Abstract
Background:
The purpose of this study was to provide updated estimates of the average lifetime medical cost per infection for chlamydia, gonorrhea, and trichomoniasis.
Methods:
We adapted a published decision tree model that allowed for 7 possible outcomes of infection: (1) symptomatic infection, treated, no sequelae; (2) symptomatic infection, not treated, sequelae; (3) symptomatic infection, not treated, no sequelae; (4) asymptomatic infection, treated, sequelae; (5) asymptomatic infection, treated, no sequelae; (6) asymptomatic infection, not treated, sequelae; and (7) asymptomatic infection, not treated, no sequelae. The base case values and ranges we applied for the model inputs (i.e., the probability and cost assumptions) were based on published studies.
Results:
The estimated lifetime medical cost per infection for men and women, respectively, was $46 (95% credibility interval: $32–62) and $262 ($127–483) for chlamydia, $78 ($36–145) and $254 ($96–518) for gonorrhea, and $5 ($1–14) and $36 ($17–58) for trichomoniasis. Cost estimates for men were most sensitive to assumptions regarding the probability that the infection is symptomatic, the probability of treatment if asymptomatic, and the cost of treatment of infection. Cost estimates for chlamydia and gonorrhea in women were most sensitive to assumptions regarding the probability and cost of subsequent pelvic inflammatory disease (PID).
Conclusions:
These estimates of the lifetime medical cost per infection can inform updated estimates of the total annual cost of sexually transmitted infections (STIs) in the United States, as well as analyses of the value and cost-effectiveness of STI prevention interventions.
Summary:
The estimated lifetime medical cost per infection for men and women, respectively, was $46 and $262 for chlamydia, $78 and $254 for gonorrhea, and $5 and $36 for trichomoniasis.
Introduction
Chlamydia and gonorrhea are the two most common nationally notifiable conditions in the United States.1 Trichomonas vaginalis (trichomoniasis) is a common sexually transmitted protozoal infection, although it is not a nationally notifiable condition.1 In 2018, there were about 1.8 million reported chlamydia cases and 600,000 reported gonorrhea cases; in 2016, there were an estimated 220,000 medical provider visits related to trichomoniasis.1 These infections can increase the risk of HIV transmission and can lead to sequelae such as epididymitis in men and pelvic inflammatory disease (PID) or adverse outcomes of pregnancy in women.1–3
In addition to substantial morbidity, these three sexually transmitted infections (STIs) impose a sizeable economic burden in terms of direct medical costs attributable to treatment of infections and sequelae in untreated or inadequately treated infections. In a previous study, the total annual medical costs of chlamydia, gonorrhea, and trichomoniasis was estimated at $703 million (in 2010 US dollars), in terms of the expected lifetime medical costs of infections acquired in 2008.3 The purpose of this study was to provide updated estimates of the average lifetime medical cost per infection for chlamydia, gonorrhea, and trichomoniasis.
Methods
Study question and perspective
For each of the three STIs we examined (chlamydia, gonorrhea, and trichomoniasis), the specific study question we addressed was: What is the average lifetime medical cost per incident infection? Specifically, we estimated the lifetime medical costs that will arise, on average, from one incident infection in an adolescent or adult (e.g., the lifetime medical cost per chlamydial infection in females). We limited our analysis to direct medical costs associated with treatment of infection and related sequelae.
We assessed costs from the health care sector perspective, thereby including all direct medical costs regardless of whether the costs were paid by the patient, an insurance company, a government agency, or any other payer. Costs beyond the health care sector perspective, such as patient time and travel costs, were excluded. We did not include costs associated with reinfection or with transmission of infection to another person, as reinfections and secondary infections were considered to be new infections and not part of the index infection. We also did not include costs of STI prevention, such as chlamydia and gonorrhea screening. However, when applicable, we did include diagnostic testing costs incurred by patients treated for infection.
Decision tree model
We used a decision tree model (Figure 1) adapted from a previous model described by Owusu-Edusei and colleagues (2013).3 We applied the same decision tree model to each of the three STIs (chlamydia, gonorrhea, and trichomoniasis), but the model inputs (i.e., the probability and cost assumptions) applied in the decision tree varied across the three STIs. The base case values and ranges we applied for the model inputs were based on numerous published studies (Table 1),1,3–13 as described in more detail below.
Figure 1: Decision tree model used to estimate the lifetime medical cost of chlamydia, gonorrhea, and trichomoniasis, per infection.
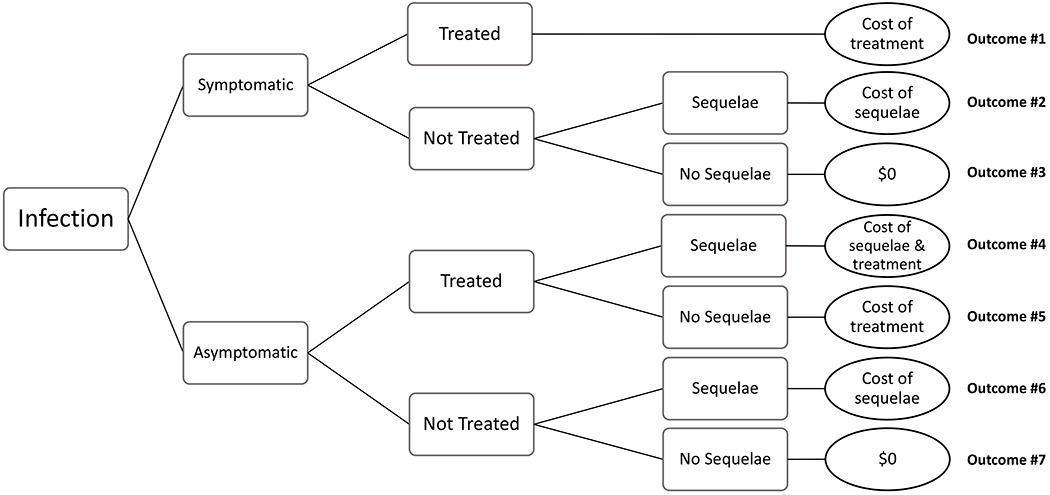
There were 7 possible outcomes in our decision tree model: (1) symptomatic infection, treated, no sequelae; (2) symptomatic infection, not treated, sequelae; (3) symptomatic infection, not treated, no sequelae; (4) asymptomatic infection, treated, sequelae; (5) asymptomatic infection, treated, no sequelae; (6) asymptomatic infection, not treated, sequelae; and (7) asymptomatic infection, not treated, no sequelae. For chlamydia and gonorrhea, we included the possibility of sequelae in men (epididymitis) and women (pelvic inflammatory disease, which included the possibility of chronic pelvic pain, ectopic pregnancy, and infertility). We assumed no sequelae attributable to trichomoniasis.
Table 1.
Probabilities, costs, and other inputs used in decision tree analysis of the lifetime medical cost of chlamydia, gonorrhea, and trichomoniasis in men and women, per infection: Base case value (range)
| Model input | Chlamydia | Gonorrhea | Trichomoniasis | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Men | Women | Men | Women | Men | Women | |
| Probabilities | ||||||
| Probability that infection is symptomatic* | 0.158 (0.082–0.262) | 0.254 (0.177–0.344) | 0.589 (0.314–0.830) | 0.314 (0.155–0.510) | 0.081 (0.051–0.224) | 0.192 (0.118–0.328) |
| Probability of treatment, symptomatic infection* | 0.936 (0.893–0.967) | 0.894 (0.855–0.931) | 0.744 (0.621–0.846) | 0.750 (0.631–0.846) | 0.406 (0.150–0.749) | 0.876 (0.586–0.949) |
| Probability of treatment, asymptomatic infection* | 0.137 (0.092–0.213) | 0.241 (0.208–0.276) | 0.020 (0.012–0.034) | 0.068 (0.043–0.111) | 0.000 | 0.000 |
| Probability of sequelae, treated symptomatic infection† | 0.003 | 0.003 | 0.003 | 0.003 | Not applicable | Not applicable |
| Probability of sequelae, treated asymptomatic infection† | 0.003 | 0.063 (0.01–0.12) | 0.003 | 0.063 (0.01–0.12) | Not applicable | Not applicable |
| Probability of sequelae, untreated infection† | 0.026 (0.01–0.04) | 0.129,10 (0.02–0.24) | 0.026 (0.01–0.04) | 0.129,10 (0.02–0.24) | Not applicable | Not applicable |
| Cost and cost-related inputs | ||||||
| Treatment of infection, non-STI clinic‡ | $1607 (134–186) | $1527 (134–170) | $1743,7 (102–319) | $1273,7 (72–237) | $1588 (145–171) | $2208 (209–231) |
| Treatment of infection, STI clinic§ | $953,11 (80–110) | $953,11 (80–110) | $953,11 (80–110) | $953,11 (80–110) | $953,11 (80–110) | $953,11 (80–110) |
| Proportion treated in STI clinic setting | 0.1011 | 0.0401 | 0.1201 | 0.0671 | 0.110 | 0.053 |
| Treatment of infection, average across settings¶ | $153 (129–178) | $150 (132–168) | $165 (99–294) | $125 (73–228) | $151 (138–164) | $213 (202–225) |
| Treatment of sequelae‖ (Epididymitis among men, PID among women) |
$37512 (232–518) |
$2,454 (1,913–3,677)3,13 |
$37512 (232–518) |
$2,454 (1,913–3,677)3,13 | Not applicable | Not applicable |
These probabilities were obtained from the models of chlamydia, gonorrhea, and trichomoniasis incidence and prevalence4,5 in this Special Issue. For each input, the lower bound, base case, and upper bound values we applied corresponded to the 2.5th percentile, median, and 97.5th percentile, respectively, from the applicable model. See the appendix and these source studies4,5 for more details.
As in the 2013 STI cost study by Owusu-Edusei and colleagues,3 we assumed (1) that the probability of sequelae following treatment of symptomatic infections was 0, under the reasoning that these infections are likely treated promptly, (2) that the probability of epididymitis was 0 for treated infections (asymptomatic and symptomatic), and (3) that no epididymitis or PID was attributable to trichomoniasis regardless of treatment status or symptom status. For chlamydia, the probability of PID was obtained from a model-based synthesis10 of data from the POPI (Prevention of Pelvic Infection) trial.9 Following the Owusu-Edusei study3 we assumed the same probability of PID for gonorrhea as for chlamydia.
For chlamydia, the base case estimate of the cost of treatment for infection was obtained from a recent study of medical claims data for chlamydia and gonorrhea treatment,7 the upper bound was obtained the Owusu-Edusei study,3 and the lower bound was selected such that the base case estimate would be the midpoint of the lower and upper bound values. These choices for the lower and upper bound values allowed us to consider a wider range of possible values than implied by the 95% confidence intervals of the recent medical claims study ($153 to $167 for men; $148 to $156 for women).7 For gonorrhea, the lower bound value was obtained from the recent medical claims study,7 the upper bound value was obtained from the Owusu-Edusei study,3 and the base case value was a weighted average of these two values, in which the estimate from the more recent medical claims study was given a weight of 2/3 and the Owusu-Edusei estimate was given a weight of 1/3. For trichomoniasis, the base case value and range was obtained from a recent study of medical claims data for trichomoniasis treatment.8
For the cost of treatment of infection in STI clinic settings, we applied the same base case value and range for all three STIs. The lower bound value reflects the physician visit cost applied in the Owusu-Edusei study,3 the base case value was approximated using data on cost projections for an clinic offering a standard set of STI services (we used a slightly lower cost per clinic visit of $95 rather the $101 estimate implied by the source study for 2,153 visits under the assumption that a higher clinic volume could reduce the average cost incurred per STI clinic visit),11 and the upper bound value was calculated such that the base case value would be the midpoint of the range.
The average cost of treatment across settings (STI-clinic and non-STI clinic) was calculated based on the cost assumptions listed in the table for the STI clinic and non-STI clinic settings, along with the assumptions shown for the percentage of treated cases that are treated in an STI clinic. For trichomoniasis, the proportion treated in an STI clinic calculated as the average of the proportions listed for chlamydia and gonorrhea.
For epididymitis, the base case cost estimate was calculated as the average of $341 and $409, which were the average cost estimates for patients in the “≥13 to <41 years” age group and patients in the “≥41 years” age group, respectively, as reported in a medical claims study.12 The upper bound was based on the cost estimates for patients in the “<13 years” age group from that same study, and the lower bound value was selected such that the base case value would be the midpoint of the range. For PID, the base case value was calculated as shown in Table 2, the lower bound value was obtained from Gift and Rein (2004),13 and the upper bound value was obtained from the Owusu-Edusei study3 as described in the manuscript text.
PID: pelvic inflammatory disease; STI: sexually transmitted infection
All costs are in 2019 US dollars.
The decision tree begins when an infection is acquired and ends when the infection is treated or when the infection is cleared naturally. For simplicity, we assumed treatment was 100% effective against infection. For most infections, the accrual of costs ended when the infection was treated or was cleared naturally. However, the decision tree did allow for the accrual of sequelae-related costs even after the infection was treated or cleared naturally.
Probabilities applied in model
The first two probabilities in the decision tree model were the (1) probability that the infection was symptomatic and (2) probability that the infection was treated. Values for these two probabilities were obtained from models used to estimate the incidence and prevalence of chlamydia, gonorrhea, and trichomoniasis in the United States.4,5 The third key probability used in the decision tree was the probability of sequelae. Specifically, for chlamydia and gonorrhea, we included the possibility of PID in women and epididymitis in men. We assumed the probability of these sequelae depended on whether the infection was symptomatic, and whether the infection was treated (Table 1).
We applied a base case probability of PID of 12% (range 2% to 24%) in women with untreated chlamydia, as suggested by a model-based synthesis10 of data from the POPI (Prevention of Pelvic Infection) trial.9 We applied this model-based estimate of 12% because it reflects the probability that an incident infection will lead to PID,10 which was more applicable to our decision tree analysis than the original POPI trial data that reflected the probability that a prevalent infection will lead to PID. We also assumed that the probability of PID for a treated asymptomatic infection was 50% that of an untreated asymptomatic infection.3 This assumption was generally consistent with a model-based synthesis of evidence from randomized controlled trials which suggested that annual screening could prevent 61% of PID attributable to chlamydia.10 Owing to limited data on the probability of sequelae attributable to gonorrhea, our assumptions regarding the probability of sequelae for gonorrhea were the same as for chlamydia.3 Similarly, due to limited data on the causal role of trichomoniasis in PID,3,14 we did not include any potential sequelae for trichomoniasis (i.e., the probability of sequelae was 0 for trichomoniasis as shown in Table 1).
Costs applied in model
The cost estimates in this analysis were updated for inflation to 2019 US dollars using the personal consumption expenditures price index for health care (https://www.bea.gov/). We assumed about 4% to 12% of treated infections would be treated at STI clinics,1 and that treatment in an STI clinic setting would be less costly than treatment in other settings (Table 1).3,11 For the cost of treatment of infection in non-STI clinic settings (cost of physician visit plus cost of medication), we applied cost estimates obtained from recent studies of medical claims data among a privately-insured population,7,8 except for gonorrhea. For gonorrhea, the cost of treatment we applied was based on estimates from two studies: (1) a more recent study ($102 for men and $72 for women) which included the cost of ceftriaxone injection and azithromycin prescription (or the cost of cefixime prescription and azithromycin prescription for those receiving the alternative regimen)7 and (2) an earlier study ($319 for men and $237 for women, updated to 2019 dollars) in which fluroquinolones made up over 95% of the prescription drug claims.3 We applied a weighted average ($174 for men, $127 for women) from these two studies in which the more recent study was given twice as much weight as the older study.
The cost estimates we applied for PID included the possibility of incurring treatment costs for chronic pelvic pain, ectopic pregnancy, and tubal factor infertility in the years after onset of PID (Table 2). The lower bound cost estimate for PID of $1,913 reflects Gift and Rein’s (2004)13 suggested conservative interpretation of the results of the PID cost study by Yeh and colleagues (2003).15 The upper bound cost estimate for PID of $3,677 is consistent with the Owusu-Edusei 2013 cost study,3 which applied a less conservative interpretation of the PID cost study by Yeh and colleagues (2003).15 The base case value of $2,454 we calculated for this study (Table 2) incorporates data from a range of sources,15–23 including data that have been published since the Owusu-Edusei 2013 cost study.3 However, this new base case value we calculated was well within the lower and upper bound values reported by previous studies.
Table 2.
Estimated average lifetime medical cost per case of pelvic inflammatory disease (PID) used in estimating the average lifetime medical cost of chlamydia and gonorrhea, per infection
| Outcome (type of treatment, if applicable) | Probability of outcome, per case of PID |
Cost per outcome (not discounted) |
Number of years outcome is discounted |
Discounted cost per outcome |
Contribution to average cost per case of PID |
|---|---|---|---|---|---|
| PID (outpatient treatment) | 0.90016 | $77616,18 | 023 | $776 | $698 |
| PID (inpatient treatment) | 0.10016 | $8,50316,18 | 023 | $8,503 | $850 |
| Ectopic pregnancy | 0.07617 | $5,04819,20 | 516 | $4,354 | $331 |
| Tubal factor infertility | 0.10017 | $12,56421,22 | 1016 | $9,349 | $421 |
| Chronic pelvic pain | 0.18017 | $90416 | 216 | $852 | $153 |
| Total of all outcomes | Not applicable | Not applicable | Not applicable | Not applicable | $2,454 |
We assumed that all PID would be treated, and that 90% would be treated on an outpatient basis and 10% inpatient.16 We assumed PID would occur within one year of infection on average, following a previous study that assumed no years of lag time between infection and PID.23 We assumed that among women with PID, 7.6% of women would have an ectopic pregnancy, 10% of women would have tubal factor infertility, and 18% of women would have chronic pelvic pain; these three outcomes of PID were expected to occur 5, 10, and 2 years after PID, respectively.16 The probabilities of ectopic pregnancy, tubal factor infertility, and chronic pelvic pain in this table are expressed per case of PID. In the review by Ong and colleagues (2016),17 these probabilities were expressed as 0.76%, 0.998%, and 1.8%, respectively, per untreated chlamydial infection; we divided these values by 10% (the probability of PID per untreated chlamydial infection in the Ong study) to arrive at the probabilities per case of PID shown in this table.
For PID and tubal factor infertility, the cost per outcome reflects the average of the two sources listed. For ectopic pregnancy, the cost per outcome reflects the value applied by Canestaro and colleagues (2017),20 which was adapted from Trussell and colleagues (2013).19
The discounted cost per outcome shows the cost of the outcome, discounted to the time of PID at a rate of 3% annually. For example, the cost of chronic pelvic pain was discounted for 2 years, and the discounted value of $852 was calculated by dividing $904 by the term [1.03 × 1.03]. For each outcome except tubal factor infertility, the value in the final column was calculated by multiplying the probability of the outcome by the discounted cost per outcome. The value in the final column for tubal factor infertility was calculated by multiplying the probability of infertility by the discounted cost of infertility, multiplied by 0.45 under the assumption that 45% of women with infertility would seek treatment for infertility.15
Costs are in 2019 US dollars.
Calculating the average, discounted lifetime medical cost per infection
There were 7 possible outcomes in our decision tree model: (1) symptomatic infection, treated, no sequelae; (2) symptomatic infection, not treated, sequelae; (3) symptomatic infection, not treated, no sequelae; (4) asymptomatic infection, treated, sequelae; (5) asymptomatic infection, treated, no sequelae; (6) asymptomatic infection, not treated, sequelae; and (7) asymptomatic infection, not treated, no sequelae (Figure 1). Of note, there would have been 8 outcomes in our decision tree had we included the possibility of sequelae among those with treated symptomatic infection. We calculated the lifetime medical cost per infection as the sum of the cost of the 7 outcomes we considered, multiplied by the probability that the outcome would occur. Specifically, the equation we used was , where the subscript i denotes the outcome number (Figure 1). This equation can be described as follows: The average lifetime medical cost per infection was calculated as (cost of outcome 1 x probability of outcome 1) plus (cost of outcome 2 x probability of outcome 2) plus (cost of outcome 3 x probability of outcome 3), and so on through all seven outcomes listed above and shown in Figure 1. These calculations were performed for each STI (chlamydia, gonorrhea, and trichomoniasis) and were stratified by sex.
Discounting of future costs
Future costs were discounted to the year of infection at an annual rate of 3%. We assumed that when treatment of infection occurred, it would typically occur within one year of infection, and thus we did not discount the costs of treatment of chlamydia, gonorrhea, and trichomoniasis. The estimated cost per case of PID that we applied (Tables 1 and 2) reflects the lifetime medical cost per case of PID discounted to the onset of PID at an annual rate of 3%. In applying the PID costs to the decision tree model, we did not further discount these costs, under the assumption that the time from infection to onset of PID would be less than 1 year on average.23 Similarly, we did not discount the cost of epididymitis, under the assumption that the time from infection to onset of epididymitis would be less than 1 year on average as well.23
One-way sensitivity analyses
We conducted one-way sensitivity analyses to examine how the lifetime medical cost estimates changed when we varied one probability or cost input at a time, while holding all other inputs at their base case values. In the one-way sensitivity analyses for chlamydia and gonorrhea, the following 7 inputs were varied one at a time: (1) the probability that the infection is symptomatic; (2) the probability of treatment for symptomatic infections; (3) the probability of treatment for asymptomatic infections; (4) the probability of sequelae for treated asymptomatic infections; (5) the probability of sequelae for untreated infections; (6) the cost of treatment of infection; and (7) the cost of treatment of sequelae. For example, when varying the cost of treating sequelae in the one-way sensitivity analyses, we first estimated the lifetime medical cost per infection when the cost of epididymitis and PID were set to their lower-bound values and all other model inputs were kept at their base case values. We then estimated the lifetime medical cost per infection when the cost of epididymitis and PID were set to their upper-bound values and all other inputs were kept at their base case values. In the one-way sensitivity analyses for trichomoniasis, we did not vary the sequelae-related parameters (i.e., cost of treating sequelae and probability of sequelae), as we assumed no sequelae attributable to trichomoniasis.
Probabilistic sensitivity analyses
We also conducted probabilistic sensitivity analyses in which we simultaneously varied all of the key model inputs listed above in our description of the one-way sensitivity analyses. Specifically, for each of the three STIs, we performed 10,000 simulations of the lifetime medical cost per infection, each time drawing a random value for all inputs according to the distributions described in the appendix for these inputs. As is common in health economic analyses, we assumed a beta distribution for the probability inputs and a lognormal distribution for the cost inputs,24,25 and distribution parameters for each input were determined according to the input’s base case value and range listed in Table 1 (see appendix for more details).
Results
Chlamydia
The estimated lifetime medical cost per chlamydial infection was $46 for men and $262 for women (Table 3) under base case assumptions. For men, most infections (71.2%) were asymptomatic and resolved without treatment and with no sequelae, and about $23 of the average $46 cost per infection was accounted for by treatment of symptomatic infection (Table 4). For women, an untreated asymptomatic infection leading to PID was a relatively uncommon outcome (6.8% of infections), but this outcome accounted for $167 of the average $262 cost per infection (Table 4). In the one-way sensitivity analyses, the lifetime medical cost estimate for men was most sensitive to assumptions regarding the probability that an infection is symptomatic, the probability of treatment among those with asymptomatic infection, and the cost of treatment of infection (Figure 2). For women, the most influential model inputs were the probability of sequelae among untreated infections and the cost of sequelae (Figure 2). In the probabilistic sensitivity analyses, the lifetime medical cost per chlamydial infection ranged from $32 to $62 for men and from $127 to $483 for women in 95% of the 10,000 calculations (Table 3, Figure 3), a range we refer to as the “95% credibility interval.”
Table 3.
Estimated lifetime medical cost of chlamydia, gonorrhea, and trichomoniasis, per infection in men and women, 2019 US dollars: Results of base case analyses and probabilistic sensitivity analyses
| STI | Results of base case analyses | Results of probabilistic sensitivity analyses |
||||
|---|---|---|---|---|---|---|
| 25th–75th percentiles of estimates | 2.5th–97.5th percentiles of estimates | |||||
| Men | Women | Men | Women | Men | Women | |
| Chlamydia | 46 | 262 | 40–51 | 195–312 | 32–62 | 127–483 |
| Gonorrhea | 78 | 254 | 58–94 | 173–313 | 36–145 | 96–518 |
| Trichomoniasis | 5 | 36 | 2–7 | 28–43 | 1–14 | 17–58 |
In estimating the average lifetime medical cost per infection, future costs were discounted to the time of infection at a rate of 3% annually. Cost estimates have been rounded to the nearest dollar.
Table 4.
Summary of results of decision tree analysis of costs of chlamydia, gonorrhea, and trichomoniasis: Components of lifetime medical cost per infection
| Outcome of infection | Percentage of infections in which outcome occurs | Contribution of outcome to average cost per infection | ||
|---|---|---|---|---|
|
| ||||
| Men | Women | Men | Women | |
| Chlamydia | ||||
| Symptomatic infection, treated, no sequelae | 14.8% | 22.7% | $22.63 | $34.06 |
| Symptomatic infection, not treated, sequelae | 0.0% | 0.3% | $0.08 | $7.93 |
| Symptomatic infection, not treated, no sequelae | 1.0% | 2.4% | $0.00 | $0.00 |
| Asymptomatic infection, treated, sequelae | 0.0% | 1.1% | $0.00 | $28.09 |
| Asymptomatic infection, treated, no sequelae | 11.5% | 16.9% | $17.65 | $25.35 |
| Asymptomatic infection, not treated, sequelae | 1.5% | 6.8% | $5.45 | $166.74 |
| Asymptomatic infection, not treated, no sequelae | 71.2% | 49.8% | $0.00 | $0.00 |
| Total of all outcomes of chlamydial infection | 100.0% | 100.0% | $45.80 | $262.17 |
| Gonorrhea | ||||
| Symptomatic infection, treated, no sequelae | 43.8% | 23.6% | $72.31 | $29.44 |
| Symptomatic infection, not treated, sequelae | 0.3% | 0.9% | $1.13 | $23.12 |
| Symptomatic infection, not treated, no sequelae | 14.8% | 6.9% | $0.00 | $0.00 |
| Asymptomatic infection, treated, sequelae | 0.0% | 0.3% | $0.00 | $7.22 |
| Asymptomatic infection, treated, no sequelae | 0.8% | 4.4% | $1.36 | $5.48 |
| Asymptomatic infection, not treated, sequelae | 0.8% | 7.7% | $3.02 | $188.28 |
| Asymptomatic infection, not treated, no sequelae | 39.5% | 56.3% | $0.00 | $0.00 |
| Total of all outcomes of gonococcal infection | 100.0% | 100.0% | $77.81 | $253.53 |
| Trichomoniasis | ||||
| Symptomatic infection, treated | 3.3% | 16.8% | $4.97 | $35.82 |
| Symptomatic infection, not treated | 4.8% | 2.4% | $0.00 | $0.00 |
| Asymptomatic infection, not treated | 91.9% | 80.8% | $0.00 | $0.00 |
| Total of all outcomes of Trichomonas vaginalis infection | 100.0% | 100.0% | $4.97 | $35.82 |
Costs are in 2019 US dollars.
Figure 2: Tornado diagram showing results of the one-way sensitivity analyses of the estimated lifetime medical cost per chlamydial infection in males (top) and females (bottom).
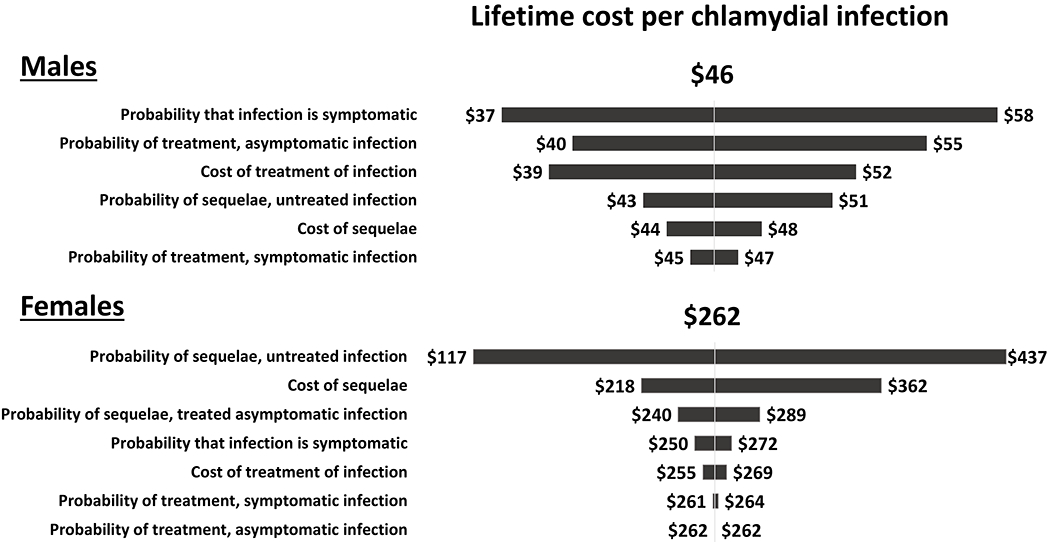
This diagram shows how the estimated lifetime medical cost per chlamydial infection changed from the base case result of $46 for males and $262 for females when one model parameter value was varied at a time and all other parameters were kept at their base case values listed in Table 1. For example, for males, the lifetime cost per infection varied from $37 to $58 when the probability that the infection was symptomatic was varied from its lower bound value of 0.082 to its upper bound value of 0.262. Note the scale is different for males and females.
Figure 3: Cumulative density function of the results of the probabilistic sensitivity analyses of the estimated lifetime cost (per infection) for chlamydia, gonorrhea, and trichomoniasis in males (left) and females (right).
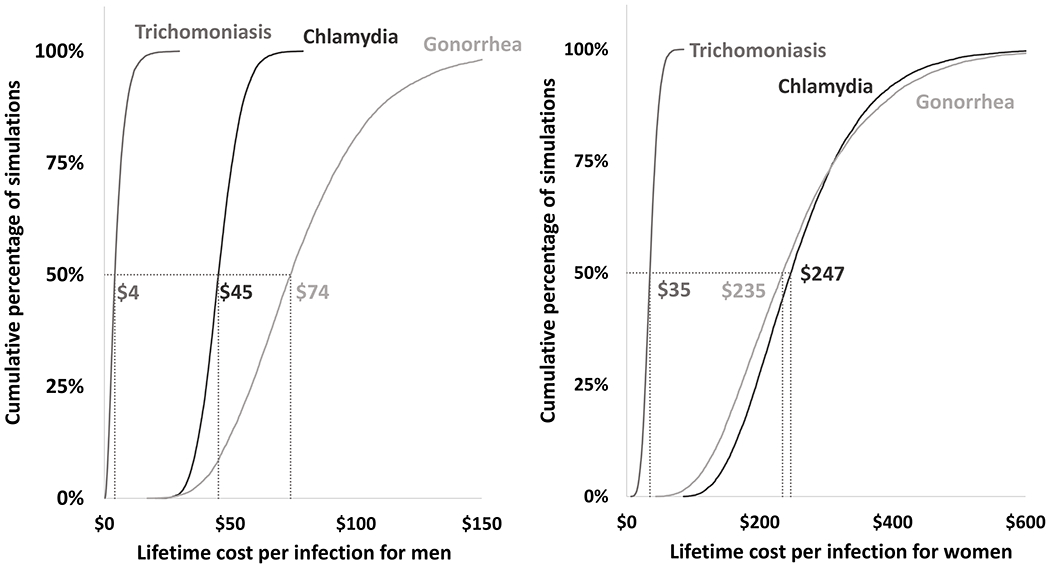
The lifetime cost per infection was estimated 10,000 times in the probabilistic sensitivity analysis. In all 10,000 simulations the model inputs listed in the tornado diagrams (7 for chlamydia and gonorrhea and 3 for trichomoniasis) were varied simultaneously according to the distributions listed in the appendix. The median results are highlighted for each STD. For example, for males, the lifetime cost per chlamydial infection was $45 or lower in 50% of the 10,000 simulations. The median results were similar to but did not precisely match the base case results in Table 3, which were calculated with all model inputs set at their base case values.
Gonorrhea
The estimated lifetime medical cost per gonococcal infection was $78 for men and $254 for women (Table 3) under base case assumptions. For men, $72 of the average $78 cost per infection was accounted for by treatment of symptomatic infection (Table 4). For women, an untreated asymptomatic infection leading to PID was a relatively uncommon outcome (7.7% of infections), but this outcome accounted for $188 of the average $254 cost per infection (Table 4). In the one-way sensitivity analyses, the lifetime medical cost estimate for men was most sensitive to the cost of treatment of infection and the probability that an infection is symptomatic (Figure 4). For women, the most influential assumptions were the probability of sequelae among untreated infections and the cost of sequelae (Figure 4). In the probabilistic sensitivity analyses, the 95% credibility interval for the lifetime medical cost per gonococcal infection was $36 to $145 for men and $96 to $518 for women (Table 3, Figure 3).
Figure 4: Tornado diagram showing results of the one-way sensitivity analyses of the estimated lifetime medical cost per gonococcal infection in males (top) and females (bottom).
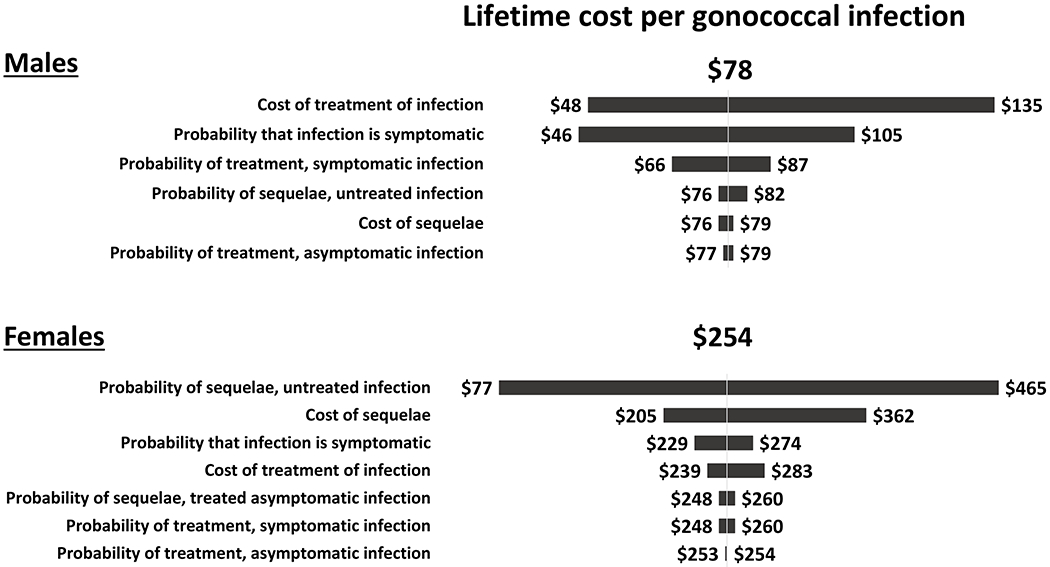
This diagram shows how the estimated lifetime medical cost per gonococcal infection changed from the base case result of $78 for males and $254 for females when one model parameter value was varied at a time and all other parameters were kept at their base case values listed in Table 1. For example, for females, the lifetime cost per infection varied from $77 to $465 when the probability of sequelae in an untreated infection was varied from its lower bound value of 0.02 to its upper bound value of 0.24. Note the scale is different for males and females.
Trichomoniasis
The estimated lifetime medical cost per Trichomonas vaginalis infection was $5 for men and $36 for women (Table 3) under base case assumptions. For men and women, treatment of symptomatic infection accounted for 100% of the lifetime medical cost per infection (Table 4), and results were most sensitive to the probability of symptomatic infection and the probability of treatment of symptomatic infections (Figure 5). In the probabilistic sensitivity analyses, the 95% credibility interval for the lifetime medical cost per Trichomonas vaginalis infection was $1 to $14 for men and $17 to $58 for women (Table 3, Figure 3).
Figure 5: Tornado diagram showing results of the one-way sensitivity analyses of the estimated lifetime medical cost per Trichomonas vaginalis infection in males (top) and females (bottom).
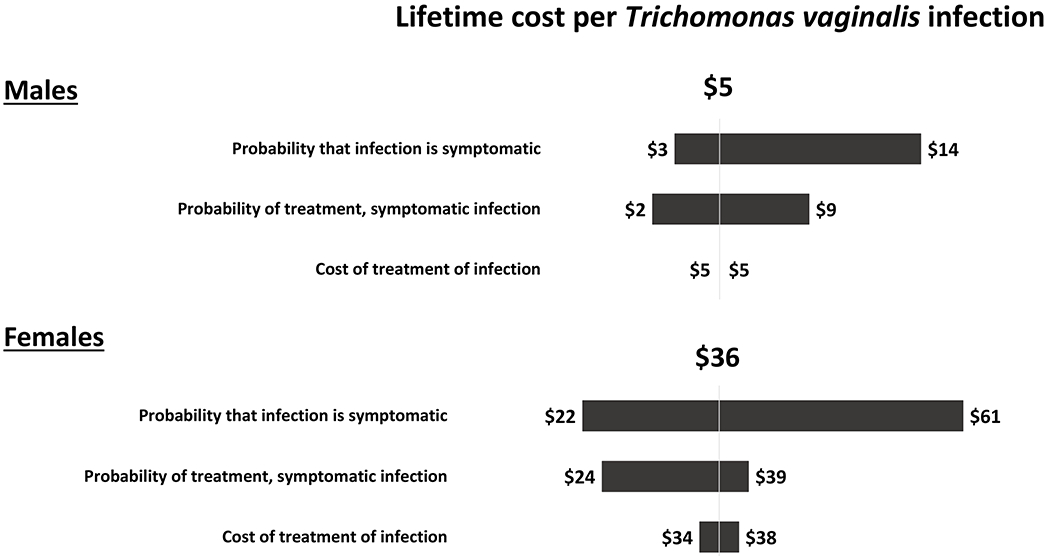
This diagram shows how the estimated lifetime medical cost per Trichomonas vaginalis infection changed from the base case result of $5 for males and $36 for females when one model parameter value was varied at a time and all other parameters were kept at their base case values listed in Table 1. For example, for females, the lifetime cost per infection varied from $22 to $61 when the probability that the infection was symptomatic was varied from its lower bound value of 0.118 to its upper bound value of 0.328. Note the scale is different for males and females.
Discussion
Our estimates of the average lifetime cost per chlamydial infection can be interpreted as the present value of the direct medical treatment costs that could be averted by preventing a single instance of chlamydia acquisition in an adolescent or adult, in the context of current chlamydia screening and prevention efforts in the United States. Our estimates for gonorrhea and trichomoniasis can be interpreted in an analogous manner. Our results are most applicable for estimating the annual burden of STIs at the national, state, or local level. That is, for a given STI (chlamydia, gonorrhea, trichomoniasis), our estimate of the lifetime cost per infection, when multiplied by the estimated number of incident infections in a given year for the given STI, yields the estimated incidence cost of the given STI.
Our results can also be used in the evaluation of STI prevention interventions and programs. For example, suppose an intervention averts 1,000 chlamydial infections in women and 100 in men, as estimated by a mathematical disease transmission model or by some other method of evaluation. The averted number of infections could be multiplied by the corresponding lifetime cost per infection ($262 in women, $46 in men) to approximate the direct medical costs averted by the intervention ($262,000 in women, $4,600 in men).
One potential test of the validity of the decision tree model we applied for chlamydia and gonorrhea in women is to compare the numbers of cases of PID each year implied by our model to other available estimates. Although we did not specifically estimate the number of cases of PID in our analysis, our decision tree analysis suggested that 8.2% of chlamydial infections and 8.9% of gonococcal infections in women result in PID under base case assumptions. These estimates, when multiplied by an estimated annual number of incident infections of 2,354,000 for chlamydia and 853,000 for gonorrhea among women aged 15–39 years,4,26 suggest about 270,000 PID cases attributable to chlamydia and gonorrhea each year. Unfortunately, limited data exist to inform accurate estimates of the annual number of PID cases resulting from chlamydial and gonococcal infections in the United States.1 The annual number of PID cases implied by our model (270,000) is lower than the range of 300,000 to 800,000 that one would estimate when assuming about one million cases of PID annually 27,28 and when assuming chlamydia and gonorrhea account for a combined 30% to 80%29,30,31s of these cases. However, the annual number of PID cases implied by our model likely exceeds the number of PID cases suggested by PID incidence rates in medical claims data32s and by self-reported lifetime PID incidence,33s depending on how these data are interpreted and extrapolated to the entire population.
Our general approach to estimating the cost per infection was similar to that of the previous cost analysis by Owusu-Edusei and colleagues,3 and we applied several of the same assumptions as applied in their study. However, we incorporated recent data in the cost and probability assumptions in our model, where available. For example, we included two studies of medical claims data completed in 2020 that informed our estimated costs of medical care for treatment of chlamydia, gonorrhea, and trichomoniasis.7,8
In addition to incorporating recent data, our analysis featured two key improvements over the previous cost study.3 First, the epidemiologic models4,5 that informed several key probability assumptions in our decision trees (e.g., the probability that an infection is symptomatic, and the probability of treatment of asymptomatic infections) were more rigorous and likely more realistic than the epidemiologic models34s that informed the previous cost study. Second, we conducted detailed sensitivity analyses, including one-way sensitivity analyses and probabilistic sensitivity analyses in which all key model assumptions were varied simultaneously.
The average lifetime medical cost per infection was difficult to estimate with precision because of uncertainties in the probability and cost inputs applied in the decision tree model. For example, the sources we used for the probability of PID for untreated chlamydia suggested a wide range of plausible values (0.02 to 0.24).9,10 When we applied this wide range of values for the probability of PID in the one-way sensitivity analyses, the estimated lifetime medical cost of chlamydia in women (per infection) ranged from $117 to $437. As another example of an uncertain but influential probability input, the probability of symptomatic infection had a notable relative effect on the estimated lifetime medical cost per infection in men for all three STIs we examined, particularly gonorrhea and trichomoniasis.
Many of the cost estimates we applied were subject to considerable uncertainty as well. A wide range of cost estimates per case of PID have been published in the United States,13,15,16,23 in part due to changing health care pracices.35s,36s The difficulty in reaching consensus on the cost of sequelae of PID, such as ectopic pregnancy and infertility, has been documented in international settings as well.17 Similarly, recent estimates of the cost of treating gonorrhea7 are notably lower than previously estimated,3,37s and the reasons for these differences are not fully understood. Accordingly, our base case value for the cost of treating gonorrhea reflected a mix of both the recent and previous estimates.
We made a number of simplifying assumptions in our analyses. For example, we did not include the possibility of sequelae among those with treated symptomatic gonococcal and chlamydial infection, under the assumption that such treatment was likely to occur within such a short period of time after infection that there would be virtually no probability of epididymitis in men and PID in women. Had we included the possibility of sequelae among those with treated symptomatic infections (e.g., due to treatment failure among those with antibiotic resistant gonorrhea), our cost estimates per gonococcal and chlamydial infection would have been higher. As another example, we made the simplifying assumption that the probability of PID for women with gonorrhea was the same as for women with chlamydia, as was done previously.3 However, there is some evidence that rates of sequelae are higher for gonorrhea than chlamydia.38s-40s Thus, our estimates of the cost of gonorrhea in women could be underestimated. The cost of gonorrhea in women (per infection) was $254 under base case assumptions and $465 when assuming a higher probability of PID for untreated infections. Although evidence suggest that repeat chlamydia infections increase the risk of PID,41s,42s we did not stratify our analysis by the lifetime number of infections. Instead, we assumed that the probability of PID we applied in our model, which was informed by a randomized controlled trial,9 reflected an approximation of the average risk of PID across all chlamydial infections (initial and repeat) in the population. Thus, our estimated lifetime cost per infection might more accurately be interpreted as a weighted average of initial and repeat infections.
We did not include all possible lifetime medical costs of chlamydia, gonorrhea, and trichomoniasis. One of the most notable omissions was the costs associated with STI-attributable HIV infections; that is, HIV infections that would not have occurred without the facilitative effects of these STIs (chlamydia, gonorrhea, and trichomoniasis) on HIV transmission and acquisition.43s The estimated STI-attributable HIV costs are indeed substantial, as discussed in more detail elsewhere in this Special Issue.44s,45s For chlamydia and gonorrhea, our estimated cost of PID included the potential costs associated with ectopic pregnancy and tubal factor infertility, but did not include neonatal sequelae, such as conjunctivitis and pneumonia attributable to chlamydia. However, in a cost-effectiveness study of chlamydia screening among women attending family planning clinics, costs associated with neonatal conjunctivitis and pneumonia accounted for less than 2% of the estimated sequelae costs of untreated chlamydial infections in women in the absence of screening.46s We did not include the possibility of sequelae attributable to trichomoniasis, which was done previously.3 Had we included potential adverse outcomes associated with trichomoniasis such as PID and infertility, 2,47s-49s the estimated cost per infection could have been notably higher, particularly for women. Finally, the costs of prevention activities like screening and partner services are not included in our lifetime medical cost estimates, and these investments in prevention likely contribute to substantial reductions in the health and medical cost burden of chlamydia, gonorrhea, and trichomoniasis.50s
In the future, new studies might be able to provide more precise estimates of the probability and cost inputs needed to estimate the lifetime medical costs of chlamydia, gonorrhea, and trichomoniasis. In the meantime, the best way to address uncertainty in the model inputs is to conduct sensitivity analyses. The one-way sensitivity analyses we conducted illustrated the need for more precise estimates of several parameter values, including the percentage of infections in men that are symptomatic, the cost and probability of PID, and the cost of treatment of gonorrhea in men. Thus, additional research in these areas would be especially helpful in developing more reliable estimates of the cost of these STIs. Further, having more reliable estimates of the annual incidence of PID in the United States would help in assessing the validity of our decision tree models for chlamydia and gonorrhea in women. The probabilistic sensitivity analyses we conducted, which simultaneously accounted for uncertainty in all of the key model inputs, yielded the most plausible range of estimates for the lifetime medical cost per infection for each of the three STIs we examined. These ranges will allow users of our cost-per-infection estimates to account for the uncertainty in our results.
Despite limitations and exclusions, our study provides useful, updated estimates of the expected lifetime medical cost per infection of chlamydia, gonorrhea, and trichomoniasis. These estimates were generated specifically to inform updated estimates of the total annual cost of incident STIs in the United States. However, our updated cost estimates are also suited for a range of additional uses, such as in cost-effectiveness analyses of STI prevention interventions and in assessments of the potential value of STI prevention activities.
Supplementary Material
Acknowledgment:
This research was supported, in part, by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention (CDC) administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and CDC.
Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflicts of Interest and Source of Funding: None declared
References
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2018. Atlanta: U.S. Department of Health and Human Services; 2019. [Google Scholar]
- 2.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 3.Owusu-Edusei K Jr., Chesson HW, Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 2013;40:197–201. [DOI] [PubMed] [Google Scholar]
- 4.Kreisel KM, Weston EJ, Cyr SB, et al. Estimates of the prevalence and incidence of chlamydia and gonorrhea among US men and women. Special Issue Sex Transm Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis FM, Spicknall IH, Flagg EW, et al. Estimates of the prevalence and incidence of trichomoniasis among US men and women. Special Issue Sex Transm Dis 2021. [Google Scholar]
- 6.Magid D, Douglas JM Jr., Schwartz JS. Doxycycline compared with azithromycin for treating women with genital Chlamydia trachomatis infections: an incremental cost-effectiveness analysis. Ann Intern Med 1996;124:389–399. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Chesson H, Gift TL. Estimating the direct medical costs and productivity loss of chlamydia and gonorrhea treatment. Sex Transm Dis, published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar S, Chesson H, Gift TL. Estimating the direct medical outpatient costs of diagnosis and treatment of trichomoniasis among commercially insured patients in the United States, 2016-2018. Sex Transm Dis, published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oakeshott P, Kerry S, Aghaizu A, et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. BMJ 2010;340:c1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price MJ, Ades AE, De Angelis D, et al. Risk of pelvic inflammatory disease following Chlamydia trachomatis infection: analysis of prospective studies with a multistate model. Am J Epidemiol 2013;178:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean LT, Montgomery MC, Raifman J, et al. The affordability of providing sexually transmitted disease services at a safety-net clinic. Am J Prev Med 2018;54:552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gift TL, Owens CJ. The direct medical cost of epididymitis and orchitis: evidence from a study of insurance claims. Sex Transm Dis 2006;33(10 Suppl):S84–88. [DOI] [PubMed] [Google Scholar]
- 13.Rein DB, Gift TL. A refined estimate of the lifetime cost of pelvic inflammatory disease. Sex Transm Dis 2004;31:325. [DOI] [PubMed] [Google Scholar]
- 14.Huntington SE, Burns RM, Harding-Esch E, et al. Modelling-based evaluation of the costs, benefits and cost-effectiveness of multipathogen point-of-care tests for sexually transmitted infections in symptomatic genitourinary medicine clinic attendees. BMJ Open 2018;8:e020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh JM, Hook EW 3rd, Goldie SJ. A refined estimate of the average lifetime cost of pelvic inflammatory disease. Sex Transm Dis 2003;30:369–378. [DOI] [PubMed] [Google Scholar]
- 16.Rein DB, Kassler WJ, Irwin KL, Rabiee L. Direct medical cost of pelvic inflammatory disease and its sequelae: decreasing, but still substantial. Obstet Gynecol 2000;95:397–402. [DOI] [PubMed] [Google Scholar]
- 17.Ong KJ, Soldan K, Jit M, Dunbar JK, Woodhall SC. Chlamydia sequelae cost estimates used in current economic evaluations: does one-size-fit-all? Sex Transm Infect 2017;93:18–24. [DOI] [PubMed] [Google Scholar]
- 18.Trent M, Ellen JM, Frick KD. Estimating the direct costs of pelvic inflammatory disease in adolescents: a within-system analysis. Sex Transm Dis 2011;38:326–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trussell J, Henry N, Hassan F, et al. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception 2013;87:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canestaro W, Vodicka E, Downing D, et al. Implications of employer coverage of contraception: Cost-effectiveness analysis of contraception coverage under an employer mandate. Contraception 2017;95:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz P, Showstack J, Smith JF, et al. Costs of infertility treatment: results from an 18-month prospective cohort study. Fertil Steril 2011;95:915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulet SL, Kawwass J, Session D, et al. US state-level infertility insurance mandates and health plan expenditures on infertility treatments. Matern Child Health J 2019;23:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Institute of Medicine. Vaccines for the 21st Century: A Tool for Decisionmaking. Washington, DC: National Academy of Sciences; 2000. [Google Scholar]
- 24.Neumann PJ, Sanders GD, Russell LB, et al. Cost-Effectiveness in Health and Medicine: Second Edition. New York, NY: Oxford University Press; 2016. [Google Scholar]
- 25.Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine 2010;28:6858–6867. [DOI] [PubMed] [Google Scholar]
- 26.Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Special Issue Sex Transm Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jichlinski A, Badolato G, Pastor W, et al. HIV and syphilis screening among adolescents diagnosed with pelvic inflammatory disease. Pediatrics 2018;142(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trent M. Status of adolescent pelvic inflammatory disease management in the United States. Curr Opin Obstet Gynecol 2013;25(5):350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am 2013;27(4):793–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price MJ, Ades AE, Welton NJ, et al. Proportion of pelvic inflammatory disease cases caused by Chlamydia trachomatis: consistent picture from different methods. J Inf Dis 2016;214(4):617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


