Abstract
Mouse models suggest that undercarboxylated osteocalcin (ucOC), produced by the skeleton, protects against type 2 diabetes development, whereas human studies have been inconclusive. We aimed to determine if ucOC or total OC is associated with incident type 2 diabetes or changes in fasting glucose, insulin resistance (HOMA-IR), or beta-cell function (HOMA-Beta). A subcohort (n = 338; 50% women; 36% black) was identified from participants without diabetes at baseline in the Health, Aging, and Body Composition Study. Cases of incident type 2 diabetes (n = 137) were defined as self-report at an annual follow-up visit, use of diabetes medication, or elevated fasting glucose during 8 years of follow-up. ucOC and total OC were measured in baseline serum. Using a case-cohort design, the association between biomarkers and incident type 2 diabetes was assessed using robust weighted Cox regression. In the subcohort, linear regression models analyzed the associations between biomarkers and changes in fasting glucose, HOMA-IR, and HOMA-Beta over 9 years. Higher levels of ucOC were not statistically associated with increased risk of incident type 2 diabetes (adjusted hazard ratio = 1.06 [95% confidence interval, 0.84–1.34] per 1 standard deviation [SD] increase in ucOC). Results for %ucOC and total OC were similar. Adjusted associations of ucOC, %ucOC, and total OC with changes in fasting glucose, HOMA-IR, and HOMA-Beta were modest and not statistically significant. We did not find evidence of an association of baseline undercarboxylated or total osteocalcin with risk of incident type 2 diabetes or with changes in glucose metabolism in older adults.
Keywords: GENERAL POPULATION STUDIES, EPIDEMIOLOGY, OTHER, SYSTEMS BIOLOGY, BONE INTERACTORS, OTHER, CELL/TISSUE SIGNALING, ENDOCRINE PATHWAYS
Introduction
Studies conducted in mouse models have reported that osteocalcin (OC), specifically the undercarboxylated form, affects glucose homeostasis.(1,2) OC is secreted from the osteoblast and is a component of the extracellular matrix, and total OC is a standard marker of bone formation in humans. OC is carboxylated posttranslationally in the presence of vitamin K, and the circulating fraction of undercarboxylated osteocalcin (ucOC) is considered a sensitive marker of vitamin K status.(3) OC knockout mice show decreased insulin secretion and decreased insulin sensitivity in the liver, muscle, and adipose tissue.(1) Conversely, mice with high levels of ucOC demonstrate enhanced β-cell proliferation, increased insulin secretion by pancreatic beta-cells, and greater insulin sensitivity.(4)
Efforts to determine if OC affects glucose metabolism and the risk of type 2 diabetes in humans have produced inconsistent results. Multiple cross-sectional studies have reported lower total OC and ucOC in those with type 2 diabetes.(5–10) However, these cross-sectional results may reflect inhibition of bone turnover by type 2 diabetes, resulting in lower ucOC, rather than an effect of ucOC on type 2 diabetes risk.(11) Only four longitudinal studies have investigated ucOC, the active form of OC, and incident type 2 diabetes, and results have been conflicting.(12–15)
To further investigate the relationship between ucOC, total OC, and type 2 diabetes, we undertook a case-cohort study in the Health Aging and Body Composition (Health ABC) study. The primary objective was to determine the associations between baseline serum OC (ucOC, %ucOC, and total OC) and incident type 2 diabetes. A secondary objective was to assess associations between baseline serum OC and changes in fasting glucose, insulin resistance, and beta-cell function. We also assessed two other serum markers of bone turnover, N-propeptide of type I procollagen (P1NP) and C-terminal crosslinking telopeptide of type I collagen (CTX), to determine if bone turnover in general is associated with incident type 2 diabetes.
Subjects and Methods
Health ABC participants
The Health ABC study was a prospective study to investigate whether changes in body composition act as a common pathway by which multiple diseases affect morbidity, disability, and risk of mortality.(16) The cohort consists of 3075 black and white men and women aged 70–79 years recruited at the University of Pittsburgh and the University of Tennessee, Memphis. Participants were excluded if they reported any difficulty with activities of daily living, walking up 10 steps without resting, or walking a quarter of a mile. Study procedures were approved by the institutional review boards and written informed consent was provided by all participants. The baseline examination took place during 1997–1998.
Incident type 2 diabetes
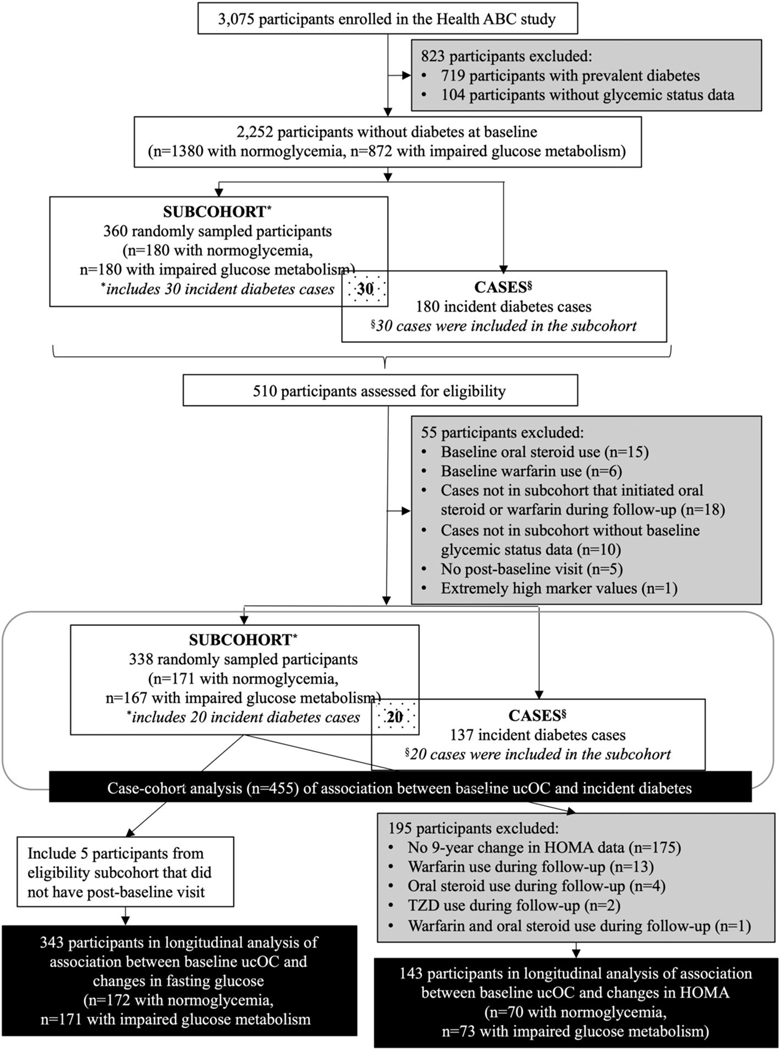
We utilized a case-cohort design to assess the association between baseline ucOC and incident diabetes (Fig. 1).(17) Those with prevalent diabetes, defined as baseline self-report of a diabetes diagnosis, use of diabetes medication, elevated serum fasting glucose (≥126 mg/dL), or elevated serum glucose 2 hours after a 75-g oral glucose tolerance test (OGTT) (≥200 mg/dL), were excluded.(18) At baseline, 719 of the 3075 participants had diabetes and were excluded. An additional 104 participants were excluded for lack of information on baseline glycemic status.
Fig. 1.
Health ABC participants included in case-cohort study of ucOC and incident type 2 diabetes.
For the subcohort, a random sample of participants without diabetes was identified, stratified by baseline glucose metabolism status. Half of the subcohort (n = 180) was randomly sampled from those with normal glucose metabolism (n = 1380), defined as a fasting glucose <100 mg/dL and a glucose of <140 mg/dL 2 hours after a 75-g OGTT. The others (n = 180) were sampled from those with impaired glucose metabolism (n = 872), defined as fasting glucose between 100 and 125 mg/dL or glucose between 140 and 199 mg/dL 2 hours after a 75-g OGTT.
Incident type 2 diabetes through year 8 was identified based on self-report at an annual follow-up visit, use of diabetes medication, or an elevated fasting glucose (≥126 mg/dL) (measured at year 1, year 3, and year 5 visits). Participants were not tested for presence of any islet cell autoantibody. In the random subcohort, 30 participants were also incident type 2 diabetes cases. An additional 150 cases were identified for a total of 180 incident cases. For the incident cases and subcohort, participants taking warfarin (n = 15) or oral steroids (n = 6) at baseline were excluded. Warfarin use reduces vitamin K and increases % ucOC(19) although effects on glucose metabolism are unknown. None of the participants in our analysis reported vitamin K supplement use at baseline. Oral steroids affect both risks of type 2 diabetes and bone turnover markers.(20) For the analyses of incident type 2 diabetes, five participants in the subcohort were excluded for not having at least one follow-up visit since diabetes status was identified at these visits. Participants who initiated warfarin or oral steroid use during study follow-up were censored at that visit. Incident cases who used warfarin or oral steroid during follow-up were excluded (n = 18). An additional 10 cases were excluded due to lack of fasting glucose data at baseline. One participant in the subcohort was excluded due to unusually high values of ucOC, total OC, and CTX more than 10 standard deviations (SDs) above the mean.
Thus, the analyses of incident type 2 diabetes include 455 participants: 171 subcohort participants with normal glucose metabolism at baseline (including three who are also cases), 167 subcohort participants with impaired glucose metabolism (including 17 who are also cases), and 137 cases of incident type 2 diabetes (of whom 20 were in the random subcohort).
Changes in fasting glucose
Fasting glucose was measured at five visits: baseline, year 1, year 3, year 5, and year 9. The association between baseline ucOC and changes in fasting glucose was assessed in the random subcohort of those with normoglycemia and those with impaired glucose metabolism included in the case-cohort analysis, plus the five participants excluded from the case-cohort analysis for lack of a follow-up visit. Analyses included 172 participants with normal glucose metabolism and 171 participants with impaired glucose metabolism at baseline.
Changes in insulin resistance and beta-cell function
HOMA-IR and HOMA-Beta were calculated using fasting glucose and fasting insulin,(21) measured at baseline and year 9. Starting with the random subcohort of 338 participants, 163 participants had data available for change in HOMA from baseline to year 9. Of these, 20 participants were excluded for use of the following medications at baseline or during follow-up: an oral steroid (n = 4), warfarin (n = 13), thiazolidinediones (TZDs) (n = 2), both oral steroid and warfarin (n = 1). The analyses of change in HOMA included 143 participants: 73 with impaired glucose metabolism and 70 with normoglycemia at baseline.
Osteocalcin and other bone turnover markers
Serum was obtained at the baseline visit after an overnight fast. The Health ABC study provided detailed instructions for obtaining, processing, and storing blood specimens. Blood drawn for serum was allowed to clot at room temperature for 40–90 minutes. Serum was then aliquotted and frozen at −70°C. Specimens were shipped on dry ice for long-term storage at McKesson Bioservices (Rockville, MD, USA). For this ancillary study, samples were retrieved fromthe storage facility and shipped on dry ice to author CMG’s lab. Samples were then placed into a −70°C freezer until assay. This ancillary study used samples without a previous freeze–thaw cycle. Hemolysis was noted at the time of the blood draw and processing; hemolyzed specimens were excluded from the serum used for this ancillary study.
Specimens were assayed for ucOC and other bone turnover markers after approximately 12 years of storage. Laboratory protocol included establishing linearity studies with every new batch of antiserum. Specific to this study, samples needing to be diluted for hydroxyapatite binding showed expected values. Assays were performed at the Department of Orthopedics, Yale University School of Medicine under the direction of author CMG.
Total OC, a 49 residue polypeptide, was measured in serum by an equilibrium radioimmunoassay utilizing a polyclonal antibody made against total OC purified from human bone in author CMG’s laboratory. The assay recognizes osteocalcin including intact OC and any fragments that contain the region from 7–42 and both carboxylated (cOC) and ucOC equivalently, providing a measure of total OC.(22) To measure ucOC, cOC was separated from ucOC by differential hydroxyapatite (HAP) binding. After adsorption of serum onto a standard suspension of HAP, ucOC is measured in the supernatant with the same equilibrium radioimmunoassay used for total OC. The binding assay depends upon both the concentration of OC in the sample and the concentration of HAP. Samples with total OC > 15 ng/mL are diluted with OC free serum to the concentration of 10 ng/mL and HAP binding repeated. Results are expressed as total OC, ucOC, and %ucOC (ucOC/total OC # 100%). Assay characteristics are computer derived for each assay. Interassay and intraassay coefficients of variation for ucOC are 6.7% and 3.2% respectively.(23)
Intact form of P1NP was measured with competitive radioimmunoassay (Orion Diagnostica UniQ, Espoo, Finland; distributed by Immunodiagnostic systems, Inc., Scottsdale, AZ, USA). Interassay and intraassay coefficients of variation were 7.7% and 2.2% respectively. CTX was quantitated by ELISA (Serum CrossLaps, Nordic Biosciences, Herlev, Denmark, distributed by Immunodiagnostic Systems). Interassay and intraassay coefficients of variation were 10.5% and 8.24%, respectively.
Fasting glucose and other laboratory assays
Laboratory measurements for Health ABC were performed at the Laboratory of Clinical Biochemistry, University of Vermont. For plasma glucose, blood was drawn after an overnight fast (≥8 hours). At baseline, participants ingested 75 g glucose in solution (glucola) immediately after blood was drawn for fasting glucose, and a second blood sample was drawn 2 hours later for the OGTT. Fasting glucose was measured at baseline, year 1, year 3, year 5, and year 9 visits. Fasting insulin was also measured on blood drawn after an overnight fast. HOMA-IR was calculated as [fasting glucose (mg/dL) × fasting insulin (μU/mL)]/405, and HOMA-Beta (%) was calculated as (360 × fasting insulin)/(fasting glucose − 63).(21) Cystatin C (cysC), a marker of renal function, was measured on baseline serum stored at −70°C for an average of 6.5 years using a BNII nephelometer (Dade Behring Inc., Deer-!eld, IL, USA) utilizing a particle-enhanced immunonephelometric assay (N Latex Cystatin C). Intraassay and interassay CVs for cysC are 2.0%−2.8% and 2.3%−3.1%, respectively.
Covariates
At the baseline and annual visits, participants completed a questionnaire. Phone visits instead of in-person visits were conducted at year 6 and year 8. Participants were asked about cigarette smoking (baseline, year 2, year 4, year 7, year 8, and year 9) and were categorized as “never, former, or current” smoker. Participants reported time spent walking and climbing stairs in the previous week at all visits. Approximate metabolic equivalent unit (MET) values were assigned to these activities to calculate a weekly energy expenditure estimate.(24) Participants were asked to bring prescription and over-the-counter medications used in the previous week to the baseline and annual visits, (except year 3, year 6, and year 8). Medications were coded according to the Iowa Drug Information System (IDIS).(25) In these analyses, bisphosphonates, calcitonin, raloxifene, fluorides, and teriparatide were grouped as “osteoporosis medications.”
At baseline, weight was measured with a calibrated balance beam scale. Height was measured with a Harpenden stadiometer. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). A composite physical performance score (range, 0–4) was calculated from tests of usual walk speed, narrow walk speed, chair stands, and standing balance, administered at baseline, year 3, year 5, and year 9.(26)
Statistical analyses
Baseline characteristics of participants by glycemia status in the random subcohort and in the incident type 2 diabetes cases were summarized using means and standard deviations (SDs) for continuous measures and counts and percentages for categorical measures. The distributions for our exposures of interest, ucOC, percent ucOC, total OC, P1NP, and CTX, were sufficiently normal, and transformation was not needed.
We determined minimum detectable effects (MDEs), assuming a type I error rate of 5%, 80% power, and correlation of 0.3 between covariates. For incident diabetes, the minimum detectable hazard ratio was 1.33 for a standardized continuous predictor. The minimum detectable correlations for the change in fasting glucose analyses was rho = 0.16 and for the change in HOMA-IR and HOMA-Beta analyses was rho = 0.24.
Incident type 2 diabetes
Cox proportional hazards models, with the Barlow weighting method and robust variance estimation to accommodate the stratified sampling and case-cohort design, were used to analyze the association between ucOC and time to incident type 2 diabetes.(27) Barlow’s method for weighting study participants in the pseudolikelihood was used to account for the stratified sampling of the subcohort. Incident diabetes cases were weighted by 1 and subcohort controls were weighted by the inverse of the sampling fraction (α= n/N, where n is the number of participants in a given glycemic group in the random subcohort, and N is the number of participants in that glycemic group among all participants at baseline, after applying medication exclusions): α = 171/1291 = 13.2% for participants with normal glucose metabolism and α = 167/819 = 20.4% for participants with impaired glucose metabolism.(28) Date of onset for incident type 2 diabetes was defined as the visit date when diabetes was identified. Minimally adjusted models included baseline age, race (black/white), sex, clinic site, and baseline glycemia stratum. To develop multivariable models, a full model was constructed that included the measures from the minimally adjusted models, plus baseline cystatin-C and the following time-varying variables: smoking status, oral estrogen use, osteoporosis medication use, statin use, thiazide diuretic use, physical activity, and physical performance score. Baseline age, race, gender, clinic site, baseline glycemia stratum, and baseline BMI were retained in the models regardless of p value; other variables were removed from the model one at a time if the p value was greater than 0.2 and if the regression coefficient for ucOC did not change by more than 10% with the removal of the variable. This approach was also used to evaluate the associations between incident type 2 diabetes and total OC, %ucOC, P1NP, and CTX. All variables except physical activity were retained in the final multivariable model. A quadratic term for ucOC or total OC was included to test for linearity and was statistically significant (p < 0.001) only for %ucOC. The risk of incident type 2 diabetes per 1 SD increase in bone turnover marker was estimated, with results presented as hazard ratios (HRs) and 95% confidence intervals (CIs). As a sensitivity analysis, we assessed the relationship between baseline markers and incident diabetes limited to 5 years of follow up, instead of 8 years.
Changes in fasting glucose
Random effects regression models were used to analyze the association between baseline marker levels and subsequent change in fasting glucose. Random effects models account for between-subject variation and within-subject correlations between repeated fasting glucose measurements. Model coefficients were estimated using the restricted maximum likelihood method. Time was modeled as a continuous covariate, measured as the number of years from the baseline to follow-up fasting glucose measurements. The distributions of fasting glucose were skewed at each visit, so the data were log-transformed for normality. Results are back-transformed, thus providing the mean difference in 9-year percent change in fasting glucose per 1 SD increase in bone turnover marker. Continuous covariates were centered at the baseline mean for those covariates that were measured only at baseline (bone turnover markers, age, BMI, cystatin-C) and at the mean across all visits for those covariates that were measured at each visit (physical performance score). Minimally adjusted models and multivariable models included the same variables as described above for the incident type 2 diabetes outcome. There was no evidence of nonlinearity.
Changes in HOMA
Linear regression models were used to analyze the associations between baseline marker levels and 9-year changes in HOMA-IR and HOMA-Beta, with results presented as the mean difference and 95% CI in 9-year change in HOMA per 1 SD increase in bone turnover marker. Minimally adjusted models and multivariable models included the same variables as described above for the incident type 2 diabetes outcome. There was no evidence of nonlinearity.
We tested for gender interactions for the biomarkers of interest and the outcomes of incident diabetes, and changes in fasting glucose, HOMA-IR, and HOMA-Beta. None of the interactions were statistically significant based on p = 0.05. All statistical analyses were performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics
Baseline characteristics for the random subcohort and the incident type 2 diabetes cases (n = 137) are provided in Table 1. Among those in the subcohort with normal glucose metabolism (n = 172), the average age was 73.4 (SD 2.7) years, 53% were women, and 33% were black. Participants with impaired glucose metabolism (n = 171) were 74.1 (SD 3.0) years, 53% were men, and 40% were black.
Table 1.
Baseline Characteristics of Participants by Glycemia Status in the Random Subcohort and in the Incident Type 2 Diabetes Cases
| Normoglycemia (n = 172) | Impaired glucose metabolism (n = 171) | Incident type 2 diabetes cases (n = 137) | |
|---|---|---|---|
|
| |||
| Age (years) | 73.5 ± 2.7 | 74.1 ± 3.0 | 73.3 ± 3.1 |
| Gender | |||
| Male | 80 (46.5) | 90 (52.6) | 66 (48.2) |
| Female | 92 (53.5) | 81 (47.4) | 71 (51.80) |
| Race | |||
| White | 115 (66.9) | 103 (60.2) | 65 (47.5) |
| Black | 57 (33.1) | 68 (39.8) | 72 (52.6) |
| Smoking status | |||
| Never | 77 (44.8) | 70 (41.2) | 58 (42.3) |
| Current | 21 (12.2) | 18 (10.6) | 11 (8.0) |
| Former | 74 (43.0) | 82 (48.2) | 68 (49.6) |
| BMI (kg/m2) | 26.4 ± 4.2 | 28.6 ± 5.4 | 29.3 ± 4.6 |
| Weight (kg) | 73.5 ± 13.4 | 79.0 ± 16.3 | 80.7 ± 14.4 |
| Physical activity from walking+stairs (kcal/kg/week) | 7.6 ± 10.7 | 5.9 ± 8.0 | 6.3 ± 11.0 |
| HABC physical performance summary score | 2.3 ± 0.5 | 2.2 ± 0.5 | 2.2 ± 0.6 |
| Serum cystatin-C (mg/L) | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.5 |
| Oral estrogen use | 24 (14.0) | 25 (14.7) | 12 (8.8) |
| Osteoporosis medication use | 10 (5.8) | 6 (3.5) | 3 (2.2) |
| Statin use | 19 (11.1) | 28 (16.5) | 27 (19.7) |
| Thiazide diuretic use | 24 (14.0) | 33 (19.4) | 29 (21.2) |
| Fasting serum glucose (mg/dL) | 89.2 ± 61 | 98.9 ± 10.0 | 101.6 ± 10.9 |
| Fasting serum insulin (μIU/mL) | 7.1 ± 4.0 | 9.6 ± 5.9 | 10.2 ± 5.3 |
| Fasting serum glucose 2 hour post-GTT (mg/dL) | 104.0 ± 19.8 | 146.2 ± 28.1 | 141.3 ± 34.6 |
| HOMA-IR | 1.6 ± 1.0 | 2.4 ± 1.6 | 2.6 ± 1.4 |
| HOMA-beta (%) | 100.8 ± 55.4 | 94.8 ± 52.3 | 102.2 ± 53.2 |
| Undercarboxylated osteocalcin (ng/mL) | 3.1 ± 2.1 | 3.0 ± 2.1 | 3.3 ± 2.0 |
| ucOC percent of total osteocalcin (%) | 32.4 ± 14.4 | 32.7 ± 13.2 | 33.9 ± 16.3 |
| Total osteocalcin (ng/mL) | 8.9 ± 3.7 | 8.8 ± 3.8 | 9.4 ± 3.7 |
| P1NP (ng/mL) | 46.4 ± 21.1 | 45.2 ± 24.6 | 51.0 ± 26.8 |
| CTX (ng/mL) | 0.56 ± 0.33 | 0.54 ± 0.31 | 0.56 ± 0.29 |
Data in table are presented as mean ± SD or n (%)
Incident type 2 diabetes
We did not find evidence that higher levels of ucOC or %ucOC were associated with risk of incident type 2 diabetes in minimally adjusted models or multivariable models (Fig. 2). For example, in the multivariable model, each 1 SD increase in serum ucOC was associated with 1.06 (95% CI, 0.84–1.34) times higher risk of incident type 2 diabetes. There was evidence of nonlinearity in the relationship between %ucOC and incident diabetes (p < 0.001). The middle tertile had the lowest risk of incident diabetes (HR 1.13 [95% CI, 0.65–1.97] comparing lowest with middle tertile and HR 1.37 [95% CI, 0.78–2.39] comparing highest with middle tertile). However, none of the associations were statistically significant. We report the linear relationship between %ucOC and incident diabetes in Fig. 2. Total osteocalcin was positively associated with incident type 2 diabetes risk in the minimally adjusted model (HR 1.27; 95% CI, 1.04–1.56, per 1 SD increase in total OC), but the association was attenuated, primarily by adjustment for renal function, and no longer statistically significant in the multivariable model (HR 1.09; 95% CI, 0.84–1.42). P1NP, a marker of bone formation, was also positively associated with incident type 2 diabetes in the minimally adjusted model (HR 1.32; 95% CI, 1.07–1.63), but results were no longer statistically significant in the multivariable model (HR 1.24; 95% CI, 0.96–1.59). Serum CTX, a bone resorption marker, was not significantly associated with incident type 2 diabetes in either model. In a sensitivity analysis we assessed the relationship between baseline markers (ucOC, %ucOC, total OC, P1NP, and CTX) and incident diabetes limited to 5 years of follow-up, reducing the number of incident DM cases to 104. The ucOC results of multivariable adjusted models for 5 years of follow-up were similar to our findings for 8-year follow-up (5-year risk for incident type 2 diabetes per 1 SD increase in ucOC was HR 1.13; 95% CI, 0.88–1.46 compared to 8-year HR 1.06; 95% CI, 0.84–1.34).
Fig. 2.
Hazard ratio (95% CI) for incident type 2 diabetes per 1 SD increase in biomarker. Minimally adjusted (min adj) models were adjusted for age, race, gender, clinic site, and baseline glycemia status. Multivariable adjusted (mv adj) models were adjusted for variables in the minimally adjusted models plus baseline cystatin-C and baseline BMI, and for the following time-varying variables: Smoking status, oral estrogen use, osteoporosis medication use, statin use, thiazide diuretic use, and physical performance score.
Changes in fasting glucose
Among participants in the random subcohort, the average increase in fasting glucose over 9 years of follow-up was 3.4% (95% CI, 0.6%–6.3%). We found no evidence that baseline ucOC, %ucOC, and total OC were associated with changes in fasting glucose (Table 2). We also found no evidence of associations for P1NP and CTX.
Table 2.
Mean Difference in 9-Year Changes in Glucose Metabolism Markers per 1 SD Increase in Bone Turnover Markers
| Fasting glucose (n = 343) | HOMA-IR (n = 143) | HOMA-Beta (n = 143) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Minimally adjusteda | Multivariable adjustedb | Minimally adjusteda | Multivariable adjustedb | Minimally adjusteda | Multivariable adjustedb | |
|
|
||||||
| Parameter | Mean difference in % change (95% Cl) | Mean difference in change (95% Cl) | Mean difference in change (95% Cl) | |||
|
| ||||||
| ucOC | 0.50 (−1.21,2.22) | 0.57 (−1.35, 2.50) | 0.28 (−0.04, 0.60) | 0.24 (−0.12, 0.59) | 2.88 (−9.63, 15.40) | 1.68 (−13.26, 16.61) |
| %ucOC | 0.41 (−1.27,2.10) | 0.51 (−1.24,2.28) | 0.17 (−0.15, 0.50) | 0.17 (−0.14, 0.48) | −0.13 (−12.75,12.49) | −3.35 (−16.59, 9.89) |
| Total OC | 0.71 (−1.14,2.56) | 0.67 (−1.49, 2.84) | 0.38 (0.06, 0.69) | 0.28 (−0.11,0.68) | 5.51 (−6.88, 17.91) | 6.34 (−10.22, 22.90) |
| PI NP | 1.89 (−0.70, 4.48) | 2.37 (−0.53, 5.29) | 0.09 (−0.23, 0.41) | 0.03 (−0.40, 0.46) | 3.89 (−8.57, 16.35) | 10.27 (−7.61, 28.14) |
| CTX | 0.77 (−1.20,2.74) | 1.17 (−0.88, 3.23) | 0.15 (−0.17, 0.47) | 0.13 (−0.24, 0.49) | 6.81 (−5.57, 19.19) | 7.72 (−7.56, 23.01) |
Adjusted for age, race, gender, clinic site, and baseline glycemia status.
Adjusted for variables in the minimally adjusted models plus baseline cystatin-C and baseline BMI, and for the following time-varying variables: smoking status, oral estrogen use, osteoporosis medication use, statin use, thiazide diuretic use, and physical performance score.
Changes in HOMA-IR and HOMA-Beta
Among the 143 participants in the random subcohort with change in HOMA data, the average 9-year increase in HOMA-IR was 0.58 (SD 1.90), and the average 9-year increase in HOMA-Beta was 5.5 (SD 76.8). There was no evidence that baseline ucOC, %ucOC, and total OC were associated with changes in HOMA-IR or HOMA-Beta (Table 2 and Supplemental Figure S1). There was also no evidence of an association between baseline levels of P1NP or CTX and changes in HOMA-IR or HOMA-Beta.
Discussion
We did not find evidence that higher levels of ucOC are protective of incident type 2 diabetes, despite rodent studies showing that ucOC regulates glucose metabolism. Furthermore, there was no evidence of ucOC affecting glucose metabolism, including changes in fasting glucose, insulin resistance, and beta-cell function, in these older black and white men and women.
There have been a number of cross-sectional studies that show lower ucOC(10) or total OC(5–9) associated with prevalent type 2 diabetes, including in our cohort.(29) These results are consistent with data from the mouse model, but they are also consistent with evidence that type 2 diabetes inhibits bone turnover.(11,30) Indeed, a recent meta-analysis of cross-sectional studies demonstrated that CTX and P1NP, in addition to OC, are lower in patients with type 2 diabetes compared to controls.(11)
Limited longitudinal studies have investigated the relation of total OC with incident type 2 diabetes, and the results have been inconsistent. A meta-analysis of three studies reported a pooled relative risk for incident type 2 diabetes of 0.89 (95% CI, 0.78–1.01) comparing the top total OC quartile to the lower quartiles.(31) Since this meta-analysis, an additional five longitudinal studies with analyses of total OC have been published.(12,15,32–34) 34) Similar to our findings for total OC, in the largest longitudinal study to date, Zwakenberg and colleagues(12) reported that total OC was not associated with incident type 2 diabetes (adjusted odds ratio [OR] 0.97; 95% CI, 0.91–1.03 for each ng/mL increase in total OC). Our study adds to the evidence that total OC does not affect type 2 diabetes risk in humans.
The more important question is the effect of ucOC, the active component of OC based on rodent models, on type 2 diabetes incidence, and this has been assessed in only four other studies. Similar to our findings, Zwakenberg and colleagues(12) found that ucOC and %ucOC were not associated with incident type 2 diabetes (adjusted OR 0.97; 95% CI, 0.88–1.08 for each ng/mL increase in ucOC; adjusted OR 1.01; 95% CI, 0.97–1.06 for each 5% increment in %ucOC). Iki and colleagues(15) also reported no association between ucOC and incident type 2 diabetes. In contrast, Diaz-López and colleagues(14) reported a protective effect of higher ucOC and Ngarmukos and colleagues(13) a trend for a protective effect.
Our results are consistent with more recent OC-deficient rodent models showing no effects of this phenotype on glucose metabolism,(35,36) in contrast to the models identifying ucOC as a hormone affecting insulin sensitivity.(1) The reasons for this discrepancy in the mouse models are not clear but are the focus of ongoing research. Possible differences include genetic background, modifier genes, and molecular genetics of the knockout alleles.(37,38)
One potential reason for the divergent results in longitudinal studies of ucOC and type 2 diabetes may be use of different assays for ucOC. In the study by Zwakenberg and colleagues(12) and our study, both reporting null associations, ucOC was measured via hydroxyapatite (HAP) binding assay. In the studies reporting a protective effect(14) or a trend for a protective effect,(13) ucOC was measured by a solid-phase enzyme immunoassay (EIA) kit specific for ucOC (TaKaRa Bio, Otsu, Japan). This assay provides a higher estimate of ucOC than the HAP-binding method, possibly due to identification of a wider range of fragments than the HAP-binding assay.(22,23,39) However, the third study reporting a null association(15) used a method that is likely to have similar performance to the Takara assay, an electrochemiluminescence immunoassay (ECLIA) (Picolumi ucOC; Sanko Junyaku Co. Ltd., Tokyo, Japan), which relies on the monoclonal antibody measuring the same epitopes as TaKaRa Bio.(40)
We considered whether differences in the underlying study populations might have contributed to the inconsistent results in the longitudinal studies. However, we could not identify a clear pattern of differences by age, sex or race/ethnicity, comparing studies that found protective versus null associations. The studies finding no protective effect of ucOC included our Health ABC study, with a study population consisting of older black and white women and men in the United States. Zwakenberg and colleagues(12) studied participants with a wider age range (21–70 years) from the Dutch EPIC-NL cohort. The study by Iki and colleagues(15) included older men in Japan. Of the two studies reporting a protective effect, Diaz-López and colleagues(14) included older men and women in Spain at high risk of cardiovascular events. Ngarmukos and colleagues(13) studied younger participants (35–55 years) in Thailand. This lack of a consistent pattern suggests that the differences in results are not explained by effect modification by age, gender, or race/ethnicity, though further comparisons are needed across age strata, race/ethnicity, and comorbid disease.
We did not find evidence of associations between other bone turnover markers (P1NP and CTX) and incident type 2 diabetes. Others have reported a negative(33) or U-shaped(41) association between serum CTX and incident type 2 diabetes. To our knowledge, there are no published studies of P1NP and incident type 2 diabetes.
Strengths of our study include a well characterized cohort with annual assessment of diabetes status as well as longitudinal measurements of fasting glucose, HOMA-IR and HOMA-Beta. UcOC was assayed using the more accurate hydroxyapatite binding assay.(23) Measurements of potential confounders, such as medication use and baseline glycemia status, were available. The bone turnover markers P1NP and CTX were also available for comparison. Our cohort included men and women, and black and white participants.
The study also has some limitations. Incident type 2 diabetes was identified based on self-report at an annual follow-up visit, use of diabetes medication, or an elevated fasting glucose (measured at year 1, year 3, and year 5 visit). However, glycated hemoglobin (HbA1c) was not available, and we might have misclassified incident diabetes status in some participants. Misclassification of diabetes status was not likely to have differed by exposure status and would tend to bias results towards the null. Our exposures were only measured at baseline which might not accurately reflect values during follow-up, resulting in an attenuation of associations. However, our sensitivity analysis, assessing associations of ucOC, %ucOC, and total OC with incident diabetes after only 5 years of follow-up, failed to find a protective effect on incident diabetes, and our point estimates were similar to 8 years of follow-up. This suggests that potential attenuation due to measurement of exposure only at baseline is not likely to account for our findings. OC was measured after approximately 12 years of storage. We are not aware of published studies on OC stability during long-term storage. However, freeze–thaw cycles appear to have little effect on OC or ucOC measurements.(42) To assess if there was a substantial degradation of osteocalcin, we compared our OC and ucOC results with other published studies. The values we obtained listed in Table 1 are within the variation reported by others.(43) Although we did not find evidence of an association with incident diabetes, we could not completely exclude a protective or a detrimental effect given our 95% confidence intervals. Health ABC participants were healthier than the general population in their 70s, as participants had to be functional at baseline recruitment, thus results may not apply to other populations.
This study adds to the evidence that ucOC does not have an effect on incident type 2 diabetes in humans. However, the number of longitudinal studies of ucOC and diabetes is limited, and the overall evidence remains inconclusive. Further longitudinal studies are needed in humans, particularly studies that assess ucOC as well as total OC.
Conclusion
We found no evidence of an association between baseline undercarboxylated or total osteocalcin and incident type 2 diabetes in our study. If there is an association, it is likely to be modest, below a level our study was powered to assess.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, National Institute of Nursing Research grant R01-NR12459, National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) grant R21 DK082848 (AVS) and T32 grant T32DK007418 (MEB). This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging.
Authors roles: All authors reviewed and approved the final version. MEB, ESS, NN, ALS, EV, CMG, and AVS conceptualized the study. AVS and EV planned and designed the study in detail. AVS acquired the funding. AVS and ESS administered the project. Under the direction of CGM ucOC and other bone turnover markers were assayed. SKE and EV formally analyzed the data. MEB, SKE, ESS, and AVS drafted the manuscript. MEB, SKE, ESS, NN, ALS, EV, CMG, and AVS reviewed and edited the manuscript. AVS is the guarantor of this work and takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Footnotes
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4519.
Disclosure
All authors declare no conflict of interest.
Data Availability Statement
The data that support the findings of this study may be requested from the National Institue of Aging on the Health ABC website at https://healthabc.nia.nih.gov/.
References
- 1.Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferron M, Wei J, Yoshizawa T, et al. Insulin signaling in osteoblastsintegrates bone remodeling and energy metabolism. Cell. 2010; 142:296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binkley NC, Krueger DC, Engelke JA, Foley AL, Suttie JW. Vitamin K supplementation reduces serum concentrations of under γ-carboxylated osteocalcin in healthy young and elderly adults. Am J Clin Nutr. 2000;72:1523–1528. [DOI] [PubMed] [Google Scholar]
- 4.Wei J, Karsenty G. An overview of the metabolic functions of osteocalcin. Rev Endocr Metab Disord. 2015;16:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choudhury AB, Sarkar PD, Sakalley DK, Petkar SB. Role of adiponectinin mediating the association of osteocalcin with insulin resistance and type 2 diabetes: a cross sectional study in pre- and postmenopausal women. Arch Physiol Biochem. 2014;120:73–79. [DOI] [PubMed] [Google Scholar]
- 6.Movahed A, Larijani B, Nabipour I, et al. Reduced serum osteocalcin concentrations are associated with type 2 diabetes mellitus and the metabolic syndrome components in postmenopausal women: the crosstalk between bone and energy metabolism. J Bone Miner Metab. 2012;30:683–691. [DOI] [PubMed] [Google Scholar]
- 7.Hwang YC, Jee JH, Jeong IK, Ahn KJ, Chung HY, Lee MK. Circulating Osteocalcin level is not associated with incident type 2 diabetes in middle-aged male subjects: mean 8.4-year retrospective follow-up study. Diabetes Care. 2012;35:1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou M, Ma X, Li H, et al. Serum osteocalcin concentrations in relationto glucose and lipid metabolism in Chinese individuals. Eur J Endocrinol. 2009;161:723–729. [DOI] [PubMed] [Google Scholar]
- 9.Lerchbaum E, Schwetz V, Nauck M, Völzke H, Wallaschofski H,Hannemann A. Lower bone turnover markers in metabolic syndrome and diabetes: the population-based study of health in Pomerania. Nutr Metab Cardiovasc Dis. 2015;25:458–463. [DOI] [PubMed] [Google Scholar]
- 10.Yeap BB, Alfonso H, Chubb SA, et al. Higher serum undercarboxylated osteocalcin and other bone turnover markers are associated with reduced diabetes risk and lower estradiol concentrations in older men. J Clin Endocrinol Metab. 2015;100:63–71. [DOI] [PubMed] [Google Scholar]
- 11.Hygum K, Starup-Linde J, Harsløf T, Vestergaard P, Langdahl BL. Mechanisms in endocrinology: diabetes mellitus, a state of low bone turnover—a systematic review and meta-analysis. Eur J Endocrinol. 2017;176:R137–R157. [DOI] [PubMed] [Google Scholar]
- 12.Zwakenberg SR, Gundberg CM, Spijkerman AM, van der A DL, van der Schouw YT, Beulens JW. Osteocalcin is not associated with the risk of type 2 diabetes: findings from the EPIC-NL study. PLoS One. 2015;10: e0138693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngarmukos C, Chailurkit L-O, Chanprasertyothin S, Hengprasith B, Sritara P, Ongphiphadhanakul B. A reduced serum level of total osteocalcin in men predicts the development of diabetes in a long-term follow-up cohort. Clin Endocrinol (Oxf). 2012;77:42–46. [DOI] [PubMed] [Google Scholar]
- 14.Díaz-López A, Bulló M, Juanola-Falgarona M, et al. Reduced serum concentrations of carboxylated and undercarboxylated osteocalcin are associated with risk of developing type 2 diabetes mellitus in a high cardiovascular risk population: a nested case-control study. J Clin Endocrinol Metabol. 2013;98:4524–4531. [DOI] [PubMed] [Google Scholar]
- 15.Iki M, Yura A, Fujita Y, et al. Circulating osteocalcin levels were not significantly associated with the risk of incident type 2 diabetes mellitus in elderly Japanese men: the Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) cohort study. Bone. 2021;147:115912. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd JT, Alley DE, Hochberg MC, et al. Changes in bone mineral density over time by body mass index in the health ABC study. Osteoporos Int. 2016;27:2109–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohortdesigns. J Clin Epidemiol. 1999;52:1165–1172. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in Diabetes-2020. Diabetes Care. 2020;43:S14–S31. [DOI] [PubMed] [Google Scholar]
- 19.Schurgers LJ, Shearer MJ, HamulyáK K, StöCklin E, Vermeer C. Effect of vitamin K intake on the stability of oral anticoagulant treatment: dose-response relationships in healthy subjects. Blood. 2004;104: 2682–2689. [DOI] [PubMed] [Google Scholar]
- 20.Devogelaer J-P, Durnez A, Gruson D, Manicourt DH. Bone Turnover Markers and Glucocorticoid Treatments. Netherlands: Springer; 2017. pp 905–932. [Google Scholar]
- 21.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. [DOI] [PubMed] [Google Scholar]
- 22.Rehder DS, Gundberg CM, Booth SL, Borges CR. Gamma-carboxylation and fragmentation of osteocalcin in human serum defined by mass spectrometry. Mol Cell Proteomics. 2015;14:1546–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gundberg CM, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metabol. 1998;83:3258–3266. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. [DOI] [PubMed] [Google Scholar]
- 25.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. [DOI] [PubMed] [Google Scholar]
- 26.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higherlevel physical function in well-functioning older adults: expanding familiar approaches in the health ABC study. J Gerontol Ser A. 2001; 56:M644–M649. [DOI] [PubMed] [Google Scholar]
- 27.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 28.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50:1064–1072. [PubMed] [Google Scholar]
- 29.Napoli N, Conte C, Eastell R, et al. Bone turnover markers do notpredict fracture risk in type 2 diabetes. J Bone Miner Res. 2020;35: 2363–2371. [DOI] [PubMed] [Google Scholar]
- 30.Napoli N, Strollo R, Paladini A, Briganti SI, Pozzilli P, Epstein S. Thealliance of mesenchymal stem cells, bone, and diabetes. Int J Endocrinol. 2014;2014:690783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunutsor SK, Apekey TA, Laukkanen JA. Association of serum totalosteocalcin with type 2 diabetes and intermediate metabolic phenotypes: systematic review and meta-analysis of observational evidence. Eur J Epidemiol. 2015;30:599–614. [DOI] [PubMed] [Google Scholar]
- 32.Shu H, Pei Y, Chen K, Lu J. Significant inverse association between serum osteocalcin and incident type 2 diabetes in a middle-aged cohort. Diabetes Metab Res Rev. 2016;32:867–874. [DOI] [PubMed] [Google Scholar]
- 33.Massera D, Biggs ML, Walker MD, et al. Biochemical markers of boneturnover and risk of incident diabetes in older women: the Cardiovascular Health Study. Diabetes Care. 2018;41:1901–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urano T, Shiraki M, Kuroda T, et al. Low serum osteocalcin concentration is associated with incident type 2 diabetes mellitus in Japanese women. J Bone Miner Metab. 2018;36:470–477. [DOI] [PubMed] [Google Scholar]
- 35.Moriishi T, Ozasa R, Ishimoto T, et al. Osteocalcin is necessary for thealignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. PLoS Genet. 2020;16:e1008586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diegel CR, Hann S, Ayturk UM, et al. An osteocalcin-deficient mouse strain without endocrine abnormalities. PLoS Genet. 2020;16:e1008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manolagas SC. Osteocalcin promotes bone mineralization but is nota hormone. PLoS Genet. 2020;16:e1008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karsenty G The facts of the matter: what is a hormone? PLoS Genet.2020;16:e1008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vergnaud P, Garnero P, Meunier PJ, BréArt G, Kamihagi K, Delmas PD. Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: the EPIDOS Study. J Clin Endocrinol Metab. 1997;82:719–724. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura J, Arai N, Tohmatsu J. Measurement of serum undercarboxylated osteocalcin by ECLIA with the “Picolumi ucOC” kit. Clin Calcium. 2007;17:1702–1708. [PubMed] [Google Scholar]
- 41.Liu T-T, Liu D-M, Xuan Y, et al. The association between the baseline bone resorption marker CTX and incident dysglycemia after 4 years. Bone Res. 2017;5:17020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lacombe J, Rifai OA, Loter L, et al. Measurement of bioactive osteocalcin in humans using a novel immunoassay reveals association with glucose metabolism and β-cell function. Am J Physiol Endocrinol Metab. 2020;318:E381–E391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shea MK, Gundberg CM, Meigs JB, et al. Gamma-carboxylation of osteocalcin and insulin resistance in older men and women. Am J Clin Nutr. 2009;90:1230–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study may be requested from the National Institue of Aging on the Health ABC website at https://healthabc.nia.nih.gov/.