Abstract
A major problem in the treatment of cocaine addiction is high rates of relapse. Relapse is often provoked by acute re-exposure to cocaine-associated cues or to cocaine itself. The lateral habenula (LHb), an epithalamic nucleus, regulates midbrain dopaminergic systems that are known to be involved in cocaine taking and seeking behaviors. However, the role of this nucleus in cocaine self-administration and reinstatement of cocaine seeking has not been entirely parsed out. We used an operant self-administration and reinstatement procedure to explore the effect of DREADD-induced transient inhibition of LHb neurons on cocaine taking and seeking. Firstly, rats were injected with adeno-associated viral vectors expressing hM4Di (a Gi/o-coupled DREADD) into the LHb, trained to self-administer cocaine (0.75 mg/kg/infusion) and the effect of clozapine-N-oxide (an inert ligand that activates DREADD’s) was assessed on cocaine self-administration. Secondly, rats were injected with hM4Di into the LHb, trained to self-administer cocaine, the operant response was extinguished, and cue- and cocaine priming-induced reinstatement was assessed. Thirdly, we tested the generality of the effect of inhibiting LHb neurons by assessing the effect of this manipulation on food-taking and seeking. hM4Di-induced inhibition of LHb neurons increased cocaine- but not food self-administration. In contrast, this manipulation decreased reinstatement of cocaine, but not food-seeking. Taken together, our data suggest that hM4Di- induced LHb inhibition specifically mediates taking and seeking behaviors reinforced by cocaine, but not by natural reinforcers. Further, our data indicate a dissociation in the role of LHb neurons on cocaine self-administration versus reinstatement of cocaine seeking.
Keywords: Lateral habenula, Cocaine self-administration, Reinstatement, Relapse, DREADD, Viral mediated gene transfer
Addiction to psychostimulant drugs such as cocaine is a worldwide epidemic with major social and economic burdens on society. An important problem in the treatment of cocaine addiction is the vulnerability of individuals to relapse months or even years after cessation of cocaine use (Dackis & O’Brien, 2001; Gossop, Green, Phillips et al., 1989). Though studies of relapse to cocaine seeking in experimental animals, primarily rodents, using the reinstatement procedure (Shalev, Grimm & Shaham, 2002) have provided invaluable information on the neurobiological bases of relapse, the neurochemical events that drive this phenomenon are still not completely understood.
The lateral habenula (LHb), an epithalamic nucleus located in the dorsal diencephalon, commonly known to be involved in behavioral flexibility (Baker & Mizumori, 2017) and the processing of aversive information (Matsumoto & Hikosaka, 2007), is an important regulator of midbrain dopaminergic systems (Balcita-Pedicino, Omelchenko, Bell et al., 2011; Brinschwitz, Dittgen, Madai et al., 2010; Hikosaka, Sesack, Lecourtier et al., 2008). The LHb receives afferent projections from the limbic forebrain, which is innervated by the cortex, basal ganglia, lateral hypothalamus and parts of the extended amygdala among other brain regions (Geisler & Trimble, 2008). LHb efferents primarily target brainstem nuclei including the dopaminergic ventral tegmental area (VTA), the GABAergic rostromedial tegmental nucleus (RMTg) (Jhou, Fields, Baxter et al., 2009), the serotonergic dorsal (DRN) and medial raphe nuclei (MRN), and the cholinergic laterodorsal tegmentum (Araki, McGeer & Kimura, 1988; Geisler & Trimble, 2008; Herkenham & Nauta, 1979) among other brain regions. Functionally, LHb lesions increase dopamine (DA) turnover in terminal regions (Geisler & Trimble, 2008; Lecourtier, Defrancesco & Moghaddam, 2008; Nishikawa, Fage & Scatton, 1986) while local LHb stimulation inhibits spontaneous firing of VTA dopamine neurons (Christoph, Leonzio & Wilcox, 1986; Ji & Shepard, 2007). Serotonin (5-HT) neurons in the DRN are also inhibited by LHb stimulation (Park, 1987; Wang & Aghajanian, 1977). Thus, the LHb forms an integrative node between the cortex and brainstem nuclei and is an important regulator of monoaminergic neuronal systems (Balcita-Pedicino, Omelchenko, Bell et al., 2011; Brinschwitz, Dittgen, Madai et al., 2010; Hikosaka, Sesack, Lecourtier et al., 2008), which are known to be involved in cocaine taking and seeking behaviors (Filip, Alenina, Bader et al., 2010; Shalev, Grimm & Shaham, 2002). However, little is known about the precise role of this nucleus in operant cocaine self-administration and reinstatement of cocaine seeking.
We assessed the effect of transient inhibition of LHb neurons, using adeno-associated viral vectors that express Gi/o-coupled DREADDs (Designer Receptors Exclusively Activated by Designer Drug) (hM4Di) on operant cocaine self-administration and reinstatement of cocaine seeking induced by a cocaine prime or re-exposure to contingent cues. These receptors, created by molecular evolution and site-directed mutagenesis have lost their affinity for their native ligand, acetylcholine, while gaining high affinity for the synthetic ligand clozapine-N-oxide (CNO) (Armbruster, Li, Pausch et al., 2007). DREADD’s are activated by CNO with nanomolar potency, allowing activation of G-protein coupled signaling depending on which DREADDs are expressed. Following infusion of the viral vectors into the LHb, systemic administration of CNO stimulates hM4Di to activate downstream Gi/o-coupled signaling. We also assessed the specificity of the effects of LHb hM4Di on cocaine self-administration and reinstatement by examining the effect of this manipulation on motor activity, operant food-self administration and reinstatement of food-seeking behavior.
A significant advantage of using the chemogenetic approach is that the same manipulation can be used to inhibit as well as stimulate neuronal activity. Under our experimental conditions, CNO-induced transient activation of LHb neurons with hM3Dq (a Gq-coupled DREADD) significantly decreased both operant cocaine and food self-administration; these effects however, were likely mediated by significant deficits in locomotor activity at the doses of CNO (1 and 3 mg/kg) used to modulate G protein-coupled signaling in the present study.
Materials and methods
Animals
For cocaine self-administration and reinstatement experiments, male, Long-Evans rats (Charles River, Raleigh, NC), weighing 325–400 g were used. Rats were double housed initially and allowed to acclimate for at least one week to the vivarium prior to the experiment. The temperature- and humidity-controlled vivarium was under a 12-h light-dark cycle (lights on at 6 a.m.). Following the acclimation period, rats were injected intracranially with viral vectors and implanted with intravenous catheters into the jugular vein. For cocaine self-administration and reinstatement experiments, food and water were available ad libitum for all rats, except during the 2–3h training, extinction and reinstatement session. For food self-administration and reinstatement experiments, rats were on a restricted diet of 18–20 g/d (about 70–75% of their regular daily Purina Rat Chow) through the duration of the experiment. All experimental procedures were approved by the University of Washington Institutional Animal Care and Use Committee and were conducted in accordance to the guidelines of the “Principles of Laboratory Animal Care” (NIH publication no. 86-23, 1996).
Drugs
CNO (National Institutes of Health, Bethesda, MD) was dissolved in sterile water with 1–2% dimethylsufloxide. The drug was administered by intraperitoneal injection in a volume of 1 ml/kg approximately twenty minutes prior to the test session.
Cocaine hydrochloride (National Institute on Drug Abuse, Bethesda, MD) was dissolved in sterile 0.9% saline and infused in a volume of 0.1ml at a dose of 0.75 mg/kg/infusion for operant self-administration training. For reinstatement experiments, cocaine (10 mg/kg) was injected intraperitoneally in a volume of 1 ml/kg.
Intracranial surgery and virus-mediated gene transfer
Adeno-associated viral vectors were obtained from the University of North Carolina viral vector core facility (AAV8-hSyn-hM4Di-mCherry [Lot # AV5360d; titer 8.3 × 10 e12] and AAV8-hSyn-hM3Dq-mCherry [Lot # AV5359d; titer 4 × 10 e12]) and Addgene (AAV8-hSyn-eGFP [Titer 3 × 10 e12]). Viral vectors were injected into the LHb stereotaxically (Ferguson, Mitchell & Neumaier, 2008; Neumaier, Vincow, Arvanitogiannis et al., 2002). Stereotaxic surgery details are provided in online supplemental methods. Rats in which at least 90% of cells expressing the reporter gene were confined to the LHb were included in the analysis. A representative image is depicted in Figure 1.
Figure 1.
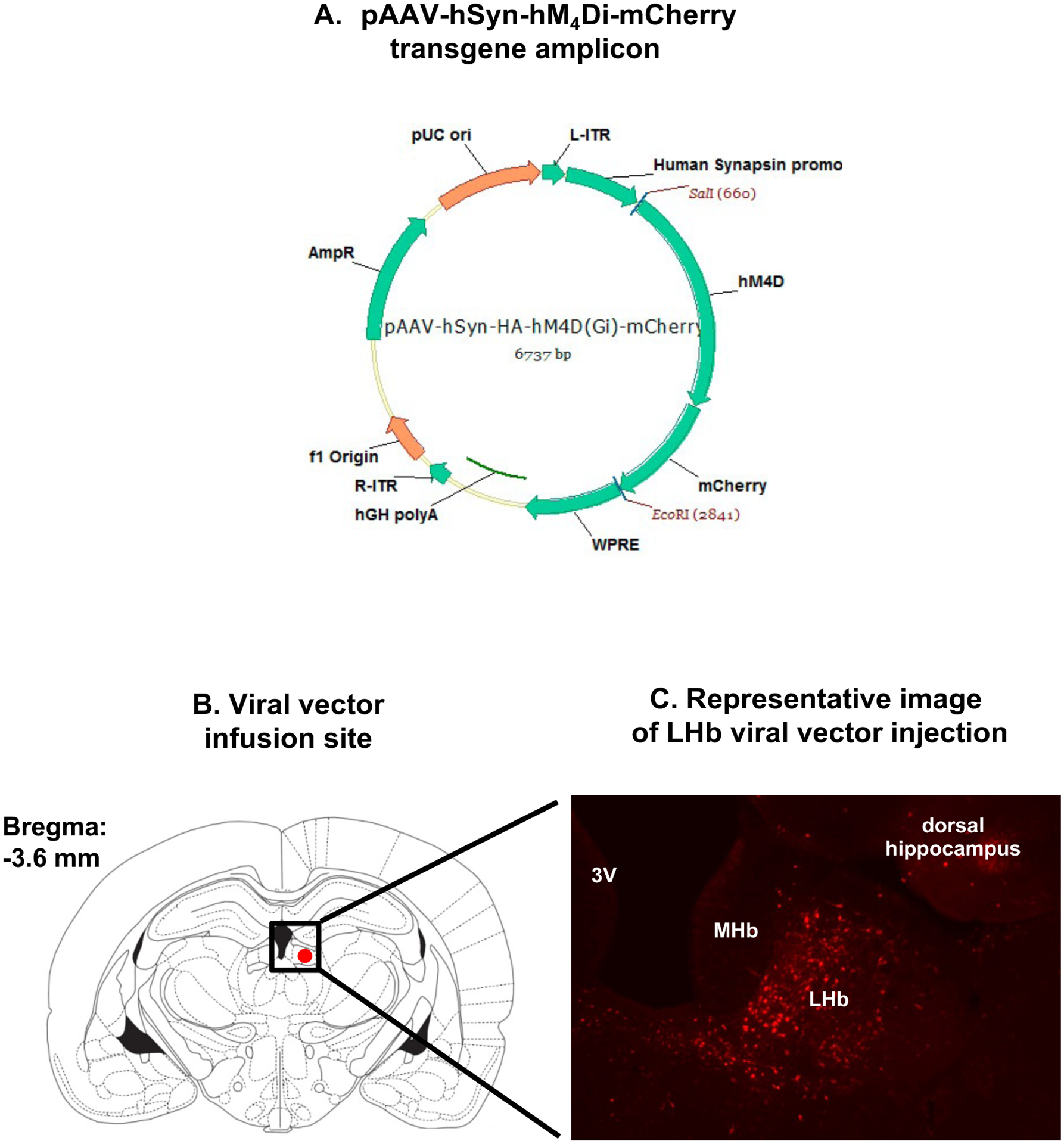
Virus mediated gene transfer (A) Illustration of the adeno-associated virus Gi/o DREADD (hM4Di) mCherry transgene amplicon (B) Illustration of rat brain coordinates (Paxinos plate, −3.6 mm) used for viral vector infusion. The red oval depicts the target zone for viral vector transduction unilaterally. 3V: Third ventricle, MHb: Medial habenula, LHb: Lateral habenula (C) Representative image of mCherry expression from a coronal section through the LHb forty days after viral vector infusion.
Intravenous surgery and behavioral testing
Intravenous surgery and behavioral testing details are provided in online supplemental methods.
Exp.1: Effect of hM4Di-mediated inhibition of the LHb on cocaine-reinforced operant responding
In Exp. 1, we examined the effect of hM4Di in the LHb on ongoing cocaine self-administration. AAV-hM4Di-hSyn-mCherry was injected into the LHb, rats were implanted with jugular catheters and trained to self-administer cocaine as described. We used a within-subjects experimental design with a within-subjects factor of Treatment (vehicle, CNO 1 or 3 mg/kg), n=6–7 per dose. Thus, each rat was injected with the vehicle or a single dose of CNO before the test sessions, which were performed in a counterbalanced order. Test days were separated by 1–2 regular training days.
Exp. 2: Effect of hM4Di-mediated inhibition of the LHb on reinstatement of cocaine seeking
Exp. 2A. Cocaine priming-induced reinstatement.
Rats were injected with AAV-hM4Di-hSyn-mCherry into the LHb, implanted with jugular catheters, trained to self-administer cocaine (3-h sessions; one session/day), and the operant response was extinguished (n=10). For reinstatement tests, we used a within-subjects experimental design with the within-subjects factors of CNO Treatment (Vehicle or CNO, 3mg) or Priming Condition (Vehicle or Cocaine, 10mg). Thus each rat was injected with Vehicle-Vehicle, CNO-Vehicle, Vehicle-Cocaine or CNO-Cocaine. The order of injections in the four experimental groups were counterbalanced. Cocaine (10 mg/kg, ip; injection volume: 1 mg/ml) or vehicle was injected immediately prior to the test session.
Exp. 2B. Cue -induced reinstatement.
For cue-induced reinstatement tests (n=9), we used a within-subjects experimental design with within-subjects factors of CNO Treatment (Vehicle or CNO, 3mg) or Priming Condition (No cue or Cue). Thus each rat was exposed to four experimental conditions; Vehicle-No Cue, CNO-No cue, Vehicle-Cue, CNO-Cue. The order of vehicle and CNO injections were counterbalanced. The order of cue condition was not counterbalanced in this experiment to allow for maximum duration between the two cue tests.
Exp. 2C: Cocaine-induced locomotor activity
The purpose of this experiment was to determine if the activation of Gi/o-coupled signaling in LHb neurons influences locomotor activity. Rats were tested approximately three weeks after AAV-hM4Di-hSyn-mCherry was injected into the LHb. Twenty minutes prior to injection with cocaine (10 mg/kg), rats were injected with either vehicle or CNO (3mg/kg) (n=8) in a between-subjects experimental design with Treatment (vehicle, CNO) as the between-subjects factor.
Exp. 3: Effect of hM4Di-mediated transient inhibition of the LHb on food-reinforced operant responding and reinstatement of food-seeking behavior
In Exp. 3A, we examined the generality of the effect of hM4Di in LHb neurons by testing the effect of this manipulation on ongoing food self-administration. AAV-hM4Di-hSyn-mCherry was injected into the LHb and rats were trained to self-administer food pellets as described. We used a within-subjects experimental design with the within-subjects factor of CNO treatment (vehicle, CNO) and CNO dose (1 or 3 mg) (n=12). Thus, each rat was injected with vehicle or a single dose of CNO before the test sessions, which were performed in a counterbalanced order. Test days were separated by 1–2 regular training days.
In Exp. 3B we examined the effect of hM4Di-mediated transient inhibition of the LHb on cue-induced reinstatement of food seeking behavior. For cue-induced reinstatement tests, we used a within-subjects experimental design with a single factor of CNO Treatment (Vehicle-Cue, CNO-Cue) (n=14). The order of vehicle and CNO (3 mg) injections was counterbalanced and the cue tests were conducted at least 72h apart with intervening extinction sessions.
Expts. 4 and 5: Effect of CNO on operant cocaine self-administration and reinstatement of cocaine seeking in rats with no viral vector injections or transduction of LHb neurons with AAV-hSyn-eGFP
Rats were either not injected with viral vectors (Exp. 4) (n=5) or injected with AAV-hSyn-eGFP in the LHb (Exp. 5) (n=4) and trained to self-administer cocaine as described. The effect of CNO (3 mg/kg) or vehicle was tested on cocaine reinforced operant responding in two counterbalanced sessions with at least 24h between the two test sessions. The operant response was then extinguished in the absence of contingent cues and rats were tested for the effect of CNO (3mg/kg) on cue-induced reinstatement as described in Exp. 2B. Subsequently, the operant response was re-extinguished in the presence of cues and the effect of CNO (3mg/kg) was tested on reinstatement of cocaine-seeking induced by a cocaine prime (10 mg/kg) as described in Exp. 2A.
Exp.6: Effect of hM3Dq-mediated activation of LHb neurons on cocaine- and food reinforced operant responding
Exp. 6A and 6B. Cocaine- and food reinforced operant responding
The experimental procedures used were identical to those described in experiments 1 and 3A with the exception that AAV-hM3Dq-hSyn-mCherry was injected into the LHb. The experimental design used to determine the effect of activation of Gq-coupled signaling in the LHb on cocaine- (n=6; Exp. 6A) or food- (n=9; Exp. 6B) reinforced operant responding was a within-subjects factor of Treatment (vehicle, CNO 1mg, CNO 3mg).
Exp. 6C Effect of hM3Dq-mediated transient activation of the LHb on motor activity
Cocaine-induced locomotor activity:
Rats were tested approximately three weeks after AAV-hM3Dq-hSyn-mCherry was injected into the LHb (n=4). Twenty minutes prior to injection with cocaine (10 mg/kg), rats (n=4) were injected with either vehicle or CNO (0.01, 0.1, 1, 3mg/kg) in a within-subjects experimental design with Treatment (vehicle, CNO 0.01mg, CNO 0.1mg, CNO 1mg, CNO 3mg) as the within-subjects factor.
Rotarod:
In this final experiment, rats injected with AAV-hM3Dq-hSyn-mCherry into the LHb (n=5) were tested for motor activity using a rotarod apparatus. Three weeks following intracranial injection of viral vector, rats were trained for 5 days (2 trials/day) to walk on the rotating rod maintained at a speed of 20 revolutions/minute for 2 minutes. Vehicle or CNO (1 or 3 mg/kg) was administered using a within-subjects counterbalanced design with factor of treatment (vehicle, CNO 1mg, CNO 3 mg), approximately 20 min prior to placing the rat on the rotarod. The latency to fall off the rotarod termed as ‘rotarod score’ was measured; maximum time allocated for test trials was 120s/trial.
Statistical analyses
Data were analyzed with the statistical program SPSS (GLM procedure). The data on the effect of activation of G-protein coupled signaling in LHb neurons on cocaine- and food self-administration were analyzed separately for the number of reinforcers (cocaine infusions or pellets earned) and active lever responding. The data from the reinstatement experiments were analyzed for non-reinforced lever responding on the previously active lever and on the inactive lever. Because the experimental manipulations had no effect on inactive lever responding, which was very low, these data are not reported.
Results
Training and extinction
The rats in Exp. 1, 2, 3, 5 and 6 were trained for 10–14 sessions and demonstrated reliable cocaine (Figs. 2A, 5A, 6A) or food self-administration (Fig. 4A). In rats trained to self-administer food, a progressive escalation of timeout responding across sessions was observed as has been previously reported (Nair, Adams-Deutsch, Epstein et al., 2009) (Fig. 4A). Significant increases in active lever responding was observed in food- (p < 0.05) but not in cocaine-trained rats. During the extinction phase, response rates decreased over time in rats previously trained to self-administer cocaine (data not shown) or food pellets (Fig. 4C).
Figure 2.
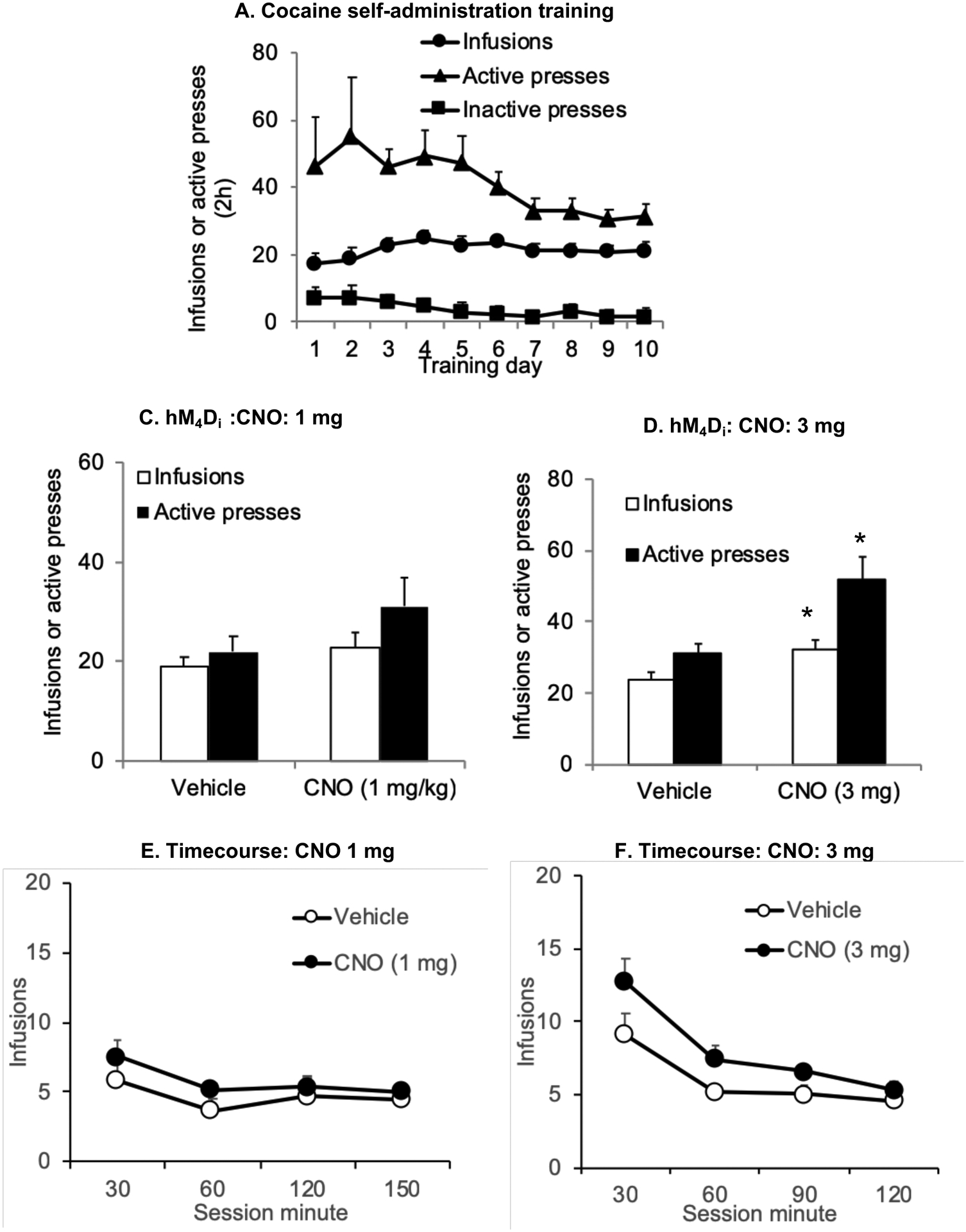
CNO enhances operant cocaine self-administration in rats with hM4Di transduction of LHb neurons. (A) Self-administration training: Mean ± SEM number of infusions, active and inactive lever responses during the first 10 days of self-administration training (one 2 h session/d) under a fixed-ratio-1 (FR-1) 20-sec timeout reinforcement schedule (B, D) Mean±SEM number of cocaine infusions self-administered and active lever presses after i.p. injections of vehicle or CNO (1mg/kg) and the corresponding time course represented at 30 minute intervals (n=6) (C, E) Mean±SEM number of cocaine infusions self-administered and active lever presses after i.p. injections of vehicle or CNO (3mg/kg) and the corresponding time course represented at 30 minute intervals (n=7) *Different from vehicle condition, p < 0.05.
Figure 5.
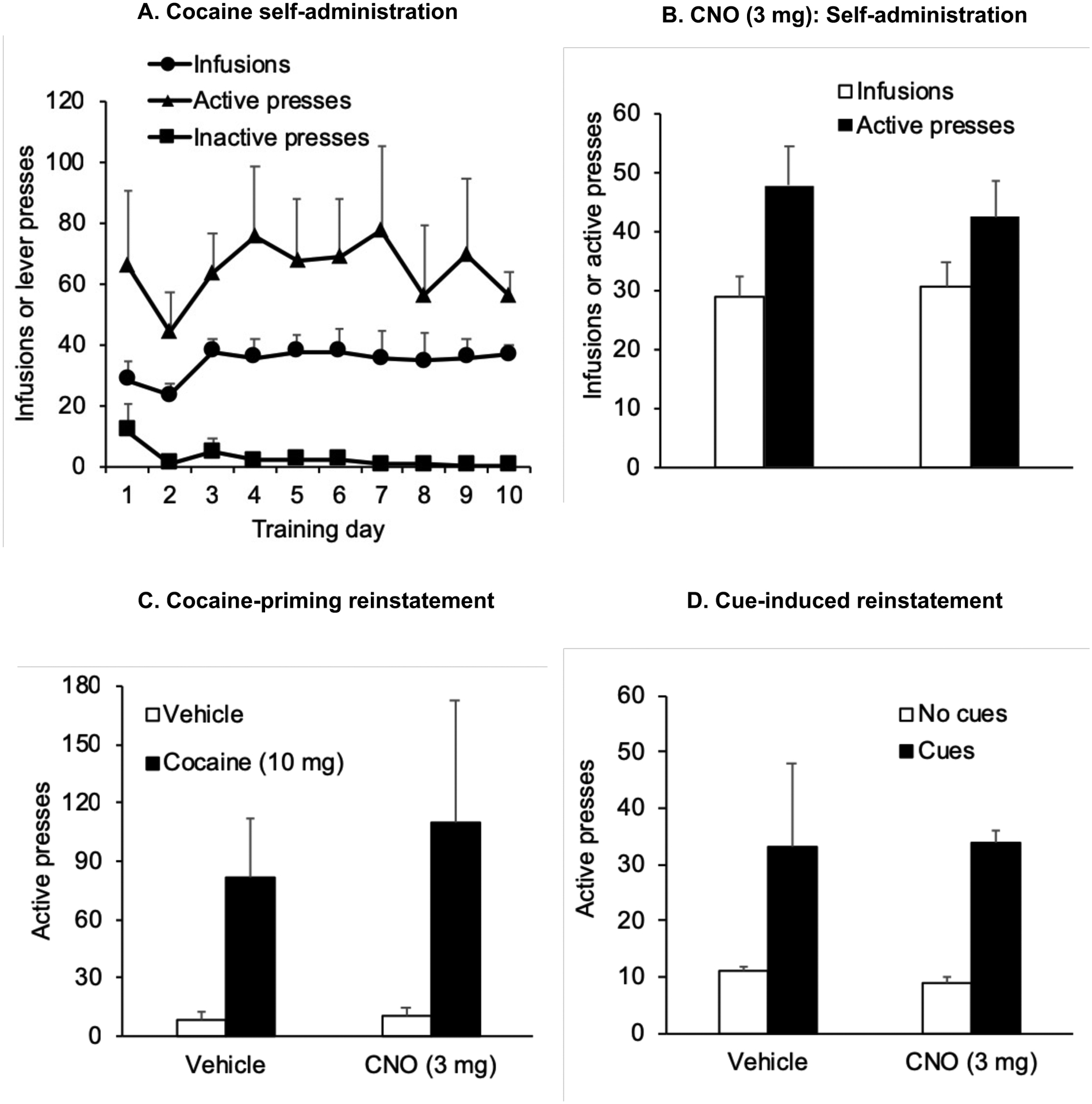
CNO has no effect on operant cocaine self-administration and cocaine-priming and cue-induced reinstatement in rats with no viral vector transduction (A) Self-administration training: Mean ± SEM number of infusions, active and inactive lever responses during 10 days of self-administration training (one 3 h session/d) under a fixed-ratio-1 (FR-1) 20-sec timeout reinforcement schedule (B) Mean±SEM number of cocaine infusions self-administered and active lever presses after i.p. injections of vehicle or CNO (3 mg/kg) (C) Mean±SEM number of active lever presses after pretreatment with CNO (3 mg) or vehicle followed by exposure to a priming injection of cocaine (10 mg) or vehicle (D) Mean±SEM number of active lever presses after pretreatment with CNO (3 mg) or vehicle followed by exposure to contingent tone-light cues or no cues under extinction conditions (n=5).
Figure 6.
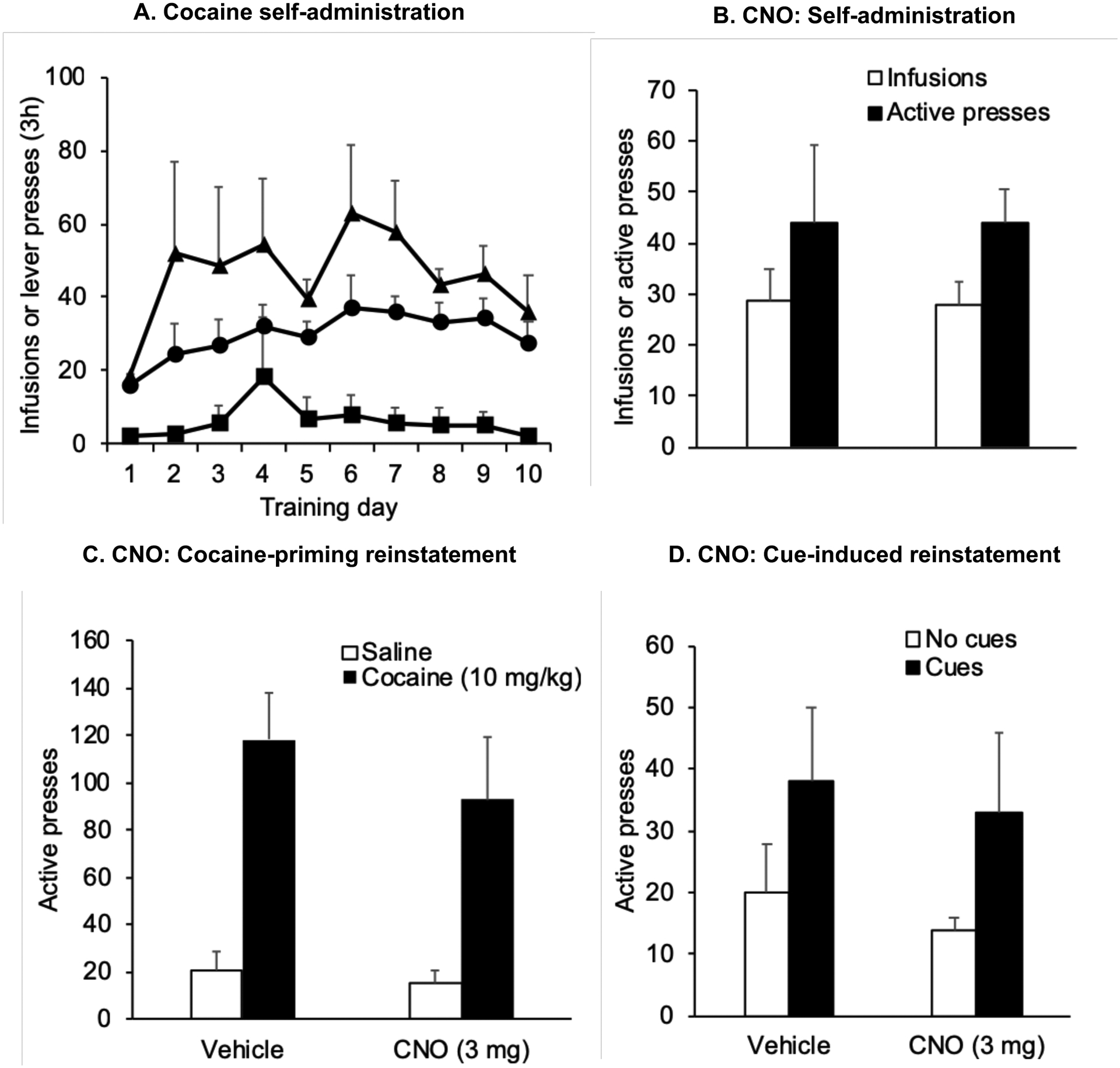
CNO has no effect on operant cocaine self-administration and cocaine-priming and cue-induced reinstatement in rats with eGFP transduction of LHb neurons (A) Self-administration training: Mean ± SEM number of infusions, active and inactive lever responses during 10 days of self-administration training (one 3 h session/d) under a fixed-ratio-1 (FR-1) 20-sec timeout reinforcement schedule (B) Mean±SEM number of cocaine infusions self-administered and active lever presses after i.p. injections of vehicle or CNO (3 mg/kg) (C) Mean±SEM number of active lever presses after pretreatment with CNO (3 mg) or vehicle followed by exposure to a priming injection of cocaine (10 mg) or vehicle (D) Mean±SEM number of active lever presses after pretreatment with CNO (3 mg) or vehicle followed by exposure to contingent tone-light cues or no cues under extinction conditions (n=4).
Figure 4.
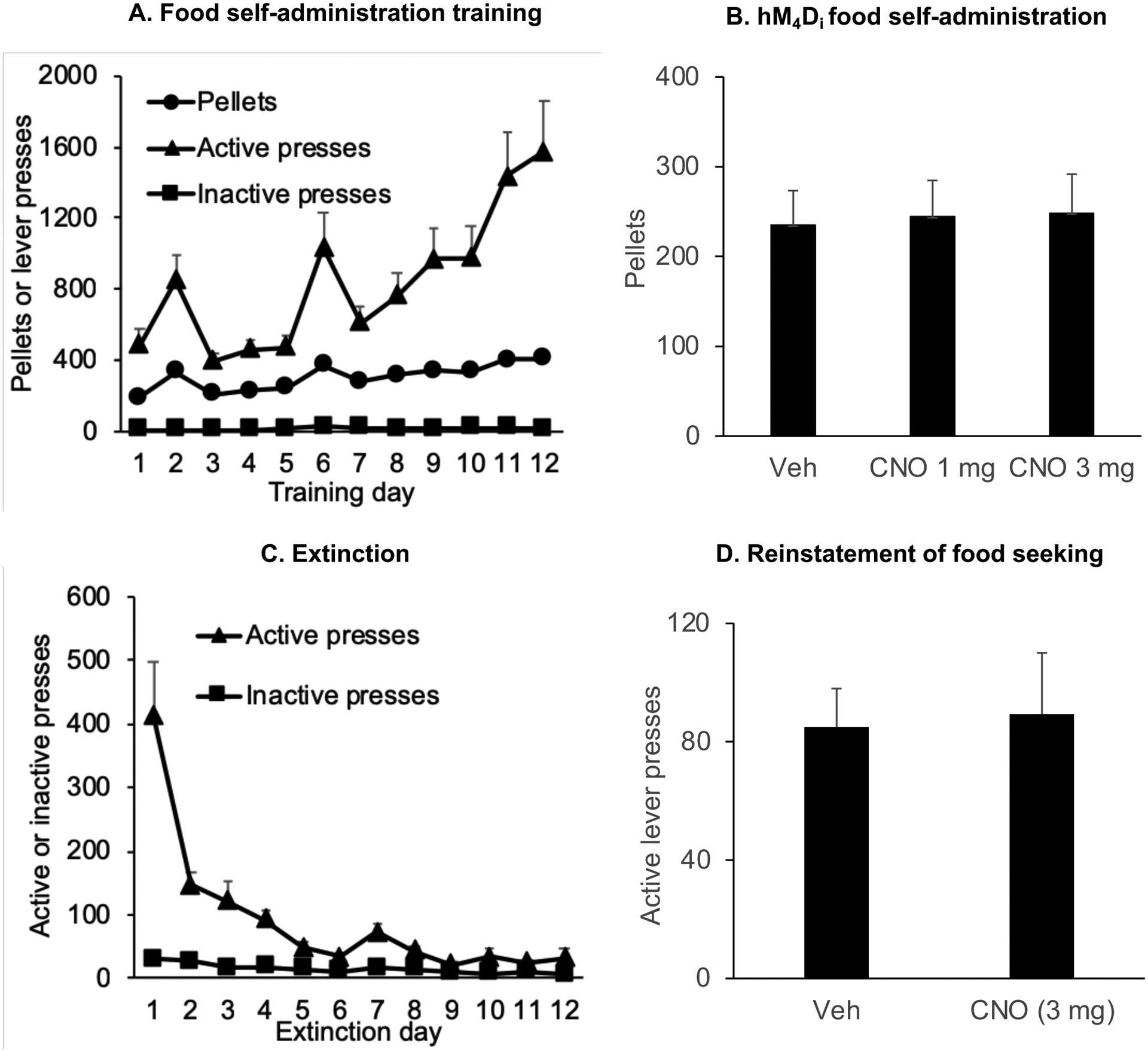
CNO has no effect on operant food self-administration and reinstatement of food seeking in rats with hM4Di transduction of LHb neurons (A) Training: Mean±SEM number of 35% fat pellets earned, active and inactive lever presses during training sessions over 12 days (one 3 h session/d) for rats that were trained under a fixed-ratio-1 (FR-1) 20-sec timeout reinforcement schedule. (B) Mean±SEM number of pellets self-administered after i.p. injections of vehicle or CNO (1 or 3 mg/kg) (n=12) (C) Extinction: Mean±SEM number of presses on the previously active lever or inactive lever during the extinction phase where pellets are not available (D) Mean±SEM number of active lever presses after pretreatment with CNO (3 mg) or vehicle followed by exposure to contingent tone-light cues/no cues under extinction conditions (n=14)
Exp. 1: Effect of hM4Di-mediated inhibition of the LHb on cocaine-reinforced operant responding
Cocaine self-administration.
hM4Di-mediated transient inhibition of the LHb significantly increased the number of cocaine infusions self-administered and active lever presses. Two groups of rats (n=6–7/group) were injected with one dose of CNO (1 or 3 mg/kg) or vehicle 20 min prior to the test session during which the rats lever pressed for the cocaine. The statistical analyses for each measure (infusions and active presses) included the within-subject’s factors of Treatment (vehicle, CNO 1 or 3 mg) and Time (Session minutes). The analysis for the effect of CNO (1 mg) on the number of infusions earned revealed a significant effect of CNO treatment (F(1,5) =10.1, p=0.024), but no significant effect of Time, or Treatment X Time interaction. The analysis for the effect of CNO (1 mg, n=6) on the number of active presses revealed no significant effect of CNO treatment, Time or Treatment X Time interaction (p > 0.05). (Figs. 2B, 2D). The analysis for the effect of CNO (3 mg) on the number of infusions earned revealed a significant effect of CNO treatment (F(1,6) =24.2, p=0.003), Time (F(3,18) =12.9, p<0.001), but no Treatment X Time interaction. The analysis for the effect of CNO (3 mg, n=7) on the number of active presses revealed a significant effect of CNO treatment (F(1,6) =59.6, p<0.001), Time (F(3,18) =8.5, p=0.001), and Treatment X Time interaction (F(1,6) =4.2, p=0.020) (Figs. 2C, 2E). In contrast, in rats with missed LHb injections (n=6, 2 in the dorsal hippocampus, 2 in thalamic nuclei lateral to the LHb, 1 in the cortex) behavioral responses were similar to controls (Mean ± SEM of infusions: Vehicle: 26 ± 5, CNO: 23 ± 11; p > 0.05).
Exp. 2: Effect of hM4Di-mediated inhibition of the LHb on reinstatement of cocaine seeking
Exp. 2A Cocaine priming-induced reinstatement
hM4Di-induced inhibition of LHb neurons significantly decreased cocaine priming-induced reinstatement of active lever responding, an effect that was pronounced in the first thirty minutes of the test session (Figs. 3A, 3C). The ANOVA revealed significant effects of CNO treatment (F(3,27) = 14.8, p<0.001), Time (F(5,45) = 29.7, p<0.001), and CNO treatment X Time interaction (F(15,135) = 17.7, p<0.001) (n=10). In contrast, in rats with missed LHb injections (n=3, 1 in the dorsal hippocampus, 2 in thalamic nuclei lateral to the LHb) behavioral responses were similar to controls (Mean ± SEM of active lever presses: Vehicle: 83 ± 8, CNO: 94 ± 11; p > 0.05) (data not shown).
Figure 3.
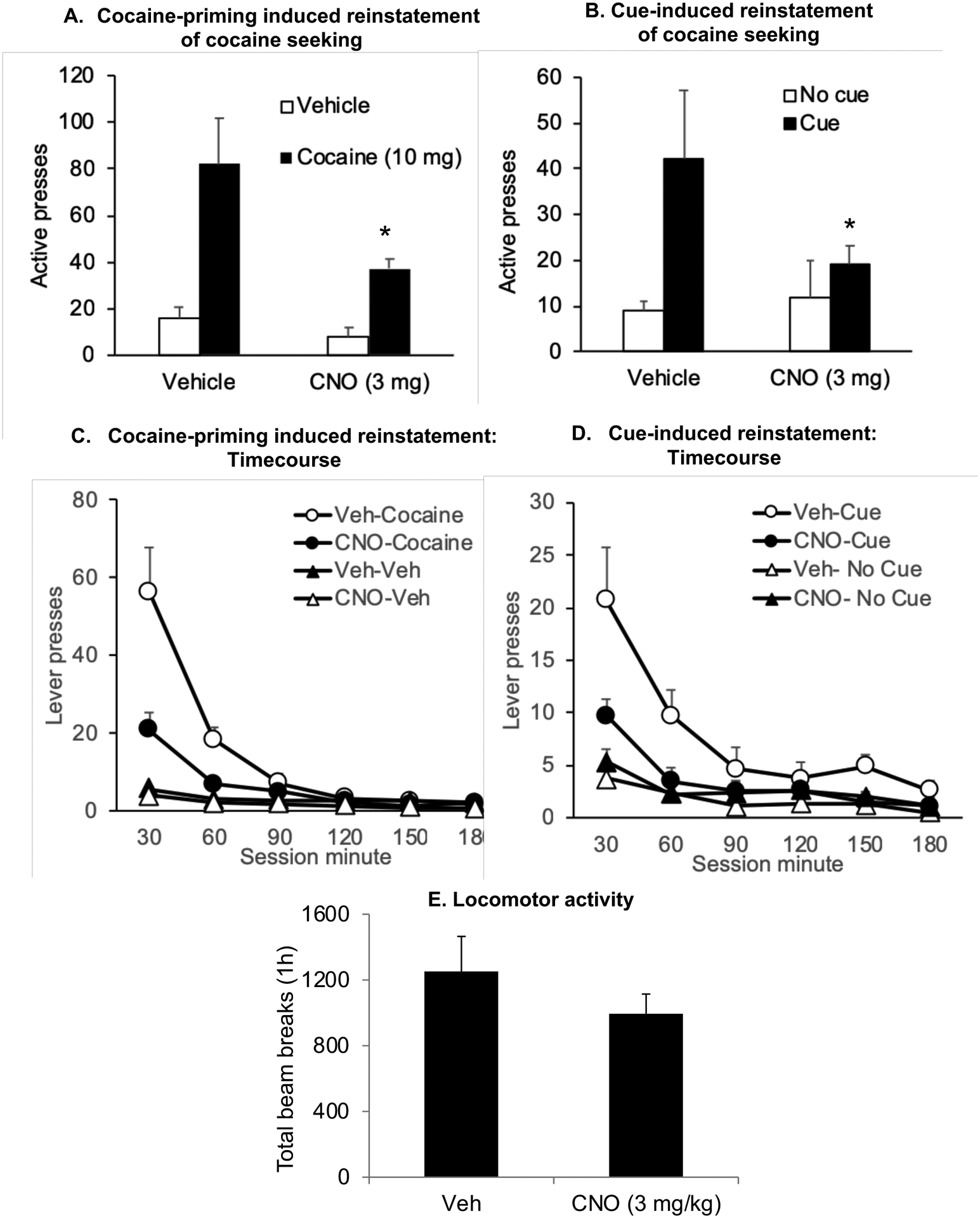
CNO decreases cocaine-priming and cue-induced reinstatement of cocaine seeking in rats with hM4Di transduction of LHb neurons (A, B) Mean±SEM number of active lever presses after pretreatment with CNO (3 mg) or vehicle followed by exposure to a priming injection of cocaine (10 mg)/vehicle (n=10) or contingent tone-light cues/no cues under extinction conditions (n=9) (C, D) Time course of the number of active lever presses during cocaine-priming and cue-induced reinstatement of lever responding represented at 30 minute intervals (E) Mean±SEM of the total number of beam breaks over 1h in rats pretreated with CNO (3 mg) or vehicle and injected with cocaine (10 mg) (n=8) *Different from vehicle condition, p < 0.05.
Exp. 2B Cue-induced reinstatement of cocaine seeking
hM4Di -induced inhibition of LHb neurons significantly decreased cue-induced reinstatement of active lever responding (Figs. 3B, 3D). The ANOVA revealed significant effects of CNO treatment (F(3,24) = 9.2, p<0.001), Time (F(5,40) = 34.3, p<0.001), and CNO treatment X Time interaction (F(15,120) = 6.1, p<0.001) (n=9).
Exp. 2C: Effect of hM4Di-mediated inhibition of the LHb on cocaine-induced locomotor activity
hM4Di-mediated transient inhibition of the LHb had no effect on cocaine (10 mg)-induced locomotor activity (Fig. 3E). The statistical analysis included the effect of Treatment (vehicle, CNO 3mg) as the between-subjects factor (p > 0.05) (n=8).
Exp. 3: Effect of hM4Di-mediated transient inhibition of the LHb on food-reinforced operant responding and reinstatement of food-seeking
Exp. 3A: Food pellet self-administration
hM4Di-mediated transient inhibition of the LHb had no effect on the number of pellets earned (Fig. 4B). The statistical analysis included a within-subjects factor of Treatment (vehicle, CNO) and CNO dose (1 or 3 mg) (p > 0.05) (n=12).
Exp. 3B Cue-induced reinstatement of food seeking
Exposure to contingent tone and light cues significantly increased active lever responding in both vehicle and CNO-treated rats. The statistical analysis (within-subjects factor of CNO treatment) revealed no significant effect of CNO Treatment (p >0.05) (Fig. 4D) (n=14).
Exp. 4 and 5: Effect of CNO on operant cocaine self-administration and reinstatement of cocaine seeking in rats with no viral vector injections or transduction of LHb neurons with AAV-hSyn-eGFP
For operant cocaine self-administration experiments, the statistical analysis included the within-subjects factor of CNO Treatment (Vehicle or CNO). CNO (3mg) had no effect on the number of infusions or active presses in rats that did not receive intracranial viral vector injections (Fig. 5B, n=5) or in rats where LHb neurons were transduced with AAV-hSyn-eGFP (Fig. 6B, n=4) (p > 0.05). For reinstatement experiments, the statistical analysis included within-subjects factors of CNO Treatment and the Reinstating Stimulus: Priming (Saline or Cocaine) or Cue reinstatement (No cue or cue). Exposure to contingent tone and light cues or a priming injection of cocaine significantly increased active lever responding in both vehicle and CNO-treated rats. Neither the effect of CNO treatment, nor the interaction between CNO Treatment and Priming condition was significantly different (p <0.05) in rats that did not receive intracranial viral vector injections (Figs. 5C, 5D), or in rats where LHb neurons were transduced with AAV-hSyn-eGFP (Figs. 6C, 6D). Together, the data depicted in Figures 5 and 6 indicate that CNO did not have non-specific effects on cocaine self-administration or seeking behavior in the absence of hM4Di expression.
Exp. 6: Effect of hM3Dq-mediated transient inhibition of the LHb on cocaine and food-reinforced operant responding
hM3Dq-mediated transient activation of the LHb significantly decreased the number of infusions (Fig. 7A) and the number of pellets earned (Fig. 7B). The statistical analysis included a within-subjects factor of Treatment (vehicle, CNO 1mg, CNO 3mg) (Infusions: (F(2,10) = 34.9, p<0.001) (n=6).; Pellets: (F=73.1, p <0.001) (n=9).
Figure 7.
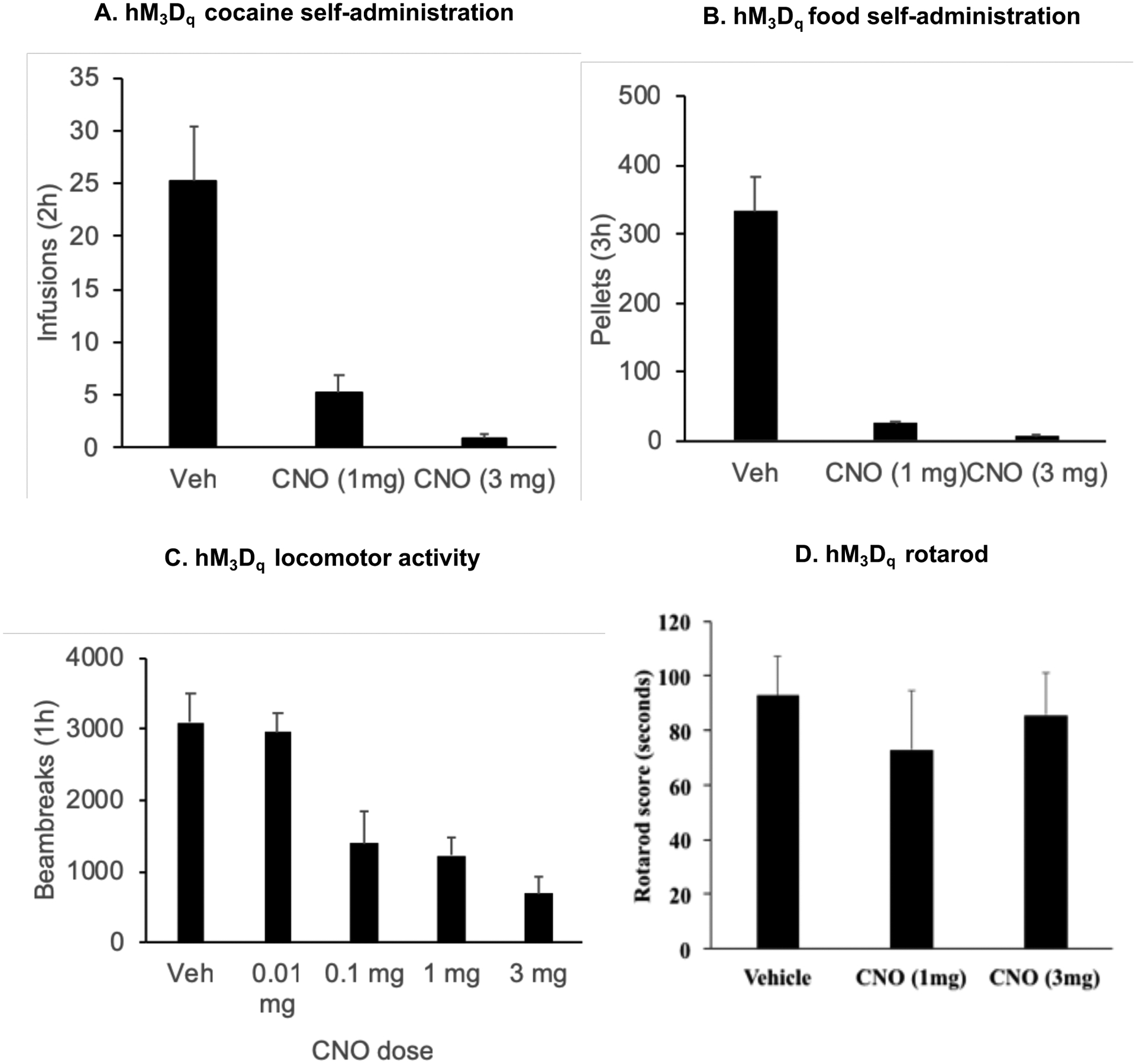
CNO decreases cocaine self-administration, food-self administration and cocaine-induced locomotor activity in rats with hM3Dq transduction of LHb neurons (A) Mean±SEM number of cocaine infusions self-administered after i.p. injections of vehicle or CNO (1 and 3 mg/kg) (n=6) (B) Mean±SEM number of food pellets self-administered after i.p. injections of vehicle or CNO (1 and 3 mg/kg) (n=9) (C) Mean±SEM of the total number of beam breaks over 1h in rats pretreated with CNO (0.01, 0.1, 1 or 3 mg) or vehicle twenty minutes prior to a single injection of cocaine (10 mg) (n=4) (D) Mean±SEM of latency of the rat to fall of the rotarod after i.p. injections of vehicle or CNO (1 and 3 mg/kg) (n=5)
Exp. 7: Effect of hM3Dq-mediated transient activation of the LHb on motor activity
Cocaine-induced locomotor activity
hM3Dq-mediated transient inhibition of the LHb dose-dependently decreased cocaine-induced locomotor activity (Fig. 7C). The statistical analysis included the effect of Treatment (vehicle, CNO 0.01, 0.1, 1, 3 mg) as the within-subjects factor (F(4,12) = 17.0, p<0.001) (n=4).
Rotarod
hM3Dq-mediated transient inhibition of the LHb had no effect on motor performance on a rotarod (Fig. 7D). The statistical analysis included the effect of treatment (vehicle, CNO dose) as the within-subjects factor (p > 0.05) (n=5).
Discussion
Results from our study support two major conclusions. Firstly, chemogenetic inhibition of LHb neurons enhances cocaine, but not food self-administration. Secondly, DREADD-mediated transient inhibition of LHb neurons decreases reinstatement of cocaine-, but not food seeking. Together, these data suggest that LHb neurons are part of the neuronal circuitries that underlie operant cocaine self-administration and reinstatement of cocaine-seeking, but not taking and seeking behaviors reinforced by non-drug reinforcers.
Role of LHb neurons in cocaine- and food-reinforced operant responding
Our study provides evidence for a role of LHb neurons in operant responding reinforced by cocaine. Our data are in agreement with previous findings implicating the LHb in drug intake and self-administration. For instance, six to ten days of intravenous cocaine self-administration increases the density of c-fos positive neurons in the LHb (Gao, Groenewegen, Vanderschuren et al., 2018; Zahm, Becker, Freiman et al., 2010). Electrolytic lesions of the LHb have been reported to increase the rate of escalation of voluntary ethanol consumption in a two-bottle choice paradigm (Haack, Sheth, Schwager et al., 2014). In addition, electrolytic lesions of the LHb also increase operant ethanol self-administration (Haack, Sheth, Schwager et al., 2014). Consistent with these results, CNO-mediated transient inhibition of LHb neuronal activity following hM4Di transduction increased cocaine intake under our experimental conditions. We have recently demonstrated that the 3 mg dose of CNO decreases cocaine (10 mg) -induced c-fos expression in LHb neurons transduced with hM4Di (Coffey, 2019). An increase in lever responding for maintenance doses of psychostimulants after an experimental manipulation can be interpreted as being due to a decrease in the rewarding effects of the self-administered drug (De Wit & Wise, 1977; Yokel & Wise, 1976). Our data suggest that transient inhibition of LHb neurons during ongoing cocaine self-administration makes the unit dose of cocaine less rewarding, which results in increased lever responding to maintain levels of cocaine at satiety. The data also indicate that this effect is pronounced in the drug loading period in the first thirty minutes of the test session. Further studies using more sophisticated behavioral techniques such as the threshold procedure (Oleson & Roberts, 2012) are warranted to fully understand the role of LHb neurons in appetitive and consummatory behaviors reinforced by cocaine. In contrast to the effect of hM4Di, transient activation of hM3Dq-transduced LHb neurons very significantly decreased cocaine intake. This effect however, is most likely due to significant locomotor deficits induced by the activation of Gq-coupled signaling in LHb neurons, despite a lack of effect on motor co-ordination observed on the rotarod.
In contrast to cocaine, it has been reported that there is a lack of effect of electrolytic lesions of the LHb on operant heroin self-administration on both fixed and progressive ratio reinforcement schedules (Wang, Zhang, Tang et al., 2009). This difference is perhaps due to the difference in motivational states produced by cocaine versus heroin (Badiani, Belin, Epstein et al., 2011). The motivational state induced by intravenous heroin self-administration is believed to be entirely appetitive, in contrast to intravenous cocaine that produces a motivational state that is both appetitive and aversive (Ettenberg & Geist, 1993; Ettenberg, Raven, Danluck et al., 1999). Since LHb neurons are known to be activated by aversive stimuli (Matsumoto & Hikosaka, 2007), it is conceivable that LHb neurons are not involved in operant behavior reinforced by heroin. Another potential interpretation of our data is that the effect of hM4Di modulation of LHb activity on cocaine self-administration may be non-specific, secondary to motor activation. However, the dose of CNO (3mg/kg) that significantly increased operant cocaine self-administration had no effect on cocaine-stimulated locomotor activity, food-reinforced operant responding, or reinstatement of food seeking in animals with hM4Di expression in LHb neurons.
In the present study, CNO was used as the exogenous ligand to activate DREADD’s. Recent work suggests that CNO may induce nonspecific behavioral effects through conversion of CNO to clozapine (Gomez, Bonaventura, Lesniak et al., 2017; MacLaren, Browne, Shaw et al., 2016). To assess the specificity of our experimental findings we examined the effect of CNO on both cocaine self-administration and reinstatement of cocaine seeking in a) rats that had no viral vector injections and b) rats that expressed eGFP in LHb neurons under the control of the same promoter (human synapsin) and the same virus serotype (AAV8) as rats injected with hM4Di. Our results indicate that CNO injections had no effect on either cocaine self-administration or reinstatement of cocaine seeking. In addition to these direct results, there are four observations which suggest that 1 and 3mg/kg injections of CNO do not induce non-specific behavioral effects under our experimental conditions. Firstly, these doses of CNO had no effect on food self-administration or reinstatement of food seeking in rats expressing hM4Di in LHb neurons. Secondly, in rats with hM4Di expression in the hippocampus or thalamic nuclei (missed injections), we found no effect of CNO on operant behavior. Thirdly, CNO-induced activation of LHb hM4Di had no effect on cocaine-induced locomotor activity. Finally, the back conversion of CNO and subsequent accumulation of clozapine occurs gradually whereas we observed the largest effects of CNO within the first 30–60 minutes of testing.
In contrast to the effect of hM4Di in the LHb on cocaine self-administration, this manipulation had no effect on operant responding reinforced by food pellets as stated above. There are two caveats that must be taken into consideration when directly comparing our result on food self-administration versus cocaine. Firstly, rats in the cocaine study were fed ad libitum, whereas rats in the food study were on a restricted diet. While, to our knowledge, there are no published parametric studies on the impact of food restriction on operant responding to food versus cocaine, it is well known that food restriction produces alterations in basal mRNA levels of a variety of feeding peptides that may subsequently impact operant behavior. Secondly, it is conceivable that the lack of effect of inhibiting the LHb on food-reinforced operant responding may be due to a ceiling effect. While rats in both the cocaine- and food- self-administration studies were trained on a fixed ratio 1 reinforcement schedule, the response rates for pellet intake are significantly higher. Despite these caveats, the lack of effect of inhibiting the LHb on food self-administration is not entirely surprising, since ingestion of small amounts of palatable food is not associated with an aversive motivational response (Ettenberg, 2004). In support of our result are two observations. One, there is minimal increase in c-fos labelled neurons in the LHb following sucrose self-administration in contrast to cocaine self-administration (Gao, Groenewegen, Vanderschuren et al., 2018). Second, electrolytic lesions of the LHb have no effect on operant responding reinforced by a 2% sucrose solution (Haack, Sheth, Schwager et al., 2014). Our findings and those of Haack et al. (2014) are somewhat in contrast to those of Friedman et al. (2011) who found that deep brain stimulation with alternating low and high frequency stimulation decreased sucrose-reinforced operant responding, while high frequency stimulation had no effect and low frequency stimulation increased sucrose self-administration.
Role of LHb neurons in reinstatement of cocaine- and food-seeking behaviors
Our current finding supports a small, but growing body of literature which suggests that in addition to ongoing operant behavior reinforced by cocaine, neurons in the LHb also mediate reinstatement of drug-seeking behaviors. Studies using c-fos as a marker for neuronal activation demonstrate an increase in density of the immediate early gene in the LHb followed cocaine-priming induced reinstatement of cocaine conditioned place preference (Brown, Short & Lawrence, 2010), discriminative stimulus- (James, Charnley, Flynn et al., 2011), as well as cue-induced induced reinstatement of cocaine-seeking behaviors (Zhang, Zhou, Liu et al., 2005). Since LHb neurons are activated in response to conditioned cues as well as a cocaine prime, it is not surprising that in the current study CNO-induced transient inhibition of LHb neurons decreased cocaine-priming, as well as cue-induced reinstatement of cocaine- seeking behavior. These results are generally in agreement with pharmacological and electrochemical studies examining the role of the LHb in the reinstatement of drug-seeking behaviors. Reversibly inactivating the LHb with GABA receptor agonists (baclofen/muscimol) attenuates yohimbine+cue-induced reinstatement of cocaine seeking (Gill, Ghee, Harper et al., 2013). Further, electrolytic lesions of the LHb decrease yohimbine-induced reinstatement of ethanol-seeking behavior (Haack, Sheth, Schwager et al., 2014). Meye et al. (2016) elegantly demonstrated that disinhibiting neuronal activity in LHb neurons driven by the entopeduncular nucleus decreases forced swim stress-induced reinstatement of cocaine conditioned place preference. Our current results taken together with the above-mentioned studies demonstrate a role for LHb neurons in the reinstatement of drug-seeking behaviors, irrespective of the reinstating stimulus. Further, this effect seems to be specific to drugs of abuse since CNO had no effect on the reinstatement of food-seeking behavior under our experimental conditions.
Concluding remarks
Cocaine addiction is a serious, life-threatening disease, treatment options for which are severely limited likely due to our limited understanding of the neuronal circuitry that underlies this disease. Here, we demonstrate a specific role for the LHb in both ongoing short-access cocaine intake (which models recreational cocaine use), as well as reinstatement of cocaine seeking (which models relapse behavior). Our results demonstrate that the LHb is part of neuronal circuitries that underlie both casual drug use as well as addiction. Since we used non-specific viral vectors in our present study, further studies are warranted to determine the role of specific populations of LHb neurons in modulating these behaviors. In addition, the LHb is broadly divided into medial and lateral subdivisions (Andres, von During & Veh, 1999; Geisler & Trimble, 2008), which have vastly different expression profiles of neurotransmitter receptors, neuropeptides etc. For instance, the dopamine transporter is primarily expressed in the medial division of the LHb complex along the rostrocaudal length of the brain region. Dissecting the role of the medial and lateral sub-divisions of the LHb in cocaine taking- and seeking behaviors will be a subject for future research.
Supplementary Material
Acknowledgements
We thank Scott Ng-Evans for maintenance of operant behavioral equipment. This work was supported by University of Washington Alcohol and Drug Abuse Institute Small Grant (SN), Brain and Behavior Research Foundation (NARSAD Young Investigator award) (SN) and NIH R21 DA 034192 (SN).
References
- Andres KH, von During M, Veh RW (1999) Subnuclear organization of the rat habenular complexes. The Journal of comparative neurology 407:130–150. [DOI] [PubMed] [Google Scholar]
- Araki M, McGeer PL, Kimura H (1988) The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain research 441:319–330. [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL (2007) Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A 104:5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y (2011) Opiate versus psychostimulant addiction: the differences do matter. Nature reviews Neuroscience 12:685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Mizumori SJY (2017) Control of behavioral flexibility by the lateral habenula. Pharmacol Biochem Behav 162:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR (2011) The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. The Journal of comparative neurology 519:1143–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinschwitz K, Dittgen A, Madai VI, Lommel R, Geisler S, Veh RW (2010) Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience 168:463–476. [DOI] [PubMed] [Google Scholar]
- Brown RM, Short JL, Lawrence AJ (2010) Identification of brain nuclei implicated in cocaine-primed reinstatement of conditioned place preference: a behaviour dissociable from sensitization. PloS one 5:e15889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS (1986) Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci 6:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey KR, Marx R, Vo E, Nair SG and Neumaier JF(2019) Chemogenetic inhibition of lateral habenula projections to the dorsal raphe nucleus reduces passive coping and persevarative reward seeking in rats. European Journal of Neuroscience In Revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, O’Brien CP (2001) Cocaine dependence: a disease of the brain’s reward centers. J Subst Abuse Treat 21:111–117. [DOI] [PubMed] [Google Scholar]
- De Wit H, Wise RA (1977) Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Canadian journal of psychology 31:195–203. [DOI] [PubMed] [Google Scholar]
- Ettenberg A (2004) Opponent process properties of self-administered cocaine. Neuroscience and biobehavioral reviews 27:721–728. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD (1993) Qualitative and quantitative differences in the operant runway behavior of rats working for cocaine and heroin reinforcement. Pharmacology, biochemistry, and behavior 44:191–198. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD (1999) Evidence for opponent-process actions of intravenous cocaine. Pharmacology, biochemistry, and behavior 64:507–512. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Mitchell ES, Neumaier JF (2008) Increased expression of 5-HT6 receptors in the nucleus accumbens blocks the rewarding but not psychomotor activating properties of cocaine. Biol Psychiatry 63:207–213. [DOI] [PubMed] [Google Scholar]
- Filip M, Alenina N, Bader M, Przegalinski E (2010) Behavioral evidence for the significance of serotoninergic (5-HT) receptors in cocaine addiction. Addict Biol 15:227–249. [DOI] [PubMed] [Google Scholar]
- Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E, Ben-Tzion M, Yadid G (2011) Electrical stimulation of the lateral habenula produces an inhibitory effect on sucrose self-administration. Neuropharmacology 60:381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Groenewegen HJ, Vanderschuren L, Voorn P (2018) Heterogeneous neuronal activity in the lateral habenula after short- and long-term cocaine self-administration in rats. Eur J Neurosci 47:83–94. [DOI] [PubMed] [Google Scholar]
- Geisler S, Trimble M (2008) The lateral habenula: no longer neglected. CNS Spectr 13:484–489. [DOI] [PubMed] [Google Scholar]
- Gill MJ, Ghee SM, Harper SM, See RE (2013) Inactivation of the lateral habenula reduces anxiogenic behavior and cocaine seeking under conditions of heightened stress. Pharmacology, biochemistry, and behavior 111:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M (2017) Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science (New York, NY) 357:503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Green L, Phillips G, Bradley B (1989) Lapse, relapse and survival among opiate addicts after treatment. A prospective follow-up study. Br J Psychiatry 154:348–353. [DOI] [PubMed] [Google Scholar]
- Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, Taha SA (2014) Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PloS one 9:e92701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ (1979) Efferent connections of the habenular nuclei in the rat. The Journal of comparative neurology 187:19–47. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sesack SR, Lecourtier L, Shepard PD (2008) Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci 28:11825–11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV (2011) Propensity to ‘relapse’ following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience 199:235–242. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC (2009) The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 61:786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD (2007) Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci 27:6923–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Defrancesco A, Moghaddam B (2008) Differential tonic influence of lateral habenula on prefrontal cortex and nucleus accumbens dopamine release. Eur J Neurosci 27:1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, Clark SD (2016) Clozapine N-Oxide Administration Produces Behavioral Effects in Long-Evans Rats: Implications for Designing DREADD Experiments. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O (2007) Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447:1111–1115. [DOI] [PubMed] [Google Scholar]
- Meye FJ, Soiza-Reilly M, Smit T, Diana MA, Schwarz MK, Mameli M (2016) Shifted pallidal co-release of GABA and glutamate in habenula drives cocaine withdrawal and relapse. Nature neuroscience 19:1019–1024. [DOI] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y (2009) The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Progress in neurobiology 89:18–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon WA Jr., (2002) Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J Neurosci 22:10856–10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Fage D, Scatton B (1986) Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain research 373:324–336. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Roberts DC (2012) Cocaine self-administration in rats: threshold procedures. Methods in molecular biology (Clifton, NJ) 829:303–319. [DOI] [PubMed] [Google Scholar]
- Park MR (1987) Monosynaptic inhibitory postsynaptic potentials from lateral habenula recorded in dorsal raphe neurons. Brain Res Bull 19:581–586. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42. [DOI] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK (1977) Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science (New York, NY) 197:89–91. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang F, Tang S, Lai M, Hao W, Zhang Y, Yang J, Zhou W (2009) Lack of effect of habenula lesion on heroin self-administration in rats. Neuroscience letters 461:167–171. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA (1976) Attenuation of intravenous amphetamine reinforcement by central dopamine blockade in rats. Psychopharmacology 48:311–318. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Becker ML, Freiman AJ, Strauch S, Degarmo B, Geisler S, Meredith GE, Marinelli M (2010) Fos after single and repeated self-administration of cocaine and saline in the rat: emphasis on the Basal forebrain and recalibration of expression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 35:445–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhou W, Liu H, Zhu H, Tang S, Lai M, Yang G (2005) Increased c-Fos expression in the medial part of the lateral habenula during cue-evoked heroin-seeking in rats. Neuroscience letters 386:133–137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


