Abstract
Objectives
To identify risk factors for progression to severe COVID-19 and estimate the odds of severe COVID-19 associated with vaccination among patients with systemic lupus erythematosus (SLE).
Methods
This retrospective cohort study identified adults with SLE in the Merative™ MarketScan® Databases. Patients were continuously enrolled the year before 1 April 2020 (baseline) and had a COVID-19 diagnosis between 1 April 2020 and the earliest of death, enrolment end or 31 December 2021. Severe COVID-19 was defined as hospitalisation with a COVID-19 diagnosis. Demographics on 1 April 2020, baseline comorbidities, corticosteroid use ≤30 days before COVID-19 diagnosis and other SLE medication use ≤6 months before COVID-19 diagnosis were assessed. Vaccination was identified by claims for a COVID-19 vaccine or vaccine administration. Backward stepwise logistic regression estimated odds of progression to severe COVID-19 associated with patient characteristics and vaccination.
Results
Among 2890 patients with SLE with COVID-19, 500 (16.4%) had a COVID-19-related hospitalisation. Significant risk factors for progression to severe COVID-19 included rituximab (OR (95% CI) 2.92 (1.67 to 5.12)), renal failure (2.15 (95% CI 1.56 to 2.97)), Medicaid (vs Commercial; 2.01 (95% CI 1.58 to 2.57)), complicated hypertension (1.96 (95% CI 1.38 to 2.77)) and time of infection, among others. Vaccination had a significant protective effect (0.68(95% CI 0.54 to 0.87)) among all patients with SLE with COVID-19, but the effect was not significant among those with prior use of belimumab, rituximab or corticosteroids.
Conclusions
Certain chronic comorbidities and SLE medications increase the odds of progression to severe COVID-19 among patients with SLE, but vaccination confers significant protection. Vaccine effectiveness may be attenuated by SLE treatments. Protective measures such as pre-exposure prophylaxis and booster vaccines should be encouraged among patients with SLE.
Keywords: COVID-19, Biological Therapy, Immune System Diseases, Vaccination
WHAT IS ALREADY KNOWN ON THIS TOPIC
Patients with immune-mediated inflammatory diseases (IMIDs), including systemic lupus erythematosus (SLE), are at greater risk of severe outcomes from COVID-19.
Response to COVID-19 vaccines may be reduced in patients with IMIDs, due in part to the use of immunosuppressive medications.
WHAT THIS STUDY ADDS
Chronic conditions such as hypertension and renal failure, as well as use of rituximab and corticosteroids, are associated with greater risk of severe COVID-19 among patients with SLE.
Vaccination is generally protective against severe COVID-19 among patients with SLE, though the use of common SLE treatments may attenuate vaccine effectiveness.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Mitigation of risk for COVID-19 infection and severe COVID-19 outcomes should be encouraged among patients with SLE, including pharmacological and non-pharmacological methods.
Introduction
By the end of 2022, there had been approximately 100.6 million cases of COVID-19 and more than 1 million COVID-19-related deaths in the USA.1 About 5% of COVID-19 cases nationwide have been severe enough to require hospitalisation, though that rate has varied over time with the emergence of different variants and the introduction of vaccines.1 2 Throughout the pandemic, many reports have indicated a greater risk of severe COVID-19 outcomes among patients with immune-mediated inflammatory diseases (IMIDs) such as systemic lupus erythematosus (SLE), as well as an increase in severe COVID-19 risk associated with IMID treatments.3–7 Age and multiple comorbidities have also been associated with worse COVID-19 outcomes in the general US population and among patients with IMIDs, including renal disease, hypertension and diabetes.6–9
The rapid development and deployment of COVID-19 vaccines represented a great step forward in protecting the US population from severe COVID-19. However, reduced vaccine response and breakthrough infections, often associated with use of immunosuppressive therapies, have been observed among individuals with IMIDs, including patients with SLE.10–17 A better understanding of the characteristics associated with severe COVID-19 and the real-world effect of vaccination among patients with SLE is necessary to counsel patients with SLE about their risk, and help triage patients with SLE for COVID-19 preventative measures and treatment. The purpose of this study was to identify risk factors for progression to hospitalisation (ie, severe COVID-19) among patients with SLE diagnosed with COVID-19, and estimate the effect of vaccination on the risk of severe COVID-19.
Methods
Study design and data sources
This retrospective observational cohort study used health insurance claims data from the Merative™ MarketScan® Commercial, Medicare and Multi-State Medicaid Research Databases between 1 January 2013 and 31 December 2021. The Commercial and Medicare Databases contain the longitudinal healthcare experience (medical and pharmacy) of approximately 30 million patients annually with employer-sponsored commercial or Medicare insurance from across all US Census regions. The Medicaid Database contains medical and pharmacy data for approximately 12 million Medicaid-eligible patients annually in geographically disperse US states. All patient records were deidentified and certified to be fully compliant with US patient confidentiality requirements set forth in the Health Insurance Portability and Accountability Act of 1996.
Patient and public involvement
Neither patients nor the public were involved in the design or conduct of this study.
Patient selection
Patients with SLE were identified by at least one inpatient or at least two non-diagnostic (ie, not for a diagnostic test or procedure) outpatient claims (30–365 days apart) between 1 January 2013 and 31 March 2020 with an SLE diagnosis (International Classification of Diseases, ninth Revision (ICD-9) code 710.0; ICD-10 codes M320, M3210–M3219, M328, M329); the earliest qualifying claim was considered the reference event. To improve the identification of patients with confirmed SLE, at least one additional inpatient or non-diagnostic outpatient SLE diagnosis claim was then required at least 365 days after the reference event. Commercial and Medicare patients meeting these criteria via outpatient claims only were also required to have an outpatient SLE diagnosis from a rheumatologist or nephrologist, or an inpatient SLE diagnosis, between the reference event and 1 April 2020. All qualifying patients were enrolled in the database and at least 18 years old on 1 April 2020, which was the index date for all patients. Qualifying patients with SLE were also required to be continuously enrolled in the database for 12 months (baseline period) prior to the index date.
Patients meeting the above criteria were then assessed for a COVID-19 diagnosis during a variable-length follow-up period, beginning with the index date and ending with the earliest of death, end of database enrolment, or end of the study period (31 December 2021 for Commercial/Medicare; 30 June 2021 for Medicaid). COVID-19 was identified by an inpatient or non-diagnostic outpatient claim with ICD-10 diagnosis code U071 or J1282. Qualifying patients with SLE with a COVID-19 diagnosis comprise the population for this analysis.
Variable assessment
The outcome of interest for this analysis was evidence of progression to severe COVID-19 among patients with SLE diagnosed with COVID-19. Among all patients with SLE with a COVID-19 diagnosis, patients with severe COVID-19 were identified as those with an inpatient medical claim with a COVID-19 diagnosis code in any position on the claim (primary or secondary) during the follow-up period. All patients without severe COVID-19 had an outpatient claim with a COVID-19 diagnosis but no inpatient COVID-19 claims. Potential risk factors for severe COVID-19 included demographic characteristics, comorbid diagnoses, SLE medication usage, evidence of COVID-19 vaccination and the time period of COVID-19 diagnosis (second quarter (Q2) 2020 through Q4 2021).
Demographic risk factors were assessed on the index date and included age, sex, payer (Commercial, Medicare, Medicaid) and urban residence. US Census region was also assessed for Commercial and Medicare patients, and race/ethnicity was assessed among Medicaid patients. The 30 comorbidities included in the Elixhauser Comorbidity Index (ECI)18 were identified by an inpatient claim or a non-diagnostic outpatient claim with a diagnosis code for the condition during the 12-month baseline period (see online supplemental table 1 for the full list of ECI diagnoses). SLE medication usage was identified by National Drug Codes (NDC) on outpatient pharmacy claims or Healthcare Common Procedure Coding System codes on medical claims for physician-administered medications. The SLE medications assessed during the 6 months prior to patients’ earliest COVID-19 diagnosis included azathioprine, belimumab, cyclosporine, hydroxychloroquine, leflunomide, methotrexate, mycophenolate, rituximab, tacrolimus and voclosporin. Use of corticosteroids was assessed during the 30 days prior to COVID-19 diagnosis, including betamethasone, cortisone, dexamethasone, fludrocortisone, hydrocortisone, methylprednisolone, prednisolone, prednisone and triamcinolone. Evidence of COVID-19 vaccination was defined as a medical claim with a Current Procedural Terminology code for COVID-19 vaccine administration, or an NDC for a COVID-19 vaccine on an outpatient pharmacy claim during patients’ variable-length follow-up.
rmdopen-2023-003250supp002.pdf (603.9KB, pdf)
Statistical analyses
Descriptive statistics are reported for all study variables. Means and SDs are reported for age and compared between patients with SLE with and without severe COVID-19 using a t-test. Counts and proportions are reported for all other variables and compared between groups using χ2 or Fisher’s exact tests. Backward stepwise logistic regression was used to estimate adjusted OR and their 95% CIs for severe COVID-19 associated with patient demographic and clinical characteristics. Candidate variables for the model included all SLE medications listed above, all ECI diagnoses, age, sex, payer, urban residence, time period of COVID-19 diagnosis and evidence of COVID-19 vaccination. Interactions between vaccination and belimumab, rituximab, steroids, and certain baseline comorbidities known to be associated with risk of severe COVID-19 (diabetes, hypertension, obesity, renal failure)19–24 were also included as candidates in the stepwise regression. A full model was first constructed containing all candidate variables and interactions, then the least significant variable was removed, and the model was fit again. This process was repeated until all remaining variables in the model were significant (p<0.05). On review of the resulting model, a post hoc backward stepwise model was constructed, with the interactions between evidence of vaccination and use of corticosteroids, rituximab and belimumab forced into the model. This was done to fully explore the effect of vaccination specifically among patients with SLE with these particular treatments, as such prior work remains scarce.
Results
Patient characteristics
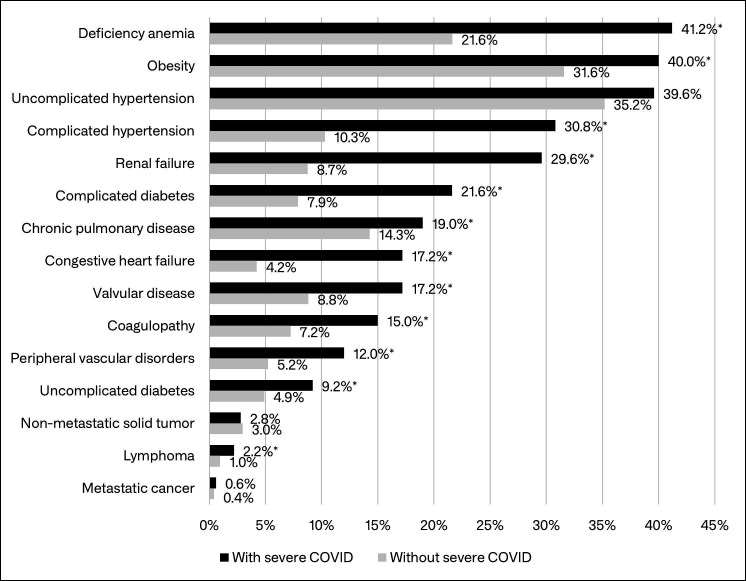
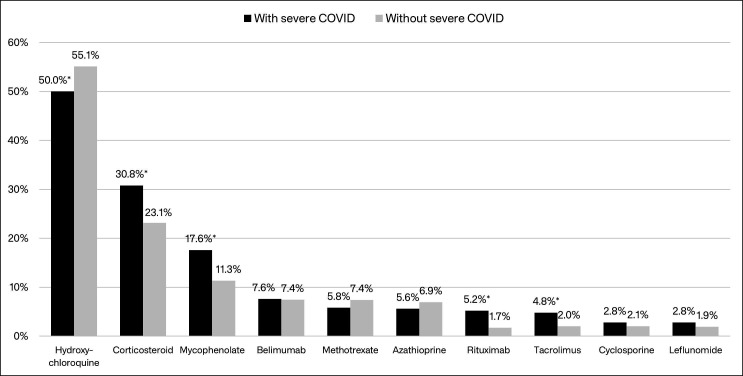
A total of 26 965 patients with confirmed SLE and at least 12 months of baseline data were identified in the MarketScan Databases; 2890 (10.7%) of those patients had a claim with a COVID-19 diagnosis and comprised the study cohort. Of the 2890 study patients, 500 (16.4%) had a COVID-19-related inpatient admission (ie, severe COVID-19) during follow-up. In unadjusted comparisons, patients whose COVID-19 required hospitalisation were about 3 years older, on average, more likely to be male, more likely to have Medicare or Medicaid insurance, and less likely to have evidence of vaccination, compared with those without severe COVID-19 (table 1). Among Medicaid patients for whom race information was available, patients with SLE with severe COVID-19 were more likely to be black than patients with SLE without severe COVID-19. Several comorbidities observed in the baseline period (12-month period before 1 April 2020) were significantly more common among patients with SLE with severe COVID-19, including congestive heart failure (17% vs 4%), renal failure (30% vs 9%), complicated hypertension (31% vs 10%), complicated diabetes (22% vs 8%), fluid and electrolyte disorders (33% vs 13%), peripheral vascular disorders (12% vs 5%) and coagulopathy (15% vs 7%) (figure 1; see online supplemental table 1 for results for all ECI diagnoses). Unadjusted comparisons also showed that COVID-19 positive hospitalised patients with SLE were less likely to have used hydroxychloroquine in the 6 months before their initial COVID-19 diagnosis (50% vs 55%), but more likely to have used mycophenolate (18% vs 11%), rituximab (5% vs 2%) and tacrolimus (5% vs 2%) (figure 2). Patients with severe COVID-19 were also more likely to have used corticosteroids in the 30 days before their initial COVID-19 diagnosis (31% vs 23%).
Table 1.
Demographic characteristics and vaccination status of all study patients, and those with and without severe COVID-19
| All patients with SLE with COVID-19 | Severe COVID-19 | No severe COVID-19 | P value | |
| N=2890 | N=500 | N=2390 | ||
| Age,* mean (SD) | 45.9 (12.1) | 48.6 (13.2) | 45.3 (11.8) | <0.001 |
| Age, N (%) | ||||
| 18–34 | 542 (18.8%) | 90 (18.0%) | 452 (18.9%) | <0.001 |
| 35–44 | 724 (25.1%) | 88 (17.6%) | 636 (26.6%) | |
| 45–54 | 876 (30.3%) | 142 (28.4%) | 734 (30.7%) | |
| 55–64 | 645 (22.3%) | 143 (28.6%) | 502 (21.0%) | |
| 65–74 | 63 (2.2%) | 23 (4.6%) | 40 (1.7%) | |
| 75+ | 40 (1.4%) | 14 (2.8%) | 26 (1.1%) | |
| Sex, N (%) | ||||
| Male | 208 (7.2%) | 60 (12.0%) | 148 (6.2%) | <0.001 |
| Female | 2682 (92.8%) | 440 (88.0%) | 2242 (93.8%) | |
| Payer, N, (%) | ||||
| Commercial | 1974 (68.3%) | 243 (48.6%) | 1731 (72.4%) | <0.001 |
| Medicare | 93 (3.2%) | 31 (6.2%) | 62 (2.6%) | |
| Medicaid | 823 (28.5%) | 226 (45.2%) | 597 (25.0%) | |
| Urban residence, N (%) | 2459 (85.1%) | 422 (84.4%) | 2037 (85.2%) | 0.636 |
| US region,† N (%) | ||||
| Northeast | 381 (18.4%) | 47 (17.2%) | 334 (18.6%) | 0.179 |
| Midwest | 353 (17.1%) | 61 (22.3%) | 292 (16.3%) | |
| South | 1137 (55.0%) | 140 (51.1%) | 997 (55.6%) | |
| West/unknown | 196 (9.4%) | 26 (9.5%) | 170 (9.5%) | |
| Race/ethnicity,‡ N (%) | ||||
| White | 272 (33.0%) | 51 (22.6%) | 221 (37.0%) | 0.001 |
| Black | 360 (43.7%) | 121 (53.5%) | 239 (40.0%) | |
| Hispanic | 68 (8.3%) | 21 (9.3%) | 47 (7.9%) | |
| Other/unknown | 123 (14.9%) | 33 (14.6%) | 90 (15.1%) | |
| Evidence of vaccination,§ N (%) | ||||
| Yes | 1016 (35.2%) | 128 (25.6%) | 888 (37.2%) | <0.001 |
| No | 1874 (64.8%) | 372 (74.4%) | 1502 (62.8%) |
*All demographic characteristics assessed on 1 April 2020.
†Region available for patients in the Commercial/Medicare database.
‡Race/ethnicity available for patients in the Medicaid database.
§Evidence of vaccination assessed during patients’ full variable-length follow-up period (1 April 2020 to earliest of death, end of database enrolment, 31 December 2021 (Commercial/Medicare), or 30 June 2021 (Medicaid)).
SLE, systemic lupus erythematosus.
Figure 1.
Presence of select Elixhauser Comorbidity Index diagnoses during the 12-month baseline period among patients with SLE with and without severe COVID-19. *Significantly different from patients without severe COVID-19 (p<0.05). See online supplemental table 1 for frequency of all Elixhauser Comorbidity Index diagnoses among patients with SLE with and without severe COVID-19. SLE, systemic lupus erythematosus.
Figure 2.
Presence of SLE treatments during the 6 months before the earliest COVID-19 diagnosis among patients with SLE with and without severe COVID-19. *Significantly different from patients without severe COVID-19 (p<0.05); voclosporin use was observed among two patients without severe COVID-19 (0.08%) and one patient with severe COVID-19 (0.2%). SLE, systemic lupus erythematosus.
Risk factors for severe COVID-19
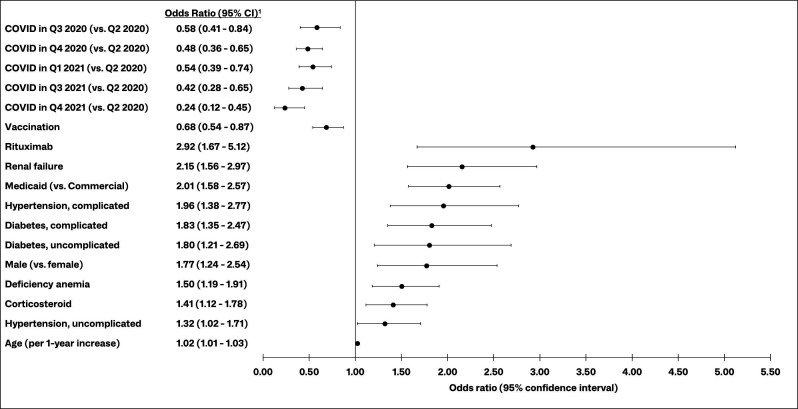
Results of backward stepwise logistic regression showed the time period of COVID-19 diagnosis to have the greatest impact on the odds of severe COVID-19 among patients with SLE with a COVID-19 diagnosis. The likelihood of severe COVID-19 generally decreased over time, as indicated by the significantly lower odds of severe COVID-19 in Q3–Q4 2020, Q1 2021 and Q3–Q4 2021, compared with Q2 2020 (figure 3). Notably, patients with SLE diagnosed with COVID-19 in Q4 2021 had 76% lower odds of severe COVID-19, compared with those diagnosed in Q2 2020 (OR (95% CI) 0.24 (0.12 to 0.45)).
Figure 3.
Risk factors for severe COVID-19 among patients with SLE with a COVID-19 diagnosis. 1Results of backward stepwise logistic regression; other candidate variables included other Elixhauser Comorbidity Index diagnoses (see online supplemental table 1), Medicare insurance (vs commercial), urban residence, COVID-19 diagnosis in Q2 2021 (vs Q2 2020), use of other SLE medications in the 6 months before COVID-19 diagnosis (azathioprine, belimumab, cyclosporine, hydroxychloroquine, leflunomide, methotrexate, mycophenolate, tacrolimus), interactions between vaccination and prior use of corticosteroids or rituximab, interactions between vaccination and select comorbidities (diabetes, hypertension, obesity, renal failure). Q, quarter; SLE, systemic lupus erythematosus.
Evidence of COVID-19 vaccination was associated with significantly lower odds of severe COVID-19 among patients with SLE. Those with evidence of COVID-19 vaccination had 32% lower odds of severe COVID-19, compared with those without evidence of vaccination (OR (95% CI) 0.68 (0.54 to 0.87)) (figure 3).
Rituximab use was the SLE treatment with the greatest impact on the odds of severe COVID-19 among patients with SLE (figure 3). Patients who used rituximab in the 6 months prior to COVID-19 diagnosis had nearly three times the odds of severe COVID-19 as those who did not use rituximab (OR (95% CI) 2.92 (1.67 to 5.12)). Additionally, corticosteroid users had 1.4 times the odds as those who did not use corticosteroids in the 30 days before COVID-19 diagnosis (OR (95% CI) 1.41 (1.12 to 1.78)).
As to comorbidity risk factors, renal failure was the strongest, more than doubling the odds of progression to severe COVID-19 (OR (95% CI) 2.15 (1.56 to 2.97)). Patients with diabetes or hypertension, with or without complications, had approximately 1.3–2.0 times the odds of severe COVID-19, compared with patients without those diagnoses, and those with anaemia had 1.5 times the odds (OR (95% CI) 1.50 (1.19 to 1.91)). Patients with SLE with Medicaid insurance had twice the odds of severe COVID-19, compared with commercially insured patients with SLE (OR (95% CI) 2.01 (1.58 to 2.97)). The odds of severe COVID-19 among males were about 1.8 times the odds among females (OR (95%) 1.77 (1.24 to 2.54)), and each additional year of age increased the odds of severe COVID-19 by 2% (OR (95% CI) 1.02 (1.01 to 1.03)). The full model containing all candidate variables and interactions yielded similar ORs for the significant predictors of severe COVID-19; no significant interactions were observed (online supplemental figure 1).
rmdopen-2023-003250supp001.pdf (570.7KB, pdf)
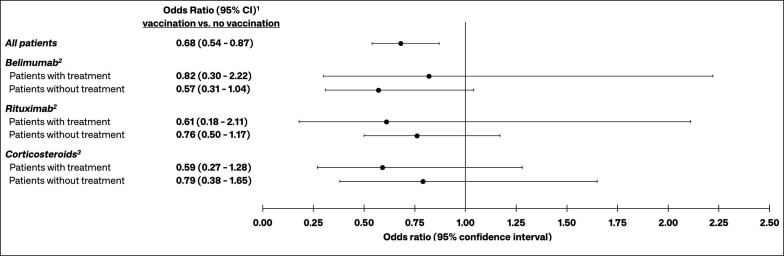
Given the significant effect on risk of severe COVID-19 observed for corticosteroids and rituximab, a post hoc backward stepwise model was constructed that examined the interactions of evidence of vaccination with corticosteroids and rituximab. The interaction between evidence of vaccination and belimumab use was also included in this model, since there is a paucity of data specifically examining the effect of belimumab use on vaccine effectiveness among patients with SLE. The results of this model showed that vaccination reduced the odds of severe COVID-19 among patients with and without these treatments, though none of these adjusted ORs were statistically significant (figure 4).
Figure 4.
ORs for the effect of vaccination on the risk of severe COVID-19 among patients with and without prior use of belimumab, rituximab or corticosteroids. 1Estimates from a backward stepwise logistic regression model containing the following variables: evidence of COVID-19 vaccination, belimumab use, rituximab use, corticosteroid use, diagnoses in the 12-month baseline period (deficiency anaemia, complicated and uncomplicated diabetes, complicated and uncomplicated hypertension, renal failure), age, sex, insurance type (Commercial, Medicare, Medicaid), time period of initial COVID-19 diagnosis, and interactions between COVID-19 vaccination and belimumab, rituximab and corticosteroids; 2Belimumab and rituximab use assessed in the 6 months prior to patients’ initial COVID-19 diagnosis; 3Corticosteroid use assessed in the 30 days prior to patients’ initial COVID-19 diagnosis.
Discussion
This retrospective analysis of a large administrative claims database identified the demographic, clinical and treatment risk factors associated with hospitalisation among patients with SLE diagnosed with COVID-19. While certain comorbidities and SLE treatments increased the likelihood of severe COVID-19, vaccination offered significant protection, as patients with evidence of COVID-19 vaccination had 32% lower odds of severe COVID-19, compared with patients without evidence of vaccination.
Our findings are consistent with prior analyses showing the risk of severe COVID-19 conferred by chronic comorbidities, immunosuppressant medications and other patient characteristics among patients with SLE and similar diseases. A small 2020 analysis showed the presence of one or more comorbidity and increased body mass index to be associated with increased risk of COVID-19 hospitalisation among patients with SLE.25 Though evidence of obesity as coded in healthcare claims was not associated with severe COVID-19 risk in our analysis, obesity-related comorbidities such as diabetes and hypertension were significant predictors of severe COVID-19. A 2022 analysis by Ugarte-Gil et al also observed elevated risk of severe COVID-19 associated with comorbidities (eg, renal failure, hypertension), timing of COVID-19 diagnosis, steroids and rituximab among patients with SLE.7 That analysis used data from the COVID-19 Global Rheumatology Alliance (C19-GRA) collected between March 2020 and June 2021 (prior to the delta variant), though it did not control for vaccination. Our finding of an increased risk of severe COVID-19 associated with anaemia is also consistent with prior studies.26 27
Gianfrancesco et al, reporting on C19-GRA data, observed twice the odds of COVID-19 hospitalisation associated with use of prednisone equivalent of ≥10 mg daily compared with no steroid use among patients with rheumatic diseases.6 Though we were unable to examine steroid dosage, our results show 41% greater odds of severe COVID-19 among patients who used steroids in the 30 days before their initial COVID-19 diagnosis, compared with those without evidence of recent steroid use. We also observed the odds of severe COVID-19 among patients with SLE who used rituximab within the prior 6 months to be 2.9 times that of those who did not use rituximab, a finding consistent with Avouac et al’s OR of 3.26 (95% CI 1.66 to 6.40) comparing patients with IMIDs who did and did not use rituximab.4
Multiple studies have demonstrated impaired response to COVID-19 vaccines among patients with IMIDs, including patients with SLE, receiving treatments such as rituximab,13 15 16 28 methotrexate,13 15 17 mycophenolate15 17 28 29 and glucocorticoids.15 28 29 However, prior examinations have shown no significant effect of belimumab use on vaccine response.13 29–31 A notable result from the current study is that adjusted ORs from a model including interactions between vaccination and certain SLE treatments showed the protective effect of vaccination against severe COVID-19 was not statistically significant among patients with SLE with COVID-19 who received belimumab, rituximab or corticosteroids.
Another notable result of this analysis is the significant drop in the odds of severe COVID-19 over time. Compared with patients with SLE diagnosed with COVID-19 in Q2 2020, those diagnosed in Q3 2020 had 42% lower odds of developing severe disease, and those diagnosed in Q4 2021 had 76% lower odds of developing severe COVID-19. This is similar to Ugarte-Gil’s observation of 50% and 60% lower odds of severe COVID-19 during Q3 2020 and Q4 2020–Q1 2021, respectively, compared with Q1–Q2 2020.7 This is likely the result of several factors, including changing variants, hybrid immunity, population uptake of vaccines, improved COVID-19 treatment regimens and other preventive measures implemented for vulnerable populations.
This analysis is the first large-scale real-world study to estimate the effect of vaccination on the risk of severe COVID-19 specifically among patients with SLE. A recent study of adolescents showed similar mRNA vaccine effectiveness between patients with and without inflammatory rheumatic diseases, including SLE, though they did not specifically assess the risk of severe COVID-19.32 Saxena et al observed fewer breakthrough COVID-19 infections among patients with SLE who received an additional vaccine dose compared with those who only received the primary vaccine series, though again, they did not assess the risk of severe COVID-19.33
Limitations
The results of this study should be interpreted in light of the following limitations. First, the identification of the underlying sample of COVID-19 cases was limited to patients with SLE with a medical claim containing a COVID-19 diagnosis; the frequency of COVID-19 infections that did not receive medical attention is unknown. For that reason, we assessed the risk of severe COVID-19 only among patients with SLE with a known COVID-19 diagnosis, rather than the risk of any COVID-19 (severe or otherwise) among all patients with SLE. It is highly likely that the number of known COVID-19 cases overall is underestimated, due in part to home testing and underreporting. Our study endpoint of severe COVID-19 required an inpatient claim with a COVID-19 diagnosis, which is a highly reliable method given that inpatient admissions will always be captured in healthcare claims.
Second, we considered a COVID-19 diagnosis code in any position (primary or secondary) on an inpatient claim to be evidence of severe disease. Some patients identified as having severe COVID-19 may have had a primary inpatient diagnosis of another condition but were also treated for COVID-19 during the same admission. Such patients may be considered as being hospitalised ‘with COVID-19’ rather than ‘for COVID-19’.
Third, our classification of patients’ vaccination status was limited to patients who had a medical or pharmacy claim for a COVID-19 vaccine or vaccine administration. An unknown number of patients with SLE are likely to have received a vaccine in a setting that did not generate an insurance claim (eg, mass vaccination site). Therefore, vaccination may offer even greater protection against progression to severe COVID-19 than observed in this study, since vaccinated patients are likely to have been classified as unvaccinated in this analysis. The same can be said regarding number of vaccines received, as our methods were unable to determine how many vaccines a patient received, and the vaccine series could have been incomplete for some, prior to COVID-19 diagnosis. Identification of patients who used various SLE medications was also limited to those with medical or pharmacy claims for those treatments; patients were assumed to take the medications as prescribed, though that cannot be confirmed, and medication dosage, including corticosteroid dose, could not be reliably assessed using claims data. We also limited our assessment of immunosuppressive agents to those more commonly used among patients with SLE. In addition, because race data are not available for Commercial and Medicare patients, race was not included in the multivariable models. There may be differences in variables not available or not controlled for in adjusted analyses (eg, race, socioeconomic status) between patients with SLE with and without severe COVID-19 that may contribute to the observed results. Finally, these results may not be generalisable to patients with SLE with insurance types other than those included in this study.
In conclusion, we observed significant effects of age, male sex, chronic comorbidities and immunosuppressive treatments on the risk of severe disease among patients with SLE diagnosed with COVID-19, and mitigation of severe COVID-19 risk by COVID-19 vaccination. These results also suggest that vaccine effectiveness against severe COVID-19 may be modified by use of certain immunosuppressive SLE treatments. These findings reinforce the importance of continued risk mitigation for patients with SLE against COVID-19, including non-pharmacological interventions (eg, masking) where appropriate, staying up to date with booster vaccines, appropriate use of preventative tools such as pre-exposure prophylaxis when available, and aggressive outpatient COVID-19 treatment.
Acknowledgments
Programming services were provided Helen Varker and Diana Stetsovsky of Merative. Statistical analysis services were provided Qiang Zhao of Merative. These services were paid for by AstraZeneca.
Footnotes
Twitter: @CCalabreseDO
Contributors: All authors contributed to the design and conduct of the study, as well as the writing and revision of the manuscript. KAE is the guarantor of this manuscript.
Funding: This study was funded by AstraZeneca.
Competing interests: S-JW and GA are employed by AstraZeneca. KAE, MM and LP are employed by Merative which received funding from AstraZeneca to conduct this study. CC is a paid consultant of AstraZeneca, as well as Eli Lilly, Pfizer and Sanofi. Results of this research have been presented in part at the 2022 American College of Rheumatology annual meeting (ACR Convergence) in Philadelphia, Pennsylvania, USA.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The data that support the findings of this study are available from Merative. Restrictions apply to the availability of these data, which were used under license for this study.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All database records are statistically deidentified and certified to be fully compliant with US patient confidentiality requirements set forth in the Health Insurance Portability and Accountability Act of 1996. Because this study used only deidentified patient records and did not involve the collection, use or transmittal of individually identifiable data, this study was exempted from institutional review board approval.
References
- 1.Centers for Disease Control and Prevention . COVID data Tracker. Available: https://covid.cdc.gov/covid-data-tracker/#datatracker-home [Accessed 06 Oct 2022].
- 2.Centers for Disease Control and Prevention . Estimated COVID-19 burden. Available: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html#est-infections [Accessed 06 Oct 2022].
- 3.Marozoff S, Lu N, Loree JM, et al. Severe COVID-19 outcomes among patients with autoimmune rheumatic diseases or transplantation: a population-based matched cohort study. BMJ Open 2022;12:e062404. 10.1136/bmjopen-2022-062404 [DOI] [Google Scholar]
- 4.Avouac J, Drumez E, Hachulla E, et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with Rituximab: a cohort study. Lancet Rheumatol 2021;3:e419–26. 10.1016/S2665-9913(21)00059-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertoglio IM, Valim JM de L, Daffre D, et al. Poor prognosis of COVID-19 acute respiratory distress syndrome in lupus erythematosus: nationwide cross-sectional population study of 252,119 patients. ACR Open Rheumatol 2021;3:804–11. 10.1002/acr2.11329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported Registry. Ann Rheum Dis 2020;79:859–66. 10.1136/annrheumdis-2020-217871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ugarte-Gil MF, Alarcón GS, Izadi Z, et al. Characteristics associated with poor COVID-19 outcomes in individuals with systemic lupus erythematosus: data from the COVID-19 global rheumatology alliance. Ann Rheum Dis 2022;81:970–8. 10.1136/annrheumdis-2021-221636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radwan NM, Mahmoud NE, Alfaifi AH, et al. Comorbidities and severity of Coronavirus disease 2019 patients. Saudi Med J 2020;41:1165–74. 10.15537/smj.2020.11.25454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Yang Q, Chi J, et al. Comorbidities and the risk of severe or fatal outcomes associated with Coronavirus disease 2019: a systematic review and meta-analysis. Int J Infect Dis 2020;99:47–56. 10.1016/j.ijid.2020.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mageau A, Ferré VM, Goulenok T, et al. Severely impaired humoral response against SARS-CoV-2 variants of concern following two doses of BNT162b2 vaccine in patients with systemic lupus erythematosus (SLE). Ann Rheum Dis 2022;81:1194–6. 10.1136/annrheumdis-2022-222498 [DOI] [PubMed] [Google Scholar]
- 11.Lee ARYB, Wong SY, Chai LYA, et al. Efficacy of COVID-19 vaccines in immunocompromised patients: systemic review and meta-analysis. BMJ 2022;376:e068632. 10.1136/bmj-2021-068632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun J, Zheng Q, Madhira V, et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med 2022;182:153–62. 10.1001/jamainternmed.2021.7024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ammitzbøll C, Bartels LE, Bøgh Andersen J, et al. Impaired antibody response to the BNT162b2 messenger RNA Coronavirus disease 2019 vaccine in patients with systemic lupus erythematosus and rheumatoid arthritis. ACR Open Rheumatol 2021;3:622–8. 10.1002/acr2.11299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izmirly PM, Kim MY, Samanovic M, et al. Evaluation of immune response and disease status in systemic lupus erythematosus patients following SARS-CoV-2 vaccination. Arthritis Rheumatol 2022;74:284–94. 10.1002/art.41937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferri C, Ursini F, Gragnani L, et al. Impaired Immunogenicity to COVID-19 vaccines in autoimmune systemic diseases. high prevalence of non-response in different patients' subgroups. J Autoimmun 2021;125:102744. 10.1016/j.jaut.2021.102744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calabrese CM, Kirchner E, Husni EM, et al. Breakthrough SARS-CoV-2 infections in immune mediated disease patients undergoing B cell depleting therapy: a retrospective cohort analysis. Arthritis Rheumatol 2022;74:1906–15. 10.1002/art.42287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moyon Q, Sterlin D, Miyara M, et al. BNT162b2 vaccine-induced humoral and cellular responses against SARS-CoV-2 variants in systemic lupus erythematosus. Ann Rheum Dis 2022;81:575–83. 10.1136/annrheumdis-2021-221097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore BJ, White S, Washington R, et al. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser comorbidity index. Med Care 2017;55:698–705. 10.1097/MLR.0000000000000735 [DOI] [PubMed] [Google Scholar]
- 19.Rey-Reñones C, Martinez-Torres S, Martín-Luján FM, et al. Type 2 diabetes mellitus and COVID-19: a narrative review. Biomedicines 2022;10:2089. 10.3390/biomedicines10092089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta P, Gasparyan AY, Zimba O, et al. Interplay of diabetes mellitus and rheumatic diseases amidst the COVID-19 pandemic: influence on the risk of infection, outcomes, and immune responses. Clin Rheumatol 2022;41:3897–913. 10.1007/s10067-022-06365-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebinger JE, Driver M, Joung S, et al. Hypertension and excess risk for severe COVID-19 illness despite booster vaccination. Hypertension 2022;79:e132–4. 10.1161/HYPERTENSIONAHA.122.19694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawadogo W, Tsegaye M, Gizaw A, et al. Overweight and obesity as risk factors for COVID-19-associated hospitalizations and death: a systematic review and meta-analysis. BMJ Nutr Prev Health 2022;5:10–8. 10.1136/bmjnph-2021-000375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancarevic I, Nassar M, Daoud A, et al. Mortality rate of COVID-19 infection in end stage kidney disease patients on maintenance hemodialysis: a systematic review and meta-analysis. World J Virol 2022;11:352–61. 10.5501/wjv.v11.i5.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh J, Malik P, Patel N, et al. Kidney disease and COVID-19 diseae severity - systematic review and meta-analysis. Clin Exp Med 2022;22:125–35. 10.1007/s10238-021-00715-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Ruiz R, Masson M, Kim MY, et al. Leveraging the United States epicenter to provide insights on COVID-19 in patients with systemic lupus erythematosus. Arthritis Rheumatol 2020;72:1971–80. 10.1002/art.41450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh SM, Skendelas JP, Macdonald E, et al. On-admission anemia predicts mortality in COVID-19 patients: a single center, retrospective cohort study. Am J Emerg Med 2021;48:140–7. 10.1016/j.ajem.2021.03.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao Z, Xu J, Chen W, et al. Anemia is associated with severe illness in COVID-19: a retrospective cohort study. J Med Virol 2021;93:1478–88. 10.1002/jmv.26444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. 10.1136/annrheumdis-2021-220647 [DOI] [PubMed] [Google Scholar]
- 29.Yuki EFN, Borba EF, Pasoto SG, et al. Impact of distinct therapies on antibody response to SARS-CoV-2 vaccine in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2022;74:562–71. 10.1002/acr.24824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boedecker-Lips SC, Claßen P, Kraus D, et al. Belimumab is not associated with COVID-19 mRNA vaccination failure in systemic lupus erythematosus. Rheumatology 2023;62:e34–5. 10.1093/rheumatology/keac459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiavoni I, Olivetta E, Natalucci F, et al. Evidence of immune response to BNT162b2 COVID-19 vaccine in systemic lupus erythematosus patients treated with belimumab. Lupus 2023;32:394–400. 10.1177/09612033221151012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziv A, Heshin-Bekenstein M, Haviv R, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine among adolescents with juvenile-onset inflammatory rheumatic diseases. Rheumatology 2023;62:SI145–51. 10.1093/rheumatology/keac408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saxena A, Engel AJ, Banbury B, et al. Breathrough SARS-CoV-2 infections, morbidity, and Seroreactivity following initial COVID-19 vaccination series and additional dose in patients with SLE in New York City. Lancet Rheumatol 2022;4:e582–5. 10.1016/S2665-9913(22)00190-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003250supp002.pdf (603.9KB, pdf)
rmdopen-2023-003250supp001.pdf (570.7KB, pdf)
Data Availability Statement
Data are available on reasonable request. The data that support the findings of this study are available from Merative. Restrictions apply to the availability of these data, which were used under license for this study.