Abstract
Background
An established treatment strategy for asymptomatic pulmonary embolism (PE) or deep vein thrombosis (DVT) remains uncertain in Japan; therefore, in this study, we clarify the characteristics and outcomes of symptomatic compared to asymptomatic patients with PE or DVT.
Methods
This prospective, multicenter sub-analysis of the J’xactly study in Japan included 1,016 patients (mean age, 68; 41% male) with venous thromboembolism (VTE) treated with rivaroxaban.
Results
Asymptomatic PE patients (47% of PE patients) were more likely to have active cancer and asymptomatic proximal DVT at lower severity than symptomatic PE patients, despite no differences in age, sex, or the proportion receiving intensive 30 mg/day-rivaroxaban. Patients with asymptomatic DVT (34% of DVT patients) were older, had higher rates of female sex, active cancer, and distal DVT, and received shorter, less intense rivaroxaban treatment. Incidences did not differ between asymptomatic and symptomatic PE patients for recurrent symptomatic VTE (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.22–1.62; P = 0.31) or major bleeding (HR, 0.68; 95% CI, 0.20–2.33; P = 0.58), nor between asymptomatic and symptomatic DVT patients for recurrent symptomatic VTE (HR, 0.56; 95% CI, 0.23–1.40; P = 0.21) and major bleeding (HR, 1.47; 95% CI, 0.54–3.97; P = 0.45).
Conclusions
The real-world composite adverse event rate for treatment with rivaroxaban, as physician-adjusted for dose and duration, was similar for asymptomatic and symptomatic patients regardless of the presence of PE or DVT, suggesting a favorable safety profile for potential rivaroxaban treatment for asymptomatic VTE.
Keywords: Anticoagulant, Bleeding, Recurrence, Rivaroxaban, Venous thromboembolism
Background
Venous thromboembolism (VTE), in the form of pulmonary embolism (PE) or deep vein thrombosis (DVT), is a common acute cardiovascular disease [1, 2] and a major medical problem worldwide [3]. PE can be detected incidentally on routine chest and abdominal computed tomography (CT) imaging studies [4, 5]. The detection of asymptomatic PE has increased with the introduction of multidirectional CT scanners that can better delineate the pulmonary arteries down to the segmental level [6]. The largest meta-analysis to date investigated more than 10,000 patients with VTE through 2009 and reported the incidence of asymptomatic PE to be 2.6% (95% confidence interval [CI] 1.9–3.4%). This was even higher in patients with VTE risk factors such as malignancy and hospitalization [7]. The optimal treatment strategy for incidentally detected asymptomatic PE remains controversial [8], although some data supports the treatment of incident PE in patients with known VTE risk factors such as malignancy [9–11]. The detection of asymptomatic distal DVT has also increased in recent years. It is considered to convey a lower risk than symptomatic distal DVT [12, 13]. Little value has been associated with uniformly administering therapeutic doses of anticoagulation for screen-detected distal DVT. Anticoagulation therapy is used for proximal DVT and PE; however, the safety and advantage of distal DVT anticoagulation treatment remain to be established.
Therefore, this study examines the background and clinical outcomes in 1,039 patients with acute symptomatic and asymptomatic DVT only (DVT group) or PE with or without DVT (PE group) treated with rivaroxaban. We used the J’xactly study (a Japanese registry) data on the efficacy and safety of rivaroXAban for the prevention of reCurrence in deep vein Thrombosis and puLmonarY Embolism patients (University Hospital Medical Information Network Clinical Trials Registry, UMIN000025072).
Methods
Study population
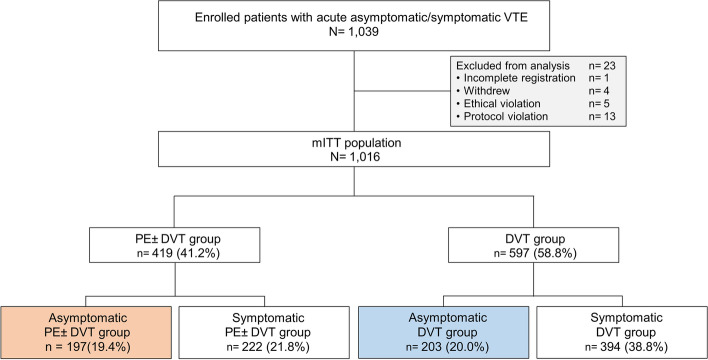
The J’xactly study was a multicenter, prospective, observational cohort study in which patients diagnosed with acute symptomatic and asymptomatic DVT, PE, or both and prescribed rivaroxaban for the treatment and prevention of VTE were enrolled from December 2016 to April 2018. Details of the study design, data collection process, and baseline characteristics of the study population have been previously described [14, 15]. The key exclusion criteria were contraindications to rivaroxaban; chronic thromboembolic pulmonary hypertension (CTEPH), except for CTEPH plus acute PE or DVT; active bleeding. All the patients provided written informed consent to participate in this study. All eligible patients were enrolled in the study within 3 weeks of starting rivaroxaban for the treatment and prevention of VTE. Data were collected until the end of the follow-up period (November 2019), regardless of whether rivaroxaban was continued, discontinued, or terminated according to either the patient’s preference or the physician’s discretion. In this study, a total of 1,016 patients evaluable by modified intention-to-treat (mITT) were included. First, patients were stratified according to the presence of PE with or without DVT (PE group) and DVT only (DVT group). Both the PE and DVT groups were evaluated for the efficacy and safety endpoints, which were compared between the symptomatic and asymptomatic patients (Fig. 1). Symptomatic patients in the PE group were defined by the presence of PE-related symptoms, including dyspnea, tachypnea, chest pain, cold sweats, fainting, palpitation, tachycardia, cough, wheezing, bloody sputum, shock, hypotension, and fever. Symptomatic patients in the DVT group were defined by the presence of DVT-related symptoms, including pain, swelling, superficial venous aggravation, color changes due to congestion, tautness, edema, tenderness, and Homan’s sign of the lower extremities. PE severity was stratified according to Japanese guidelines [2] as either cardiac arrest or collapse, massive, sub-massive or non-massive PE, in addition to the criteria described above. DVT was classified by the thrombus location as either proximal (thrombus located proximal to or involving the popliteal vein) or distal (thrombus located distal to the popliteal vein). Data regarding the initial dose of rivaroxaban (standard dosage: 30 mg/day; under-dosages: 20, 15, or 10 mg/day) were also recorded.
Fig. 1.
Flowchart of patient selection and stratification by symptoms. DVT, deep venous thrombosis; mITT, modified intention-to-treat; PE, pulmonary embolism; VTE, venous thromboembolism
The J’xactly study was conducted in accordance with the principles of the Declaration of Helsinki and all applicable legal and regulatory requirements in Japan. The study protocols and related documentation were reviewed and approved by the Institutional Review Board (IRB) of Nihon University Itabashi Hospital. All the participating institutions provided ethical approval.
Endpoints
The primary effectiveness outcome was the recurrence or aggravation of symptomatic VTE during the follow-up period [14, 15]. VTE was defined according to the established diagnostic criteria [16]. The primary safety outcome was the occurrence of a major bleeding during the treatment period and up to 2 days after rivaroxaban discontinuation. Major bleeding was defined according to the International Society on Thrombosis and Hemostasis criteria [17]. Secondary outcomes included the recurrence or aggravation of symptomatic DVT and PE, death from any cause, death related to VTE and cardiovascular disease (CVD), vascular events (acute coronary syndrome [ACS] or ischemic stroke), and non-major bleeding. Clinically relevant events were also evaluated as composite outcomes, in which each component (recurrent VTE, ACS, ischemic stroke, death from any cause, and major bleeding events) was weighted equally. An independent, blinded clinical events committee adjudicated the outcomes.
Statistical analysis
The mITT population was used for the effectiveness calculations, which included all enrolled patients except those who were excluded from the study. The on-treatment population included all patients treated with at least one dose of rivaroxaban. It was used for safety assessments. Continuous variables are reported as mean ± standard deviation, and categorical variables are reported as the number and percentage of patients. The symptomatic and asymptomatic PE and DVT groups were compared using the t-test for continuous variables and the chi-square test for categorical variables. The Kaplan–Meier method was used to estimate the cumulative event rates, with the incidence rates in each treatment group demonstrated as percentages per patient-year. A Cox proportional hazards regression model was used to compare outcomes between the two groups, and the results were expressed as hazard ratios (HRs) with 95% CIs. All statistical analyses were performed using JMP Pro 11 software (SAS Institute, Cary, NC, USA). Statistical significance was set at P < 0.05.
Results
Comparison of baseline patient characteristics in symptomatic and asymptomatic PE and DVT groups
Among the 419 patients in the PE group, 197 (47.0%) were asymptomatic and 222 (53.0%) were symptomatic, while of the 597 patients with DVT only, 203 (34.0%) were asymptomatic, and 394 (66.0%) were symptomatic. The baseline characteristics of the asymptomatic and symptomatic patients in the PE and DVT groups are summarized in Table 1. No differences in age, female sex, body weight, or creatinine clearance (CrCL) were observed between the asymptomatic and symptomatic PE groups. However, the asymptomatic PE group demonstrated a lower severity of illness than the symptomatic PE group overall, which was reflected by a lower heart rate (81.0 ± 14.8 vs. 93.9 ± 20.9 beats per minute; P < 0.001), higher oxygen saturation (97 ± 2 vs. 94 ± 6%; P < 0.001), higher rate of non-massive PE (82.2 vs. 38.7%; P < 0.001), smaller mean right ventricular (RV) diameter (32.6 ± 6.3 vs. 38.5 ± 10.4 mm; P < 0.001), lower RV/left ventricular diameter ratio (0.81 ± 0.21 vs. 1.06 ± 0.41; P < 0.001), and fewer comorbidities. Outpatient status, active cancer, history of recent surgery, use of nonsteroidal anti-inflammatory drugs, and proximal and asymptomatic DVT were more prevalent in the asymptomatic than the symptomatic PE group. Prior anticoagulation therapy was less prevalent in the asymptomatic than the symptomatic PE group. Although no differences were found in the initial dose of rivaroxaban dose, the total duration of rivaroxaban treatment tended to be longer in the asymptomatic compared to the symptomatic PE group (442 [183–661] vs. 361 [165–630] days; P = 0.08).
Table 1.
Baseline characteristics of patients stratified by baseline symptoms
| PE with/without DVT | DVT | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Total | Asymptomatic | Symptomatic | P-Value | Total | Asymptomatic | Symptomatic | P-Value |
| n = 419 | n = 197 | n = 222 | n = 597 | n = 203 | n = 394 | |||
| Age (years) | 66 ± 15 | 65 ± 14 | 66 ± 15 | 0.32 | 70 ± 15 | 72 ± 12 | 69 ± 16 | 0.017 |
| ≥ 75 years | 137 (32.7%) | 58 (29.4%) | 79 (35.6%) | 0.21 | 253 (42.4%) | 88 (43.3%) | 165 (41.9%) | 0.79 |
| Female sex | 220 (52.5%) | 94 (47.7%) | 126 (56.8%) | 0.08 | 379 (63.5%) | 151 (74.4%) | 228 (57.9%) | < 0.001 |
| Body weight (kg) | 63.0 ± 14.9 | 62.7 ± 13.8 | 63.3 ± 15.9 | 0.69 | 58.3 ± 12.9 | 55.5 ± 11.7 | 59.9 ± 13.5 | < 0.001 |
| < 50 kg | 80 (19.1%) | 33 (16.8%) | 47 (21.2%) | 0.26 | 142 (23.8%) | 63 (31.0%) | 79 (20.1%) | 0.003 |
| Body mass index (kg/m2) | 24.2 ± 4.4 | 23.8 ± 3.9 | 24.5 ± 4.8 | 0.09 | 23.5 ± 4.0 | 23.1 ± 3.9 | 23.8 ± 4.1 | 0.05 |
| Heart rate (beats per minute) | 87.9 ± 19.4 | 81.0 ± 14.8 | 93.9 ± 20.9 | < 0.001 | 78.3 ± 13.5 | 77.3 ± 13.6 | 79.0 ± 13.5 | 0.21 |
| Systolic blood pressure (mmHg) | 129.9 ± 23.5 | 129.8 ± 19.8 | 130.0 ± 26.3 | 0.93 | 128.8 ± 18.1 | 126.5 ± 15.7 | 130.1 ± 19.3 | 0.036 |
| SpO2 (%) | 95 ± 5 | 97 ± 2 | 94 ± 6 | < 0.001 | 97 ± 2 | 97 ± 2 | 97 ± 2 | 0.26 |
| Outpatient | 112 (26.7%) | 62 (31.5%) | 50 (22.5%) | 0.046 | 310 (51.9%) | 57 (28.1%) | 253 (64.2%) | < 0.001 |
| CrCL (mL/minute) | 82.1 ± 37.6 | 84.5 ± 33.7 | 79.9 ± 40.6 | 0.21 | 77.2 ± 35.0 | 74.0 ± 29.2 | 79.0 ± 37.8 | 0.045 |
| <50 mL/minute | 71 (16.9%) | 29 (14.7%) | 42 (18.9%) | 0.30 | 199 (33.3%) | 69 (34.0%) | 130 (33.0%) | 0.74 |
| D-dimer (µg/mL) | 10.0 (5.5–19.0) | 9.3 (5.3–17.5) | 10.7 (5.7–19.9) | 0.13 | 7.6 (3.5–15.1) | 7.9 (3.5–14.2) | 7.6 (3.4–16.2) | 0.84 |
| Medical history | ||||||||
| Hypertension | 171 (40.8%) | 71 (36.0%) | 98 (44.1%) | 0.11 | 211 (35.3%) | 69 (34.0%) | 142 (36.0%) | 0.65 |
| Diabetes mellitus | 54 (12.9%) | 27 (13.7%) | 27 (12.2%) | 0.66 | 64 (10.7%) | 28 (13.8%) | 36 (9.1%) | 0.09 |
| Heart failure | 18 (4.3%) | 3 (1.5%) | 15 (6.8%) | 0.008 | 16 (2.7%) | 7 (3.4%) | 9 (2.3%) | 0.43 |
| Atrial fibrillation | 16 (3.8%) | 5 (2.5%) | 11 (5.0%) | 0.21 | 10 (1.7%) | 6 (3.0%) | 4 (1.0%) | 0.10 |
| Coronary artery disease | 19 (4.5%) | 5 (2.5%) | 14 (6.3%) | 0.10 | 26 (4.4%) | 9 (4.4%) | 17 (4.3%) | 1.00 |
| Chronic heart and lung disease | 27 (6.4%) | 7 (3.6%) | 20 (9.0%) | 0.028 | 20 (3.4%) | 9 (4.4%) | 11 (2.8%) | 0.34 |
| Previous stroke | 28 (6.7%) | 13 (6.6%) | 15 (6.8%) | 1.00 | 45 (7.5%) | 16 (7.9%) | 29 (7.4%) | 0.87 |
| Previous atrial fibrillation | 8 (1.9%) | 2 (1.0%) | 6 (2.7%) | 0.29 | 15 (2.5%) | 7 (3.4%) | 8 (2.0%) | 0.41 |
| Risk factor | ||||||||
| Active cancer | 88 (21.0%) | 54 (27.4%) | 34 (15.3%) | 0.003 | 105 (17.6%) | 55 (27.1%) | 50 (12.7%) | < 0.001 |
| Recent surgery | 78 (18.6%) | 48 (24.4%) | 30 (13.5%) | 0.006 | 182 (30.5%) | 113 (55.7%) | 69 (17.5%) | < 0.001 |
| Recent injury | 31 (7.4%) | 14 (7.1%) | 17 (7.7%) | 0.85 | 62 (10.4%) | 33 (16.3%) | 29 (7.4%) | 0.001 |
| Inactivity | 130 (31.0%) | 64 (32.5%) | 66 (29.7%) | 0.60 | 237 (39.7%) | 119 (58.6%) | 118 (29.9%) | < 0.001 |
| Thrombophilia | 21 (5.0%) | 10 (5.1%) | 11 (5.0%) | 1.00 | 17 (2.8%) | 4 (2.0%) | 13 (3.3%) | 0.44 |
| Previous VTE | 28 (6.7%) | 13 (6.6%) | 15 (6.8%) | 1.00 | 55 (9.2%) | 16 (7.9%) | 39 (9.9%) | 0.46 |
| Concomitant medications | ||||||||
| Antiplatelet agents | 38 (9.1%) | 18 (9.1%) | 20 (9.0%) | 1.00 | 66 (11.1%) | 28 (13.8%) | 38 (9.6%) | 0.13 |
| Estrogen preparations | 7 (1.7%) | 3 (1.5%) | 4 (1.8%) | 1.00 | 16 (2.7%) | 1 (0.5%) | 15 (3.8%) | 0.016 |
| Anticancer agents | 43 (10.3%) | 20 (10.2%) | 23 (10.4%) | 1.00 | 45 (7.5%) | 21 (10.3%) | 24 (6.1%) | 0.07 |
| NSAIDs | 61 (14.6%) | 38 (19.3%) | 23 (10.4%) | 0.012 | 135 (22.6%) | 72 (35.5%) | 63 (16.0%) | < 0.001 |
| DVT | 320 (76.4%) | 164 (83.2%) | 156 (70.3%) | 0.002 | ||||
| Proximal | 235 (56.1%) | 133 (67.5%) | 102 (45.9%) | < 0.001 | 294 (49.3%) | 53 (26.1%) | 241 (61.2%) | < 0.001 |
| Distal | 85 (20.3%) | 31 (15.7%) | 54 (24.3%) | 0.038 | 303 (50.8%) | 150 (73.9%) | 153 (38.8%) | < 0.001 |
| Localization of right DVT | 196 (46.8%) | 97 (49.2%) | 99 (44.6%) | 0.38 | 332 (55.6%) | 137 (67.5%) | 195(49.5%) | < 0.001 |
| Localization of left DVT | 194 (46.3%) | 97 (49.2%) | 97 (43.7%) | 0.28 | 402 (67.3%) | 124 (61.1%) | 278 (70.6%) | 0.021 |
| Localization of both DVT | 70 (16.7%) | 30 (15.2%) | 40 (18.0%) | 0.51 | 137 (22.9%) | 58 (28.6%) | 79 (20.1%) | 0.024 |
| Asymptomatic DVT | 205 (48.9%) | 125 (63.5%) | 80 (36.0%) | < 0.001 | ||||
| PE | ||||||||
| Cardiac arrest or Collapse or Massive | 23 (5.5%) | 0 (0.0%) | 23 (10.4%) | < 0.001 | ||||
| Sub-massive or Non-massive | 371 (88.5%) | 175 (88.8%) | 196 (88.3%) | 0.88 | ||||
| Non-massive | 248 (59.2%) | 162 (82.2%) | 86 (38.7%) | < 0.001 | ||||
| Severer than Sub-massive | 146 (34.8%) | 13 (6.6%) | 133 (59.9%) | < 0.001 | ||||
| RV pressure indexes | ||||||||
| Mean RV diameter (mm) | 36.1 ± 9.4 | 32.6 ± 6.3 | 38.5 ± 10.4 | < 0.001 | ||||
| RV/LV diameter ratio | 0.96 ± 0.36 | 0.81 ± 0.21 | 1.06 ± 0.41 | < 0.001 | ||||
| Prior treatment | ||||||||
| Anticoagulation therapy | 171 (40.8%) | 59 (29.9%) | 112 (50.5%) | < 0.001 | 93 (15.6%) | 24 (11.8%) | 69 (17.5%) | 0.07 |
| Inferior vena cava filter | 40 (9.5%) | 23 (11.7%) | 17 (7.7%) | 0.18 | 47 (7.9%) | 6 (3.0%) | 41 (10.4%) | 0.001 |
| Thrombolytic therapy | 33 (7.9%) | 11 (5.6%) | 22 (9.9%) | 0.10 | 14 (2.3%) | 0 (0.0%) | 14 (3.6%) | 0.004 |
| Catheterization | 4 (1.0%) | 2 (1.0%) | 2 (0.9%) | 1.00 | 8 (1.3%) | 0 (0.0%) | 8 (2.0%) | 0.06 |
| Pulmonary thrombus removal | 1 (0.2%) | 0 (0.0%) | 1 (0.5%) | 1.00 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| PCPS | 3 (0.7%) | 0 (0.0%) | 3 (1.4%) | 0.25 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| Others | 14 (3.3%) | 5 (2.5%) | 9 (4.1%) | 0.43 | 12 (2.0%) | 2 (1.0%) | 10 (2.5%) | 0.36 |
| Initial rivaroxaban treatment | ||||||||
| DOSE (mg/day) | 0.37 | 0.004 | ||||||
| 30 mg/day | 341 (81.4%) | 154 (78.2%) | 187 (84.2%) | 326 (54.6%) | 90 (44.3%) | 236 (59.9%) | ||
| 20 mg/day | 6 (1.4%) | 4 (2.0%) | 2 (0.9%) | 16 (2.7%) | 7 (3.4%) | 9 (2.3%) | ||
| 15 mg/day | 64 (15.3%) | 34 (17.3%) | 30 (13.5%) | 218 (36.5%) | 92 (45.3%) | 126 (32.0%) | ||
| 10 mg/day | 8 (1.9%) | 5 (2.5%) | 3 (1.4%) | 37 (6.2%) | 14 (6.9%) | 23 (5.8%) | ||
| Treatment duration, days | ||||||||
| Median (IQR) | 388 (176–643) | 442 (183–661) | 361 (165–630) | 0.08 | 282 (106–619) | 106 (68–259) | 338 (111–637) | < 0.001 |
Data are shown as n (%), median (interquartile range), or mean ± standard deviation, unless otherwise stated
CrCL Creatinine clearance, DVT Deep vein thrombosis, IQR Interquartile range, LV Left ventricle, NSAIDs Nonsteroidal anti-inflammatory drugs, PCPS Percutaneous cardiopulmonary support, PE Pulmonary embolism, RV Right ventricle, SD Standard deviation, SpO2 Oxygen saturation, VTE Venous thromboembolism
Asymptomatic patients were older (72 ± 12 vs. 69 ± 16 years; P = 0.017) and more often female (74.4 vs. 57.9%; P < 0.001) than symptomatic patients in the DVT group. Asymptomatic patients also had lower body weight (55.5 ± 11.7 vs. 59.9 ± 13.5 kg; P < 0.001) and lower CrCL (74.0 ± 29.2 vs. 79.0 ± 37.8 mL/min; P = 0.045) as compared with symptomatic DVT group. The asymptomatic DVT group more often had active cancer complications, recent surgery, and recent trauma as a cause of VTE than the symptomatic DVT group. Moreover, this group was more likely to use nonsteroidal anti-inflammatory drugs and was more frequently diagnosed during hospitalization (71.9 vs. 35.8%; P < 0.001) than the symptomatic DVT group. The asymptomatic DVT group had a higher rate of distal DVT (73.9% vs. 38.8%; P < 0.001) than the symptomatic DVT group. The asymptomatic DVT group more often initially received a reduced dose (20, 15, or 10 mg) of rivaroxaban (55.7 vs. 40.1%; P < 0.001) with a shorter treatment duration (106 [68–259] vs. 338 [111–637] days; P < 0.001).
Clinical outcomes in asymptomatic and symptomatic PE and DVT groups
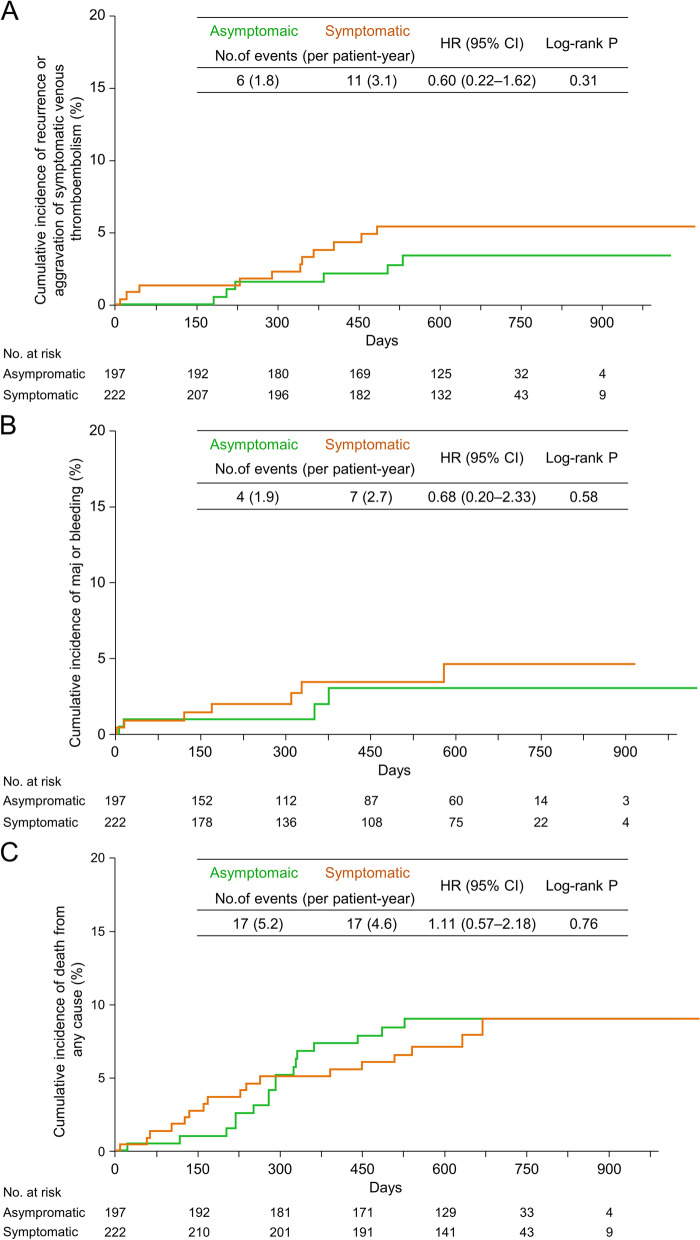
During the median follow-up period of 21.3 months (interquartile range, 18.1–24.2 months), a recurrence or aggravation of symptomatic VTE was reported in 6 (3.0%) in the asymptomatic PE group versus 11 (5.0%) in the symptomatic PE group (1.8 vs. 3.1 events per patient-year; HR, 0.60; 95% CI, 0.22–1.62; P = 0.31, Fig. 2A). No difference in major bleeding events was noted between asymptomatic and symptomatic PE groups (1.9 vs. 2.7 events per patient-year; HR, 0.68; 95% CI, 0.20–2.33; P = 0.58, Fig. 2B). However, the incidence of minor bleeding was significantly lower in the asymptomatic than the symptomatic PE group (3.4 vs. 9.5 events per patient-year; HR, 0.36; 95% CI, 0.15–0.83; P = 0.004) (Table 2). No incidents of fatal bleeding occurred in either group (Table 2). The occurrence of death from any cause (5.2 vs. 4.6 events per patient-year; HR, 1.11; 95% CI, 0.57–2.18; P = 0.76, Fig. 2C) and clinically relevant events was similar (7.8 vs. 9.5 events per patient-year; HR, 0.83; 95% CI, 0.49–1.39; P = 0.48) in the two groups (Table 2).
Fig. 2.
Results of clinical events in the PE group. Kaplan–Meier curves showing the cumulative incidence of (A) recurrence or aggravation of symptomatic VTE, (B) major bleeding, and (C) death from any cause in the PE group. CI, confidence interval; HR, hazard ratio; PE, pulmonary embolism; VTE, venous thromboembolism
Table 2.
Clinical outcomes of patients stratified by baseline symptoms
| PE with/without DVT | DVT | |||||||
|---|---|---|---|---|---|---|---|---|
| Asymptomatic | Symptomatic | HR (95% CI) | P-Value | Asymptomatic | Symptomatic | HR (95% CI) | P-Value | |
| n = 197 | n = 222 | n = 203 | n = 394 | |||||
| Recurrence or aggravation of symptomatic VTE | 1.8 (0.7–4.0) | 3.1 (1.5–5.5) | 0.60 (0.22–1.62) | 0.31 | 1.7 (0.6–3.8) | 3.1 (1.9–4.8) | 0.56 (0.23–1.40) | 0.21 |
| Recurrence or aggravation of symptomatic PE | 0.9 (0.2–2.7) | 2.5 (1.1–4.5) | 0.37 (0.10–1.35) | 0.12 | 0.3 (0.0–1.6) | 1.1 (0.4–2.2) | 0.26 (0.03–2.15) | 0.18 |
| Recurrence or aggravation of symptomatic DVT | 1.2 (0.3–3.2) | 0.8 (0.2–2.4) | 1.49 (0.33–6.67) | 0.60 | 1.4 (0.5–3.4) | 2.2 (1.2–3.6) | 0.68 (0.24–1.88) | 0.45 |
| Acute coronary syndrome | 0 | 0.3 (0.0–1.5) | - | 0.35 | 0.3 (0.0–1.6) | 0.2 (0.0–0.8) | 1.90 (0.12–30.36) | 0.64 |
| Ischemic stroke | 1.2 (0.3–3.1) | 1.1 (0.3–2.8) | 1.11 (0.28–4.45) | 0.88 | 0 | 0 | - | - |
| Death from any cause | 5.2 (3.0–8.3) | 4.6 (2.7–7.4) | 1.11 (0.57–2.18) | 0.76 | 6.5 (4.1–9.8) | 5.6 (4.0–7.7) | 1.17 (0.69–1.96) | 0.56 |
| Death related to VTE | 0.6 (0.1–2.2) | 0.5 (0.1–2.0) | 1.11 (0.16–7.89) | 0.92 | 0.3 (0.0–1.6) | 0.5 (0.1–1.4) | 0.62 (0.06–5.94) | 0.67 |
| Death related to CVD | 0.9 (0.2–2.7) | 0.5 (0.1–2.0) | 1.67 (0.28–9.99) | 0.57 | 0.3 (0.0–1.6) | 1.1 (0.4–2.2) | 0.27 (0.03–2.19) | 0.19 |
| Major bleeding | 1.9 (0.5–4.9) | 2.7 (1.1–5.7) | 0.68 (0.20–2.33) | 0.58 | 5.2 (1.9–11.4) | 3.0 (1.5–5.2) | 1.47 (0.54–3.97) | 0.45 |
| Minor bleeding | 3.4 (1.4–7.0) | 9.5 (6.0–14.3) | 0.36 (0.15–0.83) | 0.004 | 12.4 (6.8–20.8) | 9.1 (6.3–12.6) | 1.03 (0.55–1.92) | 0.94 |
| Fatal bleeding | 0 | 0 | - | - | 0 | 0.5 (0.1–1.3) | - | 0.21 |
| Clinically relevant events | 7.8 (5.1–11.6) | 9.5 (6.5–13.3) | 0.83 (0.49–1.39) | 0.48 | 9.9 (6.8–13.9) | 9.4 (7.2–12.1) | 1.05 (0.69–1.61) | 0.82 |
Data are shown as % per patient-year, unless otherwise stated
Clinically relevant events were evaluated as a composite outcome, in which each component (recurrent VTE, acute coronary syndrome, ischemic stroke, death from any cause, and major bleeding events) was weighted equally. CI Confidence interval, CVD Cardiovascular disease, DVT Deep vein thrombosis, HR Hazard ratio, PE Pulmonary embolism, VTE Venous thromboembolism
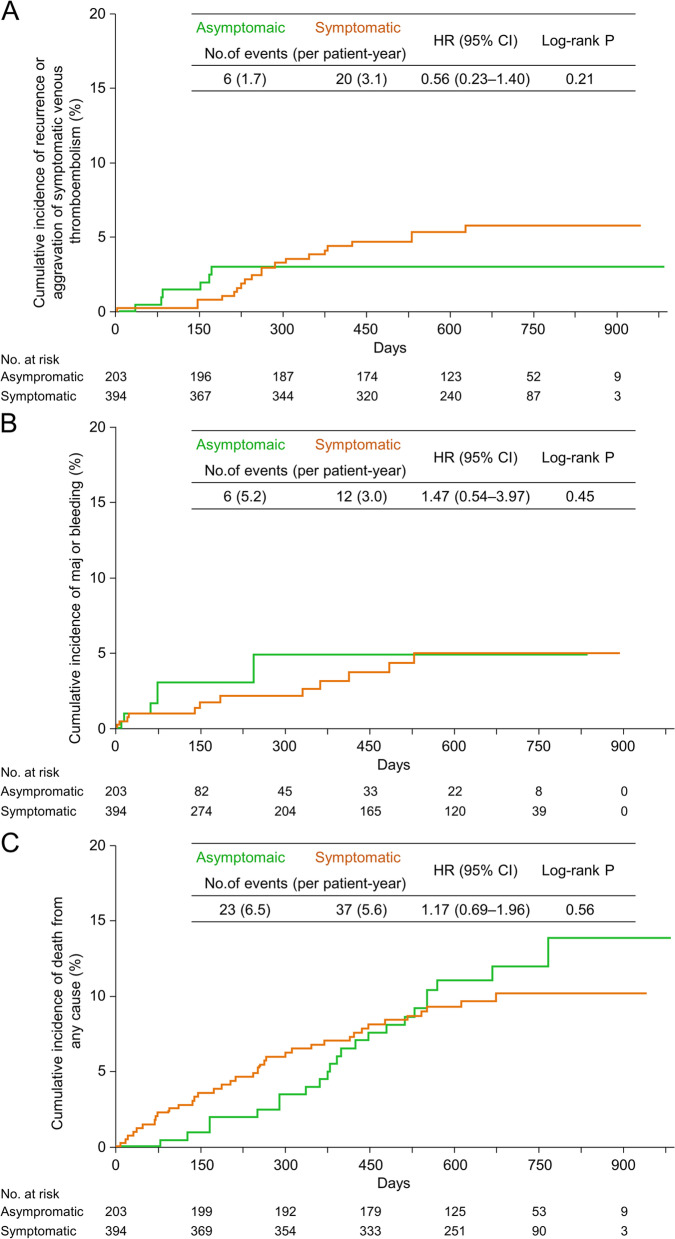
No differences were observed in the recurrence or aggravation of symptomatic VTE (1.7 vs. 3.1 events per patient-year; HR, 0.56; 95% CI, 0.23–1.40; P = 0.21, Fig. 3A), major bleeding (5.2 vs. 3.0 events per patient-year; HR, 1.47; 95% CI, 0.54–3.97; P = 0.45, Fig. 3B), fatal bleeding (not observed vs. 0.5 events per patient-year; P = 0.21), death from any cause (6.5 vs. 5.6 events per patient-year; HR, 1.17; 95% CI, 0.69–1.96; P = 0.56, Fig. 3C), or clinically relevant events (9.9 vs. 9.4 events per patient-year; HR, 1.05; 95% CI, 0.69–1.61; P = 0.82) between the asymptomatic and symptomatic DVT groups (Table 2).
Fig. 3.
Results of clinical events in the DVT group. Kaplan–Meier curves showing the cumulative incidence of (A) recurrence or aggravation of symptomatic VTE, (B) major bleeding, and (C) death from any cause in the DVT group. CI, confidence interval; DVT, deep venous thrombosis; HR, hazard ratio; VTE, venous thromboembolism
In the PE group, active cancer was a predictor of the clinically relevant events in univariate analysis (HR 4.55, 95% CI 2.72–7.62; P < 0.001). However, asymptomatic PE (HR 1.61, 95% CI 0.83–3.12; P = 0.16) was not a predictor. After multivariate adjustment, active cancer was an independent predictor of the clinically relevant events (HR 5.19, 95% CI 2.85–9.47; P < 0.001). However, asymptomatic PE (HR 1.44, 95% CI 0.74–2.81; P = 0.29) was not a predictor (Table 3). In the DVT group, active cancer (HR 3.84, 95% CI 2.53–5.82; P < 0.001) and lower CrCL (HR 2.46, 95% CI 1.61–3.74;0 P = 0.025) were predictors of clinically relevant events in univariate analysis. However, asymptomatic DVT (HR 0.95, 95% CI 0.62–1.46; P = 0.82) was not a predictor. After multivariate adjustment, active cancer (HR 4.12, 95% CI 2.65–6.38; P < 0.001) and lower CrCL (HR 2.02, 95% CI 1.20–3.42; P = 0.008) were independent predictors of the clinically relevant events. However, asymptomatic DVT (HR 1.13, 95% CI 0.56–1.78; P = 0.58) was not a predictor (Table 3).
Table 3.
Univariate and multivariate adjustment analysis for predictors of the clinically relevant events
| PE with/without DVT | DVT | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Univariate analysis HR (95% CI) | P-Value | Multivariable analysis HR (95% CI) | P-Value | Univariate analysis HR (95% CI) | P-Value | Multivariable analysis HR (95% CI) | P-Value |
| Age ≥ 75 years | 0.76 (0.43–1.35) | 0.35 | 1.55 (1.03–2.32) | 0.036 | 1.22 (0.73–2.03) | 0.44 | ||
| Female | 0.61 (0.36–1.03) | 0.06 | 0.81 (0.54–1.22) | 0.32 | ||||
| Body weight < 50 kg | 1.50 (0.83–2.70) | 0.18 | 1.92 (1.26–2.94) | 0.003 | 0.59 (0.34–1.03) | 0.06 | ||
| CrCL < 50 mL/minute | 0.73 (0.35–1.54) | 0.41 | 2.46 (1.61–3.74) | 0.025 | 2.02 (1.20–3.42) | 0.008 | ||
| Diabetes | 1.10 (0.52–2.31) | 0.81 | 0.65 (0.30–1.39) | 0.27 | ||||
| Chronic heart and lung disease | 2.11 (0.96–4.65) | 0.06 | 2.00 (0.87–4.57) | 0.10 | ||||
| Active cancer | 4.55 (2.72–7.62) | < 0.001 | 5.19 (2.85–9.47) | < 0.001 | 3.84 (2.53–5.82) | < 0.001 | 4.12 (2.65–6.38) | < 0.001 |
| Asymptomatic | 1.61 (0.83–3.12) | 0.16 | 1.44 (0.74–2.81) | 0.29 | 0.95 (0.62–1.46) | 0.82 | 1.13 (0.56–1.78) | 0.58 |
Clinically relevant events were evaluated as a composite outcome, in which each component (recurrent VTE, acute coronary syndrome, ischemic stroke, death from any cause, and major bleeding events) was weighted equally. CI Confidence interval, CrCL Creatinine clearance, DVT Deep vein thrombosis, HR Hazard ratio, PE Pulmonary embolism
Risk factors for clinically relevant events, recurrence or aggreviation of symptomatic VTE or major bleeding among the asymptomatic PE and DVT groups
Patients with asymptomatic PE who suffered from clinically relevant events were more likely to have chronic cardiopulmonary disease (12.0% vs. 2.3%; P = 0.045) and active cancer (56.0% vs. 23.3%; P = 0.001) than those who did not. Patients with asymptomatic DVT who suffered from clinically relevant events had a lower body weight (51.0 ± 10.5 vs. 56.3 ± 11.8 kg; P = 0.017) than those who did not. They were also more likely to have CrCL < 50 mL/min (39.4% vs. 20.0%; P = 0.025) and active cancer (54.5% vs. 21.8%; P < 0.001) than patients with DVT who did not suffer from clinically relevant events (Table 4). Patients with asymptomatic PE who developed clinically relevant events received rivaroxaban treatment for a shorter duration (222 [107–319] vs. 406 [173–647] days; P = 0.002) than patients with symptomatic PE. However, patients with asymptomatic DVT who developed clinically relevant events received rivaroxaban treatment for a longer duration than the symptomatic DVT group (170 [100–388] vs. 99 [55–223] days; P = 0.030) (Table 4).
Table 4.
Patient background of clinical events in the asymptomatic PE and asymptomatic DVT groups
| Asymptomatic PE with/without DVT | Asymptomatic DVT | |||||||
| Total | Occurred clinical events | Not occurred clinical events | P-value | Total | Occurred clinical events | Not occurred clinical events | P-value | |
| Clinically relevant events | n = 197 | n = 25 | n = 172 | n = 203 | n = 33 | n = 170 | ||
| Age (years) | 65 ± 14 | 67 ± 14 | 65 ± 15 | 0.47 | 72 ± 12 | 74 ± 11 | 71 ± 12 | 0.28 |
| ≥ 75 years | 58 (29.4%) | 7 (28.0%) | 51 (29.7%) | 1.00 | 88 (43.3%) | 17 (51.5%) | 71 (41.8%) | 0.34 |
| Female | 94 (47.7%) | 11 (44.0%) | 83 (48.3%) | 0.83 | 151 (74.4%) | 24 (72.7%) | 127 (74.7%) | 0.49 |
| Body weight (kg) | 62.7 ± 13.8 | 61.3 ± 13.5 | 62.9 ± 13.8 | 0.60 | 55.5 ± 11.6 | 51.0 ± 10.5 | 56.3 ± 11.8 | 0.017 |
| < 50 kg | 33 (16.8%) | 7 (28.0%) | 26 (15.1%) | 0.15 | 63 (31.0%) | 15 (45.5%) | 48 (28.2%) | 0.06 |
| CrCL (mL/minute) | 84.5 ± 33.8 | 83.7 ± 37.3 | 84.7 ± 33.3 | 0.90 | 72.9 ± 28.4 | 67.8 ± 31.4 | 73.9 ± 27.7 | 0.26 |
| < 50 mL/minute | 29 (14.7%) | 4 (16.0%) | 25 (14.5%) | 0.77 | 47 (23.2%) | 13 (39.4%) | 34 (20.0%) | 0.025 |
| Diabetes mellitus | 27 (13.7%) | 1 (4.0%) | 26 (15.1%) | 0.21 | 28 (13.8%) | 3 (9.1%) | 25 (14.7%) | 0.58 |
| Chronic heart and lung disease | 7 (3.6%) | 3 (12.0%) | 4 (2.3%) | 0.045 | 9 (4.4%) | 3 (9.1%) | 6 (3.5%) | 0.16 |
| Active cancer | 54 (27.4%) | 14 (56.0%) | 40 (23.3%) | 0.001 | 55 (27.1%) | 18 (54.5%) | 37 (21.8%) | < 0.001 |
| D-dimer (µg/mL) | 9.3 (5.3–17.5) | 9.5 (6.3–16.1) | 9.1 (5.1–17.5) | 0.65 | 6.6 (2.9–11.9) | 7.1 (3.4–11.1) | 6.4 (2.7–12.3) | 0.40 |
| Initial rivaroxaban treatment | ||||||||
| Treatment duration, days | ||||||||
| Median (IQR) | 361 (165–630) | 222 (107–319) | 406 (173–647) | 0.002 | 106 (68–259) | 170 (100–388) | 99 (55–223) | 0.030 |
| Recurrence or aggravation of symptomatic VTE | n = 197 | n = 6 | n = 191 | n = 203 | n = 6 | n = 197 | ||
| Age (years) | 65 ± 14 | 60 ± 17 | 65 ± 14 | 0.35 | 70 ± 12 | 72 ± 10 | 70 ± 12 | 0.81 |
| ≥ 75 years | 58 (29.4%) | 1 (16.7%) | 57 (29.8%) | 0.67 | 134 (42.1%) | 3 (37.5%) | 131 (42.3%) | 1.00 |
| Female | 94 (47.7%) | 4 (66.7%) | 90 (47.1%) | 0.43 | 220 (69.2%) | 6 (75.0%) | 214 (69.0%) | 1.00 |
| Body weight (kg) | 62.7 ± 13.7 | 70.0 ± 14.0 | 62.4 ± 13.7 | 0.18 | 57.3 ± 12.6 | 55.7 ± 13.5 | 57.3 ± 12.6 | 0.72 |
| < 50 kg | 33 (16.8%) | 1 (16.7%) | 32 (16.8%) | 1.00 | 90 (28.3%) | 4 (50.0%) | 86 (27.7%) | 0.23 |
| CrCL (mL/minute) | 84.5 ± 33.8 | 114.4 ± 44.0 | 83.6 ± 33.1 | 0.027 | 72.9 ± 28.4 | 67.5 ± 33.3 | 73.1 ± 28.3 | 0.64 |
| < 50 mL/minute | 29 (14.9%) | 0 (0.0%) | 29 (15.3%) | 0.59 | 69 (22.0%) | 3 (37.5%) | 66 (21.6%) | 0.38 |
| Diabetes mellitus | 27 (13.7%) | 0 (0.0%) | 27 (14.1%) | 1.00 | 41 (12.9%) | 1 (12.5%) | 40 (12.9%) | 1.00 |
| Chronic heart and lung disease | 7 (3.6%) | 0 (0.0%) | 7 (3.7%) | 1.00 | 17 (5.4%) | 1 (12.5%) | 16 (5.2%) | 0.36 |
| Active cancer | 51 (25.9%) | 1 (16.7%) | 50 (26.2%) | 1.00 | 75 (23.6%) | 2 (25.0%) | 73 (23.6%) | 1.00 |
| D-dimer (µg/mL) | 9.3 (5.3–17.5) | 8.0 (5.1–9.3) | 9.3 (5.2–17.5) | 0.30 | 7.9 (3.5–14.2) | 10.7 (3.1–16.5) | 7.8 (3.5–14.2) | 0.60 |
| Initial rivaroxaban treatment | ||||||||
| Treatment duration, days | ||||||||
| Median (IQR) | 361 (165–630) | 216 (80–477) | 362 (166–630) | 0.37 | 106 (68–259) | 170 (147–321) | 173 (85–514) | 0.82 |
| Major bleeding | n = 197 | n = 4 | n = 193 | n = 203 | n = 6 | n = 197 | ||
| Age (years) | 65 ± 14 | 55 ± 19 | 65 ± 14 | 0.16 | 70 ± 12 | 72 ± 10 | 70 ± 12 | 0.72 |
| ≥ 75 years | 58 (29.4%) | 0 (0.0%) | 58 (30.1%) | 0.32 | 134 (42.1%) | 3 (37.5%) | 131 (42.3%) | 1.00 |
| Female | 94 (47.7%) | 3 (75.0%) | 91 (47.2%) | 0.35 | 220 (69.2%) | 5 (62.5%) | 215 (69.4%) | 0.71 |
| Body weight (kg) | 62.7 ± 13.8 | 54.9 ± 13.5 | 62.8 ± 13.8 | 0.26 | 57.3 ± 12.6 | 54.3 ± 8.5 | 57.4 ± 12.7 | 0.50 |
| < 50 kg | 33 (16.8%) | 2 (50.0%) | 31 (16.1%) | 0.13 | 90 (28.3%) | 1 (12.5%) | 89 (28.7%) | 0.45 |
| CrCL (mL/minute) | 84.5 ± 33.8 | 111.9 ± 58.6 | 84.0 ± 33.1 | 0.10 | 72.9 ± 28.4 | 63.1 ± 18.7 | 73.2 ± 28.6 | 0.39 |
| < 50 mL/minute | 29 (14.9%) | 0 (0.0%) | 29 (15.2%) | 1.00 | 69 (22.0%) | 2 (25.0%) | 67 (21.9%) | 0.69 |
| Diabetes mellitus | 27 (13.7%) | 0 (0.0%) | 27 (14.0%) | 1.00 | 41 (12.9%) | 1 (12.5%) | 40 (12.9%) | 1.00 |
| Chronic heart and lung disease | 7 (3.6%) | 1 (25.0%) | 6 (3.1%) | 0.14 | 17 (5.4%) | 0 (0.0%) | 17 (5.5%) | 1.00 |
| Active cancer | 54 (27.4%) | 0 (0.0%) | 54 (28.0%) | 0.58 | 75 (23.6%) | 4 (50.0%) | 71 (22.9%) | 0.09 |
| D-dimer (µg/mL) | 9.3 (5.3–17.5) | 10.3 (9.5–15.4) | 9.1 (5.2–17.5) | 0.53 | 7.9 (3.5–14.2) | 7.1 (2.7–23.7) | 7.9 (3.6–14.2) | 0.96 |
| Initial rivaroxaban treatment | ||||||||
| Treatment duration, days | ||||||||
| Median (IQR) | 361 (165–630) | 236 (41–371) | 362 (168–631) | 0.18 | 106 (68–259) | 294 (38–595) | 173 (86–514) | 0.92 |
Data are shown as n (%), median (interquartile range), or mean ± standard deviation, unless otherwise stated
Clinically relevant events were evaluated as a composite outcome, in which each component (recurrent VTE, acute coronary syndrome, ischemic stroke, death from any cause, and major bleeding events) was weighted equally. CI Confidence interval, CVD Cardiovascular disease, DVT Deep vein thrombosis, HR Hazard ratio, PE Pulmonary embolism, VTE Venous thromboembolism, Data are presented as n (%) or mean ± SD. CrCL Creatinine clearance, DVT Deep vein thrombosis, IQR Interquartile range, PE Pulmonary embolism, SD Standard deviation
In the asymptomatic PE group, patients with recurrence or aggravation of symptomatic VTE had a significantly higher CrCL (114.4 ± 44.0 vs. 83.6 ± 33.1 mL/minute; P = 0.027) than those without it. However, no other differences were observed. There were also no significant differences between the two groups of patients who did and didn't experience major bleeding. In the asymptomatic DVT group, there were no significant differences in between the patients with and whout recurrence or aggravation of symptomatic VTE, or major bleeding (Table 4).
Relationship between active cancer and clinical events in the asymptomatic PE and DVT groups
In the asymptomatic PE group, active cancer had no effect on VTE recurrence (1.9% vs. 3.5%; P = 1.00) (Table 5). While the occurrence of major bleeding was not significantly different (0.0% vs. 2.8%; P = 0.58) between the two groups, the occurrences of all-cause death (25.9% vs. 2.1%; P < 0.001) and clinically relevant events (25.9% vs. 7.7%; P = 0.001) were significantly higher in the group with active cancer (Table 5). In the asymptomatic DVT group, active cancer had no effect on VTE recurrence (1.8% vs. 3.4%; P = 1.00). However, major bleeding (7.3% vs. 1.4%; P = 0.047), all-cause death (29.1% vs. 4.7%; P < 0.001), and clinically relevant events (32.7% vs. 10.1%; P < 0.001) were all significantly higher in the group with active cancer (Table 5).
Table 5.
Clinical outcomes in patients with and without active cancer within the asymptomatic VTE patients
| Asymptomatic PE with/without DVT | Asymptomatic DVT | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | With active cancer | Without active cancer | P-value | Total | With active cancer | Without active cancer | P-value | |
| n = 197 | n = 54 | n = 143 | n = 203 | n = 55 | n = 148 | |||
| Recurrence or aggravation of symptomatic VTE | 6 (3.0%) | 1 (1.9%) | 5 (3.5%) | 1.00 | 6 (3.0%) | 1 (1.8%) | 5 (3.4%) | 1.00 |
| Recurrence or aggravation of symptomatic PE | 3 (1.5%) | 1 (1.9%) | 2 (1.4%) | 1.00 | 1 (0.5%) | 0 (0.0%) | 1 (0.7%) | 1.00 |
| Recurrence or aggravation of symptomatic DVT | 4 (2.0%) | 0 (0.0%) | 4 (2.8%) | 0.58 | 5 (2.5%) | 1 (1.8%) | 4 (2.7%) | 1.00 |
| Acute coronary syndrome | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - | 1 (0.5%) | 0 (0.0%) | 1 (0.7%) | 1.00 |
| Ischemic stroke | 4 (2.0%) | 2 (3.7%) | 2 (1.4%) | 0.30 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| Death from any cause | 17 (8.6%) | 14 (25.9%) | 3 (2.1%) | < 0.001 | 23 (11.3%) | 16 (29.1%) | 7 (4.7%) | < 0.001 |
| Death related to VTE | 2 (1.0%) | 1 (1.9%) | 1 (0.7%) | 0.47 | 1 (0.5%) | 0 (0.0%) | 1 (0.7%) | 1.00 |
| Death related to CVD | 3 (1.5%) | 1 (1.9%) | 2 (1.4%) | 1.00 | 1 (0.5%) | 0 (0.0%) | 1 (0.7%) | 1.00 |
| Major bleeding | 4 (2.0%) | 0 (0.0%) | 4 (2.8%) | 0.58 | 6 (3.0%) | 4 (7.3%) | 2 (1.4%) | 0.047 |
| Minor bleeding | 7 (3.6%) | 3 (5.6%) | 4 (2.8%) | 0.40 | 14 (6.9%) | 3 (5.5%) | 11 (7.4%) | 0.76 |
| Fatal bleeding | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| Clinically relevant events | 25 (12.7%) | 14 (25.9%) | 11 (7.7%) | 0.001 | 33 (16.3%) | 18 (32.7%) | 15 (10.1%) | < 0.001 |
Data are shown as n (%), unless otherwise stated
Clinically relevant events were evaluated as a composite outcome, in which each component (recurrent VTE, acute coronary syndrome, ischemic stroke, death from any cause, and major bleeding events) was weighted equally. CVD Cardiovascular disease, DVT Deep vein thrombosis, PE Pulmonary embolism, VTE Venous thromboembolism
Discussion
This study had three major findings: First, while there were no differences in age, sex, body weight, or renal function, patients with asymptomatic PE had better vital signs and lower severity with a lower level of RV dysfunction but a higher prevalence of proximal DVT than patients with symptomatic PE. Patients with asymptomatic DVT were older and often female with lower body weight and renal function. However, they had a higher prevalence of distal DVT relative to those with symptomatic DVT. In both groups, active cancer and history of recent surgery were more common in asymptomatic patients than in symptomatic patients. Second, regarding rivaroxaban treatment, asymptomatic PE patients mostly had an intensive rivaroxaban treatment with a modestly longer treatment duration than symptomatic PE patients. In contrast, asymptomatic DVT patients more often received an off-label reduced dose of rivaroxaban treatment with a significantly shorter treatment duration than symptomatic DVT patients. Third, in the PE and DVT groups, the incidences of recurrence or aggravation of symptomatic VTE, major bleeding, or clinically relevant events did not differ between the asymptomatic and symptomatic groups.
Characteristics and clinical outcomes of patients with asymptomatic PE
We have clarified the differences in patient characteristics between patients with asymptomatic and symptomatic PE who received rivaroxaban treatment in Japan. No differences were observed in age, sex, body weight, or renal function between the asymptomatic and symptomatic PE groups. However, the asymptomatic PE patients were at a lower risk of PE, which was reflected by better vital signs, less frequent massive PEs, and lower RV dysfunction as assessed by the RV/LV diameter ratio. Unexpected and incidentally detected PE is reported in 3.3–5.0% of cancer patients and 1–2% of all thoracic CT scans [7, 18, 19]. Our data indicated that the rate of active cancer in the asymptomatic group was 27%, which was higher than that in the symptomatic PE group. 11–27% of all incidentally discovered PEs are confined to subsegmental vessels [9, 10, 20]. In this study, approximately 80% of the asymptomatic PE patients had non-massive PE, suggesting that some patients had subsegmental PE. The effects of treating incidental PE by direct oral anticoagulants (DOAC) have not yet been evaluated in large prospective studies. There are some real-world data supporting the treatment of incidentally detected PE in patients with known VTE risk factors such as cancer because these patients have been reported to have high rates of recurrent VTE and mortality [10], especially those who had not received anticoagulation therapy [11]. Unfortunately, Japanese guidelines have not stated a treatment strategy for particular patients with asymptomatic PE and concomitant cancer or those without cancer thus far [2]. Nonetheless, the 2019 European Society of Cardiology guidelines recommend that patients with incidentally detected PE and concomitant cancer should be managed similarly to those with symptomatic PE, also stating that no firm data exist [21], as similarly recommended by the American Society of Clinical Oncology (informal consensus, moderate strength recommendations) [22]. The higher proportion of proximal DVT and active cancer in the asymptomatic PE group in this study could be a consequence of the physicians’ motivation to prescribe rivaroxaban treatment to improve the prognosis even in these asymptomatic PE patients. Asymptomatic PE can also lead to pulmonary hypertension. In recent years, the prevalence of CTEPH after symptomatic acute PE treatment has been reported to be 3.8–5.4% [23, 24]. Theoretically, asymptomatic PE patients might be underdiagnosed and thus not receive anticoagulant therapy, leading to an increased risk of developing CTEPH. The prognoses of incidentally discovered PE and symptomatic PE are similar [25].
Hence, the American College of Chest Physicians (Grade 2B) recommends treating patients with incidentally discovered PE and symptomatic PE similarly to those with symptomatic VTE [26]. Therefore, in the real-world setting, physicians could have decided to prescribe the initial on-label dose of rivaroxaban (30 mg) to most patients with asymptomatic PE. However, the dose might have been reduced in some patients with low-VTE risk, such as subsegmental PE. Our study also demonstrated equivalent incidences of recurrent VTE, major bleeding, and a composite outcome between asymptomatic and symptomatic PE groups receiving rivaroxaban treatment. Major bleeding events were numerically, and minor bleeding events were significantly lower in the asymptomatic than the symptomatic PE group. None of the asymptomatic PE patients experienced fatal bleeding. This supports the evaluation that the benefit of improved prognosis outweighs the risk of bleeding burden from rivaroxaban treatment, even in most patients with asymptomatic PE.
Characteristics and clinical outcomes of patients with asymptomatic DVT
In our study, distal DVT was observed in 73.9% of patients with asymptomatic DVT. This was higher than that observed in patients with symptomatic DVT. Patients with asymptomatic DVT had a higher incidence of recent surgery and trauma. These results suggest that DVT was incidentally detected after general or orthopedic surgery in most patients with asymptomatic DVT. The detection of asymptomatic distal DVT by postoperative screening has increased in recent years [12]. Asymptomatic distal DVT is considered less risky than symptomatic peripheral DVT for VTE recurrence [13]. In a prospective blinded study, the incidence of PE in untreated distal DVT patients was as low as 1.6% [27]. Distal DVT is associated with fewer PE recurrences than proximal DVT [28]. A double-blind, randomized controlled study evaluating low-molecular-weight heparin treatment of symptomatic distal DVT demonstrated no benefit from anticoagulation in preventing worsening DVT or symptomatic PE (3% vs. 5%; P = 0.54) and only increased hemorrhagic complications (4% vs. 0%; P = 0.0255) [29]. Therefore, there is no evidence supporting the benefit of anticoagulant therapy, such as heparin or warfarin, in patients with asymptomatic DVT or distal DVT. The present study highlights the potential of DOAC treatment in asymptomatic DVT patients as the annual incidence of recurrent VTE in the asymptomatic DVT group did not differ from that in the symptomatic DVT group under rivaroxaban treatment (1.7 vs. 3.1 events per 100 patient-year; P = 0.21).
The annual recurrent VTE incidence reported in this study is similar to that reported for symptomatic Japanese and global VTE patients who had been treated with rivaroxaban in the J-EINSTEIN-PE/DVT [30] (1.8%) and EINSTEIN-PE/DVT [31, 32] (1.8%) studies, respectively.
Additionally, the incidence of major bleeding in the asymptomatic DVT group was not different from that in the symptomatic DVT group (5.2 vs. 3.0 events per 100 patient-year; P = 0.45). However, this incidence was modestly higher as compared with the annual rates reported in the J-EINSTEIN-PE/DVT (0.0%) and EINSTEIN-PE/DVT (1.1%) studies. Therefore, the risks and benefits should be carefully considered when initiating rivaroxaban in asymptomatic DVT patients, especially in patients with a high risk of bleeding from cancer. Major bleeding events in this study were mostly due to concomitant active cancer (67%, 4/6 patients suffering from major bleeding, Table 4). A retrospective observational study of Japanese VTE patients treated with DOACs [33] also reported that bleeding events due to active cancer complications should be considered more important than VTE-related events in patients with active cancer complicated with VTE. Therefore, in the real-world setting of this study, it is speculated that the asymptomatic DVT group as compared with the symptomatic group was more often administered off-label underdosed rivaroxaban by physicians, with a shorter treatment duration. This was possibly due to the high bleeding and low VTE risk in this group, which was also characterized by older age, a higher female sex ratio, lower body weight and CrCL, as well as a higher proportion of distal DVT and active cancer.
Clinical implications
Our data may provide a basis for using rivaroxaban to treat and prevent the recurrence of VTE in patients with asymptomatic PE (with or without DVT) and DVT-only. Patients with asymptomatic PE displayed background characteristics similar to those with incidentally detected PE as reported previously; i.e., the PE severity involved mostly low-risk non-massive and occasionally sub-massive PEs, but the concomitant cancer rate was high. Despite the use of intensive rivaroxaban treatment in the asymptomatic PE group, clinically relevant events, including VTE recurrence and mortality in this group were similar to those in the symptomatic PE group. In addition, major bleeding and fatal bleeding events were low. Our data suggest that intensive rivaroxaban treatment can be used for the prophylaxis of VTE and VTE-related complications, even in patients with asymptomatic PE, with or without concomitant cancer, as recommended previously for patients with incidentally detected PE [21, 22, 26].
The asymptomatic DVT group also demonstrated a higher rate of cancer incidence, similar to the PE group. Concomitant active cancer was one of the risk factors for clinically relevant events, mostly driven by cancer-related death rather than bleeding or VTE-related death in both the PE and DVT groups. Our study strongly recommends that concomitant active cancer should be carefully managed in both the PE and DVT-only groups. Notably, the asymptomatic DVT group had a higher risk of bleeding (related to older age, female sex, lower body weight, and lower renal function) and higher incidence of distal DVT than the asymptomatic PE group. Due to this, asymptomatic DVT patients were more likely to receive an off-label underdose rivaroxaban with a shorter treatment duration than that received by the symptomatic DVT and the symptomatic/asymptomatic PE group. The incidence of recurrent VTE was lowest in this group (1.7 events per patient-year). However, major bleeding events were highest in the asymptomatic DVT group among the four groups (5.2 vs. 3.0 events per patient-year for symptomatic DVT, 1.9 for asymptomatic PE, and 2.7 for symptomatic PE, respectively). Therefore, the decision to initiate rivaroxaban should be carefully made while considering the risks and benefits for patients with asymptomatic DVT. Once physicians decide to initiate rivaroxaban, an underdosed rivaroxaban with treatment duration adjustment based on careful monitoring of the bleeding and VTE risks could be an option for such patients [34].
Limitations
This study has some limitations. First, the sample size was relatively small, and the number of clinical events was also limited because this study only included Japanese patients. The reported incidence rates of PE and DVT in Western countries [3] seem to be higher as compared to those reported for the Japanese population [35]. This difference could be attributed to variations in the underlying causes. Factor V Leiden mutation is a common cause of DVT among Western individuals [36], while protein S deficiency is more frequently associated with DVT in Japanese patients [37], with Factor V Leiden being rarely reported in Japan. It is also important to note that the genetic predisposition to venous thrombosis differs between Japanese and Western populations. Therefore, it remains uncertain whether our study findings can be directly applied to individuals in Western countries. Second, selection bias could have occurred. Patients enrolled in this study received rivaroxaban at the discretion of their physicians; patients who were diagnosed with VTE but did not receive anticoagulation were not included in this study. However, their absence must be considered. Thus, selection bias in enrolling patients must be carefully considered when interpreting the results of this study. Finally, information was lacking regarding the enrolled patients. In particular, the lack of information on the detailed location of thrombi in pulmonary emboli and the clinical stage of patients with malignant tumors may have led to inadequate analyses.
Conclusions
This study has clarified the characteristics of patients with asymptomatic PE with and without DVT and those with DVT only. The asymptomatic PE and DVT groups were more likely to have active cancer. However, they experienced lower severity of PE and DVT as compared with patients with symptomatic PE and DVT-only there were more cases of non-massive PE in the asymptomatic PE group and more cases of distal DVT in the asymptomatic DVT group. Patients with asymptomatic DVT had the highest risk of bleeding among the four groups. They were also most often characterized by old age, female sex, low body weight, and low renal function. The real-world composite adverse event rate of rivaroxaban treatment was similar for patients with asymptomatic and symptomatic PE or DVT, as demonstrated using physician-adjusted dose and duration. This suggests a potential for safe dose- and duration-adjusted rivaroxaban treatment for asymptomatic VTE patients.
Acknowledgements
The authors thank all patients and investigators at the centers that participated in this study. The authors thank Serina Nakamoto and the other members of Mebix for their assistance with data collection, storage, and analysis. to the authors also thank Mr. John Martin and Editage (www.editage.com) for the English language editing.
Abbreviations
- ACS
Acute coronary syndrome
- CI
Confidence interval
- CrCl
Creatinine clearance
- CT
Computed tomography
- CTEPH
Chronic thromboembolic pulmonary hypertension
- CVD
Cardiovascular disease
- DOAC
Direct oral anticoagulants
- DVT
Deep vein thrombosis
- HR
Hazard ratio
- IQR
Interquartile range
- IRB
Institutional Review Board
- LV
Left ventricle
- mITT
Modified intention-to-treat
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- PCPS
Percutaneous cardiopulmonary support
- PE
Pulmonary embolism
- RV
Right ventricle
- SD
Standard deviation
- SpO2
Oxygen saturation
- VTE
Venous thromboembolism
Authors' contributions
S.M. contributed to the data analysis and interpretation. Y.O., A.H. contributed to the conception and design, data acquisition, and data analysis and interpretation. All authors contributed to the preparation of the manuscript and approved the final manuscript.
Funding
This study was supported financially by Bayer Yakuhin, Ltd (grant number: 1286981-000).
Availability of data and materials
The deidentified participant data will not be shared.
Declarations
Ethics approval and consent to participate
The analysis of the study data has been approved by the IRB of Nihon University Itabashi Hospital, Clinical Research Ethics Committee and the IRBs of the participating hospitals. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
S.M. has no relationships relevant to the content of this study. Y.O. received lecture fees from Bayer Yakuhin, Ltd., Bristol-Myers Squibb, and AstraZeneca K.K.; lecture fees, scholarship funds, and donations from Daiichi-Sankyo Co., Ltd.; scholarship funds and donations from Nihon Medi-Physics; and is associated with endowed departments sponsored by Boston Scientific Japan, Abbott Medical Japan, Medtronic Japan Co., Ltd., Nihon Kohden Co., and Japan Lifeline Co., Ltd. I.F. has no relationships relevant to the content of this study. M.N. has no relationships relevant to the content of this study. N.Y. received lecture fees from Bayer Yakuhin, Ltd., Pfizer Japan Inc., and Daiichi-Sankyo Co., Ltd. M.T. has no relationships relevant to the content of this study. H.M. has no relationships relevant to the content of this study. Ta.Y. received lecture fees, manuscript fees, and research funding from Daiichi-Sankyo Co., Ltd., Bristol-Myers Squibb, and Bayer Yakuhin Ltd.; lecture fees from Ono Pharmaceutical Co., Ltd., Toa Eiyo, Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., and Nippon Boehringer Ingelheim Co., Ltd.; and scholarships from Daiichi-Sankyo Co., Ltd. T.I. received lecture fees from Bayer Yakuhin, Ltd., Daiichi-Sankyo Co., Ltd., and Pfizer Japan Inc. and research funding from Daiichi-Sankyo Co., Ltd. M.M. received lecture fees from Bayer Yakuhin, Ltd. Ts.Y. has no relationships relevant to the content of this study. A.H. received lecture fees from Daiichi-Sankyo Co., Ltd. and Bayer Yakuhin, Ltd.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.ISTH Steering Committee for World Thrombosis Day Thrombosis: a major contributor to the global disease burden. J Thromb Haemost. 2014;12:1580–90. doi: 10.1111/jth.12698. [DOI] [PubMed] [Google Scholar]
- 2.JCS Joint Working Group Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009) Circ J. 2011;75:1258–81. doi: 10.1253/circj.cj-88-0010. [DOI] [PubMed] [Google Scholar]
- 3.Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34:2363–71. doi: 10.1161/ATVBAHA.114.304488. [DOI] [PubMed] [Google Scholar]
- 4.Di Nisio M, Ferrante N, De Tursi M, Iacobelli S, Cuccurullo F, Büller HR, et al. Incidental venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Thromb Haemost. 2010;104:1049–54. doi: 10.1160/TH10-05-0277. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Razeq HN, Mansour AH, Ismael YM. Incidental pulmonary embolism in cancer patients: clinical characteristics and outcome–a comprehensive cancer center experience. Vasc Health Risk Manag. 2011;7:153–8. doi: 10.2147/VHRM.S17947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tresoldi S, Flor N, Luciani A, Lombardi MA, Colombo B, Cornalba G. Contrast enhanced chest-MDCT in oncologic patients. Prospective evaluation of the prevalence of incidental pulmonary embolism and added value of thin reconstructions. Eur Radiol. 2015;25:3200–6. doi: 10.1007/s00330-015-3739-7. [DOI] [PubMed] [Google Scholar]
- 7.Dentali F, Ageno W, Becattini C, Galli L, Gianni M, Riva N, et al. Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: a meta-analysis. Thromb Res. 2010;125:518–22. doi: 10.1016/j.thromres.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133:454S–545S. 10.1378/chest.08-0658. [DOI] [PubMed]
- 9.O’Connell C, Razavi P, Ghalichi M, Boyle S, Vasan S, Mark L, et al. Unsuspected pulmonary emboli adversely impact survival in patients with cancer undergoing routine staging multi-row detector computed tomography scanning. J Thromb Haemost. 2011;9:305–11. doi: 10.1111/j.1538-7836.2010.04114.x. [DOI] [PubMed] [Google Scholar]
- 10.van der Hulle T, den Exter PL, Planquette B, Meyer G, Soler S, Monreal M, et al. Risk of recurrent venous thromboembolism and major hemorrhage in cancer-associated incidental pulmonary embolism among treated and untreated patients: a pooled analysis of 926 patients. J Thromb Haemost. 2016;14:105–13. doi: 10.1111/jth.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun JM, Kim TS, Lee J, Park YH, Ahn JS, Kim H, et al. Unsuspected pulmonary emboli in lung cancer patients: the impact on survival and the significance of anticoagulation therapy. Lung Cancer. 2010;69:330–6. doi: 10.1016/j.lungcan.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:I22–I30. doi: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- 13.Singh K, Yakoub D, Giangola P, DeCicca M, Patel CA, Marzouk F, et al. Early follow-up and treatment recommendations for isolated calf deep venous thrombosis. J Vasc Surg. 2012;55:136–40. doi: 10.1016/j.jvs.2011.07.088. [DOI] [PubMed] [Google Scholar]
- 14.Okumura Y, Fukuda I, Nakamura M, Yamada N, Takayama M, Maeda H, et al. A multicenter prospective observational cohort study to investigate the effectiveness and safety of Rivaroxaban in Japanese venous thromboembolism patients (the J’xactly study) Circ J. 2020;84:1912–21. doi: 10.1253/circj.CJ-20-0636. [DOI] [PubMed] [Google Scholar]
- 15.Okumura Y, Fukuda I, Nakamura M, Yamada N, Takayama M, Maeda H, et al. Design and rationale for the Japanese Registry of Rivaroxaban Effectiveness & Safety for the Prevention of recurrence in patients with deep vein thrombosis and pulmonary embolism (j’xactly) study. BMJ Open. 2018;8:e020286. doi: 10.1136/bmjopen-2017-020286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Gogh Investigators, Buller HR, Cohen AT, Davidson B, Decousus H, Gallus AS, et al. Idraparinux versus standard therapy for venous thromboembolic disease. N Engl J Med. 2007;357:1094–104. doi: 10.1056/NEJMoa064247. [DOI] [PubMed] [Google Scholar]
- 17.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 18.Browne AM, Cronin CG, English C, NiMhuircheartaigh J, Murphy JM, Bruzzi JF. Unsuspected pulmonary emboli in oncology patients undergoing routine computed tomography imaging. J Thorac Oncol. 2010;5:798–803. doi: 10.1097/JTO.0b013e3181d6153a. [DOI] [PubMed] [Google Scholar]
- 19.Di Nisio M, Carrier M. Incidental venous thromboembolism: is anticoagulation indicated? Hematology am soc Hematol Educ Program. 2017;2017:121–7. 10.1182/asheducation-2017.1.121. [DOI] [PMC free article] [PubMed]
- 20.Rouzaud D, Alexandra JF, Chauchard M, Delon M, Dossier A, Chauveheid MP, et al. Incidental venous thromboembolism diagnosed in patients admitted with a first venous thromboembolism: frequency and clinical significance (an observational study) Thromb Res. 2016;144:116–8. doi: 10.1016/j.thromres.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the european respiratory society (ERS) Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 22.Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38:496–520. doi: 10.1200/JCO.19.01461. [DOI] [PubMed] [Google Scholar]
- 23.Guérin L, Couturaud F, Parent F, Revel MP, Gillaizeau F, Planquette B, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost. 2014;112:598–605. doi: 10.1160/TH13-07-0538. [DOI] [PubMed] [Google Scholar]
- 24.Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–64. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 25.Klok FA, Huisman MV. Management of incidental pulmonary embolism. Eur Respir J. 2017;49:1700275. doi: 10.1183/13993003.00275-2017. [DOI] [PubMed] [Google Scholar]
- 26.Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545–e608. doi: 10.1016/j.chest.2021.07.055. [DOI] [PubMed] [Google Scholar]
- 27.Palareti G, Cosmi B, Lessiani G, Rodorigo G, Guazzaloca G, Brusi C, et al. Evolution of untreated calf deep-vein thrombosis in high risk symptomatic outpatients: the blind, prospective CALTHRO study. Thromb Haemost. 2010;104:1063–70. doi: 10.1160/TH10-06-0351. [DOI] [PubMed] [Google Scholar]
- 28.Galanaud JP, Sevestre MA, Genty C, Kahn SR, Pernod G, Rolland C, et al. Incidence and predictors of venous thromboembolism recurrence after a first isolated distal deep vein thrombosis. J Thromb Haemost. 2014;12:436–43. doi: 10.1111/jth.12512. [DOI] [PubMed] [Google Scholar]
- 29.Righini M, Galanaud JP, Guenneguez H, Brisot D, Diard A, Faisse P, et al. Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): a randomised, double-blind, placebo-controlled trial. Lancet Haematol. 2016;3:e556–62. doi: 10.1016/S2352-3026(16)30131-4. [DOI] [PubMed] [Google Scholar]
- 30.Yamada N, Hirayama A, Maeda H, Sakagami S, Shikata H, Prins MH, et al. Oral rivaroxaban for japanese patients with symptomatic venous thromboembolism – the J-EINSTEIN DVT and PE program. Thromb J. 2015;13:2. doi: 10.1186/s12959-015-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Investigators EINSTEIN–PE, Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, et al. Oral Rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 32.Investigators EINSTEIN, Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, et al. Oral Rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 33.Ueno Y, Ikeda S, Motokawa T, Honda T, Kurobe M, Akashi R, et al. Comparison of effectiveness and safety among 3 direct oral anticoagulants in patients with venous thromboembolism – a single-center retrospective study. Circ Rep. 2022;4:533–41. doi: 10.1253/circrep.CR-22-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Clinical Guideline Centre (UK) Venous thromboembolic Diseases: the management of venous thromboembolic diseases and the role of thrombophilia testing [Internet] London: Royal College of Physicians (UK); 2012. [PubMed] [Google Scholar]
- 35.Yamashita Y, Morimoto T, Amano H, Takase T, Hiramori S, Kim K, et al. Anticoagulation therapy for venous thromboembolism in the real world - from the COMMAND VTE Registry. Circ J. 2018;82:1262–70. doi: 10.1253/circj.CJ-17-1128. [DOI] [PubMed] [Google Scholar]
- 36.Simioni P, Prandoni P, Lensing AW, Scudeller A, Sardella C, Prins MH, et al. The risk of recurrent venous thromboembolism in patients with an Arg506–>Gln mutation in the gene for factor V (factor V Leiden) N Engl J Med. 1997;336:399–403. doi: 10.1056/NEJM199702063360602. [DOI] [PubMed] [Google Scholar]
- 37.Miyata T, Sato Y, Ishikawa J, Okada H, Takeshita S, Sakata T, et al. Prevalence of genetic mutations in protein S, protein C and antithrombin genes in japanese patients with deep vein thrombosis. Thromb Res. 2009;124:14–8. doi: 10.1016/j.thromres.2008.08.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The deidentified participant data will not be shared.