Abstract
Zoledronic acid (ZOL) as a yearly infusion is effective in reducing fracture risk. An acute-phase reaction (APR), consisting of flu-like symptoms within 3 days after infusion, is commonly seen. The objective of this analysis was to investigate whether APR occurrence influences drug efficacy. This analysis uses data from the 3-year randomized clinical trial, Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly-Pivotal Fracture Trial (HORIZON-PFT). APRs were identified as adverse events within 3 days of first infusion with higher frequency in ZOL than placebo. To compare mean 3-year change in bone mineral density (BMD) in ZOL versus placebo, among women with and without APR, t tests were used. Logistic regression was used to examine the relationship between APR occurrence and odds of incident morphometric vertebral fracture. Cox regression was used to determine the risk of nonvertebral and hip fractures for women with versus without APR. Logistic and Cox models were used to determine the risk of incident fracture in ZOL versus placebo for women with and without an APR. The analysis included 3862 women in the ZOL group and 3852 in placebo, with 42.4% in ZOL versus 11.8% in placebo experiencing an APR. The difference in BMD mean change for ZOL versus placebo was similar for women with and without an APR (all p interaction >0.10). Among ZOL women, those with APR had 51% lower vertebral fracture risk than those without (odds ratio [OR] = 0.49, p < 0.001). A similar but nonsignificant trend was observed for nonvertebral and hip fracture (relative hazard [RH] = 0.82, p = 0.10; RH = 0.70, p = 0.22, respectively). There was a greater treatment-related reduction in vertebral fracture risk among women with APR (OR = 0.19) than those without (OR = 0.38) (p interaction = 0.01). Our results suggest that women starting ZOL who experience an APR will have a larger reduction in vertebral fracture risk with ZOL.
Keywords: ANTIRESORPTIVES, CLINICAL TRIALS, FRACTURE RISK ASSESSMENT, OSTEOPOROSIS
INTRODUCTION
Bisphosphonates have been shown to be effective in reducing fracture risk and are the most commonly used therapy for treating osteoporosis.(1) Zoledronic acid (ZOL, also zoledronate) is a nitrogen-containing bisphosphonate that is used as an annual iv infusion and is approved for the treatment of postmenopausal osteoporosis,(2–5) glucocorticoid-induced osteoporosis,(6) male osteoporosis,(7) and low-trauma hip fracture worldwide.(5) It is commonly used to treat patients with a gastrointestinal contraindication to oral bisphosphonates, and also it has the potential to alleviate low compliance reported for oral treatments.(8,9)
A large 3-year international, randomized, double-blind, placebo-controlled phase 3 study, Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly-Pivotal Fracture Trial (HORIZON-PFT) randomized 7765 women with postmenopausal osteoporosis to either 5 mg ZOL or placebo.(1) The results showed that women in the ZOL group had significantly lower incidence of vertebral, hip, and non-vertebral fractures compared with those in the placebo group. ZOL was shown to be safe and well-tolerated. However, during the first 3 days following the first infusion, transient acute-phase reactions (APRs), including fever, myalgia, and flu-like symptoms, were more common in the zoledronic acid group than in the placebo group (42.4% versus 11.7%, respectively). The incidence of APRs decreased markedly with subsequent infusions.(10)
In a subsequent analysis, risk factors for and severities of APRs, and duration of the symptoms were further analysed using the results of HORIZON-PFT.(10) APRs were more common among younger participants, those using nonsteroidal anti-inflammatory drugs (NSAIDs), and Asians, whereas it was less common in smokers, those with diabetes and previous oral bisphosphonates users. However, no studies have examined the impact of APR on drug efficacy.
The objective of the present analyses of the HORIZON-PFT was to investigate whether the occurrence of APR after a first ZOL infusion was predictive of drug efficacy in terms of change in bone mineral density (BMD) or fracture reduction with treatment.
Subjects and Methods
Study design
The HORIZON-PFT was a 3-year, international, multicenter, randomized, double-blind, placebo-controlled phase 3 study that involved postmenopausal women with osteoporosis. Participants underwent randomization from February 2002 to June 2003 and were assigned to receive either ZOL (5 mg as an intravenous infusion over 15 minutes) or a placebo infusion at baseline (day 0), year 1, and year 2. In addition, all participants received oral daily calcium (1000 to 1500 mg) and vitamin D (400 to 1200 IU). Participants were monitored for 3 years with quarterly telephone interviews and clinic visits at months 6, 12, 24, and 36. Two strata of study participants were defined at baseline: stratum 1 included women not taking any osteoporosis medications at randomization, and stratum 2 included women on allowed osteoporosis medications at baseline (eg, selective estrogen receptor modulators [SERMs], calcitonin). Additional details regarding study design can be found in the main HORIZON-PFT results report.(1)
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Participants included in the analyses
Inclusion and exclusion criteria of participants are described in the previous HORIZON-PFT(1) report. In brief, postmenopausal women age 65 to 89 years were eligible if the following criteria were met; BMD T-score at the femoral neck ≤ −2.5 or T-score ≤ −1.5 with at least two mild vertebral fractures or one moderate vertebral fracture. Previous use of oral bisphosphonates was allowed, with the duration of the washout period dependent on previous bisphosphonate use. The following concomitant osteoporosis medications were allowed at baseline and during follow-up: hormone therapy, raloxifene, calcitonin, tibolone, tamoxifen, dehydroepiandrosterone, ipriflavone, and medroxyprogesterone. Participants were ineligible if any of the following criteria were met: any previous use of parathyroid hormone, strontium, or sodium fluoride; use of anabolic steroids or growth hormone within 6 months before study entry; intravenous systemic corticosteroids within 12 months; or a calculated creatinine clearance <30 mL/min.
Definition of APR
A previous secondary analysis of APR was performed from this cohort(10) and we used a similar definition for this analysis. The definition utilized adverse events, which were reported as occurring within 3 days of the first administration of study drug. The adverse events were categorized according to the Medical Dictionary for Regulatory Activities (MedDRA) version 9.(11) We analyzed specific MedDRA Preferred Terms and defined a Preferred Term as being part of the APR set if the overall frequency in the first 3 days following the first infusion was at least 0.1% (≥8 participants, both treatment groups combined) and there was a significant difference between treatment groups (p < 0.05). In a sensitivity analysis, we added any Preferred Term that differed by treatment (p < 0.5) Two physicians blinded to treatment (ALS, TYK) reviewed these additional Preferred Terms and judged whether they were synonymous with one of the Terms selected in the first step. Only a few additional Terms were added (eg, “increased body temperature” was judged to be synonymous with self-reported “fever”).
Preferred terms meeting the definition of APR were grouped into five symptom clusters as was done in the previous study(10): fever; musculoskeletal events (eg, pain and joint swelling); gastrointestinal events (eg, abdominal pain, vomiting, and diarrhea); eye inflammation; and, other events (including fatigue, nasopharyngitis, and edema).
Dual-energy X-ray absorptiometry measures of bone density
Participants had dual-energy X-ray absorptiometry (DXA) scans of the hip at baseline and at months 6, 12, 24, and 36 using a Hologic (Waltham, MA, USA), GE Lunar (Madison, WI, USA), or Norland (Trumball, CT, USA) axial bone densitometer. Measurements of bone mineral density at the lumbar spine were obtained for a subgroup of patients. All BMD measurements were corrected for variations related to machinery and site-related differences in BMD means.
Fracture ascertainment
Spinal lateral radiographs were obtained at baseline and at 12, 24, and 36 months or early termination for patients in stratum 1 and at baseline and at 36 months or early termination for patients in stratum 2. Vertebrae from T4 to L4 were evaluated by an expert reader at a central imaging laboratory (Synarc, San Francisco, CA, USA) with the use of quantitative morphometry and standard methods.(12) Incident morphometric vertebral fractures were defined as a reduction in vertebral height of at least 20% and 4 mm by quantitative morphometry, confirmed by an increase of one severity grade or more on semiquantitative analysis.(12) Prevalent vertebral fracture at baseline was defined by a height ratio of at least three standard deviations (SDs) below the vertebra-specific mean height ratio on quantitative reading with semiquantitative confirmation.(13,14)
Clinical fracture reports were obtained from participants at each contact. These fracture reports underwent central confirmation, which was performed at the UCSF Coordinating Center. Confirmation required either a radiologic or surgical procedure report or a copy of the radiograph. Excluded were fractures of the toe, facial bone, and finger and those caused by excessive trauma (assessed centrally as sufficient to cause fracture in a person without osteoporosis(15)).
Other measurements
Additional information collected at baseline included race, smoking, weight/height, current/past medication use, creatinine/alkaline phosphatase, diabetes, and active back pain. Height was measured with the use of a stadiometer, where available. Creatinine clearance was calculated using the Cockcroft-Gault equation.
Statistical analysis
The present analyses were performed using the intention-to-treat population (n = 7714) that was included in the primary report’s safety analyses.(1) Baseline characteristics of participants were summarized using means and SDs for continuous measures and counts and percentages for categorical measures. Relevant categories of adverse events were compared in ZOL versus placebo using chi-square tests.
We used two-sample t tests to compare unadjusted mean percent change in BMD (femoral neck, total hip, and lumbar spine) during the course of the 3-year study between those receiving ZOL versus placebo, among women with an APR (APR+) and among women without an APR (APR−). Linear regression models were used to estimate adjusted mean 3-year percent change in BMD in ZOL versus placebo. The adjusted models included baseline risk factors that were significantly different between ZOL versus placebo for either APR+ or APR− women: race (Asian or non-Asian), age, prior bisphosphonate usage, active back pain, diabetes, femoral neck BMD, number of prevalent vertebral fractures, creatinine clearance, and alkaline phosphatase. We tested the interaction between treatment assignment and APR development in these models to determine if the treatment-related difference in 3-year percent change in BMD differed according to APR occurrence.
Logistic regression models were used to determine the odds of incident morphometric vertebral fracture for those with APR compared to those without APR among women randomized to zoledronic acid and among women randomized to placebo; results are presented as odds ratios and 95% confidence intervals (CIs). Cox proportional hazards models were used to determine the risk of incident nonvertebral fracture and incident hip fracture for women with versus without APR, stratified by treatment assignment; results are presented as hazard ratios (HRs) and 95% CIs. Initial analyses included only APR as the predictor, but additional multivariable analyses included age then the set of confounding variables found to be significant of APR in our previous analysis(10) (“multi-adjusted” model). These confounders included race (Asian or non-Asian), age, current smoker, NSAID use at baseline, prior bisphosphonate usage, active back pain, diabetes, and prior calcitonin usage. In addition to the covariables in the Reid work,(10) we performed another level of adjustment in which we tested an additional set of baseline variables (including body mass index (BMI), femoral neck BMD, number of vertebral fractures, alkaline phosphatase, and creatinine clearance) for their relationship to APR in the ZOL group and in the placebo group. Multivariable models were then run including the variables identified in the analysis in Reid and colleagues(10) plus any variables that were significantly related to APRs in either the ZOL or placebo groups at p < 0.05 (“fully adjusted” model). We tested the interaction between treatment assignment and APR development in these models to determine if the reduction in incident fracture risk for women with versus without an APR differed according to treatment assignment.
Unadjusted and adjusted logistic and Cox regression models were used to determine the relative risk of incident fracture in ZOL versus placebo by APR occurrence. Like the change in BMD models, the adjusted models included measures that were significantly different between ZOL versus placebo for either APR + or APR− women: race (Asian or non-Asian), age, prior bisphosphonate usage, active back pain, diabetes, femoral neck BMD, number of prevalent vertebral fractures, creatinine clearance, and alkaline phosphatase. We tested the interaction between treatment assignment and APR development in these models to determine if treatment-related reduction in incident fracture risk differed according to APR occurrence.
All analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA). All tests were two-tailed and p < 0.05 was considered significant.
Results
Participant baseline characteristics
A total of 7714 women were included in this analysis (Table 1). Of these, 3852 were randomized to placebo and 3862 to zoledronic acid. Overall, at baseline, the mean ± SD age of the women was 73.1 ± 5.4, 14.1% were Asian, and mean femoral neck T-score was −2.7 ± 0.5. A total of 4878 women (63.2%) had prevalent vertebral fracture, and 1454 (18.8%) had active back pain. Less than 10% were current smokers (8.5%), and 6.2% had diabetes. Over half of the participants (56.8%) were taking NSAIDs at baseline, 1119 participants (14.6%) had prior bisphosphonate use, and 868 (11.3%) had prior calcitonin use. vertebral fracture, and 1454 (18.8%) had active back pain. Less than 10% were current smokers (8.5%), and 6.2% had diabetes. Over half of the participants (56.8%) were taking NSAIDs at baseline, 1119 participants (14.6%) had prior bisphosphonate use, and 868 (11.3%) had prior calcitonin use.
Table 1.
Baseline Characteristics by Treatment Assignment, for Those Who Developed APR and Those Who Did Nota
| Characteristic | APR+ |
APR− |
All participants (n = 7714) | ||||
|---|---|---|---|---|---|---|---|
| ZOL (n = 1639) | Placebo (n = 456) | P | ZOL (n = 2223) | Placebo (n = 3396) | P | ||
| Age (years) | 72.2 ± 4.9 | 73.0 ± 5.5 | 0.005 | 73.7 ± 5.5 | 73.0 ± 5.4 | <0.0001 | 73.1 ± 5.4 |
| BMI (kg/m2) | 24.9 ± 4.1 | 25.2 ± 4.4 | 0.17 | 25.3 ± 4.5 | 25.4 ± 4.3 | 0.36 | 25.2 ± 4.3 |
| Weight (kg) | 59.6 ± 10.8 | 59.8 ± 11.9 | 0.73 | 60.1 ± 11.3 | 60.7 ± 11.2 | 0.06 | 60.3 ± 11.2 |
| Asian race | 342 (20.9) | 118 (25.9) | 0.02 | 207 (9.3) | 420 (12.4) | 0.0004 | 1087 (14.1) |
| Femoral neck BMD T-score | −2.7 ± 0.5 | −2.7 ± 0.6 | 0.40 | −2.8 ± 0.5 | −2.7 ± 0.5 | 0.03 | −2.7 ± 0.5 |
| Prevalent vertebral fracture | |||||||
| None | 676 (41.2) | 162 (35.5) | 0.04 | 776 (34.9) | 1219 (35.9) | 0.68 | 2833 (36.7) |
| 1 fracture | 451 (27.5) | 125 (27.4) | 640 (28.8) | 947 (27.9) | 2163 (28.1) | ||
| 2+ fractures | 512 (31.2) | 169 (37.1) | 805 (36.2) | 1229 (36.2) | 2715 (35.2) | ||
| Creatinine clearance (mL/min) | 64.3 ± 17.0 | 62.8 ± 18.0 | 0.09 | 62.7 ± 17.5 | 64.0 ± 17.6 | 0.007 | 63.6 ± 17.5 |
| <60 mL/min | 709 (43.3) | 213 (46.7) | 0.19 | 1071 (48.2) | 1510 (44.5) | 0.006 | 3503 (45.4) |
| Alkaline phosphatase (IU/L) | 75.7 ± 22.3 | 77.2 ± 24.2 | 0.16 | 78.3 ± 25.7 | 76.9 ± 24.7 | 0.03 | 77.0 ± 24.5 |
| Active back pain | 328 (20.0) | 115 (25.2) | 0.02 | 381 (17.1) | 630 (18.5) | 0.18 | 1454 (18.8) |
| Diabetes | 103 (6.3) | 27 (5.9) | 0.78 | 158 (7.1) | 192 (5.6) | 0.03 | 480 (6.2) |
| Prior bisphosphonate use | 182 (11.1) | 78 (17.2) | 0.0005 | 381 (17.2) | 478 (14.1) | 0.002 | 1119 (14.6) |
| Current NSAID use | 972 (59.3) | 274 (60.1) | 0.76 | 1240 (55.8) | 1898 (55.9) | 0.94 | 4384 (56.8) |
| Prior calcitonin use | 164 (10.0) | 44 (9.7) | 0.83 | 279 (12.6) | 381 (11.3) | 0.13 | 868 (11.3) |
| Current smoker | 126 (7.7) | 25 (5.5) | 0.11 | 217 (9.8) | 289 (8.5) | 0.11 | 657 (8.5) |
APR = acute-phase reaction; BMD = bone mineral density; BMI = body mass index; NSAID = nonsteroidal anti-inflammatory drug; SD = standard deviation; ZOL = zoledronic acid.
Mean ± SD, or n (%).
Among the 2095 women who experienced an APR (APR+), there were several baseline characteristics that varied between treatment groups including age, Asian race, prevalent vertebral fractures, active back pain, and prior bisphosphonate use. Among those without an APR (APR−), age, Asian race, BMD, creatinine clearance, alkaline phosphatase, diabetes, and prior bisphosphonate use were significantly different by treatment.
Incidence of APR in ZOL versus placebo
The occurrence of any APR within 3 days of the first study drug infusion was significantly higher in the zoledronic acid compared to placebo group (42.4% versus 11.8%, p < 0.0001) (Table 2). Similar to our previous report,(10) incidence of each of the five categories of APRs also was significantly higher in ZOL versus placebo including fever, musculoskeletal (pain and joint swelling), gastrointestinal (abdominal pain, vomiting, diarrhea), and eye inflammation.
Table 2.
Adverse Events Occurring Within 3 Days of the First Study Drug Infusion
| Adverse event | ZOL (n = 3862) | Placebo (n = 3852) |
|---|---|---|
| Fever/chill/hot flush, n (%) | 778 (20.1) | 96 (2.5) |
| Musculoskeletal, n (%) | 781 (20.2) | 189 (4.9) |
| Gastrointestinal, n (%) | 292 (7.6) | 76 (2.0) |
| Ocular, n (%) | 16 (0.4) | 1 (0.0) |
| Other APR, n (%) | 852 (22.1) | 227 (5.9) |
| Any of the above, n (%) | 1639 (42.4) | 456 (11.8) |
All p values <0.001.
APR = acute-phase reaction; ZOL = zoledronic acid.
Treatment-related change in BMD by APR occurrence
The difference in mean change in BMD for ZOL versus placebo was similar for women with an APR and those without (all p for interaction >0.10) (Table 3). Among women who developed an APR, the unadjusted mean change in BMD over 3 years was +4.6% higher at the femoral neck, +5.7% higher at the total hip, and +5.4% higher at the lumbar spine for women who received ZOL compared to women who received placebo. Among those who did not develop an APR, women in the ZOL group had +5.1% greater mean change in femoral neck BMD, +6.0% greater mean change in total hip BMD, and +7.2% greater mean change in lumbar spine BMD compared to women in the placebo group. Adjusted results were similar.
Table 3.
Comparison of Mean 3-Year Percent Change in BMD in ZOL Versus Placebo for APR+ Group and for APR− Group
| Change in BMD parameter | APR + |
APR− |
p for interaction | ||||
|---|---|---|---|---|---|---|---|
| ZOL mean (95% CI) | Placebo mean (95% CI) | Difference mean (95% CI) | ZOL mean (95% CI) | Placebo mean (95% CI) | Difference mean (95% CI) | ||
| Change in FN BMD | n = 1378 | n = 368 | n = 1689 | n = 2715 | |||
| Unadjusted | 4.1% (3.8, 4.4) | −0.6% (−1.2, 0.1) | 4.6% (4.0, 5.3) | 4.1% (3.8, 4.4) | −1.0% (−1.2, −0.8) | 5.1% (4.7, 5.5) | 0.22 |
| Adjusteda | 4.1% (3.7, 4.4) | -0.5% (−1.1, 0.1) | 4.6% (3.9, 5.2) | 4.1% (3.8, 4.4) | −1.0% (−1.2, −0.8) | 5.1% (4.7, 5.5) | 0.12 |
| Change in TH BMD | n = 1377 | n = 368 | n = 1684 | n = 2709 | |||
| Unadjusted | 4.4% (4.2, 4.7) | −1.3% (−1.9, −0.7) | 5.7% (5.1, 6.3) | 4.3% (4.0, 4.5) | −1.7% (−1.9, −1.5) | 6.0% (5.7, 6.3) | 0.39 |
| Adjusteda | 4.4% (4.1, 4.6) | −1.2% (−1.7, −0.7) | 5.6% (5.0, 6.2) | 4.3% (4.0, 4.5) | −1.8% (−2.0, −1.6) | 6.1% (5.7, 6.4) | 0.14 |
| Change in LS BMD | n = 91 | n = 37 | n = 137 | n = 175 | |||
| Unadjusted | 7.1% (6.0, 8.2) | 1.7% (0.2, 3.2) | 5.4% (3.5, 7.4) | 8.1% (7.2, 8.9) | 0.9% (0.0, 1.8) | 7.2% (5.9, 8.4) | 0.16 |
| Adjusteda | 7.3% (0.5, 6.3) | 1.0% (−0.6, 2.7) | 6.2% (4.3, 8.1) | 8.1% (7.2, 9.0) | 0.9% (0.0, 1.7) | 7.2% (6.0, 8.4) | 0.38 |
APR = acute-phase reaction; BMD = bone mineral density; CI = confidence interval; FN = femoral neck; LS = lumbar spine; TH = total hip; ZOL = zoledronic acid.
Adjusted for race (Asian or non-Asian), age, prior bisphosphonate usage, active back pain, diabetes, femoral neck BMD, number of prevalent vertebral fractures, creatinine clearance, alkaline phosphatase.
Relationship of APR to incident fracture risk
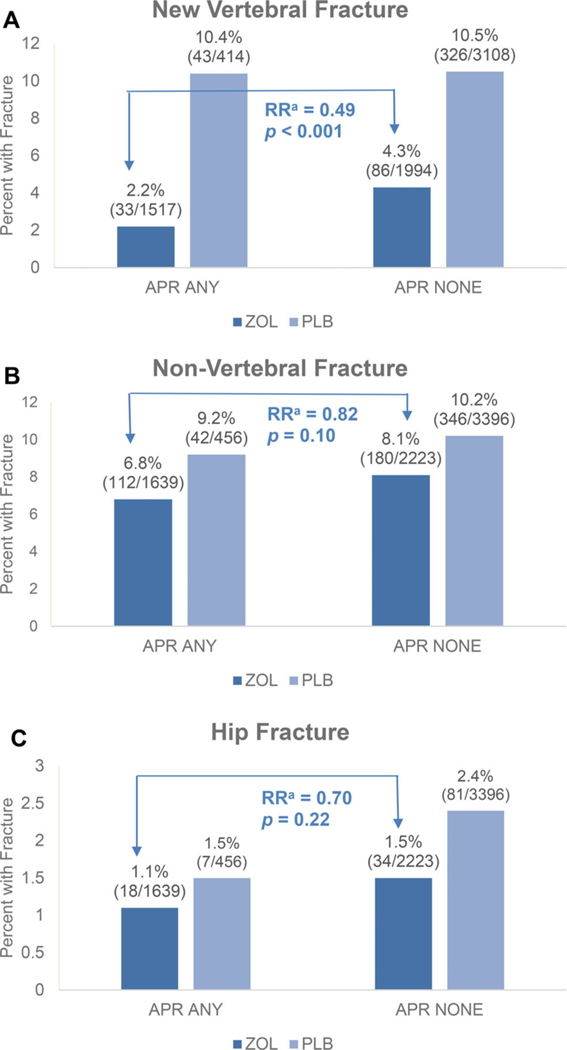
In the women who received ZOL, in unadjusted analyses, those who experienced an APR had a significantly lower risk of incident vertebral fracture than in those who did not experience an APR (Table 4, Fig. 1). The incidence of vertebral fracture was 2.2% among those with an APR compared to 4.3% among those without an APR (OR = 0.49; 95% CI, 0.33–0.74; p = 0.0007). There was also a nonsignificant trend toward a lower risk for nonvertebral and hip fracture among those who experienced an APR (nonvertebral fracture RH = 0.82; 95% CI, 0.65–1.04; p = 0.10; hip fracture RH = 0.70; 95% CI, 0.40–1.24; p = 0.22).
Table 4.
Relative Risk of Incident Fracture in APR+ Versus APR− for ZOL Group and for Placebo Group
| RRa (95% CI) of fracture for APR+ versus APR− |
||||
|---|---|---|---|---|
| Type of incident fracture | Model | ZOL group | Placebo group | p for interaction |
| Morphometric vertebral fracture | Unadjusted | 0.49 (0.33, 0.74) | 0.99 (0.71, 1.38) | 0.01 |
| Age adjusted | 0.53 (0.35, 0.80) | 0.99 (0.71, 1.38) | 0.02 | |
| Multi adjustedb | 0.52 (0.34, 0.79) | 0.90 (0.64, 1.27) | 0.02 | |
| Fully adjustedc | 0.57 (0.37, 0.87) | 0.90 (0.63, 1.28) | 0.047 | |
| Nonvertebral fracture | Unadjusted | 0.82 (0.65, 1.04) | 0.90 (0.65, 1.23) | 0.67 |
| Age adjusted | 0.86 (0.68, 1.09) | 0.90 (0.65, 1.23) | 0.77 | |
| Multi adjustedb | 0.92 (0.72, 1.17) | 0.96 (0.69, 1.33) | 0.73 | |
| Fully adjustedc | 0.91 (0.72, 1.17) | 0.93 (0.67, 1.29) | 0.88 | |
| Hip fracture | Unadjusted | 0.70 (0.40, 1.24) | 0.64 (0.30, 1.39) | 0.86 |
| Age adjusted | 0.83 (0.46, 1.47) | 0.64 (0.30, 1.39) | 0.73 | |
| Multi adjustedb | 0.90 (0.50, 1.61) | 0.70 (0.32, 1.53) | 0.71 | |
| Fully adjustedc | 1.03 (0.56, 1.87) | 0.69 (0.32, 1.51) | 0.61 | |
APR = acute-phase reaction; CI = confidence interval; RR = relative risk; ZOL = zoledronic acid.
Odds ratio for vertebral fractures using logistic regression, relative hazard for nonvertebral and hip fractures using Cox proportional hazard models.
Adjusted for variables included in Reid analysis: race (Asian or non-Asian), age, current smoker, NSAID use at baseline, prior bisphosphonate usage, active back pain, diabetes, prior calcitonin usage.
Adjusted for variables included in Reid analysis, plus body mass index, femoral neck BMD, number of prevalent vertebral fractures, creatinine clearance, alkaline phosphatase.
Fig 1.
Incident fracture according to development of APR after first infusion. aUnadjusted relative risk of incident fracture in APR+ versus APR− for ZOL group. Odds ratio for vertebral fractures using logistic regression models, relative hazard for non-vertebral and hip fractures using Cox proportional hazard models. APR = acute-phase reaction; PLB = placebo; RR = relative risk; ZOL = zoledronic acid.
Adjustment for factors significantly predictive of APR in the combined treatment groups attenuated the relationships slightly (Table 4). However, the relationship of APR to vertebral fracture among the ZOL group remained statistically significant (OR = 0.52; 95% CI, 0.34–0.79; p = 0.002).
In addition to the factors predictive of APR in the previous analysis, we found that several other factors were predictive of APR within the ZOL group: lower BMI, higher femoral neck BMD, fewer baseline vertebral fractures, higher creatinine clearance and lower baseline alkaline phosphatase (Supplemental Table S1). Addition of these covariables to the multivariate analysis did not substantially change results.
Of interest (self-reported), acute back pain at baseline was predictive of APR (also self-reported) in the placebo as well as in the ZOL group (Supplemental Table S1). This suggests that one factor influencing APR reporting may be proclivity to self-report symptoms.
Treatment-related effect on fracture risk by APR occurrence
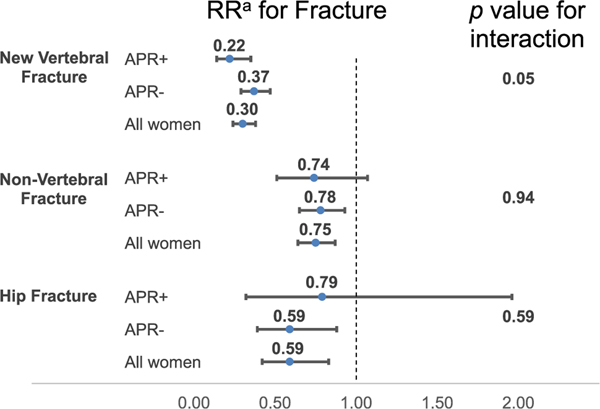
The relative risk of incident fracture for women in the ZOL group versus the placebo group by APR occurrence is displayed in Fig. 2 and Supplemental Table S2. There was a greater treatment-related reduction in vertebral fracture risk among women with an APR (unadjusted OR = 0.19; 95% CI, 0.12–0.31) than those without (unadjusted OR = 0.38; 95% CI, 0.30–0.49) (p for interaction = 0.01). Adjustment for factors significantly predictive of treatment assignment slightly attenuated the treatment-related reduction in vertebral fracture risk among APR+ women (adjusted OR = 0.22; 95% CI, 0.14–0.35) but slightly strengthened the relationship among APRCI, 0.29–0.47) (p for interaction = 0.05). There was no difference in the treatment-related reduction in non-vertebral fracture risk or hip fracture risk for women who developed an APR and those who did not in either unadjusted or adjusted analyses (all p for interaction >0.10).
Fig 2.
Relative risk (95% CI) of incident fracture in ZOL versus placebo for those who developed APR and those who did not. aOdds ratio for vertebral fractures using logistic regression models, relative hazard for nonvertebral and hip fractures using Cox proportional hazard models. The APR-stratified models were adjusted for race (Asian or non-Asian), age, prior bisphosphonate usage, active back pain, diabetes, femoral neck BMD, number of prevalent vertebral fractures, creatinine. The all-women models were unadjusted since there were no significant differences between ZOL versus placebo. APR = acute-phase reaction; CI = confidence interval; RR = relative risk; ZOL = zoledronic acid.
Discussion
APRs are common among people who are treated for the first time with iv bisphosphonates including zoledronic acid. The specific symptoms vary but almost always resolve within a few of days after the infusion. Reactions are much more common after the first compared to subsequent infusions.(1,10) In this analysis, we examined whether the occurrence of an APR after the first infusion was indicative of a differential effect of zoledronic acid on fractures or bone mineral density. We found that the treatment-related reduction in risk of vertebral fracture was significantly greater for women who experienced an APR (81% reduction in risk) than those who did not have an APR (62% reduction in risk). Among women who received ZOL, those who experienced an APR showed a 51% reduction in vertebral fractures compared to those who did not experience an APR (2.2% versus 4.3%). For nonvertebral fractures there was a similar but nonsignificant trend toward lower risk in those with an APR. These results were slightly attenuated but remained similar after adjustment for multiple covariates. For changes in BMD, there was no suggestion of a difference in efficacy based on APR occurrence.
These results may be reassuring to women who experience an APR since the occurrence of the APR indicates that the treatment which is highly effective in reducing risk of vertebral fractures may be even more efficacious in them.
It is challenging to establish some mechanism that may explain the larger reduction in vertebral fracture risk among those who had an APR. Because we saw no relationship between the occurrence of APR and changes in bone density by treatment, any potential mechanism must be independent of BMD change. In particular, a mechanism may involve bone turnover and resulting bone quality. Changes in bone turnover markers in response to osteoporosis therapy have been shown to be associated with fracture reductions,(16–20) such that those with larger decreases seem to have lower fracture risk. One might hypothesize that those with larger decreases in bone turnover markers (BTMs) might be more likely to have had an APR which may explain the association, or that differences in baseline bone turnover may play a role. These hypotheses cannot easily be tested in our database because we did not assess BTMs in the initial days after infusion and furthermore, BTMs were only assessed in HORIZON-PFT on a small subset of participants. Although we adjusted for a large set of covariables in our multivariate model, it is possible that the reduction in vertebral fractures could be due to an unmeasured confounder.
The immune system plays a role in APR: nitrogen-containing bisphosphonates inhibit farnesyl diphosphate synthase in the mevalonate pathway, which leads indirectly to activation of γδ T cells with the release of interferon-γ (IFNγ) and tumor necrosis factor (TNF) resulting in the APR.(21–23) Although subjects with higher inflammatory markers have been shown to have a higher fracture risk,(24,25) there is also evidence that zoledronate causes γδ cells to mature toward an IFNγ-producing phenotype,(26) which may induce antiresorptive responses.(27) Therefore, it is not clear if the immune system response to zoledronate might impact fracture risk.
Although the results for nonvertebral fractures were not statistically significant, the trends toward reduced risk among those with APR compared to those without APR were similar to the significant findings for vertebral fractures. These results are supportive of the validity of the reduction in vertebral fractures but are less definitive due to their nonsignificance. Future analyses examining the relationship of APR to nonvertebral fractures using other data sets or examining severity of APR would be helpful to address this question.
There are several important limitations to our data. First, APRs were self-reported and could not be objectively verified. There may be differences in the tendency to report APR depending on baseline characteristics such as age, socioeconomic status, or geographic region, but we adjusted for many of these factors in our models. In addition, some of the covariables, such as prior use of bisphosphonates and use of NSAIDs, are based only on self-report and may be incompletely reported. APR is a postrandomization variable and therefore we cannot assume that the treatment groups are comparable with respect to risk for its occurrence. However, controlling for potential confounders showed our results to be robust. Last, because HORIZON-PFT only assessed bone turnover markers in a small subset of participants, we cannot include BTMs as covariables and cannot reliably compare their change as an outcome in those with and without APR or include BTMs in the multivariable analyses.
Despite these limitations, our analysis has a number of important strengths. This large study is randomized and was conducted in 23 countries including a broad sample of women with osteoporosis internationally. Because of the nature of the treatment (an infusion), we can know for certain adherence and were able to exclude those few participants from our analysis set who did not receive the first infusion.
In summary, we found that women treated with ZOL who experienced an APR after the first infusion have a significantly greater reduction in risk of morphometric vertebral fracture compared to those who did not experience an APR. However, there was no significant difference in treatment-related reduction in risk of nonvertebral or hip fracture, or treatment-related change in BMD, according to occurrence of an APR. These results may be reassuring to patients who experience an APR, because it indicates that the treatment, which is highly effective at reducing risk of vertebral fractures, may be even more efficacious in them.
Supplementary Material
Acknowledgments
We thank Richard Eastell, MD, PhD for his assistance in reviewing and approving the paper on behalf of the HORIZON 2301 Steering Committee and Lucy Wu for her editorial assistance.
Footnotes
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4434.
Additional Supporting Information may be found in the online version of this article.
Data Availability Statement
The HORIZON-PFT datasets analyzed for the current report are proprietary to the original sponsor from whom permission is required for sharing.
References
- 1.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007; 356(18):1809–1822. [DOI] [PubMed] [Google Scholar]
- 2.Reid IR, Brown JP, Burckhardt P, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002;346(9):653–661. [DOI] [PubMed] [Google Scholar]
- 3.Devogelaer JP, Brown JP, Burckhardt P, et al. Zoledronic acid efficacy and safety over five years in postmenopausal osteoporosis. Osteporos Int. 2007;18(9):1211–1218. [DOI] [PubMed] [Google Scholar]
- 4.McClung M, Recker R, Miller P, et al. Intravenous zoledronic acid5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone. 2007; 41(1):122–1228. [DOI] [PubMed] [Google Scholar]
- 5.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid DM, Devogelaer JP, Saag K, et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2009;373(9671):1253–1263. [DOI] [PubMed] [Google Scholar]
- 7.Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012; 367(18):1714–1723. [DOI] [PubMed] [Google Scholar]
- 8.Baio G, Barbagallo M, D’Avola G, et al. Improving adherence in osteoporosis: a new management algorithm for the patient with osteoporosis. Expert Opin Pharmacother. 2011;12(2):257–268. [DOI] [PubMed] [Google Scholar]
- 9.Ringe JD. Development of clinical utility of zoledronic acid and patient considerations in the treatment of osteoporosis. Patient Prefer Adherence. 2010;4:231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab. 2010;95(9):4380–4387. [DOI] [PubMed] [Google Scholar]
- 11.Medical Dictionary for Regulatory Activities (MedDRA). McLean, VA: MedDRA Maintenance and Support Services Organization (MSSO); 2016. [Google Scholar]
- 12.Black DM, Palermo L, Nevitt MC, Genant HK, Christensen L, Cummings SR. Defining incident vertebral deformity: a prospective comparison of several approaches. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14(1):90–101. [DOI] [PubMed] [Google Scholar]
- 13.Black DM, Palermo L, Nevitt MC, et al. Comparison of methods fordefining prevalent vertebral deformities: the study of osteoporotic fractures. J Bone Miner Res. 1995;10:890–902. [DOI] [PubMed] [Google Scholar]
- 14.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137–1148. [DOI] [PubMed] [Google Scholar]
- 15.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–1541. [DOI] [PubMed] [Google Scholar]
- 16.Bauer DC, Black DM, Garnero P, et al. Change in bone turnover andhip, non-spine, and vertebral fracture in alendronate-treated women: the Fracture Intervention Trial. J Bone Miner Res. 2004;19(8):1250–1258. [DOI] [PubMed] [Google Scholar]
- 17.Bjarnason NH, Sarkar S, Duong T, Mitlak B, Delmas PD, Christiansen C. Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteporos Int. 2001;12(11):922–930. [DOI] [PubMed] [Google Scholar]
- 18.Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18(6):1051–1056. [DOI] [PubMed] [Google Scholar]
- 19.Eastell R, Hannon RA, Garnero P, Campbell MJ, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate: review of statistical analysis. J Bone Miner Res. 2007;22(11):1656–1660. [DOI] [PubMed] [Google Scholar]
- 20.Sarkar S, Reginster JY, Crans GG, Diez-Perez A, Pinette KV, Delmas PD. Relationship between changes in biochemical markers of bone turnover and BMD to predict vertebral fracture risk. J Bone Miner Res. 2004;19(3):394–401. [DOI] [PubMed] [Google Scholar]
- 21.Dunford JE, Thompson K, Coxon FP, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296(2):235–242. [PubMed] [Google Scholar]
- 22.Green JR. Zoledronic acid: pharmacologic profile of a potent bisphosphonate. J Organomet Chem. 2005;690(10):2439–2448. [Google Scholar]
- 23.Roelofs AJ, Jauhiainen M, Mönkkönen H, Rogers MJ, Mönkkönen J, Thompson K. Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144(2):245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbour KE, Lui LY, Ensrud KE, et al. Inflammatory markers and risk of hip fracture in older white women: the study of osteoporotic fractures. J Bone Miner Res. 2014;29(9):2057–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbour KE, Boudreau R, Danielson ME, et al. Inflammatory markers and the risk of hip fracture: the Women’s Health Initiative. J Bone Miner Res. 2012;27(5):1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sprini D, Rini GB, Di Stefano L, Cianferotti L, Napoli N. Correlationbetween osteoporosis and cardiovascular disease. Clin Cases Miner Bone Metab. 2014;11(2):117–119. [PMC free article] [PubMed] [Google Scholar]
- 27.Tang M, Tian L, Luo G, Yu X. Interferon-gamma-mediated osteoimmunology. Front Immunol. 2018;9:1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The HORIZON-PFT datasets analyzed for the current report are proprietary to the original sponsor from whom permission is required for sharing.