Summary
Grain size is specified by three dimensions of length, width and thickness, and slender grain is a desirable quality trait in rice. Up to now, many grain size regulators have been identified. However, most of these molecules show influence on multi‐dimensions of grain development, and only a few of them function specifically in grain width, a key factor determining grain yield and appearance quality. In this study, we identify the SLG2 (SLENDER GUY2) gene that specifically regulates grain width by affecting cell expansion in the spikelet hulls. SLG2 encodes a WD40 domain containing protein, and our biochemical analyses show that SLG2 acts as a transcription activator of its interacting WOX family protein WOX11. We demonstrate that the SLG2‐associated WOX11 binds directly to the promoter of OsEXPB7, one of the downstream cell expansion genes. We show that knockout of WOX11 results in plants with a slender grain phenotype similar to the slg2 mutant. We also present that finer grains with different widths could be produced by combining SLG2 with the grain width regulator GW8. Collectively, we uncover the crucial role of SLG2 in grain width control, and provide a promising route to design rice plants with better grain shape and quality.
Keywords: SLG2, WOX11, GW8, grain width, cell expansion, rice
Introduction
Rice is one of the most important cereal crops and the stable food of nearly half of the world's people. With the increase of the world population, the deterioration of the environment and the reduction of the arable land, increasing grain yield remains the major challenge for most rice growing areas. Rice grain yield is composed of three major factors: panicle number, grain number per panicle, and grain weight. The grain weight is determined by grain size and grain filling. Because grain size is a key determinant of yield and appearance quality, it is one of the major targets of artificial selection during domestication, and is an important trait for rational design of high‐yield and high‐quality rice varieties.
Grain size is specified by three dimensions of length, width and thickness. Based on the analysis of mutant materials and natural populations, nearly 200 genes have been cloned for their functions in grain size regulation (Jiang et al., 2022). Functional study of these genes revealed a variety of regulatory routes involved in rice grain size control. For example, SMALL GRAIN 1 (OsSMG1)/(OsMKK4), OsMAPK6 and OsMKKK10 control grain size through the mitogen‐activated protein kinase signalling pathway (Duan et al., 2014; Liu et al., 2015a,b; Xu et al., 2018), and GS3, RGB1, DEP1 and RGG2 are important grain size regulators in the G protein signalling pathway (Fan et al., 2006; Miao et al., 2019; Utsunomiya et al., 2011; Xu et al., 2016). In addition, WIDE AND THICK GRAIN 1 (WTG1)/(OsOTUB1), GW2 and OsUBP15 affect grain size by the ubiquitin‐proteasome pathway (Choi et al., 2018; Huang et al., 2017; Shi et al., 2019), while GS2, GW8, GLW7 and OsPIL15 determine grain size through transcriptional factors‐mediated pathway (Hu et al., 2015; Si et al., 2016; Sun et al., 2020; Wang et al., 2015). Besides, GS5, BG1 and GW5 are vital grain size controllers involved in the phytohormone signalling pathway (Li et al., 2011; Liu et al., 2015a,b). Moreover, regulators such as OsmiR156, OsmiR396, OsmiR408 and OsAGO17 appear to define grain size in the epigenetic pathway (Duan et al., 2015; Jiao et al., 2010; Zhang et al., 2017; Zhong et al., 2020a,b).
Because slender grains are preferred by the majority of consumers (Wang et al., 2012), isolation of regulators that only affect one of the three dimensions of grain size, especially grain width, is particularly important for rice grain yield and appearance quality (Jiang et al., 2022). So far, mutational analysis in rice has identified many genes controlling grain size (Jiang et al., 2022). However, except for GS6 (Sun et al., 2013) and GW5 (Tian et al., 2019), most of the qualitative loci isolated from grain size mutants are difficult to meet the actual production demands due to adverse effects on other agronomic traits (Chen et al., 2021). By studying germplasms with different grain shapes, about 22 quantitative trait loci (QTLs) have been identified to regulate grain size in rice (Jiang et al., 2022). However, a careful survey of the phenotypes of the loss of function mutants, but not near isogenic lines or knockdown/overexpression transgenic lines, revealed that most of these genes show influence on the development of grains in multi‐dimensions, and only four genes GS5 (Li et al., 2011), GW5 (Tian et al., 2019), TGW2 (Ruan et al., 2020) and TGW12a (Du et al., 2021) display a regulatory role confined to grain width. However, the application value of these four genes in regulating grain width remains to be evaluated, as they all seem to affect the grain filling process. Up to now, GW8, a squamosa promoter binding protein coding gene that has been artificially selected in the rice breeding program, should be the only regulatory factor that can improve the appearance quality of rice without adversely affecting other grain traits (Wang et al., 2012). The ultimate goal of studying grain size genes is undoubtedly for breeding, and pyramiding of multiple genes is usually necessary to obtain plants with ideal grain size and appearance quality (Jiang et al., 2022). However, one can imagine that if the identified regulators affect multiple dimensions of grain development, it would be difficult to achieve a balance among grain size, grain filling and grain appearance during gene pyramiding. In other words, we need to excavate more regulators that only affect one of the three dimensions of grain size, especially grain width.
The WD40 repeats are one of the most abundant domains in the eukaryotic genome, and members of the WD40 family act as scaffolds for protein–protein interactions (Jain and Pandey, 2018; Stirnimann et al., 2010). In plants, the WD40 proteins take part in diverse processes such as immune response, cell wall formation, histone modification, proteasomal degradation, and microtubule organization (Jain and Pandey, 2018). The rice genome is predicted to contain 231 genes coding for WD40 protein (Yang et al., 2021). Genetic analysis of these OsWD40s has revealed their versatile functions in multiple developmental processes, such as grain number (Chen et al., 2022), anthocyanin biosynthesis (Yang et al., 2021), pollen tube germination and elongation (Kim et al., 2021), flowering and panicle branching (Jiang et al., 2018), and fertility (Qin et al., 2016). And, mutant analysis also has uncovered the role of several OsWD40s in grain development, such as OsFIE1 (Cheng et al., 2020), OsFIE2 (Nallamilli et al., 2013), OsLIS‐L1 (Gao et al., 2012), RGB1 (Utsunomiya et al., 2011; Zhang et al., 2021a,b,c), and DRW1 (Zhang et al., 2021a,b,c). However, due to the numerous roles of WD40 proteins in cellular processes, dysfunction of these genes often leads to malformed development of not only grain size but also many other organs. Recently, Dhatt et al. (2021) reported the contribution of the allelic variation in OsFIE1 to grain width under high night temperature stress. As far as we know, this should be the only report linking WD40 protein with rice grain width so far. Because the functions of most OsWD40 members are still unclear, much effort should be made to reveal their roles in grain size regulation.
In this study, we report the identification of slg2, a rice mutant with specifically reduced grain width caused by decreased cell expansion. We show that SLG2 regulates grain width by recruiting and activating the WOX family transcription factor WOX11 to directly promote the expression of downstream cell expansion genes. We also demonstrate that the combination of SLG2 with another grain width regulator, GW8, could produce finer grains with different widths. Our results suggest that the grain width‐specific regulator SLG2 has potential value in designing rice plants with better grain shape and quality.
Results
Rice mutant slg2 shows a specific decrease in grain width
By screening the NaN3‐mutagenized M2 library in the background of ZY66 (japonica), we identified a mutant with obviously decreased grain width (Figure 1a,d), and the mutant was then designated slg2 (slender guy 2). Compared with the wild type (WT), the grain width of slg2 was reduced by about 10.5% (Figure 1a,d,e). In contrast, the other two dimensions of grain size, i.e., grain length and grain thickness, remained similar between slg2 and WT (Figure 1b,c,e). In addition, we paid special attention to grain chalkiness, which is an important trait closely related to grain quality, especially appearance quality. We found that there was no difference in the transparency of brown rice endosperm between slg2 and WT (Figure 1d; Figure S1a). We further observed the starch granules by scanning electron microscopy (SEM), and found that the starch packaging in the endosperm of slg2 was similar to that of WT (Figure S1b). We also investigated the grain filling by monitoring caryopsis development of the mutant during the whole grain maturation stage (Figure S2a). No difference was observed for ovary extension in the longitudinal direction between slg2 and WT. However, from 5 days after fertilization (DAF), the caryopsis growth of the mutant lagged behind WT in the transversal direction (Figure S2a), and the grain weight of slg2 was obviously lower than that of WT (Figure S2b,c). Nonetheless, the trend of grain weight increase during caryopsis maturation was similar between the mutant and WT (Figure S2b,c), and the grain filling rate of slg2 was identical to that of WT (Figure S2d). Taken together, these results suggest that SLG2 is a grain size regulator that acts specifically on grain width, but not on grain length, grain thichness or grain filling.
Figure 1.
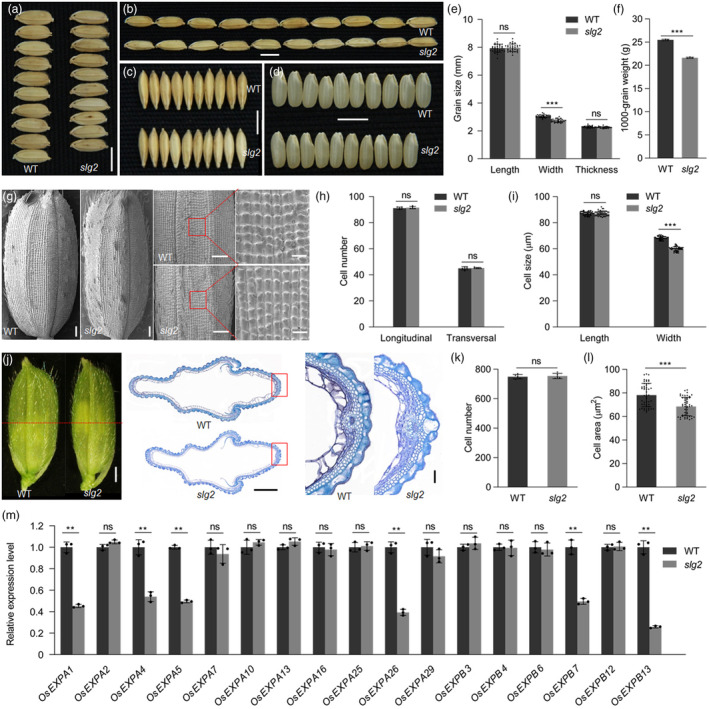
Characterization of the slg2 mutant. (a–d) Comparison of grain width (a), grain length (b), grain thickness (c) and grain quality (d) between slg2 and WT. Bars = 5 mm. (e, f) Statistical analysis of grain size (e) and 1000‐grain weight (f) between slg2 and WT. Data are means ± SD (n = 30 for e and 3 for f). ***: P < 0.001, ns: no significant difference (Student's t test). (g–i) SEM observation of outer glume surfaces (g) and statistical analysis of cell number (h) and cell size (i) in slg2 and WT. For (g), bars = 500 μm for the left images, and 50 μm for the right images. Data are means ± SD (n = 5 for h and 50 for i). ***: P < 0.001, ns: no significant difference (Student's t test). (j–l) Cross‐sections of the spikelet hulls (j) and statistical analysis of cell number (k) and cell area (l) in slg2 and WT. The spikelet hulls are sampled before anthesis. In (j), the dotted line indicates the sites of the cross‐sections shown in middle, and the boxes indicate the sites of the magnified images shown in left. Bars = 1 mm (left), 500 μm (middle) and 50 μm (right). Data are means ± SD (n = 5 for k and 50 for l). ***: P < 0.001, ns: no significant difference (Student's t test). (m) Expression analysis of cell expansion genes in slg2 and WT. RNA isolated from young panicles of 2–3 mm in length is used for RT‐qPCR. OsActin is used as the internal control. The transcript levels are normalized against WT, which is set to 1. Data are means ± SD (n = 3). **: P < 0.01, ns: no significant difference (Student's t test).
Grain size is determined by the spikelet hull composed of palea and lemma, and the development of spikelet hull is coordinately regulated by cell proliferation and cell expansion (Li et al., 2018). To understand the cellular process of SLG2 regulating grain size, we performed SEM observation of epidermal cells in the lemma of slg2 and WT (Figure 1g). We found that cell number in the central part of the lemma of slg2 was comparable to that of WT both in the longitudinal and transversal directions (Figure 1h). The length of the epidermal cells was similar between slg2 and WT. In contrast, the width of these cells in slg2 was about 12.4% less than that in WT (Figure 1g,i). The decrease of cell width in slg2 coincided well with the reduction of grain width in the mutant (Figure 1e,i). We further carried out cytological observation using cross‐sections of the central part of spikelet hulls before anthesis (Figure 1j). In agreement with the result of SEM observation, the number of cells in the outer parenchyma cell layer of slg2 remained unchanged (Figure 1k), but the size of these cells was reduced by about 12.2% compared with WT (Figure 1l).
To gain insight into the molecular basis of SLG2‐mediated grain size control, we performed expression analysis of genes related to cell division and cell expansion. We found no significant difference in the transcripts levels of genes known to function in G1‐to‐S transition (H1, E2F2, CAK1, CDKA1 and MCM3) and G2‐to‐M transition (CYCA2.1, CYCB2.1, CYCB2.2 and CDKB) between slg2 and WT (Figure S3a). Of the 17 cell expansion genes that appear to express in young panicles (https://ricexpro.dna.affrc.go.jp/GGEP/), six genes (OsEXPA1, OsEXPA4, OsEXPA5, OsEXPA26, OsEXPB7 and OsEXPB13) showed suppressed transcription in slg2 relative to WT (Figure 1m). We also explored the possible regulatory relationship between SLG2 and previously identified grain width‐related genes by detecting the expression level of these genes, including GW2 (Song et al., 2007), GS5 (Li et al., 2011), GS6 (Sun et al., 2013), GW8 (Wang et al., 2012), GW5 (Liu et al., 2017), GW5L (Tian et al., 2019), TGW2 (Ruan et al., 2020), GW6 (Shi et al., 2020) and TGW12a (Du et al., 2021). We did not find significant differences in the transcripts abundance of these genes between slg2 and WT (Figure S3b). Taken together, these results suggest that SLG2 controls grain width by affecting cell expansion in the spikelet hull in a way independent of the currently identified grain width genes.
A survey of other agronomic traits showed that the overall development of slg2 was basically normal (Figure S4a). The heading date, tillering ability and seed setting rate of slg2 were identical with those of WT (Figure S4c,d,f), while plant height of the mutant was about 3.5 cm lower than that of WT (Figure S4b). The difference of agronomic traits between slg2 and WT, in addition to grain width, was mainly the number of grains per panicle (85.3 vs. 96.6; Figure S4e). As a result, the grain yield of the mutant decreased by about 15% compared with that of WT (Figure S4g).
SLG2 encodes a WD40 domain containing protein
To understand the molecular mechanism of SLG2‐mediated grain width control, we performed map‐based cloning to isolate the causal gene. Crossing of slg2 with WT yielded an F2 population, in which the segregation ratio of WT and slender grain phenotype was 3:1 (398:128; χ2 = 0.12; 0.5 > P > 0.1). This result suggests that the slg2 mutant phenotype is caused by the recessive mutation of a single locus. By using 20 bulked WT and slg2 mutant plants from the F2 population between slg2 and KY131 (japonica), the causal gene was initially mapped to the short arm of chromosome 2 between markers M1 and M2 (Figure 2a). And, the candidate was further narrowed down to a 377‐kb region between markers M6 and M7 using 1152 F2 individuals with a mutant phenotype (Figure 2a). After sequencing the fine mapped region, we found that a point mutation of C to T occurred in slg2 in the eighth exon of the annotated gene LOC_Os02g18820 (Figure 2a; http://rice.plantbiology.msu.edu/), leading to the formation of a premature stop codon in the mutant (Figure 2b).
Figure 2.
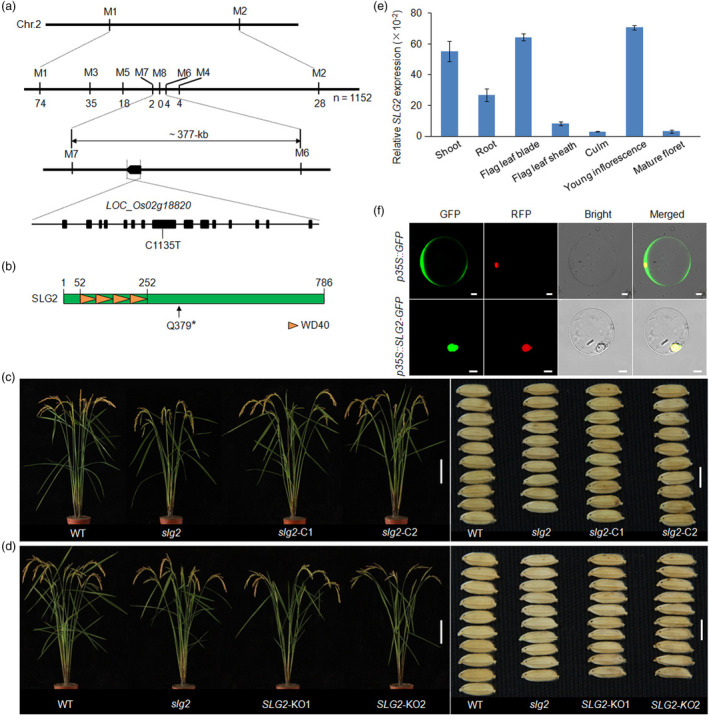
Map‐based Cloning of SLG2. (a, b) Identification of the SLG2 candidate gene. Indicating one C‐T point mutation occurred in LOC_Os02g18820 in the slg2 mutant, which generates a premature stop codon. (c, d) Phenotypes of whole plant and grain size of WT, slg2, complementation lines (slg2‐C) and knockout lines (SLG2‐KO). Bars = 15 cm for whole plant and 5 mm for grain size. (e) Expression analysis of SLG2 in various rice tissues. For RT‐qPCR, RNA is isolated from shoots and roots of 7‐day‐old seedlings, and flag leaf blade, flag leaf sheath, culm, young panicle (2–3 mm in length) and mature floret before anthesis. OsActin is used as the internal control. Data are means ± SD (n = 3). (f) Subcellular localization of SLG2. Bars = 10 μm.
To verify that the C‐T substitution was responsible for the slg2 mutant phenotype, a 17.8‐kb genomic sequence of LOC_Os02g18820, including the entire coding region, 2392‐bp upstream of ATG and 1567‐bp downstream of TGA, was amplified from WT and introduced into the slg2 mutant. We found that the overall phenotypes of all the 28 T0 positive transgenic plants, including grain width and cell width of the spikelet hull, resembled that of WT (Figure 2c; Figure S5). In addition, by selecting a specific target site in the third exon (Figure S6a), we tried to knock out the LOC_Os02g18820 locus in the ZY66 background using the CRISPR/Cas9 genome editing tool. We obtained a series of transgenic lines with multiple types of insertions or deletions in the coding region, and selected two representative lines with 1‐bp (SLG2‐KO1) and 2‐bp (SLG2‐KO2) insertions for phenotypic analysis (Figure S6a). We noticed that similar to the slg2 mutant, both SLG2‐KO lines presented slender grain phenotype (Figure 2d; Figure S6c). Moreover, we found that cell width of the spikelet hull of the SLG2‐KO plants was reduced (Figure S6b,d), and transcription of the cell expansion genes differentially expressed in slg2 were also suppressed in the SLG2‐KO lines (Figure S6e). We further created SLG2‐overexpressing transgenic lines in the background of slg2 (SLG2‐OE slg2 ). Consistent with the results of the complementation test, overexpression of SLG2 fully rescued the overall phenotypes of the mutant (Figure S7a,b), including the reduced grain width (Figure S7c,d). These observations clearly demonstrate that the slg2 phenotypes are caused by the point mutation in LOC_Os02g18820.
The transcripts of SLG2 were detected in all the tissues tested, including shoot, root, flag leaf blade, flag leaf sheath, culm, young panicle and mature floret, with the highest expression in the young panicle (Figure 2e). SLG2 is localized in the nucleus (Figure 2f), and is predicted to be a WD40 domain containing protein with four WD40 repeats in its N terminus (Figure 2b; https://www.ncbi.nlm.nih.gov/). In rice, there are 231 OsWD40s in the genome (Yang et al., 2021), and LOC_Os09g12550 is the only paralog of SLG2 (http://plants.ensembl.org/index.html). Because these two genes shared an identity of about 65% at the protein level (Figure S8), and LOC_Os09g12550 displayed a tissue‐specific expression pattern similar to SLG2 (Figure S9a), the gene was then designated HSLG2 (Homologue of SLG2). To understand whether HSLG2 also plays a role in grain size control, we knocked out this gene in the ZY66 background by specifically editing the target site in the second exon (Figure S9b). Analysis of three independent lines with differential mutations in the coding region revealed that the HSLG2‐KO plants had no any alterations in overall agronomic traits including grain size (Figure S9b–f). Moreover, we created the slg2,hslg2 double mutants by crossing HSLG2‐KO1 plants with slg2. No obvious difference was observed between slg2,hslg2 and slg2 in overall agronomic traits including grain width (Figure S9c–f), and the transcripts of SLG2 remained unchanged between HSLG2‐KO and WT (Figure S9g). These observations suggest that SLG2 and HSLG2 have divergent functions in rice plant development.
SLG2 interacts with WOX11, a positive grain width regulator
In most cases, WD40 repeat containing proteins achieve their regulatory functions by associating with other transcription factors (Jain and Pandey, 2018). In order to uncover the mechanism of SLG2‐mediated grain width control, we used a cDNA library prepared from young panicles (2–3 mm in length) to screen the interacting proteins of SLG2 by the yeast two‐hybrid system. Among the sequenced positive colonies, we were highly interested in the potential partner WOX11 (Figure 3a), a transcription factor appeared to play important roles in multiple rice developmental processes, including crown root, shoot, plant height, and panicle size (Cheng et al., 2018; Jiang et al., 2017; Zhou et al., 2017). In fact, except for the reduced plant height and panicle size (Figure S4), we also observed that at the seedling stage, the growth of shoot and root of slg2 was obviously slower than that of WT (Figure 3d–f). Moreover, we found that in the slg2 mutant, transcription of genes related to the development of shoots (such as OSH6, OSH15, OSH43 and OSH71) and roots (such as OsRAA1, OsFRDL1, OsMDP1 and OsASR3) was markedly suppressed (Figure 3g,h). All these genes have been reported to be positively regulated by WOX11 (Cheng et al., 2018; Jiang et al., 2017). Although it remained unclear whether WOX11 plays a role in grain size regulation, the highly similar functions of SLG2 and WOX11 in multiple developmental processes strongly suggest that SLG2 may control grain width by associating with the transcription factor WOX11. To verify this speculation, we first confirmed the interaction between SLG2 and WOX11 by a bimolecular fluorescence complementation (BiFC) assay in the rice protoplast (Figure 3b). In addition, in vitro pull‐down assay indicated that only GST‐SLG2, but not free GST, was able to pull down MBP‐WOX11 (Figure 3c). We thus concluded that SLG2 physically interacts with WOX11, both in vivo and in vitro.
Figure 3.
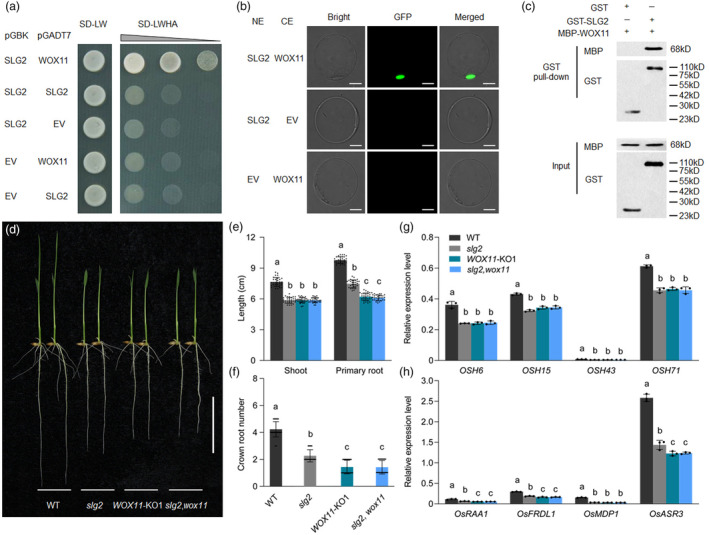
SLG2 and WOX11 interact and regulate rice seedling development. (a–c) Interaction assay between SLG2 and WOX11. Bars = 10 μm (b). (d) Morphological comparison of 7‐day‐old seedlings of WT, slg2, WOX11 knockout line (WOX11‐KO) and slg2,wox11 double mutant plants. Bar = 4 cm. (e, f) Statistical analysis of root length (e) and crown root number (f) of WT, slg2, WOX11‐KO, and slg2,wox11. Statistical analysis is performed in seedlings shown in (d). Bars followed by different letters represent significant difference at 5%. (g, h) Expression analysis of genes involved in shoot (g) and root (h) development. RNA isolated from the shoots and roots of the seedlings shown in (a) is used for RT‐qPCR. OsActin is used as the internal control. Data are means ± SD (n = 3). Bars followed by different letters represent significant difference at 5%.
To reveal the role of WOX11 in grain size regulation, we tried to knock out the locus in the ZY66 background by specifically editing a target site in the first exon (Figure 4a), and obtained dozens of transgenic plants with multiple types of mutations in the coding region. Among these transgenic plants, we selected two homozygous lines with 4‐ and 13‐bp deletions in the first exon respectively, for further analysis (Figure 4a; Figure S10). We observed that the seedling growth of WOX11‐KO lines was significantly slower than that of WT, and the shoot height, primary root length and crown root number of 7‐day‐old seedlings of WOX11‐KO and slg2 were highly similar (Figure 3d–f), and the expression level of genes related to shoot and root development was also similarly decreased (Figure 3g,h). These observations were consistent well with the previous findings (Cheng et al., 2018; Jiang et al., 2017). In addition, we found that the overall morphology of WOX11‐KO adult plants resembled that of the slg2 mutant (Figure 4b). Especially, the grain width of WOX11‐KO plants was reduced to a level similar to slg2 (Figure 4c,e), while the grain length of these knockout plants remained unchanged (Figure 4d,e). These results indicate that WOX11 also plays a role in the development of reproductive organs. We also performed cytological observation to understand the cellular processes mediated by WOX11, and found that the cell width and the cell area of spikelet hulls decreased similarly in the WOX11‐KO plants and the slg2 mutant (Figure S11). We further analysed the expression of the cell expansion genes downregulated in the slg2 mutant. As expected, transcription of all the six genes were greatly suppressed in the WOX11‐KO plants (Figure 4f). The physical interaction of the two proteins and the phenotypic similarity of the two null mutants strongly suggest that SLG2 and WOX11 act jointly to regulate grain width. To verify this speculation, we created the slg2,wox11 double mutant by crossing the slg2 mutant with the WOX11‐KO1 plants. We found that the grain width of the slg2,wox11 double mutant was completely the same as the slg2 and WOX11‐KO single mutants (Figure 4b–e). Consistently, the transcript levels of cell expansion genes were similarly downregulated in the slg2,wox11 double mutant and the two single mutants (Figures 3g,h and 4f). Taken together, these results clearly demonstrate that SLG2 functions coordinately with WOX11 in grain width regulation.
Figure 4.
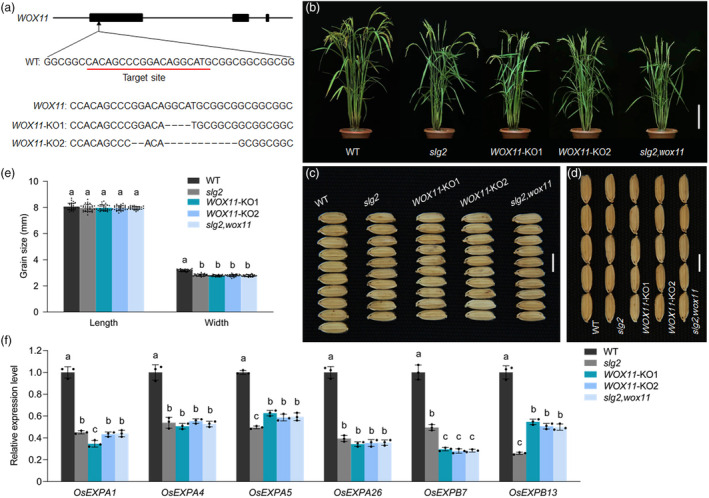
The wox11 mutant displays slender grain phenotype similar to slg2. (a) Creation of WOX11 knockout transgenic line by the CRISPR‐Cas9 genome editing system. The target site is red‐underlined. The representative transgenic lines (abbreviated as WOX11‐KO1 and WOX11‐KO2, respectively) are generated in ZY66 genetic background. The dashes indicate deleted nucleotides. (b) Plant morphology of WT, slg2, WOX11‐KO and slg2,wox11 at the maturation stage. Bar = 15 cm. (c, d) Comparison of grain width (c) and grain length (d) among WT, slg2, WOX11‐KO and slg2,wox11. Bars = 5 mm. (e) Statistical analysis of grain length and grain width in WT, slg2, WOX11‐KO and slg2,wox11. Data are means ± SD (n = 30). Bars followed by different letters represent significant difference at 5%. (f) Expression analysis of cell expansion genes in WT, slg2, WOX11‐KO and slg2,wox11. RNA isolated from young panicles of 2–3 mm in length is used for RT‐qPCR. OsActin is used as the internal control. The transcript levels are normalized against WT, which is set to 1. Data are means ± SD (n = 3). Bars followed by different letters represent significant difference at 5%.
SLG2 acts as a transcription activator of WOX11
WOX11 has been shown to specifically bind to the WOX consensus ‘TTAATGG/C’ (Jiang et al., 2017). As described above, the transcription of cell expansion genes OsEXPA1, OsEXPA4, OsEXPA5, OsEXPA26, OsEXPB7 and OsEXPB13 was suppressed considerably in both slg2 and WOX11‐KO plants (Figure 4f). To understand whether these six genes were the direct targets of WOX11, we first analysed the genomic sequences of these genes to see if there are any WOX11‐binding motifs. We found that all the six genes contain at least one conserved cis‐elements of TTAATGG/C in the 2‐kb promoter region or in the gene body (Figure 5a). Next, we explored the functional relationship between SLG2 and WOX11 by the dual‐luciferase reporter system in rice protoplasts (Figure 5b). We detected strong transactivation activity of WOX11 in our system (Figure 5c), and the activation activity of WOX11 was substantially enhanced by the SLG2 protein (Figure 5c). We further examined whether SLG2 and WOX11 jointly regulate the transcription of OsEXPB7, a potential downstream target showing the most severely suppressed transcription in the WOX11‐KO plants (Figure 4f). We found that WOX11 could effectively activate the transcription of OsEXPB7, and the combination of SLG2‐WOX11 further promoted the transcription of this gene (Figure 5d). Taken together, these results suggest that SLG2 acts as an activator of WOX11‐mediated transcriptional regulation.
Figure 5.
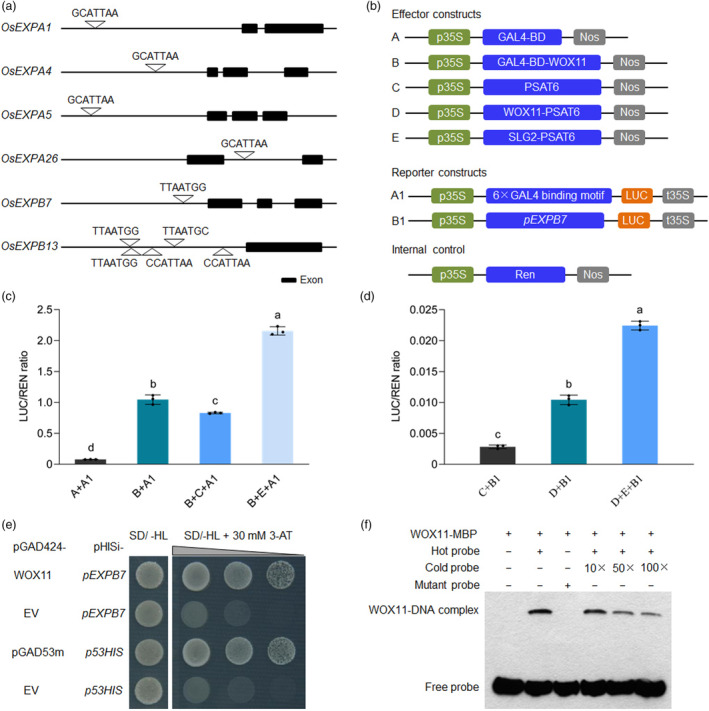
SLG2 functions as an activator of WOX11 to regulate the transcription of downstream cell expansion genes. (a) Scanning of the WOX11 binding motif ‘TTAATGG/C’ across the whole genomic sequence of the cell expansion genes downregulated in slg2 and the WOX11‐KO lines. The length of promoter sequence is 2‐kb. (b) Schematic diagram of the constructs used for transactivation activity assay. (c) Analysis of the effect of SLG2 on the transactivation activity of WOX11. Data are means ± SD (n = 3). Bars followed by different letters represent significant difference at 5%. Indicating that SLG2 significantly enhances the transactivation activity of WOX11. (d) Transcription activation of WOX11 and SLG2 on OsEXPB7. Data are means ± SD (n = 3). Bars followed by different letters represent significant difference at 5%. Indicating the coordinate function of SLG2 and WOX11 in promoting the transcription of OsEXPB7. (e, f) WOX11 directly binds to the promoter of OsEXPB7, which is tested by yeast one‐hybrid assay (e) and electrophoretic mobility shift assay (EMSA) (f). The combinations pGAD53m‐p53HIS and pGAD424‐p53HIS are used as the positive and negative controls, respectively (e). In (f), the hot probe is a biotin‐labelled fragment of the OsEXPB7 promoter sequence AGGGATCGATCGAAATTAATGGCGGGCAGGAGCAGGA, and the cold probe is a non‐labelled competitive probe. The mutant probe is the labelled hot probe sequence with two nucleotides mutated in the conserved binding site (AGGGATCGATCGAAATCCATGGCGGGCAGGAGCAGGA).
To verify whether the SLG2‐WOX11 pathway directly regulates the expression of cell expansion genes, we tested the binding of WOX11 to the promoter of OsEXPB7 by yeast one‐hybrid assay, where WOX11 was fused to GAL4 AD and the OsEXPB7 promoter fused to the HIS3 reporter gene. The result showed that WOX11 bound to the promoter, leading to activation of the HIS3 reporter (Figure 5e). We further confirmed the binding of WOX11 to the OsEXPB7 promoter by electrophoretic mobility shift assay (EMSA). We expressed the WOX11 protein in E. coli and purified it as a maltose binding protein (MBP)‐fusion protein. For the probes, we synthesized biotin‐labelled oligonucleotides containing the native (hot probe) and mutated (mutant probe) WOX binding site presented in the OsEXPB7 promoter (Figure 5a and Table S1). As shown in Figure 5f, WOX11‐MBP bound strongly the hot probe but not the mutant probe. The specificity of the binding was further confirmed by including the cold probe as the competitor, where the competitor greatly reduced binding to the probe. Together, these results clearly show that the WOX11 protein binds to TTAATGG in the OsEXPB7 promoter.
The combination of slg2 and gw8 produces more slender grains
To carry out rational design of grain size, it is necessary not only to discover a sufficient number of functional genes, but also to understand the interacting effects between these genes (Lee et al., 2015; Sun et al., 2018; Zhong et al., 2020a,b). The latter should be more important in the flexible adjustment of the target trait to meet environment and/or human needs. Therefore, we tried to reveal the combined effect of SLG2 and other grain width regulators by using CRISPR genome editing system, because this technology seems to be the most effective method in the current strategy of pyramiding favourable null alleles. To this end, we summarized the functions of the grain width‐related genes identified so far (Table S2), and selected the positive grain width regulator GW8 (Wang et al., 2012). We knocked out GW8 in the background of slg2 (GW8‐KO slg2 ) and ZY66 (GW8‐KOWT) respectively, by specifically editing a target site in the third exon (Figure 6a). After sequencing the target region of the transgenic plants, we selected two homozygous GW8‐KOWT lines with 1‐bp deletion and 1‐bp insertion in the coding region for phenotypic analysis (Figure 6a; Figure S12). We found that the obvious change in the overall morphology of the GW8‐KOWT plants was the reduction of plant height, which was about 9.5% and 5.9% lower than WT and slg2 respectively (Figure 6b; Figure S13a). Similar to the previous finding (Wang et al., 2012), we observed that the grain width of GW8‐KOWT plants decreased significantly, even about 1.6% narrower than that of slg2 (Figure 6c,e). It was reported that dysfunction of GW8 decreases grain width but increases grain length (Wang et al., 2012). However, we did not find any change in the grain length of GW8‐KOWT plants (Figure 6d,e), which may be due to the different genetic backgrounds of the receptor varieties. We also obtained two homozygous GW8‐KO slg2 lines with a C insertion and an AA deletion in the coding region (Figure 6a; Figure S12). We found that the slg2,gw8 double mutants displayed overall morphology highly resembled the slg2 single mutant, including plant height, panicle number, panicle size, and seed setting rate (Figure 6b; Figure S13). The only phenotypic difference between slg2,gw8 and slg2 was the grain size. The grain width of the double mutant was about 11.4% and 9.8% less than that of the single mutant slg2 and gw8, respectively, while the grain length did not change significantly (Figure 6c–e). Notably, similar to the slg2 mutant, the transparency of brown rice endosperm and the starch packaging in mature grains of gw8 and slg2,gw8 were completely normal (Figure S1). The reduction of grain width of the double mutants led to the decrease of 1000‐grain weight and thus grain yield per plant (Figure S13d,f). We also examined the expression of genes related to cell division and cell expansion in the spikelet hulls of the slg2,gw8 plants, and found that the transcripts of all these genes were markedly reduced in the double mutant (Figure S14). This observation is consistent with the positive functions of GW8 (Wang et al., 2012) and SLG2 (Figure 1m; Figure S6e) in these cellular processes. Collectively, our results suggest that the additive effect of slg2 and gw8 alleles is limited to the decrease of grain width, which is of potential value in improving grain appearance quality.
Figure 6.
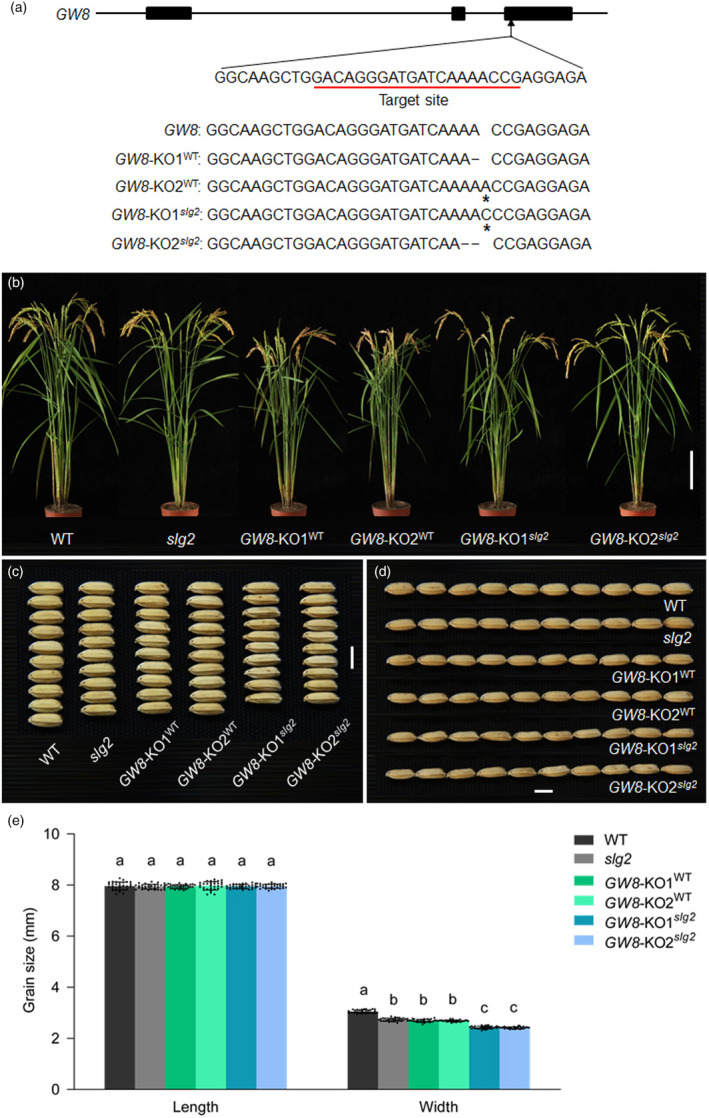
Fine regulation of grain width by slg2, gw8 and their combination. (a) Creation of GW8 knockout transgenic line by the CRISPR‐Cas9 genome editing system. The target site is red‐underlined. The representative transgenic lines (abbreviated as GW8‐KO1 and GW8‐KO2, respectively) are generated in ZY66 (GW8‐KOWT) and slg2 (GW8‐KO slg2 ) genetic background. The asterisks indicate inserted nucleotides and the dashes indicate deleted nucleotides. (b) Plant morphology of WT, slg2, GW8‐KOWT and GW8‐KO slg2 at the maturation stage. Bar = 15 cm. (c, d) Comparison of grain width (c) and grain length (d) among WT, slg2, GW8‐KOWT and GW8‐KO slg2 . Bars = 5 mm. (e) Statistical analysis of grain length and grain width in WT, slg2, GW8‐KOWT and GW8‐KO slg2 . Data are means ± SD (n = 30). Bars followed by different letters represent significant difference at 5%.
Discussion
SLG2 is a unique grain width regulator
Grain width as one of the key grain size determinants is particularly important for not only grain yield but also appearance quality, and the slender grain is more favoured by the consumers. So far, mutant analysis has identified several grain width‐related genes in rice, including HGW (Li et al., 2012), GS6 (Sun et al., 2013), OsARG (Ma et al., 2013), GW5L (Tian et al., 2019), and WG7 (Huang et al., 2020). Although loss of GS6 function increases grain width (Sun et al., 2013), it is unclear whether manipulation of GS6 expression could produce slender grains. Ectopic expression of GW5L effectively reduces grain width, but grain length of the transgenic plants also changes significantly (Tian et al., 2019). In contrast, dysfunction of HGW, OsARG and WG7 specifically decreases the grain width. However, the null mutations of the three genes all lead to fatal defects in rice development, such as severe dwarfing and late heading in hgw (Li et al., 2012), low seed setting in Osarg (Ma et al., 2013), and severe dwarfing and low seed setting in wg7 (Huang et al., 2020). On the other hand, extensive analysis of natural populations has isolated seven grain width‐related QTLs, including GW2 (Song et al., 2007), GW5 (Liu et al., 2017), GS5 (Li et al., 2011), GW8 (Wang et al., 2012), GW6 (Shi et al., 2020), TGW2 (Ruan et al., 2020), and TGW12a (Du et al., 2021). Although these genes have been reported to play a major role in regulating grain width, only four of them, GS5, GW5, TGW2 and TGW12a appear to function exclusively in grain width determination (Table S2). Moreover, in addition to regulating the grain width, all the four genes also seem to play a role in the grain filling process (Du et al., 2021; Li et al., 2011; Ruan et al., 2020; Tian et al., 2019). Regretfully, knockout of TGW12a increases the formation of chalky endosperm. Although we are not clear whether knockout of GW5 affects starch accumulation, compared with GW5L‐KO, GW5‐KO shows a wider grain but similar grain weight, which strongly suggest that GW5‐KO plants also face the problem of grain filling (Tian et al., 2019). Overexpression of TGW2 effectively reduced the grain width, however, the negative effect of this gene on grain filling makes it uncertain whether the ectopic expression would reduce the grain filling rate and deteriorate the grain quality. Similarly, we are concerned about whether there is grain filling problem in the gs5 mutant. In this study, we find that the loss of SLG2 function leads to a significant decrease in grain width, without any effects on grain length, grain thickness, grain filling and grain quality (Figure 1a–e; Figures S1 and S2). Therefore, SLG2 is a specific grain width regulator of potential value in improving rice appearance quality.
Our genetic analysis demonstrates that SLG2 is one member of the WD40 superfamily (Figure 2). In rice, some OsWD40s have been revealed to function in specific developmental processes. For example, GORI is a seven WD40 repeats protein encoding gene, its knockout transgenic plants display male sterility, while these plants do not show any growth defects during the whole developmental stages (Kim et al., 2021). OsTTG1 is a WD40 family gene that has been artificially selected during domestication. Loss of OsTTG1 function significantly reduces anthocyanin accumulation in various rice organs with no adverse effects on their development (Yang et al., 2021). Recently, the WD40 protein gene OsKRN2 has been found to present convergent selection in maize and rice (Chen et al., 2022). Knockout of OsKRN2 greatly increases rice grain yield with no apparent trade‐offs in other agronomic traits. The rice functional genomics study on grain size regulation has uncovered hundreds of related genes. Surprisingly, almost none of these genes belong to the WD40 family, although the rice genome contains 231 WD40 protein genes (Yang et al., 2021). So far, mutant analysis has uncovered a role of several OsWD40s in grain development (Cheng et al., 2020; Gao et al., 2012; Nallamilli et al., 2013; Utsunomiya et al., 2011; Zhang et al., 2021a,b,c). However, all these mutants display severe defects in multiple important agronomic traits, such as extreme dwarf, low seed setting or preharvest sprouting. The growth defects of these mutants are in sharp contrast to slg2, which displays relatively normal overall phenotypes except the reduced panicle size and grain yield. Therefore, the grain width regulator SLG2 represents a unique member of the WD40 family that could be applied in rice breeding. Further study would reveal whether there are WD40 family genes being artificially selected as grain size regulators during rice domestication.
The SLG2‐WOX11 module presents a novel pathway regulating grain width
The central role of WD40 repeats is to provide a platform for the interaction and assembly of several proteins into a signalosome (Jain and Pandey, 2018). Therefore, the WD40 proteins carry out their regulatory functions mainly by associating with other proteins. For example, OsWDR5a interacts with OsTrx1 to form the core components of the COMPASS‐like complex and regulates rice flowering and panicle branching (Jiang et al., 2018). OsTTG1 regulates anthocyanin biosynthesis by forming a MBW complex with Kala4, OsC1, OsDFR and Rc (Yang et al., 2021). The recently discovered OsKRN2 controls grain number by interacting with OsDUF1644, although the underlying mechanism of this interaction remains to be understood (Chen et al., 2022). In this study, we identify WOX11, a member of the WOX family protein, as the interactor of SLG2 (Figure 3a–c). As a central transcription factor, WOX11 has been revealed to function in root development of both rice and Arabidopsis (Sheng et al., 2017; Zhao et al., 2015), and regulates rice shoot development by recruiting a histone H3K27me3 demethylase JMJ705 (Cheng et al., 2018). Similar to the WOX11‐KO plants, the slg2 mutant also presents defects in crown root and shoot development (Figure 3d), indicating that SLG2 and WOX11 jointly act on rice seedling development. Although the WOX family genes display various biological functions in plant growth regulation (Jha et al., 2020), there is still no information on the involvement of these genes in reproductive organ development. In this study, we reveal that the SLG2‐associated WOX11 is a grain width regulator (Figure 4; Figure S11). We demonstrate that WOX11 can activate the expression of downstream cell expansion genes by directly binding to the promoters of these genes, and the transactivation activity of WOX11 can be enhanced by SLG2 (Figure 5). In addition, we find that there is no obvious difference in grain width among the slg2,wox11 double mutant, the slg2 single mutant and the WOX11‐KO plants (Figure 4). These results suggest that SLG2 is an essential component of WOX11‐mediated signalling pathways. Previous study has shown that WOX11 works as a transcription repressor of downstream RR2 gene to regulate rice crown root development (Zhao et al., 2009). The differential functions of WOX11 in grain width and crown root formation are highly similar to that of the Arabidopsis WOX family gene WUS, which acts as a bifunctional transcription factor: an inhibitor in stem cell regulation, and an activator in flower formation (Leibfried et al., 2005; Lohmann et al., 2001). And, the multifaceted functions of WOX11 in various developmental processes should be attributed to its ability to interact with various cofactors, such as the interaction with ERF3 in crown root development (Zhao et al., 2015), the interaction with ADA2 in root meristem development (Zhou et al., 2017), and the interaction with JMJ705 in shoot meristem development (Cheng et al., 2018). In this study, we present evidence that WOX11 physically interacts with SLG2, both in vivo and in vitro (Figure 3a–c). The high phenotypic similarity of the two null mutants and the double mutant, the strong interaction between the two proteins, the transcriptional regulation of the same groups of downstream genes, together with the first discovery of WOX11 involved in grain size regulation clearly indicate that the SLG2‐WOX11 module presents a novel pathway to regulate grain width.
The SLG2‐GW8 combination shows great potential in designing grain width
Grain size is one of the most complex traits controlled by a large number of genes and their interactions (Jiang et al., 2022). So far, intensive efforts have been made to understand the molecular mechanism of grain size regulation, and exploration of the combinatory effects of several major genes on grain size also have been conducted in rice during the past decade (Lee et al., 2015; Ngangkham et al., 2018; Sun et al., 2018; Yang et al., 2023; Zhong et al., 2020a,b). However, most of the studies on the interaction of grain size‐related genes were carried out in natural populations. Due to the differences in genetic backgrounds, it is difficult to accurately evaluate the interacting effects of the allelic combinations. So far, there is only one report that describes the interaction of grain size genes under the same genetic background (Sun et al., 2018). At present, rice breeders are mainly committed to developing slender rice varieties, because it is preferred by rice consumers in many countries. Unfortunately, in most cases, the increase of grain length is often accompanied by the change of grain width, and increasing grain size to make it larger than medium would compromise fitness of the rice plant (Sun et al., 2018), such as grain number reduction, quality decline, lodging, and premature senescence. Therefore, moderate improvement of grain size is of extreme importance for the balanced plant development of comprehensive agronomic traits. In this study, we describe the combined effects of SLG2 with GW8 by the CRISPR/Cas9 genome editing system, because these two genes show positive effects on grain width by regulating different cellular processes, i.e., GW8 regulates cell division and SLG2 regulates cell expansion (Figure 1; Wang et al., 2012). We find that the regulatory effects of the two null alleles slg2 and gw8 on grain width differ to some extent, with gw8 a stronger effect on grain width reduction. And, the additive effect of the two alleles produces more slender grains. Importantly, there is no obvious change in the grain length and starch accumulation of the double mutant and the two single mutants (Figure S1; Figure 6). Therefore, our results provide a promising strategy to fine tune the grain width of the receptor variety without changing other grain traits. That is, if the plant height of the receptor variety is appropriate, slg2 or slg2/gw8 combination would be a better choice to combine with the grain number genes. Alternatively, if the plant height of the receptor variety is somewhat high, the gw8 allele could be applied to reduce both the grain width and the plant height. In other words, we could reduce the grain width of the receptor variety to varying degrees by applying the slg2 and gw8 alleles individually or in combination, and maintain/enhance the grain yield through pyramiding with the genes related to grain number and/or plant height, thus improving the appearance quality without reducing the yield.
In summary, we report the identification of SLG2 as a novel and specific regulator of grain width in rice. We find that SLG2 recruits and activates WOX11 to directly promote the expression of downstream cell expansion genes. We provide a strategy to fine tune the grain width through the combination of SLG2 with the grain width regulator GW8. Our results suggest that the SLG2‐WOX11 module defines a novel pathway in rational design of rice plants with better grain appearance quality.
Methods
Plant materials and growth conditions
The slg2 mutant was identified from the NaN3‐mutagenized M2 population of ZY66 (japonica). All the materials used in this study were cultivated in the experimental fields of the Institute of Genetics and Developmental Biology in Changping (40.2°N/116.2°E), Beijing during the summer or Lingshui (18.5°N, 110.0°E), Hainan province during the winter.
For seedling culture, healthy seeds were surface‐sterilized with 3% sodium hypochlorite for 30 min, soaked at 37 °C for 3 days. Germinating seeds were sowed into 96‐well PCR plates, and water‐cultured in the phytotron (SANYO) with 12 h light (28 °C)/12 h dark (28 °C), 65%–70% relative humidity, and 150 μM/m2/s photon flux density. Morphological investigation and RNA isolation were performed on 7‐day‐old seedlings.
Map‐based cloning of SLG2
The mapping population was generated by crossing slg2 with KY131 (japonica). Whole genome polymorphic markers were designed based on resequencing data of the two parents (30×), and primers used for fine mapping are listed in Table S1. Using 20 bulked F2 plants with WT and mutant phenotypes respectively, the candidate gene was first mapped to the short arm of chromosome 2 between the markers M1 and M2. Further analysis of the F2 mutant plants subsequently fine‐mapped the causal gene to the region between the markers M6 and M7, and sequence comparison was then performed between slg2 and WT based on the resequencing data (30×).
Morphological and histological analysis
Investigation of agronomic traits was performed on plant height, days to heading, panicle number, grain number per main panicle, 1000‐grain weight, seed setting rate and grain yield per plant at the maturation stage. Caryopsis development was observed on florets marked at the time of anthesis, and developing ovaries were collected at different developmental stages. The grain filling rate was calculated as the percentage of the dry grain weight at the two adjacent indicated days. The length, width and thickness of mature grains were measured using Olympus stream software under a light microscope (SZX16; Olympus, Japan).
For histological analysis, florets before anthesis were sampled and fixed in formalin‐acetic acid alcohol (FAA) solution (50% ethanol, 5% acetic acid and 3.7% formaldehyde) overnight at 4 °C after 20 min vacuum treatment. After dehydration with a gradient of ethanol, replacement with xylene and embedding in paraffin (8002‐74‐2, Sigma‐Aldrich, Steinheim, Germany), sections (8 μm thick) were cut with a rotary microtome (RM2235, LEICA, Heidelberger, Germany), stained with 0.2% toluidine blue and observed under a microscope (BX53; Olympus). The outer parenchyma cell number and cell area were measured with Olympus stream and Image J software, respectively. For SEM observation, the outer glumes of fully mature, dry grains were sprayed with gold particles and scanned with a scanning electron microscope (S‐3000N; Hitachi, Tokyo, Japan). The outer glume cell number was counted and cell size was measured using the Olympus stream software (SZX16; Olympus, Japan). To observe the grain endosperm structure, mature grains were hand‐cut using the bladeless cutter, then the cutting surfaces were coated with gold in a vacuum with an ion sputtering device (JFC‐1100E; JEOL, Tokyo, Japan), and observed with a scanning electron microscope (S‐3000N; Hitachi, Japan).
RNA isolation and RT‐qPCR analysis
Samples were taken from shoots and roots of 7‐day‐old seedlings, and from flag leaf blade, flag leaf sheath, culm, young panicle and mature floret before anthesis. Total RNA was extracted using RNAiso PLUS reagent (Takara, Dojima, Japan), and 1 μg RNA was reverse‐transcribed by oligo (dT) primers using a reverse transcription kit (Promega, Madison, WI, USA) after digestion with RNase‐free DNaseI (Thermo Scientific, Vilnius, Lithuania). The RT‐qPCR assay was performed in triplicate with SYBR Green I Master reagent and the Light Cycler Nano system (Roche, Mannheim, Germany). OsActin was used as the internal control for normalization. The primers used are listed in Table S1.
Vector construction and transformation
For complementation test, a 17.8‐kb genomic fragment (containing the entire coding region, 2392‐bp upstream of ATG and 1567‐bp downstream of TGA) was amplified into four fragments, and cloned into the pZH2B vector by a seamless cloning kit (CloneSmarter, Fort Bend County, TX). For knockout vector, a 20‐bp carefully designed target was selected from the third exon of SLG2, the first exon of WOX11 and the third exon of GW8 by the CRISPR‐GE genome editing tool (http://skl.scau.edu.cn/) and ligated to the CRISPR/Cas9 vector pHUN4C12 (Xie et al., 2017). For overexpression construct, the coding sequence of SLG2 was amplified from ZY66 and cloned into the pZH2Bi vector driven by the ubiquitin promoter. These vectors were transformed into slg2 with the Agrobacterium tumefaciens‐mediated transformation method. For the knockout assay, the T1 plants without exogenous DNA were selected, and the target sites were sequenced. From these plants, the homozygous mutants were obtained, and the offspring of these homozygous lines were used for phenotypic analysis. The primers used for vector construction and transformant selection are listed in Table S1.
Yeast two‐hybrid assay
The coding sequences of SLG2 and WOX11 were cloned into the pGADT7 or pGBKT7 vector (Clontech, Mountain View, CA, USA), and the resulting constructs and the corresponding empty vectors were then co‐transformed into the yeast strain Golden Yeast with different combinations. Interactions were detected on SD/−Leu‐Trp‐His‐Ade medium. The transformation was conducted according to the Yeast Two‐Hybrid System User Manual (Clontech). The primers used in this assay are listed in Table S1.
Transient expression assay in rice protoplast
For subcellular localization, the coding sequence of SLG2 was inserted into the pSAT6‐EYFP‐N1 vector (PSAT6) to construct the SLG2‐EYFP (SLG2‐PSAT6) vector. The empty vector was used as a negative control. For the BiFC assay, the coding sequences of SLG2 and WOX11 were ligated into the pUC19‐VYNE (R) or pUC19‐VYCE (R) vector fused with the N‐ or C‐terminus of the Venus YFP sequence, respectively. These plasmids and the corresponding empty vectors were co‐transformed in different combinations into rice protoplasts. The fluorescent signal was detected with a confocal laser scanning microscope (Leica TCS SP5) after 28 °C incubation for 16 h in the dark.
For transactivation activity, the coding sequence of WOX11 was fused with the GAL4 DNA binding domain in the pRT‐BD vector, and the resulting construct (GAL4‐BD‐WOX11) was used as the effectors. The coding sequence of SLG2 was fused with pSAT6‐EYFP‐N1 vector to form SLG2‐PSAT6 fusion protein, and was taken as the cofactor of WOX11. The PSAT6 protein was used as the control. The LUC vector, which contains six copies of GAL4 binding motif and luciferase coding region, was used as the reporter, and the vector that expressed Renilla luciferase (pTRL) was employed as the internal control. The effectors and cofactors were then co‐transformed with the reporter and the internal control into rice protoplasts.
To detect the WOX11‐mediated activation of OsEXPB7 expression, the coding sequence of WOX11 was fused with PSAT6 (WOX11‐PSAT6) as the effector, and the promoter sequence of OsEXPB7 was cloned into the LUC vector by replacing the 35S promoter (pEXPB7‐LUC) and acted as the reporter. The above‐mentioned fused protein SLG2‐PSAT6 was used as the cofactor of WOX11, and the PSAT6 empty vector was used as the negative control. As the internal controls, the LUC and pTRL vectors were co‐transformed with the resulting constructs into rice protoplasts. All transformations were performed via the PEG (polyethylene glycol) mediated method. After 16 h incubation at 28 °C in the dark, the protoplast was centrifuged at 150 g and the pellet was used for the dual‐luciferase assay as described in the Dual‐Luciferase® Reporter Assay System Manual (Promega), and the relative LUC/REN ratio was measured with a luminometer (GLOMAX; Promega). Primers used for these assays are listed in Table S1.
Pull‐down assay
The coding sequence of SLG2 was inserted into the pGEX‐4T‐1 vector to express GST‐SLG2, and the coding sequence of WOX11 was inserted into the pMAL‐c5X vector to express MBP‐WOX11. All plasmids were transformed into Escherichia coli strain BL21. Fusion proteins were induced with 1.0 mM IPTG at 37 °C for 5 h. Anti‐GST (1:5000; Proteintech, Wuhan, China) and anti‐MBP (1:2000; Proteintech, America) antibodies were used in immunoblotting analysis. GST pull‐down assay was performed as previously described (Zhang et al., 2021a,b,c). Primers used for this assay are listed in Table S1.
Yeast one‐hybrid assay
The coding sequence of WOX11 was fused with the GAL4 activation domain of pGAD424 (Clontech), forming pGAD424‐WOX11. To generate pEXPB7‐pHISi reporter vector, the promoter fragment of OsEXPB7 was synthesized and cloned into the pHISi vector. The plasmids were co‐transformed into yeast strain YM4271, and DNA‐protein interactions were determined by the growth of the transformants on the nutrient‐deficient medium with 30 mM 3‐amino‐1,2,4‐triazole (3‐AT), following the manufacturer's manual (Clontech). Primers used in this assay are listed in Table S1.
Electrophoretic mobility shift assay (EMSA)
The LightShift Chemiluminescent EMSA Kit (No. 20148; ThermoFisher SCIENTIFIC, America) was used in this experiment. Biotin was labelled at the 5′ end of cis‐element. The biotin‐labelled DNA was synthesized by RuiBiotech (Beijing, China). The WOX11‐MBP fusion protein was expressed in Escherichia coli strain BL21 and purified using amylose resin (BioLabs, America) affinity chromatography. The detailed procedure of EMSA follows the manufacturer's instructions. Photos were taken using Charge‐coupled device (CCD) camera. Primers and probe sequences used for EMSA are listed in Table S1.
Protein sequence alignment
The protein sequence of SLG2 and HSLG2 were downloaded from Ensembl Plants (http://plants.ensembl.org/index.html), and alignments were performed using the online Clustal Omega program (https://www.ebi.ac.uk/Tools/msa/clustalo/) with manual curation.
Accession numbers
Sequence data from this article can be found in the EMBL/GenBank data libraries under the following accession numbers: SLG2, Os02g18820; WOX11, Os07g48560; HSLG2, Os09g12550; OsActin, Os03g50885; H1, Os04g18090; E2F2, Os12g06200; CAK1, Os06g07480; CDKA1, Os03g01850; MCM3, Os05g39850; CYCA2.1, Os12g39210; CYCB2.1, Os08g40170; CYCB2.2, Os06g51110; CDKB, Os05g40540; OsEXPA1, Os04g15840; OsEXPA2, Os01g60770; OsEXPA4, Os05g39990; OsEXPA5, Os02g51040; OsEXPA7, Os03g60720; OsEXPA10, Os04g49410; OsEXPA13, Os02g16730; OsEXPA16, Os06g41700; OsEXPA25, Os03g06010; OsEXPA26, Os12g36040; OsEXPA29, Os06g50400; OsEXPB3, Os10g40720; OsEXPB4, Os10g40730; OsEXPB6, Os10g40700; OsEXPB7, Os03g01270; OsEXPB12, Os03g44290; OsEXPB13, Os03g01650; GS5, Os05g06660; GS6, Os06g03710; GW2, Os02g14720; GW5, Os05g09520; GW5L, Os01g09470; GW6, Os06g15620; GW8, Os08g41940; HGW, Os06g06530; OsARG, Os04g01590; TGW2, Os02g52550; TGW12a, Os12g36660; WG7, Os07g47360.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
S.Y. conceived and supervised the project. D.X. performed the experiments, analysed the data and prepared the original draft. S.Y. designed the experiments and revised the manuscript. R.W. screened the slg2 mutant, created the mapping population and carried out field management. Y.W. contributed to the reagents and equipment management. Y.L. and G.S. assisted in the data collection.
Supporting information
Figure S1 Observation of brown rice transparency (a) and starch packaging (b) from the mature grains of WT, slg2, GW8‐KOWT and GW8‐KO slg2 .
Figure S2 slg2 shows normal grain filling.
Figure S3 Expression analysis of cell division and cell expansion genes (a) and grain width‐related genes (b) in slg2 and WT.
Figure S4 Comparison of agronomic traits between slg2 and WT.
Figure S5 The reduced grain width of slg2 is caused by decreased cell expansion.
Figure S6 Knockout of SLG2 results in plants with the slg2 mutant phenotype.
Figure S7 Overexpression of SLG2 fully rescues the slg2 mutant phenotype.
Figure S8 Protein sequence comparison between SLG2 and HSLG2.
Figure S9 The SLG2 homologue HSLG2 shows no regulatory roles in rice development.
Figure S10 Verification of the WOX11 knockout lines (WOX11‐KO) by PCR‐based sequencing.
Figure S11 The WOX11‐KO lines show reduced grain width similar to the slg2 mutant.
Figure S12 Verification of the GW8 knockout lines in the background of WT (GW8‐KOWT) and slg2 (GW8‐KO slg2 ) by PCR‐based sequencing.
Figure S13 Statistical analysis of plant height (a), panicle number (b), grain number per main panicle (c), 1000‐grain weight (d), seed setting rate (e), and grain yield per plant (f) of WT, slg2 and GW8‐KO.
Figure S14 Expression analysis of cell division and cell expansion genes in WT, slg2, GW8‐KOWT and GW8‐KO slg2 .
Table S1 Primers used in this study.
Table S2 Effects of SLG2 and known grain width genes on four grain traits.
Acknowledgements
We greatly thank Professor Shouyi Chen (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for providing the pRT‐BD and pTRL‐LUC vectors. We thank Professor Yaoguang Liu (SCAU) for generously providing the CRISPR/Cas9 genome editing vector pHUN4C12. This work was supported by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA24030201), and the State Key Laboratory of Plant Genomics (SKLPG2011B0403).
References
- Chen, K. , Łyskowski, A. , Jaremko, Ł. and Jaremko, M. (2021) Genetic and molecular factors determining grain weight in rice. Front. Plant Sci. 12, 605799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. , Chen, L. , Zhang, X. , Yang, N. , Guo, J. , Wang, M. , Ji, S. et al. (2022) Convergent selection of a WD40 protein that enhances grain yield in maize and rice. Science, 375, eabg7985. [DOI] [PubMed] [Google Scholar]
- Cheng, S. , Tan, F. , Lu, Y. , Liu, X. , Li, T. , Yuan, W. , Zhao, Y. et al. (2018) WOX11 recruits a histone H3K27me3 demethylase to promote gene expression during shoot development in rice. Nucleic Acids Res. 46, 2356–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, X. , Pan, M. , Zhiguo, E. , Zhou, Y. , Niu, B. and Chen, C. (2020) Functional divergence of two duplicated Fertilization Independent Endosperm genes in rice with respect to seed development. Plant J. 104, 124–137. [DOI] [PubMed] [Google Scholar]
- Choi, B.S. , Kim, Y.J. , Markkandan, K. , Koo, Y.J. , Song, J.T. and Seo, H.S. (2018) GW2 functions as an E3 ubiquitin ligase for rice expansin‐like 1. Int. J. Mol. Sci. 19, 1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhatt, B.K. , Paul, P. , Sandhu, J. , Hussain, W. , Irvin, L. , Zhu, F. , Adviento‐Borbe, M.A. et al. (2021) Allelic variation in rice Fertilization Independent Endosperm 1 contributes to grain width under high night temperature stress. New Phytol. 229, 335–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Z. , Huang, Z. , Li, J. , Bao, J. , Tu, H. , Zeng, C. , Wu, Z. et al. (2021) qTGW12a, a naturally varying QTL, regulates grain weight in rice. Theor. Appl. Genet. 134, 2767–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, P. , Rao, Y. , Zeng, D. , Yang, Y. , Xu, R. , Zhang, B. , Dong, G. et al. (2014) SMALL GRAIN 1, which encodes a mitogen‐activated protein kinase kinase 4, influences grain size in rice. Plant J. 77, 547–557. [DOI] [PubMed] [Google Scholar]
- Duan, P. , Ni, S. , Wang, J. , Zhang, B. , Xu, R. , Wang, Y. , Chen, H. et al. (2015) Regulation of OsGRF4 by OsmiR396 controls grain size and yield in rice. Nat Plants, 2, 15203. [DOI] [PubMed] [Google Scholar]
- Fan, C. , Xing, Y. , Mao, H. , Lu, T. , Han, B. , Xu, C. , Li, X. et al. (2006) GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 112, 1164–1171. [DOI] [PubMed] [Google Scholar]
- Gao, X. , Chen, Z. , Zhang, J. , Li, X. , Chen, G. , Li, X. and Wu, C. (2012) OsLIS‐L1 encoding a lissencephaly type‐1‐like protein with WD40 repeats is required for plant height and male gametophyte formation in rice. Planta, 235, 713–727. [DOI] [PubMed] [Google Scholar]
- Hu, J. , Wang, Y. , Fang, Y. , Zeng, L. , Xu, J. , Yu, H. , Shi, Z. et al. (2015) A rare allele of GS2 enhances grain size and grain yield in rice. Mol. Plant, 8, 1455–1465. [DOI] [PubMed] [Google Scholar]
- Huang, K. , Wang, D. , Duan, P. , Zhang, B. , Xu, R. , Li, N. and Li, Y. (2017) WIDE AND THICK GRAIN 1, which encodes an otubain‐like protease with deubiquitination activity, influences grain size and shape in rice. Plant J. 91, 849–860. [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Bai, X. , Cheng, N. , Xiao, J. , Li, X. and Xing, Y. (2020) Wide Grain 7 increases grain width by enhancing H3K4me3 enrichment in the OsMADS1 promoter in rice (Oryza sativa L.). Plant J. 102, 517–528. [DOI] [PubMed] [Google Scholar]
- Jain, B.P. and Pandey, S. (2018) WD40 repeat proteins: signalling scaffold with diverse functions. Protein J. 37, 391–406. [DOI] [PubMed] [Google Scholar]
- Jha, P. , Ochatt, S.J. and Kumar, V. (2020) WUSCHEL: a master regulator in plant growth signaling. Plant Cell Rep. 39, 431–444. [DOI] [PubMed] [Google Scholar]
- Jiang, W. , Zhou, S. , Zhang, Q. , Song, H. , Zhou, D.‐X. and Zhao, Y. (2017) Transcriptional regulatory network of WOX11 is involved in the control of crown root development, cytokinin signals, and redox in rice. J. Exp. Bot. 68, 2787–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, P. , Wang, S. , Jiang, H. , Cheng, B. , Wu, K. and Ding, Y. (2018) The COMPASS‐Like complex promotes flowering and panicle branching in rice. Plant Physiol. 176, 2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H. , Zhang, A. , Liu, X. and Chen, J. (2022) Grain size associated genes and the molecular regulatory mechanism in rice. Int. J. Mol. Sci. 23, 3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, Y. , Wang, Y. , Xue, D. , Wang, J. , Yan, M. , Liu, G. , Dong, G. et al. (2010) Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 42, 541–544. [DOI] [PubMed] [Google Scholar]
- Kim, Y.‐J. , Kim, M.‐H. , Hong, W.‐J. , Moon, S. , Kim, E.‐J. , Silva, J. , Lee, J. et al. (2021) GORI, encoding the WD40 domain protein, is required for pollen tube germination and elongation in rice. Plant J. 105, 1645–1664. [DOI] [PubMed] [Google Scholar]
- Lee, C.‐M. , Park, J. , Kim, B. , Seo, J. , Lee, G. , Jang, S. and Koh, H.‐J. (2015) Influence of multi‐gene allele combinations on grain size of rice and development of a regression equation model to predict grain parameters. Rice, 8, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibfried, A. , To, J.P.C. , Busch, W. , Stehling, S. , Kehle, A. , Demar, M. , Kieber, J.J. et al. (2005) WUSCHEL controls meristem function by direct regulation of cytokinin‐inducible response regulators. Nature, 438, 1172–1175. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Fan, C. , Xing, Y. , Jiang, Y. , Luo, L. , Sun, L. , Shao, D. et al. (2011) Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat. Genet. 43, 1266–1269. [DOI] [PubMed] [Google Scholar]
- Li, J. , Chu, H. , Zhang, Y. , Mou, T. , Wu, C. , Zhang, Q. and Xu, J. (2012) The rice HGW gene encodes a ubiquitin‐associated (UBA) domain protein that regulates heading date and grain weight. PLoS One, 7, e34231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N. , Xu, R. , Duan, P. and Li, Y. (2018) Control of grain size in rice. Plant Reprod. 31, 237–251. [DOI] [PubMed] [Google Scholar]
- Liu, L. , Tong, H. , Xiao, Y. , Che, R. , Xu, F. , Hu, B. , Liang, C. et al. (2015a) Activation of Big Grain1 significantly improves grain size by regulating auxin transport in rice. Proc. Natl Acad. Sci. USA, 112, 11102–11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S. , Hua, L. , Dong, S. , Chen, H. , Zhu, X. , Jiang, J.E. , Zhang, F. et al. (2015b) OsMAPK6, a mitogen‐activated protein kinase, influences rice grain size and biomass production. Plant J. 84, 672–681. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Chen, J. , Zheng, X. , Wu, F. , Lin, Q. , Heng, Y. , Tian, P. et al. (2017) GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat. Plants, 3, 17043. [DOI] [PubMed] [Google Scholar]
- Lohmann, J.U. , Hong, R.L. , Hobe, M. , Busch, M.A. , Parcy, F. , Simon, R. and Weigel, D. (2001) A molecular link between stem cell regulation and floral patterning in Arabidopsis . Cell 105, 793–803. [DOI] [PubMed] [Google Scholar]
- Ma, X. , Cheng, Z. , Qin, R. , Qiu, Y. , Heng, Y. , Yang, H. , Ren, Y. et al. (2013) OsARG encodes an arginase that plays critical roles in panicle development and grain production in rice. Plant J. 73, 190–200. [DOI] [PubMed] [Google Scholar]
- Miao, J. , Yang, Z. , Zhang, D. , Wang, Y. , Xu, M. , Zhou, L. , Wang, J. et al. (2019) Mutation of RGG2, which encodes a type B heterotrimeric G protein γ subunit, increases grain size and yield production in rice. Plant Biotechnol. J. 17, 650–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallamilli, B.R.R. , Zhang, J. , Mujahid, H. , Malone, B.M. , Bridges, S.M. and Peng, Z. (2013) Polycomb group gene OsFIE2 regulates rice (Oryza sativa) seed development and grain filling via a mechanism distinct from Arabidopsis . PLoS Genet. 9, e1003322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngangkham, U. , Samantaray, S. , Yadav, M.K. , Kumar, A. , Chidambaranathan, P. and Katara, J.L. (2018) Effect of multiple allelic combinations of genes on regulating grain size in rice. PLoS One, 13, e0190684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, X. , Huang, Q. , Xiao, H. , Zhang, Q. , Ni, C. , Xu, Y. , Liu, G. et al. (2016) The rice DUF1620‐containing and WD40‐like repeat protein is required for the assembly of the restoration of fertility complex. New Phytol. 210, 934–945. [DOI] [PubMed] [Google Scholar]
- Ruan, B. , Shang, L. , Zhang, B. , Hu, J. , Wang, Y. , Lin, H. , Zhang, A. et al. (2020) Natural variation in the promoter of TGW2 determines grain width and weight in rice. New Phytol. 227, 629–640. [DOI] [PubMed] [Google Scholar]
- Sheng, L. , Hu, X. , Du, Y. , Zhang, G. , Huang, H. , Scheres, B. and Xu, L. (2017) Non‐canonical WOX11‐mediated root branching contributes to plasticity in Arabidopsis root system architecture. Development, 144, 3126–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, C. , Ren, Y. , Liu, L. , Wang, F. , Zhang, H. , Tian, P. , Pan, T. et al. (2019) Ubiquitin specific protease 15 has an important role in regulating grain width and size in rice. Plant Physiol. 180, 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, C.‐L. , Dong, N.‐Q. , Guo, T. , Ye, W.‐W. , Shan, J.‐X. and Lin, H.‐X. (2020) A quantitative trait locus GW6 controls rice grain size and yield through the gibberellin pathway. Plant J. 103, 1174–1188. [DOI] [PubMed] [Google Scholar]
- Si, L. , Chen, J. , Huang, X. , Gong, H. , Luo, J. , Hou, Q. , Zhou, T. et al. (2016) OsSPL13 controls grain size in cultivated rice. Nat. Genet. 48, 447–456. [DOI] [PubMed] [Google Scholar]
- Song, X.‐J. , Huang, W. , Shi, M. , Zhu, M.‐Z. and Lin, H.‐X. (2007) A QTL for rice grain width and weight encodes a previously unknown RING‐type E3 ubiquitin ligase. Nat. Genet. 39, 623–630. [DOI] [PubMed] [Google Scholar]
- Stirnimann, C.U. , Petsalaki, E. , Russell, R.B. and Müller, C.W. (2010) WD40 proteins propel cellular networks. Trends Biochem. Sci. 35, 565–574. [DOI] [PubMed] [Google Scholar]
- Sun, L. , Li, X. , Fu, Y. , Zhu, Z. , Tan, L. , Liu, F. , Sun, X. et al. (2013) GS6, a member of the GRAS gene family, negatively regulates grain size in rice. J. Integr. Plant Biol. 55, 938–949. [DOI] [PubMed] [Google Scholar]
- Sun, S. , Wang, L. , Mao, H. , Shao, L. , Li, X. , Xiao, J. , Ouyang, Y. et al. (2018) A G‐protein pathway determines grain size in rice. Nat. Commun. 9, 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, W. , Xu, X.H. , Li, Y. , Xie, L. , He, Y. , Li, W. , Lu, X. et al. (2020) OsmiR530 acts downstream of OsPIL15 to regulate grain yield in rice. New Phytol. 226, 823–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, P. , Liu, J. , Mou, C. , Shi, C. , Zhang, H. , Zhao, Z. , Lin, Q. et al. (2019) GW5‐Like, a homolog of GW5, negatively regulates grain width, weight and salt resistance in rice. J. Integr. Plant Biol. 61, 1171–1185. [DOI] [PubMed] [Google Scholar]
- Utsunomiya, Y. , Samejima, C. , Takayanagi, Y. , Izawa, Y. , Yoshida, T. , Sawada, Y. , Fujisawa, Y. et al. (2011) Suppression of the rice heterotrimeric G protein β‐subunit gene, RGB1, causes dwarfism and browning of internodes and lamina joint regions. Plant J. 67, 907–916. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Wu, K. , Yuan, Q. , Liu, X. , Liu, Z. , Lin, X. , Zeng, R. et al. (2012) Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 44, 950–954. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Li, S. , Liu, Q. , Wu, K. , Zhang, J. , Wang, S. , Wang, Y. et al. (2015) The OsSPL16‐GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat. Genet. 47, 949–954. [DOI] [PubMed] [Google Scholar]
- Xie, X. , Ma, X. , Zhu, Q. , Zeng, D. , Li, G. and Liu, Y.‐G. (2017) CRISPR‐GE: a convenient software toolkit for crispr‐based genome editing. Mol. Plant, 10, 1246–1249. [DOI] [PubMed] [Google Scholar]
- Xu, H. , Zhao, M. , Zhang, Q. , Xu, Z. and Xu, Q. (2016) The DENSE AND ERECT PANICLE 1 (DEP1) gene offering the potential in the breeding of high‐yielding rice. Breed Sci. 66, 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, R. , Duan, P. , Yu, H. , Zhou, Z. , Zhang, B. , Wang, R. , Li, J. et al. (2018) Control of grain size and weight by the OsMKKK10‐OsMKK4‐OsMAPK6 signaling pathway in rice. Mol. Plant, 11, 860–873. [DOI] [PubMed] [Google Scholar]
- Yang, X. , Wang, J. , Xia, X. , Zhang, Z. , He, J. , Nong, B. , Luo, T. et al. (2021) OsTTG1, a WD40 repeat gene, regulates anthocyanin biosynthesis in rice. Plant J. 107, 198–214. [DOI] [PubMed] [Google Scholar]
- Yang, T. , Gu, H. , Yang, W. , Liu, B. , Liang, S. and Zhao, J. (2023) Artificially selected grain shape gene combinations in Guangdong Simiao varieties of rice (Oryza sativa L.). Rice, 16, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Yu, Y. , Feng, Y. , Zhou, Y. , Zhang, F. , Yang, Y. , Lei, M. et al. (2017) MiR408 regulates grain yield and photosynthesis via a phytocyanin protein. Plant Physiol. 175, 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Wang, R. , Xing, Y. , Xu, Y. , Xiong, D. , Wang, Y. and Yao, S. (2021a) Separable regulation of POW1 in grain size and leaf angle development in rice. Plant Biotechnol. J. 19, 2517–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. , Zhang, M. and Liang, J. (2021b) RGB1 regulates grain development and starch accumulation through its effect on OsYUC11‐mediated auxin biosynthesis in rice endosperm cells. Front. Plant Sci. 12, 585174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P. , Zhu, C. , Geng, Y. , Wang, Y. , Yang, Y. , Liu, Q. , Guo, W. et al. (2021c) Rice and Arabidopsis homologs of yeast CHROMOSOME TRANSMISSION FIDELITY PROTEIN 4 commonly interact with Polycomb complexes but exert divergent regulatory functions. Plant Cell, 33, 1417–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Hu, Y. , Dai, M. , Huang, L. and Zhou, D.‐X. (2009) The WUSCHEL‐related homeobox gene WOX11 Is required to activate shoot‐borne crown root development in rice Plant . Cell, 21, 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Cheng, S. , Song, Y. , Huang, Y. , Zhou, S. , Liu, X. and Zhou, D.‐X. (2015) The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling. Plant Cell, 27, 2469–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, H. , Liu, C. , Kong, W. , Zhang, Y. , Zhao, G. , Sun, T. and Li, Y. (2020a) Effect of multi‐allele combination on rice grain size based on prediction of regression equation model. Mol. Genet. Genomics, 295, 465–474. [DOI] [PubMed] [Google Scholar]
- Zhong, J. , He, W. , Peng, Z. , Zhang, H. , Li, F. and Yao, J. (2020b) A putative AGO protein, OsAGO17, positively regulates grain size and grain weight through OsmiR397b in rice. Plant Biotechnol. J. 18, 916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, S. , Jiang, W. , Long, F. , Cheng, S. , Yang, W. , Zhao, Y. and Zhou, D.‐X. (2017) Rice homeodomain protein WOX11 recruits a histone acetyltransferase complex to establish programs of cell proliferation of crown root meristem. Plant Cell, 29, 1088–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Observation of brown rice transparency (a) and starch packaging (b) from the mature grains of WT, slg2, GW8‐KOWT and GW8‐KO slg2 .
Figure S2 slg2 shows normal grain filling.
Figure S3 Expression analysis of cell division and cell expansion genes (a) and grain width‐related genes (b) in slg2 and WT.
Figure S4 Comparison of agronomic traits between slg2 and WT.
Figure S5 The reduced grain width of slg2 is caused by decreased cell expansion.
Figure S6 Knockout of SLG2 results in plants with the slg2 mutant phenotype.
Figure S7 Overexpression of SLG2 fully rescues the slg2 mutant phenotype.
Figure S8 Protein sequence comparison between SLG2 and HSLG2.
Figure S9 The SLG2 homologue HSLG2 shows no regulatory roles in rice development.
Figure S10 Verification of the WOX11 knockout lines (WOX11‐KO) by PCR‐based sequencing.
Figure S11 The WOX11‐KO lines show reduced grain width similar to the slg2 mutant.
Figure S12 Verification of the GW8 knockout lines in the background of WT (GW8‐KOWT) and slg2 (GW8‐KO slg2 ) by PCR‐based sequencing.
Figure S13 Statistical analysis of plant height (a), panicle number (b), grain number per main panicle (c), 1000‐grain weight (d), seed setting rate (e), and grain yield per plant (f) of WT, slg2 and GW8‐KO.
Figure S14 Expression analysis of cell division and cell expansion genes in WT, slg2, GW8‐KOWT and GW8‐KO slg2 .
Table S1 Primers used in this study.
Table S2 Effects of SLG2 and known grain width genes on four grain traits.


