Abstract
Objective:
To identify changes in the incidence and mortality of cutaneous melanoma in the fastest-growing segment of the US population, middle-aged adults.
Patients and Methods:
Using the Rochester Epidemiology Project, patients aged 40 to 60 years with a first lifetime diagnosis of cutaneous melanoma between January 1, 1970 and December 31, 2020, while a resident of Olmsted County, Minnesota were identified.
Results:
A total of 858 patients with a primary cutaneous first-time melanoma were identified. The overall age- and sex-adjusted incidence rate increased from 8.6 (95% CI 3.9–13.3) per 100,000 person-years in 1970 – 1979 to 99.1 (95% CI 89.5–108.7) per 100,000 person-years in 2011–2020 (11.6-fold increase). There was a 52.1-fold increase in females and a 6.3-fold increase in males between these two periods. In recent years (2005–2009 vs. 2015–2020), the incidence has stabilized in males (1.01-fold increase, p=0.96) and continues to significantly increase in females (1.5-fold increase, p=0.002). Among 659 patients with invasive melanoma, 43 deaths occurred due to melanoma, and male sex was significantly associated with an increased risk of death (HR 2.95, 95% CI 1.45–6.00). A more recent diagnosis of melanoma was significantly associated with a decreased risk of death due to melanoma (HR 0.66 per 5-year increase in calendar year of diagnosis, 95% CI 0.59–0.75).
Conclusion:
Melanoma incidence has significantly increased since 1970. Over the last 15 years, the incidence continues to rise in middle-aged females (approximately 50% rise in incidence) but has stabilized in males. Mortality decreased in a linear fashion throughout this time.
Keywords: Melanoma, incidence, mortality, epidemiology, middle-aged
INTRODUCTION
Cutaneous malignant melanoma is the third most common cancer diagnosed in the United States and is a significant cause of morbidity and mortality.1 The incidence in most populations have increased over the last several decades. Our team’s previous publication found a 7.6-fold increase in incidence between 1970 to 2009 (7.9 per 100,000 person-years in 1970–1979 to 60.0 per 100,000 person-years in 2000–2009). This same study found a 24-fold increase in incidence in females and a 5.4-fold increase in males.2 The worldwide increasing incidence of melanoma has also been documented by several other studies. 3,4 One study has shown a stabilization of incidence in certain populations and a down trending incidence in the younger population (25–44 years).5
Recent publications have commented on the sharp rise in diagnosis and relative stable mortality, with concern for overdiagnosis of clinically insignificant melanomas (Welch et al. New England Journal of Medicine. 2021).6 When incidence increases dramatically and mortality is stable, many consider the stable mortality a marker for true stable cancer occurrence. The so called “epidemic of diagnosis” refers to an increase in diagnosis of clinically insignificant melanomas. Some question the value of population-wide melanoma screening, given these concerns. Others feel that increased incidence represents a true rise in clinically significant melanomas, from other external factors outside of overdiagnosis. Increased tanning bed use in previous decades is postulated as a potential risk factor for rising melanoma incidence.7,8,9
The literature has been mixed regarding melanoma mortality trends. One study demonstrated a statistically insignificant steady rise in overall melanoma-specific mortality over time (over age 65 increased, under age 65 decreased over time).3 This same study demonstrated males over 65 years having the fastest rise in mortality. This data may support the notion that diagnostic practices have not been effective at reducing mortality and the epidemic of melanoma is truly an “epidemic of diagnosis”; however, our previous publication demonstrated a reduction in mortality over time (each 1-year increase in calendar year of diagnosis was associated with a statistically significantly reduced melanoma-specific risk of death (adjusted hazard ratio, 0.93; 95% CI 0.90–0.96).2
Changes in mortality over time are influenced by numerous external factors, including alterations in risk factors (sun exposure, tanning bed use), rate of diagnosis, stage at diagnosis, advanced pharmacotherapies, and refined staging practices. These factors must be considered together when drawing conclusions about mortality trends. A reduction in mortality, in the context of an increasing incidence, may in part represent successful screening practices and subsequent management. Others argue that increasing incidence without increasing mortality indicates overdiagnosis6; however, earlier diagnosis of clinically significant melanoma would presumably decrease mortality in the population while increasing incidence.
High quality data on incidence and mortality is required to properly examine these trends. This study investigates incidence and mortality of cutaneous melanoma over time in the fastest-growing segment of the US population, middle-aged adults.
PATIENTS AND METHODS
Patient Selection
After institutional review board approval, Olmsted County, Minnesota residents aged 40 to 60 years old at the time of their first lifetime diagnosis of cutaneous malignant melanoma (excluding ocular and mucosal-type melanomas) between January 1, 1970 and December 31, 2020 were identified through the resources of the Rochester Epidemiology Project (REP). All charts were manually reviewed to extract data. A previous study from our group had collected and reported incidence cases for this age group until 2009.2 The REP is a medical record linkage system that was created in 1966 and collects data regarding medical care of all residents of Olmsted County who seek care from providers affiliated with Mayo Clinic, Olmsted Medical Center, and local private practitioners. All components of medical care are available through this database; therefore, this is an excellent tool to collect epidemiology data and determine incidence.10 Patients who had denied research authorization at the time of the data abstraction were not included (approximately 95% of residents have given research authorization). The population of Olmsted County in 2020 is 162,847 according to the United States Census Bureau. This population is predominantly non-Hispanic Caucasian individuals with a comparable socioeconomic status to the general population but a higher percentage with college degrees.11 Histologic slide review of pathology was not performed, as a previous REP study demonstrated no discrepancies between the original pathology report and slide re-review.12
Statistical Analyses
Age- and sex-specific incidence rates (per 100,000 person-years) in Olmsted County were calculated as the ratio of numerator to denominator, expressed per 100,000 person-years. The numerator was the number of persons aged 40–60 with an incident melanoma diagnosis and the denominator was obtained from the REP census of persons aged 40–60 years in Olmsted County.13 Rates were age- and sex-adjusted to the total population of the United States in 2010. The 95% confidence intervals (CI) for incidence rates were calculated assuming a Poisson error distribution. Specific comparisons of the incidence rates by either sex, age group, or select calendar periods of interest were assessed by fitting Poisson regression models. The observations used in these models were either the sex, age group-, or calendar period-specific incidence counts, and corresponding person-years derived above. The natural logarithm of the corresponding total person-years was used as an offset in the Poisson models.
Cause-specific (i.e., death due to melanoma) survival rates were estimated using the Kaplan-Meier method. The duration of follow-up was calculated from the date of melanoma diagnosis to the date of death or last follow-up. Univariate associations with the risk of death due to melanoma were evaluated using Cox proportional hazards regression models and summarized with hazard ratios and 95% CIs. For each factor that was evaluated, the proportionality assumption was tested visually and by including a time-dependent coefficient in a Cox proportional hazards model; the assumption was satisfied for each factor. Calendar year of diagnosis, age, and Breslow depth were initially evaluated in univariate Cox models using smoothing splines to assess the functional form of the relationship with the risk of death due to melanoma.
All calculated p-values were two-sided, and p-values less than 0.05 were considered statistically significant. Data were analyzed using SAS version 9.4 statistical software and RStudio version 2021.09.2.
RESULTS
Patient and tumor characteristics
A total 858 patients aged 40–60 years at time of first lifetime diagnosis of cutaneous melanoma were identified between 1970 and 2009 using the REP database. Table 1 summarizes the patient and tumor characteristics. The trunk was the most commonly affected body part for males and females.
Table 1.
Summary of demographic and clinicopathologic characteristics of 858 patients
| Characteristic | Value |
|---|---|
|
| |
| Age at diagnosis (years), Mean (SD) | 51.2 (6.0) |
| Male sex | 428 (49.9%) |
| Site by Sex | |
| Female | |
| Head/neck | 35 (8.1%) |
| Trunk | 144 (33.5%) |
| Upper limb | 130 (30.2%) |
| Lower limb | 121 (28.1%) |
| Male | |
| Head/neck | 89 (20.8%) |
| Trunk | 200 (46.7%) |
| Upper limb | 105 (24.5%) |
| Lower limb | 31 (7.2%) |
| Not documented | 3 (0.7%) |
| Location | |
| Right | 353 (41.1%) |
| Left | 403 (47.0%) |
| Central | 80 (9.3%) |
| Not documented | 22 (2.6%) |
| Preexisting nevus | |
| Absent | 253 (29.5%) |
| Compound nevus | 27 (3.1%) |
| Dermal nevus | 34 (4.0%) |
| Nevus not otherwise specified | 84 (9.8%) |
| Not documented | 460 (53.6%) |
| Clark level | |
| I | 196 (22.8%) |
| II | 339 (39.5%) |
| III | 180 (21.0%) |
| IV | 120 (14.0%) |
| V | 10 (1.2%) |
| Not documented | 13 (1.5%) |
| Base transected | 804 (93.7%) |
| Positivity of margin at initial incision | |
| Yes | 298 (34.7%) |
| < 1mm | 156 (18.2%) |
| No | 348 (40.6%) |
| Not documented | 56 (6.5%) |
| Invasive or in situ | |
| Invasive | 659 (76.8%) |
| In situ | 195 (22.7%) |
| Not documented | 4 (0.5%) |
| Histogenic type of in situ melanoma | |
| (N=195) | |
| Lentigo Maligna in situ | 56 (28.7%) |
| Superficial spreading in situ | 71 (36.4%) |
| Malignant melanoma in situ | 60 (30.8%) |
| Acral lentiginous | 1 (0.5%) |
| Not documented | 7 (3.6%) |
| Among invasive melanoma only (N=659) | |
| Histogenic type | |
| Lentigo Maligna | 43 (6.5%) |
| Superficial spreading | 492 (74.7%) |
| Nodular | 38 (5.8%) |
| Desmoplastic | 3 (0.5%) |
| Acral lentiginous | 9 (1.4%) |
| Mixed | 2 (0.3%) |
| Other | 7 (1.1%) |
| Not documented | 65 (9.9%) |
| Breslow depth or thickness (mm) | |
| Median (interquartile range) | 0.5 (0.3, 0.8) |
| <1.00 | 532 (80.7%) |
| 1.01–2.00 | 68 (10.3%) |
| 2.01–4.00 | 31 (4.7%) |
| >4.00 | 14 (2.1%) |
| Not documented | 14 (2.1%) |
| Pathologic stage (AJCC 8th edition) | |
| IA | 502 (76.2%) |
| IB | 84 (12.7%) |
| IIA | 17 (2.6%) |
| IIB | 15 (2.3%) |
| IIC | 3 (0.5%) |
| III | 22 (3.3%) |
| IV | 9 (1.4%) |
| Not documented | 7 (1.1%) |
AJCC: American Joint Committee on Cancer
Incidence
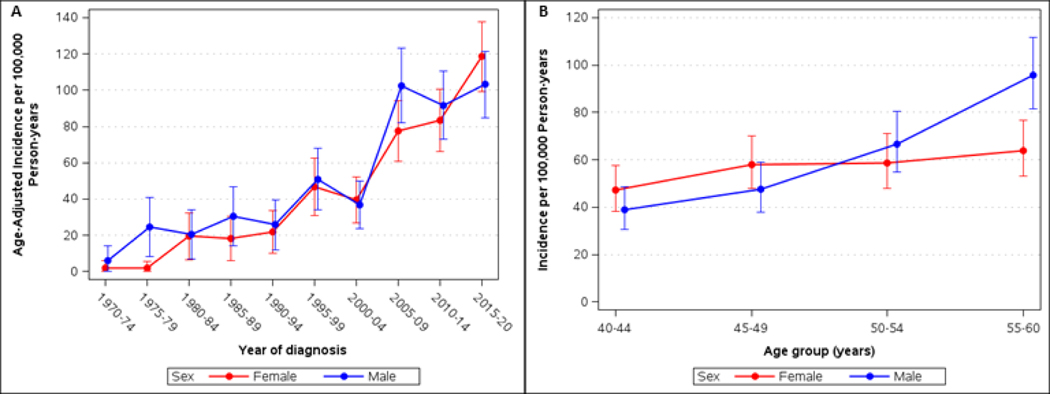
The overall age- and sex-adjusted melanoma incidence rate was 60.4 per 100,000 person-years (95% CI 56.3–64.5), and the age-adjusted incidence was not significantly different between sexes (males 63.6 [95% CI 57.6–69.7] versus females 57.4 [95% CI 51.9–62.8], P=0.13). The age- and sex-adjusted incidence rate increased from 8.6 (95% CI 3.9–13.3) per 100,000 person-years in 1970 – 1979 to 99.1 (95% CI 89.5–108.7) per 100,000 person-years in the period of 2011–2020 (11.6-fold increase).
By gender, there was a 52.1-fold increase in females and a 6.3-fold increase in males between 1970–1979 to 2011–2020. In recent years (2005–2009 vs. 2015–2020), the incidence has stabilized in males (102.6 vs. 103.2, 1.01-fold increase, P=0.96) and continues to significantly increase in females (77.5 vs. 118.6, 1.5-fold increase, P= 0.002). Age-adjusted incidence rates by sex and calendar period at diagnosis are presented in Figure 1A and Supplemental Table.
Figure 1.
(A) Age-adjusted incidence of cutaneous melanoma per 100,000 person-years in Olmsted County, Minnesota, 1970–2020, for persons 40–60 years of age, by gender and calendar period. (B) Age- and sex-specific incidence of melanoma per 100,000 person-years. Bars represent 95% Confidence intervals.
For both sexes, the incidence significantly increased across the four age groups (P<0.001 for males and P=0.035 for females; Figure 1B). In particular, when comparing the 40–44 and 55–60 age groups, there was a 2.5-fold increase from 38.8 (95% CI 30.5–48.8) to 95.7 (95% CI 81.5–111.6) for males and a 1.4-fold increase from 47.3 (95% CI 38.3–57.7) to 63.9 (95% CI 53.0–76.5) for females, (P<0.001 for the test for sex by age group interaction).
Survival
Among 659 patients with invasive melanoma, 96 deaths have been documented including 42 due to melanoma. The median time to death from melanoma was 2.5 (IQR, 1.4–6.3) years, with 38 of the 42 dying within the first 10 years of diagnosis. The median follow-up of all patients alive at last follow-up was 9.9 (IQR, 4.8–14.3) years. Cause-specific survival rates for death due to melanoma at 5 and 10 years after diagnosis of melanoma were 95.6% (95% CI, 94.0–97.3-%) and 93.0% (95% CI, 90.8–98.2%), respectively.
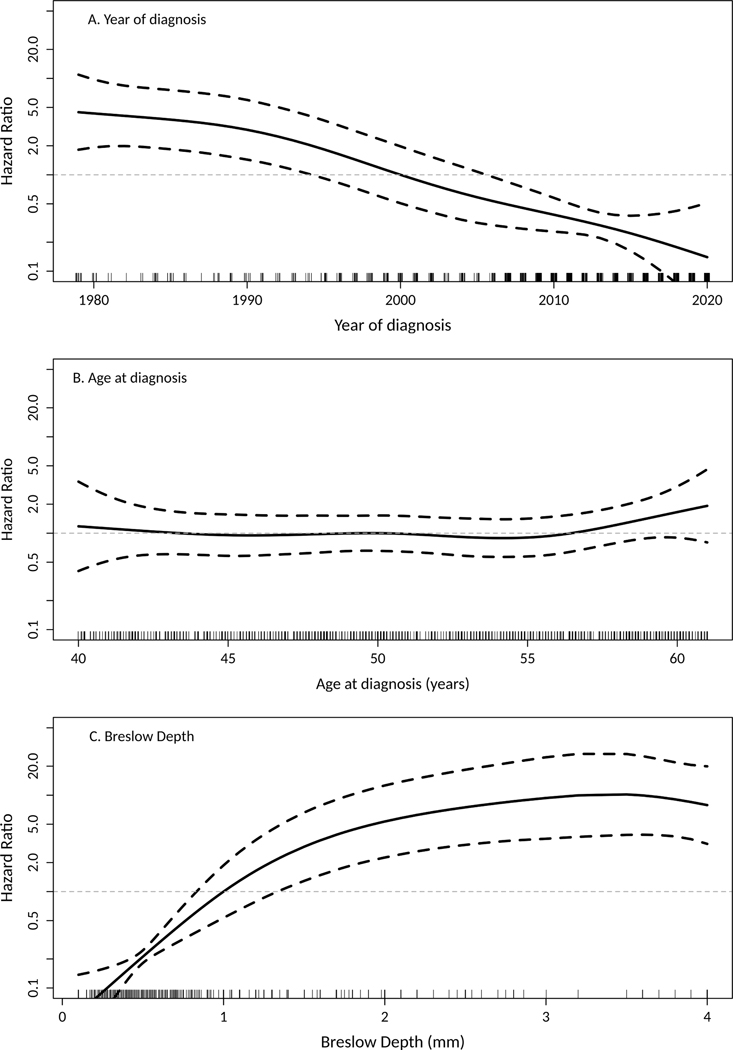
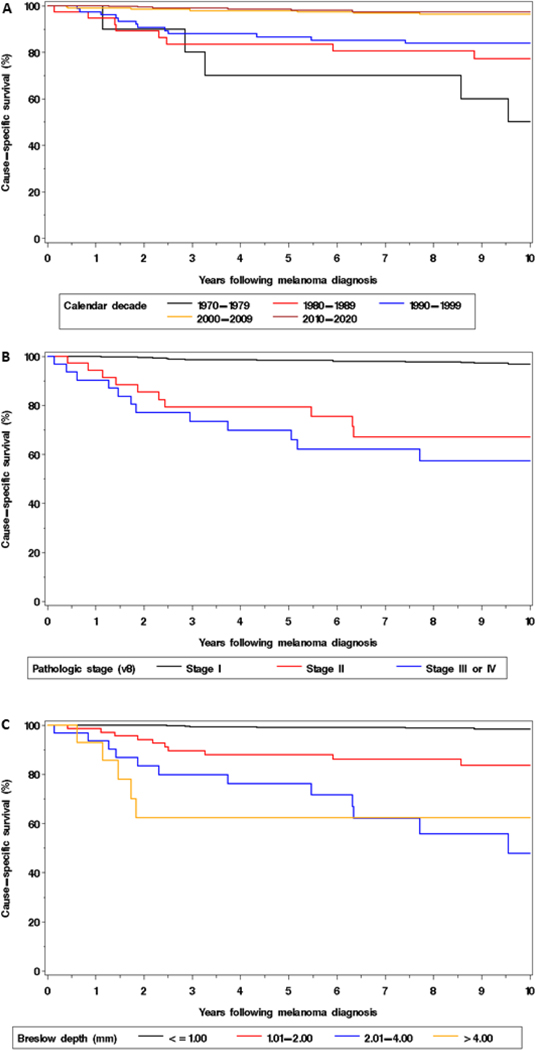
The risk of death due to melanoma decreased in a linear fashion with later calendar year of diagnosis (Figure 2A). A more recent diagnosis was significantly associated with a decreased risk of death due to melanoma (HR 0.66 per 5-year increase in calendar year of diagnosis, 95% CI 0.59–0.75). Furthermore, the risk of death due to melanoma was significantly lower for those diagnosed in the 2000–09 (HR 0.23, 95% CI 0.10–0.56) and 2010–2020 ((HR 0.14, 95% CI 0.05–0.39) compared to those diagnosed in 1990–1999 (Figure 3A).
Figure 2.
Hazard ratio (HR) for the risk of death due to melanoma according to (A) calendar year of diagnosis, (B) age at diagnosis, and (C) Breslow depth, with each analysis centered such that the HR is 1.0 at calendar year of 2000, age of 50 years, and Breslow depth of 1 mm, respectively. To address the wide 95% confidence bands (dashed lines) in the distributions’ tails, calendar years during 1970–1979 were combined as 1979 and Breslow depths >4 were combined as 4 for this illustration.
Figure 3.
Cause-specific survival according to (A) calendar decade of diagnosis, (B) pathologic stage, and (C) Breslow depth, respectively, among patients with invasive melanoma.
There was not a significant association between age and the risk of death due to melanoma (HR 1.09 per 5-year increase in age, 95% CI 0.85–1.40, Figure 2B). The relationship between Breslow depth and the risk of death due to melanoma was less linear and therefore this variable was subsequently analyzed using the standard categories (Figure 2C). Based on univariant analysis (Table 2), male sex (HR 2.95, 95% CI 1.45–6.00), pathologic stage II-IV (HR 14.96, 95% CI 8.06–27.78; Figure 3B), and Breslow depth >1 mm ((HR 19.08, 95% CI 8.72–41.76; Figure 3C) were each significantly associated with an increased risk of death among patients with invasive melanoma. The associations for pathologic stage and Breslow depth each retained statistical significance after adjusting for calendar year and sex (upon fitting two separate Cox models, results not shown).
Table 2.
Summary of factors evaluated univariately for an association with death due to melanoma among patients with invasive melanoma.
| Factor | No. of patients who died due to melanoma | Hazard ratio (95% CI) | P-value |
|---|---|---|---|
| Year of diagnosis † | 42 | 0.66 (0.59, 0.75)‡ | <0.001 |
| Age at diagnosis | 42 | 1.09 (0.85, 1.40)‡ | 0.50 |
| Sex | |||
| Female (N=317) | 10 | Referent | |
| Male (N=342) | 32 | 2.95 (1.45, 6.00) | 0.003 |
| Pathologic stage (8th edition) | |||
| I (N=586) | 17 | Referent | |
| II (N=35) | 10 | 12.29 (5.61, 26.91) | <0.001 |
| III or IV (N=31) | 13 | 19.36 (9.37, 40.02) | <0.001 |
| Not documented (N=7) | 2 | -- | |
| Breslow depth | |||
| ≤1.00 (N=532) | 8 | Referent | |
| 1.01–2.00 (N=68) | 12 | 11.81 (4.83, 28.90) | <0.001 |
| 2.01–4.00 (N=31) | 12 | 34.73 (14.14, 85.35) | <0.001 |
| >4.00 (N=14) | 5 | 32.41 (10.59, 99.20) | <0.001 |
| Not documented (N=14) | 5 | -- |
Among the patients with invasive melanoma not known to be deceased at the time of this analysis the median (IQR, interquartile range) duration of follow-up by decade of diagnosis was 44.7 (IQR, 42.8–46.7) years for 1970–79, 36.8 (IQR, 35.1–39.1) years for 1980–89, 25.1 (IQR, 22.8–26.9) years for 1990–99, 14.0 (IQR, 12.6–16.8) years for 2000–09, and 5.5 (IQR, 3.2–8.5) years for 2010–2020. A more recent diagnosis was significantly associated with a decreased risk of death due to melanoma (HR 0.64 per 5-year increase in calendar year of diagnosis, 95% CI 0.56–0.74) in a sensitivity analysis focusing on just the first 5 years of follow-up per patient.
Hazard ratio per 5-year increase in calendar year and per 5-year increase in age, respectively.
DISCUSSION
This population-based epidemiologic study demonstrates a significant rise in melanoma incidence in the middle-aged population between 1970 and 2020. Increasing incidence disproportionately affected females (5114% rise versus 529% rise in males, for 1970–1979 versus 2011–2020). This surge in incidence is supported by other population-based studies; however, the rise in female incidence is more striking than previous studies.2,3
Over the last 15 years, the incidence has continued to rise in middle-aged females (53% rise in incidence) but has stabilized in males (0.6% rise in incidence). These two populations are clearly on distinctive incidence trajectories, which is dissimilar from our previous findings on this cohort.2
The stabilization of incidence in males and continued rise in females suggests that external factors are influencing these rates and is evidence against the notion that overdiagnosis is the largest driving factor in overall increasing incidence. A divergence in incidence trends, with increased rates in females, would not be explained solely by overdiagnosis. Incidence rates in males would be predicted to increase along with females if overdiagnosis is the driving factor, as this factor is less likely to impact these groups differently.
One alternative explanation for the different trajectories of sexes, which would support the notion of overdiagnosis as a main external pressure, is a discrepancy in females versus male’s rate of screening. There is evidence which suggests females are much more likely to be screened for skin cancer compared with males; however, to our knowledge, there is no data which reports female versus male screening rate overtime.14, 15,16 To account for the discrepancy in incidence trends, females would need to be screened at increasingly higher rates, year after year, over the last 15 years. This is possible, but more data is required to support this theory.
It is unclear the driving force behind increased incidence in the female population. One possible explanation for the continued rise in females is the use of tanning beds. There is evidence that tanning bed use can increase melanoma and this population is known to have increasing rates of tanning bed use in the preceding decades. Although it is still unclear, there is active research on the role of menopause/hormonal therapy and melanoma, with a lack of current evidence.17
Cause-specific mortality continues to decrease in a linear fashion throughout this time period. The drop in mortality is likely in part due to the development of superior pharmacotherapies (immunotherapy and targeted therapy), enhanced surgical management with comprehensive margin assessment, and successful screening. The significant reduction in mortality is encouraging and perhaps refutes the notion that diagnostic measures have not impacted survival; however, it is challenging to quantify this impact in the context of other factors (i.e., improved pharmacotherapies).
Limitations of the study included the relative lack of diversity and relative greater access to healthcare, which may limit generalizability. The majority of Olmsted County residents are white and well-educated. The retrospective nature may limit the study; however, it is unlikely that true incident cases were missed due to the nature of the REP (unless patients traveled outside of this county for healthcare, which is unusual for this population). Our data did not include patient occupation, which could impact exposure to ultraviolet light and therefore risk of melanoma.
Studies are ongoing regarding melanoma incidence and mortality in younger and older populations using this same population.
CONCLUSION
Melanoma incidence in middle-aged individuals is increasing overall since 1970, with a stabilization in men and a continued rise in females over the last 15 years. This divergence may be evidence contradicting the notion of overdiagnosis as the primary driver of rising incidence. Cause-specific mortality has down trended in a linear fashion throughout the 50-year study period.
Supplementary Material
Funding status:
Mayo Clinic Department of Dermatology This study used the resources of the Rochester Epidemiology Project (REP) medical records-linkage system, which is supported by the National Institute on Aging (NIA; AG 058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users. The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health (NIH) or the Mayo Clinic.
ABBREVIATIONS
- CI
Confidence Interval
- HR
Hazard Ratio
- REP
Rochester Epidemiology Project
Footnotes
Reprint request: Elliott Campbell
IRB approval status: Exempt
Elliott Campbell: Conceptualization, Methodology, Validation, Investigation, Data Curation, Writing - Original Draft/Review and Editing, Project administration, Funding Acquisition; Jacob Reinhart: Conceptualization, Methodology, Investigation, Data Curation, Writing - Review and Editing; Olivia Crum: Conceptualization, Methodology, Investigation, Data Curation, Writing - Review and Editing; Sydney Proffer: Conceptualization, Methodology, Investigation, Data Curation, Writing - Review and Editing; Amy Weaver: Software, Validation, Resources, Data curation, Formal Analysis; Larry Gibson: Conceptualization, Methodology, Writing - Review and Editing, Supervision; Jerry Brewer: Conceptualization, Methodology, Validation, Investigation, Writing - Review and Editing, Project administration, Funding acquisition, Supervision; Addison Demer: Conceptualization, Methodology, Validation, Investigation, Writing - Review and Editing, Project administration, Funding acquisition, Supervision.
Conflict of interest (all authors): None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.National Cancer Institute. Cancer Statistics Review (CSR; ) 1975-2016. (https://seer.cancer.gov/csr/1975_2016/browse_csr.php?sectionSEL=1&pageSEL=sect_01_table.01.opensinnewtab). [Google Scholar]
- 2.Lowe GC, Saavedra A, Reed KB, Velazquez AI, Dronca RS, Markovic SN, Lohse CM, Brewer JD. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014. Jan;89(1):52–9. doi: 10.1016/j.mayocp.2013.09.014. PMID: 24388022; PMCID: PMC4389734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Investig Dermatol. 2009;129(7):1666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whiteman DC, Green AC, Olsen CM. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J Invest Dermatol. 2016. Jun;136(6):1161–1171. doi: 10.1016/j.jid.2016.01.035. Epub 2016 Feb 20. PMID: . [DOI] [PubMed] [Google Scholar]
- 5.Erdmann F, Lortet-Tieulent J, Schuz J, Zeeb H, Greinert R, Breitbart EW, et al. International trends in the incidence of malignant melanoma 1953–2008—Are recent generations at higher or lower risk? Int J Cancer. 2013;132(2):385–400. [DOI] [PubMed] [Google Scholar]
- 6.Welch HG, Mazer BL, Adamson AS. The Rapid Rise in Cutaneous Melanoma Diagnoses. N Engl J Med. 2021. Jan 7;384(1):72–79. doi: 10.1056/NEJMsb2019760. PMID: . [DOI] [PubMed] [Google Scholar]
- 7.Guy GP Jr., Zhang Y, Ekwueme DU, Rim SH, Watson M. The potential impact of reducing indoor tanning on melanoma prevention and treatment costs in the United States: An economic analysis. J Am Acad Dermatol. 2017;76(2):226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M, Qureshi AA, Geller AC, Frazier L, Hunter DJ, Han J. Use of tanning beds and incidence of skin cancer. J Clin Oncol. 2012;30(14):1588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: Systematic review and meta-analysis. J Am Acad Dermatol. 2014;70(5):847–57. e1–18. [DOI] [PubMed] [Google Scholar]
- 10.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ III. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ III, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed KB, Brewer JD, Lohse CM, Bringe KE, Pruitt CN, Gibson LE. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87(4):328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol 2011; 173(9): 1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstock MA, Martin RA, Risica PM, Berwick M, Lasater T, Rakowski W, Goldstein MG, Dubé CE. Thorough skin examination for the early detection of melanoma. Am J Prev Med. 999 Oct;17(3):169–75. doi: 10.1016/s0749-3797(99)00077-x. PMID: . [DOI] [PubMed] [Google Scholar]
- 15.Janda M, Youl PH, Lowe JB, Elwood M, Ring IT, Aitken JF. Attitudes and intentions in relation to skin checks for early signs of skin cancer. Prev Med. 2004. Jul;39(1):11-8. doi: 10.1016/j.ypmed.2004.02.019. PMID: 15207981. [DOI] [PubMed] [Google Scholar]
- 16.Davis JL, Buchanan KL, Katz RV, Green BL. Gender differences in cancer screening beliefs, behaviors, and willingness to participate: implications for health promotion. Am J Mens Health. 2012. May;6(3):211–7. doi: 10.1177/1557988311425853. Epub 2011 Nov 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill MS, Cartron AM, Burgoyne M, Driscoll MS. Hormone therapy and melanoma in women. Int J Womens Dermatol. 2021. Jun 25;7(5Part B):692–696. doi: 10.1016/j.ijwd.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.