Abstract
Members of the GATA family of zinc finger transcription factors have been shown to play important roles in the control of gene expression in a variety of cell types. GATA-1, -2, and -3 are expressed primarily in hematopoietic cell lineages and are required for proliferation and differentiation of multiple hematopoietic cell types, whereas GATA-4, -5, and -6 are expressed in the heart, where they activate cardiac muscle structural genes. Friend of GATA-1 (FOG) is a multitype zinc finger protein that interacts with GATA-1 and serves as a cofactor for GATA-1-mediated transcription. FOG is coexpressed with GATA-1 in developing erythroid and megakaryocyte cell lineages and cooperates with GATA-1 to control erythropoiesis. We describe a novel FOG-related factor, FOG-2, that is expressed predominantly in the developing and adult heart, brain, and testis. FOG-2 interacts with GATA factors, and interaction of GATA-4 and FOG-2 results in either synergistic activation or repression of GATA-dependent cardiac promoters, depending on the specific promoter and the cell type in which they are tested. The properties of FOG-2 suggest its involvement in the control of cardiac and neural gene expression by GATA transcription factors.
Members of the GATA family of zinc finger transcription factors control differentiation of a wide range of cell types (reviewed in references 5 and 24). GATA-1, -2, and -3 are expressed primarily in hematopoietic cell lineages, where GATA-1 regulates erythroid and megakaryocyte differentiation (26, 31), GATA-2 controls proliferation of hematopoietic progenitors (35), and GATA-3 controls development of T lymphocytes (33).
GATA-4, -5, and -6 have been shown to play multiple roles in cardiac muscle. Overexpression of GATA-4, -5 and -6 in Xenopus embryos can prematurely activate cardiac gene expression (12), and overexpression of GATA-6 results in an increase in heart size (7). Conversely, inhibition of GATA-4 expression in P19 cells blocks cardiac muscle differentiation (9). GATA-4 knockout mice also show severe defects in cardiac development due to a block to ventral morphogenesis of the embryo (14, 23). Recent studies have also revealed a role for GATA-4 in cardiac hypertrophy mediated, at least in part, by interaction between GATA-4 and NFAT3 (21).
The GATA factors share homology within two zinc fingers of the Cys-X2-Cys-X17-Cys-X2-Cys type and bind preferentially the DNA sequence (A/T)GATA(A/G) (6, 34). GATA binding sites are contained in the control regions of erythroid cell- and megakaryocyte-specific genes (reviewed in reference 24) and of numerous cardiac regulatory and structural genes (8, 11, 19, 22, 30, 32).
Mutational analyses of GATA-1 initially demonstrated that the carboxyl-terminal zinc finger mediates DNA binding, whereas the amino-terminal zinc finger was not required for DNA binding or transcriptional activation but was essential for erythroid differentiation (38, 39). These results suggested that the amino-terminal finger may mediate interaction with an essential transcriptional cofactor. Subsequent studies showed that the amino-terminal finger of GATA-1 interacts with a novel multitype zinc finger protein, called Friend of GATA-1 (FOG) (36). FOG is coexpressed with GATA-1 in developing hematopoietic cell lineages, and knockout mice lacking FOG fail to form megakaryocytes (37). FOG does not appear to bind DNA alone, but it can cooperate with GATA-1 to synergistically activate transcription from at least one hematopoietic cell-specific regulatory region and induce erythroid cell and megakaryocyte differentiation in vitro (37). These findings suggest an interdependent relationship between GATA-1 and FOG, such that both factors cooperate to activate hematopoietic gene expression.
Because FOG is not expressed in the cardiac lineage, we considered the possibility that the cardiac tissue-expressed GATA factors (16) cooperate with an essential FOG-related factor to regulate cardiac gene expression and development. To explore this possibility, we searched expressed sequence tag (EST) databases for FOG-related cDNA sequences that might encode cardiac tissue-restricted FOG-like proteins. Here we describe a novel FOG-related factor, FOG-2, which is expressed at highest levels in the heart, brain, and testis. FOG-2 contains eight zinc fingers which share high homology with those of FOG and interacts with members of the GATA family of transcription factors in vivo and in vitro. On certain cardiac promoters, FOG-2 enhances transcriptional activation by GATA-4, whereas on others, it inhibits transcriptional activation. The expression pattern and functions of FOG-2 suggest that it acts as an important cofactor for GATA-mediated transcriptional activation in cardiac and neural cell lineages.
MATERIALS AND METHODS
Database searches, cDNA cloning, and DNA sequencing.
The amino acid sequence of FOG was used to screen the EST database for potential related genes, and several novel human and mouse EST clones were identified. ESTs W12035 (mouse) and R57596 (human) both share homology with the first and second zinc fingers of FOG; ESTs AA231039 (mouse) and AA442019 (human) are related to the sixth zinc finger; ESTs R13039, F12732, R35921, T75115, AA247618, and AA248280 are related to the ninth zinc finger of FOG. All of these EST clones were from mouse embryo, human heart, and brain cDNA libraries.
Clone W12035 was used to further screen a mouse 10-day embryonic heart cDNA library (Stratagene, La Jolla, Calif.). Several positive clones were isolated, subcloned, and sequenced. All clones were overlapping and encoded the same gene, FOG-2. All of the other ESTs listed above were also found to correspond to FOG-2 sequences.
RNA analysis and in situ hybridization.
Whole-mount and section in situ hybridizations were performed as described previously (20). The FOG-2 3′ untranslated region and a 5′ probe corresponding to zinc fingers 4 and 5 gave the same pattern of hybridization. The 5′ probe was also used for whole-mount in situ hybridization.
A multitissue Northern blot (Clontech, Palo Alto, Calif.) was hybridized with 32P-labeled probes made from EST clones W12035, AA231039, and AA437527, using Rapid-Hyb buffer (Amersham, Ill.).
Interaction assays.
An NcoI-XhoI fragment of FOG-2 (corresponding to zinc fingers 1 to 8) and a SalI-XhoI fragment (corresponding to zinc fingers 5 to 8) were subcloned into yeast GAL4 activation domain vectors pACT2 and pACT, respectively. They were further tested in yeast two-hybrid assays, as described elsewhere (21), with different GATA baits: pAS1-GATA-4, pAS1-GATA-5 (21), and pGBT-GATA-1 (kindly provided by Stuart Orkin, Harvard University).
The full-length FOG-2 coding region was subcloned into the pCDNAI expression vector. pCDNAI-FOG-2 and pCDNAI-GATA-4 were used in coupled in vitro transcription-translation in the presence of [35S]methionine according to the TNT kit protocol (Promega, Wis.). Coimmunoprecipitations were performed as described previously (21). Briefly, 5 μl of TNT reaction mix was immunoprecipitated in a total volume of 100 μl with 5 μl of GATA-4 antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.), together with 25 μl of protein A/G-agarose.
Transfection assays.
An alpha-myosin heavy chain (α-MHC)–luciferase reporter was generated by subcloning a 5.5-kb α-MHC promoter into pGL3 (Promega, Madison, Wis.). Atrial natriuretic factor (ANF) and brain natriuretic protein (BNP) promoters, ANF3003 and BNP2501, respectively, were obtained from C. Glembotski (San Diego State University) and have been described previously (32). The ANF 638-luciferase reporter was from Young Sook Lee. GATA-4, Nkx2-5, and FOG-2 were all expressed in pCDNAI.
Transfection of COS cells was performed with FuGENE 6 (Boehringer Mannheim) according to manufacturer’s instructions. Briefly, 0.4 μg of reporter and 0.2 μg of each activator plasmid were mixed with 2 μl of FuGENE 6 and added to cells in six-well plates. Cells were harvested 48 h following transfection for luciferase assays.
10T1/2 cells were transiently transfected by calcium phosphate precipitation. Two micrograms of reporter and 1 μg of each activator plasmid were transfected into cells in six-well plates. Cells were washed once with phosphate-buffered saline after 16 h and further cultured for 24 h, followed by harvesting and performance of luciferase assays.
Primary neonatal rat cardiomyocytes were prepared from Sprague-Dawley rats obtained at 15 days of gestation. Ventricular cardiomyocytes were prepared by subjecting minced hearts from 2-day-old pups to six rounds of digestion with Pancreatin (1 mg/ml; Sigma) for 20 min at 37°C. The latter five fractions were pooled and preplated in Dulbecco modified Eagle medium-medium 199 (4:1) containing 10% horse serum and 5% fetal bovine serum. Following a 2-h incubation, nonadherent myocytes were plated at a density of 106 cells/well on gelatin-coated six-well dishes. After 18 h in culture, cells were transfected by using Lipofectamine Plus (Gibco-BRL) according to the manufacturer’s instructions. Cells were harvested 36 h posttransfection, and luciferase assays were performed with a Luciferase assay kit (Promega).
In all transfection experiments, the total amount of DNA per well was kept constant by adding the corresponding vector pCDNAI. Hsp-LacZ or CMV-LacZ (LacZ expressed under control of the cytomegalovirus [CMV] promoter) was also cotransfected to normalize for variations in transfection efficiency.
Immunostaining.
The subcellular localization of FOG-2 was determined in Cos cells transiently transfected with a pCDNA3 expression vector encoding full-length FOG-2 with a FLAG epitope tag at the amino terminus. Forty-eight hours following transfection, cells were fixed and stained with mouse anti-FLAG antibody (Kodak, IBI), followed by fluorescein isothiocyanate-labeled horse anti-mouse antibody (Vector Laboratories, Burlingame, Calif.).
Nucleotide sequence accession number. The GenBank accession number for the FOG-2 sequence is AF125166.
RESULTS
Structure of FOG-2.
In an effort to identify FOG-related proteins that might act as cofactors for GATA transcription factors in the heart, we initially searched EST databases by using the amino acid sequence of the sixth zinc finger of FOG, which has been reported to mediate interaction with GATA-1 (37). This search revealed several ESTs with the potential to encode polypeptides related to finger 6 of FOG. We also performed a database search with the full-length FOG sequence and identified additional FOG-related sequences, some of which overlapped the cDNAs encoding finger 6.
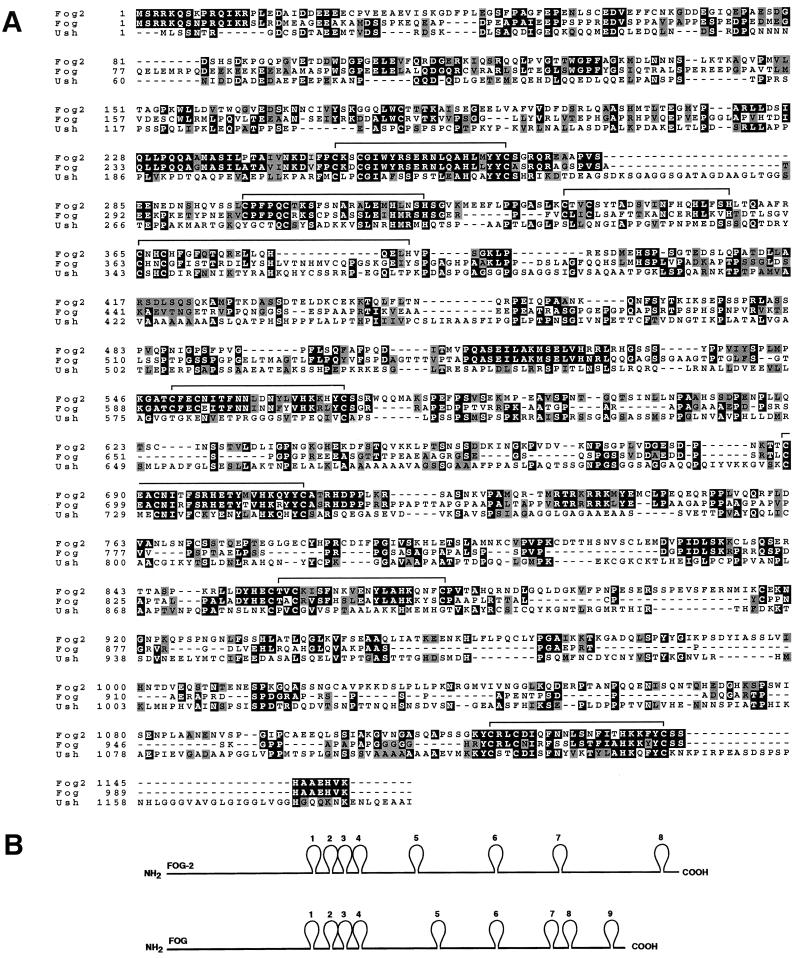
Using each of the identified ESTs, we performed Northern analysis with RNA from adult mouse tissues. All of the clones hybridized to a 5.5-kb transcript in the heart, brain, and testis (see below). We therefore used the partial EST clones to screen an embryonic day 10 (E10) mouse heart cDNA library for longer cDNAs. From this screen, we isolated a cDNA with the potential to encode a protein of 1,151 amino acids, Mr of 127,692, and pI of 6.1 (Fig. 1A). Stop codons upstream of the first methionine in this sequence indicated that it represented the entire protein. We call this protein FOG-2.
FIG. 1.
Homology between FOG-2, FOG, and Ush. (A) Amino acid alignment of FOG-2, FOG, and Ush proteins. Positions of zinc fingers in FOG-2 are overlined. (B) Schematic representation of FOG and FOG-2.
FOG-2 contains eight putative zinc fingers, four of the C2H2 type and four of the C2HC type (Fig. 1B; Table 1). By comparison, FOG contains nine zinc fingers, with finger 8 missing from FOG-2. While the zinc fingers are the most conserved domains of FOG and FOG-2, there is also substantial homology in the intervening regions, as well as at the amino and carboxyl termini. Between finger 5 and the carboxyl terminus, FOG-2 contains a greater number of amino acids separating the fingers and accounting for its larger size than FOG. Like FOG, FOG-2 shared homology with the Drosophila zinc finger protein U-shaped (Ush), which serves as a cofactor for the GATA factor Pannier (3, 10). The highest homology between FOG-2 and Ush is within zinc fingers 6 and 8 (Fig. 1A).
TABLE 1.
Zinc fingers in FOG-2
| Finger | Type | Amino acid position |
|---|---|---|
| 1 | C2HC | 252–273 |
| 2 | C2H2 | 298–320 |
| 3 | C2H2 | 337–357 |
| 4 | C2H2 | 365–385 |
| 5 | C2HC | 550–571 |
| 6 | C2HC | 689–710 |
| 7 | C2HC | 856–877 |
| 8 | C2HC | 1121–1142 |
Expression of FOG-2 mRNA in embryos and adult tissues.
By Northern analysis of adult mouse tissues, we detected a FOG-2 transcript of approximately 5.5 kb in the heart, brain, and testis (Fig. 2). Prolonged exposure to film also revealed a low level of FOG-2 transcripts in the liver. This clearly differs from FOG, which is expressed in the liver, spleen, and testis but not in the heart, brain, lung, kidney, or skeletal muscle (36). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts were measured as a control for RNA loading (Fig. 2).
FIG. 2.
Expression of FOG-2 mRNA in adult mouse tissues. FOG-2 transcripts were detected in adult tissues by Northern blotting (top). GAPDH transcripts were measured as a control for RNA loading (bottom).
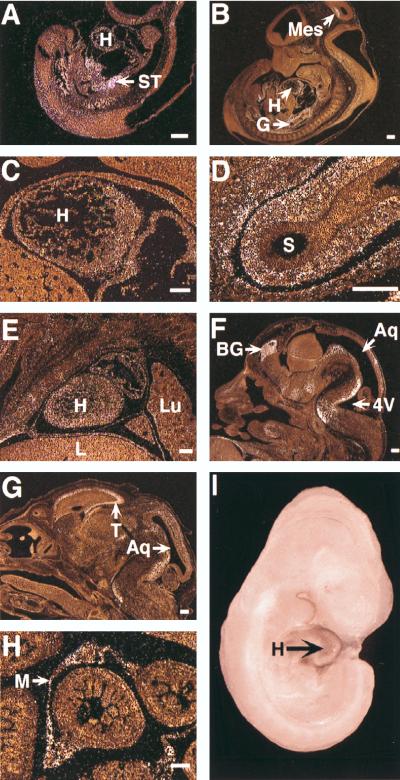
To define the expression pattern of FOG-2 during embryogenesis, we performed in situ hybridization on staged mouse embryos. At E9.5, signal was most evident in the septum transversum surrounding the developing hepatic primordium and within the undifferentiated mesenchyme surrounding the developing foregut (Fig. 3A). There was also a low level of expression in the cardiac myocytes of the ventricle and bulbus cordis at this stage. The neuroepithelium was negative, but there were scattered cells within the associated mesenchyme which were positive.
FIG. 3.
Expression of FOG-2 mRNA during mouse development. Dark-field images of in situ hybridization of sagittal sections of E9.5 (A), E11.5 (B to D), E13.5 (E and F), and E15.5 (G and H) embryos illustrate FOG-2 expression. Panels C and D are higher magnifications of panel B. At E9.5 (A), signal predominates in the septum transversum. At E11.5, signal is evident in the undifferentiated wall of the foregut (D) and in the ventricle and atrium (C). At E13.5, distinct foci of expression are evident in the brain (F) and to a lesser degree in the heart (E). At E15.5, focal expression in the telencephalon, tectum tegmentum, and pons (G) is evident. Strong expression is evident in the mesentery of the midgut (H). Whole-mount in situ hybridization of an E10.5 embryo reveals FOG-2 transcripts in the heart (I). Aq, aqueduct; BG, basal ganglia; G, gut; H, heart; L, liver; Lu, lung; M, mesentery; S, stomach; ST, septum transversum; T, telencephalon; 4V, fourth ventricle. Size bars in panels A to H represent 200 μm.
At E10.5, expression remained strong in the mesenchyme surrounding the foregut and in the cells of the septum transversum surrounding the expanding liver. Low expression persisted in the left ventricle. There was also low expression in the ventral aspect of the spinal cord and in the neuroepithelium of the mesencephalon (data not shown). Whole-mount in situ hybridization at E10.5 also clearly revealed FOG-2 transcripts in the heart (Fig. 3I).
At E11.5, FOG-2 transcripts were evident in the remnants of the septum foregut, within the myocardium, and in the tectum of the mesencephalon (Fig. 3B). In the heart, expression was evident within the cardiac myocytes of both the ventricle and atrium, but expression was lower in the atrium than in the ventricle (Fig. 3C). The signal in the gut was localized to the wall of the foregut (Fig. 3D). There was no expression in the midgut proper, but signal persisted in the mesenchyme at the root of the supporting mesentery, as well as in remnants of the septum transversum adjacent to the liver.
At E13.5, strong signal was evident within discrete foci in the basal ganglia, hypothalamus, tegmentum, and pons and at a low level in the mesenchyme of the anterior body wall, jaw, and nasal cavities (Fig. 3F). The signal persisted at low levels within the myocardium of both the ventricle and atrium (Fig. 3E).
At E15.5, strong expression was evident in the brain, specifically in the telencephalon, tectum tegmentum, and pons (Fig. 3G). Signal remained in the mesenteries supporting the midgut (Fig. 3H). Signal in the heart continued to decline thereafter.
FOG-2 interacts directly with GATA factors.
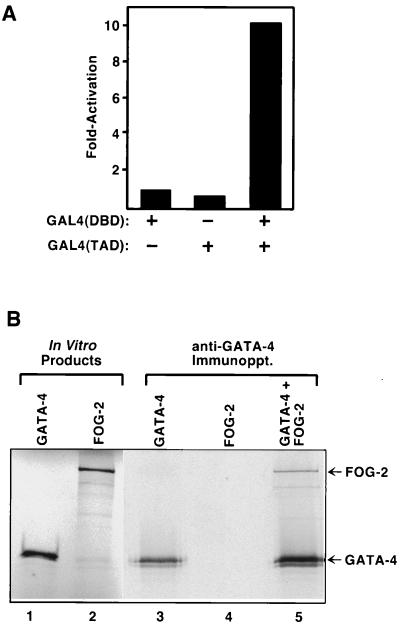
To determine whether FOG-2, like FOG, interacts with GATA transcription factors, we performed a series of yeast two-hybrid assays using various GATA factors fused to the GAL4 DNA binding domain as bait. A chimeric protein containing amino acids 130 to 409 of mouse GATA-4, encompassing the two zinc fingers, fused to the GAL4 DNA binding domain [GAL4(DBD)-GATA-4 (130–409)], failed to activate a lacZ reporter under control of GAL4 (UAS-lacZ) in yeast (Fig. 4A). Zinc fingers 1 to 8 of FOG-2 fused to the GAL4 transcription activation domain [GAL4(TAD)–FOG-2] were also inactive in this assay. However, coexpression of GAL4(DBD)–GATA-4 and GAL4(TAD)–FOG-2 resulted in strong activation of lacZ, indicative of interaction between GATA-4 and FOG-2 (Fig. 4A).
FIG. 4.
Interaction assays between GATA-4 and FOG-2. (A) Two-hybrid assays demonstrate interaction of FOG-2 with GATA factors. Activation of an integrated GAL4-dependent lacZ reporter was assayed in liquid culture as described in Materials and Methods. Yeast cells were transformed with pAS1-GATA-4 and/or pACT-FOG-2, as indicated. (B) Coimmunoprecipitation of GATA-4 and FOG-2. GATA-4 and FOG-2 were translated in vitro in the presence of [35S]methionine, and reaction products were immunoprecipitated (Immunoppt.) with anti-GATA-4 antibody, as indicated.
The C-terminal region of FOG-2, encompassing zinc fingers 5 to 8, interacted as efficiently with GATA-4 as the full-length protein (Table 2). Since FOG has been shown to associate with the N-terminal zinc finger of GATA-1, we further mapped the interacting domain in GATA-4. Two GATA-4 baits, containing amino acids 177 to 302, which contains both zinc fingers, and amino acids 212 to 262, which includes just the N-terminal finger, both interacted strongly with FOG-2. This result suggests that the N-terminal finger of GATA-4 mediates interaction with FOG-2, in a way similar to the GATA-1–FOG interaction. FOG-2 also interacted with GATA-5 and GATA-1 (Table 2), as did FOG. We reported previously that GATA-4 also interacts with NFAT3 (21). The interaction of GATA-4 with FOG-2 appeared to be stronger than that with NFAT3, based on activation of lacZ expression in the two-hybrid assay.
TABLE 2.
Interactions between GATA factors and FOGs in yeast two-hybrid assaysa
| Bait | Interaction with:
|
||
|---|---|---|---|
| GAL4(TAD)–FOG-2
|
GAL4(TAD)-NFAT3 | ||
| Fingers 1–8 | Fingers 5–8 | ||
| GAL4(DBD)–GATA-4 (130–409) | ++ | ++ | + |
| GAL4(DBD)–GATA-4 (177–302) | ++ | NDb | ND |
| GAL4(DBD)–GATA-4 (212–262) | ++ | ND | ND |
| GAL4(DBD)–GATA-5 | ++ | ++ | + |
| GAL4(DBD)–GATA-1 | + | ND | − |
Zinc fingers 1 to 8 or 6 to 8 of FOG-2 were fused to the GAL4 activation domain in pACT and tested for interaction with the indicated GATA factor fused to the GAL4 DNA binding domain, as assayed by activation of an integrated GAL4-dependent lacZ reporter (UAS-lacZ).
ND, not determined.
We also fused FOG-2 to the DNA binding domain of GAL4 and tested for the ability to activate a GAL4-dependent reporter in transfected 10T1/2 and Cos cells, but observed no transcriptional activation (data not shown). Thus, we have no evidence that FOG-2 itself acts as a transcriptional activator.
To test for direct interaction between GATA-4 and FOG-2, we performed immunoprecipitation experiments using GATA-4 and FOG-2 translated in vitro. As shown in Fig. 4B, FOG-2 was efficiently coimmunoprecipitated with GATA-4 in an assay using an anti-GATA-4 antibody, further confirming their direct interaction.
Effects of FOG-2 on transcriptional activation of cardiac promoters by GATA-4.
As a first step toward investigating the function of FOG-2, we determined its subcellular localization by using a FLAG epitope-tagged protein. In transfected Cos cells, FLAG–FOG-2 was localized to the nucleus (Fig. 5), consistent with its potential role as a modulator of GATA-dependent transcription.
FIG. 5.
Localization of FOG-2 to the nucleus. Cos cells were transiently transfected with an expression vector encoding FOG-2 with a FLAG epitope tag at the amino terminus. Staining with mouse anti-FLAG antibody followed by fluorescein isothiocyanate-conjugated horse anti-mouse antibody showed that FOG-2 was localized exclusively to the nucleus.
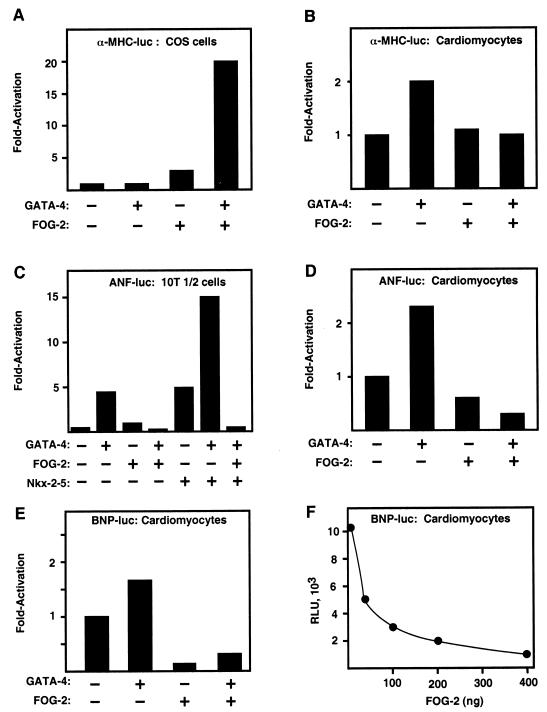
To begin to investigate the functional significance of GATA-4 interaction with FOG-2, we performed transient transfection assays with a luciferase reporter linked to the α-MHC, ANF, and BNP promoters, which have previously been shown to require GATA-4 binding for activation in cardiomyocytes (8, 22, 32). In transiently transfected Cos cells, the α-MHC promoter is expressed at background levels. GATA-4 and FOG-2 alone had little or no effect on activity of the α-MHC promoter in Cos cells, whereas together the two factors resulted in a 20-fold increase in promoter activity (Fig. 6A). The α-MHC promoter shows high activity in primary neonatal rat cardiomyocytes and was upregulated only about twofold in the presence of exogenous GATA-4 (Fig. 6B). Exogenous FOG-2 alone had no effect on MHC promoter activity, but FOG-2 prevented upregulation by GATA-4.
FIG. 6.
Effects of FOG-2 on cardiac muscle promoters. 10T1/2 cells, Cos cells, and neonatal rat cardiomyocytes were cotransfected with luciferase reporter constructs driven by sequences in the α-MHC, BNP, or ANF promoter (200 ng) in the absence or presence of the indicated expression vectors (300 ng). In all cases, transfection mixtures included a CMV-LacZ expression plasmid (200 ng) to normalize for transfection efficiency, and total input DNA was normalized by using empty pcDNA3.1 (Invitrogen). Reporter gene expression in panels A to E is depicted as the fold change in expression relative to the reporter gene without added GATA-4 or FOG-2. Panel F shows a titration of increasing amounts of FOG-2 expression plasmid, which results in dose-dependent inhibition of BNP-luciferase expression. Values in panel F are expressed as relative light units (RLU).
GATA-4 upregulated ANF promoter activity about fivefold in transiently transfected 10T1/2 cells, whereas in the presence of FOG-2, the ANF promoter failed to respond to GATA-4 (Fig. 6C). Previous studies showed that GATA-4 cooperates with the cardiac tissue-restricted homeodomain protein Nkx2-5 to activate the ANF promoter (4, 18). This cooperativity of transcriptional activation was also blocked by FOG-2 in 10T1/2 cells (Fig. 6C). GATA-4 activates the ANF promoter two- to threefold in cardiomyocytes, as reported previously (32), and this activation is prevented by FOG-2 (Fig. 6D).
The BNP promoter is highly active in primary cardiomyocytes and showed only about a 50% increase in activity in the presence of GATA-4 (Fig. 6E). FOG-2 resulted in a nearly 10-fold reduction in activity of the BNP promoter in the absence of added GATA-4, and it inhibited activity in the presence of GATA-4. Inhibition of BNP promoter activity by FOG-2 was dose dependent (Fig. 6F). In contrast to its varied effects on cardiac promoters, FOG-2 had no effect on activity of the CMV promoter, which was used to drive expression of lacZ as an internal control in transfections. Together, these results demonstrate that FOG-2 can enhance or repress transcription and that its effects are promoter and cell type specific.
DISCUSSION
FOG-2 is a novel multitype zinc finger protein expressed primarily in the heart, brain, and testis. FOG-2 interacts directly with GATA factors to modulate GATA-dependent transcriptional activation and can act as an activator or repressor, depending on promoter and cell type. Our results demonstrate that FOG-2 can cooperate with GATA-4 to activate the α-MHC promoter in Cos cells, whereas it inhibits activation of the α-MHC promoter by GATA-4 in primary cardiomyocytes. FOG-2 also inhibited activation of the ANF and BNP promoters in cardiomyocytes, Cos cells, and 10T1/2 cells. These results suggest that FOG-2 may exert different effects on different promoters, depending on the spectrum of other factors required for activation of a particular promoter. Alternatively, the repression of certain promoters by FOG-2 in transfection assays may result from overexpression, causing squelching by titrating out other factors that are limiting for transcriptional activation. We favor the idea that FOG-2 may act as a scaffold to coordinate interactions among multiple transcription factors.
FOG has been proposed to act as a positive cofactor for GATA-1, based on its ability to cooperate with GATA-1 to transactivate the erythroid cell-specific NF-E2 promoter and activate erythroid cell and megakaryocyte differentiation in GATA-1 null embryonic stem cells (36). Conversely, in the absence of FOG, there is a failure of erythropoiesis that resembles that in GATA-1 null embryos (37). However, the phenotypes of GATA-1 and FOG knockout mice are not identical, which indicates that FOG and GATA-1 can act independently. FOG mutant mice, for example, show a much more profound defect in megakaryocyte development than is seen in GATA-1 null mice, whereas the effects of GATA-1 deletion on differentiation of erythroid differentiation appear more pronounced than in FOG mutant embryos (26, 37, 39). FOG is also not required for the control of hematopoietic progenitor cell proliferation or T-lymphocyte development by GATA-2 and GATA-3, respectively, indicating that these GATA factors can act through a FOG-independent mechanism.
Consistent with our finding that FOG-2 can repress GATA-dependent transcription, there is also evidence that GATA factors play important roles as repressors of gene expression. GATA-1, for example, which induces erythroid differentiation, represses myelomonocytic markers (13). In addition, several genes involved in cell cycle progression, such as c-myb and c-myc, are downregulated during erythroid differentiation (2, 15). Thus, the ability of FOG-2 to inhibit several GATA-dependent promoters may reflect a role as a negative coregulator of certain GATA-dependent genes in cardiac and neural cell lineages. It is notable in this regard that the FOG proteins are structurally related to a Drosophila zinc finger factor, Ush, which interacts with the amino-terminal zinc finger of the Drosophila GATA factor Pannier (3, 10, 28). Pannier is a transcriptional activator required for formation of sensory bristles. Ush antagonizes the actions of Pannier by acting as a transcriptional repressor.
Under what circumstances might FOG-2 repress GATA-dependent genes in the heart? In the mouse, the α-MHC gene is expressed continuously in the atria throughout development (29). A few days before birth, α-MHC is also upregulated in the ventricle, where expression persists throughout postnatal life (29). In contrast, ANF is expressed in the atria and ventricles during embryonic and fetal development (1). However, ANF is switched off in ventricular cells postnatally, whereas the atrial expression remains high, thus establishing the adult expression pattern (1). Our results, which show that FOG-2, together with GATA-4, activates α-MHC expression but represses ANF expression, raise the possibility that FOG-2 and GATA-4 participate in chamber-specific or stage-specific transcriptional regulatory programs.
In contrast to FOG, which is restricted to developing hematopoietic cell lineages, FOG-2 is expressed predominantly in developing heart and brain during embryogenesis. FOG-2 expression is first detected in the developing cardiac tube at E9.0, and expression is maintained throughout the myocardium during embryogenesis and postnatal development. Considering the importance of GATA-4 for cardiac morphogenesis and myogenesis, it is likely that FOG-2 plays an important role in GATA-dependent transcriptional activation in the developing heart. FOG-2 expression becomes detectable in neurons within the brain and neural tube beginning at about E10.5. Several members of the GATA family have been shown to be expressed in the brain and to activate various brain-specific promoters (17, 25, 40), which also suggests a potential role for FOG-2 in regulation of neural genes.
Given the roles of GATA factors in cardiac development and hypertrophy, it will be particularly interesting to determine whether FOG-2 is an essential component of these GATA-dependent transcriptional regulatory pathways. Moreover, since upregulation of ANF and BNP is a hallmark of cardiac hypertrophy, and FOG-2 can repress both genes, we are presently investigating whether it functions as a negative regulator of hypertrophy.
ACKNOWLEDGMENTS
We thank Stuart Orkin for providing reagents and communicating results prior to publication. We also acknowledge A. Tizenor for assistance with graphics, W. Simpson for editorial assistance, J. Starke and R. Wells for histologic preparations, and Zhi-Ping Liu for two-hybrid constructs.
This work was supported by grants from NIH, the Robert A. Welch Foundation, the Texas Advanced Technology Program, and the American Heart Association to E.N.O.
ADDENDUM IN PROOF
After submission of this paper, two other papers described the cloning of FOG-2: one by Tevosian et al. (S. Tevosian, A. Deconinck, A. Cantor, H. Hir, Y. Fujiwara, G. Corfas, and S. H. Orkin, Proc. Natl. Acad. Sci. USA 96:950–955, 1999) and one by Sevensson et al. (E. C. Sevensson, R. L. Tufts, C. E. Polk, and J. M. Leiden, Proc. Natl. Acad. Sci. USA 96:956–961, 1999).
REFERENCES
- 1.Argentin S, Ardati A, Trembley S, Lihrmann I, Robitaille L, Drouin J, Nemer M. Developmental stage-specific regulation of atrial natriuretic factor gene transcription in cardiac cells. Mol Cell Biol. 1994;14:777–790. doi: 10.1128/mcb.14.1.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke M F, Kukowska-Latallo J F, Westin E, Smith M, Prochownik E V. Constitutive expression of a c-myb cDNA blocks Friend murine erythroleukemia cell differentiation. Mol Cell Biol. 1988;8:884–892. doi: 10.1128/mcb.8.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cubadda Y, Heitzler P, Ray R P, Bourouis M, Ramain P, Gelbart W, Simpson P, Haenlin M. u-shaped encodes a zinc finger protein that regulates the proneural genes achaete and scute during the formation of bristles in Drosophila. Genes Dev. 1997;11:3083–3095. doi: 10.1101/gad.11.22.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durocher D, Chen C-Y, Ardati A, Schwartz R J, Nemer M. The atrial natriuretic factor promoter is a downstream target for Nkx2.5 in the myocardium. Mol Cell Biol. 1996;16:4648–4655. doi: 10.1128/mcb.16.9.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans T. Regulation of cardiac gene expression by GATA4/5/6. Trends Cardiovasc Med. 1997;7:75–83. doi: 10.1016/S1050-1738(97)00010-8. [DOI] [PubMed] [Google Scholar]
- 6.Evans T, Felsenfeld G. The erythroid-specific transcription factor eryfl: a new finger protein. Cell. 1998;58:877–885. doi: 10.1016/0092-8674(89)90940-9. [DOI] [PubMed] [Google Scholar]
- 7.Gove C, Walmsley M, Nijjar S, Bertwistle D, Guille M, Partington G, Bomford A, Patient R. Over-expression of GATA-6 in Xenopus embryos blocks differentiation of heart precursors. EMBO J. 1997;16:355–368. doi: 10.1093/emboj/16.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grepin C, Dagnino L L, Robitaille L, Haberstroh L L, Antakely T, Nemer M. A hormone-encoding gene identifies a pathway for cardiac but not skeletal muscle gene transcription. Mol Cell Biol. 1994;14:3115–3129. doi: 10.1128/mcb.14.5.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grepin C, Robitaille L, Antakely T, Nemer M. Inhibition of transcription factor GATA-4 expression blocks in vitro cardiac muscle differentiation. Mol Cell Biol. 1995;15:4095–4102. doi: 10.1128/mcb.15.8.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haenlin M, Cubadda Y, Blondeau P, Heitzler P, Lutz P, Simpson P, Ramain P. Transcriptional activity of Pannier is regulated negatively by heterodimerization of the GATA DNA-binding domain with a cofactor encoded by the u-shaped gene of Drosophila. Genes Dev. 1997;11:3096–3108. doi: 10.1101/gad.11.22.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ip H S, Wilson D B, Heikinheimo M, Tang Z, Ting C N, Simon M C, Leiden J M, Parmacek M S. The GATA-4 transcription factor transactivates the cardiac muscle-specific troponin C promoter-enhancer in nonmuscle cells. Mol Cell Biol. 1994;14:7517–7526. doi: 10.1128/mcb.14.11.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Y, Evans T. The Xenopus GATA-4/5/6 genes are associated with cardiac specification and can regulate cardiac-specific transcription during embryogenesis. Dev Biol. 1996;174:257–270. doi: 10.1006/dbio.1996.0071. [DOI] [PubMed] [Google Scholar]
- 13.Kulessa H, Frampton J, Graf T. GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes Dev. 1995;9:1250–1262. doi: 10.1101/gad.9.10.1250. [DOI] [PubMed] [Google Scholar]
- 14.Kuo C T, Morrisey E E, Anandappa R, Sigrist K, Lu M M, Parmacek M S, Soudais C, Leiden J M. The GATA-4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1998;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 15.Lachman H M, Skoultchi A I. Expression of c-myc changes during differentiation of mouse erythroleukemia cells. Nature. 1984;310:592–594. doi: 10.1038/310592a0. [DOI] [PubMed] [Google Scholar]
- 16.Laverriere A C, MacNeill C, Mueller C, Poelmann R E, Burch J B, Evans T. GATA-4/5/6, a subfamily of three transcription factors transcribed in developing heart and gut. J Biol Chem. 1998;269:23177–23184. [PubMed] [Google Scholar]
- 17.Lawson M A, Whyte D B, Mellon P L. GATA factors are essential for activity of the neuron-specific enhancer of the gonadotropin-releasing hormone gene. Mol Cell Biol. 1996;16:3596–3605. doi: 10.1128/mcb.16.7.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y, Shioi T, Kasahara H, Jobe S M, Wiese R J, Markham B E, Izumo S. The cardiac tissue-restricted homeobox protein Csx/Nkx2.5 physically associates with the zinc finger protein GATA4 and cooperatively activates atrial natriuretic factor gene expression. Mol Cell Biol. 1998;18:3120–3129. doi: 10.1128/mcb.18.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lien C-L, Wu C, Mercer B, Webb R, Richardson J A, Olson E N. Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development. 1999;126:25–84. doi: 10.1242/dev.126.1.75. [DOI] [PubMed] [Google Scholar]
- 20.Lu J-R, Richardson J A, Olson E N. Capsulin: a novel bHLH transcription factor expressed in epicardial progenitors and mesenchyme of visceral organs. Mech Dev. 1998;73:23–32. doi: 10.1016/s0925-4773(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 21.Molkentin J D, Lu J, Antos C L, Markham B, Richardson J, Robbins J, Grant S, Olson E N. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molkentin J D, Kalvakalanou D, Markham B E. Transcription factor GATA-4 regulates cardiac muscle-specific expression of the α-cardiac myosin heavy-chain gene in vivo. Mol Cell Biol. 1994;14:4947–4957. doi: 10.1128/mcb.14.7.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molkentin J D, Lin Q, Duncan S A, Olson E N. Requirement of the transcription factor GATA-4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 24.Orkin S H. Hematopoiesis: how does it happen? Curr Opin Cell Biol. 1995;7:870–877. doi: 10.1016/0955-0674(95)80072-7. [DOI] [PubMed] [Google Scholar]
- 25.Pandolfi P P, Roth M E, Karis A, Leonard M W, Dzierzak E, Grosveld F G, Engel J D. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat Genet. 1995;11:40–44. doi: 10.1038/ng0995-40. [DOI] [PubMed] [Google Scholar]
- 26.Pevny L, Simon M C, Robertson E, Klein W H, Tsai S F, D’Agati V, Orkin S H, Constantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 27.Pevny L, Lin C S, D’Agati V, Simon M C, Orkin S H, Constantini F. Development of hematopoietic cells lacking transcription factor GATA-1. Development. 1995;121:163–172. doi: 10.1242/dev.121.1.163. [DOI] [PubMed] [Google Scholar]
- 28.Ramain P, Heitzler P, Haenlin M, Simpson P. Pannier, a negative regulator of achaete and scute in Drosophila, encodes, a zinc finger protein with homology to the vertebrate transcription factor GATA-1. Development. 1993;119:1277–1291. doi: 10.1242/dev.119.4.1277. [DOI] [PubMed] [Google Scholar]
- 29.Robbins J. Altering cardiac function via transgenesis. Trends Cardiovasc Med. 1997;7:185–191. doi: 10.1016/S1050-1738(97)00048-0. [DOI] [PubMed] [Google Scholar]
- 30.Searcy R D, Vincent E B, Liberatore C M, Yutzey K E. A GATA-dependent Nkx2-5 regulatory element activates early cardiac gene expression in transgenic mice. Development. 1998;125:4461–4470. doi: 10.1242/dev.125.22.4461. [DOI] [PubMed] [Google Scholar]
- 31.Shivdasani R A, Fujiwara Y, McDevitt M A, Orkin S H. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997;16:3965–3973. doi: 10.1093/emboj/16.13.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thuerauf D J, Hanford D S, Glembotski C C. Regulation of rat brain natriuretic peptide transcription. J Biol Chem. 1994;269:17772–17775. [PubMed] [Google Scholar]
- 33.Ting C N, Olson M C, Barton K P, Leiden J M. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature. 1996;384:474–478. doi: 10.1038/384474a0. [DOI] [PubMed] [Google Scholar]
- 34.Tsai S F, Martin D J, Zon L I, D’Andrea A D, Wong G G, Orkin S H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989;339:446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- 35.Tsai S F, Keller G, Kuo C T, Weiss M, Chen J, Rosenblatt M, Alt F W, Orkin S H. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 36.Tsang A P, Visvader J E, Turner C A, Fujiwara Y, Yu C, Weiss M, Crossley M, Orkin S H. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell. 1997;90:109–119. doi: 10.1016/s0092-8674(00)80318-9. [DOI] [PubMed] [Google Scholar]
- 37.Tsang A P, Fujiwara Y, Hom D B, Orkin S H. Failure of megakaryopoiesis and arrested erythropoiesis in mice lacking the GATA-1 transcriptional cofactor FOG. Genes Dev. 1998;12:1176–1188. doi: 10.1101/gad.12.8.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visvader J E, Crossley M, Hill J, Orkin S H, Adams J M. The C-terminal zinc finger of GATA-1 or GATA-2 is sufficient to induce megakaryocytic differentiation of an early myeloid cell line. Mol Cell Biol. 1995;15:634–641. doi: 10.1128/mcb.15.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss M, Yu C, Orkin S H. Erythroid-cell-specific properties of transcription factor GATA-1 revealed by phenotypic rescue of a gene-targeted cell line. Mol Cell Biol. 1997;17:1642–1651. doi: 10.1128/mcb.17.3.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamato M, Ko L J, Leonard M W, Beug H, Orkin S H, Engel J D. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990;4:1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]