Abstract
Recent studies indicate that phosphatidylinositide-3OH kinase (PI3K)-induced S6 kinase (S6K1) activation is mediated by protein kinase B (PKB). Support for this hypothesis has largely relied on results obtained with highly active, constitutively membrane-localized alleles of wild-type PKB, whose activity is independent of PI3K. Here we set out to examine the importance of PKB signaling in S6K1 activation. In parallel, glycogen synthase kinase 3β (GSK-3β) inactivation and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) phosphorylation were monitored as markers of the rapamycin-insensitive and -sensitive branches of the PI3K signaling pathway, respectively. The results demonstrate that two activated PKBα mutants, whose basal activity is equivalent to that of insulin-induced wild-type PKB, inhibit GSK-3β to the same extent as a highly active, constitutively membrane-targeted wild-type PKB allele. However, of these two mutants, only the constitutively membrane-targeted allele of PKB induces S6K1 activation. Furthermore, an interfering mutant of PKB, which blocks insulin-induced PKB activation and GSK-3β inactivation, has no effect on S6K1 activation. Surprisingly, all the activated PKB mutants, regardless of constitutive membrane localization, induce 4E-BP1 phosphorylation and the interfering PKB mutant blocks insulin-induced 4E-BP1 phosphorylation. The results demonstrate that PKB mediates S6K1 activation only as a function of constitutive membrane localization, whereas the activation of PKB appears both necessary and sufficient to induce 4E-BP1 phosphorylation independently of its intracellular location.
Mitogens induce the coordinated activation of a number of anabolic events which culminate in cell growth and division (41). Recent studies have defined the distinction between growth and proliferation, demonstrating the dominance of growth in this process (42, 58). An important component of the growth response is the generation of new translational machinery, required to accommodate the increased demand for additional proteins (56). The enhanced expression of protein synthetic components, most notably ribosomal proteins and elongation factors, is largely controlled at the translational level (5, 39). The transcripts for ribosomal proteins and elongation factors are characterized by an oligopyrimidine tract, termed 5′TOP, at their translational start site (5, 39). More importantly, it has been shown that the translational upregulation of these transcripts is mediated, in part, by the activation of the 40S ribosomal protein S6 kinase (S6K1) (32), presumably through the increased phosphorylation of S6 (33, 34). The importance of S6K1 in cell growth was initially inferred from the microinjection of neutralizing antibodies into cells (38, 50) and the use of the immunosuppressant rapamycin, each inhibiting mitogen-induced S6K1 activation and impeding cell growth (33, 34). Recently, the significance of S6K1 in cell growth has been emphasized by the generation of S6K1-deficient mice, which are significantly reduced in size (55), and by the discovery of a new, highly homologous S6 kinase, S6K2 (28, 55).
The signal transduction pathway which mediates S6K1 activation has received considerable attention because of its implied importance in the growth response (25, 47). Early studies demonstrated that mitogen-induced S6K1 activation is initiated at a specific growth factor receptor docking site distinct from that utilized by the mitogen-activated protein kinase-Ras signaling pathway (40). The use of the inhibitory fungal metabolite wortmannin and platelet-derived growth factor (PDGF) receptor mutants led to the identification of phosphatidylinositide-3OH kinase (PI3K) as the effector which initiates downstream signaling from the receptor (17). The activation of S6K1 appears to be mediated in a hierarchical manner, initiated by the phosphorylation of a set of sites in its autoinhibitory domain (24) that facilitate subsequent phosphorylation at T389 in the adjacent linker domain. These two sets of initial phosphorylation events act in a synergistic manner to regulate T229 phosphorylation in the catalytic domain and thus kinase activation (24). Except for the recent identification of phosphoinositide-dependent protein kinase 1 (PDK1) as the S6K1-T229 kinase (4, 49), little is known concerning the identity of the other kinases which regulate the additional phosphorylation of S6K1 (49). The key step in the activation process is T389 phosphorylation, which unlike the phosphorylation of T229, appears to be positively regulated by a wortmannin-sensitive PI3K-dependent input (23). Although the S6K1-T389 kinase has yet to be identified, recent studies have suggested that this step is mediated by PI3K through the activation of protein kinase B (PKB) (15). Like S6K1, PKB activation is mediated by PI3K through the increased phosphorylation of T308 and S473, the sites homologous to T229 and T389 in S6K1, respectively (1). The dependence of PKB activation on PI3K as well as its wortmannin sensitivity can be circumvented by constitutively anchoring PKB to the membrane (6). This leads to increased levels of T308 and S473 phosphorylation, highly activates PKB (6), and induces S6K1 activation (15). These findings have led to the hypothesis that PKB mediates PI3K-induced S6K1 activation.
Membrane-targeted alleles of PKB also induce the phosphorylation of two additional signaling components which regulate the translational machinery. One is glycogen synthase kinase 3 (GSK-3), whose direct phosphorylation by PKB leads to its inactivation (21). A key substrate of GSK-3 is the ɛ subunit of protein synthesis initiation factor eIF2B (65), whose phosphorylation suppresses eIF2B GDP-GTP exchange activity, inhibiting translation (65). The inhibition of GSK-3 activity by PKB increases eIF2B GDP-GTP exchange activity, raising the amount of active eIF2-GTP and leading to increased rates of global translation (19). The second downstream target of PKB is eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) (27, 36, 53, 60). The phosphorylation of 4E-BP1 disrupts its interaction with the mRNA m7G cap-binding protein eIF4E, allowing eIF4E to form a productive initiation complex (44). Unlike GSK-3, the effects of PKB on increased 4E-BP1 phosphorylation are indirect and may be mediated by a second kinase, possibly mTOR, the target of rapamycin (14, 53), or a kinase tightly bound to mTOR (43). Indeed, 4E-BP1 is thought to reside on the same rapamycin-sensitive PI3K-dependent signaling pathway as S6K1 (64).
Although highly activated alleles of PKB lead to S6K1 activation, the same is also true for activated oncogenic alleles of Ras (10). However, the activation of wild-type Ras by mitogens is neither sufficient nor necessary to bring about S6K1 activation (40). This raised the possibility that highly activated alleles of PKB or constitutive membrane localization may not reflect wild-type PKB signaling. To test the role of PKB signaling in S6K1 activation we have compared the abilities of specific variants of PKB to activate and confer wortmannin resistance on S6K1, as well as the ability of interfering mutants to block this response. In parallel, we have used GSK-3β inactivation and 4E-BP1 phosphorylation to independently monitor the effects of the different PKB alleles on rapamycin-sensitive and -insensitive PKB downstream signaling. The results demonstrate that constitutive membrane localization of active PKB is essential for its ability to signal S6K1 and that the pathway leading to 4E-BP1 phosphorylation, but surprisingly not S6K1 activation, is dominantly regulated by PKB.
MATERIALS AND METHODS
Construction of expression vectors.
The S6K1 construct was tagged by the insertion of the myc 9E10 epitope immediately following the S6K1 initiator ATG codon as described previously (40). Myc-S6K1-GST (63) and the pCMV constructs encoding human hemagglutinin (HA)-PKBα, T308D-S473D PKBα, myristylated and palmitylated PKBα, and kinase-dead PKB have been described (1, 6, 7). GSK-3β cDNA was amplified from a template construct and subcloned into a prk5 expression vector. The PCR primers served to add a myc tag at the C terminus of GSK-3 and provide the suitable XbaI/NotI restriction sites for subcloning. The construct was verified by DNA sequencing. Myc-PKB was generated by PCR with HA-PKBα (7) as a template. The resulting product was subcloned as a BglII-EcoRI fragment into the pECE vector and then transferred as a BglII-XbaI fragment into a pCMV5 vector. The construction of untagged PKB-CaaX (61) and HA–4E-BP1 (63) have been described previously. PKB-CaaX was subcloned into a pCMV5 expression vector.
Cell culture and transfection.
Human embryonic kidney 293 cells were maintained in Dulbecco’s modified Eagle medium containing 10% fetal calf serum as described previously (40) and seeded at 106 cells per 10-cm-diameter plate 24 h prior to transfection. Transient transfection was performed overnight by using a modified calcium phosphate procedure with 10 μg of plasmid DNA (45). The next day, cells were washed twice with Dulbecco’s modified Eagle medium to remove the serum and then made quiescent by incubation in the same medium for 24 h. Prior to lysis the cells were stimulated with specific agonists or inhibitors as described in the text. Insulin was employed at a concentration of 1 μM (stock, 10 mg/ml in 1% acetic acid). In the case of the wortmannin pretreatment, either the vehicle dimethyl sulfoxide or 250 nM wortmannin was added.
Immunoprecipitation and in vitro kinase assays.
Human 293 cell extracts were prepared by lysing cells in a buffer containing 50 mM Tris-HCl (pH 7.5), 1% Nonidet P-40, 120 mM NaCl, 1 mM EDTA, 6 mM EGTA, 1 mM benzamidine, 15 mM sodium diphosphate, 30 mM 4-nitrophenyl phosphate (disodium salt), and 0.2 mM phenylmethylsulfonyl fluoride. Lysates were centrifuged for 15 min at 12,000 × g at 4°C. PKB was immunoprecipitated with either monoclonal 12CA5 or 9E10 antibody, and S6K1 was immunoprecipitated with a monoclonal 9E10 antibody. Immunocomplexes were collected with protein G-Sepharose beads. PKBα activity was assayed with the peptide GRPRTSSFAEG as a substrate, as described previously (21). The kinase assay for S6K1 has been reported (30). Cells transfected with Myc–GSK-3β were lysed in buffer A, which contains 50 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 1% (by volume) Triton X-100, 1 mM sodium orthovanadate, 10 mM sodium glycerophosphate, 50 mM NaF, 5 mM sodium pyrophosphate, 1 μM Microcystin-LR, 0.27 M sucrose, 1 mM benzamidine, 0.2 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml, and 0.1% (vol/vol) 2-mercaptoethanol. The lysate was centrifuged as described above, and an aliquot of the supernatant was incubated for 1 h on a shaking platform with 5 μl of protein G-Sepharose coupled to 2 μg of 9E10 monoclonal antibody. The protein G–Sepharose–antibody–Myc–GSK-3β complex was washed twice with buffer A containing 0.5 M NaCl and twice with buffer B (50 mM Tris-HCl [pH 7.5], 0.1 mM EGTA, 0.1% 2-mercaptoethanol), and the immunoprecipitate was assayed for GSK-3β activity after incubation with either protein phosphatase 2A (PP2A) or microcystin in an assay buffer containing 50 mM Tris-HCl, 0.1 mM EGTA, 10 mM MgCl, 2.5 μM PKI, 100 μM ATP, and 30 μM phospho-glycogen synthase peptide 2 with the sequence YRRAAVPPSPSLSRHSSPHQpSEDEEE. For the PP2A reaction the beads were incubated for 30 min at 30°C in buffer B containing 1 mg of bovine serum albumin per ml with the catalytic subunit of PP2A (final concentration, 25 mU/ml), and the reaction was stopped by adding 5 μM microcystin to the mixture.
Phosphorylation of 4E-BP1.
Human 293 cells transfected with HA–4E-BP1 were lysed in a buffer containing 50 mM Tris-HCl (pH 7.5), 1% Nonidet P-40, 120 mM NaCl, 25 mM NaF, 40 mM β-glycerophosphate, 0.1 mM sodium vanadate, 1 mM benzamidine, and 0.2 mM phenylmethylsulfonyl fluoride. Western blot analysis of 4E-BP1 was carried out as previously described (64), except that the 12CA5 antibody was used for the detection and the N,N′-methylenebisacrylamide content of the gel was lowered to 0.25%.
Western blot analysis.
The protein concentration in samples was determined with the Bio-Rad D/C protein assay. Protein samples (40 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electrophoretically transferred to Immobilon P membranes (Millipore). Construct expression was quantified by Western blotting with monoclonal antibody 9E10 or 12CA5, rabbit anti-mouse immunoglobulin G (DAKO), and finally with fluorescein isothiocyanate (FITC)-labelled swine anti-rabbit immunoglobulin G (DAKO) or goat anti-mouse immunoglobulin G coupled to horseradish peroxidase (DAKO) as a secondary antibody. Endogenous PKB was quantified with a polyclonal anti-PKB antibody raised against the C terminus of PKB (6) and FITC-labelled swine anti-rabbit immunoglobulin G (DAKO) or mouse anti-rabbit immunoglobulin G coupled to horseradish peroxidase (DAKO) as a secondary antibody. Enhanced chemiluminescence or storage phosphorimagery and fluorimetry (Molecular Dynamics) were used to visualize expression levels and S6K1 activity as phosphate incorporation into 40S ribosomal protein S6.
Two-dimensional phosphopeptide mapping.
Human 293 cells were transiently transfected with the indicated construct and labelled with 0.2 mCi of 32Pi per ml, 3 h prior to extraction. Myc-S6K1-GST was precipitated from labelled extracts with glutathione-Sepharose (Sigma) and purified by electroelution (63). Trichloroacetic acid precipitation, performic acid oxidation, trypsin-chymotrypsin digestion, and two-dimensional phosphopeptide mapping were carried out as described previously (45). Phosphopeptides were visualized with a PhosphorImager.
Immunofluorescence.
Human 293 cells were transfected on coverslips with the indicated PKB construct, fixed, permeabilized, and incubated with a polyclonal anti-PKB antibody before being stained with an FITC-conjugated secondary antibody, as described previously (6).
RESULTS
Activation of PKB variants.
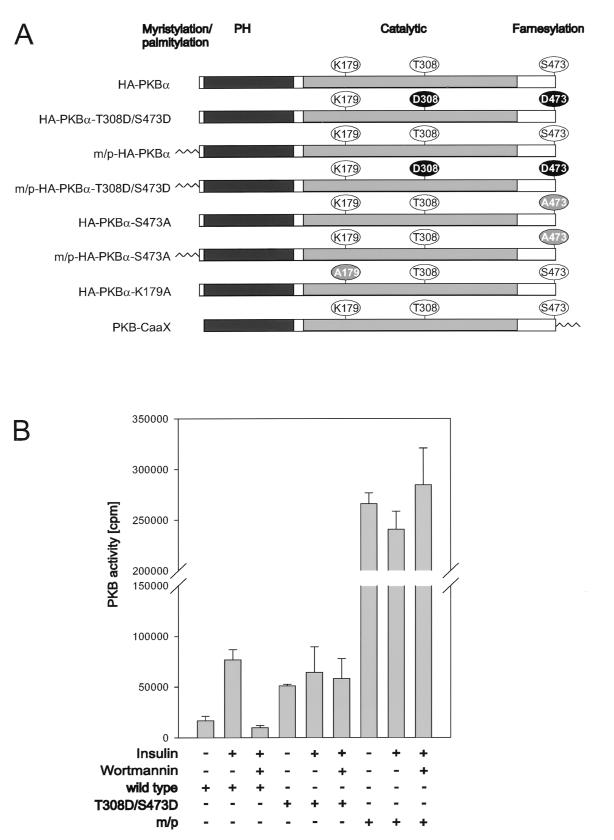
To address the importance of PKB activation in S6K1 signaling, we compared the activity of wild-type PKB and its sensitivity to wortmannin with those of two activated alleles of PKB, one of which was constitutively membrane targeted. The latter allele contains the myristylation and palmitylation signal of the proto-oncogene lck attached to its amino terminus (Fig. 1A), which constitutively localizes the PKB variant to the membrane (see reference 6 and below). The second variant of PKB contains acidic residues at the primary sites of phosphorylation associated with PKB activation, T308, in the activation loop, and S473, near the carboxy terminus (Fig. 1A) (1). Wild-type PKB and each of the variants were HA epitope tagged, transiently transfected into human 293 cells, and analyzed for basal and insulin-stimulated activity, with Crosstide (21) as a peptide substrate (Fig. 1B). In contrast to the HA epitope-tagged wild-type PKB, HA-PKBα, both variants had high basal kinase activity which was not further augmented by insulin stimulation (Fig. 1B). The activity of the double acidic variant, HA-PKBα-T308D/S473D, was equivalent to that of insulin-stimulated HA-PKBα, whereas the membrane-targeted variant, m/p-HA-PKBα, was approximately fourfold more active than insulin-stimulated HA-PKBα (Fig. 1B). Furthermore, in contrast to HA-PKBα, both HA-PKBα-T308D/S473D and m/p-HA-PKBα were resistant to wortmannin (Fig. 1B). Thus, the two activated alleles of PKB, though displaying distinct levels of activity, were unresponsive to mitogen stimulation and the effects of wortmannin.
FIG. 1.
Activation of PKB mutants. (A) Schematic presentation of the PKBα mutants employed. Membrane-targeted forms of PKBα contain either the lck myristylation and palmitylation signal (m/p) at the N terminus or the CaaX box from Ki-Ras at the C terminus. PH, pleckstrin homology domain. All the PKBα constructs are HA epitope tagged at the N terminus, except for PKBα-CaaX. (B) Ectopically expressed PKB variants from extracts of human 293 cells were assayed as described in Materials and Methods. The extracts are derived from the cotransfection experiment with S6K1 described in the legend to Fig. 2A. The cells were serum starved for 24 h and either extracted immediately, stimulated for 30 min with 1 μM insulin, or pretreated with 250 nM wortmannin for 1 h prior to stimulation with insulin, as indicated. Error bars indicate standard deviations.
Differential regulation of S6K1 by PKB.
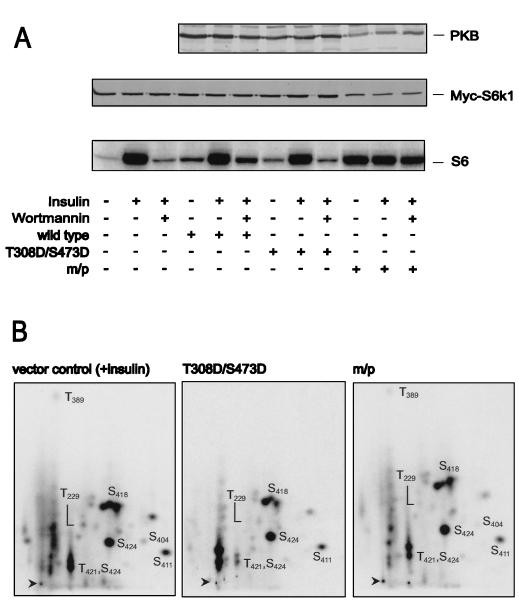
To determine the effect of PKB on S6K1 activation, each of the HA epitope-tagged PKB constructs was cotransfected with a Myc epitope-tagged S6K1 reporter, Myc-S6K1. Consistent with the results of earlier studies the wild-type construct had little effect on S6K1 activation (4), whereas m/p-HA-PKBα led to full S6K1 activation in the absence of insulin treatment (Fig. 2A) (15). In contrast to wild-type PKB, m/p-HA-PKBα also conferred wortmannin resistance on the Myc-S6K1 reporter construct (Fig. 2A). Unexpectedly, the cotransfection of the HA-PKBα-T308D/S473D variant did not raise S6K1 values above those of the wild-type construct and was ineffective at protecting S6K1 from inactivation by wortmannin treatment. The activation of S6K1 is associated with increased phosphorylation of T229, in the activation loop, and acute phosphorylation of T389 in the linker domain (23, 46, 49), as shown for the Myc-S6K1-GST reporter in 293 cells treated with insulin (Fig. 2B). Consistent with the observation that the HA-PKBα-T308D/S473D variant had no effect on S6K1 activation, it had no effect on either T229 or T389 phosphorylation, whereas the m/p-HA-PKBα was nearly as potent as insulin in inducing increased phosphorylation of the two sites (Fig. 2B). Thus, even though the HA-PKBα-T308D/S473D construct is as active as insulin-induced wild-type PKB it has no effect on S6K1 activation or on the two critical sites of phosphorylation associated with kinase activation, T229 and T389.
FIG. 2.
Effects of PKB variants on S6K1 activation. (A) The activity of ectopically expressed S6K1 from extracts of human 293 cells cotransfected with the empty vector or indicated PKB variants, as labeled in Fig. 1, was assayed. S6K1 activity is presented as 32P incorporation into 40S ribosomal protein S6 (lower panel). Cells were deprived of serum for 24 h and either extracted immediately, stimulated for 30 min with 1 μM insulin, or pretreated with 250 nM wortmannin for 1 h prior to stimulation with insulin, as indicated. Protein extracts were analyzed by Western blotting for PKB expression with the 12CA5 antibody (upper panel) and for S6K1 with the 9E10 antibody (middle panel) (see Materials and Methods). m/p, myristylation and palmitylation signal. (B) Phosphopeptide analysis of myc-tagged S6K1-GST coexpressed with the empty vector or the indicated PKB variant, as labeled in Fig. 1, in 293 cells. Prior to lysis cells were incubated for 3 h with 0.2 mCi of 32Pi per ml. In the case of the empty vector control 1 μM insulin was added 30 min before lysis. The Myc-S6K1-GST phosphopeptides were analyzed by two-dimensional thin-layer electrophoresis and chromatography as described in Materials and Methods. m/p, m/p-HA-PKBα.
Differential signaling of PKB to GSK-3.
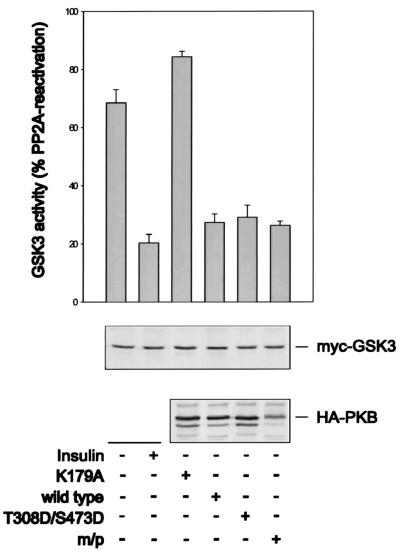
One possibility is that HA-PKBα-T308D/S473D is not capable of signaling to downstream effector molecules under the conditions employed here. To determine the effectiveness of HA-PKBα-T308D/S473D in downstream signaling, both of the activated alleles were analyzed for their abilities to inhibit the activity of an Myc epitope-tagged GSK-3β reporter (21). The results demonstrate that insulin stimulation decreased Myc–GSK-3β activity to approximately 70% of the value detected in quiescent cells (Fig. 3), in good agreement with values observed by others (54). Despite their differential effects on S6K1 activity (Fig. 2A), the coexpression of either HA-PKBα-T308D/S473D or m/p-HA-PKBα lowered Myc–GSK-3β activity to the same extent, an effect which was not further potentiated by insulin (data not shown). The coexpression of HA-PKBα was also as efficient as that of either of the two activated constructs in lowering Myc–GSK-3β activity, possibly reflecting the overexpression of HA-PKBα and the high affinity of the two kinases for one another (61). However, the kinase-inactive form of PKBα, in which K179 in the ATP binding site had been replaced by an alanine (Fig. 1A), had no effect on Myc–GSK-3β activity (Fig. 3). Thus, the overexpression of wild-type PKB is sufficient to induce GSK-3β inactivation, but not S6K1 activation, and although the double acidic mutant has no effect on S6K1 activity (Fig. 2A), it is unimpaired in its ability to signal GSK-3β (Fig. 3).
FIG. 3.
Effect of PKB variants on GSK-3 inactivation. The activity of ectopically expressed GSK-3 was assayed from extracts of human 293 cells cotransfected with the indicated PKB variants or the empty vector. GSK-3 activity is measured as a percentage of PP2A-induced activity. Cells were treated as described in the legend to Fig. 2A. Protein extracts were analyzed by Western blotting for PKB expression with the 12CA5 antibody (lower panel) and GSK-3 expression with the 9E10 antibody (middle panel) (see Materials and Methods). Error bars indicate standard deviations. m/p, myristylation and palmitylation signal.
Membrane targeting of PKB.
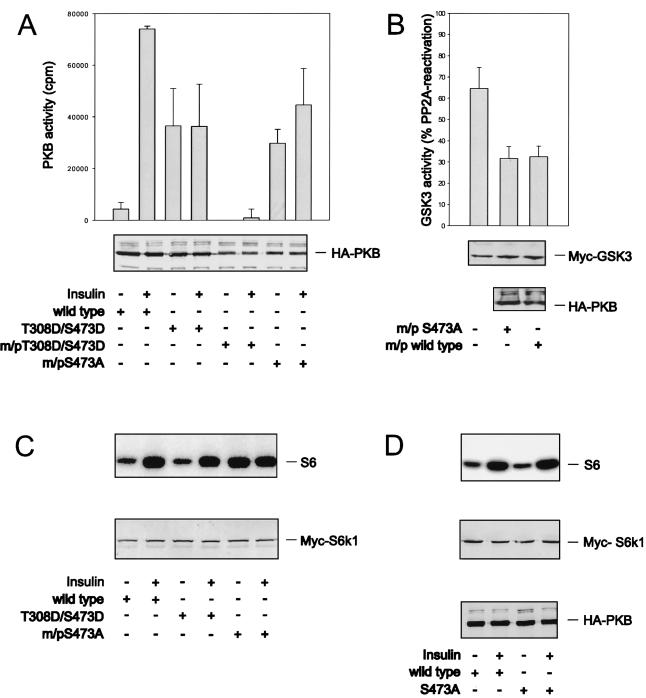
A potential explanation for the inability of the double acidic PKB to activate S6K1, versus m/p-HA-PKBα, is that such activation requires the constitutive membrane targeting of PKB. To test this possibility the double acidic mutant was constitutively targeted to the membrane by virtue of the myristylation and palmitylation signal. However, this form of the kinase was inactive, even in the presence of insulin (Fig. 4A). Therefore, to resolve this issue we exploited an m/p-HA-PKBα variant in which S473 was mutated to alanine, m/p-HA-PKBα-S473A (6). This mutant has basal activity equal to that of HA-PKBα-T308D/S473D and is significantly less active than the m/p-HA-PKBα parent construct (Fig. 4A and 1B). The addition of insulin slightly raises the kinase activity of m/p-HA-PKBα-S473A, although the level of activation is lower than that of the wild-type enzyme in the presence of insulin (Fig. 4A). Furthermore, this construct was as potent as the m/p-HA-PKBα parent construct in lowering basal Myc–GSK-3β activity (Fig. 4B). Despite the significantly lower basal activity of m/p-HA-PKBα-S473A compared with that of m/p-HA-PKBα (Fig. 4A), the constitutive targeting of this construct to the membrane led to the full activation of coexpressed Myc-S6K1, an effect not further augmented by insulin (Fig. 4C). To ensure that this effect was due to membrane targeting, the same variant lacking the myristylation and palmitylation signal, HA-PKBα-S473A, was coexpressed with Myc-S6K1. Like the double acidic mutant, HA-PKBα-S473A did not potentiate S6K1 activation beyond that of wild-type HA-PKBα (Fig. 4D). As with the double acidic mutant, we have also found that the m/p-HA-PKBα construct harboring an alanine at T308 is inactive and fails to induce S6K1 activation (data not shown). Taken together the data demonstrate that full S6K1 activation is a function of the constitutive membrane localization of active PKB rather than its specific activity.
FIG. 4.
Membrane targeting of PKB is required for S6K1 activation. (A and C) Wild-type HA-PKBα, HA-PKBα-T308D/S473D, and m/p-HA-PKBα-S473A were cotransfected into human 293 cells with an Myc-S6K1 reporter construct. Panel A shows the activity of the indicated PKB constructs as well as that of m/p-HA-PKBα-T308D/S473D. S6K1 activity is presented as in Fig. 2 (C, upper panel). Expression levels of Myc-S6K1 and the different HA-tagged PKB constructs are shown in the lower panels (A and C). (B) Myc–GSK-3β was cotransfected with the empty vector, m/p-HA-PKBα-S473A, or m/p-HA-PKBα. GSK-3 activity is presented as a percentage of PP2A-induced activity. (D) Wild-type HA-PKBα and HA-PKBα-S473A were expressed together with S6K1. S6 kinase activity is shown in the upper panel (Fig. 2), and expression levels are shown in the middle and lower panels, as indicated. Error bars indicate standard deviations.
Effect of an interfering PKB mutant on S6K1 activation.
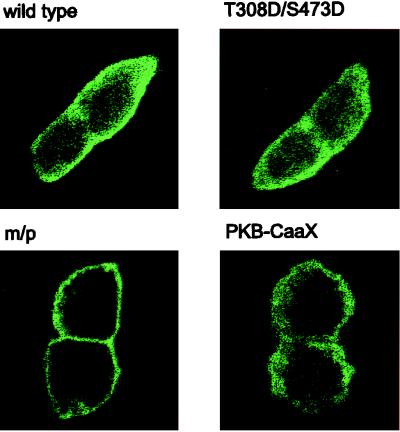
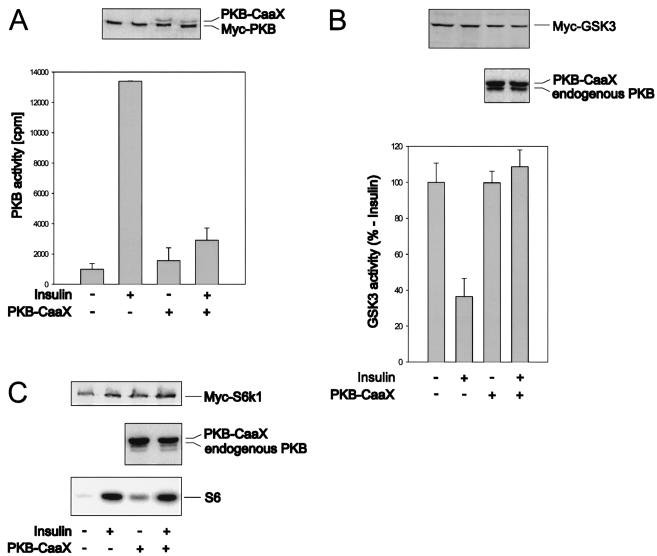
The data above indicate that PKB-mediated S6K1 activation requires the membrane localization of an active allele. Consistent with this model and their effects on S6K1 activation (Fig. 2A), immunofluorescence data show that the double acidic HA-PKBα-T308/S473D mutant, like wild-type HA-PKBα, largely resides in the cytoplasm of quiescent 293 cells, whereas the m/p-HA-PKBα construct is localized at the membrane (Fig. 5). However, recent observations have demonstrated that mitogen stimulation leads to the transient, wortmannin-sensitive membrane translocation and activation of PKB (6), a finding which would be consistent with the hypothesis that PKB mediates insulin-induced S6K1 activation. To test this hypothesis we initially analyzed the effects of a kinase-dead variant of PKBα, HA-PKBα-K179A (Fig. 1A), and a PKBα mutant having alanines at positions 308 and 473 (1) on insulin-induced S6K1 activation. However, the overexpression of either construct or the HA-PKBα-K179A construct having a myristylation and palmitylation signal failed to attenuate the insulin-induced activation of an epitope-tagged reporter PKB construct (data not shown). In recent studies it has been shown that when PKB is targeted to the membrane by a CaaX box from Ki-Ras it is catalytically inactive and blocks the insulin-induced activation of an epitope-tagged reporter PKB construct (61). As shown by immunofluorescence this construct is constitutively targeted to the membrane (Fig. 5). The coexpression of this construct with a Myc epitope-tagged PKB or GSK-3 reporter attenuates insulin-induced PKB activation and GSK-3 inactivation (Fig. 6A and B) (61), whereas it had no effect on insulin-induced S6K1 activation (Fig. 6C). These data demonstrate that insulin-induced PKB activation is not necessary for S6K1 activation.
FIG. 5.
Localization of HA-PKBα-T308D/S473D and PKBα-CaaX. Human 293 cells were transfected with 0.5 μg of the indicated HA-tagged construct per ml or 1 μg of PKB-CaaX per ml on glass coverslips. They were then processed for immunofluorescence as described in Materials and Methods. m/p, m/p-HA-PKBα.
FIG. 6.
Insulin-induced PKB activation is not necessary for S6K1 activation but is required for GSK3 inhibition. (A) Human 293 cells were transiently transfected with Myc-tagged PKBα and PKBα-CaaX or the empty vector. The serum-deprived cells were lysed directly or first were stimulated for 20 min with 1 μM insulin. Cell extracts were analyzed by Western blotting with a polyclonal antibody (1) raised against the C terminus of PKB (upper panel). The activity of Myc-tagged PKBα was measured as described in Materials and Methods with the monoclonal 9E10 antibody for immunoprecipitation (lower panel). Error bars indicate standard deviations. (B) Myc-tagged GSK-3β was cotransfected with PKBα-CaaX or the empty vector. The cells were left untreated or were stimulated as in the experiment described in panel A. GSK-3 activity was measured as described in Materials and Methods, except that the PP2A step was omitted. The expression levels of Myc–GSK-3 (upper panel) and PKBα-CaaX (middle panel) were quantified with the 9E10 and the polyclonal PKB antibodies, respectively. Error bars indicate standard deviations. (C) Human 293 cells were transiently transfected with Myc-tagged S6K1 and either the empty vector or PKBα-CaaX and treated as described for panel A. Cell extracts were analyzed by Western blotting with either the 9E10 antibody to quantify S6K1 expression (upper panel) or the polyclonal anti-PKB antibody (middle panel). S6K1 activity was measured as described in Materials and Methods (lower panel).
PKB regulation of 4E-BP1 phosphorylation.
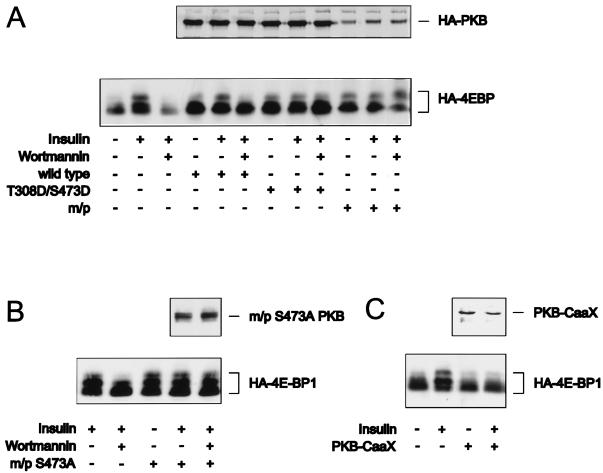
Although S6K1 and GSK-3 reside on a PI3K-dependent signaling pathway, only the S6K1 branch of this pathway is regulated by mTOR, the target of rapamycin (59). Recent studies have demonstrated that the repressor of initiation factor 4E, 4E-BP1, resides on the same PI3K-dependent, rapamycin-sensitive signaling pathway as S6K1 (64). As with S6K1, activated alleles of PKB lead to increased 4E-BP1 phosphorylation (27, 36, 60), and insulin-induced 4E-BP1 phosphorylation is blocked by interfering mutants of PKB (27). However, given their similar modes of regulation, it was reasoned that 4E-BP1 should be regulated by PKB in a manner similar to S6K1. To test this possibility we first examined the effects of insulin on 4E-BP1 phosphorylation, as demonstrated by its reduced mobility on sodium dodecyl sulfate-polyacrylamide gels (64), in the absence and presence of coexpressed wild-type PKBα. Although the coexpression of wild-type PKBα slightly raised 4E-BP1 phosphorylation levels, full activation required insulin (Fig. 7A). Furthermore, in both cases, the increases in 4E-BP1 phosphorylation were blocked by pretreatment with wortmannin (Fig. 7A). Surprisingly, in contrast to S6K1 activation (Fig. 4C), HA-PKBα-T308D/S473D alone is as potent in inducing 4E-BP1 phosphorylation as it is in the presence of insulin (Fig. 7A). Moreover, the effect of HA-PKBα-T308D/S473D on 4E-BP1 phosphorylation is equivalent to that of the constitutively membrane-targeted m/p-HA-PKBα variant, and neither effect is blocked by pretreatment with wortmannin (Fig. 7A). Consistent with these findings, we found in separate studies that the m/p-HA-PKBα-S473A variant was as potent as the two activated PKB alleles in inducing 4E-BP1 phosphorylation and that this effect was not blocked by wortmannin and was as strong in the absence of insulin (Fig. 7B). Thus, 4E-BP1 phosphorylation is affected by the activated alleles of PKB in a manner similar to that observed for GSK-3β. As the PKBα-CaaX interfering mutant has no effect on insulin-induced S6K1 activation, we also examined its effect on 4E-BP1 phosphorylation. Again, in contrast to S6K1 activation (Fig. 4C), the PKBα-CaaX interfering mutant blocked insulin-induced 4E-BP1 phosphorylation (Fig. 7C). Taken together the results demonstrate that in 293 cells PKB plays an essential role in regulating 4E-BP1 phosphorylation and GSK-3 inactivation but is dispensable for S6K1 activation.
FIG. 7.
PKB activation is necessary and sufficient for 4E-BP1 phosphorylation. (A and B) The indicated PKB constructs, as labeled in Fig. 1, were cotransfected with HA–4E-BP1 as described in the legend to Fig. 2A. After serum deprivation cells were either left untreated, stimulated with insulin for 20 min prior to lysis, or pretreated with 250 nM of wortmannin for 1 h prior to stimulation with insulin, as indicated. m/p, myristylation and palmitylation signal. (C) PKBα-CaaX or the empty vector was cotransfected in human 293 cells with HA-tagged 4E-BP1, and the cells were treated as described in the legend to Fig. 6A. 4E-BP1 electrophoretic mobility shifts were detected, as described in Materials and Methods. The upper panels show the expression of the cotransfected PKB constructs, detected with either the 12CA5 antibody (A and B) or the polyclonal anti-PKB antibody (C), as described in Materials and Methods.
DISCUSSION
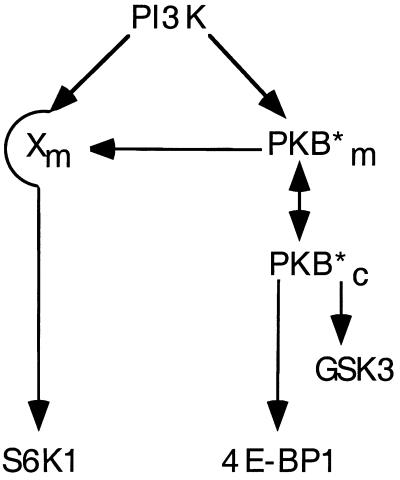
The results presented here demonstrate that activated alleles of PKB will induce S6K1 activation only when these alleles are constitutively localized to the membrane. The results further show that such membrane localization is not required for activated alleles of PKB to signal GSK-3 or 4E-BP1. In the case of 4E-BP1, this was an unexpected observation, as 4E-BP1 phosphorylation is thought to be regulated by the same rapamycin-sensitive pathway as S6K1 (64). Indeed, the results support a model in which PKB plays a dominant role in signaling 4E-BP1, whereas the input to S6K1 appears dispensable (see Fig. 8).
FIG. 8.
Model for differential PKB signaling to S6K1 and 4E-BP1. PI3K signaling to S6K1 is PKB independent, whereas 4E-BP1 and GSK-3 phosphorylation are PKB dependent. However, when PKB is constitutively targeted to the membrane it artificially activates Xm, a potential mediator of the PI3K-dependent signaling pathway to S6K1. Asterisk indicates active PKB. m, membrane; c, cytoplasm.
The finding that activated alleles of PKB, but not wild-type PKB, mediate S6K1 activation is similar to observations of the effects of activated alleles of Ras on S6K1 activation (10, 40). Activated oncogenic alleles of Ras induce S6K1 activation; however, interfering mutants of Ras do not block insulin, epidermal growth factor, or PDGF activation of S6K1 (40). Moreover, PDGF-induced activation of Ras and its downstream effector, mitogen-activated protein kinase, are unaffected in cells expressing a mutant of the PDGF receptor lacking the kinase insert domain, whereas S6K1 activation is abolished (40). Similarly, fibroblast growth factor induces potent activation of endogenous Ras but has no effect on lamellipodium formation, despite the finding that activated oncogenic Ras will induce lamellipodium formation in the same cell type (62). It has been shown that activated Ras can bind to and activate the p110 catalytic subunit of PI3Kα (51, 52); however, endogenous Ras does not appear to be a major route utilized by growth factors in signaling PI3K. In the case of activated oncogenic Ras, its increased potency in signaling is due to it being in the constitutively highly active GTP-bound state. Likewise, in the case of PKB, constructs targeted to the membrane are also much more active than their wild-type counterparts (Fig. 1) (6). However, here we demonstrate that the essential element controlling S6K1 activation is not the specific activity of PKB but its intracellular location. Consistent with this conclusion, recent studies have shown that myristylated PKBα or myristylated PKBα-S473A induce oncogenic transformation in chicken embryo fibroblasts and hemangiosarcomas in young chickens (8). In contrast, cytosolic PKBα-T308D/S473D was weakly transforming and induced no tumors, similar to wild-type PKBα, despite the fact that its level of intrinsic PKB activity was higher than that of the myristylated PKBα-S473A variant (8). In the case of S6K1, it could be envisaged that activated alleles of PKB constitutively targeted to the membrane artificially induce the activation of a membrane-localized S6K1 signaling component, leading in turn to S6K1 activation (Fig. 8). The results demonstrate that conclusions drawn from studies with constitutively membrane-targeted active alleles of PKB concerning the role of endogenous PKB in downstream signaling must be viewed with caution, as pointed out by others (3).
The findings that the insulin-induced activation of S6K1 is not blocked by interfering mutants of PKB, despite the negative effects of this mutant on 4E-BP1 phosphorylation and GSK-3 inactivation, strongly imply that PKB does not signal S6K1. Similar conclusions have been drawn from experiments carried out by Conus et al., who showed that the depletion of intracellular stores of Ca2+ had no effect on PKB activation but abolished S6K1 activation (20). In parallel, they demonstrated that an increase in intracellular Ca2+ results in full S6K1 activation, which is wortmannin sensitive, but has little to no effect on PKB. It has been shown in at least one cell type that a dominant interfering mutant of PKB inhibits S6K1 activation at very similar doses to those that block PKB activation (35). In this case, the dominant interfering mutant employed contained alanines at T308 and S473 and was delivered to the cell by utilizing an adenovirus infection strategy. In parallel, these authors observed little to no effect of the same mutant on insulin-induced S6K1 activation in a second cell type (35). As phosphoinositide-dependent protein kinase 1 is the activation loop kinase for both S6K1 and PKB (2, 4, 49, 57), it may be that the effect on S6K1 activation is accomplished not through the inhibition of PKB but through the inhibition of a parallel pathway. Indeed, recent results from this laboratory have clearly demonstrated that interfering mutants of S6K1 can affect parallel pathways (see reference 63 and below). Although the results presented here do not exclude a role for PKB in S6K1 activation, they strongly support a model whereby PKB resides on a pathway parallel to that of S6K1 (Fig. 8).
Rapamycin inhibits mitogen-induced 4E-BP1 and S6K1 phosphorylation (9, 18, 37, 48, 64), whereas rapamycin-resistant mutants of mTOR protect both S6K1 (12) and 4E-BP1 (14, 27, 31) phosphorylation from the effects of rapamycin. Although mTOR has significant homology with lipid kinases of the PI3K family (11), in vitro it autophosphorylates and can phosphorylate the rapamycin-sensitive sites in S6K1 (16) and 4E-BP1 (13). However, not all the data are consistent with the role of mTOR as the 4E-BP1 and S6K1 kinase. First, the sites of phosphorylation in 4E-BP1 contain a proline in the +1 position (14, 26), whereas in S6K1 the critical residue, T389, is flanked by large aromatic residues (45). More importantly, a mutant of S6K1 lacking both the amino and carboxy termini, although still sensitive to wortmannin inhibition, is resistant to rapamycin-induced T389 dephosphorylation (23). These latter studies led to the hypothesis that rapamycin may block S6K1 activation by activating a phosphatase which is negatively regulated by mTOR (22, 23). In the case of 4E-BP1, five phosphorylation sites were initially identified as the targets of mTOR in vitro and in vivo (26). More recent studies suggest that only two of these sites may serve as in vitro targets and that they may be highly phosphorylated in quiescent cells, acting as priming sites for an unidentified mitogen-activated kinase (see references 16 and 22). mTOR contains 16 36- to 47-amino-acid repeats, termed HEAT domains, which are characterized by a series of spaced hydrophobic amino acids (22), which have been shown in the A subunit of PP2A to serve as a protein docking site (29). Consistent with the existence of HEAT domains, recent studies have shown that other kinases may be tightly associated with mTOR (43). Obviously it will be important to identify mTOR-associated kinases and to determine whether they serve as either 4E-BP1 or S6K1 kinases.
The differential effects of PKB signaling on 4E-BP1 and S6K1 were unexpected. As stated above, 4E-BP1 and S6K1 reside on the same PI3K-dependent signaling pathway and both are sensitive to rapamycin. Furthermore, the overexpression of S6K1 blocks 4E-BP1 phosphorylation at the same sites as rapamycin, suggesting that the 4E-BP1 and S6K1 signaling pathway bifurcates just upstream of S6K1, with the most obvious point of bifurcation being mTOR (63). One possible explanation that reconciles these seemingly disparate observations is that mTOR acts as both a kinase and a scaffold for other interacting proteins, as we have recently suggested (22). In this case, PKB signaling to 4E-BP1 would be accomplished through mTOR or an associated kinase, as indicated by recent findings (43, 53), but this event would not act on the S6K1 signaling pathway. In this model, S6K1 may still require mTOR for activation but in a PKB-independent manner. The overexpression of S6K1 would sequester mTOR or a molecule which interacts with mTOR and block 4E-BP1 phosphorylation. The validation of this model will require more knowledge concerning mTOR structure and function.
ACKNOWLEDGMENTS
We thank P. B. Dennis, D. Evans, R. Meier, and N. Pullen for their critical reading of the manuscript. In addition we are grateful to D. Alessi, M. Shaw, and P. Cohen for their help in establishing the GSK-3 assays.
Part of these studies was supported by a short-term EMBO fellowship to A.D. This work was partially supported by grants from the Human Frontier Science Program and European Economic Communities to G.T.
REFERENCES
- 1.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;23:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi D R, Deak M, Casamayor A, Caudwell F B, Morrice N, Norman D G, Gaffney P, Reese C B, MacDougall C N, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 3.Alessi D R, Downes C P. The role of PI 3-kinase in insulin action. Biochim Biophys Acta. 1998;1436:151–164. doi: 10.1016/s0005-2760(98)00133-7. [DOI] [PubMed] [Google Scholar]
- 4.Alessi D R, Kozlowski M T, Weng Q P, Morrice N, Avruch J. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70S6 kinase in vivo and in vitro. Curr Biol. 1998;8:69–81. doi: 10.1016/s0960-9822(98)70037-5. [DOI] [PubMed] [Google Scholar]
- 5.Amaldi F, Pierandrei-Amaldi P. TOP genes: a translationally controlled class of genes including those coding for ribosomal proteins. Vol. 18. Berlin, Germany: Springer-Verlag; 1997. [DOI] [PubMed] [Google Scholar]
- 6.Andjelkovic M, Alessi D R, Meier R, Fernandez A, Lamb N J, Frech M, Cron P, Cohen P, Lucocq J M, Hemmings B A. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 7.Andjelkovic M, Jakubowicz T, Cron P, Ming X-F, Han J-W, Hemmings B A. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci USA. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt P K. The akt kinase: molecular determinants of oncogenicity. Proc Natl Acad Sci USA. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beretta L, Gingras A-C, Svitkin Y V, Hall M N, Sonenberg N. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 1996;15:658–664. [PMC free article] [PubMed] [Google Scholar]
- 10.Blenis J, Erikson R L. Phosphorylation of the ribosomal protein S6 is elevated in cells transformed by a variety of tumor viruses. J Virol. 1984;50:966–969. doi: 10.1128/jvi.50.3.966-969.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown E J, Albers M A, Shin T B, Ichikawa K, Keith C T, Lane W S, Schreiber S L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 12.Brown E J, Beal P A, Keith C T, Chen J, Shin T B, Schreiber S L. Control of p70 S6 kinase by kinase activity of FRAP in vivo. Nature. 1995;377:441–446. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- 13.Brunn G J, Fadden P, Haystead T A J, Lawrence J C., Jr The mammalian target of rapamycin phosphorylates sites having a (Ser/Thr)-Pro motif and is activated by antibodies to a region near its COOH terminus. J Biol Chem. 1997;272:32547–32550. doi: 10.1074/jbc.272.51.32547. [DOI] [PubMed] [Google Scholar]
- 14.Brunn G J, Hudson C C, Sekulic A, Williams J M, Hosoi H, Houghton P J, Lawrence J C, Jr, Abraham R T. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- 15.Burgering B M T, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 16.Burnett P E, Barrow R K, Cohen N A, Snyder S H, Sabatini D M. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung J, Grammer T C, Lemon K P, Kazlauskas A, Blenis J. PDGF- and insulin-dependent pp70s6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994;370:71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- 18.Chung J, Kuo C J, Crabtree G R, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- 19.Clemens M G. Protein kinases that phosphorylate eIF2 and eIF2B, and their role in eukaryotic cell translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. [Google Scholar]
- 20.Conus N M, Hemmings B A, Pearson R B. Differential regulation by calcium reveals distinct signaling requirements for the activation of Akt and p70S6k. J Biol Chem. 1998;273:4776–4782. doi: 10.1074/jbc.273.8.4776. [DOI] [PubMed] [Google Scholar]
- 21.Cross D A E, Alessi D R, Cohen P, Andjelkovic M, Hemmings B A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 22.Dennis P B, Fumagalli S, Thomas G. Target of rapamycin (TOR); balancing the opposing forces of protein synthesis and degradation. Curr Opin Genet Dev. 1999;9:49–54. doi: 10.1016/s0959-437x(99)80007-0. [DOI] [PubMed] [Google Scholar]
- 23.Dennis P B, Pullen N, Kozma S C, Thomas G. The principal rapamycin-sensitive p70s6k phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol Cell Biol. 1996;16:6242–6251. doi: 10.1128/mcb.16.11.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennis P B, Pullen N, Pearson R B, Kozma S C, Thomas G. Phosphorylation sites in the autoinhibitory domain participate in p70(s6k) activation loop phosphorylation. J Biol Chem. 1998;273:14845–14852. doi: 10.1074/jbc.273.24.14845. [DOI] [PubMed] [Google Scholar]
- 25.Downward J. Lipid-regulated kinases: some common themes at last. Science. 1998;279:673–674. doi: 10.1126/science.279.5351.673. [DOI] [PubMed] [Google Scholar]
- 26.Fadden P, Haystead T A J, Lawrence J C., Jr Identification of phosphorylation sites in the translational regulator, PHAS-I, that are controlled by insulin and rapamycin in rat adipocytes. J Biol Chem. 1997;272:10240–10247. doi: 10.1074/jbc.272.15.10240. [DOI] [PubMed] [Google Scholar]
- 27.Gingras A-C, Kennedy S G, O’Leary M A, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and activated by the Akt (PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gout I, Minami T, Hara K, Tsujishita Y, Filonenko V, Waterfield M D, Yonezawa K. Molecular cloning and characterization of a novel p70 S6 kinase, p70 S6 kinase b containing a proline-rich region. J Biol Chem. 1998;273:30061–30064. doi: 10.1074/jbc.273.46.30061. [DOI] [PubMed] [Google Scholar]
- 29.Groves M R, Hanlon N, Turowski P, Hemmings B A, Barford D. The structure of the PR65/A-subunit of protein phosphatase 2A reveals the conformation of a scaffolding molecule composed of 15 tandemly repeated HEAT motifs. Cell. 1999;96:99–110. doi: 10.1016/s0092-8674(00)80963-0. [DOI] [PubMed] [Google Scholar]
- 30.Han J W, Pearson R B, Dennis P B, Thomas G. Rapamycin, wortmannin, and the methylxanthine SQ20006 inactivate p70s6k by inducing dephosphorylation of the same subset of sites. J Biol Chem. 1995;36:21396–21403. doi: 10.1074/jbc.270.36.21396. [DOI] [PubMed] [Google Scholar]
- 31.Hara K, Yonezawa K, Weng Q-P, Kozlowski M T, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 32.Jefferies H B J, Fumagalli S, Dennis P B, Reinhard C, Pearson R B, Thomas G. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;12:3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jefferies H B J, Reinhard C, Kozma S C, Thomas G. Rapamycin selectively represses translation of the “polypyrimidine tract” mRNA family. Proc Natl Acad Sci USA. 1994;91:4441–4445. doi: 10.1073/pnas.91.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jefferies H B J, Thomas G. Ribosomal protein S6 phosphorylation and signal transduction. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 389–409. [Google Scholar]
- 35.Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, Kasuga M. Requirement for activation of the serine-threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohn A D, Barthel A, Kovacina K S, Boge A, Wallach B, Summers S A, Birnbaum M J, Scott P H, Lawrence J C, Roth R A. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J Biol Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- 37.Kuo C J, Chung J, Fiorentino D F, Flanagan W M, Blenis J, Crabtree G R. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature. 1992;358:70–73. doi: 10.1038/358070a0. [DOI] [PubMed] [Google Scholar]
- 38.Lane H A, Fernandez A, Lamb N J C, Thomas G. p70s6k function is essential for G1 progression. Nature. 1993;363:170–172. doi: 10.1038/363170a0. [DOI] [PubMed] [Google Scholar]
- 39.Meyuhas O, Avni D, Shama S. Translational control of ribosomal protein mRNAs in eukaryotes. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. [Google Scholar]
- 40.Ming X F, Burgering B M T, Wennström S, Claesson-Welsh L, Heldin C H, Bos J L, Kozma S C, Thomas G. Activation of p70/p85 S6 kinase by a pathway independent of p21ras. Nature. 1994;371:426–429. doi: 10.1038/371426a0. [DOI] [PubMed] [Google Scholar]
- 41.Nasmyth K. Another role rolls in. Nature. 1996;382:28–29. doi: 10.1038/382028a0. [DOI] [PubMed] [Google Scholar]
- 42.Neufeld T P, de la Cruz A F, Johnston L A, Edgar B A. Coordination of growth and cell division in the Drosophila wing. Cell. 1998;93:1183–1193. doi: 10.1016/s0092-8674(00)81462-2. [DOI] [PubMed] [Google Scholar]
- 43.Nishiuma T, Hara K, Tsujishita Y, Kaneko K, Shii K, Yonezawa K. Characterization of the phosphoproteins and protein kinase activity in mTOR immunoprecipitates. Biochem Biophys Res Commun. 1998;252:440–444. doi: 10.1006/bbrc.1998.9671. [DOI] [PubMed] [Google Scholar]
- 44.Pause A, Belsham G J, Gingras A-C, Donzé O, Lin T A, Lawrence J C, Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature. 1994;371:762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- 45.Pearson R B, Dennis P B, Han J W, Williamson N A, Kozma S C, Wettenhall R E H, Thomas G. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J. 1995;21:5279–5287. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearson R B, Thomas G. Regulation of p70s6k/p85s6k and its role in the cell cycle. In: Meijer L, Guidet S, Tung H Y L, editors. Progress in cell cycle research. Vol. 1. New York, N.Y: Plenum Press; 1995. pp. 21–32. [DOI] [PubMed] [Google Scholar]
- 47.Peterson R T, Schreiber S L. Translation control: connecting mitogens and the ribosome. Curr Biol. 1998;8:R248–R250. doi: 10.1016/s0960-9822(98)70152-6. [DOI] [PubMed] [Google Scholar]
- 48.Price D J, Grove J R, Calvo V, Avruch J, Bierer B E. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992;257:973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- 49.Pullen N, Dennis P B, Andjelkovic M, Dufner A, Kozma S, Hemmings B A, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- 50.Reinhard C, Fernandez A, Lamb N J C, Thomas G. Nuclear localization of p85s6k: functional requirement for entry into S phase. EMBO J. 1994;13:1557–1565. doi: 10.1002/j.1460-2075.1994.tb06418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 53.Scott P H, Brunn G J, Kohn A D, Roth R A, Lawrence J C., Jr Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of papamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci USA. 1998;95:7772–7777. doi: 10.1073/pnas.95.13.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw M, Cohen P, Alessi D R. Further evidence that the inhibition of glycogen synthase kinase-3beta by IGF-1 is mediated by PDK1/PKB-induced phosphorylation of Ser-9 and not by dephosphorylation of Tyr-216. FEBS Lett. 1997;416:3097–311. doi: 10.1016/s0014-5793(97)01235-0. [DOI] [PubMed] [Google Scholar]
- 55.Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma S C. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 1998;17:6649–6659. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sonenberg N. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. [Google Scholar]
- 57.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter G F, Holmes A B, Gaffney P R, Reese C B, McCormick F, Tempst P, Coadwell J, Hawkins P T. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 58.Su T T, O’Farrell P H. Size control: cell proliferation does not equal growth. Curr Biol. 1998;8:R687–R689. doi: 10.1016/s0960-9822(98)70436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas G, Hall M N. Tor signalling and control of cell growth. Curr Biol. 1997;9:782–787. doi: 10.1016/s0955-0674(97)80078-6. [DOI] [PubMed] [Google Scholar]
- 60.Ueki K, Yamamoto-Honda R, Kaburagi Y, Yamauchi T, Tobe K, Burgering B M, Coffer P J, Komuro I, Akanuma Y, Yazaki Y, Kadowaki T. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J Biol Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- 61.van Weeren P C, de Bruyn K M, de Vries-Smits A M, van Lint J, Burgering B M. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J Biol Chem. 1998;273:13150–13156. doi: 10.1074/jbc.273.21.13150. [DOI] [PubMed] [Google Scholar]
- 62.van Weering D H J, de Rooij J, Marte B, Downward J, Bos J L, Burgering B M T. Protein kinase B activation and lamellipodium formation are independent phosphoinositide 3-kinase-mediated events differentially regulated by endogenous Ras. Mol Cell Biol. 1998;18:1802–1811. doi: 10.1128/mcb.18.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Von Manteuffel S R, Dennis P B, Pullen N, Gingras A-C, Sonenberg N, Thomas G. The insulin-induced signalling pathway leading to S6 and initiation factor 4E binding protein 1 phosphorylation bifurcates at a rapamycin-sensitive point immediately upstream of p70s6k. Mol Cell Biol. 1997;17:5426–5436. doi: 10.1128/mcb.17.9.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Von Manteuffel S R, Gingras A-C, Ming X-F, Sonenberg N, Thomas G. 4E-BP1 phosphorylation is mediated by the FRAP-p70s6k pathway and is independent of mitogen-activated protein kinase. Proc Natl Acad Sci USA. 1996;93:4076–4080. doi: 10.1073/pnas.93.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Welsh G I, Miller C M, Loughlin A J, Price N T, Proud C G. Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett. 1998;421:125–130. doi: 10.1016/s0014-5793(97)01548-2. [DOI] [PubMed] [Google Scholar]