endoscopists, with or without the involvement of trainees prior to randomisation (that is use of the PGW technique). The generalisability of findings to low‐volume centres with less expertise in ERCP may therefore be limited.
It is important to note that successful placement of a guidewire into the PD was a requirement for enrolment in only two studies (Ito 2010a; Yoo 2013). Arguably, the PGW technique or DGT may not be achievable for people who have unfavourable pancreatic anatomy such as pancreatic ductal obstructions (for example due to malignancy, chronic pancreatitis), pancreas divisum, or tortuous main PD. The PGW technique or DGT is certainly not possible when the endoscopist fails to achieve selective PD cannulation. It is uncertain how including people who had unfavourable anatomy for the PGW technique or DGT may have impacted the results of the studies. However, it is conceivable that repeated attempts at achieving selective PD cannulation in order to carry out the PGW technique or DGT may lead to more papillary trauma and hence increased risk of PEP. Alternative techniques should probably be considered to achieve biliary cannulation in these people.
Prophylactic PD stenting has been shown to reduce the risk of PEP in high‐risk patients, including patients with difficult cannulation (Choudhary 2011; Mazaki 2010). However, the exact indications for PD stenting have not been thoroughly elucidated (Dumonceau 2010), and it remains uncertain whether prophylactic PD stenting is necessary after the use of the PGW technique or DGT (Choudhary 2011; Freeman 2005; Mazaki 2010). Nevertheless, its use was recommended for people at high risk of PEP (Dumonceau 2010), including those with difficult cannulation (Freeman 2012). Because the PGW technique or DGT is usually attempted in such a patient group, PD stenting would appear to be a logical approach for the prevention of PEP in this setting. Yet, prophylactic PD stenting was not used in most studies that did not have PD stent as a comparative arm (Angsuwatcharakon 2012; Herreros de Tejada 2009; Maeda 2003; Yoo 2013; Zheng 2010). In the one study that specifically evaluated the prophylactic effect of PD stenting in people with difficult cannulation who underwent the PGW technique or DGT (Ito 2010a), PD stenting appeared to reduce the risk of PEP (2.9% versus 23%; RR 0.13, 95% CI 0.02 to 0.95). Based on the limited evidence to date, PD stenting should probably be considered for people with difficult cannulation who underwent the PGW technique or DGT. None of the included studies reported the use of rectally administered non‐steroidal anti‐inflammatory drugs (NSAIDs), therefore it is uncertain how prophylactic use of rectally administered NSAIDs may impact the risk of PEP in people who underwent the PGW technique or DGT.
Quality of the evidence
Overall, the quality of evidence for the outcome of PEP was low because of study limitations and imprecision. We assessed none of the included studies to be at low risk of bias for all domains. Most information was obtained from studies at high risk of bias for blinding of participants and personnel (the endoscopists). Endoscopists cannot be blinded. Lack of blinding of the endoscopist may have an impact on PEP and cannulation success, depending on the experience, expertise, preference, and perseverance of the endoscopist performing the procedure. Furthermore, none of the studies reported blinding of outcome assessors. We judged random sequence generation to be adequate in three studies, unclear in three studies, and inadequate in one study. We judged allocation concealment to be adequate in four studies and unclear in three studies. Taken together, the limitations in the design and implementation of available studies suggest a high likelihood of bias. These was no significant heterogeneity for the outcome of PEP, although this should be interpreted with caution due to the small number of included studies. There was no indirectness of evidence, as the included studies assessed the appropriate population, intervention, comparisons, and outcomes. The results of the main analysis for the outcome of PEP appeared to be imprecise with wide confidence intervals.
The quality of evidence for the secondary outcomes of severity of PEP and ERCP‐related complications (bleeding, cholangitis, perforation, and mortality) was low because of study limitations and imprecision. For the secondary outcomes of CBD cannulation success with the randomised technique and overall CBD cannulation, the quality of evidence was low due to study limitations and significant heterogeneity.
Potential biases in the review process
We explored small‐study effects (a trend for the smaller studies in a meta‐analysis to show larger treatment effects), of which publication bias is one potential cause, using funnel plots (Figure 4). However, application of funnel plot asymmetry tests to detect publication bias was inappropriate or not meaningful for this review because only seven studies were included for the outcome of PEP in the main analysis (Ioannidis 2007b).
4.
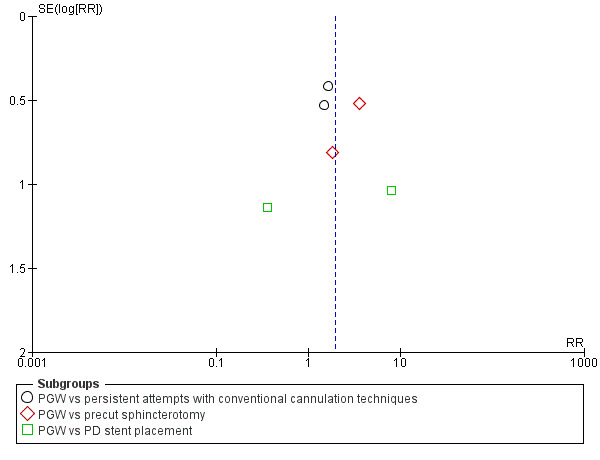
Funnel plot of comparison: 1 Pancreatic duct guidewire (PGW) vs control, main analysis, outcome: 1.1 Post‐ERCP pancreatitis.
A potential limitation of this review is the highly variable definitions of difficult cannulation used by the included studies. The heterogeneity of criteria used to define difficult cannulation may make direct comparisons of these trials difficult. However, the definition of difficult cannulation still remains a controversial issue (Freeman 1996; Testoni 2011; Udd 2010). There is currently no established time limit or limits to unsuccessful attempts before the cannulation is termed difficult (Udd 2010). The ESGE guideline's proposed definition of difficult cannulation of cannulation attempts of a duration of more than 5 minutes, more than five attempts, or two pancreatic guidewire passages has yet to be widely adopted (Dumonceau 2010; Dumonceau 2014). Nevertheless, the definitions used by all the included studies in this meta‐analysis would satisfy the criteria for difficult cannulation put forth by the ESGE guideline (Dumonceau 2010; Dumonceau 2014).
Another limitation of this review is the small number of studies per subgroup of comparators for the main outcome of PEP (N = 3 for persistent attempts with conventional cannulation techniques; N = 2 for precut sphincterotomy; N = 2 for PD stent). This may have limited our power to detect differences among subgroups. Nonetheless, the results of this meta‐analysis suggest that the PGW technique significantly increased the risk of PEP compared to other endoscopic techniques (persistent attempts with conventional cannulation, precut sphincterotomy, PD stent insertion) when considered together.
We included two studies for the subgroup of PD stent placement for the main outcome of PEP (Coté 2012; Ito 2010a): one study compared the PGW technique versus PD stent placement (Coté 2012), and the other study compared PD stent placement versus no PD stent placement in people who had undergone the PGW technique (Ito 2010a). Although the designs of the these two studies appeared to be different in that the PD stent was intended to facilitate biliary cannulation in the study by Cote et al (Coté 2012), whereas the PD stent was intended to maintain PD drainage postprocedure in the study by Ito et al (Ito 2010a), we decided to combine them for analyses due to the fact that placement of a PD stent would require deep PD guidewire placement anyway (whether to facilitate biliary cannulation or maintain PD drainage postprocedure, or both). The PGW technique would therefore have to be applied regardless of the intended use for the PD stent. Furthermore, the results of the PD stent placement would be the same (that is providing PD drainage) irrespective of its primary intended purpose (facilitating biliary cannulation or maintaining PD drainage postprocedure). Post‐hoc analysis excluding these two studies did not change the results for the main outcome of PEP (Coté 2012; Ito 2010a).
Agreements and disagreements with other studies or reviews
A systematic and comprehensive literature search yielded no other systematic reviews of the PGW technique or DGT for the prevention of PEP. The recent ESGE guideline, in Dumonceau 2010 and Dumonceau 2014, includes a couple of specific recommendations about the use of the PGW technique in cases of difficult cannulation that are partially supported by the evidence provided in this systematic review. They are as follows:
In cases of difficult biliary cannulation, PGW placement allows biliary cannulation in a proportion of cases similar to persistence in attempting cannulation with standard cannulation techniques (or precut if it is used as a backup technique), but the risk of PEP is likely higher. In such circumstances, PEP is effectively prevented by prophylactic pancreatic stenting.
The PGW technique should be restricted as a backup technique to cases with repeated inadvertent cannulation of the PD; if this method is used, deep biliary cannulation should be attempted using a guidewire rather than the contrast‐assisted method, and a prophylactic pancreatic stent should be placed.
Our systematic review found that the PGW technique significantly increased the risk of PEP compared to other endoscopic techniques (persistent attempts with conventional cannulation, precut sphincterotomy, PD stent insertion) in people with difficult cannulation. It is important to note that the ESGE recommendation of the use of PD stenting with the PGW technique to reduce the risk of PEP in cases of difficult cannulation is supported by only one RCT (Ito 2010a), which was specifically designed to address this question. Also, the effect of prophylactic rectal NSAIDs in combination with the PGW technique has not been assessed by prospective RCTs. It therefore remains uncertain what prophylactic measures (endoscopic or pharmacologic, or both) are necessary for the prevention of PEP when the PGW technique is used. Our previous systematic review provided evidence to support the use of the guidewire‐assisted cannulation technique as a first‐line primary cannulation technique as it significantly reduced the risk of PEP compared to the contrast‐assisted cannulation technique in unselected patients undergoing ERCP (Tse 2012). However, these two cannulation techniques have not been formally compared in combination with the PGW technique in people with difficult cannulation by prospective RCTs.
Authors' conclusions
Implications for practice.
Difficult cannulation has been identified as an independent procedure‐related risk factor for PEP (Cheng 2006; Freeman 2001). In such cases, endoscopists may choose to persist with conventional cannulation (contrast‐ or guidewire‐assisted) techniques. Alternatively, various advanced techniques (for example precut sphincterotomy, the PGW technique or DGT, PD stent placement, endoscopic ultrasound‐guided rendezvous technique) have been developed to facilitate biliary access and minimise the risk of PEP. Contrary to popular belief, evidence from this systematic review indicates that, compared with other endoscopic techniques (persistent attempts with conventional cannulation, precut sphincterotomy, PD stent insertion), the PGW technique not only increases the risk of PEP, it also does not appear to improve cannulation success. The increased risk of PEP may be due to irritation or injury to the PD, or both, by leaving the guidewire deep in place for prolonged times during the procedure. The use of PD stenting may reduce the risk of PEP when the PGW technique is used, however more studies are needed to confirm this finding. Furthermore, the PGW technique may not be achievable for patients who have unfavourable pancreatic anatomy such as pancreatic ductal obstructions (for example malignancy, chronic pancreatitis), pancreas divisum, or a tortuous main PD. The lack of patient‐level data also precluded us from identifying any subgroup(s) of patients where there may be a clear net benefit of the PGW technique (for example intradiverticular papilla, floppy papilla) over other endoscopic techniques. The quality of evidence for these outcomes was predominantly low. Further research is therefore very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Finally, taking into account external evidence from beyond this review regarding the time and cost of this procedure, it is possible that the use of a second guidewire with the PGW technique may increase the overall procedural time and cost without providing benefits in terms of biliary access or reducing the risks of PEP.
Implications for research.
This review has highlighted the need for further research on the optimal endoscopic interventions for the prevention of PEP in people with difficult cannulation:
Standardised definitions are important for adequate communication in clinical practice and for research. The ESGE definition for difficult cannulation has not been adopted uniformly (Dumonceau 2010; Dumonceau 2014), and studies have used different criteria to define difficult cannulation based on number or time limits of cannulation attempts, or both. This wide variation in definitions of difficult cannulation make direct comparisons of results across studies difficult. Future studies should incorporate a standardised definition of difficult cannulation to facilitate the evaluation of safety and effectiveness of endoscopic procedures.
In people with difficult cannulation, sole use of the PGW technique appears to be associated with an increased risk of PEP. Prophylactic PD stenting may reduce the risk of PEP when the PGW technique is used. Nevertheless, more studies are needed to assess the effectiveness of the PGW technique in combination with PD stenting or rectal NSAIDs, or both compared with other endoscopic techniques for the prevention of PEP in this patient population. Future studies should also include data on cost and resource utilisation.
There are many possible causes of difficult cannulation (for example peri‐ampullary diverticulum, peri‐ampullary tumour, papillary stenosis, small or large floppy papilla, variation in ductal orientation, altered anatomy, etc.). It is unlikely that the effects of the PGW technique or the risks of PEP are uniform across this patient population. There may be specific subgroups of this patient population who may benefit from this technique. Future studies should consider effects of the PGW technique among subgroups of patients with difficult cannulation. However, these subgroups may not be easy to define, and large, multicentre studies are undoubtedly required to obtain statistically significant results.
What's new
| Date | Event | Description |
|---|---|---|
| 17 May 2016 | Amended | A row (severity of PEP) was removed from the 'Summary of Findings' table as it wasn't a formal subgroup analysis. |
Acknowledgements
We would like to acknowledge the following authors for kindly providing additional data on their trials: Dr Sang‐Woo Cha (Soonchunhyang University Hospital, Seoul, Korea), Dr Gregory A Cote (Washington University School of Medicine, St. Louis, USA), Dr Alberto Herreros de Tejada (Puerta de Hierro University Hospital, Madrid, Spain), and Dr Feng‐Ping Zheng (Sun Yat‐Sen University, Guangzhou, China).
We thank Karin Dearness, Managing Editor, Cochrane Upper Gastrointestinal and Pancreatic Diseases (UGPD) Group, for providing administrative and logistical support for the conduct of the current review.
Appendices
Appendix 1. Glossary
Air insufflation: The introduction of air into a body cavity.
Amylase: An enzyme produced in the pancreas that helps in the digestion of starches.
Asymptomatic: Showing no symptoms of a condition.
Cannulation: The insertion of a small tube into a body cavity, duct, or vessel.
Catheter: A tubular medical device used for insertion into body channels such as vessels or ducts for injection or withdrawal of fluids for diagnostic or therapeutic purposes.
Cholangitis: Infection of the bile ducts.
Concomitant: Co‐existing or accompanying.
Contrast dye: A medical contrast medium (or X‐ray dye) used to enhance the contrast of structures or fluids within the body in medical imaging.
Duct: A tube in the body carrying the secretion or excretion of a gland. For example, pancreatic duct carries the secretion of the pancreas to the intestines. Bile duct carries the secretion of the liver or gallbladder (bile juices) to the intestines.
Duodenum: The first part of the small intestine.
Endoscope: An endoscope (lighted tube) is an optical instrument that allows the doctor to look inside the body, such as the oesophagus, stomach, or duodenum. It is introduced into the body through a natural opening such as the mouth or anus.
Esophagus: Part of the digestive tract through which food passes from the back of the throat to the stomach.
Fluoroscopy: A machine that uses X‐ray to produce real‐time video images.
Guidewire: A thin, usually flexible wire used to guide a larger medical device or prosthesis, such as a catheter, to a desired treatment location within the body.
Hepatobiliary: Involving the liver and bile ducts.
Hydrostatic: Pressures exerted by fluids.
Hyperamylasaemia: High levels of amylase.
Incision: Surgical cut.
Lipase: An enzyme produced by the pancreas to digest fat.
Microbiological injury: Injury caused by bacteria.
Morbidity: Illness.
Mortality: Death.
Multifactorial: Having many causes.
Oesophagus: Part of the digestive tract through which food passes from the back of the throat to the stomach.
Opacification: The process of becoming opaque for X‐ray examination.
Necrosis: Death of tissue.
Neoplasia: Tumour formation.
Papilla: Opening of the bile and pancreatic ducts located in the small intestine.
Pathogenesis: The chain of events leading to a disease.
Pathophysiological: The physiology of abnormal or diseased states.
Percutaneous transhepatic biliary drainage: Drainage of the obstructed biliary tree by the introduction of a catheter through the liver and into the biliary tree under radiological guidance.
Peri‐ampullary diverticulum: An abnormal sac or pouch formed at or around the opening of the bile and pancreatic ducts.
Pharmacological: Drug‐related.
Proteolytic enzymes: Any enzyme that catalyses the splitting of proteins into smaller peptide fractions and amino acids.
Retrograde: Going backward.
Sphincter of Oddi: The valve that controls the flow of digestive juices through the opening of the bile and pancreatic ducts.
Sphincterotome: A special catheter inserted into the bile duct or pancreatic duct to perform endoscopic retrograde cholangiopancreatography.
Stenotic: Narrowed.
Stent: A small plastic tube.
Thermal: Energy that is generated by heat.
Appendix 2. CENTRAL search strategy
Via Wiley Cochrane Library Online
pancreatitis (Word variations have been searched)
MeSH descriptor: [Pancreatitis] explode all trees
(#1 or #2)
MeSH descriptor: [Cholangiopancreatography, Endoscopic Retrograde] explode all trees
MeSH descriptor: [Sphincterotomy, Endoscopic] explode all trees
(endoscop* near sphincterotom*) (Word variations have been searched)
(endoscop* near retrograde near (cholangio‐pancreatograph* or cholangiopancreatograph*)) (Word variations have been searched)
(ERCP or EST) (Word variations have been searched)
(papillotom* or rendezvous) (Word variations have been searched)
(#4 or #5 or #6 or #7 or #8 or #9)
(#3 and #10)
(guidewire* or wireguid* or guided‐wire* or guide‐wire* or wire‐guid*) (Word variations have been searched)
((guide or guided or guid*) and (wire or wired)) (Word variations have been searched)
#12 or #13
#11 and #14
Appendix 3. MEDLINE search strategy
Via Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1946 to Present>
ERCP.mp. or exp endoscopic retrograde cholangiopancreatography/
(endoscop* adj2 retrograd* adj2 (cholangiopancreatograph* or cholangio‐pancreatograph*)).mp.
exp Sphincterotomy, Endoscopic/
((endoscop* adj3 sphincterotom*) or EST).tw,kw.
papillotom*.tw,kw. or exp papillotomy/
rendezvous.tw,kw.
or/1‐6
exp Pancreatitis/
pancreatitis.mp.
complications.ti,ab.
or/8‐10
7 and 11
(guidewir* or wireguid* or guided‐wir* or guide‐wir* or wire‐guid*).tw,kw.
(guid* and wir*).tw,kw.
(PDW or PGW or DGW or DGT).ti,ab.
or/13‐15
12 and 16
randomized controlled trial.pt.
controlled clinical trial.pt.
random*.mp.
trial.ab.
groups.ab.
or/18‐22
17 and 23
exp animals/ not humans/
24 not 25
Appendix 4. EMBASE search strategy
Via Ovid
ERCP.mp. or exp endoscopic retrograde cholangiopancreatography/
(endoscop* adj2 retrograd* adj2 (cholangiopancreatograph* or cholangio‐pancreatograph*)).mp.
exp endoscopic sphincterotomy/
((endoscop* adj3 sphincterotom*) or EST).tw,kw.
papillotom*.tw,kw. or exp endoscopic papillotomy/
rendezvous.tw,kw.
or/1‐6
exp Pancreatitis/
pancreatitis.mp.
complications.ti,ab.
or/8‐10
7 and 11
(guidewir* or wireguid* or guided‐wir* or guide‐wir* or wire‐guid*).tw,kw.
(guid* and wir*).tw,kw.
(PDW or PGW or DGW or DGT).ti,ab.
or/13‐15
12 and 16
random*.mp.
clinical trial:.mp.
exp health care quality/
double‐blind*.mp.
blind*.tw.
or/18‐22
17 and 23
exp animal/ not human/
24 not 25
Appendix 5. CINAHL search strategy
Via EBSCOhost
MH "Cholangiopancreatography, Endoscopic Retrograde"
TX endoscop* AND retrograd* AND (cholangiopancreatography OR cholangio‐pancreatography)
TX ERCP
TX (endoscop* AND sphincterotom*) OR EST
TX papillotom* OR TX rendezvous
1 or 2 or 3 or 4 or 5
(MH "Pancreatitis+") OR TX pancreatitis
6 and 7
TX guidewir* or wireguid* or guided‐wir* or guide‐wir* or wire‐guid*
TX (guid* and wir*)
TX PDW or PGW or DGW or DGT
9 or 10 or 11
8 and 12
(MH "Randomized Controlled Trials") or TX random*
13 and 14
Data and analyses
Comparison 1. Pancreatic duct guidewire (PGW) or double guidewire technique (DGT) vs other endoscopic techniques.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis | 7 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.14, 3.42] |
| 1.1 PGW vs persistent attempts with conventional cannulation techniques | 3 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.83, 3.01] |
| 1.2 PGW vs precut sphincterotomy | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 2.92 [1.24, 6.88] |
| 1.3 PGW vs PD stent placement | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.08, 37.50] |
| 2 Severity of post‐ERCP pancreatitis | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Mild PEP | 6 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 2.70 [1.27, 5.76] |
| 2.2 Moderate PEP | 6 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.27, 3.38] |
| 2.3 Severe PEP | 6 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.17, 20.34] |
| 3 CBD cannulation success with the randomised technique (before the use of rescue techniques) | 7 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.87, 1.24] |
| 4 Overall cannulation success | 7 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.91, 1.18] |
| 5 Post‐ERCP bleeding | 6 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.13, 1.79] |
| 6 Post‐ERCP perforation | 6 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 14.78] |
| 7 Post‐ERCP cholangitis | 4 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 2.71 [0.79, 9.35] |
| 8 Mortality | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.58] |
Comparison 2. PGW or DGT vs other endoscopic techniques (subgroup analysis according to trial design).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis | 7 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.14, 3.42] |
| 1.1 Studies that permitted the use of rescue techniques | 4 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.72, 4.26] |
| 1.2 Studies that did not permit the use of rescue techniques | 3 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.99, 5.40] |
| 2 Overall CBD cannulation success | 7 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.91, 1.18] |
| 2.1 Studies that permitted the use of rescue techniques | 4 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.16] |
| 2.2 Study that did not permit the use of rescue techniques | 3 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.89, 1.54] |
Comparison 3. PGW or DGT vs other endoscopic techniques according to the use of a PD stent as a rescue technique, excluding studies that used PD stent as a comparative arm (subgroup analysis according to the use of PD stent).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis | 5 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [1.06, 3.20] |
| 1.1 Studies that did not permit the use of PD stent | 4 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.96, 4.29] |
| 1.2 Studies that permitted the use of PD stent | 1 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.72, 3.73] |
Comparison 4. PGW or DGT vs other endoscopic techniques (subgroup analysis according to involvement of trainees in cannulation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis | 7 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.14, 3.42] |
| 1.1 Involvement of trainees either prior to and / or after randomisation | 3 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.72, 2.89] |
| 1.2 ERCP performed by experienced endoscopists | 3 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [1.21, 6.39] |
| 1.3 Unclear whether trainees were involved | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Overall cannulation success | 7 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.91, 1.18] |
| 2.1 Involvement of trainees either prior to and / or after randomisation | 3 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.76, 1.09] |
| 2.2 ERCP performed by experienced endoscopists | 3 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.96, 1.20] |
| 2.3 Unclear whether trainees were involved | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.14, 2.27] |
Comparison 5. PGW or DGT vs other endoscopic techniques (subgroup analysis according to risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis according to random sequence generation | 7 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.14, 3.42] |
| 1.1 Low risk for random sequence generation | 3 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.70, 4.53] |
| 1.2 Unclear risk for random sequence generation | 3 | 167 | Risk Ratio (M‐H, Random, 95% CI) | 3.35 [0.77, 14.54] |
| 1.3 High risk for random sequence generation | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.53, 4.21] |
| 2 Post‐ERCP pancreatitis according to allocation concealment | 7 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.14, 3.42] |
| 2.1 Low risk for allocation concealment | 4 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.72, 4.26] |
| 2.2 Unclear risk for allocation concealment | 3 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.99, 5.40] |
Comparison 6. PGW or DGT vs other endoscopic techniques (subgroup analysis according to publication type).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐ERCP pancreatitis | 7 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.14, 3.42] |
| 1.1 Full text | 6 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.06, 4.33] |
| 1.2 Abstract | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.53, 4.21] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Angsuwatcharakon 2012.
| Methods | Single‐centre RCT with 1 expert endoscopist in Thailand. Trainees were involved prior to randomisation | |
| Participants | Consecutive patients aged > 15 yrs with native papilla undergoing ERCP in whom cannulation of the CBD failed with conventional contrast‐assisted cannulation techniques. Excluded patients with altered anatomy of the stomach, ampulla, obstructive PD, and recent pancreatitis. Difficult cannulation was defined as the inability to cannulate the CBD after 5 minutes by trainees followed by 10 minutes by the expert endoscopist (total of 15 minutes) | |
| Interventions | PGW placement (double guidewire technique) vs precut sphincterotomy (freehand fistulotomy technique). 1. PGW: A catheter was pre‐inserted with a 0.035" guidewire (Jagwire; Boston Scientific) to facilitate PD cannulation. No contrast was injected into the PD. After the first guidewire was inserted and left in the PD, the catheter was exchanged and loaded with the second guidewire. The catheter was reinserted into the scope via the same working channel along the first guidewire. The direction of biliary cannulation was aimed to 10‐11 o’clock position, with a left, upward relation to the first guidewire. Successful cannulation was achieved after the second guidewire was left and lateral to the first one. This was confirmed by either a positive bile aspiration or a successful cholangiogram. 2. Precut sphincterotomy: A MicroKnife XL (Boston Scientific) was used to dissect the ampullary mucosa in a freehand fistulotomy fashion. PD stent was not used for prophylaxis of PEP |
|
| Outcomes | Biliary cannulation success, cannulation time, post‐ERCP serum amylase level, and complications including PEP, immediate bleeding, delayed bleeding, perforation, and cholangitis | |
| Notes | 1.'Cross‐over' design: If biliary cannulation was not achieved by the assigned technique in another 10 minutes, the other method was performed. 6 participants cross over from PGW to precut (3 inability to cannulate the PD, 3 after achieving deep PD cannulation). 4 participants in the precut group cross over to the PGW group. 2. Diagnosis for PEP was based on the consensus criteria (pancreatitis‐type abdominal pain and a serum amylase level over 3 times the upper normal limit that lasted more than 24 hours after the procedure) or pain and evidence of acute pancreatitis by computed tomography scan or magnetic resonance imaging of the abdomen. Hyperamylasaemia without abdominal pain was not recorded as PEP. Severity of pancreatitis was based on the consensus criteria (Cotton 1991) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how the randomisation list was generated. "Eligible patients were randomised by a simple randomised technique that was generated outside of the endoscopy unit" |
| Allocation concealment (selection bias) | Low risk | "The ERCP team did not know the sequence of randomisation until they declared such patients to be a case of truly difficult cannulation, and then the enveloped code was broken" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Endoscopists could not be blinded. Unclear whether participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors were blinded. All participants were admitted to the hospital for at least 24 hours to observe for post‐ERCP complications. Serum amylase concentration was measured at 24 hours after ERCP regardless of the presence or absence of abdominal pain. As a result, participants may be more likely to undergo laboratory and radiological evaluation as opposed to being discharged home following ERCP. If outcome assessors were not blinded, this could lead to differential detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up appeared to be complete |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes |
| Other bias | Low risk | No other risk of bias |
Coté 2012.
| Methods | Multicentre (2 sites) RCT with 6 expert endoscopists in USA. Trainees were involved prior to randomisation | |
| Participants | People with native papilla undergoing ERCP in whom cannulation of the CBD failed with conventional guidewire‐assisted (preferably) or contrast‐assisted cannulation techniques. Excluded people with suspected SOD, indications of endoscopic pancreatic therapeutics, unwilling or unable to provide informed consent, and postoperative anatomy. Difficult cannulation was defined as failure by the expert endoscopist to achieve biliary cannulation within 6 minutes or inadvertent cannulation or injection of the PD 3 consecutive times. If a trainee was involved, the expert endoscopist was given an additional 6 minutes or 3 inadvertent manipulations of the PD | |
| Interventions | PGW placement (double guidewire technique) vs PD stent placement followed by cannulation of the CBD with guidewire‐assisted technique. 1. PGW: A 0.025” or 0.035” guidewire was left in the PD. The depth of wire insertion was not prespecified, but ideally left beyond the genu whenever possible. Alongside the guidewire, the endoscopist used a sphincterotome preloaded with a second guidewire (0.025" or 0.035") to cannulate the bile duct using preferably guidewire‐assisted cannulation technique. 2. PD stent: A guidewire (0.025" or 0.035") was left in the mid‐body of the pancreas to facilitate stent placement. The type of stent was left to the discretion of the endoscopist with either a 4‐ or 5‐Fr stent (2 to 9 cm) in length with an external pigtail and single internal flange (Freeman pancreatic stent; Hobbs Medical Inc) or a 5‐Fr stent with a double external and single internal flange (Geenen pancreatic stent; Cook Medical). Guidewire‐assisted technique was then used to cannulate the CBD |
|
| Outcomes | Biliary cannulation success within 6 minutes, the use of precut sphincterotomy, cannulation time, and complication rates including PEP, bleeding, and cholangitis | |
| Notes | 1. 'Cross‐over' design: For the PGW group, if deep cannulation was not achieved after 6 minutes (starting from the time of first attempt after the PD wire was in position), the participant crossed over to the PD stent group. If CBD cannulation was not achieved after cross‐over and an additional 6 minutes, the endoscopists could persist in their efforts with or without performing a precut sphincterotomy. The decision to terminate the procedure was left to the treating physician. If deep PD cannulation could not be achieved after a minimum of 6 minutes after randomisation, freehand needle‐knife sphincterotomy was allowed. For the PD stent group, if deep cannulation was not achieved after 6 minutes (starting from the deployment of the PD stent), the endoscopist was allowed to persist in their efforts with or without performing a precut sphincterotomy. After 6 minutes, the decision to terminate the procedure was left to the treating physician. In total, 9 participants crossed over from the PGW group to the PD stent group. 2. Diagnostic criteria for PEP was not provided in the publication. Authors contacted and confirmed that consensus criteria was used for the diagnosis and evaluation of severity of PEP (Cotton 1991). 3. It was unclear whether trainees were involved after randomisation. Authors contacted and confirmed that trainees were not involved after randomisation. 4. No information was provided with regards to gender distribution between the 2 groups. Authors contacted and provided information: PGW group (16 males, 20 females) vs PD stent group (19 males, 26 females) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "single, blind, stratified randomisation protocol based on participating institutions to assure equal representation of both study groups at each facility"; "A randomisation list was created using a computer‐based number generator." |
| Allocation concealment (selection bias) | Low risk | "used concealed envelopes to randomise subjects in a 1:1 fashion" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Patients were blinded to their assignment group but not the treating endoscopist". Endoscopists could not be blinded. Unclear if personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up appeared to be complete |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes |
| Other bias | High risk | 1. The study design allowed 'cross‐over' from the PGW group to the PD stent group, but not vice versa. This may introduce a potential bias favouring the PD stent technique. However, this design is justified since it is not ethical to 'cross over' from PD stent to PGW group, as this would require removal of the PD stent, advancing a guidewire into the PD a second time, and then having to deploy a second PD stent for prophylaxis of PEP. 2. There was a higher prevalence of "anticipated difficult cannulation" in the PGW group vs the PD stent group (48.1% vs 38.0%) based on the endoscopist's visual inspection of the papilla. The interobserver and intraobserver variability of visual inspection of the papilla is unknown, but if a greater number of participants with "more difficult" papilla were randomised to the PGW group, this may introduce another bias favouring the PD stent technique. 2. The study terminated participant recruitment after the targeted sample size was achieved in the PGW group but not the PD stent group since the rate of cross over from the PGW group to the PD stent group was lower than anticipated. An interim analysis revealed the observed difference in efficacy (13.8%) for the primary endpoint (CBD cannulation within 6 min) would have required > 400 participants to have adequate statistical power to detect a significant difference |
Herreros de Tejada 2009.
| Methods | Multicentre (6 sites) RCT in Spain. Trainees were involved prior to randomisation and "only a minority of randomised cases were continued by the fellow" | |
| Participants | People aged 18 or older admitted as inpatients for a least 24‐hour monitoring and undergoing ERCP with the intent to cannulate the CBD were screened. People in whom cannulation of the CBD failed with conventional guidewire‐assisted cannulation techniques were included. Excluded patients with previous sphincterotomy or endoscopic papilla dilatation, previous surgical biliary‐intestinal operations, diagnosis or suspicion of pancreas divisum, use of any prophylactic drug for PEP, pancreatic or biliary stent placement within 6 months, and pregnancy or active breastfeeding. Difficult cannulation was defined as the completion of 5 unsuccessful cannulation attempts | |
| Interventions | PGW placement (double guidewire technique) vs persistent cannulation attempts with conventional guidewire‐assisted cannulation technique. 1. PGW: placement of a device preloaded with a 0.035" guidewire (Jagwire; Boston Scientific) in the ampullary orifice; insertion of the guidewire into the PD to at least half of the presumed total length of the PD (guided by fluoroscopy); withdrawal of the device, leaving the guidewire in the pancreas; insertion of the device preloaded with a new guidewire alongside the previously placed pancreatic guidewire; adjustment of the device in the papilla over the bent pancreatic guidewire, targeting the 11 o'clock position on the papillary orifice. 2. Persistent cannulation with guidewire‐assisted cannulation technique. PD stents were used for prophylaxis of PEP in selected high‐risk patients (12% in the PGW group vs 10% in the persistent cannulation group) |
|
| Outcomes | Successful CBD cannulation, number of attempts required to achieve CBD cannulation, and ERCP‐related complications | |
| Notes | 1. 'Cross‐over' design with the use of other rescue techniques: In those cases of unsuccessful CBD cannulation after completing a total of 15 attempts, endoscopists were offered the option to abort the ERCP or continue the procedure, or use any technique that they preferred without any specific limit to the number of attempts. Authors were contacted and provided information about the percentage of participants receiving "backup techniques" in each group. A backup technique was performed in 87 of 89 (98%) participants with unsuccessful CBD cannulation after the completed 15 attempts. Authors confirmed that backup techniques were used in 38 participants in the persistent‐cannulation group and 49 participants in the PGW group. Precut was the modality most often used (53%) as the backup technique. Among participants who received backup techniques, 8 crossed over from the persistent‐cannulation group to the PGW group, and 6 crossed over from the PGW group to the persistent‐cannulation group. 2. Diagnosis for PEP was based on the consensus criteria (Cotton 1991). 3. The data on the incidence and severity of PEP was based on per‐protocol analysis. Authors were contacted and provided data on PEP and overall cannulation success based on ITT analysis. 4 participants each were excluded from the persistent‐cannulation group and the PGW group due to protocol violation. 17 participants were excluded from the PGW group due to "unintentional CBD cannulation". 4. The overall cannulation success rates were not provided in the original report. Authors were contacted and provided data based on ITT |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomisation list for group allocation was generated by using computer‐based pseudo‐random number generators with variable block size stratified by centre." |
| Allocation concealment (selection bias) | Low risk | "The allocation was concealed by sequentially numbered sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Endoscopists could not be blinded. Unclear whether participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors were blinded. "There was a general coordinator in the central institution responsible for supervising all data from the participating centres. Follow‐up visits were conducted by this general coordinator before, during, and at termination of the study to supervise adherence to study protocol." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up appeared to be complete |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes |
| Other bias | Low risk | No other risk of bias |
Ito 2010a.
| Methods | Single‐centre RCT with 6 expert endoscopists in Japan. No information was provided as to whether trainees were involved | |
| Participants | People who underwent PGW placement for achieving selective biliary cannulation and: (1) difficult cannulation of the bile duct with contrast‐assisted cannulation technique, (2) successful guidewire insertion into the PD, and (3) age 18 years or older. Excluded people with inability to insert a guidewire into the PD, previous endoscopic sphincterotomy or endoscopic papillary balloon dilation, pancreas divisum, or pregnancy or breastfeeding. Difficult cannulation was defined as unsuccessful cannulation after at least 5 attempts with a cannula/sphincterotome | |
| Interventions | PGW placement (double guidewire technique) without PD stent vs PGW placement with PD stent. All participants had PGW techniques. PGW was performed with a 0.025" guidewire (Jagwire; Boston Scientific), which was inserted into the PD. A second cannula or a sphincterotome was passed into the same working channel of the scope alongside the guidewire with the 2‐devices‐in‐1‐channel method and biliary cannulation was attempted. 1. PGW + no PD stent. 2. PGW + PD stent: A 5‐Fr, 4‐cm‐long stent with a single duodenal pigtail (Pit‐stent; Cathex) was used for PD stenting |
|
| Outcomes | PEP, hyperamylasaemia, mean serum amylase level, and risk factors for PEP | |
| Notes | 1. Not a 'cross‐over' design, but permitted the use of rescue techniques including precut, second ERCP, percutaneous transhepatic biliary drainage, or a "substitute modality" such as CT/MRI. In the PD stent group, 7 participants had unsuccessful biliary cannulation, 3 underwent a second ERCP with successful cannulation, while the other 4 underwent a substitute diagnostic procedure such as CT/MRI. In the no‐PD stent group, 2 had unsuccessful biliary cannulation, 1 underwent a second ERCP, including precut, followed by successful cannulation. The other participant underwent a substitute diagnostic procedure. As overall cannulation success was defined as overall success during the same procedure, participants who had successful cannulation with the second ERCP or other procedures were excluded. 2. PGW technique was attempted in 108 (7.4%) patients with difficult cannulation of the bile duct out of 1451 patients with a native papilla. It was unclear if PGW was the only technique attempted in all patients with difficult cannulation, or if some cases received other rescue techniques and were excluded from this study. 3. Diagnosis for PEP was based on the consensus criteria (Cotton 1991). 4. 70 participants were randomised, but unclear in Table 5 why a total of 72 participants were included: 9 people with PEP vs 63 people without PEP. Authors were contacted, but did not reply. 5. All participants received an 8‐hour infusion of protease inhibitor (nafamostat mesilate, 20 mg/day) and antibiotics (cefoperazone‐sulbactam or ceftazidime, 2 g/day) for 2 days. Attempts to contact authors for additional data were unsuccessful |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients who satisfied the inclusion criteria were randomly assigned to either the PD stent placement group or the no‐stent group by means of the sealed envelope method after PGW was commenced." Unclear how the randomisation list was generated |
| Allocation concealment (selection bias) | Low risk | "Patients who satisfied the inclusion criteria were randomly assigned to either the PD stent placement group or the no‐stent group by means of the sealed envelope method after PGW was commenced." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Endoscopists could not be blinded. Unclear whether participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors were blinded. All participants were admitted to the hospital for at least 24 hours. Serum amylase concentration before the procedure and 3, 6, and 18 to 24 hours afterward. As a result, participants may be more likely to undergo laboratory and radiological evaluation as opposed to being discharged home following ERCP. If outcome assessors were not blinded, this could lead to differential detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up appeared to be complete |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes |
| Other bias | Low risk | No other risk of bias |
Maeda 2003.
| Methods | Single‐centre RCT in Japan. No information was provided about the number or the experience of the endoscopists | |
| Participants | Consecutive patients with hepatobiliary disease with difficult bile duct cannulation that was defined as using the conventional ERCP manoeuvre without a guidewire requiring more than 10 minutes | |
| Interventions | PGW placement (double guidewire technique) vs persistent cannulation attempts with conventional contrast‐assisted cannulation technique. 1. PGW: A 0.025" guidewire (Jagwire; Boston Scientific) was inserted into the PD from a cannula (Wilson Cook T‐1‐LT) after PD cholangiography. After withdrawal of the catheter, the guidewire was left in the PD and was monitored by fluoroscopy. 0.025", 0.032", and 0.035" Radifocus guidewires were also used for angled PD. A second cannula was then inserted via the same scope channel. Cholangiography and treatment were carried out through the papilla after selective cannulation into the deep bile duct in the 11 o'clock direction, while pushing the PD of the papilla underneath with the guidewire. 2. Persistent cannulation with contrast‐assisted cannulation technique |
|
| Outcomes | Successful cannulation rate and complication rate, time for bile duct cannulation | |
| Notes | 1. Not a 'cross‐over' design and did not use any rescue techniques in cases of failure to gain biliary access. 2. Diagnosis for PEP was based on the consensus criteria (Cotton 1991). Any participant graded above ”moderate” in the consensus criteria was regarded as having clinical pancreatitis. 3. All participants received pretreatment with 5 mg isosorbide dinitrate by buccal aerosol 10 min before the examination and an intravenous injection of 35 mg pethidine hydrochloride, 15 mg prifinium bromide, and an intramuscular injection of 0.5 mg atropine sulfate and 7.5 mg prifinium bromide. In the postoperative period, all participants received intravenous drip with antienzyme medication (100,000 units of the urinary trypsin inhibitor urinastatin) and antibiotics (1 g cefoperazone/sulbactam). Attempts to contact authors for additional data were unsuccessful |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned either to the pre‐insertion group or the conventional method group." Unclear how the randomisation list was generated |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information was provided. Endoscopists could not be blinded. Unclear whether participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up appeared to be complete |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes |
| Other bias | Low risk | No other risk of bias |
Yoo 2013.
| Methods | Single‐centre RCT with 1 expert endoscopist in Korea. Authors contacted and confirmed trainees were not involved | |
| Participants | Consecutive patients who underwent ERCP with clear indication of biliary access and in whom free cannulation of the CBD using guidewire‐assisted cannulation technique was not possible and selective PD cannulation was achieved without difficulty. Excluded patients age < 18 years, prior biliary or pancreatic sphincterotomy or dilatation or stenting of either duct, acute pancreatitis, and pregnancy. Difficult cannulation was defined as unsuccessful cannulation after 10 or more attempts with a cannula/sphincterotome or failure of cannulation after 10 minutes | |
| Interventions | PGW placement (double guidewire technique) vs precut sphincterotomy (transpancreatic precut sphincterotomy). 1. PGW: After PD cannulation had been achieved without difficulty, a 0.035" guidewire (Tracer Hybrid Wire Guide; Wilson Cook) was left in the PD. Another cannula or sphincterotome was passed into the same working channel of the scope alongside the guidewire using the 2‐devices‐in‐1‐channel method. The tip of the device was positioned in the papilla, and another 0.035" guidewire (Tracer Metro Direct Wire Guide; Wilson Cook) was bent over the pancreatic wire to attempt cannulation of the CBD. 2. Precut sphincterotomy: After a 0.035" guidewire (Tracer Hybrid Wire Guide; Wilson Cook) had been inserted deeply into the PD without difficulty, the tip of a standard traction sphincterotome was wedged into the pancreatic orifice, and a sphincterotomy was performed with a cutting wire along the biliary direction at 11 o'clock. The incision was made through the septum between the pancreatic and biliary duct with the aim of exposing the CBD orifice. After transpancreatic precut sphincterotomy, the guidewire placed in the PD was removed. Biliary cannulation was then attempted using a catheter or sphincterotome, either with or without a guidewire at the discretion of the endoscopist. Authors contacted and confirmed PD stent was not used for prophylaxis of PEP |
|
| Outcomes | Successful CBD cannulation, median cannulation time, and postprocedure‐related complications including PEP, bleeding, perforation, cholangitis, and cholecystitis | |
| Notes | 1. Authors contacted and confirmed that the trial was not a 'cross‐over' design. Also, rescue techniques were not used. In cases of failure, ERCP was repeated within 2 to 5 days using the same cannulation technique. 2. Participants with moderate and severe PEP were combined in 1 group in the publication. Authors contacted and provided further data with regards to the number of participants with moderate vs severe PEP in each group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomisation list for group allocation was generated using computer‐based pseudo‐random number generators." |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information was provided. Endoscopists could not be blinded. Unclear whether participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up appeared to be complete |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes |
| Other bias | Low risk | No other risk of bias was identified |
Zheng 2010.
| Methods | Single‐centre RCT in China. Authors contacted and confirmed that 2 expert endoscopists performed all procedures. Trainees were not involved | |
| Participants | People with biliary complications after liver transplantation in whom CBD cannulation was difficult to perform. Difficult CBD cannulation was defined as unsuccessful cannulation in 10 minutes. No information was provided in the conference proceeding with regards to the type of standard cannulation technique used in the study. Authors contacted and confirmed that guidewire‐assisted cannulation technique was always used as the standard technique in the study | |
| Interventions | PGW placement (double guidewire technique) vs persistent cannulation attempts with conventional guidewire‐assisted cannulation technique. 1. PGW: No information was provided with regards to the specific techniques used. 2. Persistent cannulation with guidewire‐assisted cannulation technique. Authors contacted and confirmed that PD stent was not used for prophylaxis of PEP |
|
| Outcomes | CBD cannulation success rate, time of successful CBD cannulation, PEP | |
| Notes | 1. Highly selected patients with biliary complications after liver transplantation. 2. Authors contacted and confirmed that the trial was not a 'cross‐over' design. Also, rescue techniques such as precut sphincterotomy were not used in cases of failure. 3. Diagnostic criteria for PEP was not provided in the publication. Authors contacted and confirmed that consensus criteria was used for the diagnosis and evaluation of severity of PEP (Cotton 1991). 4. Data pertaining to the severity of PEP were not provided in the abstract. Authors contacted and provided data (0 PEP in the PGW group vs 2 mild PEP in the persistent‐cannulation group) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | In the conference proceeding, it was stated that "patients were randomly assigned to". Authors contacted and provided further information: "Randomization was performed by the method of ballot of odd and even numbers into two groups." Also, 6 participants who had pancreatic guidewire inserted into the PD prior to randomisation were automatically assigned to the PGW group. The assignment of participants to treatment groups was therefore not truly randomised |
| Allocation concealment (selection bias) | Unclear risk | Conference proceeding, no information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Conference proceeding, no information was provided. Endoscopists could not be blinded. Unclear whether participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Conference proceeding, no information was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up appeared to be complete |
| Selective reporting (reporting bias) | Low risk | Reported all important outcomes |
| Other bias | Unclear risk | Conference proceeding, unable to ascertain other bias |
CBD: common bile duct CT: computed tomography ERCP: endoscopic retrograde cholangiopancreatography ITT: intention to treat MRI: magnetic resonance imaging PD: pancreatic duct PEP: post‐ERCP pancreatitis PGW: pancreatic duct guidewire placement RCT: randomised controlled trial SOD: sphincter of Oddi dysfunction
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balderas 2011 | Retrospective analysis, not an RCT |
| Cha 2012 | Not performed in people with difficult cannulation; guidewire was placed in the pancreatic duct by chance |
| Chandran 2012 | Not an RCT |
| Grönroos 2011 | Not an RCT |
| Huang 2015 | Not an RCT |
| Ito 2008 | Single‐arm observational study, not an RCT |
| Ito 2010b | Retrospective analysis, not an RCT |
| Ito 2012 | Observational study, not an RCT |
| Kim 2012 | Retrospective analysis, not an RCT |
| Kim 2013 | Inappropriate intervention |
| Kim 2014 | Not an RCT |
| Kim 2015a | Not an RCT |
| Kim 2015b | Not an RCT |
| Miao 2015 | Not an RCT |
| Nagano 2010 | Observational study for wire‐guided cannulation or pancreatic duct guidewire placement, with or without stent, not an RCT |
| Nakahara 2014 | Not an RCT |
| Ozaslan 2014 | Inappropriate intervention |
| Patel 2009 | Retrospective analysis, not an RCT |
| Sasahira 2015 | Not performed in people with difficult cannulation; guidewire was unintentionally inserted into the pancreatic duct before difficult cannulation was reached (defined as 10 attempts and 10 minutes) |
| Song 2013 | Retrospective analysis, not an RCT |
| Suzuki 2012 | Retrospective analysis, not an RCT |
| Tanaka 2013 | Divided participants chronologically according to the time period in which the procedures were performed. Not an RCT |
| Yang 2015 | Not an RCT |
| Zang 2014 | Inappropriate intervention |
RCT: randomised controlled trial
Differences between protocol and review
We intended to perform available‐case analysis versus 'worst‐case scenario' analysis as a sensitivity analysis: all participants who were lost to follow‐up in the PGW group were considered to have PEP, whereas those who were lost to follow‐up in the other comparison groups were considered to have a favourable outcome (no PEP). We did not perform this analysis because there was no loss to follow‐up in any of the trials. This version of the review now assesses the quality of the evidence using the GRADE considerations, and presents the findings in a 'Summary of findings' table.
Contributions of authors
Frances Tse, Yuhong Yuan, Grigorios I Leontiadis, Paul Moayyedi, and Alan Barkun were responsible for designing the review protocol. Yuhong Yuan developed the search strategy with collaboration of the Cochrane UGPD Group. Yuhong Yuan conducted the literature searches. Frances Tse, Yuhong Yuan and Majidah Bukhari performed data extraction. Frances Tse and Yuhong Yuan were responsible for performing eligibility checks on the search results, data analysis, quality assessment, and interpretation of data. Frances Tse and Yuhong Yuan contributed to the manuscript preparation. Grigorios I Leontiadis, Paul Moayyedi, and Alan Barkun contributed to the review of the manuscript. All review authors contributed to the final editing of the review and gave final approval.
Sources of support
Internal sources
McMaster University, Department of Gastroenterology, Canada.
External sources
No sources of support supplied
Declarations of interest
FT: none known.
YY: none known.
MB: none known.
GL: none known.
PM: has accepted speaker fees from Shire and Allergan. These companies make drugs for irritable bowel syndrome and ulcerative colitis; PM has had no involvement with pharmaceutical companies that sell drugs to treat any upper gastrointestinal disease. PM's endowed Chair is funded in part by an unrestricted donation given to McMaster University by AstraZeneca Canada.
AB: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Angsuwatcharakon 2012 {published data only}
- Angsuwatcharakon P, Rerknimitr R, Ridtitid W, Ponauthai Y, Kullavanijaya P. Success rate and cannulation time between precut sphincterotomy and double guidewire technique in truly difficult biliary cannulation. Journal of Gastroenterology and Hepatology 2012;27(2):356‐61. [DOI] [PubMed] [Google Scholar]
- Angsuwatcharakon P, Ridtitid W, Rerknimitr R, Kongkam P, Treeprasertsuk S, Kullavanijaya P. Pancreatic guide‐wire placement assisting biliary cannulation (double guide‐wire) versus precut sphincterotomy in difficult biliary cannulation: a prospective randomized study. Gastrointestinal Endoscopy 2010;71(5):AB164. Abstract No 1145. [Google Scholar]
Coté 2012 {published data only}
- Coté GA, Hovis CE, Keswani RN, Ansstas M, Edmundowicz SA, Jonnalagadda SS. A randomized clinical trial comparing the use of a pancreatic duct stent or a pancreatic duct guidewire to facilitate bile duct. Gastrointestinal Endoscopy 2010;71(5):AB163‐4. Abstract S1445. [Google Scholar]
- Coté GA, Mullady DK, Jonnalagadda SS, Keswani RN, Wani SB, Hovis CE, et al. Use of a pancreatic duct stent or guidewire facilitates bile duct access with low rates of precut sphincterotomy: a randomized clinical trial. Digestive Diseases and Sciences 2012;57(12):3271‐8. [DOI] [PubMed] [Google Scholar]
Herreros de Tejada 2009 {published data only}
- Herreros de Tejada A, Calleja JL, Díaz G, Pertejo V, Espinel J, Cacho G, et al. Double‐guidewire technique for difficult bile duct cannulation: a multicenter randomized, controlled trial. Gastrointestinal Endoscopy 2009;70(4):700‐9. [DOI] [PubMed] [Google Scholar]
- Herreros de Tejada A, Calleja JL, Dıaz G, Espinel J, Pertejo V, Cacho G, et al. Double guide wire technique compared to conventional method in selected cases of difficult common bile duct cannulation in ERCP. Preliminary data from a controlled randomized study. Gastrointestinal Endoscopy 2007;65(5):AB231. Abstract M1334. [Google Scholar]
Ito 2010a {published data only}
- Ito K, Fujita N, Noda Y, Kobayashi G, Obana T, Horaguchi J, et al. Can pancreatic duct stenting prevent post‐ERCP pancreatitis in patients who undergo pancreatic duct guidewire placement for achieving selective biliary cannulation? A prospective randomized controlled trial. Journal of Gastroenterology 2010;45(11):1183‐91. [DOI] [PubMed] [Google Scholar]
Maeda 2003 {published data only}
- Maeda S, Hayashi H, Hosokawa O, Dohden K, Hattori M, Morita M, et al. Prospective randomized pilot trial of selective biliary cannulation using pancreatic guide‐wire placement. Endoscopy 2003;35(9):721‐4. [DOI] [PubMed] [Google Scholar]
Yoo 2013 {published data only}
- Cha SW, Yoo YW, Kim A, Kim SH, Lee HL, Lee YJ, et al. Double guidewire technique versus transpancreatic precut sphincterotomy in difficult biliary cannulation; randomized prospective study. Gastrointestinal Endoscopy 2011;73(4 Suppl 1):AB348. Abstract No 1445. [Google Scholar]
- Yoo YW, Cha SW, Lee WC, Kim SH, Kim A, Cho YD. Double guidewire technique vs transpancreatic precut sphincterotomy in difficult biliary cannulation. World Journal of Gastroenterology 2013;19(1):108‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2010 {published data only}
- Zheng FP, Guo YW, Tao LI, Lin XY. Double‐guidewire technique for difficult bile duct cannulation in patients with biliary complications after liver transplantation. Journal of Gastroenterology and Hepatology 2010;25(Suppl 2):A51. Abstract 329. [Google Scholar]
References to studies excluded from this review
Balderas 2011 {published data only}
- Balderas V, Linsteadt J, Surapaneni SN, Echavarria J, Patel S, Rosenkranz L. Does implementation of the double wire technique and prophylactic pancreatic duct stenting reduce post (ERCP) pancreatitis in patients undergoing needle knife sphincterotomy. Gastrointestinal Endoscopy 2011;73(4 Suppl 1):AB268. [Google Scholar]
Cha 2012 {published data only}
- Cha SW, Kim SH, Kim A, Park ET, Yoo KS, Park MI, et al. DGT vs TPS in patients with initial PD cannulation by chance; prospective randomized multi‐center study. Gastrointestinal Endoscopy 2012;75(4 Suppl 1):AB141. [Google Scholar]
Chandran 2012 {published data only}
- Chandran S, Nikfarjam M. The utility of upfront double wire guided biliary cannulation technique following early unintentional pancreatic wire cannulation in patients undergoing endoscopic retrograde cholangiopancreatography. Journal of Gastroenterology and Hepatology 2012;27(Suppl 4):53. [DOI] [PubMed] [Google Scholar]
Grönroos 2011 {published data only}
- Grönroos JM, Vihervaara H, Gullichsen R, Laine S, Karvonen J, Salminen P. Double‐guidewire‐assisted biliary cannulation: experiences from a single tertiary referral center. Surgical Endoscopy 2011;25(5):1599‐602. [DOI] [PubMed] [Google Scholar]
Huang 2015 {published data only}
- Huang L, Yu QS, Zhang Q, Liu JD, Wang Z. Comparison between double‐guidewire technique and transpancreatic sphincterotomy technique for difficult biliary cannulation. Digestive Endoscopy 2015;27(3):381‐7. [DOI] [PubMed] [Google Scholar]
Ito 2008 {published data only}
- Ito K, Fujita N, Noda Y, Kobayashi G, Obana T, Horaguchi J, et al. Pancreatic guidewire placement for achieving selective biliary cannulation during endoscopic retrograde cholangio‐pancreatography. World Journal of Gastroenterology 2008;14(36):5595‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ito 2010b {published data only}
- Ito K, Mimura T, Hara S, Kamata I, Kishimoto Y, Okano N, et al. Device and handle of difficult bile duct cannulation. Journal of Gastroenterology and Hepatology 2010;25:A30. [Google Scholar]
Ito 2012 {published data only}
- Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, Koshita S, et al. The clinical significance of wire‐guided cannulation with pancreatic guidewire placement (double‐guidewire technique) for difficult biliary cannulation in ERCP. Gastrointestinal Endoscopy 2012;75(4 Suppl 1):AB398‐9. [Google Scholar]
Kim 2012 {published data only}
- Kim JH, Lee HS, Kang J, Wi JO, Hwang JC, Yoo BM. Wire‐guided cannulation over a pancreatic duct stent versus double‐guide wire technique for difficult bile duct cannulation. Gastrointestinal Endoscopy 2012;75(4 Suppl 1):AB389‐90. [Google Scholar]
Kim 2013 {published data only}
- Kim SJ, Kim HW, Choi CW, Park SB, Song BJ, Kang DH. Step vs 2‐step protocol for difficult biliary access with frequent pancreatic cannulation. Pancreatology 2013;13(4 Suppl):S22. [Google Scholar]
Kim 2014 {published data only}
- Kim SJ, Kang D, Choi C, Kim HW, Song B, Park SB. Early needle‐knife or double‐guidewire techniques for post‐ERCP pancreatitis in patients with unintentional repeated pancreatic cannulations. Pancreatology 2014;14(3 Suppl 1):S24. [Google Scholar]
Kim 2015a {published data only}
- Kim CW, Chang JH, Kim TH, Han SW. Sequential double‐guidewire technique and transpancreatic precut sphincterotomy for difficult biliary cannulation. Saudi Journal of Gastroenterology 2015;21(1):18‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015b {published data only}
- Kim SJ, Kang DH, Kim HW, Choi CW, Park SB, Song BJ, et al. Needle‐knife fistulotomy vs double‐guidewire technique in patients with repetitive unintentional pancreatic cannulations. World Journal of Gastroenterology 2015;21(19):5918‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miao 2015 {published data only}
- Miao L, Li QP, Zhu MH, Ge XX, Yu H, Wang F, et al. Endoscopic transpancreatic septotomy as a precutting technique for difficult bile duct cannulation. World Journal of Gastroenterology 2015;21(13):3978‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagano 2010 {published data only}
- Nagano R, Sasahira N, Isayama H, Kawakubo K, Kogure H, Sasaki T, et al. Prophylactic placement of pancreatic stent (PPPS) may induce the risk of post‐ERCP pancreatitis (PEP) under wire‐guided cannulation (WGC) technique. Journal of Gastroenterology and Hepatology 2010;25:A150. [Google Scholar]
Nakahara 2014 {published data only}
- Nakahara K, Okuse C, Suetani K, Michikawa Y, Kobayashi S, Otsubo T, et al. Need for pancreatic stenting after sphincterotomy in patients with difficult cannulation. World Journal of Gastroenterology 2014;20(26):8617‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozaslan 2014 {published data only}
- Ozaslan E, Purnak T, Efe C, Ozaslan NG, Cengiz M. The comparison of two different 5.5 fr sphincterotomes for selective cannulation of the common bile duct: a prospective, randomized study. Digestive Diseases and Sciences 2014;59(12):3078‐84. [DOI] [PubMed] [Google Scholar]
Patel 2009 {published data only}
- Patel S, Torres VJ, Gross GW. The early use of the double‐wire technique to facilitate difficult biliary cannulation and its impact on post‐ERCP pancreatitis. Gastrointestinal Endoscopy 2009;69(5):AB159. [Google Scholar]
Sasahira 2015 {published data only}
- Sasahira N, Isayama H, Kawakami H, Ito Y, Matsubara S, Ishiwatari H, et al. Can early double guidewire technique facilitate common bile duct cannulation and reduce post ERCP pancreatitis? ‐Results of a multicenter prospective randomized controlled trial: EDUCATION trial. Gastrointestinal Endoscopy 2013;77(5 Suppl 1):AB183. [DOI] [PubMed] [Google Scholar]
- Sasahira N, Isayama H, Kawakami H, Ito Y, Matsubara S, Ishiwatari H, et al. When and to whom should we apply the double guidewire cannulation method during endoscopic biliary intervention? Post hoc analysis of multicenter randomized controlled trial. United European Gastroenterology Journal 2013;1:A485. [Google Scholar]
- Sasahira N, Kawakami H, Isayama H, Uchino R, Nakai Y, Ito Y, et al. Early use of double‐guidewire technique to facilitate selective bile duct cannulation: The multicenter randomized controlled EDUCATION trial. Endoscopy 2015;47(5):421‐9. [DOI] [PubMed] [Google Scholar]
Song 2013 {published data only}
- Song BJ, Kang DH, Kim HW, Kim SJ, Hong JB, Choi CW, et al. Is double‐guidewire technique really helpful for difficult bile duct cannulation?. United European Gastroenterology Journal 2013;1:A157. [Google Scholar]
Suzuki 2012 {published data only}
- Suzuki A, Mandai K, Morikawa S, Uno K, Yasuda K. Safety and efficacy of pancreatic guidewire placement for achieving selective biliary cannulation. Gastrointestinal Endoscopy 2012;75(4 Suppl 1):AB378. [Google Scholar]
Tanaka 2013 {published data only}
- Tanaka R, Itoi T, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, et al. Is the double‐guidewire technique superior to the pancreatic duct guidewire technique in cases of pancreatic duct opacification?. Journal of Gastroenterology and Hepatology 2013;28(11):1787‐93. [DOI] [PubMed] [Google Scholar]
Yang 2015 {published data only}
- Yang MJ, Hwang JC, Yoo BM, Kim JH, Ryu HK, Kim SS, et al. Wire‐guided cannulation over a pancreatic stent versus double guidewire technique in patients with difficult biliary cannulation. BMC Gastroenterology 2015;15:150. [DOI: 10.1186/s12876-015-0381-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zang 2014 {published data only}
- Zang J, Zhang C, Gao J. Guidewire‐assisted transpancreatic sphincterotomy for difficult biliary cannulation: a prospective randomized controlled trial. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2014;24(5):429‐33. [DOI] [PubMed] [Google Scholar]
Additional references
Angsuwatcharakon 2010
- Angsuwatcharakon P, Ridtitid W, Rerknimitr R, Kongkam P, Treeprasertsuk S, Kullavanijaya P. Pancreatic guide‐wire placement assisting biliary cannulation (double guide‐wire) versus precut sphincterotomy in difficult biliary cannulation: a prospective randomized study. Gastrointestinal Endoscopy 2010;71(5):AB164. Abstract No 1145. [Google Scholar]
Artifon 2010
- Artifon EL, Chu A, Freeman M, Sakai P, Usmani A, Kumar A. A comparison of the consensus and clinical definitions of pancreatitis with a proposal to redefine post‐endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas 2010;39(4):530‐5. [DOI] [PubMed] [Google Scholar]
Badalov 2009
- Badalov N, Tenner S, Baillie J. The prevention, recognition and treatment of post‐ERCP pancreatitis. JOP 2009;10(2):88‐97. [PubMed] [Google Scholar]
Banks 2013
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis ‐ 2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62(1):102‐11. [DOI] [PubMed] [Google Scholar]
Briel 2012
- Briel M, Bassler D, Wang AT, Guyatt GH, Montori VM. The dangers of stopping a trial too early. Journal of Bone and Joint Surgery. American Volume 2012;94(Suppl 1):56‐60. [DOI] [PubMed] [Google Scholar]
Cennamo 2010
- Cennamo V, Fuccio L, Zagari RM, Eusebi LH, Ceroni L, Laterza L, et al. Can early precut implementation reduce endoscopic retrograde cholangiopancreatography‐related complication risk? Meta‐analysis of randomized controlled trials. Endoscopy 2010;42(5):381‐8. [DOI] [PubMed] [Google Scholar]
Cha 2011
- Cha SW, Yoo YW, Kim A, Kim SH, Lee HL, Lee YJ, et al. Double guidewire technique versus transpancreatic precut sphincterotomy in difficult biliary cannulation; randomized prospective study. Gastrointestinal Endoscopy 2011;73(4 Suppl 1):AB348. Abstract No 1445. [Google Scholar]
Cheng 2006
- Cheng CL, Sherman S, Watkins JL, Barnett J, Freeman M, Geenen J, et al. Risk factors for post‐ERCP pancreatitis: a prospective multicenter study. American Journal of Gastroenterology 2006;101(1):139‐47. [DOI] [PubMed] [Google Scholar]
Choudhary 2011
- Choudhary A, Bechtold ML, Arif M, Szary NM, Puli SR, Othman MO, et al. Pancreatic stents for prophylaxis against post‐ERCP pancreatitis: a meta‐analysis and systematic review. Gastrointestinal Endoscopy 2011;73(2):275‐82. [DOI] [PubMed] [Google Scholar]
Colton 2009
- Colton JB, Curran CC. Quality indicators, including complications, of ERCP in a community setting: a prospective study. Gastrointestinal Endoscopy 2009;70(3):457‐67. [DOI] [PubMed] [Google Scholar]
Conn 1991
- Conn M, Goldenberg A, Concepcion L, Mandeli J. The effect of ERCP on circulating pancreatic enzymes and pancreatic protease inhibitors. American Journal of Gastroenterology 1991;86(8):1011‐4. [PubMed] [Google Scholar]
Cortas 1999
- Cortas GA, Mehta SN, Abraham NS, Barkun AN. Selective cannulation of the common bile duct: a prospective randomized trial comparing standard catheters with sphincterotomes. Gastrointestinal Endoscopy 1999;50(6):775‐9. [DOI] [PubMed] [Google Scholar]
Cotton 1991
- Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointestinal Endoscopy 1991;37(3):383‐93. [DOI] [PubMed] [Google Scholar]
Coté 2010
- Coté GA, Hovis CE, Keswani RN, Ansstas M, Edmundowicz SA, Jonnalagadda SS. A randomized clinical trial comparing the use of a pancreatic duct stent or a pancreatic duct guidewire to facilitate bile duct cannulation. Gastrointestinal Endoscopy 2010;71(5):AB163‐4. Abstract S1445. [Google Scholar]
Dhir 2012
- Dhir V, Bhandari S, Bapat M, Maydeo A. Comparison of EUS‐guided rendezvous and precut papillotomy techniques for biliary access (with videos). Gastrointestinal Endoscopy 2012;75(2):354‐9. [DOI] [PubMed] [Google Scholar]
Dumonceau 1998
- Dumonceau JM, Deviere J, Cremer M. A new method of achieving deep cannulation of the common bile duct during endoscopic retrograde cholangiopancreatography. Endoscopy 1998;30(7):S80. [DOI] [PubMed] [Google Scholar]
Dumonceau 2010
- Dumonceau JM, Andriulli A, Deviere J, Mariani A, Rigaux J, Baron TH, et al. European Society of Gastrointestinal Endoscopy (ESGE) guideline: prophylaxis of post‐ERCP pancreatitis. Endoscopy 2010;42(6):503‐15. [DOI] [PubMed] [Google Scholar]
Dumonceau 2014
- Dumonceau JM, Andriulli A, Elmunzer BJ, Mariani A, Meister T, Deviere J, et al. Prophylaxis of post‐ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) guideline ‐ updated June 2014. Endoscopy 2014;46(9):799‐815. [DOI] [PubMed] [Google Scholar]
Elmunzer 2012
- Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, et al. A randomized trial of rectal indomethacin to prevent post‐ERCP pancreatitis. The New England Journal of Medicine 2012;366(15):1414‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forsmark 2007
- Forsmark CE, Baillie J, AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. AGA Institute technical review on acute pancreatitis. Gastroenterology 2007;132(5):2022‐44. [DOI] [PubMed] [Google Scholar]
Freeman 1996
- Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, et al. Complications of endoscopic biliary sphincterotomy. The New England Journal of Medicine 1996;335(13):909‐18. [DOI] [PubMed] [Google Scholar]
Freeman 2001
- Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, et al. Risk factors for post‐ERCP pancreatitis: a prospective, multicenter study. Gastrointestinal Endoscopy 2001;54(4):425‐34. [DOI] [PubMed] [Google Scholar]
Freeman 2004
- Freeman ML, Guda NM. Prevention of post‐ERCP pancreatitis: a comprehensive review. Gastrointestinal Endoscopy 2004;59(7):845‐64. [DOI] [PubMed] [Google Scholar]
Freeman 2005
- Freeman ML, Guda NM. ERCP cannulation: a review of reported techniques. Gastrointestinal Endoscopy 2005;61(1):112‐25. [PUBMED: 15672074] [DOI] [PubMed] [Google Scholar]
Freeman 2012
- Freeman ML. Current status of endoscopic stenting of the pancreatic duct as prophylaxis against post‐ERCP pancreatitis. Gastroenterology & Hepatology 2012;8(9):618‐20. [PMC free article] [PubMed] [Google Scholar]
Gotoh 2001
- Gotoh Y, Tamada K, Tomiyama T, Wada S, Ohashi A, Satoh Y, et al. A new method for deep cannulation of the bile duct by straightening the pancreatic duct. Gastrointestinal Endoscopy 2001;53(7):820‐2. [DOI] [PubMed] [Google Scholar]
GRADE 2008 [Computer program]
- Brozek J, Oxman A. GRADEpro. Version 3.2 for Windows. GRADE Working Group, 2008.
Gyokeres 2003
- Gyokeres T, Duhl J, Varsanyi M, Schwab R, Burai M, Pap A. Double guide wire placement for endoscopic pancreaticobiliary procedures. Endoscopy 2003;35(1):95‐6. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Herreros de Tejada 2007
- Herreros de Tejada A, Calleja JL, Dıaz G, Espinel J, Pertejo V, Cacho G, et al. Double guide wire technique compared to conventional method in selected cases of difficult common bile duct cannulation in ERCP preliminary data from a controlled randomized study. Gastrointestinal Endoscopy 2007;65(5):AB231. Abstract M1334. [Google Scholar]
Ioannidis 2007a
- Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta‐analyses. BMJ 2007;335(7626):914‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ioannidis 2007b
- Ioannidis JP, Trikalinios TA. The appropriateness of asymmetry tests for publication bias in meta‐analysis: a large survey. CMAJ 2007;176(8):1091‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iwashita 2012
- Iwashita T, Lee JG, Shinoura S, Nakai Y, Park DH, Muthusamy VR, et al. Endoscopic ultrasound‐guided rendezvous for biliary access after failed cannulation. Endoscopy 2012;44(1):60‐5. [DOI] [PubMed] [Google Scholar]
Kiriyama 2010
- Kiriyama S, Gabata T, Takada T, Hirata K, Yoshida M, Mayumi T, et al. New diagnostic criteria of acute pancreatitis. Journal of Hepato‐Biliary‐Pancreatic Sciences 2010;17(1):24‐36. [DOI] [PubMed] [Google Scholar]
Kubota 2012
- Kubota K, Sato T, Kato S, Watanabe S, Hosono K, Kobyashi N, et al. Needle‐knife precut papillotomy with a small incision over a pancreatic stent improves the success rate and reduces the complication rate in difficult biliary cannulations. Journal of Hepato‐Biliary‐Pancreatic Sciences 2012;20(3):382‐8. [DOI] [PubMed] [Google Scholar]
Laasch 2003
- Laasch HU, Tringali A, Wilbraham L, Marriott A, England RE, Mutignani M, et al. Comparison of standard and steerable catheters for bile duct cannulation in ERCP. Endoscopy 2003;35(8):669‐74. [DOI] [PubMed] [Google Scholar]
Lachin 2009
- Lachin JM. Futility interim monitoring with control of type I and II error probabilities using the interim Z‐values or confidence limit. Clinical Trials (London, England) 2009;6:565‐73. [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loperfido 1998
- Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, Berardinis F, et al. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointestinal Endoscopy 1998;48(1):1‐10. [DOI] [PubMed] [Google Scholar]
Masci 2003
- Masci E, Mariani A, Curioni S, Testoni PA. Risk factors for pancreatitis following endoscopic retrograde cholangiopancreatography: a meta‐analysis. Endoscopy 2003;35(10):830‐4. [DOI] [PubMed] [Google Scholar]
Mazaki 2010
- Mazaki T, Masuda H, Takayama T. Prophylactic pancreatic stent placement and post‐ERCP pancreatitis: a systematic review and meta‐analysis. Endoscopy 2010;42(10):842‐53. [DOI] [PubMed] [Google Scholar]
Perdue 2004
- Perdue DG, Freeman ML, ERCOST Study Group. Failed biliary ERCP: A prospective multicenter study of risk factors, complications and resource utilization. Gastrointestinal Endoscopy 59;2004:T1479. [Google Scholar]
Pezzilli 2002
- Pezzilli R, Romboli E, Campana D, Corinaldesi R. Mechanisms involved in the onset of post‐ERCP pancreatitis. Journal of the Pancreas 2002;3(6):162‐8. [PubMed] [Google Scholar]
Pocock 2006
- Pocock SJ. Current controversies in data monitoring for clinical trials. Clinical Trials (London, England) 2006;3:513‐21. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shah 2012
- Shah JN, Marson F, Weilert F, Bhat YM, Nguyen‐Tang T, Shaw RE, et al. Single‐operator, single‐session EUS‐guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointestinal Endoscopy 2012;75(1):56‐64. [DOI] [PubMed] [Google Scholar]
Siegel 1989
- Siegel JH, Ben‐Zvi JS, Pullano W. The needle knife: a valuable tool in diagnostic and therapeutic ERCP. Gastrointestinal Endoscopy 1989;35(6):499‐503. [DOI] [PubMed] [Google Scholar]
Skude 1976
- Skude G, Wehlin L, Maruyama T, Ariyama J. Hyperamylasaemia after duodenoscopy and retrograde cholangiopancreatography. Gut 1976;17(2):127‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tenner 2013
- Tenner S, Baillie J, DeWitt J, Vege SS, American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. American Journal of Gastroenterology 2013;108(9):1400‐15. [DOI] [PubMed] [Google Scholar]
Testoni 1999
- Testoni PA, Bagnolo F, Caporuscio S, Lella F. Serum amylase measured four hours after endoscopic sphincterotomy is a reliable predictor of postprocedure pancreatitis. American Journal of Gastroenterology 1999;94(5):1235‐41. [DOI] [PubMed] [Google Scholar]
Testoni 2006
- Testoni PA. Facts and fiction in the pharmacologic prevention of post‐ERCP pancreatitis: a never‐ending story. Gastrointestinal Endoscopy 2006;64(5):732‐4. [DOI] [PubMed] [Google Scholar]
Testoni 2010
- Testoni PA, Mariani A, Giussani A, Vailati C, Masci E, Macarri G, et al. Risk factors for post‐ERCP pancreatitis in high‐ and low‐volume centers and among expert and non‐expert operators: a prospective multicenter study. American Journal of Gastroenterology 2010;105(8):1753‐61. [DOI] [PubMed] [Google Scholar]
Testoni 2011
- Testoni PA, Testoni S, Giussani A. Difficult biliary cannulation during ERCP: how to facilitate biliary access and minimize the risk of post‐ERCP pancreatitis. Digestive and Liver Disease 2011;43(8):596‐603. [DOI] [PubMed] [Google Scholar]
Tse 2012
- Tse F, Yuan Y, Moayyedi P, Leontiadis GI. Guidewire‐assisted cannulation of the common bile duct for the prevention of post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD009662.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Udd 2010
- Udd M, Kylanpaa L, Halttunen J. Management of difficult bile duct cannulation in ERCP. World Journal of Gastrointestinal Endoscopy 2010;2(3):97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vandervoort 2002
- Vandervoort J, Soetikno RM, Tham TC, Wong RC, Ferrari AP Jr, Montes H, et al. Risk factors for complications after performance of ERCP. Gastrointestinal Endoscopy 2002;56(5):652‐6. [DOI] [PubMed] [Google Scholar]
Varadarajulu 2006
- Varadarajulu S, Kilgore ML, Wilcox CM, Eloubeidi MA. Relationship among hospital ERCP volume, length of stay, and technical outcomes. Gastrointestinal Endoscopy 2006;64(3):338‐47. [DOI] [PubMed] [Google Scholar]
Wang 2009
- Wang P, Li ZS, Liu F, Ren X, Lu NH, Fan ZN, et al. Risk factors for ERCP‐related complications: a prospective multicenter study. American Journal of Gastroenterology 2009;104(1):31‐40. [DOI] [PubMed] [Google Scholar]
Williams 2007a
- Williams EJ, Taylor S, Fairclough P, Hamlyn A, Logan RF, Martin D, et al. Are we meeting the standards set for endoscopy? Results of a large‐scale prospective survey of endoscopic retrograde cholangio‐pancreatograph practice. Gut 2007;56(6):821‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2007b
- Williams EJ, Taylor S, Fairclough P, Hamlyn A, Logan RF, Martin D, et al. Risk factors for complication following ERCP; results of a large‐scale, prospective multicenter study. Endoscopy 2007;39(9):793‐801. [DOI] [PubMed] [Google Scholar]
Working Group IAP/APA Acute Pancreatitis 2013
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence‐based guidelines for the management of acute pancreatitis. Pancreatology 2013;13(4 Suppl 2):e1‐15. [DOI] [PubMed] [Google Scholar]
Yadav 2002
- Yadav D, Agarwal N, Pitchumoni CS. A critical evaluation of laboratory tests in acute pancreatitis. American Journal of Gastroenterology 2002;97(6):1309‐18. [DOI] [PubMed] [Google Scholar]


